Abstract
A prominent and replicated finding is the correlation between running speed and increases in low-frequency oscillatory activity in the hippocampal local field potential. A more recent finding concerns low-frequency oscillations that increase in coherence between the hippocampus and neocortical brain areas such as prefrontal cortex during memory-related behaviors (i.e., remembering the correct arm to explore). In this review, we tie together movement-related and memory-related low-frequency oscillations in the rodent with similar findings in humans. We argue that although movement-related low-frequency oscillations, in particular, may have slightly different characteristics in humans than rodents, placing important constraints on our thinking about this issue, both phenomena have similar functional foundations. We review four prominent theoretical models that provide partially conflicting accounts of movement-related low-frequency oscillations. We attempt to tie together these theoretical proposals, and existing data in rodents and humans, with memory-related low-frequency oscillations. We propose that movement-related low-frequency oscillations and memory-related low-frequency oscillatory activity, both of which show significant coherence with oscillations in other brain regions, represent different facets of “spectral fingerprints,” or different resonant frequencies within the same brain networks underlying different cognitive processes. Together, movement-related and memory-related low-frequency oscillatory coupling may be linked by their distinct contributions to bottom-up, sensorimotor driven processing and top-down, controlled processing characterizing aspects of memory encoding and retrieval.
Keywords: hippocampus, delta, theta, spatial navigation
Research on navigation and memory-related low frequency oscillations suggest two distinct functional characterizations. Extensive empirical and theoretical work in the rodent has helped to characterize a movement-related 3–12Hz oscillation in the hippocampus. This low-frequency oscillation, also referred to as the “theta” oscillation, increases with movement speed (McFarland et al., 1975) and has a distinct relationship with the activity of “place cells,” which code spatial location (O'Keefe and Recce, 1993). We refer to these oscillations as “movement-related low-frequency oscillations”. Comparison of changes in oscillatory coherence for correct vs. incorrect retrieval of which arm is baited on a maze alternation task have also identified a potentially separate phenomenon of coherent and/or phase synchronized low-frequency oscillations (Jones and Wilson, 2005b, Benchenane et al., 2010, Fujisawa and Buzsaki, 2011). These oscillations increase in coherence between hippocampus and prefrontal cortex (PFC) during correct alternations on a maze or correct utilization of a recently learned rule but do not appear to be driven by movement-speed alone (Jones and Wilson, 2005b, Benchenane et al., 2010, Fujisawa and Buzsaki, 2011). We refer to these oscillations, and oscillatory changes driven by memory processing in general (for a review, see: Nyhus and Curran, 2010), as “memory-related low-frequency oscillations.”
Recordings in the human brain have also identified low-frequency oscillations, although with slightly different characteristics, providing an important extension to the rodent work. Humans display movement-related hippocampal low-frequency oscillations (Ekstrom et al., 2005, Watrous et al., 2011, Watrous et al., 2013a) although some of their properties – specifically, their peak frequency and mean cycle length – may differ somewhat from those characterized in rodents (Watrous et al., 2011, Lega et al., 2012, Watrous et al., 2013a). Recordings in humans from multiple cortical sites and the medial temporal lobe, similar to the rodent, have demonstrated the presence of low-frequency oscillations that increase in coherence during correct memory retrieval (Anderson et al., 2009, Watrous et al., 2013b). These results thus provide an important extension to similar findings in rodents by relating these increased oscillations specifically to successful memory encoding and retrieval (Sederberg et al., 2003, Rutishauser et al., 2010) and coding of different contexts (Watrous et al., 2013b).
Given the literature suggesting differences between movement and memory-related low-frequency oscillations, as well as the need to tie together these oscillations in rodents with humans, the purpose of this review is two-fold. First, we wish to provide a characterization of movement-related oscillations in both rodents and humans and discuss possible reasons why low-frequency oscillations in the two species may manifest differently. We will also discuss several theoretical frameworks that account for movement-related low-frequency oscillations and provide explanations for their functional relevance. We will then discuss evidence in rodents and humans that suggests that these movement-related oscillations are not just a local phenomenon within the hippocampus but interact in a coherent fashion with similar low-frequency oscillations in areas such as parietal cortex (Ekstrom et al., 2005, Sirota et al., 2008).
Second, we will discuss memory-related low-frequency coherent oscillations between hippocampus, prefrontal cortex, and other cortical and subcortical areas, and possible differences in their functional characteristics compared to movement-related oscillations. Finally, we will attempt to tie together movement-related and memory-related oscillations to provide a unitary account of their function. We suggest that movement-related oscillations represent the down-stream result of sensorimotor entrained signals (Schroeder et al., 2010) – specifically, increases in power and frequency locked to increases in optic flow and motor movements (Chen et al., 2013). In contrast, we suggest that memory-related oscillations manifest as the result of long-range synchronization underlying recruitment and cooperation of different brain regions important for decisions, including retrieval. As such, we argue that these manifest as largely top-down, internally driven signals (e.g., Buschman and Miller, 2007). While many aspects of our model remain to be validated, we believe that it represents a first step in trying to tie together disparate findings on low-frequency oscillations across mammalian species.
Rodent Hippocampal Low-frequency Oscillations and Correlation with Movement
Vanderwolf first identified rhythmic low-frequency oscillations occurring between 8–8.3 cycles/second during walking and rearing (“voluntary movements”) in the local field potential (LFP) of the rodent hippocampus. This activity was distinct from lower frequency activity (6.6–7 Hz) that emerged during handling food, licking, or grooming. Extending Vanderwolf’s findings relating theta oscillations to voluntary movements, McFarland et al. (1975), testing rats running on a treadmill at various speeds, concluded that oscillatory amplitude varied monotonically with running speed (McFarland et al., 1975). Several studies have since replicated this finding in both rats and other species, including mice, guinea pigs, rabbits, cats, and dogs (Arnolds et al., 1979, Shen et al., 1997, Czurko et al., 1999, Ekstrom et al., 2001, Geisler et al., 2007, Chen et al., 2011, Li et al., 2012, Chen et al., 2013). A similar and related finding is that oscillatory frequency also increases with running speed; this frequency shift is fairly slight (<1Hz in some cases) (Geisler et al., 2007) although consistently observed in several reports (Recce, 1994, Oddie et al., 1996, Shen et al., 1997, Woodnorth and McNaughton, 2005, Geisler et al., 2007, Chen et al., 2011, Li et al., 2012). The increases in theta power and frequency with running speed are often considered benchmark findings on low-frequency oscillations and have had a significant influence on theories detailing the functional significance of theta during both navigation and memory (Bland and Oddie, 2001, Hasselmo et al., 2002, Buzsaki, 2006).
Rodent movement-related oscillations also have a fairly precise link with the phase at which hippocampal pyramidal cell spikes occur. During navigation, pyramidal cells often fire at specific spatial locations. Within the place field of these “place cells” (O'Keefe and Dostrovsky, 1971), spikes tend to occur at earlier phases of on-going theta oscillation as the rat traverses the place field (O'Keefe and Recce, 1993), termed “phase precession.” The increase in power and frequency is central to allowing phase precession; increases in power with movement provide sufficient oscillatory signal for phase precession to occur in the first place. The increase in frequency with running speed is also important; because neural firing rate also increases with running speed (McNaughton et al., 1983), increases in theta frequency allow for a critical offset between the activity of place cells and the period of theta (Geisler et al., 2007). The simultaneous offset, yet coupled activity of theta oscillations and firing rate, is one explanation of how phase precession might emerge mechanistically within the hippocampus (O'Keefe and Recce, 1993, Geisler et al., 2007).
