Abstract
Previous studies have shown associations between SNPs in GABRA2 and adolescent conduct disorder (CD) and alcohol dependence in adulthood, but not adolescent alcohol dependence. The present study was intended as a replication and extension of this work, focusing on adolescent CD, adolescent alcohol abuse and dependence (AAD), and adult AAD. Family based association tests were run using Hispanics and non-Hispanic European American subjects from two independent longitudinal samples. Although the analysis provided nominal support for an association with rs9291283 and AAD in adulthood and CD in adolescence, the current study failed to replicate previous associations between two well replicated GABRA2 NPs and CD and alcohol dependence. Overall, these results emphasize the utility of including an independent replication sample in the study design, so that the results from an individual sample can be weighted in the context of its reproducibility.
Keywords: alcohol, association, gamma aminobutyric acid receptor alpha 2, human genetic study, single nucleotide polymorphisms
Introduction
Alcohol use is a severe and worldwide problem; statistics from the World Health Organization (WHO) showed that, as of February 2011, the harmful use of alcohol results in 2.5 million deaths each year. Alcohol is the world's 3rd largest risk factor for disease, including cardiovascular disease, liver cirrhosis, and various cancers. Furthermore, 320,000 young people between the ages of 15 and 29 die from alcohol-related causes annually (Stevens et al. 2009). Many current behavior genetic approaches seek to identify some of the underlying genetic factors that confer higher risk to genetically influenced disorders; here, we examine genetic variation in gamma aminobutyric acid receptor alpha 2 (GABRA2) for association with phenotypes relating to alcohol use disorders using two independently ascertained samples.
There is strong evidence that adolescent conduct disorder (CD) is influenced substantially by genetic factors (Rose et al. 2004; Slutske et al. 1997; Gelhorn et al. 2005). CD has been shown to be a robust predictor of both concurrent and future alcohol dependence (Kuperman et al. 2001; Moss and Lynch 2001; White et al. 2001; Palmer et al. 2013) and there is evidence for shared genetic influence between these disorders (Slutske et al. 1998; Kendler et al. 2003; Button et al. 2007). Twin and family studies have also shown evidence for a general vulnerability to substance use disorders (Button et al. 2006), and, more recently, suggested that a general externalizing liability accounts for much of the genetic risk in substance use disorder and behavior disinhibition phenotypes (Hicks et al. 2011). Therefore, one hypothesis is that the development of traits that are influenced by genetic factors can vary over time, where CD may be an adolescent manifestation of genetic factors that predispose to adult alcohol dependence (Dick et al. 2006).
The gamma aminobutyric acid, GABA, neurotransmitter is the predominant inhibitory neurotransmitter in the central nervous system and is a reasonable target for candidate gene studies on alcoholism. Not only are GABA receptors present in the mesolimbic dopamine pathway (Johnson and North 1992; Steffensen et al. 1998), widely believed to play a role in the development of addiction, but studies using both rodent and human brain samples have shown that long-term ethanol exposure, as well as ethanol withdrawal, causes alterations in GABA(A) receptor subunit expression (Lewohl et al. 1997; Mitsuyama et al. 1998; Dodd et al. 1992; Devaud et al. 1996; Grobin et al. 1998; Matthews et al. 1998; Devaud et al. 1997). GABA can also modulate emotion and response to stress, further implicating this neurotransmitter system in drug behaviors (Herman et al. 2004; Martijena et al. 2002).
Results from the Collaborative Study on the Genetics of Alcoholism demonstrated highly significant associations between alcohol dependence and single nucleotide polymorphisms (SNPs) in GABRA2 (Edenberg et al. 2004). Numerous studies have replicated these associations with GABRA2 and alcohol dependence (Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Drgon et al. 2006; Soyka et al. 2008; Covault et al. 2008; Enoch et al. 2009; Bierut et al. 2010; Olfson and Bierut 2012; Philibert et al. 2009; Ittiwut et al. 2012; Villafuerte et al. 2011; Li et al. 2013), although one study has found this effect in the opposite direction (Lind et al. 2008). While these studies have primarily focused on adult alcohol dependence, two studies have examined variation in GABRA2 for adolescent alcohol dependence and CD. These studies found no association with GABRA2 and adolescent alcohol dependence, but found evidence for an association with CD in adolescents (Dick et al. 2006; Sakai et al. 2010). These results are not altogether surprising since estimates of heritability of alcohol dependence are roughly 64% in adulthood (Heath et al. 1997), yet no genetic effects are present in adolescence, with variance in adolescent alcohol dependence being attributed to environmental influences (Rose et al. 2004). It is worth noting however, that a recent longitudinal study showed genetic influences ranging from 35-40% on general substance use disorders between ages 14-17 that decreased with age (Vrieze et al. 2012).
The study presented here was designed as a replication and extension of the Sakai (2010) study, which found some evidence for association with adolescent CD in a subset of the Colorado Center on Antisocial Drug Dependence (CADD) sample (although it did not survive statistical correction for multiple testing). In the current study, the sample size has been expanded and an independent replication sample is now available. We now have an additional wave of data collection; thus adolescent alcohol problems and CD could be evaluated at the first wave of data collection (adolescence and young adulthood) and alcohol abuse and dependence (AAD) subsequently examined in the second wave of data collection (adulthood).
Furthermore, previous studies have focused on the diagnosis of alcohol dependence as a categorical phenotype (Dick et al. 2006; Edenberg et al. 2004; Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Drgon et al. 2006; Soyka et al. 2008; Covault et al. 2008; Enoch et al. 2009; Bierut et al. 2010; Olfson and Bierut 2012; Ittiwut et al. 2012; Li et al. 2013; Sakai et al. 2010). In this study we examined the sum of AAD symptoms as a quantitative trait. There are three reasons for this approach: 1) item-response theory work that has shown that abuse and dependence symptoms provide overlapping information on severity (Gelhorn et al. 2008; Martin et al. 2006; Saha et al. 2006; Langenbucher et al. 2004), 2) this analysis is clinically relevant given the field has moved toward new DSM 5 criteria that merged abuse and dependence symptoms, and 3) continuous variables can provide a more accurate estimate of the phenotype.
