Abstract
Byproducts of normal mitochondrial metabolism and homeostasis include the buildup of potentially damaging levels of reactive oxygen species (ROS), Ca2+, etc., which must be normalized. Evidence suggests that brief mitochondrial permeability transition pore (mPTP) openings play an important physiological role maintaining healthy mitochondria homeostasis. Adaptive and maladaptive responses to redox stress may involve mitochondrial channels such as mPTP and inner membrane anion channel (IMAC). Their activation causes intra- and intermitochondrial redox-environment changes leading to ROS release. This regenerative cycle of mitochondrial ROS formation and release was named ROS-induced ROS release (RIRR). Brief, reversible mPTP opening-associated ROS release apparently constitutes an adaptive housekeeping function by the timely release from mitochondria of accumulated potentially toxic levels of ROS (and Ca2+). At higher ROS levels, longer mPTP openings may release a ROS burst leading to destruction of mitochondria, and if propagated from mitochondrion to mitochondrion, of the cell itself. The destructive function of RIRR may serve a physiological role by removal of unwanted cells or damaged mitochondria, or cause the pathological elimination of vital and essential mitochondria and cells. The adaptive release of sufficient ROS into the vicinity of mitochondria may also activate local pools of redox-sensitive enzymes involved in protective signaling pathways that limit ischemic damage to mitochondria and cells in that area. Maladaptive mPTP- or IMAC-related RIRR may also be playing a role in aging. Because the mechanism of mitochondrial RIRR highlights the central role of mitochondria-formed ROS, we discuss all of the known ROS-producing sites (shown in vitro) and their relevance to the mitochondrial ROS production in vivo.
I. INTRODUCTION
Photo-activated reactive oxygen species (ROS) may trigger mitochondrial permeability transition pore (mPTP) induction within individual mitochondria in intact cell systems. The phenomenon of ROS-triggering of the mPTP associated with further stimulation of ROS formation has been termed “ROS-induced ROS release” (RIRR) (491). mPTP opening is a mitochondrial response to an oxidative challenge resulting in an amplified ROS signal, which depending on ROS levels may result in different outcomes. In addition to ROS effects in those mitochondria (where the RIRR originated), ROS released into cytosol could trigger a complex cellular signaling response and/or RIRR in the neighboring mitochondria. In the latter case, ROS trafficking between mitochondria could constitute a positive-feedback mechanism resulting in an elevated production of ROS that could be propagated throughout the cell and may cause perceptible mitochondrial and cellular injury. Although photo-induced formation of ROS could be initially used in the experimental setting as a trigger for more massive, avalanche-like ROS release, this phenomenon is representing a more fundamental mechanism, e.g., light-independent spontaneous redox transitions associated with the induction of mPTP or other mitochondrial channel(s) that may occur under different physiological or pathological conditions with corresponding impacts on mitochondrial and cellular physiology. This review will cover the spectrum of RIRR-related phenomena, both physiological and pathological including the processes of mitochondrial ROS production and scavenging. Ultimately, the imbalance between the inflow, neutralization, and outflow of ROS with corresponding triggers in specific cell signaling pathways may result in extreme situations such as oxidative and reductive stresses with the consequent onset of numerous pathologies or even the cell and organismal death.
II. ROS: GENERAL DEFINITIONS
Eleven years ago this journal published an excellent and comprehensive review by Droge (117) on free radicals and their beneficial and detrimental roles in cell physiology and pathology. Since then, the general interest surrounding the roles of these species has constantly increased, shifting the main focus to highly potent oxidants containing oxygen, called ROS. The term ROS encompasses oxygen free radicals, such as superoxide anion radical (O2·−) and hydroxyl radical (·OH), and nonradical oxidants, such as hydrogen peroxide (H2O2) and singlet oxygen (1O2).
ROS can be interconverted from one to another (depending on ΔG of relevant processes) by enzymatic and nonenzymatic mechanisms. The primary and most abundant ROS is the superoxide anion radical that has a comparatively high oxidative capacity [standard redox potential of the oxygen/superoxide couple = −0.137 V (337) allowing single-electron reduction of molecular oxygen by certain mitochondrial oxidoreductases]. H2O2 is generated through spontaneous or superoxide dismutase (SOD)-catalyzed dismutation of O2·− (143). In mammals, three SOD isoforms were found in the living cell with precise compartmentalization: the Cu,Zn-dependent isoform (Cu,Zn SOD, SOD1) (142) is located in the mitochondrial intermembrane space and cytosol; the Mn-dependent isoform (Mn SOD, SOD2) (358, 468) is located in the mitochondrial matrix; and Cu,Zn SOD is located in the extracellular space (ecSOD, SOD3) (285).
The most potent and aggressive oxidant primarily responsible for oxidative damage of DNA bases is the hydroxyl radical, which has a relatively short half-life. ·OH can be generated through a variety of mechanisms. It is well known that ·OH is generated from H2O2 and O2·− which is catalyzed by iron ions through the Haber-Weiss reaction (169) with a specific case of Fe2+-mediated decomposition of H2O2 [the Fenton reaction (130), reviewed in Ref. 237]. Ionizing radiation causes decomposition of H2O, which also results in forming ·OH and hydrogen atoms. ·OH could be also generated by photolytic decomposition of alkylhydroperoxides (447).
In addition, a number of other oxygen-containing free radicals are capable of causing oxidation of essential cell components: nitric oxide (NO), peroxynitrite, lipid hydroperoxides (LOOH), alkoxyl radical (RO·), peroxyl radical (·OOH), nitrogen-centered radical, sulfate radical (SO4·−) and metal-oxygen complexes. These radicals combined with the previously mentioned ROS form a large and important group of active redox agents playing critical role in a number of intra- and extracellular processes.
III. ROS: FROM SIGNALING TO PATHOLOGICAL
One of our specific aims is to give an overview of a spectrum of phenomena associated with different ROS serving a “signaling” role (discussed below) which is essential for a large number of biochemical reactions. In the following sections we address the significance of reductive and oxidative stress. Under physiological conditions, the balance between ROS generation and ROS scavenging is highly controlled. Depending on circumstances, regulated oxidative stress could initiate diverse cellular responses ranging from triggering signaling pathways involved in cell protection, initiating coordinated activation of mitochondrial fission and autophagy to optimize clearance of abnormal mitochondria and cells to protect spreading the damage to the neighboring mitochondria and cells (100, 117, 490). On the other hand, unregulated oxidative and reductive stresses could result in severe cellular damage, unwanted cell death, and consequently whole organ and organism failure (102, 242, 498). Therefore, adaptive physiological redox stresses, such as those occurring under the process of a programmed removal of damaged biological systems including mitochondria and other cellular components (“physiological”), must be differentiated from maladaptive unwanted (“pathological”) oxidative damage.
Under normal physiological conditions, ROS emission (essentially, production minus scavenging) was considered to account for ∼2% of the total oxygen consumed by mitochondria (81). [A recent measurement of ROS production in mitochondria with disabled antioxidant systems revealed values fluctuating from 0.25 to 11% depending on the animal species and respiration rates (21).] A lower, as well as a higher, percentage may have deleterious consequences since ROS, when low, are unable to provide proper cellular functioning through regulation of a great number of biochemical reactions. When high, they are unable to provide a controlled regulation. It will require conditions when the flux of metabolic regulators of ROS level is finely tuned to respond to the cell demands with mitochondria playing a critical role. As for many cellular signaling elements, the principle “multet nocem” (excess is harmful) may become a key element in a switch operating between “signaling” (meaning as survival-promoting which is physiologically required for renovating a biological component) and pathological (meaning undesired death-promoting) modes (216, 218, 219, 232, 491, 493, 497). For example, the ROS-mediated ignition of a death of cells designed for long-term use (postmitotic cells such as cardiac myocytes or neurons), i.e., occurring under severe ischemic conditions causing pathologies such as myocardial infarct or stroke, under any conditions may be considered a pathological event. In other cases, apart from their pro-survival signaling role, ROS are apparently involved in a designated physiological function, eliminating unwanted mitotic cells or mitochondria and a significant rise of local ROS level within might be an efficient means to fulfill such functions. Futhermore, ROS have been shown to play a central role in regulation of the cell cycle progression (453). Whether the ROS burst can serve a signaling (survival) function will be discussed in a section describing the oscillatory behavior of mitochondria. For clarity, we will stay within definitions of signaling ROS as of those serving essential pro-survival functions and anti-survival functions including required elimination of unwanted cells (quality control, maintenance of function), while pathological ROS are considered as those causing oxidant-induced unwanted changes including unwanted cell death (loss of function).
IV. ROS: REDOX STRESS
There is an apparent heterogeneity in ROS levels and types when comparing different cells and organs (54, 173, 271, 342, 373, 401, 477). This is largely due to a heterogeneous distribution of activities of ROS producing and utilizing machineries (11, 78, 103, 290, 346). The general consensus is that overwhelming ROS production when not compensated for with their scavenging by endogenous antioxidants will lead to the rise of ROS beyond the “normal” or “physiological” threshold level. This results in a process conventionally called “oxidative stress.” Apparently, the definition of this widely used term (up to the year 2012 this term yields over 100,000 citations in PubMed) is quite broad and has “soft borders” considering the scenarios presented above. This is due to the fact that physiological levels and types of ROS in different tissues and in different parts of the same tissue under different physiological conditions are heterogeneous and highly dependent on the energy load that is met by the cell response. Even within the confines of a single cell, there are at least eight distinct organellar compartments (mitochondrial matrix, lysosomes, smooth ER/SR, rough ER, the Golgi, peroxisomes, the nucleus, the cytosol), each with its own redox poise (315). Accordingly, the term oxidative stress is often used in the literature in a very general term to define a state when the levels and types of oxidants in the cell or the organelle on average significantly exceed the ground/resting/steady-state level associated with normal homeostatic function. At the opposite end of the redox spectrum, when the reduced glutathione levels are too high, “reductive stress” occurs and demonstrates potentially detrimental consequences for the cell (154, 349). Within normal fluctuation of energy load, the productions of ROS and the ROS levels in mitochondria, cells, and the tissue are safe to perform normal activity (maintenance of function) of the particular biological system. ROS signaling and the role of ROS in vital cellular functions associated with cell proliferation, differentiation, migration, immune response, cell senescence and death, and number of inherited or acquired pathologies such as ischemia-related disease, atherosclerosis, neurodegenerative disease, malignant transformation, diabetes mellitus, rheumatoid arthritis, aging, etc., is described elsewhere (25, 117, 185, 203, 263, 348, 352, 497). However, under stress, when the ROS levels remain outside the normal range (either under conditions of enhanced antioxidative pathways associated with reductive stress or of those characterized by the rise of uncompensated ROS associated with oxidative stress), resultant instability of the redox environment may develop that could be harmful (unwanted loss of function) if it is not compensated by the feedback control mechanism.
In summary, considering these scenarios, both high levels of ROS (oxidative stress) and excessively low levels of ROS (reductive stress) are deleterious and apparently play a causative role in the pathologies caused by malfunctioning processes related to the dramatic change of redox environment. Underlying all these arguments, redox homeostasis seems to be a critical factor for normal functioning of the mitochondrion, cells, and organisms (174). Previously, it has been argued that the cell normally maintains cytosolic thiols in a highly reduced redox state, thus not supporting the existence of reductive stress (154). More recently, however, a profound increase of reduced glutathione concentration and the ratio of GSH/GSSG in cardiomyopathic animals carrying the R120G mutation in the αB-crystallin molecule was detected (349). The elevated level of reduced equivalents in these animals was accompanied by the augmented expression together with increased antioxidative enzymatic activity of glutathione peroxidase, glutathione reductase, and catalase, which supports the implication of deleterious reductive stress (349). The presence of reductive stress in yeast was also confirmed (440). A more precise term, redox stress, might be introduced reflecting both the incidence of oxidative and reductive stresses; however, this review is focused primarily on the circumstances related to oxidative stress.
V. ROS GENERATION IN MITOCHONDRIA
In 1961, Jensen was among the first investigators to demonstrate that mitochondria produce ROS (209). He observed that a small portion of the oxygen consumed by submitochondrial particles oxidizing NADH or succinate was converted to H2O2 since this consumption was catalase sensitive. Later, in 1972–1973, a classic, more general study, was done at the Johnson Research Foundation in Philadelphia by Britton Chance and co-workers (57, 58) who initiated the modern era of the mitochondrial ROS research. Since that time, scientists debated the physiological relevance of data obtained using “artificial” systems such as isolated mitochondria, inside-out submitochondrial particles, reconstituted respiratory complexes, and pure enzymes. According to the critics, these are not adequate systems to extrapolate data to the cell, organ, and organism levels (reviewed in Refs. 340, 480, 492). However, some counter-arguments support the relevance of these model systems. We are still lacking a detailed mechanistic knowledge of the architecture of mitochondrial ROS-producing systems such as of complex I or complex III and detailed insights on the mechanisms controlling their activities. We will make an attempt to partially address and clarify this scientific debate and to present the arguments in support of, and against, the importance and physiological relevance of those specific proposed mitochondrial ROS-producing components. The primary goal of this review is not a comprehensive coverage of this specific issue, but the background that needs to be addressed here to provide a basis for understanding those cases when an “innocent” molecular site (i.e., normally associated with moderate and physiological ROS production) becomes a “killer,” producing ROS levels leading to the destruction of the biological system (perhaps through some poorly understood amplification mechanism). Good reviews on the current mechanisms of ROS production in mitochondria are available elsewhere for the reader interested in a general background and for those interested in substantially detailed mechanistic depth (3, 13, 201, 202, 308, 397, 416).
A. Complex II
1. Under normal conditions
We begin this review of mitochondrial ROS-producing sites from complex II since succinate is a more frequently used oxidative substrate to explore the functioning of isolated mitochondria which became a classical object to study ROS production.
Complex II, succinate-ubiquinone oxidoreductase (EC 1.3.5.1), commonly known as succinate dehydrogenase (SDH), is a tetrameric iron-sulfur flavoprotein of the inner mitochondrial membrane and acts as part of the Krebs cycle and respiratory chain. SDH catalyzes the conversion of succinate into fumarate, yielding reduced equivalents in the form of reduced flavin adenine nucleotide (FADH2). This is followed by a reduction of ubiquinone to ubiquinol. Mammalian Complex II, as well as that from yeast, harbors a covalently bound FAD, three iron-sulfur clusters, a b-type heme, and two quinone-binding sites termed Qp and Qd, standing for proximal or distal sites correspondingly.
Typically, complex II is excluded from the list of potential candidates for important physiological contributors of ROS (347, 348, 358). It is partially due to fact that the succinate level in the tissue is low (in a range of hundreds of micromoles), while in in vitro experiments with isolated mitochondria millimolar concentrations are used. Hansford et al. (175) found that while H2O2 production can be detected in mitochondria oxidizing succinate in vitro at experimental ambient (5–10 mM) concentrations, they do not produce significant amounts of peroxide at low, more physiologically relevant succinate concentrations (175).
Some studies point to flavins (flavin adenine nucleotides, FAD) of SDH rather than other electron-carrying components (such as iron-sulfur clusters or quinones) as the site of autoxidation responsible for generating ROS (200, 293, 294). Others implicate ubisemiquinone and iron sulfur centers as these sites, although under normal and steady-state conditions these components are only partially reduced and short-lived (167, 192, 267), thus giving a low probability to transfer single electrons directly to oxygen.
2. Redox regulation of ROS production and redox buffering
During oxidation of succinate in isolated respiring mitochondria, electron flow can bifurcate forming direct (towards cytochrome oxidase) and reverse (toward NAD; rotenone-blocked) transport with the latter requiring energy input (79, 80, 187). The succinate-driven ROS generation during reverse electron transport from succinate to NAD resulting in the formation of NADH is higher when compared with that forming under direct oxidation of NAD-dependent substrates (456). The observed relationship between ROS formation and the redox state of the couple NADH/NAD resulted in the proposition that the ROS formation is directly proportional to the level of reduction of NAD. Possibly, a more generalized rule might be formulated that the more reduced the mitochondrial interior is, the more probable there will be primary ROS formation.
The redox state of the cellular milieu is mainly determined by the ratios of reduced/oxidized cofactors and proteins which carry the bulk of redox-sensitive amino acid residues and functional groups, NAD(P)H/NAD(P)+ and GSH/GSSG, which all together form a compartmentalized redox buffer where all components are in a redox equilibrium under cellular steady-state conditions (reviewed in Ref. 309). This buffer may be an important factor in determining ROS levels in the compartments such as the mitochondrial matrix or cytosol (174). High intramitochondrial redox buffering capacity, only partially represented by 3–5 mM NAD(P)H and 2–14 mM GSH (416), would resist the short-term exposure to ROS, while profound sustained ROS exposure would eventually exhaust this buffer, resulting in the elevation of intramitochondrial ROS levels (353, 416, 460). Later, we discuss in greater detail the redox dependence of ROS formation and the role of reducing equivalents and mitochondrial membrane potential on the net ROS production (see sect. VB5).
The role of complex II in maintaining and modulating the mitochondrial/cellular redox environment remains undetermined. It is unknown whether in in vivo mitochondria reverse electron transfer from complex II to complex I occurs, and whether under physiological conditions the reverse electron transport could result in substantial ROS production considering that physiological concentrations of NADH would significantly attenuate O2·− production under conditions where reverse electron transport could be observed in in vitro model systems (165). Thus it remains questionable under normal conditions if there is a significant contribution of ROS generated in complex II to the net ROS production.
3. Under pathological conditions
As we discussed previously, the question about complex II contributing to the net ROS production remains controversial. Although the tissue level of succinate is as low as 200–500 μM, under oxygen deficiency (hypoxia/ischemia) it may rise 5- to 10-fold (44, 371, 471). Recently, significantly increased levels of succinate (to a few millimolar range) were detected in macrophages exposed to lipopolysaccharide, ultimately identifying succinate as a metabolite in innate immune signaling, which stabilizes HIF-1α and enhances interleukin-1β production during inflammation (433). Activation of macrophages is known to be associated with elevation of their ROS production, but whether succinate triggers this production remains unexplored.
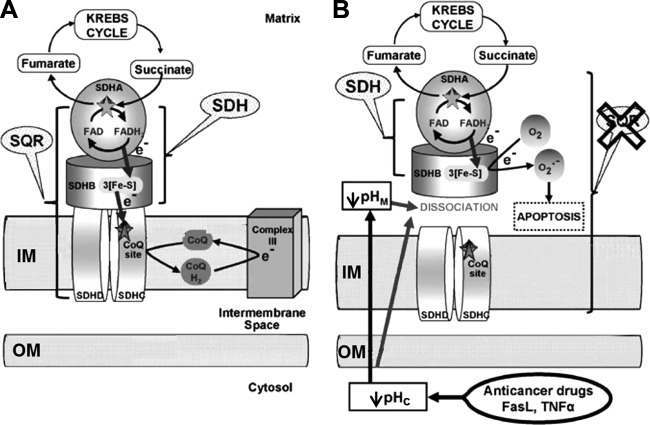
Under some circumstances of drug-induced apoptosis when intracellular pH becomes significantly acidified, impairment of complex II could correlate with ROS generation without changes in the SDH enzymatic activity which is a part of complex II activity (260). This process has been accompanied by dissociation of the SDHA (flavoprotein subunit)/SDHB (iron-sulfur protein-containing part) subunits, which encompass the SDH activity, from the membrane-bound components of complex II that are required for the SQR activity (for details, see Figure 1). Such dissociation (see Figure 1) might result in a direct single-electron reduction of oxygen by a reduced iron-sulfur cluster of complex II (113). Consequently, it has been proposed that complex II may function as a general sensor for apoptosis (162, 260). This is an example of a pathological, conformation-induced ROS production which does not happen in intact complex II.
FIGURE 1.
Model for the role of specific complex II inhibition for apoptosis induction by various proapoptotic compounds. Mitochondrial matrix, inner membrane (IM), intermembrane space, outer membrane (OM), and cytosolic space are indicated. A: in healthy cells, complex II serves to funnel electrons derived from the Krebs cycle to the respiratory chain. SDHA-mediated oxidation of succinate to fumarate by the succinate dehydrogenase activity (SDH), as part of the Krebs cycle, provides electrons to complex II. They are transferred to the iron-sulfur centers of the SDHB subunit and finally to the CoQ reduction site the succinate CoQ oxidoreductase (SQR). B: proapoptotic compounds, such as various anticancer drugs, FasL, or tumor necrosis factor (TNF)-α, induce cytosolic (pHc) and mitochondrial (pHM) acidification. These pH changes lead to the dissociation of the SDHA/B subunits from complex II and finally to the partial inhibition of the SQR activity without any impairment of the SDH reaction. This specific inhibition leads to complex II uncoupling, superoxide production, and apoptosis. [From Lemarie et al. (260). Reprinted by permission from Macmillan Publishers, Ltd.]
In a catalytic mechanism, ubiquinone receives electrons from the [3Fe-4S] center being bound at the Qp site. Since the ubiquinone is a two-electron acceptor receiving these electrons in separate steps, the intermediate state of SDH exists where after receiving a single electron the ubisemiquinone radical must be stabilized to prevent the escape of the electron to an inappropriate acceptor such as molecular oxygen. Mutations in the vicinity of the Qp site were shown (167) to compromise the ability to stabilize ubisemiquinone, and thus its unpaired electron may become more readily available to react with ambient oxygen producing its derivative, superoxide anion radical followed by dismutation to form hydrogen peroxide.
There is multiple evidence that impaired electron transport in SDH, as well as its effect on the levels of NAD(P)H through the impairment of the Krebs cycle, are the source and the cause of a substantial amount of ROS determining the onset of numerous pathologies (e.g., Refs. 32, 204, 481, 482). Malfunctioning of respiratory complexes, including complex II in brain mitochondria, is a hallmark of Huntington disease (HD), a neurodegenerative genetic disorder that elicits progressive motor, cognitive, and emotional deficits. 3-Nitropropionic acid, an irreversible inhibitor of SDH, mimics HD-like pathology and symptoms (68) and evokes an ROS increase in neurons (270). Leigh syndrome, an infantile-onset progressive neurodegenerative human disease is suggested to be caused by mutations in the SDHA gene. It appears that mutations in the SDHB, SDHC, or SDHD genes can cause paraganglioma (a neuroendocrine, highly vascularized neoplasm developing tumors in the head, neck, thorax, or abdomen) (37) or pheochromocytoma (a catecholamine-secreting neuroendocrine tumor occurring in the medulla of the adrenal glands) (37, 155; reviewed in Ref. 305). Unfortunately, it is impractical so far to estimate the contribution of the impaired Krebs cycle and reverse electron transport occurring under pathological SDH impairment to the modulating ROS production.
