Abstract
We determined the contribution of vascular large conductance Ca2+-activated K+ (BK) and L-type Ca2+ channel dysregulation to exaggerated mortality in cecal ligation/puncture (CLP)-induced septic BK channel β1-subunit knockout (BK β1-KO, smooth muscle specific) mice. CLP-induced hemodynamic changes and mortality were assessed over 7 days in wild-type (WT) and BK β1-KO mice that were either untreated, given volume resuscitation (saline), or saline + calcium channel blocker nicardipine. Some mice were euthanized 24 h post-CLP to measure tissue injury and vascular and immune responses. CLP-induced hypotension was similar in untreated WT and BK β1-KO mice, but BK β1-KO mice died sooner. At 24 h post-CLP (mortality latency in BK β1-KO mice), untreated CLP-BK β1-KO mice showed more severe hypothermia, lower tissue perfusion, polymorphonuclear neutrophil infiltration-independent severe intestinal necrosis, and higher serum cytokine levels than CLP-WT mice. Saline resuscitation improved survival in CLP-WT but not CLP-BK β1-KO mice. Saline + nicardipine-treated CLP-BK β1-KO mice exhibited longer survival times, higher tissue perfusion, less intestinal injury, and lower cytokines versus untreated CLP-BK β1-KO mice. These improvements were absent in treated CLP-WT mice, although saline + nicardipine improved blood pressure similarly in both septic mice. At 24 h post-CLP, BK and L-type Ca2+ channel functions in vitro were maintained in mesenteric arteries from WT mice. Mesenteric arteries from BK β1-KO mice had blunted BK/enhanced L-type Ca2+ channel function. We conclude that vascular BK channel deficiency exaggerates mortality in septic BK β1-KO mice by activating L-type Ca2+ channels leading to blood pressure-independent tissue ischemia.
Keywords: BK β1-subunit knockout, polymicrobial septic shock, blood flow and tissue perfusion, Ca2+ channel blocker, polymorphonuclear neutrophil infiltration and tissue injury, cytokines, vascular BK and L-type Ca2+ channel function
septic shock is the second-leading cause of death in noncoronary intensive care unit patients worldwide. Despite the international recommendation of an early goal-directed therapy based on optimization of mean arterial blood pressure (MAP), central venous pressure, urine output, and central venous oxygen saturation, mortality rates remain high in septic shock (9, 23). Although sepsis is not primarily a cardiovascular disease, 50% of septic shock patients die from circulatory failure within the first 24 h after infection and before antimicrobial therapies can become effective (9, 23, 38). Sepsis-induced circulatory failure causes reduced organ perfusion, multiple organ failure, and death. In humans and animals, polymicrobial sepsis causes a biphasic hemodynamic response. An early hyperdynamic stage (2–10 h postinfection) is characterized by increased cardiac output, tissue perfusion, oxygen delivery, and decreased vascular resistance. The later developing hypodynamic stage is characterized by declining cardiac output and increased vascular resistance, which cause reduced tissue perfusion and oxygen delivery and lactate acidosis, resulting in multiple organ failure and death (8, 13, 45, 47). Prevention of the transition from the hyperdynamic to the hypodynamic circulatory phase during the progression of polymicrobial sepsis protects against sepsis-induced organ failure and death (46, 48).
Large conductance Ca2+-activated K+ (BK) channels are assembled from pore-forming α-subunits and accessory β-subunits that modulate α-subunit Ca2+ sensitivity and channel activity (2, 26). In vascular smooth muscle cells, BK channels regulate L-type Ca2+ channel activity and vascular tone through negative-feedback modulation. Activation of BK channels causes vascular smooth muscle cell hyperpolarization, L-type Ca2+ channel closure, and vasodilation (2, 26). There are four subtypes (β1-β4) of BK channel β-subunits and β1-subunits are specific for smooth muscle cells. BK β1-knockout (KO) reduces the Ca2+ sensitivity of the α-subunit causing reduced BK channel function. These changes lead to vascular smooth muscle cell membrane depolarization, increased norepinephrine reactivity, and increased vascular tone (2, 31, 42).
Theoretically, BK channel blockers should protect against sepsis-induced vasodilation and hypotension. Early studies indicated that BK channel activation may contribute to sepsis-induced vasodilation since blockade of BK channels improves arterial reactivity to norepinephrine (NE) in endotoxemic humans and survival in endotoxemic mice (6, 30) by preventing sepsis-induced arterial relaxation (7, 12, 21, 44). However, studies using either a global BK channel α-subunit knockout (α-KO) mouse (27) or a specific smooth muscle cell BK channel-deficient (β1-KO) mouse (43) do not support this theory. These studies showed that BK channel deficiency does not protect against polymicrobial sepsis or lipopolysaccharide (LPS)-induced hypotension and instead caused shortened latency to mortality and a higher mortality rate via undefined mechanisms (27, 43). Sepsis-induced mortality is more common in elderly patients, obese individuals, diabetics, and patients with congestive heart failure (1, 5, 25, 28) who have altered vascular tone and impaired vascular BK channel function (18, 24, 37, 50), suggesting that there may be interactions between BK channel dysfunction and septic shock-induced higher mortality in these patients.
Early studies showed a benefit of Ca2+ channel blockers in protecting against endotoxin-induced organ damage and mortality (15, 17, 22, 35). Ca2+ channel blockers improved blood pressure, organ perfusion, and survival in endotoxemic animals (15, 17, 22, 35, 40), suggesting that enhanced vascular Ca2+ channel activity might partly contribute to higher peripheral vascular resistance and lower perfusion of ischemia-sensitive organs during the late stage of septic shock (19, 36). Since vascular BK channel deficiency increases arterial L-type Ca2+ channel activity and vascular tone (2, 31, 42), we hypothesized that BK channel deficiency would promote high vascular smooth muscle cell Ca2+ channel activity. Increased Ca2+ activity would accelerate the transition from the hyperdynamic to the hypodynamic circulatory stage of shock, causing more severe organ hypoperfusion, organ damage, and increased mortality in septic BK β1-KO mice. We tested this hypothesis by blocking L-type Ca2+ channels with nicardipine in cecal ligation and puncture (CLP)-induced sepsis in BK β1-KO mice.
We compared MAP, heart rate (HR), organ damage, blood flow, and mortality in CLP-treated BK β1-KO and WT mice with or without L-type calcium channel blocker treatment in vivo. CLP-induced septic shock in mice is a model for the induction of polymicrobial sepsis. We also tested the contribution of vascular BK and L-type Ca2+ channel activity to regulation of vascular tone in mesenteric arteries (MA) from septic WT and BK β1-KO mice in vitro. The role of inflammatory responses in organ damage and mortality in septic BK β1-KO mice was also determined by measurement of serum cytokine levels and organ polymorphonuclear neutrophil (PMN) infiltration.
MATERIALS AND METHODS
Animals.
BK β1-KO mice are congenic as a result of seven generations of inbreeding to the C57BL/6 line (2). Homozygous breeder male and female BK β1-KO mice were a gift from Dr. Robert Brenner (University of Texas Health Science Center, San Antonio, TX). BK β1-KO mice were maintained originally as homozygous lines in Michigan State University (42, 43). WT (C57BL/6) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Pups of BK β1-KO mice were weaned at 3 weeks, and all mice were fed a normal diet. Mice used in our studies were at 14–16 wk of age (male, 25–30 g). All of the studies were conducted in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, Revised 1996) and approved by the Michigan State University Institutional Animal Care and Use Committees.
Measurements of MAP, HR, and survival.