Materials and Methods
Samples
Colorado Center on Antisocial Drug Dependence
The sample consisted of non-Hispanic European American (EA) subjects and Hispanic subjects drawn from the Colorado Center on Antisocial Drug Dependence (CADD). The CADD is a longitudinal study currently in its 3rd wave of data collection consisting of four separate samples. The Family Study (FS) is a sample of clinical adolescent probands, ascertained while in treatment for antisocial drug dependence, their family members, and a matched set of control families (Stallings et al. 2003; Stallings et al. 2005). The Colorado Adoption Project (Petrill SA 2003), the Colorado Longitudinal Twin Study (Rhea et al. 2006), and the Colorado Community Twin Study (Rhea et al. 2006) represent community unselected samples that were used in this study for phenotypic standardization (see below). Only subjects from the FS were included in the molecular genetic study. Briefly, clinical probands were recruited from treatment facilities in the Denver area. Probands were selected from individuals who had consecutive admissions to the treatment facilities between February of 1993 and June of 2001. Controls were recruited from the community and matched to the clinical probands based on age, gender, ethnicity, and zip code. All individuals living in the same household as the proband were asked to participate in the study, which created family-based data. For this study data from both wave 1, data collection for which began in 1997 and ended in 2002, and wave 2, data collection for which began in 2002 and ended in 2008, were used. When assessed, buccal cell DNA was collected from subjects who gave voluntary consent. All recruitment, assessment, and DNA collection procedures were approved by the University of Colorado's IRB.
Genetics of Antisocial Drug Dependence
As an independent replication sample, EA subjects and Hispanic subjects from the Genetics of Antisocial Drug Dependence (GADD) were assessed, as previously described (Kamens et al. 2013). Probands in Denver, CO, and San Diego, CA, were identified from treatment programs, involvement with the criminal justice system, or special schools who met at least one of the criteria for having a substance use disorder (other than nicotine dependence) and CD. Siblings of the proband, as well as one or both biological parents, were included in the sample as well. The GADD is also a longitudinal study in the 2nd wave of data collection; data from wave 1, collected between 2001 and 2006, and wave 2, collection of which began in 2009 and is ongoing, were utilized in the analyses. DNA was obtained with consent through either buccal cells or blood. The University of California and the University of Colorado IRBs approved all subject recruitment, assessment, and DNA collection procedures.
SNP selection
The candidate SNPs were identified through review of primary literature, focusing on SNPs that have been previously associated with alcohol dependence and CD in order to replicate previous findings (Dick et al. 2006; Edenberg et al. 2004; Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Soyka et al. 2008; Covault et al. 2008; Enoch et al. 2009; Bierut et al. 2010; Philibert et al. 2009; Ittiwut et al. 2012; Li et al. 2013; Lind et al. 2008; Sakai et al. 2010). Two of the most well-replicated SNPs for alcohol dependence, rs279858 and rs279871, were chosen in addition to 3 other SNPs from GABRA2: rs567926, rs279845, and rs9291283. These three later SNPs have been associated also with alcohol dependence in the literature (Edenberg et al. 2004; Covault et al. 2004; Fehr et al. 2006; Soyka et al. 2008; Covault et al. 2008; Bierut et al. 2010; Philibert et al. 2009; Li et al. 2013; Lind et al. 2008), but with less support than rs279858 and rs279871. Rs567926, rs279845, and rs9291283 were not in high linkage disequilibrium (LD) at r2 < 0.8 with rs279858 during preliminary examination of the haplotype structure of GABRA2 in the Hapmap sample of Utah residents with ancestry from northern and western Europe using Haploview (Barrett et al. 2005). Genomic DNA extracted from buccal or blood cells was amplified with primer extension preamplification (Anchordoquy et al. 2003) or using the REPLI-g kit according to the manufacturers protocol (Qiagen, Valencia, California). Genotyping was performed in the CADD initially and it was observed that two of the SNPs, rs279858 and rs279871, were in high LD (r2 > 0.8 in both Hispanics and EAs) and thus rs279871 was not genotyped in the GADD.
Genotyping
SNP genotyping was performed with TaqMan©® assays for allelic discrimination according to manufacturer's instructions (Applied Biosystems, Foster City, California). Two thousand four hundred and ninety six subjects from CADD and 3,072 subjects from GADD were genotyped. Polymerase Chain Reaction (PCR) reactions were performed with the Biomek® 3000 Laboratory Automation Workstation (Beckman Coulter Inc, Brea, California) and the Dual 384-Well GeneAmp® PCR system 9700 (Applied Biosystems, Foster City, California). To analyze the amplified plates a 7900 Real-Time PCR System (Applied Biosystems, Foster City, California) was used. Based on all the SNPs that have been previously genotyped in these samples from the CADD (33) and GADD (12), DNA samples for subjects with overall call rates <90% were excluded. Three hundred and eighty four samples were genotyped twice for each SNP to determine concordance between replicate reactions; the percent of discordant calls for SNPs in the CADD and GADD, respectively, are shown as follows: rs567926 (0.78%, 0.00%), rs279858 (0.78%, 0.26%), rs279871 (0.52%, N/A), rs279845 (1.30%, 0.00%), rs9291283 (0.26%, 0.78%). Genotype clusters from the amplified 384 well plates were auto-called by the Applied Biosystems TaqMan® Genotyper software (Applied Biosystems, Foster City, California) and subsequently visually examined by two independent lab personnel to verify calls. Final calls were determined when both laboratory personnel agreed; if they did not agree genotypic data were excluded.
Statistical Analysis
Data descriptive
Mendelian errors were identified using FBAT (Rabinowitz and Laird 2000). If Mendelian errors were detected, the SNP genotype for that family was removed. Using Haploview (Barrett et al. 2005), pairwise LD (r2) and Hardy-Weinberg equilibrium (HWE) were evaluated.
Analysis of the CADD sample
Three phenotypes were examined: lifetime sum CD symptoms in adolescence/young adulthood, sum AAD symptoms in adolescence/young adulthood, and sum AAD symptoms in adulthood. CD was assessed in adolescents using the Diagnostic Interview Schedule for Children (DISC) (Shaffer et al. 1993). Early study participants were evaluated with DISC 2.3, which assessed DSM IIIR diagnoses. Later participants were assessed using DISC IV, which assessed DSM IV diagnoses. CD was assessed for subjects over the age of 18 utilizing the Diagnostic Interview Schedule (DIS) (Robins et al. 1981), and, similar to the DISC, began with DSM IIIR diagnoses and finished with DSM IV diagnoses. DSM IV defined AAD were assessed with the Composite International Diagnostic Interview – Substance Abuse Module (CIDI-SAM) (Cottler and Keating 1990). As comorbidity is high in this sample, subjects with comorbid drug use were not excluded (Stallings et al. 2003). Each variable was standardized and analyzed as described below.