In parasitic worms residing in an anaerobic environment in a host intestine, the energy partially is obtained from so-called fumarate respiration reflecting a reverse activity of succinate-ubiquinone reductase of complex II. Apparently, in their fumarate reductase reaction, ROS are produced in a FAD site and quinone-binding site as well. Since in the adult stage, these worms do not have either complex III or IV, their respiratory chain could serve as a good model to study the production of ROS in complex II in mitochondria (364, 436). It is noteworthy that this model with a missing cytochrome c oxidase may somehow simulate either hypoxic conditions or those induced by defective electron transfer downstream of Complex II.
B. Complex I
1. Under normal conditions
The association of complex I deficiency with a wide spectrum of pathologies such as cardiomyopathies, cataracts, Leigh disease, exercise intolerance, mitochondrial encephalomyopathy, lactic acidosis, strokelike episodes (MELAS), hepatopathy, and tubulopathy has been suggested. Prevailing dogma holds that complex I (NADH-ubiquinone oxidoreductase) is the main source of ROS in mitochondria. However, the ROS production at complex I depends on circumstances; consequently, complex I becomes a major ROS source under pathological conditions rather than being a dominant source under resting and healthy conditions.
When submitochondrial particles or isolated mitochondria oxidize NAD(P)H or glutamate plus malate, correspondingly, complex I production of superoxide is negligible. However, supplementation with the inhibitor of complex I, rotenone, results in robust production of O2·− (350, 456). This implies that the major site of ROS production in complex I is either upstream of a rotenone-binding site or it is tightly coupled to the increased level of NAD(P)H after rotenone supplementation during oxidation of NAD-dependent substrates (175, 444). According to the first alternative, rotenone would induce progressive reduction of the upstream redox groups (432) including Fe-S clusters, flavin mononucleotide (FMN), and the tightly bound pool of ubiquinone (62, 325), which can supply the oxygen molecule with a single electron yielding superoxide anion radical.
There is debate on the critical role of the components of complex I involved in superoxide production. Some consider FMN (247, 256, 287, 345, 455), while others claim that iron-sulfur clusters N1a and N2 (142, 152, 186, 254), NAD radical (245), or ubisemiquinone (241) are responsible for O2·− generation in complex I. The last point was actively challenged by Lenaz (264) who considered only hydrophilic quinones to be prooxidants, while physiological hydrophobic ubiquinones (such as CoQ10) behave more as antioxidants rather than prooxidants (264). Therefore, the question regarding the major source of superoxide in complex I under physiological conditions remains unresolved.
Recently, the physiological relevance and thus the importance of the production of ROS by complex I was questioned on the basis that NADH-supported complex I-catalyzed superoxide production by submitochondrial particles shows maximal activity at low NADH concentrations (∼50 μM) while at physiological concentrations of NADH (in the millimolar range) this reaction is severely inhibited (164, 165).
2. Under pathological conditions
It has been noticed that at least 40% of all mitochondrial disorders are associated with mutations in subunits of complex I (402). Defects in complex I are associated with a wide diversity of neurodegenerative pathologies, including Parkinson's disease (PD) which is characterized by a substantial loss of the dopaminergic neurons and cell bodies of which are in the substantia nigra pars compacta and nerve terminals in the striatum. ROS are thought to be highly involved in PD pathogenesis, triggering the loss of redox buffers (GSH and proteinaceous thiols) (336) at least partly caused by dopamine oxidation-related metabolic pathways.
Dopamine in the central nervous system, apart from being a neuronal neurotransmitter, serves as a precursor of norepinephrine and epinephrine, and is a regulator of movement (nigrostriatal pathway), and a behavior motivator (mesolimbic pathway) (425). While under normal conditions oxidative deamination of dopamine by monoamine oxidase produces hydrogen peroxide (282), it could generate toxic oxidants through alternative ways of oxidation wherein mitochondria play a role. In this pathway, dopamine is oxidized nonenzymatically by superoxide forming dopamine quinone which can be reduced by mitochondrial complex I generating semiquinone followed by a transfer of its electron to molecular oxygen to form superoxide (488), completing a vicious oxidative cycle. In addition, PD is hallmarked by elevated iron levels that may catalyze production of deadly oxidants, possibly in a self-amplifying mode (411).
PD could be mimicked by the action of complex I inhibitors such as rotenone, paraquat, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (49, 94). Exposure to the latter drug has shown to produce permanent parkinsonism in humans, non-human primates, and rodents, by exerting an effect primarily on the function of mitochondrial complex I. In patients with Friedreich ataxia, a deficient activity of the Fe-S cluster-containing subunits of mitochondrial respiratory complexes I, II, III, and aconitase was found (361). Kushnareva et al. (254) claimed that the ratio of NAD(P)H/NAD(P)+, rather than the level of NADH, determines reduction of ROS-producing sites in complex I.
Generation of ROS associated with hypoxia/reoxygenation is known as one of the most deleterious causes of oxidative damage. Three potential sources of ROS have been proposed to be responsible for this release: mitochondrial complex I, xanthine oxidase, and NADPH oxidase (2, 471, 491). However, the latter two are probably not involved since inhibition of these complexes in vivo did not afford cell protection (84, 129).
One of the very specific features of the mammalian NADH-ubiquinone oxidoreductase is the slow active/deactive state transition, suggesting gross conformational rearrangements of complex I, at least in that part which is involved in rotenone-sensitive ubiquinone reduction [which may be involved in the superoxide production (150, 454)]. It was found that complex I isolated from the heart which was exposed to a normoxic perfusion is in a fully active state, while 30-min anoxic perfusion results in a significant transformation of the enzyme into a deactive state which returns back to normal after reoxygenation (283). It has been proposed that these conformational transitions can be relevant to producing ROS by complex I after cardiac tissue is reoxygenated following a coronary occlusion (283). Using EPR spectroscopy, DeJong et al. (104) showed that NADH-coenzyme Q oxidoreductase undergoes energy-dependent structural changes in parts determining ubisemiquinone production (iron-sulfur cluster 2) (104). Thus, under pathological conditions, conformational rearrangements may be involved in the changes of the efficiency of ROS-producing machinery in complex I.
3. ROS and hypoxia
The reaction of formation of a primary ROS (superoxide only) generated in the respiratory chain from molecular oxygen is of a first order with respect to oxygen concentration. However, paradoxically, generating ROS in mitochondria in the cell remains constant or even increases when Po2 drops dramatically (i.e., under moderate hypoxic conditions). Robust ROS production under 1.5% of O2 has been recorded also (314, 374, 462).
Interestingly, in the cell the affinity of molecular oxygen to ROS-generating modules is higher than to cytochrome oxidase. This obviously takes place under conditions of partial reduction of cytochrome oxidase, i.e., when the availability of oxygen is limiting its utilization. Note that this paradox is absent in the isolated mitochondrial system (Figure 2), free from extramitochondrial signaling pathways which confirms that elevated mitochondrial ROS generation in the cell in response to hypoxia is not intrinsic to the mitochondrial respiratory chain alone but can be attributed to some involvement of extramitochondrial factors (189). Marshal et al. (286) indicated that hypoxia-induced superoxide production occurs through activation of NADPH oxidase located in the cell membrane. In addition, under moderate hypoxia, NO synthesis in mitochondria continues although being only 5–10% of the normal steady-state level (Km for oxygen of the mitochondrial NO synthase is 30–40 μM; Ref. 8). In turn, NO can partially block cytochrome oxidase (69, 70, 378), thus reducing mitochondrial electron carriers, increasing its Km for oxygen (91) and favoring generation of superoxide at hypoxic conditions (444).
FIGURE 2.
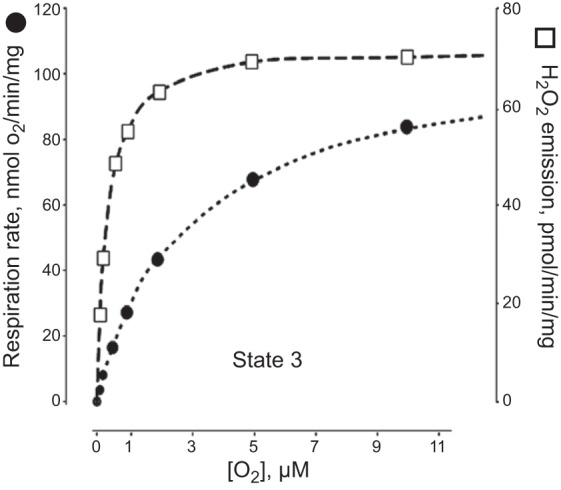
Effect of the oxygen tension on the rates of phosphorylating respiration and H2O2 emission in rat liver mitochondria. (Note that there is no ROS production increase under low Po2.) [From Starkov (416), with permission from John Wiley & Sons.]
In highly metabolizing tissues, the areas surrounding mitochondria in the cell may have higher ROS levels than remote areas. Without considerable mitochondrial ROS-quenching activities, intramitochondrial levels of ROS may potentially reach very high levels. That may happen in case of an imbalance between the oxygen supply and demand, for example, under conditions of high metabolic needs.
The nature of the tissue oxygen gradient between the source of oxygen (blood capillary) and the site of its utilization (the mitochondrion) can be explained by the Krogh cylinder model which generally serves to analyze capillary tissue exchange kinetics (246, 266) (Figure 3). The effective radius of this cylinder beyond which the tissue hypothetically is experiencing hypoxia depends in part on O2 consumption. In a tissue with high metabolic rate (such as heart muscle), capillary density during maximum or moderate exercise would not be sufficient to supply tissue with oxygen and might potentially produce more ROS in the vicinity and inside of mitochondria/mitochondrial clusters (214). However, basic theoretical assumptions of the Krogh cylinder model do not consider that the diffusion coefficient for O2 in muscle tissue may be higher due to the possibility of facilitated O2 transport by the mitochondrial network (10, 396) and/or by myoglobin molecules (475). In non-muscle tissues lacking myoglobin, the cytoglobins and neuroglobins may potentially serve as facilitators of oxygen transport (220, 327, 354, 428). Both the extended mitochondrial network and these various heme-containing proteins may be responsible for a less steep O2 gradient in the normal active cell, thus also likely flattening the ROS gradient as well. Cytoglobin and neuroglobin have been reported to not only exert an oxygen carrier function, but possibly to be oxygen sensors and ROS scavengers (220).
FIGURE 3.
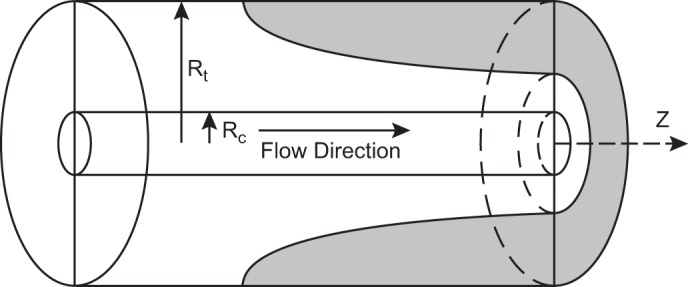
Geometry of the Krogh cylinder-type model. Inner cylinder represents the capillary. Outer cylinder corresponds to tissue cylinder. Shaded area: example of hypoxic region under conditions of high demand. Rt, tissue cylinder radius; Rc, capillary radius; z, distance along the capillary. [From McGuire and Secomb (289).]
Under experimental conditions acutely restricting the oxygen supply to an organ, such as the heart, very steep regional redox transitions have been observed across the borderline of the ischemic area (33), apparently reflecting a similarly steep oxygen gradient and thus probably the ROS gradient as well (Figure 4). On the basis of the data that ROS production is directly linked to reduced equivalents such as NADH (discussed above), hypoxic regions manifesting the highest level of NAD reduction would likely achieve much higher ROS levels than normoxic regions. The brain is the organ most vulnerable to the lack of oxygen, immediately responding by the reduction of NAD (78), thus potentially eliciting ROS within local areas adjacent to ischemic zones.
FIGURE 4.
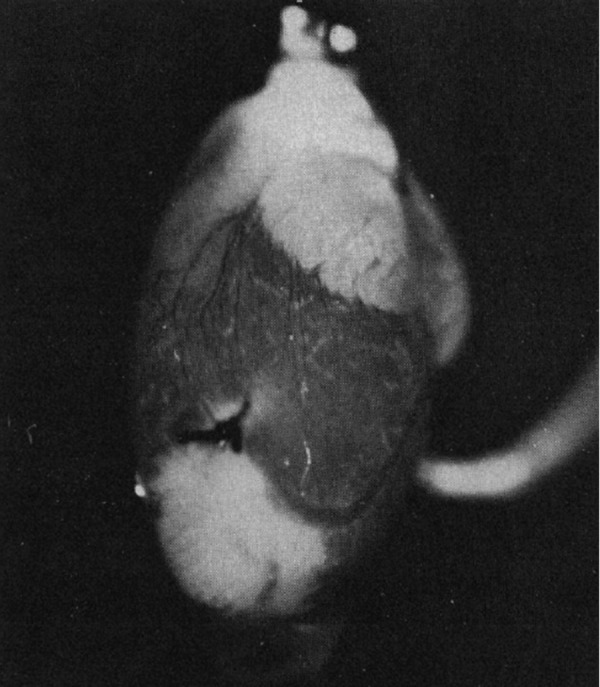
NADH fluorescence emission from perfused rat heart with a local ischemic area near the apex (seen as white areas), caused by ligation of a coronary artery. [From Korshunov et al. (33). Reprinted with permission from AAAS.]
4. Is the ROS production proton motive force sensitive?
Proton motive force (pmf, Δp) across the inner mitochondrial membrane, with Δψ as a main component, is driving ATP production (268, 298, 299, 424). Whether ROS production is dependent on this proton motive force/transmembrane potential in mitochondria has become a crucial question.
As discussed above, a reduced redox intramitochondrial environment is a prerequisite for high primary ROS formation by the mitochondrial respiratory chain. On the other hand, the same reduced redox conditions provide more buffering capacity to quench ROS activity, so it is unclear under which conditions (more reduced or more oxidized) the net level of ROS will be higher. It appears that the steady-state level of ROS in the compartment rather than the ROS-producing activity determines the level of oxidant-induced biological modifications, many of which are important because of their biological effects, both physiological and pathological.
Redox steady states of respiratory components responsible for ROS production should be in redox equilibrium with adjacent mobile and immobile redox carriers such as NAD(P)H, GSH, and the thiol groups of the proteins occupying the same compartment.
In 1996 Liu and Huang (275) and in 1997 Korshunov et al. (240) (Figure 5A) found in isolated mitochondria a strong dependence of mitochondrial ROS production on the level of the transmembrane potential (Δψ), supported by succinate oxidation (reviewed in Refs. 272–274). [It should be emphasized that mitochondrial matrix alkalinization could be the cause of increased generation of ROS due to the fact that ΔpH is an integral part of the Δp (381).] While H2O2 production was low at Δψ values at and below the phosphorylating membrane potential [the membrane potential reached under the state 3 respiration according to B. Chance's terminology when mitochondria are supplemented with an excess of ADP (82), i.e., that thermodynamically required to generate ATP from ADP and Pi], it rises dramatically above these values proportionally to the Δψ elevation (Figure 5). Accordingly, an 18% decrease in the value of the transmembrane potential inhibits 90% of mitochondrial ROS production (240) above the phosphorylating membrane potential.
FIGURE 5.
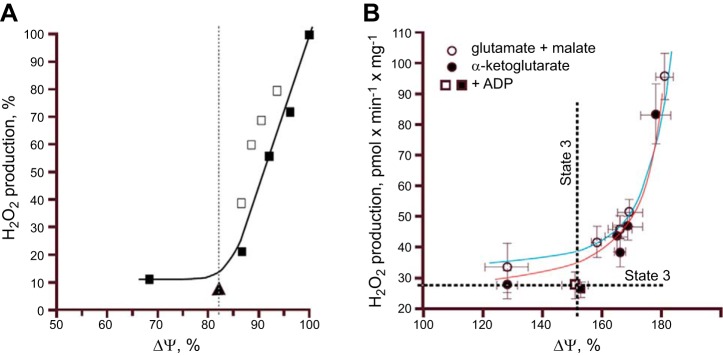
Relationship between mitochondrial ΔΨ and H2O2 production supported by succinate or NADH-linked respiratory substrates. A: rat heart mitochondria oxidizing succinate. The Δψ level was varied by adding different concentrations of uncoupler SF6847 (black squares and solid line), malonate (white squares), or 100 μM ADP and 5 mM P (triangle). Dashed line, the state 3 Δψ level. [From Korshunov et al. (240), with permission from Elsevier.] B: rat brain mitochondria oxidizing glutamate + malate or α-ketoglutarate. Differences in ΔΨ were generated by adding various concentrations of uncoupler FCCP ranging from 0 to 80 nM. Alternatively, a decrease in Δψ was induced by adding 0.8 mM ADP to mitochondria. [From Starkov and Fiskum (419), with permission from John Wiley & Sons.]
Subsequently, very steep dependence of H2O2 production on the values of Δψ exceeding the phosphorylation potential was confirmed in isolated mitochondria oxidizing NADH-dependent substrates (419) (Figure 5B). However, in another study using NADH-dependent substrates, mitochondrial respiration produced ROS in a membrane potential-independent mode (456).
5. Can ROS production be decreased in mitochondria without jeopardizing ATP production? Mild uncoupling as a possible downregulator of ROS production
As was shown in the preceding section (Figure 5), moderate lowering of Δψ could result in a lower ROS production in mitochondria without a significant effect on ATP production. This could be achieved by inducing a small proton leak through the inner mitochondrial membrane which would both stimulate oxygen consumption and, in parallel, shift the level of reduction of mitochondrial ROS-producing sites to a more oxidized state lowering the probability of ROS production in the mitochondria. While higher potential could drive increased ATP production, the higher Δψ could also result in the production of increased ROS levels and potentially unwanted oxidative consequences. Therefore, achieving a reasonable balance between ROS and ATP production by mitochondria is crucial since this reflects the current energy needs of the cell under the particular physiological state. As discussed previously, regulated moderate (“mild”) uncoupling of mitochondrial oxidative phosphorylation has been suggested as a feasible therapeutic strategy (397, 398, 415) for regulation of the intracellular and intramitochondrial ROS level (99).
Mitochondrial uncoupling proteins (UCPs) have been considered as potential mild uncouplers. The relationship between ROS production and UCPs activity was revealed in 1997 in experiments where GDP, an inhibitor of UCP1, caused an increase of Δψ and ROS production (316). Later, it was demonstrated that superoxide directly activates UCPs resulting in a negative feedback controlling both ROS production and their levels (120).
Mild uncoupling may be protective against excitotoxic injury (469) and against injury of dopaminergic neurons in substantia nigra from mitochondrial poisons such as rotenone (478). Decreasing ROS generation by uncoupling mitochondria increases longevity in healthy animals (74).
Of all the possible mild uncouplers, fatty acids are probably the most natural ones (240, 398, 476). In their protonated form they can cross the mitochondrial inner membrane followed by deprotonation in the matrix side, and then the anionic form of the fatty acid completes the cycle by returning back to the cytosolic side. The rate-limiting step of this cycle is the transport of anionic form. Different proteins such as the adenine nucleotide transporter (ANT) and glutamate/aspartate transporter are involved in fatty acid-mediated uncoupling through facilitation of the transport of the anionic form (12, 368). Thyroid hormones may also be considered as natural mild uncouplers (177, 397).
Among artificial uncouplers, 2,4-dinitrophenol (DNP) has been tested as an anti-obesity drug, but it was found to be too toxic for practical use (90, 99). Additionally, DNP was found to limit the experimental infarct size in the heart and brain, and this was interpreted to occur through diminishing the ROS level (238, 359). Recently, mild uncoupling activity was ascribed to a series of derivatives of cationic rhodamine (15).
C. Complex III
Complex III (ubiquinol-cytochrome c oxidoreductase) accepts reducing equivalents formed in complexes I and II and processes them by the Q-cycle operating mechanism. Operation of this cycle is initialized by ubiquinol, which releases its proton to the intermembrane space and donates one electron to the Riske iron-sulfur protein (which can bind to, and be inhibited by, myxothiazol) producing unstable semiquinone on the outer side of the inner mitochondrial membrane. The semiquinone serves as an electron donor for hemes of cytochrome bL, and then of cytochrome bH which is located close to the inner side of the membrane. Cytochrome bH reduces ubiquinone in an antimycin A-sensitive way producing ubisemiquinone followed by its further reduction with a second electron and protonation (442) (Figure 6).
FIGURE 6.
