Abstract
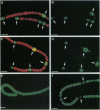
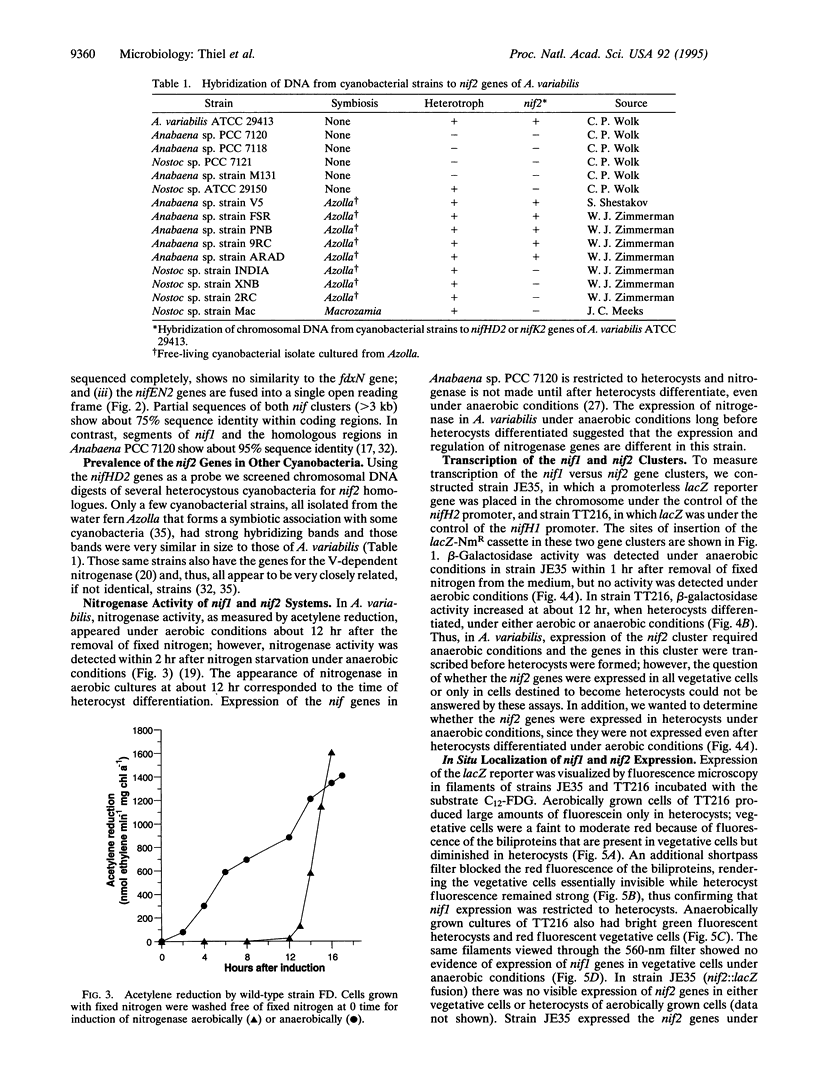
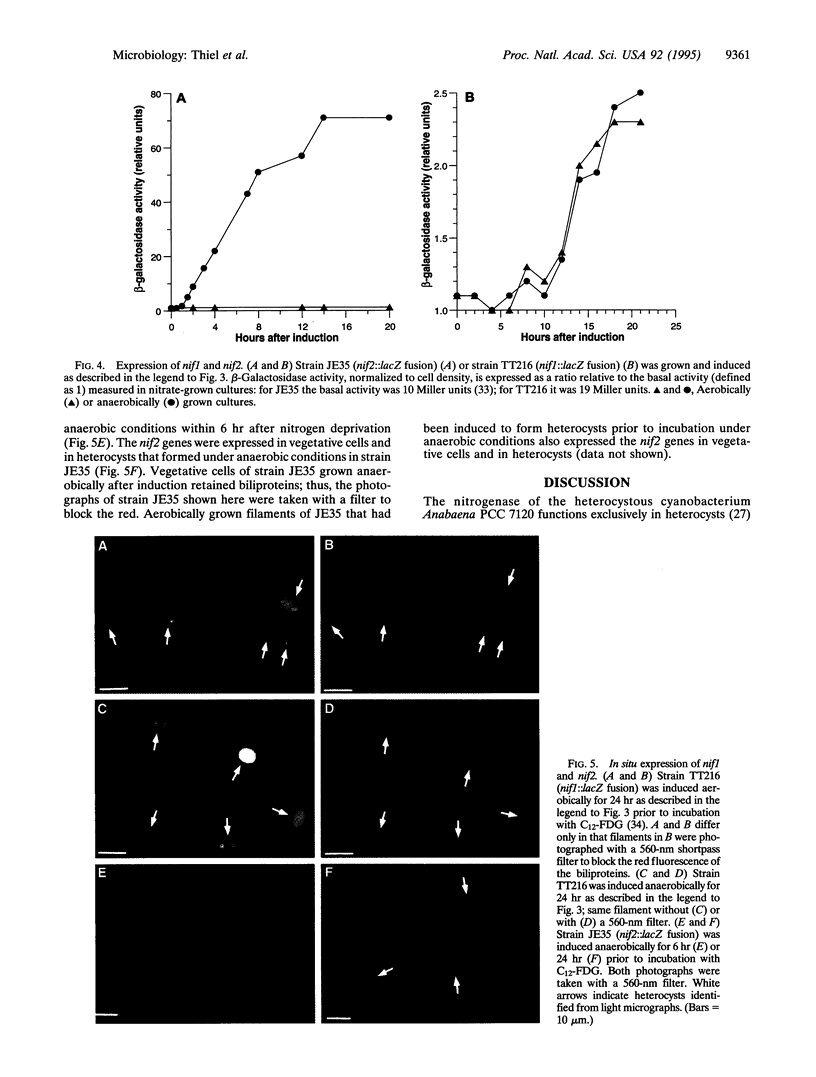
In many filamentous cyanobacteria nitrogen fixation occurs in differentiated cells called heterocysts. Filamentous strains that do not form heterocysts may fix nitrogen in vegetative cells, primarily under anaerobic conditions. We describe here two functional Mo-dependent nitrogenases in a single organism, the cyanobacterium Anabaena variabilis. Using a lacZ reporter with a fluorescent beta-galactoside substrate for in situ localization of gene expression, we have shown that the two clusters of nif genes are expressed independently. One nitrogenase functions only in heterocysts under either aerobic or anaerobic growth conditions, whereas the second nitrogenase functions only under anaerobic conditions in vegetative cells and heterocysts. Differences between the two nif clusters suggest that the nitrogenase that is expressed in heterocysts is developmentally regulated while the other is regulated by environmental factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black T. A., Cai Y., Wolk C. P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol Microbiol. 1993 Jul;9(1):77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Brusca J. S., Hale M. A., Carrasco C. D., Golden J. W. Excision of an 11-kilobase-pair DNA element from within the nifD gene in anabaena variabilis heterocysts. J Bacteriol. 1989 Aug;171(8):4138–4145. doi: 10.1128/jb.171.8.4138-4145.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund J. E., Zhang L., Haines M. A., Higgins M. L., Piggot P. J. Analysis by fluorescence microscopy of the development of compartment-specific gene expression during sporulation of Bacillus subtilis. J Bacteriol. 1994 May;176(10):2898–2905. doi: 10.1128/jb.176.10.2898-2905.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Wolk C. P. Characteristics of Anabaena variabilis influencing plaque formation by cyanophage N-1. J Bacteriol. 1979 Jul;139(1):88–92. doi: 10.1128/jb.139.1.88-92.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988 Aug 15;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990 Oct;9(10):3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992 Jun;56(2):340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Carrasco C. D., Mulligan M. E., Schneider G. J., Haselkorn R. Deletion of a 55-kilobase-pair DNA element from the chromosome during heterocyst differentiation of Anabaena sp. strain PCC 7120. J Bacteriol. 