PREAMBLE
Aims
Practice Guidelines for Management of Hepatitis C were first established in 2004. Since then, many study results have been published concerned with epidemiology, clinical outcomes and related factors, concept of response-guided therapy, therapeutic strategy, and results. Moreover, as direct acting antivirals (DAA) have been recently developed and adapted to practice, treatment of hepatitis C is rapidly evolving. Therefore, the Korean Association for the Study of the Liver (KASL) revised the guidelines based on a systematic approach that reflects evidence-based medicine and expert opinions.
The clinical practice guidelines for the management of hepatitis C have been revised to be useful for treatment, research, and education. These recommendations are not absolute standards of care, and adoption of the guidelines in clinical practice may differ for individual patients.
Target population
The target groups of these guidelines are newly or previously diagnosed patients with hepatitis C virus (HCV) infection, including not only chronic hepatitis C and cirrhosis, but also acute hepatitis C patients, hepatitis C patients under special medical conditions, such as intravenous drug use (IVDU), those with chronic kidney diseases, coinfection of human immunodeficiency virus (HIV) or hepatitis B virus (HBV), and pediatric patients.
Intended users
The guidelines are intended to provide useful information and guidance to physicians and healthcare providers involving in the diagnosis and treatment of hepatitis C, and resident physicians, practitioners, and trainers.
Development, funding, and revision
The Clinical Practice Guidelines Committee for the Management of Hepatitis C (Committee) consisting of nine hepatologists was organized according to the proposal and with approval of the KASL Board of Executives. Funding for the revision was provided by KASL. Each committee member collected and analyzed the source data in his/her own field of expertise. The members then wrote the manuscript together.
Literature review for evidence collection
The committee systematically collected and reviewed the international and domestic literature published in Pubmed, MEDLINE, KoreaMed, and other databases. The key words used were 'hepatitis C virus', 'hepatitis C', 'liver cirrhosis', 'liver cancer', and other related specific key words.
Levels of evidence and grades of recommendations
The quality of evidence was classified according to the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system (Table 1).1 Based on the types of studies, randomized, control studies were approached from a high level of evidence, while observational studies were approached from a low level of evidence. The level of evidence was adjusted by accounting for the factors influencing the quality of the studies. Through follow-up studies, the level of evidence was defined as follows: A, indicating the highest level of evidence with the smallest possibility of any changes in the conclusion; B, indicating a moderate level of potential changes; and C, indicating the lowest level of evidence with the greatest possibility of any changes.
Table 1.
Grading of Recommendations, Assessment, Development, and Evaluation (GRADE)

Of the quality levels of evidence, we excluded "very low quality (D)" in our guideline for convenience, which was originally included in the GRADE system.
The strength of a recommendation was also classified according to the GRADE system. Each study was classified as strong recommendation (1) or weak recommendation (2) under overall consideration of quality of evidence, the balance between the desirable and undesirable effect of an intervention, and socioeconomic aspects including cost or availability. A strong recommendation indicated that the interventions could be applied in most patients with high degree of certainty and that there was a greater possibility of desirable effects, high-quality evidence, and presumed patient-important outcomes, cost-effectiveness, preference, and compliance. A weak recommendation indicated a suggestion made with less certainty but that could be considered favorable for many patients, based on the level of evidence, cost, or preferences of the patients or medical practitioners.
List of key questions
The revision committee considered the following clinical questions as the key components to be covered in these guidelines.
What is the epidemiology and natural history of hepatitis C in South Korea?
How should the diagnosis and evaluation of severity of chronic hepatitis C be made?
What is the goal of treatment and who are the targets for the antiviral treatment of hepatitis C?
How is the treatment response defined, and what are predictors of the response?
How are patients with chronic HCV genotype 1 and 4 infections treated?
How are patients with chronic HCV genotype type 2, 3, and 6 infections treated?
How are patients with acute hepatitis C treated?
How are the adverse effects of antiviral drugs managed and how the patients monitored during and after antiviral treatment?
How are patients with special conditions (cirrhosis, liver transplant and other organ transplants, immunosuppressive therapy or cytotoxic chemotherapy, intravenous drug use, chronic kidney diseases, coinfection with HIV or HBV, hemophilia, and pediatric patients) treated?
Review of the manuscript and approval process
Each version of the manuscript written by committee members was reviewed, agreed, and approved through meetings of the committee. The quality of the manuscript was evaluated based on the standards suggested by AGREE II (Appraisal of Guidelines for Research and Evaluation II) along with the academic integrity of the contents. The guidelines were reviewed after counsel from three specialists, one in each division including infectious diseases, renal diseases, and pediatrics. The guidelines were reviewed at a meeting of an external review board composed of 14 KASL members, and were further modified following opinions collected at a public hearing, and a symposium open to all KASL members. The final manuscript was approved by the KASL Board of Executives.
Release of the guidelines and plan for updates
The Korean version of the KASL Clinical Practice Guidelines for the Management of Hepatitis C was released and published in December 2013 on the KASL web site (http://www.kasl.org). Future revisions will be conducted under the judgment that the revision is necessary for the promotion of health in South Korea as further research data on the management of hepatitis C accumulates. In addition, use of DAA is to be allowed in South Korea in the near future, so that updates or partial revision of the guidelines will be warranted as appropriate.
EPIDEMIOLOGY
HCV is one of the main causes of acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma.2 Hepatitis C is on the National Notifiable Infectious Disease list in South Korea and has been under surveillance since 2000. An effective HCV vaccine is yet to be discovered, so that understanding of national epidemiology and preventive strategies to block routes of HCV infection is important for public health.
Prevalence rate of HCV infection
Worldwide prevalence of HCV infection was 2.8% in 2005, equating about 185 million persons positive for antibody to hepatitis C (anti-HCV).3,4 The prevalence rate varies in different global regions; regions with high prevalence rate of over 3.5% include Central Asia covering Mongolia, China, South-East Asia covering Pakistan and Thailand, and North Africa covering Egypt. Low prevalence (below 1.5%) regions are Asia including South Korea and Japan, North America including the United States (US), and South America.4
Prevalence rate of adult health check examinees
The HCV prevalence rate in adult health check examinees was reported as 1.7% when tested by 1st-generation enzyme immunoassay (EIA) in early 1990s, just following the discovery of HCV.5 The estimated age-standardized prevalence of anti-HCV in the adult (≥40 years of age) health check examinees was reported as 1.29% (95% confidence interval 1.12-1.48) and was about 193,000 persons in a collective study including health checkup examinees from Seoul, Ulsan, Jeollanam-do, and Daegu between 1995 and 2000.6,7,8,9,10
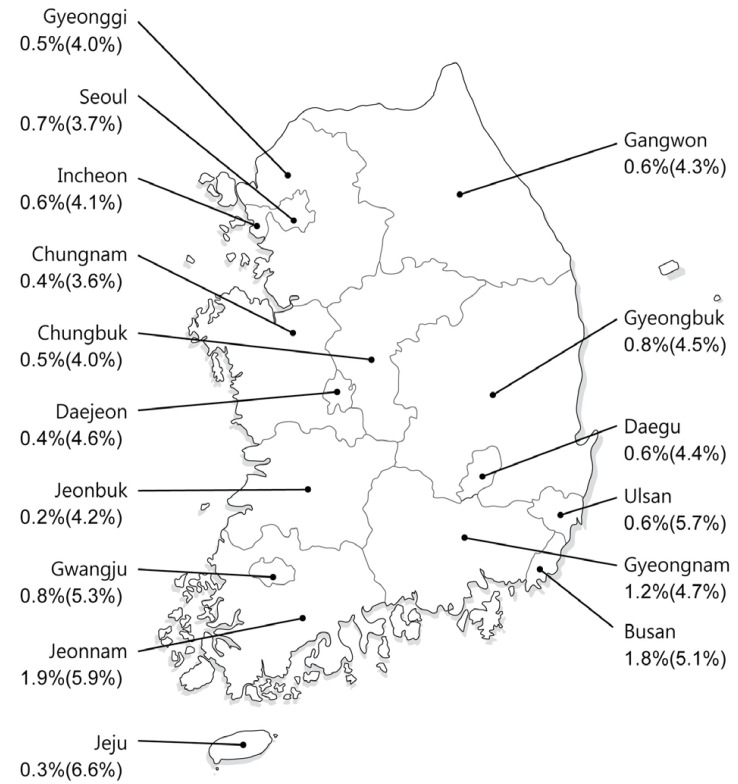
In 2009, the anti-HCV prevalence rate in health examinations of 291,314 adults ≥20 years of age from 29 health examination centers was 0.78% using 3rd-generation EIA after adjusting for age, sex, and area.11 The anti-HCV prevalence was higher in women (0.83%) than in men (0.75%) showing an age-related increase with the highest prevalence in those ≥60 years of age (20-29 years: 0.34%, 30-39 years: 0.41%, 40-49 years: 0.60%, 50-59 years: 0.80%, 60-69 years: 1.53%, ≥70 years: 2.31%). In addition, it varied in different localities; by comparison with the prevalence rate of 0.50-1.20% in most regions including Seoul and Gyeonggi-do, the prevalence rates in Pusan and Jeollanam-do were highest (1.53% and 2.07%, respectively),while Jeju Special Self-Governing Province had the lowest rate of 0.23% (Fig. 1). An update of national HCV prevalence rate in South Korea is expected soon, since the 2012 Korea National Health and Nutrition Examination Survey included anti-HCV testing.
Figure 1.
Map of South Korea showing age and sex-adjusted anti-HCV seroprevalence in each area.11
Prevalence of anti-HCV in blood donors, pregnant women, and children
The anti-HCV prevalence rate in 2,040,151 blood donors in 1997 in South Korea was 0.34% as tested by 3rd-generation EIA.12 From 2005 to 2009, the anti-HCV prevalence in 11,064,532 blood donors was 0.16%, and the HCV RNA-positive rate was 8.4 (0.0084%) of 100,000 donors, among whom 81% were young people aged 10-30 years.13
In South Korea, the risk of blood transfusion-related HCV infection decreased from 1 in 81,431 in 2000/2001 to 1 in 2,984,415 after implementation of nucleic acid test for HCV screening of donated blood beginning in February, 2005.14
The anti-HCV prevalence rates in pregnant women were reported as 0.49-1.7%,15,16,17 and a domestic report investigating over 5,000 pregnant women reported rates of 0.42-0.44%.18,19 Among anti-HCV-positive pregnant women, 57-60% were positive for HCV RNA.18,19
Domestic studies on HCV prevalence rate in children and adolescents are insufficient. A 0.82% anti-HCV-positive rate tested by 3rd generation EIA in 2,080 children between 6 and 11 years of age living in Seoul was reported.20 However, there have been no other reports or studies on children and adolescents, hindering the accurate assessment of HCV prevalence rate in the pediatric population of South Korea.
Prevalence of anti-HCV in high-risk group
High-risk groups for HCV infection include people with a history of intravenous drug use (IVDU), patients receiving hemodialysis, and those with HIV infection, hemophilia, and leprosy. However, the HCV prevalence rate in these groups has been reported mostly before 2000, with little data since then.
Domestic anti-HCV prevalence rate in the IVDU group was reported as 48.4-79.2%.21,22,23,24 Among anti-HCV-positive persons, 98.1% were HCV RNA-positive.24 Meanwhile, in case of sharing cocaine suction pipe, the anti-HCV prevalence rate was similar to IVDU group.25
Anti-HCV prevalence rate was 5.9-14.7%26,27 in previous studies that included more than 200 patients with chronic kidney diseases and the rate significantly correlated with duration of hemodialysis.
Coinfection rate of HCV was high in those infected with HIV; about 25% of westerners and 5.0-6.3% of HIV-infected individuals in South Korea were coinfected with HCV.28,29,30
Anti-HCV prevalence rate in 104 hemophilia patients tested by 3rd-generation EIA of was 42.3% in 2002 and the risk of infection correlated with age and severity of hemophilia.31 In their 2012 annual report, the Korea Hemophilia Foundation (KHF) reported that 430 of 2,148 (20.0%) hemophilia patients were anti-HCV-positive and 118 of 2,148 (5.5%) were HCV RNA-positive.32
Leprosy patients can be considered a high-risk group of HCV infection due to their skin lesions and long-term cohabitation in limited areas. The prevalence rate of 96 leprosy patients tested by 2nd-generation EIA was 67.7% in 1997 and 82% of these individuals were immunoblot-positive.33
Incidence rate of HCV infection
Studies on HCV incidence rate are rare, since only 20-30% of those with acute HCV infection develop symptoms. HCV incidence rates are decreasing in Western countries.34 In the US the incidence rate decreased from 7.4 of 100,000 people from 1982-1989 to 0.7 of 100,000 people from 1994-2006,35 and in Italy, a decreased from 2.02 of 100,000 people in 1996 to 0.55 of 100,000 people in 2006 was reported.36
The HCV infection incidence rate in South Korean blood donors was reported as 13.8 of 100,000 according to a survey conducted on those who donated blood at least twice from 1994-1996.37 The recent HCV infection incidence rates among blood donors who donated at least twice in 2 years between 2000 and 2010 were estimated 6.80 in 2001, 3.19 in 2003, 2.69 in 2005, 1.83 in 2007, and 0.80 in 2009 per 100,000 person-year, showing significant decrease of HCV incidence in this population in South Korea.14
According to surveillance sample data from the Korea Centers for Disease Control and Prevention (KCDC), the number of reported hepatitis C cases was 1,927 in 2002, 6,407 in 2008, and 4,280 in 2012. Future studies are needed for evaluation of nationwide HCV incidence rate in Korea.
Distribution of HCV genotypes
Globally, HCV genotypes 1, 2, and 3 are common and genotypes 4, 5, and 6 are localized to limited regions.38,39 Genotype 1a is most common in Northern Europe and North America, and 1b is most common in Far East Asia and Europe. Genotype 2 is less common than genotype 1. Genotype 3 is common in Southeast Asia and genotype 4 is common in the Middle East, Egypt, and Central Africa. Genotype 5 is commonly found in South Africa and genotype 6 is common in Hong Kong, Macau, and Vietnam.
Common HCV genotypes in South Korea are genotype 1b (45-59%) and 2a (26-51%); types 1a, 2b, 3, 4, and 6 are rare genotypes in South Korea.40,41
Whether genotype 1 HCV infection provokes faster progression of hepatic disease than the other genotypes is controversial. A recent meta-analysis reported that genotype 1b patients showed a 1.78-fold higher risk of developing HCC (95% CI, 1.36-2.32) compared to the non-1 genotype patients.42 Nevertheless, the HCV genotype is the most crucial factor in determining the efficacy of antiviral therapy; genotypes 1 and 4 result in lower success rates of treatment compared to genotypes 2, 3, and 6.43
PREVENTION
Route of transmission
HCV transmission occurs by parenteral exposure. The main routes of transmission include transfusion of contaminated blood or blood products, organ transplantation, IVDU, unsafe injection or medical procedures, stabs by contaminated syringe or needle, sexual contact with HCV infected person, or perinatal transmission from infected mother to newborns.
Transmission via transfusion was a main route of infection until 1991, but the possibility has become extremely low since a screening test was introduced for blood donors.44,45,46 The most important route of recent HCV transmission is the use of illicit drugs in developed countries such as the US or Europe; HCV prevalence rate is low,47 whereas anti-HCV prevalence in the IVDU group was reported as high as 50-90%.48 Meanwhile, unsafe injection with multiple-use medication vials or reused syringes, or unsanitary medical procedures including surgery, endoscopy, and dental treatment without proper disinfection are reported as the main causes of HCV transmission in developing countries.49,50,51
In addition, meta-analyses have reported that risk factors of HCV transmission include piercing, acupuncture, or tattooing without proper disinfection.52,53,54 The HCV infection risk by a small dose of percutaneous exposure, such as needle sticks, is 1.8% (0-7%)55,56,57,58 in other countries and 0.92% in South Korea.59 Heterosexual persons with chronic HCV infection in long-term monogamous relationships with a partner had little evidence for sexual transmission of HCV. However, the risk becomes higher with multiple sex partners, and unsafe sex including anal sex, sex accompanying wounds, sex carrying other sexually transmitted diseases like HIV, or in homosexuals.60,61 The percentage of perinatal transmission was reported as 1-6.2%.62,63 It was reported as 1.7% when the mothers were positive for anti-HCV regardless of HCV RNA-positivity, and as 4.3%(3.9-7.1%) in case of HCV RNA-positive mothers.63,64 The risk of perinatal transmission increased in female infants, HIV-positive mothers, and mothers with high blood HCV RNA levels.65 Cesarean section is reportedly not a preventative method for HCV transmission,65,66 and transmission via nursing was very low. Thus, it is not necessary to limit breast-feeding unless nipples are injured or are bleeding.67 Reports of horizontal transmission between siblings or family members of HCV infected person are based on a low level of evidence.68
A comparative study69 of 1,173 HCV patients and 534 control group in five university hospitals between 2007 and 2011 in South Korea reported several independent risk factors of infection including illicit use of drug, needle stick injury, transfusion before 1995, tattoo, and age.69
Counselling for prevention
Since an effective vaccine has not been developed, the main strategy of prevention is to educate people on the risk factors for HCV infection and to keep the strict standard for sanitation in every place performing the percutaneous procedures.
HCV infected persons should be counseled not to donate blood, organs, tissues, or semen, and not to share any instrument penetrating skin. They should individually use instruments including toothbrushes, oral hygiene devices, razors, or nail clippers so his/her blood are not exposed to other people. Finger stabbing needles commonly used for Korean home remedy should not be shared. IVDU should be persuaded to stop drug abuse and they should not reuse syringes, needles, injection solution, cotton swab, or alcohol sponges. They must be reminded that other people can be infected via recklessly disposed needles. Since risk of infection among monogamous couples is very low, use of barrier protections among these couples are not necessarily recommended. Nevertheless, if the partner of the infected individual request or for the infected person with multiple sex partners, it is recommended to use condoms.
Routine screening for HCV is not recommended for all pregnant women. However, for those with a risk factor, prenatal testing for HCV is needed. HCV infection does not mean a restriction of breast-feeding or a recommendation of specific delivery, such as Cesarean section.
Health care facilities should be careful to block HCV transmission. Proper disinfection, cleaning, and management of materials and instruments are essential in medical procedures and invasive procedures including tattooing, piercing, or acupuncture.
[Recommendations]
1. HCV infected persons should not donate blood, organs, tissues, or semen (A1). HCV infected persons should avoid to share toothbrushes, oral hygiene devices, razors, nail clippers, or any instrument penetrating skin, so as not to expose his/her blood to other people (C1).
2. Intravenous drug abusers should be counseled to stop illicit drug abuse (A1). They should be educated about routes of infection and tested regularly for HCV infection (B1).
3. Proper disinfection, cleaning, and management of materials and instruments are essential in medical procedures and invasive procedures including tattooing, piercing, or acupuncture (B1).
4. Since risk of infection among monogamous sexual partners is very low, use of barrier protection is not advised in these couples (B1). However, for those with multiple sex partners, it is recommended to use condoms (B1).
5. For pregnant women, if a risk factor for HCV infection is detected or HCV infection is suspected otherwise, prenatal testing for HCV infection is recommended (B1). HCV infection does not mean a restriction of breast-feeding or a recommendation of specific delivery, such as Cesarean section (B2).
NATURAL HISTORY
Acute HCV infection
After 1-3 weeks of HCV infection, HCV RNA becomes detectable in blood and rapidly increases.70,71 Serum alanine transaminase (ALT) level increases due to hepatocyte damage after 4-12 weeks of the infection. Most infection is asymptomatic (70-80%) but symptoms including flu-like symptoms, fatigue, vomiting, nausea, right upper quadrant pain, muscle pain, or pruritus may develop within 2-12 weeks. About 20% of acute infection accompanies jaundice with serum bilirubin level below 3-8 mg/dL, and acute liver failure occurs rarely in <1% of cases. Acute hepatitis progressed to chronic infection in 54-85% of patients and 20-50% of patients recovered spontaneously within 3-4 months.72,73,74 Spontaneous recovery rate is different depending on route of infection; spontaneous recovery rate in post-transfusion cases was 12%, while in the cases not related to transfusion it was 29-52%.74,75,76 Factors related to spontaneous recovery are hepatitis accompanying jaundice, female, low viral load, and genotype 3.75,76,77 A Korean study reported that among 18 acute hepatitis C patients (17 patients showed symptoms), 12 patients spontaneously recovered and 6 patients progressed to chronic hepatitis.78 Another study including 47 patients of acute hepatitis C enrolled in seven Korean institutions showed that mean age of 45.8 years, 21 of 47 (44.7%) patients recovered spontaneously, and 16 patients received antiviral therapy. All 12 patients who were treated and followed-up patients achieved a sustained virological response (SVR). Ten patients who did not receive antiviral therapy progressed to chronic hepatitis.79
A single nucleotide polymorphism (SNP) of the interleukin 28B (IL 28B) gene is strongly related to spontaneous recovery from acute hepatitis C infection.80,81,82 IL28B is located on chromosome 19 and expresses interferon-lamda-3. A study reported a spontaneous recovery rate of 53% in case with genotype CC of IL28B SNP rs12979860 and of 28% in genotype CT or TT (Odds ratio (OR)=0.33, P<10-12).83 However, future studies are needed since there have been no Korean studies of IL28B SNP in acute HCV infection.
Chronic HCV infection
About 50-80% of HCV infected patients progress to chronic infection. Once it becomes chronic hepatitis, it can cause persistent liver injury without spontaneous recovery leading to cirrhosis and HCC. Most (60-80%) patients with chronic hepatitis show no symptoms, but some can experience abdominal discomfort, fatigue, nausea, muscle pain, arthritis, or weight loss. About 60-70% of chronic HCV infected patients show chronic hepatitis accompanying steady or intermittent elevation of serum ALT. About 15-56% of chronic hepatitis may progress to cirrhosis through a period of 20-25 years.71,84,85,86 Among patients with liver cirrhosis, the annual incidence of HCC is reported as 1-4.9%,87,88,89 that of decompensated liver cirrhosis is 3-6%,71,88,89,90 and the overall annual mortality rate is 2-4%. An observational study including 1,137 Korean chronic HCV infected patients for an average follow-up of 55.2 months reported a 14.2% rate of disease progression, defined as development of HCC, spontaneous bacterial peritonitis, variceal bleeding, hepatic encephalopathy, and death due to hepatic diseases, and overall annual mortality rate was 2.0-2.5%.91 Cumulative probability of disease progression was 6.3%, 12.9%, and 26.1% at 1, 2, and 3 years, respectively. From 1,137 patients, 490 (43.0%) received antiviral treatment and 60.4% showed an SVR. Chronic infection without antiviral treatment showed significantly higher risk of disease progression compared to chronic infection with antiviral treatment (37.4% vs. 10.7%, respectively, P<0.05). A 5-year cumulative probability of disease progression was higher in a non-SVR group compared to group with SVR (13.0% vs. 3.7%, respectively, P<0.05).
Factors affecting disease progression include duration of infection, age at the time of infection (≥40 years of age), male, alcohol intake, coinfection with other viruses (HBV, HIV), insulin resistance, obesity, immune-depressed patients, organ transplantees, elevation of ALT, or genetic factors such as IL28B.71 Excessive alcohol intake by chronic hepatitis C patients is strongly related to occurrence of cirrhosis, and increases risk of HCC.86,92,93,94,95 Fatty liver, insulin resistance, and obesity increase risks of hepatic fibrosis and HCC development in chronic hepatitis C patients.96,97,98,99 Coinfection of HIV or HBV in chronic HCV infection causes faster progression of liver diseases and increases the risk of HCC compared to HCV single infection.100,101,102 In addition, coinfection of hepatitis A virus (HAV) in chronic hepatitis C increases the risk of hepatic failure.103 Pathologic stage of hepatic fibrosis at the time of chronic hepatitis C diagnosis is the most important predictor for progression to cirrhosis (refer to the Diagnosis section of this manuscript).86,100 Stage 1 hepatic fibrosis has a 10-30% incidence rate of cirrhosis over a period of 15 years, while most cases of stage 3 hepatic fibrosis are expected to progress to cirrhosis within 15 years. Therefore, patients diagnosed as having hepatic fibrosis over stage 2 must be considered for active antiviral treatment.
[Recommendations]
6. Continuous management and surveillance for development of cirrhosis and HCC is necessary in chronic hepatitis C patients (A1).
7. Chronic hepatitis C patients are recommended abstinence from alcohol or moderation in drinking, and to maintain suitable body weight through physical exercise and dietary control, since disease progression is related to alcohol, obesity, or insulin resistance (B1).
8. Patients with chronic HCV infection without antibodies against HAV and HBV should be vaccinated for HAV and HBV (C1).
DIAGNOSIS OF HCV INFECTION AND ASSESSMENT OF LIVER DISEASE SEVERITY
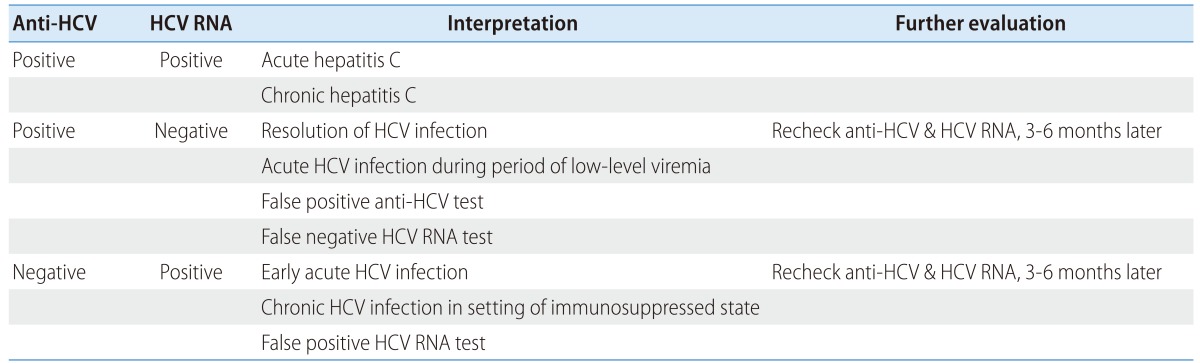
Biochemical tests, serologic assays, and HCV RNA testing are needed to confirm HCV infection. Physical examination and history taking should be done to understand the routes of transmission and block further reinfection. HCV genotyping is essential for treatment and radiologic examination, liver biopsy, or noninvasive evaluation of hepatic fibrosis can be done to determine necessity of treatment, and to assess liver disease severity. Interpretation of serological and virological test results is summarized in Table 2.
Table 2.