Conduct Disorder
For the adolescent/young adult lifetime CD symptoms score, data were drawn from wave 1. The majority of subjects were assessed using DSM IV criteria but a small proportion were assessed using DSM IIIR criteria. Sum symptom counts for CD were standardized based on the distribution of symptoms in the CADD community sample. A linear regression was performed using Statistical Analysis System (SAS) 9.3 software (SAS Institute Inc., Cary, NC) to determine residuals from sex, age, and age squared. The coefficients from the CADD community sample were applied to the CADD clinical sample (i.e. Z scores of clinical subjects were expressed as deviations from the means of the community samples). In this case, clinical subjects assessed using DSM IV criteria were standardized to the community subjects assessed with DSM IV criteria, and the same procedure was used for subjects assessed with DSM IIIR criteria. After standardization, only phenotypic data from subjects between the ages of 10 and 25 were included in the analysis. The analysis was performed on 1,789 subjects and run as described in a later section.
Adolescent/young adult alcohol abuse and dependence
For the adolescent/young adult AAD symptoms sum score, data were drawn from wave 1 of data collection. All sum AAD counts were assessed using DSM IV criteria. Only phenotypic data for subjects who had previously used alcohol were included; in other words, if a subject had never had a drink of alcohol they were excluded from the analysis. Of the 492 adolescent subjects with no dependence symptoms, 25.6% of them endorsed an abuse criterion, strengthening the argument for inclusion of abuse symptoms. Sum symptom counts were standardized in the same manner described for CD above, using residuals and coefficients derived in the community sample and applying these to the clinical subjects. After standardization, only phenotypic data from subjects between the ages of 10 and 25 were included for analysis. The analysis was performed on 1,199 subjects.
Adult alcohol abuse and dependence
For adult AAD symptoms sum score, data were drawn from wave 2. All sum AAD counts were assessed using DSM IV criteria. Only phenotypic data for subjects who had previously used alcohol were included. Of the 246 adult subjects with no dependence symptoms, 25.6% of them endorsed an abuse criterion. Sum symptom counts were standardized using the CADD community sample as above for CD and adolescent AAD. For the analysis only subjects assessed at wave 2 that were also included in the adolescent/young adult analyses of CD and AAD were analyzed. The analysis was performed on 703 subjects.
Analysis of the GADD sample
As in the CADD, CD was assessed in adolescents using the DISC and in subjects over 18 using the DIS. All study participants were evaluated using DSM IV diagnoses. DSM IV defined AAD were assessed with the CIDI-SAM. Subjects with comorbid drug use were not excluded. Each variable was standardized and analyzed as described below.
Conduct disorder
For lifetime CD symptoms, data were drawn from wave 1. All of the subjects were assessed using DSM IV criteria. The GADD sample includes only clinical subjects, so the coefficients from the standardization of the DSM IV CD symptoms from the CADD community sample were applied to the GADD sample (i.e. Z scores of GADD subjects were expressed as deviations from the means of the CADD community samples). After standardization, only phenotypic data from subjects between the ages of 10 and 25 were analyzed. The analysis was performed on 1,540 subjects.
Adolescent/young adult alcohol abuse and dependence
Adolescent/young adult AAD symptoms were drawn from wave 1 of data collection. All items were assessed using DSM IV criteria. As in the CADD, only phenotypic data for subjects who had previously used alcohol were included. Of the 572 adolescent subjects with no dependence symptoms, 10.5% of them endorsed an abuse criterion. For standardization, the coefficients from the standardization of the adolescent AAD symptoms from the CADD community sample were applied to the GADD sample. After standardization, only phenotypic data from subjects between the ages of 10 and 25 were included. The analysis was performed on 1,186 subjects.
Adult alcohol abuse and dependence
Adult AAD symptoms were drawn from wave 2. All items were assessed using DSM IV criteria. Only phenotypic data for subjects who had previously used alcohol were included. Of the 150 adult subjects with no dependence symptoms, 30.7% of them endorsed an abuse criterion. For standardization, the coefficients from the standardization of the adult AAD symptoms from the CADD community sample were applied to the GADD sample. For the analysis only standardized phenotypic data from the subjects assessed at wave 2 that were concurrently included in the GADD adolescent/young adult analyses of CD and AAD were analyzed. The analysis was performed on 873 subjects.
Combined Analysis of the CADD and GADD samples
To increase statistical power, the GADD and CADD samples were combined after the data were cleaned in each sample (e.g. removal of Mendelian errors and phenotypic standardization). The analysis of the combined sample was run as described below.
Statistical Analysis
The data were analyzed using an additive genetic model in a family-based association test performed in FBAT (Rabinowitz and Laird 2000). FBAT builds on the original Transmission Disequilibrium Test (TDT) (Spielman et al. 1993), where alleles transmitted to extreme offspring are compared to the expected distribution of alleles among offspring under Mendel's law of segregation and conditioning. Three phenotypes were analyzed using an additive test in FBAT: adolescent/young adult CD, adolescent/young adult AAD, and adult AAD.
Correction for multiple testing
We used the spectral decomposition (SNPSpD) method (Nyholt 2004) to estimate the minimum p-value required to keep experimental type I error < 0.05. This method is used as a correction for multiple testing for SNPs in linkage disequilibrium with each other. All Hispanic and EA family members were included to define a new p-value used to correct for multiple testing. The spectral decomposition was run using each sample separately to provide a corrected p-value for each analysis of the CADD, GADD, and CADD/GADD combined samples.
