Abstract
The synthesis of Vi polysaccharide, a major virulence determinant in Salmonella enterica serotype Typhi (S. Typhi), is under the control of two regulatory systems, ompR-envZ and rscB-rscC, which respond to changes in osmolarity. Some S. Typhi isolates exhibit over-expression of Vi polysaccharide, which masks clinical detection of LPS O-antigen. This variation in Vi polysaccharide and O-antigen display (VW variation) has been observed since the initial studies of S. Typhi. We have reported that the status of the rpoS gene is responsible for this phenomenon. We review the regulatory network of the Vi polysaccharide, linking osmolarity and RpoS expression. Also, we discuss how this may impact live attenuated Salmonella vaccine development.
Keywords: rpoS, VW variation, osmolarity
Introduction
Salmonella enterica serotype Typhi (hereafter S. Typhi) is a facultative intracellular pathogen that causes typhoid fever exclusively in humans and is among the most costly of human infections in terms of both morbidity and mortality [1]. The mechanism responsible for the virulence of S. Typhi is different from that of other serovars of Salmonella, and in this regard S. Typhi produces the virulence capsular polysaccharide (Vi), which is an important virulence determinant during infection [2]. Vi polysaccharide is a linear homopolymer made up of α-1,4-linked N-acetylgalactosaminuronate (GalNAcA), with 60%–70% of the monomeric units O-acetylated at the C3 position [3,4] and has a molecular mass typically over 200 kDa [5]. Virtually all strains isolated from the blood or bone marrow of patients with acute typhoid fever and from the bile or faeces of those who carry S. Typhi in the gallbladder are found to express Vi polysaccharide antigen [6,7,8]. Vi positive (Vi+) strains were shown to be more virulent than mutant strains in experiments conducted in human volunteers [2]; Vi+ strains are resistant to complement-mediated killing and phagocytosis [9] and survive in human serum [10]. In addition, Vi+ strains, but not Vi− mutant strains, can multiply in the human macrophage cell line THP-1 and in the mouse macrophage-like cell line J774.1 [11].
The Vi capsular polysaccharide is a protective antigen, with the majority of the antibody response directed toward the O-acetyl groups [9,12]. A Vi-conjugate injectable vaccine is used currently against typhoid fever in more than 92 countries [13]. However, the ability to pay for the conjugate vaccine remains a major factor driving who is and who is not vaccinated. One possible way to increase the supply of affordable Vi vaccine is to optimise the production of Vi during fermentation either by modifying growth conditions [14] or by constructing S. Typhi mutants that constitutively express high levels of Vi [15]. Therefore, a better understanding of the regulation of Vi polysaccharide synthesis will not only add to our knowledge base of S. Typhi pathogenesis, but also allow us to improve Vi antigen production and therefore provide a less expensive subunit vaccine.
The viaB locus, a part of SPI-7
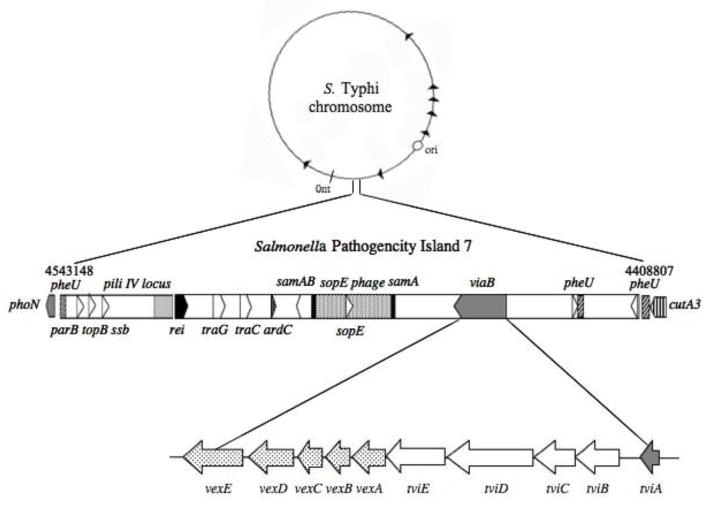
The genes required for Vi biosynthesis (viaB locus) are located in a 133.5 kb chromosomal region called Salmonella pathogenicity island 7 (SPI-7; G+C composition 49%; Figure 1). SPI-7 is bounded by direct repeats and inserted between two copies (one partial) of a tRNA gene (pheU) and contains genes encoding known pathogenicity determinants, including SopE [16] and type IV pili [17,18]. SPI-7 is an unstable genetic element that can undergo precise excision, indicating a possible role in the lateral transfer of this region among Gram negative bacteria [19]. Functional and bioinformatic analyses suggest that SPI-7 has a mosaic structure and may have evolved as a consequence of several independent insertion events [20]. Sequence analysis of the SPI-7 region from S. Typhi revealed significant synteny with clusters of genes from a variety of soil saprophytic bacteria and phytobacteria, raising the possibility that SPI-7 and viaB may have originated from soil sources [20].
Figure 1.
SPI-7 and viaB locus organisation in S. Typhi. The dark arrows in the S. Typhi chromosome indicates the rrn operons. The SPI-7 is located at 96 min or between 4408807 nt and 4543148 nt.
Biosynthesis of Vi polysaccharide
The viaB locus consists of 10 genes (Figure 1): tviBCDE for Vi polysaccharide biosynthesis and vexABCDE for export of the Vi antigen [10, 21, 22], as well as tviA, which encodes a regulatory protein that plays a role in coordinating expression of Vi antigen, flagella and a number of genes required for host invasion [23,24,25,26]. Zhang et al. have provided a detailed analysis of Vi biosynthesis [5]. They showed that Vi polysaccharide is synthesised from UDP-N-acetylglucosamine in a series of steps requiring TviB, TviC, and TviE, where tviB encodes a dehydrogenase, tviC encodes an epimerase and tviE encodes a glycosyltransferase [5]. The role of tviD is not clear, but it appears to encode a cytochrome P-450-like enzyme [5].
