SUMMARY
Functional connectivity between the prefrontal cortex (PFC) and striatum (STR) is thought critical for cognition, and has been linked to conditions like autism and schizophrenia. We recorded from multiple electrodes in PFC and STR while monkeys acquired new categories. Category learning was accompanied by an increase in beta-band synchronization of LFPs between, but not within, the PFC and STR. After learning, different pairs of PFC-STR electrodes showed stronger synchrony for one or the other category, suggesting category-specific functional circuits. This category-specific synchrony was also seen between PFC spikes and STR LFPs, but not the reverse, reflecting the direct monosynaptic connections from the PFC to STR. However, causal connectivity analyses suggested that the polysynaptic connections from STR to the PFC exerted a stronger overall influence. This supports models positing that the basal ganglia “train” the PFC. Category learning may depend on the formation of functional circuits between the PFC and STR.
Anatomical loops between the prefrontal cortex (PFC) and basal ganglia (BG) suggest a close functional relationship, but the nature of their interactions is not yet understood. It is clear that both areas are critical for learning. One hypothesis is that they have different types of plasticity: The BG (in particular the striatum or STR) are thought to rapidly acquire simple information (single associations, decision alternatives, etc) in piecemeal fashion while the PFC knits together such details into more elaborate and generalized representations (Daw et al., 2005). Interactions between these mechanisms may explain category learning (Seger and Miller, 2010). The idea is that the STR rapidly forms associations which are then fed through the BG to the PFC (Ashby et al., 2007; Djurfeldt et al., 2001). Iterations allow more gradual changes in synaptic weights in the PFC to detect and store the common features across patterns learned by the BG, thereby acquiring the categories (Miller and Buschman, 2008; Seger and Miller, 2010).
Support for this comes from human imaging studies showing that both the PFC and STR are engaged during category learning (Reber et al., 1998; Seger et al., 2000; Vogels et al., 2002). Also, computational and neurophysiological studies suggest more rapid changes in the STR than PFC during learning, as if the BG was “training” the cortex (Djurfeldt et al., 2001; Pasupathy and Miller, 2005). We recently provided more direct support in monkeys trained to learn new categories (Antzoulatos and Miller, 2011). There was the predicted reversal: Early in learning, when the associations of a few stimuli could be formed, the STR led; its activity was the earliest predictor of the behavioral choice. But then, as the animals began to truly acquire categories, the PFC became the earliest predictor of the choice.
While such results are certainly suggestive of PFC-BG functional interactions, direct evidence for functional interactions between the PFC and STR is rare. It is possible that these structures are part of different learning systems that work relatively independently. We sought to test for functional connectivity between the PFC and STR by examining synchrony between oscillations of their local field potentials (LFPs; Friston et al., 2013). Frequency-dependent synchrony between LFPs suggests neural communication and has been observed in perceptual (Hipp et al., 2011), motor (Brovelli et al., 2004), and cognitive tasks (Daitch et al., 2013). The functional connectivity between BG and PFC is of particular interest, as the network between them has been implicated in several neurological and psychiatric conditions, such as autism and schizophrenia (Padmanabhan et al., 2013; Uhlhaas and Singer, 2012; Yoon et al., 2012). We found evidence that functional connectivity between the PFC and STR increased as animals acquired new categories.
RESULTS
Learning-related enhancement of synchrony between the PFC and STR
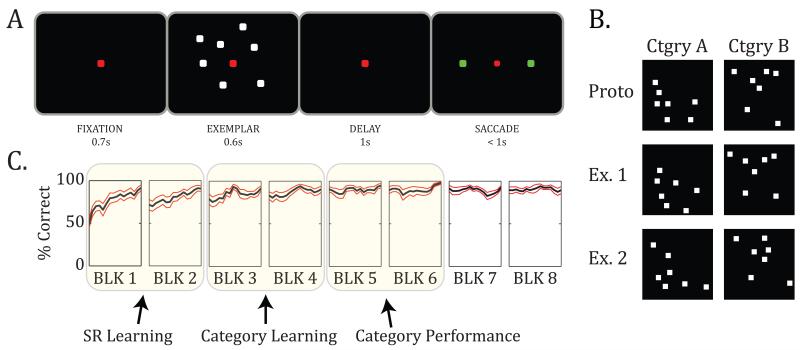
The animals were required to respond to a randomly chosen category exemplar with a saccade to the left or right target (Fig. 1A). All exemplars were created de novo each day through distortion of a new pair of prototypes (Fig. 1B). Each training session began with a single new exemplar per category, which monkeys learned as specific stimulus-response (SR) associations (Antzoulatos and Miller, 2011). Then, as learning progressed, more and more exemplars were added. This required animals to learn the categories (or fail) because sooner or later, they would be confronted with too many new exemplars to sustain performance by SR learning alone.
Figure 1.
Task design. A. The schematic illustrates the time course of a single trial. The animal had to respond to a randomly presented exemplar by choosing between a saccade to the right or left targets (green squares). B. Two example categories. New pairs of prototypes (top) were constructed for each recording session. Distortion of each prototype gave rise to hundreds of unique exemplars (only 2 of which are shown for each category). C. Average behavioral performance (% correct) ± SEM across recording sessions. The animals started by learning a few individual SR associations (SR Learning stage: always the first 2 blocks). As they progressed through the blocks, they were trained on more and more exemplars (Category Learning stage), until they eventually learned the categories and their behavior stabilized (Category Performance stage). The Category Learning and Category Performance stages are shown for illustration only: the timing of each could vary across recording sessions, based on the animals’ performance on each new set of categories. (Adapted from Antzoulatos and Miller, 2011.)
Based on the monkeys’ performance, we could distinguish three stages of learning (Antzoulatos and Miller, 2011; Fig. 1C). In stage 1 (SR Learning), monkeys learned the category of (i.e., the correct saccade for) each new exemplar individually. In stage 2 (Category Learning), the monkeys were challenged with many more exemplars, but began to perform above chance with new exemplars. This indicates the start of acquisition of category information. In stage 3 (Category Performance), learning of the categories was complete. Behavior remained at asymptote even though monkeys were mainly seeing new exemplars for the first time on most trials. We examined changes in synchrony between the PFC and STR as a function of learning stage.