Results
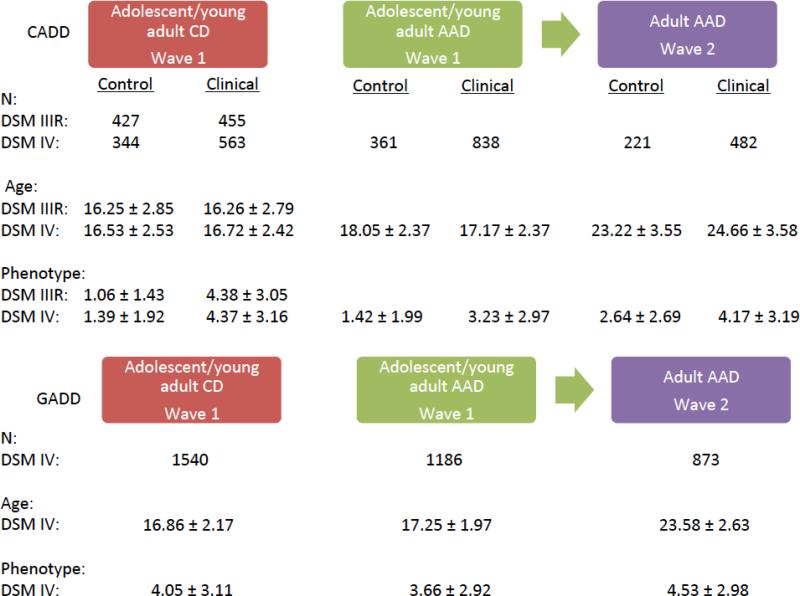
The phenotypic characteristics between the CADD clinical and GADD samples are comparable (Figure 1). Although the same subjects were used in the adolescent/young adult CD and AAD analyses, only phenotypic data from subjects that had used alcohol were included in the adolescent/young adult ADD analysis, accounting for the difference in sample size between the two adolescent/young adult analyses. This was done because most of the previous studies that found associations between GABRA2 and alcohol dependence used alcohol dependent cases and matched controls in their analyses (Edenberg et al. 2004; Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Drgon et al. 2006; Soyka et al. 2008; Covault et al. 2008; Enoch et al. 2009; Bierut et al. 2010; Olfson and Bierut 2012; Ittiwut et al. 2012), thus only subjects that had used alcohol were included in the present study in order to minimize sample differences between the current and past studies. Although our adolescent/young adult analyses include subjects of a substantially large age range, this is the approximate age range for probands in wave 1 of the CADD and GADD samples.
Figure 1.
Study sample characteristics of the CADD and GADD. Each phenotypic variable is shown with the respective wave the data were drawn from. As CADD is a sample comprised of clinical and control subjects, variable descriptives for each are shown separately. The mean ± the standard deviation is shown for age, lifetime CD symptoms, and lifetime adolescent/young adult and adult AAD symptoms for each variable used in the analysis, as well as the sample size for each variable.
The minor alleles were identical in EAs and Hispanics and the minor allele frequencies were similar for rs567926, rs279858, rs279845, and rs9291283 (Table I). Hispanic and EA subjects were combined for all analyses since the allele frequencies did not differ significantly. There were no significant deviations from the Hardy-Weinberg equilibrium at p < 0.05.
Table I.
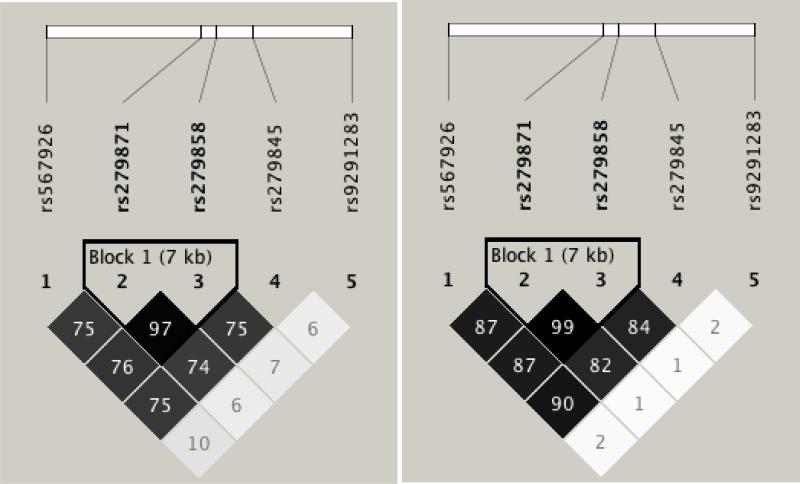
Genotypic characteristics of the CADD and GADD samples for each ethnic group. Location refers to base pair position on chromosome 4 from the UCSC genome browser. Alleles shown are major/minor allele. MAF: minor allele frequency. HWE refers to the Hardy-Weinberg equilibrium p-value. Due to high LD between rs279858 and rs279871 as shown in Figure 2, rs279871 was not genotyped in the GADD sample.
| CADD | GADD | |||||||
|---|---|---|---|---|---|---|---|---|
| Ethnicity | SNP | Location | Alleles | MAF | HWE | Alleles | MAF | HWE |
| Non-Hispanic European American | rs567926 | 46241769 | A/G | 0.48 | 0.87 | A/G | 0.42 | 0.07 |
| rs279858 | 46314593 | T/C | 0.48 | 0.86 | T/C | 0.42 | 0.21 | |
| rs279871 | 46307533 | T/C | 0.48 | 0.82 | - | - | - | |
| rs279845 | 46329723 | T/A | 0.49 | 0.98 | T/A | 0.44 | 0.59 | |
| rs9291283 | 46371833 | G/A | 0.27 | 0.87 | G/A | 0.25 | 0.98 | |
| Hispanic | rs567926 | 46241769 | A/G | 0.50 | 0.05 | A/G | 0.44 | 0.98 |
| rs279858 | 46314593 | T/C | 0.50 | 0.14 | T/C | 0.46 | 0.80 | |
| rs279871 | 46307533 | T/C | 0.50 | 0.07 | - | - | - | |
| rs279845 | 46329723 | A/T | 0.49 | 0.17 | T/A | 0.46 | 0.61 | |
| rs9291283 | 46371833 | G/A | 0.22 | 0.21 | G/A | 0.17 | 0.54 | |
In EA and Hispanic subjects in the CADD, the SNPs were somewhat correlated with an r2 > 0.50, with the exception of rs9291283 (Figure 2A and 2B, respectively). As mentioned previously, rs279858 and rs279871 were highly correlated at r2 > 0.80. Similar LD patterns were observed in EAs and Hispanics in the GADD sample as in the CADD sample (Figure 3A and 3B, respectively), which is in agreement with the similarity in allele frequencies. In addition, the r2 value between rs9291283 and all other SNPs examined was less than 0.10, indicating this SNP represents an independent signal in the gene.
Figure 2.
