ABSTRACT
BACKGROUND
Patient activation interventions (PAIs) engage patients in care by promoting increased knowledge, confidence, and/or skills for disease self-management. However, little is known about the impact of these interventions on a wide range of outcomes for adults with type 2 diabetes (DM2), or which of these interventions, if any, have the greatest impact on glycemic control.
METHODS
Electronic databases were searched from inception through November 2011. Of 16,290 citations, two independent reviewers identified 138 randomized trials comparing PAIs to usual care/control groups in adults with DM2 that reported intermediate or long-term outcomes or harms. For meta-analyses of continuous outcomes, we used a random-effects model to derive pooled weighted mean differences (WMD). For all-cause mortality, we calculated the pooled odds ratio (OR) using Peto’s method. We assessed statistical heterogeneity using the I2 statistic and conducted meta-regression using a random-effects model when I2 > 50 %. A priori meta-regression primary variables included: intervention strategies, intervention leader, baseline outcome value, quality, and study duration.
RESULTS
PAIs modestly reduced intermediate outcomes [A1c: WMD 0.37 %, CI 0.28–0.45 %, I2 83 %; SBP: WMD 2.2 mmHg, CI 1.0–3.5 mmHg, I2 72 %; body weight: WMD 2.3 lbs, CI 1.3–3.2 lbs, I2 64 %; and LDL-c: WMD 4.2 mg/dL, CI 1.5–6.9 mg/dL, I2 64 %]. The evidence was moderate for A1c, low/very low for other intermediate outcomes, low for long-term mortality and very low for complications. Interventions had no effect on hypoglycemia (evidence: low) or short-term mortality (evidence: moderate). Higher baseline A1c, pharmacist-led interventions, and longer follow-up were associated with larger A1c improvements. No intervention strategy outperformed any other in adjusted meta-regression.
CONCLUSIONS
PAIs modestly improve A1c in adults with DM2 without increasing short-term mortality. These results support integration of these interventions into primary care for adults with uncontrolled glycemia, and provide evidence to insurers who do not yet cover these programs.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2855-4) contains supplementary material, which is available to authorized users.
KEY WORDS: type 2 diabetes, patient activation, interventions
INTRODUCTION
Type 2 diabetes is common and contributes to excess morbidity, mortality, and health care costs, accounting for approximately one in five U.S. health care dollars.1,2 Behavioral interventions are one important way to improve patient outcomes,3 yet less is known about a subset of novel behavioral interventions focused on engaging patients in care4–6 (often termed “patient activation”7,8). Hibbard et al.7,9 have described patient activation interventions as those that promote motivation, knowledge, and disease self-management skills.7,9 Despite the growing evidence suggesting improved patient outcomes by engaging patients,4–6,10 these interventions are often not integrated into practice due to uncertainty about benefit, lack of resources needed for integration, lack of health insurance coverage across insurers, and lack of clarity on the best strategies to incorporate.11,12 Understanding the impact of patient activation interventions on health outcomes in adults with diabetes, and identifying which intervention strategies, if any, are most effective is vitally important to adults with diabetes and to those who develop, implement, and fund diabetes intervention programs.
Patient activating strategies, as a subset of behavioral interventions, are thought to be promising clinical tools, but have not been rigorously evaluated. Also, most prior systematic reviews of behavioral or quality improvement interventions have focused on limited clinical outcomes such as A1c and weight.3,13 We therefore conducted a systematic review and meta-analysis to evaluate the effectiveness and safety of patient activating interventions for adults with type 2 diabetes on a range of clinically relevant outcomes.
METHODS
Data Sources and Search Strategy
Our systematic review was based on a protocol developed by the authors. We searched for original articles in MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from inception to November 2011. We reviewed reference lists of included articles and relevant review articles. The search strategy for the bibliographic databases combined terms for type 2 diabetes, patient activating interventions and randomized controlled trials (RCTs), and was limited to English-language reports (Appendix Table 1). All appendix tables and figures are available online.
Study Selection
Two reviewers independently reviewed titles, abstracts, and articles to identify eligible studies. We included original RCTs in non-pregnant persons aged 18 years or older with type 2 diabetes that assessed the benefits or harms of patient activating interventions compared with usual care or a control group where minimal intervention occurred (e.g., educational brochure). Patient activation was defined as any intervention that aimed to increase patient motivation, confidence, and skills in disease self-management.7 This definition is similar to behavioral interventions, but excludes interventions with didactic education alone.
We included studies that reported on at least one major long-term clinical outcome (i.e., all-cause mortality; cardiovascular mortality; cardiovascular morbidity defined as stroke, transient ischemic attack or heart attack; retinopathy; nephropathy; foot ulcers; peripheral arterial disease; or neuropathy), intermediate endpoint [i.e., hemoglobin A1c (A1c), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), systolic blood pressure (SBP), or body weight], or safety outcome (i.e. hypoglycemia, short-term mortality, and other serious adverse events). We used the U.S. Food and Drug Administration definition of a serious adverse event as an event that may jeopardize the patient and may require medical or surgical intervention to prevent a disability or death (e.g., hypotension).14 We also excluded studies that followed patients for less than 3 months (since A1c effects require 3 months to manifest), had fewer than 40 subjects, or were limited to specific sub-populations such as patients with diabetes and comorbid hypothyroidism. The search and selection process is described in Appendix Figure 1.
Data Extraction and Quality Assessments
Both reviewers used standardized forms to extract data on study design; interventions and duration; participant characteristics (e.g., age, sex, and race); eligibility criteria; outcome measures; and outcome results along with their measures of variability. The primary reviewer entered the data while the secondary reviewer confirmed it. Both reviewers independently assessed reported quality using the validated Jadad criteria.15 Discrepancies were resolved between reviewers, or by a third reviewer if needed.
Data Synthesis and Analysis
We conducted qualitative and quantitative syntheses (when there were three or more studies with sufficiently similar populations and outcomes) for each outcome. For meta-analyses of continuous outcomes (i.e.intermediate outcomes), we used a random-effects model with the DerSimonian and Laird formula16 to derive pooled post-intervention weighted mean differences. For all-cause mortality, we calculated the pooled odds ratio using the principles of intention to treat and using Peto’s method.17 We stratified all-cause mortality by study outcome reported ≤ 2 years and > 2 years, since we decided a priori that studies were unlikely to show any impact on long-term mortality at < 2 years.18 Long-term diabetes complications and safety are described qualitatively. We synthesized results of studies that reported on diabetes complications after 2 years, since we decided a priori that studies were unlikely to show any impact at < 2 years.19 For studies with multiple arms, we used only two arms to ensure study independence, and chose to include the most intensive intervention in the meta-analysis.