Interpretation of HCV assays114
DIAGNOSIS
Serologic assays
Anti-HCV
Detection of anti-HCV in serum or plasma is used for screening of a high risk group and for diagnosis of acute or chronic hepatitis C.104 The 3rd generation EIA uses recombinant antigens including core, NS3, NS4, and NS5 of HCV protein, and its sensitivity and specificity are 97.2-99% and 99.8-100%, respectively, when tested in immune-competent individuals.105,106,107 If signal/cutoff (S/CO) ratios of 3rd generation EIA exceed 3.8, a positive result will be apparent in 95% of recombinant immunoblot assay (RIBA).108,109,110 However, a cutoff level of S/CO ratios can be different according to the types of equipment, so that high S/CO ratios do not always mean true positive.111 Recently, use of enhanced chemiluminescent immunoassay (CLIA) or electrochemiluminescence immunoassay (ECLIA) is increasing since those assays detect antigen-antibody reaction more sensitively compared to 3rd generation EIA. Meanwhile, there is point-of-care tests using saliva or fingerstick blood producing rapid results within 20 minutes.112
Average time between infection and seroconversion of anti-HCV is 8-9 weeks and anti-HCV is detectable in >97% of patients with HCV infection within 6 months.113,114 Anti-HCV is not a neutralizing antibody and persists indefinitely in chronic hepatitis C patients or even after recovery. Therefore, the differentiation of current infection from the past infection after recovery is impossible using anti-HCV positivity. Negative result for anti-HCV in combination with a positive result for HCV RNA may represent early state of acute infection, chronic infection in the setting of severe immunosuppressed condition, such as patients on hemodialysis, HIV coinfection, solid organ transplantation recipients, hypo-/a-gammaglobulinemia, and patients with HCV-associated essential mixed cryoglobulinemia.3,105,115 In these patients, HCV RNA testing is necessary for diagnosis of HCV infection. On the other hand, false-positive result for anti-HCV with negative result for HCV RNA can occur in patients with autoimmune diseases.116
Recombinant immunoblot assay (RIBA)
RIBA detects 4 HCV-specific antibodies on nitrocellulose strips.114 Borderline positive results of anti-HCV using EIA or CLIA test can be confirmed with RIBA, but RIBA has low sensitivity despite high specificity.110,117 Recently, clinical role of RIBA has disappeared because validated HCV RNA assays are sequentially conducted in patients showing positive result for anti-HCV to confirm HCV infection.
Virological assays
HCV RNA assays
HCV RNA assays are classified as quantitative and qualitative assays. Since the detection cutoff of qualitative assays is 50 IU/mL and more sensitive than previous generation quantitative assays, HCV RNA qualitative assays had been used as a diagnostic confirmation of HCV infection and HCV RNA quantification is used for pretreatment assessment and monitoring of virological response during and after antiviral therapy.104,110,118 However, recently available quantitative HCV RNA assays are using real-time polymerase chain reaction (PCR) and transcription-mediated amplification (TMA), and are very sensitive with lower detection limit of 12-15 IU/mL, while they have a broad measuring range with upper limit of 7-8 log IU/mL with 98-99% of diagnostic specificity independent of HCV genotype.104,119,120,121,122,123,124 Therefore, quantitative HCV RNA tests are now widely used both for diagnosis and evaluation of treatment response.125,126
In 1997, the World Health Organization established an international standard for HCV RNA quantification unit, IU, rather than HCV copy numbers.127,128 However, since different laboratories can vary in viral quantification results,129 it is recommended to use the same laboratory test before, during, and after-treatment for monitoring, if possible.114,125
Blood HCV RNA is detectable as early as 2 weeks after infection,74 rapidly increases to reach a plateau, and decreases along with ALT after ALT attains a maximum level.130 HCV RNA levels maintain a steady state in patients with chronic hepatitis C.130,131 HCV RNA levels do not significantly correlated with the severity of hepatic inflammation or fibrosis, and show little changes during chronic infection state without antiviral treatment.132,133
Genotyping assays
HCV genotyping is useful for epidemiologic studies as well as for predicting treatment response. Therefore, HCV genotype should be assessed before treatment for determining the optimal therapeutic duration and dose of ribavirin.134 HCV is classified into six major genotypes (1-6) and is subdivided into subtypes identified by lower-case letters, such as 1a or 1b. Differences of 31-33% at the nucleotide level among each genotype, compared with 20-25% among each subtype.135 HCV genotype does not change within a same person unless otherwise reinfected.
Determining HCV genotypes and subtypes can be performed by using direct sequence analysis, reverse hybridization, or restriction fragment mass polymorphism (RFMP).136 Most genotyping assays analyze 5'-untranslated region (UTR) and HCV core regions where nucleotide sequences are highly conserved.137 With analysis of 5'-UTR region, HCV genotyping errors occur at a rate of <3%, but HCV subtyping errors may occur in 10-25%; especially, this assay does not accurately discriminate between subtype 1a and 1b.138,139,140 Subtyping is not necessary in antiviral therapy using interferon alpha and ribavirin combination, but in the treatment including DAAs, subtypes may need to be confirmed since DAAs act differently according to genotype 1a and 1b.140 Genotyping is not possible in <5% of the patients. This results from low HCV levels, problems with the PCR amplification process, or high nucleotide variability of HCV genome itself.141
HCV drug-resistance mutation tests
HCV drug-resistance test has not been performed clinically, unlike cases with hepatitis B virus infection. As various DAAs are being used with improvement of outcomes of treatment, drug resistance tests for these drugs may be needed in the future.142
Screening test for HCV infection
Routine screening for HCV infection is recommended in populations at risk, such as those with a history of blood transfusions or organ transplantation prior to 1992; persons who have injected illicit drug; persons with HIV infection, hemophilia, or Hansen's disease; persons who have been on hemodialysis; children born to mothers infected with HCV; and health care providers after a needle stick injury or mucosal exposure to HCV positive blood (Table 3).114 In 2012, the US Centers for Disease Control and Prevention expanded the screening population to the birth cohort born between 1945-1965 and recommended to screen for HCV once in a lifetime, considering cost effectiveness.143,144,145 A Japanese study revealed that the strategy of hepatitis C screening appears cost-effective in the general population as well as in the high-risk group,146 but an European study showed that the screening test for general population is cost-effective only in HCV prevalent areas.147 Therefore, since the epidemiologic characteristics and health care system differ between nations, to adapt the optimal screening strategy in Korea, further research on its cost effectiveness is urgently needed.
Table 3.
Persons for whom HCV screening is recommended114
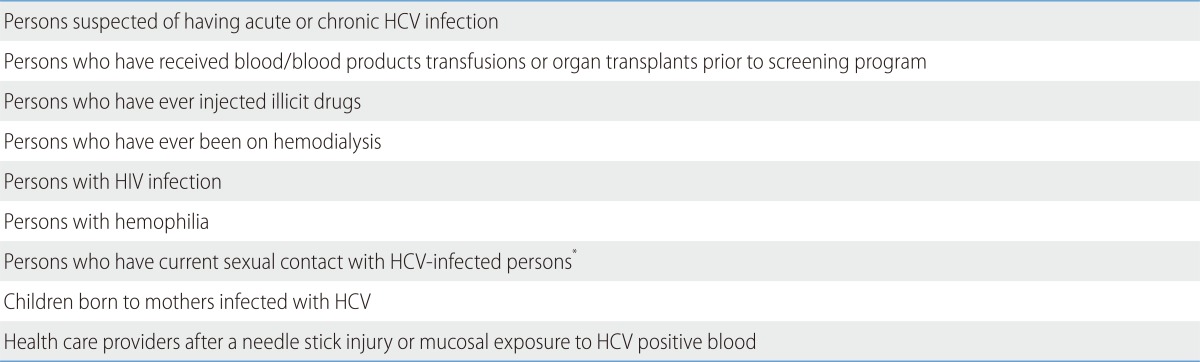
*The prevalence of infection is low.
Diagnosis in case of accidental exposure
The average incidence of anti-HCV seroconversion in healthcare providers after accidental percutaneous exposure from HCV-infected blood was reported to be 1.8% (0-7%) in other countries57,58,148,149,150,151,152 and 0.92% in South Korea.59 When a person is exposed to HCV-positive source, baseline testing for ant-HCV and serum ALT level should be performed. If anti-HCV is negative, HCV RNA assay should be performed 4-6 weeks after exposure for early diagnosis. Even if baseline tests for HCV infection were all negative, follow-up testing for anti-HCV and serum ALT level should be performed 4-6 months after the exposure.125,149 If anti-HCV is positive, a confirmative test is needed.
ASSESSMENT OF LIVER DISEASE SEVERITY
To decide the treatment for HCV infected patients, the severity of the liver disease must be evaluated through liver biopsy and/or noninvasive tests. It is important to confirm whether the patient has liver cirrhosis or not before treatment since existence of liver cirrhosis can make difference in the treatment response, its prognosis, and necessity of surveillance for HCC.
Liver biopsy
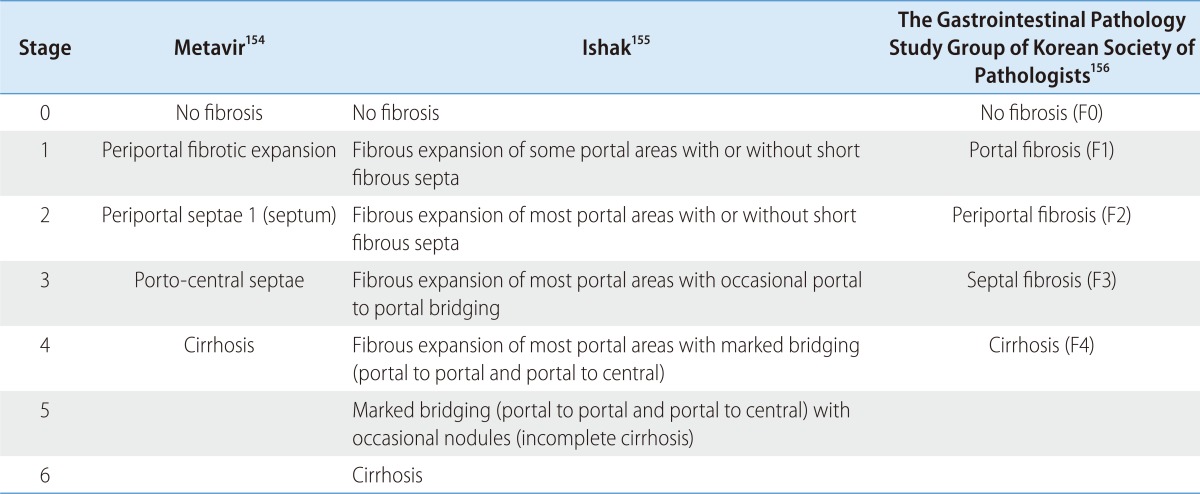
Liver biopsy is assessed for grade and stage of the hepatic injury.114,153 The Metavir154 and Ishak155 scoring systems are most widely used, and the scoring system proposed by the South Korean Study Group for the Pathology of Digestive Diseases is used in South Korea (Table 4).156 Although liver biopsy is not mandatory prior to treatment, it can help to determine when to start treatment and to provide information regarding the treatment response and prognosis. Considering the natural history of the disease, the cost of treatment and its possible adverse effects, treatment can be postponed if liver histopathology shows minimal to moderate fibrosis state <2 (Metavir stage 2 or periportal fibrosis of the South Korean Study Group for the Pathology of Digestive Diseases, F2).74,157,158 In this case, a liver biopsy should be repeated at 4-5 years later to reassess the necessity of treatment according to the progression speed of liver disease.159 About 5-30% of patients with genotype 1 with consistently normal serum ALT may have severe fibrosis160,161,162,163 and liver biopsy would be useful to determine treatment initiation in this group.86,164,165 Even though hepatic steatosis153,166,167 and liver iron load overload168 might impede treatment response, presence of these findings is not the contraindication of treatment.169,170,171 If the liver biopsy is not conducted and treatment is not undertaken, continuous monitoring is needed. Liver biopsy and start of treatment should be considered when there is elevation of serum ALT level and evidence of liver disease progression.114
Table 4.
Comparison of scoring systems for histological stage114
Noninvasive tests for evaluation of liver fibrosis
Even though liver biopsy has been widely accepted as a gold standard test for evaluation of liver fibrosis,172,173 it can cause serious complications174,175 and sampling errors,176 and it requires histopathologic specialist for accurate interpretation and also medical costs. Therefore, various blood marker panels have been developed including aspartate aminotransferase (AST)-platelet ratio index (APRI) and AST/ALT ratio (AAR), and Forns' index that use combination of AST, ALT, prothrombin time, platelet, and cholesterol; FibroTest, Hepascore, FibroMeter that use indirect fibrosis markers, such as α-2 macroglobulin and haptoglobin; FibroSpect II and Enhanced Liver Fibrosis test that use direct fibrosis markers, such as hyaluronic acid and tissue inhibitor of matrix metalloproteinase-1.177,178,179,180,181,182,183,184,185,186,187,188
APRI is calculated by the formula (AST/upper limit of normal for AST) × 100/platelet count (×109/L). The APRI is shown to be accurate in predicting both significant fibrosis (Ishak score ≥3) defined as APRI >1.5 (AUROC=0.8) and cirrhosis defined as APRI>2 (AUROC=0.89).189 A normal value of AAR is <0.8, but it increases along with hepatic fibrosis progression, so that AAR value >1.0 has a 73.7-100% positive predictive value in diagnosis.182,190,191
Liver stiffness measurement using transient elastography can be used to assess hepatic fibrosis,192,193,194,195 although it is not approved by the Food and Drug Administration as of 2013. Transient elastography cannot totally replace liver biopsy, because it often cannot produce reliable measurements in obese patients, and tends to give falsely high results in cases of acute hepatitis with severe inflammation and necrosis with mild fibrosis.196,197 In case of chronic hepatitis C, cutoff values determining significant fibrosis (≥F2) vary from study-to-study, ranging from 7.1 to 8.8 kPa, with an AUROC of 0.79-0.83.198 The AUROC of transient elastography for diagnosis of liver cirrhosis ranged from 0.95-0.97, with cutoff value of 12.5-14.6 kPa (77-78% positive predictive value, 95-97% negative predictive value).177,184,192,198,199,200,201,202
Other newly developed noninvasive tests include acoustic radiation force impulse (ARFI) imaging, real time elastography, magnetic resonance (MR) elastography, diffusion-weighted MR image, and MR spectroscopy. However, validations of their effectiveness are still needed.203,204,205
[Recommendations]
9. Anti-HCV should be tested in patients suspected of having acute or chronic HCV infection (A2).
10. HCV RNA should be tested in patients with a positive anti-HCV test for the purpose of confirmative diagnosis (A1).
11. Even with negative anti-HCV, HCV RNA testing is required when acute HCV infection is suspected or in the presence of unexplained liver disease in immunosuppressed patients (B1).
12. HCV RNA quantitative assay and genotyping should be performed prior to antiviral treatment (A1).
13. As soon as being exposed to infected blood or body fluid, testing of anti-HCV and serum ALT level should be performed. If the test result of anti-HCV is negative, HCV RNA assay is to be conducted 4-6 weeks after the exposure for early diagnosis. Even if baseline tests were all negative, follow-up testing for anti-HCV and serum ALT level should be done 4-6 months after the exposure (B2).
14. Assessment of liver disease severity is essential prior to antiviral treatment (B1).
15. Liver biopsy (B2) and/or noninvasive test for assessment of hepatic fibrosis (C2) can be done to make treatment decision and to predict prognosis.
TREATMENT GOALS
The goals of hepatitis C treatment are to eradicate HCV and to prevent complications and mortality from liver cirrhosis and HCC. It is difficult to evaluate the treatment goal in a short period of time due to slow evolution of chronic hepatitis C over several decades. Therefore, the short-term goal of hepatitis C treatment is to achieve an SVR defined as undetectable serum HCV RNA by a sensitive assay with a lower limit of detection <50 IU/mL at 24 weeks after the end of treatment. Since HCV does not reappear in 99% of the patients who achieve SVR,206,207 SVR is considered as actual eradication of HCV. In >90% of patients who achieve SVR, histological hepatic fibrosis improves or at least does not get worse,208,209 complications of cirrhosis significantly decrease,210 occurrence of hepatocellular carcinoma decreases,211,212 and survival rate improves.213,214
[Recommendations]
16. The goals of hepatitis C treatment are to eradicate HCV and to prevent complications and mortality from liver cirrhosis and hepatocellular carcinoma. (A1).
17. A short-term goal of hepatitis C treatment is to achieve an SVR defined as an undetectable serum HCV RNA by a sensitive assay with a lower limit of detection <50 IU/mL at 24 weeks after the end of treatment (A1).
INDICATIONS FOR TREATMENT
All hepatitis C patients who have no contraindications to treatment can be considered for antiviral treatment. However, treatment would be applicable in cases where benefits of treatment overweigh the risks of treatment. Generally, treatment is recommended for patients with significant hepatic fibrosis (≥stage F2), and initiation of treatment for those with advanced fibrosis (stage F3-4) is required as soon as possible. In case of mild hepatic fibrosis, treatment can be delayed after considering patient age, willingness for treatment, or perspectives of new drugs.
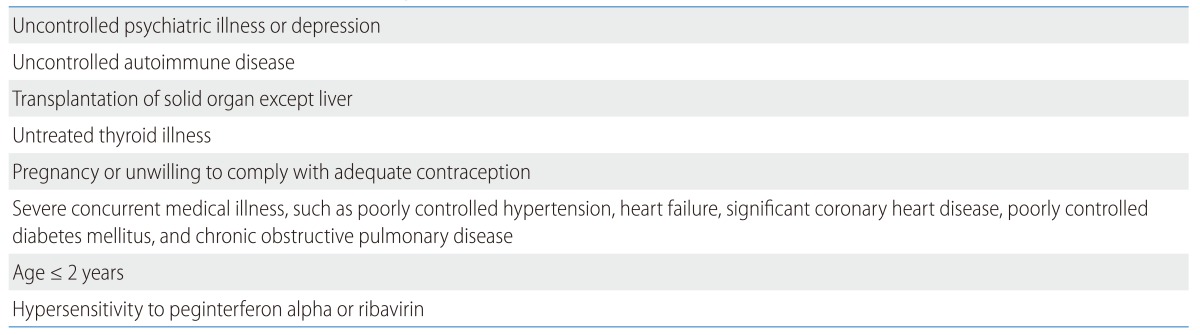
Absolute contraindications to the combination therapy of peginterferon alpha and ribavirin are summarized in Table 5.
Table 5.
Contraindications to treatment with peginterferon alpha and ribavirin114
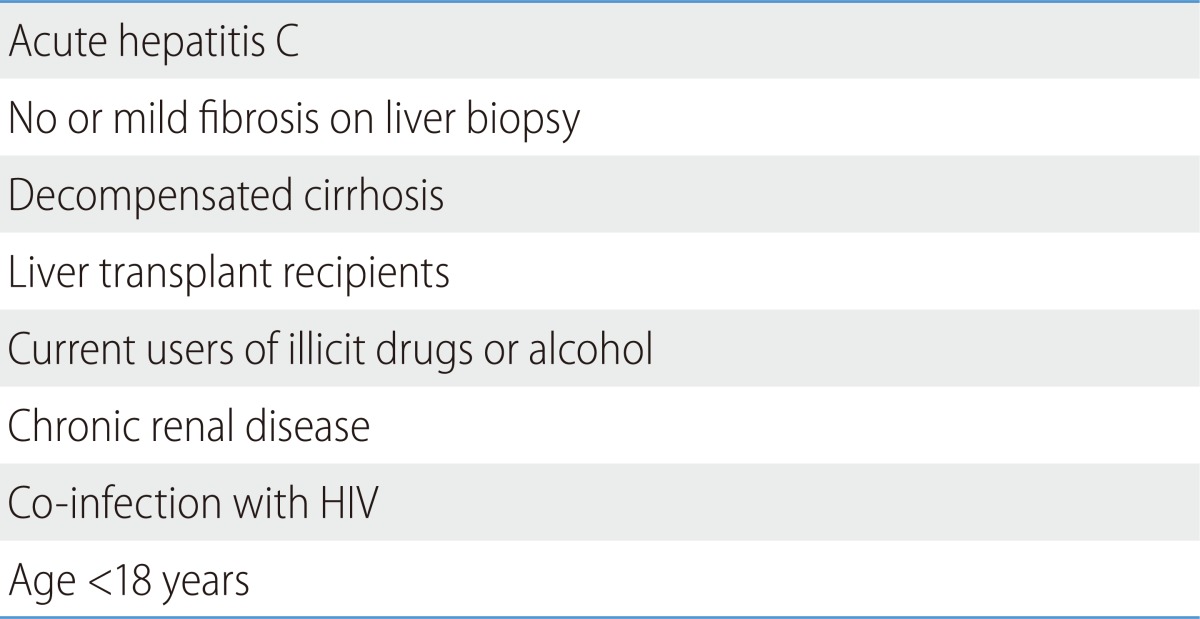
Treatment should be individualized considering benefits and risks in cases of decompensated cirrhosis, liver transplant recipients, current users of illicit drugs or alcohol, chronic renal diseases, coinfection with HIV, and no or mild fibrosis on liver biopsy (Table 6). The criteria of contraindications or individualization may change, since DAAs that are more efficient with fewer adverse effects are expected to be adapted to practice in the future.
Table 6.
Persons for whom therapy should be individualized114
A persistently normal ALT is defined as ALT value <40 IU/mL on two to three occasions separated by at least a month over a period of 6 months. Patients with persistently normal ALT have milder fibrosis compared to patients with abnormal ALT on average,215,216 but about 5-30% of patients with persistently normal ALT have advanced fibrosis and cirrhosis.217,218,219 A Korean study reported that some untreated patients with persistently normal ALT had progressive liver disease, and the risk of progressive liver disease was higher in patients with ALT value >23 IU/mL.220 Therefore, it is necessary to redefine the normal range of ALT and to treat patients with advanced fibrosis more actively, regardless ALT value. The SVR rate of normal ALT groups is similar to that of abnormal ALT groups.221,222
The elderly (>65 years of age) often have advanced liver diseases with higher necessity of treatment,223 but the SVR rates are lower and the adverse effects are more frequent.224,225 However, treatment of elderly hepatitis C patients can decrease the incidence rate of HCC and increase the survival rate.226,227 A recent study reported that the SVR rate in patients >60 years of age is similar to that of patients in 50-59 years of age.228,229 Little data are available concerning treatment in those >70 years of age. The decision of treatment in the elderly follows general rules.
[Recommendations]
18. All HCV-infected patients with no contraindications to treatment are considered as targets of treatment (A2).
19. Treatment should be individualized under overall consideration of the severity of liver diseases, chance of treatment success, risks of severe adverse effects, status of accompanying diseases, and patient willingness for treatment (B1).
DEFINITION OF TREATMENT RESPONSE
The combination therapy of peginterferon alpha and ribavirin has considerable cost and adverse effects. Meanwhile, the likelihood of SVR gets higher as the time of HCV RNA disappearance during the therapy is shorter.230 Response-guided therapy is a strategy to modify the duration of treatment based on the time of HCV RNA disappearance by measuring serum HCV RNA at weeks 4, 12, and 24 of treatment.
A rapid virological response (RVR) is defined as undetectable HCV RNA by a sensitive assay with lower limit of detection <50 IU/mL at week 4 of treatment. The SVR rate is expected to be 87.5-100% in HCV genotype 1 patients with a RVR and to be 33.3-63.8% in those without a RVR.231,232,233 The SVR rate is expected to be 85-86.5% in HCV genotype 2 and 3 patients with a RVR, and 54-58.3% in those without a RVR.232,234
An early virological response (EVR) is defined as undetectable HCV RNA using a sensitive assay with a lower limit of detection <50 IU/mL, or ≥2 log reduction of HCV RNA compared with the baseline level. The SVR rate is as low as 3% in HCV genotype 1 patients without an EVR.235,236,237 Therefore, medical costs and adverse effects can be reduced by discontinuing therapy in cases without an EVR. An EVR is classified as a complete EVR (cEVR) defined as undetectable HCV RNA and a partial EVR (pEVR) defined as an EVR with detectable HCV RNA at week 12. A delayed virological response (DVR) is defined as a pEVR that eventually resulted in undetectable HCV RNA at week 24.238,239 A null response is defined as <2 log reduction of HCV RNA level from baseline at week 12 of therapy, whereas partial nonresponse is defined as ≥2 log reduction of HCV RNA level from baseline but detectable HCV RNA at week 12 and 24.
An end-of-treatment response (ETR) is defined as undetectable HCV RNA at the end of treatment using a sensitive assay with a lower limit of detection <50 IU/mL. SVR is defined as undetectable HCV RNA by a sensitive assay with a lower limit of detection <50 IU/mL at 24 weeks after the cessation of treatment. A SVR evaluated at 12 weeks (SVR12) after the end of treatment is reported to be almost identical to a SVR at 24 weeks after the cessation of treatment,240 and recent clinical trials for new drugs tend to evaluate therapeutic efficacy by a SVR12. Viral breakthrough refers to the reappearance of HCV RNA during the treatment after virological response, and relapse is defined as the reappearance of HCV RNA after treatment is discontinued (Table 7).
Table 7.

[Recommendations]
20. HCV RNA in blood should be measured at weeks 4, 12, and 24 of treatment depending on HCV genotype to evaluate individual therapeutic responses and to modify the duration of treatment (B1).
21. HCV RNA should be measured at the end of treatment and 24 weeks after the cessation of treatment to evaluate therapeutic effects and to identify the achievement of SVR (A1).
PREDICTORS OF TREATMENT RESPONSES
Predicting the likelihood of SVR is helpful for each patient's decision to initiate therapy (Table 8). Strongest pretreatment predictors for SVR include HCV genotype,235,241,242 degree of hepatic fibrosis,243 and IL28B polymorphism.243,244 The SVR rates are 40-60% in HCV genotype 1 patients whereas they are about 70-80% in HCV genotype 2 and 3 patients.235,245 Patients with F0-F2 fibrosis have 2.7-times higher SVR rates compared to those with F3-F4 fibrosis.243 The SVR rates are higher by 2.4-2.7 times in patients with a viral load <400,000-800,000 IU/mL compared to those with a viral load exceeding 800,000 IU/mL.231,243,246 The SVR rates decrease with conditions including old age (>40 years of age),235 African-Americans,247 body weight over 70 kg,235,236,237,238,239,240,241 and insulin resistance.248,249
Table 8.
