Abstract
Background
Use of prophylactic anti-CMV therapy for 3 to 6 months after kidney transplantation can result in delayed-onset CMV disease. We hypothesized that delayed-onset CMV disease (occurring ≥ 100 days post-transplant) occurs more commonly than early-onset CMV disease, and that it is associated with death.
Methods
We assembled a retrospective cohort of 15,848 adult kidney transplant recipients using 2004 to 2010 administrative data from the California and Florida Healthcare Cost and Utilization Project State Inpatient Databases. We identified demographic data, comorbidities, CMV disease coded during readmission and inpatient death. We used multivariate Cox proportional hazards modeling to determine risk factors for delayed-onset CMV disease and inpatient death.
Results
Delayed-onset CMV disease was identified in 4.0% and early-onset CMV disease was identified in 1.2% of the kidney transplant recipients. Risk factors for delayed-onset CMV disease included previous transplant failure or rejection (HR 1.4) and residence in the lowest-income ZIP codes (HR 1.2). Inpatient death was associated with CMV disease occurring 101–365 days post-transplant (HR 1.5), CMV disease occurring > 365 days post-transplant (HR 2.1), increasing age (by decade: HR 1.5), non-white race (HR 1.2), residence in the lowest-income ZIP codes (HR 1.2), transplant failure or rejection (HR 3.2), prior solid organ transplant (HR 1.7) and several comorbidities.
Conclusions
These data showed that delayed-onset CMV disease occurred more commonly than early-onset CMV disease, and that transplant failure or rejection is a risk factor for delayed-onset CMV disease. Further research should be done to determine if delayed-onset CMV disease is independently associated with death.
Keywords: cytomegalovirus, kidney transplantation, administrative data
INTRODUCTION
Kidney transplant recipients require immunosuppressive medications to prevent allograft rejection which markedly increases their risk of developing cytomegalovirus (CMV) disease (1–3), which can manifest as CMV syndrome, or tissue-invasive CMV disease with hepatitis, enteritis, colitis, pneumonitis, nephritis and rarely retinitis (3–9). Significant research has been devoted to the prevention of CMV disease after kidney transplantation, and CMV-seronegative recipients of organs from CMV-seropositive donors (D+/R−), as well as CMV-seropositive transplant recipients (R+) in the United States now generally receive prophylactic anti-CMV therapy for at least 3 months post-transplant (3;10–14). This however has led to the emergence of delayed-onset CMV disease, typically categorized as either late-onset CMV disease (occurring between discontinuation of anti-CMV prophylaxis and 1 year post-transplant) (13;15–19), or very late-onset CMV disease (occurring more than 1 year post-transplant) (19–21).
Delayed-onset CMV disease has not been well studied, given difficulties in assembling representative study cohorts, and the propensity for up to one-third of transplant recipients to transition their care away from transplant centers to non-transplant centers nearer their homes post-transplant (22), which limits long-term follow-up. The incidence of late-onset CMV disease among D+/R− kidney transplant recipients has been reported in single-center studies to be 19% to 48%, and generally occurs 2 to 3 months after the cessation of anti-CMV prophylaxis (16–18;23;24). One study reported that late-onset CMV disease was associated with an almost 3-fold increased risk of allograft loss or death (16), while another study did not demonstrate an association with death or graft loss (18). Very late-onset CMV disease is even less well-characterized, but has been reported to present atypically (20), and has been associated with an increased risk of death compared to late-onset CMV disease in a small case-control study (21).
To expand our understanding of the scope, risk factors and outcomes associated with delayed-onset CMV disease among kidney transplant recipients, we assembled a large cohort of kidney transplant patients using the Agency for Healthcare Research and Quality – Healthcare Cost and Utilization Project (AHRQ-HCUP) – State Inpatient Databases (SID) of California and Florida. The SID are composed of demographic and billing data that capture diagnoses and procedures occurring during hospitalization through International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding. We focused on examining the SID from California and Florida given the availability of patient-level encrypted identifiers to link admissions within and across hospitals over time and the states’ population diversity. Assuming widespread use of prophylactic anti-CMV therapy for D+/R− and R+ patients for at least 3 months post-transplant, we hypothesized that delayed-onset CMV disease (> 100 days post-transplant) now occurs more commonly than early-onset CMV disease (≤ 100 days post-transplant), and that it is associated with death.
RESULTS
Our study population consisted of 15,848 adult kidney transplant recipients (Table 1). The median age was 51 and 40% were female. Fifty-eight per cent of patients were non-white, and 36% were Hispanic, Asian or Pacific Islander. The majority of kidney transplant recipients resided in large metropolitan areas, and had Medicare as their expected primary insurance payer. Patients who resided in ZIP codes with the lowest median incomes, or whose ZIP code category according to median income was missing, accounted for 34% of the study population. Approximately 4% of patients had a prior solid-organ transplant, and 2.5% had a prior kidney transplant. Thirty-seven percent of patients had pre-existing diabetes mellitus, and 13% had a Charlson comorbidity index > 4. The median duration of follow-up was 4 years (IQR 2.4–5.6 years).
Table 1.
