Abstract
The genetic circuits that regulate cellular functions are subject to stochastic fluctuations, or `noise', in the levels of their components. Noise, far from just a nuisance, has begun to be appreciated for its essential role in key cellular activities. Noise functions in both microbial and eukaryotic cells, in multicellular development, and in evolution. It enables coordination of gene expression across large regulons, as well as probabilistic differentiation strategies that function across cell populations. At the longest timescales, noise may facilitate evolutionary transitions. Here we review examples and emerging principles that connect noise, the architecture of the gene circuits in which it is present, and the biological functions it enables. We further indicate some of the important challenges and opportunities going forward.
Circuits of interacting genes and proteins implement the regulation and differentiation programs that are the basis of life. Over the past decade, experimental studies have established that many of these circuits' most critical molecular components show substantial, unavoidable stochastic fluctuations, or noise, in their levels and activities. As a result, even genetically identical cells in a homogeneous environment can behave quite differently from one another. As an impediment to the design of deterministic circuits, noise is a nuisance. But a new wave of studies is showing how noise can, and does, provide critical functions that would be difficult or impossible to achieve by (hypothetical) deterministic gene circuits.
Although the potential importance of noise for biological function was appreciated many decades ago, the development of single-cell-analysis methods in the past decade allowed the direct observation of noise in diverse organisms. Recent reviews on noise in gene circuits have focused on the sources of noise in gene expression and its mathematical representation1,2, on ways to analyse noise in the context of dynamic circuits3, and on the advantages of phenotypic variability4,5. Here we will focus on the types of dynamic behaviours that noise enables and the functional roles they have in the cell. These issues can be analysed at three distinct levels. First, noise can enable certain useful physiological regulation mechanisms, such as coordinating the expression of a large set of genes. Second, at the population level, noise permits a wide range of probabilistic differentiation strategies from microbial to multicellular organisms. Third, noise can facilitate evolutionary adaptation and developmental evolution. We will first briefly review recent work that has characterized the types and timescales of fluctuations, particularly with respect to gene expression, and then address the functional roles of noise at each of these three levels.
Gene expression noise
Many biochemical processes in the cell involve low molecule numbers or infrequent interactions and therefore give rise to stochastic fluctuations. Such effects have a critical role in diverse processes including cytoskeletal dynamics, cell polarization, signal transduction and neural activity. However, gene expression is undoubtedly the best studied example as it is both central to almost all cellular functions and, owing to the low copy number (1–2 per cell) of most genes, especially susceptible to noise1–3 (Fig. 1a–d). In fact, molecular noise is unavoidable. New theoretical work has provided fundamental limits to how well any feedback system can perform in reducing noise, and has shown that even an optimal noise-reducing feedback circuit reduces noise only with the fourth root of the number of control molecules6.
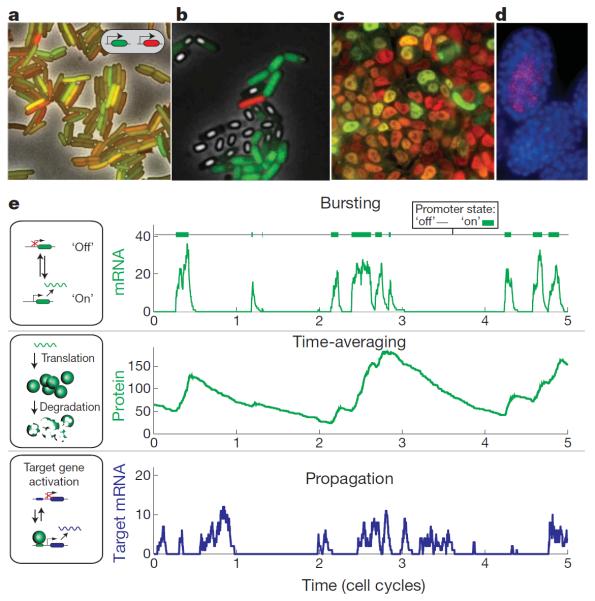
Figure 1. Gene expression noise is ubiquitous, and affects diverse systems at several levels.
a, E. coli expressing two identical promoters driving two different fluorescent proteins, in red and green, respectively. Because of noise, the ratio of red to green intensity differs from cell to cell22. b, A clonal population of B. subtilis cells differentiate into different fates in the same conditions. Here, some cells grow vegetatively or sporulate (green fluorescence), others have completed sporulation (white), and one has differentiated into a state of genetic competence (red fluorescence). Image provided by G. Süel. c, Mouse embryonic stem cells show relatively homogeneous expression of Oct4 (red nuclear protein staining), but heterogeneous expression of Nanog (green nuclear protein staining). Image provided by F. Tan. d, The C. elegans skn-1 mutant shows noise-driven partial penetrance. Two genetically identical embryos are shown. One has developed a gut (elt-2 RNA staining, red) whereas the other has not (nuclei in blue). Image provided by A. Raj. e, Mechanisms that shape noise in gene expression. Noise is characterized by bursty expression of mRNA (top). Proteins typically have longer lifetimes than bursts, leading them to time-average or `buffer' these bursts (middle). Finally, noise in one gene can propagate to generate further noise in the expression of downstream genes (bottom).
Gene expression noise can be characterized by the distribution of protein levels in individual cells and by the timescale of fluctuations, that is, the time over which a cell remains at a given position in the distribution (correlation time). Recent experimental and theoretical work has converged on a simple framework to understand gene expression noise7–10 (Fig. 1e). This framework is based on three key concepts.
The first concept is that of bursts. Proteins do not trickle out at a uniform rate, but rather are produced in stochastic bursts. This is both because each individual messenger RNA is typically translated many times to produce many proteins and also because the gene's promoter can stochastically switch between long-lived `off' and `on' states, resulting in bursts of mRNA production amplified to generate corresponding protein bursts. Whereas the mean level of expression is set by the product of promoter activity, transcription, and translation, noise depends predominantly on the first two of these processes, which work at lower molecule numbers. Examples of bursting exist in a variety of systems, including bacteria11–14, yeast15,16, mammalian cells17 and developing embryos18.
The second is time averaging. When the protein lifetime is longer than the interval between protein production bursts (as it often is), the accumulation of proteins over time tends to average out the variability generated by bursty expression, effectively buffering the protein concentration.
