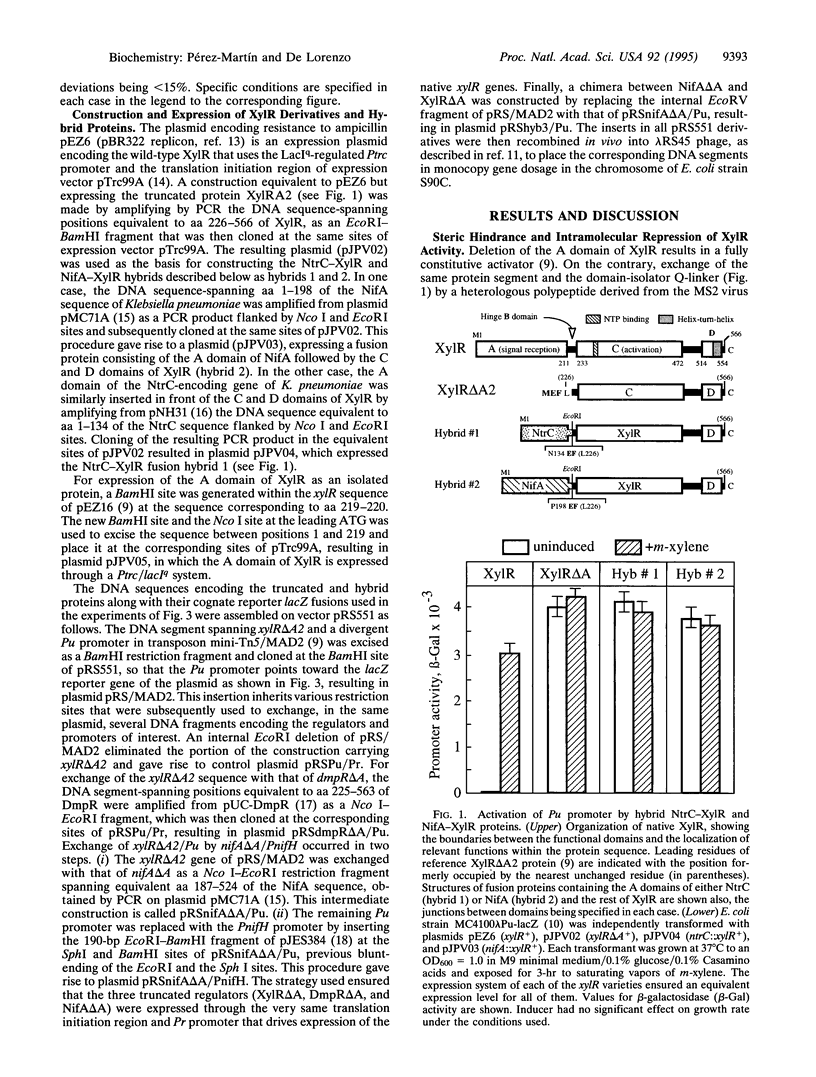
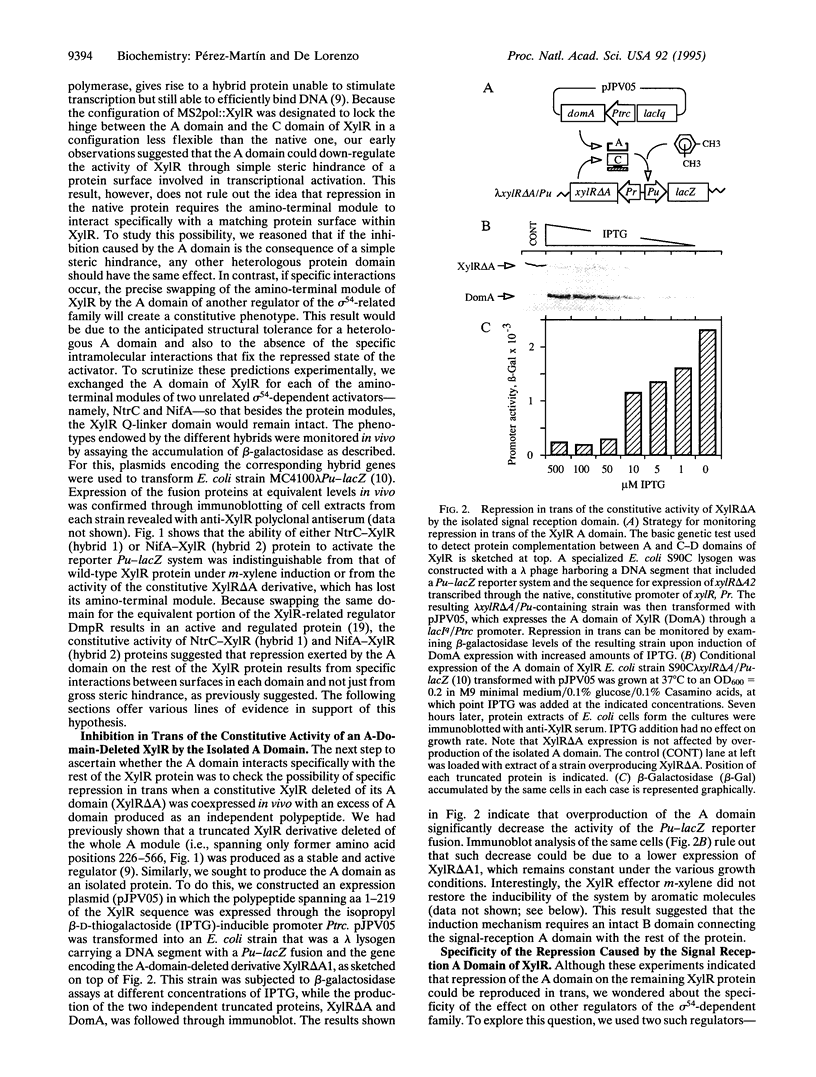
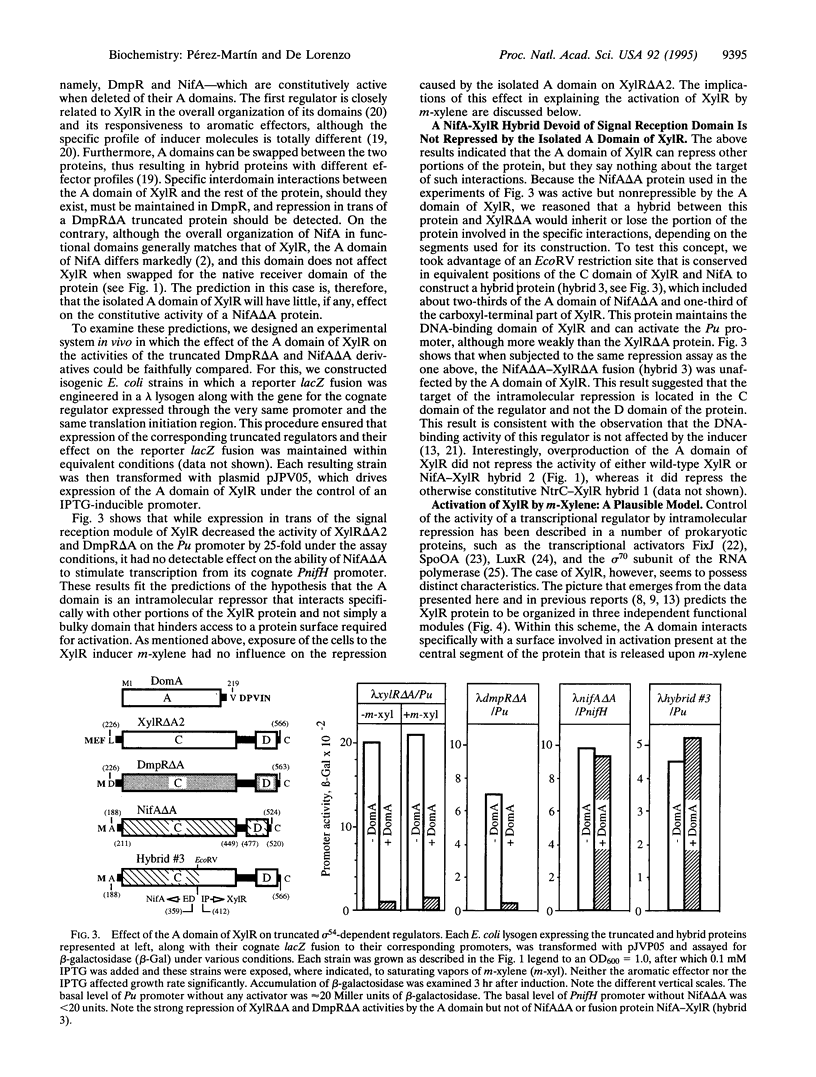
Abstract
The mechanism under which the signal-reception amino-terminal portion (A domain) of the prokaryotic enhancer-binding protein XylR controls the activity of the regulator has been investigated through complementation tests in vivo, in which the various protein segments were produced as independent polypeptides. Separate expression of the A domain repressed the otherwise constitutive activity of a truncated derivative of XylR deleted of its A domain (XylR delta A). Such inhibition was not released by m-xylene, the natural inducer of the system. Repression caused by the A domain was specific for XylR because it did not affect activation of the sigma 54 promoter PnifH by a derivative of its cognate regulator, NifA, deleted of its own A domain. The A domain was also unable to repress the activity of a NifA-XylR hybrid protein resulting from fusing two-thirds of the central domain of NifA to the carboxyl-terminal third of XylR, which includes its DNA-binding domain. The inhibitory effect caused by the A domain of XylR on XylR delta A seems, therefore, to result from specific interactions in trans between the two truncated proteins and not from mere hindering of an activating surface.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abril M. A., Buck M., Ramos J. L. Activation of the Pseudomonas TOL plasmid upper pathway operon. Identification of binding sites for the positive regulator XylR and for integration host factor protein. J Biol Chem. 1991 Aug 25;266(24):15832–15838. [PubMed] [Google Scholar]