Export of Vi polysaccharide
The Vi antigen export apparatus is composed of five polypeptides: VexA, VexB, VexC and VexD, which likely form an ABC transporter, and the VexE anchoring protein [22,27,28]. Mutants defective in VexA, VexB and VexC accumulate the Vi polysaccharide in their cytoplasm [29]. VexB and VexD have been proposed to be integral membrane proteins because they are highly hydrophobic proteins with membrane-spanning domains [27,29]. VexC is likely the ATP-binding protein [27]. VexA has a putative lipoprotein signal sequence indicating that this protein may be localised in the outer membrane and could therefore mediate the translocation of Vi polysaccharide across the outer membrane [22,25,30]. S. Typhi Ty2 vexE mutants are able to synthesise and export the Vi antigen, but do not express it on its cell surface [29]. Thus VexE is necessary for cell surface expression of the Vi capsule.
Regulation of Vi polysaccharide synthesis
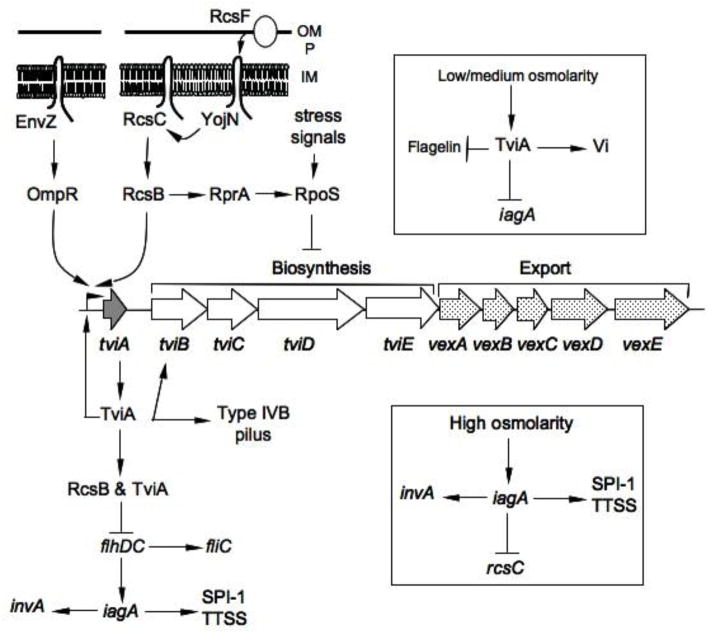
The regulation of Vi polysaccharide synthesis is complex and may play a significant role in maintaining appropriate levels of Vi production in the different host environments encountered by S. Typhi. In Figure 2, we have outlined our current understanding of this regulation. Vi antigen synthesis is subject to regulation by a pair of two-component systems, rcsB-rcsC (viaA locus) [25] and ompR-envZ (ompB locus) [26], which both respond to changes in osmolarity. A positive regulator, TviA (VipR), activates its own synthesis by binding upstream of the tviA promoter [27] and interacts with RcsB to promote optimal transcription of genes involved in Vi antigen synthesis [24, 25, 28, 29]. In the absence of RcsB or TviA, transcription initiated at the tviA promoter terminates in the tviA-tviB intergenic region, probably at a putative hairpin structure identified in this region [29].
Figure 2.
Regulatory network of Vi polysaccharide. Two two-component regulatory systems are involved in the regulation of Vi antigen expression in S. Typhi. The rcs system positively regulates transcription of tvi genes. Moreover, interaction between TviA and RcsB proteins is necessary for maximal transcription of tvi genes. OmpR/EnvZ is the second regulatory pair involved in the regulation of Vi polysaccharide expression. An increase in the environmental osmolarity leads to negative regulation of Vi antigen synthesis by inhibition of rcsC transcription. Molecular mechanisms involved in this regulation remain to be determined. OM: Outer membrane; P: Periplasm; IM: Inner membrane.
Vi is expressed preferentially at low and medium osmolarities and often masks the LPS O-antigen [25,31]. Strains of S. Typhi Ty2 grown in media with medium osmolarity (446 mosmol, ~170 mM NaCl [31]) exhibit high-level production of Vi antigen. When Vi antigen is expressed, the bacteria are less adherent and invasive into epithelial cells [30] but are more resistant to killing by macrophages [11]. Low to medium osmolarity environments might include environmental aqueous environments and certain extracellular host environments, such as blood, where the osmolarity is equivalent to 150 mM of NaCl (310 mosmol) [31,32]. It is possible that this preferential expression of Vi polysaccharide at low to medium osmolarities serves to protect bacterial cells from the complement-mediated actions of the O-antigen specific antibody in the blood [9]. Recent studies with S. Typhi Ty2 grown under LB conditions (170 mM NaCl), optimal for Vi polysaccharide synthesis, showed that Vi polysaccharide reduced TLR-dependent IL-8 production in human colonic tissue explants, suggesting that the scarcity of neutrophils in intestinal infiltrations of typhoid fever patients is due to the Vi polysaccharide [33]. In addition, at low osmolarity, RcsB, acting in association with TviA, negatively controls the transcription of flhDC, which is apparently required for activation of iagA (hilA), invF and sipB (encoding proteins involved in cell invasion) [25, 34]. However, in high osmolarity environments such as in the intestinal lumen, with values believed to be equivalent to 300 mM of NaCl and greater [31], transcription of iagA, invF, and sipB is markedly increased and the transcription of genes involved in Vi biosynthesis is markedly decreased [25]. Under these conditions, S. Typhi is more invasive into epithelial cells but less resistant to killing by macrophages [11]. Therefore, the Vi antigen of S. Typhi is a negative factor for invasion but a positive factor for surviving and multiplying inside macrophages [11]. In keeping with this observation, Zhao et al. showed that at ≥ 300 mM NaCl, but not at 10 mM NaCl, S. Typhi GIFU1007 did not express Vi antigen and exhibited a high invasion index in epithelial cells together with high secretion of SipC protein [35].