The third is propagation. Rates of gene expression are influenced directly by the levels and states of transcription factors and other upstream components that are themselves subject to bursting and time averaging. As a result, fluctuations in the expression of one gene propagate to generate fluctuations in downstream genes. In fact, this effect can be used to infer active regulatory interactions19,20. In bacteria, slow upstream fluctuations in rates of gene expression give rise to an effective cellular `memory' over cell-cycle timescales21. Mammalian cells show similar behaviour for some genes, although others fluctuate more rapidly22.
A simple way to visualize and quantify the relative importance of noise-generating bursts (intrinsic noise) versus noise propagation (extrinsic noise) is to analyse the expression of two distinguishable, but identically regulated fluorescent protein reporters in the same cell (Fig. 1a)23–25. Uncorrelated fluctuations result from bursting and time averaging, whereas correlated fluctuations reflect propagation of upstream components.
Noise and gene expression coordination
Whereas it is clear how noise can disrupt otherwise precise genetic programs, it is less obvious whether it can, counter-intuitively, improve cellular regulation. A recent paper26 examined such a case. Cai et al. studied how yeast co-regulate a large set of target genes in response to calcium using the stochastic nuclear-localization dynamics of the transcription factor Crz127 (Fig. 2a). Surprisingly, Crz1 localizes to the nucleus in short stochastic bursts lasting 1–2 min. These bursts involve the simultaneous translocation of many Crz1 molecules to the nucleus. Calcium levels, the input to this system, affect the average frequency but not the average duration or amplitude of these bursts. Thus, the operation of the system is based on frequency-modulation regulation of stochastic nuclear localization bursts.
Figure 2. Frequency modulation of stochastic nuclear localization bursts enables coordination of gene regulation.
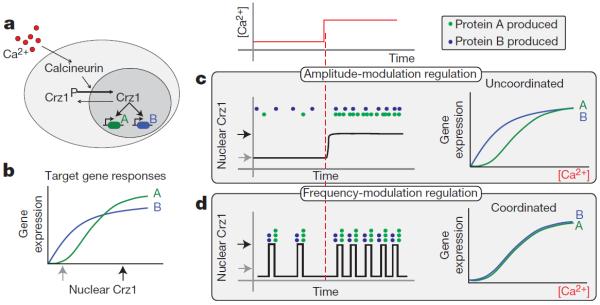
a, In yeast, calcium activates calcineurin, which in turn dephosphorylates the phosphorylated form of Crz1 (Crz1P) transcription factor, causing its localization to the nucleus where it activates over 100 target genes (two are indicated schematically). b, Response curves showing expression of the two hypothetical target genes as a function of nuclear Crz1 level. As shown here, target promoters may vary in the effective affinity and sharpness of response to Crz1. c, d, Regulation of the two target genes in amplitude-modulation and frequency-modulation schemes. c, In amplitude-modulation regulation, low levels of calcium lead to more expression of B than A, whereas the reverse is true at high levels (green and blue dots indicate newly produced proteins of genes A and B, note the step in calcium, above (red)). The resulting gene expression profiles (normalized to their own maxima) therefore differ between genes. d, In a frequency-modulation model, each burst yields (on average) the same number of proteins from each gene (blue and green dots). Increased calcium levels increase the frequency of bursts and thus the total level of expression of both A and B without affecting their ratio. Gene expression therefore follows the frequency response, regardless of the differences between promoters, enforcing coordination.
Frequency-modulation regulation can provide a functional advantage to cells, enabling coordinated (proportional) control of a large regulon. To see why, first consider a simpler, alternative amplitude-modulation scheme, in which an increase in calcium leads to a corresponding increase in the fraction of Crz1 molecules localized to the nucleus. In this hypothetical system, which corresponds to the mean behaviour of the cell population, two promoters that respond differently (with different affinities or cooperativities) to nuclear Crz1 (Fig. 2b) will have different expression ratios depending on the level of calcium (Fig. 2c). Their expression is thus `uncoordinated'. In contrast, frequency-modulation regulation maintains the products of these genes at fixed proportions across a wide range of expression levels. The explanation is shown schematically in Fig. 2d: each burst has the same characteristics (on average). Increasing calcium increases the number of bursts per unit time—the fraction of time that all promoters are `on'— without changing the relative level of expression of different genes. Analysing many endogenous targets verified that the Crz1 regulon was indeed coordinated through this mechanism. The authors also found that other stress-responsive transcription factors, such as Msn2, show stochastic bursts of nuclear localization that are uncorrelated with those of Crz1 when observed in the same cell.
From a theoretical point of view, frequency-modulation regulation is interesting as an example of a system in which the mean response to the distribution of signal levels is very different from the response to the mean of the signal levels10. It is important to note that this frequency-modulation strategy provides coordination across target genes, but does not reduce noise in their expression. In fact, bursts of nuclear localization constitute an additional, extrinsic, source of noise in the expression of target genes. The use of stochastic bursts rather than a deterministic oscillator to implement frequency modulation indicates that regularity is not critical in this context, probably because expression noise is time averaged over the much longer cell cycle. Finally, recent work indicates that other dynamic regulatory modes may also occur, such as control of the fraction of cells in a population that show nuclear–cytoplasmic oscillations28.
Functional roles of noise in probabilistic differentiations
One of the key functional advantages of noise is its ability to enable probabilistic differentiation of otherwise identical cells. This permits a number of cellular strategies such as bet-hedging and division of labour that would otherwise be difficult or impossible to implement in a deterministic system5. Recent work has identified a number of overlapping modes of probabilistic differentiation and begun to elucidate the role that noise has in the underlying gene circuits. These systems can be classified according to their dynamical characteristics (Fig. 3a). Stochastic state-switching systems switch between metastable states and are often based on positive feedback loops. Noise-triggered excitable differentiation systems allow cells to probabilistically enter a state, but return to the original state after a defined time. These systems use a combination of positive and negative feedback loops. In procrastinating differentiation systems, individual cells gradually and variably build up the level of a key regulator to generate a broad distribution of delays before committing to a new, markedly different fate.