Phase precession also provides a critical link between oscillations, neural activity, and behavior. Including phase in reconstruction of a rats location during navigation in addition to firing rate conveys more information about the rat’s spatial position than firing rate alone (Jensen and Lisman, 2000). Phase coding may also provide a critical timing mechanism for controlling what types of information the activity of single neurons in the hippocampus provides compared with rate coding (Huxter et al., 2003). Another interesting proposal relates phase precession to the binding of memories via phase offsets between different place fields (Dragoi and Buzsaki, 2006). Additionally, several in vivo and in vitro studies have linked theta oscillations to states of increased and decreased synaptic plasticity. Long-term potentiation (LTP), a measure of synaptic plasticity in the hippocampus and elsewhere, is modulated by the theta oscillation (Huerta and Lisman, 1995, Holscher et al., 1997), such that LTP is easier to induce at the peak compared to the trough of theta. This in turn has provided an important link between low-frequency oscillations as a modulator of neural activity and information coding generally (Seager et al., 2002). Together, these studies suggest the importance of low-frequency oscillations to mediating a variety of hippocampal functions, both at the cellular and behavioral level.
Hippocampal low-frequency oscillations in primates
Early reports with anesthetized monkeys noted only weak low-frequency oscillations in raw traces compared to rat and rabbit hippocampus (Green and Arduini, 1954). Later reports in anesthetized monkeys demonstrated a clear peak around 8 Hz in both the raw traces and the power spectral density (psds), which reflect the power (amplitude squared) at a given frequency (Stewart and Fox, 1991). In subsequent reports, however, inspection of raw traces during a delayed-non match to sample task did not reveal low-frequency oscillations to the extent typically reported in the rodent (Skaggs et al., 2007), leading the authors to speculate that these oscillations may be weak or absent in primates. Similarly, early reports in humans found mixed evidence for behavior-related hippocampal oscillations. Brazier (1968), recording from both epilepsy and schizophrenia patients implanted with hippocampal electrodes as part of clinical monitoring, reported low-frequency activity in both raw traces, psds, and coherence plots between different depth electrodes during resting and sleep. Halgren et al. (1978) confirmed the presence of hippocampal low-frequency oscillations during resting (Halgren et al., 1978) but suggested that low-frequency activity tended to disappear during behaviors such as remembering words or attending to stimuli (see also: Huh et al., 1990).
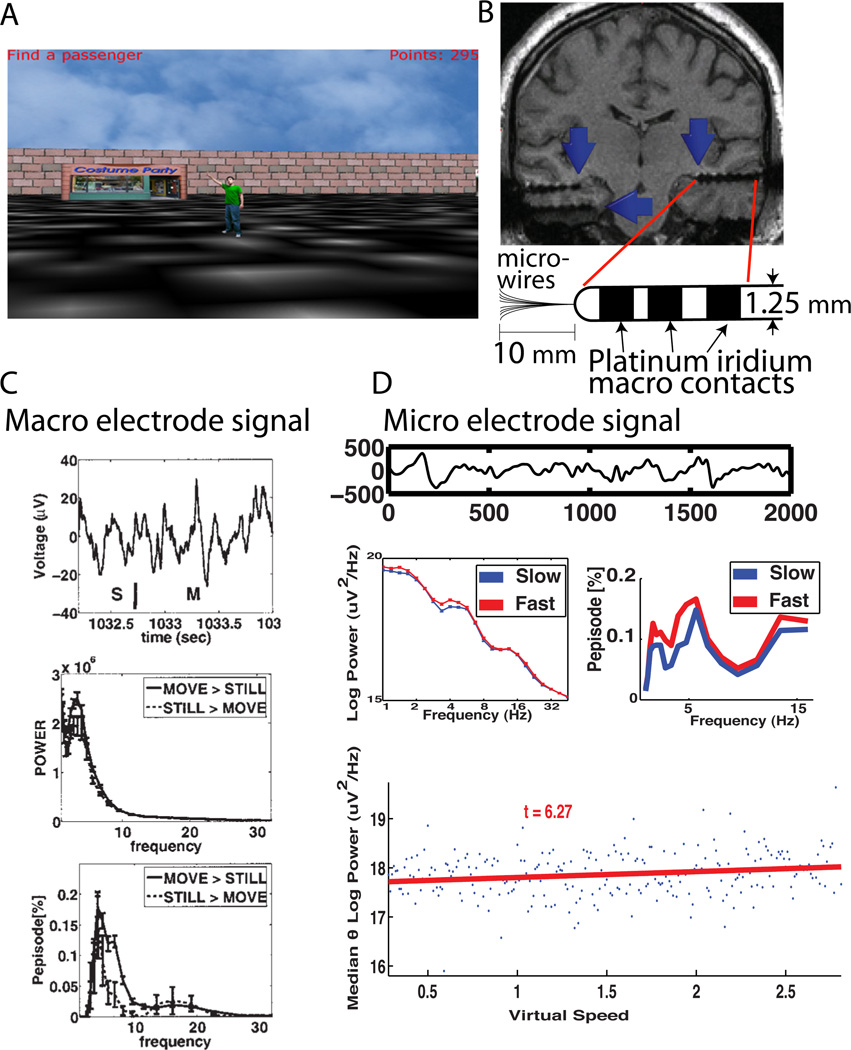
Subsequent invasive and non-invasive human recordings have demonstrated movement-related hippocampal low-frequency oscillations. Using a virtual navigation task (Figure 1A) similar to that used in rodents, we showed primarily delta (1–4 Hz) and theta (4–8 Hz), with some higher frequency (>8 Hz) oscillations present both in the power spectral density and raw traces on both macrowire and microwire recordings (Figure 1B). In one study using macroelectrodes (1.25 mm diameter) implanted in the hippocampus in 6 different patients, we compared oscillatory activity during movement vs. still periods. Our results showed increased low-frequency oscillatory activity in both raw traces and psds (Figure 1C upper and middle panel). Furthermore, we used an oscillation detection method termed Better OSCillation (BOSC) detection method to characterize these signals (Whitten et al., 2011, Hughes et al., 2012). This method employs both an amplitude and duration threshold to time varying signals, allowing statistical identification of oscillatory activity above 95% of the background noise lasting at least N number of cycles (see Figure 1 in Hughes et al, 2012). We found clear evidence for oscillations using BOSC, primarily in the human delta (1–4 Hz) and theta (4–8 Hz) bands across our population of recordings when comparing movement vs. still epochs (Figure 1C, lower panel).
Figure 1.
Movement-related theta in humans using depth electrode recordings. A) Sample image from a virtual navigation task. B) Schematic diagram of depth electrodes used to study human hippocampal oscillatory activity, illustrating both macro-contacts and micro-wires. C) Movement-related hippocampal oscillations recorded from a macroelectrode. Theta is evident in the raw trace (upper; S;still epoch, M; movement epoch), power-spectral density plots (PSD; middle), and using the Pepisode/BOSC oscillation detection algorithm (lower; see Main Text for description of algorithm). Figure modified from Ekstrom et. al, 2005. D) Movement-related hippocampal oscillations recorded from a microwire. Theta is evident in the raw trace (upper), PSD (middle-left), and using the Pepisode/BOSC detection algorithm when comparing fast vs. slow movement epochs. Virtual movement-speed was significantly correlated with theta power (lower). Figure modified from Watrous et. al 2011.
Using microelectrode recordings (40 micrometer platinum-iridium electrodes), which record from smaller fields within the hippocampus, we again found low-frequency oscillations that increased in power during faster compared to slower movements (Figure 1D). Watrous et al. (2011), analyzing 10 different patients undergoing seizure monitoring, demonstrated that a subset of electrodes showed monotonic increases in hippocampal low-frequency power with increases in virtual movement speed (Figure 1D). These findings replicate the monotonic relationship between movement speed and power shown previously in the rodent by MacFarland et al. (1975) and others. Using the same dataset, we also demonstrated that movement-related oscillations increased in frequency, albeit modestly (<1Hz), with increases in running speed (Watrous et al., 2013a).
Studies using magnetoencephalography (MEG) have also demonstrated movement-related oscillations emanating from a source localized to the MTL (de Araujo et al., 2002, Cornwell et al., 2008, Cornwell et al., 2012, Kaplan et al., 2012). Because MEG source localization to areas such as the MTL likely has an accuracy on the order of centimeters (Barnes et al., 2004), these methods cannot unambiguously pinpoint activity to specific sub-areas of the MTL. Human semi-invasive recordings that involve placing electrodes via entrance through the foramen ovale also suggest the presence of low-frequency movement-related oscillations emanating from the MTL during VR navigation (Clemens et al., 2013). A non-human primate study also demonstrated theta oscillations and spike/phase coupling in the MTL (entorhinal cortex) when monkeys looked at objects (Killian et al., 2012), which the authors related to movement-related changes. Together with our findings, reports using MEG, foramen ovale electrodes, and recordings in non-human primates suggest conservation of movement-related low-frequency oscillations in the hippocampus and medial temporal lobes.
