Abstract
As the fat body is a critical tissue for mosquito development, metamorphosis, immune and reproductive system function, characterization of regulatory modules targeting gene expression to the female mosquito fat body at distinct life stages is much needed for multiple, varied strategies for controlling vector-borne diseases such as dengue and malaria. The hexameric storage protein, Hexamerin-1.2, of the mosquito, Aedes atropalpus, is female-specific and uniquely expressed in the fat body of fourth-instar larvae and young adults. We have identified in the Hex-1.2 gene, a short regulatory module that directs female-, tissue-, and stage-specific lacZ reporter gene expression using a heterologous promoter in transgenic lines of the dengue vector, Aedes aegypti. Male transgenic larvae and pupae of one line expressed no E. coli β-galactosidase or transgene product; in two other lines reporter gene activity was highly female-biased. All transgenic lines expressed the reporter only in the fat body. However, lacZ mRNA levels were no different in males and females at all stages examined, suggesting that the gene regulatory module drives female-specific expression by post-transcriptional regulation in the heterologous mosquito. This regulatory element from the Hex-1.2 gene thus provides a new molecular tool for transgenic mosquito control as well as functional genetic analysis in aedine mosquitoes.
Keywords: hexamerin, regulated gene expression, mosquito, fat body, female specificity
Introduction
Female mosquitoes are the most prominent of blood-sucking arthropods harmful to humans, transmitting to millions worldwide the pathogens for malaria, yellow fever, West Nile virus, and dengue, as well as certain types of filariasis and encephalitides (Benedict and Robinson 2008; Carlson et al. 2006). Despite the many diverse strategies developed to combat mosquitoes and mosquito-vectored diseases, including new insecticides, vaccines, and drugs, the overall effectiveness of these approaches over time has been disappointing. Both chemical and biological means of vector and pathogen control are still subject to the development of resistance and may even contribute to disease resurgence (reviewed in Garcia et al. 2009; Raghavendra et al. 2011); they can also be particularly costly to implement. Hence, molecular genetic alternatives to pesticides for mosquito control and their spread of disease are under development (Kokoza et al. 2010; Meredith et al. 2011; Wise de Valdez et al. 2011). In the last 15 years, the success of transgenic technology (Coates et al. 1998; Jasinskiene et al. 1998) has encouraged novel approaches to modify mosquito behavior and even interfere with the pathogen life cycle within the insect (Antonova et al. 2009; Ito et al. 2002; James 2002; Li et al. 2008; Mathur et al. 2010; Raikhel et al. 2002; Rodrigues et al. 2008). The Sterile Insect Technique (SIT), applied worldwide to eradicate specific populations of disease vectors (reviewed in O’Brochta and Handler 2008), still has several persistent problems affecting successful implementation against mosquitoes. A different molecular genetic approach involving “release of insects carrying a dominant lethal” (RIDL) (Alphey 2002; Heinrich and Scott 2000; Thomas et al. 2000) depends on sex- and tissue-specific promoter elements. Targeting of gene activity to immature female mosquitoes could improve both SIT, allowing the development of genetic sexing strains and the release of only males, and RIDL, releasing males carrying a female-specific dominant lethal that acts at the most effective (pre-adult) developmental stage (Phuc et al. 2007).
Hexamerins are insect storage proteins that belong to a large arthropod protein family, including hemocyanins and prophenol-oxidases (Burmester et al. 1998; Haunerland 1996; Telfer and Kunkel 1991). In holometabolous insects, hexamerins are synthesized in the fat body, primarily at the end of larval development, secreted into the haemolymph and then taken up via receptor-mediated endocytosis into the larval fat body for storage in protein granules. Hexamerins are also expressed by the larval and adult fat body of different species of mosquitoes (Korochkina et al. 1997a; Korochkina et al. 1997b; Zakharkin et al. 1997). Previously, we characterized the expression of a mosquito hexamerin gene, Hexamerin-1.2 (Hex-1.2), which is expressed exclusively in the fat bodies of female fourth-instar (L4) larvae and female adults of the autogenous mosquito, Ae. (Ochleratatus) atropalpus (Zakharkin et al. 2001).
Molecular analysis of gene activity in the fat body of the fruit fly, Drosophila melanogaster, and the mosquito, Ae. aegypti, has suggested that its tissue-specific expression is governed by specific transcription factors, including C/EBP, GATA, BBF-2, HNF-4, and a fat body-specific repressor, AEF-1, binding to their cognate cis-acting DNA sequences (Abel et al. 1992; Abel et al. 1993; An et al. 1996; Beneš et al. 1996; Dittmer and Raikhel 1997; Falb and Maniatis 1992; Garabedian et al. 1986; Kapitskaya et al. 1998; Kokoza et al. 2000; Martin et al. 2001). In particular, the female-specific activity of the Drosophila Yolk protein (Yp) genes appears to be largely regulated by a Fat Body Enhancer (FBE) or a transcriptional regulatory module containing sequences for binding of the ‘CAAT’ Enhancer Binding Protein (C/EBP) and Doublesex (DSX) transcription factors (An et al. 1996; An and Wensink 1995a; An and Wensink 1995b; Coschigano and Wensink 1993). DSX is one of the downstream regulators of the sex determination pathways in a number of insects (Burtis 2002; Salvemini et al. 2011; including mosquitoes Scali et al. 2005; Shukla and Nagaraju 2010a). The doublesex locus encodes male-specific DSXM and female-specific DSXF isoforms produced through alternative RNA splicing in flies and mosquitoes (Burtis and Baker 1989; Nagoshi and Baker 1990; Salvemini et al. 2011; Scali et al. 2005).
In our first examination of the female- and fat body-specific activity of Hex-1.2 we observed in its 5’-flanking region putative Female-Specific regulatory Elements (FSEs; Fig. 1), which contain overlapping binding sites for C/EBP and DSX (Zakharkin et al. 2001), similar to elements dictating female specificity of the Drosophila Yp Fat Body Enhancer (FBE). We showed that the three DSX sites in the Hex-1.2 5’-flanking region bind DSX in vitro. However, 0.7 kb of the 5’flanking region (including the native promoter and transcription start site) of the Ae. atropalpus Hex-1.2 gene were only able to induce modest female-biased activity of the luciferase reporter gene in D. melanogaster (Jinwal et al. 2006). Fourth-instar (L4) female larvae expressed luciferase at a 4-fold higher level than males of the same developmental stage, while fat body- and stage-specific expression was comparable to that observed for the native Ae. atropalpus Hex-1.2 gene. Genetic experiments suggested that female specificity depended on at least one of the three functional DSX-binding sites within 220 bp of the transcription start site.
Figure 1. Schematic diagram of the Hex-1.2 5’-flanking region and structure of the 0.7HexEnh-hsp-lacZ transgene.
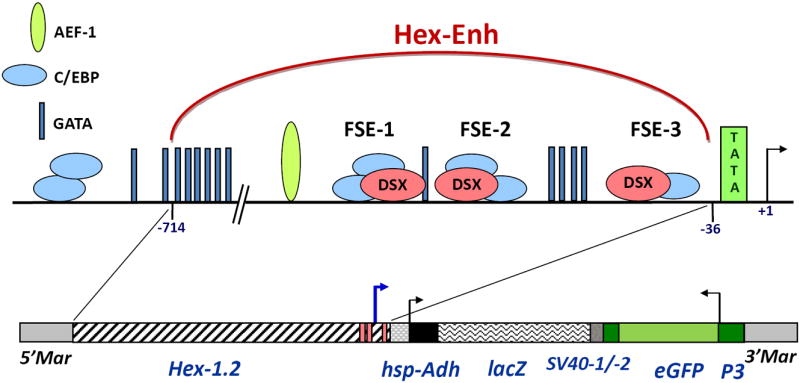
Putative binding sites for AEF-1 (Adult Enhancer Factor), C/EBP (“CAAT” Enhancer Binding Protein) and GATA factors and female-specific elements (FSE-1, -2 and -3) were previously proposed (Zakharkin et al. 2001); the Doublesex (DSX) binding sites were proven functional in vitro (Jinwal et al. 2006). Construction of the HexEnh-hsp-lacZ fusion gene is described in Experimental procedures and contains the following elements: Adh, 5’UTR and N-terminal coding sequences of the Drosophila Alcohol dehydrogenase gene; hsp, the Drosophila heat shock protein 70 minimal promoter; lacZ, the E. coli β-galactosidase coding region with SV40 polyadenylation signal sequences (SV40-1). Parts of the pMos transformation vector are indicated: Mar, the mariner inverted repeats and the transformation selection marker, 3XP3-eGFP with its SV40 sequences (SV40-2). The anomalous transcription start site (at -171, relative to the transcription Hex-1.2 start site in Ae. atropalpus larvae (Zakharkin et al. 2001)) is shown as a blue arrow.