We tested for statistical heterogeneity by using a chi-square test with a significance threshold for alpha of ≤ 0.10 and an I2 statistic greater than 50 %.20 We conducted meta-regression using a random effects model to explore possible sources of heterogeneity using a priori study-level characteristics. We divided our meta-regression analyses into two sets of variables. The first set were variables we felt were most likely to explain the between-study variability and included baseline outcome values, study duration, the Jadad quality score,15 intervention leader (e.g., physician, pharmacist), and types of intervention strategies. Since no standard taxonomy exists to classify patient activating strategies, we developed a taxonomy based on a combination of expert opinion, prior literature, and common categories seen in the first 15 articles we evaluated. The strategies included: financial incentives; audit and feedback; psychological counseling; theory-based counseling; problem solving; skill building; lay health advisors/community health workers; peer/family support; and individualized care plans (Table 1).
Table 1.
Definitions of Patient Activating Strategies Used in the Interventions
| 1. Problem solving: Studies have used problem solving as a multidimensional construct, comprising both effective and ineffective problem-solving strategies, emotional and cognitive orientation to problem solving, ability to learn from past experience, and environmental context.21 Problem solving was checked only if problem solving was specifically mentioned in the article, even if a description of problem solving was not provided. |
| 2. Audit and feedback: Any summary of clinical performance over a specified period of time that reported objectively measured professional practice in a healthcare setting or healthcare outcomes.22 This category also includes any kind of diary maintained by a participant during the intervention period. |
| 3. Individualized care plans: At least one aspect of the intervention involved tailoring it to the needs of each individual participant. For example, individualized care plans could include individualized meal plans, diet, exercise regimens, diabetes care recommendations, and self-management counseling. |
| 4. Financial incentive: Any free or low-cost offering used to motivate the patient to participate in a diabetes intervention study. Examples included free or low cost food, device, counseling, or gym or other membership. This category could also include cash, vouchers, lottery tickets, or gifts. |
| 5. Peer support/family: The provision of emotional, appraisal, and informational assistance by a created social network member who possesses experiential knowledge of a specific behavior or stressor and similar characteristics as the target population, to address a health-related issue of the participant. Peer support or family was checked if peer support or family was fully part of an intervention. If peer or family involvement was optional, then it was not checked. If the peer was functioning as a lay health advisor, then lay health advisor was checked instead of peer support. |
| 6. Lay health advisor/community health worker: Lay members of communities who work either for pay or as volunteers in association with the local health care system in both urban and rural environments and who usually share ethnicity, language, socioeconomic status, and life experiences with the community members they serve.23 |
| 7. Psychological counseling: Psychological counseling referred to more formal counseling done by psychiatrists or psychologists, such as cognitive behavioral therapy. |
| 8. Theory-based counseling: Theory-based counseling was defined as counseling conducted to motivate behavior change and based on the work of a behavioral change theory.24 This category often included motivational interviewing. |
| 9. Skill building: Learning activities/protocols that are content-driven and intended to develop core skills. These activities may build upon a previously acquired skill and/or develop and hone a new skill. Skill building activities included demonstration/return demonstration, simulation, role play, inoculation, or activism participation.25 For example, participants engaged in the following: communication; self-management and self-care; coping; Life Skills; listening; mindfulness; meditation; and more. |
We also explored a second set of variables in the meta-regression (from our list of a priori study-level characteristics) in order to better assess between-study variability. These variables included country, intervention location (primary care clinic, diabetes clinic, other/NR), mode (group, one-on-one, other), type (in-person, phone, internet, other), intensity (number of sessions), intervention focus (e.g. healthy eating, exercise), study funding (for-profit or non-profit), and intervention theory. The final meta-regression model included all of the first set of variables plus two additional variables (country and setting) from the second set that were found to be potentially significant in univariable meta-regression models (p value close to 0.05).
We conducted sensitivity analyses by omitting one study at a time to assess the influence of any single study on the pooled estimate. Publication bias was tested using the Begg and Mazumdar26 and the Egger test.27 If publication bias was present, we used the trim and fill technique28 to assess the impact of publication bias on the point estimate. All statistical analyses were performed using STATA, version 12.1 (StataCorp, College Station, Texas).
Grading of the Evidence
Two reviewers independently graded the quantity, quality, and consistency of the results; directness of the measures used for each outcome; precision of the results; and magnitude of the effect using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) working group criteria.29 Risk of bias across studies was determined by evaluating the Jadad quality scores for the group of studies for each outcome. Since double blinding is often impossible in patient activation interventions, the highest expected quality score would be a 3 instead of a 5. Therefore, a score of 3 was considered high quality, and a score of ≤ 1 was considered low quality. If > 30 % of the studies were graded low quality for an outcome, then we downgraded the evidence strength one level (from high to moderate strength). If >50 % were graded low quality, then we downgraded the evidence to low strength. Any disagreements were resolved by group consensus. “High” strength of evidence indicates that the evidence probably reflects the true effect; “moderate” strength indicates that further research may change the result; “low” strength indicates low confidence that the evidence reflects the true effect and further research is very likely to change the result, and “very low” indicates that any estimate of effect is very uncertain.
RESULTS
Study Characteristics and Quality
Out of 16,291 citations, 138 studies with 33,124 participants (mean 244 participants per RCT) met the inclusion criteria (Appendix Figure 1). A full reference list can be found in the Online Appendix. All included studies were RCTs and most occurred in the US (48 %) or Europe (32 %). An appropriate method of randomization was only found in 44 % of the studies, since most studies did not report the method of randomization in sufficient detail (54 %). About one-third of the studies (34 %) did not report an adequate description of withdrawals or dropouts. The mean Jadad quality score was 2 (range 0 to 3). Most studies were considered moderate (42 % with score of 2) or high quality (31 % with score of 3) (Appendix Table 2). The mean study duration was 12 months, and only 7 % of studies lasted more than 2 years.
Participants were mainly middle-aged (weighted mean age 59 years in the 112 studies reporting age), overweight or obese adults [weighted mean baseline body mass index (BMI) 33 kg/m2 in the 89 studies that reported BMI] with fair baseline glucose and blood pressure control (weighted mean A1c 8.1 % and weighted mean SBP 140 mmHg, in 119 and 71 studies reporting these measures, respectively). In the 60 studies that reported diabetes duration, the weighted mean diabetes duration was 10 years. About a quarter of studies (24 %) excluded patients with mental illness or those with diabetes-associated complications such as cardiovascular disease (Appendix Table 3).