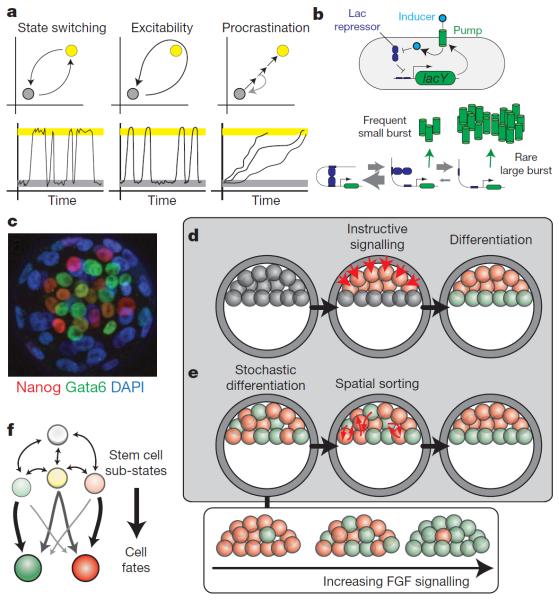
Figure 3. Probabilistic differentiation.
a, Schematic illustrations of three distinct modes of probabilistic differentiation (top) and corresponding time traces (below), as indicated. b, Noise in the lac system. Top, schematic view of the lac-positive feedback loop: increased expression of the LacY transporter (green cylinder) increases intracellular inducer levels (light blue circle), inhibiting the LacI repressor (dark blue) and further increasing expression. Bottom, lacY expression is blocked upon simultaneous binding of LacI to two operator sites on the lac promoter, which forms a DNA loop. Unbinding from one of these sites leads to transcription of at most one mRNA before re-looping, causing small increases in LacY. More rarely, LacI will be completely released from both sites, resulting in a large burst of mRNA and proteins that may lead to a switch of the positive feedback loop. c–f, Probabilistic differentiation in embryonic stem cells. c, Expression of Nanog (red) and Gata6 (green) in individual cells in the inner cell mass of a mouse embryo. Note the predominantly exclusive but spatially disorganized expression of the two genes. DAPI, 4',6-diamidino-2-phenylindole. Image adapted with permission from ref. 59. d, e, Two models for patterning of the inner cell mass (ICM). d, In a positional model cell, fate is determined by position through signalling from neighbouring cells. e, In the stochastic sorting model, cells first differentiate randomly, and subsequently move to appropriate positions based on their identity. f, Stem cell populations are not homogeneous but rather consist of a dynamic distribution of sub-states (dim green and red circles). Some sub-states resemble the differentiated states to which they are more prone to differentiate (bright green and red circles).
Stochastic state switching
Cells show a rich diversity of stochastic state-switching systems in the context of physiology, development, stress response, cancer and pathogenicity4. Theoretical and experimental analysis has shown that random switching between states can be advantageous in unpredictable environments and is optimal when the switching rate is tuned to the typical timescale for environmental fluctuations29–32. For example, Escherichia coli switch spontaneously in and out of a slow-growing `persister' state in which they are insensitive to antibiotics33. At the same time, synthetic biology and other studies have established that positive feedback loops are sufficient to generate such transitions34,35. These observations provoke the question of how actual state-switching circuits function.
Noise can lead to the coexistence of distinct states in positive feedback systems even without bistability per se. In yeast, a synthetic auto-regulatory transcription factor was recently shown to generate a bimodal distribution of activation levels in a cell population without a significantly nonlinear response function. To et al. showed that bimodality results when the transcription factor is both unstable and produced in a burst-like fashion36. The bursts enable spontaneous transitions to the high-expressing state, whereas the instability of the transcription factor enables a stochastic return to the low-expressing state. Thus, co-existing states can be generated in a remarkably simple way.
Perhaps the most comprehensively analysed bistable system (see refs 37 and 38 from the 1950s) is the lactose utilization system, governed by the lac operon, the expression of which is bistable when induced by non-metabolizable lactose analogues such as thiomethylgalactoside (TMG). The lac operon is repressed by tetramers of LacI, which simultaneously bind to two sites in the lac promoter, forming a DNA loop (Fig. 3b). Inducers inhibit LacI binding to DNA, allowing lac operon transcription. Bistability arises from positive feedback: addition of TMG induces the expression of the lac operon including the LacY transporter, leading to higher intracellular TMG concentrations, which result in further induction39 (Fig. 3b).
Examination of lac expression8,11,12 has indicated that noise in the `off' state stems from rare lac transcription events (less than one per cell cycle) that lead to small bursts of proteins. A recent paper by Choi et al. has re-examined the noise characteristics of lac in the context of state switching from `off' to `on' states40. They determined first the threshold of LacY needed to engage the positive feedback. By pre-inducing LacY and letting it dilute out by cell growth before adding inducer, they identified a sharp threshold at ~375 LacY proteins, far above LacY levels in the `off' state. How can these cells ever build up enough LacY to cross the threshold? The answer lies in the dynamics of the LacI-mediated loops (Fig. 3b). Most of the time, LacI dissociates from at most one site, but quickly reassociates because it remains bound at a neighbouring site, producing brief, basal bursts of expression. Very rarely, however, LacI will dissociate from both operators simultaneously, leading to a much longer burst41. When inducer is present, the duration of this large burst is extended further, enabling transition to the `on' state. This example shows clearly that cell-state transitions can depend on rare stochastic events at the level of the promoter. A number of issues remain unclear even in this well-characterized system. For example, how is the `off' state maintained throughout the cell cycle when replication suddenly doubles the number of lac operons?
The issue of promoter state switching and stability is particularly pertinent to eukaryotic cells, where recent work showed that eukaryotic promoter-switching events may arise from infrequent changes in the position of nucleosomes in the promoter42,43. Recent work indicates that cancer cells show a stochastic persistence phenotype. Individual cells within clonal populations can spontaneously and reversibly switch to a long-lived, drug-tolerant state44. Intriguingly, stochastic switching to persistence depends on histone-modifying enzymes, and the appearance of persisters can therefore be eliminated by inhibiting these histone modifications.