Predictors of SVR
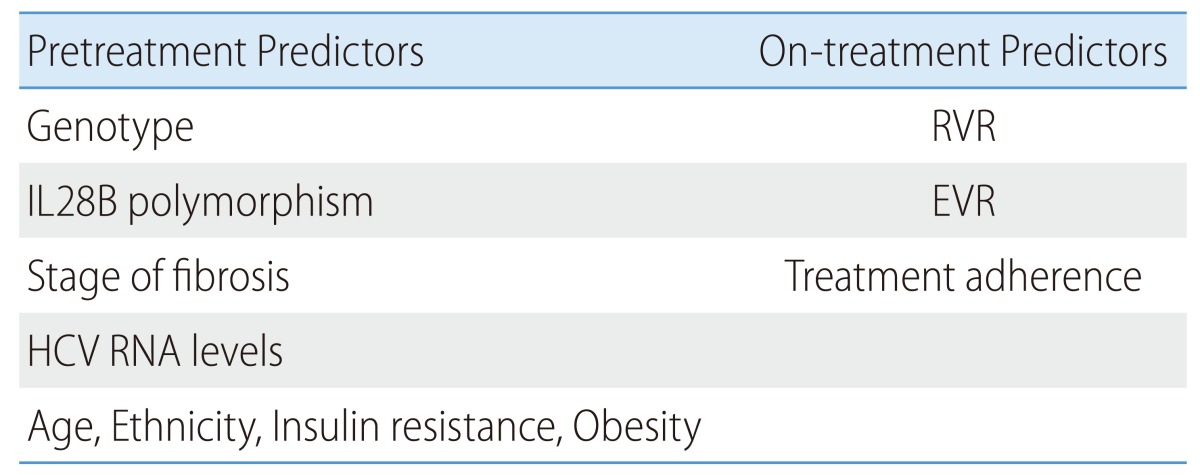
Recent studies have revealed that host IL28B polymorphism is a strong predictor for SVR.243,244,250 SVR rates vary depending on SNP of IL28B; in other words, C or T allele of the rs12979869 locus. The SVR rates of HCV genotype 1 Caucasian patients are 69%, 33%, and 27% in CC homozygote, CT heterozygote, and TT homozygote, respectively. The SVR rates of HCV genotype 1 African-American patients are 48%, 15%, and 13%, respectively.243,250 The SVR rates of HCV genotype 1 Korean patients are 73-88% in CC homozygote and 0-40% in CT heterozygote.251,252,253 IL28B polymorphism is variable depending on race. CC homozygote population in Korea accounts for 88-89%,251,252,253 compared to 17% in African-Americans and 37% in Caucacinas243. Further study is needed to evaluate the usefulness of pretreatment determination of IL28B polymorphism in Korea, where about 90% of the population has the CC homozygote, although many institutions in western countries determine IL28B polymorphisms to predict SVR prior to the initiation of treatment. Polymorphism of the rs8099917 locus near IL28B also affects SVR, and a SVR rate is higher in the TT or TG genotype than in the GG genotype.254
RVR is the strongest on-treatment predictor for SVR,231,232 and the likelihood of a SVR increases by 9-times with a RVR.243 Meanwhile, an EVR is a strong negative predictor for a SVR and the SVR rate is very low (3%) without an EVR.236 In addition, SVR increases when medication adherence exceeds 80%,255 so efforts to assess and maintain the medication adherence can increase the SVR rate.
TREATMENT OF CHRONIC HEPATITIS C
Treatment of hepatitis C is rapidly evolving. SVR rates have increased from about 10% with conventional interferon monotherapy for 6 months, to about 54-56% with combination therapy of peginterferon alpha and ribavirin, and further to 75% with triple therapy in which boceprevir or telaprevir is added to the peginterferon alpha and ribavirin combination therapy.235,241,256,257,258,259
In South Korea, the dual combination therapy of peginterferon alpha and ribavirin has been the standard therapy in 2013, since DAA is still not approved for practice. Response-guided therapy is used to reduce adverse effects and to increase therapeutic effects. It is expected that drugs including DAAs with a strong antiviral effect and relatively few adverse effects will be introduced soon in South Korea. DAA acts on a specific step of viral life cycle. DAAs including NS3/4A protease inhibitors, NS5A inhibitors, and NS5B polymerase inhibitors and host-targeting antiviral agents, such as the cyclophyllin A inhibitor and miR-122, are under development. It is expected that triple or quadruple therapy in which one or two DAAs plus peginterferon alpha and ribavirin, or interferon-free therapy with combination of oral agents would be available soon.260,261 Nearly 90% of a SVR rate and shortening of treatment duration with those new therapeutic strategies have been reported,262,263 although more evidence is needed. However, the high cost of the drugs, drug resistance, drug interactions, and new adverse effects must be considered.
TREATMENT OF GENOTYPES 1 AND 4 CHRONIC HEPATITIS C
Optimal treatment of genotypes 1 and 4
The standard of care for HCV genotypes 1 and 4 infected patients in 2013 in South Korea is combination therapy of peginterferon alpha and ribavirin for 48 weeks.235,241,242 Currently, two types of peginterferon alpha are approved for the treatment of chronic hepatitis C: peginterferon alpha-2a (pegasys; Hoffman-La Roche, USA) and peginterferon alpha-2b (peg-Intron; MSD, USA). Peginterferon alpha is a polyethylene glycol (PEG)-modified interferon alpha (40 kD PEG for peginterferon alpha-2a, 12 kD PEG for peginterferon alpha-2b), which has a longer half-life and higher blood concentration for an extended period of time. Therefore, peginterferon alpha is more effective and is more convenient for once-weekly injection compared to thrice-weekly injections of conventional interferon alpha.264 Peginterferon alpha-2a should be injected at a uniform dose of 180 µg once a week, regardless of the patient body weight, whereas peginterferon alpha-2b is to be injected at a dose of 1.5 µg/kg once a week. Ribavirin is to be given in weight-based dose. Most studies suggested 1,000 mg/day of ribavirin in patients with a body weight under 75 kg and 1,200 mg/day for a body weight over 75 kg when given with peginterferon alpha-2a, and 800 mg/day of ribavirin for bodyweight <65 kg, 1,000 mg/day for 65-80 kg, 1,200 mg/day for 85-105 kg, and 1,400 mg/d for >105 kg when given with peginterferon alpha-2b.
SVR rates in Europe and the US were reported as 40-50% when HCV genotype 1 patients were treated with peginterferon alpha and ribavirin.265 SVR rates in South Korea were reportedly higher; 53.6-69.5%228,245,266,267,268 and 62.7% in pooled analysis of 10 studies.269 This is related to the higher frequency of favorable IL28B genotypes in Koreans than in Whites or Blacks.81,244,252,254,270,271
Standard of care for HCV genotype 4 infected patients is the combination therapy of peginterferon alpha and ribavirin for 48 weeks, the same as the standard of care for HCV genotype 1. SVR rate was reported as 72%.272 There are no reports on therapeutic outcome for chronic HCV genotype 4 in South Korea.
Response-guided therapy in genotypes 1 and 4
Several studies have investigated whether treatment duration could be shortened from 48 to 24 weeks when a RVR is achieved using combination therapy of peginterferon alpha and ribavirin for HCV genotype 1 patients. SVR rates of 24- and 48-week treatment groups were 77.2-100% and 85-92.4%, respectively,230,231,273,274,275,276 which were not statistically significant. However, the upper limit of lower level of HCV RNA concentration was inconsistent, ranging from 400,000 to 800,000 IU/mL and the number of recruited patients was not large enough in those studies. Recently, two meta-analyses of randomized controlled studies including the patients with a RVR showed that a lower rate of SVR and higher recurrence rate were observed in the 24-week treatment group when compared with that in the 48-week treatment group. However, in pooled analysis in patients with low baseline HCV viral load (<400,000 IU/mL) SVR rates were not statistically different between the 24- and 48-week treatment groups.277,278 The results support the conclusion, patients with genotype 1 who achieve a RVR and low baseline viral load (<400,000 IU/mL) may have their duration of therapy shortened to 24 weeks if there are no negative predictors of response, such as advanced liver fibrosis, cirrhosis, obesity, or insulin resistance.230 Meanwhile, in patients with genotype 4 with a RVR, the 24-week treatment group had a similar SVR rate to that of the 48-week treatment group, regardless of baseline viral load.275,279
Treatment should be stopped in patients who do not achieve an EVR, as a SVR rate in these patients with standard treatment duration is <3%, and in patients with pEVR and detectable HCV RNA at week 24, as a SVR rate is 2-4%.235,236,280 Meanwhile, an additional 10-20% increase in SVR rate was reported in an extended treatment to 72 weeks when patients achieved a pEVR with negative HCV RNA at week 24.238,239,278,281 However, extension of duration of treatment should be carefully determined after considering many aspects of patients, such as adverse effects or compliance. In case of a cEVR without RVR, SVR rate was reported as 62-70%,230,237,239,275 and extension of the treatment duration did not increase the SVR rate.238,278
Retreatment of HCV genotype 1 and 4 patients who fail to respond to previous treatment
Patients who have failed prior treatment can be classified as relapsers and non-responders. Retreatment with peginterferon alpha and ribavirin can be considered for relapsers who have previously been treated with conventional interferon alpha with or without ribavirin, or peginterferon alpha monotherapy, since SVR rates are reported as 31-47%.282,283 However, as for relapsers after peginterferon alpha and ribavirin therapy, a SVR rate after re-treatment with the same regimen was reported as only 23%, and retreatment should be carefully determined after discussion with the patient.284
In non-responders to conventional interferon alpha with or without ribavirin, retreatment with peginterferon alpha and ribavirin should be carefully determined considering that the SVR rate is very low (8-24%) in these patients.282,283,284,285,286 Retreatment with same regimen in non-responders to peginterferon alpha and ribavirin is not recommended, since SVR rates are only 4-8%. The retreatment should be postponed until DAAs are available.
During retreatment, SVR rates of patients with a cEVR is 35.1-49%, with a pEVR is 3.5-12%, and those without an EVR is 0-1%. Therefore, cEVR can be a useful marker in determining cessation of the retreatment.284,287,288
A maintenance therapy with a low dose of peginterferon alpha is not recommended because it cannot reduce long-term complications in patients with advanced liver fibrosis or cirrhosis.285,289
New therapies including DAAs in genotypes 1 and 4
Triple therapy including boceprevir or telaprevir
The standard therapy in Europe and the US during 2011-2013 was a triple therapy combining peginterferon alpha, ribavirin, and oral protease inhibitor, such as boceprevir or telaprevir. However, these two protease inhibitors have not yet been approved in South Korea.
In two phase III clinical trials using boceprevir257,290 and three phase III clinical trials using telaprevir,258,259,291 SVR rates were reported as 63-75%, 69-88%, and 29-33% in treatment-naïve patients, relapsers, and non-responders to peginterferon alpha and ribavirin, respectively. There were additional improvement in SVR by 25-30% in naïve patients and 25-60% in treatment experienced patients compared to combination therapy with peginterferon alpha and ribavirin.
However, more adverse effects were reported in triple therapy including boceprevir or telaprevir compared to the dual combination therapy.257,259,290,291 In the boceprevir trials, dysgeusia, anemia, and neutropenia were more common, while in the telaprevir trials, rashes, anemia, and anorectal symptoms (discomfort and pruritus) were more common. In addition, drug resistance should be considered because of the reduced drug compliance due to the discomfort and inconvenience in taking numerous drugs three times a day. Drug-drug interaction should also be considered, since boceprevir and telaprevir are metabolized in cytochrome P450 system (CYP2C, CYP3A4, and CYP1A) causing interaction with many other drugs. Information of drug interactions is provided in various websites (e.g., www.hep-druginteractions.org). Another issue of increasing concern is differentiating patients who require immediate treatment with the triple therapy from those who can wait until drugs with improved adverse effects or lower prices are available, because the triple therapy imposes considerable expense.
Present situation for other newly developed drugs
Peginterferon lamda acts on receptors that are different from those of peginterferon alpha. The receptors of interferon lamda are found mainly on hepatocytes. A clinical trial reported a higher RVR rate and significantly lower occurrence of adverse effects including hematologic side effects, flu-like symptoms, and muscular pain compared to peginterferon alpha.292 New DAAs being evaluated in clinical trials include NS3/4A protease inhibitors (asunaprevir, faldaprevir, ABT-450, etc.), NS5A polymerase inhibitors (daclatasvir, etc.), NS5B polymerase inhibitors (sofosbuvir, deleobuvir, ABT-333, etc.), and host-acting antiviral agents include cyclophilin A inhibitor, miR-122 inhibitor (miravirsen).221,260,261,262,263,293,294,295 These drugs can be simply administrated orally (except miravirsen, which is injected) with fewer adverse effects and stronger antiviral effects. A SVR12 rate (SVR rate at 12 weeks after the cessation of treatment) was 90% in a phase III clinical trial with sofosbuvir plus peginterferon alpha and ribavirin in 327 treatment naïve patients with chronic HCV (including 17% of cirrhosis patients) genotype 1, 4, 5, and 6 (seven patients of type 5 and 6).263
Many clinical trials with interferon-free, DAA combination regimens have been done or are ongoing. These have reported different therapeutic outcomes depending on the combination of drugs and subtypes (1a vs. 1b) of HCV.261,262,293,295,296 Therefore, a therapeutic strategy with more effective combination regimen, less adverse effect, and reduced drug resistance is expected to become available.
[Recommendations]
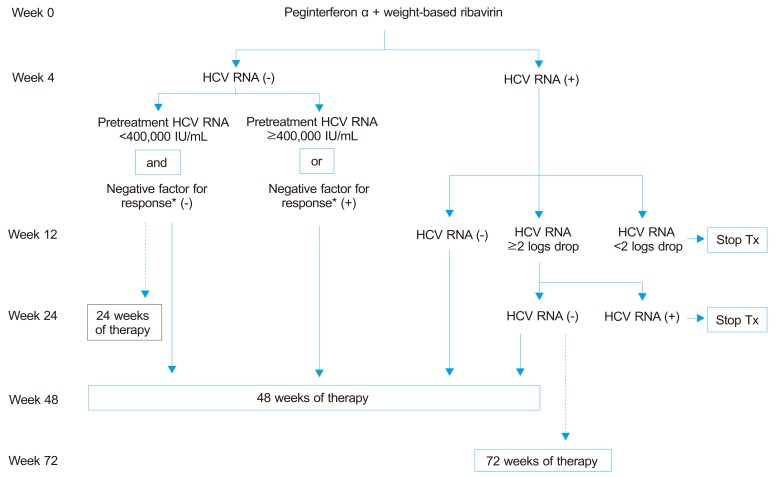
22. Optimal treatment of genotypes 1 and 4 (Fig. 2)
Figure 2.
Treatment algorithm for patients with genotype 1 chronic HCV infection. This algorithm applies to genotype 4 at a B2 grade of evidence. The dotted lines indicated weaker strength of recommendation compared with the solid lines.
*Negative factors for response include advanced liver fibrosis or cirrhosis, obesity, and insulin resistance.
1) Treatment with one of two peginterferon alpha molecules in combination with ribavirin should be planned for 48 weeks (A1). Peginterferon alpha-2a should be injected 180 µg subcutaneous once a week, regardless of patient body weight with ribavirin using doses of 1,000 mg/d for those ≤75 kg in weight and 1,200 mg/day for those > 75 kg. Peginterferon alpha-2b is to be injected at 1.5 µg/kg/week with ribavirin using doses of 800 mg for those <65 kg in weight, 1,000 mg for 65-85 kg, 1,200 mg for 85-105 kg, and 1,400 mg for >105 kg (A1).
2) In patients with a RVR and low baseline HCV viral load (<400,000 IU/mL), and without any negative predictors for SVR (advanced liver fibrosis, cirrhosis, obesity or insulin resistance), shortening of treatment duration to 24 weeks can be considered (B1).
3) In patients of genotype 4 with a RVR, 24-week treatment can be considered, regardless of baseline viral load (B2).
4) Treatment should be stopped in patients who fail to achieve an EVR (A1). Patients who achieve a cEVR can be treated for 48 weeks (A1). Patients with a pEVR should be re-tested at week 24; if HCV RNA remains positive, treatment should be stopped (A1), while if HCV RNA test becomes negative, extending therapy to 72 weeks can be considered (B2).
23. Retreatment of HCV genotypes 1 and 4 patients who failed to respond to previous treatment
1) Retreatment with peginterferon alpha plus ribavirin can be considered for relapsers or non-responders that were previously treated with conventional interferon with or without ribavirin, or peginterferon monotherapy (B2). Patients who failed to achieve a SVR after peginterferon alpha and ribavirin combination therapy are not recommended to be retreated by the same regimen (A2).
2) A low-dose maintenance therapy with peginterferon alpha is not recommended for patients who have failed a combination therapy with peginterferon alpha and ribavirin (A1).
Triple therapy with peginterferon alpha and ribavirin plus either boceprevir or telaprevir is recommended for treatment naïve or experienced HCV genotype 1 patients (A1). It is desirable that more effective regimens including DAAs are adapted to Korean patients after further studies.
TREATMENT OF GENOTYPES 2 AND 3 CHRONIC HEPATITIS C
Optimal treatment of genotypes 2 and 3
The first-line treatment of HCV genotypes 2 and 3 patients is combination therapy of any one of two peginterferon alpha and ribavirin for 24 weeks.235,241,242,297,298,299 Peginterferon alpha-2a should be injected 180 µg subcutaneously once a week, regardless of body weight, whereas peginterferon alpha-2b is to be injected 1.5 µg/kg subcutaneously once a week. Ribavirin is to be given at a flat dose of 800 mg daily, regardless of the type of peginterferon alpha used. There is insufficient evidence to show whether a weight-based dose of ribavirin is more effective in achieving a SVR for HCV genotype 2 and 3 patients.241,242,297 The SVR rate of Korean patients with HCV genotype 2 treated with the first-line therapy exceeded 80%.245, 300 Although a SVR rate of HCV genotype 3 in Korean patients is hardly been reported, reports from other ethnicities show a lower SVR rate in HCV genotype 3 patients by 10-20% than that of genotype 2.235,242,246,278,297,299,301,302,303
Response-guided therapy in genotype 2 and 3 patients
Although several studies investigated whether the treatment duration could be shortened according to the on-treatment virological response,246,301,302,303,304,305,306,307 the results of these studies should not be compared directly since factors affecting SVR rates, such as duration of the shorted treatment, dose of ribavirin, proportion of patients with a RVR, are heterogeneous. A study comparing 16-week therapy with 24-week therapy each including about 350 patients reported a lower SVR rate of 65% in the 16-week treatment group compared to 82% in the 24-week treatment group.303 However, shortening of the treatment duration was not performed according to the on-treatment response, RVR, but was randomly assigned in this study. Meanwhile, in HCV genotype 3 patients, the same study reported a SVR rate of 61% in the 16-week treatment group and 71% in the 24-week treatment group that was not statistically significantly different. Another study compared 16-week and 24-week treatment groups of 200 HCV genotype 2 patients for each group, and reported a SVR rate of 81% in the 16-week group and 92% in the 24-week group, with no statistically significant difference.305 However, the 16-week treatment group had a relapse rate of 17%, which was significantly higher than that of the 5% rate of the 24-week treatment group. In addition, this study used weight-based dose of ribavirin from 1,000 to 1,200 mg in combination with peginterferon alpha and resulted in a higher relapse rate, despite an equivalent SVR rate, as that of the 24-week therapy. As for the factors predicting relapse after treatment other than the duration of the therapy, existence of cirrhosis, baseline high viral load, body weight, gender, and old age have been suggested.246,305,306 However, studies contradicting these results also exist, and further evidences are required.301,303
In conclusion, patients with genotype 2 and 3 who achieve RVR may have their duration of therapy shortened to 16 weeks if there are no negative predictors of response, such as advanced liver fibrosis, cirrhosis, and high baseline viral load, at the expense of a higher chance of post-treatment relapse. If negative predictors of response exist, such as advanced liver fibrosis or cirrhosis, there is a lack of evidence supporting equal efficacy of shortened therapy. Meanwhile, studies testing the efficacy of extended duration of treatment up to 48 weeks in patients with negative predictors for response, such as lack of RVR, high baseline HCV RNA level, or accompanying advanced liver fibrosis or cirrhosis. A study on 1,311 HCV genotype 2 or 3 patients with negative predictors for SVR reported no benefit of extended treatment duration to 48 weeks on achieving SVR.242
Retreatment of HCV genotype 2 and 3 patients who fail to respond to previous treatment
Retreament with peginterferon alpha and ribavirin combination therapy may be given to HCV genotype 2 or 3 patients that were previously treated with conventional interferon with or without ribavirin, or peginterferon alpha without ribavirin, and failed to achieve SVR.282,283,284,285,286,308,309,310 The SVR rate after retreatment with peginterferon alpha and ribavirin combination therapy in relapsers is reported to be 54.8-67.0%, whereas the SVR rate of non-responders after retreatment is 39.3-53.0%.282,283 There is insufficient evidence for adequate duration of retreatment in HCV genotype 2 and 3 patients, and previous studies on retreatment arbitrarily applied treatment duration of either 24 weeks or 48 weeks.282,283,284,285,308,310 A study including relapsers after 24-week of peginterferon alpha and ribavirin therapy who were retreated with the same regimen for the extended period of 48 weeks (n=92) reported a SVR rate of 57%.284 Therefore, for those who fail to achieve a SVR after peginterferon alpha and ribavirin treatment, there is still not enough evidence for the retreatment using the same regimen and it is not recommended, especially in non-responders.
New therapies in genotypes 2 and 3
Although a relatively high SVR rate can be achieved by 24-week combination therapy with peginterferon alpha and ribavirin in HCV genotype 2 and 3 patients, new therapeutic strategies may be required for patients that fail to achieve a SVR, and those who cannot tolerate interferon-based treatment. A phase III clinical trial investigating the efficacy of 12-week treatment of ribavirin and sofosbuvir combination therapy on 70 treatment naïve HCV genotype 2 or 3 patients reported a SVR12 rate of 97%.263 In addition, a SVR12 rate of 94% was achieved after 16 weeks of sofosbuvir and ribavirin combination therapy in 32 treatment experienced chronic HCV genotype 2 patients.311 Therefore, interferon-free DAA combination regimens are effective in both treatment failure and interferon intolerant patients, and are expected to become available in Korea.
[Recommendations]
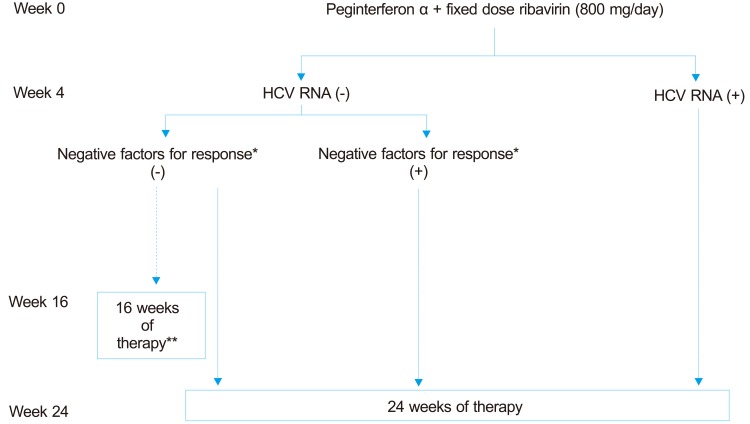
24. Optimal treatment of genotypes 2 and 3 (Fig. 3)
Figure 3.
Treatment algorithm for patients with genotype 2, 3 chronic HCV infection. The dotted lines indicate weaker strength of recommendation compared with the solid lines.
*Negative factors for response may include advanced fibrosis, cirrhosis and others.
**The shortened therapy may result in higher relapse rate.
1) Treatment with one of two peginterferon alpha molecules in combination with ribavirin should be planned for 24 weeks (A1).
2) The dose for peginterferon alpha-2a is 180 µg subcutaneously once a week and peginterferon alpha-2b is 1.5 µg/kg per week (A1). Daily administration of 800 mg of ribavirin should be done, regardless of body weight (A2).
3) In patients with an RVR and without any negative predictors for SVR, shortening of treatment duration to 16 weeks can be considered (B2). However, shortening of treatment duration should be done in caution, since this can result in higher relapse rate (A2).
25. Retreatment of HCV genotype 2 and 3 patients who fail to respond to previous treatment.
1) Retreatment with peginterferon alpha plus ribavirin can be considered for relapsers or non-responders that were previously treated with conventional interferon with or without ribavirin, or peginterferon alpha monotherapy (B2).
2) Non-responders to a full course of treatment with peginterferon alpha plus ribavirin are not recommended to be retreated by the same regimen (B2).
TREATMENT OF GENOTYPE 6 CHRONIC HEPATITIS C
HCV genotype 6 is limited mostly to Southeast Asia, Southern China, Hong Kong, and Macau. It comprises about 1% of total chronic HCV patients in South Korea.312 SVR rate of chronic HCV genotype 6 treated with combination of peginterferon alpha and ribavirin is 70.0-85.7%, which is comparable with that of HCV genotype 3 and higher than that of HCV genotype 1.313,314,315
A study comparing the efficacy of fixed dose ribavirin and weight based ribavirin for HCV genotype 6 patients is not available. All studies of peginterferon alpha based treatment for HCV genotype 6 have adapted weight based doses of ribavirin.314,315,316,317,318,319,320
A study comparing the efficacy of fixed dose ribavirin and weight based ribavirin for HCV genotype 6 patients is not available. All studies of peginterferon alpha based treatment for HCV genotype 6 have adapted weight based doses of ribavirin.314,315,316,317,318,319,320
Two randomized control studies on combination therapy of peginterferon alpha and ribavirin reported no statistical difference in SVR between 24-week and 48-week treatment.72,80,318,320,321,322,323 No study has been conducted about retreatment for HCV genotype 6 patients who failed previous treatment.
[Recommendations]
26. Optimal treatment of genotype 6
1) Treatment with one of two peginterferon alpha molecules in combination with ribavirin should be planned for 24 weeks (A1).
2) The dose for peginterferon alpha-2a is 180 µg subcutaneously once a week and for peginterferon alpha-2b is 1.5 µg/kg per week(A1). Ribavirin is to be orally administered 1,000 mg in patients under 75 kg, and 1,200 mg in patients over 75 kg when administered with peg-interferon alpha-2a and 800 mg for under 65kg, 1,000 mg for between 65-85 kg, 1,200 mg for between 85-105 kg, and 1,400 mg for over 105 kg when given with peginterferon alpha-2b (B2).
TREATMENT OF ACUTE HEPATITIS C
Spontaneous recovery rate of acute hepatitis C varies from 20-50%.72,80,321,322,323 Treatment can be initiated immediately after the diagnosis of acute hepatitis C. However, evidence supports a therapeutic strategy of delaying treatment for 8-12 weeks to allow spontaneous remission.72,324,325 According to a randomized control study comparing an immediate treatment with a delayed treatment for 12 weeks, the SVR rate of the delayed treatment is not inferior to the immediate treatment considering spontaneous recovery rate and treatment-induced SVR.326 Nevertheless, diagnosis of acute hepatitis C is not always straightforward and treatment can be done in accordance with the treatment of chronic HCV infection when the differentiation of acute hepatitis C and acute exacerbation of chronic hepatitis is difficult.
Anti-HCV antibody starts to appear at the time of highest ALT and of decreasing point of blood HCV RNA, which is about 8-12 weeks after the infection when most patients may not show any specific symptoms.130 Therefore, testing for serum HCV RNA is useful for diagnosis and treatment when acute hepatitis C is suspected but showing a negative result for anti-HCV.