Why have behavior-related low-frequency oscillations been elusive in primates?
Previous research reporting little or no primate hippocampal low-frequency oscillations typically relied on a limited number of raw traces, making it difficult in some cases to observe oscillations. But given the prominence of these oscillations in the raw trace in the rodent (Skaggs et al., 2007), why are theta oscillations often difficult to observe? One interesting proposal is that human low-frequency oscillatory activity often manifests within a lower frequency band than rodent hippocampal theta, with a peak around 3 Hz rather than 8 Hz (Jacobs et al., 2007, Lega et al., 2012). In a study designed to address this idea directly, we compared microwire recordings from rats running on a Barnes maze, a comparable task to the taxi-driver task used in our previous work (Ekstrom et al., 2005, Watrous et al., 2011), with microwire recordings from our patients in this previous study (Watrous et al., 2011). All electrodes analyzed were placed in the hippocampus and we employed the BOSC method to better characterize oscillations (Whitten et al., 2011, Hughes et al., 2012). Using this method, we determined that rodent movement-related oscillations typically peaked at around 8 Hz and lasted about 4.3 cycles while those from humans peaked around 3.4 Hz and lasted typically about 2.7 cycles (Watrous et al., 2013a). These quantitative findings mirror previous qualitative observations in the literature suggesting human low-frequency oscillations are more transient and may peak at a lower frequency than that characterized in the rodent (Brazier, 1968, Arnolds et al., 1980, Kahana et al., 1999, Bodizs et al., 2001, Cantero et al., 2003, Lega et al., 2012).
Based on these findings, we suggest several possible reasons why primate low-frequency oscillations may have been elusive in the past. Given that they are more transient in humans, they may often be difficult to detect in the raw trace. Simply put, an oscillation lasting consistently fewer cycles will be less obvious in a raw trace than one that is more sustained. Also, using analytic methods that do not allow unambiguous identification of signal from the background 1/f noise spectrum, such as Fourier methods, may often miss these oscillatory signals, particularly given their transient nature (van Vugt et al., 2007). Specifically, increases oscillatory power within fairly narrow frequency bands or frequency shifts close in time are more easily identified with methods such as BOSC compared to looking at the raw trace or using Fourier-based methods (van Vugt et al., 2007, Cruikshank et al., 2012). Another possible reason why low-frequency oscillations may have been elusive in primates compared to rodents is the substantial difference in size between the primate and rodent brain. Larger brains would likely involve greater number of sources converging onto a single recording electrode, the summation of which would involve greater degrees of oscillatory interference. This in turn could explain why oscillatory frequency may show an inverse relationship with brain size across species (Robinson, 1980, Herculano-Houzel, 2009). We note, though, that both non-invasive and semi-invasive recording approaches have also identified oscillations in the delta-theta band emanating from the MTL (de Araujo et al., 2002, Cornwell et al., 2008, Cornwell et al., 2012, Kaplan et al., 2012, Clemens et al., 2013). If greater number of phase cancelling sources alone is responsible for the difference in low-frequency oscillations between rats and primates, the presence of oscillations as far away as the scalp seems to weigh against this argument.
What is the function of hippocampal movement-related oscillations?
Several decades of work in the rodent attempted to determine the precise behavioral correlate of hippocampal low frequency oscillations. Past studies have suggested roles ranging from attention, memory, arousal, navigation, sensory integration, and sexual behavior (for a review, see: Buzsaki, 2005). One important suggestion is that there is no one behavioral correlate of hippocampal theta oscillations and that they likely serve a more general role in coordinating ensembles within the hippocampus during behavior (Buzsaki, 2005). According to this conceptualization, movement may thus be one example where this is particularly important need for coordination of ensembles of neurons in order to navigate to the correct location (Buzsaki, 2006, Montgomery et al., 2009). But what then is the precise function of hippocampal movement-related low-frequency oscillations?
One particularly influential idea, that accounts nicely for movement-related oscillations in rats and humans, is the sensorimotor integration hypothesis (Bland, 1986, Bland and Oddie, 2001, Caplan et al., 2003, Ekstrom et al., 2005, Cruikshank et al., 2012). This proposal suggests that these oscillations serve to organize incoming sensory information for correct output of motor responses (Bland and Oddie, 2001). The crux of the theory derives from the fact that behavioral and pharmacological studies suggest a functional division between movement-related 7–8Hz oscillations (Type 1, sensitive to serotonin antagonism) and immobility, movement-initiation-related 6–7Hz oscillations (type 2, sensitive to cholinergic antagonism) (Kramis et al., 1975, Bland and Oddie, 2001, Bland, 2008). Later versions of the proposal have suggested that the two oscillations may exist in parallel during movement, with type 2 oscillations handling sensory processing and type 1 handling motor movements. Their simultaneous presence in in the hippocampus during movement thus allows sensorimotor integration necessary for navigation (Bland, 2008).
In assessing the sensorimotor integration hypothesis, it is clear that movement-related low-frequency oscillations fit well with this idea, as greater optic flow requires more coordinated activity to allow faster running speed, i.e., greater motor output. Low-frequency oscillations, in human hippocampus and elsewhere, however, have also been related to correct memory encoding and retrieval (Sederberg et al., 2003, Nyhus and Curran, 2010, Addante et al., 2011) and spatial updating during navigation (Watrous et al., 2011). Sensorimotor-related oscillations, though, may manifest as a reflection of necessary computational inputs for memory-related processing and are not therefore exclusive of a larger role in memory processing (Bland, 2004). Somewhat more problematic for the sensorimotor integration hypothesis, hippocampal lesions do not significantly impair sensorimotor integration in human patients (Shadmehr et al., 1998, Corkin, 2002) although the same lesions do impact the ability to encode and retrieve recently acquired memories (Scoville and Milner, 1957, Yonelinas et al., 2002). A recent scalp EEG study demonstrated increased low-frequency oscillations related to movement-initiation and sensory processing that were distinct from purely motor-related mu activity (Cruikshank et al., 2012). Given that scalp EEG is likely capturing primarily neocortical, rather than hippocampal activity (Nunez and Silberstein, 2000) and parietal lesions impair sensorimotor processing (Sirigu et al., 2004), one intriguing possibility is that sensorimotor integration is primarily a cortical function. Sensorimotor-related oscillations may nonetheless manifest in the hippocampus via phase synchronization with cortical and subcortical brain areas. We return to this idea later when we discuss coherence of low-frequency oscillations between the hippocampus and cortical areas.
Another influential idea, which we refer to as the ensemble phase coding hypothesis, suggests that low-frequency oscillations serve to coordinate disparate ensembles of neurons within the hippocampus to organize them during encoding and retrieval of memories (Buzsaki, 2006, Mizuseki et al., 2009). As opposed to the sensorimotor integration hypothesis, this idea has focused on a role for these oscillations primarily in memory function (Caplan and Glaholt, 2007, Nyhus and Curran, 2010, Buzsaki and Moser, 2013). A basic prediction of the proposal, which has generally been validated, is that disruption of low-frequency oscillations should profoundly impair memory. Lesions of the septal nucleus, which disrupt low-frequency oscillations, do indeed affect place coding and spatial memory (Winson, 1978, Leutgeb and Mizumori, 1999). This proposal also naturally accounts for the sizeable literature in both rodents and humans suggesting that low-frequency oscillations also play roles in memory encoding and retrieval (Sederberg et al., 2003, Jeewajee et al., 2007, Montgomery et al., 2009, Nyhus and Curran, 2010, Watrous et al., 2011).
