Abstract
Atherosclerosis, the major pathological process through which arterial plaques are formed, is a dynamic chronic inflammatory disease of large and medium sized arteries in which the vasculature, lipid metabolism, and the immune system all play integral roles. Both the innate and adaptive immune systems are involved in the development and progression of atherosclerosis but myeloid cells represent the major component of the burgeoning atherosclerotic plaque. Various myeloid cells, including monocytes, macrophages, and dendritic cells can be found within the healthy and atherosclerotic arterial wall, where they can contribute to or regulate inflammation. However, the precise behaviors and functions of these cells in situ are still active areas of investigation that continue to yield exciting and surprising new data. Here, we review recent progress in understanding of the complex biology of macrophages and dendritic cells, focusing particularly on the dynamic regulation of these subsets in the arterial wall and novel, emerging functions of these cells during atherogenesis.
Keywords: atherosclerosis, dendritic cell, macrophage, inflammation, immune response
Introduction
Cardiovascular diseases (CVD) are the most frequent cause of mortality and morbidity worldwide,1 however atherosclerosis, the major etiological process that accompanies CVD, has plagued humanity at least since classical antiquity.2 The specific term atherosclerosis was introduced by Felix Marchand in 1904, who suggested that atherosclerosis was responsible for almost all obstructive processes in the arteries.3 With pioneering work from I. Ignatowski, A. Windaus, and N. Anichkov in the early 1900s, a critical link between cholesterol and atherosclerosis was proposed.3 With the turn of the century, numerous studies have demonstrated that cholesterol and lipoproteins play a critical role in the development of atherosclerosis. The conceptual association between immune cells and the pathogenesis of atherosclerosis was raised by Virchow as early as 1856;4 however, this hypothesis did not crystallize until the latter half of the 20th century. In 1973, R. Ross and colleagues suggested the response to injury model, in which, localized injury within the artery was proposed to initiate the accumulation of smooth muscle cells, resulting in narrowing the arterial lumen.5 Conceptually, today atherosclerosis is a multifactorial disease of large and medium size vessels6-9 in which cholesterol rich lipoproteins are slowly retained within the arterial wall in conjunction with localized activation of vascular cells and the accumulation of immune cells (Fig.1).
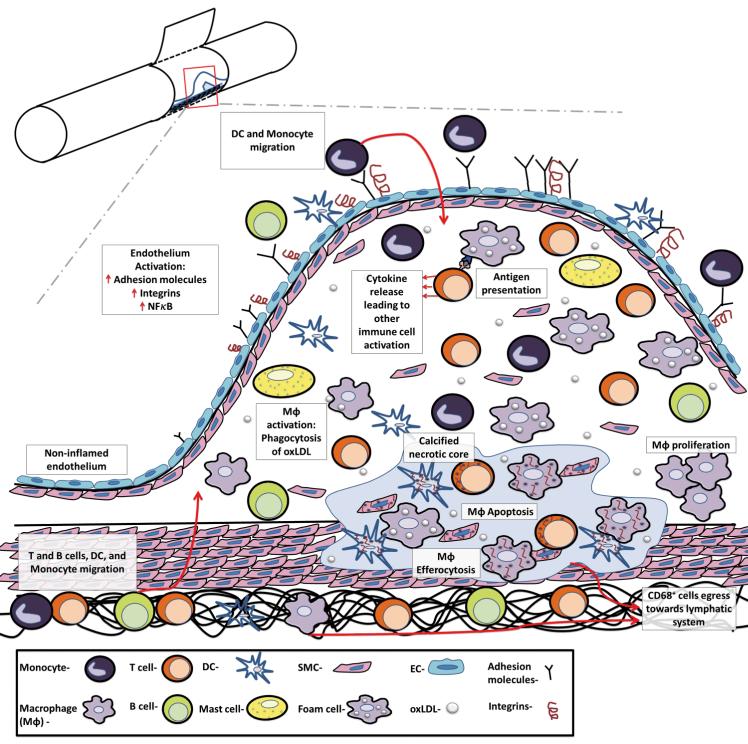
Figure 1.
The major steps in the development and progression of atherosclerosis. Atherosclerosis is a progressive, multifactorial inflammatory disease that encompasses endothelial activation, leukocyte recruitment, MΦ activation and proliferation, and antigen presentation to T cells and ultimately results in elevated pro-inflammatory cytokine production, inflammation, and necrosis. Atherosclerosis is initiated by the deposition, retention, and modification of LDL in the intima, resulting in NF-κB–dependent activation of the endothelium, the expression of adhesion molecules, chemokines, and the recruitment of Ly6Chi monocytes and pre-DCs to the intimal layer. Ly6Chi monocytes may subsequently differentiate to MΦ or possibly DCs. MΦs are the prominent cell type within the arterial wall, and several arterial macrophage subsets (M1, M2, M4, and Mox MΦ, Figure 2) have been observed. Dendric cells and DC subsets (Flt3-dependent DCs, Flt3-independent DCs, and pDCs, not shown), while far less abundant, are similarly present. MΦs and DCs scavenge for modified lipoproteins (or other potential self-antigens) within the arterial lumen by scavenger receptors and Toll-like receptors, and may present antigens to T cells to activate intra-plaque T helper (Th) 1 and Th17 cells, and thus to further support leukocyte recruitment to the nascent plaque. Once activated, arterial MΦs become either pro-inflammatory proteolytic M1 MΦs, releasing IL-12, IL-1β, and TNF-α, or less inflammatory M2 MΦs, releasing IL-10 and TGF-β. DC subsets also support atherosclerotic low grade inflammation by the release of type 1 interferons, and activation/modulation of T cell responses. B cells, which are also present in atherosclerotic plaques, may support or suppress inflammation through the production of antibodies and cytokines. As MΦs start to proliferate and as a result of persistent foam cells formation and reduced efferocytosis, the nascent plaque becomes an advanced atherosclerotic lesion, resulting in the formation of a calcified necrotic core. Upon the resolution of hypercholersterolemia, CD68+ cell egression from the plaque through adventitial lymphatic vessels and efficient efferocytosis might result in regression of the lesion.
Several populations of immune cells including B and T lymphocytes, eosinophils, macrophages (MΦ), dendritic cells (DCs), neutrophils, and mast cells are involved in the progression of atherosclerosis, both within the arterial wall and secondary lymphoid tissues.10 Atherosclerosis develops in several stages. One of the initial steps in atherosclerosis is the accumulation of modified low density lipoproteins (LDLs) within the intima of arteries and their uptake by arterial MΦ, which quickly become the major leukocyte population within nascent atheroclerotic plaques.9 These events trigger the subsequent activation of endothelial and smooth muscle cells, resident lymphocytes, DCs, and elicits massive production of pro-inflammatory mediators, which accelerates leukocyte recruitment, induces chronic, low-grade inflammation, and likely an antigen-specific adaptive immune response.8 To date, it remains unclear whether the antigen-presentation occurs primarily in the arterial wall or in secondary lymphoid tissues, or at both sites. Advanced atherosclerotic lesions are complex and characterized by large necrotic cores, elevated levels of cytokines, tissue factors, matrix proteases, and lesional apoptotic cells—mostly macrophages.11 Unstable calcified necrotic cores may subsequently rupture, leading to the formation of an intravascular blood clot, which may result in a stroke or myocardial infarction.
In this review, we will discuss our current understanding and recent advances on the roles of MΦs and DCs in atherosclerosis.