We first calculated synchrony of LFPs between recording sites in the PFC and STR (n=426 electrode pairs). Each site’s LFP signal was decomposed to its frequency components using wavelet analysis (Torrence and Compo, 1998) and then a phase-locking value (PLV) was determined for each pair of simultaneously recorded LFPs (Lachaux et al., 1999). We subtracted out any phase-locking due to external events (e.g., stimulus onset) so that we could isolate true neural synchrony (i.e., the PLV values shown are the difference between observed PLV and surrogate-data PLV; see Supplemental Experimental Procedures). Analysis was focused on two critical task epochs, the last 500 ms of the 600-ms long exemplar presentation (exemplar epoch) and the last 500 ms preceding the behavioral response (decision epoch). Similar results were obtained from other trial epochs and using diverse measures of synchrony (i.e., coherence and pairwise phase consistency). We first limited this analysis to correctly performed trials; Error trials will be considered further below.
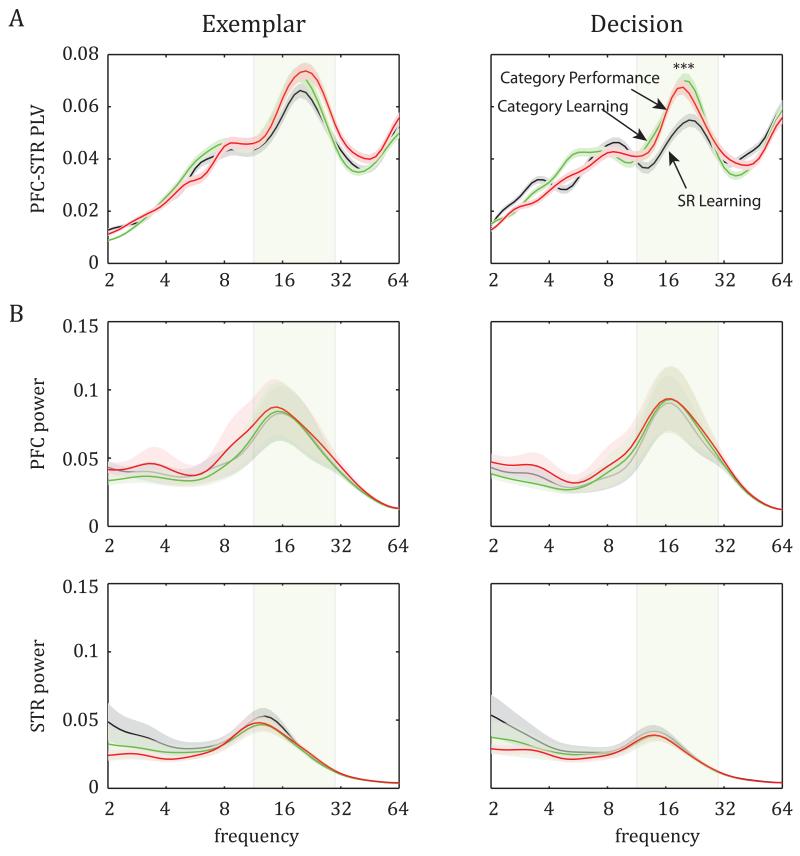
This analysis revealed a peak of PFC-STR synchrony in the beta band (defined as 12-30 Hz) during the exemplar and decision epochs (peak at approx. 20Hz; see Fig 2A). After the switch from SR Learning to Category Learning (Stage 1 to 2), there was a significant increase in decision-epoch average beta-band PLV between the PFC and STR (Fig 2A, right; ANOVA, F(2,1277)=11.23, p=1.5×10−5; post-hoc comparison: SR Learning PLV less than Category Learning and Category Performance PLV, p=0.0005). Correspondingly, during the decision epoch, there was a learning-related increase in the percentage of pairs of PFC-STR recording sites that showed significant beta-band PLV (greater than the 95th percentile of the PLV expected by chance): More pairs showed significant PLV during Category Learning (57.3%, Stage 2) and Category Performance (55.9%, Stage 3) than during SR Learning (42.3%, Stage 1; p=0.003, chi-square test). Learning-related changes in PFC-STR synchrony were limited to the decision epoch. The PFC-STR beta PLV during the exemplar epoch did not significantly increase across learning stages (Fig. 2A, left; ANOVA across stages: F(2,1277)=1.06, p=0.35). Likewise, the number of pairs of PFC-STR recording sites with significant PLV was not different across learning stages for the exemplar epoch (SR Learning: 48.6%, Category Learning: 55.9%, Category Performance: 50.5%; p=0.31, chi-square test). There were also no significant learning-related changes in PLV for baseline activity (middle 500-ms time segment from the 3-s-long inter-trial interval; ANOVA: F(2,1277)=1.04, p=0.35; Fig. S2A). The phase relationship between PFC and STR remained stable at 0° phase-lag across all trial epochs and learning stages (Fig. S2B).
Figure 2.
Frequency-specific oscillations in PFC and striatum (STR) during two trial epochs (exemplar and decision) across the three stages of learning. A. Average PLV ± SEM as a function of frequency: Peak synchrony between PFC and STR beta-band oscillations (in this and all figures, shaded rectangle indicates the 12-30 Hz beta band), and learning-induced enhancement of this synchrony during the decision epoch (see also Figs. S1, S2, and Table S1). B. Average spectral power (±SEM) in PFC (top) and STR (bottom) is high at the beta band, but does not change across learning stages.
No changes in oscillatory power within the PFC or STR
The learning-related increase in PFC-STR synchrony was independent of changes in oscillatory power, i.e., the synchrony changes were not a by-product of increased oscillations per se. Note that the synchrony measure we employed (PLV) is computed only from the phase of the wave, independently from its amplitude (and thus oscillatory power). However, we also computed the frequency-dependent power of PFC and STR LFPs. To correct for the LFP’s power-law decay, power was normalized to 1/frequency.
Both PFC (n=84 electrodes) and STR (n=65 electrodes) LFPs displayed a peak in beta (STR LFPs also displayed strong power in the 2-4 Hz delta band; Fig.2B). Beta power was stronger in the PFC than STR, with a peak at a somewhat higher frequency (peak at 16 Hz vs. 13 Hz, respectively, Fig. 2B). However, there was no change in beta oscillatory power across learning stages in either area for either the exemplar or decision epoch (Fig 2B, ANOVA in PFC: exemplar epoch F(2,251)=0.004, p=0.99, decision epoch F(2,251)=0.001, p=0.99; STR: exemplar epoch F(2,194)=0.49, p=0.62, decision epoch F(2,194)=0.18, p=0.82). This suggests that the learning-related changes in synchrony between the PFC and STR reflected changes in functional connectivity per se, rather than just a general change in oscillatory dynamics. Indeed, as we will see next, learning-related changes in synchrony only occurred between the PFC and STR; there was no learning-related change in synchrony within either area.
