Abstract
Cucurbitacin I is a hydrophobic molecule that exerts a degree of polarity, which is expected to complicate its loading in PLGA nanoparticles by the classical emulsion solvent evaporation technique. In the current study, variants of emulsion solvent evaporation method were used to prepare PLGA nanoparticles of cucurbitacin: CI-NP1 (single emulsion starting with 1000 μg drug), CI-NP2 (double emulsion starting with 250 μg drug), and CI-NP3 (double emulsion starting with 500 μg drug). On the other hand, CI-NP4 was prepared by nanoprecipitation (starting with 1000 μg drug). In CI-NP1, cucurbitacin I encapsulation efficiency (EE) was 1.29%. The employment of double emulsion, in CI-NP2 and CI-NP3, increased cucurbitacin I EE to 4.8% and 7.96%, respectively. Nanoprecipitation significantly increased the EE of cucurbitacin I to 48.79% in CI-NP4. It is likely that cucurbitacin I escapes with the organic solvent after the emulsification step to the aqueous phase leading to ineffective entrapment in the polymeric matrix. Avoiding emulsification seems efficient in increasing cucurbitacin I disposition in the instantly-precipitating NPs. Therefore, nanoprecipitation method increases cucurbitacin I entrapment in PLGA NPs and possibly other water-insoluble polar drugs.
Keywords: PLGA, Cucurbitacin I, Nanoprecipitation, Nanoparticles, Emulsion solvent evaporation
1. Introduction
Poly(d,l-lactic-co-glycolic acid) (PLGA) (Fig. 1) is an aliphatic polyester that is approved by the FDA for human use (Jain, 2000; Bala et al., 2004). It has been utilized in research and in clinic as a vehicle for a wide range of compounds (Mundargi et al., 2008). Although emulsion solvent evaporation is the most commonly used method to prepare PLGA nanoparticles (NPs), this method is affected to a great extent by the payload physical and chemical properties. Generally, the single emulsion process is most suited for water-insoluble drugs such as steroids, while the double emulsion process is considered ideal to encapsulate water-soluble drugs such as peptides (Makadia and Siegel, 2011). However, the encapsulation of polar yet water-insoluble drugs, such as cucurbitacin I (Fig. 2), in PLGA NP by emulsion solvent evaporation technique is quite challenging. Cucurbitacin I is a triterpene hydrocarbon that is isolated from plants of the families Cucurbitaceae and Cruciferae, which have been part of Chinese and Indian folk medicine for centuries (Jing and Tweardy, 2005; Blaskovich et al., 2003). The relative polarity of cucurbitacin I along with its hydrophobicity is expected to make its physical loading in PLGA NPs quite challenging. So far, no studies have been conducted to examine or improve the physical loading of cucurbitacin I in PLGA NPs, although a previous attempt to conjugate cucurbitacin I covalently to PLGA chain had been reported (Molavi et al., 2010). In this study, emulsion solvent evaporation technique was evaluated against nanoprecipitation for the physical encapsulation of cucurbitacin I in PLGA NPs. Different variants of emulsion solvent evaporation methods were applied, while the nanoprecipitation method of a similar amount of cucurbitacin I and PLGA was tested and evaluated for particle size, encapsulation efficiency, and drug loading.
Figure 1.
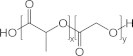
Chemical structure of poly(d,l-lactic-co-glycolic acid).
Figure 2.
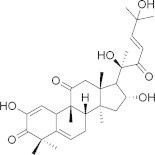
Chemical structure of cucurbitacin I.
2. Materials and methods
2.1. Materials
Cucurbitacin I was purchased from EMD Millipore (Billerica, MA). Poly(vinyl alcohol) (PVA, Mw ∼31–50 kDa) and Pluronic® F-68 were purchased from Sigma–Aldrich (St. Louis, MO). Ester-terminated 50:50 DL-PLGA (inherent viscosity 0.15–0.25 dL/g) was purchased from LACTEL Absorbable Polymers (Birmingham, AL).