Here we report that a 679-bp fragment of the Hex-1.2 5’-flanking region, tentatively termed a putative Hex-1.2 Enhancer (HexEnh), is able to drive very robust female- and fat body-specific expression of the lacZ reporter gene in transgenic Ae. aegypti mosquitoes. While reporter gene expression was found to be specific to L4 larvae, pupae and young adult females, lacZ mRNA levels were no different in males and females at all stages examined, suggesting that the HexEnh gene regulatory module operates in post-transcriptional control and not as in the native mosquito, Ae. atropalpus. However, given its ability to drive female-specific gene expression in the heterologous Ae. aegypti, the HexEnh should prove valuable in future development of SIT and RIDL strategies for control of aedine mosquito populations.
Results and Discussion
Construction of a fusion gene to test enhancer function
We hypothesized that a subset of the Hex-1.2 5’-flanking sequences, previously tested in Drosophila (Jinwal et al. 2006) and containing DSX-, C/EBP, GATA and AEF-binding sites (Fig. 1), should function as a gene regulatory module to direct heterologous gene activity in the fat body of female Ae. aegypti. We choose 679 bp of the Hex-1.2 5’-flanking region, specifically sequences from -714 to -36 relative to the transcription start site at +1, and tentatively termed this region a putative Hex-1.2 Enhancer (HexEnh; Fig. 1). Using the Drosophila pCaSpeR-hs43-lacZ vector (Pirrotta 1994), we constructed a 0.7HexEnh-hsp-lacZ fusion gene (Fig. 1) containing a basal promoter (from the Drosophila hsp70 gene) and the E. coli lacZ gene (encoding β-galactosidase) as a reporter gene. Between the Hex-1.2 sequences and the hsp70 promoter (starting at position -43 of hsp70), there was a 53-bp polylinker sequence. As originally designed in the Drosophila pCaSpeR-hs43-lacZ vector, the 5’UTR (untranslated region) of the fusion gene consisted of 136 bp of the hsp70 5’UTR followed by 37 bp of the Drosophila Alcohol dehydrogenase (Adh) 5’UTR. The coding region included 31 N-terminal codons for ADH preceding the β-galactosidase coding sequence, followed by the 850-bp of SV40 DNA containing a polyadenylation signal sequence. For simplicity in subsequent data presentation or discussion, the reporter gene or its mRNA will be referred to as lacZ; and the protein, as β-galactosidase, the final gene product that was measured.
Generation of transgenic Aedes aegypti
To test the function of HexEnh in a closely related culicine mosquito species, Ae. aegypti, the 0.7HexEnh-hsp-lacZ fusion gene was subcloned into the mariner-based pMos vector and injected into embryos of the WE (khw) strain of Ae. aegypti. From the screening of G1 L4 larvae for the EGFP marker gene expression in the optic lobes, three (3) stable, transgenic lines, 2M2, F16 and I1, were obtained and verified for the presence of the HexEnh-hsp-lacZ transgene by PCR on genomic DNA. Southern blot analysis of genomic DNA from each line was performed after >10 generations of selection for the EGFP transformation marker. In addition to a common 5.5-kb BamHI DNA fragment (representing the entire hsp-lacZ transgene and most of the 3xP3-eGFP gene; Fig. 1), each transgenic line exhibited a single, uniquely hybridizing fragment indicating the presence of a single insertion of the 0.7HexEnh-hs-lacZ transgene in each line (data not shown).
Female-specific expression of the β-galactosidase reporter gene in transgenic Aedes aegypti
According to the profiles of hexamerin expression in holometabolous insects (Telfer and Kunkel 1991) and mosquitoes in particular (Burmester et al. 1998; Korochkina et al. 1997a; Zakharkin et al. 2001), the hexamerin mRNA levels are highest at the end of the last larval instar; the highest level of protein accumulates within 5-10 hours of pupation. As a first estimation of lacZ reporter gene activity driven by the HexEnh DNA sequences, male and female 5-h transgenic pupae were assayed for the transgene product, β-galactosidase activity. Female pupae of all three transgenic lines exhibited very high levels of β-galactosidase activity: ranging from highly female-biased expression (84-fold higher in I1 females or 295-fold higher in F16 females vs. males) to complete female-specific expression in the 2M2 lines (Fig. 2A). Indeed, for the 2M2 and F16 male pupae, crude β-galactosidase activities were equal to or at most 2-fold higher than in control WE males. The almost 20-fold range in reporter gene expression among female transgenic pupae of the three lines suggests that chromosomal position of transgene insertion may play a significant role in determining the maximum level of female-specific expression. Only a very poor polyclonal anti-β-galactosidase antibody is available commercially; hence we were unable to confirm sex-specific expression of the β-galactosidase protein by western blot analysis.
Figure 2. Female-specific expression of the β-galactosidase reporter in transgenic Ae. aegypti.
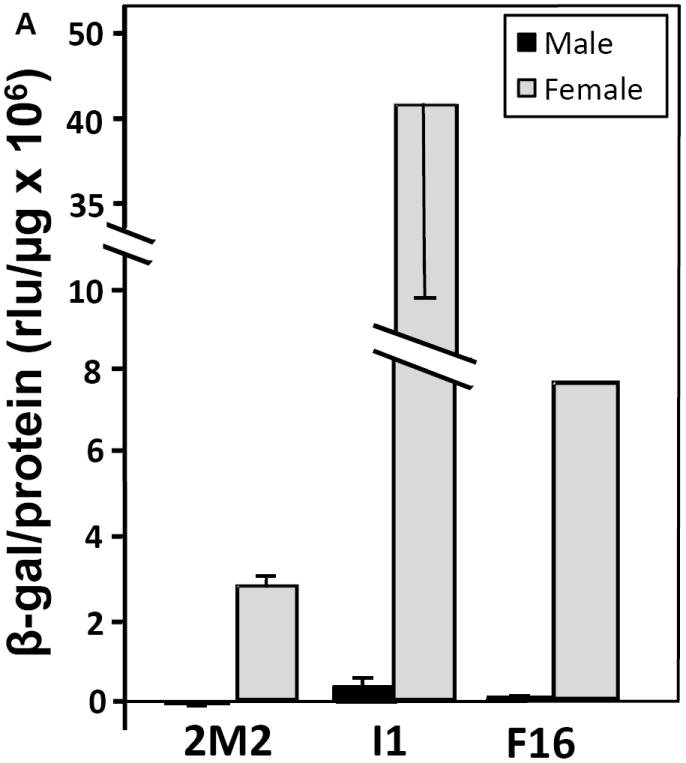
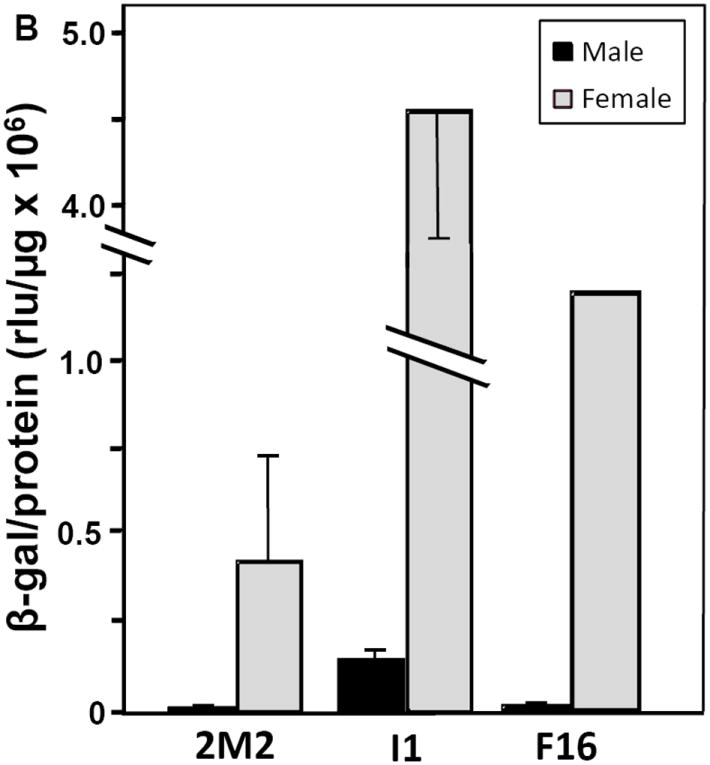
(A) Female-specific expression of the β-galactosidase reporter in transgenic pupae. (B) Female-biased expression of β-galactosidase in young transgenic adults. Individual 5-h pupae or five pooled 5-d adults of each sex from the 2M2, F16 and I1 transgenic lines and the WE control were collected and assayed in triplicate (technical replicates) for β-galactosidase (β-gal) activity (normalized to protein concentration). Low non-specific background activity detected in male or female WE samples was subtracted from means of transgenic β-galactosidase levels for the corresponding sex. Differences between females and males of each transgenic line were significant, with p-values of 9×10-3, 1×10-3 and 1×10-4 for 2M2, I1 and F16 pupae, respectively, and 0.06 and 5×10-4 for 2M2 and I1 adults.