SVR rate is as high as 80-90% when acute hepatitis C is treated by conventional interferon alpha or by peginterferon alpha monotherapy for 24 weeks.327,328,329,330,331,332,333 Peginterferon alpha-2b and ribavirin combination therapy did not increase an SVR rate compared to that of peginterferon alpha-2b monotherapy.326,329 No clear additional benefits of combining ribavirin with interferon alpha or peg-interferon alpha are apparent to date.
The optimal treatment duration for acute hepatitis C is not definitely established. A randomized control study (n=34 in each group) reported no significant difference between SVR rates (82.4% in the 12-week treatment group and 91.2% in the 24-week treatment group) regardless of HCV genotypes.333 However, studies reporting good therapeutic outcomes of acute hepatitis C have tended to adopt a 24-week treatment, this length of treatment is recommended until contrary evidence is presented.327,328,332,333
[Recommendations]
27. Antiviral therapy is to be considered for treatment of acute hepatitis C (A1).
28. Initiation of treatment can be postponed for 8-12 weeks after onset of acute hepatitis C to allow spontaneous recovery (B2).
29. Peginterferon alpha monotherapy is preferentially considered in treatment of acute hepatitis C (B1), and duration of treatment is to be 24 weeks (B2).
MANAGEMENT OF ADVERSE EFFECTS OF ANTIVIRAL TREATMENT FOR HEPATITIS C
Monitoring adverse effects of antiviral treatment
During combination therapy of peginterferon alpha and ribavirin, many patients experience adverse effects; 10-20% of patients discontinue the treatment and 20-30% of patients experience dose reduction.334,335 Patients who received ≥80% of both their planned peginterferon alpha and ribavirin doses for ≥80% of the expected duration show an SVR rate of 63%, which was significantly higher than that (52%) of the patients who received reduced dose (<80%) of one or both drugs.255 Therefore, meticulous monitoring and management of adverse effects can improve therapeutic outcome by preventing from drug discontinuation or dose reduction.
Adverse effects of antiviral therapy and its management
More than 20% of patients treated with the peginterferon alpha and ribavirin combination therapy experience headache, fever, myalgia, muscular rigidity, arthralgia, nausea, anorexia, weight loss, diarrhea, hair loss, skin rash, pruritus, inflammation on sites of injection, dyspnea, fatigue, insomnia, irritability, or depression (Table 9).235,241,242,336 However, severity or frequency of these adverse effects may vary, since these adverse reactions have been reported from patients chosen for clinical trials.336
Table 9.
Adverse events of peginterferon alpha or interferon alpha and ribavirin
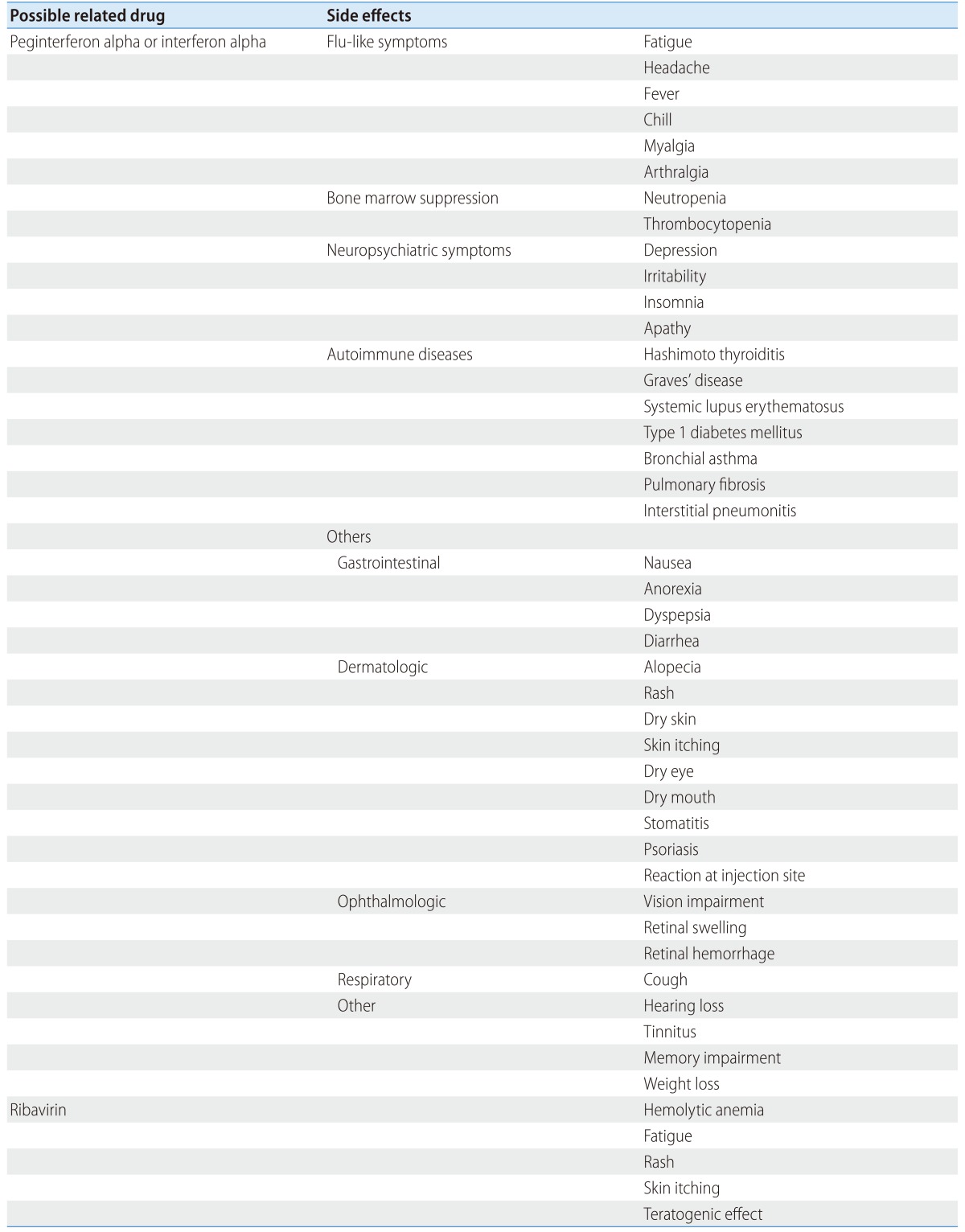
Adverse effects after peginterferon alpha injection can be classified as flu-like symptoms, myelosuppression, neuropsychological problems, and autoimmune dysfunction. Flu-like symptoms including fever, fatigue, myalgia, or nausea occur in about 37% of the patients,235,241,242 but these symptoms can be alleviated by administration of analgesics and usually lessened 4-6 weeks after the treatment.336 Myelosuppression causes neutropenia and thrombocytopenia, the main causes of dose reduction, and often set the therapeutic limit in cirrhosis. Dose of peginterferon alpha should be reduced or skipped in case of severe adverse effects. Especially, when absolute neutrophil count decreases to under 750/mm3 or platelet count decreases to <50,000/mm3, dose reduction should be considered; when absolute neutrophil count decreases under 500/mm3 or platelet count decreases under 25,000/mm3, drug discontinuation should be considered. Later, re-administration of the drugs can be considered following adequate recovery of absolute neutrophil count and platelet count; for example, 50% of the previous dose can be administered when absolute neutrophil count recovers up to1,000/mm3 or over, and platelet count up to 75,000/mm3or over, with continuous monitoring of those cell counts. Although evidences for the role of granulocyte colony stimulating factor (G-CSF) on lowering infection rate and on improving SVR are not enough, use of G-CSF can be considered in some patients with cirrhosis.337 Meanwhile, treatment is to be halted in case of acute deterioration of hepatitis with elevation of ALT to over 10 times of upper-normal level or severe bacterial infection, such as sepsis. Even though thrombopoietin receptor agonist can raise platelet count in cirrhosis prior to treatment,338 use of this drug should be done very carefully, since evidence for improved SVR rate by this drug remain insufficient, whereas the risk of thrombosis can cause portal vein thrombosis.339
Neuropsychological problems including insomnia, difficulty in concentrating, memory impairment, irritability, or apathy can be caused by peginterferon alpha. Especially, severe depression can provoke a suicide attempt, which requires careful observation during antiviral treatment.340 Past history of depression should be checked for, since presence of uncontrolled depression is a contraindication to the treatment. Depression occurs in about 28% of patients during the treatment,341 and antidepressants like serotonin uptake inhibitors can be used to maintain the treatment.342 Preventive administration of antidepressants can reduce occurrence of depression during the treatment but cannot increase SVR rate.341,342,343
Thyroid complications can occur in about 15-20% of patients, due to immunomodulatory function of peginterferon alpha,336,344 which may be from autoimmune or non-autoimmune causes; autoimmune thyroid diseases are classified as Graves' disease, Hashimoto's disease, and auto-antibody generation against thyroid gland345 and non-autoimmune thyroid disease results from thyroid damage by HCV itself.344,345,346 Hashimoto's disease is the most common and starts with hyperthyroidism and may progress to hypothyroidism. Thyroid function may not be recovered even after the cessation of treatment.347,348,349 Discontinuation of treatment should be considered in case of severe hyperthyroidism during interferon administration, while treatment can be maintained with careful observation if hyperthyroidism is not severe.350 In case of hypothyroidism at the beginning, interferon therapy can be maintained by administrating thyroxine.344 Meanwhile, thyroid gland dysfunction can occur even after the end of treatment336 and it is desirable to check thyroid stimulating hormone (TSH) and free thyroxine levels at 2-4-month intervals during treatment and regularly for 1 year after the termination of treatment.
Various kinds of autoimmune diseases, such as systemic lupus erythematosus, type 1 diabetes mellitus, asthma, interstitial pulmonary fibrosis, or thyroid diseases, can be induced by interferon therapy.351 Therefore, baseline evaluation of these diseases is necessary prior to the initiation of treatment, although existence of these diseases is not an absolute contraindication to the treatment especially when these diseases are well controlled.336,344 Other adverse effects related to peginterferon alpha, such as visual field defect, retinal hemorrhage and edema, hearing defect, tinnitus, vomiting, nausea, pruritus, weight loss or hair loss, improve after termination of treatment.336 Frequency of retinal defect is reported as about 3.8-30.9%352,353,354,355,356 with variable clinical course from severe visual field defect to no symptoms, and it is desirable to check the retina prior to the treatment in cases with risk factors such as old age, hypertension, or diabetes356,357,358 even though pretreatment and regular follow-up evaluation of retina remains debatable. Hearing loss occurs in <1% of patients and it cannot be recovered completely even after the termination of treatment.359
A common adverse effect of ribavirin is hemolytic anemia due to dose-dependent direct toxicity of ribavirin to erythrocytes and it may be a barrier to successful treatment.360 Anemia due to ribavirin can deteriorate ischemic heart or pulmonary diseases in patients with existing cardiac or pulmonary diseases.360 Immediate dose reduction by 200 mg should be considered when hemoglobin level decreases to under 10 g/dL and drug discontinuation should be considered in case of anemia with under 8.5 g/dL hemoglobin, but re-administration of the reduced dose is possible when anemia improves. Recombinant erythropoietin can be used in case of severe anemia, not to stop ribavirin or to prevent dose reduction, although the evidence of erythropoietin raising SVR rate is lacking.337,361 Meanwhile, it is apprehended that ribavirin causes congenital deformity during pregnancy, therefore thorough contraception is essential during treatment and for 6 months after treatment for both male and female patients.362 Other adverse effects related to ribavirin include fatigue, pruritus, rashes, sinusitis, and gout.
Educating patients on treatment related adverse effects and their management helps to maintain the therapy. Detection of the adverse reactions during the first 2-4 weeks of the treatment is important and monitoring at 4-12 week intervals thereafter is required even if the patients seem to tolerate the antiviral therapy well.
[Recommendations]
30. A pretreatment evaluation of depression, cardiac and pulmonary diseases, hypertension, diabetes mellitus, thyroid diseases, or anemia is needed to monitor adverse effects of treatment (B1).
31. Monitoring of adverse effects at 2-4 week interval after the initiation of the treatment and thereafter at 4-12 week intervals during the treatment (C1).
32. When absolute neutrophil count decreases to <750/mm3 or platelet count decreases to <50,000/mm3, dose reduction of peginterferon alpha should be considered; when absolute neutrophil count decreases to <500/mm3 or platelet count decreases to <25,000/mm3, discontinuation of peginterferon alpha should be considered. Later, re-administration of peginterferon alpha with reduced dose can be considered following adequate recovery of absolute neutrophil count and platelet count and continuous monitoring of those counts is needed (C2).
33. Dose reduction of ribavirin should be considered when anemia with hemoglobin level <10 g/dL occurs and discontinuation of ribavirin should be considered in case of hemoglobin level <8.5 g/dL. Later, when anemia improves, re-administration of ribavirin with reduced dose is possible and continuous monitoring of hemoglobin level is needed (C2).
34. Monitoring of TSH and free thyroxine levels at 2-4-month intervals is recommended to investigate the occurrence of thyroid abnormality (C1).
35. Appropriate management is needed when depression develops during the antiviral treatment and antiviral treatment should be halted in case of severe depression (C1).
MONITORING AFTER THE END OF TREATMENT
Continuous observation of undetectable HCV RNA after reaching SVR can be regarded as complete eradication of HCV. Re-infection of HCV is possible even after reaching SVR, mainly involving IVDU.363,364,365,366 Therefore, follow-up is needed to check re-infection or relapse of HCV after reaching SVR. A risk of HCC remained even after reaching SVR in case of accompanying cirrhosis or advanced hepatic fibrosis prior to the treatment.214 In these patients, monitoring for HCC according to the surveillance strategy and management of general complications of cirrhosis are needed. If SVR is not achieved, the incidence of HCC and progress of the disease is significantly higher compared to the cases with SVR,214,367 and continuous management of chronic hepatitis is necessary in cases without SVR.
[Recommendations]
36. Continuous observation of undetectable HCV RNA after reaching SVR can be regarded as a complete eradication of HCV (C1).
37. A risk of hepatocellular carcinoma or complication of chronic liver disease still exist even after achieving SVR in patients with cirrhosis or advanced hepatic fibrosis, and continuous management and surveillance following the strategies for chronic liver diseaseare needed (B1).
38. If SVR is not achieved, continuous management of chronic liver disease is necessary (B1).
TREATMENT OF SPECIAL POPULATIONS
Considering that clinical trials on patients in specific medical conditions have many limitations, antiviral treatment in these populations should be individualized.
CIRRHOSIS
Treatment of patients with compensated cirrhosis
Compensated cirrhosis has a high probability of progression to decompensated cirrhosis or development of HCC. Thus, antiviral treatment is strongly recommended unless there are absolute contraindications. The acquisition of SVR in patients with cirrhosis or advanced hepatic fibrosis leads to the reduction of liver disease-related mortality and incidence of HCC.211,214,368,369 However, SVR rate of a combination therapy with peginterferon alpha plus ribavirin is significantly lower in patients with cirrhosis or advanced hepatic fibrosis compared to those patients with mild fibrosis. A Korean study reported SVR rates of 20.8% in genotype 1 HCV infected patients and 52.6% in genotype 2 HCV infected patients with Child-Turcotte-Pugh (CTP) class A cirrhosis.370 Also, meticulous monitoring and management of complications are necessary, since cirrhotic patients are usually elderly and treatment-related complications occur more frequently. Cirrhotic patients have low neutrophil and platelet counts due to portal hypertension and splenomegaly, so hematological problems including anemia, neutropenia, or thrombocytopenia often occur during treatment. Therefore, growth factors such as recombinant erythropoietin or G-CSF could be helpful overcoming these complications.371 Triple therapy using boceprevir or telaprevir has tended to produce a higher SVR rate than that of the standard combination therapy and a 48-week triple therapy resulted in higher SVR rate than that of 'response-guided therapy' in genotype 1 HCV-infected compensated cirrhotic patients.258,291,372 Therefore, the therapeutic efficacy of cirrhotic patients should be improved in the upcoming DAA era. Continuous monitoring for complications of cirrhosis is needed, even after reaching SVR, since there is still a possibility of development of HCC.
Treatment of patients with decompensated cirrhosis
Therapeutic outcome in decompensated cirrhotic patients is poorer with more frequent treatment-related complications compared to compensated cirrhosis. A small study (n=10, 8 genotype 1 HCV patients, 2 genotype non-1 HCV patients) reported A SVR rate of 20.0% in treating decompensated cirrhotic patients by a combination therapy with peginterferon alpha plus ribavirin.373 Therefore, antiviral treatment of CTP class B cirrhotic patients can be tried by experienced specialists with careful monitoring. Good therapeutic outcome is expected in case of low HCV RNA concentration, or HCV genotype 2 or 3. Although drug administration can be started with standard doses, more than half of the patients experience drug discontinuation or dose reduction. Thus, a careful approach starting with low accelerated dose regimen(starting with 90 µg/week of peginterferon alpha-2a or 0.5 µg/kg/week of peginterferon alpha-2b and 600 mg/day of ribavirin, and gradual increase of dose every 2 weeks up to the maximum tolerable dose)374 might be helpful.
The current standard treatment regimen is contraindicated in patients of CTP class C due to the likely possibility of severe complications including death.374 Efficacy and safety of DAAs have not been proven yet in treating decompensated cirrhosis.
[Recommendations]
39. Antiviral treatment is strongly recommended in CTP class A patients unless there are absolute contraindications, since HCV eradication decreases the risk of long-term complications, such as progression to decompensated cirrhosis or development of hepatocellular carcinoma (A1).
40. Meticulous monitoring and management of treatment-related complications are necessary, since cirrhotic patients often have hematological problems due to portal hypertension and splenomegaly (A2). Growth factors can be helpful to overcome these complications (C2).
41. Continuous monitoring for appearance of cirrhosis-related complications and HCC is needed even after reaching SVR in cirrhotic patients (B1).
42. Antiviral treatment of CTP class B cirrhotic patients can be tried by experienced specialists with careful and meticulous monitoring (C2). Treatment can be started with standard doses or low doses of peginterferon alpha and ribavirin (C2).
43. The current standard treatment regimen is contraindicated in patients of CTP class C due to the likely possibility of severe complications including death (C1).
LIVER TRANSPLANTATION AND OTHER ORGAN TRANSPLANTS
Treatment following liver transplantation
Patients with HCV infection at the time of liver transplantation have higher graft failure rate (hazard ratio (HR), 1.30; 95% CI, 1.21-1.39) and mortality rate (HR, 1.23; 95% CI, 1.12-1.35) compared to patients without HCV infection.375 HCV reinfection occurs within several hours after transplantation in most of patients with detectable HCV RNA at the time of the transplantation.376 HCV-related liver diseases rapidly deteriorate following liver transplantation and around one-third of the patients progress to cirrhosis within 5 years after transplantation.375,377 Successful elimination of HCV after transplantation improves survival rate of the graft and patients.378
Treatment of HCV reinfection is recommended after histological confirmation of chronic hepatitis C at least 6 months after transplantation. The reason for this 6-month interval is that shortly after transplantation patients are heavily immunosuppressed and incompletely recovered from the surgery, resulting in high probability of drug intolerability as well as allograft rejection during interferon use. Antiviral treatment should be started as soon as possible when advanced fibrosis (numerous septa without cirrhosis) or portal hypertension is noted, since these conditions predict a rapid progression of liver diseases and graft failure.379,380 In case of fibrosis limited to the portal tract without portal hypertension, antiviral treatment should be determined in consideration of treatment efficacy and risk of treatment-related complications.
The SVR rate of antiviral treatment after transplantation is 30-40%, and genotype 2 or 3 has better therapeutic outcome compared to genotype 1.378,381,382 Post-transplant patients reportedly show similar therapeutic outcomes (33% and 38% of SVR rates in each therapy) using either peginterferon alpha plus ribavirin combination therapy or peginterferon alpha monotherapy, which may be explained by frequent dose reduction or discontinuation of ribavirin due to complications.383 Anemia is the most common cause of treatment discontinuation and recombinant erythropoietin is recommended in this case.381,382 Allograft rejection can occur related to interferon alpha use, and liver biopsy is required to differentiate the cause of liver function deterioration during antiviral treatment. The on-treatment virological response of triple therapy using boceprevir or telaprevir in patients with recurrent chronic hepatitis C after liver transplantation is reported to be 50-60% at week 24 of treatment.384
Treatment following other organ transplants
Patients of renal transplant with HCV infection display rapidly progressing hepatic fibrosis and show high mortality related to hepatic failure. Thus, the presence of cirrhosis must be screened for prior to kidney transplantation.385 Sometimes liver biopsy is necessary to confirm cirrhosis. As hemorrhagic tendency exists in patients with end stage kidney disease (ESKD) due to platelet dysfunction, there is a concern that liver biopsy may cause procedure-related hemorrhagic complications. However, severe complications related to percutaneous liver biopsy have been rarely reported in patients with ESKD and the incidence of complications was not significantly higher than that of patients with normal renal function.386 Administration of 0.3 µg/kg desmopressin (1-deamino-8-D-arginine vasopressin, DDAVP) can be used prior to liver biopsy when hemorrhagic complications are concerned.387
The combination therapy with peginterferon alpha plus ribavirin causes graft rejection in over 30% of patients, leading to graft failure and death. Thus, use of interferon-including regimen is an absolute contraindication in renal transplant patients except life-threatening fibrosing cholestatic hepatitis. Fundamentally, treatment of HCV is recommended prior to renal transplantation.388
There have been two successful cases of anti-HCV treatment using interferon in heart transplant setting.389 However, treatment of HCV in heart transplant patients is not recommended except life-threatening fibrosing cholestatic hepatitis because of limited information. There are no available data on the situation of transplants of lung, pancreas, small intestine, or cornea.
[Recommendations]
44. Treatment of HCV reinfection after liver transplantation is recommended with histological confirmation of chronic hepatitis C (B1). Antiviral treatment should be started as soon as possible when advanced fibrosis or portal hypertension is noted, since these conditions predict a rapid progression of liver diseases and graft failure (B2). Treatment regimen could be either combination therapy with peginterferon alpha plus ribavirin or monotherapy with peginterferon alpha (B2).
45. Liver biopsy is often required to differentiate causes of liver function deterioration during antiviral treatment (C1).
46. Use of interferon based regimen is absolutely contraindicated in kidney, heart, and lung transplants except in life-threatening fibrosing cholestatic hepatitis (C1).
PATIENTS RECEIVING IMMUNOSUPPRESSANTS OR CYTOTOXIC CHEMOTHERAPY
There is no universal consensus on definition of HCV reactivation, although the commonly used criteria include blood level of ALT and HCV RNA. One study defined HCV reactivation as reemergence or increase of HCV RNA plus elevation of ALT up to 3 times of the upper normal limit.390
The incidence of HCV reactivation in patients taking immunosuppressants or under cytotoxic chemotherapy is lower compared to that of HBV.390,391,392,393,394 For example, the reactivation rate of HCV was 0% (0 of 11) compared to 38% (3 of 8) of HBV in a study including 98 non-Hodgkin's lymphoma patients receiving chemotherapy.395 However, another study of B cell non-Hodgkin's lymphoma reported higher incidence (26.3% vs. 2.1%) of significantly elevated ALT in HCV infected patients compared to patients without HCV infection, indicating that HCV reactivation does occur and may cause clinically significant morbidity.396
Risk factors predicting HCV reactivation have not clearly identified. However reactivation has been reported to occur more frequently in patients with hematological malignancies.392,397 HCV reactivation has also been reported in patients with solid cancer or stem cell transplantation.398,399,400,401 Although death due to HCV reactivation has rarely been documented,402 the mortality is similar to that of HBV once severe hepatitis occurs with HCV reactivation.403,404,405
Strategies to prevent HCV reactivation in these patients have not been established. Conservative therapy and discontinuation of offending drugs are currently recommended options. However, one should take into account hepatic morbidity from HCV reactivation and disadvantages from immunosuppressive drug discontinuation, and decisions should be individualized. Further studies are needed to explore how to prevent and treat HCV reactivation by using DAAs.
TREATMENT OF ACTIVE INTRAVENOUS DRUG USERS
Intravenous drug abuse is the main route of HCV transmission and the abusers show significantly higher HCV infection rate compared to those without a history of drug abuse.114,406 Anti-HCV positive rate of Korean intravenous drug users has been reported as 48.4-79.2%.22,24,407 Meanwhile, high HCV infection rate also has been reported in case of sharing cocaine inhalation tubes.25
A meta-analysis including over 2,800 injection drug users showed SVR rates as 44.9% in HCV genotype 1 and 70.0% in HCV genotype 2 and 3 patients treated by combination therapy of peginterferon alpha and ribavirin.408
The standard therapy is recommended only to the patients with a history of drug abuse but not for those that are actively using ilicit drugs. Active drug users often show low willingness for HCV treatment, diminished ability to adhere to the treatment or abide by precautions regarding contraception, and higher possibilities of re-infection due to reuse of IV drugs. Therefore, it is important to clearly evaluate willingness of each patients for antiviral treatment and suspension from abusing ilicit drugs for 6-12 months is usually needed despite of weak evidence supporting it.125 Multidisciplinary cooperative treatment among medical and psychiatric counseling services, and social support showed a significant decrease of treatment interruption rate408 and resulted in cost-effectiveness by inhibition of disease progression.409
[Recommendations]
47. Multidisciplinary cooperative treatment among medical and psychiatric counseling services and social support by specialists about drug abuse and improvement of social environment can raise degree of compliance to treatment in intravenous drug users (B2).
TREATMENT OF PATIENTS WITH CHRONIC KIDNEY DISEASES
HCV infection rate is high in chronic kidney disease patients. Yet, anti-HCV screening may not be needed for these patients. Screening should be selectively conducted when HCV-related glomerulonephritis clinically presenting as hematuria, albuminuria, or cryoglobulinemia is suspected. However, anti-HCV should be tested for in patients taking maintenance dialysis for the first time or transferred from other dialysis units. In addition, when unexplained abnormal liver-related biochemical tests is found or HCV exposure is suspected, anti-HCV should be tested and HCV RNA assay is needed in case of continuous negative detection of anti-HCV antibody.378 HCV prevalence among the patients taking dialysis is reported variously ranging from 3-80% depending on location;410 the prevalence rate in South Korea is reported as 5-15%.26,27 The optimal surveillance interval for HCV infection in anti-HCV negative patients in dialysis unit should be between 6-12 months, considering the HCV infection rate of the dialysis unit where the patients are taken care of. Dialysis patients infected with HCV show higher mortality rate and rapid progress to cirrhosis or hepatocellular carcinoma compared to non-dialysis patients.411,412,413 Patients scheduled for kidney transplantation should receive an anti-HCV assay. Survival rate after the kidney transplantation tends to be low with a possibility of graft rejection when interferon therapy were to be initiated after the transplantation and also with a possibility of increased occurrence of diabetes and membranous nephritis.414,415,416,417,418,419,420 Therefore, interferon based antiviral therapy is recommended when HCV infection is confirmed via HCV RNA assay before impending necessity of kidney transplantation.378
Indications of HCV treatment in chronic kidney disease patients are to be determined considering liver disease condition and therapeutic complications. However, dose adjustment is needed depending on severity of kidney disease, since the clearance of both peginterferon alpha and ribavirin are reduced according to the degree of impaired kidney function. Moreover, ribavirin should be carefully used in case of creatinine clearance under 50 mL/min since ribavirin can cause severe hemolytic anemia.421
Patients with mild kidney disease (glomerular filtration rate (GFR) ≥60 mL/min) can be administered the same dose of the therapeutic drugs as patients without kidney disease. If the patient has severe kidney disease (GFR of 15-59 mL/min), 135 µg of peginterferon alpha-2a or 1 µg/kg of peginterferon alpha-2b along with 200-800 mg/day of ribavirin twice a day with gradual dose increase is recommended.278 Patients on dialysis can take either interferon alpha or peginterferon alpha although combination with ribavirin is not recommended. SVR rate was variable as 7-97% in a study conducted using the combination therapy of peginterferon alpha (135 µg/week) and low-dose ribavirin (200 mg/day) in patients on dialysis, and most studies reported a high rate of treatment discontinuation.
