SUMMARY
The tumor microenvironment plays a critical role in cancer progression, but the precise mechanisms by which stromal cells influence the epithelium are poorly understood. Here we show that p62 levels were reduced in the stroma of several tumors, and that its loss in the tumor microenvironment or stromal fibroblasts resulted in increased tumorigenesis of epithelial prostate cancer cells. The mechanism involves the regulation of cellular redox through an mTORC1/c-Myc pathway of stromal glucose and amino acid metabolism, resulting in increased stromal IL-6 production, which is required for tumor promotion in the epithelial compartment. Thus, p62 is an anti-inflammatory tumor suppressor that acts through modulation of metabolism in the tumor stroma.
INTRODUCTION
Primary tumors are initiated as a result of the stepwise acquisition of genetic alterations within the epithelial compartment (Shen and Abate-Shen, 2010). However, increasing evidence supports the notion that the tumor microenvironment also plays a critical role in cancer progression in many types of neoplasias, including prostate cancer (PCa), although relatively little is known about the signaling pathways that mediate communication between the stromal and epithelial compartments (Ammirante et al., 2010; Erez et al., 2010; Santos et al., 2009; Trimboli et al., 2009). Inflammation and metabolism are two critical factors contributing to the pro-tumorigenic properties of the stroma (DeBerardinis and Thompson, 2012; Grivennikov et al., 2010; Hanahan and Coussens, 2012; Metallo and Vander Heiden, 2013; Vander Heiden, 2013). Although not totally understood, some evidence suggests that the metabolic state of the tumor stroma can decisively influence the tumorigenic potential of the tumor epithelial compartment (Lisanti et al., 2013). Here we have addressed this fundamental biological question in the context of p62 deficiency in the non-epithelial tumor compartment. Our laboratory initially identified p62, also known as sequestosome-1, as a scaffold protein for the atypical PKCs (aPKCs), and later implicated p62 in other cell stress responses (Diaz-Meco and Moscat, 2012; Moscat and Diaz-Meco, 2012; Moscat et al., 2007; Sanchez et al., 1998). p62 binds Raptor, a key component of the mTOR-orchestrated nutrient-sensing complex and an important activator of anabolic pathways that are instrumental in metabolic reprogramming during cell transformation (Duran et al., 2011; Moscat and Diaz-Meco, 2011). Nonetheless, nothing is known about the signaling cascades that p62 regulates in stromal cells, or to what extent these pathways influence the epithelial-stromal interaction in the tumor microenvironment. Cancer-associated fibroblasts (CAFs) have been proposed to be key mediators of the crosstalk between malignant tumor cells and their microenvironment (Barron and Rowley, 2012; Franco and Hayward, 2012). CAFs and the complex set of signaling molecules they secrete generate an environment conducive to inflammation, and this in turn maintains the pro-tumorigenic status of the stromal cells. Among these proteins, interleukin-1β (IL-1β), IL-8, and IL-6 have been implicated as part of the pro-inflammatory signature of the PCa stroma (Erez et al., 2010; Franco and Hayward, 2012; Schauer et al., 2008). Furthermore, IL-6 has received increasing attention as a key pro-inflammatory and pro-tumorigenic molecule in many types of cancer, including PCa (Azevedo et al., 2011; De Marzo et al., 2007; Guo et al., 2012; Schafer and Brugge, 2007). Here we address the role of p62 in the stroma in the control of the inflammatory environment in PCa.
RESULTS
p62 expression levels in the tumor microenvironment
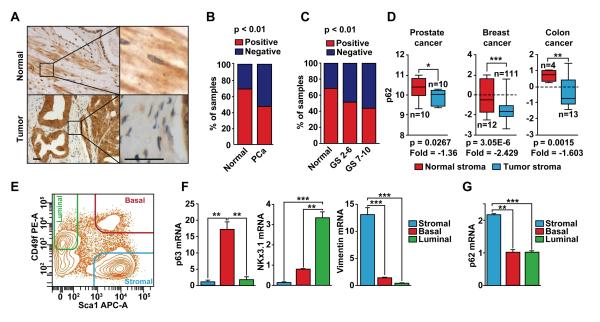
The initial evidence suggesting that p62 plays a role in the regulation of the tumor microenvironment in PCa came from the histological analysis of a tissue panel comprising 202 primary human PCa tumors, 8 metastases, and 22 adjacent normal prostate tissue samples. This study revealed that p62 was expressed in the prostate epithelium and also in the stroma (Figure 1A). p62 protein levels were downregulated in the stroma of human primary PCa tumors as compared with the stroma of normal samples (Figures 1A and B). Furthermore, when the tumor samples were grouped based on low (GS 2-6) or high (GS 7-10) Gleason score, p62 levels in the stroma were significantly reduced upon progression to the most aggressive stage (Figure 1C). p62 was also overexpressed in the epithelial compartment of the PCa human samples (Figure 1A and S1A and S1B). This is consistent with previous observations suggesting that p62 is upregulated in many cancers including lung (Duran et al., 2008; Inoue et al., 2012), liver (Inami et al., 2011), glioblastoma (Galavotti et al., 2013), breast (Rolland et al., 2007; Thompson et al., 2003) and kidney (Li et al., 2013). However, since those studies did not report on expression in the stromal component, it is not clear whether p62 was downregulated in the stroma in those samples, as we have shown in the samples analyzed here. Moreover, bioinformatics analysis of public datasets of stromal gene expression also demonstrated that p62 was significantly downregulated in the tumor stroma, as compared with normal stroma, in several types of cancers including prostate, breast, and colon (Figure 1D). In addition, fluorescence-activated cell sorting (FACS) analysis of adult mouse prostates showed that p62 is more highly expressed in cells of the stroma than in those of basal or luminal lineages (Figures 1E-1G). Quantitative RT-PCR analyses of the sorted prostate cell populations showed that transcripts for the basal marker p63, the luminal cell marker Nkx3.1, and the stroma marker vimentin were enriched in their corresponding cell populations demonstrating successful cell fractionation (Figures 1E and 1F). Of note, p62 expression was highly enriched in the stromal compartment as compared with the other two cell populations (Figure 1G). These results suggest that p62 could exert its effect as a tumor suppressor in the tumor microenvironment, likely in the stroma.
Figure 1. p62 levels are reduced in the stroma of human prostate tumors.