Procedures used for telemeter implantation in mice have been described previously (42, 43). In mice under isoflurane anesthesia (2–3%) a catheter attached to a radiotelemetry transmitter (Data Sciences International, St. Paul, MN) was placed into the abdominal aorta via the left femoral artery. The transmitter was placed subcutaneously. Depth of anesthesia during surgery was assessed as stability of respiratory movement, pupil size, and paw-pinch reflexes. After the mice recovered from surgery (>3 days), they were maintained on a 12:12-h light-dark cycle. Blood pressure and HR were sampled continuously for 10 s every 10 min. Two weeks after implantation of telemetry, blood pressure and HR were collected at 1-h intervals 48 h before and 7 days after CLP procedures. The latency to mortality was defined as the time from CLP surgery until the first death within samples.
Cecal ligation/puncture.
With the mice under isoflurane anesthesia (2–3%), the cecum was exposed through an abdominal midline incision (32). Cecal puncture was performed by using 24-gauge hypodermic needles and punctures were made in the mesenteric to antimesenteric direction (through-through). The abdominal incision was closed, and all mice received warm saline (50 ml/kg sc, only once in untreated groups) immediately to induce a hyperdynamic circulatory phase (32). Mice were returned to their cages and checked at 3- to 6-h intervals for 7 days. Buprenorphine (0.05 mg/kg im, once) was used as analgesia after the CLP surgery.
Volume and/or nicardipine resuscitation.
In treated groups, 2 h after CLP surgery, mice received 30 ml/kg (low) or 50 ml/kg (intermediate) warm saline (37°C) (9, 49) with or without nicardipine (1.25 or 2.5 mg/kg sc) thereafter every 6 h (q.6.h., sc) for 72 h post-CLP.
Measurement of peripheral blood flow and rectal temperature.
Paw blood flow was measured by scanning laser Doppler (PeriScan PIM 3, Perimed, Stockholm, Sweden). While the mouse was under anesthesia, the paw was exposed. The scanning laser Doppler was positioned ∼19 cm above the surface of paw, and blood flow was measured in both paws. The wavelength of the laser light is set at 670–690 nm with a penetrating depth of 0.5–1 mm. A total of four consecutives scans were performed at each time point. The measurements were taken before and at 24 h post-CLP (the latency to mortality in BK β1-KO mice). Mean perfusion in each paw was measured using LDPIwin 3.1 software (Perimed).
To minimize the influence of body temperature on blood flow measurement, rectal temperature was constantly measured using a thermometer (BAT-12, Physitem Instruments) during the measurements. The power of the heating pad was adjusted to maintain the rectal temperature at premeasurement levels.
Blood sample and tissue collections.
At 24 h post-CLP, WT and BK β1-KO mice were euthanized with pentobarbital sodium (80–100 mg/kg ip), and blood was collected through cardiac puncture and centrifuged to separate the serum, which was frozen at −80°C. Left kidney, left lobes of liver, and small intestine segments were taken and fixed in 4% paraformaldehyde for 24 h before hematoxylin and eosin and PMN staining.
Measurement of vascular contractility in vitro.
A branch of MA was isolated from septic WT and BK β1-KO mice at 24 h post-CLP for contractile studies. MAs (60 mmHg pressurized inner diameter ≈100–150 μm) were mounted in a pressure myograph with Krebs solution equilibrated with compressed air (5% CO2-21% O2-74% N2, 37°C) (42). Changes in MA inner diameter (ID) caused by paxilline (0.5 μM, a selective BK channel antagonist) and BAY K 8644 (0.1 mM, a selective L-type Ca2+ channel agonist) were recorded.
Morphological assessment of organ damage.
The liver, kidney, and small intestine were removed immediately after mice were euthanized at 24 h after CLP. These tissue specimens were fixed overnight in 4% buffered formaldehyde, processed by standard methods, and stained with hematoxylin and eosin. One observer, who was blinded to the treatment of animals, performed the tissue analysis. The severity of the organ injury was determined as described previously (43). The severity of small intestine injury was scored from 0 to 3 as follows: 0, normal; no damage; 1, mild; focal epithelial edema and necrosis; 2, moderate; diffuse swelling or necrosis of the villi; and 3, severe; diffuse necrosis of the villi with evidence of neutrophil infiltration in the submucosa and/or hemorrhage. The severity of liver injury observed in the tissue sections was scored as follows: 0, minimal or no evidence of injury; 1, mild injury consisting of cytoplasmic vacuolation and focal nuclear pyknosis; 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and 3, severe necrosis with disintegration of the hepatic cords, hemorrhage, and neutrophil infiltration. The severity of renal tubular injury was scored by estimating the percentage of tubules in the cortex or the outer medulla that showed epithelial necrosis or had luminal necrotic debris, tubular dilation, and hemorrhage, as follows: 0, none; 1, <5%; 2, 5–25%; 3, 25–75%, and 4, >75%. All evaluations were made on 5 fields per section and 5 sections per organ.
Immunohistochemistry staining of PMN accumulation in small intestinal tissues, liver, and kidney.
PMN accumulation was determined by immunohistochemistry staining of PMN in small intestinal tissues, liver, and kidney. The paraffin-embedded tissue slices from the liver, kidney, and small intestine were cut into 6-μm thick slices and stained for PMNs using a rabbit anti-PMN antibody (1:2500, Abtu-neutrophil Clone 7/4) (43). After incubation with the primary antibody, tissue sections were incubated with biotinylated goat anti-rabbit IgG, avidin-conjugated alkaline phosphatase, and Vector Red substrate to stain PMNs.
Assays for MPO, TNF-α, and IL-6 production.
Production of myeloperoxidase (MPO) in small intestine was measured using ELISA with a mouse MPO kit (USCN, Life Science, Wuhan, Hubei, China). Serum TNF-α and IL-6 levels were measured using ELISA with mouse TNF-α and IL-6 kits (BD Biosciences).
Statistics.
Data are means ± SE from n mice. Paired and unpaired t-tests were used for two-group comparisons. All two-group comparisons were against a two-tailed alternative hypothesis. Heart rate (HR) and MAP were compared using a mixed design two-way ANOVA followed by Student-Newman-Keuls test. Survival curves of WT and BK β1-KO mice were fitted and compared using the Kaplan-Meier method. Data were analyzed using GraphPad Prism 5.0 software. P < 0.05 was considered statistically different.
RESULTS
Hypotension-independent shortened latency to mortality and lower survival rate in CLP-BK β1-KO mice.
