Abstract
Recent advances in genetically encoded fluorescent sensors enable the monitoring of cellular events from genetically defined groups of neurons in vivo. In this protocol, we describe how to use a time-correlated single-photon counting (tcspc)–based fiber optics system to measure the intensity, emission spectra and lifetime of fluorescent biosensors expressed in deep brain structures in freely moving mice. When combined with cre-dependent selective expression of genetically encoded ca2+ indicators (GecIs), this system can be used to measure the average neural activity from a specific population of cells in mice performing complex behavioral tasks. as an example, we used viral expression of GcaMps in striatal projection neurons (spns) and recorded the fluorescence changes associated with calcium spikes from mice performing a lever-pressing operant task. the whole procedure, consisting of virus injection, behavior training and optical recording, takes 3–4 weeks to complete. With minor adaptations, this protocol can also be applied to recording cellular events from other cell types in deep brain regions, such as dopaminergic neurons in the ventral tegmental area. the simultaneously recorded fluorescence signals and behavior events can be used to explore the relationship between the neural activity of specific brain circuits and behavior.
INTRODUCTION
Monitoring neural activity in specific brain circuits in behaving animals is a key approach to decode neural correlates of behavior and to explore the mechanisms underlying neuropsychiatric disorders in humans. Information obtained from traditional methodologies has often been limited by the lack of definitive methods to select or determine the identities of recorded cells. With recent advances in genetically encoded neural activity reporters (e.g., Ca2+ indicators and voltage sensors) and optical technologies, it has become possible to express these fluorescent sensors in specific identified groups of neurons and measure the fluorescence changes in these neurons to report neural activity in vivo1. However, it remains challenging to apply such a strategy to study deep brain structures in freely moving animals. Brain surface–mounted two-photon microscopy has limited penetration depth (<1 mm) and requires head fixation2. Gradient index (GRIN) lens–based intracranial microendoscopy is a potential solution but may cause substantial tissue damage owing to the large diameter (≥1 mm) of the implanted objective and/or relay lens3. Tissue damage is expected to be a quadratic function of the probe diameter, and thus even a small reduction in the diameter of an imaging probe may result in a marked reduction in tissue damage.
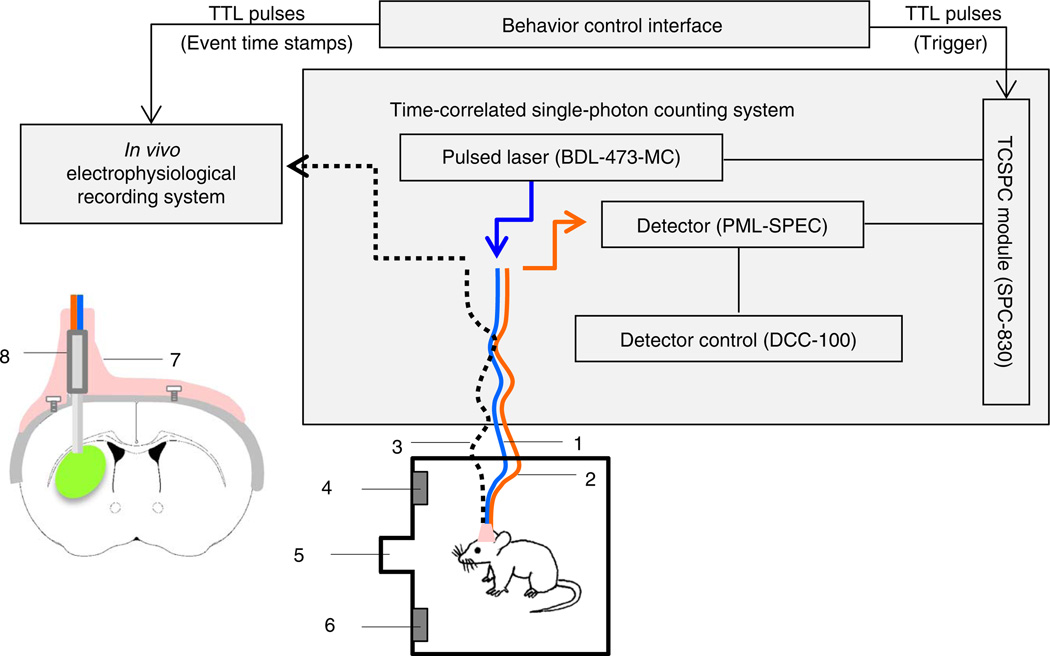
To circumvent these issues and achieve optical recording of neural activity from deep brain nuclei in freely moving animals, we developed an in vivo photometry method that uses TCSPC-based fiber optics4 (Fig. 1). In this method, the animal is tethered to a pair of light and flexible optical fibers: a single-mode fiber for delivering excitation laser pulses and a multimode fiber for collecting emitted photons. The two fibers join together at the end to form a parallel dual-fiber probe (125 µm in diameter for each fiber), which is implanted into the targeted structure in mouse brain. The main rationale behind the dual-fiber design is to provide a stable illumination using a single-mode fiber (the laser output through a multimode fiber is very sensitive to bending) while achieving a higher photon collection efficiency using a multimode fiber for detection. Photons collected by the TCSPC system can be plotted as a series of 3D time-resolved spectra (Fig. 2). Each time-resolved spectrum contains information on the emission spectrum (Fig. 2d), the fluorescence lifetime of each spectral component (Fig. 2e) and the integrated fluorescence intensity (Fig. 2a). In this protocol, as an example of the type of experiment that can be performed with this approach, we describe how to use changes in GCaMP fluorescence intensity (‘fluorescent transients’) as a readout of neural activity in SPNs when the animals are performing a lever-pressing operant task.
Figure 1.
General scheme for using TCSPC-based photometry to measure the fluorescence of genetically encoded biosensors in vivo. Schematic drawing of a TCSPC-based fiber optic system to measure local fluorescence signals in the dorsal striatum of a mouse performing a lever-pressing operant task. (1) Jacketed single-mode fiber for excitation. (2) Jacketed multimode fiber for photon collection. (3) Optional electrical cable for simultaneous electrophysiological recording. (4) Right lever. (5) Food magazine. (6) Left lever. The inset illustrates a hybrid fiber probe (8) lowered into the dorsal striatum and fixed in place by dental acrylic (7). This figure is adapted with permission from ref. 4, Nature Publishing Group.
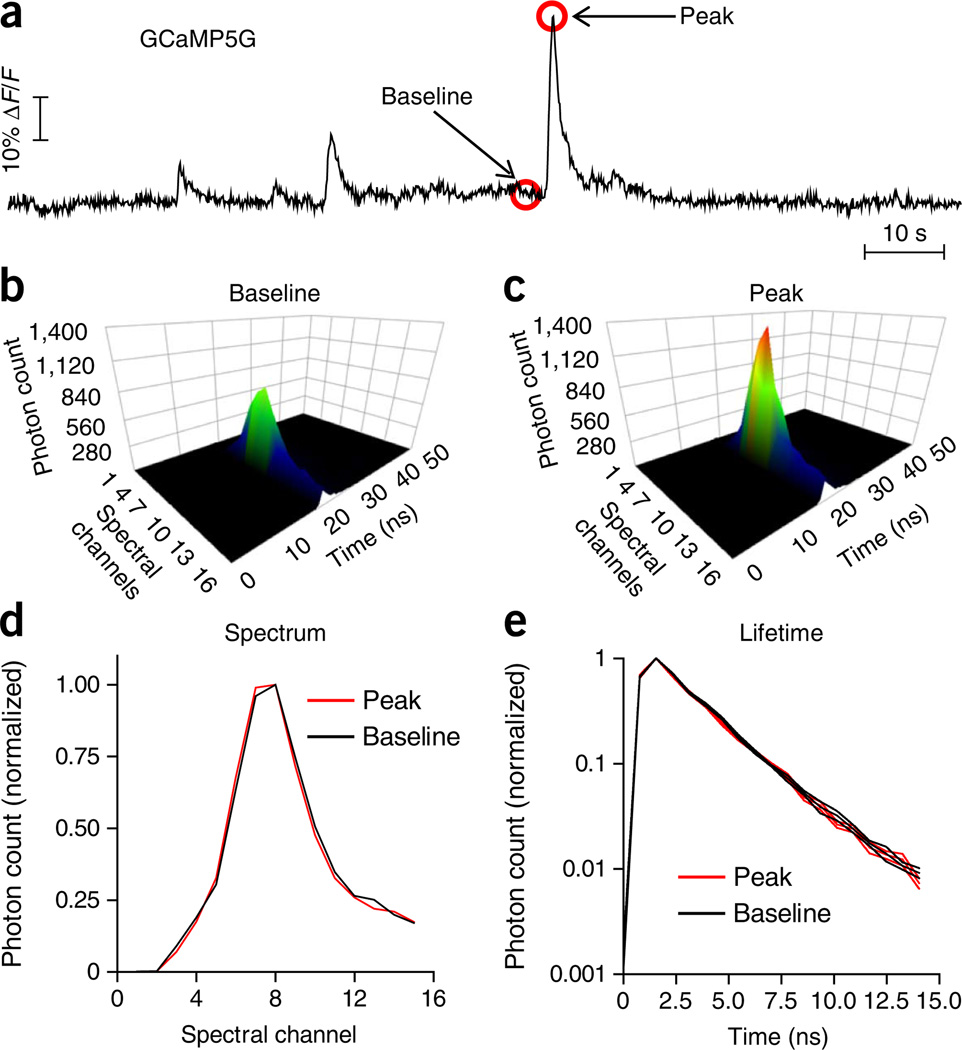
Figure 2.