(A) Representative examples of p62 staining of normal and primary prostate cancer (tumor) samples. Scale bars, 25 μm (B) Quantification of p62 staining in the stroma of primary PCa tumors as compared to normal; n = 22 (normal); n = 202 (PCa). Fisher test, p < 0.01. (C) p62 levels are reduced upon PCa progression; n = 22 (normal); n = 70 (GS 2-6); n = 132 (GS 7-10). Chi-square, p < 0.01. (D) p62 mRNA levels in stroma of human cancer samples. Data was collected from public datasets of gene expression in the tumor stroma of several human cancers. GSE34312 (prostate cancer), GSE9014 (breast cancer), and GSE35602 (colon cancer). (E) FACS-sorted adult murine prostate cell linages. Prostate basal, luminal, and stromal cells are Lin−Sca-1+CD49fHi, Lin−Sca-1−CD49fLow, and Lin−Sca-1+CD49f−, respectively. (F) RT-PCR of specific markers for each prostate cell population (n = 3): p63 (basal), Nkx3.1 (luminal), and vimentin (stromal). (G) RT-PCR for p62 in prostate cell populations (n = 3). *p < 0.05, **p < 0.01. ***p < 0.001. Results are presented as mean ± SEM. See also Figure S1.
p62 is a suppressor of inflammation and the CAF phenotype in the tumor microenvironment
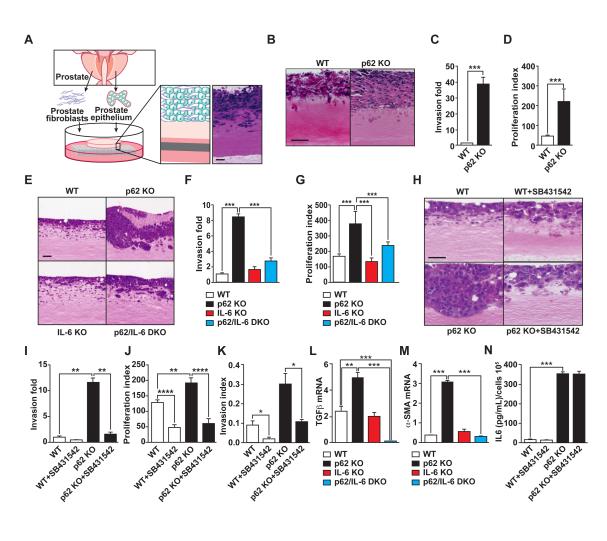
To test whether p62 deficiency in the tumor microenvironment is relevant to the transforming properties of epithelial cells, we performed orthotopic injections of syngeneic murine PCa cells (TRAMP-C2Re3) (Olson et al., 2006) into the prostates of wild-type (WT) and p62 knockout (KO) mice and then assessed tumor growth. The resulting tumors were bigger in the prostates of p62 KO mice than in those of WT mice (Figures 2A-2C), supporting the notion that a loss of p62 in the tumor microenvironment promotes PCa growth. We next carried out transcriptomic profiling of the orthotopic tumors in the WT and p62 KO mice. NextBio analysis revealed important correlations between genes upregulated in the p62 KO orthotopic tumors with a gene signature in the category of “response to wounding” (Figure S2A). In addition, gene set enrichment analysis (GSEA) also identified “response to wounding” as significantly enriched of the gene ontology (GO) biological-process categories (Figures 2D, S2B and S2C), and “stromal stimulation” in the C2 curated gene set library (Figures S2D and S2E). Since CAFs acquire an “activated phenotype” during tumor progression that resembles that of fibroblasts during the wound-healing repair process, these results suggested that the p62 KO stroma is likewise activated (Barron and Rowley, 2012; Bissell and Radisky, 2001; Franco and Hayward, 2012; Schafer and Werner, 2008), and has a more CAF-like phenotype than the WT stroma. In support of this notion, we observed an increase in the expression of α-smooth muscle actin (αSMA) in sections from orthotopic tumors in p62 KO mice as compared to WT controls (Figure 2E), as well as an increase in transforming growth factor β (TGFβ) transcripts as determined by RT-PCR in the same samples (Figure 2F). TGFβ and α-SMA are two bona fide markers of the CAF phenotype (Barron and Rowley, 2012; Franco and Hayward, 2012). Consistent with this, Ingenuity Pathway Analysis (IPA) identified TGFβ1 as a predicted upstream regulator in the p62 KO orthotopic tumors (P=1.47E-07; activation z-score= 3.890). To determine the potential cell-autonomous effect of p62 in this important function, we used FACS to isolate prostate stromal cells from mice of both genotypes, as described in Figures 1E-1F. Interestingly, we found that p62-deficient stromal cells also showed characteristics of CAFs as determined by increased expression levels of α-SMA, TGFβ, and vimentin (Figure 2G). To facilitate subsequent studies, we generated prostate fibroblasts from WT and p62 KO mice and determined their “CAF activation” state. In these cells the loss of p62 resulted in increased CAF transcript markers (Figure 2H), as well as in the secretion of TGFβ, as determined by ELISA (Figure 2I). This is important because TGFβ is essential for the acquisition and maintenance of the CAF/myofibroblast phenotype (Kojima et al., 2010; Ostman and Augsten, 2009). Therefore, p62 loss modifies the stroma by inducing a CAF phenotype, which, in turn, drives tumor progression.
Figure 2. IL-6 is required for p62’s role in the tumor microenvironment.
(A) Orthotopic injection of TRAMP-C2Re3 cells into the prostates of syngeneic WT and p62 KO mice. Orthotopic tumors were allowed to grow for two months. (B and C) Genitourinary (GU) tract weight (B) and pictures (C) from (A); n = 5 or 6 mice per genotype. Scale bar, 1 cm. (D) “Response to wounding” GSEA plot of enrichment of gene expression in p62 KO orthotopic tumors. (E) α-SMA staining of orthotopic tumors from WT and p62 KO mice. Scale bars, 25 μm (F) RT-PCR of TGFβ in orthotopic tumors from WT and p62 KO mice (n = 3). (G and H) RT-PCR of CAF markers (α-SMA, TGFβ and Vimentin) in FACS-sorted prostate stromal fraction from WT and p62 KO mice (G) and in WT and p62 KO prostate fibroblasts (H); n = 3. (I) TGFβ production in prostate fibroblasts was determined by ELISA. (J) GSEA plots of enrichment of gene expression in p62 KO orthotopic tumors. (K) RT-PCR of inflammatory cytokines in orthotopic tumors of WT and p62 KO mice. n = 5 or 6 animals per group versus WT. (L) IL-6 ELISA in fibroblasts. (M and N) Orthotopic injection of TRAMP-C2Re3 cells into the prostates of mice of different genotypes (n = 5 or 6 mice). GU weights (M) and pictures (N). Scale bar, 1 cm. *p < 0.05, **p < 0.01. ***p < 0.001. Results are presented as mean ± SEM. See also Figure S2.
Further bioinformatics GSEA revealed a hyper-inflammatory phenotype in the p62 KO orthotopic tumors. That is, we found “humoral immune response” and “inflammatory response” as second GO categories enriched in the p62 KO transcriptome profile (Figures 2J, S2F and S2G). RT-PCR analysis of the tumors from p62 KO mice showed an increase in the transcripts of inflammatory cytokines such as IL-6, IL-1β and KC (Figure 2K), as well as in the secretion of IL-6 as determined by ELISA (Figure 2L). We hypothesized that IL-6 could be an important mediator of the stromal p62-dependent signals that influence PCa progression in the epithelium. To test this possibility, we carried out an orthotopic injection experiment using p62/IL-6 double KO (DKO) mice as hosts. Notably, the increased tumor growth observed in p62 KO mice was completely reversed in the DKO mice (Figures 2M and 2N), demonstrating that p62 plays a tumor-suppressive role in the tumor microenvironment during PCa progression by inhibiting CAF activation and blocking inflammation.
