Abstract
Introduction
Muscle-directed gene therapy is rapidly gaining attention primarily because muscle is an easily accessible target tissue and is also associated with various severe genetic disorders. Localized and systemic delivery of recombinant adeno-associated virus (rAAV) vectors of several serotypes results in very efficient transduction of skeletal and cardiac muscles, which has been achieved in both small and large animals, as well as in humans. Muscle is the target tissue in gene therapy for many muscular dystrophy diseases, and may also be exploited as a biofactory to produce secretory factors for systemic disorders. Current limitations of using rAAVs for muscle gene transfer include vector size restriction, potential safety concerns such as off-target toxicity and the immunological barrier composing of pre-existing neutralizing antibodies and CD8+ T-cell response against AAV capsid in humans.
Areas covered
In this article, we will discuss basic AAV vector biology and its application in muscle-directed gene delivery, as well as potential strategies to overcome the aforementioned limitations of rAAV for further clinical application.
Expert opinion
Delivering therapeutic genes to large muscle mass in humans is arguably the most urgent unmet demand in treating diseases affecting muscle tissues throughout the whole body. Muscle-directed, rAAV-mediated gene transfer for expressing antibodies is a promising strategy to combat deadly infectious diseases. Developing strategies to circumvent the immune response following rAAV administration in humans will facilitate clinical application.
Keywords: adeno-associated virus, gene therapy, gene transfer, muscle
1. Introduction
Adeno-associated viruses (AAVs), a diverse collection of non-pathogenic, naturally replication-deficient parvoviruses, were originally isolated as contaminants in stocks of human and simian adenovirus [1]. Currently, 12 AAV serotypes and > 100 variants have been identified in human and nonhuman primate (NHP) populations [2]. The AAV is composed of a linear single-stranded (ss) DNA genome and a small non-enveloped capsid [3]. The AAV genome is ~ 4.7 kb long, and comprises the rep and cap genes flanked by two 145 bp long inverted terminal repeat (ITR) sequences on the ends. By utilizing two transcription initiation sites and alternative splicing, the rep gene dictates the expression of four Rep proteins (Rep78, Rep68, Rep 52 and Rep40) that are critical for the AAV life cycle. Expression of the cap gene is regulated by alternative splicing and different translation initiation sites, resulting in three capsid proteins (VP1, VP2 and VP3) that form an icosahedral capsid of ~ 3.9 kD [4]. In addition to Rep and capsid proteins, a nested and alternative open reading frame buried in the cap gene encodes the assembly-activating protein that is required for capsid formation [5]. The capsid crystal structures of the most popular AAVs have been determined [6–8]. The intact AAV capsid is ~ 26 nm in diameter and contains 60 capsid protein subunits at the ratio of 1:1:10 (VP1:VP2:VP3) [6]. Optimal AAV replication is dependent on a helper virus, such as adenovirus [9], herpes simplex virus [10] or vaccinia virus [11]. While in cell culture systems and in the absence of a helper virus, wild-type AAV (wtAAV) genome integrates into human chromosome 19q13 in a Rep protein-dependent manner [12] to establish a latent infection, no site-specific integration events have been identified in the animals manifesting natural infections of wtAAVs.
Since the wtAAV genome is capable of persisting in tissues for long durations without pathogenic effects, the use of recombinant AAV (rAAV) vectors as gene transfer vehicles has become popular [13,14]. Capsids of different AAV serotypes can package recombinant viral genomes flanked by AAV2 ITRs to form `pseudotyped' vectors, which have been extensively developed for different gene delivery applications [4]. The versatility and utility of rAAV vectors were further expanded by the natural or artificially evolved [15,16] diversity of AAV capsid proteins, which dictate the biological properties of rAAV such as cell or tissue tropism, biodistribution, host immune responses and so on. To bypass the rate-limiting step in rAAV-mediated transduction, that is, converting the single-stranded and transcriptionally inactive vector genome to a transcriptionally active double-stranded form, the self-complementary AAV (scAAV) vector containing double-stranded viral genome was developed, which can achieve much higher transduction efficiency in vivo compared with the conventional single-stranded AAV (ssAAV) vector [17,18]. rAAV vectors have been successfully used to transfer a variety of therapeutic genes into many cell types in vitro, and into different small and large animal models in vivo. Currently, rAAV vectors are being evaluated in Phase I/II clinical trials for gene therapy of a number of diseases such as Pompe disease [19], cystic fibrosis, α-1 anti-trypsin deficiency, Parkinson's disease, Batten's disease, muscular dystrophy, Leber's congenital amaurosis (LCA) and hemophilia [20]. Recently, the first human gene therapy drug Glybera® (uniQure Biopharma B.V., Meibergdreef, Amsterdam, The Netherlands) (a rAAV vector expressing lipoprotein lipase) was approved for the treatment of lipoprotein lipase deficiency (LPLD) in Europe [21].
rAAV vector is ideal for gene transfer directed to muscle tissues including skeletal muscle and cardiac muscle. rAAV genome predominantly exists as episomes after entering host cells, thus minimizing the risk of insertional mutagenesis. The episomal rAAV genomes concatemerize to form a stable circular configuration [22], allowing for potentially long-term gene expression in terminally differentiated tissues such as muscle. In addition, Kaeppel et al. recently demonstrated a largely random rAAV integration profile after intramuscular LPLD gene therapy, and concluded that rAAV integration is extremely rare and potentially safe [23]. rAAV vectors can be delivered into both skeletal and cardiac muscles in either localized or widespread manner. Multiple serotypes, such as rAAV1, 2, 5, 6, 7, 8 and 9 were developed for efficient muscle transduction [24–26]. Moreover, minimal immune toxicity of rAAV as compared with other viral vectors, such as adenovirus, retrovirus and herpes simplex virus, is a major advantage for rAAV gene therapy. Some devastating muscle diseases caused by lack of certain proteins are incurable with currently available treatments, such as Duchenne muscular dystrophy (DMD), limb girdle muscular dystrophies (LGMD) and Pompe disease. Using gene therapy strategy to reconstitute deficient muscle structural proteins or enzymes offers great hopes for a large population of patients [25,27,28]. In addition, muscle can be targeted as a biofactory to synthesize and secrete therapeutic agents to treat diabetes, atherosclerosis, hemophilia, cancer and infectious diseases [29]. Although successful animal studies have led to multiple promising clinical trials involving muscle-directed rAAV gene therapy (Table 1), it should be noted that hurdles toward broad clinical applications still exist, such as rAAV genome size limitation, potential toxic effects due to uncontrolled transgene expression, pre-existing neutralizing antibodies (NAbs) in the host, potential CD8+ T cell response induced by rAAV vectors and difficulty in delivering rAAV to large muscle mass in human. In this article, we will review basic rAAV vector biology pertaining to muscle-directed gene delivery, advantages and disadvantages of rAAV-mediated gene delivery to muscle, potential vector design strategies to overcome certain obstacles in different muscle gene therapy applications and immune biology of rAAV-mediated muscle gene transfer.
Table 1.
Muscle-directed gene transfer using rAAV vectors has been characterized in vertical translation studies from animal preclinical models to human clinical trials.
| Serotypes | Target issue/organ | Preclinical study in small animal models | Translational study in large animal models | Clinical trials |
|---|---|---|---|---|
| AAV1 | Skeletal muscle | Mouse [154] | Rabbit [157], cat [158] dog [36,96], NHP [32] | A1ATD [161], LGMD2D [162], LPLD [163] |
| Heart | Mouse [155] | Pig [159] | Heart failure [39] | |
| Rat [156] | Sheep [160] | |||
| Diaphragm | Mouse | Pompe disease [19] | ||
| AAV2 | Skeletal muscle | Mouse [164], Rat [165] | Dog [101], NHP [32] | A1ATD [169] |
| Hemophilia B [104] | ||||
| Heart | Mouse [155], Hamster [166] | Pig [168] | ||
| Rat [167] | ||||
| AAV5 | Skeletal muscle | Mouse [170] | ||
| Rat [171] | ||||
| AAV6 | Skeletal muscle | Mouse [172–174] | Dog [178] | |
| Heart | Mouse [175] | Dog [179, 180] | ||
| Rat [156, 176, 177] | Pig [181] | |||
| Sheep [182, 183] | ||||
| NHP [37] | ||||
| AAV7 | Skeletal muscle | Mouse [184, 185] | ||
| AAV8 | Skeletal muscle | Mouse [41, 186] | Dog [189, 190] | |
| Heart | Hamster [41] | NHP [191] | ||
| Mouse [41, 187] | NHP [37] | |||
| Hamster [41] | ||||
| Rat [188] | ||||
| AAV9 | Skeletal muscle | Mouse [42,47,192] | Dog [194] | |
| Heart | Mouse [43,47,56,188] | Dog [35] | ||
| Rat [188,193] | Pig [195] | |||
| NHP [37] | ||||
| AAV12 | Skeletal muscle | Mouse [196] | ||
| AAV2.5 | Skeletal muscle | DMD [34] |
A1ATD: α-1 anti-trypsin deficiency; AAV: Adeno-associated virus; DMD: Duchenne muscular dystrophy; LGMD2D: Limb-girdle muscular dystrophy type 2D; LPLD: Lipoprotein lipase deficiency; NHP: Nonhuman primate; rAAV: Recombinant adeno-associated virus.
2. rAAV vector delivery to muscle for gene transfer
rAAV vector of various serotypes have recently gained attention as potentially useful gene transfer vehicles, many of which can deliver therapeutic transgenes to cardiac and skeletal muscles (Table 1). Multiple routes of vector administration proved to achieve either localized or widespread muscle gene transfer, both of which have potential applications and drawbacks in clinical use. The biodistribution profile of recombinant viral genome following different routes of administration suggests possible sources of toxic effects following rAAV administration and potential ways to minimize them.
2.1 Routes of vector administration: localized versus systemic gene transfer to muscle
Route of administration significantly influences the biodistribution and transduction efficiency of rAAV vectors in vivo. Currently, many routes of rAAV administration can achieve either localized or systemic muscle gene transfer for different therapeutic purposes [24], such as intramuscular injection, retrograde transvenous limb perfusion, intracoronary infusion, intrapericardial injection, transendocardial injection, recirculating delivery, intraperitoneal injection and intravenous delivery (Table 2).
Table 2.