In vivo measurement of GCaMP5G fluorescence using TCSPC-based photometry. (a) An example trace of GCaMP5G fluorescence intensity in the format of ΔF/F over time in a freely moving A2A–Cre mouse expressing GCaMP5G specifically in striatal indirect-pathway SPNs. Fluorescence intensity was calculated by integrating the photon count of peak GCaMP5G spectral channels (channels 6–13) in each time-resolved spectrum (b,c). Individual spectra were acquired at 20 Hz. (b,c) Examples of individual time-resolved GCaMP5G spectra at the baseline level (b) and at the peak of a fluorescence transient (c). (d,e) Normalized GCaMP5G spectra (d) and fluorescence decay curves (e) acquired at the baseline (as in b) and at the peak of a fluorescence transient (as in c). Despite the large difference in fluorescence intensity (b versus c), there is no difference between normalized spectra (d) and fluorescence lifetime (e), consistent with previous studies20. All animal protocols used in this study were approved by the US National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
Potential applications of the protocol
Genetically encoded fluorescent tags and sensors have made it possible to achieve in vivo real-time monitoring of molecular and cellular events such as gene expression5, protein-protein interaction6, enzyme activity7, and membrane potential change8, as well as to measure the intracellular and extracellular concentrations of various ions and molecules1,9. Optical fibers are an excellent choice for collecting fluorescent signals from deep brain tissues in freely moving animals because they are light, mechanically flexible and relatively small in diameter. The fiber optics method described in this protocol features simultaneous recording of fluorescence spectral and lifetime changes. The equipment is straightforward to set up and the experimental procedures are easy to perform.
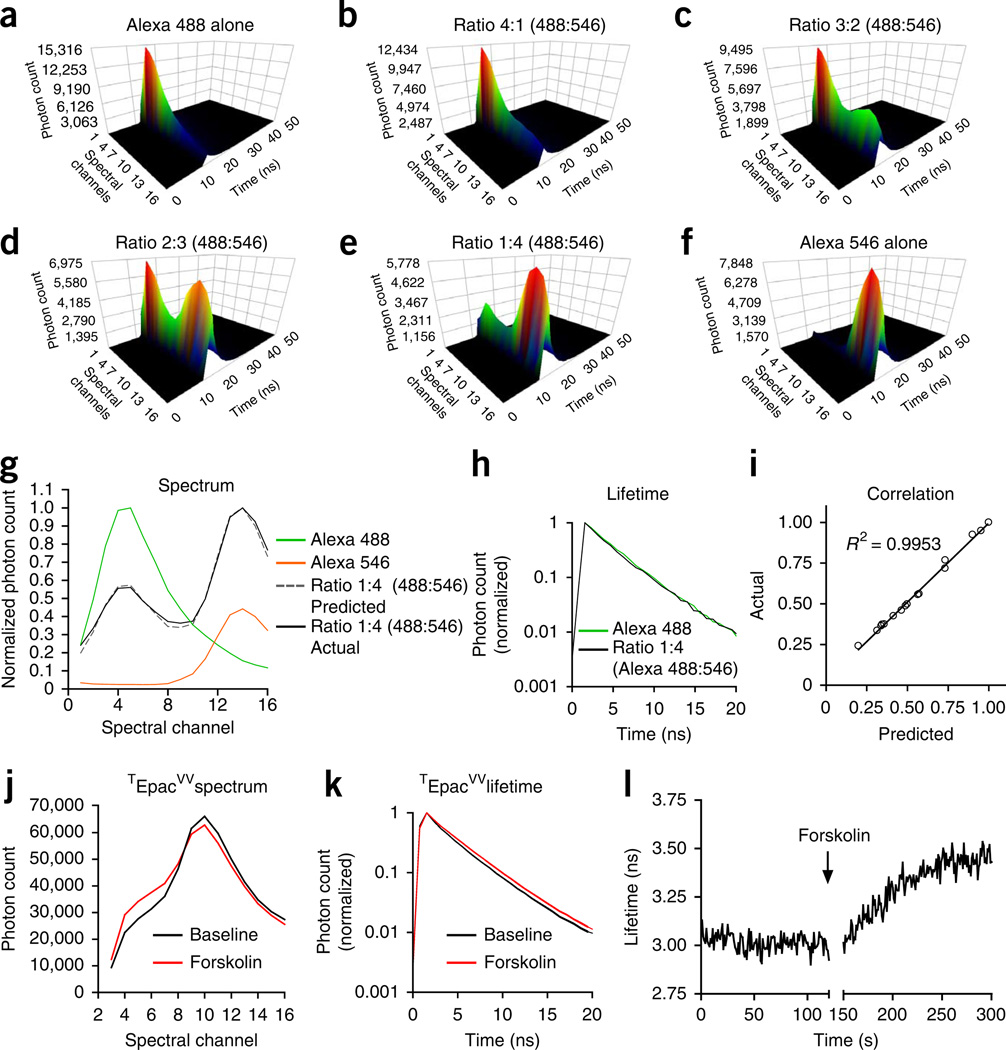
In addition to being used with intensity-based fluorescent biosensors such as the GCaMPs shown in this protocol, the same multispectral TCSPC-based optical system can also be used for spectral unmixing in dual-fluorophore assays10 and for measuring Förster resonance energy transfer (FRET) by fluorescence lifetime imaging microscopy (FLIM)11 (Fig. 3). A typical application of spectral unmixing is ratiometric measurement of the relative abundance of two fluorescent proteins that have partially overlapping emission spectra. Spectral unmixing can also be used to control for movement artifacts during in vivo measurement by co-expressing a fiduciary fluorescent protein in the same cells that express event-reporting fluorescent biosensors (such as Ca2+-reporting GCaMPs). By deconvolving the complex spectrum formed by the two fluorescent proteins and calculating the peak ratio of the reporter fluorophore spectrum over the control fluorophore spectrum, the influences of movement artifacts during the measurement may be minimized. In both of these applications, it is essential to know whether FRET is occurring between the two fluorophores before simple linear unmixing can be applied for deconvolution. If FRET is occurring (i.e. part of the energy from the excited donor fluorophore (shorter wavelength) is transferred to the acceptor fluorophore (longer wavelength)), there will be an apparent decrease in donor fluorescence intensity and an increase in acceptor fluorescence intensity compared with the values predicted on the basis of simple abundance. One way to determine whether FRET is occurring and to correct for it, if necessary, is to monitor the fluorescence lifetime of the donor fluorophore simultaneously with the spectral measurement. A decrease in donor fluorescence lifetime is indicative of FRET. If FRET is occurring, the linear unmixing algorithm needs to be modified to accurately estimate the relative abundance of the two fluorophores10. The principles of spectral unmixing are illustrated in Figure 3a–i using solutions containing a mixture of fluorescent dyes Alexa Fluor 488 and Alexa Fluor 546 at different volume ratios. In this example, the lifetime of Alexa Fluor 488 measured at its peak spectral channel does not change in the 1:4 mixture of Alexa Fluor 488 and Alexa Fluor 546 (Fig. 3h), indicating that FRET is not occurring between the two fluorophores, the application of a simple linear unmixing algorithm is justified and yields an accurate measure of the relative abundance of these flurophores (Fig. 3g,i).
Figure 3.
Potential applications of a TCSPC-based fiber optics system for ratiometric measurement in a dual-fluorophore system and lifetime measurement in FRET-based biosensors. (a–f) Examples of time-resolved spectra measured in solutions containing different volume ratios of Alexa Fluor 488 and Alexa Fluor 546 fluorescent dyes. (g) Normalized spectra of Alexa Fluor 488 (green), Alexa Fluor 546 (orange) and a mixture of Alexa Fluor 488 and Alexa Fluor 546 with the volume ratio of 1:4 (black). The dashed gray trace is a reconstructed spectrum using the equation Y = a × Y1 + b × Y2, where Y1 is the normalized spectrum of Alexa Fluor 488, Y2 is the normalized spectrum of Alexa Fluor 546, a = 0.2 and b = 0.8. (h) Normalized fluorescence decay curve of Alexa Fluor 488 and the 1:4 mixture of Alexa Fluor 488 and Alexa Fluor 546. (i) Goodness of fit to show the reconstructed spectrum (gray in g) and the measured spectrum (black in g) has a nearly perfect match. (j,k) Examples of fluorescence spectra (j) and lifetime (k) acquired from HEK cells expressing FRET-based cAMP sensor TEpacVV before (black) and after (red) adding the adenylyl cyclase activator forskolin (100 µM) into the medium. (l) Continuous measurement of fluorescence lifetime of TEpacVV before and after the addition of forskolin.
As mentioned above, FRET can be problematic for accurate spectral unmixing. It can also be used as a tool for studying protein-protein interactions and receptor activation, as well as for designing fluorescent sensors. Typically FRET-based sensors have donor and acceptor fluorophores tagged to different locations on the molecules being studied. As the FRET efficiency is inversely proportional to the sixth power of the distance between the donor and acceptor, a small activation-induced conformational change in the molecule typically can cause a large change in the FRET efficiency, which can be monitored by fluorescence lifetime measurement. Still, it is important to realize that the absence of a change in FRET (donor lifetime) does not necessarily mean that a conformational change did not occur. An example of using a FRET-based sensor is illustrated in Figure 3j–l in which TEpacVV (an engineered FRET sensor for measuring cAMP levels) is used to measure the change of cAMP levels in cultured HEK cells12.
Advantages
Compared with other in vivo imaging methods, the photometry method described here has the following advantages. First, TCSPC-based optical systems can, by definition, detect single photons, and thus they have superb sensitivity for detecting low-level light signals. Practically, this can allow for the use of markedly reduced illumination power (~0.1 mW) and accordingly it can minimize photodamage to cells and photobleaching of fluorescent biosensors. Thus, the TCSPC system described here is well suited for extended continuous recordings of neuronal activity. Second, the capability to measure fluorescence intensity, emission spectra and fluorescence lifetime makes this system very flexible for monitoring different types of fluorescent biosensors while studying varied types of cellular events in vivo. As we discussed above, in addition to working with intensity-based GECIs such as the GCaMPs described in this protocol, the same system can also be used for FRET-based fluorescent sensors to study protein-protein interactions and enzyme and receptor activation using fluorescence-lifetime imaging11,13,14. One further advantage of the system described here is that the dual-fiber probe used in this system provides stable illumination with a single-mode fiber (the laser output through a multimode fiber is more sensitive to bending) while achieving a high photon-collection efficiency with a multimode fiber for detection. Furthermore, it is low-cost, simple to fabricate in the laboratory and has a relatively small diameter, thus minimizing the tissue damage during surgery and data acquisition. No behavioral deficits were observed in the close to 30 mice we implanted with the fiber probe for this study (data not shown).