Demographic and clinical characteristics of 15,848 kidney transplant recipients at the time of organ transplantation.
| Variables | All recipients |
|---|---|
| Age, years | |
| Mean ± SD | 50.28 ± 13.68 |
| Median (interquartile range) | 51 (41 – 61) |
|
| |
| Female sex (%) | 39.82 |
|
| |
| Race (%) | |
| White | 42.31 |
| Black | 15.64 |
| Hispanic | 24.96 |
| Asian or Pacific Islander | 10.68 |
| Other or missing | 6.42 |
|
| |
| Patient location (urban-rural) (%) 1 | |
| Large metropolitan | 72.60 |
| Small metropolitan | 23.31 |
| Micropolitan | 2.85 |
| Not metropolitan or micropolitan | 0.88 |
| Missing | 0.36 |
|
| |
| Median income of patient ZIP code (%) 2 | |
| First quartile (poorest) | 21.47 |
| Second quartile | 21.18 |
| Third quartile | 23.24 |
| Fourth quartile (wealthiest) | 21.31 |
| Missing | 12.79 |
|
| |
| Expected primary insurance payer (%) | |
| Medicare | |
| < 65 years of age | 50.15 |
| ≥ 65 years of age | 14.39 |
| Private insurance | 30.18 |
| Medicaid, self-pay, no charge or other | 5.28 |
|
| |
| Prior transplant (%) | 3.98 |
| Kidney | 2.51 |
| Liver | 0.73 |
| Heart | 0.40 |
| Lung | 0.11 |
| Pancreas | 0.44 |
| Heart-Lung | <0.01 |
| Intestine | <0.01 |
|
| |
| Comorbidities (%) | |
| Hypertension | 95.85 |
| Diabetes mellitus | 37.21 |
| Congestive heart failure | 10.92 |
| Hypothyroidism | 9.42 |
| Obesity | 9.40 |
| Coagulopathy | 9.09 |
| Peripheral vascular disease | 8.99 |
| Chronic pulmonary disease | 8.11 |
| Depression | 7.43 |
| Valvular disease | 6.99 |
| Neurologic disorders | 5.64 |
| Rheumatoid arthritis or collagen vascular disease | 5.43 |
| Liver disease | 4.87 |
| Pulmonary circulation disease | 3.04 |
| Psychoses | 1.92 |
| Drug abuse | 1.89 |
| Paralysis | 1.12 |
| Alcohol abuse | 1.05 |
| Solid tumor without metastasis | 0.42 |
| HIV | 0.29 |
| Lymphoma | 0.16 |
|
| |
| Charlson comorbidity index (%) | |
| ≤ 4 | 87.37 |
| > 4 | 12.63 |
|
| |
| Duration of follow-up, years | |
| Mean | 4.1 |
| Median (interquartile range) | 4 (2.4 – 5.6) |
Large metropolitan – at least 1 million residents; Small metropolitan – less than 1 million residents; Micropolitan – adjacent to large or small metropolitan area.
Based on quartiles of the estimated median household income of the patient’s ZIP code for the state. Values vary by year and state. According to the U.S. Census Bureau, in 2011: first quartile: <$5,000-$29,999; second quartile: $30,000-$54,999; third quartile: $55,000-$94,999; fourth quartile: >$95,000.
New-onset CMV disease coded at hospital readmission occurred in 5.2% of kidney transplant recipients (Table 2). Coding for CMV disease likely represents microbiological or histopathologic evidence of CMV replication along with signs and symptoms consistent with CMV disease. Approximately 1.2% of transplant recipients had early-onset (≤ 100 days post-transplant), 2.5% had late-onset (101 to 365 days post-transplant) and 1.5% had very late-onset (> 365 days post-transplant) CMV disease. Among patients hospitalized with newly-coded CMV disease (first readmission), 26% were coded for esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy or colonoscopy; 12% were coded for pneumonia or hepatitis; and 35% were coded with EGD, flexible sigmoidoscopy, colonoscopy, pneumonia or hepatitis, possibly reflecting tissue-invasive CMV disease. A greater proportion of hospitalizations coded with late and very late-onset CMV disease had codes indicating possible tissue-invasion, compared to hospitalizations coded with early-onset CMV disease. Approximately 55% were coded for transplant failure or rejection; 13% were coded for percutaneous kidney biopsy; and 11% were coded for hemodialysis.
Table 2.
Number of patients coded for new-onset CMV disease during hospitalization 1, coincident conditions and procedures, and subsequent inpatient death in a cohort of 15,848 kidney transplant recipients.
| All CMV | Early | Late | Very late | |
|---|---|---|---|---|
| Number of patients (%) | 825 (5.21) | 184 (1.16) | 400 (2.52) | 241 (1.52) |
|
| ||||
| EGD, flexible sigmoidoscopy or colonoscopy | 213 (25.82) | 42 (22.83) | 106 (26.50) | 65 (26.97) |
| Pneumonia or hepatitis | 99 (12.00) | 11 (5.98) | 54 (13.50) | 34 (14.11) |
| Possible tissue-invasive CMV disease 2 | 288 (34.91) | 50 (21.17) | 143 (35.75) | 95 (39.42) |
| Transplant failure or rejection | 451 (54.67) | 101 (54.89) | 214 (53.50) | 136 (56.43) |
| Percutaneous kidney biopsy | 107 (12.97) | 29 (15.76) | 51 (12.75) | 27 (11.20) |
| Hemodialysis | 88 (10.67) | 15 (8.15) | 30 (7.50) | 43 (17.84) |
| Died | 109 (13.21) | 18 (9.78) | 45 (11.25) | 46 (19.09) |
| Median time to death (interquartile range) | 175 (33–642) | 315 (31 – 1,212) | 367 (46 – 1,075) | 72 (33 – 234) |
The first readmission coded for CMV disease was used for analysis.
Coding for EGD, flexible sigmoidoscopy, colonoscopy, pneumonia or hepatitis.
Univariate and multivariate Cox proportional hazard ratios for risk factors associated with delayed-onset CMV disease coded during hospitalization are shown in Table 3. Previous transplant failure or rejection was significantly associated with a 43% increased risk of delayed-onset CMV disease. Previous transplant failure or rejection preceded delayed-onset CMV disease by a median of 7.2 months (IQR, 3.9–15.5 months). Residence in ZIP codes with the lowest median incomes, or with missing information, was significantly associated with a 19% increased risk of delayed-onset CMV disease. Diabetes mellitus was modestly associated with delayed-onset CMV disease on univariate analysis (HR 1.2), and approached statistical significance on multivariate analysis (p=0.055). Non-white race was significantly associated on multivariate analysis with a 19% decreased risk of delayed-onset CMV disease.