p62 in stromal fibroblasts regulates an IL-6/TGFβ cascade essential for tumor invasion
We next set up a three dimensional (3D) organotypic culture model that recapitulates, in a genetically accessible system, the tumor microenvironment and its interactions with the tumor epithelial cell, closely mimicking the physiological situation and the cellular architecture (Gaggioli et al., 2007; Kim et al., 2013; Nystrom et al., 2005; Ridky et al., 2010). Since our genome-wide transcriptomic analysis suggested that the loss of p62 in the tumor microenvironment is associated with a CAF-like signature, and because fibroblasts are a critical component of the stroma, we next tested whether p62 KO prostate fibroblasts were able to recapitulate the in vivo phenotype in 3D organotypic cultures. To do this, we co-cultured in this organotypic system, prostate fibroblasts from p62 KO and WT mice with TRAMP-C2Re3 PCa cells (Figure 3A). Importantly, p62 KO prostate fibroblasts (versus WT counterparts) enhanced the invasiveness and proliferation index of PCa epithelial tumor cells (Figure 3B-3D). Similar results were obtained with other PCa cell lines such as mouse Myc-CaP (Figure S3A), or human PC3 cells (Figure S3B). Mouse fibroblasts from p62 KO mice also enhanced the invasiveness and proliferation index of human normal prostate epithelial cells as compared to similar organotypic cultures with WT fibroblasts (Figure S3C). Altogether, this indicates that p62 deficiency in the stromal fibroblasts has a pivotal role in mediating cancer cell proliferation and invasion.
Figure 3. p62-deficient stroma-mediated invasion is IL-6 and TGFβ dependent.
(A) Schematic representation of 3D organotypic cultures. (B) H&E-stained sections of TRAMP-C2Re3 cells cultured in an organotypic system in the presence of primary prostate fibroblasts from WT and p62 KO mice. (C and D) Quantification of PCa cell invasion (C) and proliferation index (D) of experiment shown in (B); n = 4. (E) H&E staining of organotypic gels combining Myc-CaP cells with prostate fibroblasts from mice of different genotypes (n = 4). (F and G) Quantification of PCa cell invasion (F) and proliferation index (G) of experiment shown in (E); n = 4. (H) H&E staining of organotypic gels combining Myc-CaP cells with prostate fibroblasts from mice WT and p62 KO mice in the presence or absence of the TGFβ inhibitor, SB431542 (10 μM). (I and J) PCa cell invasion quantification (I) and proliferation index (J) of (H); n = 4. (K) Invasion index determined by modified Boyden chamber assay with conditioned media from WT and p62 KO fibroblasts in the presence or absence of SB431542 (10 μM); n = 3. (L and M) RT-PCR of TGFβ (L) and α-SMA (M) mRNA levels in fibroblasts of mice of different genotypes (n = 4). (N) IL-6 production by WT and p62 KO fibroblasts in the presence or absence of the TGFβ inhibitor, SB431542 (n = 4). *p < 0.05, **p < 0.01. ***p < 0.001, ****p < 0.0001. Results are presented as mean ± SEM. Scale bars, 100 μm. See also Figure S3.
To follow up on our findings that IL-6 levels were increased in orthotopically injected tissues and that increased IL-6 expression was associated with enhanced tumorigenicity in vivo (Figures 2K-2N), we further investigated the role of this cytokine in the protumorigenic microenvironment created by p62 deficiency in the stroma. The two major sources of IL-6 in the tumor microenvironment are macrophages and stromal fibroblasts (Hanahan and Coussens, 2012). Notably, 3D organotypic culture experiments established that fibroblasts (Figure 3B and 3C), but not macrophages (Figure S3D), from p62 KO mice recapitulated the p62 KO phenotype in the orthotopic tissue grafting experiment, and this effect was abolished when p62/IL-6 DKO fibroblasts were used in the 3D system (Figures 3E-3G). Furthermore, when TGFβ signaling was inhibited by incubating the organotypic cultures with the TGFβ inhibitor SB431542 (Inman et al., 2002), the pro-tumorigenic phenotype of p62-deficient fibroblasts was reverted, consistent with the notion that TGFβ is important for the CAF phenotype and PCa proliferation (Figures 3H-3J). Likewise, the TGFβ inhibitor reverted the increased invasion index of PCa cells incubated with p62-deficient fibroblast conditioned medium in a Boyden chamber invasion assay (Figure 3K). Results of Figure S3E demonstrate the effectiveness of this inhibitor to block the TGFβ pathway. Moreover, the enhanced TGFβ production observed in the p62 KO fibroblasts, as well as that of α-SMA, was completely abrogated in the DKO fibroblasts (Figures 3L and 3M). Consistently, the knockdown of IL-6 in p62 KO fibroblasts impaired IL-6 secretion and, more importantly, also reverted TGFβ production and PCa invasion (Figures S3F-S3H). Furthermore, incubation of p62/IL-6 DKO fibroblasts with exogenously added IL-6 restored TGFβ levels to those of p62 KO cells as well as PCa invasion (Figure S3I and S3J). All this is consistent with a cell autonomous role of the p62-IL-6 axis in the control of the CAF phenotype. However, incubation of p62 KO fibroblasts with the TGFβ signaling inhibitor SB431542 did not affect the overproduction of IL-6 in p62 KO fibroblasts (Figure 3N). These are important observations that establish a sequential p62/IL-6/TGFβ axis in the tumor fibroblastic compartment contributing to the control of epithelial tumorigenesis during PCa progression.
p62 controls IL-6 levels by repressing ROS production through metabolic reprogramming
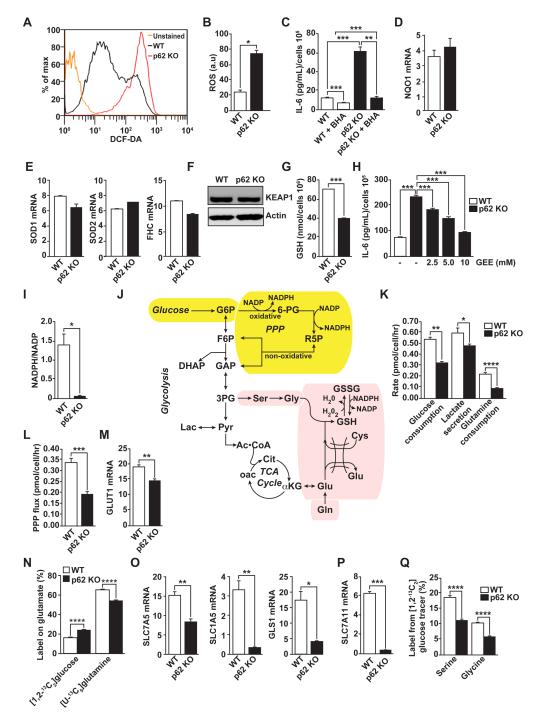
We next sought to determine how p62 controls IL-6 production in fibroblastic stromal cells, and whether the mechanisms mediating IL-6 production are relevant to stroma-driven tumorigenesis. It should be noted that p62 KO fibroblasts have increased levels of reactive oxygen species (ROS) (Figure 4A and B); and that the inhibition of ROS production (by the ROS scavenger BHA) completely reverts the IL-6 hyperproduction phenotype (Figure 4C). This indicates that the mechanism whereby p62 represses IL-6 production in fibroblasts involves the control of ROS levels. It has previously been reported that p62 can activate NF-κB and NRF2 (Duran et al., 2008; Komatsu et al., 2010; Moscat and Diaz-Meco, 2009), which suggests that these molecules could play a role in the ability of p62 to repress ROS production and the subsequent activation of IL-6. The expression of critical detoxifying NF-κB- or NRF2-dependent genes (Figures 4D and E), as well as the levels of the NRF2 inhibitor Keap1 (Figure 4F) were not affected by the loss of p62 in fibroblasts. However, we found that p62 KO fibroblasts displayed lower levels of reduced glutathione (GSH) than the WT controls (Figure 4G). These are important observations because GSH is central to the control of ROS levels. In fact, treatment of p62 KO fibroblasts with the glutathione analogue glutathione-reduced ethyl ester (GEE) reduced IL-6 to levels comparable to those of WT fibroblasts (Figure 4H). These results demonstrate that the loss of p62 results in lower glutathione levels, thus promoting ROS accumulation, which is required for IL-6 overproduction in p62-deficient fibroblasts.