Linkage Disequilibrium (LD) plot generated by Haploview (Barrett et al. 2005) for the SNPs chosen in the CADD sample. Numbers in the boxes are r2 x 100. A) LD plot for EAs. Note that rs279858 and rs279871 are in high LD. B) LD plot for Hispanics.
Figure 3.
Linkage Disequilibrium (LD) plot generated by Haploview (Barrett et al. 2005) for the SNPs chosen in the GADD sample. Numbers in the boxes are r2 x 100. A) LD plot for EAs. B) LD plot for Hispanics.
As expected from previous analyses of these SNPs, no significant associations were detected with AAD in adolescence/young adulthood in the CADD, GADD, or combined sample (results not shown).
Rs9291283 was suggestively associated with AAD in adulthood in the CADD sample (Table II). Results from the spectral decomposition to correct for multiple SNP testing indicated the significance threshold required to keep Type I error rate at 5% is 0.017. One should bear in mind an additional correction for the four multiple phenotypes, but since the phenotypes are correlated we present results as comparison to the 0.017 cut-off. Although rs279871 was included in the spectral decomposition and FBAT analysis it was not significantly associated with the phenotypes of interest (results not shown). In this analysis rs9291283 showed a trend toward association with AAD in adulthood at p = 0.063, with the A allele conferring risk to AAD.
Table II.
Results from the FBAT analysis in the CADD. Only the results for the risk allele are shown. The number of informative families used in the analysis is shown to the right of the p-value.
| Phenotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD at adolescence/young adulthooda | AAD at adulthooda | |||||||
| SNP | Risk Allele | Z score | P-value | # Fam | Risk Allele | Z score | P-value | # Fam |
| rs567926 | A | 0.387 | 0.699 | 214 | G | 1.625 | 0.104 | 164 |
| rs279858 | C | 0.180 | 0.857 | 232 | C | 0.525 | 0.599 | 170 |
| rs279845 | T | 1.283 | 0.199 | 202 | A | 1.100 | 0.271 | 159 |
| rs9291283 | G | 0.997 | 0.319 | 172 | A | 1.859 | 0.063b | 129 |
The sign of the Z score indicates which allele is over transmitted; a positive Z score indicates an allele is over transmitted and is interpreted as the risk allele.
Indicates trending p-values at p < 0.1.
In the GADD sample, rs9291283 was associated with CD (Table III). Results from spectral decomposition indicated a multiple SNP significance threshold required to keep Type I error rate at 5% is 0.019. In this sample, rs9291283 was significantly associated with CD in adolescence/young adulthood at p = 0.005, a finding that was not seen in the CADD sample. Again, the A allele was shown to be over-transmitted to extreme offspring.
Table III.
Results from the FBAT analysis using subjects in the GADD. Only the results for the risk allele are shown. The number of informative families used in the analysis is shown to the right of the p-value.
| Phenotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD at adolescence/young adulthooda | AAD at adulthooda | |||||||
| SNP | Risk Allele | Z score | P-value | # Fam | Risk Allele | Z score | P-value | # Fam |
| rs567926 | G | 1.503 | 0.133 | 242 | G | 0.925 | 0.355 | 132 |
| rs279858 | C | 0.885 | 0.376 | 258 | C | 0.863 | 0.388 | 146 |
| rs279845 | 1.050 | 0.294 | 258 | A | 0.700 | 0.484 | 142 | |
| rs9291283 | A | 2.809 | 0.005b | 184 | A | 0.734 | 0.463 | 95 |
The sign of the Z score indicates which allele is over transmitted; a positive Z score indicates an allele is over transmitted and is interpreted as the risk allele.
Indicates significant p-values after correction for multiple SNP testing at p = 0.019.
In the combined CADD and GADD analysis, rs9291283 was nominally associated with AAD in adulthood (p = 0.057; Table IV). Results from the spectral decomposition set the multiple SNP significance threshold required to keep Type I error rate at 5% at 0.019. In this analysis rs9291283 showed a trend toward association with AAD in adulthood at p = 0.057, replicating the trending association between rs9291283 and adult AAD in the CADD sample. However, neither of these associations was statistically significant.
Table IV.
Results from the FBAT analysis using subjects in the CADD and GADD combined sample. Only the results for the risk allele are shown. The number of informative families used in the analysis is shown to the right of the p-value.
| Phenotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD at adolescence/young adulthooda | AAD at adulthooda | |||||||
| SNP | Risk Allele | Z score | P-value | # Fam | Risk Allele | Z score | P-value | # Fam |
| rs567926 | G | 0.934 | 0.350 | 456 | G | 1.809 | 0.070b | 296 |
| rs279858 | C | 0.787 | 0.431 | 490 | C | 0.973 | 0.331 | 316 |
| rs279845 | A | 0.071 | 0.943 | 460 | A | 1.270 | 0.204 | 301 |
| rs9291283 | A | 1.494 | 0.135 | 356 | A | 1.902 | 0.057b | 224 |
The sign of the Z score indicates which allele is over transmitted; a positive Z score indicates an allele is over transmitted and is interpreted as the risk allele.
Indicates trending p-values at p < 0.1.
Discussion
The goals of this study were to expand on previous work demonstrating strong associations of GABRA2 gene SNPs with alcohol dependence in adulthood (Edenberg et al. 2004; Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Drgon et al. 2006; Soyka et al. 2008; Covault et al. 2008; Enoch et al. 2009; Bierut et al. 2010; Olfson and Bierut 2012; Philibert et al. 2009; Ittiwut et al. 2012; Villafuerte et al. 2011; Li et al. 2013; Lind et al. 2008), and replicate work showing a suggestive association with CD in younger samples (Dick et al. 2006; Sakai et al. 2010). This study was an important extension of the Sakai (2010) study; not only was adolescent CD and AAD assessed in a larger sample, but AAD in adulthood as well. This study design enabled the examination of genetic influences on the development of three traits over time.