Table 3.
Cox proportional hazard model for risk factors associated with delayed-onset CMV disease coded during hospitalization.
| Risk factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Increasing age at time of transplantation per decade | 1.07 (1.01–1.13) | 0.021 | 1.05 (0.99–1.12) | 0.098 |
|
| ||||
| Female sex | 1.02 (0.87–1.20) | 0.805 | ||
|
| ||||
| Non-white race | 0.84 (0.72–0.98) | 0.027 | 0.81 (0.69–0.95) | 0.009 |
|
| ||||
| Patient location non-metropolitan | 1.12 (0.76–1.66) | 0.561 | ||
|
| ||||
| Patient residence in lowest-income ZIP codes, or missing | 1.18 (1.01–1.38) | 0.042 | 1.19 (1.01–1.40) | 0.036 |
|
| ||||
| Primary payer | ||||
| Private insurance or Medicare ≥ 65 years of age | 1.00 | |||
| Medicare < 65 years of age, Medicaid, self-pay, no charge or other | 1.07 (0.91–1.25) | 0.403 | ||
|
| ||||
| Previous transplant failure or rejection | 1.44 (1.23–1.69) | <0.001 | 1.43 (1.22–1.68) | <0.001 |
|
| ||||
| Prior transplant | 1.01 (0.68–1.50) | 0.944 | ||
| Kidney | 0.98 (0.60–1.61) | 0.934 | ||
| Liver | 0.21 (0.03–1.50) | 0.119 | ||
| Heart | 2.53 (1.13–5.64) | 0.024 | ||
| Pancreas | 1.44 (0.54–3.86) | 0.462 | ||
|
| ||||
| Diabetes mellitus | 1.24 (1.06–1.45) | 0.007 | 1.17 (1.00–1.38) | 0.055 |
|
| ||||
| Charlson comorbidity index > 4 | 1.29 (1.04–1.61) | 0.021 | ||
Ten per cent of patients with early-onset and 11% of patients with late-onset CMV disease died in-hospital, whereas 19% of patients with very late-onset CMV disease died in-hospital. The median time between coding for CMV disease and death for patients who had early and late-onset CMV disease was 315 days and 367 days respectively, while the median time between coding for CMV disease and death for patients who had very late-onset CMV disease was 72 days. In-hospital death occurred in 5.8% (919/15,848) of the study cohort, with 597 (3.8%) of the deaths occurring > 365 days post-transplant. On Kaplan-Meier analysis, patients with CMV disease coded during readmission had lower survival compared to patients with no coding for CMV disease (Figure 1). Approximately 5.4% of patients with no coding for CMV disease died (median time to death post-transplant, 724 days, IQR 169 to 1,330 days), whereas 13.2% of patients coded with CMV disease died (median time to death post-transplant 746 days, IQR 407 to 1,698 days).
Figure 1.
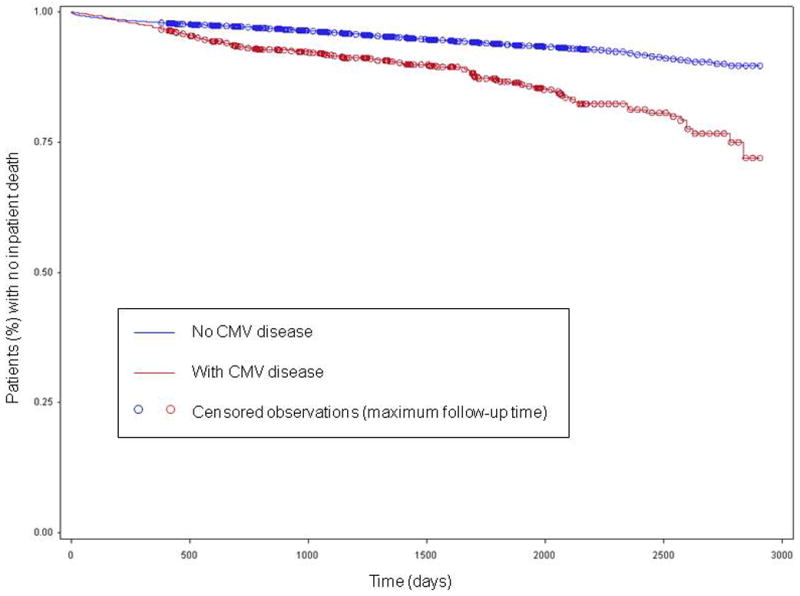
Time (days) to inpatient death in a cohort of 15,848 adult kidney transplant recipients, stratified according presence or absence of CMV disease coded during hospitalization.
Univariate and multivariate Cox proportional hazard ratios for risk factors associated with death are shown in Table 4. Late and very late-onset CMV disease were significantly associated on multivariate analysis with a 1.5 and 2.1-fold increased risk of death after transplantation respectively, whereas early-onset CMV disease was not. Other risk factors for inpatient death identified on multivariate analysis were increasing age (HR 1.5); non-white race (HR 1.2); residence in ZIP codes with the lowest median incomes, or with missing information (HR 1.2); transplant failure or rejection (HR 3.2); prior solid-organ transplant (HR 1.7); diabetes mellitus (HR 1.5); congestive heart failure (HR 1.5); coagulopathy (HR 1.3); peripheral vascular disease (HR 1.5); chronic pulmonary disease (HR 1.2); and pulmonary circulation disease (HR 1.5). CMV disease coded during readmission with no codes for possible tissue-invasion was associated with a 1.4-fold increased risk of death (p=0.010), whereas CMV disease coded during readmission with codes for possible tissue-invasion was associated with a 2.1-fold increased risk of death (p <0.001), adjusted for the same covariates.