VW variation a subtle regulation of Vi polysaccharide by RpoS
The synthesis of Vi antigen increases as the osmolarity decreases (Figure 3), masking the O antigen (Table 1 and [24]). At osmolarities of 676 mosmol (300 mM of NaCl) or higher, the Vi antigen no longer blocks detection of the O-antigen (Table 1). Since the Vi polysaccharide can block access of antibodies to the underlying O-antigen, sometimes agglutination with Salmonella somatic D1 antiserum cannot be demonstrated until the bacterial cells are boiled to remove the Vi polysaccharide [36].
Figure 3.
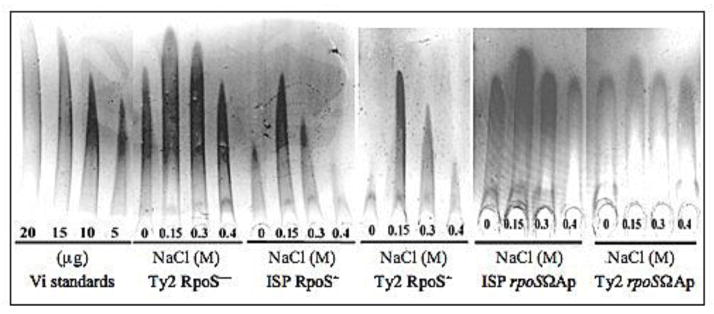
Evaluation of the effect of RpoS in the synthesis of Vi polysaccharide in S. Typhi by rocket immune electrophoresis. S. Typhi Ty2 RpoS−; S. Typhi ISP1820 RpoS+; S. Typhi Ty2 RpoS+ dervivative; S. Typhi Ty2 rpoSΩAp (RpoS−) derivative; S. Typhi ISP1820 rpoSΩAp (RpoS−) derivative; The strains were grown in LB media with 0, 0.15, 0.3 and 0.4 M NaCl. Reproduced with permission from Santander et al. [51].
Table 1.
O9 and Vi slide agglutination reactions of S. Typhi strains grown on LB agar (pH 7) supplemented with different amounts of NaCl at 37°C overnight (18–24 h).
| Environment | Blood-Fluid tissues | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NaCl (mM) | 0 | 10 | 85 | 150 | ||||
|
| ||||||||
| Strains | O9 | Vi | O9 | Vi | O9 | Vi | O9 | Vi |
| Ty2 RpoS− | − | +++ | − | +++ | − | +++ | − | +++ |
| ISP RpoS+ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ |
| Ty2 RpoS+ | ++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ |
| Ty2 rpoSΩAp | − | +++ | − | +++ | − | +++ | − | +++ |
| ISP rpoSΩAp | − | +++ | − | +++ | − | +++ | − | +++ |
| Intestinal lumen | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| NaCl (mM) | 300 | 400 | 500 | |||
|
| ||||||
| Strains | O9 | Vi | O9 | Vi | O9 | Vi |
| Ty2 RpoS− | ++ | +++ | +++ | + | +++ | − |
| ISP RpoS+ | +++ | − | +++ | − | +++ | − |
| Ty2 RpoS+ | +++ | − | +++ | − | +++ | − |
| Ty2 rpoSΩAp | ++ | +++ | +++ | + | +++ | − |
| ISP rpoSΩAp | ++ | +++ | +++ | + | +++ | − |
O9 agglutination reactions were carried out without prior boiling of cells. The degree of agglutinations ranged from not detectable (−) to weak (+) to strong (+++); ± and ++ indicate intermediate degrees. Adapted with permission from Santander et al. [51].
Starting from the initial studies on S. Typhi, variation in Vi and O-antigen detection has been observed. S. Typhi strains non-agglutinable with O-antisera and agglutinable only with Vi antisera, are called V form while S. Typhi strains that lack the Vi antigen and agglutinate only with O-antisera are called W form [37]. Observations recorded by Kauffman [38] and confirmed by Felix and Pitt [39] demonstrated the concept of VW variation in Vi and O antigen relationships. The VW form, which is the most common form observed in clinical laboratories, is defined when both Vi and O-antigen are detected by agglutination with the respective antisera [37]. Coincident with the early work of Felix and Pitt [39], and since verified by others [11,40,41,42], most virulent strains of S. Typhi, were the VW form.
Effect of rpoS on Vi synthesis
In Salmonella, the rpoS gene encodes an alternative sigma factor (σs/RpoS) that is the master regulator in the general stress response and is required for survival under extreme conditions, including osmotic and oxidative stress, transition to stationary phase, acid shock, [43,44] and for virulence of S. Typhimurium [45,46,47, 48,49]. RpoS controls expression of the S. Typhimurium virulence plasmid genes, spvRABCD [46, 47]. In addition, RpoS regulates chromosomal genes required for colonisation of Peyer’s patches and for persistence in mice [48,49]. S. Typhi does not contain a virulence plasmid and the role of rpoS in the virulence of this serotype has not been rigorously studied. However, rpoS might also contribute to the virulence of this serotype because RpoS− strains of S. Typhi are less cytotoxic than RpoS+ strains, but RpoS− strains survive better inside resting THP-1 macrophages without apoptosis induction [50]. Recent studies in our laboratory indicate that there is a correlation between Vi polysaccharide over-expression and the allelic state of the rpoS gene [51].