In considering the SNPs in high LD and comparing results to previous reports, our findings are in agreement with Dick (2006) and Sakai (2010) in not finding an association with adolescent AAD and rs279871 or rs279858 in GABRA2, yet our results contrast with theirs in findings with CD. In the GADD sample, rs9291283 was associated with CD in adolescence/young adulthood (p = 0.005). This result was not seen in the CADD sample, or in the combined CADD and GADD sample. This may be due in part to the finding that, for CD, the G allele was over-transmitted to affected offspring in the CADD sample. Although this finding was not significant, it could explain why, when the two samples were combined, rs9291283 was not associated with CD despite the fact it was the strongest association in the study. In the Dick (2006) and Sakai (2010) studies, rs279871 was found to be associated with adolescent CD, yet here neither rs279871 nor the SNP we examined in high LD (r2 > 0.8) with this SNP, rs279858, was associated with CD. Interestingly, the Sakai (2010) study used two association approaches: a case control and a family based approach. While the case control approach indicated evidence of an association between rs279871 and adolescent CD, the family based approach did not. This is particularly note-worthy in that there was some overlap between our wave 1 sample and the sample used by Sakai; eight hundred and thirty four Hispanic and EA subjects were used in both the present study and the study by Sakai et al. (2010). In addition to using different statistical approaches, non-replication may be related to the inclusion of both adolescents and young adults since both previous studies used only adolescents. Furthermore, both Dick (2006) and Sakai (2010) used primarily DSM IIIR criteria for establishing CD symptoms while our study used primarily DSM IV criteria. Although the DSM IV and DSM IIIR classifications contain many of the same criteria for CD, the DSM IV, compared to DSM IIIR, introduced two new criteria, staying out late without permission (prior to age 13) and bullying, and also required that truancy began before age 13 to be counted. Recently however, a study by Dick and colleagues found evidence for association between rs279871 and subclinical self-reports of externalizing behavior, yet no evidence for association between rs279871 and DSM IV CD symptoms in adults or adolescents (Dick et al. 2013), lending support to our findings.
Rs9291283 was nominally associated with adult AAD in the CADD and combined CADD and GADD samples. Although rs9291283 has been previously shown to be nominally associated with alcohol dependence (Covault et al. 2004; Soyka et al. 2008; Bierut et al. 2010), rs279858 has been the most well replicated SNP in GABRA2 associated with adult alcohol dependence (Edenberg et al. 2004; Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Bierut et al. 2010; Villafuerte et al. 2011; Li et al. 2013), a finding which failed to replicate in our study. This result may be related to slight differences in the alcohol phenotypes examined. Most of the previous studies (Edenberg et al. 2004; Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Drgon et al. 2006; Soyka et al. 2008; Covault et al. 2008; Enoch et al. 2009; Bierut et al. 2010; Olfson and Bierut 2012; Philibert et al. 2009; Ittiwut et al. 2012; Li et al. 2013) have focused specifically on alcohol dependence. However, recent item response theory work has shown that AAD symptoms provide overlapping information on severity (Gelhorn et al. 2008; Martin et al. 2006; Saha et al. 2006; Langenbucher et al. 2004), so we included both alcohol abuse and dependence scores in our analysis. Although many studies have replicated GABRA2 SNP associations with adult alcohol dependence, there have been a few studies whose findings parallel our own (Matthews et al. 2007; Lydall et al. 2011; Onori et al. 2010) or contrast in directionality from the majority (Lind et al. 2008). Matthews et al. (2007) found no association between rs279871 and rs279858 with alcohol dependence in a linkage study. Similarly, Lydall et al. (2011) found no association between rs279871, rs279858, or rs9291283 and alcohol dependence using a case control design. Of particular interest is the study by Onori et al. (2010) examining alcohol use disorders; no significant associations were found with rs567926, rs279871, rs279858, or rs279845. Finally, Lind et al. (2008) found evidence for association with rs279858 with quantitative sum alcohol dependence symptoms, as well as a single principal component factor score from the DSMIV alcohol dependence symptoms. However, the direction of effect was opposite from that previously observed and replicated (Edenberg et al. 2004; Covault et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006; Bierut et al. 2010; Villafuerte et al. 2011; Li et al. 2013) and furthermore did not survive correction for multiple testing. Thus the overall results for association of this region with alcohol disorders remain mixed.
There are some important strengths, as well as limitations, in our study. In particular, the use of a family-based design protects against population stratification. With the exception of the Sakai (2010) study, prior analyses used primarily subjects of one ethnicity. While many ethnicities have been represented, including European American, Japanese, and African American populations, our study represents one of the first to include subjects of two ethnicities. Furthermore, the inclusion of the Hispanics provided a larger sample size. While it is possible that distributional differences in CD and AAD may be culturally influenced and affect our results, we do not believe this is the case because the distributions for CD and adult AAD between EAs and Hispanics were comparable in our samples. In addition, we used a continuous variable to examine CD and AAD, rather than dichotomous variable. Continuous variables provide a more accurate estimate of the phenotype, as dichotomizing variables can cause a loss of information. Moreover, our incorporation of both AAD symptoms is unique. This study, however, is not without limitations. Specifically, there is low power to detect and replicate genetic associations when the CADD and GADD samples are analyzed separately, a fact that could explain false positives. In addition, there might exist unidentified ascertainment or sample characteristics differences between the CADD and GADD samples, again leading to inconsistent results. High comorbid drug use is also present in our samples, which could confound results. Although we attempted to capture all the genetic signals in the region, some may have been missed. Furthermore, it is becoming clear that the genetic risk for alcohol disorders is likely due to common variants in many genes, each of small effect, as well as rare variants with potentially large effects (Enoch 2013). The advent of next generation sequencing methods may allow identification of novel rare variants in future studies.
The analyses presented here were run in several different ways, all of which showed consistent results. Although we limited our sample size in the adult AAD analyses by only including those subjects who where included in the adolescent/young adult CD and AAD, when we did not impose this restriction and included all subjects from waves 1 and 2 the results yielded the same findings. The goal of limiting the samples was to ensure that each subject in the analysis was represented at each time point. Furthermore, when subjects who had never had alcohol were included in the analysis, the results were comparable.
The ideal end goal of many of these human molecular genetic studies is to inform future pharmacogenetic studies of the role of certain polymorphisms in genetically influenced disorders. While it is likely to take time for these approaches to be implemented in clinical practice, molecular genetic studies serve to identify possible targets for novel treatments. Although our findings provide limited evidence for a role of GABRA2 in CD and AD, GABRA2 is also a likely candidate for disorders such as anxiety, general substance abuse, depression, and schizophrenia (Engin et al. 2012). For example, a recent haplotype analysis of rs9291283, the SNP with which we found the strongest evidence of association with CD in the GADD sample, revealed a significant association with cocaine addiction, and it has been postulated that rs894269, a SNP in high LD with rs9291283, lies in a cis-enhancer region of the gene. Future studies examining the possible function of rs894269 should be pursued in order to examine whether it may represent the underlying causal variant contributing the association seen with its correlates, including rs9291283 (Dixon et al. 2010).