Table 4.
Cox proportional hazard model for risk factors associated with inpatient death.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| New-onset CMV disease | ||||
| Early-onset (≤ 100 days post-transplant) | 1.77 (1.11–2.83) | 0.016 | 1.34 (0.84–2.15) | 0.217 |
| Late-onset (101 to 365 days post-transplant) | 2.08 (1.54–2.82) | <0.001 | 1.47 (1.09–1.99) | 0.012 |
| Very late-onset (> 365 days post-transplant) | 3.14 (2.34–4.23) | <0.001 | 2.08 (1.54–2.81) | <0.001 |
|
| ||||
| Increasing age at time of transplantation per decade | 1.64 (1.56–1.74) | <0.001 | 1.54 (1.46–1.63) | <0.001 |
|
| ||||
| Female sex | 0.83 (0.72–0.95) | 0.007 | ||
|
| ||||
| Non-white race | 1.12 (0.98–1.28) | 0.092 | 1.18 (1.03–1.35) | 0.018 |
|
| ||||
| Patient location non-metropolitan | 0.99 (0.70–1.41) | 0.961 | ||
|
| ||||
| Patient residence in lowest-income ZIP codes, or missing | 1.21 (1.06–1.38) | 0.006 | 1.15 (1.00–1.32) | 0.049 |
|
| ||||
| Primary payer | ||||
| Private insurance or Medicare ≥ 65 years of age | 1.00 | |||
| Medicare < 65 years of age, Medicaid, self-pay, no charge or other | 0.97 (0.85–1.11) | 0.674 | ||
|
| ||||
| Transplant failure or rejection | 3.77 (3.19–4.46) | <0.001 | 3.20 (2.70–3.79) | <0.001 |
|
| ||||
| Prior transplant | 1.66 (1.27–2.16) | <0.001 | 1.66 (1.27–2.17) | <0.001 |
| Kidney | 1.55 (1.11–2.16) | 0.010 | ||
| Liver | 2.02 (1.17–3.49) | 0.012 | ||
| Heart | 3.06 (1.64–5.71) | <0.001 | ||
| Lung | 2.75 (0.89–8.55) | 0.080 | ||
| Pancreas | 0.99 (0.37–2.64) | 0.981 | ||
|
| ||||
| Comorbidities | ||||
| Hypertension | 0.98 (0.71–1.33) | 0.874 | ||
| Diabetes mellitus | 2.34 (2.05–2.66) | <0.001 | 1.48 (1.29–1.70) | <0.001 |
| Congestive heart failure | 2.55 (2.18–2.97) | <0.001 | 1.52 (1.29–1.79) | <0.001 |
| Hypothyroidism | 1.38 (1.13–1.70) | 0.002 | ||
| Obesity | 1.36 (1.11–1.67) | 0.003 | ||
| Coagulopathy | 1.58 (1.30–1.93) | <0.001 | 1.30 (1.06–1.59) | 0.011 |
| Peripheral vascular disease | 2.67 (2.27–3.14) | <0.001 | 1.51 (1.27–1.79) | <0.001 |
| Chronic pulmonary disease | 1.68 (1.38–2.04) | <0.001 | 1.24 (1.02–1.52) | 0.035 |
| Depression | 1.25 (0.99–1.57) | 0.057 | ||
| Valvular disease | 1.96 (1.60–2.39) | <0.001 | 1.23 (0.99–1.53) | 0.059 |
| Neurologic disorders | 1.05 (0.79–1.39) | 0.752 | ||
| Rheumatoid arthritis or collagen vascular disease | 0.77 (0.56–1.06) | 0.113 | ||
| Liver disease | 1.51 (1.17–1.95) | 0.002 | ||
| Pulmonary circulation disease | 2.71 (2.09–3.52) | <0.001 | 1.48 (1.12–1.96) | 0.006 |
| Psychoses | 1.27 (0.82–1.96) | 0.275 | ||
| Drug abuse | 0.86 (0.51–1.46) | 0.576 | ||
| Paralysis | 0.98 (0.53–1.83) | 0.956 | ||
| Alcohol abuse | 1.70 (1.04–2.79) | 0.035 | ||
| Solid tumor without metastasis | 1.69 (0.76–3.78) | 0.199 | ||
| HIV | 1.29 (0.42–4.01) | 0.658 | ||
| Lymphoma | 1.53 (0.38–6.11) | 0.546 | ||
|
| ||||
| Charlson comorbidity index > 4 | 2.78 (2.40–3.23) | <0.001 | ||
DISCUSSION
We assembled a large cohort of adult kidney transplant patients who received transplants from 2004 to 2010 using inpatient hospital billing data from California and Florida and followed them over time to determine the incidence of CMV disease coded during hospital readmission. We hypothesized that late and very late-onset CMV disease occurred more commonly than early-onset CMV disease, and that delayed-onset CMV disease was associated with death. Analysis of these billing data with regards to kidney transplantation and CMV disease has never been done, and provides more generalizable information regarding the incidence, risk factors and outcomes associated with CMV disease coded during readmision.