Limitations
Unlike imaging techniques that can resolve individual neurons3,15, our photometry method does not provide information on the activity of individual cells. The integrated photon count (fluorescence intensity) collected from the optical probe reflects the average activity from a large population of neurons residing near the probe tip (within ~500 µm)4. Thus, if only a small fraction of GCaMP-expressing neurons in the detection volume are activated, the system may not be able to detect changes in fluorescence intensity.
Another caveat associated with the procedures described in this protocol is that the mice need to be tethered to the fibers once the fiber probe has been implanted. For chronic experiments lasting for days or weeks, special care is required to maintain the welfare of the mice and to keep the fibers from being damaged. There are two potential improvements that can be explored to solve the tethering issue. The first option is to use a guide cannula that is permanently cemented to the skull. The optical fiber probe can be inserted through the guide during the measurement and removed after the experiment. The major issues associated with this option are the local tissue damage caused by repeated probe insertions and variations in the final tip location between each insertion. An alternative design is to use detachable connectors between the implanted fibers and the fibers attached to the laser and the detector. To reduce the size and weight of the connector, a viable approach is to use a ceramic or metal mating sleeve to form a temporary ferrule-to-ferrule connection (Fig. 4). Another possibility is to use only one fiber (a single-mode, a multimode or a double-clad fiber16) for both excitation and photon collection with a ferule-to-ferule connection (Supplementary Fig. 1). Furthermore, modification of the fiber tip by etching or pulling methods can further reduce the tip size and may produce a more focused excitation and detection volume, similarly to the probes used in near-field scanning optical microscopy17.
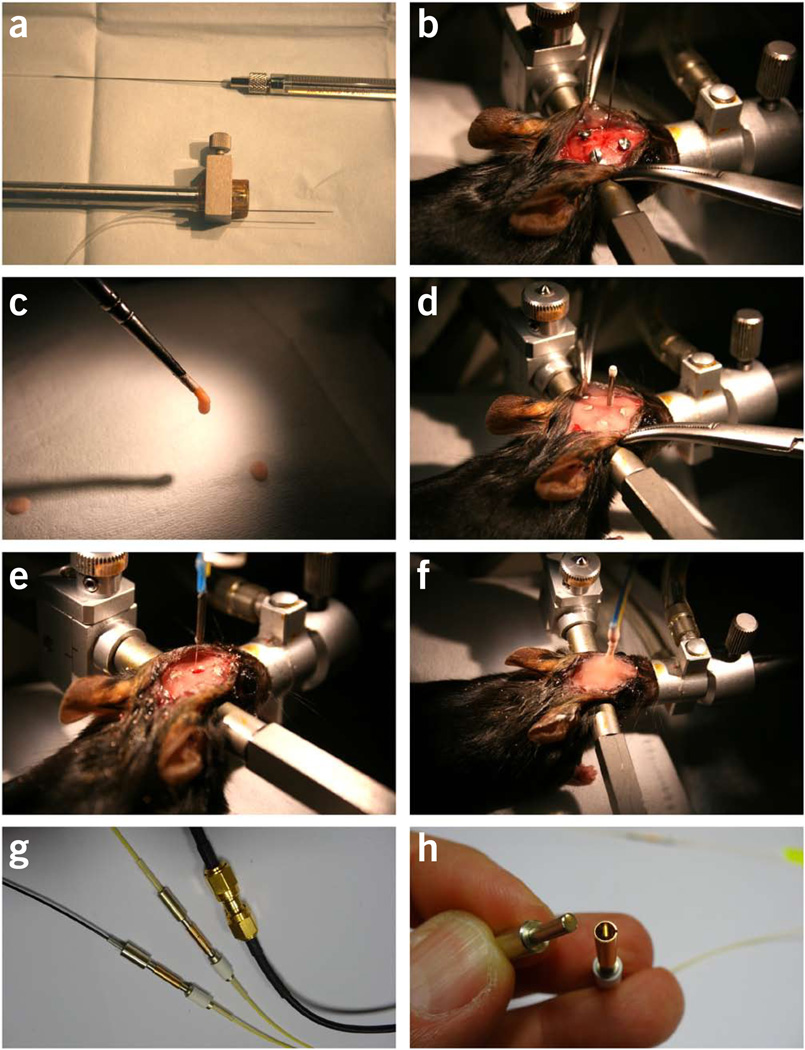
Figure 4.
Surgical procedures for intra-striatal virus injection and optical fiber implantation. (a–d) The first surgery for virus injection and creating a dental cement base. (a) A virus injection kit attached to the vertical arm of a stereotaxis. The kit consists of a 1-µl (or 2-µl) Hamilton syringe, connection polyethylene tubing (filled with saline) and a 30-gauge needle. (b) Picture showing the virus injection needle placed above the burr hole targeting the left striatum. Three anchoring screws have been driven into the skull. (c) Dental cement is applied with a paintbrush. (d) Picture showing the final step of the first surgery. A dummy cannula (marking the position of the burr hole) is fixed in place by dental cement. A thin dental cement base is created to cover the skull surface. Animals are allowed at least 2 weeks of recovery time before proceeding to next steps. (e,f) The second surgery for fiber implantation on the day of optical measurement. (e) The dummy cannula has been removed. The burr hole made during the first surgery has been re-exposed for lowering the fiber probe into the brain. (f) The final step of the second surgery. The fiber probe is fixed in place with a generous amount of dental cement. (g) Available options for connection between the implanted fibers and the fibers to the laser and the detector. (h) A detachable ferrule-sleeve connection for single-mode fibers. Panels g and h are courtesy of L. Bergann at Becker & Hickl. All animal protocols used in this study were approved by the US National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
Experimental design
We first developed this protocol to achieve in vivo cell type–specific neural activity recordings in striatal direct- and indirect-pathway SPNs4. Adeno-associated virus (AAV) vectors containing Flip-excision (FLEX)-GCaMP5G (or GCaMP3) were injected into the dorsal striatum of D1-Cre (direct pathway–specific) and A2A–Cre (indirect pathway–specific) BAC-transgenic mice to express GCaMPs in specific groups of SPNs18. In this protocol, we describe the specific procedure we used in ref. 4 as an example application of this method. To record fluorescence changes and the operant behavior simultaneously, the TCSPC-based optical recording system, the Med-Associates operant box and the Plexon multichannel acquisition processor (MAP) system are linked together (Fig. 1 and Supplementary Fig. 2). To synchronize the three systems, we added an ‘Acquisition start’ command in the Med PC code (controlling the Med-Associates operant box) to send out a transistor-transistor logic (TTL) triggering signal to the TCSPC system to start the optical recording, and to the Plexon system to create a corresponding time stamp. The time stamps of all behavioral events detected by the Med-Associates system were also fed into the Plexon system. The mouse’s behavior was also recorded by a video camera for detailed analysis. At the end of the experiments, the optical recording results (time-lapse values of integrated photon counts) were imported into the Plexon file that contains all the behavioral time stamps for post hoc analysis.
Equipment considerations
It should be noted that the key component in this protocol is the TCSPC-based fiber optics system. The multispectral TCSPC system can be replaced by a band-pass emission filter plus a photomultiplier tube (PMT) or a sensitive camera if only the fluorescence intensity at a specific wavelength range is desired instead of a time-resolved spectrum. The Med-Associates behavior system and the Plexon system that were used in the original study can be replaced by other electrophysiological and behavioral recording systems. Indeed, although it adds convenience and an option for simultaneous optical and electrophysiological recordings, the Plexon system (or any other recording system) is not an absolute requirement for completing the experiments we describe here. Any device that can record continuous analog and digital signals with multiple TTL inputs can be used as a time-stamp recorder to align the behavioral events and the optical data. Another option that does not require an external time-stamp recorder is to use the behavioral control hardware and software that can generate event time stamps. For example, one can use the array function in the Med-PC code to record the inter-response times and later stitch these intervals together to form a continuous file.
Choice of controls
As the protocol described here is designed for in vivo measurement in freely moving mice, it is crucial to carry out control experiments to rule out movement-induced artifacts in the recorded fluorescence responses. A key control experiment is to repeat all optical recordings in mice expressing constitutive fluorescent tags (e.g., eGFP, whose fluorescence intensity is not influenced by neural activity change) in the same population of neurons.
MATERIALS
REAGENTS
-
• Cre mouse lines of interest (e.g., D1-Cre/EY217 and A2A–Cre/KG139 from GENSAT to target striatal direct and indirect pathways, respectively)
! CAUTION Experiments involving live rodents must conform to all relevant institutional and governmental rules and regulations.
AAV vectors, see Reagent Setup ! CAUTION Follow institutional procedures to properly handle and dispose of AAV vectors.