Interventions are described in Appendix Table 4, and generally included a team composed of physicians (48 %), nurses (44 %), dieticians (28 %), and/or diabetes educators (17 %). The initial intervention occurred mainly in a primary care clinic (31 %), diabetes clinic (11 %), home (19 %; either online, by phone or in-person), or was not reported (26 %). For the 127 intervention groups that reported the intended number of sessions, the median number and interquartile range (IQR) of intended sessions was 9 (IQR 6 to 14). For the 29 % of studies with fixed intervention session duration, the median duration was 1.5 hours per session (IQR 1 to 2 hours per session).
Long-Term Clinical Outcomes
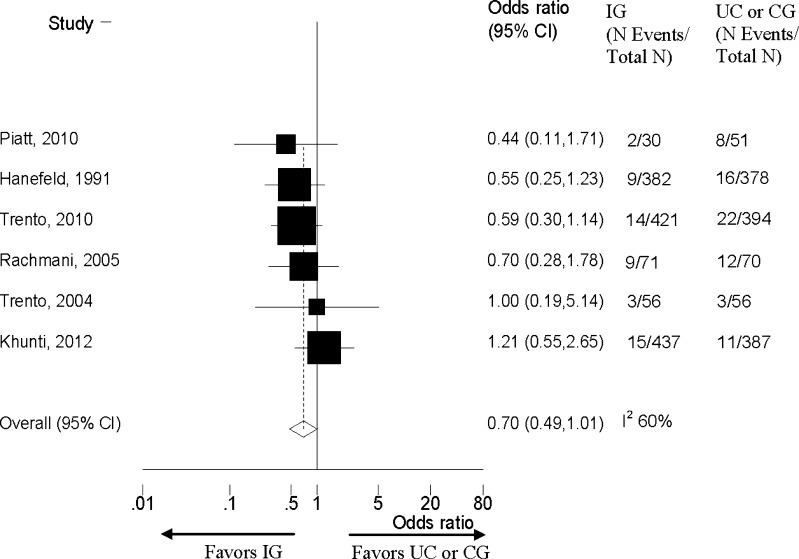
The six patient activation intervention studies reporting deaths with more than 2 years of follow-up showed no significant differences in mortality (Fig. 1 and Table 2).30–35 We summarize the key study characteristics for these studies30–35 in Appendix Table 5. No study evaluated mortality as a primary outcome. Due to few events and inconsistency in these studies, we graded the strength of the evidence as low (Table 2).
Figure 1.
Effects of longer duration (> 2 years) patient activation intervention studies on all cause mortality in adults with Type 2 diabetes. IG=Intervention group; UC= Usual care; CG = Minimal control group; N = Number; CI= Confidence Interval.
Table 2.
Evidence Summary and GRADE Analysis
| Outcome | N RCTs (N participants) | Summary of results | I2 (%) | Risk of bias* | Consistency | Directness | Precision | Conclusion | Evidence strength |
|---|---|---|---|---|---|---|---|---|---|
| Intermediate outcomes | |||||||||
| A1c (%) | 111 (12,780) | WMD −0.37 (CI −0.45 to −0.28) | 83 | 0 | −1 | 0 | 0 | Small improvement | Moderate |
| SBP (mmHg) | 54 (7,630) | WMD −2.2 (CI −3.5 to −1.0) | 72 | 0 | −1 | 0 | −1 | Small improvement | Low |
| LDL-c (mg/dL) | 37 (4,845) | WMD −4.2 (CI −6.9 to −1.5) | 64 | 0 | −1 | −1 | −1 | Small improvement | Very low |
| HDL-c (mg/dL) | 34 (4,908) | WMD 0.03 (CI −0.8 to 0.8) | 52 | 0 | −1 | −1 | 0 | No difference | Low |
| TG (mg/dL) | 38 (5,021) | WMD −8.5 (CI −15.0 to −2.3) | 51 | 0 | −1 | −1 | −1 | Small improvement | Very low |
| Body weight (pounds) | 43 (5,749) | WMD −2.3 (CI −3.2 to −1.3) | 64 | 0 | −1 | 0 | −1 | Small improvement | Low |
| Long-term outcomes (studies with ≥ 2 years of follow-up) | |||||||||
| Long-term mortality | 6 (2,733) | OR 0.70 (CI 0.49-1.01) | 60 | 0 | −1 | 0 | −1 | No difference | Low |
| CVD morbidity | 1 (141) | RD: 20 % less CVD morbidity in IG | NA | 0 | NA | 0 | −1 | Small improvement | Very low† |
| Nephropathy | 1 (141) | RD: 10 % less patients with proteinuria and 4 % less ESRD in IG | NA | 0 | NA | 0 | −1 | Modest improvement | Very low† |
| Retinopathy | 2 (251) | 1) RD: 20 % more patients developed retinopathy in UC; 2) OR 5.4 (1.2-25.1) for UC vs IG | NA | −1 | 0 | 0 | −1 | Modest improvement | Very low† |
| Safety | |||||||||
| Short-term mortality | 38 (8,791) | OR 0.85 (CI 0.61-1.17) | 6 | 0 | −1 | 0 | 0 | No difference | Moderate |
| Hypoglycemia | 18 (3,579) | No clear differences but low event rates | NA | 0 | −1 | 0 | −1 | No difference | Low |
* While the Jadad quality score goes from 0 to 5, most behavioral interventions would not be expected to be double blinded. The highest quality score these studies could receive if not double blinded would be a score of 3. Therefore, we rated risk of bias across studies as having a serious limitation (−1) in study quality if > 30 % of the studies were rated as ≤ 1. No significant or meaningful publication bias was detected
†We rated the long-term complications as very low due to the scarcity of data on these outcomes. Consistency was not rated when only one RCT reported outcomes
A1c hemoglobin A1c; SBP systolic blood pressure; LDL-c low density lipoprotein cholesterol; HDL-c high density lipoprotein cholesterol; TG triglycerides; WMD weighted mean difference; CI 95 % confidence interval, RD absolute risk difference; CVD cardiovascular disease; IG intervention group; UC usual care; OR odds ratio
Four diabetes complications were reported in two studies lasting greater than 2 years,31,36 including nephropathy (N = 1), retinopathy (N = 2), and non-fatal cardiovascular events (N = 1). While both studies showed greater absolute improvements in the intervention groups,31,36 too few studies existed to draw any firm conclusions about any particular complication (Table 2 and Appendix Table 6).
