Abstract
Macrophages play critical roles in innate immune defense by sensing microbes using pattern-recognition receptors. Lipopolysaccharide (LPS) stimulates macrophages via TLR, which leads to activation of downstream signaling cascades. In this study, we investigated the roles of a conserved signaling pathway, Notch signaling, in regulating the downstream signaling cascades of the LPS/TLR4 pathways in macrophages. Using a phosphoproteomic approach and a gamma-secretase inhibitor (GSI) to suppress the processing and activation of Notch signaling, we identified regulator of G protein signaling 19 (RGS19) as a target protein whose phosphorylation was affected by GSI treatment. RGS19 is a guanosine triphosphatase (GTPase)-activating protein that functions to negatively regulate G protein-coupled receptors via Gαi/Gαq-linked signaling. Stimulation of RAW264.7 cells with LPS increased the level of the phosphorylated form of RGS19, while LPS stimulation in the presence of GSI decreased its level. GSI treatment did not alter the mRNA level of rgs19. Treatment with GSI or silencing of rgs19 in macrophages impaired the phosphorylation of Akt Thr308 upon LPS stimulation. Furthermore, targeted deletion of a DNA-binding protein and binding partner of the Notch receptor, RBP-Jκ/CSL, in macrophages resulted in delayed and decreased Akt phosphorylation. Because the PI3K/Akt pathway regulates cell survival in various cell types, the cell cycle and cell death were assayed upon GSI treatment, phosphatidylinositol 3 kinase (PI3K) inhibitor treatment or silencing of rgs19. GSI treatment resulted in decreased cell populations in the G1 and S phases, while it increased the cell population of cell death. Similarly, silencing of rgs19 resulted in a decreased cell population in the G1 phase and an increased cell population in the subG1 phase. Inhibition of Akt phosphorylation by PI3K inhibitor in LPS-stimulated macrophages increased cell population in G1 phase, suggesting a possible cell cycle arrest. Taken together, these results indicate that Notch signaling positively regulates phosphorylation of Akt, possibly via phosphorylation of RGS19, and inhibition of both molecules affects the cell survival and cell cycle of macrophages upon LPS stimulation.
Keywords: Akt, Lipopolysaccharide, Macrophage, Notch signaling, RGS19
Introduction
Notch signaling plays critical roles in regulating the innate and adaptive immune responses (Radtke et al., 2013). The activation of Notch signaling begins when the extracellular domain of Notch receptor interacts with the Notch ligand, which results in the cleavage of the receptor by the enzymes tumor necrosis factor alpha converting enzyme and gamma secretase. These enzymatic cleavages allow the intracellular domain of the Notch receptor to translocate into the nucleus and bind to a transcription factor, RBP-Jk/CSL (Maillard et al., 2003). Previously, it was reported that Notch signaling was activated upon TLR ligation in macrophages (Hu et al., 2008a,b; Palaga et al., 2008). The activation of Notch signaling in macrophages regulates effector functions, such as pro-inflammatory cytokine production and antigen presentation (Monsalve et al., 2006; Palaga et al., 2008). Notch signaling directly or indirectly regulates diverse macrophage responses to TLR ligands, and it directly fine-tunes the responses to LPS by inducing HES1 and HEY1 to decrease expression of some pro-inflammatory cytokines (Hu et al., 2008a,b). Furthermore, Notch signaling directly regulates il-6 promoter activity and the transcription of IL-6 (Wongchana and Palaga, 2012). In contrast, Notch signaling indirectly regulates inflammatory responses in macrophages by cross talking with other signaling pathways such as the NF-κB, IRF and MAPK pathways (Foldi et al., 2010; Palaga et al., 2008; Xu et al., 2012).
Proteomics analysis reveals that TLR signaling is involved in more than 340 signaling proteins, and LPS-stimulated macrophages Exhibit 24% more phosphoproteins (Weintz et al., 2010). Recently, it was reported that TLR4 signaling modifies G-protein coupled receptors (GPCRs) by altering the expression of the regulator of G protein signaling (RGS) family of proteins in dendritic cells and controlling the response of dendritic cells to chemokines (Shi et al., 2004). RGS is a protein family of more than 30 members that all contain RGS or RGS-like domains. RGS acts as a GTPase activating protein, which accelerates the intrinsic hydrolysis of GTP to GDP during GPCR activation to attenuate GPCR signaling pathways (Cho and Kehrl, 2009). Recent evidence also suggest that RGS proteins are involved in other cellular events besides regulating GPCR signaling, such as regulating cell proliferation and differentiation (Ji et al., 2010; Tso et al., 2011).
RGS19, also known as Gα-interacting protein (GAIP), is one of the first members of the RGS protein family to be discovered to interact with subunits of inhibitory G proteins (Gαi, Gαq) and enhance the GTPase activity of the inhibitory subunits of GPCR (De Vries et al., 1995). Furthermore, RGS19 was shown to cross talk with other signaling pathways, such as the Wnt-β-catenin, Akt and SAPK/JNK signaling pathways (Feigin and Malbon, 2007; Ip et al., 2012; Tso et al., 2011). RGS19 is expressed in dendritic cells, and, unlike other RGS members, the expression level did not change upon LPS treatment (Shi et al., 2004). However, most studies on the role of RGS19 were carried out in cancer cells/cell lines, and the involvement of this protein in the function of macrophages was not reported.
In this study, we identified RGS19 as a molecule with altered phosphorylation status when Notch signaling is inhibited by GSI in LPS-stimulated macrophages. The cross talk between RGS19 and the Notch and Akt signaling pathways was analyzed.
Materials and methods
Animals and cells
RAW264.7 cells, a mouse macrophage cell line, (ATCC TIB-71), were maintained in Dulbecco’s Modified Eagle Media (DMEM) (Hyclone, Logan, UT, USA) supplemented with 10% (v/v) fetal bovine serum (Hyclone), 1% (w/v) sodium pyruvate (Hyclone), 1% (w/v) HEPES (Hyclone) and penicillin and streptomycin (M&H Manufacturing Co. Ltd., Bangkok, Thailand). Bone marrow-derived macrophages were generated as described previously (Palaga et al., 2008). Macrophages with targeted deletion of RBP-Jκ/CSL were derived from bone marrow from Mx-Cre+/−; CSLfl/fl or Mx-Cre−/−; CSLfl/fl mice. The mice were injected with polyI:C daily for 7 days and allowed to rest for 1 month before bone marrow extraction. All procedures involving laboratory animals were carried out according to the guidelines issued by Chulalongkorn University and University of Massachusetts at Amherst. All animal protocols were reviewed by the IACUC (protocol review No. 0923013). Cells were maintained in media as described above.