No changes in synchrony within the PFC or STR
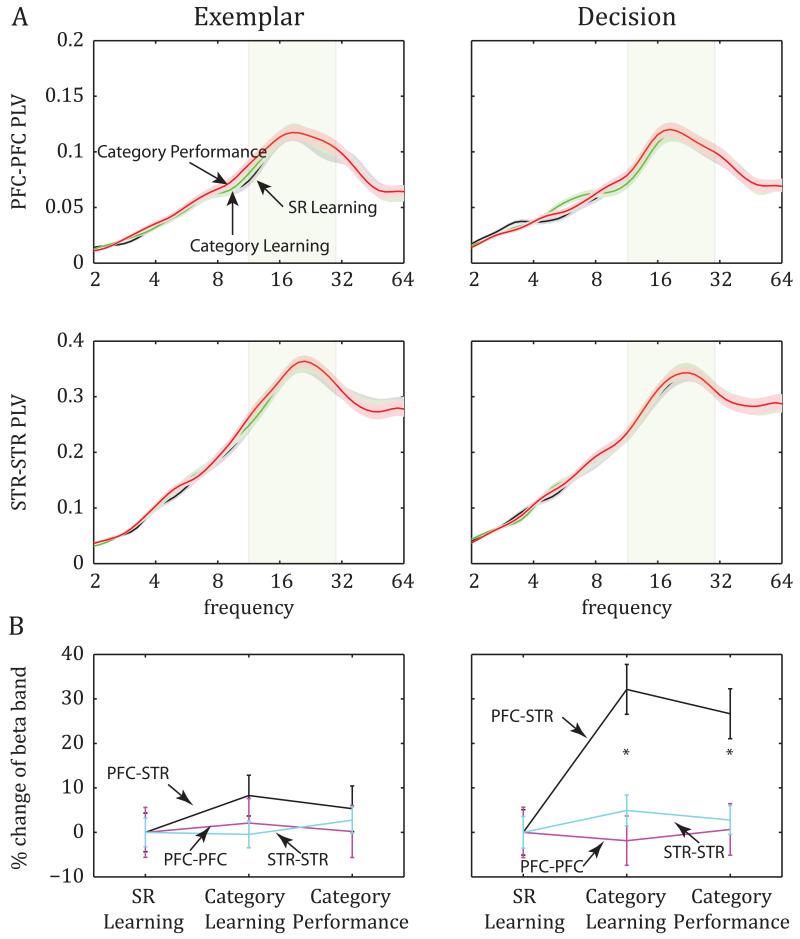
The learning-related increase in beta synchrony was limited to interactions between the PFC and STR; there was no learning-related change in beta (or any other frequency band) synchrony within either area. We performed the same analyses as above on pairs of recording sites within each area (Fig. 3A and Fig. S1A). Synchrony between recording sites within the PFC (n=240 electrode pairs) or STR (n=141 electrode pairs) were overall greater than those between PFC and STR (within-STR average PLV was also greater than within-PFC average PLV) with a peak in the beta band (at approx. 20Hz). However, beta-band PLV values within the PFC and STR did not change across learning stages in either the exemplar epoch (ANOVA across stages in beta-specific PLV within PFC: F(2,719)=0.05, p=0.95; within STR: F(2,422)=0.23, p=0.79; Fig. 3A, left) or the decision epoch (within PFC: F(2,719)=0.06, p=0.94; within STR: F(2,422)=0.42, p=0.66; Fig 3A, right). Decision-epoch PLV within the PFC and within the STR was similar during SR Learning, Category Learning and Category Performance (only 0.5%-2.12% difference across learning within PFC and 2.2%-4.4% within STR). Compare this to learning-related increases in beta-band PLV of around 30% between the PFC and STR (Fig. 3B, right).
Figure 3.
Synchrony between intrinsic pairs of electrodes in PFC and STR. A. Average PLV (±SEM): Although intrinsic connectivity peaked at the beta band both in PFC (top) and in STR (bottom), it did not change with learning (see also Fig. S2). B. The percent increase of synchrony between PFC and STR after the SR Learning stage during the decision epoch (right) was significantly greater than the corresponding change in synchrony of intrinsic PFC pairs (PFC-PFC) and STR pairs (STR-STR) of electrodes.
Decrease in PFC-STR synchrony during error trials
To determine whether the PFC-STR synchrony was related to task performance, we examined PLV from trials in which the monkeys made the incorrect behavioral choice at the end of the trial. This analysis was necessarily focused only on the SR Learning and Category Learning stages because the animals’ asymptotic performance during Category Performance did not include sufficient error trials for their analysis.
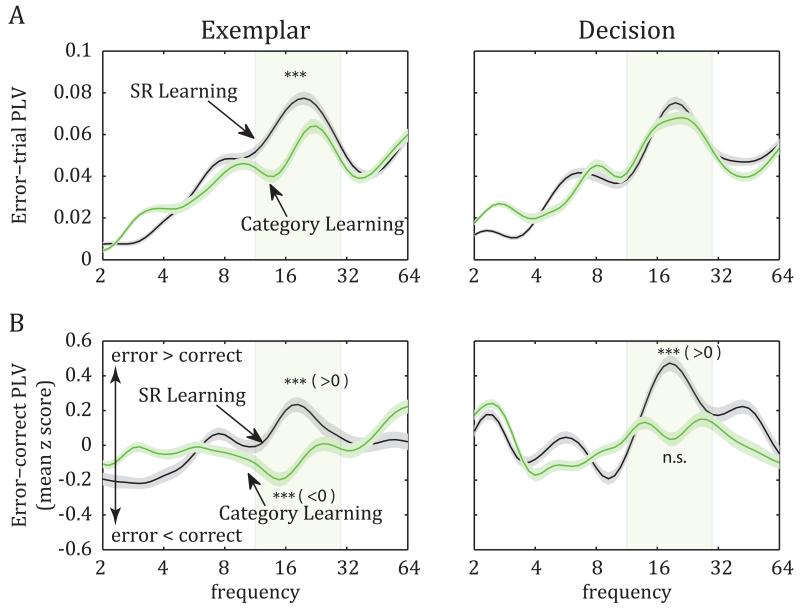
While there was strong beta-band synchrony during error trials (Fig. 4A), there was no learning-related increase in beta synchrony (PLV), unlike what was seen for correct trials (see above). On the contrary, there was a significant decrease of PFC-STR beta-band PLV from SR Learning to Category Learning during the exemplar epoch (Fig. 4A, left; ANOVA across stages: F(1,810)=28.83, p=10−7). Synchrony did not change significantly across the two stages in the decision epoch (F(1,810)=3.48, p=0.06; Fig 4A, right).