2.2. PLGA NPs preparation
2.2.1. Emulsion solvent evaporation method
Three variants of cucurbitacin I-loaded PLGA NPs were prepared. In the first formulation (CI-NP1), 1000 μg of cucurbitacin I was dissolved with 100 mg PLGA in 300 μL chloroform. The organic phase was emulsified in 2 mL of 9% PVA by microtip sonicator for 45 s. The emulsion was added drop wise into 8 mL of stirring 9% PVA solution (continuous phase). The emulsion was left on gentle stirring for 3 h to allow for solvent evaporation. Then, suspended NPs were collected by ultracentrifugation at 40,000g for 15 min at 4 °C. The NPs were washed three times with cold double-deionized water and then freeze-dried for 48 h. In the second formulation (CI-NP2), 250 μg of cucurbitacin I was dissolved first in 50 μL ethanol:water solution (1:4). Using a microtip sonicator for 15 s, this solution was emulsified in 300 μL chloroform containing dissolved 100 mg PLGA to form the primary (w/o) emulsion. Thereafter, the primary (w/o) emulsion was further emulsified in 2 mL of 9% PVA solution and the following preparation steps continued as mentioned earlier. The third formulation (CI-NP3) was prepared exactly like CI-NP2 except that 500 μg was used in this formulation instead of 250 μg.
2.2.2. Nanoprecipitation method
The fourth formulation (CI-NP4), was prepared by nanoprecipitation technique as follows: 1000 μg cucurbitacin I and 100 mg PLGA were co-dissolved in 10 mL acetone. The organic phase was added drop wise to 20 mL stirring double-deionized water (1000 rpm) containing 1% Pluronic® F-68. The solution was kept for 6 h to allow for acetone evaporation. After that, suspended NPs were freeze-dried for 48 h.
2.3. PLGA NPs characterization
Hydrodynamic diameter of PLGA NPs was determined by dynamic light scattering. In order to evaluate cucurbitacin I entrapment, 5 mg of each NP formulation was placed in a sterile capped Eppendorf tube. To each tube, 50 μL of chloroform was added to completely dissolve PLGA and cucurbitacin I. The chloroform was evaporated under nitrogen stream pressure and the precipitant was suspended in 200 μL of 70% ethanol to dissolve cucurbitacin I only, while precipitating PLGA. Then, cucurbitacin I concentration in the supernatant was determined by the liquid chromatography/mass spectrometry (LC–MS) using a Waters Micromass ZQ 4000 spectrometer coupled to a Waters 2795 separations module as previously described in (Molavi et al., 2006). The encapsulation efficiency (EE) and drug loading (DL) were calculated as follows:
2.4. Statistical analysis
The data were analyzed for statistical significance (p < 0.05) by the one-way ANOVA (IBM SPSS Statistics 19).
3. Results and discussion
The relative polarity of cucurbitacin I, in spite of its poor water solubility, is expected to impede physical loading of cucurbitacin I in PLGA NPs using emulsion solvent evaporation technique. Therefore, this study aims to examine different variants of that method in comparison to nanoprecipitation for cucurbitacin I encapsulation. Although all formulations were within a close size range, the EE and DL values of cucurbitacin I were significantly different. As shown in Table 1, CI-NP1 demonstrated the lowest EE among all formulations reaching as low as 1.29%. Because cucurbitacin I is freely soluble in chloroform and exhibits a degree of polarity that reduces the chance of its interaction with the hydrophobic core of PLGA, it is highly likely carried with the chloroform during the quick solvent escape to the aqueous phase in the single (o/w) emulsification process. Therefore, the low EE in this case is quite predictable.
Table 1.
Determination of cucurbitacin I contents in PLGA NPs.
| Formulation | CI-NP1 | CI-NP2 | CI-NP3 | CI-NP4 |
|---|---|---|---|---|
| Emulsion type | (o/w) | (w/o/w) | (w/o/w) | – |
| Amount of drug added (μg) | 1000 | 250 | 500 | 1000 |
| Average particle size (nm) | 399 ± 61 | 403 ± 23 | 411 ± 13 | 386 ± 57 |
| Encapsulation efficiency (%) | 1.286 ± 0.05 | 4.8 ± 0.31a | 7.96±.056 a,b | 48.79 ± 6.18 a,b,c |
| Drug loading (μg drug/mg NP) | 0.37 ± 0.07 | 0.35 ± 0.12 | 0.99 ± 0.08 a,b | 12.2 ± 2.37 a,b,c |
(p < 0.05) Compared to CI-NP1.
(p < 0.05) Compared to CI-NP2.
(p < 0.05) Compared to CI-NP3.