High osmolarity is one of the environmental signals that induces rpoS through the RcsB-RprA pathway [43,48]. Since the osmolarity of the growth media influences the synthesis of Vi antigen, we examined strains with different rpoS genotypes, in media with different osmolarities, for levels of Vi antigen synthesis. RpoS+ strains grown at osmolarities less than 676 mosmol (~300 mM NaCl [31]) showed over-expression of Vi antigen sufficient to cover the somatic O-antigen (Table 1). The phenotypically O-antigen negative strains were boiled and the O-antigen was subsequently detected in all cases, indicating that Vi was masking the intact LPS. The level of Vi polysaccharide synthesis was higher in RpoS− strains than in RpoS+ strains, although Vi synthesis was responsive to changes in osmolarity for both genotypes (Table 1; Figure 3). Pickard et al. [24] showed in S. Typhi vaccine strains that the ompB locus is required for Vi synthesis and is influenced by osmolarity. These authors also observed that S. Typhi Ty2 and S. Typhi ISP1820 have different levels of Vi antigen synthesis when these strains were grown in different osmolarities. They suggested that this difference could be due to a mutation in Ty2 owing to in vitro passage, since it is an older isolate. In fact, it was subsequently shown that S. Typhi Ty2 carries an rpoS frame-shift mutation [52]. However, it is not clear whether this mutation was present in the original isolate or is a result of laboratory passage. In one recent study, 36% of fresh human S. Typhi isolates were found to be rpoS mutants [53]. We confirmed that the rpoS allelic state is in fact responsible for these observations by constructing an rpoS deletion in ISP1820 [51]. In addition, we constructed an RpoS+ derivative of Ty2. RpoS+ Ty2 had the same Vi phenotype as ISP1820 and the RpoS− derivative of ISP1820 had the same phenotype as Ty2 (Table 1).
Rocket immune electrophoresis assays indicated that RpoS+ strains down-regulate Vi antigen expression (Figure 3). Maximum Vi polysaccharide levels were observed at 150 mM NaCl for both Ty2 (RpoS−) and ISP1820 (RpoS+), but Ty2 produced more Vi than ISP1820 at all osmolarities tested (Figure 3). These results support our interpretation of the agglutination results in Table 1 and indicate that RpoS down-regulates Vi polysaccharide synthesis in S. Typhi. The molecular mechanism governing how and why RpoS accomplishes this is still an open question.
Effect of rpoS on Vi antigen synthesis and the effect on Hd detection
The allelic variant of the fliC gene present in S. Typhi strains encodes one type of flagellin, designated Hd [25]. S. Typhi GIFU10007 appeared to require intrinsic, intact motility for invading cultured epithelial cells, as non-motile mutants were not invasive [54]. The production of the Hd flagellin, as well as Vi antigen, is modulated by the RcsB-RcsC regulatory system in response to changes in the osmolarity of the growth medium [25]. We have examined Hd synthesis in strains with different rpoS genotypes grown in media with different osmolarities [51]. The synthesis of Hd flagellar antigen in RpoS+ strains was detected at all osmolarities tested. In contrast, the Hd flagellar antigen was not detected in RpoS strains at osmolarities of 10 mM NaCl or lower (Table 2) unless the samples were first boiled, indicating that the Vi antigen masks flagellar antigens at low osmolarities in RpoS strains. Western blot analysis revealed a low level of Hd flagellin synthesis when cells were grown in a low osmolarity medium, as expected (Figure 4). Similar results were obtained using arabinose inducible rpoS strains [51], where masking of flagellar antigens at low osmolarity was only observed when cells were grown in the absence of arabinose [51].
Table 2.
Hd flagellar antigen slide agglutination reactions of S. Typhi strains grown on LB agar (pH 7) [76] supplemented with different amounts of NaCl at 37°C overnight (18–24 h).
| Strains | NaCl (mM) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 85 | f | 300 | 400 | 500 | |
| Ty2 RpoS− | − | − | +++ | +++ | +++ | +++ | +++ |
| ISP RpoS+ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Ty2 RpoS+ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Ty2 rpoSΩ Ap | − | − | +++ | +++ | +++ | +++ | +++ |
| ISP rpoSΩ Ap | − | − | +++ | +++ | +++ | +++ | +++ |
The degree of agglutinations ranged from not detectable (−) to weak (+) to strong (+++);± and ++ indicate intermediate degrees. Adapted with permission from Santander et al. [51]
Figure 4.
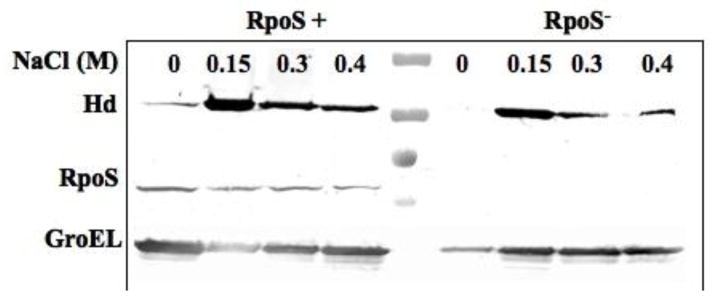
Evaluation of Hd (S. Typhi flagella factor; 55 kDa) and RpoS expression in different osmolarities by western blot. S. Typhi Ty2 RpoS−; S. Typhi ISP1820 RpoS+; The strains were growth in LB media with 0, 0.15, 0.3 and 0.4 M of NaCl. Reproduced with permission from Santander et al. [51].