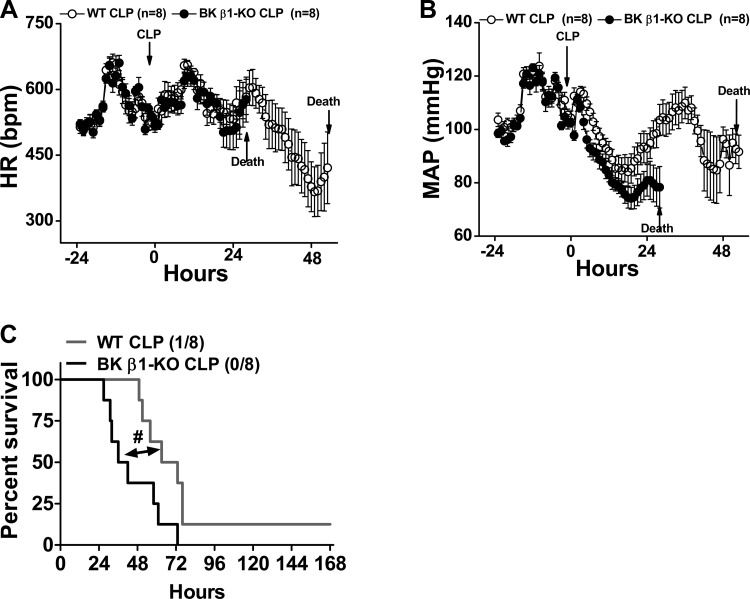
At 15 days after telemeter implantation, CLP-induced sepsis was induced in WT and BK β1-KO mice. MAP, HR, and survival rate were followed for up to 7 days (Fig. 1). Baseline MAP and HR were collected as 48-h averages before CLP, and they were similar in the two groups of mice. The latency to mortality post-CLP was 25 h in BK β1-KO mice and 52 h in WT mice (Fig. 1C); therefore, MAP and HR from each group was averaged only up to 25 h and 52 h post-CLP, respectively (Fig. 1, A and B). CLP caused a progressively developing hypotension (Fig. 1B), and the peak fall in MAP was at 18 h post-CLP in both groups. Although the peak fall of MAP in CLP-BK β1-KO mice was slightly larger than WT mice (102 ± 2 to 74 ± 3 mmHg vs. 104 ± 3 to 84 ± 5 mmHg), the overall progression of hypotension during the 24-h post-CLP was similar in both groups (P = 0.98). Hypotension, without a change in HR, persisted until death in BK β1-KO mice (Fig. 1A). After the early fall in MAP, CLP-WT mice developed a slow elevation of MAP and a progressive decrease in HR. At 36 h post-CLP, MAP recovered in CLP-WT mice to pre-CLP levels (109 ± 5 mmHg), and thereafter fell again. The peak fall of HR was at 48 h post-CLP (575 ± 24 to 366 ± 56 beats/min, P = 0.009). Bradycardia and hypotension persisted to 52 h post-CLP, the latency to earliest mortality in CLP-WT mice (Fig. 1, A and B).
Fig. 1.
Continuous measurement of heart rate (HR), mean arterial pressure (MAP), and mortality in telemetry implanted untreated wild-type (WT) and large conductance Ca2+-activated K+ (BK) β1-knockout (KO) mice before and up to 7 days after cecal ligation/puncture (CLP) surgery. MAP and HR were sampled for 10 s every 10 min and reported as hourly. Averaged HR (A) and MAP (B) from WT and BK β1-KO mice before and 51 and 24 h post-CLP are shown; the parameters cannot be averaged after this time point in each group because of decreased numbers of septic mice. C: 7-day survival rate in untreated CLP-WT and BK β1-KO mice. #Significantly different from CLP-WT mice (P < 0.05, Kaplan-Meier method).
The 7 days survival was significantly lower in septic BK β1-KO mice (P = 0.02) (Fig. 1C). All BK β1-KO mice died before 72 h post-CLP (Fig. 1C). One WT mouse survived up to 7 days (Fig. 1C). The median survival time in BK β1-KO mice was 39 h, which was shorter than WT mice (68 h). Since septic BK β1-KO mice had a shorter latency to mortality, we chose 24 h post-CLP as the time point to investigate the consequences of BK channel deficiency in septic shock.
Volume resuscitation improved survival in CLP-WT but not CLP-BK β1 KO mice.
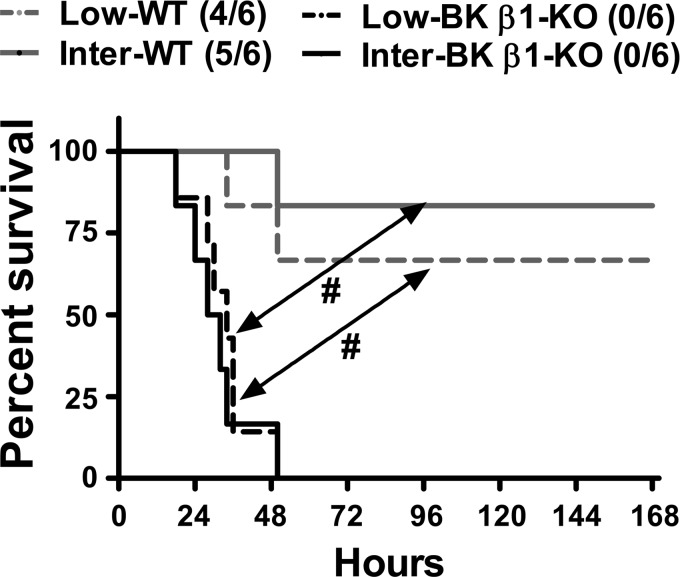
To determine whether hypovolemia contributes to the exacerbated mortality in septic BK β1-KO mice, after CLP procedures, WT and BK β1-KO mice received low (30 ml/kg) or intermediate (50 ml/kg) fluid resuscitation regimens (49), warm saline (37°C, 30 or 50 ml/kg sc) at the time of CLP, and then q.6.h. for up to 72 h. Seven-day survival in CLP-WT mice was volume dependently improved by saline treatment (Fig. 1C vs. Fig. 2, P = 0.025). Volume treatment neither delayed the latency to mortality (25 h vs. 24 h) nor improved the median survival time (39 h vs. 41 h) or 7-day survival in CLP-BK β1-KO mice (Fig. 1C vs. Fig. 2, P = 0.079).
Fig. 2.
Mortality and 7 days survival in volume-treated CLP-WT and BK β1-KO mice. Low-, treated with 30 ml/kg saline, Inter-; treated with 50 ml/kg saline. #Significantly different from CLP-WT mice (P < 0.05, Kaplan-Meier method).
Volume resuscitation combined with L-type Ca2+ channel blocker treatment improves survival time in CLP-BK β1 KO mice.
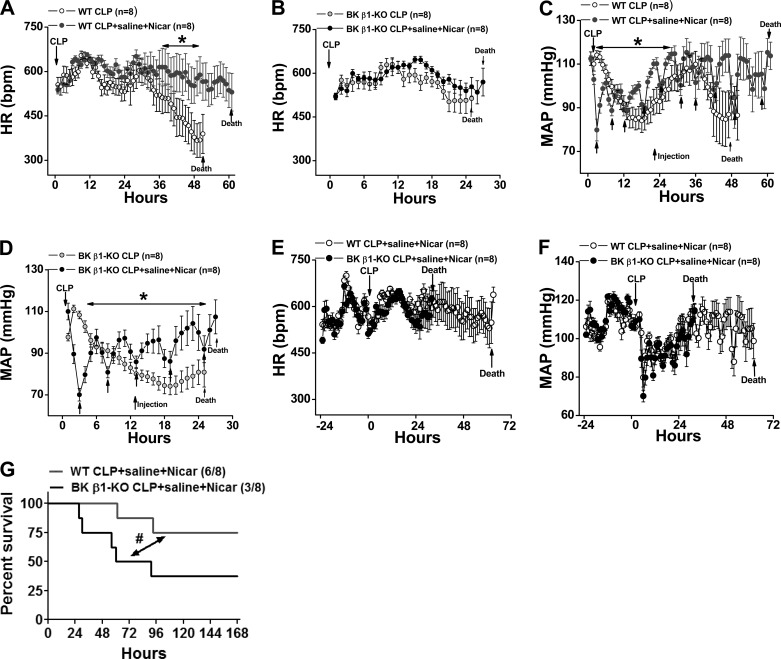
We hypothesized that BK channel dysfunction accelerates the transition from the hyperdynamic to the hypodynamic stage of septic shock by increasing vascular smooth muscle cell Ca2+ channel activity. This could cause hyposensitivity to vasodilators and reduced organ blood flow. If so, blocking L-type Ca2+ channels should improve the survival rate in BK β1-KO mice. We tested the effect of the dihydropyridine calcium channel blocker nicardipine on MAP, HR, and mortality in septic WT and BK β1-KO mice. Fifteen days posttelemetry implantation, WT and BK β1-KO mice received CLP surgery and saline (50 ml/kg) plus nicardipine (2.5 mg/kg sc) treatment at 2 h post-CLP and thereafter q.6.h. for up to 72 h. MAP, HR, and survival rate were followed for up to 7 days (Fig. 3). The nicardipine dose and volume were selected based on our primary studies showing that this combined treatment improved survival time in CLP-BK β1-KO mice. We found that the survival time was significantly prolonged in CLP-BK β1-KO mice when these mice were treated with 2.5 mg/kg nicardipine + 50 ml/kg saline, but not with 1.25 mg/kg nicardipine + 50 ml/kg saline (data not shown).