We found that late and very late-onset CMV disease coded during readmission occurred more commonly than early-onset CMV disease. Notably, our study cohort was comprised of a subset of kidney transplant recipients in the United States, where anti-lymphocyte antibodies are used frequently for induction immunosuppressive therapy (16;23). Anti-lymphocyte antibodies are infrequently used as induction therapy in other countries (e.g., United Kingdom), due to concerns over its side effects (25). A study from the United Kingdom reported the incidence of late-onset CMV disease among D+/R− kidney transplant recipients to be 19%, of which only 10% had tissue-invasive disease (24). In contrast, 2 studies from the U.S. reported the incidence of late-onset CMV disease among D+/R− kidney transplant patients to be 26% and 27%, of which 34% and 51% had tissue-invasion (16;23). Prophylactic anti-CMV therapy with ganciclovir or valganciclovir was used for at least 3 months in all 3 studies. Anti-lymphocyte antibodies were frequently used as induction therapy in the U.S. studies, whereas it was not used in the U.K. study. In our current study, 35% of cases of CMV disease coded during hospital readmission were assessed as having possible tissue-invasive CMV disease, consistent with the proportion in the U.S. studies. Interestingly, EGD, flexible sigmoidoscopy or colonoscopy were commonly performed during hospitalizations in which CMV disease was coded, indicating significant gastrointestinal symptomatology during the hospitalization. This is not unexpected, given that CMV disease among kidney transplant recipients commonly manifests as esophagitis, gastritis, enteritis or colitis (16;18;26). In contrast, other potential indicators of tissue invasion by CMV, such as codes for pneumonia and hepatitis, were found less commonly, possibly reflecting the relative infrequency of CMV pneumonitis and hepatitis in kidney transplant recipients (16;18). Indicators of allograft dysfunction, such as coding for transplant failure or rejection, performance of percutaneous kidney biopsy and hemodialysis frequently co-occurred with CMV disease coding, possibly reflecting a bi-directional relationship between CMV and kidney failure (27). CMV can cause nephritis (3;28), trigger upregulation of alloantigens thereby promoting allograft rejection (27), or cause gastrointestinal tract disease resulting in emesis and/or diarrhea and subsequent dehydration and acute tubular necrosis (16;18;26). Conversely, kidney failure can cause decreased clearance of immunosuppressive medications, or prompt the administration of anti-lymphocyte antibodies or high-dose steroids if acute allograft rejection is proven or suspected, thereby increasing the net state of immunosuppression and promoting CMV disease (15;27;29;30).
Factors associated with increased risk of delayed-onset CMV disease coded during readmission in this cohort were previous transplant failure or rejection and patient residence in ZIP codes with the lowest median incomes. The association between allograft rejection and the subsequent development of CMV disease is well-documented (8;15;31), and is presumably due to the administration of lymphocyte depleting therapy or high-dose steroids to treat acute cellular rejection (3;27;29;30). More recent studies however, do not describe an association between allograft rejection and subsequent CMV disease (18;19;23), and one in particular, found a seemingly protective effect of acute graft rejection against CMV disease (16). This change may be due to academic transplant centers restarting prophylactic anti-CMV therapy (valganciclovir, oral ganciclovir or intravenous ganciclovir) for 1 to 3 months, or instituting a pre-emptive anti-CMV strategy concurrent with treatment for acute allograft rejection, as recommended by the American Society of Transplantation (3). Our study shows that coding for transplant failure or rejection was associated with an increased risk for delayed-onset CMV disease, possibly indicating that medical providers do not always reinstitute prophylactic or preemptive strategies to prevent CMV disease upon commencing treatment for allograft rejection. Patient residence in the lowest-income ZIP codes was modestly associated with CMV disease. The mechanisms that link low socioeconomic status to inferior transplant outcomes are likely multifactorial, and include contextual and individual-level factors (32;33). It is possible that the expense of preventive anti-CMV therapy leads to non-adherence in patients with lower socioeconomic status (34), or that access to outpatient continuity care is more difficult (32). Our current study relied on receipt of inpatient care, since the SID consist of billing data from hospitalizations. However, these hospital admissions may have resulted from impaired access to continuity outpatient care, leading to the development of CMV disease requiring hospitalization.
Diabetes mellitus was modestly associated with delayed-onset CMV disease coded during hospitalization on univariate analysis, and approached statistical significance on multivariate analysis. This is an observation that has not been previously demonstrated in other studies presumably because of lack of power (16;19;21). Patients with poorly-controlled diabetes mellitus are presumed to be immunocompromised because of multiple hyperglycemia-related immune system impairments (35–39), and most relevantly may have decreased production of interferon-gamma and tumor necrosis factor-alpha by T-cells (40), which may result in increased risk of CMV disease.
Non-white race appeared protective against delayed-onset CMV disease. Non-white persons have a CMV-seropositivity prevalence of approximately 75% compared to approximately 40% among whites, according to the National Health and Nutrition Examination Surveys (NHANES) from 1988 to 2004 (41). A consequence of this would be that white patients are more likely to be D+/R− compared to persons of other races, and therefore as a group would be more likely to develop CMV disease (3;8;27). It has been shown that failure to develop effective CMV-specific T-cell immunity predicts CMV disease (42), a condition presumably more likely to develop in CMV-naïve patients than those with immune memory.
We found that late and very late-onset CMV disease coded during readmission was associated with inpatient death, while early-onset CMV disease was not. The underlying reason for the association between late and very late-onset CMV disease and death is most likely the development of allograft failure. Acute cellular rejection necessitates the administration of anti-lymphocyte antibodies or high-dose steroids which markedly increases the risk of CMV disease (15;27;29;30). Acute cellular rejection may culminate in allograft failure and hemodialysis, which increase the risk of death (43;44). Delayed-onset CMV disease therefore, may merely reflect the occurrence of allograft failure, and it is likely that allograft failure, not CMV disease, is directly associated with death. We attempted to control for allograft failure using coding for prior transplant failure or rejection; however, residual confounding is likely present. In addition, delayed-onset CMV disease coded during hospitalization may represent a subset of cases with more tissue-invasive disease, compared to all cases of CMV disease that occur in kidney transplant recipients. In our study, we found that more than one-third of hospitalizations in which CMV disease was coded had evidence for possible tissue invasion by CMV, and that delayed-onset CMV disease had a greater frequency of these codes compared to early-onset CMV disease. We also found that tissue-invasive CMV disease had a greater association with death than CMV disease with no evidence for tissue invasion. It has been reported that delayed-onset CMV disease presents atypically (20) and may not be recognized until it has resulted in tissue-invasion. It is possible that severe tissue-invasive CMV disease contributes to the causal pathway for death.
