Abstract
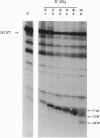
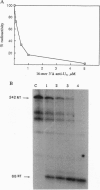
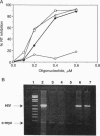
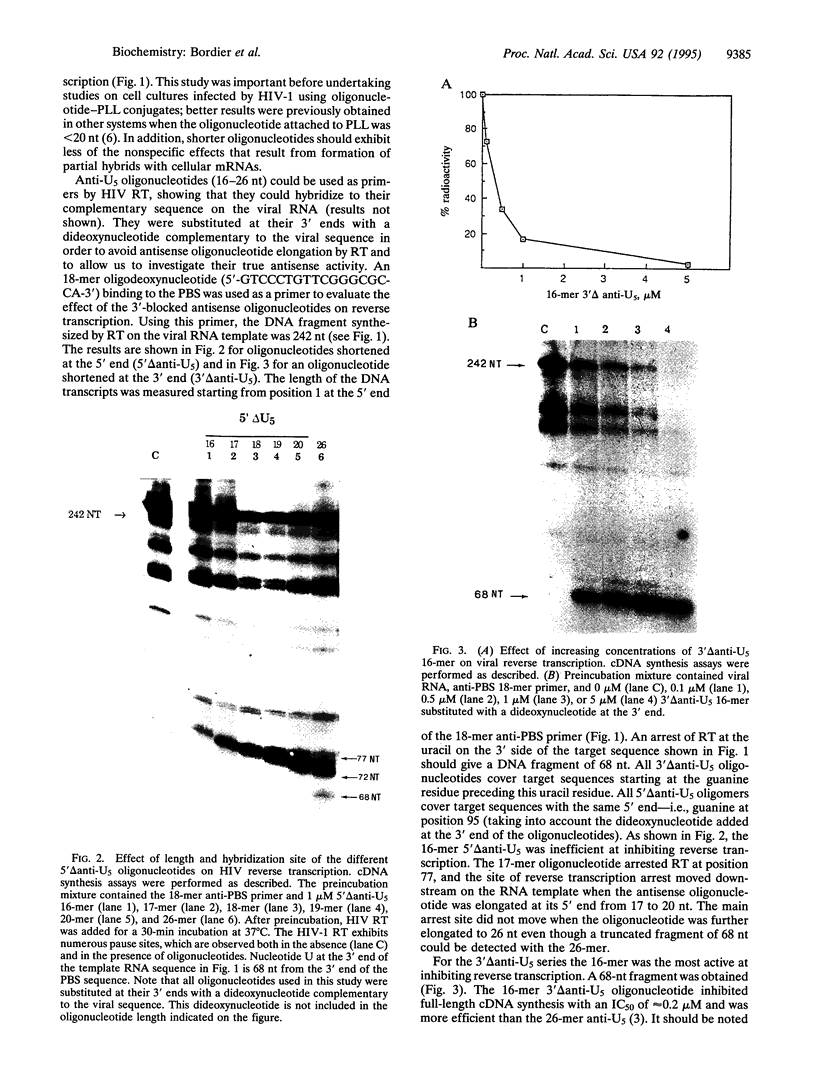
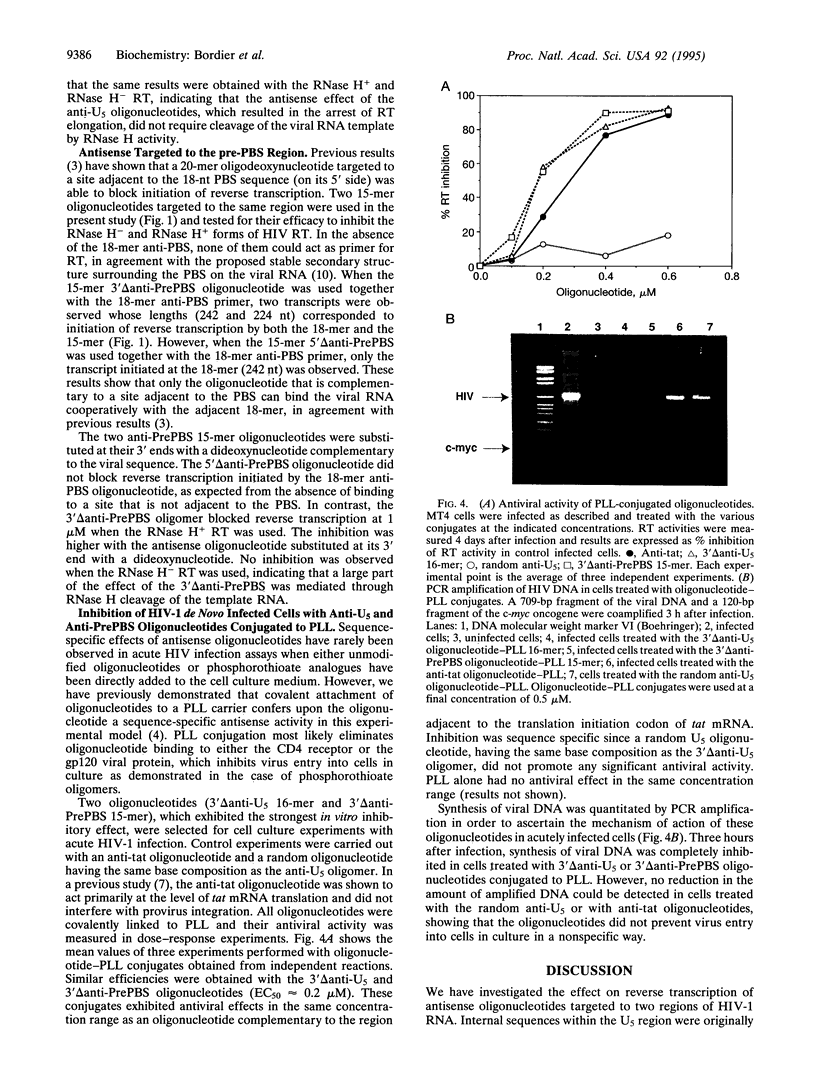
We have investigated two regions of the viral RNA of human immunodeficiency virus type 1 (HIV-1) as potential targets for antisense oligonucleotides. An oligodeoxynucleotide targeted to the U5 region of the viral genome was shown to block the elongation of cDNA synthesized by HIV-1 reverse transcriptase in vitro. This arrest of reverse transcription was independent of the presence of RNase H activity associated with the reverse transcriptase enzyme. A second oligodeoxynucleotide targeted to a site adjacent to the primer binding site inhibited reverse transcription in an RNase H-dependent manner. These two oligonucleotides were covalently linked to a poly(L-lysine) carrier and tested for their ability to inhibit HIV-1 infection in cell cultures. Both oligonucleotides inhibited virus production in a sequence- and dose-dependent manner. PCR analysis showed that they inhibited proviral DNA synthesis in infected cells. In contrast, an antisense oligonucleotide targeted to the tat sequence did not inhibit proviral DNA synthesis but inhibited viral production at a later step of virus development. These experiments show that antisense oligonucleotides targeted to two regions of HIV-1 viral RNA can inhibit the first step of viral infection--i.e., reverse transcription--and prevent the synthesis of proviral DNA in cell cultures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudin F., Marquet R., Isel C., Darlix J. L., Ehresmann B., Ehresmann C. Functional sites in the 5' region of human immunodeficiency virus type 1 RNA form defined structural domains. J Mol Biol. 1993 Jan 20;229(2):382–397. doi: 10.1006/jmbi.1993.1041. [DOI] [PubMed] [Google Scholar]
- Boiziau C., Thuong N. T., Toulmé J. J. Mechanisms of the inhibition of reverse transcription by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):768–772. doi: 10.1073/pnas.89.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier B., Hélène C., Barr P. J., Litvak S., Sarih-Cottin L. In vitro effect of antisense oligonucleotides on human immunodeficiency virus type 1 reverse transcription. Nucleic Acids Res. 1992 Nov 25;20(22):5999–6006. doi: 10.1093/nar/20.22.