In conclusion, our results provide limited support for an association between rs9291283 and CD in adolescence/young adulthood and AAD in adulthood. Unfortunately, we did not replicate previous findings showing associations between rs279871 and rs279858 with adolescent CD and adult AAD, respectively. However, perhaps the more important implication here is the importance of replication and combining studies. There is an increasing literature on negative and non-replicated findings and the field of behavior genetics has yet to identify the reasons for this (Hewitt 2012). There are many possibilities, including differences in sample ascertainment, assessment, as discussed above. It is equally likely that many of the initial positive associations are false positives, and this study demonstrates the utility of including an independent replication sample in the study design, so that the results from an individual sample can be weighted in the context of their reproducibility.
Acknowledgements
Thanks to Thomas Crowley, an important contributor to the CADD and GADD data collection. This work supported by AA019447, DA011015, DA012845, and DA017637.
References
- Anchordoquy HC, McGeary C, Liu L, Krauter KS, Smolen A. Genotyping of three candidate genes after whole-genome preamplification of DNA collected from buccal cells. Behavior genetics. 2003;33(1):73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr., Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107(11):5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Res Hum Genet. 2006;9(1):38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- Button TM, Rhee SH, Hewitt JK, Young SE, Corley RP, Stallings MC. The role of conduct disorder in explaining the comorbidity between alcohol and illicit drug dependence in adolescence. Drug and alcohol dependence. 2007;87(1):46–53. doi: 10.1016/j.drugalcdep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Keating SK. Operationalization of alcohol and drug dependence criteria by means of a structured interview. Recent Dev Alcohol. 1990:869–83. [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B(1):104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5′-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2008;33(4):837–848. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. Journal of neurochemistry. 1997;69(1):126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. The Journal of pharmacology and experimental therapeutics. 1996;278(2):510–517. [PubMed] [Google Scholar]
- Dick DM, Aliev F, Latendresse S, Porjesz B, Schuckit M, Rangaswamy M, Hesselbrock V, Edenberg H, Nurnberger J, Agrawal A, Bierut L, Wang J, Bucholz K, Kuperman S, Kramer J. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin Res Hum Genet. 2013;16(3):661–669. doi: 10.1017/thg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr., Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dixon CI, Morris HV, Breen G, Desrivieres S, Jugurnauth S, Steiner RC, Vallada H, Guindalini C, Laranjeira R, Messas G, Rosahl TW, Atack JR, Peden DR, Belelli D, Lambert JJ, King SL, Schumann G, Stephens DN. Cocaine effects on mouse incentive-learning and human addiction are linked to alpha2 subunit-containing GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2289–2294. doi: 10.1073/pnas.0910117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd PR, Thomas GJ, Harper CG, Kril JJ. Amino acid neurotransmitter receptor changes in cerebral cortex in alcoholism: effect of cirrhosis of the liver. Journal of neurochemistry. 1992;59(4):1506–1515. doi: 10.1111/j.1471-4159.1992.tb08467.x. [DOI] [PubMed] [Google Scholar]
- Drgon T, D'Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(8):854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr., O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Liu J, Rudolph U. alpha2-containing GABA(A) receptors: a target for the development of novel treatment strategies for CNS disorders. Pharmacology & therapeutics. 2012;136(2):142–152. doi: 10.1016/j.pharmthera.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. Genetic influences on the development of alcoholism. Curr Psychiatry Rep. 2013;15(11):412. doi: 10.1007/s11920-013-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan Q, Albaugh B, Virkkunen M, Goldman D. GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology. 2009;34(5):1245–1254. doi: 10.1038/npp.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16(1):9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Gelhorn H, Hartman C, Sakai J, Stallings M, Young S, Rhee SH, Corley R, Hewitt J, Hopfer C, Crowley T. Toward DSM-V: an item response theory analysis of the diagnostic process for DSM-IV alcohol abuse and dependence in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1329–1339. doi: 10.1097/CHI.0b013e318184ff2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelhorn HL, Stallings MC, Young SE, Corley RP, Rhee SH, Hewitt JK. Genetic and environmental influences on conduct disorder: symptom, domain and full-scale analyses. J Child Psychol Psychiatry. 2005;46(6):580–591. doi: 10.1111/j.1469-7610.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139(1-2):2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27(6):1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Annals of the New York Academy of Sciences. 2004:101835–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Hewitt JK. Editorial policy on candidate gene association and candidate gene-by-environment interaction studies of complex traits. Behavior genetics. 2012;42(1):1–2. doi: 10.1007/s10519-011-9504-z. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior genetics. 2011;41(4):459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittiwut C, Yang BZ, Kranzler HR, Anton RF, Hirunsatit R, Weiss RD, Covault J, Farrer LA, Gelernter J. GABRG1 and GABRA2 variation associated with alcohol dependence in African Americans. Alcoholism, clinical and experimental research. 2012;36(4):588–593. doi: 10.1111/j.1530-0277.2011.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Corley RP, McQueen MB, Stallings MC, Hopfer CJ, Crowley TJ, Brown SA, Hewitt JK, Ehringer MA. Nominal association with CHRNA4 variants and nicotine dependence. Genes Brain Behav. 2013 doi: 10.1111/gbb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. Am J Psychiatry. 2001;158(12):2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Langenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L, Chung T. An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSMIV. J Abnorm Psychol. 2004;113(1):72–80. doi: 10.1037/0021-843X.113.1.72. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29(4):493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Crane DI, Dodd PR. Expression of the alpha 1, alpha 2 and alpha 3 isoforms of the GABAA receptor in human alcoholic brain. Brain Res. 1997;751(1):102–112. doi: 10.1016/s0006-8993(96)01396-0. [DOI] [PubMed] [Google Scholar]