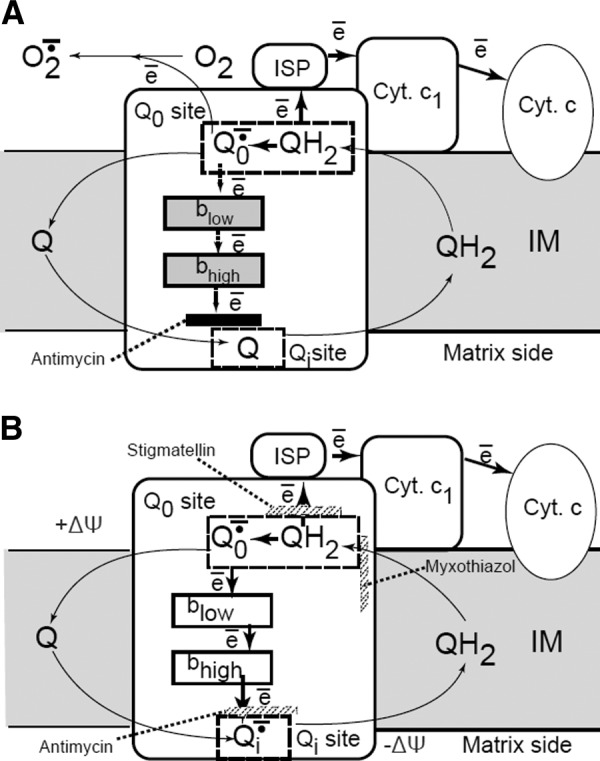
Q-cycle model. The mechanism of superoxide formation in complex III (bcl1 complex). The reaction starts from the oxidation of the CoQ quinol (QH2) in a bifurcated electron transfer reaction at the Qo site of the complex III. The first electron is transferred to a high reduction potential chain consisting of the iron-sulfur protein (ISP, aka Rieske protein), cytochrome c1 (cyt c1), cytochrome c (cyt c), and further to cytochrome c oxidase (not shown). The remaining semiquinone (Qo−·) is unstable. It donates the second electron to the low reduction potential chain consisting of two cytochromes b, cyt bL and cyt bH, which serve as a pathway conducting electrons to the Qi site. There these electrons reduce another CoQ molecule. To provide two electrons required for the complete reduction of CoQ quinone at the Qi-site, the Qo-site oxidizes two QH2 molecules in two successive steps. The first electron at the Qi-site generates a stable semiquinone (Qi−) that is reduced to a quinol (QH2) by the second electron. Most frequently used inhibitors of complex III, stigmatellin and myxothiazol, prevent the transfer of the first electron to ISP and the binding of the quinol at the Qo site correspondingly. [Modified from Starkov and Fiskum (418), with permission from Elsevier.]
Under normal conditions, the probability of existence of unstable semiquinone (Q_.) is low due to its fast oxidation; therefore, the probability of donation of one electron to molecular oxygen in this system is relatively low. Only the block of the electron flow by antimycin A results in a high superoxide release apparently due to the reduction of both hemes of the cytochrome c in parallel with the elevation of the steady-state level of semiquinone, thus giving a higher chance of one-electron reduction of oxygen (Figure 6). Among all of the mechanisms presented in the scheme, inhibitors of bc1 complex (antimycin A, myxothiazol, and stigmatellin), antimycin is the only effective ROS inducer, although some low level of superoxide production could be detected in the presence of other inhibitors (418) in the site of bc1 complex different from that induced by antimycin A. Therefore, the potential role of complex III as a cause of gross mitochondrial ROS production under the physiological steady-state mode of operation remains uncertain considering that substantial mitochondrial ROS release occurs only after application of a drug having no natural analogs in animal physiology. Noteworthy, as in the case of ROS production in complex I, conformational changes detected in bcl1 complex after antimycin A binding (45, 95, 193, 355) may be a prerequisite for dramatic molecular rearrangements in the complex resulting in a marked amount of ROS production.
Overproduction of ROS by complex III may result from acquired and genetic defects in the mitochondrial respiratory chain in close proximity to an antimycin A-binding site. B. Chance's lab performed a study on a patient who was diagnosed with a deficiency of cytochrome b in complex III, resulting in muscle weakness associated with a ragged-red fiber myopathy and lactic acidosis (122). The total succinate-cytochrome c reductase activity in skeletal muscle of this patient was only ∼5% of normal. It was already known that menadione can shuttle at least a portion of electrons over an antimycin-sensitive site (319). The treatment of this patient with menadione bridging electrons from coenzyme Q directly to cytochrome c, thus bypassing the defective cytochrome b, resulted in a significant therapeutic effect with partially normalized muscle exercise tests. This approach, named “redox therapy,” demonstrates the importance of detailed understanding of mitochondrial redox-related pathogenesis.
D. Importance of Redox State of NAD(P)H/NAD(P)+ in Mitochondrial ROS Production
A comprehensive analysis of the relevance of NADH in managing mitochondrial production of ROS has been performed by Vinogradov's group (163, 165). With the use of submitochondrial particles oxidizing NADH, it has been found that a substantial amount of superoxide production took place only when 50 μM NADH was used, while in the presence of 1 mM NADH, the production was remarkably suppressed. NAD+ revealed the same superoxide suppressive ability (165). Considering that physiological concentration of the couple NAD+ + NADH in the mitochondrial matrix is in range of a few millimolar (474), with a significant fraction of it existing in a free form, the gross generation of ROS mediated by complex I may be almost negligible. Similar experiments with isolated permeabilized mitochondria and the soluble protein fraction of the mitochondrial matrix showed the same result. The authors concluded that complex I was not a primary source of ROS in mitochondria under physiological conditions. Instead, they hypothesized that some oxidoreductases poised in equilibrium with NAD(P)/NAD(P)H may be that primary mitochondrial source of ROS (165).
In isolated permeabilized mitochondria, the same authors detected quite high NADH-dependent H2O2 production when they supplemented the system with ammonium salts (163). The mitochondrial H2O2 release was insensitive to dicumarol (inhibitor of NADH-quinone oxidoreductase, D,T diaphorase) and NAD-OH (inhibitor of complex I), suggesting the matrix localization of H2O2-producing activity. This ROS-generating activity depended on the ratio of NAD(P)+/NAD(P)H. It was concluded that a specific ammonium-sensitive NADH oxidase activity in the mitochondrial matrix is responsible for this H2O2 production, but the in vivo relevance of this process is still unknown. [An alternative explanation for an ammonium effect could be the hypothesis that mitochondrial matrix alkalinization (caused by ammonium entry) increases superoxide production by stabilizing semiquinone radical (381).]
Further analysis of the nature of this ammonium-stimulated enzyme producing primary mitochondrial ROS revealed that the questioned enzyme possessed NADH:lipoamide oxidoreductase activity and later was identified as dihydrolipoyl dehydrogenase (224). Dihydrolipoyl dehydrogenase is an essential component (called E3 component) of two mitochondrial redox complexes: α-ketoglutarate dehydrogenase complex (KGDHC) and pyruvate dehydrogenase complex (PDHC). This mitochondrial enzyme contains FAD [which contributes mostly to the overall mitochondrial autofluorescence signal originating from cellular flavins (178, 250)] with the redox state in equilibrium with the environmental NAD(P)H/NAD(P)+. It has been found that E3 is responsible for superoxide and hydrogen peroxide generation in purified KGDHC and PDHC as well as in KGDHC operating in mitochondria in vitro (71, 151, 417, 420, 438).
It is not surprising that ammonium has an effect on the component of α-KGDHC since α-ketoglutarate, instead of converting into succinyl CoA in the citric acid cycle (the Krebs cycle), can be transformed into glutamate by glutamate dehydrogenase. Typically, this reaction does not take place in mammals, since the equilibrium of the reaction is shifted toward the reverse direction, but it may occur in toxic levels of ammonia. Ammonia metabolism is important in all tissues. However, in the brain for which a high level of ammonium is extremely toxic (59), it becomes a critical element involved not only in the detoxification process (by astrocytic glutamine synthase and the all-mitochondria-located urea cycle) but also in a number of essential biochemical reactions in the cell as part of the brain signaling modules (i.e., glutaminase reaction to maintain optimal cycling of glutamine/glutamate; Refs. 421, 422, 448). With the consideration of results mentioned above, the ammonium toxicity might be at least partially mediated by the mitochondria-formed ROS.
E. Other Mitochondrial ROS-Producing Sites
1. NADPH-oxidase
The prototypic NADPH-oxidase (Nox) has been found in the plasma membrane of phagocytes and B lymphocytes, and it is involved in the phagocytic activity by ejecting superoxide radical which is a primary element igniting antibacterial defense. Its membrane domain is represented by a protein gp91PHOX (PHOX for phagocyte oxidase) which can be organized as a heterodimer in combination with other cytosolic proteins from the PHOS family which all together form flavocytochrome b558 complex (6, 24).
So far, seven isoforms of Nox (Nox1–5, Duox1–2) have been identified, with all having distinct catalytic domains. The above-mentioned form is called Nox2. It has been found not only in the cell membrane, but also in the cell interior. Other isoforms reside in different specialized tissues and different intracellular loci. Nox4 is the only member of the family found to have a mitochondrial localization [in cultured mesangial cells (55), rat kidney cortex (55) and cardiac myocytes (251)]. Nox4 differs from the other members of the Nox family in that it preferentially produces H2O2 rather than O2·− (356). The discovery of its mitochondrial localization conflicts with data that shows that the natural cytosolic partner of Nox4 complex, p22PHOX, whose presence affords NADPH oxidation was not found in mitochondria, and furthermore, that the specific activity of Nox in mitochondria is not measurable (111). Considering the high importance of NADPH-oxidase, specifically Nox4, in angiogenesis (483) and pathogenesis of atherosclerosis, diabetic injury (279) and other pathologies (280) including aging (4), further experimental research is needed to resolve this apparent conflict and gain a better understanding of the relevant aspects of Nox-mediated redox signaling.
An interesting mode of interaction between Nox and mitochondria was shown recently in cancer cells where glucose deprivation provoked a signaling-based positive-feedback loop that amplifies ROS levels above a toxicity threshold resulting in cell death (159). This positive-feedback loop involved the complex integration of homeostatic control mechanisms for metabolism (particularly, redox balance established by Nox and mitochondria) and tyrosine kinase signaling through regulation of protein tyrosine phosphatases. According to the authors, glucose withdrawal activates supraphysiological phosphotyrosine signaling and ROS-mediated cell death. In cancer cells that are highly dependent on glucose for survival, glucose and pyruvate deprivation induces oxidative stress driven by Nox and mitochondria. This oxidative stress provokes a positive-feedback loop in which Nox and mitochondria generate ROS and inhibit tyrosine phosphatases by oxidation. With the negative regulators turned off, tyrosine kinase activates Nox, further amplifying ROS generation and provoking cell death.
2. Monoaminoxidase
Monoaminoxidase (MAO) resides in the outer mitochondrial membrane and serves as a marker there. This flavoenzyme has two isoforms A and B with different substrate specificity and sensitivity to inhibitors (121). Their substrates are biogenic amines whose oxidation yields in the generation of corresponding aldehydes, H2O2, and ammonium base. MAO activity has special importance in the brain where peroxidase and catalase activities are low to fully decompose H2O2 formed during oxidative deamination of neurotransmitters (e.g., dopamine, serotonin), thus significantly depleting the endogenous pool of reduced glutathione (370).
The H2O2-generating activity of MAO might be the highest among all mitochondrial ROS generators. Isolated rat brain mitochondria produce H2O2 during oxidation of the exogenous amine, tyramine (at supraphysiological 2 mM concentration) at a rate 45.2 μM/s (179), while H2O2 production during succinate oxidation in the presence of antimycin (considered to be the “gold standard” method for mitochondrial ROS production) is 0.95 μM/s (179, 334), i.e., MAO activity is 48 times more H2O2-generating than complex III. Oxidation of tyramine by brain mitochondria results in oxidative damage of mitochondrial DNA which is abolished by MAO inhibitors (179). Compared with normal conditions, MAO produces much more H2O2 during ischemia/reperfusion of the brain (389), the kidney (249), and the heart (50, 221). MAO activity in cardiac mitochondria of 24-mo-old rats was about eight times higher than that in 1-mo-old rats, demonstrating that MAO may be an important source of ROS in the aging heart (109, 288). However, the basic MAO activity in normal tissue is quite low due to the limitation of the availability of endogenous substrates of oxidation (such as serotonin, epinephrine, norepinephrine, dopamine, and others present in the brain in only nanomolar concentrations). On the other hand, chemical inhibitors of MAO elevate ROS production in cells (50–52). Paradoxically, the ablation of MAO causes a very slight rise of its endogenous substrates in the brain tissue (386). Because the levels remain in the nanomolar range, it is doubtful that these substrates could contribute significantly to overall ROS production in tissue. However, close proximity to the sites where biologically active amines are formed and released (such as synapses and extraneuronal compartments such as astrocytes and glial cells) and the high mitochondrial density in and around these areas, may render mitochondrial MAO an important component in both inactivation of amines and the local rather than overall ROS production in such areas. It has been speculated that under normal physiological activity, ROS produced by MAO in these areas performs metabolic and signaling functions in the brain (31). In addition to ROS, the by-products of biologically active amine conversion by MAO may play a direct role in degenerative processes, e.g., the dopamine molecule after entering MAO reaction produces a reactive quinone that could modify and damage cellular components (425).
3. p66shc
On the basis of the observation that p66shc-deficient mice display extended life span, remarkably reduced levels of ROS, and increased tolerance to oxidative stress, it has been suggested that p66shc could have an important role in ROS production and aging (295, 328, 439). Normally p66shc resides in the cytosol, while under oxidative stress (e.g., under ischemia/reperfusion insult), it could be translocated in the mitochondria in a PKCβ-dependent way (341) where it serves as an important source of ROS (109). In mitochondria, this adapter molecule has been suggested to function as a redox enzyme possibly oxidizing cytochrome c and generating H2O2 in the amino-terminal portion of p66shc containing sequence similar to that of certain redox enzymes (156).
It was hypothesized that p66shc in mitochondria exists within a high-molecular-weight complex which includes mtHSP70 and TIM-TOM complex. Inside of such a complex, p66shc is inactive. After propagation of apoptotic signal, the complex is dissociated resulting in the release of free p66shc, which becomes activated and capable of participating in the electron transport which generates H2O2. Mutations in the redox active sequence of p66shc abolish its pro-apoptotic activity apparently through the inability to interact with cytochrome c (156). Recent data show that p66shc-generated ROS regulate insulin signaling (48), T-cell and B-cell signaling pathways (133), and expression and activity of the ROS-generating enzyme NADPH oxidase (NOX4) as well as activate NF-κB (226, 291), thereby amplifying oxidative stress and inflammation.
4. α-Glycerophosphate dehydrogenase
Another potential source of ROS in mitochondria could be α-glycerophosphate dehydrogenase that occupies the outer surface of the inner mitochondrial membrane, but its activity is relatively low in the liver, heart, and brain but high in brown adipose tissue (304). However, isolated mitochondria supplemented with α-glycerophosphate in the presence of antimycin A produce hydrogen peroxide which, when normalized to an enzymatic activity, exceeds that originating from complexes I or II (116). This source could represent one of the most efficient ROS generators in mitochondria. It is almost insensitive to the presence of an uncoupler or rotenone, implying that ROS generation by α-glycerophosphate oxidation is not dependent on mitochondrial Δψ contrary to, e.g., succinate oxidation (304). The detailed mechanism of ROS production by this enzyme is not defined yet.
In addition to previously mentioned sources of mitochondrial ROS production, it was shown that cytochrome b5 reductase (470) and dihydroorotate dehydrogenase (112, 140, 276) produce ROS on the outer surface of the inner membrane. However, the significance of these enzymes in the total ROS production remains questionable.
5. Electron transfer flavoprotein (ETF) and ETF quinone oxidoreductase (ETF dehydrogenase)
In 1972 Boveris et al. (58) found that rat liver mitochondria can produce a substantial amount of hydrogen peroxide when oxidizing palmitoyl carnitine or octanoate. This production was ceased in the presence of an uncoupler. The fatty acid-induced ROS generation was comparable to that in the presence of glutamate plus malate and was slightly lower than with succinate (58). Thirty years later, similar results were obtained with skeletal muscle and heart mitochondria (414). Increased lipid metabolism was correlated with upregulated UCPs expression/activities (73, 369), possibly to salvage the system from excessive ROS production (414). Since ROS generation was almost insensitive to external SOD, it has been proposed that the fatty acid β-oxidation may result in ROS generation at a distinct mitochondrial matrix site which is different from o-center of complex III. In addition, it has been proposed that a significant contribution to ROS production during fatty acid oxidation comes from ETF which accepts electrons from different dehydrogenases including those involved in β-oxidation (41) and transfers them to the ubiquinone pool in the inner mitochondrial membrane by a reaction catalyzed by ETF-ubiquinone oxidoreductase (ETF-QO) residing in the matrix side of the inner mitochondrial membrane (153). In β-oxidation, the sequence of reactions is as follows: acyl CoA dehydrogenase → ETF → ETF-QO → Ubiquinone → complex III.
ETF-QO contains flavin (FAD) and a [4Fe-4S] cluster which makes it vulnerable to ROS (363), and it serves as a convergence point for electrons flowing from nine flavoprotein acyl-CoA dehydrogenases and two N-methyl dehydrogenases (153, 227). Recently, it was found that oxidation in muscle mitochondria of long-chain fatty acids in physiological (low) concentration is associated with higher rates of ROS formation than oxidation of NADH-linked substrates while exhibiting relatively low dependence on the mitochondrial membrane potential (380). The authors suggest that this enzymatic activity may be responsible for Δψ-independent ROS production.
Deficiency of ETF-QO in most cases is caused by single point mutations around the FAD-ubiquinone interface (326) and results in a human genetic disorder known as multiple acyl-CoA dehydrogenase deficiency (MADD) or glutaric acidemia type II (141). This is characterized by impaired fat and protein metabolism. It may be associated with acidosis or hypoglycemia and accompanied by other symptoms such as general weakness, liver enlargement, increased risk of heart failure, and carnitine deficiency, and could result in a fatal metabolic crisis (144, 390, 443).
6. Aconitase
Aconitase catalyzes transformation of citrate to isocitrate in the Krebs cycle. It contains a cubane-type [4Fe-4S] center with three iron atoms interacting with cysteine residues and inorganic sulfur atoms, while the fourth iron, Fe-α, is exposed to the solvent that allows the catalytic dehydration of citrate to form the intermediate cis-aconitate, as well as the subsequent hydration of cis-aconitate to form isocitrate (42, 138). The prosthetic group of aconitase is highly susceptible to inactivation by superoxide anion radical yielding an inactive [3Fe-4S] form, Fe2+-α and H2O2 (138, 267, 452). Interaction of the latter two ignites Fenton's reaction resulting in the release of ·OH radical (452). It has been proposed that aconitase would be an ideal sensor for ROS in cells (147, 148). Subsequently, superoxide toxicity in mitochondria could be explained by enhanced aconitase inactivation and related processes. For example, doxorubicin cardiotoxicity was explained mainly by an aconitase inactivation accompanied by hydroxyl radical release (296). Aconitase inactivation is an example of a “ROS cross-talk” where one type of ROS (superoxide) is inducing the release of another, potentially more damaging, ROS (the hydroxyl radical). An alternative point of view is that aconitase inactivation may serve a protective role by diminishing electron flow through the ROS-generating respiratory chain. In addition, the accumulation of citrate as a result of a decreased Krebs cycle flux promotes chelation of Fe2+ which is then irreversibly oxidized to a more stable complex citrate-Fe3+, thus preventing catalysis of Fenton's reaction by free Fe2+ (400).
VI. ROS-INDUCED ROS RELEASE ASSOCIATED WITH THE mPTP
A. Fundamentals of the Discovery
The importance of cellular redox homeostasis in progression of inherited and acquired pathologies, including those associated with an aggressive oxidative environment, was postulated elsewhere (reviewed in Refs. 72, 174). As we discussed earlier, the redox homeostasis is determined by the balance between ROS generation matching metabolic needs and ROS quenching capacity. Undoubtedly, tipping the balance in favor of increased ROS production within the cellular microenvironment can severely alter the cellular redox equilibrium, potentially resulting in oxidative stress which when mild can cause oxidation of essential mitochondrial components. In extreme cases it can irreversibly damage these components resulting in a cell death. Within the great diversity of types of cell death, which to date comprise as much as 13 types (145), at least some of them could be associated with induction of the mPTP.
mPTP opening is a phenomenon known in the field of mitochondrial research for many decades, but for the first time described in details in a set of three consecutive papers by Haworth and Hunter in 1979 (182, 195, 196). This phenomenon [also recognized as an opening of a megachannel (228, 229, 430)], originally studied in isolated mitochondria, represents a sudden change in the permeability of the inner mitochondrial membrane allowing not only protons but also other ions and solutes of a size up to ∼1.5 kDa to go through this membrane. There are many reviews on the tentative nature and identity of the mPTP (64, 489, 494) with details which are far beyond the scope of the present review. Previously, many candidates were considered to serve as the core of the pore [i.e., mitochondrial VDAC, cyclophilin D (cyPD), ANT (108, 170, 217, 351, 457, 489, 494)], but largely they have been all dismissed because of various reasons, but still leaving for them important pore-modulating functions. Recent evidence suggests that a dimer of mitochondrial ATP-synthase is essential to form a core of the mitochondrial pore (157) and c subunit of the mitochondrial ATP synthase complex may be required for mPTP-dependent mitochondrial fragmentation and cell death (56).