- Amann E., Ochs B., Abel K. J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988 Sep 30;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Austin S., Dixon R. The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent. EMBO J. 1992 Jun;11(6):2219–2228. doi: 10.1002/j.1460-2075.1992.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Choi S. H., Greenberg E. P. The C-terminal region of the Vibrio fischeri LuxR protein contains an inducer-independent lux gene activating domain. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11115–11119. doi: 10.1073/pnas.88.24.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Re S., Bertagnoli S., Fourment J., Reyrat J. M., Kahn D. Intramolecular signal transduction within the FixJ transcriptional activator: in vitro evidence for the inhibitory effect of the phosphorylatable regulatory domain. Nucleic Acids Res. 1994 May 11;22(9):1555–1561. doi: 10.1093/nar/22.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado A., Ramos J. L. Genetic evidence for activation of the positive transcriptional regulator Xy1R, a member of the NtrC family of regulators, by effector binding. J Biol Chem. 1994 Mar 18;269(11):8059–8062. [PubMed] [Google Scholar]
- Delgado A., Salto R., Marqués S., Ramos J. L. Single amino acids changes in the signal receptor domain of XylR resulted in mutants that stimulate transcription in the absence of effectors. J Biol Chem. 1995 Mar 10;270(10):5144–5150. doi: 10.1074/jbc.270.10.5144. [DOI] [PubMed] [Google Scholar]
- Dixon R., Eydmann T., Henderson N., Austin S. Substitutions at a single amino acid residue in the nitrogen-regulated activator protein NTRC differentially influence its activity in response to phosphorylation. Mol Microbiol. 1991 Jul;5(7):1657–1667. doi: 10.1111/j.1365-2958.1991.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Dombroski A. J., Walter W. A., Gross C. A. Amino-terminal amino acids modulate sigma-factor DNA-binding activity. Genes Dev. 1993 Dec;7(12A):2446–2455. doi: 10.1101/gad.7.12a.2446. [DOI] [PubMed] [Google Scholar]