Dose adjustment of new drugs, such as boceprevir and telaprevir, is not necessary since they are metabolized in the liver and eliminated mostly through feces and negligibly through urine.422 However, SVR rates after the treatment with these drugs have not been reported yet in chronic kidney disease patients.
Antiviral therapy for HCV can be conducted in patients having HCV-related cryoglobulinemia or membranous glomerulonephritis. Immunosuppressive therapy or plasma exchange can be done prior to the antiviral treatment in case of nephrotic syndrome or rapid decrease of kidney function among these patients.423,424,425
[Recommendations]
48. Anti-HCV should be tested to plan further treatment and management in patients preparing kidney replacement therapy, such as dialysis or kidney transplantation (B1).
49. HCV RNA should be tested to confirm HCV infection in patients having idiopathic liver diseases despite of negative anti-HCV results or in patients with positive anti-HCV (B1).
50. Combination therapy of peginterferon alpha (135 µg of alpha-2a or 1 µg/kg alpha-2b) and ribavirin (200-800 mg/day) or therapy of interferon alpha and ribavirin can be used in chronic hepatitis C patients with severe kidney disease not undergoing hemodialysis (15-59 mL/min of glomerular filtration rate) (C2).
51. Treatment of HCV in patients on dialysis may be considered with either interferon alpha (2a or 2b, 3,000,000 units, three times a week), or reduced dose of peginterferon alpha (2a, 135 µg/week or 2b, 1 µg/kg/week) (C2).
TREATMENT OF PATIENTS WITH HIV OR HBV COINFECTION
Chronic hepatitis C patients with HIV coinfection
Coinfection rate of HIV and HCV is reported to be 25% in western countries28 and 5.0-6.6% in South Korea.29,30,426 Since the frequency of coinfection is relatively high, all HIV infected patients should receive HCV testing consisting primarily of an anti-HCV assay. However, antibody formation may fail to appear in about 6% of HIV infected patients and a HCV RNA assay should be conducted in patients with idiopathic liver disease and negative anti-HCV.427,428 Chronic hepatitis C patients with risk factors of HIV infection should be tested for HIV.
HIV coinfected patients show rapid progress of liver disease and 2-fold higher incidence rate of cirrhosis compared to HCV monoinfection. Therefore, liver diseases due to HCV is regarded as an important factor affecting morbidity and mortality rate in HIV infected patients, since highly active antiretroviral therapy (HAART) was introduced in 1996.429,430,431 Especially, progression of liver disease speeds up as CD4+ lymphocyte count is lower and immune disorder is more severe.432 Recovery of immune function by antiretroviral therapy can delay the progress of liver disease.433,434,435 Generally, antiretroviral therapy is recommended in HIV/HCV coinfected patients regardless of CD4+ lymphocyte count, since the benefits from antiretroviral therapy are bigger than risk of drug toxicity. However, antiretroviral therapy should be conducted carefully due to high risk of liver toxicity, especially in HIV/HCV coinfected patients with progressed liver disease.436,437 It is desirable to adapt antiretroviral therapy first when CD4+ lymphocyte count is low and to adapt HCV treatment after the recovery of CD4+ lymphocyte count. Meanwhile, antiretroviral therapy can be delayed in patients with a CD4+ lymphocyte count >500 cells/mm3.438
Peginterferon alpha can be used at same dose recommended for treating HCV monoinfection and ribavirin dose can be adjusted depending on body weight (1,000 mg/day for under 75 kg, 1,200 mg/day for over 75 kg),439 regardless of HCV genotype in HIV coinfection. Treatment duration is usually recommended as 48 weeks, regardless of HCV genotype. A shortened duration of therapy down to 24 weeks can be effective in genotype 2 and 3 with RVR, and an extended duration of therapy up to 60-72 weeks can be helpful in genotype 1 and 4 with pEVR and no RVR.114,439,440,441,442,443
SVR rate of the combination therapy in HIV coinfection was reported as 29% in genotype 1 and 62% in genotype 2 and 3.444 Lower SVR rate compared to that of HCV monoinfection may be related to the high blood concentration of HCV RNA in HIV coinfected patients compared to that of HCV monoinfection.444,445,446,447,448
Anemia related to ribavirin is a raising problem in treatment of HIV coinfection and especially it is more frequent and severe in patients taking zidovudine (AZT).449 Ribavirin can deteriorate didanosine (ddI) toxicity by inhibition of inosine-5-monophosphate dehydrogenase and severe lactic acidosis has been reported in patients taking ddI along with ribavirin.450,451,452,453 Therefore, patients receiving AZT and especially ddI should be switched to an equivalent antiretroviral agent before beginning combination therapy containing ribavirin.
SVR rate of triple therapy using boceprevir was 60.7%, which is superior to the 26.5% reported in the dual combination therapy, and triple therapy using telaprevir showed higher SVR rate (74%) compared to 45% in the combination therapy. Adverse effects of the triple therapy in HIV coinfection have been reported to be similar to those occurring in HCV monoinfection although further studies are needed.454 In addition, detailed monitoring is necessary when using the regimens including boceprevir or telaprevir due to possible drug-drug interactions.422
[Recommendations]
52. All HIV infected patients should take anti-HCV test (B1).
53. HCV RNA assay should be conducted in patients having idiopathic liver disease even with negative anti-HCV or in patients with positive anti-HCV (B1).
54. Peginterferon alpha should be used at same dose recommended for treating HCV monoinfection and ribavirin dose can be adjusted depending on body weight for 48 weeks regardless of HCV genotype in HIV co-infected patients (B1).
Chronic hepatitis C patients with HBV coinfection
The number of HBV/HCV coinfected patients worldwide is estimated as 15,000,000,455 and 2.37% of the anti-HCV positive patients are reported to be coinfected with HBV in South Korea.456 A 10-year follow-up study of HCV monoinfected patients reported HCC occurrence rate of 28%, whereas HCV/HBV coinfected patients showed a occurrence rate of 45%, which was significantly higher.457 In addition, risks of severe and fulminant hepatitis increase and the incidence rate of cirrhosis and HCC increases in case of HBV/HCV coinfected patients compared to HBV monoinfection.458,459,460
In patients with HBV/HCV coinfection, blood levels of HCV RNA and HBV DNA representing replicative status of each virus should be evaluated, and if HCV infection is the dominant cause of the liver disease, the same antiviral therapy as for HCV monoinfection is recommended for an SVR rate, similar to that of HCV monoinfection.461,462,463 Reactivation of HBV is possible during or after HCV treatment,456 and administration of oral antiviral agents may be indicated when significant proliferation of HBV is confirmed in this case.464
[Recommendation]
55. After confirming the dominant cause of liver diseases in HBV/HCV coinfection, treatment based on the standard therapy of HCV monoinfection is recommended and oral administration of anti-HBV agents may be indicated when significant proliferation of HBV is confirmed (B1).
TREATMENT OF PATIENTS WITH HEMOPHILIA OR THALASSEMIA
Accompanying HCV infection in patients with hemophilia or thalassemia causes significant increases of morbidity and mortality rates compared to patients without HCV infection.456,457,458,459,460,461,462,463,464,465,466,467,468,469 Therefore, aggressive treatment of HCV infection should be considered and the combination therapy of peginterferon alpha and ribavirin is recommended. Therapeutic outcomes in both HCV infected cases with or without hemophilia were similar and there was no increase in complications regarding bleeding tendency.470 Severe anemia can occur due to ribavirin in thalassemia and up to 30-40% of cases may require blood transfusion to maintain 9-10 g/dL of hemoglobin at 3-4 week intervals. Therefore careful monitoring is required to confirm hematological complications. However, discontinuation of treatment or incidence of other main complications did not increased in these patients.465
[Recommendation]
56. The combination therapy of peginterferon alpha and ribavirin is recommended in treating chronic hepatitis C patients accompanying hemophilia or thalassemia (B1).
TREATMENT OF CHILDREN WITH CHRONIC HEPATITIS C
A Korean study recruiting 2,080 children reported an anti-HCV positive rate of 0.82% in 1998.20 Transfusion of infected blood components or vertical transmission is the most common cause of HCV infection in children,471 although transfusion-related HCV transmission has been rarely reported after the introduction of screening test in 1991 in South Korea. The global HCV infection rate of pregnant women has been reported as 0.49-1.7%.15,16,17 Korean studies including 5,000 pregnant women and another study on 20,000 pregnant women showed anti-HCV positive rate of 0.42-0.44%, where 57-60% of anti-HCV positive pregnant woman resulted in HCV RNA positive.18,19 Transmission of HCV was reported as 1-6.2% during the perinatal period65,67,472,473 and the evidence of lowering the risk of vertical HCV transmission by Cesarean section is weak.474 An anti-HCV assay in children is recommended at over 18 months of age, since maternal antibodies can be delivered to newborns.474,475,476 HCV RNA assay may be performed at 1 or 2 months of age if an earlier diagnosis is desired, although the sensitivity of this assay is as low as 22% at that time; it is desirable to conduct the HCV RNA assay at the age over 6 months when the sensitivity reaches 85%.475,477
Spontaneous recovery is more frequent in children having high tendency of a normal ALT level478 along with slow progress of hepatic fibrosis and, rarely, severe hepatic damage. However, aggressive treatment instead of waiting until the children grow has been suggested, since children usually have regular life cycle and show higher therapeutic compliance. Aggressive treatment is considered in case of continuously elevated serum AST/ALT or when progressed hepatic fibrosis is confirmed by liver biopsy. In addition, treatment can also be considered when serum AST/ALT is normal or fibrosis is mild by liver biopsy since evaluating tools of predicting disease progression are not sufficient in children.479
Although in the past studies on HCV infected children, treatment was limited to interferon alpha monotherapy due to the potential teratogenic effects of ribavirin, higher SVR rates have been reported with the addition of ribavirin to treatment recently.480,481,482,483,484 Therefore, most studies have adapted the combination therapy in children, since this approach is standard in adults. Use of peginterferon alpha in children over 3 years of age was approved in North America and Europe.479 The dose of peginterferon is 60 µg/m2/week for alpha-2b and 180 µg/1.73m2/week for alpha-2a and the dose of ribavirin is 15 mg/kg twice a day. Genotype 1 and 4 patients should be treated for 48 weeks and genotype 2 and 3 patients should be treated for 24 weeks, similar to adults.479 SVR rate after the combination therapy of peginterferon alphaand ribavirin (47-53% in genotype 1 and 80-100% in genotypes 2 and 3) is superior to that of the combination therapy of interferon alpha and ribavirin.482,483,484 Factors predicting SVR include genotype 2 and 3, and HCV RNA titer <600,000 IU/mL.480,483,484 Efficacy and safety of boceprevir or telaprevir have not been proved yet in children <18 years of age.422
[Recommendations]
57. Diagnosis and evaluation of HCV in children should proceed following the similar steps as in adults (B1).
58. Anti-HCV assay in children is recommended at the age over 18 months since maternal antibodies can be delivered to newborns. If an earlier assay is required, HCV RNA assay may be considered after 6 months of age (B2).
59. HCV infected children aged 3-17 years should be considered as appropriate treatment candidates according to the same criteria used in adults (B1).
60. The dose of peginterferon alpha is 60 µg/1.73 m2/week for 2b and 180 µg/1.73 m2/week for 2a and the dose of ribavirin is 15 mg/kg/day. Genotype 1 and 4 patients should be treated for 48 weeks and genotype 2 and 3 patients should be treated for 24 weeks (B1).
Footnotes
The authors have no conflicts to disclose.
References
- 1.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh DJ, Jeong SH. Current status of hepatitis C virus infection in Korea. Intervirology. 2006;49:70–75. doi: 10.1159/000087266. [DOI] [PubMed] [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Pai CH, Chi HS, Kim DW, Min YI, Ahn YO. Prevalence of hepatitis C virus antibody among Korean adults. J Korean Med Sci. 1992;7:333–336. doi: 10.3346/jkms.1992.7.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong TH, Jeon TH. PCR prevalence and risk factors of hepatitis C virus infection in the adult population of Ulsan. J Korean Acad Fam Med. 1998;19:364–373. [Google Scholar]
- 7.Na HY, Park MH, Park KS, Sohn YH, Joo YE, Kim SJ. Geographic characteristics of positivity of anti-HCV and Chonnam province: survey data of 6,790 Health screenees. Korean J Gastroenterol. 2001;38:177–184. [Google Scholar]
- 8.Park KS, Lee YS, Lee SG, Hwang JY, Chung WJ, Cho KB, et al. A study on markers of viral hepatitis in adults living in Daegu and Gyungbuk area. Korean J Gastroenterol. 2003;41:473–479. [Google Scholar]
- 9.Seo WT, Lee SS. A study on positive rate of HBsAg, HBsAb and anti-HCV in Korean adults. Korean J Blood Transfus. 1998;9:259–272. [Google Scholar]
- 10.Shin HR. Epidemiology of hepatitis C virus in Korea. Intervirology. 2005;49:18–22. doi: 10.1159/000087258. [DOI] [PubMed] [Google Scholar]
- 11.Kim do Y, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013;33:586–594. doi: 10.1111/liv.12108. [DOI] [PubMed] [Google Scholar]
- 12.Oh HB, Hwang YS, Cho YJ, Kim DS, Kim SI. Experience of anti-HCV antibody immunoblot test in Korean blood donors. Korean J Blood Transfus. 1997;8:1–8. [Google Scholar]
- 13.Oh DJ, Park YM, Seo YI, Lee JS, Lee JY. Prevalence of hepatitis C virus infections and distribution of hepatitis C virus genotypes among Korean blood donors. Ann Lab Med. 2012;32:210–215. doi: 10.3343/alm.2012.32.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MJ, Park Q, Min HK, Kim HO. Residual risk of transfusion-transmitted infection with human immunodeficiency virus, hepatitis C virus, and hepatitis B virus in Korea from 2000 through 2010. BMC Infect Dis. 2012;12:160. doi: 10.1186/1471-2334-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriya T, Sasaki F, Mizui M, Ohno N, Mohri H, Mishiro S, et al. Transmission of hepatitis C virus from mothers to infants: its frequency and risk factors revisited. Biomed Pharmacother. 1995;49:59–64. doi: 10.1016/0753-3322(96)82587-x. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto M, Nagata I, Murakami J, Kaji S, Iitsuka T, Hoshika T, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511–1514. doi: 10.1086/315883. [DOI] [PubMed] [Google Scholar]
- 17.Claret G, Noguera A, Esteva C, Munoz-Almagro C, Sanchez E, Fortuny C. Mother-to-child transmission of hepatitis C virus infection in Barcelona, Spain: a prospective study. Eur J Pediatr. 2007;166:1297–1299. doi: 10.1007/s00431-006-0392-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim YW, Lee JM, Kim GJ, Lee HM, Kim SY, Lee JS, et al. Hepatitis C virus infection in pregnancy. Korean J Obstet Gynecol. 2000;43:597–603. [Google Scholar]
- 19.Kang MJ, Kim HJ, Park KJ, Kang KH, Ahn HS. Prevalence of HCV infection in pregnant woman and vertical transmission. Korean J Obstet Gynecol. 2004;47:2045–2050. [Google Scholar]
- 20.Lee JM, Lee JM, Yoo HS, Jang UK, Kim DJ, Kim YB, et al. The prevalence of anti-HCV positivity in healthy Korean children. Korean J Hepatol. 1996;2:160–165. [Google Scholar]
- 21.Kim HS, Choo DH. Prevalence of hepatitis C, B and human immunodeficiency virus among drug users and chronic alcoholic patients in Korea. Korean J Med. 1997;52:754–762. [Google Scholar]
- 22.Lee SW, Kim SY, Kim JK. Seropositivity of anti-HCV in intravenous drug abusers. J Korean Acad Fam Med. 1997;18:1508–1518. [Google Scholar]
- 23.Yun H, Kim D, Kim S, Kang S, Jeong S, Cheon Y, et al. High prevalence of HBV and HCV infection among intravenous drug users in Korea. J Med Virol. 2008;80:1570–1575. doi: 10.1002/jmv.21255. [DOI] [PubMed] [Google Scholar]
- 24.Min JA, Yoon Y, Lee HJ, Choi J, Kwon M, Kim K, et al. Prevalence and associated clinical characteristics of hepatitis B, C, and HIV infections among injecting drug users in Korea. J Med Virol. 2013;85:575–582. doi: 10.1002/jmv.23523. [DOI] [PubMed] [Google Scholar]
- 25.Macias J, Palacios RB, Claro E, Vargas J, Vergara S, Mira JA, et al. High prevalence of hepatitis C virus infection among noninjecting drug users: association with sharing the inhalation implements of crack. Liver Int. 2008;28:781–786. doi: 10.1111/j.1478-3231.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Kim KT, Yoo JH, Kim BI, Lee SJ, Lee EJ, et al. Prevalence and risk factors of hepatitis C virus infectioin in chronic hemodialysis patients (multi-center study) Korean J Med. 1997;52:833–840. [Google Scholar]
- 27.Shin YH, Kim HK, Choi SD, Kim YS, Shin HS, Won YJ, et al. Prevalence of anti-HCV in hemodialysis patients in Taegu and Kyeongbuk, Korea. Korean J Med. 1998;54:640–646. [Google Scholar]
- 28.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS clinical trials group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 29.Kim O, Kim SS, Park MS, Suh SD, Lee MW, Kim KS, et al. Seroprevalence of sexually transmitted viruses in Korean populations including HIV-seropositive individuals. Int J STD AIDS. 2003;14:46–49. doi: 10.1258/095646203321043264. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Kim KH, Lee SG, Chen DH, Jung DS, Moon CS, et al. Trends of mortality and cause of death among HIV-infected patients in Korea, 1990-2011. J Korean Med Sci. 2013;28:67–73. doi: 10.3346/jkms.2013.28.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SY, Kook JH, Choi IS, Kim SJ, Kook H, Hwang TJ. Viral hepatitis and change of lymphocyte subpopulation in hemophiliacs in Chonnam Kwangju area. Korean J Blood Transfus. 2002;13:43–51. [Google Scholar]
- 32.Korea Hemophilia Association. 2012 Korean hemophilia annual report. Seoul: Korea Hemophilia Association; 2012. [Google Scholar]
- 33.Choi SH. The prevalence of hepatitis C virus infection in leprous patients. Korean J Gastroenterol. 1997;30:486–494. [Google Scholar]
- 34.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 35.Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Arch Intern Med. 2011;171:242–248. doi: 10.1001/archinternmed.2010.511. [DOI] [PubMed] [Google Scholar]
- 36.Torre GL, Gualano MR, Semyonov L, Nicolotti N, Ricciardi W, Boccia A. Hepatitis C virus infections trends in Italy, 1996-2006. Hepat Mon. 2011;11:895–900. doi: 10.5812/kowsar.1735143X.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh HB, Hwang YS, Kim DS, Kim SI, Lee SY, Han KS. Study on the seroincidence of hepatitic C virus infection among blood donors in Korea. Korean J Blood Transfus. 1997;8:33–41. [Google Scholar]
- 38.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148–162. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 39.Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007;42:513–521. doi: 10.1007/s00535-007-2064-6. [DOI] [PubMed] [Google Scholar]
- 40.Shin HR, Kim JY, Kim JI, Lee DH, Yoo KY, Lee DS, et al. Hepatitis B and C virus prevalence in a rural area of South Korea: the role of acupuncture. Br J Cancer. 2002;87:314–318. doi: 10.1038/sj.bjc.6600436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho EJ, Jeong SH, Han BH, Lee SU, Yun BC, Park ET. Hepatitis C virus (HCV) genotypes and the influence of HCV subtype 1b on the progression of chronic hepatitis C in Korea: a single center experience. Clin Mol Hepatol. 2012;18:219–224. doi: 10.3350/cmh.2012.18.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142–1154. doi: 10.1016/j.jhep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Hnatyszyn HJ. Chronic hepatitis C and genotyping: the clinical significance of determining HCV genotypes. Antivir Ther. 2005;10:1–11. [PubMed] [Google Scholar]
- 44.Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, et al. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45:254–264. doi: 10.1111/j.1537-2995.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 45.Vermeulen M, Lelie N, Sykes W, Crookes R, Swanevelder J, Gaggia L, et al. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion. 2009;49:1115–1125. doi: 10.1111/j.1537-2995.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 46.Shan H, Ren FR, Zhao HY, Zhang YZ, Wen GX, Yao FZ, et al. A multi-Chinese blood center study testing serologic-negative donor samples for hepatitis C virus and human immunodeficiency virus with nucleic acid testing. Transfusion. 2007;47:2011–2016. doi: 10.1111/j.1537-2995.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 47.Papatheodoridis G, Hatzakis A. Public health issues of hepatitis C virus infection. Best Pract Res Clin Gastroenterol. 2012;26:371–380. doi: 10.1016/j.bpg.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 50.Deterding K, Wiegand J, Gruner N, Hahn A, Jackel E, Jung MC, et al. The German Hep-Net acute hepatitis C cohort: impact of viral and host factors on the initial presentation of acute hepatitis C virus infection. Z Gastroenterol. 2009;47:531–540. doi: 10.1055/s-0028-1109149. [DOI] [PubMed] [Google Scholar]
- 51.Gutelius B, Perz JF, Parker MM, Hallack R, Stricof R, Clement EJ, et al. Multiple clusters of hepatitis virus infections associated with anesthesia for outpatient endoscopy procedures. Gastroenterology. 2010;139:163–170. doi: 10.1053/j.gastro.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 52.Hayes MO, Harkness GA. Body piercing as a risk factor for viral hepatitis: an integrative research review. Am J Infect Control. 2001;29:271–274. doi: 10.1067/mic.2001.114402. [DOI] [PubMed] [Google Scholar]
- 53.Ernst E, Sherman KJ. Is acupuncture a risk factor for hepatitis? Systematic review of epidemiological studies. J Gastroenterol Hepatol. 2003;18:1231–1236. doi: 10.1046/j.1440-1746.2003.03135.x. [DOI] [PubMed] [Google Scholar]
- 54.Jafari S, Copes R, Baharlou S, Etminan M, Buxton J. Tattooing and the risk of transmission of hepatitis C: a systematic review and meta-analysis. Int J Infect Dis. 2010;14:e928–e940. doi: 10.1016/j.ijid.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(Suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 56.Tomkins SE, Elford J, Nichols T, Aston J, Cliffe SJ, Roy K, et al. Occupational transmission of hepatitis C in healthcare workers and factors associated with seroconversion: UK surveillance data. J Viral Hepat. 2012;19:199–204. doi: 10.1111/j.1365-2893.2011.01543.x. [DOI] [PubMed] [Google Scholar]
- 57.Puro V, Petrosillo N, Ippolito G Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Am J Infect Control. 1995;23:273–277. doi: 10.1016/0196-6553(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 58.Lanphear BP, Linnemann CC, Jr, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol. 1994;15:745–750. doi: 10.1086/646851. [DOI] [PubMed] [Google Scholar]
- 59.Ryoo SM, Kim WY, Kim W, Lim KS, Lee CC, Woo JH. Transmission of hepatitis C virus by occupational percutaneous injuries in South Korea. J Formos Med Assoc. 2012;111:113–117. doi: 10.1016/j.jfma.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52:1497–1505. doi: 10.1002/hep.23808. [DOI] [PubMed] [Google Scholar]
- 61.Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect. 2012;88:558–564. doi: 10.1136/sextrans-2012-050566. [DOI] [PubMed] [Google Scholar]
- 62.Prasad MR, Honegger JR. Hepatitis C virus in pregnancy. Am J Perinatol. 2013;30:149–159. doi: 10.1055/s-0033-1334459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology. 2002;36(Suppl 1):S106–S113. doi: 10.1053/jhep.2002.36792. [DOI] [PubMed] [Google Scholar]
- 64.Indolfi G, Resti M. Perinatal transmission of hepatitis C virus infection. J Med Virol. 2009;81:836–843. doi: 10.1002/jmv.21437. [DOI] [PubMed] [Google Scholar]
- 65.European Paediatric Hepatitis C Virus Network. A significant sex--but not elective cesarean section--effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872–1879. doi: 10.1086/497695. [DOI] [PubMed] [Google Scholar]
- 66.Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, Alavian SM. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255–260. doi: 10.1007/s00404-010-1588-9. [DOI] [PubMed] [Google Scholar]
- 67.European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. European Paediatric Hepatitis C Virus Network. BJOG. 2001;108:371–377. [PubMed] [Google Scholar]
- 68.Indolfi G, Nesi A, Resti M. Intrafamilial transmission of hepatitis C virus. J Med Virol. 2013;85:608–614. doi: 10.1002/jmv.23522. [DOI] [PubMed] [Google Scholar]
- 69.Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, et al. Clinical and epidemiological features of hepatitis C virus infection in South Korea: A prospective, multicenter cohort study. J Med Virol. 2013;85:1724–1733. doi: 10.1002/jmv.23661. [DOI] [PubMed] [Google Scholar]
- 70.Farci P, Alter HJ, Wong D, Miller RH, Shih JW, Jett B, et al. A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med. 1991;325:98–104. doi: 10.1056/NEJM199107113250205. [DOI] [PubMed] [Google Scholar]
- 71.Maasoumy B, Wedemeyer H. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol. 2012;26:401–412. doi: 10.1016/j.bpg.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Corey KE, Mendez-Navarro J, Gorospe EC, Zheng H, Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17:201–207. doi: 10.1111/j.1365-2893.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol. 2008;49:625–633. doi: 10.1016/j.jhep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 75.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 76.Tremolada F, Casarin C, Alberti A, Drago C, Tagger A, Ribero ML, et al. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol. 1992;16:273–281. doi: 10.1016/s0168-8278(05)80657-9. [DOI] [PubMed] [Google Scholar]
- 77.Lehmann M, Meyer MF, Monazahian M, Tillmann HL, Manns MP, Wedemeyer H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J Med Virol. 2004;73:387–391. doi: 10.1002/jmv.20103. [DOI] [PubMed] [Google Scholar]
- 78.Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, Steindl-Munda P, et al. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology. 2003;37:60–64. doi: 10.1053/jhep.2003.50019. [DOI] [PubMed] [Google Scholar]
- 79.Kim KA, Lee JS, Yang JH, Moon YS, Lee WJ. Natural history of acute symptomatic hepatitis C in Korea. Korean J Gastroenterol. 2005;46:105–109. [PubMed] [Google Scholar]
- 80.Kim JY, Won JE, Jeong SH, Park SJ, Hwang SG, Kang SK, et al. Acute hepatitis C in Korea: different modes of infection, high rate of spontaneous recovery, and low rate of seroconversion. J Med Virol. 2011;83:1195–1202. doi: 10.1002/jmv.22100. [DOI] [PubMed] [Google Scholar]