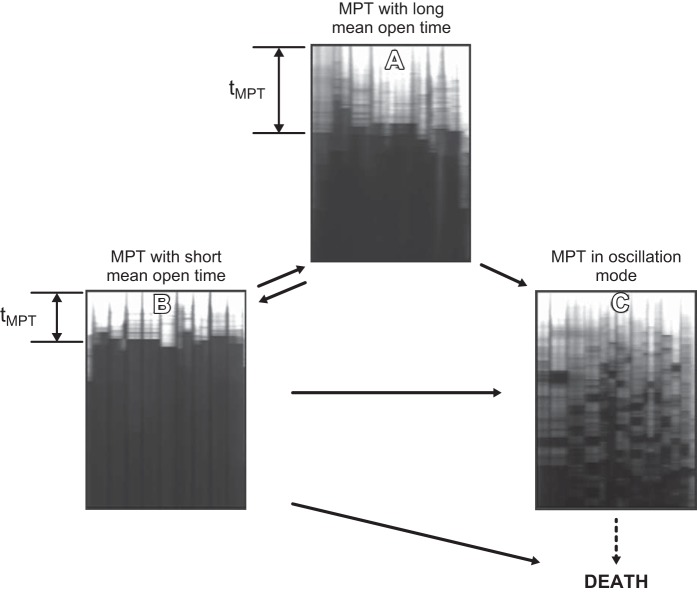
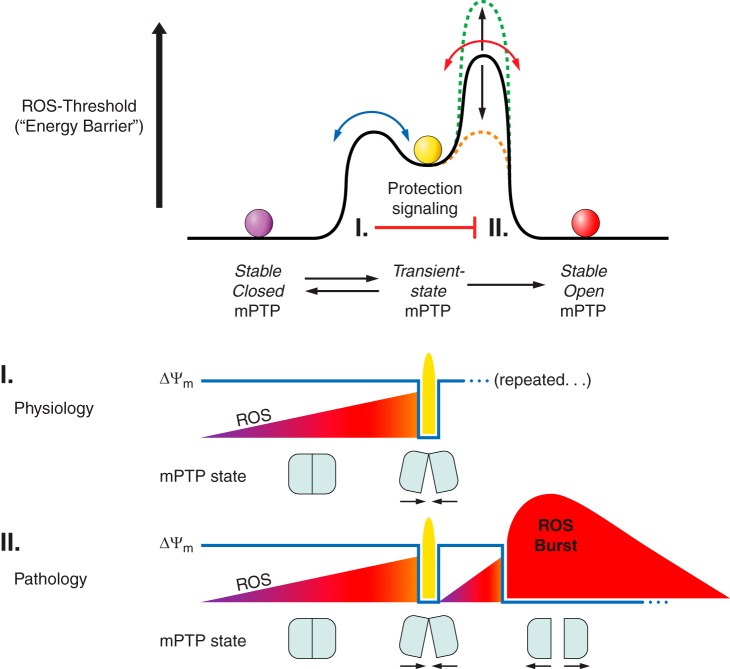
Although mPTP induction is typically referred to as a pathological event very often resulting in the degradation of mitochondria or the cell (which will be discussed later), there is multiple evidence and assumptions that in fact it can also serve physiological functions. It has been postulated that mPTP could serve as a release valve for quick release of cations constantly leaking into the mitochondrial matrix due to the mitochondrial membrane potential. A flickering mode of mPTP may serve this purpose with the mPTP opening for a time not sufficient for the onset of complete mitochondrial depolarization (239). Another support of a physiological role of the mPTP was obtained in CyPD knockout mice which demonstrated an obvious maladaptive phenotype in their hearts (124). While the role of CyPD as a core of the mPTP has been dismissed, there is a general consensus that CyPD can serve a modulatory role in the process of the mPTP induction. It was shown that CyPD knockout mice exhibit substantially greater cardiac hypertrophy, fibrosis, and reduction in myocardial function in response to pressure overload stimulation than control mice while cardiomyocyte-specific transgene expression of CyPD in these mice helped to rescue from the named pathologies. Also, in mice lacking CyPD ischemic preconditioning was augmented (239) while mPTP openings in wild-type mitochondria were much more frequent than in mitochondria of knockout mice. This supports the notion that the mPTP might have an important physiological role, possibly through regulation of intramitochondrial Ca2+.
The role of Ca2+ in the induction of the mPTP has been already mentioned above; similarly, the role of oxidants in generation of the mPTP pore is essential too, although both resulting in the same phenomenological outcome (255, 277). It has been recognized that the mPTP induction represents a highly complex phenomenon (322, 409). Particularly, isolated mitochondria exposed to Ca2+ plus Pi demonstrate the collapse of Δψ preceding mitochondrial swelling, suggesting that the generation of a smaller, low-conductance pore occurs with permeability for ions but not for solutes prior to induction of the mPTP (full size) pore (5, 67, 244, 339). Oxidants such as hydrogen peroxides, organic peroxides, and some other inducers generate both pores, but the insensitivity of induction of low-conductance pore to Ca2+ and insensitivity of fully induced mPTP to conventional inhibitor, cyclosporine A and sometimes to EGTA (65, 243, 262, 284), makes them different from classical pore inducers (255; reviewed in Ref. 489). Therefore, the mitochondrial pore phenomenon appears to be multifaceted.
Light is known to be a potent oxidant inducer when it interacts with photosensitizing agents, and such a property is successfully utilized in photodynamic therapy (114, 317). It is based on the ability of the excited fluorophore to generate primary ROS which after release inside of the biological sample may become destructive for cellular components. In principle, many biological molecules and cell compartments can be fluorescently labeled and oxidatively modified after interaction with excitation light. The mitochondrion is an easy target for such modification since it can accumulate fluorescent cations to a great extent using its intrinsic proton motive force, namely, its Δψ (211, 298). Mitochondrial Δψ provides selectivity for photodynamic action localized exclusively in mitochondria without potential impact on other intracellular compartments (301, 303, 366, 367, 403). After exposure to light, the photosensitizer, e.g., tetramethyl rhodamine methyl ester (TMRM) which is a conventional probe for mitochondrial membrane potential widely used for visualization of energized mitochondria in the cell, generates various ROS in water including very strong oxidants such as superoxide anion radical and hydrogen anion radical (491).
Because mitochondria are critical intracellular loci of ROS production, together with the fact that ROS exposure can lead to the mPTP, it was hypothesized that under certain circumstances the mPTP could become self-amplifying and unstable (491). This hypothesis was tested in cardiac myocytes, taking advantage of the unique organization of mitochondria between myofilaments in an ordered three-dimensional latticelike array forming straight lines thus allowing specific applications of line-scan confocal microscopy to address this question (491, 493). For this purpose, rat cardiac myocytes are double stained with the probe for mitochondrial membrane potential (TMRM or ethyl derivative, TMRE having excitation maximum in a green region of the spectrum) and the ROS probe, 2,7-dichlorodihydorfluorescein diacetate (DCF-H2). DCF-H2 itself is nonfluorescing and unreactive toward oxidants; however, after base hydrolysis of the ester bonds, the resulting nonfluorescing compound becomes reactive toward ROS, and after oxidation it is transformed into fluorescing DCF with maximum excitation in the blue region of the spectrum. The first scan of a cardiac myocyte previously unexposed to light frequently reveals the area(s) in which mitochondria are expected to occupy, but unstained with TMRM (suggesting that mitochondrial Δψ had collapsed in these mitochondria) but also demonstrating high DCF fluorescence. The size of the area is dependent on the physiological status of the cell (Figure 7), possibly reflecting the cellular level of tolerance to ROS. Therefore, isolated adult rat cardiac myocytes are a convenient model to study cellular oxidative stress.
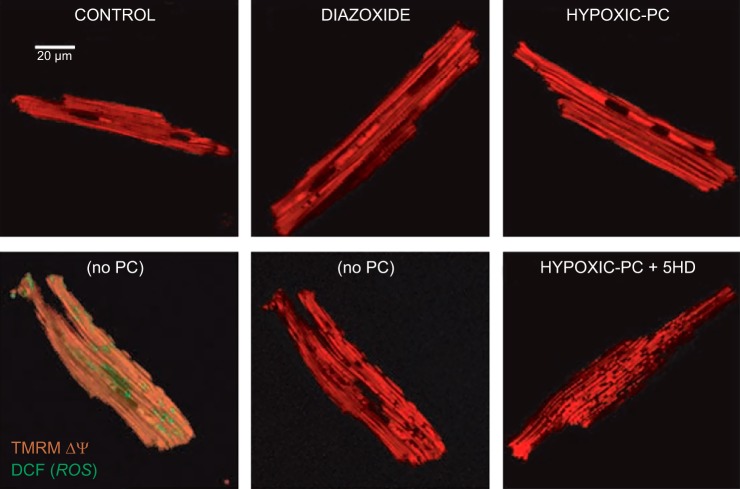
FIGURE 7.
Δψ loss in a significant fraction of mitochondria, caused by hypoxia/reoxygenation. Depolarized mitochondria (red-fluorescence ”holes“; bottom panels) are associated with increased ROS (green; bottom left panel). Hypoxic PC or pharmacological preconditioning (PC), represented by diazoxide (Dz), prevents mitochondrial depolarization, and 5-hydroxydecanoate (5HD) accentuates the loss. [From Hoffman et al. (189).]
B. mPTP and Ischemia/Reperfusion Injury
For the last 50 years it has been well recognized that coronary reperfusion of infarcted myocardium is associated with increased necrotic death of irreversibly injured cardiac myocytes. Jennings et al. (208) were first to report harmful, both structural and functional, changes associated with reperfusion. Therefore, early reperfusion while crucial for preserving ventricular function, preventing infarct expansion and potential development of heart failure, may also contribute to the pathogenesis of reperfusion arrhythmias and myocardial stunning manifested by reversible contractile dysfunction. The biological basis of reperfusion injury has been extensively studied since then and consequently the notion of reperfusion as a double-edged sword was formulated in 1985 by Braunwald and Kloner (63). In 1986 ischemic preconditioning was described as a means to render the heart more resistant to ischemia/reperfusion injury (311). The importance and the clinical potential of these discoveries has prompted the research community to focus on deciphering the molecular mechanisms that underlie reperfusion-induced cellular injury as well as cardioprotection. Thousands of research papers have already been published, and a detailed picture has emerged, e.g., recently reviewed in References 181, 205, 207, 375. The significant role of so-called oxygen paradox was recognized (183) early on and was based on the finding that substantial injury occurs when molecular oxygen is reintroduced into ischemic tissue. A burst of ROS production on reperfusion has been detected (149, 500). Immediately after exposure of the ischemic organ to oxygen, superoxide anion radicals dominate among the emerging ROS in the effluent perfusate, with further formation of hydroxyl radical (499) pointing to the occurrence of iron-mediated Fenton chemistry. This can be explained by the likelihood that in oxygen-depleted medium the iron ions are mostly reduced (redox potential of the couple Fe2+/Fe3+ = +0.77 V), but since hydrogen peroxide is absent, the Fenton reaction does not yet occur. Reperfusion ignites the formation of superoxide anions which dismutate resulting in formation of H2O2 and immediately reacting with iron ion [still in a reduced form (Fe2+)], thus generating highly reactive ·OH (400).
Mitochondria have been implicated as a potential source as well as a target of the generated ROS resulting in an observed loss in mitochondrial function during ischemia/reperfusion and consequent irreversible cellular injury (9, 101, 329). It has been noted that not only these radicals play a significant role in the tissue damage observed following ischemia/reperfusion but that this injury can be mitigated by oxygen radical scavengers (e.g., Ref. 213). In the early 1990s, Crompton and colleagues demonstrated in isolated mitochondria that the derangement of mitochondrial bioenergetics that develop on reoxygenation, when resting cytosolic Ca2+ is high and ATP low, could lead to excessive mitochondrial Ca2+ uptake and consequent induction of mPTP (96, 97). This results in mitochondrial uncoupling and activation of ATP hydrolysis by F1Fo-ATP-synthase (96, 97, 118).
The cardiomyocytes exposed to the hypoxia-reoxygenation cycle have much larger areas occupied with fully deenergized mitochondria and high levels of ROS, and these levels were diminished and Δψ was regained in most mitochondria after ischemic or pharmacological preconditioning of the cell (Figure 7) (218). Based on the previously expressed assumption that the cardiac ischemia-reperfusion injury is associated with the induction of the mPTP (97, 161), the speculation was put forth that the found abnormal subcellular loci in cardiac myocytes were indeed occupied by mitochondria which had undergone permeability transition. The most striking detail was that mitochondrial deenergization, if caused by the mPTP opening, was associated with higher, rather than lower, ROS production as follows from Figure 5.
C. RIRR: Experimental Demonstration
To further explore this puzzling phenomenon, confocal microscopy was employed in line-scan mode allowing repeated excitation of a row of mitochondria along the selected line in cardiac myocyte (Figure 8, A–C). Within the light-exposed mitochondria, a sudden loss of Δψ with corresponding rise of ROS generation was revealed, suggesting mPTP induction. Although these transitions were modestly sensitive to cyclosporine A, they were dramatically delayed by another mPTP inhibitor, bongkrekic acid, and by a ROS scavenger, Trolox. Furthermore, redistribution of the inert fluorescent probe calcein (mol wt 620) from cytosol to mitochondria at the moment of mPTP induction also confirmed opening of a pore permeable to a 620-Da compound (Figure 8B). Thus it was concluded that the collapse of the mitochondrial Δψ occurs due to the mPTP pore opening.
FIGURE 8.
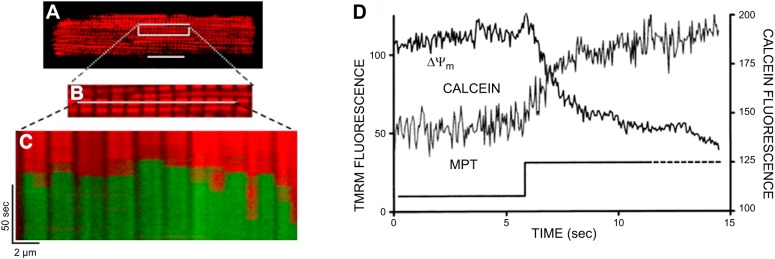
RIRR and MPT induction. A: confocal microscope fluorescence imaging of cardiac myocytes loaded with TMRM (red) and DCF-H2 (green). A, top: fluorescence image of TMRM-loaded cardiac myocyte. B: enlarged portion of the TMRM-loaded cardiac myocyte. Line drawn on image shows position scanned for experiment in bottom panel. C: row of mitochondria were line-scan imaged at 2 Hz with excitation at both 488 for DCF and 543 nm for TMRM and collecting simultaneous fluorescence emission at 510–550 nm and >560 nm, respectively. The sudden loss of Δψ and rise of ROS generation in individual mitochondria are indicated by the loss of TMRM fluorescence and increase in DCF fluorescence. B: cells dual-loaded with TMRM (Δψ) and calcein-AM (the latter loaded under conditions that results in a cytosolic distribution initially in excess over that in mitochondria); line-scan imaging at 2 Hz. For details, see Refs. 491, 493.
Fine analysis of the ROS burst kinetics associated with the mPTP induction showed that ROS generation in the affected mitochondrion proceeds in two distinct phases: the initial, slow rise due to the accumulation of a photochemistry-associated production of ROS (called “trigger ROS”) and the subsequent ROS burst associated with dissipation of the mitochondrial membrane potential. This biphasic process was named “ROS-induced ROS release” (abbreviated RIRR). In addition to the ROS burst and the mPTP induction, it was accompanied by the burst of nitric oxide production; thus the term ROS-induced ROS/RNS release where RNS stands for reactive nitrogen species is also relevant (491).
The identical phenomenon using the same instrumentation as in original RIRR study (491) was later described in cells infected by adenovirus carrying mitochondria targeted circularly permuted yellow fluorescent protein (cpYFP) as the ROS sensor. The term superoxide flashes was used to describe the RIRR in transfected cardiac myocytes (461). Recently, this approach was reexamined and challenged by observation that the cpYFP is very sensitive to pH changes, therefore rendering this interpretation inconclusive (376, 377, 466).
The complex I inhibitor rotenone (at low concentrations not affecting TMRM sequestration) significantly decreased the ROS burst magnitude, confirming the mitochondrial nature of the ROS burst during mPTP-associated RIRR (491). The few second delay of NADH oxidation following the collapse of the mitochondrial ΔΨ suggests that NADH is the redox-energy store driving the electron donor necessary to support the single-electron reduction of molecular oxygen that produces superoxide as an initiating radical for generation of primary ROS followed by the mPTP induction. As we have already mentioned, the ROS burst accompanying ΔΨ collapse is against the existing “dogma” that ROS production is inversely related to the mitochondrial membrane potential magnitude (Figure 5). This dogma seems to be true when the classical uncoupling is considered, i.e., the drop of ΔΨ due to the enhanced proton conductance of the inner mitochondrial membrane. It seems to be irrelevant to the extreme dysequilibrium situation when a megachannel is opened in this membrane. The enhanced ROS production in mitochondria undergoing mPTP opening has been confirmed in vitro when isolated mitochondria were supplemented with NADH to compensate for its loss as a result of opening a megachannel (35).
The paradoxical aberration of established relations between ROS production and mitochondrial transmembrane potential (240, 275) may have the same nature that we suggested when discussing the ROS production in complexes I and II. We concluded that, at least partially, it is determined by unusual conformational changes within these complexes. It is known that at least some components demonstrate conformational changes within mitochondria undergoing mPTP (171, 426, 427, 463, 464). Such conformational changes have been described, e.g., for ANT. It was shown that the c-conformation of ANT (when ADP is bound to the cytosolic side) is more preferred for the open state of the mPTP than the m-conformation (when ATP is bound to the matrix side), and it corresponds to the closed state of the pore. Accordingly, atractylates stabilize the c-conformation of the ANT and promote the mPTP while bongkrekic acid (184) stabilizes ANT in the m-conformation and inhibits the mPTP (172, 233, 320). However, ANT was proposed to be a pore modulator (494) rather than a component of the core of the pore (235).
Mitochondrial ROS rise simultaneously with the mPTP-induced drop of ΔΨ. However, quite often it has been possible to observe a very brief phase of mitochondrial hyperpolarization coinciding with the onset of the mPTP opening. The analysis proved that the hyperpolarization phase also coincided with the start of the excessive ROS generation (493) (Figure 9). Although a hyperpolarization spike has not been observed in all recorded mPTP opening events in the cardiac myocyte, the flicker could be sufficiently brief and be below the kinetic resolution of the fluorescent signal from TMRM belonging to the class of “slow-response” (distributed or accumulated) probes for the mitochondrial ΔΨ (458, 459). The flickering mitochondrial hyperpolarization mode was found to be a frequent phenomenon during photo-excitation of TMRM in loaded cardiac myocytes observed in the confocal microscope during line-scanning. Not every flicker could ignite the mPTP opening; nevertheless, each flicker initiated a small burst of ROS production that was insensitive to the mPTP inhibitor bongkrekic acid. Also these hyperpolarization flickers were not associated with the entry of the inert probe calcein from the cytosol into the mitochondria, suggesting these flickers do not involve a long opening of the mPTP. So, apparently there were at least two different modes of RIRR, one of which was accompanied by the large-conductor mPTP opening, while another was too brief to establish the conductance. It is noteworthy that the flicker-induced ROS production associated with mitochondrial hyperpolarization theoretically obeyed the conventionally established relations between ROS production and mitochondrial transmembrane potential (Figure 5) (240, 275), while the mPTP-associated ROS production did not. Both were caused by the triggering ROS formed during the photoexcitation process.
FIGURE 9.
Transient Δψ hyperpolarization and depolarization (flickering) preceding MPT induction can be observed. A: overlay showing the 20-Hz linescan image in a cardiomyocyte loaded with TMRM (red) and DCF (green). B: the TMRM channel from A; arrows indicate examples of transient hyperpolarization immediately preceding Δψ loss (MPT induction). C: the directional derivative of the TMRM intensity with respect to time, to enhance the identification of intensity transients (transient hyperpolarization prior to MPT induction); arrows indicate examples of transient hyperpolarization (positive slope maxima) immediately preceding MPT. D: intensity plot of the TMRM responses of the mitochondrial pair indicated by the asterisk in B; inset shows an enlargement of the area inside the red box and shows transient (relative) hyperpolarization immediately preceding MPT. [From Zorov et al. (493), with permission from Elsevier.] E–G: ROS production (green) occurs during transient mitochondrial hyperpolarization flickering (red image, arrows in E). H–I: 2-Hz line scan image in a cardiomyocyte loaded with TMRM. Transient hyperpolarization and depolarization spikes are seen as white and black vertical lines (shown by the arrows from 1 to 7). TMRM fluorescence intensity (Δψ) in the region of the cardiomyocyte denoted by the arrow-bracket in H (∼6 μm in width, consisting of 3 sarcomere-associated pairs of mitochondria) within which mitochondria display concerted flickerings of the membrane potential. Note that these transient depolarizations, during the time interval between arrows 1–7, are accompanied by progressive mitochondrial swelling (as seen by the lateral displacement of adjacent mitochondria) consistent with repeated transient MPT-induction episodes.
VII. RIRR AND Ca2+
RIRR was named for the analogy with the process of Ca2+-induced Ca2+ release (with acronym CICR) which has been known since the 1970s (125, 127, 128, 139) and which plays a critical role in excitation-contraction coupling. In this process, an action potential depolarizes the cell membrane providing a small influx of Ca2+ through the plasma membrane, which by itself is insufficient to provide contractile activation. Moreover, since the contractile apparatus is at a distance from the sites of inward Ca2+ transport, Ca2+-induced muscle fibers contraction would be retarded if it depended only on Ca2+ diffusion within the muscle cell. CICR serves two goals. First, it significantly facilitates the propagation of the Ca2+ signal in the cell, and second, it amplifies Ca2+ concentration to reach Ca2+ levels in the vicinity of contractile apparatus essential to provide a contraction. Then, both RIRR and CICR contain signaling amplification loops to reach the threshold necessary for the transition. CICR and RIRR are also similar in spatial terms; CICR includes different intracellular compartments and covers the space between the plasma membrane Ca2+-channels and ER/SR and fibers, and RIRR can span between mitochondria and thus spread across and between cells.
Although there have been some attempts in the past to ascribe mitochondrial mPTP to the existence of mitochondrial CICR (194) or even strontium-induced strontium release (190), eventually the essential role of ROS has been established in this process. In fact, mitochondria have quite a high Ca2+-buffering capacity (maximal calcium uptake), explaining the fact that a number of consecutive Ca2+ pulses could be sequestered and tolerated by isolated mitochondria or permeabilized cells until mPTP opening occurs resulting in a robust release from mitochondria of both sequestered and intrinsic amounts of Ca2+ (or Sr2+) (77, 166, 321, 331). Such mitochondrial ability to sequester Ca2+ could be modified by a number of factors (7, 30, 132, 306, 465).
Consequently, “X-induced X release” may be a general mechanism for any determined (such as RIRR and CICR) and still undetermined signaling amplification loops. Meanwhile, reciprocal amplification of ROS and Ca2+ signals or any other “X-induced Y” release processes representing solitary events or additional complex processes within a signaling cascade, probably also occur, e.g., in the case when ROS generated in mitochondria may be a source of Ca2+ unitary releases from ryanodine receptor of SR (Ca2+-sparks) (491) (see Figure 10). The process of the spontaneous ROS-induced Ca2+ release by the SR ryanodine receptor described in Reference 491 has been recently analyzed in a model of sustained mitochondrial ΔΨ oscillations driven by the mitochondrially produced ROS. Dynamic changes in mitochondrial energy state resulted in altered frequency and properties of the Ca2+ sparks (485).
FIGURE 10.