Noise-triggered excitable circuits for transient differentiation
Bistable systems typically generate an exponential distribution of durations for both states (Fig. 3a, left). But some cell types probabilistically initiate transient differentiation episodes that last for a well-defined period of time. The dynamical mechanism of excitability enables such behaviours (Fig. 3a, middle). An excitable system shows a large-amplitude, transient and stereotyped dynamic response to a threshold-crossing perturbation, much as a toilet goes through a specific sequence of events (flush) in response to a sufficiently large press of its handle. Recently, it was shown that differentiation into a genetically competent state in Bacillus subtilis fits the description of a noise-triggered excitable system (Fig. 1b). The master regulator ComK positively autoregulates its own expression, facilitating rapid activation of the system45, which leads to downregulation of ComS, an indirect inhibitor of ComK degradation, forming a slow negative feedback loop46. Whereas the positive feedback controls the threshold, and thus the frequency of initiation, the negative feedback controls the duration of competence events. Thus, the system enables independent tuning of its two key properties47. Recent work has shown how noise affects both the initiation and duration of competence.
Noise is required for initiation, as shown recently in two different ways. Süel et al. used a global strategy, specifically blocking septation to produce elongated bacterial filaments47. Because the absolute number of copies of any molecular species is proportional to the size of the filament, protein fluctuations, but not mean protein concentrations, are reduced. Maamar et al. specifically increased comK transcription and decreased its translation to reduce noise without changing mean expression level48. Both methods led to a reduction in the rate of competence initiation with less noise, as predicted by noise-dependent models. These two approaches are complementary: the first (recently applied in yeast cells49) quantifies the global effects of noise, whereas the second can be used to test candidate noise sources.
In addition to allowing probabilistic initiation of competence episodes, noise also increases variability in their duration. A recent paper showed how variability in the duration of competence episodes depends on the architecture of the ComS negative feedback loop50. Whereas in the endogenous system ComK represses its own activator (ComS), an alternative negative feedback design can allow ComK to activate its own inhibitor. These two designs, which are similar in the deterministic limit, can behave very differently when one considers noise. In the wild-type system, competence episodes end when ComS levels are very low and subject to relatively large fluctuations, leading to substantial variability in duration. In the alternative system, competence episodes end at high levels of the inhibitor, which are relatively less susceptible to noise, leading to more uniform durations. Indeed, synthetic circuits of the second type were functional and showed the same mean duration as wild type, but reduced variability. This indicates that variability might be adaptive. What function could such variability provide? One advantage of variable durations is that they enable cells to take up DNA more efficiently across a broad range of extracellular DNA concentrations. Variability could therefore be advantageous when environmental DNA concentration is unpredictable.
Procrastinating differentiation
Many terminal differentiation processes show large variability in the time from sensing an initial inducing signal to the final commitment to their new fate. Recent work has analysed several such systems, including the transformation of bacterial cells to spores (bacterial sporulation)51,52, meiosis during yeast sporulation53, and, perhaps most irreversibly, the initiation of cell death (apoptosis) in mammalian cells54. In each case, individual cells initiate differentiation at widely varying times owing to the variable rate of accumulation of a master regulator before commitment. This variable `procrastination' strategy enables cells to defer commitment for differing lengths of time. It could be advantageous when the environment has a significant probability of reverting to a condition that no longer favours differentiation.
Procrastinating differentiation requires a noisy initiation process with a long correlation time. Two recent papers suggest that this long correlation time stems from persistent extrinsic variation. Nachman et al. examined the onset of sporulation in yeast53, controlled by the variable accumulation of the master regulator Ime1. They showed that the accumulation rate depended on cell size, which shows significant cell-to-cell variability but is constant throughout the initiation process, resulting in stable differences between cells. Similarly, Spencer et al. used time-lapse microscopy to study temporal variability in the activation of apoptosis54. They found that stable differences between cells, specifically in the rate of caspase-mediated activation of the pro-apoptotic protein BID, explained much of this variability. Sister-cell correlations in the timing of apoptosis were increased when protein synthesis was inhibited, indicating that new protein production is necessary to erase these stable correlations.
Positive feedback may also extend the correlation time of fluctuations, even without bistability. Two cells that start out with different component levels can accumulate master regulators at different rates. For example, positive feedback of the sporulation master-regulator Spo0A on its own rate of accumulation was recently shown to be important for generating variability in sporulation timing51. It will be interesting (but not urgent) to develop a more comprehensive picture of how timing and variability are regulated together in procrastinated differentiation systems.
Probabilistic differentiation in stem cells
Through their dual capacity to both self-renew (proliferate) and differentiate, stem cells enable regulation of cell type and number during development. It has long been recognized that fate choice can occur in an apparently stochastic fashion55. For example, in neural differentiation, only a fraction of cells in an apparently equivalent population adopt the neural fates56. Transforming growth factor β (TGF-β) signals were shown to control the size of this fraction. Recent work is beginning to indicate a heterogeneous and dynamic picture of the stem cell state, in which cell–cell variability functionally impacts the determination of individual cell fates in response to stimuli.
A prime example is found in the early mouse embryo, where the inner cell mass gives rise to distinct epiblast and primitive endoderm lineages. The epiblast develops into embryonic tissues and expresses the pluripotency regulator Nanog, whereas the primitive endoderm produces extraembryonic tissue and expresses the transcription factor Gata6. Before any morphological separation between the two fates, individual cells begin to express only one transcription factor or the other, but not both, in a heterogeneous `salt and pepper' fashion57,58 (Fig. 3c). Intercellular signalling (through the FGF pathway) seems to bias the relative frequencies of the two states (Fig. 3e, bottom), but maintains the early binary distinction between them59. These and other results have indicated a noise-dependent developmental patterning mechanism. Cell fates are initially specified in a stochastic and spatially disorganized manner (though lineage-biased60); subsequently, cells of the same type `sort out' to generate the correct spatial arrangement (Fig. 3e). This contrasts with more deterministic models in which spatial position or lineage history determines cell fate (Fig. 3d). However, the small number of cells in the inner cell mass provokes a further question: is the stochastic cell-fate-determination process cell autonomous, and therefore subject to binomial fluctuations in the number of cells of each fate? Or, might further feedback loops and a period of reversibility of the two fates59 enable the embryo to regulate the percentage of each cell type? Interestingly, similar issues are also being explored in simpler systems, such as fruiting body formation in Dictyostelium discoideum61.