In CI-NP2 and CI-NP3, a primary emulsion (w/o) of 250 and 500 μg cucurbitacin I in ethanol:water, respectively, was prepared followed by further emulsification (w/o/w) in the continuous aqueous phase. This technique is usually used to encapsulate hydrophilic drugs and peptides in PLGA microspheres and NPs (Jain, 2000; Mundargi et al., 2008). The relative solubility of cucurbitacin I in ethanol:water mixture, owing to its relative polarity, allows the drug to be emulsified, rather than dissolved, in chloroform. When a double emulsion was prepared (w/o/w), the EE marginally increased (Table 1). This was in correlation to the original amount of cucurbitacin I used in the primary emulsion where 3.7-fold and 5.4-fold increase were noticed with CI-NP2 and CI-NP3, respectively. It is noteworthy that 1000 μg cucurbitacin I could not be used in the double-emulsion approach because of the limited solubility of cucurbitacin I in the small volume of the ethanol:water mixture during the primary (w/o) emulsification step. This primary emulsion is thought to reduce the inevitable microcrystallization of cucurbitacin I following chloroform escape and enhances drug retention during the hardening of the oil droplet. Consequently, the results indicated a marginal, yet significant, increase in the EE and DL.
Since cucurbitacin I follows chloroform to the aqueous phase, which reduces its retention in the monolithic matrix during the NPs formation, the omission of the emulsification step is quite plausible. Nanoprecipitation was employed instead of emulsification solvent evaporation technique in order to further improve the EE and DL of cucurbitacin I. This technique is based on dissolving the polymer and the drug in a water-miscible solvent such as acetone followed by drop-wise addition to an excess volume of stirring aqueous phase containing a stabilizer such as Pluronic® F-68. The particles are formed and precipitated during solvent evaporation. Compared to the previous formulations, a dramatic increase in cucurbitacin I EE (∼49%) has been recorded for CI-NP4. Similarly, the DL value was at least 12 times higher than the formulations prepared by emulsification solvent-evaporation method. This might be attributed to both the hydrophobicity and the relative polarity of cucurbitacin I. Although cucurbitacin I is not soluble in water, its relative polarity might drive its diffusion to the aqueous phase along with chloroform during the preparation of CI-NP1, CI-NP2, and CI-NP3. On the contrary in CI-NP4 preparation, acetone was used as an organic solvent. Since acetone is miscible with water, there is no emulsification step involved but solvent evaporation rather starts immediately. In this case, the hydrophobicity of cucurbitacin I will retain it in the instantly-precipitating NPs without the need for complete evaporation of the solvent. Nonetheless, it is worth noting that the employed method caused about 30% polymer loss, which can be reduced by increasing the viscosity of the aqueous phase using higher concentration of the stabilizer or other thickening agents such as glycerol. Such polymer loss has also been noticed with protein encapsulation using nanoprecipitation technique (Bilati et al., 2005).
4. Conclusion
PLGA nanoprecipitation can be a better alternative to emulsification solvent-evaporation technique for the physical loading of cucurbitacin I. Further evaluation of nanoprecipitation to entrap other polar water-insoluble drugs is worth testing.
Acknowledgment
The author is grateful to the College of Pharmacy Research Centre and the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for logistic and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bala I., Hariharan S., Kumar M.N. PLGA nanoparticles in drug delivery: the state of the art. Crit. Rev. Ther. Drug Carrier Syst. 2004;21:387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- Bilati U., Allemann E., Doelker E. Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech. 2005;6:E594–E604. doi: 10.1208/pt060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovich M.A., Sun J., Cantor A., Turkson J., Jove R., Sebti S.M. Discovery of JSI-124 (Cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Jain R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Jing N., Tweardy D.J. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molavi O., Mahmud A., Hamdy S., Hung R.W., Lai R., Samuel J., Lavasanifar A. Development of a poly(d,l-lactic-co-glycolic acid) nanoparticle formulation of STAT3 inhibitor JSI-124: implication for cancer immunotherapy. Mol. Pharm. 2010;7:364–374. doi: 10.1021/mp900145g. [DOI] [PubMed] [Google Scholar]
- Molavi O., Shayeganpour A., Somayaji V., Hamdy S., Brocks D.R., Lavasanifar A., Kwon G.S., Samuel J. Development of a sensitive and specific liquid chromatography/mass spectrometry method for the quantification of cucurbitacin I (JSI-124) in rat plasma. J. Pharm. Pharm. Sci. 2006;9:158–164. [PubMed] [Google Scholar]
- Mundargi R.C., Babu V.R., Rangaswamy V., Patel P., Aminabhavi T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J. Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]