Figure 4.
Analyses of PFC-STR synchrony in error trials of SR Learning and Category Learning stages. A. Average (±SEM) PLV in error trials: In contrast to the increase of beta-band synchrony observed in correct trials (Fig. 2), synchrony between PFC and STR did not increase across learning stages; rather, it decreased significantly at least during the exemplar epoch. B. The average (±SEM) z-transformed difference (d’) between error- and correct-trial PLV. During both trial epochs, error trials displayed stronger PFC-STR synchrony than did correct trials in the SR Learning stage, but, in the Category Learning stage, this difference was either eliminated (decision epoch) or reversed (exemplar epoch).
In order to compare synchrony between correct and error trials, we employed the discrimination index d’, which quantifies the difference between the mean of two sets of trials (i.e., error and correct trials), normalized to their pooled standard deviation (Dayan and Abbott, 2001). This quantity was transformed into a z score, based on 200 random shuffles of the trials between the correct and error groups. The average z-transformed d’ indicated a significant decline in error-correct synchrony for the beta band from SR Learning to Category Learning (15-20 Hz; ANOVA on exemplar epoch d’ between SR Learning and Category Learning: F(1,810)=54.11, p=4.7×10−13; during decision epoch: F(1,810)=60.97, p=1.8×10−14). Figure 4B plots the z-scores for correct trials subtracted from error trials (i.e., the average z-transformed d’). During the exemplar epoch (Fig 4B, left), note that the scores were significantly above zero in beta for SR Learning (t-test for d’ relative to zero discrimination: p=1.7×10−8), indicating greater synchrony on error trials and significantly below zero for Category Learning (p=2.8×10−5), indicating greater beta synchrony on correct trials. For the decision epoch (Fig 4B, right), error-correct values were significantly greater than zero for SR Learning, indicating greater beta synchrony on error trials (t-test, p=3×10−21). However, during Category Learning, there was no difference in beta synchrony between correct and error trials, i.e., error-correct PLV values did not differ from zero (p=0.81). Therefore, we see that the shift from SR Learning to Category Learning led to changes in PFC-STR synchrony that depended on trial epoch and task performance. In the exemplar epoch, there was a significant decline of PLV during error trials (Fig. 4A, left) but no change in correct trials (Fig. 2A, left). In the decision epoch, there was a significant increase of PLV during correct trials (Fig. 2A, right) but no change in error trials (Fig. 4A, right). Note that the net effect is similar: for both exemplar and decision epochs, the transition from SR Learning to Category Learning preferentially favored the PFC-STR synchrony during correct, relative to erroneous, categorization.
Emergence of category-specific patterns of synchrony between the PFC and STR with learning
A recent study of PFC LFPs reported rule-specific patterns of beta-band synchrony between different recording sites, suggesting that beta synchrony can help form network ensembles for rules (Buschman et al., 2012). We examined whether category-specific rhythmic networks formed in the process of category learning.
For each pair of electrodes, we computed differences in synchrony (PLV) for exemplars from the two categories using the discrimination index d’ (described above), as in our previous study of neural activity (Antzoulatos and Miller, 2011). To correct biases of the d’ metric due to variable and unequal numbers of trials, and to evaluate its statistical significance, trials were randomly shuffled 200 times between the two categories, thus generating a randomization distribution for the d’ quantity, which was then used to z-transform each electrode-pair’s d’. In short, we used this measure to determine whether different electrode pairs showed different levels of synchrony for the two different categories (i.e., category selectivity).
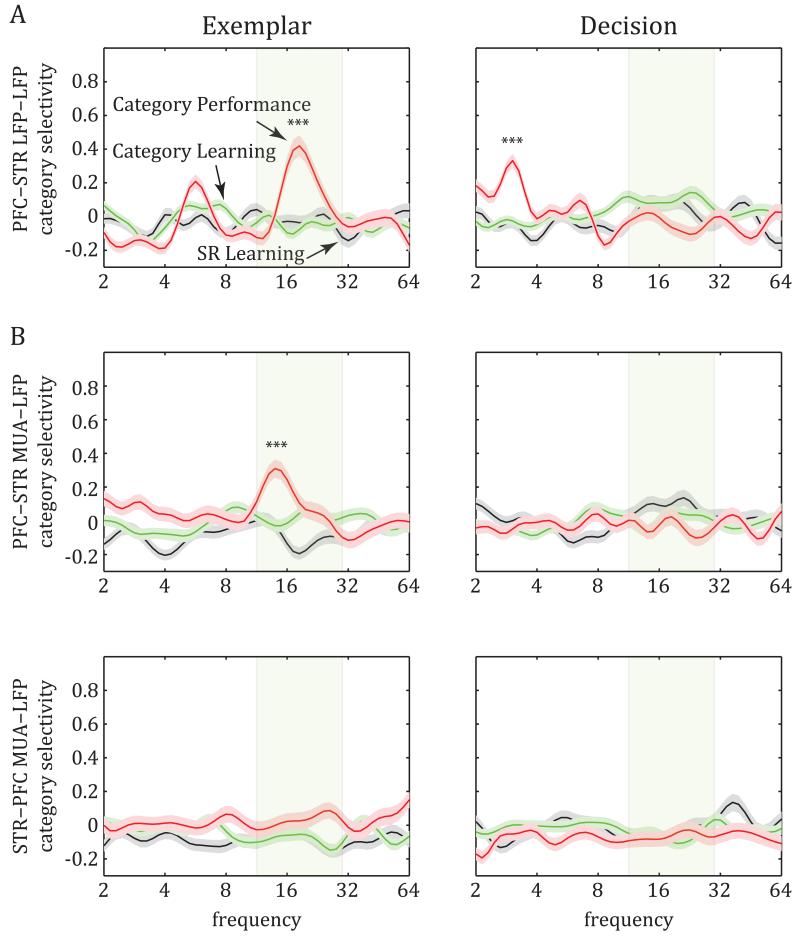
Significant category-selective synchrony was observed, but only after the animals had learned the categories. During Category Performance, there was a significant increase of exemplar epoch category-selective synchrony in the beta-band (peak at approx. 19 Hz) between the PFC and STR (Fig. 5A, left; ANOVA on category selectivity across learning stages, F(2,1277)=21.88, p=4.5×10−10; post-hoc comparisons: selectivity during Category Performance greater than during SR Learning and Category Learning, p=5×10−7). During the decision epoch, there was a significant increase of delta-band category selectivity (peak at approx. 3 Hz, Fig. 5A, right; F(2,1277)=39.37, p=2.6×10−17; post-hoc comparisons: selectivity during Category Performance greater than during SR Learning and Category Learning: p=10−11). Correspondingly, category-selective PFC-STR synchrony was not different from that expected by chance during the SR Learning or Category Learning stages but was significantly different from chance during Category Performance (t-test for selectivity greater than zero in beta band of exemplar epoch: SR Learning, p=0.99; Category Learning, p=0.99; Category Performance, p=7.2×10−8; in delta band of decision epoch: SR Learning, p=0.99; Category Learning, p=0.99; Category Performance, p=5.7×10−16).