The strengths of our study are the size of the study population, the long duration of follow-up and the generalizability of its findings. However, it has several limitations. Administrative data based on ICD-9-CM codes recorded for medical billing may be inaccurate. There is only one procedure code for kidney transplantation, which precludes differentiation between cadaveric and living related donor transplantation. Only conditions coded during a hospitalization within the state can be identified using the SID. Conditions managed in the outpatient setting, and the site of outpatient follow-up cannot be captured, as are important risk factors for CMV disease such as donor and recipient CMV serostatus, induction and maintenance immunosuppressive therapy, and the choice, route and duration of anti-CMV therapy.
In summary, we have shown using a large cohort of adult kidney transplant recipients observed over an extended period of time that late and very late-onset CMV disease coded during readmission occurs more frequently than early-onset CMV disease, and that previous transplant failure or rejection is a risk factor for delayed-onset CMV disease. Future studies should focus on determining the accuracy of ICD-9-CM coding for CMV disease coded during hospitalization in both transplant centers and non-transplant centers, and determining whether severe tissue-invasive CMV disease is independently associated with death.
METHODS
Study design and patient population
We conducted a retrospective cohort study of patients ≥ 18 years of age who underwent kidney transplantation, identified using ICD-9-CM code 55.69. We included kidney transplant recipients from 2004 to 2010 in the California SID and 2006 to 2010 in the Florida SID (n=15,848). These years were used to accrue 1 year of preexisting data to determine comorbidities, and at least 1 year of follow-up data. We excluded patients coded for CMV disease in the year prior to transplantation, and during the transplant hospitalization (n=168) because we were interested only in new-onset CMV disease. In addition, we excluded patients who received another solid-organ transplant during the same hospital stay (n=1,019) and patients coded for kidney transplant at a pediatric hospital (n=46) or a hospital coding for less than 1 transplant per year (n<11). The American Hospital Association (AHA) Annual Hospital Survey was used to determine whether transplant centers were pediatric or non-pediatric hospitals. This study was considered exempt from Human Research Protection Office oversight by the Institutional Review Board of Washington University in St. Louis.
Demographic data, comorbidities and prior transplantation
We ascertained the demographic characteristics of our study cohort at the time of transplantation (age, sex, race, patient location, median income of patient ZIP code and expected primary insurance payer), history of prior solid-organ transplantation and comorbidities. We identified prior solid-organ transplantation using ICD-9-CM codes V42.0, V42.7, V42.1, V42.6, V42.83 and V42.84 from 1 year before the transplant and through the transplant admission and using ICD-9-CM procedure codes 50.59, 37.51, 33.50 to 33.52, 33.6, 52.80 and 46.97 prior to the transplant admission. Comorbidities within 1 year before transplantation and during the transplant hospitalization were identified using the comorbidity software provided by HCUP (45). The Charlson comorbidity index was computed using software adapted from the Surveillance, Epidemiology and End Results (SEER) Program (46).
Follow-up
Subsequent inpatient admissions of the study cohort were identified from the SID using the encrypted patient-level identifier to link across admissions. Newly-coded CMV disease occurring after transplantation was identified using ICD9-CM diagnosis code 078.5, and was classified according to time of onset. Assuming that most transplant centers in the United States provide prophylactic anti-CMV therapy for at least 3 months after transplantation, early-onset CMV disease was defined as occurring at readmissions ≤ 100 days post-transplant, while delayed-onset CMV disease was defined as occurring > 100 days post-transplant. Delayed-onset CMV disease was further categorized into late-onset CMV disease (occurring 101 to 365 days post-transplant) and very late-onset CMV disease (occurring > 365 days post-transplant). New-onset transplant failure or rejection was identified using code 996.81 at readmission or during the transplant admission if the patient did not have a history of prior kidney transplantation. Repeat solid-organ transplantation on a subsequent admission was identified using ICD-9-CM procedure codes 55.69, 50.59, 37.51, 33.50 to 33.52, 33.6, 52.80 and 46.97. The hospitalization during which CMV disease was coded was further characterized by identifying codes for esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy or colonoscopy (45.12, 45.13, 45.14, 45.16, 45.23, 45.24 and 45.25), which may have been used to diagnose gastrointestinal tract CMV disease; pneumonia (484.1); hepatitis (573.1); and percutaneous kidney biopsy (55.23) and hemodialysis (39.95), which indicate allograft dysfunction. Time to death (during an inpatient hospital stay) was determined using the discharge status variable.
Statistical analysis
Descriptive statistics were used to describe the demographic and clinical characteristics of the study cohort. Potential risk factors for delayed-onset CMV disease and inpatient death coded during hospitalization, including age, sex, race, patient location, median income of patient ZIP code, primary expected payer, previous transplant failure or rejection, prior solid-organ transplantation and selected comorbidities were analyzed using univariate and multivariate Cox proportional hazard modeling. Only patients who survived > 100 days post-transplant and were never coded with CMV disease ≤ 100 days post-transplant were included in the analysis for risk factors for delayed-onset CMV disease. The proportional hazards assumption was evaluated for each variable using visual inspection of log-log survival curves and the correlation between Schoenfeld residuals for a particular covariate and the ranking of individual failure times (47). Statistical significance was set at a p-value ≤ 0.05. All analyses were done using SAS version 9.2 (Cary, North Carolina).
Acknowledgments
We would like to acknowledge Harini Subramaniam, Cherie Hill and Anita Hellstrom for help with coding, database management and administrative support. This study was supported by the Center for Administrative Data Research, which is funded in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ), and Grant Number KM1CA156708 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH). CAQ Santos is supported by the ICTS Multidisciplinary Clinical Research Career Development Program funded by NIH grant KL2 TR000450.