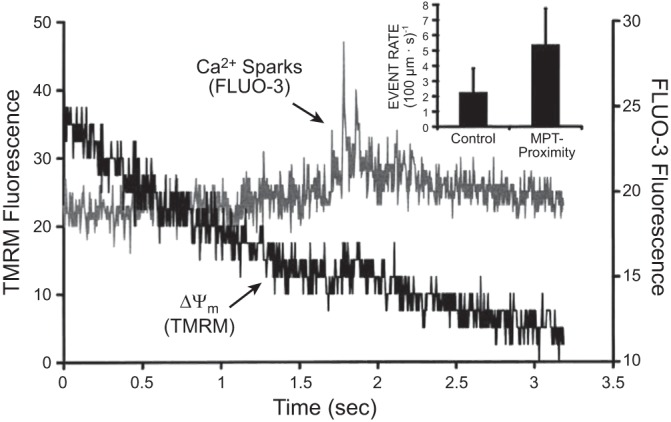
Induction of Ca2+ sparks after the mPTP. Cell is dual-loaded with TMRM (Δψ) and fluo-3 (Ca2+) and line-scan imaged at 230 Hz. Representative example showing the dissipation of TMRM fluorescence from a single mitochondrion and a cluster of Ca2+ sparks in the immediate vicinity, within seconds of MPT induction. Inset: comparison of Ca2+ spark rate in proximity of the mPTP occurrence, i.e., within the sarcomere containing the involved mitochondria and within 3 s after the mPTP occurrence. [From Zorov et al. (491).]
Isolated mitochondria possess an ability to sequester very large amounts of Ca2+ from the medium (85, 106, 259). Although proton motive force potentially drives cations to negatively charged mitochondrial matrix forcing cations to be accumulated inside of mitochondria, it has been found that at resting conditions in rat cardiac myocytes the intramitochondrial free Ca2+ level is <100 nM. Mitochondrial Ca2+ concentration is maintained by inward and outward Ca2+-transporting systems in the inner mitochondrial membrane. One of these, the uniporter (231, 372, 450, 451), has low affinity but high capacity for transporting Ca2+, whereas Ca2+/2H+ and Ca2+/2Na+ exchangers (60, 61, 75, 98, 136, 137) have much lower capacity. In stimulated cells at higher pacing rates, the increased cytosolic Ca2+ will steadily raise the [Ca2+]m. Over the course of many contractions, [Ca2+]m can rise up to 600 nM (300) and potentially results in activation of mitochondrial dehydrogenases. Pyruvate and α-ketoglutarate dehydrogenases show the greatest dependence on [Ca2+]m (107, 176, 278, 302). Such activation of the key mitochondrial dehydrogenases results in activation of respiration and ATP synthesis to meet rising energy demands under increased work load.
Interestingly, calcium ions target and activate those mitochondrial enzymes (pyruvate dehydrogenase and α-ketoglutarate dehydrogenase) that are considered to be a key source of ROS in mitochondria (see above). However, while the crucial role of Ca2+ in mitochondrial metabolism is established (reviewed in Refs. 166, 434) with recent molecular identification of the Ca2+-uniporter (36, 105) and Ca2+/2Na+ exchanger (332), the role of Ca2+ in ROS production in mitochondria remains controversial. In isolated mitochondria, the mPTP is opened by elevated Ca2+. This led to the conventional assumption that mPTP induction by mitochondrial Ca2+ loading may be the cause of many types of cell death, such as those induced by ischemia-reperfusion injury and chemical toxins, in the heart and brain. In intact cardiomyocytes and neurons, however, normal excitability achieves Ca2+ elevations to levels comparable to those believed to induce the mPTP based on in vitro data. This suggests a serious paradox that will have to be reconciled regarding the role of Ca2+ in mPTP induction in vitro with the empirical evidence of health and longevity of these postmitotic cells in vivo (218, 494). Although Ca2+ is a typical tool for induction of permeability transition in isolated mitochondria (182, 195, 196; reviewed in Ref. 489), experiments with intact cardiac myocytes and neurons demonstrated that the mPTP is largely insensitive to increased cytosolic Ca2+ (see supplements to Ref. 218). In these experiments, however, after cell permeabilization, the Ca2+ sensitivity of the mPTP is unmasked and is similar to that observed in mitochondria isolated from these cells (Figure 11, A and B). Probably, some cytosolic factors that are lost after permeabilization and mitochondrial isolation are responsible for Ca2+ insensitivity of the mPTP in the intact excitable cell such as the cardiac myocyte or neuron. These facts themselves put into question the role of Ca2+ in cell death correlated with mPTP induction after, e.g., hypoxia/reoxygenation which may be overestimated due to limitation of the biological model (212) (see Figure 11C).
FIGURE 11.
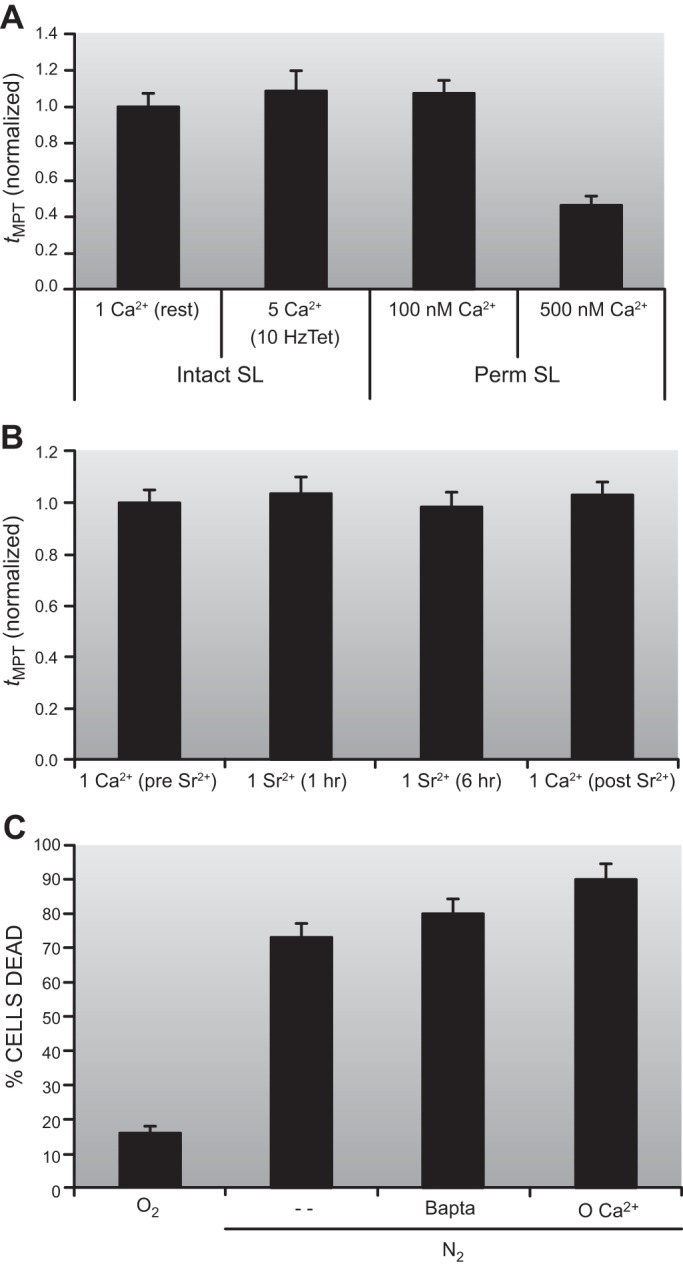
Ca2+ dependence of the mPTP in rat adult cardiac myocytes. A: mPTP ROS threshold during a “clamp” of intracellular Ca2+ >500nM for >20 min (using 10-Hz electrical tetanization in 5 mM bathing Ca2+ in the presence of thapsigargin or cyclopiazonic acid to disable the sarcoplasmic reticulum) is identical to that in unstimulated cells (with a cytoplasmic Ca2+ of ∼100 nM). B: complete equimolar replacement of Ca2+ for Sr2+ (for 6 h) in intact cardiac myocytes resulted in the same mPTP ROS threshold as seen in cells with normal Ca2+. C: buffering intracellular Ca2+ (with BAPTA) or limiting Ca2+ influx (in nominally Ca2+-free buffer) does not limit cardiac myocyte death after hypoxia/reoxygenation. [From Juhaszova et al. (218). Republished with permission from the American Society for Clinical Investigation; permission conveyed through Copyright Clearance Center, Inc.]
There is also a puzzling discrepancy between a very high apparent Km (250–350 μM) for endogenous ADP in the control of mitochondrial respiration in permeabilized muscle cells and that in isolated mitochondria where the apparent Km equals 15–20 μM (248). One of the possible explanations for this inconsistency is that isolated mitochondria are stripped from cytoskeletal proteins that maintain mitochondrial integrity within the intracellular energetic unit, which might be involved in fine regulation of enzymatic parameters in the live cell and be responsible for observed differences (22).
VIII. MITOCHONDRIAL COMPARTMENTATION
The reviewed data raise the question about the adequacy of isolated mitochondria or permeabilized cells to the actual processes taking place in an intact live cell, as all three objects have different levels of structural and organizational complexity. One of the structural parameters missing in isolated mitochondria is a close proximity of mitochondria to ER/SR found in the cell (119, 379, 384), thus making easier shuttling of small signaling elements (e.g., ROS, H+, or Ca2+) and tightening the spatial and functional compartmentation of two organelles (357). The loss of spatial compartmentation due to the isolation procedure may dramatically change kinetics and, thus, enzymatic parameters of interactive systems. The compartmentalized system might greatly diminish the diffusion-controlled step(s) when the product of one enzymatic reaction almost immediately becomes a substrate of the second enzyme without the step of a product release in the bulk phase followed by extraction from this phase.
It is beyond the scope of this review, but the dramatic difference between two theories of oxidative phosphorylation, one proposed by Peter Mitchell and another by Robert Williams, deserves mentioning. Both theories were named chemiosmotic with one principal difference: while Mitchell's theory considered the delocalized proton ejected from a proton pump into the bulk phase to be a substrate for ATP synthase complex (298, 299), Williams considered a localized proton to be consumed by ATP synthase without an intermediate step of going into the bulk phase (472, 473). Although there are some more or less sketchy observations supporting the second mechanism which apparently requires organization of supramolecular protein complexes (reviewed in Ref. 131), the first mechanism was generally accepted, and its founder was awarded the Nobel prize in 1978.
The problem of intramitochondrial compartmentation has many supporters (e.g., Refs. 412, 413; reviewed in Ref. 265). However, recent data has led to an interpretation highly critical of the organization of mitochondrial respiratory chain into supramolecular complexes (organized protein assemblies) (441), leaving the compartmentation issue still rather controversial.
Another important issue is the intermitochondrial compartmentation. The possibility that mitochondrial units may work in concert unifying energy transmission and signaling has been highly explored. Concerted work of individual mitochondria in striated muscles interconnected by electrically permeable junctions (28) was proposed (394) and later confirmed for cardiac myocytes (10). Such electrical unification of mitochondria was postulated to adequately allow fast transportation of energy along the mitochondrial reticulum to all cellular regions remote from the initial energy source. Later the mitochondrial matrix lumen continuity was confirmed by using photoactivated GFP (445), supporting the idea of mitochondrial organization in networks (115, 199, 395) which play an important role in intracellular signaling (46, 215, 395). Cooperation of mitochondria in terms of synchronous response to oxidative challenge as part of the RIRR process seems to open a new door for exploring alternative roles of mitochondria in the cell apart from their energetic function (491, 493).
IX. MITOCHONDRIAL RIRR IN OSCILLATING MODE
It is noteworthy that fluctuations of ROS within the cell in the vicinity of mitochondria can elicit instability of the intracellular redox state which, as we pointed out earlier, is greatly maintained by homeostatic mitochondrial functioning. Figure 12 demonstrates ROS-induced fluctuations of the mitochondrial Δψ (one recorded in adult and another in neonatal cardiac myocyte) showing that mPTP in these cells can be reversed and the mitochondrial inner membrane be sealed and Δψ regained with many repetitive cycles of the mPTP induction and closure. The first demonstration in intact cells of such an oscillatory mode of the mPTP triggered by photodynamically induced ROS accompanied by the RIRR was given in 2000 (491) and further explored in 2006 (493).
FIGURE 12.
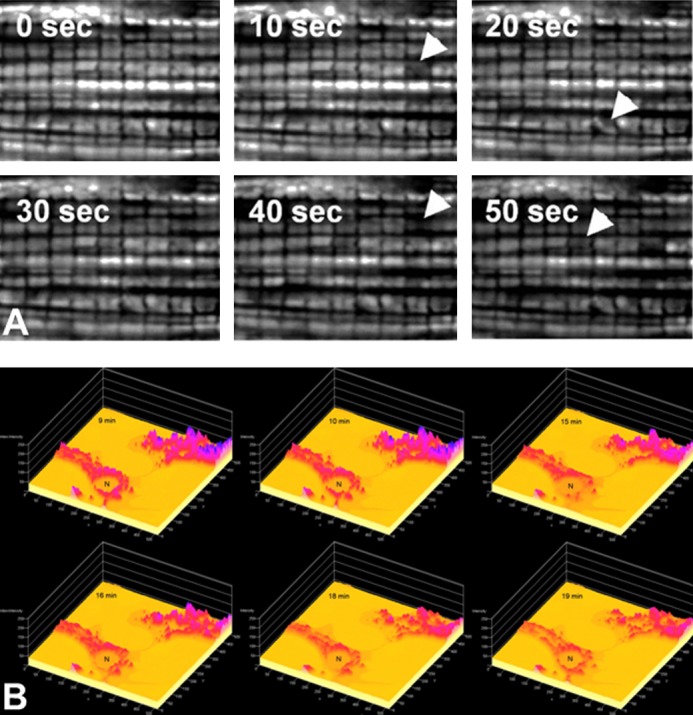
Oscillations of Δψ in individual mitochondria over time in a portion of a cardiac myocyte induced by 10 μM clotrimazole. Panels of consecutive fluorescence frames with a different interval indicated in each plane 10 s acquired from either a 18 × 15 μm region of an adult cardiac myocyte [A; from Zorov et al. (493), with permission from Elsevier.] or neonatal cardiac myocytes loaded with TMRM (B). Arrows indicate individual mitochondria or mitochondrial clusters displaying periodic changes in the membrane potential.
The regulation of mitochondrial ROS production and their levels is exerted by a number of factors, such as the redox state of respiratory components, oxygen tension, ionic environment and the activity of redox buffers, etc. However, the mitochondrial environment may become unstable, which may cause the instability of mitochondrial functioning, and potential episodes of fluctuating mitochondrial ROS production are not an exception. Several researchers described synchronized mitochondrial oscillations including rhythmic changes of ion fluxes, respiration, and mitochondrial volume in vitro in suspensions of isolated mitochondria (particularly, under conditions of energized ion transport) (23, 83, 158, 168, 198, 312, 330). Typically, these oscillations had the form of damped sinusoidal rhythmic changes. Under these conditions, within each cycle, the mitochondrial shrinkage phase was associated with increased respiration, a more highly oxidized state of pyridine nucleotides, a stimulation of ATP hydrolysis, an inhibition of proton release, and stimulation of cation release. The shrinkage phase is followed by a swelling phase, and when these changes were reversed, it also showed evidence of damping. It was concluded that the oscillatory states of electron and energy transfer pathways might be under the control of mitochondrial swelling and ion transport by a feedback mechanism.
Later, such oscillatory behavior of different mitochondrial parameters including those induced by ROS has been detected in intact cells and tissues (18, 92, 126, 281, 297, 360, 491, 493) showing that structural peculiarities in organization of mitochondria within the cell do not play the most important role in induction and propagation of oscillations.
X. IMAC-ASSOCIATED RIRR
Another model of RIRR was generated and explored in B. O'Rourke's lab at Johns Hopkins University. The study was preceded by an investigation of oscillations in the sarcolemmal current in cardiac myocytes caused by substrate deprivation resulting in spontaneous fluctuations of ATP-sensitive K+ current in parallel with changes of the redox state of NADH/NAD (324). This implies the critical role of energy in generating oscillations. Since metabolic oscillations were in concert with the shortening and suppression of the action potential, it has been concluded that these metabolic oscillations may be relevant to pathologies such as arrhythmias as a result of ischemia/reperfusion insult (reviewed in Refs. 20, 323). Later, it was found that a local two-photon excitation of cardiac myocyte covering a few mitochondria loaded with a mitochondrial membrane potential-sensitive fluorescent dye triggered oscillation of the membrane potential within these mitochondria. It also triggered the oscillations in intramitochondrial redox potential measured by a ratio of reduced-to-oxidized flavins. The igniting impulse was triggered by ROS generated by the local excitation resulting in production of primary ROS. The oscillations spread in three dimensions along all interconnected mitochondria forming a lattice with the primary oscillators consisting of only a few mitochondria. These could later spread over the entire mitochondrial network and possibly extend beyond the boundaries of the single cell.
Mitochondrial inhibitors such as rotenone and bongkrekic acid suppressed mPTP-associated RIRR and ROS to the level below the threshold implicating the source of ROS as mitochondrial (491). However, these inhibitors as well as others including cyclosporine A, cyanide, myxothiazol, nigericin, and oligomycin did not block RIRR associated with transient mitochondrial hyperpolarizations (mitochondrial membrane potential flickers described above) (Figure 9) (493). In contrast to mPTP-associated RIRR, in the RIRR mode associated with hyperpolarization flickers, the brief changes of the membrane potential were not accompanied by significant redistribution of an inert fluorescent agent of 620 Da (calcein) between mitochondria and cytosol. Therefore, the mPTP is either too transient for sufficient movement of calcein to be measured, or of low conductance, or possibly even not involved in this type of oscillatory mode. In a comprehensive search for ion channels responsible for the various types of non-mPTP-related mitochondrial oscillations, the focus moved to the so-called IMAC because the oscillations were blocked by the IMAC inhibitor DIDS (38–40). In addition, these oscillations were significantly attenuated by ligands of the mitochondrial (peripheral) benzodiazepine receptor (PBR) such as Ro5–4864 and PK11195 (18, 323). It has been speculated that PBR (which presumably consists of ANT, VDAC, and 18-kDa protein of the outer mitochondrial membrane, TSPO) could be responsible for IMAC (20) since some ligands of PBR can block the mitochondrial inner membrane 107-pS channel (230) which has moderate anion selectivity (410). However, PBR ligands (230) as well as the mPTP inhibitor cyclosporine A (430) both block mitochondrial giant MCC (multiple conductance channel), implying that mPTP might be related to the functional state of PBR. However, this speculation needs additional experimental support with elucidation of the molecular identity of IMAC.
Thus, in addition to mPTP-associated RIRR, another independent mode was proposed for RIRR which involves IMAC. According to this model, mitochondrial respiratory chain is the main oscillatory source of ROS which can be released to the cytosol through IMAC due to its permeability for superoxide (449) produced in complex III (Figure 13).
FIGURE 13.
Hypothetical scheme of the IMAC-associated ROS-induced ROS release mechanism underlying the synchronization of mitochondrial activity during the oscillations. [Modified from Aon et al. (18), with permission from The American Society for Biochemistry and Molecular Biology.]
Recently, the Δψ oscillations and RIRR induced by local oxidative stress (401) and by perfusion with hydrogen peroxide (53) have been detected in live, intact myocardium. Perfusion of rat hearts with hydrogen peroxide, depending on the concentration of the oxidant, elicited two peaks of superoxide in live myocardium measured by optical mapping of the fluorescent ROS probe, with the second peak being much more intense than the first one (53). Spatiotemporal ROS mapping during the second ROS peak revealed a propagation of superoxide signal within the cardiac tissue with a velocity ∼20 μm/s. The hearts with the second peak displayed a much greater arrhythmia compared with those where the second peak was absent. PBR ligand, Ro5–4864, and superoxide dismutase mimetic but not cyclosporine A abolished RIRR and ventricular tachycardia and ventricular fibrillation, suggesting IMAC involvement. These findings further extended the concept of RIRR as a key factor in the incidence of postischemic arrhythmias.
A computational model with superoxide as a trigger of mitochondrial Δψ depolarization has been developed (484, 486). The model is based on the percolation theory to explain how neighbor-to-neighbor interaction defines propagation of the signals along the spatially organized excitable matrix, arising from a spanning cluster of oxidatively stressed mitochondria united in the network (19, 20). In addition, the dynamic spatiotemporal properties of individual mitochondrial oscillators within stress-induced synchronized oscillatory clusters of cardiac mitochondrial network have been characterized by applying wavelet-based analysis (252, 253). Individual oscillating mitochondria within clusters have been identified and their frequency correlated with the size of the cluster. As the cluster grew larger, they required more time to achieve full synchronization of all mitochondria within the cluster. Additionally, the percentage of cluster size has been inversely correlated with the percentage of amplitude. Nonlinear characteristics of the mitochondrial network, particularly in the heart, make mitochondria prone to the local disturbances including those involving oxidative stress. Once these local disturbances are formed, they spread over the network causing loss of its normal functioning. They cause a mismatch of the finely tuned balance of available energy production to energy expenditures (93, 365, 435, 479), resulting in an inability of mitochondrial network to completely repolarize between oscillations. Finally, it reaches a point of no return that results in pathologies and ultimately cell death.
XI. RIRR-RELATED PATHOLOGIES: THE IMPORTANCE OF Δψ HOMEOSTASIS
Mitochondria are not only the energy source but also one of the primary sources of a vast number of deadly pathologies. As to cardiovascular problems, a striking example of “misbehavior” of a mitochondrial network extending over the whole heart and affecting the whole organism is ischemia/reperfusion-induced oxidative injury which in extreme cases results not only in cell death, but also organ failure, and eventually organismal death. Experiments with single cardiac myocytes exposed to transient hypoxia, followed by a reoxygenation phase, demonstrated substantial mitochondrial instability caused by the introduction of oxygen into the system. In these cells, transient mitochondrial hyperpolarization was followed by a progressive rise of the ROS level in deteriorating mitochondria to the point of mPTP introduction and accompanying large ROS burst (Figure 14A), i.e., typical of RIRR. The incidence of cardiac cell death depended on the proportion of damaged mitochondria having developed mPTP to their total number in the cell and on the physiological status of the cell (218) (Figure 14B).