β-galactosidase reporter activity was also examined in adult mosquitoes of all three transgenic lines (Fig. 2B). Overall expression levels were reduced about 7- to 10-fold as compared to 5-h pupae (Fig. 2A). Young adult females exhibited clearly much higher expression than males, ranging from 141-fold higher in the 2M2 line, 104-fold in the F16 line to 26-fold higher in the I1 line (Fig. 2B). However, expression appeared derepressed in 5-day old adult males, resulting in female-biased expression that also reflected chromosomal position effects on each transgenic line: highest levels appeared in I1 and lowest again in the 2M2 line. In future studies, we will use the phi C31 viral integrase system (Nimmo et al. 2006) to neutralize position effects and study the molecular basis for female-specific activity of HexEnh sequences at the pre-adult stages.
Stage-specific expression of the β-galactosidase reporter
Previously, we characterized the temporal profile of Hex-1.2 mRNA expression in Ae. atropalpus (Zakharkin et al. 2001) and found highest expression in female L4 larvae, a reduction in expression in late pupae and a minor increase in young adult females; no mRNA was detected in L1 or L2 larvae or older adult females. Examination of the Ae. aegypti 2M2 line (carrying the 0.7HexEnh-hsp-lacZ transgene) confirmed that the HexEnh sequences targeted reporter gene expression to the same specific developmental stages as the native Hex-1.2 gene. Late L4 female larvae and 5-h pupae (Fig. 3) exhibited the highest level of β-galactosidase activity with no detectable expression in males. Female-specific expression was still maintained in older 46-h pupae. However, in repeated experiments, β-galactosidase activity rose slightly in young adult females at the same time as very low expression appeared in males. Female-biased expression was still preserved in 10-day old adults although it was lower than in younger females; male expression appeared to rise, suggesting depression of reporter gene activity with age. Except for the minor derepression in older male adults, lacZ reporter gene activity in the 2M2 line appeared to completely replicate the expression observed for the native Hex-1.2 gene (Zakharkin et al. 2001). It was not possible to examine the effect of bloodfeeding on 0.7HexEnh-hsp-lacZ transgene expression as β-galactosidase activity, due to the presence of mouse hemoglobin which masked β-galactosidase enzyme activity (as detected by the chemiluminescent assay; see Experimental procedures) even 48-hours after bloodfeeding. We looked at the effect of bloodfeeding on lacZ mRNA levels, reported below.
Figure 3. Stage-specific expression of the β-galactosidase reporter in the 2M2 transgenic line.
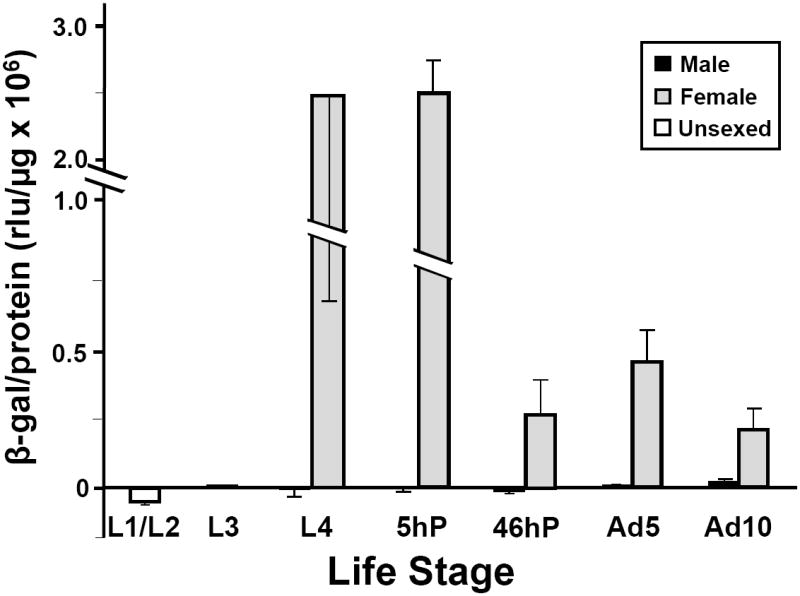
Frozen mosquitoes of the indicated developmental stages from the 2M2 line and the WE control were assayed for β-galactosidase (β-gal) activity (as in Fig. 2). Third-instar (L3) larvae (3 samples of ~30 larvae each) and a mixture of L1 and L2 larvae were collected. Individual L4 larvae (~48 h after L3/L4 molt; n=3) were dissected and the abdomens assayed for β-gal activity; RNA isolated from the head and thorax of each larva was used to assay its sex by RT-PCR as described in Experimental procedures. Individual pupae (n=3) were assayed in repeated experiments at 5 and 46 h after the larval/pupal molt. Three (3) groups of 5 adult animals pooled were assayed at days 5 and 10 after eclosion.
Fat body-specific activity of the β-galactosidase reporter
The 679-bp Hex-1.2 sequences in the 0.7HexEnh-hsp-lacZ fusion gene directed fat body tissue specificity in L4 larvae from all three of our Ae. aegypti transgenic lines and confirmed the sex specificity in 2M2 larvae (Fig. 4A). No staining was detected in male larvae from the other two transgenic lines (data not shown). However, this finding was not surprising as the histochemical assay is not as sensitive as the chemiluminescent assay for β-galactosidase activity. In some L4 larvae, we occasionally detected in the midgut ectopic staining (*, in Fig. 4A) which has also been observed in many different, transgenic Drosophila lines, generated by transformation with the pCaSpeR-hs43-lacZ vector. Gut bacteria can be responsible for expression of a prokaryotic β-galactosidase, depending on feeding and digestion conditions. Fat body-specific expression was preserved in transgenic adult females (only the 2M2 line shown in Fig. 4B), with again no detectable histochemical β-galactosidase activity in males due to a less sensitive assay.
Figure 4. Female- and fat body-specific expression of the β-galactosidase reporter in transgenic mosquitoes.
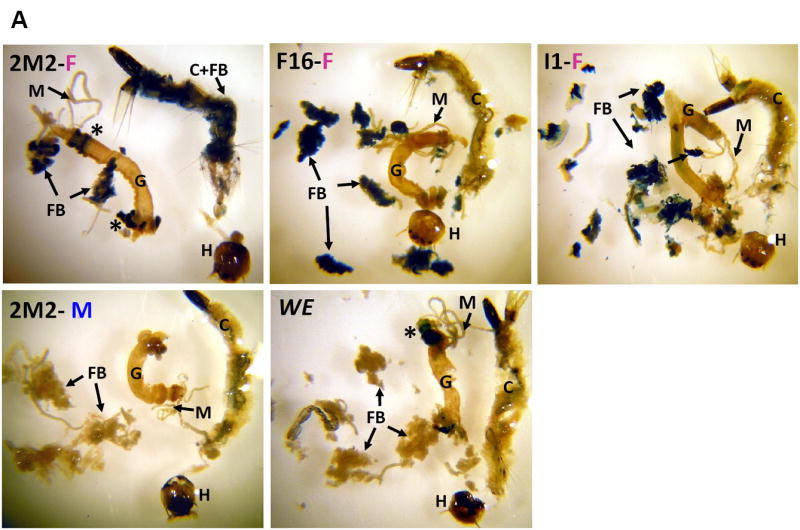
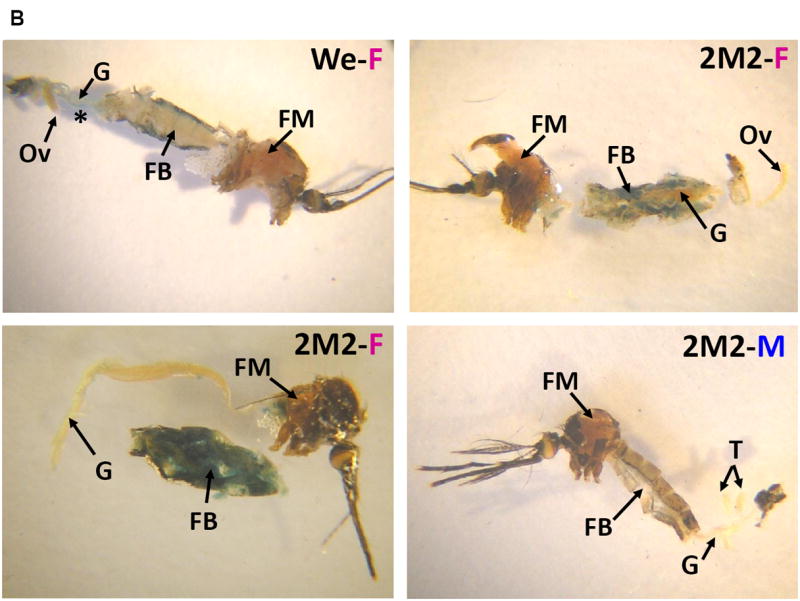
(A) L4 larvae from the indicated transgenic lines or the WE control. (B) Adults from the 2M2 line compared to a female WE control. Animals were dissected and stained for β-galactosidase activity as previously described (Beneš et al. 1996). C, cuticle; FB, fat body; FM, flight muscle; G, gut; H, head; M, Malpighian tubules; Ov, ovary; T, testes; *, non-specific staining in the midgut.