Routes of administration for muscle-directed rAAV gene transfer.
| Advantages | Disadvantages | Route | Serotypes | Preclinical and clinical applications | |
|---|---|---|---|---|---|
| Localized | Easy to administer High transduction in muscle | Not able to transduce large muscle mass | i.m. | AAV1 | A1ATD [161], DMD [36,154] Hemophilia B [96], LGMD2D [162], |
| Low off-target vector delivery | LPLD [163], XLMTM [197], Pompe disease [19] | ||||
| Low immune response | AAV2 | A1ATD [169], DMD [164], McArdle disease [198] | |||
| AAV6 | Hemophilia B [104] | ||||
| AAV7 | DMD [173,178], Pompe disease [172] | ||||
| AAV8 | Heart failure [176] | ||||
| AAV9 | Hemophilia B [185] | ||||
| DMD [189] | |||||
| CMD [192] | |||||
| t.v.p | AAV8 | DMD [190] | |||
| AAV2.5 | DMD [34] | ||||
| i.c.i | AAV1 | Heart failure [39] | |||
| AAV2 | Heart failure and LGMD [166] | ||||
| AAV6 | Heart failure [177] | ||||
| Myocardial infarction [182] | |||||
| AAV9 | Heart failure [195] | ||||
| r.c.d. | AAV1 | Heart failure [160] | |||
| AAV6 | Heart failure [183] | ||||
| t.e.c. | AAV6 | DMD [179], heart failure [180] | |||
| Systemic | Widespread transduction of large muscle mass, potentially the muscles of whole body | Off-target vector delivery Immune responses Need of high doses of vector Limited to certain rAAV serotypes |
i.v. | AAV6 | DMD [199], FSHD [174] |
| AAV8 | Heart failure [187], mouse [41] | ||||
| AAV9 | DMD [45], mouse [56], heart failure [193], myocardial infarction [200] | ||||
| i.p. | AAV8 | DMD [186] | |||
| AAV9 | LGMD [47] |
A1ATD: α-1 anti-trypsin deficiency; AAV: Adeno-associated virus; CMD: Congenital muscular dystrophies; DMD: Duchenne muscular dystrophy; FSHD: Facioscapulohumeral muscular dystrophy; i.c.L: Intracoronary infusion; LGMD2D: Limb-girdle muscular dystrophy type 2D; LGMD: Limb-girdle muscular dystrophy; LPLD: Lipoprotein lipase deficiency; rAAV: recombinant adeno-associated virus; r.c.d: Recirculating delivery; t.e.c: Transendocardial injection; t.v.p.: Transvenous limb perfusion; XLMTM: X-linked myotubular myopathy.
Direct intramuscular injection is a simple method that allows for local vector delivery to a targeted area of skeletal muscle. rAAV1 and rAAV2 are commonly used in directly targeting skeletal muscle for local gene transfer. Intramuscularly delivered rAAV vectors of other serotypes, such as rAAV6, 7, 8 and 9, can also lead to robust transgene expression in the injected muscle as well as some other tissues to which those vectors are spread via blood circulation (Table 1 and references therein). Since gene therapy for most muscle diseases requires whole-body muscle gene transfer, localized intramuscular vector delivery is not practical in these cases. However, if muscle-targeted gene transfer is used to produce secretory proteins, such as blood clotting factor VIII and IX, human α1 antitrypsin (A1AT) and erythropoietin (EPO) [30–32], intramuscular administration of vectors is indeed valuable. The second route for regional muscle delivery is retrograde transvenous limb perfusion, which targets a larger number of muscle groups. It delivers rAAV vectors to the entire muscle mass of the limb [33], which could be useful for the treatment of diseases that affect large muscle mass. This method of rAAV delivery has been used in clinical trials for muscle gene transfer to treat muscle diseases such as DMD [34]. Intrapericardial injection of rAAV is usually performed with neonatal animals, and can be used to transduce the heart and diaphragm simultaneously [24]. Transendocardial delivery of rAAV vector to achieve global cardiac gene transfer has been reported in canine models [35,36] and NHP [37]. Although the latter two approaches demonstrated proven practicability in animal models, no clinical applications have been reported. However, intracoronary infusion of rAAV vector to deliver therapeutic gene into the heart has been used in several clinical trials for the treatment of heart failure [38,39].
Systemic gene transfer to muscles by intravenous injection holds great advantages to treat diseases that disable nearly all muscle fibers throughout the body. This method not only avoids the requirement for multiple injections to saturate large muscles, but also potentially targets all the muscles of the body including cardiac muscle and diaphragm. Most of the popular rAAV serotypes have been evaluated for systemic muscle gene transfer via intravenous administration, many of which can transduce skeletal muscles under certain circumstances. For instance, with transient permeabilization of the peripheral microvasculature, rAAV6 leads to widespread skeletal muscle transgene expression after a single intravenous administration into mice [40]. Under unperturbed physiological conditions, rAAV8 and rAAV9 demonstrate the most prominent features relevant to therapeutic use among all serotypes examined so far. rAAV8 outperformed rAAVs 1, 2, 5 and 6 in achieving efficient and persistent transgene expression in skeletal muscle and heart following intravenous injection in both neonatal and adult mice [41]. After intravenous delivery, rAAV9 can efficiently transduce mouse skeletal muscles [42,43]. Moreover, rAAV9 following intravenous injection leads to robust and sustained whole body skeletal muscle transduction in neonatal dogs without pharmacological intervention or immune suppression [44]. The systemic muscle gene transfer by rAAV8 and rAAV9 vectors has shown great promise in the treatment of several muscle diseases in animal models, such as DMD [45,46], LGMD [47] and Pompe disease [48]. In addition, rAAV9 more efficiently transduces cardiac muscle than rAAV serotypes 4, 6, 7 and 8 after intravenous injection to mice, leading to the highest transgene expression in heart [49,50]. The intravenous delivery of rAAV9 was already used to treat heart diseases in animal models. For example, intravenous injection of rAAV9 vector was used to express microdystrophin in heart for ameliorating electrocardiographic abnormalities in mdx mice [51], and to transfer the delta-sarcoglycan gene in cardiac muscles for preventing cardiomyopathy in delta-sarcoglycan knockout mice [52]. In addition, intravenous administration of a rAAV9 vector in NHP preferentially transduced cardiac tissue [50]. These studies suggest that rAAV9 vector by intravenous administration can be a viable therapeutic approach for cardiac disorders. Importantly, intravenous injection of rAAV9 vector holds the potential to treat some muscle diseases such as DMD and Pompe disease, which affect both skeletal and cardiac muscles, and require correction in both organs to achieve maximal therapeutic benefit. However, it should be noted that rAAV9 vector delivered by intravenous injection in neonatal dogs surprisingly did not transduce heart [44], suggesting that unknown differences among species may influence transduction outcome following the same route of administration.
The intraperitoneal delivery of rAAV vectors can also be used for systemic gene transfer to muscle in animals, in which case rAAV8 and rAAV9 demonstrate advantages over other serotypes. Following intraperitoneal injection, rAAV8 is superior to rAAV1, 2, 5, 6 and 7 in crossing the blood vessel barrier, and in achieving systemic gene expression in skeletal and cardiac muscles in neonatal mice [41]. The transduction initiates as early as 3 days post injection and sustains up to adulthood in the vast majority of muscles [41]. In adult mice, rAAV8 by intraperitoneal injection also results in efficient skeletal and cardiac muscle transduction [53]. In addition, it was reported that the intraperitoneal delivery of rAAV9 vectors encoding shRNA to neonate mice efficiently silenced gene expression in skeletal and cardiac muscles [54].
Although the routes of intravenous and intraperitoneal administration of rAAV have achieved widespread gene transfer to muscles, complications also arose including off-target transduction of non-muscle tissues and immunotoxicity associated with the large vector doses [55]. These shortcomings may be overcome by using certain strategies such as the development of novel rAAV vectors with muscle-specific tropism, the use of muscle-specific promoters and the addition of microRNA binding sites to de-target transgene expression in non-muscle tissues and antigen presenting cells (see discussion below).
2.2 Biodistribution of rAAV genome after muscle-directed gene transfer
Characterization of rAAV genome biodistribution in vivo not only guides the optimization of muscle-directed vector development, but also provides insights into the potential sources of off-target toxicity, thus suggesting possible solutions to such toxic effects. The biodistribution pattern of rAAV genome after in vivo administration is mainly dependent on the route of administration and the serotype [49].
Following a direct intramuscular injection of rAAV of most serotypes, the rAAV genomes were found to be largely restricted within the injected region. However, some serotypes such as rAAV9 can achieve highly efficient widespread gene transfer after localized intramuscular injection (LZ & GG, unpublished data). After initially entering muscle cells, such vectors are able to transcytose through multiple layers, including the basal lamina and the endothelial cells lining blood essels. The vectors finally reach the bloodstream, and the circulatory system carries the vectors to the whole body resulting in widespread biodistribution such as in liver.
The intravenous and intraperitoneal routes of administration aiming at systemic muscle gene transfer inevitably lead to genome biodistribution in non-muscle tissues. The biodistribution of vector genome in mice after tail vein injection of rAAV1 – 9 was systemically studied [49]. Different AAV serotypes show distinct tissue distribution patterns after intravenous administration, although liver is the most common organ harboring a large amount of rAAV vectors. Regarding the most commonly used muscle-targeting rAAV8 and rAAV9 vectors by intravenous route, the highest vector genome copy numbers were observed in the liver by tail vein delivery into adult mice [56]. The skeletal and cardiac muscles, pancreas and adrenal gland are the next common tissues containing abundant rAAV genomes after peripheral vein injection of rAAV8 and rAAV9 vectors [49,56]. The intraperitoneal delivery of rAAV8 vectors to adult mice yielded slightly different biodistribution pattern compared with the intravenous route. While liver still contains the most abundant genome copies followed by abdominal muscle, pancreas shows higher genome biodistribution than arm muscle and heart [53].
3. rAAV vector design for muscle-directed gene therapy
Gene therapy to treat muscle diseases often requires the replacement of large genes, whereas the packaging capacity of rAAV vector is quite limited. In this regard, several strategies have been developed to deliver large therapeutic genes using rAAV vectors. After the transgene is successfully delivered into host, toxic effects may occur due to uncontrolled gene expression. In this case, expression of the rAAV transgene cassette has to be amenable to regulation for safe clinical use.
3.1 Vectorology to overcome size restriction of rAAV vectors
wtAAV contains an ssDNA viral genome that is ~ 4.7 kb long [3]. Similarly, the maximal size of viral genome including the gene expression cassette and ITRs encapsidated in an infectious rAAV particle should be no larger than 5 kb [57]. An intriguing study reported that AAV5 can encapsidate a viral genome up to 8.9 kb [58]. However, several groups were unsuccessful in reproducing the result and confirmed that the viral genomes encapsidated into AAV particles were truncated, thus supporting the fact that the size limitation of rAAV genome packaging is ~ 5 kb [59,60]. The size limit of the gene expression cassette in scAAV vector is further reduced to half, because a scAAV genome contains two complementary sequences of the same gene expression cassette. Since cDNAs of many therapeutic muscle proteins are large, devising strategies to deliver large transgenes using rAAV vector can significantly expand the clinical application of rAAV-mediated muscle gene therapy. To this end, multiple creative approaches involving both dual vector and single vector designs have been developed.
The most attractive strategy is dual vector design, which is based on the propensity of intermolecular recombination between two separate rAAV genomes. Four approaches are based on dual vector design: cis-activation [61], trans-splicing [62,63], overlapping [62] and hybrid dual vector (Figure 1) [64]. The first two approaches utilize ITR-mediated AAV genome intermolecular concatamerization; the overlapping method exploits homologous recombination of the overlapping regions in two separate rAAV genomes to rebuild a large full-length transgene; the hybrid dual vector design combines the trans-splicing and overlapping strategies, from which a large expression cassette can be reconstituted by both ITR-dependent and -independent mechanisms.
Figure 1. Schematics depicting four dual vector designs for delivering large genes using rAAV vector.
In all designs, two rAAV DNA genomes from separate vectors join together through either ITR-dependent or ITR-independent recombination. The recombined DNA is transcribed into pre-mRNA (not shown), which further undergoes splicing in trans-splicing and hybrid dual vector designs. The mature mRNA is translated into therapeutic proteins. See text for details.