In vitro, stem cell populations show similar heterogeneity to their in vivo counterparts. For example, in embryonic stem cells, Nanog levels are distributed bimodally62,63 (Fig. 1c). Cells from both high and low Nanog sub-populations self-renew and, under appropriate conditions, differentiate, but with differing propensities (Fig. 3f). Cells also slowly switch between sub-states over long timescales63,64 (~1 week or more). Such dynamics seem to be quite general, as they have been observed in other genes in embryonic stem cells and in other stem cell types, such as haematopoietic progenitors65. Moreover, recent work has now identified further sub-states within the embryonic stem cell population that differ not just in gene expression but also in function, making different contributions to embryonic and extraembryonic tissues when introduced into embryos66. Together these data indicate a dynamically heterogeneous view of the stem cell `state.'
In the context of probabilistic differentiation during development, slow fluctuations in gene expression levels or between sub-states could help coordinate two related objectives. On short timescales, a signal could induce a response, such as differentiation, in a fraction of otherwise identical cells. On a longer timescale, re-equilibration would maintain a fully responsive stem cell population that could again be fractionally induced. Understanding this and other potential roles for stem cell fluctuations will require comprehensive knowledge of what states and transitions occur within the stem cell type, which proteins are fluctuating and how they work together to affect cell fate decisions.
Noise and evolution
In addition to its roles in physiology and differentiation, noise can also have an integral role in evolution by expanding the range of phenotypes that can result from a given genotype. This effect can be seen both in the evolution of quantitative phenotypes and in qualitative transitions such as those that occur in the evolution of development. Here we discuss examples of both.
First consider a simple quantitative phenotype, such as the level of expression of a gene in a unicellular organism. This phenotype is characterized by both its mean and its noise (variability between individuals), both of which are genetically controlled and subject to evolutionary selection. Fluctuating environments present an obvious selection for increased noise29–31. But how do noise traits respond to a simple directional selection on gene expression level? Interestingly, the result depends on the strength of selection. A tight threshold selection leads to increased noise (as well as increased mean) in expression (Fig. 4a, c). In contrast, a lower threshold, which still selects for increased mean expression, selects for lower noise (Fig. 4b, c). Based on these general results, one might expect increased phenotypic noise during periods of adaptation to new environments, followed by reduction in noise when selection becomes stabilizing67.
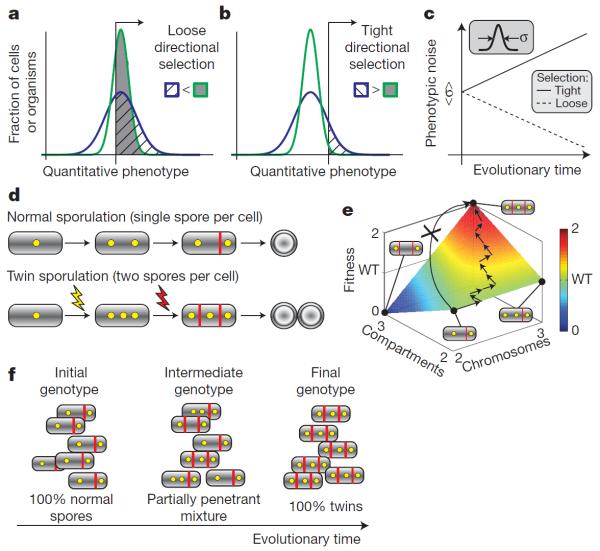
Figure 4. Roles of noise in evolution.
a–c, How genetically controlled noise in a quantitative trait could respond to directional selection. a, Directional selection for values of the trait above a threshold (black line and arrow) can lead to reduced noise when the threshold is low. Thus, the noisier distribution (blue line) has less area above the threshold (cross-hatch) than a less noisy distribution (green line, grey shading) with the same mean. b, By contrast, when selection is tighter, the noisier distribution is favoured, as shown by the larger above-threshold area under the blue distribution compared to the green distribution. c, Over evolutionary timescales, noise (σ, defined as the standard variation of the distribution) would thus be expected to increase under tight selection and decrease under weak selection. In both cases, selection would also increase the mean value of the trait (not shown). d–f, Noise enables the generation of partially penetrant alternative cell fates, which facilitate discrete evolutionary changes. d, Wild-type B. subtilis cells (top) contain two chromosomes (yellow circles) when they initiate sporulation by an asymmetric division (red line). This event leads to differentiation of the forespore (smaller compartment) followed by the mother cell (larger compartment), and eventually results in formation of a single spore (white circle). Mutations can increase the frequency with which cells acquire an extra chromosome (yellow lightning) and/or an extra compartment (red lightning). Cells with both characteristics form two mature spores from a single sporulating cell (twins). e, Depending on the number of chromosomes and compartments, single cells show four distinct fates, each of which has a specific fitness (the corners of the square), assumed to be proportional to the expected number of spores it will produce. Evolution from mono-spores to twins would be difficult with a single mutation (curved arrow) as it would have to affect both septation and replication. However, several mutations affecting the penetrance of extra chromosomes and extra compartments can allow a gradual increase in the mean fitness of the population (path with multiple arrows). WT, wild type. f, This allows a gradual evolutionary transition from a homogenous population of mono-spores, to a partially penetrant intermediate population of multiple fates, to a homogenous population of twin spores.
Actual results in real organisms could deviate from this idealized model for many reasons. Arecent laboratory study examined fluorescent protein expression as a phenotype in E. coli68. The reporter gene was subjected to rounds of mutagenesis and selection for high expression in individual cells. The authors indeed found increases in mean expression or noise in individual clones under tight selection. Developments in laboratory evolution approaches will be able to elucidate further the relationship between evolutionary selection and noise.
Noise in developmental evolution
Many mutations cause qualitative changes in development but do so only in a fraction of individuals, even in an isogenic population. For example, recent work showed how mutations in the Caenorhabditis elegans gut-development pathway generated partially penetrant effects owing to noise69 (Fig. 1d). Partial penetrance has also been observed in isogenic Arabidopsis thaliana mutant populations70. The prevalence of such effects provokes the fundamental question of what role noise-dependent partially penetrant mutant phenotypes might have in developmental evolution.