lacZ reporter gene expression at the RNA level
To begin to elucidate the mechanism by which the HexEnh sequences from the Ae. atropalpus Hex-1.2 gene drive female-specific reporter gene activity in transgenic mosquitoes of the closely related species, Ae. aegypti, we performed real-time PCR analysis to examine lacZ mRNA levels in the three transgenic lines. In repeated experiments, using primer pairs targeted to the 3’ end of the transcript, we could detect no statistically significant difference between mRNA levels in female and male 5-h pupae (Table 1). We also examined different sections of the entire 4.2-kb hsp-Adh-lacZ-SV40 fusion gene transcription unit, using primer pairs targeted to the 5’UTR, the Adh-lacZ junction, internal sequences of the lacZ coding region, the lacZ-SV40 junction and the SV40 polyadenylation signaling region and could not demonstrate any sex-specific accumulation of the transgene transcript (see Supporting Information). This is in direct contrast to our original findings for the Hex-1.2 mRNA which is only synthesized in female L4 larvae and young pupae of Ae. atropalpus, as detected by northern blot analysis (Zakharkin et al. 2001) and more recently by qRT-PCR (data not shown). For the 2M2 line, we examined male and female mRNA levels at different developmental stages: L4 larvae, 5-hr pupae and 5-day old adults. Despite differences in protein expression (Figs. 3 and 4), we could detect no statistically significant differences in lacZ mRNA levels at the L4 and P5 stages (Table 2). Only in young adults (Ad5), a 3.6-fold difference in lacZ mRNA was statistically significant and could only partially account for the difference between female and male transgene expression (141-fold difference, Fig. 2B) at this specific stage in 2M2. In contrast, bloodfed females appeared to have 2-fold lower lacZ mRNA levels than unfed females; but this difference did not achieve significance. Since we observed differences in CT values between the L4 and Ad5 stages versus the P5 stage (Table. S3), we performed a 2-way ANOVA to examine a potential group (male vs. female) and stage interaction; however, this interaction proved to be only marginally significant (p = 0.09).
Table 1.
Comparison of relative lacZ mRNA expression in transgenic Ae. aegypti lines.
| Line | Fold difference between females and males | p |
|---|---|---|
| 2M2 | 1.07 | 0.84 |
| I1 | -1.16 | 0.62 |
| F16 | -1.53 | 0.27 |
Levels of the Adh-lacZ-SV40 transcript in female vs. male transgenic 5-h pupae were analyzed by real-time PCR, relative to α-tubulin mRNA levels. Primers (lacZ-SV40 bridge) and qPCR conditions are described in Experimental procedures and Table S1. No significant p values were observed in a 2-way ANOVA.
Table 2.
Comparison of relative lacZ mRNA expression at different developmental stages of the 2M2 transgenic line.
| Developmental stage | Fold difference between females and males | p |
|---|---|---|
| L4 larvae | 1.11b | 0.85 |
| P5 pupae | 1.3a | 0.48 |
| Ad5 adults | 3.58b | 0.03* |
| Bloodfed females* | -2.49a | 0.22 |
| -1.91b | 0.45 |
Levels of the Adh-lacZ-SV40 transcript in female vs. male 2M2 mosquitoes, of the indicated developmental stages, were analyzed by real-time PCR, relative to α-tubulin or β-actin mRNA levels. Primers (lacZ-SV40 bridge) and qPCR conditions are described in Experimental procedures and Table S1.
In a 1-way ANOVA performed for each stage, a significant p value was only observed for 5-day old adults. Bloodfed females were compared to unfed females.
Δ tub data used in statistical analysis;
Δ act data.
To explain the observation of similar reporter gene mRNA levels in males and females, in contrast to the dramatic differences in β-galactosidase activities (reflecting protein levels) between males and females at all stages examined (Figs. 2, 3 and 4), we considered two possible mechanisms. There might be read-through transcription from the EGFP transgene through to the lacZ transcription unit. By conventional RT-PCR using the eGFP-lacZ bridge pair of primers, we could detect no cDNA product, although the primers amplified a DNA product of the predicted size from the plasmid used to generate transgenic mosquitoes. We also examined the possibility of an alternate promoter and transcription start site, producing a different 5’UTR than the expected hsp70-Adh fusion sequence.
We undertook a preliminary bioinformatic analysis of the Hex-1.2 DNA sequences fused just upstream of the Drosophila hsp70 promoter (see Fig. 1) and discovered a putative “TATA” box and transcription start site at positions -202 and -171, respectively, relative to the transcription start site of the Hex-1.2 gene in Ae. atropalpus larvae (Zakharkin et al. 2001; ). Furthermore, an initiator (Inr) element (Cherbas and Cherbas 1993) was found at the expected interval (-10, +10) relative to the anomalous transcription start site (at -171; see Fig. 1, blue arrow) and may allow the discrimination between two promoters, the putative one at -202 and the Drosophila hsp70 promoter downstream. Upon closer inspection, we found that the hsp70 promoter does not have any Inr element corresponding to the most conserved sequence or its cognates (see Cherbas and Cherbas, 1993) and may be less likely to be used in Ae. aegypti. Furthermore, between the anomalous “TATA” box (at -202) and the first codon (ATG) of the Drosophila Adh coding sequence, there is no other “ATG” sequence. Hence, use of this anomalous upstream promoter and transcription start site should still produce the same functional reporter gene product: a β-galactosidase fusion protein and enzyme that would result in the same enzymatic and biochemical activities that we measure at the L4, pupal and early adult stages.
We examined the cDNAs from 5-hr pupae (P5) of the 2M2 line and were able to detect, by RT-PCR, a 360-bp fragment from the predicted 405-bp “Anomalous 5’UTR” (Fig. S2); its identity was confirmed by DNA sequencing. This 405-bp 5’UTR could be the binding site of non-coding regulatory RNAs (Kaikkonen et al. 2011) such as microRNAs (miRNAs or miRs) or long-non-coding RNAs (lncRNAs) which could prevent (or dramatically reduce in lines F16 and I1) translation of the lacZ mRNA in males at the L4 larval, pupal and adult stages. While miRNAs were originally thought to regulate translation by binding to 3’UTRs of mRNAs, more recently miR binding sites have been discovered in the 5’UTR and coding regions (Gebauer et al. 2012; Li et al. 2010; Medenbach et al. 2011; Moretti et al. 2010). Hence, the sex-specific expression of the fusion reporter gene (lacZ), as seen at the protein level with β-galactosidase activities (Figs. 2, 3, 4), is likely to be post-transcriptionally regulated in Ae. aegypti. We are currently examining the 5’end of the lacZ transcript in all three transgenic lines and will screen an Ae. aegypti miRNA database (Li et al. 2009) to determine if there might be binding sites in this “anomalous” 405-bp 5’UTR. A more focused study of sex-specific miRNA or lncRNA expression in Ae. aegypti will be needed to elucidate and confirm a potentially novel, post-transcriptional mechanism for sex-specific regulation of gene expression.
Conclusions
We report here the discovery of a gene regulatory module, HexEnh., a DNA sequence from an heterologous hexamerin gene (Hex-1.2 of Ae. atropalpus), that produces female-specific, stage-specific and fat body-specific expression of a reporter transgene in immature Ae. aegypti. This gene regulatory module was tested before, in its natural state, with the Hex-1.2 promoter and 5’UTR, and was only able to induce low-level female-biased reporter gene activity in transgenic Drosophila lines (Jinwal et al. 2006). In the fruit fly, reporter gene activity resulted in female-based mRNA synthesis, indicating that the HexEnh sequences together with the homologous Ae. atropalpus promoter (“TATA box”) induced transcriptional regulation in the fly. However, our current findings suggest that when the HexEnh DNA sequences are fused to an heterologous Drosophila promoter and 5’UTR, they are used in the related aedine mosquito, Ae. aegypti, in a stage-specific manner (Fig. 3) to produce female-specific gene expression (Fig. 2A and 2B), which is influenced by transgene chromosomal position and may be regulated post-transcriptionally. The Drosophila hsp70 basal promoter (without heat shock elements) has been used extensively for low-level, consistent expression of transposase activity in transgenic Ae. aegypti (Adelman et al. 2004; Morris et al. 1989; Pinkerton et al. 2000) However, to our knowledge the hsp70 promoter has not been coupled with a tissue-specific enhancer as we tested here.