Treatment of cells with LPS and inhibitors
A GSI and DAPT (Sigma–Aldrich, St. Louise, MO, USA) were dissolved in DMSO at a stock concentration of 50 mM and stored at −20 °C until use. LY294002 was used as a specific inhibitor of phosphatidylinositol 3 kinase (PI3K) (Cell Signaling Technology, USA). The cells were pretreated with GSI at the indicated concentration or with the vehicle (DMSO) for 1 h before subjecting them to stimulation with Salmonella LPS (100 ng/ml) (Sigma Aldrich) for the indicated periods.
Two dimension gel electrophoresis (2D-GE) and protein identification
Lysates prepared from RAW264.7 cells were treated as indicated and extracted using the previously described buffers and method (Palaga et al., 2008). The protein concentration was measured using the BCA™ protein assay kit (PIERCE, USA) according to the manufacturer’s instruction. One hundred and fifty micrograms of proteins from each condition were subjected to the removal of lipid, nucleic acids, salts and other contaminants using a 2D clean-up kit (Bio-Rad, USA). The cleared solutions were loaded onto IPG strips, pH 4–7 (GE Healthcare Bio-Sciences, UK), and subjected to 2D-GE using PROTEAN® IEF and PROTEIN® II xi Cell (Bio-Rad, USA). After electrophoresis, the phosphoproteins in the gels were stained using Pro-Q® Diamond phosphoprotein gel stain (Invitrogen, UK) according to the manufacturer’s instructions. Fluorescent signals were visualized using Phosphorimager Typhoon Trio™ (GE Healthcare). After Pro-Q® Diamond staining, the gels were stained with Coomassie blue R-250 (Bio-Rad, USA) for total protein detection and visualized using an ImageScanner™ II (GE Healthcare). The intensity of the phosphoproteins and total proteins in the 2D-GE gel were analyzed using an ImageMaster™ 2D Platinum v7.0. The in-gel digestion procedure was performed as described by Shevchenko et al. (2007), and the tryptic peptides were analyzed using an HRESIMS nano-LC (EASY-nLCII, Bruker Daltonics, Germany)-MS/MS (microTOF-QII, Bruker Daltonics, Germany). The results from LC–MS/MS were subjected to a database search using an online Mascot database, and the outputs of these results were compared between conditions and cross-checked using the iso-electric focusing point and molecular weight of the excised spots.
Western blot
After separating the protein lysates by SDS-PAGE, the proteins were transferred onto a PVDF membrane using the semi-dry transfer Transfer-Blot® SD (Bio-Rad, USA). The primary antibodies that were used in this study were as follows: rabbit anti-cleaved Notch1 Ab (Val1477) (Cell Signaling Technology, MA, USA), rabbit anti-Notch1 Ab (Santa Cruz Biotech, Santa Cruz, CA, USA), rabbit anti-RGS19 Ab (Abcam, USA), rabbit anti-pAkt Ab (Cell Signaling Technology), rabbit anti-Akt Ab (Cell Signaling Technology) and mouse anti-β-actin Ab (Millipore, USA). The secondary antibodies that were used in this study were as follows: horseradish peroxidase conjugated goat anti-mouse IgG Ab (GE Healthcare) and horseradish peroxidase conjugated goat anti-rabbit IgG Ab (GE Healthcare). The signal was detected using the Amersham Hyperfilm™ ECL chemiluminescent detection method (Amersham Bioscience, UK).
Realtime quantitative RT-PCR (qPCR)
The cells were treated as indicated, and the total RNA was extracted using TRIzol® (Invitrogen, UK). One hundred nanograms to 1 μg of RNA was used as a template to synthesize cDNA using reverse transcriptase (Fermentas, Canada). The qPCR was carried out using 1xMaxima™ SYBR Green/ROX qPCR (Fermentas, Canada) or iQ™ SYBR® Green (Supermix, BioRad, USA) according to manufacturer’s protocols, and the qPCR was performed using the MJ Mini personal Thermal Cycler (Bio-Rad, USA). Expression of β- actin mRNA was used as a reference. The relative expressions of the mRNA levels were calculated and analyzed using the 2−ΔΔCP method, as described previously (Livak and Schmittgen, 2001).
Intracellular staining for RGS19 and apoptosis assay
For intracellular staining and detection by flow cytometry, cells were harvested, fixed in 1.5% formaldehyde and permeated by cold methanol treatment. After this step, cells were stained with anti-RGS19 antibody followed by anti-Rabbit IgG (H+L), F(ab′)2 fragment (phycoerythrin conjugate), and RGS19 expression was determined using flow cytometry (FC500, Beckman Coulter, USA). For apoptosis assay, the RAW264.7 cell line were pretreated with indicated inhibitors or transfected with indicated siRNA and then stimulated with LPS (100 ng/ml) for 48 h. Apoptosis assay was performed according to the manufacturer’s instruction (Biolegend, CA). Staining of annexinV and 7AAD was analyzed by flow cytometry. The acquired data were analyzed using FlowJo data analysis software (Tree Star, Inc.).
Silencing of RGS19 in macrophages
siRNAs were custom synthesized by Qiagen (QIAGEN, Germany). The transfection of siRNA was carried out using HiPerFect Transfection (QIAGEN) according to the manufacturer’s instruction, and silencing of RGS19 was confirmed using qPCR and Western blotting.
Cell cycle analysis
Cells were treated and harvested at the indicated times. After washing in cold PBS, the cell pellet was resuspended in 200 μl cold 70% ethanol and fixed on ice for at least 4 h. The cells were then washed once with 500 μl PBS, and the cell pellet was resuspended in 250 μl PBS containing 0.1 mg/ml RNaseA (Fermentas, Canada) and incubated at 37 °C for 30 min. Propidium iodide (1 mg/ml) was added into each sample, and the cells were incubated at room temperature in the dark for at least 30 min before subjecting them to cell cycle analysis using a flow cytometer FC500 (Beckman Coulter, USA). The acquired data were analyzed using the CXP software (Beckman Coulter).
Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) and the SPSS software. A p-value of less than 0.05 was determined as statistically significant.
Results
Alteration in RGS19 by the inhibition of Notch signaling
A proteomics approach was used to identify the phosphorylated proteins whose phosphorylated levels were affected by Notch signaling in the LPS-activated macrophage cell line, RAW264.7. Pre-treatment with GSI and DAPT, was carried out before stimulation with LPS. One of the proteins that were identified in this study was RGS19 (Supplementary data: Figures S1 and S2). As shown in Fig. 1, the levels of total RGS19 after separation by 2D-GE were similar in cells treated with the vehicle control (DMSO) or DAPT. However, the phosphorylation level, as detected by Pro-Q® Diamond phosphoprotein staining, in DAPT-treated cells were lower than that of the control. The effectiveness in inhibiting the processing of the Notch receptor by DAPT was verified by decreased levels of cleaved Notch1 (Val1744) (Fig. 2A).
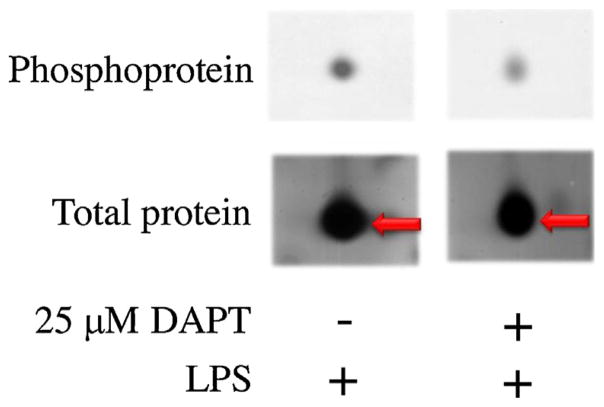
Fig. 1.
Protein spot of RGS19 by 2D-PAGE. RAW264.7 cells were pretreated with DAPT (25 μM) or vehicle control DMSO for 1 h before stimulation by LPS (100 ng/ml) for 1 h. Protein lysates were separated using 2D-GE, and the gels were stained for phospho-and total protein. One of the spots shown here was identified as RGS19.
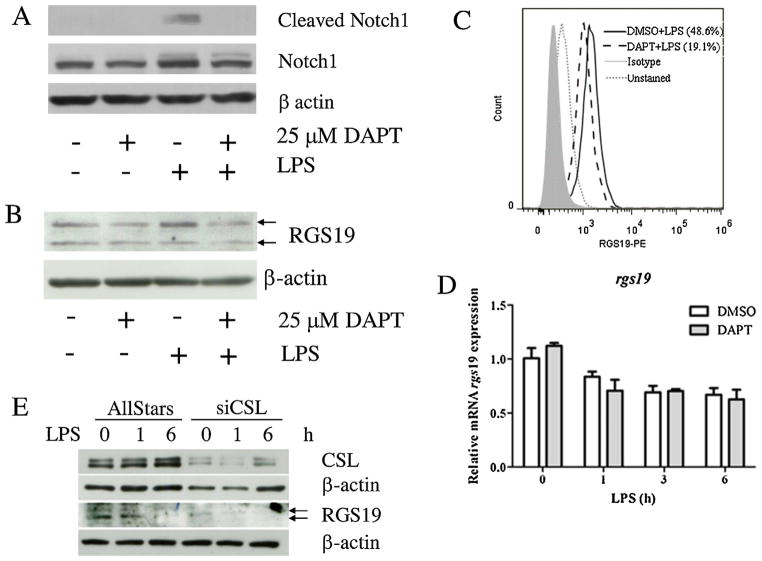
Fig. 2.
Effect of DAPT treatment on RGS19 in LPS-stimulated macrophages. (A) The RAW264.7 cell line was pretreated with DAPT (25 μM) for 1 h before stimulation with 100 ng/ml LPS for 1 h. Cleaved (Val1744) and total Notch1 were detected using Western blotting, and β-actin was used as a loading control. (B) Protein lysates were collected to investigate the presence of RGS19 by Western blotting, as described above. Arrows indicated the two bands corresponding to phosphor-RGS19 and total RGS19. (C) The RAW264.7 cell line was treated as indicated, harvested and stained with anti-RGS19 antibody. The level of RGS19 was detected using flow cytometry. (D) The mRNA levels of rgs19 were measured using qPCR. Total RNA was harvested from LPS-stimulated RAW264.7 cells after treatment with DAPT or DMSO for 0, 1, 3 and 6 h after LPS stimulation. (E) The RAW264.7 cell line was transfected with a control scramble siRNA or CSL-specific siRNA and subjected to LPS stimulation. The expression of RGS19 and CSL were detected by Western blotting.
To investigate whether RGS19 is affected by inhibition of Notch signaling, Western blotting and flow cytometry using an RGS19-specific Ab were used. As shown in Fig. 2B and C, decreased levels of RGS19 was detected using both techniques. Using Western blotting, two bands appeared as specific bands for RGS19 that were later verified by specific gene silencing (Fig. 4B). The band with higher molecular weight, which corresponded to approximately 29 kDa, was considered phosphorylated RGS19 (Feigin and Malbon, 2007). LPS treatment increased the band intensity of the phosphorylated RG19 and GSI treatment decreased its level. LPS stimulation or GSI treatment did not affect the level of the unphosphorylated RGS19 (Fig. 2B). Using intracellular staining, the significant decreased level of RGS19 was observed. Because intracellular staining cannot distinguish between phosphorylated and unphosphorylated form, this decrease in the staining may indicate that antibody used in our study recognizes a phosphorylated form better (Fig. 2C). When the mRNA levels of rgs19 were compared using qPCR, LPS treatment gradually decreased the rgs19 mRNA level, but no differences were detected between the DAPT and DMSO treatments (Fig. 2D).