Abbreviations
- CMV
cytomegalovirus
- AHRQ-HCUP
Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project
- SID
State Inpatient Databases
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- SEER
Surveillance, Epidemiology and End Results
- AHA
American Hospital Association
- EGD
esophagogastroduodenoscopy
- IQR
interquartile range
- HR
hazard ratio
- NHANES
National Health and Nutrition Examination Surveys
Footnotes
- Carlos A. Q. Santos – Participated in research design, performance of research, data analysis and writing of the paper; supported by the Washington University Institute of Clinical and Translational Sciences Multidisciplinary Clinical Research Career Development Program funded by National Institutes of Health grant KL2 TR000450; no conflicts of interest to disclose.
- Daniel C. Brennan – Participated in research design, performance of research, data analysis and writing of the paper; no conflicts of interest to disclose.
- Victoria J. Fraser – Participated in research design and writing of the paper; no conflicts of interest to disclose.
- Margaret A. Olsen – Participated in research design, performance of research, data analysis and writing of the paper; no conflicts of interest to disclose.
References
- 1.Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005 Feb 27;79(4):381–6. doi: 10.1097/01.tp.0000148239.00384.f0. [DOI] [PubMed] [Google Scholar]
- 2.Green M. Introduction: Infections in solid organ transplantation. Am J Transplant. 2013 Mar;13( Suppl 4):3–8. doi: 10.1111/ajt.12093. [DOI] [PubMed] [Google Scholar]
- 3.Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013 Mar;13( Suppl 4):93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002 Apr 15;34(8):1094–7. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 5.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004 Jul;66(1):329–37. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann A, Sagedal S, Hjelmesaeth J. The natural course of cytomegalovirus infection and disease in renal transplant recipients. Transplantation. 2006 Jul 27;82(2 Suppl):S15–S17. doi: 10.1097/01.tp.0000230460.42558.b0. [DOI] [PubMed] [Google Scholar]
- 7.Eid AJ, Bakri SJ, Kijpittayarit S, Razonable RR. Clinical features and outcomes of cytomegalovirus retinitis after transplantation. Transpl Infect Dis. 2008 Feb;10(1):13–8. doi: 10.1111/j.1399-3062.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 8.Cordero E, Casasola C, Ecarma R, Danguilan R. Cytomegalovirus disease in kidney transplant recipients: incidence, clinical profile, and risk factors. Transplant Proc. 2012 Apr;44(3):694–700. doi: 10.1016/j.transproceed.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 9.Sagedal S, Nordal KP, Hartmann A, Degre M, Holter E, Foss A, et al. A prospective study of the natural course of cytomegalovirus infection and disease in renal allograft recipients. Transplantation. 2000 Oct 27;70(8):1166–74. doi: 10.1097/00007890-200010270-00007. [DOI] [PubMed] [Google Scholar]
- 10.Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004 Apr;4(4):611–20. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 11.Doyle AM, Warburton KM, Goral S, Blumberg E, Grossman RA, Bloom RD. 24-week oral ganciclovir prophylaxis in kidney recipients is associated with reduced symptomatic cytomegalovirus disease compared to a 12-week course. Transplantation. 2006 Apr 27;81(8):1106–11. doi: 10.1097/01.tp.0000204048.90367.97. [DOI] [PubMed] [Google Scholar]
- 12.Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006 Sep;6(9):2134–43. doi: 10.1111/j.1600-6143.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 13.Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010 May;10(5):1228–37. doi: 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- 14.Hodson EM, Ladhani M, Webster AC, Strippoli GF, Craig JC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2013;2:CD003774. doi: 10.1002/14651858.CD003774.pub4. [DOI] [PubMed] [Google Scholar]
- 15.Razonable RR, Rivero A, Rodriguez A, Wilson J, Daniels J, Jenkins G, et al. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir. J Infect Dis. 2001 Dec 1;184(11):1461–4. doi: 10.1086/324516. [DOI] [PubMed] [Google Scholar]
- 16.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008 Mar 15;46(6):840–6. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 17.Helantera I, Lautenschlager I, Koskinen P. Prospective follow-up of primary CMV infections after 6 months of valganciclovir prophylaxis in renal transplant recipients. Nephrol Dial Transplant. 2009 Jan;24(1):316–20. doi: 10.1093/ndt/gfn558. [DOI] [PubMed] [Google Scholar]
- 18.Helantera I, Kyllonen L, Lautenschlager I, Salmela K, Koskinen P. Primary CMV infections are common in kidney transplant recipients after 6 months valganciclovir prophylaxis. Am J Transplant. 2010 Sep;10(9):2026–32. doi: 10.1111/j.1600-6143.2010.03225.x. [DOI] [PubMed] [Google Scholar]
- 19.Cervera C, Fernandez-Ruiz M, Valledor A, Linares L, Anton A, Angeles MM, et al. Epidemiology and risk factors for late infection in solid organ transplant recipients. Transpl Infect Dis. 2011 Dec;13(6):598–607. doi: 10.1111/j.1399-3062.2011.00646.x. [DOI] [PubMed] [Google Scholar]
- 20.Boobes Y, Al HM, Dastoor H, Bernieh B, Abdulkhalik S. Late cytomegalovirus disease with atypical presentation in renal transplant patients: case reports. Transplant Proc. 2004 Jul;36(6):1841–3. doi: 10.1016/j.transproceed.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Blyth D, Lee I, Sims KD, Gasink LB, Barton TD, Van Deerlin VM, et al. Risk factors and clinical outcomes of cytomegalovirus disease occurring more than one year post solid organ transplantation. Transpl Infect Dis. 2012 Apr;14(2):149–55. doi: 10.1111/j.1399-3062.2011.00705.x. [DOI] [PubMed] [Google Scholar]
- 22.Israni AK, Snyder JJ, Skeans MA, Tuomari AV, Maclean JR, Kasiske BL. Who is caring for kidney transplant patients? Variation by region, transplant center, and patient characteristics. Am J Nephrol. 2009;30(5):430–9. doi: 10.1159/000239220. [DOI] [PubMed] [Google Scholar]
- 23.Boudreault AA, Xie H, Rakita RM, Scott JD, Davis CL, Boeckh M, et al. Risk factors for late-onset cytomegalovirus disease in donor seropositive/recipient seronegative kidney transplant recipients who receive antiviral prophylaxis. Transpl Infect Dis. 2011 Jun;13(3):244–9. doi: 10.1111/j.1399-3062.2011.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvala H, Stewart C, Muller K, Burns S, Marson L, MacGilchrist A, et al. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol. 2013 May;85(5):893–8. doi: 10.1002/jmv.23539. [DOI] [PubMed] [Google Scholar]
- 25.Immunosuppressive therapy for renal transplanation in adults. NHS National Institute for Clinical Excellence. 2013 Available from: URL: www.nice.org.uk/TA085guidance.