Fig. 3.
Continuous measurement of HR, MAP, and mortality in telemetry implanted saline + nicardipine (Nicar)-treated WT and BK β1-KO mice before and up to 7 days after CLP surgery. MAP and HR were sampled for 10 s every 10 min and reported as hourly. Averaged HR (A, B) and MAP (C, D) from WT and BK β1-KO mice were compared with its untreated mice (adapted from Fig. 1); averaged HR (E) and MAP (F) from treated WT and BK β1-KO mice before and 61 and 24 h post-CLP; the parameters cannot be averaged after this time point in each group because of decreased numbers of septic mice. G: 7-day survival rate in saline + Nicar-treated CLP-WT and BK β1-KO mice. *Significantly different from its untreated CLP mice (P < 0.05, two-way ANOVA). #Significantly different from treated CLP-WT mice (P < 0.05, Kaplan-Meier method).
Since latency to mortality in treated groups was 28 and 61 h post-CLP in treated CLP-BK β1-KO and WT mice (Fig. 3), MAP and HR for each group were averaged for up to 28 and 61 h, respectively. In both treated groups, blood pressure was lower 6–8 h post-CLP; thereafter, blood pressure was significantly higher than untreated mice (Fig. 3, C and D), although MAP transiently dropped by 10 to 15 mmHg after each nicardipine treatment (Fig. 3, C and D). Treatment greatly improved HR in CLP-WT (Fig. 3A) but did not affect HR in BK β1-KO mice (Fig. 3B).
We also compared MAP and HR in treated WT versus BK β1-KO mice. The transient depressor responses caused by the first (at 2 h) and second (at 8 h) nicardipine treatments were slightly larger in BK β1-KO mice than septic control mice: the blood pressure nadir occurred at 70 ± 3 mmHg vs. 80 ± 4 mmHg (P = 0.047) and 80 ± 2 mmHg vs. 89 ± 2 mmHg, respectively (P = 0.045) (Fig. 3F). However, overall MAP and HR during 24 h post-CLP were similar in treated WT and BK β1-KO mice (Fig. 3, E and F).
When compared with saline-treated mice, the combined treatment increased the latency to mortality from 51 to 61 h without improving 7-day survival (5/8 vs. 6/8, P = 0.79) (Fig. 2 vs. Fig. 3G) in CLP-WT mice. In CLP-BK β1-KO mice, the 7-day survival time was significantly improved compared with saline-treated BK β1-KO mice (Fig. 2 vs. Fig. 3G, P = 0.0075). The combined treatment did not delay the latency to mortality (24 h vs. 28 h), but median survival time improved from 39 to 77 h. Treatment improved overall 7-day survival from 1/8 to 3/8 (Fig. 2 vs. Fig. 3G), which was still significantly lower than that of treated WT mice (Fig. 3G, P = 0.042).
Ca2+ channel blocker and volume resuscitation protect against hypoperfusion in CLP-BK β1-KO mice.
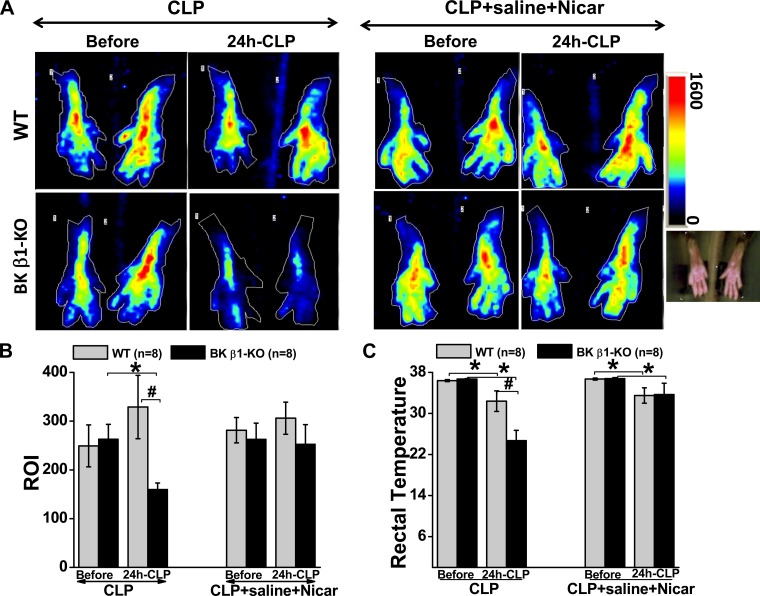
To test the hypothesis that L-type Ca2+ channel-dependent vascular mechanisms mediate CLP-induced organ damage and mortality in BK β1-KO mice, we measured blood flow before and 24 h post-CLP with or without combination treatment. We measured paw blood flow as an index of peripheral tissue perfusion using Doppler and also measured rectal body temperature simultaneously as an indirect index of internal organ blood flow (20). Paw blood flow was measured bilaterally but we report data from the left side only (the flow rate was similar on both sides). We found that CLP reduced peripheral blood flow in untreated BK β1-KO mice, but not in untreated WT mice at 24 h post-CLP, the latency to mortality in BK β1-KO mice (Fig. 4, A-C). In addition, we also found that CLP caused more severe hypothermia in untreated BK β1-KO mice compared with untreated WT mice (Fig. 4C), suggesting that organ perfusion was lower in these mice. Importantly, the combination treatment improved blood flow and body temperature in CLP-BK β1-KO but not in CLP-WT mice (Fig. 4, A and B).
Fig. 4.
Measurements of paw blood flow (A, B) and rectal temperature (C) with or without saline + Nicar treatment in WT and BK β1-KO mice before and 24 h post-CLP. ROI, region of interest. *Significantly different from before CLP surgery (P < 0.05, paired t-test). #Significantly different from untreated CLP-WT mice (P < 0.05, unpaired t-test).
Ca2+ channel blocker and volume resuscitation protect against organ damage in CLP-BK β1-KO mice.