Evaluation of Vi polysaccharide synthesis in S. Typhi RpoS+ strains during growth
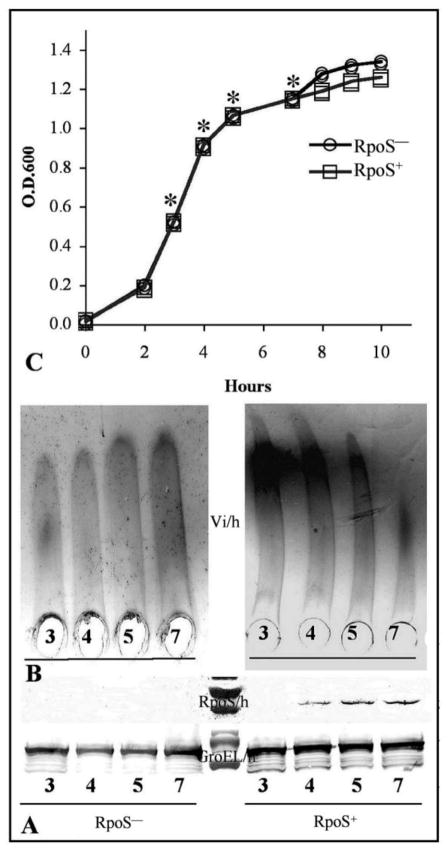
RpoS is a key factor in the stress response during the transition from the exponential growth phase to the stationary growth phase [43]. Very little RpoS is detected in exponentially growing E. coli cells, due to either low levels of expression or protein instability [56,57]. To determine what impact this might have on Vi expression, we evaluated the effect of growth phase on Vi and RpoS synthesis in S. Typhi. RpoS was not detected in the early exponential phase, but was detectable from the middle exponential growth phase cultures and into the early stationary phase (Figure 5), although bubble production in the catalase test was positive only in the stationary phase. Notably, Vi polysaccharide synthesis decreased as RpoS accumulated (Figure 5). There was no growth phase dependent reduction in Vi antigen synthesis in strain Ty2 (RpoS−). These results indicate that the RpoS allelic state is responsible for the VW and V variation in S. Typhi. RpoS− strains over-express the Vi polysaccharide without RpoS regulation, leading to a permanent V form. RpoS+ strains exhibit both forms, the V form during the early exponential growth, when RpoS is not expressed or expressed at low levels, and the VW form, when RpoS is expressed. The W form (Vi−) can be caused by spontaneous deletion of SPI-7, where the viaB loci is located [19].
Figure 5.
Evaluation of Vi polysaccharide synthesis in S. Typhi RpoS+ during growth. A. RpoS expression during the growth curve. GroEL was used as control. The strains were growth in LB medium with 150 mM of NaCl; B. Vi polysaccharide expression during the growth; C. Growth curve. S. Typhi Ty2 RpoS−; S. Typhi ISP1820 RpoS+. Reproduced with permission from Santander et al. [51].
Vaccine development
The live typhoid vaccine Ty21a, which is an RpoS, GalE− and Vi− [57] derivative of S. Typhi Ty2 [58], has been evaluated in several clinical trials and found to be well tolerated, although only modesty immunogenic; three or four doses are required to confer protection [59,60,61,62]. The rpoS mutation could affect the immunogenicity in recombinant vaccines [63]. In fact, live typhoid vaccines derived from Ty2 (RpoS−) have yielded poor results when used as a live recombinant S. Typhi vaccine (RAStyVs) expressing protective antigens from a diversity of pathogens [64,65,66,67,68,69]. It is thus possible that the poor immunogenicity observed for Ty21a may be the result of the rpoS mutation rather than Vi antigen deletion. On the other hand, over-expression of Vi antigen in Ty2 could decrease adherence to and invasion into intestinal tissues necessary to colonise more internal lymphoid effector tissues [64]. In S. Typhimurium, it has been demonstrated that chromosomal RpoS-regulated genes are necessary for invasion into and colonisation of the gut-associated lymphoid tissue (GALT) [49]. In accord with this, RpoS− S. Typhimurium mutants exhibit diminished immunogenicity [70,71]. The RpoS-regulated genes carried on the S. Typhimurium virulence plasmid appear to play no role in this effect [70,71].
Furthermore, S. Typhi ISP1820 (RpoS+) seems to be more virulent in humans than S. Typhi Ty2 (RpoS−) [72]. In concordance, S. Typhi CVD906, a live vaccine, derived from ISP1820 with deletion mutations caused fever and other adverse reactions in humans in aroC and aroD [73], is highly immunogenic but [72,74]. This is in contrast to the S. Typhi CVD908 Ty2 ΔaroC ΔaroD vaccine strain, which did not cause any adverse effects [72]. These results collectively imply that RpoS+ S. Typhi, with or without ability to produce the Vi capsular antigen, might be superior to RpoS− strains as a vector in the development of recombinant attenuated Salmonella vaccines for humans [75,76], although they will require more effective means of attenuation that has been used previously in S. Typhi Ty2 vaccine constructions. We are currently testing this hypothesis in human volunteers.
Acknowledgments
This work was supported by National Institutes of Health R01 AI24533, R01 AI057885, R01 AI056289, the Bill and Melinda Gates Foundation #37863 and CONICYT, Chile.
Footnotes
Conflict of interest: No conflict of interest is declared.