 : ITR; E: Enhancer; I: Intron; ITR: Inverted terminal repeat; P: Promoter; pA: Polyadenylation signal; rAAV: recombinant adeno-associated virus; SA: Splicing acceptor; SD: Splicing donor; Tg: Transgene.
: ITR; E: Enhancer; I: Intron; ITR: Inverted terminal repeat; P: Promoter; pA: Polyadenylation signal; rAAV: recombinant adeno-associated virus; SA: Splicing acceptor; SD: Splicing donor; Tg: Transgene.
The cis-activation approach (Figure 1) was first reported by Duan et al., in which transgene and regulatory elements are packaged into two rAAV vectors, respectively [61]. After co-infection of host cells, the two rAAV genomes undergo ITR-dependent intermolecular recombination to form a large expression cassette with the regulatory elements joined with the transgene. This mechanism provides cis-activation of transgene expression in fibroblasts in vitro and in murine muscle in vivo [61]. This strategy is useful for rAAV gene delivery when the therapeutic transgene is already close to the maximum packaging capacity of AAV, while regulatory elements are still missing. However, this approach requires that the transgene itself is small enough for rAAV encapsidation. To deliver transgenes that already exceed the AAV packaging capacity, the trans-splicing approach was developed (Figure 1) [62,63]. Briefly, two separate rAAV vectors deliver two parts of a large transgene into target cells, one part containing a splicing donor signal and the other part harboring a splicing acceptor signal. Intermolecular recombination between the two vector genomes generates an intervening ITR junction, which is excluded by the cellular splicing mechanism to form a complete full-length transgene cassette. Initially the transduction efficiency of this approach was low. However, this system was dramatically improved later by overcoming a rate-limiting step in splicing, thus achieving robust transgene expression in murine muscles in vivo [65,66].
The overlapping approach (Figure 1) provides another option to deliver large therapeutic genes using rAAV vector [67]. In this design, a large transgene is split in such a way that the upstream and downstream parts contain an overlapping region. The two portions of the transgene are packaged into two separate rAAV vectors. After co-infection, homologous recombination between the shared regions occurs, regenerating a complete full-length transgene cassette. Ghosh et al. showed that rAAV6 overlapping vectors for alkaline phosphatase gene achieved transduction efficiency as high as 96% of that of the intact gene vector in mouse skeletal muscle [68]. Using overlapping rAAV6 vectors, Odom et al. successfully delivered a 7.3 kb cassette expressing minidystrophin protein in the mdx mice [69]. More recently, this approach was highlighted in a retinal gene therapy trial to deliver a 7.9 kb MYO7A cDNA with AAV2 and AAV5 single vectors for the treatment of Usher 1 patients [70]. Although great progress has been made, limitations of the aforementioned approaches should also be kept in mind. For example, an optimal gene-splitting site for efficient splicing is required for the trans-splicing approach, and a highly recombinogenic region within the transgene is a prerequisite for the overlapping approach. To deliver large transgenes lacking these features, a hybrid dual vector system combining these two approaches was established, which significantly outperformed the other dual vector systems in mouse muscle in vivo [64]. In this system, more efficient transgene reconstitution can be achieved through both ITR-dependent and intron-dependent recombination followed by splicing (Figure 1). This novel strategy is not restricted to certain sequence-related features in transgenes such as an optimal gene-splitting site or a highly recombinogenic region, thus allowing for more flexible vector designs. Zhang et al. used the dual vector system to efficiently deliver a mini-dystrophin gene [71] and a neuronal nitric oxide synthase gene [72] to mouse muscle, respectively, both of which are about 6 kb long.
Besides the dual vector approach, some strategies utilizing single rAAV vectors can also expand encapsidation capacity. The AAV packaging capacity is influenced by the capsid structure. For example, most residues of the capsid protein VP2 N terminus localize inside AAV virions [6,73], which may reduce AAV packaging capacity. It is well known that VP2 plays a minimum role in AAV transduction, and infectious VP2-null AAV vectors can be packaged efficiently [73]. Therefore, deletion of VP2 may create more space for AAV packaging without affecting transduction capability. This hypothesis was confirmed by several studies [60,74]. A viral genome up to 6 kb can be successfully encapsidated in the VP2-null rAAV serotype 1 – 5 vectors [74]. A 5.8 kb genome was also packaged efficiently into the VP2-null rAAV6 vector, which achieved moderate transgene expression in mouse muscle in vivo [75]. These studies suggest that the VP2-null rAAV vectors can potentially deliver certain large genes for muscle gene therapy.
3.2 Regulated expression after in vivo transgene delivery
Uncontrolled transgene expression in off-target tissue types or prolonged transgene expression in target tissue may lead to toxicity. A recent study aimed at treating limb girdle muscular dystrophy type 2A (LGMD2A) exemplified such a problem. Briefly, LGMD2A is a skeletal muscle disease due to loss of calpain-3, an intracellular protease encoded by the CAPN3 gene that is predominantly expressed in skeletal muscle. The intravenous injection of a rAAV9 vector containing CAPN3 driven by a pan-muscle-specific promoter in mice caused severe fibrosis in heart and mortality, which was related to the transduction in heart and unregulated proteolytic activity of calpain-3 therein [76]. To prevent the detrimental effects related to both off-target and prolonged transgene expression, a number of strategies have been developed to regulate gene expression at both transcriptional and post-transcriptional levels.
First, utilizing tissue-specific promoters to drive transcription is a straightforward way to avoid off-target transgene expression in undesired tissue/cell types. There are several well-characterized muscle-specific promoters amenable to the limited packaging capacity of rAAV vector, such as the desmin promoter [77] and muscle creatine kinase (MCK) promoter [78]. For example, promoters derived from the regulatory regions of murine MCK gene can yield therapeutic levels of gamma-sarcoglycan [79] and show microdystrophin [40] expression in skeletal muscles of two muscular dystrophy mouse models, respectively. These MCK promoters exhibit strong activity in skeletal muscle but not in cardiac muscle [80]. To target both skeletal and cardiac muscle involvement in diseases such as DMD and Pompe disease, promoters driving high-level transgene expression in both muscle types were successfully developed [80]. One of these promoters, hybrid alpha-myosin heavy chain enhancer-/MCK enhancer-promoter (MHCK7), was assessed in a later study involving Pompe disease in mice [48]. Pompe disease is caused by deficiency in acid alpha-glucosidase (GAA) and lysosomal accumulation of glycogen in both cardiac and skeletal muscle cells. rAAV vectors expressing GAA under the control of MHCK7 promoter reduced glycogen content in both muscle types to a greater extent than that using the original MCK promoter [48].
Second, transgene expression in off-target tissues can also be prevented at the post-transcriptional level. MicroRNAs (miRNAs) are a class of ~ 22 nt long small RNAs with broad and potent effect on gene expression. Most often, they down-regulate gene expression by binding to the 3′-untranslated region (3'-UTR) of mRNAs, and triggering mRNA degradation and/or translation inhibition. There are miRNA species whose expression shows a highly tissue/cell type-specific pattern. For example, miR-122 and miR-142-3p are predominantly found in liver and macrophage, respectively [81,82]. Therefore, to prevent undesired tissue/cell types from expressing transgene, one can design the 3′-UTR of transgene to carry the target sequence of miRNAs that are expressed specifically in that tissue/cell type. The first proof-of-concept study of such an endogenous miRNA-based de-targeting strategy was reported by Brown et al. [82]. In this study, the authors constructed a lentiviral vector consisting of a green fluorescent protein (GFP) expression cassette with miRNA-142-3p target sequence incorporated in the 3′-UTR. This vector design suppressed GFP expression in antigen-presenting cells (APCs) where endogenous miRNA-142-3p is expressed, which avoided immune-mediated vector clearance and maintained stable gene expression in other cell types. The same strategy has been adopted in rAAV-mediated gene transfer studies. For example, rAAV vectors incorporating target sequences of miR-142-3p [83] and miR-122 [84] in 3′-UTRs have been shown to effectively reduce transgene expression in APCs and liver, respectively, whereas transgene expression from muscle tissue was maintained. Importantly, the endogenous miRNA expression profile is not detectably perturbed due to ectopic miRNA target sequences [84]. Tissue-specific promoter and miRNA-based de-targeting design can be combined to further restrict transgene expression in muscle. In the LGMD2A study described at the beginning of this section, the authors developed new rAAV vectors that carry skeletal muscle-specific promoters and cardiac-specific miRNA-208a target sequence [76]. This vector design successfully suppressed calpain-3 expression in heart, thus preventing cardiac toxicity, and resulted in the correction of LGMD2A disease phenotype in skeletal muscle.
Third, inducible promoters responsive to pharmacologic regulators provide a flexible toolkit to control the level of transgene expression not only in a temporally on-demand fashion, but also within a certain therapeutic window. The most widely used is the tetracycline-regulated system, which is derived from prokaryotes and renders bacteria resistant to the antibiotic tetracycline [85]. Other inducible transcription systems include, but are not limited to, promoters induced by the antibiotic rapamycin [86] and the insect steroid hormone ecdysone [87]. Detailed mechanisms of these and other inducible gene expression designs were recently reviewed in Jazwa et al. [88]. Such inducible promoters proved to be effective in controlling rAAV-mediated, muscle-directed gene transfer to express secretory proteins such as EPO [32,85], growth hormone [86] and IL-10 [89]. Here we provide an example to highlight the necessity and value of utilizing an inducible promoter in the context of rAAV gene therapy targeting muscle as a biofactory.
β-thalassemia is a hemolytic disease caused by a deficiency in β-globin that pairs with α- globin in adult human. This leads to excess unpaired α-globin and its toxic aggregation in red blood cells. Patients suffer from hemolytic anemia, which often leads to death [90]. One approach to treating this disease is to reactivate the expression of fetal hemoglobin, which can pair with α-globin and neutralize the imbalance caused by the lack of β-globin. The secretory protein erythropoietin (EPO at high doses is capable of stimulating fetal hemoglobin expression in baboons [91], and studies have shown that EPO overexpression in the bloodstream can alleviate beta-thalassemia phenotype. For example, β-thalassemic mice treated with viral vector-mediated Epo gene transfer showed an improvement in hematologic abnormalities [92]. However, uncontrolled overexpression of EPO in mice was accompanied by severe and lethal polycythemia (overproduction of red blood cells) [93]. Intramuscular injection of an rAAV vector constitutively expressing Epo resulted in high level of Epo in serum, and 7 of 10 mice died within 10 weeks post injection [93]. To overcome this side effect, Johnston et al. devised an rAAV gene therapy strategy consisting of two rAAV vectors for expressing inducible Epo (Figure 2) [93]. In this design, one rAAV vector expresses two chimeric proteins that dimerize in the presence of rapamycin to form an active transcription factor. The second rAAV vector carries Epo gene driven by a promoter that is specifically recognized by the dimerized, active transcription factor. These two rAAV vectors were co-injected intramuscularly into beta-thalassemic mice. In the absence of rapamycin induction, baseline Epo expression was negligible and hematologic abnormalities in these mice were unchanged. Transient induction by weekly high doses of rapamycin for 7 weeks correlated with high level of Epo secretion into circulation, along with severe polycythemia and mortality in 5 of 13 animals. In contrast, when the mice surviving the high-dose rapamycin induction were treated with weekly low doses of rapamycin for 7 weeks, abnormal hematologic characteristics associated with thalassemia were improved without additional mortality (except for one mouse euthanized due to a skin condition). This study illustrated such a scenario that a pharmacologically inducible system can yield therapeutic benefit, while avoiding the side effects caused by uncontrolled, prolonged transgene expression.