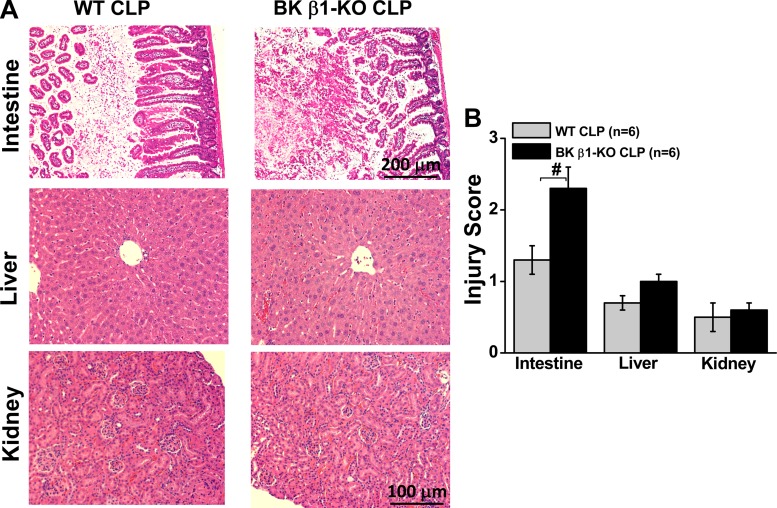
To determine whether vascular BK channel deficiency exaggerates organ damage in septic mice, we evaluated the tissue injury scores in untreated mice. Small intestine, liver, and kidney were taken at 24 h post-CLP surgery. Tissue injury scores were calculated from hematoxylin and eosin-stained tissue slices (43). At 24 h post-CLP, untreated CLP-BK β1-KO mice displayed more severe injury in the mucosa of small intestine than the tissues from untreated WT mice (Fig. 5). Injury to the liver and kidney was similar in WT and β1-KO mice (Fig. 5). In our preliminary studies, the plasma creatinine and alanine aminotransferase levels were very low at 24 h post-CLP in control and BK β1-KO mice (data were not shown) indicating that sepsis-induced kidney and liver damage may not contribute to mortality in BK β1-KO mice. Therefore, intestinal injury was used to evaluate organ damage caused by CLP in subsequent studies.
Fig. 5.
Hematoxylin and eosin-stained slices (A) and injury scores (B) in small intestine, liver, and kidney tissues from untreated WT and BK β1-KO mice at 24 h post-CLP. #Significantly different from untreated CLP-WT mice (P < 0.05, unpaired t-test).
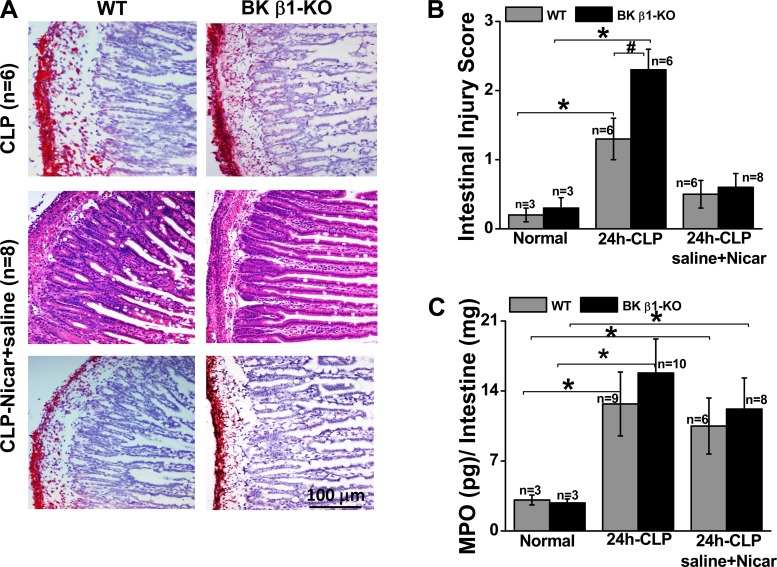
To determine the role of L-type Ca2+ channel activation in CLP-induced organ damage, we evaluated intestinal injury scores in saline + nicardipine-treated WT and BK β1-KO mice. We found that intestinal injury was reduced in both treatment groups, but improvement was greater in BK β1-KO mice (Fig. 6B). We then evaluated PMN infiltration into the small intestine. At 24 h post-CLP the small intestines from untreated CLP-BK β1-KO mice did not show higher PMN infiltration and MPO levels compared with CLP-WT mice (Fig. 6, A and C). We also found that most PMNs infiltrated the adventitial layer of the intestine, not the mucosa where tissue damage was most prominent (Fig. 6A). Saline + nicardipine treatment did not change PMN infiltration or MPO levels in the small intestine of CLP-WT and CLP-BK β1-KO mice (Fig. 6, A and C).
Fig. 6.
Comparison of polymorphonuclear neutrophil (PMN) infiltration (A), tissue injury score (B), and myeloperoxidase (MPO) levels (C) in small intestine from CLP-WT and BK β1-KO mice with or without saline + Nicar treatment. *Significantly different from its normal mice (P < 0.05, paired t-test). #Significantly different from untreated CLP-WT mice (P < 0.05, unpaired t-test).
BK channel function is maintained in MA at 24 h after CLP in WT mice.
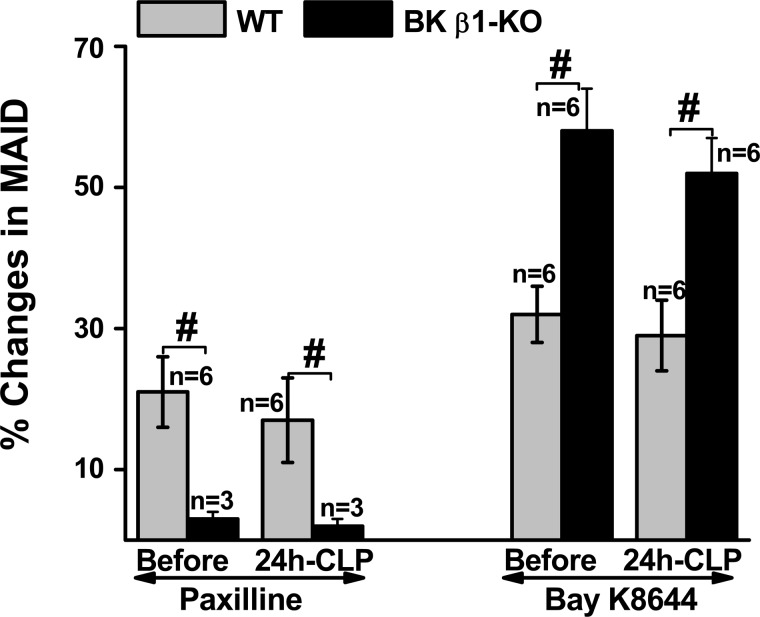
Finally, we compared BK and L-type Ca2+ channel function in MA from untreated WT and BK β1-KO mice at 24 h post-CLP (the latency to mortality in BK β1-KO, but not in WT mice). BK and L-type Ca2+ channel function in MA were tested using paxilline and BAY K 8644 (Fig. 7). Higher BK and lower L-type Ca2+ channel functions were maintained in WT MA before 24 h post-CLP, but as expected there was an impaired BK and enhanced L-type Ca2+ channel function in BK β1-KO MA.
Fig. 7.
Comparison the contractile responses to paxilline and BAY K 8644 in mesenteric arteries (MA) from normal and untreated septic WT and BK β1-KO mice at 24 h-post CLP. MAID, mesenteric arterial inner diameter. #Significantly different from WT MA (P < 0.05, unpaired t-test).
Ca2+ channel blocker and volume resuscitation reduced serum cytokine levels.
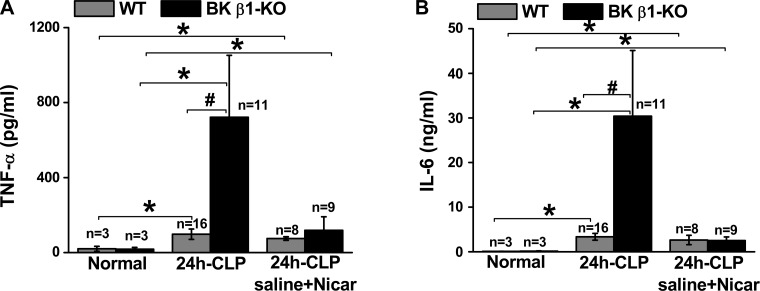
To determine the role of immune activation in organ damage and mortality in septic BK β1-KO mice, we measured serum TNF-α and IL-6 levels from untreated and saline + nicardipine-treated CLP-WT and BK β1-KO mice at 24 h post-CLP (Fig. 8). We found that both TNF-α and IL-6 levels in untreated CLP-BK β1-KO mice were higher than untreated CLP-WT mice (Fig. 8). Interestingly, these higher levels of cytokines in CLP-BK β1-KO mice were sensitive to L-type Ca2+ channel blockade since the cytokine levels were reduced in treated mice. Treatment did not affect cytokine levels in CLP-WT mice.