Intermediate Outcomes
Patient activation interventions modestly decreased A1c, SBP, body weight, and LDL-c and triglycerides more than usual care or control groups (Table 2 and Appendix Table 4). No single study influenced any of these results. The strength of the evidence was moderate for A1c, given that results were considered direct and precise with a low risk of bias. All other intermediate outcomes were rated as low or very low since results were inconsistent, imprecise, and/or indirect (Table 2). Studies with higher mean baseline values for A1c, SBP, and LDL-c had a greater reduction in these outcomes (Table 3).
Table 3.
Effects of Interventions on Intermediate Outcomes Stratified by Median Baseline Value
| Baseline value | N of studies | WMD (95 % CI) | I2 |
|---|---|---|---|
| A1c < 8 % | 55 | −0.28 (−0.40 to −0.16) | 88 % |
| A1c ≥ 8 % | 56 | −0.48 (−0.60 to −0.35) | 69 % |
| SBP < 137 mmHg | 26 | −1.3 (−3.0 to 0.4) | 52 % |
| SBP ≥ 137 mmHg | 28 | −2.9 (−4.7 to −1.2) | 79 % |
| LDL-c < 112 mg/dL | 18 | −2.6 (−5.4 to 0.1) | 34 % |
| LDL-c ≥ 112 mg/dL | 19 | −5.6 (−10 to −1.3) | 68 % |
| HDL-c < 46.5 mg/dL | 17 | −0.2 (−1.1 to 0.6) | 73 % |
| HDL-c ≥ 46.5 mg/dL | 17 | 0.12 (−1.2 to 1.5) | 63 % |
| TG < 176 mg/dL | 19 | −9.2 (−18.3 to −0.1) | 72 % |
| TG ≥ 176 mg/dL | 19 | −4.2 (−11.6 to 3.2) | 45 % |
| Weight < 202 lbs | 20 | −2.5 (−3.9 to −1.1) | 66 % |
| Weight ≥ 202 lbs | 23 | −2.0 (−3.4 to −0.6) | 62 % |
WMD weighted mean difference; CI confidence interval; N number; A1c hemoglobin A1c; SBP systolic blood pressure; LDL-c low density lipoprotein cholesterol; TG triglycerides; HDL-c high density lipoprotein cholesterol
Safety
Short-Term Mortality
Thirty-eight studies lasting ≤ 24 months reported deaths,5,35–71 and showed no significant differences in all-cause mortality between the intervention and usual care or control groups (pooled OR 0.90, 95 % CI 0.64–1.28; Appendix Figure 2). No single study strongly influenced the results. The strength of the evidence for short-term mortality was graded as moderate, given that results were precise, direct, and had low risk of bias (Table 2).
Hypoglycemia
Of the 138 studies, only 18 reported on hypoglycemia (Appendix Table 7),4,21,49,61,62,69,72–83 showing no clear differences between groups in hypoglycemia. The strength of evidence was graded as low, given the inconsistency and imprecision of the results (Table 2). Most studies reported hypoglycemia as total hypoglycemic events75–77(N = 5) or participants experiencing hypoglycemia (N = 8).61,62,69,73,74,78,80,82 Hypoglycemic outcomes were defined heterogeneously, with some studies based on self report4,21,62,72,73,77,82 and others on glucose readings.74,76,78 Twelve of the 18 studies reported measures of variability.4,49,61,62,69,72,73,74,76,78,80,82 Most (66 %) of these studies showed no statistically significant differences between groups (N = 8)49,61,69,73,74,78,80,82; however, studies had limited power due to small numbers of events. Three of the remaining four studies showed significantly less hypoglycemia in the intervention groups,4,72,76 while one study showed significantly less hypoglycemia in the usual care group.62
Other Serious Adverse Events
Studies rarely and inconsistently reported other serious adverse events (three of 138 articles; Appendix Table 8).4,49,84 The only other serious adverse events reported were hyperglycemia and hypotension. Too few studies existed for us to draw conclusions regarding hyperglycemia or hypotension (Evidence Grade: Very Low).
Unadjusted Stratified Meta-Analyses and Adjusted Meta-regression
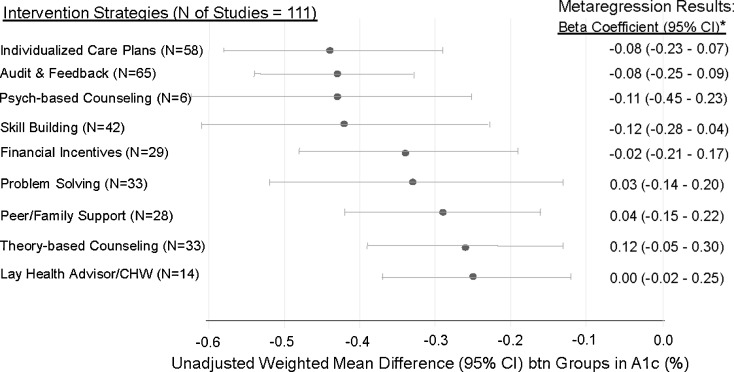
In Figure 2, we show the unadjusted stratified meta-analyses and the final adjusted meta-regression results by intervention strategy for A1c. All of the intervention strategies modestly improved A1c with audit and feedback, skill building, psych-based counseling, and individualized care plans having the largest unadjusted effect. However, no single intervention strategy showed significantly larger or smaller between group differences in A1c after adjustment. Similarly, the meta-regression results for all other intermediate outcomes showed no differences in outcome by intervention strategy (data not shown). Higher baseline A1c (Beta coefficient (B) -0.11, CI −0.19 to −0.02), longer study duration (B −0.01 per month, CI −0.01 to 0.00), pharmacist-led interventions (B −0.50, CI −0.85 to −0.15), and study location outside the US, Europe or Canada (B −0.40, CI −0.61 to −0.18) were associated with larger between group differences in A1c. The final meta-regression model (variables listed under Fig. 2) explained 46 % of the between-study heterogeneity (adjusted r-squared 0.46).
Figure 2.