FIGURE 14.
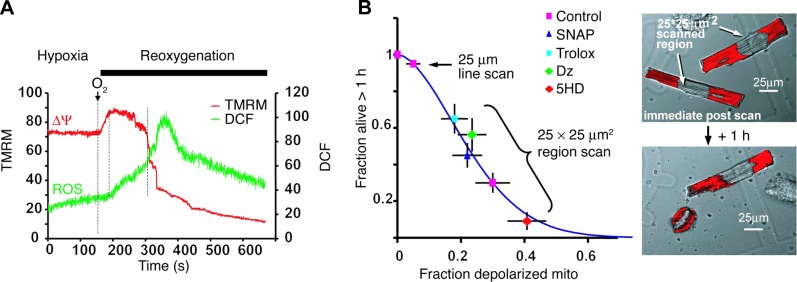
ROS are involved in mitochondria and cell deterioration. A: hypoxia/reoxygenation-induced mitochondrial hyperpolarization leads to increased ROS. Mitochondria stained with TMRM (Δψ, red) and DCF (ROS, green) were laser line-scanned (2 Hz) during hypoxia and the reoxygenation phase. The ROS burst is delayed after reoxygenation and starts at the maximum Δψ. Mitochondrial hyperpolarization lasts for ∼2 min, followed by loss of Δψ. B: cell survival after constant-energy photoexcitation of a 25 × 25 μm2 region. Right panels show TMRM-stained cardiomyocyte (red) immediately after, and 1 h after, irradiation. Survival is inversely related to the fraction of mitochondria (mito) undergoing mPTP induction and is improved by ROS scavenger (Trolox), NO donor (SNAP), and the KATP channel opener diazoxide (Dz) and is impaired by 5-hydroxydecanoate (5HD), the blocker of KATP channel. [From Juhaszova et al. (218). Republished with permission from the American Society for Clinical Investigation; permission conveyed through Copyright Clearance Center, Inc.]
To assess the degree of system readiness to resist an oxidative challenge, a simple approach was developed using time as a factor. Time quantifies the titrated amount of ROS (delivered incrementally by successive photoactivated exposures during linescan imaging) required to achieve the threshold for mPTP induction in a particular mitochondrion (218, 491). Apparently, mitochondria with higher resistance to oxidative stress require a longer time (i.e., higher total oxidant exposure level) to open the pore while those with a low tolerance require shorter time (i.e., lower total oxidant exposure level) for the mPTP induction. The timing of mPTP opening (tmPTP) is an integral factor, depending on the rate of oxidant quenching (the level of intra- and extramitochondrial antioxidants, e.g., glutathione) and the physicochemical state of the system responsible for the generation of the mPTP as an event. While the molecular identity of the mPTP needs to be validated in detail (157, 217, 494), the presence of bcl-2/bcl-xL or phosphorylated glycogen synthase kinase 3β (P-GSK-3β) in the vicinity of the pore or attachment of hexokinase II desensitize the pore to oxidants (86, 217, 218) and extend the time necessary to overcome the desensitization and open the pore. The conventional line-scan confocal microscopy has been employed as a model to determine the ROS threshold for inducing the mPTP. For this purpose, an ideal experimental object is a cardiac myocyte with mitochondria arranged in regular latticelike arrays rendering the line-scan instrumentation straightforward (491, 493, 496) (Figure 15A). Furthermore, the same approach has been successfully applied to neuronal mitochondria in situ and cultured neonatal cardiac myocytes (217) and potentially could be applied to mitochondria in other cell types with cell motility or intracellular mitochondrial motion during the line-can procedure being carefully considered.
FIGURE 15.
Linescan technique applied to mitochondria in rat cardiac myocytes. A: definition of the mPTP ROS threshold as an average time over population of mitochondria (shown by a dashed red line) in myocyte stained with fluorescent mitochondrial probe for the membrane potential to induce the mPTP (shown by arrow) (491, 493). B: dependence of the mPTP induction in the cell on ambient molecular oxygen.
It is not surprising that the mPTP threshold is highly dependent on ambient molecular oxygen, since ROS production in the system is a reaction of the first order with respect to molecular oxygen. In cardiac myocytes incubated in media with low O2, tmPTP was much greater than in mitochondria incubated in normoxic or hyperoxic media (Figure 15B). Consequently, adequate oxygenation of cellular mitochondria is important for unambiguous interpretation of tmPTP.
The value of tmPTP determined by the above-mentioned approach, the inherent resistance of mitochondria to mPTP induction by ROS, and can be significantly prolonged by ischemic or pharmacological preconditioning. This approach has been applied for the detailed elucidation of the architecture of the signaling pathways engaged by ischemic/pharmacological preconditioning (218, 385).
The term mitochondrial criticality was coined (16, 17) for the situation when the mitochondrial network in the cardiac myocyte becomes highly sensitive to the changes of ambient/intramitochondrial ROS, for example, by responding as synchronized oscillatory depolarizations of the mitochondrial Δψ which could be scaled to the whole cell or cluster of myocytes (401). When the mitochondrial system reaches the point of criticality, oscillations of Δψ are ignited, eventually creating spatial and temporal heterogeneity of excitability in a heart exposed to oxidative challenge, e.g., under hypoxia/reoxygenation. This inflicts dramatic pathological changes to the heart involving lethal cardiac arrhythmias possibly followed by cardiac arrest and an individual's death.
Based on the discussion above, we would tentatively suggest the existence of specific in vivo mitochondrial states that may contribute to the fate of the cell (see Figure 16). The relatively stable state (state A) has a comparatively high tolerance to oxidative stress and is able to sustain the Δψ for a longer time (with longer tmPTP). This is characteristic of the healthy cell where the mPTP formation is delayed. States B and C characterize mitochondria which have been stressed (prior to experimental procedure of the mPTP-induction protocol has been employed) and thus are unable to sustain the ROS challenge and to maintain their Δψ for an extended period. In both cases (B and C), the first mean tmPTP value is similar, characterized by a shorter tmPTP compared with mitochondria in state A, but there are some obvious and significant differences between the states B and C. While in state B mitochondria remain depolarized after mPTP induction, mitochondria in state C are characterized by dynamic instability revealed by Δψ oscillations which progressively deteriorate and ultimately cease. These differences probably reflect not only differences in the state of the antioxidant defense system in B versus C but also intrinsic differences in the function/activities of the pore complex subunits itself triggered in response to posttranslational modifications, the signaling status of the immediate pore modulators, and/or changes in the pore complex arrangement at the protein level (Figure 16).
FIGURE 16.
Basic mitochondrial redox states (see explanation in the text). A: unstressed mitochondria with high tolerance to ROS (high survival). B: stressed mitochondria with low tolerance to ROS (low survival). C: stressed mitochondria with high tolerance to ROS (unknown survival).
A preponderance of data point to the fact that mitochondrial membrane potential is an essential attribute of mitochondria, and its homeostasis is a prerequisite for the health of mitochondria and the preservation of normal cell and tissue function. Under hypoxic conditions, when mitochondria are incapable of sustaining ΔΨ driven by respiration, the mitochondrial membrane potential is maintained at the expense of cytosolic ATP hydrolysis by mitochondrial ATPase (110). Sufficient buffering of the mitochondrial Δψ by cytosolic ATP may explain why the intracellular concentration of ATP (a few mM) exceeds the Km for intracellular ATP-utilizing enzymes by orders of magnitude. Thus, under physiological conditions, these enzymes are fully saturated and do not depend on ATP concentration for their function.
The homeostasis of mitochondrial Δψ is critical for the mitochondrial function. Only small vital variations in a range (approximately between 160 and 220 mV) were tolerated in isolated mitochondria at states 3 and 4 according to the terminology of B. Chance (82). These small variations preserve the minimal energy that may be sufficient for phosphorylation of ADP by mitochondrial ATP synthase but may dramatically influence ROS production within mitochondria (see Figure 5). In case of a drop of the membrane potential below the phosphorylating membrane potential, especially when it is nulled after induction of mPTP, mitochondria could initiate a process of self-destruction involving Ca2+-independent degrading hydrolases and phospholipases (66, 261, 495, 496). This process is called mitophagy (reviewed in Refs. 88, 234, 258) and further substantiates the vital importance of maintaining Δψ homeostasis to keep mitochondria structurally and functionally intact. The questions regarding a mitochondrial Δψ sensor, or which processes in mitochondria could be considered as vital, the cessation of which will result in mitochondrial degradation, remain unknown. One possible candidate for such a process is the energy (Δψ)-dependent transport of peptides into mitochondria (reviewed in Ref. 338). This machinery is highly important for the quality control of mitochondria (362) through the Δψ-dependent ubiquitin labeling of the impaired mitochondrion resulting in the initiation of mitophagy (see explanations in the Figure 17; Ref. 210).
FIGURE 17.
Tentative model of regulation of the mitochondrial quality control by Δψ. Parkin is an E3 ubiquitin ligase located in the cytosol, while PINK1 is a mitochondrial kinase carrying mitochondrial import sequence to be accumulated inside of mitochondria followed by degradation by PARL. Δψ drives PINK1 into mitochondria where it is processed by mitochondrial processing protease (MMP). When mitochondrial membrane potential is dissipated, PINK1 accumulates on the outer mitochondrial membrane, where it can recruit Parkin to impaired mitochondria followed by its ubiquitination as a starting point for mitophagy. OM, outer membrane; IM, inner membrane. TOM and TIM are transport outer and inner membrane proteins correspondingly. (From Ref. 210: Copyright 2010 Jin et al. Originally published in Journal of Cell Biology. 191: 933–942. doi:10.1083/jcb.201008084.)
XII. PRACTICAL ASPECTS OF USING MITOCHONDRIAL Δψ AS A DRIVER AND REPORTER
The mitochondrial membrane potential drives natural cationic substances into mitochondria, and this property could be utilized for intramitochondrial transport of man-made drugs.
In principle, the delivery of drugs to the mitochondrial interior can be executed by using two targeting vehicles: 1) mitochondrial targeting signal sequences and 2) mitochondriaphillic compounds. While the first approach implies specific transport of the vehicle through the TIM/TOM mitochondrial protein transport system (318), the second uses positively charged lipophilic compounds, for example, constructed in a way to delocalize the charge over the set of coupled double bonds (268). Due to negative charge of the mitochondrial interior, the latter nonspecifically accumulates these cations to the thermodynamically permissive level which theoretically exceeds extramitochondrial levels by about three orders of magnitude. The idea of driving such charged compounds by virtue of the electrogenic nature of mitochondrial energetics is fully consistent with Mitchell's chemiosmotic theory (268, 298, 299). Nowadays, after establishment of this mechanism (1), the approach started to serve two experimental missions: to deliver to the mitochondrial interior a desired compound which is bound to a mitochondriatropic vehicle (225, 393, 407) and, on the other hand, to discriminate between the mitochondrial and nonmitochondrial nature of some process under study. In this context, mitochondriatropic antioxidants are not only potentially beneficially targeted drugs to quench unwanted excessive ROS and other reactive species in mitochondria, but they may also serve as excellent reporters providing an answer as to whether a given mechanism involves mitochondrial ROS. In the first case, in vitro data and animal experiments have suggested the possible beneficial effect of mitochondria-targeted antioxidants (such as MitoQ, SkQ1, SkQR1, and others) in treatment of a great number of model systems associated with oxidative stress. These include stroke, arrhythmias, ischemia/reoxygenation, chemical toxicity, infection, inflammation, and some inherited and acquired age-related and unrelated diseases (such as Alzheimer's, diabetes, hepatitis, metabolic syndrome, hearing loss, etc.) (14, 27, 146, 206, 223, 236, 269, 292, 307, 343, 388, 391, 399, 404–406, 408).
Apart from the practical issue of using mitochondria-targeted chimeric compounds as potential therapeutic agents, their specific effects uncovered the fundamental role of mitochondria and mitochondrial ROS in the onset and propagation of different pathologies. What we have learned is that ROS originating from mitochondrial rather than from other intracellular sources, when they exceed the homeostatic level, are among the most pathogenic factors (222, 382, 383, 437, 467). In this context, targeted normalization of ROS levels (specifically in mitochondria) may be beneficial in preventing a number of ROS-related pathologies including that involving RIRR (310, 392). The requirement of mitochondria-derived ROS for the propagation of RIRR together with its concomitant pathological cell death has received support from experiments wherein 1) the primary extramitochondrial ROS source induced a secondary intramitochondrial ROS release, and 2) both of these processes were prevented by specific mitochondria-targeted antioxidants (344).
XIII. CONCLUDING COMMENTS
ROS-induced ROS release is a fundamental process originating in mitochondria (18, 491, 493) responding to an increased oxidative stress by a positive feedback loop resulting in a regenerative, autocatalytic cascade which in a vast majority of cases is terminated, presumably by the local redox environment together with a change of functional properties of the “release valve.” Indeed, for this mechanism to serve a physiological role, its underlying properties of chain-reaction-like propagation must be efficiently terminated to prevent the unwanted spread and widespread destruction of mitochondria risking unintended and unwanted loss of the cell. These considerations are entirely analogous to the control of a nuclear chain reaction in power plants (e.g., by control rods) or that of CICR in excitable cells by intrinsic ryanodine receptor- and SR-related mechanisms (423). When RIRR is appropriately not terminated, it may lead to the wanted removal of a mitochondrion or cell, e.g., damaged beyond repair. When RIRR is inappropriately not terminated, it may lead to unwanted cell loss such as after stroke or myocardial infarction.
We propose that RIRR may result in a cellular response that can be considered either adaptive (e.g., promoting signaling resulting in the elevation of the tolerance to oxidative stress or the removal of unwanted organelles and cells) or maladaptive (e.g., the pathological destruction and death of functional mitochondria and vital, essential cells). Adaptive cell elimination may involve cell death executed by necrotic or apoptotic mechanisms (for example, elimination of nonfunctional or damaged cells). It remains an open question whether RIRR mechanisms would be recruited to serve the adaptive elimination of cells necessary to achieve proper organ size, shape and functions related to embryogenesis, self-antigen-recognizing immune cells that would be dangerous, cells no longer needed (e.g., nonlactating breast involution after pregnancy), activated lymphocytes after infection is resolved, or pre-cancer and cancer cells.
The RIRR phenomenon provides an opportunity to see the mitochondrion as machinery designed not only to trigger but also to facilitate important signaling mechanisms, further extending the concept of the mitochondrion as an excitable organelle capable of generating and conveying redox, electrical (10), and Ca2+ (199) signals. Therefore, mitochondria are not only efficient cellular energy powerhouses, they are significant signal transmitters and signal resonators, by which we mean the mitochondrial ability to respond to and to enhance, internal and external signals, in a manner analogous to the propagation, and Ca2+-activated reaction triggering of CICR (125, 127, 128, 139).
We propose that the mPTP may be involved in at least four conceptual modes of pathophysiological function. The first role is to serve as a potential release valve which transiently opens a gate for controlled release of accumulated ions or newly formed low-molecular-weight toxic substances including elevated ROS and Ca2+. This likely maintains mitochondrial (and cellular) redox homeostasis, which is a critical point in normal cell functioning. The characteristics of this mode include brief and reversible episodes of the mPTP pore opening or activation of some other mitochondrial pores/channels/leaks constituting a low-conductance state of the mPTP which have been demonstrated in in vitro systems (5, 29, 244, 255). We conceive that this serves a housekeeping function, and at low energy demand (rest), it is likely relatively inactive. At high stress/energy demand conditions, this function plays a significant role (123). This is borne out by the study (124) whereby the genetic loss of CyPD (Ppif−/−) and acute cyclosporine A treatment (presumably causing mPTP-desensitization to ROS-induction) result in elevated resting levels of Ca2+ in the mitochondrial matrix of cardiomyocytes and an increase in the time of decay to baseline following continuous pacing. Also, Ppif−/− mice display increased mortality and heart failure in pathological and physiological models of hypertrophy (124) presumably due to an inappropriate high “set-point” for the physiological activation of transient mPTP-related RIRR events leading to the inappropriate mitochondrial accumulation of toxic levels of Ca2+ and ROS.
The second mode may also include mPTP openings that prevent the accumulation of ROS and Ca2+ (etc.) inside mitochondria that would cause damage to that organelle if not released into the cytosol where a large buffer pool might better deal with that stress factor. In this scenario, mPTP openings, although much more frequent, are still brief enough not to cause irreversible mitochondrial or cellular changes. These brief episodes of mPTP [or some other channel(s)] opening may coincide with the flickering mode of the mitochondrial membrane potential which when integrated may cause a small albeit significant rise of ROS in the immediate vicinity of mitochondria, thus activating local pools of redox-sensitive enzymes, such as protein kinase C (218). In this mode, for example, the levels of released ROS are enough to precondition the cell to upcoming more drastic oxidative challenge (e.g., long-lasting ischemic insult; Refs. 180, 218, 335). The potential protective significance of the second mode of the mPTP is to adaptively desensitize the mPTP pore complex and to delay the transition to the third and fourth destructive modes of mPTP associated with much higher levels of oxidative stress.
The third mode constitutes a persistent, generally irreversible mPTP-associated large conductance of the inner mitochondrial membrane sufficient to activate the mechanisms of destruction of mitochondria destined for elimination (for example, by mitophagy, because they do not meet the necessary mitochondrial “quality control” standards). Apparently, the existence of impaired mitochondria could be a threat for the proper functioning of the whole mitochondrial population (because they are connected in a functional network), and opening of the mPTP may be interpreted as the switch leading to programmed mitochondrial elimination (mitophagy) (66, 76, 88, 210, 234, 258, 261, 446, 495, 496). The signal initiating the process of mitochondrial execution may arise from the consistent and long-lasting drop of the mitochondrial membrane potential (210) (Figure 17), which may reinforce the transition to irreversible mPTP opening (i.e., the point of no return) (88, 188). The timing to reach this point is controlled in part by the depletion of the intramitochondrial redox buffer caused by the flux of external (or internally produced) oxidants (491). This point may correspond to the term mitochondrial criticality (17) by reaching a redox crisis in an oxidatively stressed cell after which the behavior of mitochondrial energetics becomes unstable. By this principle, criticality determines ROS-induced organization of an excitable mitochondrial cluster spreading oscillations of Δψ over the cell.
The fourth mode of the mPTP is the terminal destructive step resulting in the elimination of the cell (which could be wanted or unwanted). As for mitochondria, impaired cells can be programmed for the destruction with the mPTP playing a critical role in that process. In general, this mechanism of cell elimination would encompass a large fraction of mitochondria, undergoing the process described above, involving significant releases of ROS from mitochondria undergoing mPTP (218).
Schematically, Figure 18 represents a hierarchical model of the proposed pathophysiological roles and functions of the mPTP. According to this model, the stable state with the closed mPTP can undergo a transition to a metastable (transient) state of the mPTP. By analogy to an energy barrier (threshold) necessary to overcome in order to undergo a physico-chemical transition, the reaction probability and rate is determined by a redox threshold (process I) that normally is lower than the threshold of process II determining the transition to the fully open, stable mPTP. Such a metastable state of the pore (preceding its potential transition to a long open state) occurs as a result of intra- and extramitochondrial changes of the redox buffer still compromising the dynamic balance between the influx/production of oxidants and antioxidants, and the stability of the “gating architecture” of the mPTP complex. As discussed above, the RIRR-mPTP process may serve to provide an adaptive release of excess Ca2+ and ROS from mitochondria. The latter may be responsible for redox-activated protective signaling. The stable-open mPTP state is a result of the redox transition to the point of no return (long-lasting mPTP-opening) (188) leading to the destruction of mitochondria (210), and when involving a sufficient fraction of the entire mitochondrial population, it results in the death of the cell. The height of both ROS thresholds (Figure 18) is under redox control as well as the sensitivity of the mPTP-gating mechanism (which is regulated by certain enzymatic posttranslational modifications), both of which may be targets for pharmacological intervention. It has been shown that the height of the second threshold is age dependent, being higher in mitochondria of young, and lower in mitochondria of old animals (216, 257, 487). Various mPTP-related cytoprotective signaling cascades (e.g., through preconditioning, activation of PKC, receptor triggers including adenosine, opioid and β-receptors, mitochondrial ATP-dependent potassium channels, inhibition of glycogen synthase kinase-3β, and other effectors; described in Ref. 218) as well as more enhanced redox buffering (491), high deacetylase activity, specifically resulting in deacetylation of CyPD (387), attachment of hexokinase II to the mPTP complex (86, 333) restore or even drive this threshold up, but this protective signaling also fails with aging (reviewed in Ref. 216). Therefore, it is possible that the beneficial “anti-aging” effect of increased deacetylation (191) (particularly by mitochondrial SIRT3–5; Refs. 43, 160, 429) of critical mPTP-regulatory elements such as CyPD (172) may partially be explained by a relative desensitization of the mPTP complex to ROS induction which could restore the age-dependent decline of mPTP ROS threshold (i.e., “aged” state) (257) back towards to the basal level (“young” state).
FIGURE 18.
Two-ROS thresholds scheme for the induction of the mPTP. Resting state (shown by a pink ball) is a state with a stable closed mPTP. After depletion of a redox buffer, the pore may undergo a transition to a metastable flickering state (I) (shown by yellow color). The flickering mode of the pore is accompanied by a small release of ROS from mitochondria, and when low it serves a physiological housekeeping purposes as a release valve and when higher it is enough to precondition the system for better output after the following, more extensive oxidative challenge (180). If the oxidative stress is severe, the phase I is changed by a state II (shown by red) characterized by a stable open mPTP accompanied by a ROS burst. The pathological ROS burst results in a programmed elimination of unwanted organelles or cells. The critical height of the ROS threshold is determined by a balance between ROS production and quenching within mitochondria. It can be raised by protective signaling [through preconditioning (218), cyclosporin A (180, 218), sanglofehrin (89), acidosis (47), cyclophilin D acetylation or its knockout (26, 34, 313, 387)] shown by a green dotted line. Low redox buffering (491), low SIRT3 activity (387), hexokinase II detachment from mitochondria (86), as well as high age (216, 487) drive the threshold II down (shown by a brown dotted line). Note that one of the most important elements of the scheme is that the long occupancy in state I hardens the onset of the state II.