Figure 5.
Category selectivity in the strength of PFC-STR synchrony. A. Synchrony between PFC and STR LFPs (average z-transformed d’ ±SEM) displayed significant category selectivity during the Category Performance stage. Category-specific synchrony was observed at the beta band during the exemplar epoch and the delta band during the decision epoch. B. Similar to the LFP-LFP synchrony above, MUA-LFP synchrony (average z-transformed d’ ± SEM) between PFC-STR (spikes in PFC, LFP in STR; top) also displayed significant category selectivity at the beta band of the exemplar epoch: Again this was evident for the first time during the Category Performance stage. In contrast, the reverse direction (spikes in STR and LFP in PFC; bottom) did not show any category selectivity (see also Fig. S3).
As was seen for the learning-related general increase in beta synchrony, significant category-selective synchrony was only seen between the PFC and STR. There was no significant category-selective synchrony within the PFC or STR (t-test for selectivity greater than zero: SR Learning stage: exemplar epoch, within PFC p=0.99, within STR p=0.99, decision epoch, PFC p=0.14, STR p=0.99; Category Learning stage: exemplar PFC p=0.99, STR p=0.06, decision PFC p=0.99, STR p=0.08; Category Performance stage: exemplar PFC p=0.99, STR p=0.99, decision PFC p=0.27, STR p=0.92). Thus, it seems that acquisition of the categories is accompanied by development of category-specific patterns of synchrony between, but not within, the PFC and STR.
Significant category-selective beta synchrony (at approx. 14 Hz) during Category Performance was also seen between spikes and LFPs during the exemplar epoch, specifically between PFC multiunit spiking activity (MUA) and STR LFPs (Fig. 5B; ANOVA across stages: F(2,1239)=19.71, p=3.8×10−9; post-hoc comparisons: selectivity during Category Performance stage greater than during SR Learning and Category Learning stages, p=5×10−6). This spike-LFP synchrony was significantly greater than that expected by chance during Category Performance (t-test: p=5.3×10−8) but not during SR Learning (p=0.49) or Category Learning (p=0.90). Importantly, spike-LFP category-selective synchrony was asymmetric. It was seen between PFC spikes and STR LFPs (above) but not between STR spikes and PFC LFPs at any of the learning stages (Fig 5B and Fig. S2A, t-test: SR Learning, p=0.82; Category Learning, p=0.96; Category Performance, p=0.59). As was seen for LFP-LFP synchrony, there was no evidence for category-specific spike-LFP synchrony within PFC or STR (Fig. S2B; SR Learning stage: PFC p=0.96, STR p=0.99; Category Learning stage: PFC p=0.52, STR p=0.86; Category Performance stage: PFC p=0.11, STR p=0.23). Thus, it seemed that patterns of category-selective synchrony were from the PFC to the STR, not the other way around and not within either area.
In our task, each category was uniquely associated with a saccade to the left or the right. Most cortical areas show activity that is biased toward processing of, and actions to, the contralateral hemifield. We therefore sought to determine whether the category-selective synchrony was primarily associated with categories signaling contralateral saccades. While LFP-LFP synchrony was seen for categories associated with both contralateral and ipsilateral saccades, there was a significant bias toward contralateral saccades for delta-band category-selective synchrony during the decision epoch, when the monkeys prepared to execute the saccade (63.9% of all electrode pairs preferred contralateral saccades: p=0.0001, chi-square test). By contrast, there was no contralateral or ipsilateral bias for beta-band category-selective synchrony during the exemplar epoch (53.5% of all pairs preferred contralateral saccades: p=0.16). Category-specific beta-band spike-LFP synchrony between PFC-STR was also not biased for ipsilateral vs. contralateral saccades (50.8% of all pairs preferred contralateral saccades: p=0.81).
STR exerts larger causal influence on PFC than the reverse
The measure of synchrony utilized above (PLV) is a measure of functional, not causal, connectivity, because it provides no information on the causal influence of one area on the other. Granger’s test of causal connectivity can be used to indicate the degree of influence each area has on another. It evaluates how much of one area’s LFP variance can be explained by the other area’s LFP variance. Furthermore, this analysis can be performed at the frequency domain, to identify causal influence specific to brain rhythms (Friston et al., 2013). Because Granger causality can also be affected by areas that provide common input to PFC and STR and/or intervene between the two (e.g. the rest of BG), it is also a more global measure of influence than the spike-LFP synchrony we showed above (which is more sensitive to direct neurophysiological connections between the 2 areas).