Unadjusted meta-analyses and adjusted meta-regression results for A1c by intervention strategy. *Meta-regression results show beta coefficients adjusted for all other listed intervention strategies and for significant variables found in univariate meta-regression analyses, or for key variables felt to be important for adjustment: mean baseline A1c, pharmacist-led interventions (Y vs. N), country of origin (Other vs. Europe, Canada and the US), Jadad quality score, and initial intervention setting (diabetes clinic vs. primary clinic vs. other/NR). N= Number of studies, CI = 95 % confidence interval, and CHW= community health worker, NR=Not reported.
While unadjusted meta-regression analyses showed larger improvements in A1c in diabetes versus primary care clinic settings (B −0.04, CI −0.34 to −0.26) and smaller improvements in studies with allocation concealment (B 0.18, CI 0.01 to 0.35), these differences were non-significant in adjusted models. Unadjusted meta-regression analyses showed no significant effects of several other factors, including the intervention theory, number of intended intervention sessions, intervention mode and setting, intervention focus, and study funding.
Publication Bias
Funnel plots for all intermediate clinical outcomes did not demonstrate evidence of publication bias except for A1c, p = 0.04. However, a sensitivity analysis using the trim and fill technique showed no change in point estimate or confidence interval for A1c.
DISCUSSION
In our study, we evaluated nine different types of patient activating strategies in 138 studies with over 33,000 adults with type 2 diabetes. We found moderate strength of evidence that these interventions improve A1c (range in WMD from −0.25 % to −0.44 %) without increasing short-term mortality. In addition, over 30 studies showed small improvements in other intermediate outcomes (SBP, LDL-c, triglycerides, and body weight). The strength of evidence was rated low or very low for these outcomes, due to inconsistency among the studies and concerns about the clinical benefit of these small improvements. Multiple strategies were used in these patient activating interventions to engage patients and improve patient outcomes. Although psych-based counseling, audit and feedback, individualized care plans and problem solving skills had the largest unadjusted improvements in A1c, no single strategy significantly outperformed any other. Higher baseline A1c, pharmacist-led interventions, longer follow-up, and having the study occur outside the US, Canada or Europe all were associated with larger improvements in A1c. A reduction in A1c in adults with type 2 diabetes of 1 absolute percentage point has been associated with a 21 % reduction in mortality;19 therefore, these interventions could have a strong impact on long-term morbidity and mortality, especially if they can improve multiple outcomes.
We had a limited number of studies with sufficient duration to evaluate the impact on diabetes complications and mortality. The six studies lasting > 2 years that reported few deaths showed no clear differences between groups (pooled OR 0.70, CI 0.49–1.01). The two studies reporting on diabetes complications at > 2 years reported a benefit in the intervention compared to control groups. Our review showed no evidence of differences between groups in several harms, including hypoglycemia and short-term mortality. Other potential harms were not reported consistently.
An updated search through December 2013 in PubMed found one additional RCT that deserves mention. The Look AHEAD trial compared an intensive lifestyle intervention for weight loss through decreased caloric intake and increased physical activity versus diabetes support and education among 5,145 overweight or obese adults with type 2 diabetes.85 Audit and feedback was used as a strategy for self-monitoring diet and exercise. After a median of 9.6 years of follow-up, they reported a 2.5 % difference in weight between groups, and found no differences in the primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina.85 This RCT focused solely on weight reduction in relatively well-controlled adults with type 2 diabetes (mean baseline A1c 7.2 %). Consistent with our findings, it is still unclear which patient activation interventions will impact long-term outcomes.
Prior systematic reviews have evaluated the effects of behavioral interventions mainly on the outcomes of A1c and weight, showing similar results to our findings.3,86 This study adds to the literature by developing a taxonomy of patient activating strategies, and evaluating these nine different strategies on a host of clinically relevant outcomes. Our study was novel in several additional ways. First, we evaluated which strategy had the greatest impact on A1c. No one strategy had a significantly larger impact on A1c, suggesting that we should integrate the most efficient and least expensive interventions. Second, we found that pharmacist-led interventions and studies with longer duration were associated with larger improvements in A1c. Lastly, we found no evidence that interventions conducted in diabetes clinics versus primary care settings achieve better outcomes.
Our systematic review and meta-regression have several limitations based on the literature reviewed and inherent to meta-regression. First, we may have found no differences between strategies in the meta-regression due to insufficient reporting of intervention details. Second, many articles did not report outcomes or measures of variability consistently, making it difficult to combine studies to evaluate a specific outcome or adverse event. Third, significant factors associated with larger improvements in A1c in the meta-regression may be confounded with other variables. For instance, authors may be more likely to report on studies with longer follow-up if the study showed a significant between-group difference in A1c. However, we did not note any significant effect of publication bias in our analyses. Fourth, most studies were small and short, limiting our ability to detect clinically important harms and long-term benefits most important to patients. Fifth, the large I-squared values in the meta-analyses suggests substantial heterogeneity, which is not surprising given the differing countries and usual care/control groups. Our meta-regression variables explained 46 % of the between-study heterogeneity, which suggests that inclusion of unmeasured variables or more clear reporting of existing variables could better explain this heterogeneity. Sixth, meta-regression analyses are often limited in power to detect differences where they might exist; however, we did have over 100 studies in the A1c meta-regression analyses. Lastly, because meta-regression analyses are observational in nature, significant associations do not imply causation.
Our study has several important implications for clinical care, research and policy. First, our findings reinforce the evidence base to promote integration of disease self-management programs in health care and community settings, especially for those adults with A1c > 8 %. While the American Diabetes Association endorses disease self-management as a standard of care87 and the Center for Medicare Services reimburses for these services, many private insurers still do not cover disease self-management for adults with diabetes.88 If reimbursement is unavailable or insufficient, then practices will not be able to build, sustain or provide these services to patients who need them, especially in underserved communities who often have no coverage or are underinsured. Future cost effectiveness studies will be useful to help support policy reform, which could mandate insurers to offer coverage for patients who are not well controlled. Second, using pharmacists to help lead and create interventions that combine patient activation strategies and medication intensification protocols will likely yield greater improvements in patient outcomes. Third, we found that studies with longer duration had larger improvements in A1c; therefore, we should ensure programs are sustained and accessible to patients over time. Lastly, diabetes intervention research can be improved by clearer reporting of intervention details to allow accurate classification and replication of intervention strategies (possibly using the taxonomy of strategies reported in this article), standardized methods for defining and reporting adverse effects, attention to and reporting of allocation concealment, and consideration of a priori subgroup analyses to determine which populations to target with specific interventions. To address these concerns, journals should consider requiring authors to publish the expanded CONSORT checklist for nonpharmacologic RCTs in an Online appendix, along with intervention strategies to allow for more accurate future analyses.89
In conclusion, patient activating interventions modestly improve A1c without increasing short-term mortality. Actively engaging adults with uncontrolled diabetes in self-management within the health care and community setting is likely to have a strong cumulative impact on both morbidity and mortality. Future clinical and research efforts should encourage and evaluate dissemination of patient activating interventions within health care settings and the community with specific assessment of long-term outcomes and cost. Lastly, future policy efforts should consider mandatory coverage of self-management programs by insurers for patients with uncontrolled glycemia.