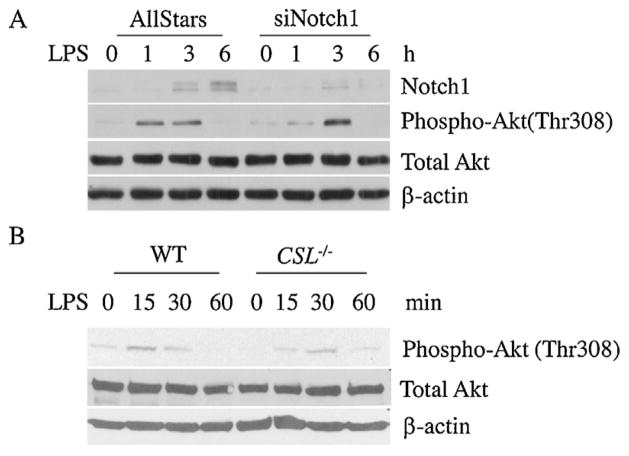
Fig. 4.
The role of CSL and Notch1 in regulating Akt phosphorylation. (A) The RAW264.7 cell line was transfected with the control scrambled siRNA or notch1-specific siRNA. After 24 h of transfection, the cells were stimulated with LPS (100 ng/ml) for the indicated time, and phosphorylated and total Akt were detected using Western blotting. (B) BMMs from CSL conditional knockout mice were stimulated with LPS (100 ng/ml) for the indicated times, and the protein lysates were harvested to investigate the phosphorylation status of Akt.
To test the specific effect of Notch signaling on RGS19, silencing of CSL was carried out. As shown in Fig. 2E, silencing of CSL significantly decreased the RGS19 levels at all time points tested both unphosphorylated and phosphorylated forms. Immunofluorescence staining revealed that RGS19 remained exclusively in the cytoplasm with or without LPS stimulation, and DAPT treatment did not affect the cellular localization of this protein (data not shown). These results strongly indicated that Notch signaling regulates phosphorylation of RGS19 in a canonical, CSL-dependent manner in macrophages upon LPS stimulation.
Effect of silencing rgs19 and inhibition of Notch signaling on the activation of Akt
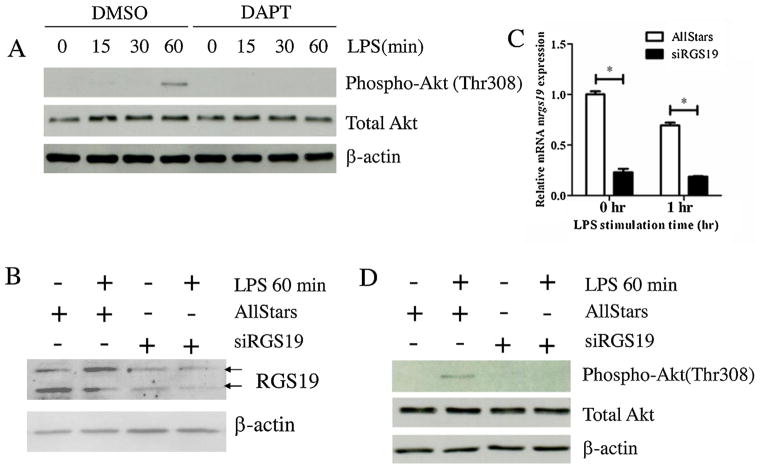
RGS19 cross regulates multiple pathways, including the MAPK and PI3K/Akt pathways. We investigated whether this cross regulation is partially mediated through Notch signaling in LPS-stimulated macrophages. First, the effect of DAPT treatment was monitored for its effect on the phosphorylation of Akt Thr308. As shown in Fig. 3A, DAPT treatment significantly decreased the level of Akt phosphorylation as early as 15 min after LPS stimulation. To verify that RGS19 also regulates Akt phosphorylation, a silencing approach using siRNA was carried out. As shown in Fig. 3B and C, RGS19 was successfully knock down, and the intensities of the two bands corresponding to unphosphorylated and phosphorylated RGS19 were lower. Furthermore, the known down of rgs19 at the level of mRNA was also confirmed. Silencing rgs19 in macrophages also decreased Akt Thr308 phosphorylation upon LPS stimulation, similar to DAPT treatment. In addition, silencing rgs19 increased the phosphorylation of SAPK/JNK upon stimulation of the macrophages (data not shown: Figure S3). This result suggested that Notch signaling and RGS19 may cross talk at the activation of Akt.
Fig. 3.
The Effect of DAPT and silencing of RGS19 on Akt phosphorylation in LPS-stimulated macrophages. (A) The RAW264.7 cell line was pretreated with DAPT (25 μM) or the vehicle control (DMSO) for 1 h before the cells were stimulated with LPS (100 ng/ml) for the indicated times. Phosphorylation of Akt in cell lysates was detected using Western blotting. (B) RAW264.7 cells were transfected with the control scrambled siRNA or rgs19-specific siRNA. After the transfection, cells were stimulated with 100 ng/ml LPS for 0 and 60 min, and protein lysates were collected to examine the level of RGS19 expression using Western blotting. Arrow heads indicated the two bands corresponding to phosphor-RGS19 and total RGS19. (C) RAW264.7 cell line was transfected with siRNA, as described above, and stimulated with LPS (100 ng/ml) for 0 and 60 min. Total RNA was harvested to investigate the expression level of rgs19 mRNA by qPCR. * indicates statistical significance (p < 0.05). (D) RAW264.7 cell were transfected with siRNA, as described above, and stimulated with LPS (100 ng/ml) for 0, 15, 30 and 60 min. Phosphorylated (Thr308) and total Akt were detected using Western blotting.
Notch1 and CSL is indispensable for Akt phosphorylation in LPS-stimulated macrophages
Multiple Notch receptors are expressed in macrophages, and the most predominant are Notch1 and Notch2. Furthermore, Notch signaling is transduced in either CSL-dependent or CSL-independent manners. To investigate the requirement of Notch1 for Akt phosphorylation, gene silencing using siRNA was carried out. Silencing Notch1 negatively affected early Akt phosphorylation (1 h after LPS stimulation) (Fig. 4A). However, 3 h after LPS stimulation, Akt phosphorylation was recovered to the control level, which suggested that Notch1 is responsible for regulating early Akt phosphorylation. To determine the dependency on CSL, targeted deletion of CSL in BMMs was carried out. CSL deficiency led to a delay in Akt phosphorylation upon LPS stimulation (Fig. 4B). Taken together, these results suggested that Notch1 regulates early Akt phosphorylation in a canonical, CSL-dependent manner in macrophages upon LPS stimulation.