The ensemble phase coding hypothesis, though, does not appear to provide a natural explanation of movement-related increases in oscillatory power compared to the sensorimotor integration hypothesis. Why might increases in movement alone necessitate greater memory demands and the need for presumably greater coordination of disparate ensembles within the hippocampus? Also, several studies that have demonstrated a memory-related function for low-frequency oscillations have also suggested that these oscillations manifest independently from movement-related variables and movement-related oscillations (Caplan et al., 2000, Jones and Wilson, 2005b, Jeewajee et al., 2007, Montgomery et al., 2009, Benchenane et al., 2010, Watrous et al., 2011). Specifically, these studies showed that memory-related low-frequency oscillations manifest on different electrodes from those showing movement-related increases (Watrous et al., 2011), that memory and movement-related effects derive from different neural sources (Montgomery et al., 2009), and that memory and task related changes in low-frequency oscillations cannot be explained by changes in movement alone (Jones and Wilson, 2005b, Benchenane et al., 2010). If the two are functionally independent, how does a memory-related explanation account for movement-driven oscillatory activity (and vice versa for the sensorimotor integration hypothesis)? A final issue is that the ensemble phase coding hypothesis (as well as the sensorimotor integration hypothesis), would seem to have difficulty explaining why low-frequency oscillations might manifest differently in humans than rodents. For example, it seems reasonable to assume that humans might have greater need for ensemble coordination than rodents, given the complexity of human behavior and the detail of our memory (Tulving, 2002), and thus less continuous oscillations in humans in general might appear to be detrimental to ensemble coordination.
Hippocampal low-frequency movement-related oscillatory activity as an entrainment phenomenon
Several reports studying oscillatory activity, both intracranially with humans and monkeys and using scalp EEG with humans, suggest that rhythmic sensory and motor activity may often entrain activity in specific brain areas. Based on these findings, the active sensing model advances the idea that actively processing stimuli, be it listening to, touching, or viewing them, imposes rhythmicity that is subsequently coded within the brain (Schroeder et al., 2010). This proposal is distinct from the sensorimotor integration hypothesis in that 1) it does not hypothesize a specific brain region as integral to this function 2) states that either sensory or motor rhythmicity will manifest as oscillatory entrainment (rather than their integration).
In support of this proposal, Lakotos et al. (Lakatos et al., 2005, Lakatos et al., 2008) tested monkeys attending to either flashing lights or repeated sounds. Attention to either rhythmic auditory or visual stimuli entrained oscillations in monkey visual cortex at approximately a similar frequency (within the 1–4Hz delta range), a finding also demonstrated with human invasive recordings (Besle et al., 2011). Other studies have suggested that rhythmic motor movements induce oscillatory entrainment in motor areas (e.g.,Murthy and Fetz, 1992, Baker et al., 1997). As evidence that entrainment may reach as far as the hippocampus, neurons in the hippocampus (and elsewhere) are triggered by saccade initiations and subsequently display rhythmicity based on the frequency of saccade movements (Ringo et al., 1994, Rajkai et al., 2008).
One possibility then is that hippocampal movement-related oscillations may occur as a byproduct of rhythmicity imposed by sensory inputs and/or motor processing (Ringo et al., 1994). In support of this idea, both visual input and locomotion appear to modulate low-frequency oscillations independently. Chen et al. (2011) found that passive displacement of a rat through a tube, which would result in vestibular input but not motor related activity, caused a significant reduction in low-frequency oscillatory power, suggesting that motor activity represents a component of rodent low-frequency hippocampal oscillations (see also: Gavrilov et al., 1995). Removal of visual information, by having the animals navigate the tube in the dark, though, resulted in only a marginal decrease in oscillatory power. While on the surface this might seem to suggest that visual input has only a small effect on low-frequency oscillations compared to locomotion, because the authors did not independently manipulate the rate of optic flow (visual input) from vestibular input, the experiment does not allow us to conclude whether visual input alone does in fact modulate oscillatory activity in the hippocampus.
Papers by Chen et al. (2013) and Terrazas et al. (2005) in fact support the idea that the rate of optic flow, independent of locomotion, modulates low-frequency oscillatory activity. Terrazas et al. (2005) showed that rats trained to navigate around a track on a car activated by a lever press, which resulted in movement-related low-frequency oscillations, nonetheless showed low-frequency oscillations simply when the visual environment was passively moved around them (which would be likely to induce vection). Chen et al. (2013) trained restrained mice to run on a ball whose movement was either yoked or not fixed to the rate of optic flow appearing on a computer monitor. Consistent with earlier reports (McFarland et al., 1975), faster movement and optic flow on a real track led to increases in theta power and frequency compared to slower movement. Critically, increases in optic flow alone, in the absence of any motor movement, also led to increases in low-frequency oscillations, albeit at a slightly (~1 Hz) lower frequency and power than observed when combined with motor movements. These data suggest that both visual and motor components likely entrain theta oscillations, consistent with the ideas proposed by Schroeder et al. (2010). These studies though do not allow us to conclude that entrainment with motor output during locomotion necessarily indicates that oscillations are phase locked specifically to the output itself; it could also be the case that prioprioreceptive/sensory feedback or even visual feedback in observing limb movement may also contribute to entrainment. In this way, entrainment to motor activity could still represent bottom-up processing of sensory feedback information, and not necessarily output itself.
Reconciling the Ensemble Phase Coding Hypothesis and Active Sensing
One potential problem for the active sensing model emerges from findings suggesting that low-frequency oscillations, in human hippocampus and elsewhere relate to correct memory encoding and retrieval (Sederberg et al., 2003, Nyhus and Curran, 2010, Addante et al., 2011) and spatial updating during navigation (Watrous et al., 2011). How do movement-driven oscillations emerge when there are no obvious synchronizing stimuli? One possibility is that mental imagery involves it own internal temporal dynamics (Tulving, 2002), although this is idea is difficult to test directly without self-report and introspection-based measures. Another possibility is that memory-related oscillatory activity could represent a distinct phenomenon from movement-related oscillations (Lega et al., 2012), which may emerge due to influences from other top-down brain areas, such as prefrontal cortex. As we will consider in more detail shortly, memory-related oscillations often manifest at different frequencies than movement-related oscillations and may represent a top-down, intrinsically driven signal rather than a largely movement-driven one.
Another issue for the active sensing model regards the differences in frequency at which low-frequency oscillations emerge between rats and humans (Lega et al., 2012, Watrous et al., 2013a). The exact frequency band in which oscillations manifest may depend on numerous factors, including frequency of sensory input and sensory processing (Schroeder et al., 2010), overall brain/body size (Robinson, 1980), and intrinsic factors of brain networks, such as the prevalence and connectivity amongst neurons with different resonant properties (Buzsaki, 2002). Because humans receive strong modulatory input from visual areas via parahippocampal cortex compared to rodents (Van Hoesen, 2002), it is intriguing to consider that differences in transmission of visual input could account for some of these differences. It is as yet unclear though precisely how differences in sensory input could account for the observed lower frequency activity overall.
An intermediate position related to both the active sensing model and the ensemble phase coding hypothesis is the idea that different phases of hippocampal theta oscillations underlie memory encoding and retrieval. Specifically, entorhinal input into the hippocampus, reflecting information processed during encoding vs. retrieval, occurs at opposite phases of oscillatory activity (Hasselmo et al., 2002, Manns et al., 2007). Thus, information related to encoding occurs at the trough of the hippocampal theta oscillation and that related to retrieval occurs at the peak, helping to prevent the two processes from interfering with each other. Consistent with this idea, retrieval probes result in significant phase resetting of ongoing theta oscillatory activity in both the neocortex and hippocampus (Rizzuto et al., 2003, Mormann et al., 2005). Furthermore, this reset occurs at different oscillatory phases during encoding vs. retrieval, providing some support for the idea that the two processes involve different phases of theta oscillations (Rizzuto et al., 2006). Rodent studies have also observed similar phase resetting phenomenon during working memory tasks (Givens, 1996, McCartney et al., 2004). The magnitude of phase resetting, however, does not appear to predict successful memory encoding (Rutishauser et al., 2010). Another potential issue is that navigation often does not involve clear segregation of encoding and retrieval, and often the two processes may occur in concert (Sturz et al., 2009, Zhang and Ekstrom, 2012).