References
- 1.Pang T. Genetic dynamics of Salmonella typhi-diversity in clonality. Trends Microbiol. 1998;6:339–342. doi: 10.1016/s0966-842x(98)01341-9. [DOI] [PubMed] [Google Scholar]
- 2.Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. Typhoid fever: pathogenesis and immunologic control. N Engl J Med. 1970;283:739–746. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 3.Daniels EM, Schneerson R, Egan WM, Szu SC, Robbins JB. Characterization of the Salmonella paratyphi C Vi polysaccharide. Infect Immun. 1989;57:3159–3164. doi: 10.1128/iai.57.10.3159-3164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyns K, Kiessling G. Strukturaufklarung des Vi-antigens aus Citrobacter freundii (E. coli) 5396/38. Carbohydr Res. 1967;3:340–352. [Google Scholar]
- 5.Zhang H, Zhou Y, Bao H, Liu H. Vi Antigen Biosynthesis in Salmonella typhi: Characterization of UDP-N-acetylglucosamine C-6 dehydrogenase (TviB) and UDP-N-acetylglucosaminuronic acid C-4 epimerase (TviC) Biochemistry. 2006;45:8163–8173. doi: 10.1021/bi060446d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jesudason MV, Sridharan G, Mukundan S, John TJ. Vi-specific latex agglutination for early and rapid detection of Salmonella serotype typhi in blood cultures. Diagn Microbiol Infect Dis. 1994;18:75–78. doi: 10.1016/0732-8893(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 7.Lesmana M, Rockhill RC, Sanborn WR. A coagglutination method for presumptive identification of Salmonella typhi. Southeast Asian J Trop Med Public Health. 1980;11:302–307. [PubMed] [Google Scholar]
- 8.Qadri A, Ghosh S, Talwar GP. Monoclonal antibodies against two discrete determinants on Vi capsular polysaccharide. J Immunoassay. 1990;11:235–250. doi: 10.1080/01971529008053271. [DOI] [PubMed] [Google Scholar]
- 9.Robbins JD, Robbins JB. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984;150:436–449. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Li N, Yokoyama H, Ezaki T. Complete nucleotide sequence and molecular characterization of ViaB region encoding Vi antigen in Salmonella typhi. J Bacteriol. 1993;175:4456–4465. doi: 10.1128/jb.175.14.4456-4465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirose K, Ezaki T, Miyake M, Li T, Khan AQ, Kawamura Y, Yokoyama H, Takami T. Survival of Vi-capsulated and Vi-deleted Salmonella typhi strains in cultured macrophage expressing different levels of CD14 antigen. FEMS Microbiol Lett. 1997;147:259–265. doi: 10.1111/j.1574-6968.1997.tb10251.x. [DOI] [PubMed] [Google Scholar]
- 12.Szu SC, Bystricky S. Physical, chemical, antigenic, and immunologic characterization of polygalacturonan, its derivatives, and Vi antigen from Salmonella typhi. Method Enzymol. 2003;363:552–567. doi: 10.1016/S0076-6879(03)01079-6. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Background document: the diagnosis, treatment and prevention of typhoid fever. Geneva: World Health Organization; 2003. (WHO/V&B/03.07) [Google Scholar]
- 14.Jang H, Yoona YK, Kima JA, Kima HS, Ana SJ, Seob JH, Cuia C, Carbisa R. Optimization of Vi capsular polysaccharide production during growth of Salmonella enterica serotype Typhi Ty2 in a bioreactor. J Biotechnol. 2008;135:71–77. doi: 10.1016/j.jbiotec.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Wang JY, Noriega FR, Galen JE, Barry E, Levine MM. Constitutive expression of the Vi polysaccharide capsular antigen in attenuated Salmonella enterica serovar Typhi oral vaccine strain CVD 909. Infect Immun. 2000;68:4647–4652. doi: 10.1128/iai.68.8.4647-4652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardt W, Urlaub H, Galán JE. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc Natl Acad Sci USA. 1998;95:2574–2579. doi: 10.1073/pnas.95.5.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XL, Tsui IS, Yip CM, Fung AW, Wong DK, Dai X, Yang Y, Hackett J, Morris C. Salmonella enterica serovar Typhi uses type IVB pili to enter human intestinal epithelial cells. Infect Immun. 2000;68:3067–3073. doi: 10.1128/iai.68.6.3067-3073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XL, Morris C, Hackett J. Molecular cloning, nucleotide sequence, and function of a site-specific recombinase encoded in the major ‘pathogenicity island’ of Salmonella typhi. Gene. 1997;202:139–146.81. doi: 10.1016/s0378-1119(97)00466-6. [DOI] [PubMed] [Google Scholar]
- 19.Bueno SM, Santiviago CA, Murillo AA, Fuentes JA, Trombert AN, Rodas PI, Youderian P, Mora GC. Precise excision of the large pathogenicity island, SPI7, in Salmonella enterica serovar Typhi. J Bacteriol. 2004;186:3202–3213. doi: 10.1128/JB.186.10.3202-3213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickard D, Wain J, Baker S, Line A, Chohan S, Fookes M, Barron A, Gaora PÓ, Chabalgoity JA, Thanky N, Scholes C, Thomson N, Quail M, Parkhill J, Dougan G. Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. J Bacteriol. 2003;18:5055–5065. doi: 10.1128/JB.185.17.5055-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto Y, Li N, Yokoyama H, Ezaki T. Molecular cloning of the ViaB region of Salmonella typhi. FEMS Microbiol Lett. 1991;90:53–56. doi: 10.1016/0378-1097(91)90645-q. [DOI] [PubMed] [Google Scholar]
- 22.Kolyva S, Waxin H, Popoff MY. The Vi antigen of Salmonella typhi: molecular analysis of the viaB locus. J Gen Microbiol. 1992;138:297–304. doi: 10.1099/00221287-138-2-297. [DOI] [PubMed] [Google Scholar]