Figure 4. Metabolic reprogramming in p62-deficient stroma.
(A and B) Total intracellular levels of ROS in WT and p62 KO fibroblasts (A) and quantification (B); n = 4. (C) IL-6 ELISA of WT and p62 KO fibroblasts treated with vehicle or ROS scavenger BHA (100 μM) for 12 hr (n = 4). (D and E) RT-PCR of NQO1 (D) and SOD1, SOD2 and FHC (E) mRNA levels in fibroblasts (n = 4). (F) Immunoblot analysis of KEAP1 in cell lysates from WT and p62 KO fibroblasts. Results are representative of three experiments. (G) Cellular GSH levels in WT and p62 KO fibroblasts (n = 4). (H) IL-6 ELISA of fibroblasts treated with increasing concentrations of glutathione analog GEE (n = 4). (I) Cellular NADPH/NADP levels in WT and p62 KO fibroblasts (n = 4). (J) Metabolic scheme depicting biosynthetic routes to NAPDH (yellow shading) and GSH (pink shading) from glucose and glutamine. (K) Glucose consumption, lactate secretion, and glutamine consumption rates determined by spent medium analysis from WT and p62 KO fibroblasts (n = 3). (L) PPP flux estimates from metabolic flux analysis in WT and p62 KO fibroblast cultures labeled with [1,2-13C2]glucose (n = 3). (M) RT-PCR of GLUT1 mRNA (n = 4). (N) Glutamate labeling in WT and p62 KO fibroblasts grown in either [1,2-13C2]glucose and unlabeled glutamine or [U-13C5]glutamine and unlabeled glucose (n = 3). (O and P) RT-PCR of SLC7A5, SLC1A5 and GLS1 (O) and SLC7A11 (P) mRNA levels in WT and p62 KO fibroblasts (n = 3). (Q) Labeling of serine and glycine from [1,213C2]glucose (n=3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Results are presented as mean ± SEM.
We observed a striking decrease in the NADPH/NADP ratio in p62-deficient fibroblasts (Figure 4I). This ratio provides additional information on the cellular redox status, as the relative concentration of GSH versus oxidized glutathione (GSSG) depends on the cellular content of NADPH. Glycolytic metabolism plays a critical role in maintaining NADPH production through the oxidative pentose phosphate pathway (PPP) (Figure 4J yellow shading). Indeed, p62 KO cells exhibited decreased glucose uptake and lactate secretion (Figure 4K). This reduction in glycolytic rate resulted in decreased flux through the oxidative PPP, as determined by stable isotope tracing with [1,2-13C2]glucose (Figure 4L). These metabolic changes correlated with a reduction in GLUT1 levels in p62-deficient fibroblasts (Figure 4M), providing evidence that transcriptional changes associated with p62 loss influence metabolic flux.
Amino acids are critical for the production of glutathione, a peptide composed of glutamate, cysteine, and glycine (Figure 4J, pink shading). Glutamine serves as an important precursor for glutamate, and loss of p62 in fibroblasts leads to lower glutamine consumption compared to WT cells (Figure 4K). We also observed a decrease in the direct conversion of [U-13C5] glutamine to glutamate in p62 KO fibroblasts, with a relative increase in the fraction of glutamate derived from [1,2-13C2]glucose (Figure 4N). In good agreement with these changes in glutamine metabolism, we observed reduced levels of the glutamine transporters SLC7A5 and SLC1A5, as well as glutaminase-1 (GLS1) (Figure 4O), a critical enzyme in the pathway that catalyzes the conversion of glutamine into glutamate (Figure 4J). Consistent with reduced levels of glutathione, p62 KO fibroblasts also exhibit a dramatic reduction in the levels of SLC7A11, the xCT cystine/glutamate antiporter, which is the major driver of cystine uptake, a critical and rate-limiting step in the synthesis of glutathione in several cell types, including fibroblasts (Figure 4P) (Bannai and Tateishi, 1986; Gout et al., 1997). Finally, we observed significant decreases in labeling of both serine and glycine from [1,2-13C2]glucose (Fig 4Q). Serine serves as a precursor to glycine and cysteine (when synthesized from methionine), so this decrease in label transfer provides evidence that there is less demand for GSH synthesis in p62-deficient cells. These results collectively demonstrate that loss of p62 in fibroblasts influences metabolic pathways controlling cellular redox, including NADPH production in the PPP and GSH synthesis.
p62 is a critical regulator of c-Myc levels
Previous data from other laboratories have established the critical role of c-Myc in the regulation of glutamine and glucose metabolism (Dang, 2012). We found significantly reduced levels of c-Myc in p62 KO fibroblasts as well as in WT fibroblasts in which p62 has been knocked down by shRNA (Figures 5A and 5B), and a reduction in the levels of the key glutamine transporters SLC7A5 and SLC1A5, and GLS1 (Figure 4O), which are targets of c-Myc (Dang, 2012). Interestingly, ectopic expression of c-Myc in p62 KO fibroblasts (Figure 5C) reverted the p62-deficient phenotype in terms of IL-6 production (Figure 5D), and the levels of glutamine transporters and GLS1 (Figure 5E) and GSH (Figure 5F). On the contrary, c-Myc knockdown in WT fibroblasts (Figure 5G) resulted in increased IL-6 production at the mRNA and protein levels (Figures 5H and 5I). c-Myc knockdown in fibroblasts led to decreased levels of GSH (Figure 5J), as well as enhanced PCa cell invasion and proliferation in organotypic cell cultures (Figures 5K-5M). Also, the knockdown of c-Myc in fibroblasts resulted in increased PCa cell invasion index in a Boyden chamber assay (Figure 5N). Of note, this cause-and-effect correlation between p62 deficiency, c-Myc expression, and IL-6 production was also found in FACS-isolated prostate stromal cells from WT and p62 KO mice (Figure 5O). Collectively these results demonstrate that p62 repression of c-Myc expression in the stroma fibroblasts accounts for its tumor suppressive role in PCa.
Figure 5. c-Myc-mediated metabolism in p62-deficient stroma.