- 81.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586–1592. doi: 10.1053/j.gastro.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Rao HY, Sun DG, Jiang D, Yang RF, Guo F, Wang JH, et al. IL28B genetic variants and gender are associated with spontaneous clearance of hepatitis C virus infection. J Viral Hepat. 2012;19:173–181. doi: 10.1111/j.1365-2893.2011.01497.x. [DOI] [PubMed] [Google Scholar]
- 84.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 85.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 86.Poynard T, Bedossa P, Opolon P The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 87.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 89.Gomez EV, Rodriguez YS, Bertot LC, Gonzalez AT, Perez YM, Soler EA, et al. The natural history of compensated HCV-related cirrhosis: a prospective long-term study. J Hepatol. 2013;58:434–444. doi: 10.1016/j.jhep.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 90.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 91.Sinn DH, Paik SW, Kil JS, Kang P, Song SM, Gwak GY, et al. Incidence and risk factors for disease progression in Korean patients with chronic hepatitis C [Abstract] Korean J Hepatol. 2007;13(Suppl 3):S19. [Google Scholar]
- 92.Zarski JP, Mc Hutchison J, Bronowicki JP, Sturm N, Garcia-Kennedy R, Hodaj E, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307–314. doi: 10.1016/s0168-8278(02)00387-2. [DOI] [PubMed] [Google Scholar]
- 93.Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med. 2001;134:120–124. doi: 10.7326/0003-4819-134-2-200101160-00012. [DOI] [PubMed] [Google Scholar]
- 94.Wiley TE, McCarthy M, Breidi L, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- 95.Noda K, Yoshihara H, Suzuki K, Yamada Y, Kasahara A, Hayashi N, et al. Progression of type C chronic hepatitis to liver cirrhosis and hepatocellular carcinoma--its relationship to alcohol drinking and the age of transfusion. Alcohol Clin Exp Res. 1996;20(Suppl):95A–100A. doi: 10.1111/j.1530-0277.1996.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 96.Ortiz V, Berenguer M, Rayon JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 97.Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 98.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 99.Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036–3043. doi: 10.1002/cncr.11427. [DOI] [PubMed] [Google Scholar]
- 100.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 101.Imazeki F, Yokosuka O, Fukai K, Hiraide A, Saisho H. Significance of prior hepatitis B virus infection in the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci. 2003;48:1786–1792. doi: 10.1023/a:1025459431613. [DOI] [PubMed] [Google Scholar]
- 102.Tsai JF, Jeng JE, Ho MS, Chang WY, Lin ZY, Tsai JH. Independent and additive effect modification of hepatitis C and B viruses infection on the development of chronic hepatitis. J Hepatol. 1996;24:271–276. doi: 10.1016/s0168-8278(96)80004-3. [DOI] [PubMed] [Google Scholar]
- 103.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 104.Pawlotsky JM. Molecular diagnosis of viral hepatitis. Gastroenterology. 2002;122:1554–1568. doi: 10.1053/gast.2002.33428. [DOI] [PubMed] [Google Scholar]
- 105.Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat. 2001;8:87–95. doi: 10.1046/j.1365-2893.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 106.Abdel-Hamid M, El-Daly M, El-Kafrawy S, Mikhail N, Strickland GT, Fix AD. Comparison of second- and third-generation enzyme immunoassays for detecting antibodies to hepatitis C virus. J Clin Microbiol. 2002;40:1656–1659. doi: 10.1128/JCM.40.5.1656-1659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36(Suppl 1):S65–S73. doi: 10.1053/jhep.2002.36815. [DOI] [PubMed] [Google Scholar]
- 108.Pawlotsky JM, Lonjon I, Hezode C, Raynard B, Darthuy F, Remire J, et al. What strategy should be used for diagnosis of hepatitis C virus infection in clinical laboratories. Hepatology. 1998;27:1700–1702. doi: 10.1002/hep.510270632. [DOI] [PubMed] [Google Scholar]
- 109.Stramer SL, Caglioti S, Strong DM. NAT of the United States and Canadian blood supply. Transfusion. 2000;40:1165–1168. doi: 10.1046/j.1537-2995.2000.40101165.x. [DOI] [PubMed] [Google Scholar]
- 110.Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–13. 15. quiz CE11-14. [PubMed] [Google Scholar]
- 111.Moretti M, Pieretti B, Masucci A, Sisti D, Rocchi M, Delprete E. Role of signal-to-cutoff ratios in hepatitis C virus antibody detection. Clin Vaccine Immunol. 2012;19:1329–1331. doi: 10.1128/CVI.00175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee SR, Kardos KW, Schiff E, Berne CA, Mounzer K, Banks AT, et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods. 2011;172:27–31. doi: 10.1016/j.jviromet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 113.Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, et al. The natural history of community-acquired hepatitis C in the United States. N Engl J Med. 1992;327:1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 114.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thio CL, Nolt KR, Astemborski J, Vlahov D, Nelson KE, Thomas DL. Screening for hepatitis C virus in human immunodeficiency virus-infected individuals. J Clin Microbiol. 2000;38:575–577. doi: 10.1128/jcm.38.2.575-577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meyer zum Buschenfelde KH, Gerken G, Manns M. Hepatitis C virus (HCV) and autoimmune liver diseases. Arch Virol Suppl. 1992;4:201–204. doi: 10.1007/978-3-7091-5633-9_42. [DOI] [PubMed] [Google Scholar]
- 117.Dufour DR, Talastas M, Fernandez MD, Harris B, Strader DB, Seeff LB. Low-positive anti-hepatitis C virus enzyme immunoassay results: an important predictor of low likelihood of hepatitis C infection. Clin Chem. 2003;49:479–486. doi: 10.1373/49.3.479. [DOI] [PubMed] [Google Scholar]
- 118.Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA. 2007;297:724–732. doi: 10.1001/jama.297.7.724. [DOI] [PubMed] [Google Scholar]
- 119.Sarrazin C, Teuber G, Kokka R, Rabenau H, Zeuzem S. Detection of residual hepatitis C virus RNA by transcription-mediated amplification in patients with complete virologic response according to polymerase chain reaction-based assays. Hepatology. 2000;32:818–823. doi: 10.1053/jhep.2000.17709. [DOI] [PubMed] [Google Scholar]
- 120.Martinot-Peignoux M, Boyer N, Le Breton V, Le Guludec G, Castelnau C, Akremi R, et al. A new step toward standardization of serum hepatitis C virus-RNA quantification in patients with chronic hepatitis C. Hepatology. 2000;31:726–729. doi: 10.1002/hep.510310324. [DOI] [PubMed] [Google Scholar]
- 121.Pradat P, Chossegros P, Bailly F, Pontisso P, Saracco G, Sauleda S, et al. Comparison between three quantitative assays in patients with chronic hepatitis C and their relevance in the prediction of response to therapy. J Viral Hepat. 2000;7:203–210. doi: 10.1046/j.1365-2893.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- 122.Chevaliez S, Bouvier-Alias M, Pawlotsky JM. Performance of the Abbott real-time PCR assay using m2000sp and m2000rt for hepatitis C virus RNA quantification. J Clin Microbiol. 2009;47:1726–1732. doi: 10.1128/JCM.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vermehren J, Kau A, Gartner BC, Gobel R, Zeuzem S, Sarrazin C. Differences between two real-time PCR-based hepatitis C virus (HCV) assays (RealTime HCV and Cobas AmpliPrep/Cobas TaqMan) and one signal amplification assay (Versant HCV RNA 3.0) for RNA detection and quantification. J Clin Microbiol. 2008;46:3880–3891. doi: 10.1128/JCM.00755-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fytili P, Tiemann C, Wang C, Schulz S, Schaffer S, Manns MP, et al. Frequency of very low HCV viremia detected by a highly sensitive HCV-RNA assay. J Clin Virol. 2007;39:308–311. doi: 10.1016/j.jcv.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 125.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 126.Vermehren J, Yu ML, Monto A, Yao JD, Anderson C, Bertuzis R, et al. Multi-center evaluation of the Abbott RealTime HCV assay for monitoring patients undergoing antiviral therapy for chronic hepatitis C. J Clin Virol. 2011;52:133–137. doi: 10.1016/j.jcv.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 127.Pawlotsky JM, Bouvier-Alias M, Hezode C, Darthuy F, Remire J, Dhumeaux D. Standardization of hepatitis C virus RNA quantification. Hepatology. 2000;32:654–659. doi: 10.1053/jhep.2000.16603. [DOI] [PubMed] [Google Scholar]
- 128.Saldanha J, Lelie N, Heath A WHO Collaborative Study Group. Establishment of the first international standard for nucleic acid amplification technology (NAT) assays for HCV RNA. Vox Sang. 1999;76:149–158. doi: 10.1159/000031040. [DOI] [PubMed] [Google Scholar]
- 129.Chevaliez S, Pawlotsky JM. Hepatitis C virus: virology, diagnosis and management of antiviral therapy. World J Gastroenterol. 2007;13:2461–2466. doi: 10.3748/wjg.v13.i17.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 131.Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79:9369–9380. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguyen TT, Sedghi-Vaziri A, Wilkes LB, Mondala T, Pockros PJ, Lindsay KL, et al. Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection. J Viral Hepat. 1996;3:75–78. doi: 10.1111/j.1365-2893.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 133.Ferreira-Gonzalez A, Shiffman ML. Use of diagnostic testing for managing hepatitis C virus infection. Semin Liver Dis. 2004;24(Suppl 2):9–18. doi: 10.1055/s-2004-832923. [DOI] [PubMed] [Google Scholar]
- 134.Pawlotsky JM, Prescott L, Simmonds P, Pellet C, Laurent-Puig P, Labonne C, et al. Serological determination of hepatitis C virus genotype: comparison with a standardized genotyping assay. J Clin Microbiol. 1997;35:1734–1739. doi: 10.1128/jcm.35.7.1734-1739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 136.Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol. 2008;22:1031–1048. doi: 10.1016/j.bpg.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 137.Smith DB, Mellor J, Jarvis LM, Davidson F, Kolberg J, Urdea M, et al. The International HCV Collaborative Study Group. Variation of the hepatitis C virus 5' non-coding region: implications for secondary structure, virus detection and typing. J Gen Virol. 1995;76:1749–1761. doi: 10.1099/0022-1317-76-7-1749. [DOI] [PubMed] [Google Scholar]
- 138.Park JC, Kim JM, Kwon OJ, Lee KR, Chai YG, Oh HB. Development and clinical evaluation of a microarray for hepatitis C virus genotyping. J Virol Methods. 2010;163:269–275. doi: 10.1016/j.jviromet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 139.Chen Z, Weck KE. Hepatitis C virus genotyping: interrogation of the 5' untranslated region cannot accurately distinguish genotypes 1a and 1b. J Clin Microbiol. 2002;40:3127–3134. doi: 10.1128/JCM.40.9.3127-3134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chevaliez S, Bouvier-Alias M, Brillet R, Pawlotsky JM. Hepatitis C virus (HCV) genotype 1 subtype identification in new HCV drug development and future clinical practice. PLoS One. 2009;4:e8209. doi: 10.1371/journal.pone.0008209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Germer JJ, Rys PN, Thorvilson JN, Persing DH. Determination of hepatitis C virus genotype by direct sequence analysis of products generated with the Amplicor HCV test. J Clin Microbiol. 1999;37:2625–2630. doi: 10.1128/jcm.37.8.2625-2630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Romano KP, Ali A, Royer WE, Schiffer CA. Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc Natl Acad Sci U S A. 2010;107:20986–20991. doi: 10.1073/pnas.1006370107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–822. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moyer VA. Screening for hepatitis C virus infection in adults: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 145.Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakamura J, Terajima K, Aoyagi Y, Akazawa K. Cost-effectiveness of the national screening program for hepatitis C virus in the general population and the high-risk groups. Tohoku J Exp Med. 2008;215:33–42. doi: 10.1620/tjem.215.33. [DOI] [PubMed] [Google Scholar]
- 147.Sroczynski G, Esteban E, Conrads-Frank A, Schwarzer R, Muhlberger N, Wright D, et al. Long-term effectiveness and cost-effectiveness of screening for hepatitis C virus infection. Eur J Public Health. 2009;19:245–253. doi: 10.1093/eurpub/ckp001. [DOI] [PubMed] [Google Scholar]
- 148.Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis. 1997;1:559–568. vi–vii. doi: 10.1016/s1089-3261(05)70321-4. [DOI] [PubMed] [Google Scholar]
- 149.U.S. Public Health Service. Updated U.S. public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50:1–52. [PubMed] [Google Scholar]
- 150.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 151.Mitsui T, Iwano K, Masuko K, Yamazaki C, Okamoto H, Tsuda F, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology. 1992;16:1109–1114. [PubMed] [Google Scholar]
- 152.Kubitschke A, Bahr MJ, Aslan N, Bader C, Tillmann HL, Sarrazin C, et al. Induction of hepatitis C virus (HCV)-specific T cells by needle stick injury in the absence of HCV-viraemia. Eur J Clin Invest. 2007;37:54–64. doi: 10.1111/j.1365-2362.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 153.Kleiner DE. The liver biopsy in chronic hepatitis C: a view from the other side of the microscope. Semin Liver Dis. 2005;25:52–64. doi: 10.1055/s-2005-864781. [DOI] [PubMed] [Google Scholar]
- 154.Bedossa P, Poynard T The METAVIR Cooperative Study Group. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 155.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 156.Park YN, Kim H, Chon CY, Park JB, Sohn JH, Yang SH, et al. Histological grading and staging of chronic hepatitis: standardized guideline proposed by the Korean study group for the pathology of digestive diseases. Korean J Pathol. 1999;33:337–346. [Google Scholar]
- 157.Levine RA, Sanderson SO, Ploutz-Snyder R, Murray F, Kay E, Hegarty J, et al. Assessment of fibrosis progression in untreated Irish women with chronic hepatitis C contracted from immunoglobulin anti-D. Clin Gastroenterol Hepatol. 2006;4:1271–1277. doi: 10.1016/j.cgh.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 158.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398. vi. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 159.Wong JB, Koff RS. Watchful waiting with periodic liver biopsy versus immediate empirical therapy for histologically mild chronic hepatitis C.A cost-effectiveness analysis. Ann Intern Med. 2000;133:665–675. doi: 10.7326/0003-4819-133-9-200011070-00008. [DOI] [PubMed] [Google Scholar]
- 160.Martinot-Peignoux M, Boyer N, Cazals-Hatem D, Pham BN, Gervais A, Le Breton V, et al. Prospective study on anti-hepatitis C virus-positive patients with persistently normal serum alanine transaminase with or without detectable serum hepatitis C virus RNA. Hepatology. 2001;34:1000–1005. doi: 10.1053/jhep.2001.28458. [DOI] [PubMed] [Google Scholar]
- 161.Shiffman ML, Diago M, Tran A, Pockros P, Reindollar R, Prati D, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol. 2006;4:645–652. doi: 10.1016/j.cgh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 162.Boccato S, Pistis R, Noventa F, Guido M, Benvegnu L, Alberti A. Fibrosis progression in initially mild chronic hepatitis C. J Viral Hepat. 2006;13:297–302. doi: 10.1111/j.1365-2893.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 163.Persico M, Persico E, Suozzo R, Conte S, De Seta M, Coppola L, et al. Natural history of hepatitis C virus carriers with persistently normal aminotransferase levels. Gastroenterology. 2000;118:760–764. doi: 10.1016/s0016-5085(00)70145-4. [DOI] [PubMed] [Google Scholar]
- 164.Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–1340. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 165.Fontaine H, Nalpas B, Poulet B, Carnot F, Zylberberg H, Brechot C, et al. Hepatitis activity index is a key factor in determining the natural history of chronic hepatitis C. Hum Pathol. 2001;32:904–909. doi: 10.1053/hupa.2001.28228. [DOI] [PubMed] [Google Scholar]
- 166.Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E, et al. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004;53:406–412. doi: 10.1136/gut.2003.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 168.Olynyk JK, Reddy KR, Di Bisceglie AM, Jeffers LJ, Parker TI, Radick JL, et al. Hepatic iron concentration as a predictor of response to interferon alfa therapy in chronic hepatitis C. Gastroenterology. 1995;108:1104–1109. doi: 10.1016/0016-5085(95)90209-0. [DOI] [PubMed] [Google Scholar]
- 169.Westin J, Lagging M, Dhillon AP, Norkrans G, Romero AI, Pawlotsky JM, et al. Impact of hepatic steatosis on viral kinetics and treatment outcome during antiviral treatment of chronic HCV infection. J Viral Hepat. 2007;14:29–35. doi: 10.1111/j.1365-2893.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 170.Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallee M, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484–490. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 171.Fontana RJ, Israel J, LeClair P, Banner BF, Tortorelli K, Grace N, et al. Iron reduction before and during interferon therapy of chronic hepatitis C: results of a multicenter, randomized, controlled trial. Hepatology. 2000;31:730–736. doi: 10.1002/hep.510310325. [DOI] [PubMed] [Google Scholar]
- 172.Reiss G, Keeffe EB. Role of liver biopsy in the management of chronic liver disease: selective rather than routine. Rev Gastroenterol Disord. 2005;5:195–205. [PubMed] [Google Scholar]
- 173.Crockett SD, Kaltenbach T, Keeffe EB. Do we still need a liver biopsy? Are the serum fibrosis tests ready for prime time? Clin Liver Dis. 2006;10:513–534. viii. doi: 10.1016/j.cld.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 174.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36(Suppl 1):S152–S160. doi: 10.1053/jhep.2002.36381. [DOI] [PubMed] [Google Scholar]
- 175.Cadranel JF, Rufat P, Degos F For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Practices of liver biopsy in France: results of a prospective nationwide survey. Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 176.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 177.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43(Suppl 1):S113–S120. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 178.Poynard T, Ngo Y, Munteanu M, Thabut D, Massard J, Moussalli J, et al. Biomarkers of liver injury for hepatitis clinical trials: a meta-analysis of longitudinal studies. Antivir Ther. 2010;15:617–631. doi: 10.3851/IMP1570. [DOI] [PubMed] [Google Scholar]
- 179.Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, et al. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838–845. doi: 10.1002/hep.20814. [DOI] [PubMed] [Google Scholar]
- 180.Halfon P, Bourliere M, Deydier R, Botta-Fridlund D, Renou C, Tran A, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol. 2006;101:547–555. doi: 10.1111/j.1572-0241.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 181.Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18:23–31. doi: 10.1111/j.1365-2893.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 182.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 183.Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 184.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 185.Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Myers RP, Thabut D, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. doi: 10.1186/1476-5926-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 187.Boursier J, Bacq Y, Halfon P, Leroy V, de Ledinghen V, de Muret A, et al. Improved diagnostic accuracy of blood tests for severe fibrosis and cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21:28–38. doi: 10.1097/MEG.0b013e32830cebd7. [DOI] [PubMed] [Google Scholar]
- 188.Patel K, Benhamou Y, Yoshida EM, Kaita KD, Zeuzem S, Torbenson M, et al. An independent and prospective comparison of two commercial fibrosis marker panels (HCV FibroSURE and FIBROSpect II) during albinterferon alfa-2b combination therapy for chronic hepatitis C. J Viral Hepat. 2009;16:178–186. doi: 10.1111/j.1365-2893.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 189.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 190.Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol. 2000;15:386–390. doi: 10.1046/j.1440-1746.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 191.Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 192.Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 193.Castera L. Transient elastography and other noninvasive tests to assess hepatic fibrosis in patients with viral hepatitis. J Viral Hepat. 2009;16:300–314. doi: 10.1111/j.1365-2893.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- 194.Jung KS, Kim SU. Clinical applications of transient elastography. Clin Mol Hepatol. 2012;18:163–173. doi: 10.3350/cmh.2012.18.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim BK, Fung J, Yuen MF, Kim SU. Clinical application of liver stiffness measurement using transient elastography in chronic liver disease from longitudinal perspectives. World J Gastroenterol. 2013;19:1890–1900. doi: 10.3748/wjg.v19.i12.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380–384. doi: 10.1002/hep.22007. [DOI] [PubMed] [Google Scholar]
- 197.Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592–595. doi: 10.1002/hep.22056. [DOI] [PubMed] [Google Scholar]
- 198.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 199.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 200.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 201.Sebastiani G, Halfon P, Castera L, Pol S, Thomas DL, Mangia A, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1821–1827. doi: 10.1002/hep.22859. [DOI] [PubMed] [Google Scholar]
- 202.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 203.Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008;47:332–342. doi: 10.1002/hep.21972. [DOI] [PubMed] [Google Scholar]
- 204.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 205.Lee YJ, Lee JM, Lee JE, Lee KB, Lee ES, Yoon JH, et al. MR elastography for noninvasive assessment of hepatic fibrosis: Reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging. 2014;39:326–331. doi: 10.1002/jmri.24147. [DOI] [PubMed] [Google Scholar]
- 206.Rino Y, Tarao K, Morinaga S, Ohkawa S, Miyakawa K, Hirokawa S, et al. Reduction therapy of alanine aminotransferase levels prevent HCC development in patients with HCV-associated cirrhosis. Anticancer Res. 2006;26:2221–2226. [PubMed] [Google Scholar]
- 207.Puoti C, Bellis L, Castellacci R, Montagnese F, Bergami N, Petrone De. HCV carriers with persistently normal aminotransferase levels. Hepatology. 2004;40:266–267. doi: 10.1002/hep.20311. author reply 267-268. [DOI] [PubMed] [Google Scholar]
- 208.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 209.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 210.Pradat P, Tillmann HL, Sauleda S, Braconier JH, Saracco G, Thursz M, et al. Long-term follow-up of the hepatitis C HENCORE cohort: response to therapy and occurrence of liver-related complications. J Viral Hepat. 2007;14:556–563. doi: 10.1111/j.1365-2893.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 211.Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105–114. doi: 10.7326/0003-4819-142-2-200501180-00009. [DOI] [PubMed] [Google Scholar]
- 212.Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Maruyama T, et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol. 2013;58:495–501. doi: 10.1016/j.jhep.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 213.Deuffic-Burban S, Deltenre P, Louvet A, Canva V, Dharancy S, Hollebecque A, et al. Impact of viral eradication on mortality related to hepatitis C: a modeling approach in France. J Hepatol. 2008;49:175–183. doi: 10.1016/j.jhep.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 214.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 215.Brillanti S, Foli M, Gaiani S, Masci C, Miglioli M, Barbara L. Persistent hepatitis C viraemia without liver disease. Lancet. 1993;341:464–465. doi: 10.1016/0140-6736(93)90210-8. [DOI] [PubMed] [Google Scholar]
- 216.Nutt AK, Hassan HA, Lindsey J, Lamps LW, Raufman JP. Liver biopsy in the evaluation of patients with chronic hepatitis C who have repeatedly normal or near-normal serum alanine aminotransferase levels. Am J Med. 2000;109:62–64. doi: 10.1016/s0002-9343(00)00381-8. [DOI] [PubMed] [Google Scholar]
- 217.Puoti C, Castellacci R, Montagnese F. Hepatitis C virus carriers with persistently normal aminotransferase levels: healthy people or true patients? Dig Liver Dis. 2000;32:634–643. doi: 10.1016/s1590-8658(00)80850-6. [DOI] [PubMed] [Google Scholar]
- 218.Puoti C, Guido M, Mangia A, Persico M, Prati D. Clinical management of HCV carriers with normal aminotransferase levels. Dig Liver Dis. 2003;35:362–369. doi: 10.1016/s1590-8658(03)00185-3. [DOI] [PubMed] [Google Scholar]
- 219.Puoti C, Magrini A, Stati T, Rigato P, Montagnese F, Rossi P, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology. 1997;26:1393–1398. doi: 10.1053/jhep.1997.v26.pm0009397976. [DOI] [PubMed] [Google Scholar]
- 220.Sinn DH, Gwak GY, Shin JU, Choi MS, Lee JH, Koh KC, et al. Disease progression in chronic hepatitis C patients with normal alanine aminotransferase levels. World J Gastroenterol. 2013;19:2256–2261. doi: 10.3748/wjg.v19.i14.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Arora S, O'Brien C, Zeuzem S, Shiffman ML, Diago M, Tran A, et al. Treatment of chronic hepatitis C patients with persistently normal alanine aminotransferase levels with the combination of peginterferon alpha-2a (40 kDa) plus ribavirin: impact on health-related quality of life. J Gastroenterol Hepatol. 2006;21:406–412. doi: 10.1111/j.1440-1746.2005.04059.x. [DOI] [PubMed] [Google Scholar]
- 222.Bini EJ, Mehandru S. Sustained virological response rates and health-related quality of life after interferon and ribavirin therapy in patients with chronic hepatitis C virus infection and persistently normal alanine aminotransferase levels. Aliment Pharmacol Ther. 2006;23:777–785. doi: 10.1111/j.1365-2036.2006.02819.x. [DOI] [PubMed] [Google Scholar]
- 223.Thabut D, Le Calvez S, Thibault V, Massard J, Munteanu M, Di Martino V, et al. Hepatitis C in 6,865 patients 65 yr or older: a severe and neglected curable disease. Am J Gastroenterol. 2006;101:1260–1267. doi: 10.1111/j.1572-0241.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- 224.Huang CF, Yang JF, Dai CY, Huang JF, Hou NJ, Hsieh MY, et al. Efficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis C. J Infect Dis. 2010;201:751–759. doi: 10.1086/650470. [DOI] [PubMed] [Google Scholar]