1988 Nov;170(11):5034–5041. doi: 10.1128/jb.170.11.5034-5041.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Mulligan M. E., Haselkorn R. Different recombination site specificity of two developmentally regulated genome rearrangements. Nature. 1987 Jun 11;327(6122):526–529. doi: 10.1038/327526a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Helber J. T., Johnson T. R., Yarbrough L. R., Hirschberg R. Regulation of nitrogenase gene expression in anaerobic cultures of Anabaena variabilis. J Bacteriol. 1988 Feb;170(2):552–557. doi: 10.1128/jb.170.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A., Wolk C. P. Genetic mapping of the chromosome of the cyanobacterium, Anabaena variabilis. Proximity of the structural genes for nitrogenase and ribulose-bisphosphate carboxylase. J Biol Chem. 1986 Jun 15;261(17):7748–7754. [PubMed] [Google Scholar]
- Lyons E. M., Thiel T. Characterization of nifB, nifS, and nifU genes in the cyanobacterium Anabaena variabilis: NifB is required for the vanadium-dependent nitrogenase. J Bacteriol. 1995 Mar;177(6):1570–1575. doi: 10.1128/jb.177.6.1570-1575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Joseph C. M., Haselkorn R. Organization of the nif genes in cyanobacteria in symbiotic association with Azolla and Anthoceros. Arch Microbiol. 1988 May;150(1):61–71. doi: 10.1007/BF00409719. [DOI] [PubMed] [Google Scholar]
- Parke D. Construction of mobilizable vectors derived from plasmids RP4, pUC18 and pUC19. Gene. 1990 Sep 1;93(1):135–137. doi: 10.1016/0378-1119(90)90147-j. [DOI] [PubMed] [Google Scholar]
- Thiel T., Bramble J., Rogers S. Optimum conditions for growth of cyanobacteria on solid media. FEMS Microbiol Lett. 1989 Oct 1;52(1-2):27–31. doi: 10.1016/0378-1097(89)90164-x. [DOI] [PubMed] [Google Scholar]
- Thiel T. Characterization of genes for an alternative nitrogenase in the cyanobacterium Anabaena variabilis. J Bacteriol. 1993 Oct;175(19):6276–6286. doi: 10.1128/jb.175.19.6276-6286.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Genetic analysis of cyanobacterial development. Curr Opin Genet Dev. 1991 Oct;1(3):336–341. doi: 10.1016/s0959-437x(05)80297-7. [DOI] [PubMed] [Google Scholar]