Electronic supplementary material
(DOCX 221 kb)
Acknowledgements
We would like to thank the following people who received compensation for assisting with title, abstract or article reviews and/or data extraction: Hardeep Phull MD1, Amir Raed MPH2, and Caitlin Mocarski MPH2. We would like to thank Shriva Shrotriya MD, MPH2 who also helped with title, abstract, and article review and data abstraction without compensation. We would like to thank James Werner PhD3 who without compensation assisted with title and abstract reviews, and contributed to our discussions of patient activating interventions. Finally, we would like to thank Randall Cebul MD4,5 and Shirley Moore PhD6 for their uncompensated time giving advice at key study junctures.
Affiliations of Contributors Who are Not Authors
1. The Cleveland Clinic Lerner College of Medicine, Case Western Reserve University (CWRU), Cleveland, OH
2. Department of Biostatistics and Epidemiology, CWRU, Cleveland, OH
3. Department of Family Medicine, CWRU, Cleveland, OH
4. Center for Health Care Research and Policy, MetroHealth Medical Center/CWRU, Cleveland, OH
5. Department of Medicine, MetroHealth Medical Center/CWRU, Cleveland, OH
6. The School of Nursing, CWRU, Cleveland, OH
Funding Support and Role of the Sponsor
The project funding was supported by MetroHealth Medical Center using Dr. Bolen’s start-up research funds. Dr. Bolen’s time was supported by the National Center for Research Resources (grant #KL2 RR024990) and the National Center for Advancing Translational Sciences (grant # KL2TR000440), National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Bolen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest and Financial Disclosures
The authors declare that they do not have any conflict of interest.
REFERENCES
- 1.National Diabetes Fact Sheet, 2011. Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. 2011. Accessed 3-5-2014.
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 3.Gary TL, Genkinger JM, Guallar E, Peyrot M, Brancati FL. Meta-analysis of randomized educational and behavioral interventions in type 2 diabetes. Diabetes Educ. 2003;29:488–501. doi: 10.1177/014572170302900313. [DOI] [PubMed] [Google Scholar]
- 4.Lorig K, Ritter PL, Villa F, Piette JD. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabetes Care. 2008;31:408–414. doi: 10.2337/dc07-1313. [DOI] [PubMed] [Google Scholar]
- 5.Cooper H, Booth K, Gill G. A trial of empowerment-based education in type 2 diabetes—global rather than glycaemic benefits. Diabetes Res Clin Pract. 2008;82:165–171. doi: 10.1016/j.diabres.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Brown SA, Hanis CL. A community-based, culturally sensitive education and group-support intervention for Mexican Americans with NIDDM: a pilot study of efficacy. Diabetes Educ. 1995;21:203–210. doi: 10.1177/014572179502100307. [DOI] [PubMed] [Google Scholar]
- 7.Hibbard JH, Collins PA, Mahoney E, Baker LH. The development and testing of a measure assessing clinician beliefs about patient self-management. Health Expect. 2010;13:65–72. doi: 10.1111/j.1369-7625.2009.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remmers C, Hibbard J, Mosen DM, Wagenfield M, Hoye RE, Jones C. Is patient activation associated with future health outcomes and healthcare utilization among patients with diabetes? J Ambul Care Manag. 2009;32:320–327. doi: 10.1097/JAC.0b013e3181ba6e77. [DOI] [PubMed] [Google Scholar]
- 9.Stepleman L, Rutter MC, Hibbard J, Johns L, Wright D, Hughes M. Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disabil Rehabil. 2010;32:1558–1567. doi: 10.3109/09638280903567885. [DOI] [PubMed] [Google Scholar]
- 10.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 11.Crossing the quality chasm: a new health system for the 21st century. Committee on Quality Health Care in America, IOM. Available at: http://www.nap.edu/openbook.php?record_id=10027&page=13. Washington D.C.: National Academy Press, 2012. Accessed 03-05-2014.
- 12.Timbie JW, Schneider EC, Van BK, Fox DS. Five reasons that many comparative effectiveness studies fail to change patient care and clinical practice. Health Aff (Millwood) 2012;31:2168–2175. doi: 10.1377/hlthaff.2012.0150. [DOI] [PubMed] [Google Scholar]
- 13.Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 14.US food and drug administration. Protecting and promoting your health. Available at: http://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm. 8-23-2014. Accessed 03-05-2014.
- 15.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/S0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 18.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 19.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen SD, Kang J, Kline GA. Portion control plate for weight loss in obese patients with type 2 diabetes mellitus: a controlled clinical trial. Arch Intern Med. 2007;167:1277–1283. doi: 10.1001/archinte.167.12.1277. [DOI] [PubMed] [Google Scholar]
- 22.Ivers N, Jamtvedt G, Flottorp S et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;6:CD000259. [DOI] [PMC free article] [PubMed]
- 23.U.S. Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Professions: Community health worker national workforce study, March 2007. Available at http://bhpr.hrsa.gov/healthworkforce/supplydemand/publichealth/communityhealthworkforcebibliography.pdf. 2007. Accessed 03-05-2014.
- 24.Miller RW, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: The Guilford Press; 1991. [Google Scholar]
- 25.Public Health Interventions. Applications for public health nursing practice. Minnesota Department of Health. Available at: http://www.health.state.mn.us/divs/opi/cd/phn/docs/0301wheel_manual.pdf. 2001. Accessed 03-05-2014.