Fig. 8.
Comparison of plasma TNF-α (A) and IL-6 (B) levels in normal, untreated, and saline + Nicar-treated WT and BK β1-KO mice at 24 h post-CLP. *Significantly different from its normal mice (P < 0.05, paired t-test). #Significantly different from untreated CLP-WT mice (P < 0.05, unpaired t-test).
DISCUSSION
Exacerbated hypoperfusion, organ damage, and mortality in septic BK β1-KO mice are blood pressure independent.
Previous work has shown exacerbated mortality in septic BK channel knockout/deficient mice compared with its WT mice, but the mechanisms were not determined (27, 43). Similar mortality data were obtained in the present study done in CLP-induced polymicrobial sepsis, where we found that hypotension was similar in untreated septic WT and BK β1-KO mice. However, we found that untreated septic BK β1-KO mice had a shorter latency to mortality and a lower survival rate than did untreated WT mice. These results indicate that maintenance of BK channel function protects against mortality in septic BK β1-KO mice but this protection is blood pressure independent.
Movement of the circulation from the hyperdynamic to the hypodynamic phase and prolonged organ hypoperfusion contribute to sepsis-induced organ damage and mortality. Maintaining organ perfusion with volume resuscitation and/or maintenance of cardiac output is more critical than controlling blood pressure in prevention of organ damage and mortality during septic shock (9, 23, 49). Vasoconstrictors are used to maintain blood pressure only if mean blood pressure is consistently lower than 65 mmHg after initial fluid resuscitation. Presumably this is because most critical tissues require a minimum driving pressure of at least 65 mmHg to maintain adequate blood flow. Clearly, vasoconstrictors do not increase vascular resistance in critical tissues (kidney, heart, brain) would be preferable, but there are no known vasoconstrictors with such high tissue specificity. In our studies, hypotension occurred in all untreated septic mice, and untreated septic BK β1-KO mice died at 24 h before the transition to the hypodynamic phase. Untreated septic WT mice showed a progressive increase in MAP and decrease in HR, suggesting that there was an increase in peripheral resistance and a decrease in cardiac output (it is unlikely that higher stroke volume occurs in septic mice) between 24 and 51 h post-CLP (latency to mortality). Untreated septic WT mice showed signs of transition to the hypodynamic circulatory phase 24 h post-CLP. Progression of hypotension up to 24 h post-CLP was similar in WT and BK β1-KO mice. However, untreated BK β1-KO mice may progress more quickly to the later stage of septic shock, which includes severe hypoperfusion, hyperthermia, and intestinal ischemia. These data suggest that normal vascular BK channel function is crucial in protecting against the transition between the hyperdynamic and hypodynamic hemodynamic states in septic BK β1-KO mice. Definitive proof of this accelerated transition in septic BK β1-KO mice will require direct measurements of cardiac output, stroke volume, and peripheral resistance in conscious septic mice.
Increased L-type Ca2+ channel activity exacerbates hypoperfusion, organ damage, and mortality in septic BK β1-KO mice.
Volume resuscitation can reduce mortality in septic shock by maintaining organ perfusion and cardiac output (9, 23, 49). In our study, most septic WT mice were rescued by volume resuscitation, even at 30 ml/kg of volume replacement (9, 49), a volume that is considered a low level of volume resuscitation for septic shock patients. This result indicates that hypovolemia is responsible for sepsis-induced mortality in WT mice. However, volume resuscitation did not rescue septic BK β1- KO mice. These results suggest that organ perfusion may be limited by higher vascular resistance and reduced tissue perfusion in septic BK β1-KO mice. In the later stage of septic shock, high peripheral vascular resistance would reduce perfusion of ischemia-sensitive tissues (for example, the mucosa of small intestine), possibly via enhanced vascular Ca2+ channel activity. Blocking L-type Ca2+ channels improves blood pressure, organ perfusion, and survival in endotoxemic septic shock (15, 17, 22, 33, 35, 40). Dysregulated L-type Ca2+ channel function is more prominent in BK β1-KO mice due to the absence of counterregulation by BK channels (2, 31, 42). In our previous studies, we found that the fall in MAP caused by the calcium channel blocker nifedipine showed modest dose dependency, and depressor responses to nifedipine were slightly (∼10 mmHg at peak) larger in BK β1-KO than WT mice (42). Therefore, we tested the hypothesis that increased L-type Ca2+ channel activity in vascular smooth muscle cells was a cause of reduced organ perfusion and increased mortality in septic BK β1-KO mice.
Higher vascular L-type Ca2+ channel activity in septic BK β1-KO mice has been reported previously (43) and in the current study. In current study, lower BK and higher L-type Ca2+ channel activity occurred in mesenteric resistance arteries from septic BK β1-KO mice until death. Higher vascular BK channel and lower L-type Ca2+ channel activity were maintained in septic WT mice at least up to 24 h post-CLP, the time of death in BK β1-KO mice. The transient depressor responses caused by the first (at 2 h) and second (at 8 h) nicardipine treatments were also slightly larger in BK β1-KO mice than septic WT mice. Our data indicate that BK β1-KO mice have enhanced L-type Ca2+ channel activity controlling vascular tone and blood pressure. Nicardipine treatment (combined with volume resuscitation) increased survival time in septic BK β1-KO mice compared with untreated and volume-treated septic BK β1-KO mice. Importantly, this treatment also improved peripheral perfusion (blood flow) and body temperature and reduced tissue injury in septic BK β1-mice. Improvements caused by combined treatment in septic BK β1-KO mice were blood pressure independent, since blood pressures were improved similarly in both treated groups. Improvement in blood pressure was largely mediated by the volume resuscitation, but nicardipine treatment did cause transient depressor responses in all mice, likely due to reduced peripheral resistance, which could increase tissue perfusion. Interestingly, the combinational treatment did not provide additional survival benefit in septic WT mice compared with volume resuscitation alone. These data indicate that septic WT mice may not have an impairment of the coupling between BK and L-type Ca2+ channels. Furthermore, we did not find that impaired interactions between BK and L-type Ca2+ channel function (if there is) will have clinical significance on the overall outcomes in sepsis-induced mortality in WT mice. Therefore, our data indicate that both hypovolemia and L-type Ca2+ channel-mediated increases in vascular resistance contributed to hypoperfusion and mortality in septic BK β1-KO mice. Direct measurements of peripheral resistance are needed to support our hypothesis that increased L-type Ca2+ channel activity in septic BK β1-KO mice is responsible for reduced tissue perfusion.
Higher cytokines levels in septic BK β1-KO mice are also sensitive to Ca2+ channel blocker: a role for BK channels in the immune system?