Isoflurane (e.g., Baxter, cat. no. 1001936060, available via institutional veterinarian)
Medical-grade oxygen (e.g., Airgas, cat. no. OX USPE)
Betadine surgical scrub (e.g., McGuff, cat. no. 003577)
70% (vol/vol) isopropyl rubbing alcohol (e.g., CVS, cat. no. 152074)
Sterile normal saline, 0.9% (wt/vol) NaCl (e.g., Hopsira, cat. no. H04888-10)
3% (wt/vol) hydrogen peroxide solution (e.g., CVS, cat. no. 209478)
Instant glue (e.g., Krazy Glue, liquid form, cat. no. KG585)
Dental cement and curing liquid (cat. nos. 525000 and 526000, A–M Systems)
EQUIPMENT
Surgery equipment
Isoflurane anesthesia system for small animals (e.g., E–Z anesthesia system, Model EZ-7000)
Stereotaxis for small animals (KOPF, Model 962 Dual Ultra Precise small-animal stereotaxic instrument, with Model 923-B mouse gas anesthesia head holder and Model 921 zygoma ear cups)
Surgical microscope (e.g., Zeiss Ompi 1)
Dental drill (e.g., Medidenta Denti-Lab electric unit with E-type handpiece)
Round-tip carbide burr, size no. 1 (Medidenta, cat. no. CBRA1)
Stainless steel screws (size no. 00–90, length 0.0625 inches) (e.g., Small Parts, cat. no. B000FN43FK)
Sterile surgical drape (Moore Medical, cat. no. 14170)
Sterile cotton applicator (Dynarex, cat. no. 4305)
Scalpel handle, no. 3 (WPI, cat. no. 500236)
Scalpel blades, no. 10 (WPI, cat. no. 504169)
Iris forceps (WPI, cat. no. 15915)
Dumont forceps, no. 5B (WPI, cat. no. 500234)
Wire retractor (WPI, cat. no. 501994)
BD PrecisionGlide needles: 1/2 inch, 19 gauge (cat. no. 305187); 1 ½ inch, 20 gauge (cat. no. 305176); 1 inch, 23 gauge (cat. no. 305145)
Surgical skin marker (e.g., Precision Dynamics, cat. no. SP1620)
Parts for making intracranial microinjection kit
30 G disposable dental needle (0.3 mm diameter × 25 mm, cat. no. 20–30GL, J. Morita USA)
PE-10 tubing (cat. no. 64–0750, Warner Instruments)
Hamilton syringe, 1.0 µl (or 2.0 µl, depending on the volume of injection) (Hamilton 7000 Series, SKU 20731, Sigma-Aldrich)
TCSPC equipment
Simple-Tau 830 system with PML-SPEC multiwavelength detector (Becker & Hickl) ▲ CRITICAL There are several options (three different polychromator gratings, two choices for the detector cathode, two choices for the input port and different user-selected emission filters) for the PML-SPEC multichannel detector. We choose the 1,200 lines/mm grating to have a total 106-nm wavelength coverage over 16 spectral channels (6.65 nm per channel) and the multi-alkali cathode to have a detector spectral response range between 300 nm and 850 nm. A hand screw per micrometer is used to select the actual wavelengths (by adjusting the angle of the grating) projected to each detector channel. Depending on the emission spectra of the fluorescence indicators used, users can freely select any 106 nm wavelength window between 300 nm and 850 nm by turning the hand screw. Wider (208 nm or 320 nm) wavelength windows are available by choosing different types of gratings (600 lines per mm or 400 lines per mm). For the light input port and emission filter, we choose the SMA connector receptacle to accommodate a multimode fiber with a SMA905 connector, and a 485-nm long-pass filter for green-to-red fluorescence detection.
Excitation laser (BDL-473-SMC, Becker & Hickl)
Polarization maintaining a single-mode optical fiber patchcord (core/cladding diameter 3.5/125 µm, with a 0.9-mm jacket, 2.5 m long; users can decide the actual length that fits their own study), with an FC connector at one end and a flat-cleaved free end at the other. To reduce cost, we order a 5-m-long patchcord with FC connectors at both ends and cut it in half to obtain two usable patchcords. OZ optics, part no. QPMJ-A3A,X-488-3.5/125-1–5-1)
Multimode fiber patchcord (core/cladding diameter 105/125 µm, 0.9 mm jacket, 5 m long, with SMA connectors on both ends, Thorlabs, cat. no. AFS105/125Y-CUSTOM)
No-Nik fiber optic stripper (Clauss, cat. no. NN203)
High-precision fiber cleaver (Thorlabs, cat. no. XL411)
Operant behavioral testing equipment (see Equipment Setup for further details)
In vivo multichannel electrophysiological recording system. We use the MAP system (or OmniPlex system, Plexon) ▲ CRITICAL The Plexon system can be replaced by other generic equipment that can record continuous files with multiple analog, digital and TTL inputs.
Video monitor control package (cat. no. MED-SYST-VMO-D, Med-Associates) ▲ CRITICAL This is optional; it is useful for capturing a video of the mouse’s behavior.
Key software for data acquisition and analysis
MED-PC IV, video monitor, optional (Med-Associates)
SPCM and DCC (Beck & Hickl)
Sortclient, Neuroexplorer (Plexon)
MATLAB (optional)
REAGENT SETUP
AAV vectors
The following vectors are required: AAV2/9.hSynap.Flex. GCaMP3.3.SV40 (titer: 1.26 × 1013 genomic copies (GCs)/ml); AAV2/9. hSynap.Flex.GCaMP5G.WPRE.SV40 (titer: 7.45 × 1012 GCs/ml); and AAV1. CAG.Flex.GCaMP6s.WPRE.SV40 (titer: 1.19 × 1013 GCs/ml). We obtain our vectors from Penn Vector Core (http://www.med.upenn.edu/gtp/vectorcore/Catalogue.shtml). ▲ CRITICAL Multiple factors should be considered when choosing different versions of GCaMP. Although GCaMP6s has the highest sensitivity, its slow kinetics make it difficult to resolve fast events such as indiviual spikes during a burst. Its relatively high affinity compared with other GCaMPs also raises the concern of causing excessive Ca2+ buffering. Of all the popular GCaMPs, GCaMP6f has the fastest kinetics, whereas GCaMP5G has the lowest affinity and a very good dynamic range19.
EQUIPMENT SETUP
Operant behavioral testing equipment
This protocol describes using the Standard Med-Associates mouse operant behavior testing package. The following essential components are required: the operant chamber with two retractable levers; one pellet dispenser for Bio-Serv dustless precision pellets; a food magazine with IR beam-break detector; one house light. In addition, the SmartCtrl interface module (DIG-716) and SuperPort 16 output module, TTL (DIG726-TTL) are required, housed in a compact module cabinet. Intracranial microinjection kit Connect the kit components as shown in Figure 4a.
Interfacing the equipment
To synchronize the optical recording with recorded behavior events, TTL pulses coding different events and triggering signals are sent from the Med-Associates interface card (DIG726-TTL, Supplementary Fig. 2a) to the digital signal processor (DSP) card on the Plexon MAP system (Supplementary Fig. 2b) and to the Becker & Hickl SPC830 module (pin 13 on connector 2, Supplementary Fig. 2c). As the Med-Associates system only synchronizes the start of the optical acquisition, which then runs on its own clock, it is important to empirically test the accuracy of the timing between the recorded optical file and the behavioral events at the end of each recorded optical file. An example of such tests is to place the optical probe in the operant box and measure the intensity of the ambient light controlled by the house light on/off commands in Med-PC IV. The timing of the ambient light intensity change recorded in the optical file should match the timing programmed in Med-PC IV (Supplementary Fig. 3). A practical solution to avoid timing errors in long experimental sessions is to record the optical data in multiple 1-min-long segments with a 5-s-long pause between individual segments. Each segment is triggered by the synchronizing pulses from the behavioral control module (see Supplementary Methods for an example of the MedPC code).
PROCEDURE
surgery for intrastriatal AAV vector injection and constructing the dental cement base • TIMING 60 min
Thaw one aliquot of AAV vector on ice. (The volume of each aliquot should be chosen on the basis of the dose for each mouse and the number of animals receiving injections.)
Turn on the main valve of the oxygen tank connected to the isoflurane vaporizer and adjust the regulator to set the flow rate at around 1.5 liter/min. Switch on the isoflurane vaporizer to 5%.
-
Open the lid of the induction chamber. Place the mouse in the induction chamber and cover the lid. Wait until the animal has been adequately anesthetized (respiratory rate drops to ~60/min, loss of toe pinch reflex).
! CAUTION Avoid leaving the mouse in the induction chamber with 5% isoflurane for too long (>5 min).
Take the mouse out of the chamber and shave the hair off the top of the head.
-
Divert the isoflurane flow to the gas anesthesia head holder on the stereotaxis by plugging the connector on the inlet tubing into one of the ports on the vaporizer. Reduce the vaporizer setting from 5% to 1–2%.
▲ CRITICAL STEP Monitor the vital signs of the mouse during the whole surgical procedure and adjust the depth of anesthesia by changing the setting of the vaporizer (usually between 1% and 2% isoflurane). Loss of response to the toe pinch reflex indicates sufficient depth of anesthesia. The respiratory rate should be kept between 55 and 65 per min. Labored breathing (gasping) and darkened (blue) skin color at the paws and lips indicate hypoxia.
Fix the head of the mouse on the stereotaxis. Protect the eyes from drying by covering eyes with ophthalmic ointment.
Disinfect the surgical area by applying generous amounts of Betadine. Wipe off Betadine with cotton applicators soaked with 70% (vol/vol) ethanol in a spiral pattern from the center to the periphery of the surgical area.
Make a 6–8-mm-long single incision with a scalpel through the skin along the midline of the skull, starting from the line across the eyes and toward the line across the ears. Place the wire retractor in the skin pocket to expose the surface of the skull.
Apply hydrogen peroxide solution with a cotton applicator and rub the skull surface to remove residual soft tissue and expose the skull. Dry the area with cotton applicators. Further reduce the isoflurane vaporizer setting to 1.5 and maintain it between 1 and 1.5 during the rest of the surgery.
Make final adjustments to align the head and level the top of the skull. To do this, first reposition the ear bars (by loosening and tightening one at a time) to make sure that the sagittal suture falls within the projection line from the head holder when observed from above. Second, level the y axis (anterior-posterior) by adjusting the screw on the mouse head holder to keep the line across bregma and lambda horizontal. Finally, level the x axis by adjusting the screw on the left ear bar.