- Li D, Sulovari A, Cheng C, Zhao H, Kranzler HR, Gelernter J. Association of Gamma-Aminobutyric Acid A Receptor alpha2 Gene (GABRA2) with Alcohol Use Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Agrawal A, Montgomery GW, Heath AC, Martin NG, Whitfield JB. The role of GABRA2 in alcohol dependence, smoking, and illicit drug use in an Australian population sample. Alcohol Clin Exp Res. 2008;32(10):1721–1731. doi: 10.1111/j.1530-0277.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall GJ, Saini J, Ruparelia K, Montagnese S, McQuillin A, Guerrini I, Rao H, Reynolds G, Ball D, Smith I, Thomson AD, Morgan MY, Gurling HM. Genetic association study of GABRA2 single nucleotide polymorphisms and electroencephalography in alcohol dependence. Neurosci Lett. 2011;500(3):162–166. doi: 10.1016/j.neulet.2011.05.240. [DOI] [PubMed] [Google Scholar]
- Martijena ID, Rodriguez Manzanares PA, Lacerra C, Molina VA. Gabaergic modulation of the stress response in frontal cortex and amygdala. Synapse. 2002;45(2):86–94. doi: 10.1002/syn.10085. [DOI] [PubMed] [Google Scholar]
- Martin CS, Chung T, Kirisci L, Langenbucher JW. Item response theory analysis of diagnostic criteria for alcohol and cannabis use disorders in adolescents: implications for DSM-V. J Abnorm Psychol. 2006;115(4):807–814. doi: 10.1037/0021-843X.115.4.807. [DOI] [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: within-family association and linkage analyses. J Stud Alcohol Drugs. 2007;68(5):625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABA(A) receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. Journal of neurochemistry. 1998;70(3):1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- Mitsuyama H, Little KY, Sieghart W, Devaud LL, Morrow AL. GABA(A) receptor alpha1, alpha4, and beta3 subunit mRNA and protein expression in the frontal cortex of human alcoholics. Alcoholism, clinical and experimental research. 1998;22(4):815–822. [PubMed] [Google Scholar]
- Moss HB, Lynch KG. Comorbid disruptive behavior disorder symptoms and their relationship to adolescent alcohol use disorders. Drug and alcohol dependence. 2001;64(1):75–83. doi: 10.1016/s0376-8716(00)00233-7. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. American journal of human genetics. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson E, Bierut LJ. Convergence of Genome-Wide Association and Candidate Gene Studies for Alcoholism. Alcoholism, clinical and experimental research. 2012 doi: 10.1111/j.1530-0277.2012.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onori N, Turchi C, Solito G, Gesuita R, Buscemi L, Tagliabracci A. GABRA2 and alcohol use disorders: no evidence of an association in an Italian case-control study. Alcohol Clin Exp Res. 2010;34(4):659–668. doi: 10.1111/j.1530-0277.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Knopik VS, Rhee SH, Hopfer CJ, Corley RC, Young SE, Stallings MC, Hewitt JK. Prospective effects of adolescent indicators of behavioral disinhibition on DSM-IV alcohol, tobacco, and illicit drug dependence in young adulthood. Addict Behav. 2013;38(9):2415–2421. doi: 10.1016/j.addbeh.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SAPR, DeFries JC, Hewitt JK. Nature, nurture, and the transition to early adolescence. Oxford University Press. 2003 [Google Scholar]
- Philibert RA, Gunter TD, Beach SR, Brody GH, Hollenbeck N, Andersen A, Adams W. Role of GABRA2 on risk for alcohol, nicotine, and cannabis dependence in the Iowa Adoption Studies. Psychiatric genetics. 2009;19(2):91–98. doi: 10.1097/YPG.0b013e3283208026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz D, Laird N. A unified approach to adjusting association tests for population admixture with arbitrary pedigree structure and arbitrary missing marker information. Hum Hered. 2000;50(4):211–223. doi: 10.1159/000022918. [DOI] [PubMed] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado Twin Registry. Twin Res Hum Genet. 2006;9(6):941–949. doi: 10.1375/183242706779462895. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38(4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcoholism, clinical and experimental research. 2004;28(10):1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36(7):931–941. doi: 10.1017/S003329170600746X. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Stallings MC, Crowley TJ, Gelhorn HL, McQueen MB, Ehringer MA. Test of association between GABRA2 (SNP rs279871) and adolescent conduct/alcohol use disorders utilizing a sample of clinic referred youth with serious substance and conduct problems, controls and available first degree relatives. Drug Alcohol Depend. 2010;106(2-3):199–203. doi: 10.1016/j.drugalcdep.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Schwab-Stone M, Fisher P, Cohen P, Piacentini J, Davies M, Conners CK, Regier D. The Diagnostic Interview Schedule for Children-Revised Version (DISC-R): I. Preparation, field testing, interrater reliability, and acceptability. J Am Acad Child Adolesc Psychiatry. 1993;32(3):643–650. doi: 10.1097/00004583-199305000-00023. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Modeling genetic and environmental influences in the etiology of conduct disorder: a study of 2,682 adult twin pairs. J Abnorm Psychol. 1997;106(2):266–279. doi: 10.1037//0021-843x.106.2.266. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107(3):363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42(3):184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). American journal of human genetics. 1993;52(3):506–516. [PMC free article] [PubMed] [Google Scholar]
- Stallings MC, Corley RP, Dennehey B, Hewitt JK, Krauter KS, Lessem JM, Mikulich-Gilbertson SK, Rhee SH, Smolen A, Young SE, Crowley TJ. A genome-wide search for quantitative trait Loci that influence antisocial drug dependence in adolescence. Arch Gen Psychiatry. 2005;62(9):1042–1051. doi: 10.1001/archpsyc.62.9.1042. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Corley RP, Hewitt JK, Krauter KS, Lessem JM, Mikulich SK, Rhee SH, Smolen A, Young SE, Crowley TJ. A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug and alcohol dependence. 2003;70(3):295–307. doi: 10.1016/s0376-8716(03)00031-0. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(19):8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens G, Mascarenhas M, Mathers C. Global health risks: progress and challenges. Bull World Health Organ. 2009;87(9):646. doi: 10.2471/BLT.09.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Wendy Yau WY, Majczenko K, Zubieta JK, Zucker RA, Burmeister M. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Molecular psychiatry. 2011 doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Hicks BM, Iacono WG, McGue M. Decline in genetic influence on the cooccurrence of alcohol, marijuana, and nicotine dependence symptoms from age 14 to 29. Am J Psychiatry. 2012;169(10):1073–1081. doi: 10.1176/appi.ajp.2012.11081268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Xie M, Thompson W, Loeber R, Stouthamer-Loeber M. Psychopathology as a predictor of adolescent drug use trajectories. Psychol Addict Behav. 2001;15(3):210–218. [PubMed] [Google Scholar]