None of the endogenous hexamerin genes of Ae. aegypti exhibit pure female-specific activity; however, expression of the Hex-1γ protein (the homolog of the Ae. atropalpus Hex-1.2 protein) in Ae. aegypti shows a weak female bias (Korochkina et al. 1997b). Further studies are on-going to determine if regulated Hex-1γ expression in Ae. aegypti females is transcriptional or post-transcriptional. Our data reported here suggest that the Ae. aegypti fat body has the molecular machinery to recognize signals from a female-specific Ae. atropalpus gene. These signals may be transcription factor binding sites, such as the putative GATA, C/EBP and AEF-1 sites observed in the HexEnh sequences and known to be important for stage-specific and fat body-specific expression in dipteran insects (Abel et al. 1992; Abel et al. 1993; An et al. 1996; Beneš et al. 1996; Dittmer and Raikhel 1997; Falb and Maniatis 1992; Garabedian et al. 1986; Kapitskaya et al. 1998; Kokoza et al. 2000; Martin et al. 2001) or, as yet uncharacterized, non-coding RNA (ncRNAs) binding sites necessary for post-transcriptional regulation. ncRNAs may be targeted by transcription factors, thereby coupling transcriptional and translational regulation (Kaikkonen et al. 2011). From our qRT-PCR analysis, sex-specific regulation dictated by the HexEnh sequences appears to be post-transcriptional, at the L4 and P5 stages; however, a different or additional regulatory mechanism may operate at the adult stage. This difference in stage-specific regulation is not unprecedented, and could be mediated by miRNAs or lncRNAs acting very specifically at each developmental or physiological stage (Behura et al. 2011; Campbell et al. 2008; Mead et al. 2012). In Ae. aegypti transgenic adults described here, the adult female specificity of the Ae. atropalpus Hex-1.2 gene (Zakharkin et al., 2001) is lost, possibly because of a stage-specific difference in post-transcriptional regulation: the same ncRNA may not be expressed at all or to the same level in transgenic L4 larvae and Ad5 adults. The adult fat body of aedine mosquitoes is derived from the larval fat body and undergoes some remodeling (Clements 1992) which clearly allows a very specific response to hormonal changes, the blood meal and parasite infection in females, in contrast to males. The role of miRNAs has been well demonstrated to play a role in response to signaling in different mosquito tissues and in response to a blood meal (Bryant et al. 2010; Campbell et al. 2008; Mead et al. 2012; Winter et al. 2007).
While our studies to date suggest that the HexEnh sequences identified in the Ae. atropalpus Hex-1.2 gene operate to dictate female bias or specificity, the molecular mechanisms used may be quite different in Ae. aegypti, Ae. atropalpus and the more distantly related D. melanogaster. We observe transcriptional regulation in Ae. atropalpus and Drosophila, using the Hex-1.2 HexEnh and promoter sequences (Jinwal et al. 2006; Zakharkin et al. 2001). Preliminary studies suggest a role for DSX in female-biased transcription in Drosophila (Umesh Jinwal and Helen Beneš, unpublished observations). Doublesex also appears to play an important role in the female-specific activity of a typically lepidopteran female-specific hexamerin gene (Telfer 2009; Telfer and Pan 2003). Expression of the female isoform of doublesex mRNA induced anomalous Storage Protein 1 expression in male silkworms (Bombyx mori) (Suzuki et al. 2003). More recently, knockdown of the dsx transcripts by RNA interference in two wild silkmoths produced complete abolition of both hexamerin and vitellogenin expression (Shukla and Nagaraju 2010b). As Ae. atropalpus is a facultatively autogenous mosquito (the Hex-1.2 gene was isolated from a constituitively autogenous strain), it may have evolved different mechanisms than Ae. aegypti to achieve female-biased (for its other hexamerins; (Zakharkin et al. 2001) or female-specific expression of the Hex-1.2 gene. In Ae. aegypti post-transcriptional, specifically translational control, mechanisms could involve miRNAs or lncRNAs that bind to the 5’UTR, in our case a composite including Hex-1.2 sequences from the HexEnh. Indeed, species-specific differences in upstream elements have been implicated in differences in regulatory control among mosquito and other dipiteran species (Behura et al. 2011; Bryant et al. 2010; Li et al. 2009; Mead and Tu 2008; Severson and Behura 2012; Skalsky et al. 2010).
Further studies are in progress to understand the mechanism of female-specific activity of our lacZ fusion gene in transgenic Ae. aegypti and will test the roles of the individual DSX-binding sites and potential miRNA or lncRNA binding sites in this putative Hex enhancer using the phi C31/attP system to mitigate the effects of chromosomal position (Nimmo et al. 2006). Studies are in progress to determine if female-specific expression in the transgenic 2M2 line of Ae.aegypti is due to DSX binding sites or similar mechanisms that underlie female-biased expression of the endogenous Hex-1γ gene of this anautogenous mosquito. Chromatin context in aedine mosquitoes will also need to be explored, given the wide range of expression levels in the transgenic Ae. aegypti lines reported here.
Since our characterization of HexEnh activity in both Drosophila and Ae. aegypti showed that it completely recapitulated the spatial and temporal specificity of the original gene activity (Jinwal et al. 2006), we expect this regulatory module to be functional in many mosquito species. The HexEnh sequence targets reporter gene expression both uniquely in females and to a critical insect tissue, the fat body. To our knowledge, no other gene regulatory element identified in mosquitoes to date targets gene expression to the late-larval and pupal fat body. The fat body is essential for coordinating metabolism through different hormonal and nutritional signals regulating insect development, metamorphosis, and reproduction (Aguila et al. 2007; Canavoso et al. 2001; Colombani et al. 2005; King-Jones and Thummel 2005; Nelliot et al. 2006). Indeed, a strong Drosophila hexamerin promoter (previously characterized by us; Beneš et al. 1996) was successfully used for transgenic ablation of the larval fat body and proved its indispensability for pupal metamorphosis and adult development (Liu et al. 2009). Using mathematical modeling, Phuc et al. showed that a late-acting lethality was more effective in a population limited by density-dependent effects (e.g., a larval mosquito population) because delaying the time of death until after the larval/pupal transition phase actually proved beneficial (Phuc et al. 2007). Hence, female-specific targeting of gene expression by the HexEnh regulatory element could be used in strategies for controlling lepidopteran agricultural pests by transgenic sexing for elimination of females or even inducing transgenic sterility by driving genes affecting female fertility, as previously suggested (Nolan et al. 2011). However, our present report suggests that differences even among closely related species will need to be taken into account in the use of transgenes for SIT or RIDL-based insect control.
Experimental procedures
Mosquitoes
Aedes aegypti mosquitoes of the White-eyed (WE or khw) strain (Cornel et al. 1997), obtained from the Insect Transformation Facility (ITF; http://ibbr.umd.edu/insect_transformation) at the Institute for Bioscience and Biotechnology Research, University of Maryland (IBBR, Rockville, MD, USA) where transgenic mosquitoes were generated. Mosquitoes were reared at 26.5°C under standard conditions (Shapiro and Hagedorn 1982), with adults maintained on a solution of 10% sucrose; females were allowed to bloodfeed on mice. Fourth-instar larvae (L4) were sexed by RT-PCR as described below. Male and female pupae, collected as “white” pupae at the L4/pupal molt, were staged at indicated hours after the molt and sexed by observation of differently shaped primordia for external genitalia. Adult mosquitoes were staged relative to eclosion and selected according to sex-specific antennal morphology.
Fusion gene construction
From a portion of the 5’-flanking region of the Hex-1.2 gene, previously isolated and subcloned as described (Zakharkin et al. 2001), a 696-bp DNA fragment was amplified using primers to introduce restriction sites (underlined) for subcloning: Hex-0.7EcoRI-Forward 5’-TTGGAATTCTGCATAAAAAAGATATGACTATCAAGG-3’ and Hex1.2Enh-BamHI-Reverse 5’-CGCGGATCCAAAAATGTTCTCGATCTCACTGC-3’. PCR conditions included an initial denaturation at 93°C for 3 min, followed by 30 cycles of 93°C, 10 s; 53°C, 30 s; 72°C, 1 min. Following restriction digestion, the 683-bp fragment was subcloned into the EcoRI and BamHI sites of the pCaSpeR-hs43-lacZ vector (obtained from the Drosophila Genomics Resource Center, https://dgrc.cgb.indiana.edu; (Pirrotta 1994)), generating pCaSpeR-0.7HexEnh-hs43-lacZ. This plasmid was then digested with HindIII and EcoRI to subclone the 0.7HexEnh-hs43-lacZ fusion gene into the pSLfa1180fa shuttle vector (Horn and Wimmer 2000); digestion of the pSL0.7HexEnh-hs43-lacZ plasmid with AscI allowed subcloning of the 0.7HexEnh-hs43-lacZ fusion gene in the insect pMos3xP3-EGFP transformation vector (Horn and Wimmer 2000), producing pMos-0.7HexEnh-hs43-lacZ. Sequence analysis of the 0.7HexEnh-hs43-lacZ fusion gene in the pMos vector was performed by the DNA Sequencing Core Facility at the University of Arkansas for Medical Sciences with sequence-specific synthetic primers (produced by Integrated DNA Technologies, Inc., Coralville, IA, USA).