Figure 2. Rapacymin-mediated inducible gene expression system.
The viral genome delivered by Vector 1 encodes the fusion proteins FRB-p65 and FKBP-ZFHD1, which dimerize in the presence of rapamycin. ZFHD1 tethers the dimerized transcription factor to the ZFHD1 DNA binding domains (BD) in the viral genome of Vector 2, when p65 activates promoter and Epo expression.
CMV: Cytomegalovirus promoter; E: Epitope tag; FKBP: FK-binding protein; FRB: Rapamycin binding domain of FKBP-rapamycin associated protein; IRES: Internal ribosome entry site; minP IL-2: Minimal IL-2 promoter; N: Nuclear localization signal; pA: Polyadenylation signal; ZFHD1: Zinc finger homeodomain 1.
It should be noted that translation of these regulatable systems to human use suffers from several obstacles. For example, the bacteria-derived tetracycline regulator is a potential antigen in human; the use of antibiotics such as tetracycline and rapamycin has to be strictly regulated to prevent antibiotic misuse; the immune suppression capacity of rapamycin may complicate treatment. Developing safe regulatable gene expression systems for human use will greatly expedite the translation of gene therapy to the clinic.
4. Muscle as a biofactory to produce secretory therapeutic agents
Skeletal muscle has been targeted in gene therapy not only for muscle diseases, but also as a biofactory to produce and secrete therapeutic proteins for the treatment of diseases in trans in distant organs. After rAAV transduction, muscle cells have the ability to express recombinant proteins with bona fide post-translational modifications and to secrete these proteins into the bloodstream for whole-body delivery. These characteristics of muscle tissue make it a versatile platform to develop gene therapy strategies for delivering genes that are defective in diseases [94–96], for expressing immunoadhesins and antibodies to combat infections [97–99] and for supplementing cytokine IL-10 with broad anti-inflammatory effects [29].
Kessler et al. first demonstrated the concept that intramuscular administration of rAAV vector can result in secretion of functional proteins [100]. In this study, a rAAV vector containing a gene encoding human EPO, a hormone that stimulates erythropoiesis, was delivered into adult BALB/c mice through a single intramuscular injection. Dose-dependent secretion of EPO into serum and corresponding increases in red blood cell production persisted for ≤ 40 weeks. Shortly after this proof-of-concept study, intramuscular injection of rAAV vector to express factor IX (F.IX) in both immunodeficient Rag1 mice [94] and canine models [101] of hemophilia B proved to yield sustained F.IX secretion into plasma at therapeutic levels. The bleeding diathesis in the treated hemophilia B dogs was ameliorated [101]. F.IX is normally synthesized in liver, where it also undergoes post-translational modifications that are important for its biological properties. Arruda et al. demonstrated that myotube-synthesized F.IX has similar post-translational modifications as those of liver-derived F.IX, such as cleavage of the propeptide and γ-carboxylation of the N-terminal glutamic acid residues. Importantly, both forms have comparable specific activity [102]. These and other [103] pre-clinical studies fostered a Phase I clinical trial involving severe hemophilia B patients with missense mutations in the gene encoding F.IX [104]. Multiple intramuscular injections of rAAV expressing F.IX resulted in detectable gene expression at injection sites, but circulating F.IX levels did not reach the target therapeutic level of > 1% of normal. Despite the limited efficacy from the first clinical trial, recent pre-clinical studies suggested ways to improve muscle-directed rAAV-F.IX gene therapy. For example, Arruda et al. adopted an intravenous route to achieve widespread muscular transduction in hemophilia B dogs, which resulted in circulating F.IX levels tenfold higher than those achieved by direct intramuscular administration [105]. Along with higher expression levels of F.IX, immune response against the transgene becomes a bottleneck of achieving high gene therapy efficacy, which will be discussed in Section 5.
Expressing immunoadhesins (antibody-like molecules) and antibodies in muscle tissue for systemic delivery through the circulatory system recently emerged as a novel approach to battling against infectious diseases. Lewis et al. injected immunodeficient Rag1 mice with a rAAV2 vector containing the gene encoding a human broad HIV-neutralizing antibody IgG1b12 [106]. After a single intramuscular injection, biologically active IgG1b12 was detected in mouse serum and persisted for > 6 months. In their follow-up study in the NHP model of simian immunodeficiency virus (SIV) infection, genes encoding chimeric immunoadhesin molecules that are based on broad SIV-neutralizing antibodies or CD4 binding regions of SIV [97] were constructed and packaged into rAAV1 vectors, followed by intramuscular injections into rhesus macaques. This approach generated sustained neutralizing activity in serum for ≥ 1 year, and the immunized monkeys were protected against aggressive intravenous challenge with SIV. However, immunoadhesin-specific antibodies were detected in 3 out of 9 immunized monkeys, which proved to correlate with poor protection. To circumvent immune response against immunoadhesins (i.e., anti-antibody response) and to achieve higher protective antibody production from transduced muscles, Balazs et al. packaged individual genes encoding full-length human broad HIV-neutralizing antibodies into rAAV8 vectors [98]. After a single intramuscular injection into mice with humanized immunity, high concentrations of secreted antibodies were detected in serum, and both immuno-competent and immunodeficient mice were fully protected when challenged intravenously with lethal doses of virulent HIV. The same strategy, termed vectored immunoprophylaxis (VIP), was recently reported to be successful in protecting humanized mice from influenza [99]. Together, these studies demonstrated a host immune system-independent approach to providing protection against infectious diseases. Based on these encouraging pre-clinical studies, two separate Phase I clinical trials using muscle-targeted rAAV vectors to express HIV-neutralizing antibodies are currently under development [107].
IL-10 is a pleiotropic immunoregulator with potent anti-inflammatory activity. In rodent models, intramuscular injection of rAAV vectors expressing IL-10 results in high levels of IL-10 in circulation, which dampens inflammation under several disease conditions, including atherosclerosis in apolipo-protein E-deficient mice, monocrotaline-induced pulmonary arterial hypertension in rats, hypertensive organ damage in Dahl salt-sensitive rats [29] and allergic response in a cystic fibrosis (CF) mouse model [108]. However, the CF mice injected with rAAV1-IL-10 showed thrombocytopenia and splenomegaly due to prolonged secretion of IL-10 from transduced muscle tissue [108]. The side effects of long-term transgene expression emphasize the necessity to utilize controllable rAAV gene therapy strategies.
5. To overcome immunological hurdles towards successful muscle-directed rAAV gene transfer
Translation of rAAV gene therapy from animal study to clinical trial unexpectedly revealed that immunotoxicity in humans is a major obstacle towards successful treatment [109]. Three components in rAAV administration are potential antigens, namely, the recombinant viral genome, the transgene product and the capsid protein. These factors interact with the human immune system in multiple facets, posing a significant barrier to achieving high transduction efficiency and long-term therapeutic efficacy. Current research focuses heavily on elucidating how rAAV administration shapes host immune responses, and identifying viable approaches to bypassing the destructive reaction.
5.1 Immune biology of muscle
Skeletal muscle is the largest cellular compartment of human body in mass and can actively participate in many local immune reactions [110]. Human myoblasts and myotubes are capable of expressing various immunophenotypes, including MHC and costimulatory molecules [111]. An emerging body of evidence suggests that muscle fibers actively generate a local inflammatory milieu by secreting proinflammatory cytokines [112]. Human myotubes express various chemokines such as CXCL8, CXCL9 and CXCL10 as well as the β-chemokines CCL3, CCL20 and CCL5 in response to cytokines, notably IFN-γ [112]. Myoblasts can present exogenous and endogenous antigens through MHC molecules, leading to the stimulation of antigen-specific CD4+ T cells [113]. Therefore, muscle cells are considered as facultative antigen-presenting cells (APCs). Additionally, CD8+ T cells can recognize muscle cells in an MHC I-restricted fashion [114].
Consistent with the muscle's ability to generate immunological reactions, muscle-directed rAAV gene transfer has met with several immune obstacles including both transgene-and capsid-induced immune responses. For example, intramuscular injection with high titer rAAV2 vectors showed long-term expression of hF.IX in SCID mice; however, it was not seen in immune-competent mice due to the development of anti-hF.IX antibodies [94,115]. In addition, pre-existing NAbs against AAV capsid can limit transduction efficiency in the case of systemic rAAV delivery to muscle [116]. High prevalence of pre-existing anti-AAV capsid immunity, memory T cell effect and transgene immunity in humans are among the major obstacles that must be addressed for broad clinical application of muscle-directed rAAV gene therapy.
5.2 Pre-existing antibodies to AAV capsid
Prior exposure to wtAAVs during childhood is prevalent, which results in NAbs against rAAV vectors in global human populations [117]. The seroprevalence of different AAV serotypes is varied [118], with NAbs to AAV2 and AAV1 being the most abundant and detected in 2 of 3 among the healthy population [119]. The circulating immunoglobulin raised from natural wtAAV infection can neutralize rAAV vectors of various serotypes, thus compromising or even preventing in vivo gene transfer. Although NAbs do not always cause problems in some cases of localized rAAV administration such as intramuscular injection [95], they clearly impede gene transfer through intravascular route such as intravenous injection [120]. The effect of NAb was experimentally evaluated in SCID mice with adoptive transfer of pooled human immunoglobulin of pre-calculated titers, and challenged with a rAAV2-F.IX vector [121]. In this study, AAV2 NAb titers higher than 1:10 sufficiently abolished F.IX expression following systemic delivery of rAAV2 vectors at the dose of 2.0 × 1012 viral genomes per kilogram body weight (vg/kg). In another NHP study, an AAV8 NAb titer as low as 1:5 resulted in complete absence of transgene expression as well as vector DNA in all tissues examined after 5 ± 1012 vg/kg rAAV8-F.IX administration via the hepatic artery [122]. In contrast, animals with NAb titer < 1:1 were effectively transduced by the same vector dose [122].
Simply excluding AAV seropositive patients from treatment is not an ideal approach to avoiding NAbs, since NAbs are highly prevalent in humans. In addition, NAbs can cross-react with multiple AAV serotypes, rendering serotype switch inefficient in escaping NAbs [118]. However, chemical modification [123] and genetic engineering of capsid [124] have been shown to partially protect rAAV vector from neutralization. Circulating NAbs can be reduced to some extent by plasmapheresis [125], or separation from administered rAAV vector by balloon catheterization with saline flushing [126]. However, these two methods only proved to be effective in dealing with low-to-moderate NAb titers. Recently, Mingozzi et al. reported using excess empty capsid decoys to absorb NAbs and to overcome their inhibitory effect in both mouse and NHP [127]. In the same study, they further created a mutant AAV2 empty capsid that retained NAb binding capability but failed to enter target cells, thus improving the safety of this approach by reducing capsid antigen load.