Whether or not BK channels are expressed in immune cells is controversial (10, 11), although early studies reported that BK channels were required for LPS-induced macrophage cytokine release (29). Our studies show that serum cytokine levels were much higher in septic BK β1-KO mice at 24 h postsepsis when compared with septic control mice. Higher serum cytokine levels in septic mice were reduced by saline + nicardipine treatment in BK β1-KO but not in control mice. Higher serum cytokine levels in the late stage of septic BK β1-KO mice may be caused by diminished clearance rather than enhanced release. This conclusion is consistent with our observation of similar inflammatory responses in the small intestine from septic control and BK β1-KO mice with or without combined treatment. It has been reported that the increase in local concentration of cytokines is the most critical factor causing organ injury in sepsis (15, 17). Ca2+ channel blockers improve survival in endotoxemic shock at least in part by blocking the oxidative burst, nitrite formation, and cytokine release from macrophages (3, 4, 14, 16, 41). However, Ca2+ channel blockers may increase mortality in sepsis due to impaired bacterial killing (15, 17). In our study, the nicardipine-induced increase in blood flow and perfusion and extended survival time were more prominent in septic BK β1-KO mice than in WT mice, and increased blood flow would be expected to increase cytokine clearance. We also showed that tissue injury and inflammation were not uniform across all organs, as more severe injury occurred in the small intestine, but without enhanced intestinal inflammatory responses, in septic BK β1-KO mice. PMNs infiltrated mostly into the intestinal adventitia, while the intestinal injury occurred mostly at the mucosa. We also found that the PMN infiltration was similar in the liver, but PMN infiltration was absent in the kidney, in septic WT and BK β1-KO mice (data not shown), and the sepsis-induced visual tissue injury was minor in these mice. These results argue against a broad contribution of immune system activation to organ damage and mortality in septic BK β1-KO mice. Our animal model separate vascular from other systemic responses that may be involved in organ damage and hemodynamic regulation in septic shock. Direct measurements of cytokine/bacterial clearance are needed to support our hypothesis.
Role of BK and L-type Ca2+ channel dysregulation in human cardiovascular diseases-clinical relevance.
We did not find that acute septicemia impairs BK channel function during the early stage of CLP-induced sepsis, although our previous studies in endotoxemic control mice showed impaired vascular BK channel activity at 22 h post-LPS treatment (43). Impaired vascular BK channel function may develop more slowly in CLP-induced septic WT mice. However, our data indicate that impaired interactions between BK and L-type Ca2+ channels may not affect outcomes in sepsis-induced mortality in humans with normal vascular BK channel function as most of septic WT mice are rescued by volume replacement without nicardipine or antimicrobial therapy. We used 30 ml/kg of volume replacement as this is the recommendation for first line treatment of septic shock patients (9, 49). However, sepsis-induced mortality is much higher in elderly patients (25), obese individuals (28), diabetic patients (5), and patients with congestive heart failure (1). These patients have higher vascular tone and impaired vascular BK channel function via downregulation of BK β1-subunit expression and function (18, 24, 37, 50). We propose that BK β1-KO mice mimic the pathophysiological condition occurring in these patients. Our studies indicate that the Ca2+ channel blocker treatment may benefit populations of septic patients who have impaired vascular BK channel function and do not respond adequately to volume resuscitation. A promising clinical study showed that coadministration of a Ca2+ channel blocker and immunosuppressive drugs not only decreased the rate of organ failure in transplant patients but also reduced the incidence of septic episodes and death in diabetic patients receiving a kidney transplant (39).
Limitations and further studies.
We cannot rule out contributions of immune activation or other systemic responses in exacerbated organ damage and mortality in septic BK β1-KO. Nicardipine extended the survival time, but the overall 7-day survival rate was still significantly lower in treated BK β1-KO mice compared with WT mice. The higher cytokine levels in septic BK β1-KO mice may be explained by diminished cytokine clearance, but this hypothesis needs to be substantiated by direct measurements. Future studies should determine whether BK and L-type Ca2+ channels function in immune cells and if their dysregulation affects sepsis-induced immune responses. An evaluation of cytokine concentrations within the organs will help to distinguish between BK channel dysfunction in vascular smooth muscle versus immune cells. Additional studies need to directly measure organ blood flow, cardiac output, and peripheral resistance, although these hemodynamic measurements are very difficult in septic mice due to hemodynamic lability during sepsis. Finally, no animal model recapitulates the complex pathophysiology of sepsis in humans. This point is highlighted by a study showing marked differences in the profile of activation of genes controlling the inflammatory response to endotoxemia in humans and mice (34). Results from studies of septic shock need to be interpreted with caution when attempting to extrapolate data obtained using mouse models to human diseases (34).
In conclusion, maintenance of BK channel function delays the hemodynamic transition into the hypodynamic circulatory phase (the late stage of septic shock causing mortality) by stabilizing vascular L-type Ca2+ channel function. Dysregulated BK and L-type Ca2+ channel function are responsible for hypoperfusion, organ damage, and mortality in septic BK β1-KO mice. L-type Ca2+ channel blocker treatment is a potential therapeutic approach to rescue septic shock patients who have primary diseases associated with reduced vascular BK channel function.
Perspectives and Significance
Septic shock is associated with high morbidity and mortality, and there are no effective treatments (9, 23). Sepsis and sepsis-induced mortality are more common in elderly patients, obese individuals, diabetic patients, and patients with congestive heart failure who have altered vascular tone and impaired vascular BK channel function, suggesting that there are interactions between BK channel dysfunction and sepsis-induced mortality (1, 5, 25, 28). Our studies provide new information about the causes of hemodynamic dysfunction and mortality in septic shock and support the use of L-type Ca2+ channel blockers for treatment of septic shock in groups of patients known to have BK channel dysfunction. Tissue hypoperfusion is an important factor in the development of multiple organ failure. Understanding these hemodynamic abnormalities and cellular mechanisms responsible for tissue hypoperfusion will help develop treatments that will reduce organ injury and mortality in septic shock.
GRANTS
This study was supported, in part, by National Heart, Lung and Blood Institute Grant P01 HL-070687 (to H. Xu, J. J. Galligan, and G. D. Fink) and American Heart Association Midwest Affiliate 11GRNT6690010 (to H. Xu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.X., J.J.G., and G.D.F. conception and design of research; H.X., H.G., and R.F. performed experiments; H.X. and R.F. analyzed data; H.X., J.J.G., and G.D.F. interpreted results of experiments; H.X. and R.F. prepared figures; H.X. drafted manuscript; H.X., J.J.G., and G.D.F. edited and revised manuscript; H.X., H.G., R.F., J.J.G., and G.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Robert Brenner (University of Texas Health Science Center at San Antonio, TX) for the gift of BK β1-KO mice. We thank staffs in Histopathology laboratory at Michigan State University for the tissue processing and histological staining.