Generation of transgenic Aedes aegypti
To produce transgenic lines carrying single copies of the 0.7HexEnh-hs43-lacZ fusion gene, Transgenic Aedes aegypti were generated at the Insect Transformation Facility at the University of Maryland by established methods (Morris et al. 1989). Preblastoderm embryos of the white-eyed (WE) strain were microinjected with 150 ng/μl of pMos-0.7HexEnh-hs43-lacZ (the donor plasmid), 200 ng/μl of the pKhsp82M helper (Coates et al. 1998), 50 ng/μl of an ITF pBac-ECFP control plasmid and 200 ng/μl of the phsp pBac (Handler and Harrell 1999). The control vector, ITF-pBac-ECFP, was included to assure the microinjection quality as determined by obtaining ECFP-expressing, stably transformed mosquitoes. G1 offspring were screened for the presence of EGFP expression from the transformation marker of pMos-0.7HexEnh-hs43-lacZ. Three transgenic lines, F16-N-G, I1-N-G and 2M2-N-G, were confirmed by PCR, and then sequencing, of genomic DNA to contain the Hex portion of the 0.7HexEnh-hs43-lacZ transgene, using the forward primer MosLf (5’-CAACTGTGAAAACGTGTGAACGG-3’) and the reverse primer Hsp43r (5’-GCTTAGCTTTCGCTTAGCGACG-3’) from the hsp70 basal promoter sequence. To confirm that each transgenic line contained only a single transgene insertion, Southern blot analysis was performed as previously described for Drosophila (Beneš et al. 1996). Southern blots were probed with a 683-bp radioactive DNA fragment, containing the entire Hex-1.2 sequence from the HexEnh-hs43-lacZ fusion gene.
β-Galactosidase activity and histochemical assays
Individual animals (or groups as indicated) were collected at different developmental stages, rapidly frozen and stored at -80°C. Assays for transgene expression as β-galactosidase (β-gal) activity were performed on a minimum of 3 animals per assay, with 3 technical replicates per sample; each developmental stage was repeated at least 3 times. For L4 larvae, assays were performed on only the abdomen, as the head and thorax were used for sexing each larva by an RT-PCR assay (see below). β-galactosidase activity was assayed using the Galacto-Light Plus kit, according the manufacturer’s protocol (Applied Biosystems, Foster City, CA, USA), and a PerkinElmer (Boston, MA, USA) Wallac VICTORLight 1420 Luminescence Counter. Protein concentrations were determined on an aliquot of the lysate using the Bradford reagent (Sigma, St. Louis, MO, USA). Activities were normalized to protein and expressed as Relative Light Units (RLU)/μg protein; averages of non-specific activity, measured in the same experiment in the WE host strain of the same age and sex, were subtracted from results for the transgenic lines to plot results representing only lacZ transgene activity. Statistical calculations to determine significance by a Student’s t test were carried out in Microsoft Excel 2007.
For analysis of tissue-specific lacZ gene expression, or β-galactosidase activity, fourth-instar larvae and 3-day old adults from the transgenic lines and the WE recipient strain were dissected and stained as previously reported for Drosophila larvae and adults (Beneš et al. 1996). Staining times were 6-8 hrs for larvae and 16-18 hrs for adults.
Conventional and real-time PCR
Total RNA was isolated from individual, frozen L4 larvae, individual sexed pupae or 5 pooled adults using the TRIzol reagent in a protocol scaled down from the manufacturer’s specifications (Invitrogen/Life Technologies, Carlsbad, CA, USA). RNA purity and concentration were assessed using a Nanodrop NO-1000 (Nanodrop Technologies, Wilmington, DE, USA). cDNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA) which includes a vendor-specific RNase-free deoxyribonuclease I and uses 0.8 μg of RNA, oligo-dT20 and random hexanucleotide primers.
To sex L4 larvae, we developed a simple RT-PCR assay based on our previous cloning and characterization of cDNAs encoding the male and female isoforms of the Doublesex transcription factor (Jinwal et al., unpublished data). As in other insects, splicing results in the expression of sex-specific dsx mRNAs that can be detected by RT-PCR. Primers (synthesized by IDT, Coralville, IA, USA) were used as follows: Common Forward, 5’-GATGGTAAAGATGTCCGAGG-3’; Male-Specific Reverse, 5’-GAGGGAAAGGGAATGCTAGTG-3’ in reactions initial denaturation at 93°C for 3 min, followed by 30 cycles of 93°C, 10 s; 50°C, 30 s; 72°C, 1 min. This sexing assay was validated in 5-h male and female pupae whose sex was determined by the appearance of external morphological criteria (genital primordial). In male pupae, PCR resulted in a single 1198-bp product (confirmed by DNA sequencing as a male dsx splice variant), while there were multiple non-specific products in a consistent pattern from females of the same stage (Fig. S1).
RT-PCR was also used to demonstrate the presence of an anomalous 5’-UTR. Primers (synthesized by IDT, Coralville, IA, USA and used as follows) produced a 360-bp product (Fig. S2): Forward, 5’-GATGGTAAAGATGTCCGAGG-3’; Male-Specific Reverse, 5’-GAGGGAAAGGGAATGCTAGTG-3’ in reactions initial denaturation at 93°C for 3 min, followed by 30 cycles of 93°C, 10 s; 56°C, 30 s; 72°C, 1 min.
Real-time quantitative PCR (q-PCR) amplification reactions, to assess mRNA levels, were performed using the SYBR Green PCR Mix (Applied Biosystems, Warrington, U.K.). For each primer set reported (see Supporting Information), we obtained extension efficiencies within 10% of the perfect efficiency of 2. A number of different primer pairs were used to screen for expression across the entire Adh-lacZ-SV40 transcript (see Supporting Information and Table S1). Repeated experiments were performed to amplify the 3’ end of the transcript using a lacZ-SV40 bridge primer pair: lacZ forward: 5’-CCACCGCTGTTTGGTCTGCTTTCT-3’; SV40 reverse: 5’-GTCACACCACAGAAGTAAGGTTCC-3’. Duplicate samples of 3 individual pupae of each sex, or male and female adults (with and without blood-feeding) were analyzed using the DNA Engine Opticon 2 Detection System (MJ Research, Waltham, MA, USA) with Opticon2 Monitor software. Amplifications were performed as follows: initial denaturation at 95°C for 15 min, followed by 40 cycles of 15s at 95°C, 30s at 58°C and 30s at 72°C. The β-actin and α-tubulin transcripts served as internal controls to normalize RNA levels from different samples and were detected using the following primers: α-tubulin transcript, forward: 5’-AGGCTGTCGGTTGACTATGG-3’; α-tubulin reverse: 5’-TATCGACCATGAACGCACAG-3’; and β-actin primers (Zhu et al. 2003). Average CT values across all stages of development examined for these reference gene products were as follows: α-tubulin, males – 24.73±1.40, females – 23.87±1.21; β-actin, males – 17.42±0.86, females – 16.40±0.94.
To confirm our ability to detect female-specific transcripts, Vitellogenin (Vg) primer sequences were used as previously reported (Zhu et al. 2003). Data were analyzed using the comparative ΔΔCT method (Livak & Schmittgen, 2001) to calculate differences in transcript levels between samples. Foreach developmental stage, each experiment was repeated three times, for a total of 9 female and 9 male pupae assayed technically in duplicate, allowing an ANOVA to test for significance. Where appropriate, one-way or two-way ANOVA was performed to determine if there were any statistically significant differences in sex-specific expression among all three transgenic lines (Table 1) or at different developmental stages of the 2M2 transgenic line.
Supplementary Material
Fig. S1. Sexing individual pupae by detection of a male-specific dsx mRNA variant.
Fig. S2. Detection of upstream sequences from the HexlacZ reporter gene transcript in pupae.
Acknowledgments
This research was supported by grants from the National Institutes of Health, USA (AI046738 to H.B.) and a Pilot Study Grant (to H.B.) from the College of Medicine Research Council at the University of Arkansas for Medical Sciences. We thank Kristina Pilitt (University of Maryland) for her reading of the manuscript, and Eric R. Siegel (University of Arkansas for Medical Sciences) for statistical advice. This report is dedicated to the memory of William H. Telfer, the “father” of insect hexamerin research.