Monocyte maturation and migration to and potentially from atherosclerotic plaques
As MΦs and DCs play key roles in the pathology of atherosclerosis, monocytes and the processes underlying their recruitment to the arterial wall have been the subject of intense research.12 Monocytes originate from monocyte-dendritic cell precursors13 during hematopoiesis within the bone marrow, and they subsequently emigrate from the bone marrow to the blood in a CCL2-14 and CCL7-dependent manner.15 Following their maturation, several subsets of monocytes exists both within mice and humans, subsets that can be characterized based on their functions in vivo and on variable expression of surface markers.15 In mice, two distinct monocyte subsets exist: Ly6Chigh pro-inflammatory monocytes that are CD11b+ CD115int F4/80int-low CD68int Gr1+ (Ly6G/Ly6C) and express high levels of CCR2, CCR5, CCR1, low levels of CX3CR1, CD62L, and rely on CCL2/CCR2 for egression from the bone marrow;14 and a Ly6Clow “patrolling” subset of monocytes that are CD11b+ CD115int F4/80int-low CD68int and express high levels of CX3CR1 and low levels of CCR2.16 These two murine monocyte subsets and their activities are functionally reminiscent of human CD14+ CD16− and CD14dim CD16+ monocytes, respectively.15
Ly6Chi monocytes have been a major subset of interest in atherosclerosis as Ly6Chi monocytes are the first subset recruited to developing atherosclerotic plaques.17,18 Indeed, immigrated Ly6Chi monocytes, upon differentiation in situ, may produce TNF-α, IL-12, IL-6, and iNOSs,18 and many CD11b+ CD11c+ cells that arise from immigrated Ly6Chi monocytes strongly express MHCII, CD80, and CD86 and may become professional antigen-presenting cells.18;19 In contrast, Ly6Clo patrolling monocytes do not efficiently extravasate through the arterial wall. Instead, Ly6Clo monocytes crawl along the luminal surface of arteries and capillaries in a CX3CL1/CX3CR1– and LFA-1–dependent manner.20 The function of Ly6Clo monocytes seems to be surveillance, as Ly6Clo monocytes actively sense for nucleic acids and viruses via Toll-like receptors (TLR) 7 and 8, and importantly they help recruit neutrophils to infected sites of the endothelium.21 However, the role of Ly6Clo monocytes in atherosclerosis is unclear, as mice that lack Ly6Clo monocytes due to deficiency of Nur77 (Nr4a1−/−) and which are prone to athrosclerosis due to deficiency of apolipoprotein E (Apoe−/−) display accelerated atherosclerosis;22 on the other hand, Nr4a1−/− Ldlr−/− (i.e., Nur77- and low density lipoprotein–deficient) bone marrow chimeric mice demonstrate no change in plaque burden.23 Interestingly, the deficiency of NR4A orphan nuclear receptor NOR1 reduces atherogenesis24. Future work should be focused at delineating the cell subsets-specific effects of NR4A nucler receptors in monocytes and vascular cells.
In the context of atherosclerosis and hypercholesterolemia, several lines of evidence have demonstrated that the development of monocytosis, and monocyte recruitment to the artery, play key roles in the initiation and progression of atherosclerosis.10;12;15 Seminal studies examining the role of monocytes in atherosclerosis through the use of knockout mice demonstrated that the recruitment of Ly6Chi monocytes via CCR2, CX3CR1, and CCR5, are necessary for the initial establishment of atherosclerotic plaques.17;25;26 Several reports suggested a unique role for the CX3CL1/CX3CR1 axis in the regulation of both survival and recruitment of monocyte subsets into aortas.27;28 Additionally, in murine models of experimental atherosclerosis, hypercholesterolemia induces monocytosis within the bone marrow, blood, and in the spleen.29 In atherosclerosis-prone Apoe−/− mice and Ldlr−/− mice, hypercholesterolemic conditions can promote colonization of hematopoietic stem cells (HSCs) and extramedullary hematopoiesis of monocytes within the spleen that may give rise to functional Ly6Chi monocytes.29;30 In addition, recent work with Abca1−/− Abcg1−/− (two genes expressing ATP-binding cassette transporters) mice and Apoe−/− mice has demonstrated that components of the cholesterol efflux pathway, notably ApoE, in addition to Abca1 and Abcg1 may promote hypercholesterolemia-induced monocytosis.31;32 Together, these data further demonstrate the importance of monocytes recruitment to site(s) of vascular inflammation.
While the contribution of Ly6Chi monocytes in atherosclerosis is clear, the potential role(s) of Ly6Clo monocytes are uncertain and future work should help to clarify the extent of their involvement. Recently, however, the paradigm that MΦs in atherosclerotic aortas arise primarily from recruited monocytes has been challenged. Several lines of emerging data suggest that while monocyte recruitment is required for the formation of early atherosclerotic lesions, in situ proliferation33 and survival27 of MΦs may account for the majority of these cells found in advanced atherosclerotic plaques. In addition, a recent study by Jakubzick, et al. demonstrated that not all lung- and skin-infiltrating monocytes differentiate into MΦs or DCs in homeostasis.34 Instead, these tissue monocytes acquire antigen and migrate to the draining lymph nodes. The potential importance of monocyte infiltration and antigen-laden monocyte efflux to draining lymph nodes in the context of chronic or robust inflammation is unclear, as monocytes are not efficient antigen presenting cells and antigen-laden monocytes do not seem to differentiate to MΦs within the draining lymph node. To date, the stimuli that induce the differentiation of tissue-monocytes to either DCs or MΦs need to be investigated. The impact of this newly discovered phenomenon on the understanding of chronic inflammatory diseases, including atherosclerosis, remains to be elucidated.
Another important question pertaining to the condition of atherosclerosis and monocyte recruitment concerns the removal of plaque-associated CD68+ myeloid cells during the resolution of inflammation. In atherosclerotic mice, lesion regression through the normalization of hypercholesterolemia or the transplantation of atherosclerotic aortic segments to wild-type aortas35-38 has been hypothesized to occur either by reduced monocyte accumulation37 or by increased efflux of CD68+ myeloid cells—reminiscent of MΦs—from the plaque via CCR7/CCL19 and CCL21.39-41 Additional data within the last few years have strengthened both hypothesized mechanisms of action. Gautier and colleagues demonstrated that in an acute model of peritonitis, MΦ removal occurs primarily through apoptosis and that only a minor number of cells migrated to draining lymph nodes in a CCR7-dependent manner.42 In contrast, other studies have proposed that the neural guidance cues Netrin-1, Semaphorin 3A, and ephrin B might play a role in retaining CD68+ MΦs within atherosclerotic plaques, thereby preventing their egress via CCR7.43-46 These neural guidance cues are likely hypoxia-induced; and several lines of data indicate that they serve as a trap signals for MΦs to stay within the atherosclerotic aorta. Interestingly, as netrin-1 deficiency in endothelial cells supports transendothelial migration, endothelial netrin-1 may potentially be atheroprotective.44
While the above reports propose opposite mechanisms of action for the accumulation of myeloid cells within atherosclerotic plaques, alternative hypotheses might also explain the results. Accelerated monocyte and DC migration plays a key role in the establishment of early atherosclerotic plaques, whereas low grade chronic plaque inflammation also enables CD68+ cell egress from the arterial wall. Given recent data that monocytes may, in homeostatic conditions, enter and leave peripheral tissues via the lymphatics,34 and that early atherogenesis depends heavily on monocyte recruitment, Netrin-1 and neural guidance cues may play a role in initially attracting and retaining monocytes within early atherosclerotic plaques, thereby preventing their egression via the lymphatics. Alternatively, Netrin-1 might play a subset-specific role on retaining immigrating monocytes or emigrating CD68+ macrophage-like cells during atheroregression, thereby affecting efferocytosis in the resolving plaque. Ultimately, further studies examining both proposed mechanisms of action, and how they might relate to recent studies concerning monocyte recruitment and emigration34 and lesional MΦ proliferation,33 are required to clarify the mechanisms behind arterial myeloid cell accumulation within the aorta.