- 225.Kim HI, Kim IH, Jeon BJ, Lee S, Kim SH, Kim SW, et al. Treatment response and tolerability of pegylated Interferon-alpha plus ribavirin combination therapy in elderly patients (≥ 65 years) with chronic hepatitis C in Korea. Hepat Mon. 2012;12:430–436. doi: 10.5812/hepatmon.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ikeda K, Arase Y, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, et al. Necessities of interferon therapy in elderly patients with chronic hepatitis C. Am J Med. 2009;122:479–486. doi: 10.1016/j.amjmed.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 227.Arase Y, Ikeda K, Suzuki F, Suzuki Y, Saitoh S, Kobayashi M, et al. Long-term outcome after interferon therapy in elderly patients with chronic hepatitis C. Intervirology. 2007;50:16–23. doi: 10.1159/000096308. [DOI] [PubMed] [Google Scholar]
- 228.Sinn DH, Shin SR, Kil JS, Kim J, Gwak GY, Choi MS, et al. Efficacy of peg-interferon-alpha-2a plus ribavirin for patients aged 60 years and older with chronic hepatitis C in Korea. J Gastroenterol Hepatol. 2011;26:469–476. doi: 10.1111/j.1440-1746.2010.06478.x. [DOI] [PubMed] [Google Scholar]
- 229.Frei P, Leucht AK, Held U, Kofmehl R, Manser CN, Schmitt J, et al. Elderly age is not a negative predictive factor for virological response to therapy with pegylated interferon-α and ribavirin in chronic hepatitis C virus patients. Liver Int. 2014;34:551–557. doi: 10.1111/liv.12279. [DOI] [PubMed] [Google Scholar]
- 230.Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci GL, Carretta V, et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology. 2008;47:43–50. doi: 10.1002/hep.22061. [DOI] [PubMed] [Google Scholar]
- 231.Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954–960. doi: 10.1002/hep.21159. [DOI] [PubMed] [Google Scholar]
- 232.Yu JW, Wang GQ, Sun LJ, Li XG, Li SC. Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J Gastroenterol Hepatol. 2007;22:832–836. doi: 10.1111/j.1440-1746.2007.04904.x. [DOI] [PubMed] [Google Scholar]
- 233.Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, et al. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47:1884–1893. doi: 10.1002/hep.22319. [DOI] [PubMed] [Google Scholar]
- 234.Andriulli A, Mangia A, Iacobellis A, Ippolito A, Leandro G, Zeuzem S. Meta-analysis: the outcome of anti-viral therapy in HCV genotype 2 and genotype 3 infected patients with chronic hepatitis. Aliment Pharmacol Ther. 2008;28:397–404. doi: 10.1111/j.1365-2036.2008.03763.x. [DOI] [PubMed] [Google Scholar]
- 235.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 236.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 237.Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Goncales FL, Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 238.Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–1097. doi: 10.1053/j.gastro.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 239.Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1-infected slow responders. Hepatology. 2007;46:1688–1694. doi: 10.1002/hep.21919. [DOI] [PubMed] [Google Scholar]
- 240.Chen J, Florian J, Carter W, Fleischer RD, Hammerstrom TS, Jadhav PR, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144:1450–1455.e2. doi: 10.1053/j.gastro.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 241.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 242.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 243.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–129. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 244.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 245.Park SH, Park CK, Lee JW, Kim YS, Jeong SH, Kim YS, et al. Efficacy and tolerability of peginterferon alpha plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Korea: a multi-center, retrospective observational study. Gut Liver. 2012;6:98–106. doi: 10.5009/gnl.2012.6.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.von Wagner M, Huber M, Berg T, Hinrichsen H, Rasenack J, Heintges T, et al. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005;129:522–527. doi: 10.1016/j.gastro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 247.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 248.Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, et al. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712–718. doi: 10.1016/j.jhep.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 249.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 250.Mangia A, Thompson AJ, Santoro R, Piazzolla V, Tillmann HL, Patel K, et al. An IL28B polymorphism determines treatment response of hepatitis C virus genotype 2 or 3 patients who do not achieve a rapid virologic response. Gastroenterology. 2010;139:821–827. 827.e1. doi: 10.1053/j.gastro.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 251.Lyoo K, Song MJ, Hur W, Choi JE, Hong SW, Kim CW, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol. 2011;52:363–366. doi: 10.1016/j.jcv.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 252.Jung YK, Kim JH, Ahn SM, Yang JW, Park SJ, Kim JW, et al. Role of interleukin 28B-related gene polymorphisms in chronic hepatitis C and the response to antiviral therapy in Koreans. J Clin Gastroenterol. 2013;47:644–650. doi: 10.1097/MCG.0b013e3182896abf. [DOI] [PubMed] [Google Scholar]
- 253.Jeong SH, Jung YK, Yang JW, Park SJ, Kim JW, Kwon OS, et al. Efficacy of peginterferon and ribavirin is associated with the IL28B gene in Korean patients with chronic hepatitis C. Clin Mol Hepatol. 2012;18:360–367. doi: 10.3350/cmh.2012.18.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 255.McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 256.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 257.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 259.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012;61(Suppl 1):i36–i46. doi: 10.1136/gutjnl-2012-302144. [DOI] [PubMed] [Google Scholar]
- 262.Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 263.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 264.Zeuzem S, Welsch C, Herrmann E. Pharmacokinetics of peginterferons. Semin Liver Dis. 2003;23(Suppl 1):23–28. doi: 10.1055/s-2003-41631. [DOI] [PubMed] [Google Scholar]
- 265.Afdhal NH, McHutchison JG, Zeuzem S, Mangia A, Pawlotsky JM, Murray JS, et al. Hepatitis C pharmacogenetics: state of the art in 2010. Hepatology. 2011;53:336–345. doi: 10.1002/hep.24052. [DOI] [PubMed] [Google Scholar]
- 266.Kang MJ, Jung EU, Park SW, Choi P, Kim JH, Park SJ, et al. Effects of pegylated interferon and ribavirin in Korean patients with chronic hepatitis C virus infection. Korean J Hepatol. 2008;14:318–330. doi: 10.3350/kjhep.2008.14.3.318. [DOI] [PubMed] [Google Scholar]
- 267.Jeong SW, Kim JD, Woo HY, You CR, Lee SW, Song MJ, et al. Impact of adherence to peginterferon-ribavirin combination therapy in chronic hepatitis C patients on achieving a sustained virologic response. Korean J Hepatol. 2009;15:338–349. doi: 10.3350/kjhep.2009.15.3.338. [DOI] [PubMed] [Google Scholar]
- 268.Kwon JH, Bae SH, Choi JY, Yoon SK, Byun KS, Paik SW, et al. Assessment of the efficacy of reducing peginterferon alpha-2a and ribavirin dose on virologic response in Koreans with chronic hepatitis C. Korean J Intern Med. 2009;24:203–211. doi: 10.3904/kjim.2009.24.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Park SY, Rim MY, Yo IK, Ha MS, Kim JS, Lee JW, et al. Efficacy of peginterferon and ribavirin combination therapy of chronic hepatitis C: a pooled analysis. Korean J Gastroenterol. 2012;60:306–314. doi: 10.4166/kjg.2012.60.5.306. [DOI] [PubMed] [Google Scholar]
- 270.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 271.Sinn DH, Kim YJ, Lee ST, Gwak GY, Choi MS, Lee JH, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in Asian patients. J Gastroenterol Hepatol. 2011;26:1374–1379. doi: 10.1111/j.1440-1746.2011.06744.x. [DOI] [PubMed] [Google Scholar]
- 272.Khuroo MS, Dahab ST. Meta-analysis: a randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Aliment Pharmacol Ther. 2004;20:931–938. doi: 10.1111/j.1365-2036.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- 273.Zeuzem S, Buti M, Ferenci P, Sperl J, Horsmans Y, Cianciara J, et al. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006;44:97–103. doi: 10.1016/j.jhep.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 274.Moon SS, Kang HG, Seo JA, Jung EU, Lee SH, Park SJ, et al. 24 weeks treatment with pegylated interferon alfa plus ribavirin may be possible in genotype 1 chronic hepatitis C patients with rapid virological response who have low pretreatment viremia. Korean J Gastroenterol. 2010;56:33–38. doi: 10.4166/kjg.2010.56.1.33. [DOI] [PubMed] [Google Scholar]
- 275.Ferenci P, Laferl H, Scherzer TM, Gschwantler M, Maieron A, Brunner H, et al. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135:451–458. doi: 10.1053/j.gastro.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 276.Zeuzem S, Pawlotsky JM, Lukasiewicz E, von Wagner M, Goulis I, Lurie Y, et al. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J Hepatol. 2005;43:250–257. doi: 10.1016/j.jhep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 277.Moreno C, Deltenre P, Pawlotsky JM, Henrion J, Adler M, Mathurin P. Shortened treatment duration in treatment-naive genotype 1 HCV patients with rapid virological response: a meta-analysis. J Hepatol. 2010;52:25–31. doi: 10.1016/j.jhep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 278.Di Martino V, Richou C, Cervoni JP, Sanchez-Tapias JM, Jensen DM, Mangia A, et al. Response-guided peg-interferon plus ribavirin treatment duration in chronic hepatitis C: meta-analyses of randomized, controlled trials and implications for the future. Hepatology. 2011;54:789–800. doi: 10.1002/hep.24480. [DOI] [PubMed] [Google Scholar]
- 279.Kamal SM, El Kamary SS, Shardell MD, Hashem M, Ahmed IN, Muhammadi M, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: the role of rapid and early virologic response. Hepatology. 2007;46:1732–1740. doi: 10.1002/hep.21917. [DOI] [PubMed] [Google Scholar]
- 280.Berg T, Sarrazin C, Herrmann E, Hinrichsen H, Gerlach T, Zachoval R, et al. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology. 2003;37:600–609. doi: 10.1053/jhep.2003.50106. [DOI] [PubMed] [Google Scholar]
- 281.Buti M, Lurie Y, Zakharova NG, Blokhina NP, Horban A, Teuber G, et al. Randomized trial of peginterferon alfa-2b and ribavirin for 48 or 72 weeks in patients with hepatitis C virus genotype 1 and slow virologic response. Hepatology. 2010;52:1201–1207. doi: 10.1002/hep.23816. [DOI] [PubMed] [Google Scholar]
- 282.Sherman M, Yoshida EM, Deschenes M, Krajden M, Bain VG, Peltekian K, et al. Peginterferon alfa-2a (40KD) plus ribavirin in chronic hepatitis C patients who failed previous interferon therapy. Gut. 2006;55:1631–1638. doi: 10.1136/gut.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jacobson IM, Gonzalez SA, Ahmed F, Lebovics E, Min AD, Bodenheimer HC, Jr, et al. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol. 2005;100:2453–2462. doi: 10.1111/j.1572-0241.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 284.Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136:1618–1628. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 285.Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 286.Taliani G, Gemignani G, Ferrari C, Aceti A, Bartolozzi D, Blanc PL, et al. Pegylated interferon alfa-2b plus ribavirin in the retreatment of interferon-ribavirin nonresponder patients. Gastroenterology. 2006;130:1098–1106. doi: 10.1053/j.gastro.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 287.Jensen DM, Marcellin P, Freilich B, Andreone P, Di Bisceglie A, Brandao-Mello CE, et al. Re-treatment of patients with chronic hepatitis C who do not respond to peginterferon-alpha2b: a randomized trial. Ann Intern Med. 2009;150:528–540. doi: 10.7326/0003-4819-150-8-200904210-00007. [DOI] [PubMed] [Google Scholar]
- 288.Marcellin P, Craxi A, Brandao-Mello CE, Di Bisceglie AM, Andreone P, Freilich B, et al. Predicting early and sustained virological responses in prior nonresponders to pegylated interferon alpha-2b plus ribavirin retreated with peginterferon alpha-2a plus ribavirin and the benefit-risk ratio of retreatment. J Clin Gastroenterol. 2013;47:786–793. doi: 10.1097/MCG.0b013e31827b9b45. [DOI] [PubMed] [Google Scholar]
- 289.Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990–1999. doi: 10.1053/j.gastro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 290.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 292.Zeuzem S, Arora S, Bacon B, Box T, Charlton M, Diago M, et al. Pegylated interferon-lambda (PegIFN-λ) shows superior viral response with improved safety and tolerability versus pegIFN-α-2a in HCV patients (G1/2/3/4): EMERGE phase IIb through week 12 [Abstract] J Hepatol. 2011;54(Suppl 1):S538. [Google Scholar]
- 293.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 294.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 295.Welsch C, Zeuzem S. Clinical relevance of HCV antiviral drug resistance. Curr Opin Virol. 2012;2:651–655. doi: 10.1016/j.coviro.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 296.Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Mullhaupt B, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369:630–639. doi: 10.1056/NEJMoa1213557. [DOI] [PubMed] [Google Scholar]
- 297.Jacobson IM, Brown RS, Jr, Freilich B, Afdhal N, Kwo PY, Santoro J, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46:971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 298.Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993–999. doi: 10.1016/j.jhep.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 299.Ferenci P, Brunner H, Laferl H, Scherzer TM, Maieron A, Strasser M, et al. A randomized, prospective trial of ribavirin 400 mg/day versus 800 mg/day in combination with peginterferon alfa-2a in hepatitis C virus genotypes 2 and 3. Hepatology. 2008;47:1816–1823. doi: 10.1002/hep.22262. [DOI] [PubMed] [Google Scholar]
- 300.Lee S, Kim IH, Kim SH, Kim SW, Lee SO, Lee ST, et al. Efficacy and tolerability of pegylated interferon-alpha2a plus ribavirin versus pegylated interferon-alpha2b plus ribavirin in treatment-naive chronic hepatitis C patients. Intervirology. 2010;53:146–153. doi: 10.1159/000274975. [DOI] [PubMed] [Google Scholar]
- 301.Mangia A, Santoro R, Minerva N, Ricci GL, Carretta V, Persico M, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609–2617. doi: 10.1056/NEJMoa042608. [DOI] [PubMed] [Google Scholar]
- 302.Dalgard O, Bjoro K, Ring-Larsen H, Bjornsson E, Holberg-Petersen M, Skovlund E, et al. Pegylated interferon alfa and ribavirin for 14 versus 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological response. Hepatology. 2008;47:35–42. doi: 10.1002/hep.21975. [DOI] [PubMed] [Google Scholar]
- 303.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola R, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 304.Yu ML, Dai CY, Huang JF, Hou NJ, Lee LP, Hsieh MY, et al. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007;56:553–559. doi: 10.1136/gut.2006.102558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Diago M, Shiffman ML, Bronowicki JP, Zeuzem S, Rodriguez-Torres M, Pappas SC, et al. Identifying hepatitis C virus genotype 2/3 patients who can receive a 16-week abbreviated course of peginterferon alfa-2a (40KD) plus ribavirin. Hepatology. 2010;51:1897–1903. doi: 10.1002/hep.23531. [DOI] [PubMed] [Google Scholar]
- 306.Lagging M, Langeland N, Pedersen C, Farkkila M, Buhl MR, Morch K, et al. Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology. 2008;47:1837–1845. doi: 10.1002/hep.22253. [DOI] [PubMed] [Google Scholar]
- 307.Mecenate F, Barbaro G, Pellicelli A, Barlattani A, Mazzoni E, Bonaventura ME, et al. Comparison of peg-interferon alfa-2a and ribavirin for 12 or 24 weeks in patients with HCV genotype 2 or 3: the CLEO trial [Abstract] Hepatology. 2007;46(Suppl 1):828A. [Google Scholar]
- 308.Goncales FL, Jr, Moma CA, Vigani AG, Angerami AF, Goncales ES, Tozzo R, et al. Retreatment of hepatitis C patients with pegylated interferon combined with ribavirin in non-responders to interferon plus ribavirin. Is it different in real life? BMC Infect Dis. 2010;10:212. doi: 10.1186/1471-2334-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sagir A, Heintges T, Akyazi Z, Oette M, Erhardt A, Haussinger D. Relapse to prior therapy is the most important factor for the retreatment response in patients with chronic hepatitis C virus infection. Liver Int. 2007;27:954–959. doi: 10.1111/j.1478-3231.2007.01508.x. [DOI] [PubMed] [Google Scholar]
- 310.Krawitt EL, Ashikaga T, Gordon SR, Ferrentino N, Ray MA, Lidofsky SD. Peginterferon alfa-2b and ribavirin for treatment-refractory chronic hepatitis C. J Hepatol. 2005;43:243–249. doi: 10.1016/j.jhep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 311.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 312.Nguyen MH, Keeffe EB. Chronic hepatitis C: genotypes 4 to 9. Clin Liver Dis. 2005;9:411–426. vi. doi: 10.1016/j.cld.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 313.Yuen MF, Lai CL. Response to combined interferon and ribavirin is better in patients infected with hepatitis C virus genotype 6 than genotype 1 in Hong Kong. Intervirology. 2006;49:96–98. doi: 10.1159/000087270. [DOI] [PubMed] [Google Scholar]
- 314.Tsang OT, Zee JS, Chan JM, Li RS, Kan YM, Li FT, et al. Chronic hepatitis C genotype 6 responds better to pegylated interferon and ribavirin combination therapy than genotype 1. J Gastroenterol Hepatol. 2010;25:766–771. doi: 10.1111/j.1440-1746.2009.06163.x. [DOI] [PubMed] [Google Scholar]
- 315.Nguyen NH, VuTien P, Garcia RT, Trinh H, Nguyen H, Nguyen K, et al. Response to pegylated interferon and ribavirin in Asian American patients with chronic hepatitis C genotypes 1 vs 2/3 vs 6. J Viral Hepat. 2010;17:691–697. doi: 10.1111/j.1365-2893.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 316.Fung J, Lai CL, Hung I, Young J, Cheng C, Wong D, et al. Chronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirin. J Infect Dis. 2008;198:808–812. doi: 10.1086/591252. [DOI] [PubMed] [Google Scholar]
- 317.Nguyen MH, Trinh HN, Garcia R, Nguyen G, Lam KD, Keeffe EB. Higher rate of sustained virologic response in chronic hepatitis C genotype 6 treated with 48 weeks versus 24 weeks of peginterferon plus ribavirin. Am J Gastroenterol. 2008;103:1131–1135. doi: 10.1111/j.1572-0241.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- 318.Lam KD, Trinh HN, Do ST, Nguyen TT, Garcia RT, Nguyen T, et al. Randomized controlled trial of pegylated interferon-alfa 2a and ribavirin in treatment-naive chronic hepatitis C genotype 6. Hepatology. 2010;52:1573–1580. doi: 10.1002/hep.23889. [DOI] [PubMed] [Google Scholar]
- 319.Tangkijvanich P, Komolmit P, Mahachai V, Poovorawan K, Akkarathamrongsin S, Poovorawan Y. Response-guided therapy for patients with hepatitis C virus genotype 6 infection: a pilot study. J Viral Hepat. 2012;19:423–430. doi: 10.1111/j.1365-2893.2011.01566.x. [DOI] [PubMed] [Google Scholar]
- 320.Thu Thuy PT, Bunchorntavakul C, Tan Dat H, Rajender Reddy K. A randomized trial of 48 versus 24 weeks of combination pegylated interferon and ribavirin therapy in genotype 6 chronic hepatitis C. J Hepatol. 2012;56:1012–1018. doi: 10.1016/j.jhep.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 321.Licata A, Di Bona D, Schepis F, Shahied L, Craxi A, Camma C. When and how to treat acute hepatitis C? J Hepatol. 2003;39:1056–1062. doi: 10.1016/s0168-8278(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 322.Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474–1482. doi: 10.1086/522608. [DOI] [PubMed] [Google Scholar]
- 323.McGovern BH, Nagami EH, Birch CE, Bowen MJ, Reyor LL, Chung RT, et al. Rate of sustained virologic response in relation to baseline hepatitis C virus (HCV) RNA level and rapid virologic clearance in persons with acute HCV infection. J Infect Dis. 2009;200:877–881. doi: 10.1086/605444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Al Tawil A, Khalifa KE, et al. Peginterferon alfa-2b therapy in acute hepatitis C: Impact of onset of therapy on sustained virologic response. Gastroenterology. 2006;130:632–638. doi: 10.1053/j.gastro.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 325.Deuffic-Burban S, Castel H, Wiegand J, Manns MP, Wedemeyer H, Mathurin P, et al. Immediate vs. delayed treatment in patients with acute hepatitis C based on IL28B polymorphism: a model-based analysis. J Hepatol. 2012;57:260–266. doi: 10.1016/j.jhep.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 326.Deterding K, Gruner N, Buggisch P, Wiegand J, Galle PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis. 2013;13:497–506. doi: 10.1016/S1473-3099(13)70059-8. [DOI] [PubMed] [Google Scholar]
- 327.Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 328.Nomura H, Sou S, Tanimoto H, Nagahama T, Kimura Y, Hayashi J, et al. Short-term interferon-alfa therapy for acute hepatitis C: a randomized controlled trial. Hepatology. 2004;39:1213–1219. doi: 10.1002/hep.20196. [DOI] [PubMed] [Google Scholar]
- 329.Kamal SM, Ismail A, Graham CS, He Q, Rasenack JW, Peters T, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology. 2004;39:1721–1731. doi: 10.1002/hep.20266. [DOI] [PubMed] [Google Scholar]
- 330.Broers B, Helbling B, Francois A, Schmid P, Chuard C, Hadengue A, et al. Barriers to interferon-alpha therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005;42:323–328. doi: 10.1016/j.jhep.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 331.Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005;42:329–333. doi: 10.1016/j.jhep.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 332.Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250–256. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]
- 333.Kamal SM, Moustafa KN, Chen J, Fehr J, Abdel Moneim A, Khalifa KE, et al. Duration of peginterferon therapy in acute hepatitis C: a randomized trial. Hepatology. 2006;43:923–931. doi: 10.1002/hep.21197. [DOI] [PubMed] [Google Scholar]
- 334.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(Suppl 1):S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 335.Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124:1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 336.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Thevenot T, Cadranel JF, Di Martino V, Pariente A, Causse X, Renou C, et al. A national French survey on the use of growth factors as adjuvant treatment of chronic hepatitis C. Hepatology. 2007;45:377–383. doi: 10.1002/hep.21517. [DOI] [PubMed] [Google Scholar]
- 338.McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227–2236. doi: 10.1056/NEJMoa073255. [DOI] [PubMed] [Google Scholar]
- 339.Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367:716–724. doi: 10.1056/NEJMoa1110709. [DOI] [PubMed] [Google Scholar]
- 340.Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241–243. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 341.Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, Forns X, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73:1128–1138. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- 342.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 343.de Knegt RJ, Bezemer G, Van Gool AR, Drenth JP, Hansen BE, Droogleever Fortuyn HA, et al. Randomised clinical trial: escitalopram for the prevention of psychiatric adverse events during treatment with peginterferon-alfa-2a and ribavirin for chronic hepatitis C. Aliment Pharmacol Ther. 2011;34:1306–1317. doi: 10.1111/j.1365-2036.2011.04867.x. [DOI] [PubMed] [Google Scholar]
- 344.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36:1051–1066. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: toward a new classification. Hepatology. 2006;43:661–672. doi: 10.1002/hep.21146. [DOI] [PubMed] [Google Scholar]
- 346.Marazuela M, Garcia-Buey L, Gonzalez-Fernandez B, Garcia-Monzon C, Arranz A, Borque MJ, et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44:635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 347.Lisker-Melman M, Di Bisceglie AM, Usala SJ, Weintraub B, Murray LM, Hoofnagle JH. Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology. 1992;102:2155–2160. doi: 10.1016/0016-5085(92)90348-3. [DOI] [PubMed] [Google Scholar]
- 348.Martocchia A, Labbadia G, Paoletti V, Gargano S, Grossi A, Trabace S, et al. Hashimoto's disease during interferon-alpha therapy in a patient with pre-treatment negative anti-thyroid autoantibodies and with the specific genetic susceptibility to the thyroid disease. Neuro Endocrinol Lett. 2001;22:49–52. [PubMed] [Google Scholar]
- 349.Carella C, Mazziotti G, Morisco F, Manganella G, Rotondi M, Tuccillo C, et al. Long-term outcome of interferon-alpha-induced thyroid autoimmunity and prognostic influence of thyroid autoantibody pattern at the end of treatment. J Clin Endocrinol Metab. 2001;86:1925–1929. doi: 10.1210/jcem.86.5.7459. [DOI] [PubMed] [Google Scholar]
- 350.Wong V, Fu AX, George J, Cheung NW. Thyrotoxicosis induced by alpha-interferon therapy in chronic viral hepatitis. Clin Endocrinol (Oxf) 2002;56:793–798. doi: 10.1046/j.1365-2265.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- 351.Gutkowski K, Gutkowska D, Bilkiewicz T. Interferon therapy in chronic viral hepatitis; an autoimmunity dilemma. Przegl Lek. 2007;64:148–152. [PubMed] [Google Scholar]
- 352.Panetta JD, Gilani N. Interferon-induced retinopathy and its risk in patients with diabetes and hypertension undergoing treatment for chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2009;30:597–602. doi: 10.1111/j.1365-2036.2009.04071.x. [DOI] [PubMed] [Google Scholar]
- 353.Malik NN, Sheth HG, Ackerman N, Davies N, Mitchell SM. A prospective study of change in visual function in patients treated with pegylated interferon alpha for hepatitis C in the UK. Br J Ophthalmol. 2008;92:256–258. doi: 10.1136/bjo.2006.106278. [DOI] [PubMed] [Google Scholar]
- 354.Chisholm JA, Williams G, Spence E, Parks S, Keating D, Gavin M, et al. Retinal toxicity during pegylated alpha-interferon therapy for chronic hepatitis C: a multifocal electroretinogram investigation. Aliment Pharmacol Ther. 2005;21:723–732. doi: 10.1111/j.1365-2036.2005.02365.x. [DOI] [PubMed] [Google Scholar]
- 355.Sene D, Touitou V, Bodaghi B, Saadoun D, Perlemuter G, Cassoux N, et al. Intraocular complications of IFN-alpha and ribavirin therapy in patients with chronic viral hepatitis C. World J Gastroenterol. 2007;13:3137–3140. doi: 10.3748/wjg.v13.i22.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Vujosevic S, Tempesta D, Noventa F, Midena E, Sebastiani G. Pegylated interferon-associated retinopathy is frequent in hepatitis C virus patients with hypertension and justifies ophthalmologic screening. Hepatology. 2012;56:455–463. doi: 10.1002/hep.25654. [DOI] [PubMed] [Google Scholar]
- 357.Okuse C, Yotsuyanagi H, Nagase Y, Kobayashi Y, Yasuda K, Koike K, et al. Risk factors for retinopathy associated with interferon alpha-2b and ribavirin combination therapy in patients with chronic hepatitis C. World J Gastroenterol. 2006;12:3756–3759. doi: 10.3748/wjg.v12.i23.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Schulman JA, Liang C, Kooragayala LM, King J. Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmology. 2003;110:437–442. doi: 10.1016/S0161-6420(02)01741-4. [DOI] [PubMed] [Google Scholar]