There is new information concerning the identification of ATP synthase complex as the core structure necessary to form the mPTP that is certain to be relevant to the RIRR mechanism. The important role of mitochondrial ATP synthase complex (complex V) in the mPTP functioning has been proposed in a number of studies over more than two decades (56, 87, 197, 322), in part based on the fact that oligomycin prevents mPTP in isolated mitochondria (321). However, this fact was explained by a probable indirect effect of changed levels of adenine nucleotides rather than by a direct effect on the pore structure itself. Recent evidence indicates that a dimer of complex V is necessary and sufficient to form the mitochondrial pore with the apparent modulating role of CyPD (157). It is tempting to speculate how this could potentially confer the gating mechanism of the mPTP and explain bimodal behavior of ATP synthase in terms of induction of the mPTP. Such bimodality (opened/closed pore) may correlate with a bimodality of ATP synthase (dimer/monomer and higher order oligomers). Quick and sometimes reversible transitions from closed to open state of the pore (mPTP oscillations or flickers) may be speculated to stem from a bimodal output of CyPD enzymatic activity which is a rotamase (peptidyl prolyl cis-trans isomerase; Refs. 135, 431) determining two modes of conformation of proline residues in the targeted protein(s) (134) (e.g., such as ATP synthase complex dimer), forming the mitochondrial pore. Additionally, this may also explain why CyPD acetylation critically appears to be affecting this process, for example, during aging (387).
In addition to the mPTP-associated RIRR, the important contribution of the IMAC-associated mode of RIRR functioning (20, 323), especially in the mitochondrial network propagation properties, and its possible role in arrhythmogenesis, needs to be established. Resolving the molecular identity of the IMAC will enable us to better appreciate its role in physiology and pathology. It seems that IMAC-associated redox instability during pathological stress is quite different from a safe, physiological flickering mode of the mPTP, and it is possible that IMAC-related mechanisms may contribute to a transitional state between mPTP-associated signaling and pathological modes of RIRR.
In conclusion, mPTP and IMAC are the main known players in the mechanism of RIRR which is a multifaceted process important in the physiology and pathology of the cell. The mitochondrial role in this mechanism is above and beyond its well-recognized energy-producing activity, but includes signaling potentially regulating both positive and negative destructive roles. Mitochondrially produced ROS are elements that may activate/deactivate a diverse set of signals igniting as well as terminating signaling cascades. Apparently, the “fine-tuning” regulation of ROS levels in mitochondria is an essential function of RIRR, and mitochondria are important players in the propagation of ROS signals within a cell. Hypoxia/ischemia raises basal production of ROS which may activate the mPTP and/or IMAC and, if not appropriately terminated, is involved in the conversion of signaling to pathological ROS. The primary ROS signal to ignite RIRR may originate in as well as outside of mitochondria and may in turn be amplified by the RIRR mechanism. The ROS amplification site may coincide with one or more already known mitochondrial sites of ROS production. The full understanding of the RIRR mechanism will require a more comprehensive and detailed mechanistic knowledge of the ROS-producing sites operating in situ and in vivo. It is certain that at least some of the mechanisms of mitochondrial ROS production presented in this review are relevant to both physiological and pathological ROS production in the cell.
GRANTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, Russian Foundation of Basic Research (14-04-00542) and Russian Science Foundation (14-15-00147, 14-24-00107). The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
D. B. Zorov and S. J. Sollott contributed equally to this paper.
Address for reprint requests and other correspondence: D. B. Zorov, A. N. Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, Russia 119992 (e-mail: zorov@genebee.msu.su).
REFERENCES
- 1.A man driven by proticity: Peter Mitchell Nobel prize for Chemistry 1978. Nature 276: 8–9, 1978 [PubMed] [Google Scholar]
- 2.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27: 1129–1138, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci 27: 639–645, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging 2: 1012–1016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Nasser I, Crompton M. The reversible Ca2+-induced permeabilization of rat liver mitochondria. Biochem J 239: 19–29, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51: 1271–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol 262: H1699–H1704, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Alvarez S, Valdez LB, Zaobornyj T, Boveris A. Oxygen dependence of mitochondrial nitric oxide synthase activity. Biochem Biophys Res Commun 305: 771–775, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993 [PubMed] [Google Scholar]
- 10.Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB. Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J Cell Biol 107: 481–495, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RE, Meyer FB. In vivo fluorescent imaging of NADH redox state in brain. Methods Enzymol 352: 482–494, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Andreyev A, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, Tsofina LM, Volkov NI, Vygodina TV. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur J Biochem 182: 585–592, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry 70: 200–214, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Anisimov VN, Egorov MV, Krasilshchikova MS, Lyamzaev KG, Manskikh VN, Moshkin MP, Novikov EA, Popovich IG, Rogovin KA, Shabalina IG, Shekarova ON, Skulachev MV, Titova TV, Vygodin VA, Vyssokikh MY, Yurova MN, Zabezhinsky MA, Skulachev VP. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging 3: 1110–1119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonenko YN, Avetisyan AV, Cherepanov DA, Knorre DA, Korshunova GA, Markova OV, Ojovan SM, Perevoshchikova IV, Pustovidko AV, Rokitskaya TI, Severina II, Simonyan RA, Smirnova EA, Sobko AA, Sumbatyan NV, Severin FF, Skulachev VP. Derivatives of rhodamine 19 as mild mitochondria-targeted cationic uncouplers. J Biol Chem 286: 17831–17840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aon MA, Cortassa S, Akar FG, Brown DA, Zhou L, O'Rourke B. From mitochondrial dynamics to arrhythmias. Int J Biochem Cell Biol 41: 1940–1948, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aon MA, Cortassa S, Akar FG, O'Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta 1762: 232–240, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Aon MA, Cortassa S, O'Rourke B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys J 91: 4317–4327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aon MA, Cortassa S, O'Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol 641: 98–117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol 139: 479–491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appaix F, Kuznetsov AV, Usson Y, Kay L, Andrienko T, Olivares J, Kaambre T, Sikk P, Margreiter R, Saks V. Possible role of cytoskeleton in intracellular arrangement and regulation of mitochondria. Exp Physiol 88: 175–190, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Azzi A, Azzone GF. Swelling and shrinkage phenomena in liver mitochondria. II. Low amplitude swelling-shrinkage cycles. Biochim Biophys Acta 105: 265–278, 1965 [DOI] [PubMed] [Google Scholar]
- 24.Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 25.Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells 32: 491–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Bakeeva LE, Barskov IV, Egorov MV, Isaev NK, Kapelko VI, Kazachenko AV, Kirpatovsky VI, Kozlovsky SV, Lakomkin VL, Levina SB, Pisarenko OI, Plotnikov EY, Saprunova VB, Serebryakova LI, Skulachev MV, Stelmashook EV, Studneva IM, Tskitishvili OV, Vasilyeva AK, Victorov IV, Zorov DB, Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochemistry 73: 1288–1299, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 501: 349–369, 1978 [DOI] [PubMed] [Google Scholar]
- 29.Balakirev MY, Zimmer G. Gradual changes in permeability of inner mitochondrial membrane precede the mitochondrial permeability transition. Arch Biochem Biophys 356: 46–54, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Bambrick LL, Chandrasekaran K, Mehrabian Z, Wright C, Krueger BK, Fiskum G. Cyclosporin A increases mitochondrial calcium uptake capacity in cortical astrocytes but not cerebellar granule neurons. J Bioenerg Biomembr 38: 43–47, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, Rice ME. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J Neurosci 29: 9002–9010, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochim Biophys Acta 1807: 1432–1443, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Barlow CH, Chance B. Ischemic areas in perfused rat hearts: measurement by NADH fluorescence photography. Science 193: 909–910, 1976 [DOI] [PubMed] [Google Scholar]
- 34.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 280: 18558–18561, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem 279: 17197–17204, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baysal BE, Willett-Brozick JE, Lawrence EC, Drovdlic CM, Savul SA, McLeod DR, Yee HA, Brackmann DE, Slattery WH, 3rd, Myers EN, Ferrell RE, Rubinstein WS. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet 39: 178–183, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beavis AD. On the inhibition of the mitochondrial inner membrane anion uniporter by cationic amphiphiles and other drugs. J Biol Chem 264: 1508–1515, 1989 [PubMed] [Google Scholar]
- 39.Beavis AD. Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr 24: 77–90, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Beavis AD, Garlid KD. The mitochondrial inner membrane anion channel. Regulation by divalent cations and protons. J Biol Chem 262: 15085–15093, 1987 [PubMed] [Google Scholar]
- 41.Beckmann JD, Frerman FE. Reaction of electron-transfer flavoprotein with electron-transfer flavoprotein-ubiquinone oxidoreductase. Biochemistry 24: 3922–3925, 1985 [DOI] [PubMed] [Google Scholar]
- 42.Beinert H, Kennedy MC, Stout CD. Aconitase as iron − sulfur protein, enzyme, and iron-regulatory protein. Chem Rev 96: 2335–2374, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, Franceschi C, Passarino G, De Benedictis G. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85: 258–263, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Benzi G, Pastoris O, Dossena M. Relationships between gamma-aminobutyrate and succinate cycles during and after cerebral ischemia. J Neurosci Res 7: 193–201, 1982 [DOI] [PubMed] [Google Scholar]
- 45.Berden JA, Slater EC. The allosteric binding of antimycin to cytochrome b in the mitochondrial membrane. Biochim Biophys Acta 256: 199–215, 1972 [DOI] [PubMed] [Google Scholar]
- 46.Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech 27: 198–219, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgio M. p66Shc-generated oxidative signal promotes fat accumulation. J Biol Chem 283: 34283–34293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry C, La Vecchia C, Nicotera P. Paraquat and Parkinson's disease. Cell Death Differ 17: 1115–1125, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112: 3297–3305, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J 19: 641–643, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Bianchi P, Seguelas MH, Parini A, Cambon C. Activation of pro-apoptotic cascade by dopamine in renal epithelial cells is fully dependent on hydrogen peroxide generation by monoamine oxidases. J Am Soc Nephrol 14: 855–862, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Biary N, Xie C, Kauffman J, Akar FG. Biophysical properties and functional consequences of reactive oxygen species (ROS)-induced ROS release in intact myocardium. J Physiol 589: 5167–5179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 278: 9796–9801, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12: 674–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134: 707–716, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J 128: 617–630, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braissant O. Current concepts in the pathogenesis of urea cycle disorders. Mol Genet Metab 100 Suppl 1: S3–S12, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Brand MD. Electroneutral efflux of Ca2+ from liver mitochondria. Biochem J 225: 413–419, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brand MD. The stoichiometry of the exchange catalysed by the mitochondrial calcium/sodium antiporter. Biochem J 229: 161–166, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brandt U. Energy converting NADH:quinone oxidoreductase (complex I). Annu Rev Biochem 75: 69–92, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest 76: 1713–1719, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brdiczka DG, Zorov DB, Sheu SS. Mitochondrial contact sites: their role in energy metabolism and apoptosis. Biochim Biophys Acta 1762: 148–163, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 264: 7826–7830, 1989 [PubMed] [Google Scholar]
- 66.Broekemeier KM, Iben JR, LeVan EG, Crouser ED, Pfeiffer DR. Pore formation and uncoupling initiate a Ca2+-independent degradation of mitochondrial phospholipids. Biochemistry 41: 7771–7780, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Broekemeier KM, Klocek CK, Pfeiffer DR. Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry 37: 13059–13065, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Brouillet E, Conde F, Beal MF, Hantraye P. Replicating Huntington's disease phenotype in experimental animals. Prog Neurobiol 59: 427–468, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res 75: 283–290, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356: 295–298, 1994 [DOI] [PubMed] [Google Scholar]
- 71.Bunik VI, Sievers C. Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur J Biochem 269: 5004–5015, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 111: 1091–1106, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Cadenas S, Buckingham JA, Samec S, Seydoux J, Din N, Dulloo AG, Brand MD. UCP2 and UCP3 rise in starved rat skeletal muscle but mitochondrial proton conductance is unchanged. FEBS Lett 462: 257–260, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 7: 552–560, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Carafoli E, Tiozzo R, Lugli G, Crovetti F, Kratzing C. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol 6: 361–371, 1974 [DOI] [PubMed] [Google Scholar]
- 76.Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy 6: 462–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chance B. The energy-linked reaction of calcium with mitochondria. J Biol Chem 240: 2729–2748, 1965 [PubMed] [Google Scholar]
- 78.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science 137: 499–508, 1962 [DOI] [PubMed] [Google Scholar]
- 79.Chance B, Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J Biol Chem 236: 1534–1543, 1961 [PubMed] [Google Scholar]
- 80.Chance B, Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. IV. The pathway of electron transfer. J Biol Chem 236: 1562–1568, 1961 [PubMed] [Google Scholar]
- 81.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979 [DOI] [PubMed] [Google Scholar]
- 82.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 217: 409–427, 1955 [PubMed] [Google Scholar]
- 83.Chance B, Yoshioka T. Sustained oscillations of ionic constituents of mitochondria. Arch Biochem Biophys 117: 451–465, 1966 [DOI] [PubMed] [Google Scholar]
- 84.Chaudhari T, McGuire W. Allopurinol for preventing mortality and morbidity in newborn infants with suspected hypoxic-ischaemic encephalopathy. Cochrane Database Syst Rev CD006817, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Chen CH, Greenawalt JW, Lehninger AL. Biochemical and ultrastructural aspects of Ca2+ transport by mitochondria of the hepatopancreas of the blue crab Callinectes sapidus. J Cell Biol 61: 301–315, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M, Bernardi P, Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One 3: e1852, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chinopoulos C, Adam-Vizi V. Modulation of the mitochondrial permeability transition by cyclophilin D: moving closer to F(0)-F(1) ATP synthase? Mitochondrion 12: 41–45, 2013 [DOI] [PubMed] [Google Scholar]
- 88.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell 79: 13–21, 1994 [DOI] [PubMed] [Google Scholar]
- 89.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 277: 34793–34799, 2002 [DOI] [PubMed] [Google Scholar]
- 90.Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol 48: 115–117, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Cooper CE, Davies NA. Effects of nitric oxide and peroxynitrite on the cytochrome oxidase K(m) for oxygen: implications for mitochondrial pathology. Biochim Biophys Acta 1459: 390–396, 2000 [DOI] [PubMed] [Google Scholar]
- 92.Corkey BE, Tornheim K, Deeney JT, Glennon MC, Parker JC, Matschinsky FM, Ruderman NB, Prentki M. Linked oscillations of free Ca2+ and the ATP/ADP ratio in permeabilized RINm5F insulinoma cells supplemented with a glycolyzing cell-free muscle extract. J Biol Chem 263: 4254–4258, 1988 [PubMed] [Google Scholar]
- 93.Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J 84: 2734–2755, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortopassi G, Wang E. Modelling the effects of age-related mtDNA mutation accumulation; complex I deficiency, superoxide and cell death. Biochim Biophys Acta 1271: 171–176, 1995 [DOI] [PubMed] [Google Scholar]
- 95.Crofts AR, Barquera B, Gennis RB, Kuras R, Guergova-Kuras M, Berry EA. Mechanism of ubiquinol oxidation by the bc(1) complex: different domains of the quinol binding pocket and their role in the mechanism and binding of inhibitors. Biochemistry 38: 15807–15826, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Crompton M, Andreeva L. On the involvement of a mitochondrial pore in reperfusion injury. Basic Res Cardiol 88: 513–523, 1993 [DOI] [PubMed] [Google Scholar]
- 97.Crompton M, Costi A. A heart mitochondrial Ca2+-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem J 266: 33–39, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crompton M, Kunzi M, Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur J Biochem 79: 549–558, 1977 [DOI] [PubMed] [Google Scholar]
- 99.Cunha FM, Caldeira da Silva CC, Cerqueira FM, Kowaltowski AJ. Mild mitochondrial uncoupling as a therapeutic strategy. Curr Drug Targets 12: 783–789, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Das DK, George A, Liu XK, Rao PS. Detection of hydroxyl radical in the mitochondria of ischemic-reperfused myocardium by trapping with salicylate. Biochem Biophys Res Commun 165: 1004–1009, 1989 [DOI] [PubMed] [Google Scholar]
- 102.De Bie MK, Buiten MS, Rabelink TJ, Jukema JW. How to reduce sudden cardiac death in patients with renal failure. Heart 98: 335–341, 2012 [DOI] [PubMed] [Google Scholar]
- 103.De Cavanagh EM, Fraga CG, Ferder L, Inserra F. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol 272: R514–R518, 1997 [DOI] [PubMed] [Google Scholar]
- 104.De Jong AM, Kotlyar AB, Albracht SP. Energy-induced structural changes in NADH:Q oxidoreductase of the mitochondrial respiratory chain. Biochim Biophys Acta 1186: 163–171, 1994 [DOI] [PubMed] [Google Scholar]
- 105.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci USA 47: 1744–1750, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Denton RM, McCormack JG, Edgell NJ. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J 190: 107–117, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Lisa F, Carpi A, Giorgio V, Bernardi P. The mitochondrial permeability transition pore and cyclophilin D in cardioprotection. Biochim Biophys Acta 1813: 1316–1322, 2011 [DOI] [PubMed] [Google Scholar]
- 109.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol 104: 131–139, 2009 [DOI] [PubMed] [Google Scholar]
- 110.Di Lisa F, Silverman HS, Hansford RG. Mitochondrial function and cell injury in single cardiac myocytes exposed to anoxia and reoxygenation. Transplant Proc 27: 2829–2830, 1995 [PubMed] [Google Scholar]
- 111.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dileepan KN, Kennedy J. Complete inhibition of dihydro-orotate oxidation and superoxide production by 1,1,1-trifluoro-3-thenoylacetone in rat liver mitochondria. Biochem J 225: 189–194, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dong LF, Jameson VJ, Tilly D, Cerny J, Mahdavian E, Marin-Hernandez A, Hernandez-Esquivel L, Rodriguez-Enriquez S, Stursa J, Witting PK, Stantic B, Rohlena J, Truksa J, Kluckova K, Dyason JC, Ledvina M, Salvatore BA, Moreno-Sanchez R, Coster MJ, Ralph SJ, Smith RA, Neuzil J. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J Biol Chem 286: 3717–3728, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst 90: 889–905, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drachev VA, Zorov DB. [Mitochondria as an electric cable. Experimental testing of a hypothesis]. Dokl Akad Nauk SSSR 287: 1237–1238, 1986 [PubMed] [Google Scholar]
- 116.Drahota Z, Chowdhury SK, Floryk D, Mracek T, Wilhelm J, Rauchova H, Lenaz G, Houstek J. Glycerophosphate-dependent hydrogen peroxide production by brown adipose tissue mitochondria and its activation by ferricyanide. J Bioenerg Biomembr 34: 105–113, 2002 [DOI] [PubMed] [Google Scholar]
- 117.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 118.Duchen MR, McGuinness O, Brown LA, Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res 27: 1790–1794, 1993 [DOI] [PubMed] [Google Scholar]
- 119.Duvert M, Verna A. Ultrastructure and architecture of the sarcoplasmic reticulum in frog sino-atrial fibres: a comparative study with various preparatory procedures. J Mol Cell Cardiol 17: 43–56, 1985 [DOI] [PubMed] [Google Scholar]
- 120.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96–99, 2002 [DOI] [PubMed] [Google Scholar]
- 121.Ekstedt B. Substrate specificity of the different forms of monoamine oxidase in rat liver mitochondria. Biochem Pharmacol 25: 1133–1138, 1976 [DOI] [PubMed] [Google Scholar]
- 122.Eleff S, Kennaway NG, Buist NR, Darley-Usmar VM, Capaldi RA, Bank WJ, Chance B. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc Natl Acad Sci USA 81: 3529–3533, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J 77: 1111–1122, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 120: 3680–3687, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev 57: 71–108, 1977 [DOI] [PubMed] [Google Scholar]
- 126.Evtodienko Yu V, Teplova V, Khawaja J, Saris NE. The Ca2+-induced permeability transition pore is involved in Ca2+-induced mitochondrial oscillations. A study on permeabilised Ehrlich ascites tumour cells. Cell Calcium 15: 143–152, 1994 [DOI] [PubMed] [Google Scholar]
- 127.Fabiato A, Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann NY Acad Sci 307: 491–522, 1978 [DOI] [PubMed] [Google Scholar]