Attach a 1 1/2-inch 19-gauge needle to the vertical arm of the stereotaxis. Adjust the x, y and z coordinates to place the tip of the needle gently touching the bregma. Use the x, y coordinates of bregma as the reference and calculate the x, y coordinates of the targeted brain area. For example, use xbregma+ 1.5 mm, ybregma+ 0.5 mm for the left dorsal striatum. Move the needle tip right above the targeted location. Dye the tip of the needle with a surgical skin marker pen and move down the needle along the z axis to mark the drilling point on the skull.
Drill a small burr hole (1.1 mm in diameter, can fit a 19-gauge blunt needle) centered at the marked point. Drill an extra small burr hole (slightly smaller than the diameter of the anchoring screws) on each side of the posterior parietal lobe for anchoring screws (Fig. 4b).
-
Screw two anchoring screws into two predrilled burr holes on the parietal bones.
▲ CRITICAL STEP These two screws provide the sole source of anchoring force to hold the dental cement cap on the skull (dental cement itself does not form a strong bond to the skull surface). This step is critical for stable recording. A third anchoring screw can be used if there is room on the skull (as shown in Fig. 4b).
Open a new vial of sterilized normal saline. Immerse the tip of the injector needle in the saline and draw saline to fill the needle and the extension tubing with a 5-cc syringe attached to the free end of the tubing. Disconnect the 5-cc syringe and replace it with a 2-µl Hamilton syringe.
To leave a small air gap between the AAV vector buffer and the filling saline, pull back the piston of the Hamilton syringe to draw 0.2 µl of air into the needle before immersing the tip into the AAV vector buffer. Draw 1.2 µl of AAV vector buffer into the needle.
-
Attach the injector needle to the vertical arm on the stereotaxis and place it above the center of the burr hole (Fig. 4b). To make final check on the patency of the injector, slightly push the piston of the Hamilton syringe to eject ~0.2 µl of AAV vector buffer while observing the tip of the needle under the dissection scope. The appearance of a small drop of shiny liquid indicates good patency. Gently remove the extra AAV buffer from the tip of the needle with a piece of disposable non-absorbing material, for example, a sterilized bamboo tooth pick.
! CAUTION Follow institutional procedures to properly handle and dispose of AAV vector-contaminated materials.
Remove the dura under the burr hole with the tip of fine forceps or a sharp hook made from a 23-gauge needle. Lower the injector needle until the tip touches the brain surface. Record the z axis coordinate and move 2.5 mm down below this point (into the dorsal striatum).
-
Slowly inject the 0.8-µl viral vectors over the course of 5–10 min. This can be done manually in small steps (0.05 µl per step) at even intervals (~30 s), or by using a commercial microinjection pump.
▲ CRITICAL STEP The exact volume of injection (from a few tens of nanoliters up to microliters) should be determined on the basis of the animal species; the size of the targeted structure and cell type; and the serotype, promoter and titer of vectors.
Leave the needle in place for an additional 5–10 min before lifting it. While the needle tip is in the air, eject 0.2 µl of vectors while observing under the dissection scope to confirm that the patency is good.
-
Attach a 23-gauge needle (serving as a guide for the 19-gauge tube) to the vertical arm of the stereotaxis. Move the needle above the injection burr hole. Use forceps to slide a piece of 5-mm-long 19-gauge metal tube (cut from a 19-gauge needle) onto the 23-gauge needle. Lower the vertical arm while holding the 19-gauge tube with forceps until the tip of the 23-gauge needle is ~1 mm above the burr hole. Slide down the 19-gauge tube to gently fit into the burr hole without depressing the cortex.
▲ CRITICAL STEP The 19-gauge tube serves as a landmark of the burr hole for future insertion of the optical fiber probe.
Fill two small rubber cups with dental cement and dental cement cure liquid, respectively. Use a small (no. 0 size) paintbrush to apply dental cement paste to cover the skull (Fig. 4c). Keep applying dental cement paste after the previous layer is dry until an ~2-mm-thick base is formed.
Wait for at least 15 min for the dental cement to solidify. Slowly lift the 23-gauge needle guide without disturbing the 19-gauge tube that is fixed in place by the dental cement.
Apply some dental cement to seal the opening of the 19-gauge tube (Fig. 4d).
Place the mouse in a new recovery cage on a warm water blanket.
-
Follow institutional protocols for postsurgical animal care.
Behavior training for the lever-pressing operant task ● TIMING 30–90 min per d for ~7 d
▲ CRITICAL Wait for the mice to fully recover from the surgery (~2 weeks) before starting their behavior training (Step 26 and onward).
Start food restriction 1–4 d before the training starts. Record the body weight of each mouse before food restriction starts as the baseline body weight. Restrict food throughout the training days and maintain mice at 85% of their baseline body weight (or follow recommended institutional procedures) by adjusting the amount of regular laboratory chow given at the end of each day.
Start with a two-lever free-choice fixed-ratio 1 schedule on the first training day, in which the mice obtain one food reward (20 mg of purified pellets from Bio-Serv) after each left- or right-lever press. Each fixed-ratio 1 session should last 90 min or until the mouse receives 30 rewards. (See supplementary Methods for an example of Med PC code for a two-lever free-choice fixed-ratio 10 task. Change the value ‘10’ to ‘1’ in S2 of S.S.3 to turn it into a fixed-ratio 1 task. Change the value ‘30’ to ‘90’ in S2 of S.S.7 to define the session time as 90 min.)
Repeat Step 27 daily or twice per day until the mice are able to obtain 30 rewards within 90 min for 2 consecutive days.
Start training on a two-lever free-choice fixed-ratio 5 schedule, in which a total of five lever presses, regardless of left or right, are needed for each reward delivery. (See supplementary Methods for an example of Med PC code for a two-lever free-choice fixed-ratio 10 task. Change the value ‘10’ to ‘5’ in S2 of S.S.3 to turn it into a fixed-ratio 5 task.)
Repeat Step 29 daily or twice per day until the mice are able to obtain 30 rewards within 30 min for 2 consecutive days.
Start training on a two-lever free-choice fixed-ratio 10 schedule in which a total of ten lever presses, regardless of left or right, are needed for each reward delivery. (See supplementary Methods for an example of Med PC code for a two-lever free-choice fixed-ratio 10 task.)
-
Repeat Step 31 daily or twice per day until the mice are able to obtain 30 rewards within 30 min for 2 consecutive days.
▲ CRITICAL STEP The mice should receive daily fixed-ratio 10 training until the imaging experiment is carried out. To achieve high expression levels of GCaMPs, we find it best to wait for 4 weeks from the time of injection before starting the imaging experiments. Note that the two-lever free-choice fixed-ratio 10 task used in this protocol serves as a basic example. Any operant design can be used here for studying different questions.
? TROUBLESHOOTING
Construction of the excitation and detection hybrid optical fiber probe ● TIMING 15 min
▲ CRITICAL It is best to construct the optical fiber probe on the same day as the imaging experiment (i.e., perform Steps 33–42 immediately before Step 43).
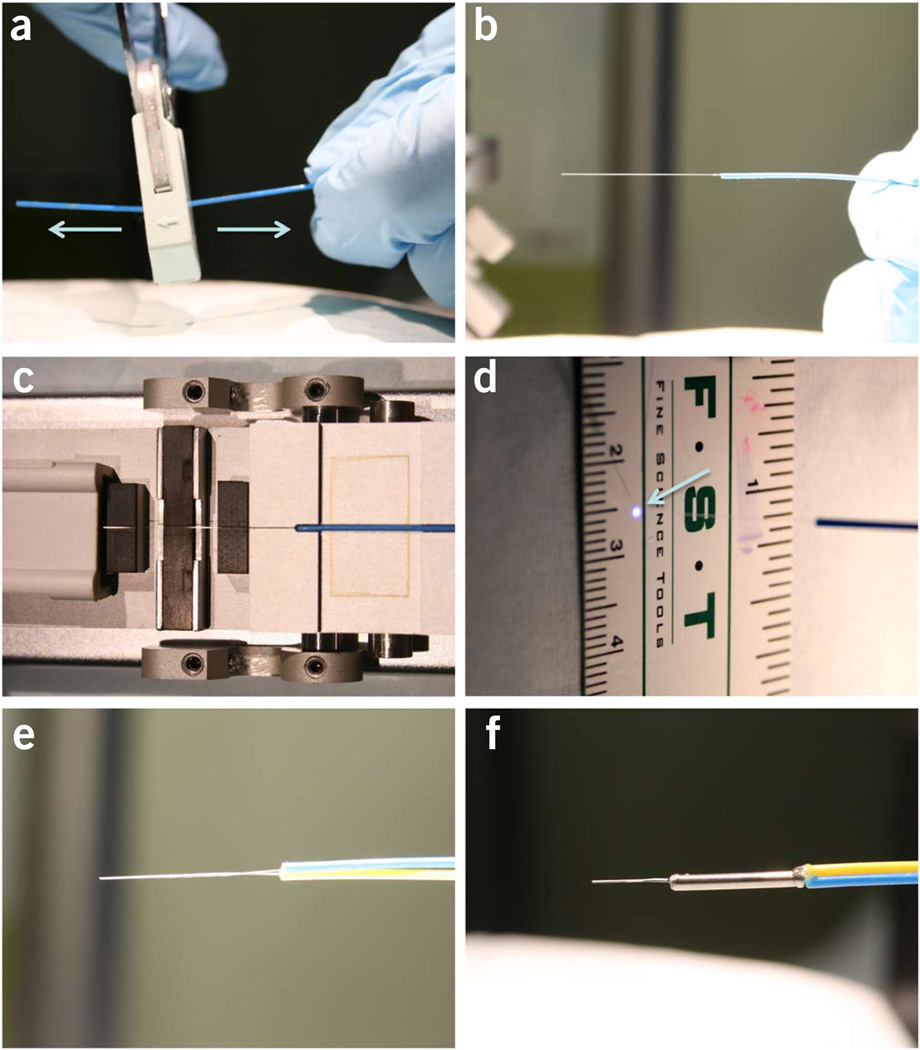
Strip the 15–20-mm-long protection jacket and acrylic buffer off the single-mode fiber patch cord with a single pull by using Clauss No-Nik fiber optic stripper to expose the bare fiber (cladding + core) (Fig. 5a,b).