Many faces of macrophages
MΦs are key participants in both the induction of an innate immune response and the resolution of inflammation. Although there are several cell surface markers commonly expressed by MΦs, the phenotype of MΦs within different tissues varies, and is likely affected by the local milieu.47;48 There are at least two major subsets of MΦs that can be induced by a distinct set of stimuli; these subsets can enriched into relatively pure populations based on the expression of pro-inflammatory cytokines, scavenger and chemokine receptors, and their phagocytic, microbicidal, and tumoricidal activities.49;50 M1 macrophages are classically activated by interferon gamma (IFN-γ) and/or microbial products such as lipopolysaccharides (LPS);49;51 these macrophages play an important role in defending against a broad spectrum of pathogens but also are involved in some autoimmune diseases.50 The M1 phenotype is characterized by high expression of inducible nitric oxide synthase (iNOS), MHCII, IL-12, IL-1β, tumor necrosis factor α (TNF-α), and costimulatory molecules CD80 and CD86.52-55 M2 macrophages are alternatively activated and induced in vitro from exposure to Th2 cytokines IL-4 and IL-13, to immune complexes, or to glucocorticoids;51;56 they are characterized by high expression of macrophage mannose receptor (CD206), IL-10, arginase-1, increased secretion of collagen, and low expression of IL-12, co-stimulatory molecules, and MHCII;49;56;57 and they are involved in tissue remodeling, resolution of inflammation, and tumorgenesis.49;50
An increasing body of evidence suggests MΦ heterogeneity within the aortic wall, as there are a variety of expressed markers and functions of MΦs within atherosclerotic plaques.58 Although in vitro–generated MΦs display a distinct phenotype, MΦs isolated from multiple peripheral tissues demonstrate complex, mixed phenotypes, which probably reflects the diversity of local environmental factors and the potential plasticity of MΦs in vivo. The origins of M1 and M2 MΦs within atherosclerosis have been an area of debate. Several reports have suggested that Ly6Chigh monocytes might preferentially differentiate into M1s,17;18;25 while Ly6Clow monocytes may give rise to wound-healing M2s within the arterial wall. However, there is only limited evidence that supports this hypothesis; further studies are needed to confirm this concept. While, numerous reports suggest the existence of MΦs subsets within the aorta, the mechanisms that govern how MΦs differentiate and polarize in situ are unclear. Microenvironmental cues, such as pathogens, foreign substances, apoptotic cells, modified LDL, and local cytokines, likely play key roles in MΦ subset polarization. Within aortas, macrophage colony-stimulating factor (M-CSF) released from SMCs59 and local pro-inflammatory cytokines likely drive monocyte differentiation into either aortic MΦs or subsets of aortic DCs in the early stages of atherosclerosis. While M2 MΦs are detected within early atherosclerotic lesions, more advanced lesions of Apoe−/− mice contain an enlarged population of aortic M1 MΦs.60 M1 MΦs are also found aortas of high fat diet–fed Ldlr−/− mice,61 and CCL2+ CD206− M1 MΦs are detected in human endartectomy specimens.62 Recently, two additional MΦ phenotypes were described: M4 and Mox MΦs. In response to CXCL4, macrophages expressed a unique profile comprised of elevated IL-6, TNF-α, CCL18, and CCL22, but low IL-10, a profile distinct from M1 and M2 MΦ transcriptomes. These CXCL4-treated macrophages were termed M4 macrophages.63;64 Interestingly, CXCL4 deficiency results in attenuated atherosclerotic plaque burden without affecting platelet activation, suggesting a pro-atherogenic role for both CXCL4 and M4 macrophages.65
Oxidized phospholipids may induce another emerging MΦ subset: Mox.61 Mox MΦs express a variety of genes, notably including heme oxygenase-1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2)–dependent redox regulatory genes. Mox MΦs are found within the atherosclerotic aortas of Ldlr−/− mice, and constitute 30% of the total aortic MΦ population.61 The deficiency of Nrf2 protects against atherogenesis in Apoe−/− mice;66-68 however, Nrf2 deficiency in bone marrow results in earlier accelerated atherosclerosis, possibly via increased formation of foam cells.69 Further studies are needed to address the functions of Mox MΦs, particularly whether Mox MΦs are an important component of the efferocytosis machinery within the necrotic core; whether M1 and M2 macrophages might “re-differentiate” into Mox MΦs in vivo in the presence of oxidized lipids; and what the precise role(s) of Mox MΦs are during atherogenesis.
Involvement of macrophage subsets in atherosclerosis
The important role of MΦs in the development and progression of atherosclerosis has been established for many years. However, recent advances in monocyte and MΦ cell biology have provided new insights on the impact of arterial MΦs.
MΦs were the first immune cells associated with atherosclerosis. Aortic MΦs express a broad range of pattern recognition receptors (PRRs), including TLRs,70 and under inflammatory conditions MΦs may cooperatively induce an innate immune response. Thus, lipoprotein uptake by aortic MΦs within the atherosclerosis-prone areas of the lesser curvature and arterial branching points is one of the first, crucial steps in the initiation of atherosclerosis. Scavenger receptors, particularly scavenger receptor A1 (SR-A1) and CD36, are primarily responsible for the uptake of modified LDL and formation of foam cells.71;72 The uptake of moderately oxidized LDL by CD36 activates heterodimeric TLR4/TLR6 complexes and activates the NF-κB pathway,73 whereas oxidized phospholipids and saturated fatty acids induce endoplasmic reticulum (ER)-stress and apoptosis via activation of CD36/TLR2-dependent pathways.74 SR-A1 (Msr1−/−) and CD36 (Cd36−/−)-deficient Apoe−/− mice display increased aortic sinus lesions, characterized by elevated MΦ foam cell content,75 suggesting alternative MΦ lipid scavenging mechanisms. In contrast, Manning-Tobin et al. demonstrated that the loss of SR-A and CD36 activity reduced atherosclerotic lesion complexity but had no effects on foam cell formation.76 The traditional view is that modified LDL uptake by macrophages induces MΦ activation and up-regulation of macrophage-derived pro-inflammatory stimuli, such as pro-inflammatory cytokines, chemokines, matrix metalloproteinases, as well as apoptosis and defective efferocytosis (Fig. 2). Accumulation of intracellular cholesterol also results in the activation of the inflammasome and subsequent IL-1β maturation.77;78 Interestingly, a recent study demonstrated that murine peritoneal foam cell formation deactivates pro-inflammatory gene expression, resulting in the suppression of inflammation.79 However, it is unclear whether this phenomenon occurs within the arterial wall; additional studies are needed.
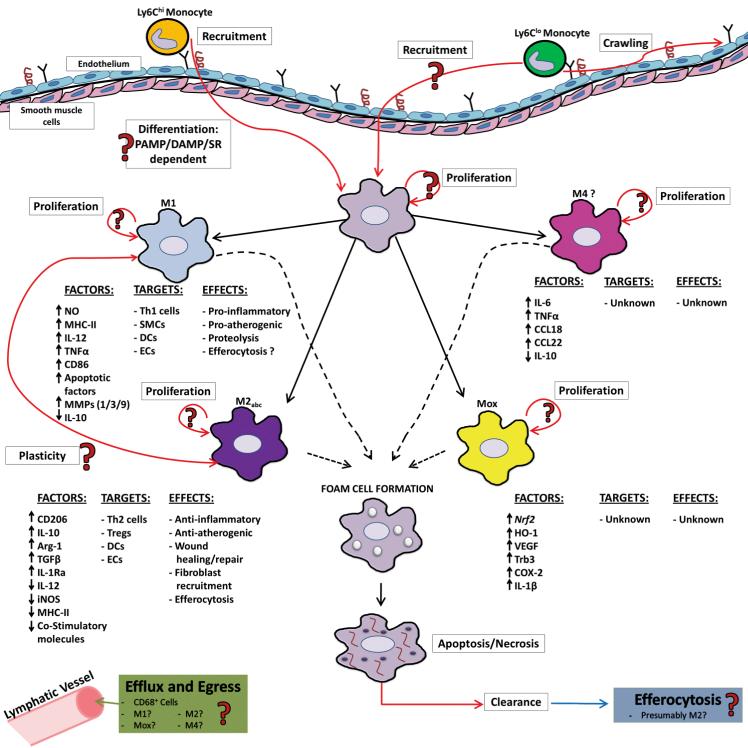
Figure 2.
The many faces of arterial macrophages. Upon atherosclerosis-prone conditions, monocyte subsets, which display different migratory behaviors, are recruited to atherosclerotic lesions. While Ly6Chi monocytes have been demonstrated to migrate to the intima, whether Ly6Clo monocytes enter the intimal layer is unclear. Based on local microenviromental cues, recruited monocytes differentiate into intimal MΦs (or DCs); although the efficiency of this process is not entirely clear. Intimal macrophages may then polarize to different MΦ subsets, namely M1, M2, Mox, or M4 MΦ subsets, where they presumably play different roles in atherosclerosis. The predominate subset of MΦs within the lesions are M1 MΦs, which produce pro-inflammatory factors that may lead to the activation of Th1 cells, SMC, DCs, and ECs, thereby supporting atherogenesis. M2 MΦs up-regulate anti-inflammatory factors and down-regulate pro-inflammatory factors, suggesting that M2 MΦs are anti-atherogenic. The potential functions of the newly discovered macrophage subsets Mox and M4 are largely unknown. Plaque MΦs, upon phagocytizing oxLDL, become foam cells; however, it is unknown whether all MΦ subsets can become foam cells. Foam cells eventually undergo apoptosis and then necrosis, resulting in the formation of a necrotic core. Efferocytes within the plaque, likely M2 MΦs, may play a role in the removal of apoptotic cells. In the atheroregressive environment, CD68+ cells may potentially egress from the plaque through adventitial lymphatic vessels. Similarly, recent data suggests that MΦs may proliferate within atherosclerotic plaques, although it is unclear which subset(s) of MΦs are proliferative.