(A-C) Immunoblot analysis of c-Myc levels in WT and p62 KO fibroblasts (A); in WT fibroblasts lentivirally infected with shRNA non-targeted control (shNT) or shRNA specific for p62 (shp62) (B); and in p62 KO fibroblasts retrovirally infected with control vector (control) or with c-Myc expression vector (c-Myc) (C). Results are representative of three experiments. (D) IL-6 ELISA in control and c-Myc cells (n = 4) as in (C). (E) RT-PCR of SLC7A5, SLC1A5, and GLS1 mRNA in control and c-Myc cells (n = 3). (F) Intracellular GSH levels in control and c-Myc cells (n = 3). (G) Immunoblot analysis of c-Myc and p-STAT3 in WT fibroblasts infected with non-targeting shRNA (shNT) or shRNA for c-Myc (shMyc). (H and I) RT-PCR of IL-6 mRNA (H) and IL-6 ELISA (I) in the same cells (n = 3) as in (G). (J) Quantification of intracellular GSH levels in WT shNT and shMyc cells (n = 3). (K) H&E-stained organotypic gels of TRAMP-C2Re3 cells with shNT or shMyc fibroblasts. Scale bar, 100 μm. (L and M) Quantification of PCa cell invasion (L) and proliferation index (M) of experiment shown in (K). (N) Invasion index determined by modified Boyden chamber assay of Myc-CaP cells co-cultured with shNT and shMyc fibroblasts. (O) RT-PCR of c-Myc and IL-6 mRNA levels in FACS-isolated prostate stromal cells from WT and p62 KO mice (n = 3). (P) Immunoblot analysis with the indicated antibodies of cell lysates of WT and p62 KO fibroblasts. (Q) Immunoblot analysis for the specified proteins of cell lysates from p62 KO fibroblasts retrovirally infected with control vector (control) or FLAG-RagBGTP expression vector (RagBGTP). Results are representative of three experiments. (R) IL-6 ELISA (n = 3) in cells shown in (Q). (S and T) Immunoblot analysis of c-Myc and p-S6K in fibroblasts treated with rapamycin (S) or Torin1 (T) for 12 hr. (U) Intracellular GSH levels in fibroblasts treated with Torin1. (V) IL-6 ELISA in fibroblasts treated with Torin1 (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Results are presented as mean ± SEM.
IL-6 is regulated by a p62/mTORC1/c-Myc cascade
Consistent with previously published observation (Duran et al., 2011), we found that p62 KO cells displayed reduced mTORC1 activity (Figure 5P). We hypothesized that the reduction in c-Myc levels found in p62 KO fibroblasts could be the consequence of mTORC1 inhibition. Importantly, we rescued c-Myc inhibition in p62 KO fibroblasts by expressing a permanently active mutant of the small-GTPase RagB, which is a critical activator of mTORC1 (Figure 5Q). IL-6 levels were likewise reduced under these conditions (Figure 5R). These results demonstrate that reduced mTORC1 activity in p62 KO fibroblasts accounts for the low levels of c-Myc and the subsequent increase in IL-6 production in these mutant cells. Treatment of WT fibroblasts with rapamycin or Torin, two different inhibitors of mTORC1, effectively reduced c-Myc levels (Figures 5S and 5T), promoting a significant reduction in GSH levels (Figure 5U), and a concomitant increase in IL-6 production (Figure 5V). Therefore, p62’s ability to regulate mTORC1 in the stroma is essential for its control of the c-Myc/GSH/IL-6 axis.
p62 KO mice develop prostate hyperplasia and PIN upon aging
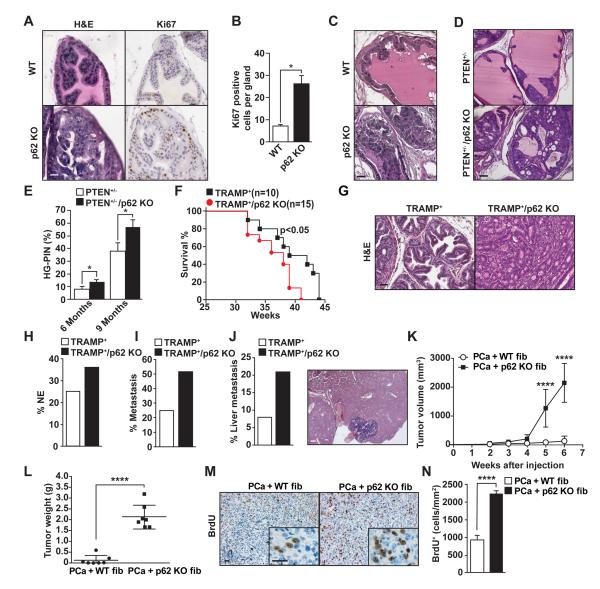
Based on these results we hypothesized that the loss of p62 at an organismal level, which would include both the prostate stroma and epithelium, might be sufficient to drive prostate epithelium towards neoplasia. We characterized the prostates of p62 KO mice by histological analysis, which revealed no abnormalities in development or morphology at early stages. However, at 9 months of age prostates from p62 KO mice developed hyperplasia with a concomitant increase in Ki67 staining (Figures 6A and 6B). These lesions progressed to prostatic intraepithelial neoplasia (PIN) at 1 year of age (Figure 6C). This indicated that, whereas in xenograft experiments PCa epithelial cells with reduced p62 displayed inhibited tumorigenesis (Duran et al., 2011), the total loss of p62 in vivo promoted prostate epithelial cell growth. These observations are in good agreement with our model whereby p62 in the stromal fibroblasts normally acts as a tumor suppressor, and the total KO of p62 results in p62-deficient stromal fibroblasts that drive the prostate epithelium to a malignancy-prone state. To further test this hypothesis, we crossed total p62 KO mice with two well-established mouse models of PCa (PTEN+/− and TRAMP+) (Di Cristofano et al., 1998; Greenberg et al., 1995) and asked whether total ablation of p62 inhibited or promoted prostate tumor development. Figures 6D and 6E show H&E analyses of PTEN+/−/p62 KO prostrates demonstrating an increase in the percentage of glands with high-grade PIN at the age of 6 months. Furthermore, TRAMP+/p62 KO mice had reduced survival (Figure 6F), increased percentage of poorly differentiated adenocarcinoma (Figure 6G) and neuroendocrine tumors (Figure 6H), as well as a higher number of metastases (Figure 6I) of which a higher percentage were in the liver (Figure 6J). Consistent with our model, prostate fibroblasts from PTEN+/−/p62 KO mice showed increased IL-6 and reduced c-Myc expression as compared to those from p62-proficent PTEN+/− mice (Figure S4A-S4C). Interestingly, immunohistochemical (IHC) analysis of prostates from PTEN+/− mice confirmed reduced expression of p62 in the stromal compartment as compared to those from WT mice (Figure S4D). To further support the role of p62 deficiency in the stroma in driving tumorigenesis in vivo, we coinjected syngeneic PCa cells (TRAMP-C2Re3) with WT or p62 KO fibroblasts and assessed the effect that fibroblasts exert on tumor growth. Tumors coinjected with p62 KO fibroblasts grew significantly faster and were larger than tumors in mice injected with WT fibroblasts, consistent with the cell-autonomous tumor promoting activity of p62-deficient fibroblasts on epithelial PCa cells (Figures 6K and 6L). In agreement with this, bromodeoxyuridine (BrdU) incorporation was increased in the p62 KO fibroblast-driven tumors (Figures 6M and 6N).