- 359.Formann E, Stauber R, Denk DM, Jessner W, Zollner G, Munda-Steindl P, et al. Sudden hearing loss in patients with chronic hepatitis C treated with pegylated interferon/ribavirin. Am J Gastroenterol. 2004;99:873–877. doi: 10.1111/j.1572-0241.2004.30372.x. [DOI] [PubMed] [Google Scholar]
- 360.De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997–1004. doi: 10.1053/he.2000.5789. [DOI] [PubMed] [Google Scholar]
- 361.Afdhal NH, Dieterich DT, Pockros PJ, Schiff ER, Shiffman ML, Sulkowski MS, et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–1311. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 362.Kochhar DM, Penner JD, Knudsen TB. Embryotoxic, teratogenic, and metabolic effects of ribavirin in mice. Toxicol Appl Pharmacol. 1980;52:99–112. doi: 10.1016/0041-008x(80)90252-5. [DOI] [PubMed] [Google Scholar]
- 363.Backmund M, Meyer K, Edlin BR. Infrequent reinfection after successful treatment for hepatitis C virus infection in injection drug users. Clin Infect Dis. 2004;39:1540–1543. doi: 10.1086/425361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Dalgard O. Follow-up studies of treatment for hepatitis C virus infection among injection drug users. Clin Infect Dis. 2005;40(Suppl 5):S336–S338. doi: 10.1086/427449. [DOI] [PubMed] [Google Scholar]
- 365.Currie SL, Ryan JC, Tracy D, Wright TL, George S, McQuaid R, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus. Drug Alcohol Depend. 2008;93:148–154. doi: 10.1016/j.drugalcdep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 366.Grebely J, Knight E, Ngai T, Genoway KA, Raffa JD, Storms M, et al. Reinfection with hepatitis C virus following sustained virological response in injection drug users. J Gastroenterol Hepatol. 2010;25:1281–1284. doi: 10.1111/j.1440-1746.2010.06238.x. [DOI] [PubMed] [Google Scholar]
- 367.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516.e1. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 368.Hung CH, Lee CM, Lu SN, Wang JH, Hu TH, Tung HD, et al. Long-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat. 2006;13:409–414. doi: 10.1111/j.1365-2893.2005.00707.x. [DOI] [PubMed] [Google Scholar]
- 369.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 370.Kim KH, Jang BK, Chung WJ, Hwang JS, Kweon YO, Tak WY, et al. Peginterferon alpha and ribavirin combination therapy in patients with hepatitis C virus-related liver cirrhosis. Korean J Hepatol. 2011;17:220–225. doi: 10.3350/kjhep.2011.17.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Schmid M, Kreil A, Jessner W, Homoncik M, Datz C, Gangl A, et al. Suppression of haematopoiesis during therapy of chronic hepatitis C with different interferon alpha mono and combination therapy regimens. Gut. 2005;54:1014–1020. doi: 10.1136/gut.2004.057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Bruno S, Vierling JM, Esteban R, Nyberg LM, Tanno H, Goodman Z, et al. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/cirrhosis. J Hepatol. 2013;58:479–487. doi: 10.1016/j.jhep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 373.Cheong HR, Woo HY, Heo J, Yoon KT, Kim DU, Kim GH, et al. Clinical efficacy and safety of the combination therapy of peginterferon alpha and ribavirin in cirrhotic patients with HCV infection. Korean J Hepatol. 2010;16:38–48. doi: 10.3350/kjhep.2010.16.1.38. [DOI] [PubMed] [Google Scholar]
- 374.Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255–262. doi: 10.1002/hep.20793. [DOI] [PubMed] [Google Scholar]
- 375.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 376.Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680–687. doi: 10.1053/jhep.2002.31773. [DOI] [PubMed] [Google Scholar]
- 377.Prieto M, Berenguer M, Rayon JM, Cordoba J, Arguello L, Carrasco D, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999;29:250–256. doi: 10.1002/hep.510290122. [DOI] [PubMed] [Google Scholar]
- 378.Berenguer M, Palau A, Aguilera V, Rayon JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679–687. doi: 10.1111/j.1600-6143.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- 379.Neumann UP, Berg T, Bahra M, Seehofer D, Langrehr JM, Neuhaus R, et al. Fibrosis progression after liver transplantation in patients with recurrent hepatitis C. J Hepatol. 2004;41:830–836. doi: 10.1016/j.jhep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 380.Blasco A, Forns X, Carrion JA, Garcia-Pagan JC, Gilabert R, Rimola A, et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006;43:492–499. doi: 10.1002/hep.21090. [DOI] [PubMed] [Google Scholar]
- 381.Samuel D, Bizollon T, Feray C, Roche B, Ahmed SN, Lemonnier C, et al. Interferon-alpha 2b plus ribavirin in patients with chronic hepatitis C after liver transplantation: a randomized study. Gastroenterology. 2003;124:642–650. doi: 10.1053/gast.2003.50095. [DOI] [PubMed] [Google Scholar]
- 382.Carrion JA, Navasa M, Garcia-Retortillo M, Garcia-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746–1756. doi: 10.1053/j.gastro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 383.Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, et al. A randomized study on Peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol. 2007;46:1009–1017. doi: 10.1016/j.jhep.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 384.Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, et al. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690–700. doi: 10.1002/lt.23669. [DOI] [PubMed] [Google Scholar]
- 385.Gane E, Pilmore H. Management of chronic viral hepatitis before and after renal transplantation. Transplantation. 2002;74:427–437. doi: 10.1097/00007890-200208270-00001. [DOI] [PubMed] [Google Scholar]
- 386.Pawa S, Ehrinpreis M, Mutchnick M, Janisse J, Dhar R, Siddiqui FA. Percutaneous liver biopsy is safe in chronic hepatitis C patients with end-stage renal disease. Clin Gastroenterol Hepatol. 2007;5:1316–1320. doi: 10.1016/j.cgh.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 387.Sterling RK, Sanyal AJ, Luketic VA, Stravitz RT, King AL, Post AB, et al. Chronic hepatitis C infection in patients with end stage renal disease: characterization of liver histology and viral load in patients awaiting renal transplantation. Am J Gastroenterol. 1999;94:3576–3582. doi: 10.1111/j.1572-0241.1999.01649.x. [DOI] [PubMed] [Google Scholar]
- 388.Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol. 2008;49:613–624. doi: 10.1016/j.jhep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 389.Fagiuoli S, Cooper DK, Zuhdi N. Hepatitis C status of heart transplant recipients. Clin Transplant. 1998;12:5–10. [PubMed] [Google Scholar]
- 390.Ozguroglu M, Bilici A, Turna H, Serdengecti S. Reactivation of hepatitis B virus infection with cytotoxic therapy in non-Hodgkin's lymphoma. Med Oncol. 2004;21:67–72. doi: 10.1385/MO:21:1:67. [DOI] [PubMed] [Google Scholar]
- 391.Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 392.Kawatani T, Suou T, Tajima F, Ishiga K, Omura H, Endo A, et al. Incidence of hepatitis virus infection and severe liver dysfunction in patients receiving chemotherapy for hematologic malignancies. Eur J Haematol. 2001;67:45–50. doi: 10.1034/j.1600-0609.2001.067001045.x. [DOI] [PubMed] [Google Scholar]
- 393.Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology. 1999;46:2925–2930. [PubMed] [Google Scholar]
- 394.Vento S, Cainelli F, Longhi MS. Reactivation of replication of hepatitis B and C viruses after immunosuppressive therapy: an unresolved issue. Lancet Oncol. 2002;3:333–340. doi: 10.1016/s1470-2045(02)00773-8. [DOI] [PubMed] [Google Scholar]
- 395.Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, et al. Acute hepatic toxicity during cyclic chemotherapy in non Hodgkin's lymphoma. Haematologica. 1997;82:38–42. [PubMed] [Google Scholar]
- 396.Nosotti L, D'Andrea M, Pitidis A, Pimpinelli F, Dessanti ML, Pisani F, et al. Hepatitis C virus infection prevalence and liver dysfunction in a cohort of B-cell non-Hodgkin's lymphoma patients treated with immunochemotherapy. Scand J Infect Dis. 2012;44:70–73. doi: 10.3109/00365548.2011.611819. [DOI] [PubMed] [Google Scholar]
- 397.Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, et al. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158–165. doi: 10.1111/j.1600-0609.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 398.de Pree C, Giostra E, Galetto A, Perrin L, Zulian GB. Hepatitis C virus acute exacerbation during chemotherapy and radiotherapy for oesophageal carcinoma. Ann Oncol. 1994;5:861–862. doi: 10.1093/oxfordjournals.annonc.a059022. [DOI] [PubMed] [Google Scholar]
- 399.Melisko ME, Fox R, Venook A. Reactivation of hepatitis C virus after chemotherapy for colon cancer. Clin Oncol (R Coll Radiol) 2004;16:204–205. doi: 10.1016/j.clon.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 400.Fan FS, Tzeng CH, Hsiao KI, Hu ST, Liu WT, Chen PM. Withdrawal of immunosuppressive therapy in allogeneic bone marrow transplantation reactivates chronic viral hepatitis C. Bone Marrow Transplant. 1991;8:417–420. [PubMed] [Google Scholar]
- 401.Kanamori H, Fukawa H, Maruta A, Harano H, Kodama F, Matsuzaki M, et al. Case report: fulminant hepatitis C viral infection after allogeneic bone marrow transplantation. Am J Med Sci. 1992;303:109–111. doi: 10.1097/00000441-199202000-00009. [DOI] [PubMed] [Google Scholar]
- 402.Vento S, Cainelli F, Mirandola F, Cosco L, Di Perri G, Solbiati M, et al. Fulminant hepatitis on withdrawal of chemotherapy in carriers of hepatitis C virus. Lancet. 1996;347:92–93. doi: 10.1016/s0140-6736(96)90212-3. [DOI] [PubMed] [Google Scholar]
- 403.Nakamura Y, Motokura T, Fujita A, Yamashita T, Ogata E. Severe hepatitis related to chemotherapy in hepatitis B virus carriers with hematologic malignancies. Survey in Japan, 1987-1991. Cancer. 1996;78:2210–2215. doi: 10.1002/(sici)1097-0142(19961115)78:10<2210::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 404.Locasciulli A, Bruno B, Alessandrino EP, Meloni G, Arcese W, Bandini G, et al. Hepatitis reactivation and liver failure in haemopoietic stem cell transplants for hepatitis B virus (HBV)/hepatitis C virus (HCV) positive recipients: a retrospective study by the Italian group for blood and marrow transplantation. Bone Marrow Transplant. 2003;31:295–300. doi: 10.1038/sj.bmt.1703826. [DOI] [PubMed] [Google Scholar]
- 405.Hamaguchi M, Yamada H, Gondo H, Takemoto Y, Morishima Y, Kodera Y. Retrospective study on the impact of hepatitis B and hepatitis C virus infection on hematopoietic stem cell transplantation in Japan. Int J Hematol. 2002;75:324–331. doi: 10.1007/BF02982051. [DOI] [PubMed] [Google Scholar]
- 406.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 407.Kim HS, Choo DH. Prevalence of anti-HCV among drug users in Korea. Korean J Med. 1996;50:194–200. [Google Scholar]
- 408.Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56:806–816. doi: 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Martin NK, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55:49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 410.Liu CH, Kao JH. Treatment of hepatitis C virus infection in patients with end-stage renal disease. J Gastroenterol Hepatol. 2011;26:228–239. doi: 10.1111/j.1440-1746.2010.06488.x. [DOI] [PubMed] [Google Scholar]
- 411.Marcelli D, Stannard D, Conte F, Held PJ, Locatelli F, Port FK. ESRD patient mortality with adjustment for comorbid conditions in Lombardy (Italy) versus the United States. Kidney Int. 1996;50:1013–1018. doi: 10.1038/ki.1996.403. [DOI] [PubMed] [Google Scholar]
- 412.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 413.Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11:1896–1902. doi: 10.1681/ASN.V11101896. [DOI] [PubMed] [Google Scholar]
- 414.Bruchfeld A, Wilczek H, Elinder CG. Hepatitis C infection, time in renal-replacement therapy, and outcome after kidney transplantation. Transplantation. 2004;78:745–750. doi: 10.1097/01.tp.0000131948.29742.24. [DOI] [PubMed] [Google Scholar]
- 415.Aroldi A, Lampertico P, Montagnino G, Passerini P, Villa M, Campise MR, et al. Natural history of hepatitis B and C in renal allograft recipients. Transplantation. 2005;79:1132–1136. doi: 10.1097/01.tp.0000161250.83392.73. [DOI] [PubMed] [Google Scholar]
- 416.Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002;13:1374–1380. doi: 10.1097/01.asn.0000012382.97168.e0. [DOI] [PubMed] [Google Scholar]
- 417.Kamar N, Mariat C, Delahousse M, Dantal J, Al Najjar A, Cassuto E, et al. Diabetes mellitus after kidney transplantation: a French multicentre observational study. Nephrol Dial Transplant. 2007;22:1986–1993. doi: 10.1093/ndt/gfm011. [DOI] [PubMed] [Google Scholar]
- 418.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5:2433–2440. doi: 10.1111/j.1600-6143.2005.01040.x. [DOI] [PubMed] [Google Scholar]
- 419.Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, et al. De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation. 1995;59:1676–1682. doi: 10.1097/00007890-199506270-00006. [DOI] [PubMed] [Google Scholar]
- 420.Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant. 2006;6:2535–2542. doi: 10.1111/j.1600-6143.2006.01502.x. [DOI] [PubMed] [Google Scholar]
- 421.Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat. 2006;13:316–321. doi: 10.1111/j.1365-2893.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 422.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB American Association for Study of Liver Diseases. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Garini G, Allegri L, Carnevali L, Catellani W, Manganelli P, Buzio C. Interferon-alpha in combination with ribavirin as initial treatment for hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis. Am J Kidney Dis. 2001;38:E35. doi: 10.1053/ajkd.2001.29291. [DOI] [PubMed] [Google Scholar]
- 424.Mazzaro C, Zorat F, Caizzi M, Donada C, Di Gennaro G, Maso LD, et al. Treatment with peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study. J Hepatol. 2005;42:632–638. doi: 10.1016/j.jhep.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 425.Saadoun D, Resche-Rigon M, Thibault V, Piette JC, Cacoub P. Antiviral therapy for hepatitis C virus--associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum. 2006;54:3696–3706. doi: 10.1002/art.22168. [DOI] [PubMed] [Google Scholar]
- 426.Lee SH, Kim KH, Lee SG, Cho H, Chen DH, Chung JS, et al. Causes of death and risk factors for mortality among HIV-infected patients receiving antiretroviral therapy in Korea. J Korean Med Sci. 2013;28:990–997. doi: 10.3346/jkms.2013.28.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr. 2001;26:340–344. doi: 10.1097/00126334-200104010-00008. [DOI] [PubMed] [Google Scholar]
- 428.Marcellin P, Martinot-Peignoux M, Elias A, Branger M, Courtois F, Level R, et al. Hepatitis C virus (HCV) viremia in human immunodeficiency virus-seronegative and -seropositive patients with indeterminate HCV recombinant immunoblot assay. J Infect Dis. 1994;170:433–435. doi: 10.1093/infdis/170.2.433. [DOI] [PubMed] [Google Scholar]
- 429.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 430.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 431.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 432.Qurishi N, Kreuzberg C, Luchters G, Effenberger W, Kupfer B, Sauerbruch T, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 433.Tien PC Veterans Affairs Hepatitis C Resource Center Program; National Hepatitis C Program Office. Resource Center Program, National Hepatitis C Program Office. Management and treatment of hepatitis C virus infection in HIV-infected adults: recommendations from the Veterans Affairs Hepatitis C Resource Center Program and National Hepatitis C Program Office. Am J Gastroenterol. 2005;100:2338–2354. doi: 10.1111/j.1572-0241.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 434.Avidan NU, Goldstein D, Rozenberg L, McLaughlin M, Ferenci P, Masur H, et al. Hepatitis C viral kinetics during treatment with peg IFN-alpha-2b in HIV/HCV coinfected patients as a function of baseline CD4+ T-cell counts. J Acquir Immune Defic Syndr. 2009;52:452–458. doi: 10.1097/QAI.0b013e3181be7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21:2209–2216. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 436.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 437.Macias J, Berenguer J, Japon MA, Giron JA, Rivero A, Lopez-Cortes LF, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056–1063. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 438.A working group of the Office of AIDS Research Council (OARAC) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescent. AIDSinfo website; [Accessed 2013]. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 439.Nunez M, Miralles C, Berdun MA, Losada E, Aguirrebengoa K, Ocampo A, et al. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res Hum Retroviruses. 2007;23:972–982. doi: 10.1089/aid.2007.0011. [DOI] [PubMed] [Google Scholar]
- 440.Soriano V, Puoti M, Sulkowski M, Cargnel A, Benhamou Y, Peters M, et al. Care of patients coinfected with HIV and hepatitis C virus: 2007 updated recommendations from the HCV-HIV International Panel. AIDS. 2007;21:1073–1089. doi: 10.1097/QAD.0b013e3281084e4d. [DOI] [PubMed] [Google Scholar]
- 441.Cargnel A, Angeli E, Mainini A, Gubertini G, Giorgi R, Schiavini M, et al. Open, randomized, multicentre Italian trial on PEG-IFN plus ribavirin versus PEG-IFN monotherapy for chronic hepatitis C in HIV-coinfected patients on HAART. Antivir Ther. 2005;10:309–317. [PubMed] [Google Scholar]
- 442.Santin M, Shaw E, Garcia MJ, Delejido A, de Castro ER, Rota R, et al. Efficacy and safety of pegylated interferon-alpha2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. AIDS Res Hum Retroviruses. 2006;22:315–320. doi: 10.1089/aid.2006.22.315. [DOI] [PubMed] [Google Scholar]
- 443.Nunez M, Camino N, Ramos B, Berdun MA, Barreiro P, Losada E, et al. Impact of ribavirin exposure on early virological response to hepatitis C therapy in HIV-infected patients with chronic hepatitis C. Antivir Ther. 2005;10:657–662. [PubMed] [Google Scholar]
- 444.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 445.Soriano V, Garcia-Samaniego J, Bravo R, Gonzalez J, Castro A, Castilla J, et al. Hepatitis-HIV Spanish Study Group. Interferon alpha for the treatment of chronic hepatitis C in patients infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:585–591. doi: 10.1093/clinids/23.3.585. [DOI] [PubMed] [Google Scholar]
- 446.Laguno M, Murillas J, Blanco JL, Martinez E, Miquel R, Sanchez-Tapias JM, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27–F36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 447.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 449.Alvarez D, Dieterich DT, Brau N, Moorehead L, Ball L, Sulkowski MS. Zidovudine use but not weight-based ribavirin dosing impacts anaemia during HCV treatment in HIV-infected persons. J Viral Hepat. 2006;13:683–689. doi: 10.1111/j.1365-2893.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 450.Hoggard PG, Kewn S, Barry MG, Khoo SH, Back DJ. Effects of drugs on 2',3'-dideoxy-2',3'-didehydrothymidine phosphorylation in vitro. Antimicrob Agents Chemother. 1997;41:1231–1236. doi: 10.1128/aac.41.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Lafeuillade A, Hittinger G, Chadapaud S. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet. 2001;357:280–281. doi: 10.1016/S0140-6736(00)03618-7. [DOI] [PubMed] [Google Scholar]
- 452.Salmon-Ceron D, Chauvelot-Moachon L, Abad S, Silbermann B, Sogni P. Mitochondrial toxic effects and ribavirin. Lancet. 2001;357:1803–1804. doi: 10.1016/S0140-6736(00)04921-7. [DOI] [PubMed] [Google Scholar]
- 453.Bristol Myers Squibb. Patient Information. 2003. [Google Scholar]
- 454.Sulkowski MS. Current management of hepatitis C virus infection in patients with HIV co-infection. J Infect Dis. 2013;207(Suppl 1):S26–S32. doi: 10.1093/infdis/jis764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Fernandez-Montero JV, Soriano V. Management of hepatitis C in HIV and/or HBV co-infected patients. Best Pract Res Clin Gastroenterol. 2012;26:517–530. doi: 10.1016/j.bpg.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 456.Kim YJ, Lee JW, Kim YS, Jeong SH, Kim YS, Yim HJ, et al. Clinical features and treatment efficacy of peginterferon alfa plus ribavirin in chronic hepatitis C patients coinfected with hepatitis B virus. Korean J Hepatol. 2011;17:199–205. doi: 10.3350/kjhep.2011.17.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, et al. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132–2137. [PubMed] [Google Scholar]
- 458.Lee LP, Dai CY, Chuang WL, Chang WY, Hou NJ, Hsieh MY, et al. Comparison of liver histopathology between chronic hepatitis C patients and chronic hepatitis B and C-coinfected patients. J Gastroenterol Hepatol. 2007;22:515–517. doi: 10.1111/j.1440-1746.2006.04547.x. [DOI] [PubMed] [Google Scholar]
- 459.Sagnelli E, Pasquale G, Coppola N, Scarano F, Marrocco C, Scolastico C, et al. Influence of chronic coinfection with hepatitis B and C virus on liver histology. Infection. 2004;32:144–148. doi: 10.1007/s15010-004-3080-6. [DOI] [PubMed] [Google Scholar]
- 460.Benvegnu L, Fattovich G, Noventa F, Tremolada F, Chemello L, Cecchetto A, et al. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer. 1994;74:2442–2448. doi: 10.1002/1097-0142(19941101)74:9<2442::aid-cncr2820740909>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 461.Potthoff A, Manns MP, Wedemeyer H. Treatment of HBV/HCV coinfection. Expert Opin Pharmacother. 2010;11:919–928. doi: 10.1517/14656561003637659. [DOI] [PubMed] [Google Scholar]
- 462.Potthoff A, Wedemeyer H, Boecher WO, Berg T, Zeuzem S, Arnold J, et al. The HEP-NET B/C co-infection trial: A prospective multicenter study to investigate the efficacy of pegylated interferon-alpha2b and ribavirin in patients with HBV/HCV co-infection. J Hepatol. 2008;49:688–694. doi: 10.1016/j.jhep.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 463.Liu CJ, Chuang WL, Lee CM, Yu ML, Lu SN, Wu SS, et al. Peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. Gastroenterology. 2009;136:496–504.e3. doi: 10.1053/j.gastro.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 464.Potthoff A, Berg T, Wedemeyer H. Late hepatitis B virus relapse in patients co-infected with hepatitis B virus and hepatitis C virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol. 2009;44:1487–1490. doi: 10.3109/00365520903329585. [DOI] [PubMed] [Google Scholar]
- 465.Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working Party. McCaughan GW, Omata M, Amarapurkar D, Bowden S, Chow WC, et al. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:615–633. doi: 10.1111/j.1440-1746.2007.04883.x. [DOI] [PubMed] [Google Scholar]
- 466.Vento S, Cainelli F, Cesario F. Infections and thalassaemia. Lancet Infect Dis. 2006;6:226–233. doi: 10.1016/S1473-3099(06)70437-6. [DOI] [PubMed] [Google Scholar]
- 467.Plug I, Van Der Bom JG, Peters M, Mauser-Bunschoten EP, De Goede-Bolder A, Heijnen L, et al. Mortality and causes of death in patients with hemophilia, 1992-2001: a prospective cohort study. J Thromb Haemost. 2006;4:510–516. doi: 10.1111/j.1538-7836.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 468.Zhang M, Rosenberg PS, Brown DL, Preiss L, Konkle BA, Eyster ME, et al. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 2006;107:892–897. doi: 10.1182/blood-2005-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Maor Y, Bashari D, Kenet G, Lubetsky A, Luboshitz J, Schapiro JM, et al. Non-invasive biomarkers of liver fibrosis in haemophilia patients with hepatitis C: can you avoid liver biopsy? Haemophilia. 2006;12:372–379. doi: 10.1111/j.1365-2516.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 470.Honda T, Katano Y, Kuzuya T, Hayashi K, Ishigami M, Itoh A, et al. Comparison of the efficacy of ribavirin plus peginterferon alfa-2b for chronic hepatitis C infection in patients with and without coagulation disorders. J Med Virol. 2013;85:228–234. doi: 10.1002/jmv.23444. [DOI] [PubMed] [Google Scholar]
- 471.Mohan P, Barton BA, Narkewicz MR, Molleston JP, Gonzalez-Peralta RP, Rosenthal P, et al. Evaluating progression of liver disease from repeat liver biopsies in children with chronic hepatitis C: a retrospective study. Hepatology. 2013;58:1580–1586. doi: 10.1002/hep.26519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Pembrey L, Newell ML, Tovo PA EPHN Collaborators. The management of HCV infected pregnant women and their children European paediatric HCV network. J Hepatol. 2005;43:515–525. doi: 10.1016/j.jhep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 473.Airoldi J, Berghella V. Hepatitis C and pregnancy. Obstet Gynecol Surv. 2006;61:666–672. doi: 10.1097/01.ogx.0000238671.13495.33. [DOI] [PubMed] [Google Scholar]
- 474.American Academy of Pediatrics, Committee on Infectious Diseases. Hepatitis C virus infection. Pediatrics. 1998;101:481–485. [PubMed] [Google Scholar]
- 475.Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880–1889. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- 476.Palomba E, Manzini P, Fiammengo P, Maderni P, Saracco G, Tovo PA. Natural history of perinatal hepatitis C virus infection. Clin Infect Dis. 1996;23:47–50. doi: 10.1093/clinids/23.1.47. [DOI] [PubMed] [Google Scholar]
- 477.Polywka S, Pembrey L, Tovo PA, Newell ML. Accuracy of HCV-RNA PCR tests for diagnosis or exclusion of vertically acquired HCV infection. J Med Virol. 2006;78:305–310. doi: 10.1002/jmv.20540. [DOI] [PubMed] [Google Scholar]
- 478.Guido M, Rugge M, Jara P, Hierro L, Giacchino R, Larrauri J, et al. Chronic hepatitis C in children: the pathological and clinical spectrum. Gastroenterology. 1998;115:1525–1529. doi: 10.1016/s0016-5085(98)70032-0. [DOI] [PubMed] [Google Scholar]
- 479.Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, et al. NASPGHAN practice guidelines: diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr. 2012;54:838–855. doi: 10.1097/MPG.0b013e318258328d. [DOI] [PubMed] [Google Scholar]
- 480.González-Peralta RP, Kelly DA, Haber B, Molleston J, Murray KF, Jonas MM, et al. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology. 2005;42:1010–1018. doi: 10.1002/hep.20884. [DOI] [PubMed] [Google Scholar]
- 481.Wirth S, Lang T, Gehring S, Gerner P. Recombinant alfa-interferon plus ribavirin therapy in children and adolescents with chronic hepatitis C. Hepatology. 2002;36:1280–1284. doi: 10.1053/jhep.2002.36495. [DOI] [PubMed] [Google Scholar]
- 482.Wirth S, Pieper-Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology. 2005;41:1013–1018. doi: 10.1002/hep.20661. [DOI] [PubMed] [Google Scholar]
- 483.Wirth S, Ribes-Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, et al. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol. 2010;52:501–507. doi: 10.1016/j.jhep.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 484.Schwarz KB, Gonzalez-Peralta RP, Murray KF, Molleston JP, Haber BA, Jonas MM, et al. The combination of ribavirin and peginterferon is superior to peginterferon and placebo for children and adolescents with chronic hepatitis C. Gastroenterology. 2011;140:450–458. doi: 10.1053/j.gastro.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]