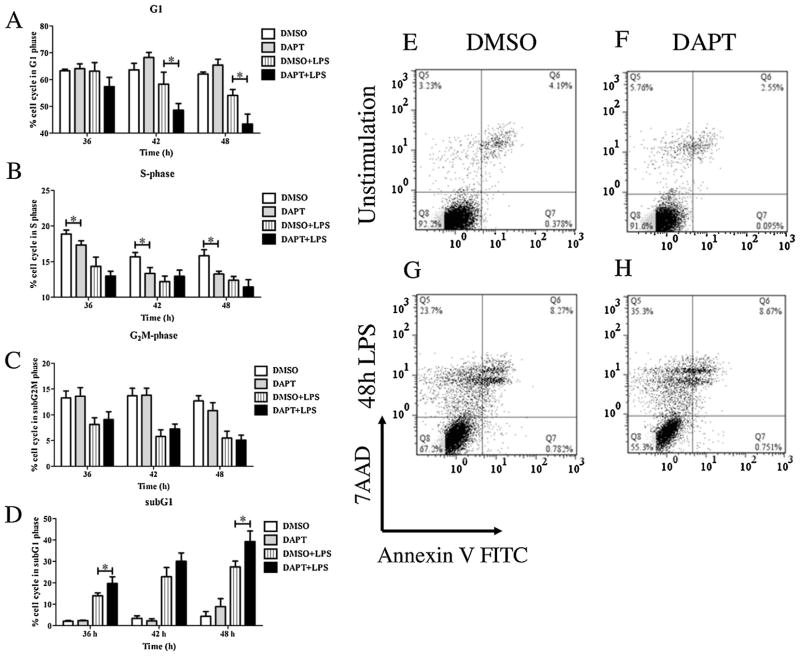
Effects of inhibiting Notch signaling and silencing rgs19 on the cell cycle and cell death profiles
Notch signaling and RGS19 stimulate cell proliferation and cell survival by multiple pathways, including PI3K/Akt pathways. We investigated whether interfering with these two pathways resulted in similar consequences on the cell cycle and cell death profile. As shown in Fig. 5A–D, DAPT treatment resulted in a decreased subset of cells in the G1 phase and increased cell subset in the sub G1 phase in LPS-stimulated macrophages. During the time pointes examined, no increase in cell subset of the G1 phase was detected, suggesting that there is no cell cycle arrest. To determine whether cells in the sub G1 phase are dead cells, apoptosis assay was carried out. As shown in Fig. 5E–H, LPS stimulation of macrophages for 48 h increased percentages of dead cells (both apoptosis and necrosis). Inhibition of Notch signaling increasing the percentages of dead cells (Fig. 5E–H). More necrotic cells were detected in DAPT-treated macrophages, possibly as the results of long incubation times before cell death assay.
Fig. 5.
Effect of DAPT on cell cycle progression and cell death of macrophages during LPS stimulation. (A–D) The RAW264.7 cell line was pretreated with DAPT (25 μM) or the vehicle control (DMSO) for 1 hr and then stimulated with LPS (100 ng/ml) for 36, 42 and 48 h. The cell cycle profile was determined using propidium iodine staining and flow cytometry. The results represent three independent experiments in triplicate. * indicates statistical significance (p < 0.05). (E–H) The RAW264.7 cell line was pretreated with DAPT (25 μM) or the vehicle control (DMSO) for 1 h and then stimulated with LPS (100 ng/ml) for 48 h. Cell death was determined using annexinV-FITC and 7-AAD staining. The results represented three independent experiments.
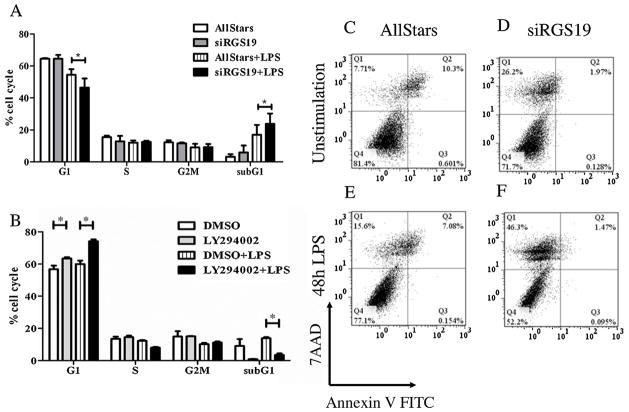
Similarly, silencing rgs19 mRNA expression in macrophages also decreased the percentage of cells in G1 phase and instead increased cells in the subG1 phase (Fig. 6A). Cell death assay revealed the drastic increased necrotic cells upon silencing of rgs19 with or without LPS treatment (Fig. 6C–D). These results strongly link the cell cycle and cell death to Notch signaling and RGS19 in LPS-stimulated macrophages. To determine whether PI3K/Akt pathway which is the target of Notch/RGS19 signaling cascade is involved in cell cycle and cell death of macrophages, PI3K specific inhibitor was used. As shown in Fig. 6B, inhibition of Akt phosphorylation by PI3K resulted in significant increased cells in the G1 phase and decreased cells in the subG1 phase, suggesting a possible cell cycle arrest. Apoptotic assay also revealed that inhibition of Akt significantly decreased cell death upon LPS stimulation (data not shown). Taken together, interfering with Notch/RGS19 pathways results in increasing cell death upon LPS stimulation in macrophages while inhibition of PI3K/Akt pathways decreased cell death by cell cycle arrest at G1 phase.
Fig. 6.