Figure 6. p62 deficiency accelerates prostate tumor progression in different mouse models of prostate cancer.
(A) Hyperplasia in the prostatic anterior lobe of p62 KO mice. H&E and Ki67 staining of prostates from 9-month-old WT and p62 KO mice (n = 5). (B) Quantification of Ki67-positive cells in the prostate sections shown in A. Results are the means ± SD of counts from 10 different fields per mouse (n = 5). (C) PIN in the dorsolateral lobes of prostates from 12-month-old p62 KO mice. (D) Representative examples of H&E staining of dorsolateral lobes of prostates from PTEN+/− and PTEN+/−/p62 KO mice at 6 months of age (n = 5). (E) Percentage of glands with HG-PIN (n = 5 mice). (F) Kaplan-Meier survival curve of TRAMP+ mice (n = 10), as compared with TRAMP+/p62 KO mice (n = 15). (G) Representative H&E staining of mouse prostate sections from TRAMP+ and TRAMP+/p62 KO mice (n = 10). (H) Incidence of neuroendocrine tumors in TRAMP+ (n = 12) and TRAMP+/p62 KO mice (n = 19). (I and J) Incidence of metastasis (I) and liver metastasis (J) in TRAMP+ compared with TRAMP+/p62 KO mice. (K-M) Coinjection of syngeneic TRAMPC2-Re3 PCa cells with either WT or p62 KO fibroblasts in C57BL/6 mice. (K) Tumor volume assessed at different time points after injection (n = 7 mice). (L) Tumor weight at 6 weeks after injection (n = 7 mice). (M) BrdU staining in tumor sections. (N) Quantification of BrdU positive cells of (M). Results are the means ± SEM (n = 10). *p < 0.05, ****p < 0.001. Scale bars, 25 μm. See also Figure S4.
p62/mTORC1/c-Myc connection in human cancer stroma
To determine whether the identified link between p62 and c-Myc through mTORC1 has relevance to the role of the stroma in human cancer, we used bioinformatics to analyze c-Myc transcript levels in two sets of human gene-expression arrays from prostate and breast cancer stroma. Stroma of human tumors displayed reduced levels of c-Myc (Figure 7A), and there was a statistically significant correlation between c-Myc and p62 expression in tumor stroma (Figure 7B), emphasizing the clinical relevance of the p62-Myc connection in the stroma. To further explore the link between p62 and mTORC1 in the tumor stroma of human cancers, we identified expression neighbors of p62. We developed this gene signature by using the human cancer stroma data set of Figure 7A in which we classified tumors based on p62 expression levels and selected for analysis only those samples in the top and bottom 25%. Interestingly, this analysis revealed a statistically significant correlation between p62 expression and that of genes previously reported to be controlled by mTORC1 activity (Figures 7C and S5) (Pena-Llopis et al., 2011). We determined the expression levels of a selection of these genes by RT-PCR and found that their expression was reduced in p62 KO fibroblasts as compared with WT (Figure 7D). The same results were obtained when these were analyzed in prostate stromal cell preparations from p62 KO and WT mice (Figure 7E). Furthermore, we found a clear statistically significant correlation between p62 expression and that of these genes in human cancer stroma (Figures S5B-S5H). Altogether these results demonstrate that the p62/mTORC1/c-Myc connection is not only relevant in the mouse prostate stroma but it is also important in human cancer stroma.
Figure 7. The p62-Myc-mTORC1 cassette is downregulated in prostate tumor associated stroma in human samples.
(A) Myc levels are downregulated in the tumor stroma of human tissue samples. Data was collated from public datasets of gene expression in tumor stroma in several human cancers. GSE34312 (prostate cancer) and GSE9014 (breast cancer). p value, fold change of expression, and size of sample (n) for each study is indicated in the corresponding panels. (B) Positive correlation between p62 and Myc levels in the stroma. (C) Heat map of mTORC1 signature selected from p62 neighboring genes in human stroma. p62 levels are indicated as SQSTM1. (D and E) RT-PCR analysis of mTORC1 genes in WT and p62 KO fibroblasts (D) and in FACS sorted mouse prostate stromal fraction (E). *p < 0.05, **p < 0.01. ***p < 0.001. Results are presented as mean ± SEM (n = 3). See also Figure S5.
DISCUSSION
Tumorigenesis is a slow process that is initiated by the successive accumulation of genetic and epigenetic changes that result in the activation of cell growth and survival genes and the inactivation of tumor suppressors (Hanahan and Weinberg, 2011). However, for tumor development to take place, initiation is not sufficient. Other signals are required to drive tumor promotion and progression and the development of the fully malignant stage. The progression phase is most likely orchestrated via the tumor microenvironment by non-epithelial cells in which metabolic stress and inflammation create an environment in which epithelial tumor-derived cells propagate and acquire more aggressive phenotypes (Hanahan and Coussens, 2012; Hanahan and Weinberg, 2011). Immune cells, such as tumor-associated macrophages, are among the cell types in the tumor microenvironment that contribute to inflammation (Coussens and Werb, 2002; Johansson et al., 2008). On the other hand, a cross-talk between metabolic pathways in the stromal and epithelial compartments of the tumor may drive the survival and growth of epithelial cancer cells (Lisanti et al., 2013). However, it has not been thoroughly investigated whether metabolic reprogramming in the stromal cells of the tumor microenvironment exerts any control over inflammation and the malignant characteristics of the transformed epithelium.
Here we demonstrate that the inactivation of mTORC1 in p62-deficient stromal fibroblasts results in metabolic reprogramming through c-Myc inactivation (Figure 8). This reprogramming leads to increased levels of IL-6, which promotes epithelial cell invasion and proliferation. Therefore, because of its regulation of mTORC1, p62 emerges as a tumor suppressor that acts by regulating c-Myc and thus inducing an inflammatory response. These results are in marked contrast to the role played by p62 and mTORC1 in epithelial cancer cells. That is, we have recently demonstrated that p62 inactivation in PCa and lung adenocarcinoma epithelial cells inhibits the proliferation and tumorigenic properties of these cells and correlates with decreased mTORC1 activation. Moreover, the increased IL-6 phenotype can be reverted by expression of a permanently active mutant of the mTORC1 activator RagB. This has important implications from a therapeutic point of view since inhibition of p62 and/or mTORC1 may result in opposite effects in the stroma and the epithelium of the tumor, thus reducing the efficacy of broadly applied mTORC1-based chemotherapeutic approaches. In this regard, these results are reminiscent of the dual role that mTORC1 might play as a regulator of autophagy, which can have a tumor-suppressing or a tumor-promoting effect, depending of the stage of the tumor (Guo et al., 2013; Levine and Kroemer, 2008), and also on whether the manipulation takes place in the epithelium or in the stroma (Lisanti et al., 2013). Our data using KO mice clearly reveal that p62 deficiency creates a protumorigenic environment for p62-proficient PCa cells in orthotopic experiments, and also show that, even under normal conditions, it drives PIN formation in the endogenous epithelium in the absence of any other induced mutations. Furthermore, in two PCa models the lack of p62 at an organismal level results in increased tumorigenesis, despite the fact that p62 is absent not only in the stroma but also in the transformed epithelium. These results are very important because they demonstrate that, even though p62 is required for epithelial cancer cells to proliferate in vitro and in xenografts (Duran et al., 2011), the p62-deficient tumor microenvironment overrides the requirement for p62 in the epithelium. Our in vitro and in vivo findings establish that increased IL-6 levels generated by stromal fibroblasts are a critical event in that process. Therefore, it can be predicted that total ablation of p62 at an organismal level, either genetically or pharmacologically, may increase tumorigenesis, rather than inhibiting it, depending on the contribution of the stroma and the ability of p62 deficiency to reprogram stromal metabolism to generate ROS and inflammation. Our data shown here demonstrate that this is the case in prostate tumorigenesis, and suggest that it could be a relevant mechanism in other tumor types as well. The pro-inflammatory microenvironment in the p62-deficient stroma results in a CAF-activated phenotype that is maintained by stromal TGFβ production. This is consistent with previous results in colon cancer demonstrating that a TGFβ-activated tumor microenvironment is critical for fully aggressive cancer cells to metastasize (Calon et al., 2012).