5.3 Cytolytic T-cell response against rAAV capsid
In humans, initial infection with wtAAV takes place in the context of a helper virus coinfection such as adenovirus, which causes a robust inflammatory response likely initiating the formation of memory CD8+ T cells to both AAV and adenovirus. In support of the presence of capsid-specific memory CD8+ T cells in general population, previous studies have shown that AAV2 capsid-specific CD8+ T cells in healthy donors are capable of quick expansion and mediating potent cytolytic activity against cells displaying the capsid antigen [109]. After in vitro expansion with capsid antigen, 60% of splenocyte samples isolated from healthy donors were positive for T-cell responses to AAV2 [128], suggesting the high prevalence of CD8+ T cells due to wtAAV infection. Upon re-exposure to capsid during rAAV gene transfer in vivo, these memory CD8+ T cells are activated to expand and eliminate transduced cells that display rAAV capsid epitopes on MHC I in a vector dose-dependent manner. Such a CD8+ T-cell response has been observed in several clinical trials, including the liver-directed hemophilia trials [95,129], muscle-directed LPLD trial [130] and DMD trial [131]. Recently, Martino et al. established a mouse model to test the effect of capsid-specific CD8+ T cells on therapeutic gene expression and provided direct evidence showing that the CD8+ T-cell response eliminates transduced hepatocytes in mice, resulting in reduced transgene expression [132].
CD8+ T cells specifically recognize cells flagged by MHC I antigen presentation for destruction. In target cells such as hepatocytes and muscle fibers, the capsid epitopes likely reach the MHC I molecules via cross-presentation [133], which occurs when rAAV capsid escapes endosome and gets degraded by proteasome (Figure 3). This is evident by observing that AAV capsids undergo ubiquitination, a process that may target exogenous capsids to proteasomal degradation followed by MHC I presentation [134]. Furthermore, Pien et al. demonstrated the presence of capsid epitope-MHC I complexes on the surface of AAV-transduced hepatocytes by confocal microscopy [135]. Recently, Samulski and colleagues demonstrated in vitro that capsid antigen presentation is dependent on endosomal escape in AAV-permissive cells [136]. Together, these studies suggest that preventing capsid endosomal escape and/or proteasomal degradation may reduce capsid antigen cross-presentation, dampen T-cell response and preserve transduced target cells. Indeed, the proteasomal inhibitor bortezomib is effective in reducing AAV2 capsid epitope presentation on MHC I [137]. Preventing proteasomal degradation of capsid and thus reducing antigen presentation can also be achieved by capsid modification. Martino et al. showed that an AAV2 capsid mutant that is less prone to proteasomal degradation [16] reduced MHC I-restricted presentation and minimized cytolytic killing of transduced hepatocytes by CD8+ T cells compared with AAV2 [132].
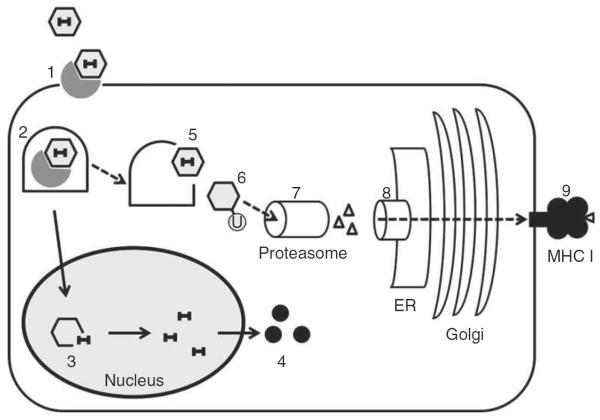
Figure 3. Cross-presentation of rAAV capsid epitope in transduced cell.
(1) rAAV capsid binds to receptor on cell surface. (2) rAAV enters the cell by receptor-mediated endocytosis and is transported in endosome. (3) rAAV vector undergoes uncoating in the nucleus to release the genome. (4) Transgene expression leads to protein production in cytoplasm. (5) rAAV can escape from endosome in cytoplasm, where (6) the capsid is tagged by ubiquitylation for (7) proteasomal degradation. (8) The resulting peptide is transported to the endoplasmic reticulum (ER), loaded onto MHC I and transported through ER and Golgi. (9) The MHC I presents the epitope on cell surface and tags the cell for CD8+ T-cell recognition.
Dashed arrows: Cross-presentation of capsid epitope; rAAV: Recombinant adeno-associated virus; Solid arrows: Transgene expression pathway.
Similar to the cross-reactivity of NAbs, AAV2-specific human T cells proliferated upon exposure to alternate AAV serotypes (AAV8 or AAV1) [109], indicating that serotype switching is unlikely to evade capsid-specific T-cell immune response. Transient immune suppression by a short course of high dose prednisolone has been shown to ameliorate capsid-directed T-cell response, and to partially rescue transgene expression in the rAAV8-hFIX clinical trial [129].
5.4 Transgene immunity
Protein product of transgene represents another potential source of antigen in gene replacement therapy, yet rAAV generally shows weak transgene immunity compared with other viral vectors such as adenovirus. This is largely because rAAV is inefficient in transducing professional APCs such as dendritic cells (DCs) and macrophages [138]. Nevertheless, once APC is transduced, transgene-encoded protein can be processed by proteasome and resulting epitopes are loaded on MHC I molecules. MHC I-restricted antigen presentation eventually leads to cytolytic CD8+ T-cell response to target cells. Direct intramuscular rAAV administration for treating muscle diseases rarely induced detectable transgene-specific CD8+ T-cell response [116]. However, Mendell et al. reported detection of dystrophin-specific CD4+ and CD8+ T cells in four out of six patients receiving rAAV vectors carrying a truncated dystrophin gene, whereas two patients had dystrophin-specific T cells before vector treatment [131]. Instead of being directly transduced by rAAV, APCs can take up secreted proteins or engulf transduced cells and process them through either direct presentation on MHC II molecules to induce B cell immunity and antibody formation, or cross-presentation on MHC I molecules to trigger CD8+ T-cell immunity. Both responses have been observed in animal studies and human trials [139]. For example, liver-directed rAAV delivery expressing secretory GAA in Pompe mice could induce anti-GAA antibody, which will hamper cross-correction in muscle [140].
To reduce transgene immunity, one can design rAAV vector for restricted muscle gene expression by using tissue-specific promoter or miRNA-based de-targeting strategy as mentioned in Section 4.2. The goal is to minimize APC transduction and antigen presentation. Switching to an intravascular route to target muscle can also reduce immune response compared with direct intramuscular injection [105,141,142].
6. Conclusion
Among a handful of viral vectors, rAAV emerged as the most promising for safe and effective in vivo gene delivery. The natural diversity of AAV serotypes combined with recent technologies to engineer AAV capsid has provided a plethora of rAAV vectors with distinct biological profiles concerning not only tissue tropism, but also immune reactivity with host, many of which possess superb capability in delivering genes to muscle tissues with ease. The transgene delivered by rAAV predominantly exists episomally in post-mitotic cells such as muscle fibers, thus mediating long-term gene expression without the risk of insertional mutagenesis. Preclinical studies in animal models and clinical trials have consistently demonstrated the feasibility of using rAAV to deliver therapeutic genes to muscle tissue to treat human diseases in two scenarios: muscle diseases per se, and non-muscle diseases that are treatable by muscle-synthesized, secretory therapeutic factor in bloodstream.
Similar to other therapeutic developments, muscle-directed rAAV gene delivery is not without limitations. The relatively small cargo capacity of rAAV is not amenable to delivering many large muscle genes involved in human diseases. Uncontrolled transgene expression both in off-target tissues and at inappropriate times may cause toxicity. In addition, as a natural host of AAV, the human body has the potential to interact with rAAV vector and transgene product through immune system in multiple ways, posing another major barrier to successful gene transfer. To address these limitations, research has been directed to study the basic vectorology of AAV and how rAAV administration shapes the unique immune responses in humans. Furthermore, advances in basic research have fostered the development of novel vector design strategies and immune-modulatory approaches to overcome these limitations.
7. Expert opinion
Rodent models are the most frequently used platforms for preclinical testing of therapeutic strategies, including muscle-directed rAAV gene delivery. Translation from rodent models to larger animals and clinical application poses the challenge that a significantly larger muscle mass needs to be transduced to achieve optimal therapeutic outcome. This is arguably the most urgent unmet demand in treating diseases affecting muscles throughout the body such as DMD. This challenge has to be tackled on two levels: identifying a safe rAAV delivery route for systemic muscle transduction and developing a scalable, good manufacturing practice (GMP)-compatible rAAV production method. A single intravenous injection of several rAAV serotypes can result in effective systemic skeletal and cardiac muscle transduction in mice [40,49]. However, this could be hampered by destructive immune response to both capsid and transgene product in large animals [143]. Hydrodynamic limb vein infusion proved successful in delivering rAAV vectors to isolated skeletal muscle in dogs [144] and monkeys [145] without obvious immune responses. Nevertheless, this method does not target other muscle types such as cardiac muscle [44], an important disease-relevant organ in many myopathies. Further development of systemic delivery routes, likely in combination with special vector design that can bypass immune surveillance, will greatly enhance the efficacy and safety of rAAV muscle gene therapy. Currently, lack of a scalable rAAV vector production method limits preclinical studies in large animals and clinical trials for muscle gene therapy. Vector yield from the widely used triple-transfection method [146] well satisfies the need for small animal studies. However, to achieve widespread muscle transduction in humans, ~ 1,000 times more vector will be required than the amount for transducing an entire mouse. Because transfection-based method is difficult to scale up, several infection-based rAAV production methods potentially amenable to large-scale manufacture are under extensive development, including using stable cell lines [147], adenovirus-AAV hybrid system [148], herpes virus-based system [149] and a baculovirus-insect cell system [150], each with unresolved technical hurdles towards successful clinical application. Further improvement of GMP-compatible, large-scale rAAV vector production system will expedite preclinical testing in large animal models and clinical investigation.
The VIP strategy described in Section 3 is a novel application of muscle-directed rAAV gene delivery. In essence, transgene encoding a broad neutralizing antibody is delivered to muscle cells using rAAV vector, which leads to potentially sustained expression and secretion of the antibody to the bloodstream and protects the host from infection. Protection mediated by VIP is independent of the host immune system. This is of particular importance for protecting individuals with compromised immune system, who are often the most vulnerable during a pandemic and poorly respond to immune system-dependent vaccination. As clinical trials to test VIP are developing [107], it would be exciting to examine whether this strategy is able to successfully combat deadly infectious diseases such as HIV infection in humans.
The unique destructive immune response following rAAV administration in humans is another major obstacle to effective gene transfer. Although the adaptive immunity described in Section 5 plays a critical role, the effect of innate immunity to rAAV should not be neglected due to its ability to promote adaptive immunity. The low immunogenic profile of rAAV is in part due to its inability to stimulate a robust innate immune response [151]. However, myopathies are usually accompanied by inflammation due to tissue damage, which is thought to lower the threshold for rAAV to trigger adaptive immune response [151]. Studies showed that innate immunity to rAAV is mainly mediated by TLR9/MyD88 pathway-mediated response to viral genome DNA and TLR2-mediated response to capsid [151]. In line with these finding, Wilson and colleagues recently reported that depleting TLR9 ligand, the unmethylated CpG in viral genome, allows a highly immunogenic vector rAAVrh32.33 to evade immune response and to achieve persistent transgene expression after intramuscular injection in mice [152]. Future studies may facilitate vector development combined with host immune-modulatory approaches to dodge multiple branches of host immune surveillance to rAAV administration.