Taking into account their pro-inflammatory properties, M1 MΦs are thought to play a pro-atherogenic role via the release of pro-apoptotic factors TNF-α and NO, thereby supporting smooth muscle cell and lymphocyte apoptosis80;81 and the formation of a calcified necrotic core. In addition, potential M1-released TNF-α, IL-1β, and IL-6 can activate endothelial and smooth muscle cells, with a subsequent chemokine release by vascular cells, induce endothelial dysfunction via down-regulation of endothelial eNOS expression, and support ROS/RNS-driven oxidative stress.82 M1 MΦ may also contribute to fibrous cap thinning and plaque destabilization through the release of matrix metalloproteinases (MMPs), mainly MMP1, 3 and MMP9, which assist in extracellular matrix degredation.83 Although it is unclear whether antigen presentation occurs primarily within the aortic wall or within secondary lymphoid organs, or both, one of the major M1 macrophage cytokines, IL-12, helps to direct pro-atherogenic T helper 1 (Th1) differentiation and maintenance.49-51 Recently, significant advances in the network of transcriptional factors that support the differentiation of macrophage-specific subsets has been made, and several transcriptional factors which are necessary for M1 or M2 polarization were identified. IRF5 plays a critical role in M1 MΦ polarization and functions as a transcriptional repressor for the IL-10 gene.84 Further studies with the use of specific knock-out models, for example, with IRF5-deficient mice, may help to identify precise roles of M1 MΦ in atherosclerosis.
The role of M2s in atherosclerosis is more controversial. M2 MΦs mainly produce anti-inflammatory cytokines, such as IL-10 and TGF-β, which may potentially alleviate the severity of inflammation.52;82 Furthermore, M2-related cytokines may support the differentiation or maintenance of Th2 or regulatory T cell subsets. M2 MΦ-derived IL-4, IL-13, and IL-10, through their immunosuppressive effects on T cell and MΦ activation, may result in decreased endothelial cell and smooth muscle cell activation; leading to decreased pro-inflammatory chemokine expression, increased endothelial eNOS, vasorelaxation, and smooth muscle cell proliferation.82 The transcription factor Kruppel-like factor 4 (KLF4) supports the M2 MΦ polarization and suppresses the M1 activation program via cooperation with Stat6 and attenuation of NF-κB activation.85 Importantly, KLF4 deficiency in Apoe−/− mice enhanced pro-inflammatory activation and foam cell formation in response to oxidized lipids, and consequently, increased plaque burden. Thus, there is an increasing body of evidence suggesting an important atheroprotecitve role for M2 MΦs. One interesting topic that remains to be investigated is whether MΦs display plasticity and may alter their phenotype in situ (Fig. 2). MΦ phenotypes can be reversed in vitro and in vivo;86;87 however, the potential degree of macrophage plasticity in atherosclerosis is unclear. Future studies using macrophage subset-specific reporter or lox-cre atherosclerotic mouse models may lead to a better understanding of MΦ differentiation and plasticity in the context of atherosclerosis.
Re-emerging myeloid cell paradigms in atherosclerosis: proliferation and efferocytosis
Seminal studies demonstrating that atherosclerosis results in the development of monocytosis within circulation, and that monocytes may infiltrate atherosclerotic lesions to give rise to new arterial macrophages,18;25;26 have solidified the conclusion that monocytes and MΦ play an important role in atherogenesis. Indeed, the roles of monocytes—their recruitment and resulting effect(s)—on macrophage content within inflammatory foci has been a major area of research for decades.88 However, the assumption that all in situ MΦs in inflammation arise from the differentiation of recruited monocytes has recently been challenged. Several lines of emerging data suggest that MΦs may strongly proliferate in situ.
Seminal work by Jenkins et al., demonstrated that local MΦ proliferation, specifically M2 MΦ proliferation rather than monocyte recruitment, is the primary mechanism responsible for the accumulation of MΦ in the context of pleural infection by Litomosoides sigmodontis, rodent filarial nematode.89 Several other groups have similarly demonstrated that MΦ proliferation occurs in situ during both homeostatic and inflammatory conditions89-92 and might lead to the outgrowth of MΦs beyond physiologic levels,91;92 as seen in Th1- and Th17-driven pathologies.90;93 Data from the aforementioned studies suggest that MΦ proliferation can be either macrophage-colony stimulation factor (M-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF)–dependent or IL-4–dependent; however the precise mechanisms governing MΦ proliferation are still being investigated.
Given the preponderance of MΦs in atherosclerosis, the strong association of MΦ accumulation and plaque growth, the early evidence demonstrating that arterial macrophages may proliferate,94-96 and the emerging data demonstrating that tissue-resident macrophages may strongly proliferate in situ rather than relying on monocyte recruitment, Robbins and colleagues33 recently re-examined the importance of this phenomenon in atherosclerosis. Using Apoe−/− mice and pulse-chase BrdU labeling of proliferating cells, Robbins et al. demonstrated that proliferating aortic MΦs are a major component of the plaque MΦ depot, ranging from 28 ± 3% of the proliferating MΦs in relatively early atherosclerotic plaques to 92 ±1% of the proliferating MΦs in established atherosclerotic plaques. The accumulation of aortic MΦs within established atherosclerotic plaques is largely due to in situ proliferation, as monocytes do not readily differentiate to macrophages within atherosclerotic aortas or in the system of parabiotic congenic CD45.1 and CD45.2 Apoe−/− mice. Importantly, in Apoe−/− mice with early atherosclerotic lesions monocyte recruitment was found to be the primary mechanism through which MΦs accumulate, suggesting that, at some point, there is a switch from active recruitment of monocytes to local in situ MΦ proliferation and, possibly, CX3CL1-dependent survival27 as the major mechanism by which MΦs accumulate in atherosclerotic plaques.
The signals that induce MΦ proliferation are puzzling; however, oxLDL may be involved. A recent report suggested that SRA may play a role in aortic MΦ proliferation in atherosclerosis;33 but it is likely not the sole mechanism, as SRA-deficient aortic MΦs were found to proliferate, albeit at a greatly reduced rate.
Taken together, these emerging data sets demonstrate that aortic MΦs survive and proliferate in a lesion stage-dependent manner during atherogenesis. However, the precise kinetics of this process, and whether proliferating macrophages are a particular macrophage subset or are tissue-resident or monocyte-derived macrophages, are exciting questions that have yet to be addressed.