Effects of silencing rgs19 and PI3K inhibitor on cell cycle progression and cell death of macrophages during LPS stimulation. (A) Cell cycle profiles of RAW264.7 cells silenced for rgs19 was analyzed after LPS simulation for 48 hr as described above. The result represents three independent experiments in triplicate. * indicates statistical significance (p < 0.05). (B) The RAW264.7 cell line was pretreated with LY294002 (10 μM) or the vehicle control (DMSO) for 1 h and stimulated with LPS (100 ng/ml) for 48 h. The cell cycle profiles were determined as described above. The results represent three independent experiments in triplicate. * indicates statistical significance (p < 0.05). (C–F) The RAW264.7 cell line silenced for rgs19 as described above was treated with LPS (100 ng/ml) after 28 h of transfection and cell death was determined using annexinV-FITC and 7-AAD staining at 48 h after stimulation. The results represented three independent experiments.
Discussion
Notch signaling crosses paths with multiple signaling pathways in macrophages upon TLR ligation (Fung et al., 2007; Hu et al., 2008a,b; Monsalve et al., 2006; Palaga et al., 2008). In this study, we reported, for the first time, cross talk between RGS and Notch signals in macrophages. Members of the RGS family of proteins have been reported to regulate differentiation and osteoclastogenesis in monocyte/macrophage precursors (Yang and Li, 2007; Yang et al., 2013). In addition, microarray analysis revealed that RGS1 and RGS2 are transcriptionally controlled by TLR ligation in macrophages (Riekenberg et al., 2009).
Expression of some members of the RGS family of proteins was altered in DCs upon TLR activation. Activation of TLR3 or TLR4 in monocyte-derived DCs upregulated RGS1, RGS16 and RGS20, but downregulated RGS18 and RGS14 at the transcriptional level (Shi et al., 2004). The RGS19 mRNA is constitutively expressed in DCs. Furthermore, it was demonstrated that RGS proteins in DCs regulate chemokine-induced migration.
RGS19 was identified using a proteomics approach as a phosphorylated protein with an altered level of phosphorylation upon GSI treatment in LPS-stimulated macrophages. However, the level of transcription was not affected by Notch signaling. This result supports the microarray data of macrophages stimulated with bacterial lipopeptides and LPS which reports a slight decrease at the mRNA level of rgs19 upon stimulation (Riekenberg et al., 2009). RGS19 is a member of the RGS protein family subfamily RZ based on the conserved sequence within the catalytic RGS domain (Ross and Wilkie, 2000). Phosphorylation of RGS19 was described in mouse teratocarcinoma cells, where the band that corresponded to a molecular mass of 29 kDa was found exclusively in the membrane fraction, and alkaline phosphatase treatment reduced the size of the band to 27 kDa (Feigin and Malbon, 2007). Our immunofluorescence staining patterns correlated well with membrane-associated RGS19 in macrophages.
RGS19 is reported to contain a phosphorylated form exclusively in the membrane-anchored pool. Detailed analysis revealed that RGS19 can be phosphorylated by casein kinase 2 (CK2) in vitro and Ser24 is the potential phosphorylation site (Fischer et al., 2000). In our study, activation of macrophages by LPS increased phosphorylated RGS19 and inhibition of Notch signaling decreased its level. How Notch signaling regulates phosphorylation of RGS19 is unclear. The effect of inhibition of Notch signaling on RGS19 phosphorylation can be either direct or indirect. The effect of Notch signaling on the level of RGS19 that was observed in this study was suppressed by GSI treatment and by knock down of Notch1 and CSL, which suggests that cleavage of a Notch receptor, likely Notch1, is essential for this effect and that CSL is indispensable. Because Notch/CSL forms a transcriptional activation complex that drives gene expression, it is possible that Notch signaling may turn on an unknown kinase that is responsible for RGS19 phosphorylation (Radtke et al., 2013). Interestingly, Notch signaling and CK2 are reported to be involved in regulating stabilization of phosphatase and tensin homolog (PTEN), an inhibitor of Akt activation (Silva et al., 2010). Furthermore, recent evidence suggests that intracellular domain of Notch is a novel target of phosphorylation by CK2 (Ranganathan et al., 2011). The phosphorylation of intracellular domain of Notch decreases the transcriptional activities of Notch/CSL complex, suggesting that CK2 negatively regulates canonical Notch signaling. Therefore, Notch/CK2/RGS19 may interact in LPS-stimulated macrophages.
Multiple reports link RGS19 with the PI3K/Akt pathway. RGS19 stimulates the proliferation of teratocarcinomas and enhancement of Akt phosphorylation (Tso et al., 2011). In cancer models, RGS19 increased serum-stimulated PTEN and Akt phosphorylation, which are responsible for cyclin D1/3 upregulation. The outcome of increasing RGS19 is deregulation of cell cycle control. In another study, RGS19-overexpressing transgenic mice were generated and exhibited enhanced phosphorylation of Akt and β-catenin in cardiomyocytes that led to defects in heart development and function (Ji et al., 2010). Consistent with these observations, we detected a decreased level of Akt phosphorylation when RGS19 was knocked down in macrophages, which implies that similar cross talk between RGS19 and the Akt pathways operates in innate immune cells.
Several lines of evidence indicate that Notch signaling regulates the PI3K/Akt pathways in several cancer and immune cells (Calzavara et al., 2008; Cornejo et al., 2011; Efferson et al., 2010; Wang et al., 2010). In macrophages, ligation of the Notch receptor by co-culturing with the Notch ligand Dll4 increased Akt phosphorylation, suggesting a link between the Notch and Akt pathways (Fung et al., 2007). One of the key molecules that is responsible for Notch/Akt cross talk is PTEN (Gutierrez and Look, 2007). In developing thymocytes and Notch1-induced leukemia, one of the target genes of Notch signaling, the transcriptional repressor hairy and enhancer of split-1 (HES-1), is responsible for repressing PTEN and activating Akt (Palomero et al., 2008; Wong et al., 2012).
One of the biological outcomes of the cross talk between Notch/RGS19/Akt is the disruption of cell cycle profiles and cell death of macrophages (Quan et al., 2013). The Akt pathway is shown to be one of the major regulators of cell survival and cell death in macrophage. Therefore, one of the novel findings of this study is the role of Notch signaling in regulating cell survival of macrophages, possibly via the Akt pathway.