Wipe the debris off the exposed bare fiber with a piece of an alcohol prep pad.
Place the fiber onto the high-precision fiber cleaver and make a flat-cleaved end following the steps printed on the cleaver (Fig. 5c). A correctly cleaved single-mode fiber should produce a perfectly round bright spot when the blue laser light is launched through the fiber (Fig. 5d).
Repeat Steps 33–35 for the multimode fiber.
Place the two exposed fiber ends in parallel with each other and press them tightly together.
Under a dissection microscope, make small adjustments to align the tips so that the two fibers stop at the same plane without staggering.
Apply a thin layer of acrylic glue to the fiber ends 5 mm away from the tips (Fig. 5e).
Insert the bound fiber ends through a 5-mm-long 20-gauge protective metal tube until the metal tube meets with the fiber jackets, leaving ~5-mm-long naked fiber ends protruding from the other end of the metal tube.
Apply more acrylic glue to both ends of the metal tube to ensure a tight bond between the jackets, fibers and the metal tube (Fig. 5f).
-
Tape the hybrid-fiber probe to the vertical arm of the stereotaxis. Make sure that the projection line from the tip of the probe is parallel with the arm (Fig. 4e).
Surgery for implanting the hybrid optical fiber probe ● TIMING 60 min plus recovery time
▲ CRITICAL If the optical recording experiments are scheduled to be completed on the same day as the surgery, the surgery should start early in the morning to allow sufficient time for the mouse to recover. We recommend that the surgery (Steps 43–67) and the recording sessions (Steps 68–76) be carried out on the same day to avoid leaving fiber-tethered mice unsupervised overnight. Please refer to institutional policy on this point. Alternatively, connectors (Fig. 4g,h and supplementary Fig. 1) can be added between the implanted fibers and the fibers connected to the laser and the detector to allow the mice to be free from tethering after each recording session.
Repeat Steps 2 and 3 to anesthetize the mouse.
Take the mouse out of the induction chamber. Pull the implanted 19-gauge metal tube straight away from the skull, using pliers, to expose the burr hole.
Repeat Steps 5 and 6 to fix the mouse head onto the stereotaxis.
-
Under the dissection scope, use fine forceps to remove blood clots and any other debris covering the burr hole until the soft brain surface is clearly visible under the scope.
▲ CRITICAL STEP If the dental cement surrounding the burr hole is too thick and blocks the view, carefully thin the dental cement around the burr hole by using a Dremel tool before this step.
Attach the vertical arm with the fiber probe to the stereotaxis. Adjust the coordinates of the vertical arm to place the hybrid probe above the cleaned burr hole (Fig. 4e).
Power on the components of the TCSPC system by switching on the power of the extension box housing the SPC-830 card and the DCC-100 card, powering on the laptop and turning the laser on by turning the key clockwise on the laser power control box.
Start the DCC and SPCM programs by double-clicking the icons on the desktop. In SPCM software, name and select or create a folder for the optical file, and then configure the SPCM settings and choose ‘None’ for ‘Trigger’ (see supplementary Methods and supplementary Fig. 4 for an example).
-
Lower the hybrid probe until the tip is in the burr hole but has not yet touched the brain. Adjust the x and y axes to place the tip close to the lateral edge of the burr hole (fnal location of the tip: ~2 mm medial-lateral, 0.5 mm anterior-posterior). Slightly lower the tip until it touches the brain surface. Record the z axis coordinates as the reference.
▲ CRITICAL STEP The fiber probe should be lateral to the center of the burr hole (1.5 mm medial-lateral, 0.5 mm anterior-posterior) to avoid damaged tissue left by the viral injection needle during previous surgery. Another reason not to measure at the injection site is that the GCaMP expression tends to be too high around the injection site, and it becomes more uniform when the probe tip is further away.
Dim the ambient light around the surgical field and start measurement in SPCM with a low gain setting for the detector. A nonspecific spectrum from ambient light is expected to show up in the display (Fig. 6a).
-
Make a single quick twisting move on the hand-screw controlling the z axis to help the tip penetrate the brain surface. Gradually increase the gain of the detector on the DDC-100 control panel to ~95–100%, and maintain it at the same value throughout the rest of the experiment. An example of the measured spectrum seen at this step is shown in Figure 6b.
▲ CRITICAL STEP To avoid overloading the detector, do not use high gain before the tip is submerged under the brain surface.
Keep lowering the fiber probe toward the dorsal striatum at ~200 µm per step until the GCaMP spectrum is detected (Fig. 6c). Next, start lowering the probe at 50 µm per step while closely monitoring the spectrum.
-
Stop at the depth at which further lowering of the probe no longer increases the peak value (Fig. 6d). The typical final tip location is anterior-posterior +0.5 mm, medial-lateral +2.0 mm (for left, or −2.0 mm for right) from bregma, and dorsal-ventral 2.0–2.5 mm from the brain surface.
? TROUBLESHOOYING
Fill two small rubber cups with dental cement and dental cement cure liquid, respectively.
Make a very thin (liquid-like) drop of dental cement with a small (size 0 to size 3/0) paintbrush. Gently apply the drop to the metal tube on the probe (avoid touching the probe with the brush itself, if possible) and let it flow down along the probe to cover the burr hole.
Keep applying dental cement until the lower half of the metal tube and the bare fibers are fully imbedded in a cone-shaped dental cement crust.
Apply thick (paste-like) dental cement to the joint between the plastic fiber jacket and the metal tube to reinforce it. The dental cement usually will flow down and fuse with the dental cement cone covering the lower half of the probe.
Wait for 30 min, until the dental cement is fully solidified, before removing the fiber-tethered mouse from the stereotaxis
-
Transfer the mouse to an open-top container with high walls (e.g., a 5-liter plastic beaker) with fresh bedding and water Monitor the mouse’s behavior until the next steps.
▲ CRITICAL STEP Wait until the mouse has fully recovered from anesthesia (>2 h) before proceeding to next steps.
Isoflurane test (optional) ● TIMING 30 min plus recovery time
▲ CRITICAL The results from this section are a good indicator of how well the previous steps have been performed and whether the GCaMP responses will be robust during the operant task.
-
Power on the TCSPC system by repeating Steps 48 and 49. Shut down the laser output by clicking ‘digital 6’ on the DCC-100 controller (not the main laser power supply controlled by the turnkey). In SPCM software, name and select or create a folder for the optical file, and then configure the SPCM settings and choose ‘None’ for ‘Trigger’ (see supplementary Methods and supplementary Fig. 4 for an example).
▲ CRITICAL STEP To avoid unnecessary extended illumination of the brain tissue, the laser output should only be turned on immediately before the recording starts.
Repeat Steps 2 and 3 to fill the induction chamber with isoflurane.
-
Place the fiber-tethered mouse in an arena (e.g., a large open-top rat cage) with fresh bedding and/or novel objects to encourage movement. Immediately start the optical recording by using automated cycles.
▲ CRITICAL STEP Turn off ‘display each step’ during all behavior-correlated optical recordings. Displaying each step will slow down the acquisition and cause significant error in timing between the recorded optical data and behavior.
After five or six cycles of recording with baseline locomotor activity, transfer the mouse to the isoflurane induction chamber. Slide back the chamber cover leaving a small slit that just allows the fibers to pass through.
Continue recording for five more cycles under the isoflurane anesthesia, and then transfer the mouse back to the arena to recover.
Shut down the isoflurane system.
-
Continue recording for ten more cycles during recovery.
▲ CRITICAL STEP Wait until the mouse has fully recovered from anesthesia (>2 h) before proceeding to the next steps. Closely monitor the mouse and prevent severe twisting of fibers.
? TROUBLESHOOTING
Optical recording of GcaMp fluorescence during a lever-pressing operant task ● TIMING 30 min
-
Power on the TCSPC system by repeating Steps 48 and 49. In SPCM software, name and select or create a folder for the optical file, and then configure the SPCM settings and choose the downward pulse for ‘Trigger’ (see supplementary Methods and supplementary Fig. 4 for examples).
▲ CRITICAL STEP The ‘trigger’ setting should be set to ‘arrow down’ and ‘Each start of sequence’ in the ‘system parameters’ setting. This tells the SPCM software to wait for external triggers from the Med-Associates system to start the optical recording. The time stamp of the triggering signal will also be recorded by the Sortclient software as a reference in order to align the optical data with behavior events offline.
Power on the Plexon MAP system. Start the Sortclient software. Open previously saved template file with proper settings to record different event timestamps (TTL pulses via selected digital input channels). Click the ‘Start’ button to start running the template without recording the file.
Power on the Med-Associates operant behavior test system. Start the Med-PC IV software. Load the previously saved MPC file (see supplementary Methods for example).
Start the video capturing software if you are using such software.
Carefully place the fiber-tethered mouse through the opening on the roof of the sound attenuation box into the operant chamber.
Click the ‘Start-recording’ button in Sortclient to manually start recording the time stamps.
Click the ‘Start’ button in SPCM to start optical measurement. The ‘Measurement’ indicator light at the bottom of the SPCM software window will appear yellow, indicating that it is waiting for external trigger.
Start the loaded MPC file in Med-PC IV. The periodically occurring ‘Acquisition start’ command programmed in the file (supplementary Methods) will send triggering TTL pulses to the TCSPC system every 65 s, allowing for a 60-s optical recording segment and a 5-s pause. The time stamps of these triggering signals, as well as various behavioral events detected by the Med-Associates operant box, will be recorded in Sortclient.
-
After the mouse has obtained the 30 rewards or when the 30-min session time is reached, the Med-PC program will automatically stop. Manually stop the optical recording in the SPCM software. Continue with the next mouse or quit all the programs and power down the systems.
? TROUBLESHOOTING
▪ PAUSE POINT The saved optical data and the behavior files can be combined and analyzed at a later date.