Macrophages and efferocytosis
The prompt and efficient clearance of apoptotic cells from sites of injury or inflammation is necessary for preventing secondary necrosis of apoptotic cells, avoiding immune responses to self-antigens, and resolving inflammation. As atherosclerosis is characterized by the accumulation of macrophages, foam cells, and the development of a large necrotic core, the removal of apoptotic and necrotic cells via efferocytosis has been a keen field of interest. Indeed, atherosclerosis was intially associated with abnormal efferocytosis. Bennett, M. et al.97 observed high rates of apoptotic vascular smooth muscle cells, and Geng, Y. et al.98 demonstrated impairment in the clearance of necrotic cells within atherosclerotic lesions. Efferocytosis, derived from the latin word effero meaning to take to the grave or bury, is the process through which phagocytic cells, notably macrophages and DCs, clear apoptotic cells in situ.11;99;100 In general, the process involves several important communicative steps between apoptotic cells and efferocytes: namely the “find-me”, “eat-me”, and phagocytosis/engulfment steps. When cells start to undergo apoptosis, several find-me signals, such as the lipid lysophosphatidylcholine (LPC), sphingosine 1-phosphate (S1P), CX3CL1, and nucleotides such as ATP and UTP,101-105 are secreted to advertise their presence and to establish a chemotactic gradient for phagocyte recruitment. In addition to these immunologically quiet early apoptosis find-me cues, the leaky cellular membrane of late apoptotic cells or necrotic cells results in the release of damage-associated alarmins, such as heat shock proteins 70 and 90 or nDNA binding protein high-mobility group box 1 protein, which result in the activation of the immune system. Notably, most of these molecules have been associated with the pathology of atherosclerosis.10
In addition to chemoattractant find-me signals, eat-me and don’t-eat-me signals are up- and down-regulated, respectively, by apoptotic cells. Several protective don’t-eat-me signals, including CD31 and CD47, are expressed by healthy cells to ward off phagocytes and to protect themselves from engulfment.100 In contrast, eat-me signals, including phosphatidyl serine (PtdSer), exposure of the endoplasmic reticulum protein calreticulin, changes in membrane charge and glycosylation patterns, and alteration of intercellular adhesion molecule-1 (ICAM-1) epitopes, serve to activate phagocytes and to encourage engulfment of the apoptotic cell.100 Several phagocyte receptors may recognize PtdSer, either directly or indirectly, through adaptor proteins. Following recognition of PtdSer or other eat-me signals, the phagocyte and apoptotic cell form a phagocytic synapse, after which the apoptotic cell is internalized, processed, and ultimately degraded. Subsequently, depending on the stage of cellular death and the availability of “danger” antigens, antigen processing and presentation by macrophages or DCs may result in the activation of the adaptive immune system.106
Given the preponderance of MΦs, necrotic material, and apoptotic cells within atherosclerotic plaques, MΦ apoptosis and efferocytosis have been keen areas of interest within the atherosclerosis community. MΦs containing apoptotic material are detectable, and apoptosis occurs in macrophage-rich regions of the atherosclerotic plaque.11;99 However, as extracellular apoptotic debris and apoptotic cells are also readily detectable, the ability of MΦs to remove or handle the number of apoptotic cells may be insufficient. To date, the majority of studies on efferocytosis in atherosclerosis have relied primarily on histological examination of co-localizing apoptotic cells and phagocytes,107 due primarily to the technical challenges of directly monitoring efferocytosis in situ. In early atherosclerotic lesions, genetic manipulations to either increase or decrease efferocytosis have demonstrated that apoptotic cells are associated with smaller lesions and slower plaque progression.108-111 In contrast, in later-stage atherosclerotic plaques, evidence suggests that increased MΦ apoptosis corresponds with plaque necrosis, suggesting defective or inefficient clearance via efferocytosis in established plaques.11;99 Indeed, the importance of efferocytotic receptors has been demonstrated by recent studies examining the effects of Lrp, G2a, Tg, Mfge8, Mertk, and C1 genetic manipulation in murine atherosclerosis,112-117 with the general consensus being that altering only one efferocytotic pathway results in elevated apoptotic macrophage content, larger necrotic cores, and larger atherosclerotic plaques. However, while genetic studies provide an excellent starting point for studying efferocytosis pathways in atherosclerosis, recent data suggest that post-translational regulatory mechanisms may play important roles, in situ. Recent studies indicate that Mertk can become inactivated during inflammation116;117 via cleavage, which occurs in advanced atherosclerotic plaques, and may potentially compete for the PtdSer bridging molecules Gas6 and Protein S. Likewise MFG-E8, another PtdSer bridging molecule, was shown to be downregulated in mouse model of sepsis within splenic macrophages, in a TLR4-dependent manner.118 Several studies have also demonstrated that CD36,119 Mertk,120 and Lox-1121 may be cleaved by ADAM17, thereby affecting MΦ efferocytotic capacity. In the case of scavenger receptor (CD36), several cleavage sites and products were identified; and genetic deficiency of ADAM17 enhanced efferocytosis, in vivo, in a CD36-dependent manner.119 Similarly, cathepsin G–deficient Apoe−/− mice demonstrated that heterozygous Ctsg+/− Apoe−/− mice, compared with Ctsg+/+ Apoe−/− mice, display reduced complex aortic root lesions with fewer apoptotic cells, in addition to enhanced efferocytosis of Ctsg−/− bone marrow-derived MΦs.122 While cathepsin G has a plethora of functions in vivo, several eat-me signals are also targets of cathepsin G, supporting the notion that post-translational modification or cleavage of efferocytotic molecules may play important regulatory roles in chronic inflammation and atherogenesis.122
Taken together, these studies demonstrate the importance of efferocytosis in atherosclerosis and suggest that, in complex lesions, counter-regulatory mechanisms may hamper the efforts of macrophages to clear apoptotic materials. However, the exact mechanisms of counter-regulation in inflammation, the relationship between MΦ proliferation, efferocytosis, and plaque progression, and the potential efferocytotic capacities of aortic MΦ subsets remain to be investigated.
Vascular dendritic cells
The total number of leukocytes, including the number of MΦs and DCs, increases progressively during atherogenesis.10;123 Activation of the endothelium and the resulting chemokine gradients support the recruitment of monocytes and, likely, pre-DCs into the aortic wall.124 To date, the extent to which different monocyte subsets give rise to monocyte-derived arterial DCs is poorly understood. Nevertheless, it is clear that both Ly6Clo and Ly6Chi monocytes can differentiate into aortic DCs,17 and that dendritic marker–bearing cells are present even in early murine atherosclerotic plaques.125 To date, while there is no single marker that can uniquely identify all subsets of tissue DCs and MΦs,47;126 a combination of surface markers may be employed to identify DC populations. In both healthy and atherosclerotic aortas, two major subsets of CD11c+ DCs have been observed.82 CD11c+ CD11b+ CD103− F4/80+ DCs, which are likely Ly6Chi monocyte-derived DCs, and CD11c+ CD11b− CD103+ F4/80− DCs, which resemble classical DCs and proliferate independently from Ly6Chi monocytes.127 After a short–term high cholesterol diet, the numbers of aortic CD11c+ CD11b− CD103+ F4/80− DCs is elevated, in part due to elevated GM-CSF–dependent proliferation,127 suggesting that atherosclerosis-prone conditions dynamically affect DC recruitment to arterial walls, therefore potentially impacting the magnitude of the arterial adaptive immune response.
DC functions in atherosclerosis
DCs, which bridge the adaptive and innate responses, effectively orchestrate the differentiation and modulation of effector Th1, Th17, and T regulatory cell (Treg) responses.128;129 In the context of atherosclerosis, one of the important initial questions raised was whether the functions of DCs are altered during hypercholesterolemia. Although hypercholesterolemia alters DC migration from peripheral tissues to draining lymph nodes,130 a detailed analysis of DC functions revealed that DCs isolated from atherosclerosis-prone Ldlr−/− mice or Apoe−/− mice are capable of priming naive T cells.131 However, until recently, the mechanisms and consequences of oxLDL uptake by DCs were undefined. Nickel et al. demonstrated that oxLDL uptake by human DCs is mediated by the scavenger-receptors LOX-1, CD36, and CD205, which leads to DC maturation and the release of pro-inflammatory cytokines.132 This study is in line with earlier observations demonstrating that oxLDL may induce the maturation of monocyte-derived DCs that secrete IL-12 but not IL-10, and support both syngeneic and allogeneic T cell stimulation.133 In contrast, oxidized phospholipids (ox-PLs) prevent DC maturation via TLR3/TLR4-dependent blockade of CD40, CD80, and CD83 up-regulation.134 However, whether all DC subsets may efficiently engulf modified LDL, and function similarly after they acquire potential LDL-derived antigens, is unclear.
To further address the potential implication of DCs in atherosclerosis, several knock-out and transgenic mouse models have been used. CD11c deficiency reduces atherosclerosis in Cd11c−/− Apoe−/− mice, at least partly due to reduced accumulation of DCs and some CD11c+ monocytes/macrophages within the aorta.135 Although CD11c deficiency would impact both DC and a subpopulation of CD11c+ monocytes/macrophages, this study clearly demonstrates the potential impact of DCs on atherogenesis. Conditional depletion of CD11c+ cells in CD11c-DTR-mice reduced the intimal area of nascent atherosclerotic lesions by 55% in Ldlr−/− mice,125 suggesting an important role of CD11c-expressing cells in the initiation of atherosclerosis. Gautier et al. used another approach and generated a mouse model that over-expressed the anti-apoptotic gene hBcl-2 under the control of the CD11c promoter.136 In this model, conventional DCs (CD11chigh MHCII+), expressed Bcl-2, whereas plasmacytoid DCs (CD11cint PDCA-1+) did not. Expansion of these DCs in either Ldlr−/− or Apoe−/− mice was associated with elevated expression of Th1- and Th17-related cytokines and increased production of Th1-dependent IgG2c autoantibodies. Unexpectedly, elevated DC content was also associated with diminished levels of total circulating cholesterol, making the interpretation of the impact of expanded DCs on atherogenesis difficult.136
With evidence for a pro-atherogenic role for at least a subset of DCs, DC function as potential antigen-presenting cells has become a recent topic of interest. Evidence suggests that there are a few sources of potential atherogenic antigens, including oxLDL, beta-2 glycoprotein I (β2GPI), and heat shock proteins HSP-60 and HSP-65, which can induce a T cell–specific response.123 Unexpectedly, recent data also suggests that native LDL and purified ApoB100 may similarly induce a MHCII-restricted T cell response, suggesting that LDL-derived peptides play multi-faceted roles on the atherosclerotic adaptive immune response.137 While an increasing body of evidence suggests that DCs are involved in antigen presentation, it was, until recently, unclear whether DCs could present antigen locally within the aortic wall. Using two-photon microscopy and Cd11c-YFP+ mice, in which yellow fluorescent protein (YFP) is expressed under the Cd11c promoter,138 Koltsova and colleagues demonstrated that YFP+ DCs may interact, present antigen, and subsequently induce T cell activation and IFN-γ and TNF-α release within the aorta.19 Interestingly, as the number of T cells and CD11c+ cells is starkly elevated during atherogenesis, more aortic T/DC interactions, and likely a stronger antigen-dependent response, may elicit chronic inflammation in the aorta. Further evidence for the impact of DCs as antigen presenting cells was suggested by several studies demonstrating that deficiency of co-stimulatory molecules expressed by APCs, such as B7.1/B7.2,139 TNF–TNFR-associated CD137L,140 and CD134 (OX40L), reduced atherosclerosis at different stages of the disease.141;142 Although not formally proven because the above studies used gene knockouts in the whole mouse, these reports provide evidence for the involvement of antigen presenting cells in atherosclerosis.