The common thread amongst these three models is that memory networks may be optimally suited for encoding incoming stimuli during specific time windows dictated by the local oscillation. Although the active sensing model relies on either sensory or motoric rhythmicity to synchronize brain networks, the Hasselmo and ensemble phase coding models allow for gating of information flow based on a single, task-relevant stimulus, which in turn may regulate periods of heightened sensory processing in the absence of rhythmic sensory input. We believe, though, that there is sufficient evidence to suggest that movement-related oscillations may emerge, in part, due to sensory and motoric entrainment, at least for consideration of theoretical possibilities in the section.
Low-frequency oscillations as a means of long-range coordination of neural ensembles
Somewhat in contrast to the above theories, which primarily focus on a role for movement-related low-frequency oscillations in coordinating activity locally, evidence suggests that low-frequency oscillations are not solely a local phenomenon but reflect synchronization with more distant brain areas. Computational models and experimental findings suggest that lower frequency oscillations may be better suited for long-range coordination of distant brain regions while higher frequency oscillations may be more involved in local computations (Kopell et al., 2000, von Stein and Sarnthein, 2000, for a review, see: Siegel et al., 2012). Thus, we focus here on a role for low-frequency oscillations in coordinating distant brain regions (but see: Varela et al., 2001). Consistent with this idea, simultaneous recordings from hippocampus and prefrontal cortex in rodents has shown single neuron activity phase locked to hippocampal movement-related low frequency oscillations (Hyman et al., 2005, Jones and Wilson, 2005a, Siapas et al., 2005). These studies suggest that low-frequency oscillations are often coherent during behavior between hippocampus and prefrontal cortex (Jones and Wilson, 2005a, b) and hippocampus and parietal cortex (Sirota et al., 2008).
In one such study, Sirota et al. investigated low-frequency movement-related coherence between hippocampus and parietal cortex. They found that movement-related coherent low-frequency oscillations could be detected in both hippocampus and parietal cortex. Parietal cortex pyramidal and interneurons phase locked with hippocampal oscillatory activity, which might seem to argue against the idea that the observed coherence was due only to volume condition (Sirota et al., 2008). Using macroelectrode recordings in hippocampus and neocortex, we also showed that bouts of increased movement-related oscillations were correlated with similar such bouts in our neocortical recordings. These data also suggested that the two signals tended to co-occur during movement (Ekstrom et al., 2005). Together, these data support the possibility that movement-related oscillations are also present in neocortex and could possibly originate in the hippocampus, in part, from entrainment with the cortex. Thus, hippocampal movement-related oscillations could be a reflection of interactions with areas more directly involved in processing sensorimotor information, optic flow, and path integration information, such as parietal cortex (Sirigu et al., 2004, Wolbers et al., 2008). In this way, hippocampal movement-related low-frequency oscillations can be considered an example of a bottom-up signal, driven primarily by visual, vestibular, sensory, or proprioreceptive feedback.
Memory-related low-frequency oscillations as distinct from movement-related low-frequency oscillations
As mentioned previously, several studies have shown low-frequency oscillations also increase during encoding and retrieval of recently acquired memories. These findings seem slightly at odds with numerous studies suggesting roles for low-frequency oscillations in sensory and locomotion components of navigation. Several of these studies have also compared electrodes on which the two different effects manifest and reported that these oscillatory changes emerge from different electrodes sites and sources (Montgomery et al., 2009, Watrous et al., 2011). This raises the interesting possibility that movement-related and memory-related oscillations may in many cases emerge independently. Indeed, several studies looking at long-range coherence in hippocampal low-frequency oscillations also support this idea.
In one study looking at low-frequency coupling between hippocampus and PFC in rodents (Benchenane et al., 2010), rats were trained to follow two different rules while running on a Y maze to receive reward. During the task, rats utilized either a spatial rule (i.e., run left or right) or a cue rule (i.e. choose the illuminated or dark arm). Hippocampal-PFC coherence was predictive of correct rule acquisition and was higher for correct decisions than incorrect (error) trials. Heightened coherence states were also associated with the formation of cell assemblies in PFC following learning, providing a direct link between inter-regional coherence and memory encoding processes. Importantly, this increase in 5–10 Hz coherence between hippocampus and PFC could not be accounted for by changes in running speed between the different conditions. These data suggest that memory-related low-frequency oscillations may exist as a mechanism independent from changes in movement-related hippocampal low-frequency oscillations. In a similar study by Jones and Wilson study, the authors found hippocampal-PFC 4–12 Hz oscillatory coherence was higher during correct choices than forced turns and lower during error trials during a maze alternation task. The authors found that running speed did not differ between correct choices, forced turns, nor error trials (Jones and Wilson, 2005, Figure 4A), suggesting that these oscillations did not derive from movement-related effects alone.
In an elegant experiment that provided evidence for both a dissociation but also a link between memory-related and movement-related hippocampal low-frequency oscillations, Fujisawa et al. (2011) trained rats to run to one of two different arms of a maze based on sampling a specific odor (Fujisawa and Buzsaki, 2011). Rats also performed a control task in which they ran on one arm of the maze for reward every trial. Simultaneous recording from the hippocampus, prefrontal cortex (PFC), and the ventral tegmental area (VTA) revealed two dominant oscillations – a 4 Hz oscillation showing greater coherence between VTA and PFC during correct choices and movement-related low-frequency (“theta”) oscillations in the hippocampus. Movement-related hippocampal oscillations (7–8 Hz) did not show a difference between the working memory and control task, and correlated most robustly with running speed. In contrast, coherence was highest in the 4Hz band between PFC and VTA during the working memory task and did not correlate with running speed. Hippocampal theta, 4Hz VTA-PFC coherence, gamma band activity, and single neuron activity in hippocampus and PFC, though, showed significant phase coupling during the working memory task. The Fujisawa and Buzsaki 2011 study thus provides the clearest evidence to date that movement-related oscillations are distinct from memory-related oscillatory activity. The data also suggest, though, that they may simultaneously interface via phase coupling at different frequencies.
Memory-related and movement-related low-frequency oscillations and the spectral fingerprint hypothesis
If movement and memory-related oscillations both involve coordination between hippocampus and neocortex, in what way are these two signals then dissociable? One perspective is that these two signals are not unique oscillations per se but examples instead of “spectral fingerprinting.” The spectral fingerprint hypothesis states that multiple frequencies may operate within the same network to accomplish different cognitive operations (Siegel et al., 2012). Specifically, the spectral fingerprint hypothesis helps address the issue of how multiple cognitive operations can occur in parallel, even involving the same brain regions, by arguing that the frequency of interactions determines what ensembles are recruited for a specific component of a task (Knight, 2007, Siegel et al., 2012). Although the idea of simultaneously active oscillations underlying different components of information flow is not in itself new (e.g., Lisman and Idiart, 1995, Bland, 2008), the spectral fingerprint hypothesis deals with the specific problem of how a relatively limited number of brain regions can interact in parallel to process and output different components of behavior simultaneously.
Several studies provide some preliminary support for the idea of spectral fingerprinting. Colgin et al. (2009) found task-independent frequency variations in the gamma band and suggested that these may play different roles in gating information flow within the medial temporal lobe (Colgin et al., 2009). Cruikishank et al. (2012) showed that mu and theta rhythms exist closely in time yet subserve different elements of behavior (motor preparation and sensorimotor integration, respectively). Several lines of evidence from attentional and perceptual studies also support the idea that lower and higher (beta and gamma) frequencies may exist in the same neocortical regions. Their coordinated activity between different cortical locations could in turn subserve different task demands (Buschman and Miller, 2007, Hipp et al., 2011). But what about the idea that different aspects of memory (such as “where” vs. “when” information) may be characterized by different frequency interactions within the same brain networks?