- 23.Houng HS, Noon KF, Ou JT, Baron LS. Expression of Vi antigen in Escherichia coli K-12: characterization of ViaB from Citrobacter freundii and identity of ViaA with RcsB. J Bacteriol. 1992;174:5910–5915. doi: 10.1128/jb.174.18.5910-5915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickard D, Li J, Roberts M, Maskell D, Hone D, Levine M, Dougan G, Chatfield S. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun. 1994;62:3984–3993. doi: 10.1128/iai.62.9.3984-3993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey PS, Popoff MY. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. doi: 10.1046/j.1365-2958.1998.00976.x. [DOI] [PubMed] [Google Scholar]
- 26.Winter SE, Raffatellu M, Wilson RP, Russmann H, Bäumler A. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell Microbiol. 2008;10:247–261. doi: 10.1111/j.1462-5822.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, Quayum KA, Ezaki T. Positive autoregulation of vipR expression in ViaB region-encoded Vi antigen of Salmonella typhi. J Bacteriol. 1996;178:1430–1436. doi: 10.1128/jb.178.5.1430-1436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virlogeux I, Waxin H, Ecobichon C, Lee JO, Popoff MY. Characterization of the rcsA and rcsB genes from Salmonella typhi: rcsB through tviA is involved in regulation of Vi antigen synthesis. J Bacteriol. 1996;178:1691–1698. doi: 10.1128/jb.178.6.1691-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virlogeux I, Waxin H, Ecobichon C, Popoff MY. Role of the viaB locus in synthesis, transport and expression of Salmonella typhi Vi antigen. Microbiology. 1995;141:3039–3047. doi: 10.1099/13500872-141-12-3039. [DOI] [PubMed] [Google Scholar]
- 30.Waxin H, Virologeux I, Kolyva S, Popoff MY. Identification of six open reading frames in the Salmonella enterica subsp. enterica ser. Typhi viaB locus involved in Vi antigen production. Res Microbiol. 1993;144:363–371. doi: 10.1016/0923-2508(93)90193-6. [DOI] [PubMed] [Google Scholar]
- 31.Tartera C, Metcalf ES. Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun. 1993;61:3084–3089. doi: 10.1128/iai.61.7.3084-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kugelmass IN. Biochemistry of blood in health and disease. Springfield: Charles C. Thomas Publisher IL; 1959. [Google Scholar]
- 33.Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Bäumler AJ. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun. 2005;73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winter SE, Russmann H, Bäumler AJ. TviA, in conjunction with RcsB, coordinates expression of the invasion associated type 3 secretion system, the Vi capsular antigen, and the flagella in response to the osmolarity. American Society for Microbiology 108th General meeting Abstract B-098; 2008. p. 50. [Google Scholar]
- 35.Zhao L, Ezak T, Li ZY, Kawamura Y, Hirose K, Watanabe H. Vi-suppressed wild strain Salmonella typhi cultured in high osmolarity is hyperinvasive toward epithelial cells and destructive of Peyer’s patches. Microbiol Immunol. 2001;45:149–158. doi: 10.1111/j.1348-0421.2001.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 36.Edwards PR, Ewing WH. Identification of Enterobacteriaceae. 3. Minneapolis: Burgess Publishing Co; 1972. [Google Scholar]
- 37.Tully JG, Currie JA. Vi-negative strains of Salmonella typhosa: attempts to induce W-V reversion and the use of non-Vi strains in evaluating typhoid vaccines. J Bacteriol. 1962;84:747–753. doi: 10.1128/jb.84.4.747-753.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kauffmann F. Uber einen neuen serologischen formenwechsel der Typhusbacillen. Z Hyg Infektionskrankh. 1935;117:617–651. [Google Scholar]
- 39.Felix A, Pitt RM. A new antigen of B. typhosus. Its relation to virulence and to active and passive immunization. Lancet. 1934;227:186–191. [Google Scholar]
- 40.Gaines S, Tully JG. Comparison of intracerebral and intraperitoneal infective techniques for assaying mouse virulence of Salmonella typhosa strains. Am J Hyg. 1961;74:60–66. doi: 10.1093/oxfordjournals.aje.a120201. [DOI] [PubMed] [Google Scholar]
- 41.Gaines S, Tully JG, Tigertt WD. Enhancement of the mouse virulence of a non-Vi variant of Salmonella typhosa by Vi antigen. J Immunol. 1961;86:543–551. [PubMed] [Google Scholar]
- 42.Tully JG, Gaines S. Comparison of intraperitoneal and intracerebral infective techniques for evaluating typhoid vaccines in mouse protection tests. Am J Hyg. 1961;74:259–266. doi: 10.1093/oxfordjournals.aje.a120218. [DOI] [PubMed] [Google Scholar]
- 43.Loewen PC, Hengge-Aronis R. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Ann Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 44.Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang FC, Libby SJ, Buchmeier NA, Loewen PC, Switala J, Harwood J, Guiney DG. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norel F, Robbe-Saule V, Popoff MY, Coynault C. The putative sigma factor KatF (RpoS) is required for the transcription of the Salmonella typhimurium virulence gene spvB in Escherichia coli. FEMS Microbiol Lett. 1992;78:271–276. doi: 10.1016/0378-1097(92)90039-q. [DOI] [PubMed] [Google Scholar]
- 47.Kowarz L, Coynault C, Robbe-Saule V, Norel F. The Salmonella typhimurium katF (rpoS) gene: cloning, nucleotide sequence, and regulation of spvR and spvABCD virulence plasmid genes. J Bacteriol. 1994;176:6852–6860. doi: 10.1128/jb.176.22.6852-6860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (sigma S) regulon. Mol Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 49.Nickerson CA, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan AQ, Zhao L, Hirose K, Miyake M, Li T, Hashimoto Y, Kawamura Y, Ezaki T. Salmonella typhi rpoS mutant is less cytotoxic than the parent strain but survives inside resting THP-1 macrophages. FEMS Microbiol Lett. 1998;161:201–208. doi: 10.1111/j.1574-6968.1998.tb12949.x. [DOI] [PubMed] [Google Scholar]