While DCs play key roles in the processes of antigen presentation and initiating a T helper response, they are also essential in maintaining immunological tolerance. One important question about the role of DCs in atherosclerosis has been whether different subsets of DCs might play unique roles in atherosclerosis. DCs are generated at least by two major pathways that differ in their requirement for the Flt3/Flt3L (Flt3L, fms-like tyrosine kinase 3 ligand) axis.16 The development of DCs from monocyte-independent precursors is Flt-3/Flt3L-dependent, whereas the generation of DCs from monocytes is Flt3/Flt3L-independent. Using Flt-3−/− Ldlr−/− mice, Choi et al. demonstrated that Flt3 deficiency resulted in the depletion of CD11c+ CD11b− CD103+ DCs, increased levels of aortic IFN-γ and TNF-α without significant alterations in the lipid levels, and aggravated plaque burden.143 Importantly, the number of Treg cells was reduced in Flt-3−/− Ldlr−/− mice, suggesting either direct or indirect-regulation of Treg cells by Flt-3-dependent DCs. Additional studies also support the notion that some subsets of DCs in atherosclerosis may be immuno-suppressive. TGF-β regulates DC apoptosis and dampens the effects of TNF-α, IL-12, CCL5, and CD40, and decreases the expression of CD80, and CD86, resulting in a more tolerogenic DC phenotype. Interestingly, the absence of TGF-βRII signaling in DCs causes an increase in atherosclerotic plaque size despite a 50% reduction in plasma cholesterol levels, suggesting an atheroprotective role for TGF-β–dependent DCs in atherosclerosis.144 In contrast to the protective role of aortic Flt-3-dependent CD11c+ cells, another subset of DCs expressing CCL17 displayed a pro-atherogenic phenotype. CCL17-expressing DCs have been shown to limit Treg cell expansion and increase atherosclerotic plaque burden.145 The importance of DC-mediated regulation of the immune response has recently been highlighted in a study focusing on the PD-1/PDL-1 axis. PD-L1/2 deficiency led to significantly increased atherosclerotic burden throughout the aorta, which was accompanied by APC-induced activation of T cells.146
To further understand the role of CD11c+ cells in atherosclerosis, Subramanian and colleagues investigated the impact of MyD88-dependent signaling in CD11c+ DCs.147 Surprisingly, MyD88 deficiency in CD11c+ cells increased the plaque burden of western diet-fed Ldlr−/− mice, despite decreased aortic infiltration of both effector T cells and Treg cells overall. Thus, the net effect of MyD88 signaling in CD11c+ DCs appears to be atheroprotective. However, it should be noted that some MΦs also express CD11c, and MyD88-dependent signaling might also have an impact on the observed phenotype. Since some TLRs may signal through MyD88-independent pathways, it will be important to determine the impact of TRIF-signaling on DC functions. There is still an open question whether tolerogenic DCs are a lineage-specific subset of DCs or subset at a unique activation state with suppressive potential. To date, mechanisms that control the number and functions of different DC subsets are not well understood, but recently discovered diverse functions of DCs in atherosclerosis suggest the existence of a complex network of DCs that controls the arterial immune response during homeostasis and atherogenesis.
Plasmacytoid dendritic cells
It has become increasingly apparent that several subsets of DCs might play opposite roles in atherogenesis. Plasmacytoid (pDCs) rapidly produce type 1 interferon (interferon α/β, IFN) and other cytokines following activation through nucleic acid-sensing TLRs, such as TLR7 and TLR9. pDCs are closely related to classical antigen-presenting DCs, as they have some common developmental origin and genetic similarity; however, under homeostatic conditions, pDCs are weak antigen presenting cells compared with classical DCs.148 pDCs are found within the shoulder region of atherosclerotic plaque areas.149;150 Interestingly, reduced numbers of circulating pDCs can be a predictor of cardiovascular events in coronary artery disease.151
The importance of pDCs in atherosclerosis has recently been highlighted by a number of studies focusing on the potential mechanisms of pDC involvement in a low grade chronic inflammation within the aortic wall. Niessener and colleagues uncovered a potential role of pDCs on SMC apoptosis. pDCs, via the production of type I IFNs, induced the expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) on CD4+ T cells, enabling them to effectively kill vSMCs.150 Therefore, in vivo, pDCs likely sense TLR7/9-dependent pathogens, might provoke cytolytic T cell functions, and would likely induce immune-mediated complications in the aortic wall. Surprisingly, recent study by Salagianni and colleagues questioned the pro-atherogenic role of TLR7 signaling and, therefore, a role of pDC in atherogenesis.152 TLR7 deficiency in Apoe−/− mice resulted in elevated levels of CCL2, increased accumulation of M1 macrophages, and accelerated plaque burden. Based on the analysis of human carotid endarterectomy specimens, TLR7 levels were consistently associated with an M2 anti-inflammatory macrophage signature, suggesting that TLR7 signaling might be important in regulating the M1/M2 balance.
Recent studies using blocking Abs have provided controversial results about the impact of pDCs on atherosclerosis. Depletion of pDCs with Abs which recognizes PDCA-1 (marrow stromal cell antigen 2, BST2), a marker exclusively expressed on mouse pDC, increased T cell proliferation, elevated IFN-γ production and reduced expression of indoleamine 2,3-dioxygenase (IDO) in lymphoid pDCs and aggravated atherosclerosis in Ldlr−/− recipient mice.149 In contrast, pDC depletion reduced atherosclerosis and anti-double-stranded DNA antibody titers in Apoe−/− mice.153 Moreover, the specific activation of pDCs and IFN-α treatment promoted plaque growth associated with enhanced anti-double-stranded–DNA antibody titers. Additional work by Macritchie et al. has also demonstrated that pDC depletion leads to the attenuated T cell activation, a low profile of pro-inflammatory cytokines, and overall reduced content of macrophages in atherosclerotic plaques of Apoe−/− mice.154 Thus, the exact role of pDCs in atherosclerosis remains to be established; further studies are needed in order to identify a tolerogenic potential of pDC in atherosclerosis.
Concluding remarks
Many exciting recent advances in the parallel fields of MΦ and DC biology have augmented our understanding on the behavior and functions of myeloid cells in situ. Recent discoveries that MΦ proliferation dominates established arterial plaques and that blood or extramedullary-derived monocytes may, in homeostatic conditions, traffic through peripheral tissues to lymphatic vessels without differentiating to MΦ will result in exciting changes in the way that the atherosclerosis community thinks and studies arterial myeloid cells. In addition, emerging data concerning plaque M4 and Mox MΦ subsets, pDCs, Flt3-dependent and -independent aortic DC subsets and their functions in vivo, and the impact of the MyD88-dependent pathway have resulted in a dynamic model of atherosclerosis in which the balance of MΦ and DC subsets, which are simultaneously present within the atherosclerotic plaque, may play decidedly pro- or anti-inflammatory roles to control athero-progression or regression. However, many questions concerning the balance of these subsets, their precise functions, and the kinetics of their accumulation, proliferation, and removal remain to be answered. In addition, as local antigen presentation can occur within the arterial wall, the precise contributions of aortic versus secondary lymphoid organ antigen presentation to the etiology of atherosclerosis are unclear. Further work on the importance of systemic lymphoid organs and the changes that may occur during atherogenesis may provide valuable new insights into the pathophysiology of atherosclerosis. Additionally, several groups have recently started to study whether vaccines for LDL particles or LDL peptides might protect against the development of atherosclerosis.7;155 These studies have been successful to varying degrees within mice, but further understanding the biology of intra-plaque antigen presentation and arterial DCs may help to target specific DC subsets to develop better atherosclerosis vaccines.
Thus, despite the considerable progress in our the understanding of the behavior and functions of subsets of arterial MΦs, DCs, and monocytes, there is still much to learn in order to eventually help treat and prevent atherosclerosis.
Table 1.