Plotting time-lapsed GcaMp intensity changes and combining them with behavioral data ● TIMING 30 min for a 30-min recorded file
To convert the recorded optical data (SDT files) into ASCII files, click ‘convert’ in the ‘main’ menu of the SPCM software and select ‘SDT files’. In the pop-up window, select all the files to be converted. Then input ‘1024’ for the ‘Number of values per line’ at the bottom of the window and click ‘convert’.
Open the ASCII files in Excel or MATLAB. The final output of each file (after deleting the header portion at the top rows) will be a matrix containing 1,200 (rows) × 1,024 (columns) values. The 1,024 values in each row are the photon counts recorded for 16 spectral channels at temporal (ADC) resolution of 64 channels (per 50 ns) (16 × 64 = 1,024) connected head to tail for each spectral channel. The 1,200 rows represent continuously recorded time-resolved spectra (each row) at 20 Hz for 60 s (20 × 60 = 1,200).
To calculate the time-lapsed fluorescence intensity (integrated photons), sum up the photon counts of the eight peak spectral channels for GCaMPs in each row and plot them (as y value) against the 1,200 rows (60 s, x axis).
-
Transform the fluorescence intensity into ΔF/F and save it as a single column text file. Ideally, F should be defined as the baseline fluorescence and calculated by averaging the fluorescence intensity values from segments without transients. However, it can be challenging to select baselines when there are many transients. On those occasions, we define F as the mean of the total 1,200 fluorescence intensity values per min to simplify the data processing.
? TROUBLESHOOTING
-
To combine the behavior time stamps data and the optical data in a single file for analysis, open the Plexon file containing the behavioral time stamps. Import the text files containing corresponding ΔF/F data as analog data. Defne the starting time of each text file on the basis of the recorded ‘Acquisition start’ time stamp, and select 0.05 s as the interval between each data point. Save each of these files as a ‘Nex’ file. Merge all the saved ‘Nex’ files on the basis of the time, which will give a single Nex file containing all the segments of 1-min ∆F/F data aligned to the same time clock of the files containing the behavioral events. Copy all the behavior time stamp data from the original Plexon file to the new file containing the ∆F/F data.
? TROUBLESHOOTING
? TROUBLESHOOTING
Figure 5.
Procedures to make a hybrid optical fiber probe. (a) A fiber optic stripper is used to remove the jacket and acrylic coating of a single-mode fiber. (b) A single pull of the fiber stripper can remove both the jacket and coating. (c) The stripped single-mode fiber is placed on a high-precision fiber cleaver to create a flat-cleaved end. (d) A perfectly round bright spot is formed by the blue light coming out of a single-mode fiber that has been stripped and cleaved through the steps shown in a–c. (e) The multimode and single-mode fibers are glued together. (f) A metal tube is added to reinforce the probe.
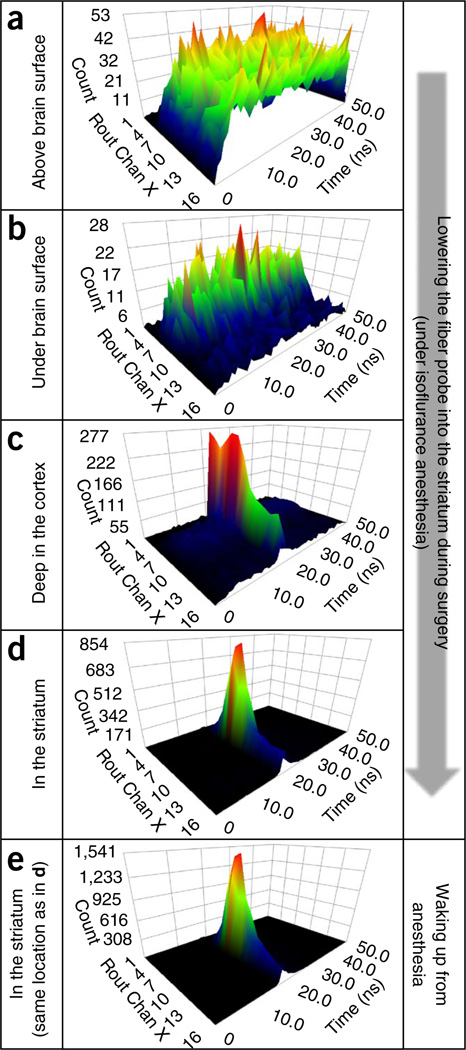
Figure 6.
A series of snapshots of time-resolved spectra acquired during the process of lowering the fiber probe into the striatum. (a) Detected photons are mostly from the ambient light when the fiber probe is placed above the brain surface. (b) The photon count decreases after the tip of the probe is submerged below the brain surface. (c) The laser-excited GCaMP5G spectrum starts to appear while the probe is going down through the cortex. (d) The GCaMP5G spectrum becomes dominant when the tip of the probe is in the dorsal striatum. (e) The intensity of GCaMP5G (measured at the same place as in d) is markedly increased after the animal wakes up from anesthesia (note the peak values highlighted by red dashed ovals). In all time-resolved spectrum plots, the x axis (‘Rout Chan X’) depicts the wavelength channels for detecting the fluorescence spectrum; the y axis depicts the time channels (in ns) for measuring fluorescence decay time; and the z axis is the photon count. All animal protocols used in this study were approved by the US National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 32 | Some of the animals trained in the two-lever free-choice task may develop a strong bias toward one of the two levers during the training, making it difficult to obtain within-subject comparison of left-lever and right-lever pressing behaviors | Naturally occurring, though not often | Further train the mice in single-lever fixed-ratio 10 sessions during which either the left or right lever is presented. For each animal trained in this way, the optical measurements should be carried out in two consecutive sessions with either left or right lever presented. To minimize the effect of satiety levels between the two consecutive sessions, only ten rewards should be used in each session |
| 54 | Poor GCaMP spectrum (e.g., the spectrum shown in Fig. 6c) during the fiber implantation surgery | Low GCaMP expression | Use AAV serotypes that have high selectivity for the cells of interest. Use strong promoters such as the CAG promoter. Wait for a longer time (4 weeks or more after injection) or increase the injection dose |
| Fiber tip is not in the targeted structure | Further lower the probe or retract the probe and change the x-y coordinates and lower the fiber again | ||
| Poorly constructed probe | Make sure the fiber ends are precisely flat-cleaved, aligned parallel, without staggering ends | ||
| 67, 80 | Good GCaMP spectrum but no fluorescence transients are seen in freely moving animals, and isoflurane does not induce reversible decrease in GCaMP fluorescence | GCaMP expression is too high and causes toxicity | Choose a weaker promoter or AAV serotype. Reduce the AAV injection dose. Reduce the interval between injection and measurement. Do not measure at the injection site. The GCaMP expression becomes more uniform and less intense when moving away from the injection site |
| The GCaMP fluorescence is from damaged cells | Move further away from the injection site | ||
| The targeted cell type may not show transient activation in freely moving animals. They may only activate in response to specific stimuli that are not present | Use the specific stimuli that are known to activate the cells of interest | ||
| Isoflurane anesthesia may not inhibit the neural activity of the targeted cells | Normal phenomenon, no solution needed | ||
| 76 | Broken fibers | Occasionally, some animals may display turning behavior strongly biased toward one direction after the fiber probe has been implanted, especially if they are not pre-trained with tethering. The jacketed fibers can usually tolerate twisting very well when the twisting force is evenly distributed through the whole length of fibers. However, strong twisting of fibers may cause a small diameter local loop to form when there is extra slack, which may break the fiber | Pre-train the animals with dummy fibers in later stages of operant training to accustom them to being tethered |
| Avoid extra slack after the mouse has been implanted with the fiber probe | |||
| Closely supervise the fiber-tethered mouse after surgery and manually rotate the housing container to release the twisting when necessary | |||
| Use connectors between the implanted fibers and the fibers connected to the laser and the detector (Fig. 4g,h and supplementary Fig. 1). Disconnect the mice from the rest of the system after each experiment | |||
| Select thicker furcation tube for the fibers, such as 1/16-inch-diameter polyolefin heat-shrink tubing | |||
| 81 | Timing error between the recorded optical data, behavior time stamps and video files | Multiple independent systems with different clocks, streaming and buffering causing variable delays in different systems | Send synchronizing TTL pulses to all the systems at the beginning of the recording and periodically throughout the recording. Later, align these reference marks in different files to calibrate for time. (e.g., supplementary Fig. 3) |
● TIMING
The total time for completing a typical experiment, from the viral injection to the imaging experiment, is ~4 weeks.