To address this question, we recently analysed B. subtilis sporulation, arguably one of the simplest developmental systems71. Early in sporulation, the cell divides asymmetrically into two compartments. The smaller forespore compartment will develop into a spore with the help of the larger, `mother cell' compartment. Signalling from the forespore to the mother cell is required to initiate mother-cell-specific gene expression. Mutations that attenuate this signal generate a mixture of discrete sporulation morphologies with variable numbers of chromosomes and compartments. (Extra compartments arise because in the absence of a signal the mother cell divides asymmetrically again, see Fig. 4d.) At very low penetrance (<1%), cells undergo both a further replication and a further division, and two `twin' forespores successfully develop within a single mother cell (Fig. 4d). Whereas twin sporulation was not previously observed in Bacillus it does appear in some Clostridia species, indicating that it could be adaptive under some conditions72.
In wild-type cells, the signalling step occurs fast, immediately inhibiting division and replication in the mother cell and thereby guaranteeing normal sporulation. When signalling is attenuated by the mutation, however, noise enables alternative event sequences and hence alternative phenotypes (including twins) in some cells (Fig. 4d). Time-lapse analysis of sporulation in individual mutant cells showed that most of this noise was not variation in the signalling step itself. Evidently other sources of noise, which normally do not affect development, do contribute to fate variability in the mutant. The penetrance of the twin fate can be strongly and deliberately enhanced (to ~50%) by additional mutations that increase the probability of chromosome replication and septation.
In conditions that select for twins, evolution to a fully penetrant twin phenotype would require simultaneous changes in several processes (for example, replication and division), which are individually deleterious or non-advantageous (Fig. 4e). However, owing to partial penetrance, twins can evolve gradually through smaller mutations that progressively increase the penetrance of the twin fate and thereby increase overall fitness (Fig. 4e, f). In fact, this is a plausible evolutionary mechanism for twin formation as a distantly related Clostridia species forms twins in a qualitatively similar manner to these B. subtilis mutants, and does so in a partially penetrant manner.
Outlook
Noise is not merely a quirk of biological systems, but a core part of how they function and evolve5. However, critical questions about the function of noise in gene circuits remain unanswered.
First, how does noise originate? Despite our understanding of bursting in bacterial and eukaryotic gene expression, further regulatory layers could, and probably do, have a big impact. For example, microRNA has a critical role in regulating diverse processes and could substantially alter the noise characteristics of its regulated genes73,74 owing to its direct interaction with mRNA targets and expected burst-like production. Perhaps most importantly, the effect of epigenetic chromatin modifications on the stability and transition rates of promoter-activity states requires further investigation.
Second, which systems use noise in physiological processes? Stochastic burst-modulated regulatory systems could be used quite generally for coordination. For example, membrane potential spikes with regulated frequency (or duration) have been shown to be part of the response to glucose in pancreatic β cells75. Stochastic bursting could enable coordination at other physiological levels, such as in metabolic networks and hormone regulation of tissues. Improved ability to monitor diverse biochemical reactions in individual living cells should help to identify new burst-modulated or noise-driven systems.
Third, a better understanding of probabilistic differentiation processes will require knowledge of the cellular states and sub-states that occur in seemingly homogeneous cell types. Single-cell transcriptional profiling76, RNA fluorescence in situ hybridization17 and other techniques may be able to provide a broader `snapshot' of cellular states, whereas more comprehensive use of fluorescent protein reporters may provide insight into dynamic variation. In complex systems, such as stem cells, a major challenge is to disentangle the relative effects of noise, intercellular signalling, intracellular regulatory dynamics and chromatin effects to understand both how and why cells switch dynamically among states or sub-states.
Last, recent studies are beginning to indicate that the role of noise could extend to the evolutionary level. However, more laboratory evolution experiments and evolution-of-development analyses will be required to address this issue77. In some cases, such as the evolution of drug resistance in cancer, the importance of noise in the evolution of new traits must be carefully compared to alternative effects, such as those of micro-environmental niches78 or genetic heterogeneity.
The fundamental principles governing when and how genetic circuits can usefully employ noise are beginning to emerge. As the earlier examples show, the question of how cells and organisms use and control random variation in their own components to grow, develop and evolve goes right to the heart of many fundamental biological problems. We anticipate that future work will continue to reveal unexpected, and essential, roles for noise in diverse biological systems.
Acknowledgements
We thank G. Süel, A. Raj, F. Tan and J. Rossant for providing images. We thank N. Wingreen, D. J. Anderson, R. Kishony, J.-G. Ojalvo, G. Süel, H. Y. Kueh and members of the Elowitz laboratory for discussions. Work in M.B.E.'s laboratory was supported by NIH grants R01GM079771, P50 GM068763, NSF CAREER Award 0644463 and the Packard Foundation. A.E. was supported by EMBO, the International Human Frontier Science Organization and a Baxter fellowship.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Maheshri N, O'Shea EK. Living with noisy genes: how cells function reliably with inherent variability in gene expression. Annu. Rev. Biophys. Biomol. Struct. 2007;36:413–434. doi: 10.1146/annurev.biophys.36.040306.132705. [DOI] [PubMed] [Google Scholar]
- 2.Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke J, Elowitz M. Using movies to analyse gene circuit dynamics in single cells. Nature Rev. Microbiol. 2009;7:383–392. doi: 10.1038/nrmicro2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson C, Surette M. Individuality in bacteria. Annu. Rev. Genet. 2008;42:253–268. doi: 10.1146/annurev.genet.42.110807.091601. [DOI] [PubMed] [Google Scholar]
- 5.Losick R, Desplan C. Stochasticity and cell fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lestas I, Vinnicombe G, Paulsson J. Fundamental limits on the suppression of molecular fluctuations. Nature. doi: 10.1038/nature09333. doi:10.1038/nature09333 (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams HH, Arkin A. Stochastic mechanisms in gene expression. Proc. Natl Acad. Sci. USA. 1997;94:814–819. doi: 10.1073/pnas.94.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman N, Cai L, Xie X. Linking stochastic dynamics to population distribution: an analytical framework of gene expression. Phys. Rev. Lett. 2006;97:168302. doi: 10.1103/PhysRevLett.97.168302. [DOI] [PubMed] [Google Scholar]