- Fernández S., Shingler V., De Lorenzo V. Cross-regulation by XylR and DmpR activators of Pseudomonas putida suggests that transcriptional control of biodegradative operons evolves independently of catabolic genes. J Bacteriol. 1994 Aug;176(16):5052–5058. doi: 10.1128/jb.176.16.5052-5058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández S., de Lorenzo V., Pérez-Martín J. Activation of the transcriptional regulator XylR of Pseudomonas putida by release of repression between functional domains. Mol Microbiol. 1995 Apr;16(2):205–213. doi: 10.1111/j.1365-2958.1995.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Gu B., Lee J. H., Hoover T. R., Scholl D., Nixon B. T. Rhizobium meliloti DctD, a sigma 54-dependent transcriptional activator, may be negatively controlled by a subdomain in the C-terminal end of its two-component receiver module. Mol Microbiol. 1994 Jul;13(1):51–66. doi: 10.1111/j.1365-2958.1994.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Huala E., Stigter J., Ausubel F. M. The central domain of Rhizobium leguminosarum DctD functions independently to activate transcription. J Bacteriol. 1992 Feb;174(4):1428–1431. doi: 10.1128/jb.174.4.1428-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Rudner D. Z., Siranosian K. J., Grossman A. D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993 Feb;7(2):283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- Kustu S., North A. K., Weiss D. S. Prokaryotic transcriptional enhancers and enhancer-binding proteins. Trends Biochem Sci. 1991 Nov;16(11):397–402. doi: 10.1016/0968-0004(91)90163-p. [DOI] [PubMed] [Google Scholar]
- Marqués S., Ramos J. L. Transcriptional control of the Pseudomonas putida TOL plasmid catabolic pathways. Mol Microbiol. 1993 Sep;9(5):923–929. doi: 10.1111/j.1365-2958.1993.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Morett E., Segovia L. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993 Oct;175(19):6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S. C., North A. K., Wedel A. B., Kustu S. Oligomerization of NTRC at the glnA enhancer is required for transcriptional activation. Genes Dev. 1993 Nov;7(11):2258–2273. doi: 10.1101/gad.7.11.2258. [DOI] [PubMed] [Google Scholar]