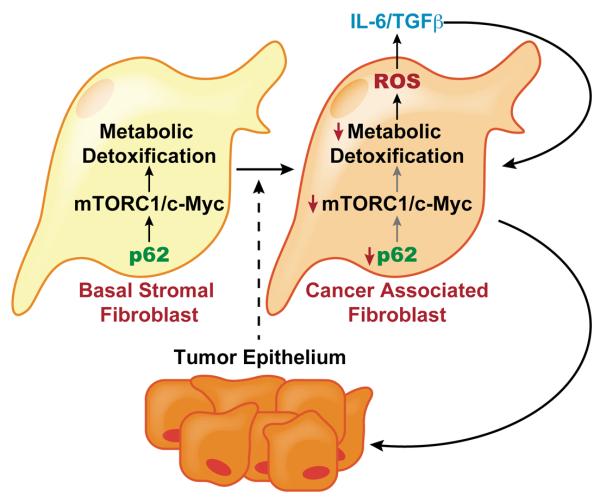
Figure 8. Stromal activation by p62 deficiency in cancer.
Tumor epithelium promotes the downregulation of p62 in stromal fibroblasts leading to reduced mTORC1 activity and c-Myc expression, which results in impaired metabolic detoxification and the subsequent release of ROS and IL-6. An autocrine pathway promotes TGFβ and the induction of CAF phenotype that further increases epithelial invasion and tumorigenesis.
Metabolic reprogramming in cancer is emerging as a central process in tumor cell survival and growth (DeBerardinis and Thompson, 2012; Metallo and Vander Heiden, 2013; Vander Heiden, 2013). The so-called Warburg effect supports the importance of an atypical glucose metabolism tailored to the cancer cell’s need for efficient anabolic utilization of nutrients (Vander Heiden et al., 2009). More recently, different types of reprogramming events have been unveiled that constitute specific responses of the tumor cell to a nutrient-deficient environment. These include the metabolism of serine or the utilization of the pentose phosphate pathway to alleviate oxidative stress conditions during tumorigenesis (Locasale, 2013; Ma et al., 2013; Possemato et al., 2011; Vander Heiden et al., 2010). In the current study, we show that metabolic reprogramming triggered by p62 deficiency in the tumor stroma is critical for the creation of a protumorigenic inflammatory environment driven by IL-6. Moreover, we have shown that this involves a previously unanticipated mTORC1/c-Myc/ROS cascade that is controlled by p62. In this regard, previous results from our and other laboratories have shown that p62 represses ROS by inducing the activation of NF-κB or NRF2-dependent detoxifying molecules (Duran et al., 2008; Komatsu et al., 2010; Ling et al., 2012). Surprisingly, neither of these two transcription factors nor Keap1 levels was affected in p62-deficient stromal fibroblasts, indicating that p62 may use diverse cascades in different cellular compartments of the tumor. Interestingly, our previous data demonstrate that, under conditions of Ras-induced transformation, p62 deficiency leads to increased cell death and reduced tumorigenesis due to enhanced ROS production (Duran et al., 2008). In contrast, we show here that the enhanced ROS observed in the untransformed stromal fibroblasts does not result in increased cell death but, rather, in the creation of a pro-inflammatory phenotype. The main conclusion of these results is that increased ROS production induced by p62 deficiency has different outcomes depending on the cell type and the mechanisms whereby ROS is produced. The outcome also depends on whether or not the levels of ROS are high enough to engage a JNK-driven cell-death pathway, as found in the Ras-tumor cell, as opposed to increased IL-6 production and a pro-tumorigenic effect on epithelial cells, as we demonstrated in the stromal non-transformed fibroblasts.
Importantly, we were able to show that the implication of the p62/mTORC1/c-Myc cascade is not only relevant in mouse model systems but also in human samples where this pathway is inactivated in the tumor stroma. Therefore, our findings support a more comprehensive approach when devising therapeutic strategies in cancer, which should take into account not only the altered pathways in the transformed epithelial compartment but also how the inhibition of these cascades might impact the surrounding stroma. Our observations suggest that pharmacological inhibition of IL-6 and/or TGFβ to target stromal activation could be beneficial in combination with epithelial-targeted therapies.
EXPERIMENTAL PROCEDURES
Mice
Wild-type, p62 KO, PTEN+/−, and TRAMP+ mice were previously described (Duran et al., 2004) (Di Cristofano et al., 1998; Greenberg et al., 1995). All mouse strains were generated in a C57BL/6 background. All mice were born and maintained under pathogen-free conditions. All genotyping was done by PCR. Mice were sacrificed and genitourinary (GU) sections were dissected. Mice were injected with 5-bromo-2′-deoxyuridine intraperitoneally and sacrificed 2 hr after injection. Animal handling and experimental procedures conformed to institutional guidelines (Sanford-Burnham Medical Research Institute Institutional Animal Care and Use Committee).
Cell Lysis and Western immunoblotting
Cells were rinsed once with ice-cold PBS and lysed in RIPA buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM phenyl methyl sulfonyl fluoride, and protease inhibitors). Cell extracts were denatured, subjected to 8%-14% SDS-PAGE, transferred to nitrocellulose-ECL membranes (GE Healthcare) and immunoblotted with the specific antibodies. Chemiluminescence was used to detect the proteins (Thermo Scientific).
Statistical Analysis
Significant differences between groups were determined using the Student’s t-test. Scoring of immunostaining of human prostate tissue microarrays (TMA) was analyzed using Fisher’s exact test. The significance level for statistical testing was set at p < 0.05.
Supplementary Material
HIGHLIGHTS.
p62 levels are reduced in mouse and human tumor stroma
p62 loss in stromal fibroblasts resulted in increased epithelial tumorigenesis cells
p62 regulates stromal inflammation by mTor/c-Myc metabolic reprogramming
Stromal metabolic reprogramming is essential for IL-6-driven epithelial tumorigenesis
SIGNIFICANCE.