- 9.Paulsson J. Summing up the noise in gene networks. Nature. 2004;427:415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- 10.Paulsson J, Berg O, Ehrenberg M. Stochastic focusing: fluctuation-enhanced sensitivity of intracellular regulation. Proc. Natl Acad. Sci. USA. 2000;97:7148. doi: 10.1073/pnas.110057697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Xiao J, Ren X, Lao K, Xie XS. Probing gene expression in live cells, one protein molecule at a time. Science. 2006;311:1600–1603. doi: 10.1126/science.1119623. [DOI] [PubMed] [Google Scholar]
- 13.Cai L, Friedman N, Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440:358–362. doi: 10.1038/nature04599. [DOI] [PubMed] [Google Scholar]
- 14.>Ozbudak EM, Thattai M, Kurtser I, Grossman AD, van Oudenaarden A. Regulation of noise in the expression of a single gene. Nature Genet. 2002;31:69–73. doi: 10.1038/ng869. [DOI] [PubMed] [Google Scholar]
- 15.Zenklusen D, Larson DR, Singer RH. Single-RNA counting reveals alternative modes of gene expression in yeast. Nature Struct. Mol. Biol. 2008;15:1263–1271. doi: 10.1038/nsmb.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake WJ, KÆrn M, Cantor CR, Collins JJ. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- 17.Raj A, Peskin C, Tranchina D, Vargas D, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paré A, et al. Visualization of individual Scr mRNAs during Drosophila embryogenesis yields evidence for transcriptional bursting. Curr. Biol. 2009;19:2037–2042. doi: 10.1016/j.cub.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunlop M, Cox R, III, Levine J, Murray R, Elowitz M. Regulatory activity revealed by dynamic correlations in gene expression noise. Nature Genet. 2008;40:1493–1498. doi: 10.1038/ng.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox C, McCollum J, Allen M, Dar R, Simpson M. Using noise to probe and characterize gene circuits. Proc. Natl Acad. Sci. USA. 2008;105:10809–10814. doi: 10.1073/pnas.0804829105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenfeld N, Young JW, Alon U, Swain PS, Elowitz MB. Gene regulation at the single-cell level. Science. 2005;307:1962–1965. doi: 10.1126/science.1106914. [DOI] [PubMed] [Google Scholar]
- 22.Sigal A, et al. Variability and memory of protein levels in human cells. Nature. 2006;444:643–646. doi: 10.1038/nature05316. [DOI] [PubMed] [Google Scholar]
- 23.Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 24.Bar-Even A, et al. Noise in protein expression scales with natural protein abundance. Nature Genet. 2006;38:636–643. doi: 10.1038/ng1807. [DOI] [PubMed] [Google Scholar]
- 25.Newman J, et al. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441:840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- 26.Cai L, Dalal CK, Elowitz MB. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature. 2008;455:485–490. doi: 10.1038/nature07292. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that the yeast calcium-response system uses frequency modulation of stochastic nuclear localization bursts of the Crz1 transcription factor to enable coordination (proportional expression) across large regulons.
- 27.Cyert M. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu. Rev. Genet. 2001;35:647–672. doi: 10.1146/annurev.genet.35.102401.091302. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran H, et al. Rapid and sustained nuclear–cytoplasmic ERK oscillations induced by epidermal growth factor. Mol. Syst. Biol. 2009;5:332. doi: 10.1038/msb.2009.90. doi:10.1038/msb.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thattai M, van Oudenaarden A. Stochastic gene expression in fluctuating environments. Genetics. 2004;167:523–530. doi: 10.1534/genetics.167.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kussell E, Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309:2075–2078. doi: 10.1126/science.1114383. [DOI] [PubMed] [Google Scholar]
- 31.Wolf D, Vazirani V, Arkin A. Diversity in times of adversity: probabilistic strategies in microbial survival games. J. Theor. Biol. 2005;234:227–253. doi: 10.1016/j.jtbi.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Acar M, Mettetal JT, van Oudenaarden A. Stochastic switching as a survival strategy in fluctuating environments. Nature Genet. 2008;40:471–475. doi: 10.1038/ng.110. [DOI] [PubMed] [Google Scholar]; A synthetic study demonstrating that the rate of stochastic switching between phenotypic states is optimized when it matches the rate of environmental fluctuations.
- 33.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 34.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 35.Kashiwagi A, Urabe I, Kaneko K, Yomo T. Adaptive response of a gene network to environmental changes by fitness-induced attractor selection. PLoS ONE. 2006;1:e49. doi: 10.1371/journal.pone.0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.To T, Maheshri N. Noise can induce bimodality in positive transcriptional feedback loops without bistability. Science. 2010;327:1142. doi: 10.1126/science.1178962. [DOI] [PubMed] [Google Scholar]
- 37.Novick A, Weiner M. Enzyme induction as an all-or-none phenomenon. Proc. Natl Acad. Sci. USA. 1957;43:553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benzer S. Induced synthesis of enzymes in bacteria analyzed at the cellular level. Biochim. Biophys. Acta. 1953;11:383–395. doi: 10.1016/0006-3002(53)90057-2. [DOI] [PubMed] [Google Scholar]
- 39.Ozbudak EM, Thattai M, Lim HN, Shraiman BI, Van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427:737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
- 40.Choi PJ, Cai L, Frieda K, Xie XS. A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science. 2008;322:442–446. doi: 10.1126/science.1161427. [DOI] [PMC free article] [PubMed] [Google Scholar]; This detailed analysis of stochastic state transitions in the lac operon identified stochastic promoter state switching as the origin of phenotypic changes in E. coli.
- 41.Elf J, Li GW, Xie XS. Probing transcription factor dynamics at the single-molecule level in a living cell. Science. 2007;316:1191–1194. doi: 10.1126/science.1141967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boeger H, Griesenbeck J, Kornberg RD. Nucleosome retention and the stochastic nature of promoter chromatin remodeling for transcription. Cell. 2008;133:716–726. doi: 10.1016/j.cell.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degenhardt T, et al. Population-level transcription cycles derive from stochastic timing of single-cell transcription. Cell. 2009;138:489–501. doi: 10.1016/j.cell.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Sharma S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; A small sub-population resists drug treatment with no genetic variability. Specific inhibitors eliminate the resistant sub-population while keeping the larger sensitive population.