The roles of monocytes and dendritic cell subsets in atherosclerosis
| Subsets | Migration to atherosclerotic lesions | Functions | Locations | |
|---|---|---|---|---|
| In | Out | |||
| Ly6Chi | CCL2/5 dependent14 | CCR7 dependent36;39-41 | Pro-atherogenic14;17;18
Potential Mϕs polarization (M1, M2, M4, Mox)17;18;25;29;61 Surveillance and egression(?)34 Traffic out(?)37;39-41;43;44 |
Atherosclerotic plaques17;18
Blood17;18;29;39 Spleen29;30 |
| Ly6Clo | Potential CX3CL1/ CX3CRI–and LFA- l–dependent crawling(?)20 |
CCR7-dependent(?)39-41 crawling(?) | Patrolling monocytes (nucleic acids and viruses)21 Protective22 or no effects23 Differentiate to Mϕs or DCs(?)34 |
Arterial lumen20
Blood20 |
| Pre-DCs | Unknown | Unknown | Monocyte-independent cDC precursors While not formally demonstrated, may potentially differentiate to FLT3-dependent DCs in atherosclerosis?16,143 |
Bone marrow Blood Spleen16 |
|
CD11C+
DCs/Mϕs |
CX3CR1, CCR2, and CCR5 dependent17;124 |
Unknown Might be CCR7 dependent36;39;40 |
Intra-plaque antigen presentation19 Potential site(s) of antigen presentation: lymph nodes and/or arterial wall?19;131;143
Pro-atherogenic136 or protective147 |
Peripheral tissues47
Atherosclerotic plaques and secondary lymphoid organs 19;131;143 Blood (CD11c+ monocytes)135 |
| Monocyte- dependent cDCs, Flt3 independent |
Likely CX3CR1, CCR2, and CCR5 dependent17,127 |
Unknown Might be CCR7 dependent?36 |
Pro-atherogenic143
Potential site(s) of antigen presentation: lymph nodes and/or arterial wall? |
Peripheral tissue Atherosclerotic aorta143 |
| Monocyte- independent cDCs, Flt3 dependent |
Unknown | Unknown Might be CCR7 dependent?36 |
Athero-protective143
Potential site(s) of antigen presentation: lymph nodes and/or arterial wall? |
Peripheral tissue Atherosclerotic aorta143 |
| pDCs | Unknown | Unknown Might be CCR7 dependent?36 |
Pro-atherogenic150;153;
154 or athero- protective149;152 Type 1 IFN-production Antigen presentation? |
Atherosclerotic plaques, (shoulder regions)149;150 Secondary lymphoid organs? Blood151 |
| CCL17+ DCs | Unknown | Unknown Might be CCR7 dependent?36 |
Pro-atherogenic Antigen presentation Negatively regulates Treg cell homeostasis145 |
Atherosclerotic plaques, 145 secondary lymphoid organs? |
Acknowledgements
This work was supported by American Heart Association Pre-doctoral Fellowship grant 11PRE7520041 (to Matthew Butcher), NHLBI HL112605 supplemental grant 02S1 (to Paresa Taghavie-Moghadam) and by NHLBI HL107522 (to Elena Galkina).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Reference List
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Thompson RC, Allam AH, Lombardi GP, Wann LS, Sutherland ML, Sutherland JD, Soliman MA, Frohlich B, Mininberg DT, Monge JM, Vallodolid CM, Cox SL, bd el-Maksoud G, Badr I, Miyamoto MI, Nur el-Din el-Halim, Narula J, Finch CE, Thomas GS. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet. 2013;381:1211–1222. doi: 10.1016/S0140-6736(13)60598-X. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinov IE, Mejevoi N, Anichkov NM, Nikolai N. Anichkov and his theory of atherosclerosis. Tex. Heart Inst. J. 2006;33:417–423. [PMC free article] [PubMed] [Google Scholar]
- 4.Mayerl C, Lukasser M, Sedivy R, Niederegger H, Seiler R, Wick G. Atherosclerosis research from past to present--on the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch. 2006;449:96–103. doi: 10.1007/s00428-006-0176-7. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J. Clin. Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 14.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 15.Hilgendorf I, Swirski FK. Making a difference: monocyte heterogeneity in cardiovascular disease. Curr. Atheroscler. Rep. 2012;14:450–459. doi: 10.1007/s11883-012-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van RN, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von VS, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 21.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ. Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao LC, Soto E, Hong C, Ito A, Pei L, Chawla A, Conneely OM, Tangirala RK, Evans RM, Tontonoz P. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J. Lipid Res. 2013;54:806–815. doi: 10.1194/jlr.M034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Howatt DA, Gizard F, Nomiyama T, Findeisen HM, Heywood EB, Jones KL, Conneely OM, Daugherty A, Bruemmer D. Deficiency of the NR4A orphan nuclear receptor NOR1 decreases monocyte adhesion and atherosclerosis. Circ. Res. 2010;107:501–511. doi: 10.1161/CIRCRESAHA.110.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 26.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 28.White GE, Greaves DR. Fractalkine: a survivor's guide: chemokines as antiapoptotic mediators. Arterioscler. Thromb. Vasc. Biol. 2012;32:589–594. doi: 10.1161/ATVBAHA.111.237412. [DOI] [PubMed] [Google Scholar]
- 29.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van RN, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van RN, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van RN, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desurmont C, Caillaud JM, Emmanuel F, Benoit P, Fruchart JC, Castro G, Branellec D, Heard JM, Duverger N. Complete atherosclerosis regression after human ApoE gene transfer in ApoE-deficient/nude mice. Arterioscler. Thromb. Vasc. Biol. 2000;20:435–442. doi: 10.1161/01.atv.20.2.435. [DOI] [PubMed] [Google Scholar]
- 36.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potteaux S, Gautier EL, Hutchison SB, van RN, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van RN, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS. ONE. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautier EL, Ivanov S, Lesnik P, Randolph GJ. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013;122:2714–2722. doi: 10.1182/blood-2013-01-478206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, varez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Gils JM, Ramkhelawon B, Fernandes L, Stewart MC, Guo L, Seibert T, Menezes GB, Cara DC, Chow C, Kinane TB, Fisher EA, Balcells M, varez-Leite J, Lacy-Hulbert A, Moore KJ. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler. Thromb. Vasc. Biol. 2013;33:911–919. doi: 10.1161/ATVBAHA.112.301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanschel A, Seibert T, Hewing B, Ramkhelawon B, Ray TD, van Gils JM, Rayner KJ, Feig JE, O'Brien ER, Fisher EA, Moore KJ. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler. Thromb. Vasc. Biol. 2013;33:886–893. doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramkhelawon B, Yang Y, van Gils JM, Hewing B, Rayner KJ, Parathath S, Guo L, Oldebeken S, Feig JL, Fisher EA, Moore KJ. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler. Thromb. Vasc. Biol. 2013;33:1180–1188. doi: 10.1161/ATVBAHA.112.301008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 49.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 53.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verreck FA, de BT, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziegler HK, Staffileno LK, Wentworth P. Modulation of macrophage Ia-expression by lipopolysaccharide. I. Induction of Ia expression in vivo. J. Immunol. 1984;133:1825–1835. [PubMed] [Google Scholar]
- 56.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shnyra A, Brewington R, Alipio A, Amura C, Morrison DC. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J. Immunol. 1998;160:3729–3736. [PubMed] [Google Scholar]
- 58.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clinton SK, Underwood R, Hayes L, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am. J. Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- 60.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS. ONE. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouhlel MA, Derudas B, Rigamonti E, DiFvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPAR[gamma] Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metabolism. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Gleissner CA, Shaked I, Erbel C, Bockler D, Katus HA, Ley K. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ. Res. 2010;106:203–211. doi: 10.1161/CIRCRESAHA.109.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J. Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sachais BS, Turrentine T, wicki McKenna JM, Rux AH, Rader D, Kowalska MA. Elimination of platelet factor 4 (PF4) from platelets reduces atherosclerosis in C57Bl/6 and apoE−/− mice. Thromb. Haemost. 2007;98:1108–1113. [PubMed] [Google Scholar]
- 66.Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, Wang X, Castellani LW, Reue K, Lusis AJ, Araujo JA. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur. J. Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 68.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS. ONE. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruotsalainen AK, Inkala M, Partanen ME, Lappalainen JP, Kansanen E, Makinen PI, Heinonen SE, Laitinen HM, Heikkila J, Vatanen T, Horkko S, Yamamoto M, Yla-Herttuala S, Jauhiainen M, Levonen AL. The absence of macrophage Nrf2 promotes early atherogenesis. Cardiovasc. Res. 2013;98:107–115. doi: 10.1093/cvr/cvt008. [DOI] [PubMed] [Google Scholar]
- 70.Cole JE, Georgiou E, Monaco C. The expression and functions of toll-like receptors in atherosclerosis. Mediators. Inflamm. 2010;2010:3939–46. doi: 10.1155/2010/393946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 73.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El KJ, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, varez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grebe A, Latz E. Cholesterol crystals and inflammation. Curr. Rheumatol. Rep. 2013;15:313. doi: 10.1007/s11926-012-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr., Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler. Thromb. Vasc. Biol. 2003;23:1553–1558. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- 81.Boyle JJ, Weissberg PL, Bennett MR. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler. Thromb. Vasc. Biol. 2002;22:1624–1630. doi: 10.1161/01.atv.0000033517.48444.1a. [DOI] [PubMed] [Google Scholar]