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanefeld M, Fischer S, Schmechel H, et al. Diabetes Intervention Study. Multi-intervention trial in newly diagnosed NIDDM. Diabetes Care. 1991;14:308–317. doi: 10.2337/diacare.14.4.308. [DOI] [PubMed] [Google Scholar]
- 31.Rachmani R, Slavacheski I, Berla M, Frommer-Shapira R, Ravid M. Treatment of high-risk patients with diabetes: motivation and teaching intervention: a randomized, prospective 8-year follow-up study. J Am Soc Nephrol. 2005;16(Suppl 1):S22–S26. doi: 10.1681/ASN.2004110965. [DOI] [PubMed] [Google Scholar]
- 32.Trento M, Passera P, Borgo E, et al. A 5-year randomized controlled study of learning, problem solving ability, and quality of life modifications in people with type 2 diabetes managed by group care. Diabetes Care. 2004;27:670–675. doi: 10.2337/diacare.27.3.670. [DOI] [PubMed] [Google Scholar]
- 33.Trento M, Gamba S, Gentile L, et al. Rethink Organization to iMprove Education and Outcomes (ROMEO): a multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care. 2010;33:745–747. doi: 10.2337/dc09-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piatt GA, Anderson RM, Brooks MM, et al. 3-year follow-up of clinical and behavioral improvements following a multifaceted diabetes care intervention: results of a randomized controlled trial. Diabetes Educ. 2010;36:301–309. doi: 10.1177/0145721710361388. [DOI] [PubMed] [Google Scholar]
- 35.Khunti K, Gray LJ, Skinner T, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ. 2012;344:e2333. doi: 10.1136/bmj.e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettitt DJ, Wollitzer AO, Jovanovic L, He G, Ipp E. Decreasing the risk ofdiabeticretinopathy in a study ofcasemanagement: The California medi-caltype 2diabetesstudy. Diabetes Care. 2005;28:2819–2822. doi: 10.2337/diacare.28.12.2819. [DOI] [PubMed] [Google Scholar]
- 37.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378:129–139. doi: 10.1016/S0140-6736(11)60442-X. [DOI] [PubMed] [Google Scholar]
- 38.Dejesus RS, Chaudhry R, Leutink DJ, Hinton MA, Cha SS, Stroebel RJ. Effects of efforts to intensify management on blood pressure control among patients with type 2 diabetes mellitus and hypertension: a pilot study. Vasc Health Risk Manag. 2009;5:705–711. doi: 10.2147/VHRM.S5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keogh KM, Smith SM, White P, et al. Psychological family intervention for poorly controlled type 2 diabetes. Am J Manage Care. 2011;17:105–113. [PubMed] [Google Scholar]
- 40.Keyserling TC, Samuel-Hodge CD, Ammerman AS, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabetes Care. 2002;25:1576–1583. doi: 10.2337/diacare.25.9.1576. [DOI] [PubMed] [Google Scholar]
- 41.Cavanaugh K, Wallston KA, Gebretsadik T, et al. Addressing Literacy and Numeracy to Improve Diabetes Care: two randomized controlled trials. Diabetes Care. 2009;32:2149–2155. doi: 10.2337/dc09-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker EA, Shmukler C, Ullman R, Blanco E, Scollan KM, Cohen HW. Results of a successful telephonic intervention to improve diabetes control in urban adults: a randomized trial. Diabetes Care. 2011;34:2–7. doi: 10.2337/dc10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiss RG, Armbruster BA, Gillard ML, McClure LA. Nurse care manager collaboration with community-based physicians providing diabetes care: a randomized controlled trial. Diabetes Educ. 2007;33:493–502. doi: 10.1177/0145721707301349. [DOI] [PubMed] [Google Scholar]
- 44.Groeneveld Y, Petri H, Hermans J, Springer M. An assessment of structured care assistance in the management of patients with type 2 diabetes in general practice. Scand J Prim Health Care. 2001;19:25–30. doi: 10.1080/028134301300034585. [DOI] [PubMed] [Google Scholar]
- 45.Wakefield BJ, Holman JE, Ray A, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E Health. 2011;17:254–261. doi: 10.1089/tmj.2010.0176. [DOI] [PubMed] [Google Scholar]
- 46.Heller SR, Clarke P, Daly H, et al. Group education for obese patients with type 2 diabetes: greater success at less cost. Diabet Med. 1988;5:552–556. doi: 10.1111/j.1464-5491.1988.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 47.Thoolen BJ, de Ridder D, Bensing J, Gorter K, Rutten G. Beyond good intentions: the role of proactive coping in achieving sustained behavioural change in the context of diabetes management. Psychol Health. 2009;24:237–254. doi: 10.1080/08870440701864504. [DOI] [PubMed] [Google Scholar]
- 48.Sperl-Hillen JM, Solberg LI, Hroscikoski MC, Crain AL, Engebretson KI, O’Connor PJ. The effect of advanced access implementation on quality of diabetes care. Prev Chron Dis. 2008;5:A16. [PMC free article] [PubMed] [Google Scholar]
- 49.Rothman RL, Malone R, Bryant B, et al. A randomized trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med. 2005;118:276–284. doi: 10.1016/j.amjmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Clark M, Hampson SE, Avery L, Simpson R. Effects of a tailored lifestyle self-management intervention in patients with type 2 diabetes. Br J Health Psychol. 2004;9:365–379. doi: 10.1348/1359107041557066. [DOI] [PubMed] [Google Scholar]
- 51.Samuel-Hodge CD, Keyserling TC, Park S, Johnston LF, Gizlice Z, Bangdiwala SI. A randomized trial of a church-based diabetes self-management program for African Americans with type 2 diabetes. Diabetes Educ. 2009;35:439–454. doi: 10.1177/0145721709333270. [DOI] [PubMed] [Google Scholar]
- 52.Sarkadi A, Rosenqvist U. Experience-based group education in Type 2 diabetes: a randomised controlled trial. Patient Educ Couns. 2004;53:291–298. doi: 10.1016/j.pec.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Adolfsson ET, Starrin B, Smide B, Wikblad K. Type 2 diabetic patients’ experiences of two different educational approaches–a qualitative study. Int J Nurs Stud. 2008;45:986–994. doi: 10.1016/j.ijnurstu.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Sturt J. One-to-one structured education using the Diabetes Manual: evidence of effectiveness. J Diabetes Nurs. 2008;12:368. [Google Scholar]
- 55.Gary TL, Batts TM, Yeh HC, et al. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2009;169:1788–1794. doi: 10.1001/archinternmed.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gary TL, Bone LR, Hill MN, et al. Randomized controlled trial of the effects of nurse case manager and community health worker interventions on risk factors for diabetes-related complications in urban African Americans. Prev Med. 2003;37:23–32. doi: 10.1016/S0091-7435(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 57.Lincoln NB, Radford KA, Game FL, Jeffcoate WJ. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia. 2008;51:1954–1961. doi: 10.1007/s00125-008-1110-0. [DOI] [PubMed] [Google Scholar]