- Pérez-Martín J., Timmis K. N., de Lorenzo V. Co-regulation by bent DNA. Functional substitutions of the integration host factor site at sigma 54-dependent promoter Pu of the upper-TOL operon by intrinsically curved sequences. J Biol Chem. 1994 Sep 9;269(36):22657–22662. [PubMed] [Google Scholar]
- Santero E., Hoover T. R., North A. K., Berger D. K., Porter S. C., Kustu S. Role of integration host factor in stimulating transcription from the sigma 54-dependent nifH promoter. J Mol Biol. 1992 Oct 5;227(3):602–620. doi: 10.1016/0022-2836(92)90211-2. [DOI] [PubMed] [Google Scholar]
- Shingler V., Bartilson M., Moore T. Cloning and nucleotide sequence of the gene encoding the positive regulator (DmpR) of the phenol catabolic pathway encoded by pVI150 and identification of DmpR as a member of the NtrC family of transcriptional activators. J Bacteriol. 1993 Mar;175(6):1596–1604. doi: 10.1128/jb.175.6.1596-1604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingler V., Moore T. Sensing of aromatic compounds by the DmpR transcriptional activator of phenol-catabolizing Pseudomonas sp. strain CF600. J Bacteriol. 1994 Mar;176(6):1555–1560. doi: 10.1128/jb.176.6.1555-1560.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Batut J., Klose K. E., Keener J., Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991 Oct 4;67(1):155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- Wootton J. C., Drummond M. H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989 May;2(7):535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Herrero M., Metzke M., Timmis K. N. An upstream XylR- and IHF-induced nucleoprotein complex regulates the sigma 54-dependent Pu promoter of TOL plasmid. EMBO J. 1991 May;10(5):1159–1167. doi: 10.1002/j.1460-2075.1991.tb08056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]