- 82.Butcher MJ, Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol. 2012;3:44. doi: 10.3389/fphys.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Unemori EN, Lark MW, Amento E, Libby P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ. Res. 1994;75:181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- 84.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 85.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, Jain MK. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. "Re-educating" tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int. J. Cancer. 2009;125:367–373. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 88.Robbins CS, Swirski FK. The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol. Life Sci. 2010;67:2685–2693. doi: 10.1007/s00018-010-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van RN, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur. J. Immunol. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van RN, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 94.Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc. Res. 1999;41:473–479. doi: 10.1016/s0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 96.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- 97.Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am. J. Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 99.Van Vre EA, it-Oufella H, Tedgui A, Mallat Z. Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:887–893. doi: 10.1161/ATVBAHA.111.224873. [DOI] [PubMed] [Google Scholar]
- 100.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a "come-and-get-me" signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 104.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, D.Gregory C. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 105.Weigert A, Cremer S, Schmidt MV, von KA, Angioni C, Geisslinger G, Brune B. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood. 2010;115:3531–3540. doi: 10.1182/blood-2009-10-243444. [DOI] [PubMed] [Google Scholar]
- 106.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 108.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boesten LS, Zadelaar AS, van NA, Hu L, Teunisse AF, Jochemsen AG, Evers B, van de WB, Gijbels MJ, van Vlijmen BJ, Havekes LM, de Winther MP. Macrophage p53 controls macrophage death in atherosclerotic lesions of apolipoprotein E deficient mice. Atherosclerosis. 2009;207:399–404. doi: 10.1016/j.atherosclerosis.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 111.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 112.Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am. J. Pathol. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boisvert WA, Rose DM, Boullier A, Quehenberger O, Sydlaske A, Johnson KA, Curtiss LK, Terkeltaub R. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler. Thromb. Vasc. Biol. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- 114.Bolick DT, Skaflen MD, Johnson LE, Kwon SC, Howatt D, Daugherty A, Ravichandran KS, Hedrick CC. G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circ. Res. 2009;104:318–327. doi: 10.1161/CIRCRESAHA.108.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 116.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 117.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J. Immunol. 2009;182:581–587. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Driscoll WS, Vaisar T, Tang J, Wilson CL, Raines EW. Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype. Circ. Res. 2013;113:52–61. doi: 10.1161/CIRCRESAHA.112.300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK) J. Biol. Chem. 2011;286:33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao XQ, Zhang MW, Wang F, Zhao YX, Li JJ, Wang XP, Bu PL, Yang JM, Liu XL, Zhang MX, Gao F, Zhang C, Zhang Y. CRP enhances soluble LOX-1 release from macrophages by activating TNF-alpha converting enzyme. J. Lipid Res. 2011;52:923–933. doi: 10.1194/jlr.M015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rafatian N, Karunakaran D, Rayner KJ, Leenen FH, Milne RW, Whitman SC. Cathepsin G deficiency decreases complexity of atherosclerotic lesions in apolipoprotein E-deficient mice. Am. J. Physiol Heart Circ. Physiol. 2013;305 doi: 10.1152/ajpheart.00618.2012. [DOI] [PubMed] [Google Scholar]
- 123.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 124.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 125.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 126.Niessner A, Weyand CM. Dendritic cells in atherosclerotic disease. Clin. Immunol. 2010;134:25–32. doi: 10.1016/j.clim.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J. Exp. Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 131.Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, H.Lichtman A. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ. Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nickel T, Schmauss D, Hanssen H, Sicic Z, Krebs B, Jankl S, Summo C, Fraunberger P, Walli AK, Pfeiler S, Weis M. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205:442–450. doi: 10.1016/j.atherosclerosis.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 133.Perrin-Cocon L, Coutant F, Agaugue S, Deforges S, Andre P, Lotteau V. Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. J. Immunol. 2001;167:3785–3791. doi: 10.4049/jimmunol.167.7.3785. [DOI] [PubMed] [Google Scholar]
- 134.Bluml S, Kirchberger S, Bochkov VN, Kronke G, Stuhlmeier K, Majdic O, Zlabinger GJ, Knapp W, Binder BR, Stockl J, Leitinger N. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J. Immunol. 2005;175:501–508. doi: 10.4049/jimmunol.175.1.501. [DOI] [PubMed] [Google Scholar]
- 135.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ, Lesnik P. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 137.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, C.Nussenzweig M. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 139.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 140.Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, Park JE, Park HY, Kwack K, Choi BK, Kwon BS, Oh GT. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–1133. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 141.Nakano M, Fukumoto Y, Satoh K, Ito Y, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. OX40 ligand plays an important role in the development of atherosclerosis through vasa vasorum neovascularization. Cardiovasc. Res. 2010;88:539–546. doi: 10.1093/cvr/cvq211. [DOI] [PubMed] [Google Scholar]
- 142.Foks AC, van Puijvelde GH, Bot I, Ter Borg MN, Habets KL, Johnson JL, Yagita H, van Berkel TJ, Kuiper J. Interruption of the OX40-OX40 Ligand Pathway in LDL Receptor-Deficient Mice Causes Regression of Atherosclerosis. J. Immunol. 2013;191:4573–4580. doi: 10.4049/jimmunol.1200708. [DOI] [PubMed] [Google Scholar]
- 143.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 Signaling-Dependent Dendritic Cells Protect against Atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 144.Lievens D, Habets KL, Robertson AK, Laouar Y, Winkels H, Rademakers T, Beckers L, Wijnands E, Boon L, Mosaheb M, it-Oufella H, Mallat Z, Flavell RA, Rudling M, Binder CJ, Gerdes N, Biessen EA, Weber C, Daemen MJ, Kuiper J, Lutgens E. Abrogated transforming growth factor beta receptor II (TGFbetaRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur. Heart J. 2012;34(48):3717–27. doi: 10.1093/eurheartj/ehs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weber C, Meiler S, Doring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E, van Zandvoort MA, Binder CJ, Jelinek I, Hristov M, Boon L, Jung S, Korn T, Lutz MB, Forster I, Zenke M, Hieronymus T, Junt T, Zernecke A. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Invest. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Daissormont IT, Christ A, Temmerman L, Sampedro MS, Seijkens T, Rousch M, Poggi M, Boon L, van der LC, Daemen M, Lutgens E, Halvorsen B, Aukrust P, Janssen E, Biessen EA. 2011;9(109(12)):1387–95. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 151.Doring Y, Zernecke A. Plasmacytoid dendritic cells in atherosclerosis. Front Physiol. 2012;3:230. doi: 10.3389/fphys.2012.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Salagianni M, Galani IE, Lundberg AM, Davos CH, Varela A, Gavriil A, Lyytikainen LP, Lehtimaki T, Sigala F, Folkersen L, Gorgoulis V, Lenglet S, Montecucco F, Mach F, Hedin U, Hansson GK, Monaco C, Andreakos E. Toll-like receptor 7 protects from atherosclerosis by constraining "inflammatory" macrophage activation. Circulation. 2012;126:952–962. doi: 10.1161/CIRCULATIONAHA.111.067678. [DOI] [PubMed] [Google Scholar]
- 153.Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J, Daemen MJ, Lutgens E, Zenke M, Binder CJ, Weber C, Zernecke A. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125:1673–1683. doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- 154.Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M, McInnes IB, Brewer JM, Garside P, Maffia P. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:2569–2579. doi: 10.1161/ATVBAHA.112.251314. [DOI] [PubMed] [Google Scholar]
- 155.Ketelhuth DF, Gistera A, Johansson DK, Hansson GK. T cell-based therapies for atherosclerosis. Curr. Pharm. Des. 2013;19:5850–5858. doi: 10.2174/1381612811319330003. [DOI] [PubMed] [Google Scholar]