Importantly, rAAV transfer to liver induces immune tolerance to transgene product, which does not occur in muscle [139]. A subclass of regulatory CD4+CD25+Foxp3+ T cell (Treg) population in liver is responsible for suppressing CTL response by creating an anti-inflammatory cytokine microenvironment including IL-10 [153]. Interestingly, Haurigot et al. detected a population of canine F.IX (cF.IX) antigen-specific, expandable CD4+Foxp3+IL-10+ cells in muscle following peripheral transvenular administration of rAAV-cF.IX in dog with immune suppression [141]. This study suggested that Treg-mediated immune suppression might not be restricted to the liver, whereas under certain circumstances this mechanism can also take place in muscle. Future studies need to further elucidate the role of Treg in immune tolerance to rAAV, and to utilize its modulatory function to ease muscle gene transfer by rAAV.
Article highlights.
Different rAAV serotypes can deliver genes to muscle tissues in either a localized or a systemic manner.
rAAV-mediated gene transfer to muscle has proved to be effective in treating a series of human diseases in animal models.
Muscle can be exploited as a biofactory to produce secretory therapeutic agents such as antibodies.
Strategies to deliver large genes using rAAV vectors expand the application of rAAV-mediated muscle gene therapy.
Gene expression after delivery can be regulated for safety and efficacy.
Immune response following rAAV administration poses a significant barrier to gene transfer in humans.
This box summarizes key points contained in the article.
Acknowledgment
Declaration of interest This work was supported by Public Health Service grants UL1RR031982, 2P01 HL059407, 1P01AI100263-01 and 2R01NS076991-01 from the National Institutes of Health (to GG), and partially supported by a grant from National High Technology Research and Development Program (`863' Program) of China (2012AA020810) (to GG) and by a UMMS internal grant (also to GG).
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Blacklow NR, Hoggan MD, Rowe WP. Isolation of adenovirus-associated viruses from man. Proc Natl Acad Sci USA. 1967;58(4):1410–15. doi: 10.1073/pnas.58.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report on isolation of AAV from human.
- 2.Gao G, Zhong L, Danos O. Exploiting natural diversity of AAV for the design of vectors with novel properties. Methods Mol Biol. 2011;807:93–118. doi: 10.1007/978-1-61779-370-7_4. [DOI] [PubMed] [Google Scholar]; • The review summarizes how the naturally evolved AAV capsid genes are used to derive novel rAAV vectors for gene transfer.
- 3.Muzyczka N, Berns K. Parvoviridae: the viruses and their replication. In: Knipe D, Howley P, Griffin D, et al., editors. Fields virology. Lippincott Williams and Wilkins; Philadelphia, PA: 2001. pp. 2327–59. [Google Scholar]; •• A classic book chapter on AAV biology.
- 4.Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 2012;507:229–54. doi: 10.1016/B978-0-12-386509-0.00012-0. [DOI] [PubMed] [Google Scholar]
- 5.Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci USA. 2010;107(22):10220–5. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Q, Bu W, Bhatia S, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA. 2002;99(16):10405–10. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkatakrishnan B, Yarbrough J, Domsic J, et al. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J Virol. 2013;87(9):4974–84. doi: 10.1128/JVI.02524-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMattia MA, Nam HJ, Van Vliet K, et al. Structural insight into the unique properties of adeno-associated virus serotype 9. J Virol. 2012;86(12):6947–58. doi: 10.1128/JVI.07232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci USA. 1966;55(6):1467–74. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buller RM, Janik JE, Sebring ED, et al. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J Virol. 1981;40(1):241–7. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlehofer JR, Ehrbar M, zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986;152(1):110–17. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- 12.Kotin RM, Siniscalco M, Samulski RJ, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87(6):2211–15. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 14.Zhong L, Jayandharan GR, Aslanidi GV, et al. Development of novel recombinant AAV vectors and strategies for the potential gene therapy of hemophilia. J Genet Synd Gene Ther. 2012;S1 doi: 10.4172/2157-7412.S1-008. Article number 008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Li J, Xiao X. Directed evolution of adeno-associated virus (AAV) as vector for muscle gene therapy. Methods Mol Biol. 2011;709:127–39. doi: 10.1007/978-1-61737-982-6_8. [DOI] [PubMed] [Google Scholar]
- 16.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105(22):7827–32. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty DM, Fu H, Monahan PE, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–18. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]; • One of the first reports on the development of scAAV vector.
- 18.Wang Z, Ma HI, Li J, et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10(26):2105–11. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]; • One of the first reports on the development of scAAV vectors.
- 19.Smith BK, Collins SW, Conlon TJ, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther. 2013;24(6):630–40. doi: 10.1089/hum.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341–55. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 21.Bryant LM, Christopher DM, Giles AR, et al. Lessons learned from the clinical development and market authorization of Glybera. Hum Gene Ther Clin Dev. 2013;24(2):55–64. doi: 10.1089/humc.2013.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan D, Sharma P, Yang J, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72(11):8568–77. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article provided direct evidence that the episomal rAAV genomes concatermerize to form a stable circular configuration, allowing for potentially long-term gene expression.
- 23.Kaeppel C, Beattie SG, Fronza R, et al. A largely random AAV integration profile after LPLD gene therapy. Nat Med. 2013;19(7):889–91. doi: 10.1038/nm.3230. [DOI] [PubMed] [Google Scholar]
- 24.Gruntman AM, Bish LT, Mueller C, et al. Gene transfer in skeletal and cardiac muscle using recombinant adeno-associated virus. Curr Protoc Microbiol. 2013;14(14D):3. doi: 10.1002/9780471729259.mc14d03s28. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article describes technical details of muscle-directed rAAV gene delivery in mice.
- 25.Miyagoe-Suzuki Y, Takeda S. Gene therapy for muscle disease. Exp Cell Res. 2010;316(18):3087–92. doi: 10.1016/j.yexcr.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Duan D, Yan Z, Yue Y, et al. Enhancement of muscle gene delivery with pseudotyped adeno-associated virus type 5 correlates with myoblast differentiation. J Virol. 2001;75(16):7662–71. doi: 10.1128/JVI.75.16.7662-7671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrne BJ, Falk DJ, Pacak CA, et al. Pompe disease gene therapy. Hum Mol Genet. 2011;20(R1):R61–8. doi: 10.1093/hmg/ddr174. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The article reviews the use of rAAV vectors for the treatment of Pompe disease.
- 28.Mendell JR, Rodino-Klapac L, Sahenk Z, et al. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci Lett. 2012;527(2):90–9. doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mah CS, Pacak CA, Byrne BJ. Muscle as a metabolic factory for gene therapy. In: Duan D, editor. Muscle gene therapy. Springer; New York, London: 2010. pp. 219–30. [Google Scholar]
- 30.Chuah MK, Evens H, VandenDriessche T. Gene therapy for hemophilia. J Thromb Haemost. 2013;11(Suppl 1):99–110. doi: 10.1111/jth.12215. [DOI] [PubMed] [Google Scholar]
- 31.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther. 2011;22(10):1239–47. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera VM, Gao GP, Grant RL, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105(4):1424–30. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 33.Fan Z, Kocis K, Valley R, et al. Safety and feasibility of high-pressure transvenous limb perfusion with 0.9% saline in human muscular dystrophy. Mol Ther. 2012;20(2):456–61. doi: 10.1038/mt.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowles DE, McPhee SW, Li C, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20(2):443–55. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article reports the first DMD clinical trial using rAAV gene therapy.
- 35.Bish LT, Sleeper MM, Brainard B, et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol Ther. 2008;16(12):1953–9. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- 36.Vulin A, Barthelemy I, Goyenvalle A, et al. Muscle function recovery in golden retriever muscular dystrophy after AAV1-U7 exon skipping. Mol Ther. 2012;20(11):2120–33. doi: 10.1038/mt.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao G, Bish LT, Sleeper MM, et al. Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum Gene Ther. 2011;22(8):979–84. doi: 10.1089/hum.2011.042. [DOI] [PubMed] [Google Scholar]
- 38.Jaski BE, Jessup ML, Mancini DM, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15(3):171–81. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessup M, Greenberg B, Mancini D, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10(8):828–34. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23(3):321–8. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]; •• First report on rAAV8-mediated muscle and heart transduction by systemic delivery.
- 42.Pacak CA, Sakai Y, Thattaliyath BD, et al. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther. 2008;6:13. doi: 10.1186/1479-0556-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katwal AB, Konkalmatt PR, Piras BA, et al. Adeno-associated virus serotype 9 efficiently targets ischemic skeletal muscle following systemic delivery. Gene Ther. 2013;20(9):930–8. doi: 10.1038/gt.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yue Y, Ghosh A, Long C, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16(12):1944–52. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kornegay JN, Li J, Bogan JR, et al. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther. 2010;18(8):1501–8. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao C, Li J, Jiang J, et al. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther. 2008;19(3):241–54. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- 47.Xu L, Lu PJ, Wang CH, et al. Adeno-associated virus 9 mediated FKRP gene therapy restores functional glycosylation of alpha-dystroglycan and improves muscle functions. Mol Ther. 2013;21(10):1832–40. doi: 10.1038/mt.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun B, Young SP, Li P, et al. Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol Ther. 2008;16(8):1366–71. doi: 10.1038/mt.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zincarelli C, Soltys S, Rengo G, et al. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]; •• This article reports systemic study on the biodistribution and transduction efficiency of rAAV serotypes 1–9 folllowing i.v. injection in mice.
- 50.Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99(4):e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 51.Bostick B, Yue Y, Lai Y, et al. AAV-9 micro-dystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther. 2008;19(8):851–6. doi: 10.1089/hum.2008.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goehringer C, Rutschow D, Bauer R, et al. Prevention of cardiomyopathy in delta-sarcoglycan knockout mice after systemic transfer of targeted adeno-associated viral vectors. Cardiovasc Res. 2009;82(3):404–10. doi: 10.1093/cvr/cvp061. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Zhu T, Rehman KK, et al. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes. 2006;55(4):875–84. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- 54.Mayra A, Tomimitsu H, Kubodera T, et al. Intraperitoneal AAV9-shRNA inhibits target expression in neonatal skeletal and cardiac muscles. Biochem Biophys Res Commun. 2011;405(2):204–9. doi: 10.1016/j.bbrc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Garikipati D, Chamberlain JS. Muscle gene therapy. Springer; New York, London: 2010. Systemic gene delivery for muscle gene therapy. In: Duan D, editor; pp. 163–79. [Google Scholar]
- 56.Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7(17):2101–12. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 58.Allocca M, Doria M, Petrillo M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118(5):1955–64. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong B, Nakai H, Xiao W. Characterization of genome integrity for oversized recombinant AAV vector. Mol Ther. 2010;18(1):87–92. doi: 10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome > or = 8.2 kb. Mol Ther. 2010;18(1):75–9. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan D, Yue Y, Yan Z, et al. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat Med. 2000;6(5):595–8. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]; • First report on dual vector design to deliver large genes using rAAV.