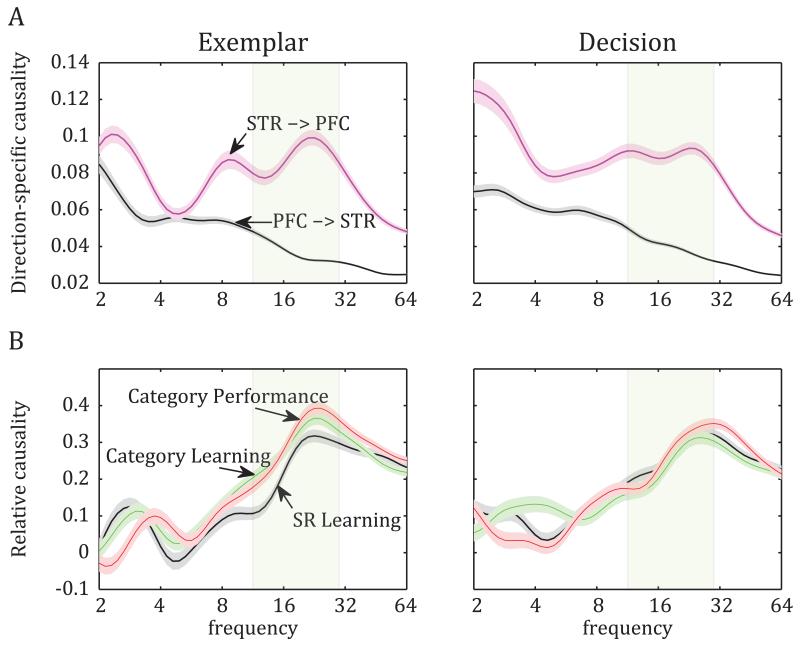
We analyzed the causality between PFC and STR LFPs with a non-parametric variant of the Granger causality test so the results would not hinge on the order of the multivariate autoregressive model that a parametric test would require (Dhamala et al., 2008; Roberts et al., 2013). This analysis revealed that, although both areas had causal influence on each other, STR exerted a significantly stronger causal influence on PFC than PFC on STR (i.e., STR LFPs were better predictors of PFC LFPs rather than the reverse), and this was evident across the largest part of the frequency spectrum (Fig. 6A; e.g., 20-Hz beta band at SR Learning stage, exemplar epoch: t-test on magnitude of Granger causality between the 2 directions: p=10−45; Decision epoch: p=10−41). Indeed, most of the PFC-STR electrode pairs showed a stronger influence from STR to the PFC than the other way around (exemplar epoch: 80.8% of all pairs: p=0.0001, chi-square test; decision epoch: 77.2%; p=0.0001). In addition to their difference in magnitude of causal connectivity, the two areas also differed in the spectral profile of their causal influence on one another: PFC had the strongest influence on the low frequencies of STR LFPs (e.g., delta band), whereas STR displayed clear peak influence on both the delta and beta bands of the prefrontal LFPs (Fig. 6A).
Figure 6.
Analyses of Granger causal connectivity between PFC and STR. A. Average Granger connectivity index ± SEM: The two directions of causal connectivity during the two trial epochs of the SR Learning stage. Striatum exerts stronger influence on the prefrontal LFPs (STR->PFC) than the other way around (PFC->STR). This difference is seen across the frequency spectrum, but especially at the beta band (shaded rectangle). B. Average (± SEM) relative causality (STR->PFC direction normalized to the PFC->STR direction) across learning stages. In contrast to the robust enhancement of functional connectivity at the beta band (20 Hz) with learning (Fig. 2), causal connectivity did not increase significantly, suggesting that the relative influence of one area on the other did not change.
In order to evaluate learning-induced changes in the relative causal connectivity between PFC and STR, we computed a composite causality index from both directions (PFC->STR and STR->PFC) for each learning stage and trial epoch: (A−B)/(A+B), wherein A is STR->PFC causality and B is PFC->STR causality. There was little change in the direction of influence between the PFC and STR (Fig. 6B) and no significant change in the 20-Hz beta band that displayed the aforementioned synchrony changes (ANOVA on causality of exemplar epoch across stages of learning: F(2,1277)=2.49, p=0.08; decision epoch: F(2,1277)=1.37, p=0.25). This suggests that the relative weight of one area’s influence on the other did not change as a result of learning. Thus, while the analysis on category-selective spike-LFP synchrony (above) suggested a one-way PFC-STR synchrony, consistent with the monosynaptic connections from the PFC to STR, it appears that the polysynaptic connections from the STR back to the PFC had a greater influence on the PFC oscillations.
DISCUSSION
We found that category learning was accompanied by increased synchronization between, but not within, the prefrontal cortex and striatum. Synchrony is thought to play a role in establishing functional circuitry (Engel et al., 2001; Fries, 2005; Miller and Buschman, 2013; Uhlhaas et al., 2009). Supporting this, we found that once the categories were learned, different pairs of PFC-STR recording sites showed increased synchrony for one or the other category, suggesting functional circuits for mapping category representations in the PFC to the appropriate motor program in the BG. Spike-LFP synchrony did suggest that the category-specific synchrony was, in fact, asymmetric between the PFC and STR, reflecting the asymmetric monosynaptic projections between them. However, causal connectivity analysis suggested that the polysynaptic projections from the STR back to the PFC exerted a greater influence. This is consistent with models positing that the STR (through the BG) continually “trains” the PFC (Antzoulatos and Miller, 2011; Ashby et al., 2007; Djurfeldt et al., 2001; Houk and Wise, 1995; Miller and Buschman, 2008; Pasupathy and Miller, 2005; Seger and Miller, 2010).
The learning-related increases in PFC-STR synchrony seemed functional. First, they were not simply due to an overall increase in oscillatory power. Second, they were only seen during the trial and not in baseline activity. Third, they were specific to synchrony between the areas; there were no synchrony changes within PFC or STR. Finally, error trials (incorrect choices) did not display the same increase in beta synchrony that correct trials did. Curiously, error trials during SR Learning displayed stronger PFC-STR beta synchrony than did correct trials. This reversed once the animals advanced to Category Learning. SR learning is well known to rely on the BG (Packard and Knowlton, 2002), and striatal neurons display rapid acquisition of SR associations (Antzoulatos and Miller, 2011; Pasupathy and Miller, 2005). It is possible that stronger error-trial beta synchrony between the STR and PFC interfered with the ability of STR to map the stimulus to the correct motor response during SR Learning. Indeed, excessive synchrony of cortex-BG networks is seen during Parkinsonian motor symptoms (Hammond et al., 2007; Marreiros et al., 2013).
Learning-related effects in the beta band are consistent with prior observations that beta band (12-30 Hz) oscillations are prominent in frontal cortex (Puig and Miller, 2012; Siegel et al., 2009) while gamma band oscillations predominate in posterior cortex (Fries, 2009), that cortical beta vs. gamma are associated with top-down (feedback) vs. bottom-up (feedforward) processing (Buschman and Miller, 2007; Engel and Fries, 2010), and beta band oscillations synchronize striatal neurons in monkeys performing oculomotor tasks (Courtemanche et al., 2003). It should be noted that “beta band” may include more than one type of oscillations with distinct neurophysiological mechanisms and functions (Cannon et al., 2013). Indeed, the different results from this study showed peaks at different frequencies within the beta band. A dissection of the contributions of different beta sub-bands was beyond the scope of our study.