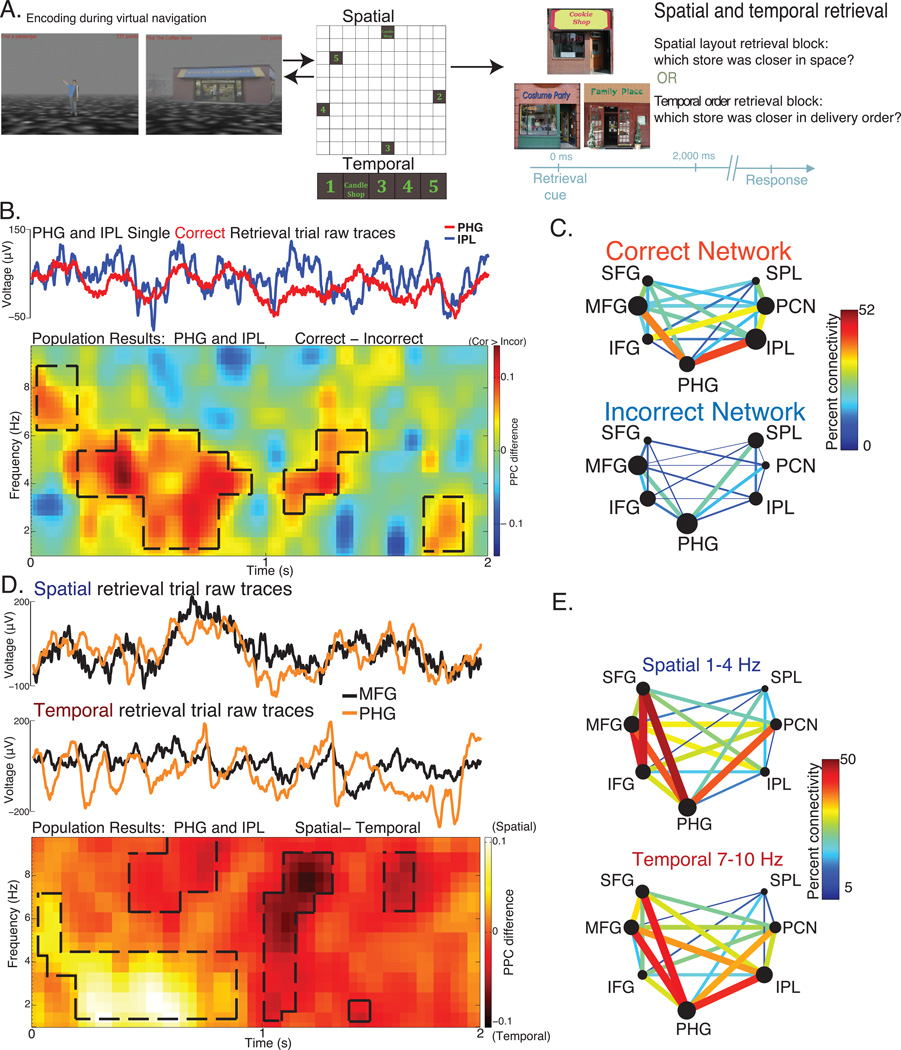
In a recent study, we had patients undergoing seizure monitoring navigate a virtual environment and encode both the location of stores as well as the order in which they encountered the stores (Watrous et al., 2013b). They then performed a memory task in which they retrieved either the location of the store within the spatial layout or the order in which they encountered that store relative to other stores (Figure 2a). We recorded simultaneously from electrode grids implanted along the parahippocampal gyrus, prefrontal cortex, and parts of parietal cortex. Consistent with other memory studies (e.g., Anderson et al., 2009, Benchenane et al., 2010), we found prominent low-frequency (1–10Hz) coherent oscillations between the parahippocampal gyrus, prefrontal cortex, and parietal cortex during correct compared to incorrect memory retrieval (Figure 2b–c). When comparing retrieval of the store within the spatial layout vs. its temporal order, which we have shown in previous reports to be a statistically independent behavioral processes (Ekstrom et al., 2011), we found that temporal order retrieval was characterized by higher frequency coherence between parahippocampal, prefrontal, and parietal recordings sites than spatial layout retrieval (Figure 2d–e). These data provide support for the idea that different frequencies of coherence between the same brain regions may aid in different memory processes, providing novel evidence for the spectral fingerprint hypothesis during memory retrieval (Watrous et al., 2013b).
Figure 2.
Inter-regional theta phase synchronization during the retrieval of spatial and temporal information in humans. A) Diagram of task structure. Patients encoded spatial and temporal information by navigating a novel virtual environment (left) before performing a blocked retrieval task (right). B) Increased theta-band phase synchronization during correct memory retrieval, evident in simultaneously recorded raw traces from a parahippocampal gyrus (PHG) and inferior parietal lobe (IPL; upper) electrode from a single patient. Similar findings of enhanced theta phase synchronization during correct retrieval were found at the population level between all PHG-IPL electrode pairs pooled across subjects (lower). D) Networks for correct and incorrect retrieval, defined at 5Hz, demonstrate increased functional connectivity via theta phase synchronization. D-E) Spatial and temporal retrieval were characterized by phase-synchronization at different frequencies, providing evidence for the spectral fingerprint hypothesis during human memory retrieval. D) Increased 1–4Hz phase synchronization during spatial memory retrieval (upper) and 7–10Hz synchronization during temporal retrieval (middle), evident in simultaneously recorded raw traces from a parahippocampal gyrus (PHG) and middle frontal gyrus (MFG) electrode from a single patient. Similar findings were found at the population level between all PHG-MFG electrode pairs pooled across subjects (lower). E) Networks for spatial and temporal retrieval, defined at their peak frequencies, demonstrate increased functional connectivity via theta phase synchronization. Other abbreviations: SFG: Superior Frontal Gyrus, IFG: Inferior Frontal Gyrus, SPL: Superior Parietal Lobule, PCN: Precuneus, PPC: Pairwise Phase Consistency. Figure modified from Watrous et. al 2013.
Synthesis: Movement and memory-related low-frequency coherent oscillations as examples of spectral fingerprinting
An intriguing possibility is that movement and memory-related low frequency coherent oscillations may be another example of globally coherent spectral fingerprinting (Figure 3). But what then might spectral fingerprinting accomplish in the context of memory and navigation? As suggested by Buschman and Miller (2007), different frequencies of oscillations may co-exist within the same brain networks to accomplish bottom-up sensory processing and top-down, rule-guided processing (Buschman and Miller, 2007). According to this logic, sensory and motor processing entrains cortical networks based on rate of optic flow and motor movements (Schroeder et al., 2010), which in turn will bias downstream networks, such as the hippocampus (via the septum and other nuclei), to also oscillate at this frequency. Simultaneously, lower frequency oscillations interact in a top-down fashion via PFC- hippocampal mediated connections to impose constraints on hippocampal processing (Fujisawa and Buzsaki, 2011). Memory retrieval then may involve a combination of top-down, “control-based” processing (Mitchell and Johnson, 2009) as well as sensory-driven replay, which may manifest at a slightly different frequency. As we outline in Figure 3, bottom-up movement-related low frequency oscillations may coordinate input of sensorimotor information from parietal cortex to hippocampus and prefrontal cortex. Top-down memory-related low-frequency oscillations may coordinate internal dynamics critical for retrieval between hippocampus and cortical areas.
Figure 3.
Proposed model of spectral fingerprints in navigation and memory. "Bottom up" movement related low-frequency oscillations and "Top down" choice related low-frequency oscillations are manifest as frequency-specific patterns of oscillatory coupling. Bottom up oscillations derive primarily from external sensory rhythmicity and motor entrainment while memory-related low-frequency oscillations are derived largely based on internal network dynamics.