- 58.Smith SM, Paul G, Kelly A, Whitford DL, O’Shea E, O’Dowd T. Peer support for patients with type 2 diabetes: cluster randomised controlled trial. BMJ. 2011;342:d715. doi: 10.1136/bmj.d715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor CB, Miller NH, Reilly KR, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care. 2003;26:1058–1063. doi: 10.2337/diacare.26.4.1058. [DOI] [PubMed] [Google Scholar]
- 60.Anderson DR, Christison LJ, Villagra V, Liu H, Dziura J. Managing the space between visits: a randomized trial of disease management for diabetes in a community health center. J Gen Intern Med. 2010;25:1116–1122. doi: 10.1007/s11606-010-1419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edelman D, Fredrickson SK, Melnyk SD, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: a randomized trial. Ann Intern Med. 2010;152:689–696. doi: 10.7326/0003-4819-152-11-201006010-00001. [DOI] [PubMed] [Google Scholar]
- 62.Farmer AJ, Wade AN, French DP, et al. Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial. Health Technol Assess. 2009;13:iii–xi. doi: 10.3310/hta13150. [DOI] [PubMed] [Google Scholar]
- 63.Di LC, Fanelli C, Lucidi P, et al. Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care. 2003;26:404–408. doi: 10.2337/diacare.26.2.404. [DOI] [PubMed] [Google Scholar]
- 64.Fornos JA, Andres NF, Andres JC, Guerra MM, Egea B. A pharmacotherapy follow-up program in patients with type-2 diabetes in community pharmacies in Spain. Pharm World Sci. 2006;28:65–72. doi: 10.1007/s11096-006-9003-0. [DOI] [PubMed] [Google Scholar]
- 65.Shibayama T, Kobayashi K, Takano A, Kadowaki T, Kazuma K. Effectiveness of lifestyle counseling by certified expert nurse of Japan for non-insulin-treated diabetic outpatients: a 1-year randomized controlled trial. Diabetes Res Clin Pract. 2007;76:265–268. doi: 10.1016/j.diabres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Anderson RM, Funnell MM, Aikens JE, et al. Evaluating the efficacy of an empowerment-based self-management consultant intervention: results of a two-year randomized controlled trial. Ther Patient Educ. 2009;1:3–11. doi: 10.1051/tpe/2009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen LB, Taveira TH, Khatana SA, Dooley AG, Pirraglia PA, Wu WC. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educ. 2011;37:801–812. doi: 10.1177/0145721711423980. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Idigoras MI, Sepulveda-Munoz J, Sanchez-Garrido-Escudero R, et al. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol Ther. 2009;11:431–437. doi: 10.1089/dia.2008.0114. [DOI] [PubMed] [Google Scholar]
- 69.Crasto W, Jarvis J, Khunti K, et al. Multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: the Microalbuminuria Education and Medication Optimisation (MEMO) study. Diabetes Res Clin Pract. 2011;93:328–336. doi: 10.1016/j.diabres.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health. 2011;101:2253–2260. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rygg LO, Rise MB, Gronning K, Steinsbekk A. Efficacy of ongoing group based diabetes self-management education for patients with type 2 diabetes mellitus. A randomised controlled trial. Patient Educ Couns. 2012;86:98–105. doi: 10.1016/j.pec.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ. 2009;35:641–651. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- 73.Negri C, Bacchi E, Morgante S, et al. Supervised walking groups to increase physical activity in type 2 diabetic patients. Diabetes Care. 2010;33:2333–2335. doi: 10.2337/dc10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim S, Kang SM, Shin H, et al. Improved glycemic control without hypoglycemia in elderly diabetic patients using the ubiquitous healthcare service, a new medical information system. Diabetes Care. 2011;34:308–313. doi: 10.2337/dc10-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoo HJ, Park MS, Kim TN, et al. A ubiquitous chronic disease care system using cellular phones and the internet. Diabet Med J Br Diabet Assoc. 2009;26:628–635. doi: 10.1111/j.1464-5491.2009.02732.x. [DOI] [PubMed] [Google Scholar]
- 76.Bujnowska-Fedak MM, Puchala E, Steciwko A. The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland. Telemed J E Health. 2011;17:153–163. doi: 10.1089/tmj.2010.0113. [DOI] [PubMed] [Google Scholar]
- 77.Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2:203–211. doi: 10.1111/j.1753-0407.2010.00081.x. [DOI] [PubMed] [Google Scholar]
- 78.Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34:262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with Type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011;28:789–796. doi: 10.1111/j.1464-5491.2011.03268.x. [DOI] [PubMed] [Google Scholar]
- 80.Closing the gap: effect of diabetes case management on glycemic control among low-income ethnic minority populations: the California Medi-Cal type 2 diabetes study. Diabetes Care 2004;27:95–103. [DOI] [PubMed]
- 81.Luley C, Blaik A, Reschke K, Klose S, Westphal S. Weight loss in obese patients with type 2 diabetes: effects of telemonitoring plus a diet combination - the Active Body Control (ABC) Program. Diabetes Res Clin Pract. 2011;91:286–292. doi: 10.1016/j.diabres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 82.Lu J, Bu RF, Sun ZL, et al. Comparable efficacy of self-monitoring of quantitative urine glucose with self-monitoring of blood glucose on glycaemic control in non-insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2011;93:179–186. doi: 10.1016/j.diabres.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Partapsingh VA, Maharaj RG, Rawlins JM. Applying the Stages of Change model to Type 2 diabetes care in Trinidad: a randomised trial. J Negat Results Biomed. 2011;10:13. doi: 10.1186/1477-5751-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sacco WP, Malone JI, Morrison AD, Friedman A, Wells K. Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. J Behav Med. 2009;32:349–359. doi: 10.1007/s10865-009-9209-4. [DOI] [PubMed] [Google Scholar]
- 85.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 87.Funnell MM, Brown TL, Childs BP, et al. National standards for diabetes self-management education. Diabetes Care. 2012;35(Suppl 1):S101–S108. doi: 10.2337/dc12-s101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carpenter DM, Fisher EB, Greene SB. Shortcomings in public and private insurance coverage of diabetes self-management education and support. Popul Health Manag. 2012;15:144–148. doi: 10.1089/pop.2011.0042. [DOI] [PubMed] [Google Scholar]
- 89.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 221 kb)