References
- Abel T, Bhatt R, Maniatis T. A Drosophila CREB/ATF transcriptional activator binds to both fat body- and liver-specific regulatory elements. Genes Dev. 1992;6:466–80. doi: 10.1101/gad.6.3.466. [DOI] [PubMed] [Google Scholar]
- Abel T, Michelson AM, Maniatis T. A Drosophila GATA family member that binds to Adh regulatory sequences is expressed in the developing fat body. Development. 1993;119:623–33. doi: 10.1242/dev.119.3.623. [DOI] [PubMed] [Google Scholar]
- Adelman ZN, Jasinskiene N, Vally KJ, Peek C, Travanty EA, Olson KE, Brown SE, Stephens JL, Knudson DL, Coates CJ, James AA. Formation and loss of large, unstable tandem arrays of the piggyBac transposable element in the yellow fever mosquito, Aedes aegypti. Transgenic Res. 2004;13:411–25. doi: 10.1007/s11248-004-6067-2. [DOI] [PubMed] [Google Scholar]
- Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK. The role of larval fat cells in adult Drosophila melanogaster. J Exp Biol. 2007;210:956–63. doi: 10.1242/jeb.001586. [DOI] [PubMed] [Google Scholar]
- Alphey L. Re-engineering the sterile insect technique. Insect Biochem Mol Biol. 2002;32:1243–1247. doi: 10.1016/s0965-1748(02)00087-5. [DOI] [PubMed] [Google Scholar]
- An W, Cho S, Ishii H, Wensink PC. Sex-specific and non-sex-specific oligomerization domains in both of the doublesex transcription factors from Drosophila melanogaster. Mol Cell Biol. 1996;16:3106–11. doi: 10.1128/mcb.16.6.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An W, Wensink PC. Integrating sex- and tissue-specific regulation within a single Drosophila enhancer. Genes Dev. 1995a;9:256–66. doi: 10.1101/gad.9.2.256. [DOI] [PubMed] [Google Scholar]
- An W, Wensink PC. Three protein binding sites form an enhancer that regulates sex- and fat body-specific transcription of Drosophila yolk protein genes. EMBO J. 1995b;14:1221–30. doi: 10.1002/j.1460-2075.1995.tb07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova Y, Alvarez KS, Kim YJ, Kokoza V, Raikhel AS. The role of NF-kappaB factor REL2 in the Aedes aegypti immune response. Insect Biochem Mol Biol. 2009;39:303–14. doi: 10.1016/j.ibmb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behura SK, Haugen M, Flannery E, Sarro J, Tessier CR, Severson DW, Duman-Scheel M. Comparative genomic analysis of Drosophila melanogaster and vector mosquito developmental genes. PLoS One. 2011;6:e21504. doi: 10.1371/journal.pone.0021504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ, Robinson AS. Impact of technological improvements on traditional control strategies. Adv Exp Med Biol. 2008;627:84–92. doi: 10.1007/978-0-387-78225-6_7. [DOI] [PubMed] [Google Scholar]
- Beneš H, Neal KC, Willis RL, Gadde D, Castleberry AB, Korochkina SE. Overlapping Lsp-2 gene sequences target expression to both the larval and adult Drosophila fat body. Insect Mol Biol. 1996;5:39–49. doi: 10.1111/j.1365-2583.1996.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Bryant B, Macdonald W, Raikhel AS. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2010;107:22391–8. doi: 10.1073/pnas.1016230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester T, Massey HC, Jr, Zakharkin SO, Beneš H. The evolution of hexamerins and the phylogeny of insects. J Mol Evol. 1998;47:93–108. doi: 10.1007/pl00006366. [DOI] [PubMed] [Google Scholar]
- Burtis KC. Development. Doublesex in the middle. Science. 2002;297:1135–6. doi: 10.1126/science.1074492. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Campbell CL, Black WCt, Hess AM, Foy BD. Comparative genomics of small RNA regulatory pathway components in vector mosquitoes. BMC Genomics. 2008;9:425. doi: 10.1186/1471-2164-9-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu Rev Nutr. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- Carlson J, Suchman E, Buchatsky L. Densoviruses for control and genetic manipulation of mosquitoes. Adv Virus Res. 2006;68:361–92. doi: 10.1016/S0065-3527(06)68010-X. [DOI] [PubMed] [Google Scholar]
- Cherbas L, Cherbas P. The arthropod initiator: the capsite consensus plays an important role in transcription. Insect Biochem Mol Biol. 1993;23:81–90. doi: 10.1016/0965-1748(93)90085-7. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. 1. Vol. 1. Chapman & Hall; New York: 1992. [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 1998;95:3748–51. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–70. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- Cornel AJ, Benedict MQ, Rafferty CS, Howells AJ, Collins FH. Transient expression of the Drosophila melanogaster cinnabar gene rescues eye color in the white eye (WE) strain of Aedes aegypti. Insect Biochem Mol Biol. 1997;27:993–7. doi: 10.1016/s0965-1748(97)00084-2. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993;7:42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Dittmer NT, Raikhel AS. Analysis of the mosquito lysosomal aspartic protease gene: an insect housekeeping gene with fat body-enhanced expression. Insect Biochem Mol Biol. 1997;27:323–35. doi: 10.1016/s0965-1748(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Falb D, Maniatis T. Drosophila transcriptional repressor protein that binds specifically to negative control elements in fat body enhancers. Mol Cell Biol. 1992;12:4093–103. doi: 10.1128/mcb.12.9.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian MJ, Shepherd BM, Wensink PC. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986;45:859–67. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Garcia GP, Flores AE, Fernandez-Salas I, Saavedra-Rodriguez K, Reyes-Solis G, Lozano-Fuentes S, Guillermo Bond J, Casas-Martinez M, Ramsey JM, Garcia-Rejon J, Dominguez-Galera M, Ranson H, Hemingway J, Eisen L, Black WCt. Recent rapid rise of a permethrin knock down resistance allele in Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2009;3:e531. doi: 10.1371/journal.pntd.0000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Preiss T, Hentze MW. From Cis-Regulatory Elements to Complex RNPs and Back. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a012245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM, Harrell RA., 2 Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol. 1999;8:449–57. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- Haunerland NH. Insect storage proteins: gene families and receptors. Insect Biochem Mol Biol. 1996;26:755–65. doi: 10.1016/s0965-1748(96)00035-5. [DOI] [PubMed] [Google Scholar]
- Heinrich JC, Scott MJ. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc Natl Acad Sci U S A. 2000;97:8229–32. doi: 10.1073/pnas.140142697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev Genes Evol. 2000;210:630–7. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–5. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- James AA. Engineering mosquito resistance to malaria parasites: the avian malaria model. Insect Biochem Mol Biol. 2002;32:1317–23. doi: 10.1016/s0965-1748(02)00094-2. [DOI] [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci U S A. 1998;95:3743–7. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinwal UK, Zakharkin SO, Litvinova OV, Jain S, Benes H. Sex-, stage- and tissue-specific regulation by a mosquito hexamerin promoter. Insect Molec Biol. 2006;15:301–311. doi: 10.1111/j.1365-2583.2006.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikkonen MU, Lam MT, Glass CK. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc Res. 2011;90:430–40. doi: 10.1093/cvr/cvr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitskaya MZ, Dittmer NT, Deitsch KW, Cho WL, Taylor DG, Leff T, Raikhel AS. Three isoforms of a hepatocyte nuclear factor-4 transcription factor with tissue- and stage-specific expression in the adult mosquito. J Biol Chem. 1998;273:29801–10. doi: 10.1074/jbc.273.45.29801. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Developmental biology. Less steroids make bigger flies. Science. 2005;310:630–1. doi: 10.1126/science.1120410. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Cho WL, Jasinskiene N, James AA, Raikhel A. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 2000;97:9144–9. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2010;107:8111–6. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korochkina SE, Gordadze AV, York JL, Beneš H. Mosquito hexamerins: characterization during larval development. Insect Mol Biol. 1997a;6:11–21. doi: 10.1046/j.1365-2583.1997.00150.x. [DOI] [PubMed] [Google Scholar]
- Korochkina SE, Gordadze AV, Zakharkin SO, Beneš H. Differential accumulation and tissue distribution of mosquito hexamerins during metamorphosis. Insect Biochem Mol Biol. 1997b;27:813–24. doi: 10.1016/s0965-1748(97)00053-2. [DOI] [PubMed] [Google Scholar]