The learning-related changes in PFC-STR synchrony parallel the changes in category learning-related changes in single-neuron activity previously seen in this data set (Antzoulatos and Miller, 2011). During SR Learning, STR spiking activity was an earlier predictor of the corresponding saccade than the PFC. However, when monkeys advanced to Category Learning, PFC neurons began predicting the saccade associated with each category before the STR. One result of this was that PFC and STR neurons showed more overlap of their task-related spiking activity during and after Category Learning, relative to SR Learning. This overlap was in the decision epoch, just before the behavioral response. This is when we also first observed the learning-related increase in PFC-STR beta synchrony.
Category-selective beta synchrony could serve to communicate the categorical decision from the PFC to the STR. It occurred well before the motor response, during exemplar presentation, and did not show a contralateral motor bias. By contrast, category-specific delta synchrony occurred when the monkeys were about to make their motor response and it was contralaterally biased. This could reflect recruitment of PFC and striatum in a larger network for motor acts. Low frequency oscillations (like delta and theta) have been associated with long-range synchronization among spatially diverse systems in the context of decision making, attention and memory (Haegens et al., 2011; Schroeder and Lakatos, 2009; Watrous et al., 2013). Delta band synchronization (at least in visual cortex) is also observed during eye movements (Bosman et al., 2009; Ito et al., 2013). It should be noted that an emergence of category selectivity in the absence of a change in general synchrony suggests that synchrony during the preferred category increases, while synchrony during the non-preferred category decreases, thus offsetting each other when synchrony across all trials is computed.
Dopamine may play a role in learning-related changes in synchrony. It mediates plasticity of excitatory corticostriatal connections. Because the phasic dopamine release that signals reward-prediction errors induces long-term potentiation of active cortical synapses onto medium spiny striatal neurons of the direct pathway (Gerfen and Surmeier, 2011; Lerner and Kreitzer, 2011), i.e., the pathway that closes the PFC-BG-thalamus-PFC loop, it may also be responsible for increasing the synchronization between PFC and STR. Although dopamine is also known to affect the activity of prefrontal neurons during SR learning (Puig and Miller, 2012) and working memory (Arnsten et al., 2012), it is thought to be of less consequence for corticocortical than for corticostriatal synapses (Ashby et al., 2007; Miller and Buschman, 2008). This may be why we found that corticostriatal synchrony was enhanced while corticocortical synchrony was not. It is also possible that corticocortical (and striatostriatal) connections require more experience for functional circuitry to be established. Rule-specific beta synchronization within the PFC has been observed (Buschman et al., 2012) but it was for highly familiar rules, not during new learning as in this study.
The lack of learning-related changes in synchrony within the PFC and STR was in contrast to changes between them. Although we cannot exclude a ceiling effect for intrinsic synchrony, it is unlikely. The PFC-PFC synchrony was weaker than STR-STR synchrony, and yet it did not change with learning. Interestingly, the lateral connections between the STR medium spiny neurons are sparse, with high failure rate (Plenz, 2003). The stronger intrinsic STR synchrony, therefore, may arise from a common signal external to the STR, such as the substantia nigra pars compacta (SNpc): Its dopaminergic neurons fire spikes at highly regular intervals and could have pacemaking functions (Surmeier et al., 2005). Recent studies have also suggested that striatal synchrony can be regulated by the subthalamic nucleus (Marreiros et al., 2013).
We found that the STR had a stronger net influence on the PFC than PFC on STR. This causal influence may be task-dependent. In a stimulus-stimulus association task, the PFC was reported to exert larger causal influence on STR, consistent with their monosynaptic connections (Ma et al., 2013). However, the BG is indeed thought to exert a strong influence on frontal cortex (Ashby et al., 2007; Seger, 2008). The globus pallidus (which receives direct projections from the STR) affects the timing and presumably strength of thalamocortical communication (Goldberg et al., 2013) and also sends monosynaptic feedback signals to STR (Gerfen and Surmeier, 2011; Lerner and Kreitzer, 2011). The dopaminergic projection from SNpc is denser (i.e., presumably stronger) to STR than to the PFC (Lynd-Balta and Haber, 1994). Any of these signals, therefore, could shape both the PFC but mostly the STR rhythms, thus making the STR LFPs better predictors of PFC signals. The greater causal influence of the STR on the PFC is consistent with the hypothesis that STR learns about individual exemplars and then, via the rest of the BG, “trains” their categories in the PFC (Antzoulatos and Miller, 2011; Pasupathy and Miller, 2005; Seger and Miller, 2010). However, this process is continual and recursive: Once the categories are learned, they can be fed into the STR for further learning, which may explain why, after learning, we found category-specific synchrony from the PFC to the STR.
EXPERIMENTAL PROCEDURES
Animals
Data were acquired from two adult female macaque monkeys, maintained in accordance with the National Institutes of Health guidelines and the policies of the Massachusetts Institute of Technology Committee for Animal Care.
Task
The details of the task have been presented previously (Antzoulatos and Miller, 2011). Briefly, the animals initiated a trial by fixating on a central target. While the animals maintained fixation, a randomly chosen category exemplar from either category was presented for 0.6 s. Trials from both categories were randomly interleaved throughout the session. One second after the exemplar display offset, 2 saccade targets appeared on the left and right of the center of fixation, and the animal had to make a single, direct saccade to the correct target for reward. Category exemplars were random 7-dot constellations, generated through distortion of the corresponding prototype (Fig. 1).