We observed that interfering with Notch/RGS19/Akt results in either increased in cell death or disruption of cell cycle in LPS-activated macrophages. One possible explanation is the indirect effect of cytokines produced by activated macrophages. Silencing rgs19 or GSI treatment both resulted in increased in TNFα production (data not shown). In contrast, suppression of Akt activation resulted in decreased TNFα level (Rajaram et al., 2006). TNFα can have a negative impact on cell survival of macrophages which can explain the contrast effect of inhibition of Notch/RGS19 and Akt pathways observed in our study.
In this study, we also confirmed the negative affect of Notch/RGS19 on the activation of SAPK/JNK. Interfering with both pathways similarly increased phosphorylation of SAPK/JNK upon LPS stimulation. It was reported that upregulation of RGS19 severely disrupts mitogenic response of SAPKs via small GTPase (Ip et al., 2012). Therefore, inhibition of Notch signaling may influence the activation of SAPK/JNK by decreasing phosphorylation of RGS19. Interestingly, Notch signaling in macrophages was shown to be activated by SAPK/JNK pathway via upregulation of one of its ligand (Tsao et al., 2011).
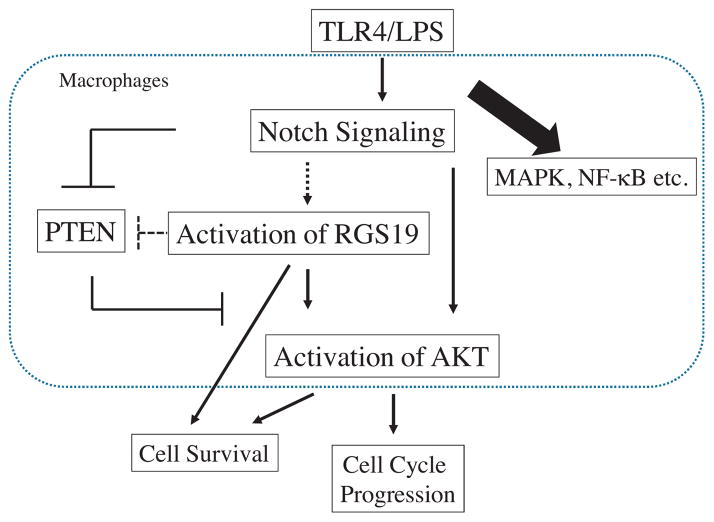
Taken together, we propose a model of cross talk between the Notch/RGS19/Akt pathways in macrophages, as shown in Fig. 7. Ligation of TLR4 with LPS induces the activation of the Notch receptor and the formation of the Notch/CSL transcriptional complex. This activation, in turn, switches on target genes, including Hes-1. HES-1 suppresses the expression of PTEN, which leads to more phosphorylation of Akt. Activation of Notch signaling, through an unknown mechanism, increases the phosphorylation of RGS19, which can directly or indirectly affect the phosphorylation state of Akt. Phosphorylated Akt, in turn, controls cell survival and cell cycle progression by phosphorylating apoptosis regulators and cell cycle regulators. Furthermore, Notch and RGS19 may regulate cell death independently of activation of Akt pathway.
Fig. 7.
Model of the cross talk between Notch/RGS19/Akt in macrophages. See text for details. Bold lines indicated the links that have been documented and the dotted lines indicated the possible links.
Supplementary Material
Acknowledgments
This work was supported by the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (RES560530144-AS) and the Special Task Force for Activating Research (STAR) from the Centenary Academic Development Project (Chulalongkorn University). BAO and TP were supported by the Fogarty International Research Collaborative Award (Grant No. R03 TW008420-01A1, NIH, USA). The equipment used in this study was purchased with funds from the Thai Government Stimulus Package 2 (TKK2555) under the Project for the Establishment of Innovative Food and Health Products and Agriculture and A1B1 Strategic Grant from Faculty of Science, Chulalongkorn University. NS was supported by the Thailand Research Fund (TRF) through the Royal Golden Jubilee Ph.D. Program (PHD/0268/2553).
Abbreviations
- Ab
antibody
- BMM
bone marrow-derived macrophages
- DMSO
dimethyl sulfoxide
- GSI
gamma secretase inhibitor
- GTPase
guanosine triphosphatase
- GPCR
G-protein couple receptor
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- PI3K
phosphatidylinositol 3 kinase
- qPCR
quantitative real-time RT-PCR
- RGS19
regulator of G protein signaling 19
- 2D-GE
two-dimensional gel electrophoresis
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.imbio.2014.03.020.
Footnotes
Conflict of interest
All authors declared no conflict of interest.