The time course of a typical experiment is as follows:
Steps 1–25, day 1, surgery for viral injection: 60 min
Steps 26–32, days 15–28, daily behavior training: 30–90 min per d
Steps 33–42, day 29, construction of fiber probe: 15 min
Steps 43–60, day 29, surgery for implanting fiber probe: 60 min plus a 120-min recovery
Steps 61–67, day 29, isoflurane test (optional): 30 min plus a 120-min recovery
Steps 68–76, day 29, optical recording during operant task: 30 min
Steps 77–81, day of choosing after day 29, data analysis: 30 min for 30-min recorded file
ANTICIPTED RESULTS
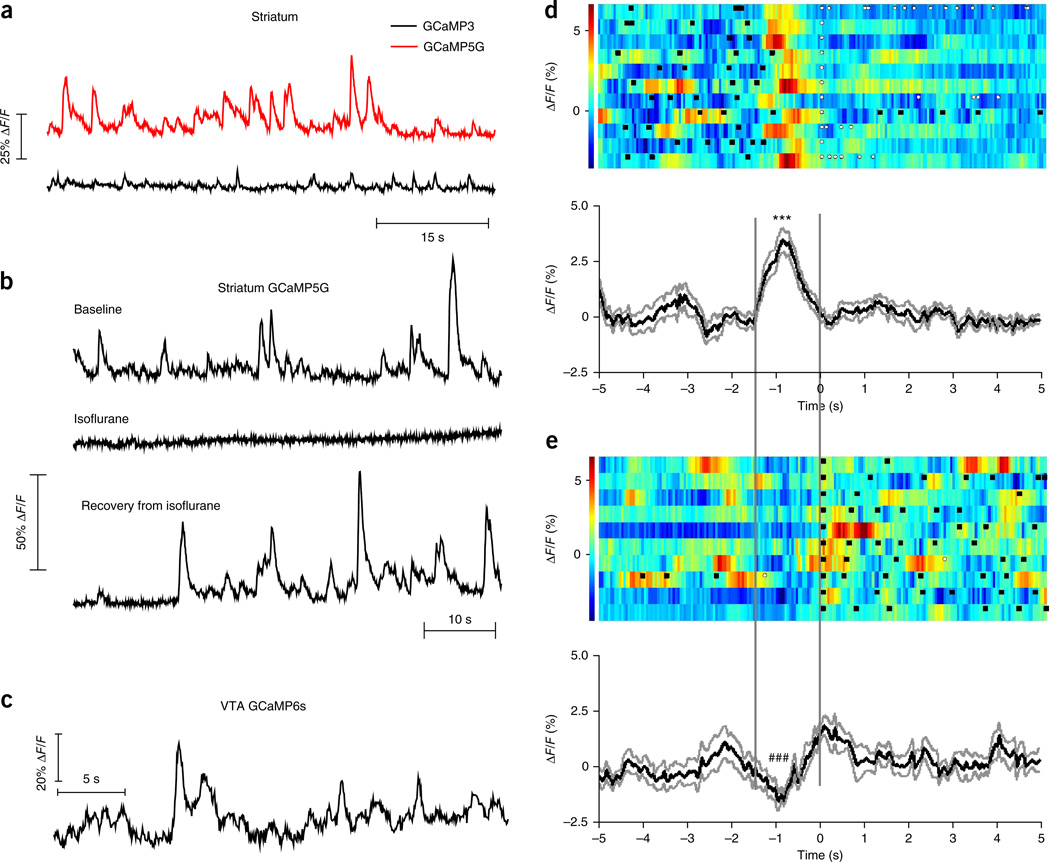
By properly following the procedures described in this protocol, it is expected that the GCaMP spectrum should be distinct from autofluorescence in the striatum when measured 1–2 months after the virus injection (Fig. 6c–e). After the implanted mice recover from anesthesia and start to move around, transient increases in fluorescence should be seen in both direct- and indirect-pathway SPNs expressing GCaMPs, but not in mice expressing constitutive GFP4. We found that GCaMP5G had a much lower baseline fluorescence level and higher ΔF/F compared with GCaMP3 (Fig. 7a), consistent with previous studies using two-photon imaging microscopy20. These fluorescence transients are blocked by isoflurane anesthesia, suggesting that they are dependent on neural activity in targeted cells (Fig. 7b). Furthermore, by following similar procedures, fluorescence transients can also be detected in other deep brain areas that are not easily accessible by traditional methods in behaving mice. For example, Figure 7c shows a trace containing fluorescence transients measured from the ventral tegmental area in a freely moving TH-Cre mouse expressing GCaMP6s in dopaminergic neurons18,19.
Figure 7.
Examples of fluorescence changes in freely moving mice expressing GCaMPs. (a) Comparison between GCaMP3 and GCaMP5G fluorescence transients recorded in freely moving mice expressing GCaMP3 or GCaMP5G in indirect-pathway SPNs. (b) The GCaMP5G transients (expressed in indirect-pathway SPNs) are abolished by isoflurane anesthesia and come back after the mouse wakes up. (c) Fluorescence transients detected in the ventral tegmental area (VTA) in TH-Cre mice after viral injection of AAV-FLEX-GCaMP6s. (d,e) GCaMP3 (expressed in direct-pathway SPNs) fluorescence aligned to the first food magazine entry made by the mouse after left lever presses (d), and its reversal behavior: the first left lever press after food magazine entries (e). Top, individual trials with color-coded fluorescence intensity. Black squares indicate left lever presses. White dots indicate food magazine entries. Bottom, averaged response from all the trials expressed as mean (black trace) ± s.e.m. (gray traces). *** and ### indicate P < 0.0001, paired t test between the fluorescence at 0 s and the peak (in d) or trough (in e). The peak (d) and trough (e) are calculated by averaging the fluorescence between −1 s and −0.8 s. Two vertical lines are drawn to highlight the area of interest in d and e. All animal protocols used in this study were approved by the US National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
After aligning the fluorescence data to behavioral time stamps, various plots and statistical analysis can be made (using software such as NeuroExplorer or MATLAB) to study the relationship between the recorded fluorescence (as a readout for neural activity) and specific behaviors. For example, to examine the activity of striatal indirect-pathway SPNs during different behavioral events, GCaMP (expressed in indirect-pathway SPNs) fluorescence can be plotted in graphs analogous to peri-event time histograms by using different behavioral events as references (Fig. 7d,e). When recorded in the left striatum, GCaMP fluorescence increases before the mouse is making the first food magazine entries after pressing the left lever (Fig. 7d), but not before the mouse is making the first left lever presses after food magazine entries (Fig. 7e). Further examination with video tracking suggests that the SPNs in both direct and indirect pathways activate during contraversive movements4.
ACKNOWLEDGMENTS
We thank V. Jayaraman, R.A. Kerr, D.S. Kim, L.L. Looger, and K. Svoboda from the GENIE Project, Janelia Farm Research Campus, Howard Hughes Medical Institute for allowing us to use GCaMP6s vectors. We thank L.L. Looger, J. Akerboom and D.S. Kim from the GENIE Project, Janelia Farm Research Campus, Howard Hughes Medical Institute for permission to use GCaMP5G vectors; L.L. Looger and Janelia Farm Research Campus of the Howard Hughes Medical Institute for permission to use GCaMP3 vectors; C.R. Gerfen for gifts of multiple bacterial artificial chromosome transgenic mouse lines; and K. Jalink for gifts of DNA constructs of EPAC (exchange proteins directly activated by cAMP) sensors. This work was supported by US government funding from the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism to D.M.L., S.S.V. and R.M.C., by the European Research Council (STG 243393), and by an International Early Career Scientist grant from the Howard Hughes Medical Institute to R.M.C.; by a National Research Foundation of Korea grant (2011-0029485, 2012-0004003) and a Smart IT Convergence System Research Center grant (SIRC-2011-0031866) from the Korean government (MEST) to S.B.J.; and by an Ellison Medical Foundation grant (AG-NS-0944-12) to X.J.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS R.M.C. and S.S.V. conceived the original idea of using the TCSPC technique for optical equipment, and optimized procedures for in vivo optical recording. G.C. and S.B.J. carried out the in vivo experiments and analyzed data. X.J. helped with programming and data analysis. G.L. performed the DNA transfection in HEK cells and helped in maintaining mouse lines. M.D.P. performed initial in vitro experiments by using the TCSPC system and analyzed data. G.C., D.M.L., S.S.V. and R.M.C. wrote the paper.
COMPETING FINANCIAL INTERSESTS The authors declare no competing financial interests.
References
- 1.Looger LL, Griesbeck O. Genetically encoded neural activity indicators. Curr. Opin. Neurobiol. 2012;22:18–23. doi: 10.1016/j.conb.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JN, Denk W. Imaging in vivo: watching the brain in action. Nat. Rev. Neurosci. 2008;9:195–205. doi: 10.1038/nrn2338. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh KK, et al. Miniaturized integration of a fluorescence microscope. Nat. Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui G, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang KH, et al. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Lleres D, Swift S, Lamond AI. Detecting protein-protein interactions in vivo with FRET using multiphoton fluorescence lifetime imaging microscopy (FLIM) Curr. Protoc. Cytometry. 2007;12 doi: 10.1002/0471142956.cy1210s42. 12.10. [DOI] [PubMed] [Google Scholar]
- 7.Kettling U, Koltermann A, Schwille P, Eigen M. Real-time enzyme kinetics monitored by dual-color fluorescence cross-correlation spectroscopy. Proc. Natl. Acad. Sci. USA. 1998;95:1416–1420. doi: 10.1073/pnas.95.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Y, Li JZ, Schnitzer MJ. Enhanced archaerhodopsin fluorescent protein voltage indicators. PLoS ONE. 2013;8:e66959. doi: 10.1371/journal.pone.0066959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimley JS, et al. Visualization of synaptic inhibition with an optogenetic sensor developed by cell-free protein engineering automation. J. Neurosci. 2013;33:16297–16309. doi: 10.1523/JNEUROSCI.4616-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel SS, Blank PS, Koushik SV, Thaler C. In: Laboratory Techniques in Biochemistry and Molecular Biology. Gadella TWJ, editor. Vol. 33. Elsevier; 2009. pp. 351–394. [Google Scholar]
- 11.Elangovan M, Day RN, Periasamy A. Nanosecond fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy to localize the protein interactions in a single living cell. J. Microsc. 2002;205:3–14. doi: 10.1046/j.0022-2720.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 12.Klarenbeek JB, Goedhart J, Hink MA, Gadella TW, Jalink K. A mTurquoise-based cAMP sensor for both FLIM and ratiometric read-out has improved dynamic range. PLoS ONE. 2011;6:e19170. doi: 10.1371/journal.pone.0019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann C, et al. A FlAsH-based FRET approach to determine G protein–coupled receptor activation in living cells. Nat. Methods. 2005;2:171–176. doi: 10.1038/nmeth742. [DOI] [PubMed] [Google Scholar]
- 15.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yelin D, Bouma BE, Yun SH, Tearney GJ. Double-clad fiber for endoscopy. Optics Lett. 2004;29:2408–2410. doi: 10.1364/ol.29.002408. [DOI] [PubMed] [Google Scholar]
- 17.Dunn RC. Near-field scanning optical microscopy. Chem. Rev. 1999;99:2891–2928. doi: 10.1021/cr980130e. [DOI] [PubMed] [Google Scholar]
- 18.Gong S, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akerboom J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]