REFERENCES
- 1.Bauer ME, Bateman BT, Bauer ST, Shanks AM, Mhyre JM. Maternal sepsis mortality and morbidity during hospitalization for delivery: temporal trends and independent associations for severe sepsis. Anesth Analg 117: 944–950, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bosson S, Kuenzig M, Schwartz SI. Verapamil improves cardiac function and increases survival in canine E. coli endotoxin shock. Circ Shock 16: 307–316, 1985 [PubMed] [Google Scholar]
- 4.Bosson S, Kuenzig M, Schwartz SI. Increased survival with calcium antagonists in antibiotic-treated bacteremia. Circ Shock 19: 69–74, 1986 [PubMed] [Google Scholar]
- 5.Carton JA, Maradona JA, Nuño FJ, Fernandez-Alvarez R, Pérez-Gonzalez F, Asensi V. Diabetes mellitus and bacteraemia: a comparative study between diabetic and non-diabetic patients. Eur J Med 1: 281–287, 1992 [PubMed] [Google Scholar]
- 6.Cauwels A, Brouckaert P. Critical role for small and large conductance calcium-dependent potassium channels in endotoxemia and TNF toxicity. Shock 29: 577–582, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chen SJ, Wu CC, Yang SN, Lin CI, Yen MH. Hyperpolarization contributes to vascular hyporeactivity in rats with lipopolysaccharide-induced endotoxic shock. Life Sci 68: 659–668, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock 9: 1–11, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit Care Med 41: 580–637, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Essin K, Salanova B, Kettritz R, Sausbier M, Luft FC, Kraus D, Bohn E, Autenrieth IB, Peschel A, Ruth P, Gollasch M. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol Cell Physiol 293: C45–C54, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Essin K, Gollasch M, Rolle S, Weissgerber P, Sausbier M, Bohn E, Autenrieth IB, Ruth P, Luft FC, Nauseef WM, Kettritz R. BK channels in innate immune functions of neutrophils and macrophages. Blood 113: 1326–1331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farias NC, Borelli-Montigny GL, Fauaz G, Feres T, Borges AC, Paiva TB. Different mechanism of LPS-induced vasodilation in resistance and conductance arteries from SHR and normotensive rats. Br J Pharmacol 137: 213–220, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hohlfeld T, Klemm P, Thiemermann C, Warner TD, Schrör K, Vane JR. The contribution of tumour necrosis factor-alpha and endothelin-1 to the increase of coronary resistance in hearts from rats treated with endotoxin. Br J Pharmacol 116: 3309–3315, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Osborne DF, Lappas GD, Karl IE. Calcium antagonists decrease plasma and tissue concentrations of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 alpha in a mouse model of endotoxin. Shock 3: 337–342, 1995 [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Karl IE. Calcium: a regulator of the inflammatory response in endotoxemia and sepsis. New Horiz 4: 58–71, 1996 [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Bowling WM, Karl IE, Osborne DF, Flye MW. Calcium antagonists inhibit oxidative burst and nitrite formation in lipopolysaccharide-stimulated rat peritoneal macrophages. Shock 8: 170–178, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Karl IE. Ca2+, a regulator of the inflammatory response–the good, the bad, and the possibilities. Shock 7: 308–310, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Howitt L, Grayson TH, Morris MJ, Sandow SL, Murphy TV. Differential effects of diet-induced obesity on BKCa β1-subunit expression and function in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol Heart Circ Physiol 301: H29–H40, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Jones JJ, Rapps JA, Sturek M, Mattox ML, Adams HR, Parker JL. Contractile function and myoplasmic free Ca2+ (Cam) in coronary and mesenteric arteries of endotoxemic guinea pigs. Shock 11: 64–71, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Kuttila K, Niinikoski J, Haglund U. Visceral and peripheral tissue perfusion after cardiac surgery. Scand J Thorac Cardiovasc Surg 25: 57–62, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Kuo JH, Chen SJ, Shih CC, Lue WM, Wu CC. Abnormal activation of potassium channels in aortic smooth muscle of rats with peritonitis-induced septic shock. Shock 32: 74–79, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Lee HC, Hardman JM, Lum BK. Effects of nicardipine in rats subjected to endotoxic shock. Gen Pharmacol 23: 71–74, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36: 222–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res 88: 210–216, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 34: 15–21, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 27.O'Brien AJ, Terala D, Orie NN, Davies NA, Zolfaghari P, Singer M, Clapp LH. BK Large Conductance Ca2+-activated K+ channel deficient mice are not resistant to hypotension and display reduced survival benefit following polymicrobial sepsis. Shock 35: 485–491, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien JM, Jr, Aberegg SK, Ali NA, Diette GB, Lemeshow S. Results from the national sepsis practice survey: predictions about mortality and morbidity and recommendations for limitation of care orders. Crit Care 13: R96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papavlassopoulos M, Stamme C, Thon L, Adam D, Hillemann D, Seydel U, Schromm AB. MaxiK blockade selectively inhibits the lipopolysaccharide-induced I kappa B-alpha/NF-kappa B signaling pathway in macrophages. J Immunol 177: 4086–4093, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Pickkers P, Dorresteijn MJ, Bouw MP, et al. In vivo evidence for nitric oxide-mediated calcium-activated potassium-channel activation during human endotoxemia. Circulation 114: 414–421, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4: 31–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayeed MM. Alterations in calcium signaling and cellular responses in septic injury. New Horiz 4: 58–71, 1996 [PubMed] [Google Scholar]
- 34.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation, and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirmagul B, Kilic FS, Tunc O, Yildirim E, Erol K. Effects of verapamil and nifedipine on different parameters in lipopolysaccharide-induced septic shock. Heart Vessels 21: 162–168, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Song SK, Karl IE, Ackerman JJ, Hotchkiss RS. Increased intracellular Ca2+: a critical link in the pathophysiology of sepsis? Proc Natl Acad Sci USA 90: 3933–3937, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan E, Kushner JS, Zakharov S, Nui XW, Chudasama N, Kelly C, Waase M, Doshi D, Liu G, Iwata S, Shiomi T, Katchman A, D'Armiento J, Homma S, Marx SO. Reduced vascular smooth muscle BK channel current underlies heart failure-induced vasoconstriction in mice. FASEB J 27: 1859–1867, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med 35: 1928–1936, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Weinrauch LA, D'Elia JA, Gleason RE, Shaffer D, Monaco AP. Role of calcium channel blockers in diabetic renal transplant patients: preliminary observations on protection from sepsis. Clin Nephrol 44: 185–192, 1995 [PubMed] [Google Scholar]
- 40.Wu CC, Wang JH, Chiao CW, Yen MH. Comparison between effects of dantrolene and nifedipine on lipopolysaccharide-induced endotoxemia in the anesthetized rats. Chin J Physiol 42: 211–217, 1999 [PubMed] [Google Scholar]
- 41.Wyska E. Pretreatment with R(+)-verapamil significantly reduces mortality and cytokine expression in murine model of septic shock. Int Immunopharmacol 9: 478–490, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Garver H, Galligan JJ, Fink GD. Large-conductance Ca2+-activated K+ channel β1-subunit knockout mice are not hypertensive. Am J Physiol Heart Circ Physiol 300: H476–H485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Wang Y, Garver H, Galligan JJ, Fink GD. Vascular BK channel deficiency exacerbates organ damage and mortality in endotoxemic mice. J Cardiovasc Pharmacol 59: 207–214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakubovich N, Eldstrom JR, Mathers DA. Lipopolysaccharide can activate BK channels of arterial smooth muscle in the absence of iNOS expression. Biochim Biophys Acta 1514: 239–252, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Yang S, Cioffi WG, Bland KI, Chaudry IH, Wang P. Differential alterations in systemic and regional oxygen delivery and consumption during the early and late stages of sepsis. J Trauma 47: 706–712, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Zhou M, Koo DJ, Chaudry IH, Wang P. Pentoxifylline prevents the transition from the hyperdynamic to hypodynamic response during sepsis. Am J Physiol Heart Circ Physiol 277: H1036–H1044, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Yang S, Chung CS, Ayala A, Chaudry IH, Wang P. Differential alterations in cardiovascular responses during the progression of polymicrobial sepsis in the mouse. Shock 17: 55–60, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Yang S, Zhou M, Chaudry IH, Wang P. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg 236: 625–633, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanotti-Cavazzoni SL, Guglielmi M, Parrillo JE, Walker T, Dellinger RP, Hollenberg SM, et al. , et al. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Med 35: 748–754, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Zhang DM, He T, Katusic ZS, Lee HC, Lu T. Muscle-specific f-box only proteins facilitate bk channel β(1) subunit downregulation in vascular smooth muscle cells of diabetes mellitus. Circ Res 107: 1454–1459, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]