Critical aspects of this proposal remain to be tested. While the Fujisawa et al. (2011) study showed that movement-related low-frequency oscillations were present simultaneously with memory-related low-frequency oscillations in VTA-PFC, and that these oscillations showed significant phase coupling, no study, to our knowledge, has shown that movement-related and memory-related oscillations are present simultaneously in the same brain regions. A critical test of this idea would be to record from a rat in a situation in which movement-related and memory-related oscillations are induced simultaneously in the hippocampus. This issue would best be studied using simultaneous recordings from multisite arrays, such as the Fujisawa et al. (2011) study. For example, if a rat runs faster prior to a choice-point, and subsequently makes a correct choice, are there “fingerprints” related to both increases in running speed and making the correct choice? The oscillatory “fingerprints” (i.e., frequencies) could first be established by testing choice independent of changes in running speed and running speed independent of changes in choice. Changes in coherence between hippocampus and cortex during increases in running speed and increases in coherence between hippocampus and cortex related to correctly (vs. incorrectly) choosing the turn could then be investigated at the respective frequencies. Even if the two cognitive operations do not depend on different frequencies, if one could demonstrate different sources within the same brain regions responsive to the different cognitive operations (bottom-up sensorimotor processing related to running speed changes and top-down memory-related processing), this would provide additional evidence for spectral fingerprinting.
Another critical test involves the issue of directionality and causality: we hypothesize that top down vs. bottom up signals should originate primarily in different brain areas (i.e., prefrontal cortex vs. parietal cortex). While coherence only allows measure of correlation and not directionality, methods such as granger causality may hold some promise in resolving this issue (Anderson et al., 2009). Focal and/or transient lesions may also help shed some light on this issue: if one region is entraining or directly modulating another region, what happens when this region is removed from the network? Another important issue is how disruption of specific oscillations affects cognition. Is it possible that selective disruption of movement-related oscillations, without complete disruption of the brain region, can affect only one subset of memory, leaving top-down processing intact? Experiments disrupting theta during phase precession suggest the technical abilities exist to address this issue (Zugaro et al., 2005). A final issue is how spectral fingerprinting relates to BOLD, which is unable to capture activity within specific electrophysiological frequency bands. Recent evidence suggests that at least part of what is captured by functional connectivity may reflect theta band activity in resting monkeys and humans (de Pasquale et al., 2010, Wang et al., 2012). Increases in movement-related low-frequency oscillations also correlate with increases in the parahippocampal cortical BOLD signal (Ekstrom et al., 2009). Thus, it is possible that different functionally connected networks defined using fMRI may also reflect different spectral fingerprints, suggesting a critical link between fMRI and electrophysiological studies in rodents.
Conclusion
Hippocampal movement-related low-frequency oscillations are one of the most well-characterized and replicated findings across species. Previous literature has demonstrated that movement-related oscillations are distinct phenomenon from memory-related low-frequency oscillations. We suggest that movement-related oscillations arise, in part, due to entrainment from sensorimotor information inherited from upstream cortical areas and thus may represent largely bottom-up input. We also discuss memory-related low-frequency oscillations and their emergence when rodents retrieve information about which part of a maze to explore. These memory-related oscillations often appear coordinated between hippocampus and cortex and may reflect top-down, or rule based processing. The presence of both bottom-up and top-down low-frequency oscillations may be yet another example of spectral fingerprinting, in which different cognitive operations manifest in the same brain networks via different frequencies of synchronization. While many aspects of this idea remains to be tested, particularly in the context of navigation, we believe that these ideas represent one step in bringing some synchrony to an often desynchronized field on the study of neural oscillations.
Acknowledgements
This work was supported by the Sloan Foundation, Hellman Young Investigator Award, Grant Sponsor: NINDS; Grant Number: R01NS076856. We thank Andrew Yonelinas and Frank Hsieh for comments on an early version of this manuscript.
Footnotes
The authors declare no conflicts of interest.
References
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10702–10707. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex. 2009;20:1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- Arnolds DE, Lopes da Silva FH, Aitink JW, Kamp A. Hippocampal EEG and behaviour in dogIHippocampal EEG correlates of gross motor behaviour. Electroencephalography and clinical neurophysiology. 1979;46:552–570. doi: 10.1016/0013-4694(79)90009-9. [DOI] [PubMed] [Google Scholar]
- Arnolds DE, Lopes da Silva FH, Aitink JW, Kamp A, Boeijinga P. The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalography and clinical neurophysiology. 1980;50:324–328. doi: 10.1016/0013-4694(80)90160-1. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. The Journal of physiology. 1997;501(Pt 1):225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Hillebrand A, Fawcett IP, Singh KD. Realistic spatial sampling for MEG beamformer images. Human Brain Mapping. 2004;23:120–127. doi: 10.1002/hbm.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Besle J, Schevon CA, Mehta AD, Lakatos P, Goodman RR, McKhann GM, Emerson RG, Schroeder CE. Tuning of the human neocortex to the temporal dynamics of attended events. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3176–3185. doi: 10.1523/JNEUROSCI.4518-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bland BH. The power of theta: providing insights into the role of the hippocampal formation in sensorimotor integration. Hippocampus. 2004;14:537–538. doi: 10.1002/hipo.20027. [DOI] [PubMed] [Google Scholar]
- Bland BH. Anatomical, Physiological, and Pharmacological Properties Underlying Hippocampal Sensorimotor Integration. In: Holscher C, Munk M, editors. Information Processing by Neuronal Populations. Cambridge University Press; 2008. [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Bodizs R, Kantor S, Szabo G, Szucs A, Eross L, Halasz P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001;11:747–753. doi: 10.1002/hipo.1090. [DOI] [PubMed] [Google Scholar]
- Brazier MA. Studies of the EEG activity of limbic structures in man. Electroencephalography and clinical neurophysiology. 1968;25:309–318. doi: 10.1016/0013-4694(68)90171-5. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. Oxford, U.K.: Oxford University Press; 2006. [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature neuroscience. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23:10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, Glaholt MG. The roles of EEG oscillations in learning relational information. Neuroimage. 2007;38:604–616. doi: 10.1016/j.neuroimage.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Kahana MJ, Sekuler R, Kirschen M, Madsen JR. Task dependence of human theta: The case for multiple cognitive functions. Neurocomputing. 2000;32–33:659–665. [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yang CC, Lin YY, Kuo TB. Locomotion-induced hippocampal theta is independent of visual information in rats during movement through a pipe. Behavioural brain research. 2011;216:699–704. doi: 10.1016/j.bbr.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Chen G, King JA, Burgess N, O'Keefe J. How vision and movement combine in the hippocampal place code. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:378–383. doi: 10.1073/pnas.1215834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens Z, Borbely C, Weiss B, Eross L, Szucs A, Kelemen A, Fabo D, Rasonyi G, Janszky J, Halasz P. Increased mesiotemporal delta activity characterizes virtual navigation in humans. Neuroscience research. 2013 doi: 10.1016/j.neures.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Corkin S. What's new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012;22:1848–1859. doi: 10.1002/hipo.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank LC, Singhal A, Hueppelsheuser M, Caplan JB. Theta oscillations reflect a putative neural mechanism for human sensorimotor integration. Journal of Neurophysiology. 2012;107:65–77. doi: 10.1152/jn.00893.2010. [DOI] [PubMed] [Google Scholar]
- Czurko A, Hirase H, Csicsvari J, Buzsaki G. Sustained activation of hippocampal pyramidal cells by 'space clamping' in a running wheel. Eur J Neurosci. 1999;11:344–352. doi: 10.1046/j.1460-9568.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- de Araujo DB, Baffa O, Wakai RT. Theta oscillations and human navigation: a magnetoencephalography study. J Cogn Neurosci. 2002;14:70–78. doi: 10.1162/089892902317205339. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M. Temporal dynamics of spontaneous MEG activity in brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S. Correlation Between BOLD fMRI and Theta-Band Local Field Potentials in the Human Hippocampal Area. J Neurophysiol. 2009;101:2668–2678. doi: 10.1152/jn.91252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 2011;2011:18. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal "place fields". Neuron. 2001;31:631–638. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Buzsaki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov VV, Wiener SI, Berthoz A. Enhanced hippocampal theta EEG during whole body rotations in awake restrained rats. Neuroscience letters. 1995;197:239–241. doi: 10.1016/0304-3940(95)11918-m. [DOI] [PubMed] [Google Scholar]