- Li C, Marrelli MT, Yan G, Jacobs-Lorena M. Fitness of transgenic Anopheles stephensi mosquitoes expressing the SM1 peptide under the control of a vitellogenin promoter. J Hered. 2008;99:275–82. doi: 10.1093/jhered/esn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mead EA, Liang S, Tu Z. Direct sequencing and expression analysis of a large number of miRNAs in Aedes aegypti and a multi-species survey of novel mosquito miRNAs. BMC Genomics. 2009;10:581. doi: 10.1186/1471-2164-10-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mine T, Ioannides CG. Short GC-rich RNA similar to miR 1909 and 1915 folds in silico with the 5’-UTR and ORF of Notch and responders: potential for the elimination of cancer stem cells. Oncol Rep. 2010;24:1443–53. doi: 10.3892/or_00001004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Liu S, Wang S, Jiang RJ, Li S. Hormonal and nutritional regulation of insect fat body development and function. Arch Insect Biochem Physiol. 2009;71:16–30. doi: 10.1002/arch.20290. [DOI] [PubMed] [Google Scholar]
- Martin D, Piulachs MD, Raikhel AS. A novel GATA factor transcriptionally represses yolk protein precursor genes in the mosquito Aedes aegypti via interaction with the CtBP corepressor. Mol Cell Biol. 2001;21:164–74. doi: 10.1128/MCB.21.1.164-174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur G, Sanchez-Vargas I, Alvarez D, Olson KE, Marinotti O, James AA. Transgene-mediated suppression of dengue viruses in the salivary glands of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2010;19:753–63. doi: 10.1111/j.1365-2583.2010.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EA, Li M, Tu Z, Zhu J. Translational regulation of Anopheles gambiae mRNAs in the midgut during Plasmodium falciparum infection. BMC Genomics. 2012;13:366. doi: 10.1186/1471-2164-13-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EA, Tu Z. Cloning, characterization, and expression of microRNAs from the Asian malaria mosquito, Anopheles stephensi. BMC Genomics. 2008;9:244. doi: 10.1186/1471-2164-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medenbach J, Seiler M, Hentze MW. Translational control via protein-regulated upstream open reading frames. Cell. 2011;145:902–13. doi: 10.1016/j.cell.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Meredith JM, Basu S, Nimmo DD, Larget-Thiery I, Warr EL, Underhill A, McArthur CC, Carter V, Hurd H, Bourgouin C, Eggleston P. Site-specific integration and expression of an anti-malarial gene in transgenic Anopheles gambiae significantly reduces Plasmodium infections. PLoS One. 2011;6:e14587. doi: 10.1371/journal.pone.0014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti F, Thermann R, Hentze MW. Mechanism of translational regulation by miR-2 from sites in the 5’ untranslated region or the open reading frame. RNA. 2010;16:2493–502. doi: 10.1261/rna.2384610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Eggleston P, Crampton JM. Genetic transformation of the mosquito Aedes aegypti by micro-injection of DNA. Medical and Veterinary Entomology. 1989;3:1–7. doi: 10.1111/j.1365-2915.1989.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Nagoshi RN, Baker BS. Regulation of sex-specific RNA splicing at the Drosophila doublesex gene: cis-acting mutations in exon sequences alter sex-specific RNA splicing patterns. Genes Dev. 1990;4:89–97. doi: 10.1101/gad.4.1.89. [DOI] [PubMed] [Google Scholar]
- Nelliot A, Bond N, Hoshizaki DK. Fat-body remodeling in Drosophila melanogaster. Genesis. 2006;44:396–400. doi: 10.1002/dvg.20229. [DOI] [PubMed] [Google Scholar]
- Nimmo DD, Alphey L, Meredith JM, Eggleston P. High efficiency site-specific genetic engineering of the mosquito genome. Insect Mol Biol. 2006;15:129–136. doi: 10.1111/j.1365-2583.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T, Papathanos P, Windbichler N, Magnusson K, Benton J, Catteruccia F, Crisanti A. Developing transgenic Anopheles mosquitoes for the sterile insect technique. Genetica. 2011;139:33–9. doi: 10.1007/s10709-010-9482-8. [DOI] [PubMed] [Google Scholar]
- O’Brochta DA, Handler AM. Perspectives on the state of insect transgenics. Adv Exp Med Biol. 2008;627:1–18. doi: 10.1007/978-0-387-78225-6_1. [DOI] [PubMed] [Google Scholar]
- Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, Fu G, Condon KC, Scaife S, Donnelly CA, Coleman PG, White-Cooper H, Alphey L. Late-acting dominant lethal genetic systems and mosquito control. BMC Biology. 2007;5:1–11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton AC, Michel K, O’Brochta DA, Atkinson PW. Green fluorescent protein as a genetic marker in transgenic Aedes aegypti. Insect Mol Biol. 2000;9:1–10. doi: 10.1046/j.1365-2583.2000.00133.x. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. D.melanogaster P element CaSpeR-hs43-lacZ gene transformation vector. GenBank/EMBL/DDBJ. 1994;X81643 [Google Scholar]
- Raghavendra K, Barik TK, Reddy BP, Sharma P, Dash AP. Malaria vector control: from past to future. Parasitol Res. 2011;108:757–79. doi: 10.1007/s00436-010-2232-0. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang SF, Li C, Sun G, Ahmed A, Dittmer N, Attardo G. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem Mol Biol. 2002;32:1275–86. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- Rodrigues FG, Santos MN, de Carvalho TX, Rocha BC, Riehle MA, Pimenta PF, Abraham EG, Jacobs-Lorena M, Alves de Brito CF, Moreira LA. Expression of a mutated phospholipase A2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Insect Mol Biol. 2008;17:175–83. doi: 10.1111/j.1365-2583.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini M, Mauro U, Lombardo F, Milano A, Zazzaro V, Arca B, Polito LC, Saccone G. Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti. BMC Evol Biol. 2011;11:41. doi: 10.1186/1471-2148-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scali C, Catteruccia F, Li Q, Crisanti A. Identification of sex-specific transcripts of the Anopheles gambiae doublesex gene. J Exp Biol. 2005;208:3701–9. doi: 10.1242/jeb.01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson DW, Behura SK. Mosquito genomics: progress and challenges. Annu Rev Entomol. 2012;57:143–66. doi: 10.1146/annurev-ento-120710-100651. [DOI] [PubMed] [Google Scholar]
- Shapiro JP, Hagedorn HH. Juvenile hormone and the development of ovarian responsiveness to a brain hormone in the mosquito, Aedes aegypti. Gen Comp Endocrinol. 1982;46:176–83. doi: 10.1016/0016-6480(82)90199-x. [DOI] [PubMed] [Google Scholar]
- Shukla JN, Nagaraju J. Doublesex: a conserved downstream gene controlled by diverse upstream regulators. J Genet. 2010a;89:341–56. doi: 10.1007/s12041-010-0046-6. [DOI] [PubMed] [Google Scholar]
- Shukla JN, Nagaraju J. Two female-specific DSX proteins are encoded by the sex-specific transcripts of dsx, and are required for female sexual differentiation in two wild silkmoth species, Antheraea assama and Antheraea mylitta (Lepidoptera, Saturniidae) Insect Biochem Mol Biol. 2010b;40:672–82. doi: 10.1016/j.ibmb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Singh SP, Coronella JA, Benes H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur J Biochem. 2001;268:2912–23. doi: 10.1046/j.1432-1327.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- Skalsky RL, Vanlandingham DL, Scholle F, Higgs S, Cullen BR. Identification of microRNAs expressed in two mosquito vectors, Aedes albopictus and Culex quinquefasciatus. BMC Genomics. 2010;11:119. doi: 10.1186/1471-2164-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MG, Funaguma S, Kanda T, Tamura T, Shimada T. Analysis of the biological functions of a doublesex homologue in Bombyx mori. Dev Genes Evol. 2003;213:345–54. doi: 10.1007/s00427-003-0334-8. [DOI] [PubMed] [Google Scholar]
- Telfer WH. Egg formation in lepidoptera. J Insect Sci. 2009;9:1–21. doi: 10.1673/031.009.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer WH, Kunkel JG. The function and evolution of insect storage hexamers. Annu Rev Entomol. 1991;36:205–28. doi: 10.1146/annurev.en.36.010191.001225. [DOI] [PubMed] [Google Scholar]
- Telfer WH, Pan ML. Storage hexamer utilization in Manduca sexta. J Insect Sci. 2003;3:1–6. doi: 10.1093/jis/3.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287:2474–6. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- Winter F, Edaye S, Huttenhofer A, Brunel C. Anopheles gambiae miRNAs as actors of defence reaction against Plasmodium invasion. Nucleic Acids Res. 2007;35:6953–62. doi: 10.1093/nar/gkm686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise de Valdez MR, Nimmo D, Betz J, Gong HF, James AA, Alphey L, Black WCt. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci U S A. 2011;108:4772–5. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharkin SO, Gordadze AV, Korochkina SE, Mathiopoulos KD, Della Torre A, Beneš H. Molecular cloning and expression of a hexamerin cDNA from the malaria mosquito, Anopheles gambiae. Eur J Biochem. 1997;246:719–26. doi: 10.1111/j.1432-1033.1997.t01-1-00719.x. [DOI] [PubMed] [Google Scholar]
- Zakharkin SO, Headley VV, Kumar NK, Buck NA, Wheeler DE, Beneš H. Female-specific expression of a hexamerin gene in larvae of an autogenous mosquito. European Journal of Biochemistry. 2001;268:5713–22. doi: 10.1046/j.0014-2956.2001.02514.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen L, Raikhel AS. Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2003;100:13338–43. doi: 10.1073/pnas.2234416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Sexing individual pupae by detection of a male-specific dsx mRNA variant.
Fig. S2. Detection of upstream sequences from the HexlacZ reporter gene transcript in pupae.