Neurophysiology
Simultaneous recordings from PFC and STR were performed using multi-electrode arrays, lowered at different PFC and STR sites every day. LFPs were decomposed to their individual frequency components using wavelet analysis. Functional connectivity (i.e., frequency-specific synchrony) between pairs of LFP signals was computed as a phase-locking value (PLV) over two 500-ms trial epochs: the exemplar epoch (last 500ms of exemplar display) and the decision epoch (last 500 ms before the animals’ saccade). PLV computes the circular mean of a sample of phase differences (phase-lags) and varies between 0 (when all phase-lags are uniformly distributed across 360 degrees) and 1 (when all phase-lags are concentrated at a single phase). Similar results on synchrony were obtained when we computed coherence or pairwise phase consistency. For differences in synchrony between 2 sets of trials (e.g., correct vs. error, or category A vs. B), we used the same selectivity metric (discrimination index d’) we employed previously (Antzoulatos and Miller, 2011). To correct for sampling bias, we randomly shuffled the trials between the two sets 200 times, thus generating a randomization distribution that was used as surrogate data. The observed d’ values were subsequently transformed into z scores based on the surrogate dataset, and averaged across the population of electrode pairs. Finally, causal connectivity analyses relied on a non-parametric Granger test, which evaluates the degree to which signal A can predict (i.e., explain the variance of) the frequency-specific oscillations of signal B. All computations were done using MATLAB (see Supplemental Materials for more detail).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank B. Gray, S. Koopman, and D. Ouellette for technical assistance, S. Brincat, J. Donoghue, S. Kornblith, R. Loonis, M. Lundqvist, M. Moazami, V. Puig, J. Rose, J. Roy, and M. Silver for helpful discussions, and M. Wicherski for comments on the manuscript.
This work was funded by the National Institute of Mental Health (5R01MH065252-12)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antzoulatos EG, Miller EK. Differences between neural activity in prefrontal cortex and striatum during learning of novel abstract categories. Neuron. 2011;71:243–249. doi: 10.1016/j.neuron.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Ennis JM, Spiering BJ. A neurobiological theory of automaticity in perceptual categorization. Psychol Rev. 2007;114:632–656. doi: 10.1037/0033-295X.114.3.632. [DOI] [PubMed] [Google Scholar]
- Bosman CA, Womelsdorf T, Desimone R, Fries P. A microsaccadic rhythm modulates gamma-band synchronization and behavior. J Neurosci. 2009;29:9471–9480. doi: 10.1523/JNEUROSCI.1193-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U A. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J, McCarthy MM, Lee S, Lee J, Borgers C, Whittington MA, Kopell N. Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci. 2013 doi: 10.1111/ejn.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch AL, Sharma M, Roland JL, Astafiev SV, Bundy DT, Gaona CM, Snyder AZ, Shulman GL, Leuthardt EC, Corbetta M. Frequency-specific mechanism links human brain networks for spatial attention. Proc Natl Acad Sci U A. 2013;110:19585–19590. doi: 10.1073/pnas.1307947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Dayan P, Abbott LF. Theoretical Neuroscience. The MIT Press; Cambridge: 2001. [Google Scholar]
- Dhamala M, Rangarajan G, Ding M. Analyzing information flow in brain networks with nonparametric Granger causality. Neuroimage. 2008;41:354–362. doi: 10.1016/j.neuroimage.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurfeldt M, Ekeberg O, Graybiel AM. Cortex-Basal Ganglia Interaction and Attractor States. Neurocomputing. 2001;38-40:573–579. [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Friston K, Moran R, Seth AK. Analysing connectivity with Granger causality and dynamic causal modelling. Curr. Opin. Neurobiol. 2013;23:172–178. doi: 10.1016/j.conb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Farries MA, Fee MS. Basal ganglia output to the thalamus: still a paradox. Trends Neurosci. 2013;36:695–705. doi: 10.1016/j.tins.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Hernandez A, Luna R, Jensen O, Romo R. Beta oscillations in the monkey sensorimotor network reflect somatosensory decision making. Proc Natl Acad Sci U A. 2011;108:10708–10713. doi: 10.1073/pnas.1107297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69:387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Ito J, Maldonado P, Grun S. Cross-frequency interaction of the eye-movement related LFP signals in V1 of freely viewing monkeys. Front Syst Neurosci. 2013;7:1. doi: 10.3389/fnsys.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Kreitzer AC. Neuromodulatory control of striatal plasticity and behavior. Curr Opin Neurobiol. 2011;21:322–327. doi: 10.1016/j.conb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994;59:609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Ma C, Pan X, Wang R, Sakagami M. Estimating causal interaction between prefrontal cortex and striatum by transfer entropy. Cogn. Neurodyn. 2013;7:253–261. doi: 10.1007/s11571-012-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreiros AC, Cagnan H, Moran RJ, Friston KJ, Brown P. Basal ganglia-cortical interactions in Parkinsonian patients. Neuroimage. 2013;66C:301–310. doi: 10.1016/j.neuroimage.2012.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Buschman TJ. Rules through recursion: How interactions between the frontal cortex and basal ganglia may build abstract, complex rules from concrete, simple ones. In: Bunge SA, Wallis JD, editors. Neuroscience of Rule-Guided Behavior. Oxford University Press; New York: 2008. [Google Scholar]
- Miller EK, Buschman TJ. Cortical circuits for the control of attention. Curr. Opin. Neurobiol. 2013;23:216–222. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci. 2013;7:814. doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Puig MV, Miller EK. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron. 2012;74:874–886. doi: 10.1016/j.neuron.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Stark CE, Squire LR. Cortical areas supporting category learning identified using functional MRI. Proc Natl Acad Sci U A. 1998;95:747–750. doi: 10.1073/pnas.95.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MJ, Lowet E, Brunet NM, Ter Wal M, Tiesinga P, Fries P, De Weerd P. Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron. 2013;78:523–536. doi: 10.1016/j.neuron.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neurosci Biobehav Rev. 2008;32:265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Miller EK. Category learning in the brain. Annu Rev Neurosci. 2010;33:203–219. doi: 10.1146/annurev.neuro.051508.135546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Poldrack RA, Prabhakaran V, Zhao M, Glover GH, Gabrieli JD. Hemispheric asymmetries and individual differences in visual concept learning as measured by functional MRI. Neuropsychologia. 2000;38:1316–1324. doi: 10.1016/s0028-3932(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci U A. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Mercer JN, Chan CS. Autonomous pacemakers in the basal ganglia: who needs excitatory synapses anyway? Curr Opin Neurobiol. 2005;15:312–318. doi: 10.1016/j.conb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998;79:61–78. [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R, Sary G, Dupont P, Orban GA. Human brain regions involved in visual categorization. Neuroimage. 2002;16:401–414. doi: 10.1006/nimg.2002.1109. [DOI] [PubMed] [Google Scholar]
- Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci. 2013;16:349–356. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Raouf S, D’Esposito M, Carter CS. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol Psychiatry. 2012;74:122–129. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.