- 62.Duan D, Yue Y, Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther. 2001;4(4):383–91. doi: 10.1006/mthe.2001.0456. [DOI] [PubMed] [Google Scholar]
- 63.Nakai H, Storm TA, Kay MA. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat Biotechnol. 2000;18(5):527–32. doi: 10.1038/75390. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh A, Yue Y, Lai Y, et al. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol Ther. 2008;16(1):124–30. doi: 10.1038/sj.mt.6300322. [DOI] [PubMed] [Google Scholar]
- 65.Lai Y, Yue Y, Liu M, et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol. 2005;23(11):1435–9. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghosh A, Yue Y, Long C, et al. Efficient whole-body transduction with trans-splicing adeno-associated viral vectors. Mol Ther. 2007;15(4):750–5. doi: 10.1038/sj.mt.6300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun L, Li J, Xiao X. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat Med. 2000;6(5):599–602. doi: 10.1038/75087. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh A, Yue Y, Duan D. Viral serotype and the transgene sequence influence overlapping adeno-associated viral (AAV) vector-mediated gene transfer in skeletal muscle. J Gene Med. 2006;8(3):298–305. doi: 10.1002/jgm.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Odom GL, Gregorevic P, Allen JM, et al. Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther. 2011;19(1):36–45. doi: 10.1038/mt.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopes VS, Boye SE, Louie CM, et al. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther. 2013;20(8):824–33. doi: 10.1038/gt.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum Gene Ther. 2012;23(1):98–103. doi: 10.1089/hum.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Yue Y, Li L, et al. Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum Mol Genet. 2013;22(18):3720–9. doi: 10.1093/hmg/ddt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warrington KH, Jr, Gorbatyuk OS, Harrison JK, et al. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J Virol. 2004;78(12):6595–609. doi: 10.1128/JVI.78.12.6595-6609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79(15):9933–44. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai Y, Yue Y, Bostick B, et al. Delivering large therapeutic gene for muscle gene therapy. In: Duan D, editor. Muscle gene therapy. Springer; New York, London: 2010. pp. 205–18. [Google Scholar]
- 76.Roudaut C, Le Roy F, Suel L, et al. Restriction of calpain3 expression to the skeletal muscle prevents cardiac toxicity and corrects pathology in a murine model of limb-girdle muscular dystrophy. Circulation. 2013;128(10):1094–104. doi: 10.1161/CIRCULATIONAHA.113.001340. [DOI] [PubMed] [Google Scholar]
- 77.Falk DJ, Mah CS, Soustek MS, et al. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther. 2013;21(9):1661–7. doi: 10.1038/mt.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toscano MG, Romero Z, Munoz P, et al. Physiological and tissue-specific vectors for treatment of inherited diseases. Gene Ther. 2011;18(2):117–27. doi: 10.1038/gt.2010.138. [DOI] [PubMed] [Google Scholar]; • Comprehensive review on regulatory gene expression systems.
- 79.Cordier L, Gao GP, Hack AA, et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12(2):205–15. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 80.Salva MZ, Himeda CL, Tai PW, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther. 2007;15(2):320–9. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 81.Lagos-Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 82.Brown BD, Venneri MA, Zingale A, et al. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12(5):585–91. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 83.Majowicz A, Maczuga P, Kwikkers KL, et al. Mir-142-3p target sequences reduce transgene directed immunogenicity following intramuscular AAV1 vector-mediated gene delivery. J Gene Med. 2013;15(6–7):219–32. doi: 10.1002/jgm.2712. [DOI] [PubMed] [Google Scholar]
- 84.Xie J, Xie Q, Zhang H, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol Ther. 2011;19(3):526–35. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bohl D, Salvetti A, Moullier P, et al. Control of erythropoietin delivery by doxycycline in mice after intramuscular injection of adeno-associated vector. Blood. 1998;92(5):1512–17. [PubMed] [Google Scholar]
- 86.Rivera VM, Ye X, Courage NL, et al. Long-term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc Natl Acad Sci USA. 1999;96(15):8657–62. doi: 10.1073/pnas.96.15.8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci USA. 1996;93(8):3346–51. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jazwa A, Florczyk U, Jozkowicz A, et al. Gene therapy on demand: site specific regulation of gene therapy. Gene. 2013;525(2):229–38. doi: 10.1016/j.gene.2013.03.093. [DOI] [PubMed] [Google Scholar]; •• Comprehensive review on strategies to control transgene expression in gene therapy.
- 89.Apparailly F, Millet V, Noel D, et al. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther. 2002;13(10):1179–88. doi: 10.1089/104303402320138961. [DOI] [PubMed] [Google Scholar]
- 90.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379(9813):373–83. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 91.Al-Khatti A, Veith RW, Papayannopoulou T, et al. Stimulation of fetal hemoglobin synthesis by erythropoietin in baboons. N Engl J Med. 1987;317(7):415–20. doi: 10.1056/NEJM198708133170704. [DOI] [PubMed] [Google Scholar]
- 92.Villeval JL, Rouyer-Fessard P, Blumenfeld N, et al. Retrovirus-mediated transfer of the erythropoietin gene in hematopoietic cells improves the erythrocyte phenotype in murine beta-thalassemia. Blood. 1994;84(3):928–33. [PubMed] [Google Scholar]
- 93.Johnston J, Tazelaar J, Rivera VM, et al. Regulated expression of erythropoietin from an AAV vector safely improves the anemia of beta-thalassemia in a mouse model. Mol Ther. 2003;7(4):493–7. doi: 10.1016/s1525-0016(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 94.Herzog RW, Hagstrom JN, Kung SH, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94(11):5804–9. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A breakthrough in using muscle as a biofactory to produce secretory therapeutic agents in mice.
- 95.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–72. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 96.Arruda VR, Schuettrumpf J, Herzog RW, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103(1):85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson PR, Schnepp BC, Zhang J, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15(8):901–6. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first report on a novel approach to delivering genes to muscle for preventing infection.
- 98.Balazs AB, Chen J, Hong CM, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–4. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balazs AB, Bloom JD, Hong CM, et al. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat Biotechnol. 2013;31(7):647–52. doi: 10.1038/nbt.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kessler PD, Podsakoff GM, Chen X, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93(24):14082–7. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5(1):56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]; • A breakthrough in using muscle as a biofactory to produce secretory therapeutic agents in large animals.
- 102.Arruda VR, Hagstrom JN, Deitch J, et al. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001;97(1):130–8. doi: 10.1182/blood.v97.1.130. [DOI] [PubMed] [Google Scholar]
- 103.High KA. Gene therapy for haemophilia: a long and winding road. J Thromb Haemost. 2011;9(Suppl 1):2–11. doi: 10.1111/j.1538-7836.2011.04369.x. [DOI] [PubMed] [Google Scholar]
- 104.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24(3):257–61. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 105.Arruda VR, Stedman HH, Haurigot V, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115(23):4678–88. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lewis AD, Chen R, Montefiori DC, et al. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol. 2002;76(17):8769–75. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balazs AB, West AP., Jr Antibody gene transfer for HIV immunoprophylaxis. Nat Immunol. 2013;14(1):1–5. doi: 10.1038/ni.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mueller C, Braag SA, Martino AT, et al. The pros and cons of immunomodulatory IL-10 gene therapy with recombinant AAV in a Cftr−/− - dependent allergy mouse model. Gene Ther. 2009;16(2):172–83. doi: 10.1038/gt.2008.156. [DOI] [PubMed] [Google Scholar]
- 109.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–22. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 110.Hohlfeld R, Engel AG. The immunobiology of muscle. Immunol Today. 1994;15(6):269–74. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 111.Nagaraju K. Immunological capabilities of skeletal muscle cells. Acta Physiol Scand. 2001;171(3):215–23. doi: 10.1046/j.1365-201x.2001.00823.x. [DOI] [PubMed] [Google Scholar]
- 112.Loell I, Lundberg IE. Can muscle regeneration fail in chronic inflammation: a weakness in inflammatory myopathies? J Intern Med. 2011;269(3):243–57. doi: 10.1111/j.1365-2796.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 113.Garlepp MJ, Chen W, Tabarias H, et al. Antigen processing and presentation by a murine myoblast cell line. Clin Exp Immunol. 1995;102(3):614–19. doi: 10.1111/j.1365-2249.1995.tb03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hohlfeld R, Engel AG. Coculture with autologous myotubes of cytotoxic T cells isolated from muscle in inflammatory myopathies. Ann Neurol. 1991;29(5):498–507. doi: 10.1002/ana.410290509. [DOI] [PubMed] [Google Scholar]
- 115.Nathwani AC, Davidoff A, Hanawa H, et al. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97(5):1258–65. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 116.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Recent review on immunological barriers to rAAV gene transfer.
- 117.Chirmule N, Propert K, Magosin S, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574–83. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 118.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 119.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.High KA. The gene therapy journey for hemophilia: are we there yet? Hematology Am Soc Hematol Educ Program. 2012;2012:375–81. doi: 10.1182/asheducation-2012.1.375. [DOI] [PubMed] [Google Scholar]
- 121.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107(5):1810–17. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 122.Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321–8. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee GK, Maheshri N, Kaspar B, et al. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng. 2005;92(1):24–34. doi: 10.1002/bit.20562. [DOI] [PubMed] [Google Scholar]
- 124.Huttner NA, Girod A, Perabo L, et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther. 2003;10(26):2139–47. doi: 10.1038/sj.gt.3302123. [DOI] [PubMed] [Google Scholar]
- 125.Monteilhet V, Saheb S, Boutin S, et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19(11):2084–91. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mimuro J, Mizukami H, Hishikawa S, et al. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther. 2013;21(2):318–23. doi: 10.1038/mt.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mingozzi F, Anguela XM, Pavani G, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5(194):194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This report demonstrated a highly innovative appoach to overcoming NAbs.
- 128.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7(5):316–24. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 129.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mingozzi F, Meulenberg JJ, Hui DJ, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077–86. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363(15):1429–37. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121(12):2224–33. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1(2):126–34. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 134.Yan Z, Zak R, Luxton GW, et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76(5):2043–53. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119(6):1688–95. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li C, He Y, Nicolson S, et al. Adeno-associated virus capsid antigen presentation is dependent on endosomal escape. J Clin Invest. 2013;123(3):1390–401. doi: 10.1172/JCI66611. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The research article demonstrates pathways leading to rAAV capsid epitope presentation, thus suggesting possible ways to circumvent capsid-specific immune response.
- 137.Finn JD, Hui D, Downey HD, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010;18(1):135–42. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jooss K, Yang Y, Fisher KJ, et al. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72(5):4212–23. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther. 2011;19(1):16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review on transgene-specific T cell response following rAAV gene transfer.
- 140.Cresawn KO, Fraites TJ, Wasserfall C, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther. 2005;16(1):68–80. doi: 10.1089/hum.2005.16.68. [DOI] [PubMed] [Google Scholar]
- 141.Haurigot V, Mingozzi F, Buchlis G, et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther. 2010;18(7):1318–29. doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Toromanoff A, Adjali O, Larcher T, et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther. 2010;18(1):151–60. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Z, Tapscott SJ, Chamberlain JS, et al. Immunity and AAV-mediated gene therapy for muscular dystrophies in large animal models and human trials. Front Microbiol. 2011;2:201. doi: 10.3389/fmicb.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This review article summerizes the outcomes from muscle-directed rAAV gene therapy in large animals, focusing on immunological perspectives.