- Geisler C, Robbe D, Zugaro M, Sirota A, Buzsaki G. Hippocampal place cell assemblies are speed-controlled oscillators. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8149–8154. doi: 10.1073/pnas.0610121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens B. Stimulus-evoked resetting of the dentate theta rhythm: relation to working memory. Neuroreport. 1996;8:159–163. doi: 10.1097/00001756-199612200-00032. [DOI] [PubMed] [Google Scholar]
- Green JF, Arduini AA. Hippocampal electrical activity in arousal. Journal of Neurophysiology. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Halgren E, Babb TL, Crandall PH. Human hippocampal formation EEG desynchronizes during attentiveness and movement. Electroencephalography and clinical neurophysiology. 1978;44:778–781. doi: 10.1016/0013-4694(78)90212-2. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural computation. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Frontiers in human neuroscience. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69:387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Holscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Hughes AM, Whitten TA, Caplan JB, Dickson CT. BOSC: A better oscillation detection method, extracts both sustained and transient rhythms from rat hippocampal recordings. Hippocampus. 2012;22:1417–1428. doi: 10.1002/hipo.20979. [DOI] [PubMed] [Google Scholar]
- Huh K, Meador KJ, Lee GP, Loring DW, Murro AM, King DW, Gallagher BB, Smith JR, Flanigin HF. Human hippocampal EEG: effects of behavioral activation. Neurology. 1990;40:1177–1181. doi: 10.1212/wnl.40.8.1177. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O'Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain Oscillations Control timing of Single Neuron Activity in Humans. Journal of Neuroscience. 2007;27:3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewajee A, Lever C, Burton S, O'Keefe J, Burgess N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus. 2007 doi: 10.1002/hipo.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Position reconstruction from an ensemble of hippocampal place cells: contribution of theta phase coding. J Neurophysiol. 2000;83:2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005a;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS biology. 2005b;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Doeller CF, Barnes GR, Litvak V, Duzel E, Bandettini PA, Burgess N. Movement-related theta rhythm in humans: coordinating self-directed hippocampal learning. PLoS biology. 2012;10:e1001267. doi: 10.1371/journal.pbio.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian NJ, Jutras MJ, Buffalo EA. A map of visual space in the primate entorhinal cortex. Nature. 2012;491:761–764. doi: 10.1038/nature11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT. Neuroscience. Neural networks debunk phrenology. Science. 2007;316:1578–1579. doi: 10.1126/science.1144677. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. Journal of Neurophysiology. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Mizumori SJ. Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single-unit representations. J Neurosci. 1999;19:6661–6672. doi: 10.1523/JNEUROSCI.19-15-06661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Kuo TB, Hsieh IT, Yang CC. Changes in hippocampal theta rhythm and their correlations with speed during different phases of voluntary wheel running in rats. Neuroscience. 2012;213:54–61. doi: 10.1016/j.neuroscience.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiology of learning and memory. 2007;87:9–20. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- McCartney H, Johnson AD, Weil ZM, Givens B. Theta reset produces optimal conditions for long-term potentiation. Hippocampus. 2004;14:684–687. doi: 10.1002/hipo.20019. [DOI] [PubMed] [Google Scholar]
- McFarland WL, Teitelbaum H, Hedges EK. Relationship between hippocampal theta activity and running speed in the rat. J Comp Physiol Psychol. 1975;88:324–328. doi: 10.1037/h0076177. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsaki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Betancur MI, Buzsaki G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J Neurosci. 2009;29:1381–1394. doi: 10.1523/JNEUROSCI.4339-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernandez G. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB. On the relationship of synaptic activity to macroscopic measurements: does co-registration of EEG with fMRI make sense? Brain Topogr. 2000;13:79–96. doi: 10.1023/a:1026683200895. [DOI] [PubMed] [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34:1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Stefanek W, Kirk IJ, Bland BH. Intraseptal procaine abolishes hypothalamic stimulation-induced wheel-running and hippocampal theta field activity in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:1948–1956. doi: 10.1523/JNEUROSCI.16-05-01948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkai C, Lakatos P, Chen CM, Pincze Z, Karmos G, Schroeder CE. Transient cortical excitation at the onset of visual fixation. Cerebral Cortex. 2008;18:200–209. doi: 10.1093/cercor/bhm046. [DOI] [PubMed] [Google Scholar]
- Recce ML. The representation of space in the rat hippocampus as revealed using new computer-based methods. vol. Ph.D. London: University College; 1994. [Google Scholar]
- Ringo JL, Sobotka S, Diltz MD, Bunce CM. Eye movements modulate activity in hippocampal, parahippocampal, and inferotemporal neurons. Journal of Neurophysiology. 1994;71:1285–1288. doi: 10.1152/jn.1994.71.3.1285. [DOI] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. Neuroimage. 2006;31:1352–1358. doi: 10.1016/j.neuroimage.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Seelig D, Aschenbrenner-Scheibe R, Kahana MJ. Reset of human neocortical oscillations during a working memory task. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE. Hippocampal rhythmic slow activity (RSA; theta): a critical analysis of selected studies and discussion of possible species-differences. Brain research. 1980;203:69–101. doi: 10.1016/0165-0173(80)90004-1. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. Dynamics of Active Sensing and perceptual selection. Current opinion in neurobiology. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci U S A. 2002;99:1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Brandt J, Corkin S. Time-dependent motor memory processes in amnesic subjects. J Neurophysiol. 1998;80:1590–1597. doi: 10.1152/jn.1998.80.3.1590. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA, McNaughton BL, Skaggs WE, Weaver KL. The effect of aging on experience-dependent plasticity of hippocampal place cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:6769–6782. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nature reviews Neuroscience. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Daprati E, Ciancia S, Giraux P, Nighoghossian N, Posada A, Haggard P. Altered awareness of voluntary action after damage to the parietal cortex. Nature neuroscience. 2004;7:80–84. doi: 10.1038/nn1160. [DOI] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, Barnes CA. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. Journal of Neurophysiology. 2007;98:898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Hippocampal theta activity in monkeys. Brain research. 1991;538:59–63. doi: 10.1016/0006-8993(91)90376-7. [DOI] [PubMed] [Google Scholar]
- Sturz BR, Brown MF, Kelly DM. Facilitation of learning spatial relations among locations by visual cues: implications for theoretical accounts of spatial learning. Psychon Bull Rev. 2009;16:306–312. doi: 10.3758/PBR.16.2.306. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. The Human Parahippocampal Region in Alzheimer's Disease, Dimentia, and Ageing. In: Witter MP, Wouterlood F, editors. The Parahippocampal Region: Organization and Role in Cognitive Function. Oxford: Oxford University Press; 2002. pp. 271–295. [Google Scholar]
- van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. J Neurosci Methods. 2007;162:49–63. doi: 10.1016/j.jneumeth.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76:1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Fried I, Ekstrom AD. Behavioral Correlates of Human Hippocampal Delta and Theta Oscillations During Navigation. Journal of Neurophysiology. 2011 doi: 10.1152/jn.00921.2010. [DOI] [PubMed] [Google Scholar]
- Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013a doi: 10.1002/hipo.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nature neuroscience. 2013b doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten TA, Hughes AM, Dickson CT, Caplan JB. A better oscillation detection method robustly extracts EEG rhythms across brain state changes: the human alpha rhythm as a test case. Neuroimage. 2011;54:860–874. doi: 10.1016/j.neuroimage.2010.08.064. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M, Buchel C, Loomis JM. Spatial updating: how the brain keeps track of changing object locations during observer motion. Nat Neurosci. 2008;11:1223–1230. doi: 10.1038/nn.2189. [DOI] [PubMed] [Google Scholar]
- Woodnorth MA, McNaughton N. Different systems in the posterior hypothalamic nucleus of rats control theta frequency and trigger movement. Behavioural brain research. 2005;163:107–114. doi: 10.1016/j.bbr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ekstrom A. Human neural systems underlying rigid and flexible forms of allocentric spatial representation. Human Brain Mapping. 2012 doi: 10.1002/hbm.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro MB, Monconduit L, Buzsaki G. Spike phase precession persists after transient intrahippocampal perturbation. Nature neuroscience. 2005;8:67–71. doi: 10.1038/nn1369. [DOI] [PMC free article] [PubMed] [Google Scholar]