- 26.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2010 Jan;10(1):157–61. doi: 10.1111/j.1600-6143.2009.02861.x. [DOI] [PubMed] [Google Scholar]
- 27.Eid AJ, Razonable RR. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010 May 28;70(8):965–81. doi: 10.2165/10898540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Wong KM, Chan YH, Chan SK, Mak CK, Chau KF, Li CS. Cytomegalovirus-induced tubulointerstitial nephritis in a renal allograft treated by foscarnet therapy. Am J Nephrol. 2000 May;20(3):222–4. doi: 10.1159/000013592. [DOI] [PubMed] [Google Scholar]
- 29.Hibberd PL, Tolkoff-Rubin NE, Conti D, Stuart F, Thistlethwaite JR, Neylan JF, et al. Preemptive ganciclovir therapy to prevent cytomegalovirus disease in cytomegalovirus antibody-positive renal transplant recipients. A randomized controlled trial. Ann Intern Med. 1995 Jul 1;123(1):18–26. doi: 10.7326/0003-4819-123-1-199507010-00002. [DOI] [PubMed] [Google Scholar]
- 30.Conti DJ, Freed BM, Singh TP, Gallichio M, Gruber SA, Lempert N. Preemptive ganciclovir therapy in cytomegalovirus-seropositive renal transplants recipients. Arch Surg. 1995 Nov;130(11):1217–21. doi: 10.1001/archsurg.1995.01430110075014. [DOI] [PubMed] [Google Scholar]
- 31.Abbott KC, Hypolite IO, Viola R, Poropatich RK, Hshieh P, Cruess D, et al. Hospitalizations for cytomegalovirus disease after renal transplantation in the United States. Ann Epidemiol. 2002 Aug;12(6):402–9. doi: 10.1016/s1047-2797(01)00283-6. [DOI] [PubMed] [Google Scholar]
- 32.Patzer RE, Pastan SO. Measuring the disparity gap: quality improvement to eliminate health disparities in kidney transplantation. Am J Transplant. 2013 Feb;13(2):247–8. doi: 10.1111/ajt.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patzer RE, McClellan WM. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012 Sep;8(9):533–41. doi: 10.1038/nrneph.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pescovitz MD. Formulary considerations for drugs used to prevent cytomegalovirus disease. Am J Health Syst Pharm. 2003 Dec 1;60(23 Suppl 8):S17–S21. doi: 10.1093/ajhp/60.suppl_8.S17. [DOI] [PubMed] [Google Scholar]
- 35.Amano H, Yamamoto H, Senba M, Oishi K, Suzuki S, Fukushima K, et al. Impairment of endotoxin-induced macrophage inflammatory protein 2 gene expression in alveolar macrophages in streptozotocin-induced diabetes in mice. Infect Immun. 2000 May;68(5):2925–9. doi: 10.1128/iai.68.5.2925-2929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997 Jan;14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 37.Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes. 2000 Sep;49(9):1451–8. doi: 10.2337/diabetes.49.9.1451. [DOI] [PubMed] [Google Scholar]
- 38.Mazade MA, Edwards MS. Impairment of type III group B Streptococcus-stimulated superoxide production and opsonophagocytosis by neutrophils in diabetes. Mol Genet Metab. 2001 Jul;73(3):259–67. doi: 10.1006/mgme.2001.3185. [DOI] [PubMed] [Google Scholar]
- 39.Ilyas R, Wallis R, Soilleux EJ, Townsend P, Zehnder D, Tan BK, et al. High glucose disrupts oligosaccharide recognition function via competitive inhibition: a potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011 Jan;216(1–2):126–31. doi: 10.1016/j.imbio.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price CL, Hassi HO, English NR, Blakemore AI, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. 2010 Jun;14(6B):1806–15. doi: 10.1111/j.1582-4934.2009.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010 Jun 1;50(11):1439–47. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009 May;9(5):1214–22. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 43.Matas AJ, Gillingham KJ, Payne WD, Najarian JS. The impact of an acute rejection episode on long-term renal allograft survival (t1/2) Transplantation. 1994 Mar 27;57(6):857–9. doi: 10.1097/00007890-199403270-00015. [DOI] [PubMed] [Google Scholar]
- 44.Opelz G, Dohler B. Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation. 2008 Mar 15;85(5):661–6. doi: 10.1097/TP.0b013e3181661695. [DOI] [PubMed] [Google Scholar]
- 45.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 46.SEER Medicare. 2013 http://healthservices.cancer.gov/seermedicare/program/charlson.comorbidity.macro.txt.
- 47.Schoenfeld David. Partial Residuals for The Proportional Hazards Regression Model. Biometrika. 1982;69(1):239–241. [Google Scholar]