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Soskey L., Leis J. A retroviral RNA secondary structure required for efficient initiation of reverse transcription. J Virol. 1988 Oct;62(10):3622–3630. doi: 10.1128/jvi.62.10.3622-3630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G., Devaux C., Lebleu B. Oligonucleotide-poly(L-lysine)-heparin complexes: potent sequence-specific inhibitors of HIV-1 infection. Bioconjug Chem. 1994 Jan-Feb;5(1):8–13. doi: 10.1021/bc00025a002. [DOI] [PubMed] [Google Scholar]
- Degols G., Leonetti J. P., Benkirane M., Devaux C., Lebleu B. Poly(L-lysine)-conjugated oligonucleotides promote sequence-specific inhibition of acute HIV-1 infection. Antisense Res Dev. 1992 Winter;2(4):293–301. doi: 10.1089/ard.1992.2.293. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Jacobo-Molina A., Ding J., Nanni R. G., Clark A. D., Jr, Lu X., Tantillo C., Williams R. L., Kamer G., Ferris A. L., Clark P. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti J. P., Rayner B., Lemaitre M., Gagnor C., Milhaud P. G., Imbach J. L., Lebleu B. Antiviral activity of conjugates between poly(L-lysine) and synthetic oligodeoxyribonucleotides. Gene. 1988 Dec 10;72(1-2):323–332. doi: 10.1016/0378-1119(88)90159-x. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J., Sun D., Metelev V., Zamecnik P., Gallo R. C., Agrawal S. Long-term treatment of human immunodeficiency virus-infected cells with antisense oligonucleotide phosphorothioates. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3860–3864. doi: 10.1073/pnas.90.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger W., Hermann T., Schatz O., Le Grice S. F., Heumann H. Hydroxyl radical footprint analysis of human immunodeficiency virus reverse transcriptase-template.primer complexes. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5909–5913. doi: 10.1073/pnas.90.13.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallafranque-Andreola M. L., Robert D., Barr P. J., Fournier M., Litvak S., Sarih-Cottin L., Tarrago-Litvak L. Human immunodeficiency virus reverse transcriptase expressed in transformed yeast cells. Biochemical properties and interactions with bovine tRNALys. Eur J Biochem. 1989 Sep 15;184(2):367–374. doi: 10.1111/j.1432-1033.1989.tb15028.x. [DOI] [PubMed] [Google Scholar]