References
- Calzavara E, Chiaramonte R, Cesana D, Basile A, Sherbet GV, Comi P. Reciprocal regulation of Notch and PI3K/Akt signalling in T-ALL cells in vitro. J Cell Biochem. 2008;103:1405. doi: 10.1002/jcb.21527. [DOI] [PubMed] [Google Scholar]
- Cho H, Kehrl JH. Regulation of immune function by G protein-coupled receptors, trimeric G proteins, and RGS proteins. Prog Mol Biol Transl Sci. 2009;86:249. doi: 10.1016/S1877-1173(09)86009-2. [DOI] [PubMed] [Google Scholar]
- Cornejo MG, Mabialah V, Sykes SM, Khandan T, Lo Celso C, Lopez CK, Rivera-Munoz P, Rameau P, Tothova Z, Aster JC, DePinho RA, Scadden DT, Gilliland DG, Mercher T. Crosstalk between NOTCH and AKT signaling during murine megakaryocyte lineage specification. Blood. 2011;118:1264. doi: 10.1182/blood-2011-01-328567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries L, Mousli M, Wurmser A, Farquhar MG. GAIP, a protein that specifically interacts with the trimeric G protein G alpha i3, is a member of a protein family with a highly conserved core domain. Proc Natl Acad Sci U S A. 1995;92:11916. doi: 10.1073/pnas.92.25.11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE, Buser C, Yeatman T, Coppola D, Winter C, Clark EA, Draetta GF, Strack PR, Majumder PK. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res. 2010;70:2476. doi: 10.1158/0008-5472.CAN-09-3114. [DOI] [PubMed] [Google Scholar]
- Feigin ME, Malbon CC. RGS19 regulates Wnt-beta-catenin signaling through inactivation of Galpha(o) J Cell Sci. 2007;120:3404. doi: 10.1242/jcs.011254. [DOI] [PubMed] [Google Scholar]
- Fischer T, Elenko E, Wan L, Thomas G, Farquhar MG. Membrane-associated GAIP is a phosphoprotein and can be phosphorylated by clathrin-coated vesicles. Proc Natl Acad Sci U S A. 2000;97:4040. doi: 10.1073/pnas.97.8.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldi J, Chung AY, Xu H, Zhu J, Outtz HH, Kitajewski J, Li Y, Hu X, Ivashkiv LB. Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1. J Immunol. 2010;185:5023. doi: 10.4049/jimmunol.1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung E, Tang SM, Canner JP, Morishige K, Arboleda-Velasquez JF, Cardoso AA, Carlesso N, Aster JC, Aikawa M. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115:2948. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Look AT. NOTCH and PI3K-AKT pathways intertwined. Cancer Cell. 2007;12:411. doi: 10.1016/j.ccr.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008a;226:41. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji JD, Tateya T, Kang YJ, Han J, Gessler M, Kageyama R, Ivashkiv LB. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008b;29:691. doi: 10.1016/j.immuni.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip AK, Tso PH, Lee MM, Wong YH. Elevated expression of RGS19 impairs the responsiveness of stress-activated protein kinases to serum. Mol Cell Biochem. 2012;362:159. doi: 10.1007/s11010-011-1138-1. [DOI] [PubMed] [Google Scholar]
- Ji YR, Kim MO, Kim SH, Yu DH, Shin MJ, Kim HJ, Yuh HS, Bae KB, Kim JY, Park HD, Lee SG, Hyun BH, Ryoo ZY. Effects of regulator of G protein signaling 19 (RGS19) on heart development and function. J Biol Chem. 2010;285:28627. doi: 10.1074/jbc.M109.073718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity. 2003;19:781. doi: 10.1016/s1074-7613(03)00325-x. [DOI] [PubMed] [Google Scholar]
- Monsalve E, Perez MA, Rubio A, Ruiz-Hidalgo MJ, Baladron V, Garcia-Ramirez JJ, Gomez JC, Laborda J, Diaz-Guerra MJ. Notch-1 up-regulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen-presenting capacity and cytotoxic activity. J Immunol. 2006;176:5362. doi: 10.4049/jimmunol.176.9.5362. [DOI] [PubMed] [Google Scholar]
- Palaga T, Buranaruk C, Rengpipat S, Fauq AH, Golde TE, Kaufmann SH, Osborne BA. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol. 2008;38:174. doi: 10.1002/eji.200636999. [DOI] [PubMed] [Google Scholar]
- Palomero T, Dominguez M, Ferrando AA. The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle. 2008;7:965. doi: 10.4161/cc.7.8.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan JH, Cha GH, Zhou W, Chu JQ, Nishikawa Y, Lee YH. Involvement of PI 3 kinase/Akt-dependent Bad phosphorylation in Toxoplasma gondii-mediated inhibition of host cell apoptosis. Exp Parasitol. 2013;133:462. doi: 10.1016/j.exppara.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- Ranganathan P, Vasquez-Del Carpio R, Kaplan FM, Wang H, Gupta A, VanWye JD, Capobianco AJ. Hierarchical phosphorylation within the ankyrin repeat domain defines a phosphoregulatory loop that regulates Notch transcriptional activity. J Biol Chem. 2011;286:28844. doi: 10.1074/jbc.M111.243600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekenberg S, Farhat K, Debarry J, Heine H, Jung G, Wiesmuller KH, Ulmer AJ. Regulators of G-protein signalling are modulated by bacterial lipopeptides and lipopolysaccharide. FEBS J. 2009;276:649. doi: 10.1111/j.1742-4658.2008.06813.x. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2007;1:2856. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Shi GX, Harrison K, Han SB, Moratz C, Kehrl JH. Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: implications for G protein-coupled receptor signaling. J Immunol. 2004;172:5175. doi: 10.4049/jimmunol.172.9.5175. [DOI] [PubMed] [Google Scholar]
- Silva A, Jotta PY, Silveira AB, Ribeiro D, Brandalise SR, Yunes JA, Barata JT. Regulation of PTEN by CK2 and Notch1 in primary T-cell acute lymphoblastic leukemia: rationale for combined use of CK2- and gamma-secretase inhibitors. Haematologica. 2010;95:674. doi: 10.3324/haematol.2009.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Wei SC, Huang MT, Lee MC, Chou HC, Chen CY, Hsieh WS. Lipopolysaccharide-induced Notch signaling activation through JNK-dependent pathway regulates inflammatory response. J Biomed Sci. 2011;18:56. doi: 10.1186/1423-0127-18-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso PH, Yung LY, Wang Y, Wong YH. RGS19 stimulates cell proliferation by deregulating cell cycle control and enhancing Akt signaling. Cancer Lett. 2011;309:199. doi: 10.1016/j.canlet.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. 2010;109:726. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- Weintz G, Olsen JV, Fruhauf K, Niedzielska M, Amit I, Jantsch J, Mages J, Frech C, Dolken L, Mann M, Lang R. The phosphoproteome of toll-like receptor-activated macrophages. Mol Syst Biol. 2010;6:371. doi: 10.1038/msb.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GW, Knowles GC, Mak TW, Ferrando AA, Zuniga-Pflucker JC. HES1 opposes a PTEN-dependent check on survival, differentiation, and proliferation of TCRbeta-selected mouse thymocytes. Blood. 2012;120:1439. doi: 10.1182/blood-2011-12-395319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongchana W, Palaga T. Direct regulation of interleukin-6 expression by Notch signaling in macrophages. Cell Mol Immunol. 2012;9:155. doi: 10.1038/cmi.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, Outtz H, Kitajewski J, Shi C, Weber S, Saftig P, Li Y, Ozato K, Blobel CP, Ivashkiv LB, Hu X. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13:642. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li YP. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 2007;21:1803. doi: 10.1101/gad.1544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li YP, Liu T, He X, Yuan X, Li C, Cao J, Kim Y. Mx1-cre mediated Rgs12 conditional knockout mice exhibit increased bone mass phenotype. Genesis. 2013;51:201. doi: 10.1002/dvg.22373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.