- 51.Santander J, Wanda SY, Nickerson CA, Curtiss R., III Role of RpoS in fine-tuning the synthesis of Vi capsular polysaccharide in Salmonella enterica serotype Typhi. Infect Immunol. 2007;75:1382–1392. doi: 10.1128/IAI.00888-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbe-Saule V, Norel F. The rpoS mutant allele of Salmonella typhi Ty2 is identical to that of the live typhoid vaccine Ty21a. FEMS Microbiol Lett. 1999;170:141–143. doi: 10.1111/j.1574-6968.1999.tb13366.x. [DOI] [PubMed] [Google Scholar]
- 53.Robbe-Saule V, Algorta G, Rouilhac I, Norel F. Characterization of the RpoS status of clinical isolates of Salmonella enterica. Appl Environ Microbiol. 2003;69:4352–4358. doi: 10.1128/AEM.69.8.4352-4358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56:1967–1973. doi: 10.1128/iai.56.8.1967-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jishage M, Iwata A, Ueda S, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J Bacteriol. 1996;178:5447–5451. doi: 10.1128/jb.178.18.5447-5451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muffler A, Traulsen DD, Lange R, Hengge-Aronis R. Posttranscriptional osmotic regulation of the sigma(s) subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1996;178:1607–13. doi: 10.1128/jb.178.6.1607-1613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbe-Saule V, Coynault C, Norel F. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol Lett. 1995;126:171–178. doi: 10.1111/j.1574-6968.1995.tb07412.x. [DOI] [PubMed] [Google Scholar]
- 58.Germanier R, Furer E. Isolation and characterization of galE mutant Ty21a of Salmonella typhi: a candidate strain for a live oral typhoid vaccine. J Infect Dis. 1975;141:553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 59.Wahdan MH, Serie C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live Salmonella typhi strain Ty21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982;145:292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- 60.Levine MM, Ferreccio C, Black RE, Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet. 1987;1:1049–1052. doi: 10.1016/s0140-6736(87)90480-6. [DOI] [PubMed] [Google Scholar]
- 61.Simanjuntak CH, Paleologo FP, Punjabi NH, Darmowigoto R, Soeprawoto S, Totosudirjo H, Haryanto P, Suprijanto E, Witham ND, Hoffman SL. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet. 1991;338:1055–1059. doi: 10.1016/0140-6736(91)91910-m. [DOI] [PubMed] [Google Scholar]
- 62.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17:S22–S27. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 63.Coynault C, Norel F. Comparison of the abilities of Salmonella typhimurium rpoS, aroA and rpoS aroA strains to elicit humoral immune responses in BALB/c mice and to cause lethal infection in athymic BALB/c mice. Microb Pathog. 1999;26:299–305. doi: 10.1006/mpat.1999.0273. [DOI] [PubMed] [Google Scholar]
- 64.Black RE, Levine MM, Clements ML, Losonsky G, Herrington D, Berman S, Formal SB. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155:1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 65.Orme IM. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 66.Hohmann EL, Oletta CA, Killeen KP, Miller SI. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 67.DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine. 1999;18:449–459. doi: 10.1016/s0264-410x(99)00246-7. [DOI] [PubMed] [Google Scholar]
- 68.Dilts DA, Riesenfeld-Orn I, Fulginiti JP, Ekwall E, Granert C, Nonenmacher J, Brey RN, Cryz SJ, Karlsson K, Bergman K, Thompson T, Hu B, Bruckner AH, Lindberg AA. Phase I clinical trials of aroA aroD and aroA aroD htrA attenuated S. typhi vaccines; effect of formulation on safety and immunogenicity. Vaccine. 2000;18:1473–1484. doi: 10.1016/s0264-410x(99)00424-7. [DOI] [PubMed] [Google Scholar]
- 69.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FA, Everest P, Wu T, Pickard D, Holden DW, Dougan G, Griffin GE, House D, Santangelo JD, Khan JA, Shea JE, Feldman RG, Lewis DJ. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002;70:3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gulig PA, Curtiss RIII. Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987;55:2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardon P, Popoff MY, Coynault C, Marly J, Miras I. Virulence-associated plasmids of Salmonella serotype typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986;137B:47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- 72.Tacket CO, Hone DM, Curtiss R, III, Kelly SM, Losonsky G, Guers L, Harris AM, Edelman R, Levine MM. Comparison of the safety and immunogenicity of ΔaroC ΔaroD and Δcya Δcrp Salmonella typhi strains in adult volunteers. Infect Immun. 1992;60:536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hone DM, Tacket CO, Harris AM, Kay B, Losonsky G, Levine MM. Evaluation in volunteers of a candidate live oral attenuated Salmonella typhi vector vaccine. J Clin Invest. 1992;90:412–420. doi: 10.1172/JCI115876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hone DM, Harris AM, Chatfield S, Dougan G, Levine MM. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine. 1991;9:810–816. doi: 10.1016/0264-410x(91)90218-u. [DOI] [PubMed] [Google Scholar]
- 75.Curtiss R., III Bacterial infectious disease control by vaccine development. J Clin Invest. 2002;110:1061–1066. doi: 10.1172/JCI16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curtiss R., III . In: Antigen delivery systems: Development of live recombinant attenuated bacterial antigen and DNA vaccine delivery vector vaccines. Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. Mucosal Immunology Academic Press; 2005. pp. 1009–1037. [Google Scholar]