Inappropriate activation of the stroma as a consequence of the tumorigenic process can potentiate the growth and transformation of epithelial tumor cells, thus facilitating the progression of cancers towards more malignant stages. Using prostate cancer as a model system we show that the loss of the signaling adapter, p62, in stromal cells triggers an inflammatory response that leads to activation of cancer-associated fibroblasts that enhances tumorigenesis in vitro and in vivo. Deficiency in p62 results in reduced mTORC1 activity and deregulation of metabolic pathways controlling inflammation. Since the stroma is increasingly recognized as a potential source of therapeutic targets, this study suggests that targeting stromal metabolic reprogramming can decisively influence the tumorigenic potential of the tumor epithelial compartment.
ACKNOWLEDGEMENTS
NIH Grants R01CA132847 (J.M.), R01CA172025 (J.M.), R01CA134530 (M.T.D.-M.), and 5P30CA030199 (M.T.D-M. and J.M.) funded this work. Additional support was provided by DoD Grants W81XWH-13-1-0353 (M.T.D-M.), W81XWH-13-1-0354 (J.M.) and W81XWH-13-1-0105 (C.M.M.). We thank Maryellen Daston for editing this manuscript, Diantha LaVine for the artwork, and Tomoko Yajima, Tom Hudson, Jessica Leung and the personnel of the Cancer Metabolism, Flow Cytometry, Cell Imaging, Animal Facility, Histology, Functional Genomics and Viral Vectors Shared Resources at SBMRI for technical assistance. We thank Neil Bhowmick for assistance in preparation of prostate fibroblasts.
Footnotes
ACCESSION NUMBERS The GEO accession number for the microarray data reported in this paper is GSE55587.
AUTHOR CONTRIBUTIONS T.V., J.Y.K., S.A.-B. and A.D. performed experiments; J.M.-P. and M.R.-C. performed bioinformatics analysis; E.A. provided pathologist expertise for histological analysis; C.S.A. and T.V. performed the metabolic experiments; C.M.M., M.D.M., and J.M. designed and analyzed metabolic data; M.D.M. and J.M conceived and supervised the project with equal contribution; M.D.M. and J.M. wrote the manuscript with assistance from all the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo A, Cunha V, Teixeira AL, Medeiros R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World journal of clinical oncology. 2011;2:384–396. doi: 10.5306/wjco.v2.i12.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. The Journal of membrane biology. 1986;89:1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer. 2012;19:R187–204. doi: 10.1530/ERC-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nature reviews Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nature reviews Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco MT, Moscat J. The atypical PKCs in inflammation: NF-kappaB and beyond. Immunol Rev. 2012;246:154–167. doi: 10.1111/j.1600-065X.2012.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 Is a Key Regulator of Nutrient Sensing in the mTORC1 Pathway. Mol Cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The Signaling Adaptor p62 Is an Important NF-[kappa]B Mediator in Tumorigenesis. Cancer Cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Duran A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP, Moscat J, Diaz-Meco MT. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004;6:303–309. doi: 10.1016/s1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Franco OE, Hayward SW. Targeting the tumor stroma as a novel therapeutic approach for prostate cancer. Adv Pharmacol. 2012;65:267–313. doi: 10.1016/B978-0-12-397927-8.00009-9. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Galavotti S, Bartesaghi S, Faccenda D, Shaked-Rabi M, Sanzone S, McEvoy A, Dinsdale D, Condorelli F, Brandner S, Campanella M, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32:699–712. doi: 10.1038/onc.2012.111. [DOI] [PubMed] [Google Scholar]
- Gout PW, Kang YJ, Buckley DJ, Bruchovsky N, Buckley AR. Increased cystine uptake capability associated with malignant progression of Nb2 lymphoma cells. Leukemia. 1997;11:1329–1337. doi: 10.1038/sj.leu.2400739. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–1219. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer treatment reviews. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. The Journal of cell biology. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Molecular pharmacology. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Inoue D, Suzuki T, Mitsuishi Y, Miki Y, Suzuki S, Sugawara S, Watanabe M, Sakurada A, Endo C, Uruno A, et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760–766. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Valencia T, Abu-Baker S, Linares J, Lee SJ, Yajima T, Chen J, Eroshkin A, Castilla EA, Brill LM, et al. c-Myc phosphorylation by PKCzeta represses prostate tumorigenesis. Proc Natl Acad Sci U S A. 2013;110:6418–6423. doi: 10.1073/pnas.1221799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shen C, Nakamura E, Ando K, Signoretti S, Beroukhim R, Cowley GS, Lizotte P, Liberzon E, Bair S, et al. SQSTM1 Is a Pathogenic Target of 5q Copy Number Gains in Kidney Cancer. Cancer Cell. 2013;24:738–750. doi: 10.1016/j.ccr.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle. 2013;12:2723–2732. doi: 10.4161/cc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature reviews Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, Castilla EA, Chen J, Yajima T, Porollo A, et al. Control of Nutrient Stress-Induced Metabolic Reprogramming by PKCzeta in Tumorigenesis. Cell. 2013;152:599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Vander Heiden MG. Understanding metabolic regulation and its influence on cell physiology. Mol Cell. 2013;49:388–398. doi: 10.1016/j.molcel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. Feedback on fat: p62-mTORC1-autophagy connections. Cell. 2011;147:724–727. doi: 10.1016/j.cell.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–236. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Nystrom ML, Thomas GJ, Stone M, Mackenzie IC, Hart IR, Marshall JF. Development of a quantitative method to analyse tumour cell invasion in organotypic culture. J Pathol. 2005;205:468–475. doi: 10.1002/path.1716. [DOI] [PubMed] [Google Scholar]
- Olson MV, Lee J, Zhang F, Wang A, Dong Z. Inducible nitric oxide synthase activity is essential for inhibition of prostatic tumor growth by interferon-beta gene therapy. Cancer Gene Ther. 2006;13:676–685. doi: 10.1038/sj.cgt.7700941. [DOI] [PubMed] [Google Scholar]
- Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, Xie XJ, Corey DR, Brugarolas J. Regulation of TFEB and V-ATPases by mTORC1. Embo J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med. 2010;16:1450–1455. doi: 10.1038/nm.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland P, Madjd Z, Durrant L, Ellis IO, Layfield R, Spendlove I. The ubiquitin-binding protein p62 is expressed in breast cancers showing features of aggressive disease. Endocr Relat Cancer. 2007;14:73–80. doi: 10.1677/erc.1.01312. [DOI] [PubMed] [Google Scholar]
- Sanchez P, De Carcer G, Sandoval IV, Moscat J, Diaz-Meco MT. Localization of atypical protein kinase C isoforms into lysosome- targeted endosomes through interaction with p62. Mol Cell Biol. 1998;18:3069–3080. doi: 10.1128/mcb.18.5.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008;72:205–213. doi: 10.1016/j.urology.2007.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes & development. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HG, Harris JW, Wold BJ, Lin F, Brody JP. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene. 2003;22:2322–2333. doi: 10.1038/sj.onc.1206325. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG. Exploiting tumor metabolism: challenges for clinical translation. J Clin Invest. 2013;123:3648–3651. doi: 10.1172/JCI72391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.