- 144.Sun B, Li S, Bird A, et al. Hydrostatic isolated limb perfusion with adeno-associated virus vectors enhances correction of skeletal muscle in Pompe disease. Gene Ther. 2010;17(12):1500–5. doi: 10.1038/gt.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodino-Klapac LR, Montgomery CL, Mendell JR, et al. AAV-mediated gene therapy to the isolated limb in rhesus macaques. Methods Mol Biol. 2011;709:287–98. doi: 10.1007/978-1-61737-982-6_19. [DOI] [PubMed] [Google Scholar]
- 146.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72(3):2224–32. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yuan Z, Qiao C, Hu P, et al. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum Gene Ther. 2011;22(5):613–24. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang H, Xie J, Xie Q, et al. Adenovirus-adeno-associated virus hybrid for large-scale recombinant adeno-associated virus production. Hum Gene Ther. 2009;20(9):922–9. doi: 10.1089/hum.2009.125. [DOI] [PubMed] [Google Scholar]
- 149.Clement N, Knop DR, Byrne BJ. Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies. Hum Gene Ther. 2009;20(8):796–806. doi: 10.1089/hum.2009.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Negrete A, Yang LC, Mendez AF, et al. Economized large-scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. J Gene Med. 2007;9(11):938–48. doi: 10.1002/jgm.1092. [DOI] [PubMed] [Google Scholar]
- 151.Rogers GL, Martino AT, Aslanidi GV, et al. Innate Immune Responses to AAV Vectors. Front Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article comprehensively reviews innate immune responses to rAAV vectors.
- 152.Faust SM, Bell P, Cutler BJ, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest. 2013;123(7):2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cao O, Dobrzynski E, Wang L, et al. Induction and role of regulatory CD4 +CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110(4):1132–40. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This report provides direct evidence showing the function of Treg in rAAV-mediated immune tolerance.
- 154.Lorain S, Gross DA, Goyenvalle A, et al. Transient immunomodulation allows repeated injections of AAV1 and correction of muscular dystrophy in multiple muscles. Mol Ther. 2008;16(3):541–7. doi: 10.1038/sj.mt.6300377. [DOI] [PubMed] [Google Scholar]
- 155.Du L, Kido M, Lee DV, et al. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol Ther. 2004;10(3):604–8. doi: 10.1016/j.ymthe.2004.06.110. [DOI] [PubMed] [Google Scholar]
- 156.Palomeque J, Chemaly ER, Colosi P, et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14(13):989–97. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- 157.Flotte TR, Conlon TJ, Poirier A, et al. Preclinical characterization of a recombinant adeno-associated virus type 1-pseudotyped vector demonstrates dose-dependent injection site inflammation and dissemination of vector genomes to distant sites. Hum Gene Ther. 2007;18(3):245–56. doi: 10.1089/hum.2006.113. [DOI] [PubMed] [Google Scholar]
- 158.Ross CJ, Twisk J, Bakker AC, et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006;17(5):487–99. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- 159.Karakikes I, Hadri L, Rapti K, et al. Concomitant intravenous nitroglycerin with intracoronary delivery of AAV1. SERCA2a enhances gene transfer in porcine hearts. Mol Ther. 2012;20(3):565–71. doi: 10.1038/mt.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Byrne MJ, Power JM, Preovolos A, et al. Recirculating cardiac delivery of AAV2/ 1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15(23):1550–7. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 161.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106(38):16363–8. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66(3):290–7. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gaudet D, Methot J, Dery S, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20(4):361–9. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article reported a clinical trial for LPLD using muscle-directed delivery of rAAV1 vector, which led to the administrative approval of the first gene therapy drug in Europe.
- 164.Yoshimura M, Sakamoto M, Ikemoto M, et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther. 2004;10(5):821–8. doi: 10.1016/j.ymthe.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 165.Zaratiegui M, Castilla-Cortazar I, Garcia M, et al. IGF1 gene transfer into skeletal muscle using recombinant adeno-associated virus in a rat model of liver cirrhosis. J Physiol Biochem. 2002;58(3):169–76. doi: 10.1007/BF03179854. [DOI] [PubMed] [Google Scholar]
- 166.Li J, Wang D, Qian S, et al. Efficient and long-term intracardiac gene transfer in delta-sarcoglycan-deficiency hamster by adeno-associated virus-2 vectors. Gene Ther. 2003;10(21):1807–13. doi: 10.1038/sj.gt.3302078. [DOI] [PubMed] [Google Scholar]
- 167.Tuuminen R, Nykanen AI, Krebs R, et al. PDGF-A, -C, and -D but not PDGF-B increase TGF-beta1 and chronic rejection in rat cardiac allografts. Arterioscler Thromb Vasc Biol. 2009;29(5):691–8. doi: 10.1161/ATVBAHA.108.178558. [DOI] [PubMed] [Google Scholar]
- 168.Raake PW, Hinkel R, Muller S, et al. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008;15(1):12–17. doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- 169.Flotte TR, Brantly ML, Spencer LT, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004;15(1):93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- 170.Grose WE, Clark KR, Griffin D, et al. Homologous recombination mediates functional recovery of dysferlin deficiency following AAV5 gene transfer. PLoS ONE. 2012;7(6):e39233. doi: 10.1371/journal.pone.0039233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lai NC, Tang T, Gao MH, et al. Improved function of the failing rat heart by regulated expression of insulin-like growth factor I via intramuscular gene transfer. Hum Gene Ther. 2012;23(3):255–61. doi: 10.1089/hum.2011.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sun B, Zhang H, Franco LM, et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther. 2005;11(6):889–98. doi: 10.1016/j.ymthe.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 173.Hollinger K, Gardan-Salmon D, Santana C, et al. Rescue of dystrophic skeletal muscle by PGC-1alpha involves restored expression of dystrophin-associated protein complex components and satellite cell signaling. Am J Physiol Regul Integr Comp Physiol. 2013;305(1):R13–23. doi: 10.1152/ajpregu.00221.2012. [DOI] [PubMed] [Google Scholar]
- 174.Bortolanza S, Nonis A, Sanvito F, et al. AAV6-mediated systemic shRNA delivery reverses disease in a mouse model of facioscapulohumeral muscular dystrophy. Mol Ther. 2011;19(11):2055–64. doi: 10.1038/mt.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Towne C, Raoul C, Schneider BL, et al. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol Ther. 2008;16(6):1018–25. doi: 10.1038/mt.2008.73. [DOI] [PubMed] [Google Scholar]
- 176.Rengo G, Lymperopoulos A, Zincarelli C, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119(1):89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pleger ST, Most P, Boucher M, et al. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115(19):2506–15. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 178.Wang Z, Storb R, Halbert CL, et al. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Mol Ther. 2012;20(8):1501–7. doi: 10.1038/mt.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bish LT, Sleeper MM, Forbes SC, et al. Long-term restoration of cardiac dystrophin expression in golden retriever muscular dystrophy following rAAV6-mediated exon skipping. Mol Ther. 2012;20(3):580–9. doi: 10.1038/mt.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bish LT, Sleeper MM, Reynolds C, et al. Cardiac gene transfer of short hairpin RNA directed against phospholamban effectively knocks down gene expression but causes cellular toxicity in canines. Hum Gene Ther. 2011;22(8):969–77. doi: 10.1089/hum.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Raake PW, Schlegel P, Ksienzyk J, et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34(19):1437–47. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Beeri R, Chaput M, Guerrero JL, et al. Gene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitation. Circ Heart Fail. 2010;3(5):627–34. doi: 10.1161/CIRCHEARTFAILURE.109.891184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Katz MG, Fargnoli AS, Swain JD, et al. AAV6-betaARKct gene delivery mediated by molecular cardiac surgery with recirculating delivery (MCARD) in sheep results in robust gene expression and increased adrenergic reserve. J Thorac Cardiovasc Surg. 2012;143(3):720–6. e3. doi: 10.1016/j.jtcvs.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Louboutin JP, Wang L, Wilson JM. Gene transfer into skeletal muscle using novel AAV serotypes. J Gene Med. 2005;7(4):442–51. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- 185.Wang L, Louboutin JP, Bell P, et al. Muscle-directed gene therapy for hemophilia B with more efficient and less immunogenic AAV vectors. J Thromb Haemost. 2011;9(10):2009–19. doi: 10.1111/j.1538-7836.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Charan RA, Niizawa G, Nakai H, et al. Adeno-associated virus serotype 8 (AAV8) delivery of recombinant A20 to skeletal muscle reduces pathological activation of nuclear factor (NF)-kappaB in muscle of mdx mice. Mol Med. 2012;18:1527–35. doi: 10.2119/molmed.2012.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gao MH, Lai NC, Miyanohara A, et al. Intravenous adeno-associated virus serotype 8 encoding urocortin-2 provides sustained augmentation of left ventricular function in mice. Hum Gene Ther. 2013;24(9):777–85. doi: 10.1089/hum.2013.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bish LT, Morine K, Sleeper MM, et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008;19(12):1359–68. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohshima S, Shin JH, Yuasa K, et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther. 2009;17(1):73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qiao C, Li J, Zheng H, et al. Hydrodynamic limb vein injection of adeno-associated virus serotype 8 vector carrying canine myostatin propeptide gene into normal dogs enhances muscle growth. Hum Gene Ther. 2009;20(1):1–10. doi: 10.1089/hum.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rodino-Klapac LR, Montgomery CL, Bremer WG, et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther. 2010;18(1):109–17. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yu M, He Y, Wang K, et al. Adeno-associated viral-mediated LARGE gene therapy rescues the muscular dystrophic phenotype in mouse models of dystroglycanopathy. Hum Gene Ther. 2013;24(3):317–30. doi: 10.1089/hum.2012.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Karakikes I, Chaanine AH, Kang S, et al. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc. 2013;2(2):e000078. doi: 10.1161/JAHA.113.000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shin JH, Pan X, Hakim CH, et al. Microdystrophin ameliorates muscular dystrophy in the canine model of duchenne muscular dystrophy. Mol Ther. 2013;21(4):750–7. doi: 10.1038/mt.2012.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fish KM, Ladage D, Kawase Y, et al. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circ Heart Fail. 2013;6(2):310–17. doi: 10.1161/CIRCHEARTFAILURE.112.971325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schmidt M, Voutetakis A, Afione S, et al. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82(3):1399–406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Buj-Bello A, Fougerousse F, Schwab Y, et al. AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis. Hum Mol Genet. 2008;17(14):2132–43. doi: 10.1093/hmg/ddn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Howell JM, Walker KR, Davies L, et al. Adenovirus and adeno-associated virus-mediated delivery of human myophosphorylase cDNA and LacZ cDNA to muscle in the ovine model of McArdle's disease: expression and re-expression of glycogen phosphorylase. Neuromuscul Disord. 2008;18(3):248–58. doi: 10.1016/j.nmd.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 199.Gregorevic P, Allen JM, Minami E, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12(7):787–9. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Konkalmatt PR, Beyers RJ, O'Connor DM, et al. Cardiac-selective expression of extracellular superoxide dismutase after systemic injection of adeno-associated virus 9 protects the heart against post-myocardial infarction left ventricular remodeling. Circ Cardiov Imag. 2013;6(3):478–86. doi: 10.1161/CIRCIMAGING.112.000320. [DOI] [PMC free article] [PubMed] [Google Scholar]