- 45.Maamar H, Dubnau D. Bistability in the Bacillus subtilis K-state (competence) system requires a positive feedback loop. Mol. Microbiol. 2005;56:615–624. doi: 10.1111/j.1365-2958.2005.04592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Süel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–550. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 47.Süel GM, Kulkarni RP, Dworkin J, Garcia-Ojalvo J, Elowitz MB. Tunability and noise dependence in differentiation dynamics. Science. 2007;315:1716–1719. doi: 10.1126/science.1137455. [DOI] [PubMed] [Google Scholar]
- 48.Maamar H, Raj A, Dubnau D. Noise in gene expression determines cell fate in Bacillus subtilis. Science. 2007;317:526–529. doi: 10.1126/science.1140818. [DOI] [PMC free article] [PubMed] [Google Scholar]; By reciprocally perturbing transcription and translation rates, the authors showed that noise in the expression of a master transcription factor directly regulates the frequency of differentiation into the competent state.
- 49.Di Talia S, Skotheim J, Bean J, Siggia E, Cross F. The effects of molecular noise and size control on variability in the budding yeast cell cycle. Nature. 2007;448:947–951. doi: 10.1038/nature06072. [DOI] [PubMed] [Google Scholar]
- 50.Çagğatay T, Turcotte M, Elowitz M, Garcia-Ojalvo J, Süel GM. Architecture-dependent noise discriminates functionally analogous differentiation circuits. Cell. 2009;139:512–522. doi: 10.1016/j.cell.2009.07.046. [DOI] [PubMed] [Google Scholar]; Two seemingly equivalent architectures for the competence transient differentiation system differ principally in their variability, with the wild-type version more sensitive to noise, enhancing the range of environments in which the system can function.
- 51.Veening JW, et al. Bet-hedging and epigenetic inheritance in bacterial cell development. Proc. Natl Acad. Sci. USA. 2008;105:4393–4398. doi: 10.1073/pnas.0700463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujita M, Losick R. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 2005;19:2236–2244. doi: 10.1101/gad.1335705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nachman I, Regev A, Ramanathan S. Dissecting timing variability in yeast meiosis. Cell. 2007;131:544–556. doi: 10.1016/j.cell.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 54.Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459:428–432. doi: 10.1038/nature08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suda T, Suda J, Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc. Natl Acad. Sci. USA. 1983;80:6689. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah N, Groves A, Anderson D. Alternative neural crest cell fates are instructively promoted by TGFβ superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 57.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- 59.Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- 60.Morris SA, et al. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proc. Natl Acad. Sci. USA. 2010;107:6364–6369. doi: 10.1073/pnas.0915063107. [DOI] [PMC free article] [PubMed] [Google Scholar]; The above two references show that differentiation in the early mouse embryo seems to occur through a stochastic process with a lineage bias, supporting the stochastic sorting model of patterning.
- 61.Kay RR, Thompson CRL. Forming patterns in development without morphogen gradients: scattered differentiation and sorting out. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001503. doi:10.1101/cshperspect.a001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh AM, Hamazaki T, Hankowski KE, Terada N. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells. 2007;25:2534–2542. doi: 10.1634/stemcells.2007-0126. [DOI] [PubMed] [Google Scholar]
- 63.Chambers I, et al. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 64.Kalmar T, et al. Regulated fluctuations in nanog expression mediate cell fate decisions in embryonic stem cells. PLoS Biol. 2009;7:e1000149. doi: 10.1371/journal.pbio.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang H, Hemberg M, Barahona M, Ingber D, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Canham MA, Sharov AA, Ko MSH, Brickman JM. Functional heterogeneity of embryonic stem cells revealed through translational amplification of an early endodermal transcript. PLoS Biol. 2010;8:e1000379. doi: 10.1371/journal.pbio.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hill W, Zhang X. Effects on phenotypic variability of directional selection arising through genetic differences in residual variability. Genet. Res. 2004;83:121–132. doi: 10.1017/s0016672304006640. [DOI] [PubMed] [Google Scholar]
- 68.Ito Y, Toyota H, Kaneko K, Yomo T. How selection affects phenotypic fluctuation. Mol. Syst. Biol. 2009;5 doi: 10.1038/msb.2009.23. doi:10.1038/msb.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raj A, Rifkin S, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature. 2010;463:913–918. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- 71.Eldar A, et al. Partial penetrance facilitates developmental evolution in bacteria. Nature. 2009;460:510–514. doi: 10.1038/nature08150. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single-cell analysis of a bacterial developmental pathway reveals how new morphologies can be produced at low penetrance and then stabilized by additional mutations, providing a gradual pathway for discrete evolutionary transitions.
- 72.Angert ER. Alternatives to binary fission in bacteria. Nature Rev. Microbiol. 2005;3:214–224. doi: 10.1038/nrmicro1096. [DOI] [PubMed] [Google Scholar]
- 73.Mehta P, Goyal S, Wingreen N. A quantitative comparison of sRNA-based and protein-based gene regulation. Mol. Syst. Biol. 2008;4 doi: 10.1038/msb.2008.58. doi:10.1038/msb.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levine E, Zhang Z, Kuhlman T, Hwa T. Quantitative characteristics of gene regulation by small RNA. PLoS Biol. 2007;5:e229. doi: 10.1371/journal.pbio.0050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valdeolmillos M, Gomis A, Sanchez-Andres J. In vivo synchronous membrane potential oscillations in mouse pancreatic β-cells: lack of co-ordination between islets. >J. Physiol. (Lond.) 1996;493:9–15. doi: 10.1113/jphysiol.1996.sp021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang F, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 77.Beaumont H, Gallie J, Kost C, Ferguson G, Rainey P. Experimental evolution of bet hedging. Nature. 2009;462:90–93. doi: 10.1038/nature08504. [DOI] [PubMed] [Google Scholar]
- 78.Snijder B, et al. Population context determines cell-to-cell variability in endocytosis and virus infection. Nature. 2009;461:520–523. doi: 10.1038/nature08282. [DOI] [PubMed] [Google Scholar]