- 128.Fabiato A, Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res 40: 119–129, 1977 [DOI] [PubMed] [Google Scholar]
- 129.Feng Y, Shi W, Huang M, LeBlanc MH. Oxypurinol administration fails to prevent hypoxic-ischemic brain injury in neonatal rats. Brain Res Bull 59: 453–457, 2003 [DOI] [PubMed] [Google Scholar]
- 130.Fenton HJH. The oxidation of tartaric acid in presence of iron. J Chem Soc Proc 9: 113, 1893 [Google Scholar]
- 131.Ferguson SJ. Fully delocalised chemiosmotic or Iocalised proton flow pathways in energy coupling? A scrutiny of experimental evidence. Biochim Biophys Acta 811: 47–95, 1985 [Google Scholar]
- 132.Fernandez-Gomez FJ, Galindo MF, Gomez-Lazaro M, Gonzalez-Garcia C, Cena V, Aguirre N, Jordan J. Involvement of mitochondrial potential and calcium buffering capacity in minocycline cytoprotective actions. Neuroscience 133: 959–967, 2005 [DOI] [PubMed] [Google Scholar]
- 133.Finetti F, Pellegrini M, Ulivieri C, Savino MT, Paccagnini E, Ginanneschi C, Lanfrancone L, Pelicci PG, Baldari CT. The proapoptotic and antimitogenic protein p66SHC acts as a negative regulator of lymphocyte activation and autoimmunity. Blood 111: 5017–5027, 2008 [DOI] [PubMed] [Google Scholar]
- 134.Fischer G, Schmid FX. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry 29: 2205–2212, 1990 [DOI] [PubMed] [Google Scholar]
- 135.Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337: 476–478, 1989 [DOI] [PubMed] [Google Scholar]
- 136.Fiskum G, Cockrell RS. Ruthenium red sensitive and insensitive calcium transport in rat liver and Ehrlich ascites tumor cell mitochondria. FEBS Lett 92: 125–128, 1978 [DOI] [PubMed] [Google Scholar]
- 137.Fiskum G, Lehninger AL. Regulated release of Ca2+ from respiring mitochondria by Ca2+/2H+ antiport. J Biol Chem 254: 6236–6239, 1979 [PubMed] [Google Scholar]
- 138.Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem 268: 22369–22376, 1993 [PubMed] [Google Scholar]
- 139.Ford LE, Podolsky RJ. Regenerative calcium release within muscle cells. Science 167: 58–59, 1970 [DOI] [PubMed] [Google Scholar]
- 140.Forman HJ, Kennedy J. Superoxide production and electron transport in mitochondrial oxidation of dihydroorotic acid. J Biol Chem 250: 4322–4326, 1975 [PubMed] [Google Scholar]
- 141.Frerman FE, Goodman SI. Deficiency of electron transfer flavoprotein or electron transfer flavoprotein:ubiquinone oxidoreductase in glutaric acidemia type II fibroblasts. Proc Natl Acad Sci USA 82: 4517–4520, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol 41: 35–97, 1974 [DOI] [PubMed] [Google Scholar]
- 143.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Galloway JH, Cartwright IJ, Bennett MJ. Abnormal myocardial lipid composition in an infant with type II glutaric aciduria. J Lipid Res 28: 279–284, 1987 [PubMed] [Google Scholar]
- 145.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19: 107–120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 30: 1019–1026, 2010 [DOI] [PubMed] [Google Scholar]
- 147.Gardner PR, Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem 267: 8757–8763, 1992 [PubMed] [Google Scholar]
- 148.Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci USA 91: 12248–12252, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garlick PB, Davies MJ, Hearse DJ, Slater TF. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res 61: 757–760, 1987 [DOI] [PubMed] [Google Scholar]
- 150.Gavrikova EV, Vinogradov AD. Active/de-active state transition of the mitochondrial complex I as revealed by specific sulfhydryl group labeling. FEBS Lett 455: 36–40, 1999 [DOI] [PubMed] [Google Scholar]
- 151.Gazaryan IG, Krasnikov BF, Ashby GA, Thorneley RN, Kristal BS, Brown AM. Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J Biol Chem 277: 10064–10072, 2002 [DOI] [PubMed] [Google Scholar]
- 152.Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett 505: 364–368, 2001 [DOI] [PubMed] [Google Scholar]
- 153.Ghisla S, Thorpe C. Acyl-CoA dehydrogenases. A mechanistic overview. Eur J Biochem 271: 494–508, 2004 [DOI] [PubMed] [Google Scholar]
- 154.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol 63: 69–172, 1990 [DOI] [PubMed] [Google Scholar]
- 155.Gimm O, Armanios M, Dziema H, Neumann HP, Eng C. Somatic and occult germ-line mutations in SDHD, a mitochondrial complex II gene, in nonfamilial pheochromocytoma. Cancer Res 60: 6822–6825, 2000 [PubMed] [Google Scholar]
- 156.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122: 221–233, 2005 [DOI] [PubMed] [Google Scholar]
- 157.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA 110: 5887–5892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gooch VD, Packer L. Adenine nucleotide control of heart mitochondrial oscillations. Biochim Biophys Acta 245: 17–20, 1971 [DOI] [PubMed] [Google Scholar]
- 159.Graham NA, Tahmasian M, Kohli B, Komisopoulou E, Zhu M, Vivanco I, Teitell MA, Wu H, Ribas A, Lo RS, Mellinghoff IK, Mischel PS, Graeber TG. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol Syst Biol 8: 589, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Green MF, Hirschey MD. SIRT3 weighs heavily in the metabolic balance: a new role for SIRT3 in metabolic syndrome. J Gerontol A Biol Sci Med Sci 68: 105–107, 2013 [DOI] [PubMed] [Google Scholar]
- 161.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol 25: 1461–1469, 1993 [DOI] [PubMed] [Google Scholar]
- 162.Grimm S. Respiratory chain complex II as general sensor for apoptosis. Biochim Biophys Acta 1827: 565–572, 2013 [DOI] [PubMed] [Google Scholar]
- 163.Grivennikova VG, Cecchini G, Vinogradov AD. Ammonium-dependent hydrogen peroxide production by mitochondria. FEBS Lett 582: 2719–2724, 2008 [DOI] [PubMed] [Google Scholar]
- 164.Grivennikova VG, Kareyeva AV, Vinogradov AD. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim Biophys Acta 1797: 939–944, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta 1757: 553–561, 2006 [DOI] [PubMed] [Google Scholar]
- 166.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol 258: C755–C786, 1990 [DOI] [PubMed] [Google Scholar]
- 167.Guo J, Lemire BD. The ubiquinone-binding site of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase is a source of superoxide. J Biol Chem 278: 47629–47635, 2003 [DOI] [PubMed] [Google Scholar]
- 168.Gylkhandanyan AV, Evtodienko YV, Zhabotinsky AM, Kondrashova MN. Continuous Sr2+-induced oscillations of the ionic fluxes in mitochondria. FEBS Lett 66: 44–47, 1976 [DOI] [PubMed] [Google Scholar]
- 169.Haber F, Weiss J. über die Katalyse des Hydroperoxydes. Naturwissenshaften 20: 948–950, 1932 [Google Scholar]
- 170.Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38: 841–860, 2010 [DOI] [PubMed] [Google Scholar]
- 171.Halestrap AP. The regulation of the oxidation of fatty acids and other substrates in rat heart mitochondria by changes in the matrix volume induced by osmotic strength, valinomycin and Ca2+. Biochem J 244: 159–164, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268: 153–160, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hall AM, Crawford C, Unwin RJ, Duchen MR, Peppiatt-Wildman CM. Multiphoton imaging of the functioning kidney. J Am Soc Nephrol 22: 1297–1304, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol 46: 215–234, 2006 [DOI] [PubMed] [Google Scholar]
- 175.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr 29: 89–95, 1997 [DOI] [PubMed] [Google Scholar]
- 176.Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol Cell Biochem 184: 359–369, 1998 [PubMed] [Google Scholar]
- 177.Harper ME, Ballantyne JS, Leach M, Brand MD. Effects of thyroid hormones on oxidative phosphorylation. Biochem Soc Trans 21: 785–792, 1993 [DOI] [PubMed] [Google Scholar]
- 178.Hassinen I, Chance B. Oxidation-reduction properties of the mitochondrial flavoprotein chain. Biochem Biophys Res Commun 31: 895–900, 1968 [DOI] [PubMed] [Google Scholar]
- 179.Hauptmann N, Grimsby J, Shih JC, Cadenas E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch Biochem Biophys 335: 295–304, 1996 [DOI] [PubMed] [Google Scholar]
- 180.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 109: 1714–1717, 2004 [DOI] [PubMed] [Google Scholar]
- 181.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol 8: 619–629, 2011 [DOI] [PubMed] [Google Scholar]
- 182.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467, 1979 [DOI] [PubMed] [Google Scholar]
- 183.Hearse DJ, Garlick PB, Humphrey SM. Ischemic contracture of the myocardium: mechanisms and prevention. Am J Cardiol 39: 986–993, 1977 [DOI] [PubMed] [Google Scholar]
- 184.Henderson PJ, Lardy HA. Bongkrekic acid. An inhibitor of the adenine nucleotide translocase of mitochondria. J Biol Chem 245: 1319–1326, 1970 [PubMed] [Google Scholar]
- 185.Hernandez-Garcia D, Wood CD, Castro-Obregon S, Covarrubias L. Reactive oxygen species: a radical role in development? Free Radic Biol Med 49: 130–143, 2010 [DOI] [PubMed] [Google Scholar]
- 186.Herrero A, Barja G. Localization of the site of oxygen radical generation inside the complex I of heart and nonsynaptic brain mammalian mitochondria. J Bioenerg Biomembr 32: 609–615, 2000 [DOI] [PubMed] [Google Scholar]
- 187.Hinkle PC, Butow RA, Racker E, Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J Biol Chem 242: 5169–5173, 1967 [PubMed] [Google Scholar]
- 188.Hirsch T, Susin SA, Marzo I, Marchetti P, Zamzami N, Kroemer G. Mitochondrial permeability transition in apoptosis and necrosis. Cell Biol Toxicol 14: 141–145, 1998 [DOI] [PubMed] [Google Scholar]
- 189.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol 292: H101–H108, 2007 [DOI] [PubMed] [Google Scholar]
- 190.Holmuhamedov EL, Teplova VV, Chukhlova EA, Evtodienko YV, Ulrich RG. Strontium excitability of the inner mitochondrial membrane: regenerative strontium-induced strontium release. Biochem Mol Biol Int 36: 39–49, 1995 [PubMed] [Google Scholar]
- 191.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003 [DOI] [PubMed] [Google Scholar]
- 192.Huang J, Lemire BD. Mutations in the C. elegans succinate dehydrogenase iron-sulfur subunit promote superoxide generation and premature aging. J Mol Biol 387: 559–569, 2009 [DOI] [PubMed] [Google Scholar]
- 193.Huang LS, Cobessi D, Tung EY, Berry EA. Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: a new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. J Mol Biol 351: 573–597, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang X, Zhai D, Huang Y. Study on the relationship between calcium-induced calcium release from mitochondria and PTP opening. Mol Cell Biochem 213: 29–35, 2000 [DOI] [PubMed] [Google Scholar]
- 195.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys 195: 453–459, 1979 [DOI] [PubMed] [Google Scholar]
- 196.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys 195: 468–477, 1979 [DOI] [PubMed] [Google Scholar]
- 197.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 251: 5069–5077, 1976 [PubMed] [Google Scholar]
- 198.Huser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J 74: 2129–2137, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89: 1145–1153, 1997 [DOI] [PubMed] [Google Scholar]
- 200.Imlay JA. A metabolic enzyme that rapidly produces superoxide, fumarate reductase of Escherichia coli. J Biol Chem 270: 19767–19777, 1995 [PubMed] [Google Scholar]
- 201.Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Cross talk of nitric oxide, oxygen radicals, and superoxide dismutase regulates the energy metabolism and cell death and determines the fates of aerobic life. Antioxid Redox Signal 5: 475–484, 2003 [DOI] [PubMed] [Google Scholar]
- 202.Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem 10: 2495–2505, 2003 [DOI] [PubMed] [Google Scholar]
- 203.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275: 1649–1652, 1997 [DOI] [PubMed] [Google Scholar]
- 204.Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res 65: 203–209, 2005 [PubMed] [Google Scholar]
- 205.Ivanes F, Mewton N, Rioufol G, Piot C, Elbaz M, Revel D, Croisille P, Ovize M. Cardioprotection in the clinical setting. Cardiovasc Drugs Ther 24: 281–287, 2010 [DOI] [PubMed] [Google Scholar]
- 206.Jankauskas SS, Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Skulachev VP, Zorov DB. Mitochondria-targeted antioxidant SkQR1 ameliorates gentamycin-induced renal failure and hearing loss. Biochemistry 77: 666–670, 2012 [DOI] [PubMed] [Google Scholar]
- 207.Jennings RB. Commentary on selected aspects of cardioprotection. J Cardiovasc Pharmacol Ther 16: 340–348, 2011 [DOI] [PubMed] [Google Scholar]
- 208.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 70: 68–78, 1960 [PubMed] [Google Scholar]
- 209.Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. II. Steroid effects. Biochim Biophys Acta 122: 167–174, 1966 [DOI] [PubMed] [Google Scholar]
- 210.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci USA 77: 990–994, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature 491: 269–273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res 54: 277–285, 1984 [DOI] [PubMed] [Google Scholar]
- 214.Jones DP. Intracellular diffusion gradients of O2 and ATP. Am J Physiol Cell Physiol 250: C663–C675, 1986 [DOI] [PubMed] [Google Scholar]
- 215.Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature 377: 438–441, 1995 [DOI] [PubMed] [Google Scholar]
- 216.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res 66: 233–244, 2005 [DOI] [PubMed] [Google Scholar]
- 217.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann NY Acad Sci 1123: 197–212, 2008 [DOI] [PubMed] [Google Scholar]
- 218.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res 104: 1240–1252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kakar S, Hoffman FG, Storz JF, Fabian M, Hargrove MS. Structure and reactivity of hexacoordinate hemoglobins. Biophys Chem 152: 1–14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta 1813: 1323–1332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kaminski MM, Sauer SW, Kaminski M, Opp S, Ruppert T, Grigaravicius P, Grudnik P, Grone HJ, Krammer PH, Gulow K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep 2: 1300–1315, 2012 [DOI] [PubMed] [Google Scholar]
- 223.Kapay NA, Popova OV, Isaev NK, Stelmashook EV, Kondratenko RV, Zorov DB, Skrebitsky VG, Skulachev VP. Mitochondria-targeted plastoquinone antioxidant SkQ1 prevents amyloid-beta-induced impairment of long-term potentiation in rat hippocampal slices. J Alzheimers Dis 36: 377–383, 2013 [DOI] [PubMed] [Google Scholar]
- 224.Kareyeva AV, Grivennikova VG, Cecchini G, Vinogradov AD. Molecular identification of the enzyme responsible for the mitochondrial NADH-supported ammonium-dependent hydrogen peroxide production. FEBS Lett 585: 385–389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596, 2001 [DOI] [PubMed] [Google Scholar]
- 226.Khanday FA, Santhanam L, Kasuno K, Yamamori T, Naqvi A, Dericco J, Bugayenko A, Mattagajasingh I, Disanza A, Scita G, Irani K. Sos-mediated activation of rac1 by p66shc. J Cell Biol 172: 817–822, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kim JJ, Miura R. Acyl-CoA dehydrogenases and acyl-CoA oxidases. Structural basis for mechanistic similarities and differences. Eur J Biochem 271: 483–493, 2004 [DOI] [PubMed] [Google Scholar]
- 228.Kinnally KW, Campo ML, Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr 21: 497–506, 1989 [DOI] [PubMed] [Google Scholar]
- 229.Kinnally KW, Zorov D, Antonenko Y, Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem Biophys Res Commun 176: 1183–1188, 1991 [DOI] [PubMed] [Google Scholar]
- 230.Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci USA 90: 1374–1378, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364, 2004 [DOI] [PubMed] [Google Scholar]
- 232.Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog Neuropsychopharmacol Biol Psychiatry 23: 359–376, 1999 [DOI] [PubMed] [Google Scholar]
- 233.Klingenberg M, Grebe K, Scherer B. Opposite effects of bongkrekic acid and atractyloside on the adenine nucleotides induced mitochondrial volume changes and on the efflux of adenine nucleotides. FEBS Lett 16: 253–256, 1971 [DOI] [PubMed] [Google Scholar]
- 234.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kolosova NG, Stefanova NA, Muraleva NA, Skulachev VP. The mitochondria-targeted antioxidant SkQ1 but not N-acetylcysteine reverses aging-related biomarkers in rats. Aging 4: 686–694, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Koppenol WH. The centennial of the Fenton reaction. Free Radic Biol Med 15: 645–651, 1993 [DOI] [PubMed] [Google Scholar]
- 238.Korde AS, Pettigrew LC, Craddock SD, Maragos WF. The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J Neurochem 94: 1676–1684, 2005 [DOI] [PubMed] [Google Scholar]
- 239.Korge P, Yang L, Yang JH, Wang Y, Qu Z, Weiss JN. Protective role of transient pore openings in calcium handling by cardiac mitochondria. J Biol Chem 286: 34851–34857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416: 15–18, 1997 [DOI] [PubMed] [Google Scholar]
- 241.Kotlyar AB, Sled VD, Burbaev DS, Moroz IA, Vinogradov AD. Coupling site I and the rotenone-sensitive ubisemiquinone in tightly coupled submitochondrial particles. FEBS Lett 264: 17–20, 1990 [DOI] [PubMed] [Google Scholar]
- 242.Kozlov AV, Bahrami S, Calzia E, Dungel P, Gille L, Kuznetsov AV, Troppmair J. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care 1: 41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Krasnikov BF, Avad AS, Zorov DB, Yaguzhinsky LS. Effects of amyl ester of unsubstituted rhodamine on respiration and Ca2+ transport in rat liver mitochondria. Biochem Biophys Res Commun 175: 1010–1016, 1991 [DOI] [PubMed] [Google Scholar]
- 244.Krasnikov BF, Zorov DB, Antonenko YN, Zaspa AA, Kulikov IV, Kristal BS, Cooper AJ, Brown AM. Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim Biophys Acta 1708: 375–392, 2005 [DOI] [PubMed] [Google Scholar]
- 245.Krishnamoorthy G, Hinkle PC. Studies on the electron transfer pathway, topography of iron-sulfur centers, and site of coupling in NADH-Q oxidoreductase. J Biol Chem 263: 17566–17575, 1988 [PubMed] [Google Scholar]
- 246.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 52: 457–474, 1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279: 4127–4135, 2004 [DOI] [PubMed] [Google Scholar]
- 248.Kummel L. Ca,Mg-ATPase activity of permeabilised rat heart cells and its functional coupling to oxidative phosphorylation of the cells. Cardiovasc Res 22: 359–367, 1988 [DOI] [PubMed] [Google Scholar]
- 249.Kunduzova OR, Bianchi P, Parini A, Cambon C. Hydrogen peroxide production by monoamine oxidase during ischemia/reperfusion. Eur J Pharmacol 448: 225–230, 2002 [DOI] [PubMed] [Google Scholar]
- 250.Kunz WS, Kunz W. Contribution of different enzymes to flavoprotein fluorescence of isolated rat liver mitochondria. Biochim Biophys Acta 841: 237–246, 1985 [DOI] [PubMed] [Google Scholar]
- 251.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Spatio-temporal oscillations of individual mitochondria in cardiac myocytes reveal modulation of synchronized mitochondrial clusters. Proc Natl Acad Sci USA 107: 14315–14320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Wavelet analysis reveals heterogeneous time-dependent oscillations of individual mitochondria. Am J Physiol Heart Circ Physiol 299: H1736–H1740, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J 368: 545–553, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kushnareva YE, Sokolove PM. Prooxidants open both the mitochondrial permeability transition pore and a low-conductance channel in the inner mitochondrial membrane. Arch Biochem Biophys 376: 377–388, 2000 [DOI] [PubMed] [Google Scholar]
- 256.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci USA 103: 7607–7612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lakatta EG, Sollott SJ. The “heartbreak” of older age. Mol Interv 2: 431–446, 2002 [DOI] [PubMed] [Google Scholar]
- 258.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441: 523–540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lehninger AL, Carafoli E, Rossi CS. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol 29: 259–320, 1967 [DOI] [PubMed] [Google Scholar]
- 260.Lemarie A, Huc L, Pazarentzos E, Mahul-Mellier AL, Grimm S. Specific disintegration of complex II succinate:ubiquinone oxidoreductase links pH changes to oxidative stress for apoptosis induction. Cell Death Differ 18: 338–349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366: 177–196, 1998 [DOI] [PubMed] [Google Scholar]
- 262.Lenartowicz E, Bernardi P, Azzone GF. Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J Bioenerg Biomembr 23: 679–688, 1991 [DOI] [PubMed] [Google Scholar]
- 263.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52: 159–164, 2001 [DOI] [PubMed] [Google Scholar]
- 264.Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta 1366: 53–67, 1998 [DOI] [PubMed] [Google Scholar]
- 265.Lenaz G, Genova ML. Supramolecular organisation of the mitochondrial respiratory chain: a new challenge for the mechanism and control of oxidative phosphorylation. Adv Exp Med Biol 748: 107–144, 2012 [DOI] [PubMed] [Google Scholar]
- 266.Levitt DG. Capillary-tissue exchange kinetics: an analysis of the Krogh cylinder model. J Theor Biol 34: 103–124, 1972 [DOI] [PubMed] [Google Scholar]
- 267.Liang LP, Patel M. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson's disease. J Neurochem 90: 1076–1084, 2004 [DOI] [PubMed] [Google Scholar]
- 268.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature 222: 1076–1078, 1969 [DOI] [PubMed] [Google Scholar]
- 269.Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, Woo JT, Kim YS, Murphy MP, Ali L, Ha J, Kim SS. Mitochondria-targeted antioxidants protect pancreatic beta-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cell Physiol Biochem 28: 873–886, 2011 [DOI] [PubMed] [Google Scholar]
- 270.Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ 16: 899–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Liu R, Liu W, Doctrow SR, Baudry M. Iron toxicity in organotypic cultures of hippocampal slices: role of reactive oxygen species. J Neurochem 85: 492–502, 2003 [DOI] [PubMed] [Google Scholar]
- 272.Liu SS. Cooperation of a ”reactive oxygen cycle“ with the Q cycle and the proton cycle in the respiratory chain–superoxide generating and cycling mechanisms in mitochondria. J Bioenerg Biomembr 31: 367–376, 1999 [DOI] [PubMed] [Google Scholar]
- 273.Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci Rep 17: 259–272, 1997 [DOI] [PubMed] [Google Scholar]
- 274.Liu SS. Mitochondrial Q cycle-derived superoxide and chemiosmotic bioenergetics. Ann NY Acad Sci 1201: 84–95, 2010 [DOI] [PubMed] [Google Scholar]
- 275.Liu SS, Huang JP. Co-existence of ”reactive oxygen species“ with Q cycle and proton cycle in respiratory chain fo mitochondria. In: Proceedings of the International Symposium on Natural Antioxidants: Molecular Mechanisms and Health Effects, edited by Parker L, Traber MG, Xin WJ. Champaign, IL: AOCS, 1996, p. 511–529 [Google Scholar]
- 276.Loffler M, Becker C, Wegerle E, Schuster G. Catalytic enzyme histochemistry and biochemical analysis of dihydroorotate dehydrogenase/oxidase and succinate dehydrogenase in mammalian tissues, cells and mitochondria. Histochem Cell Biol 105: 119–128, 1996 [DOI] [PubMed] [Google Scholar]
- 277.Lotscher HR, Winterhalter KH, Carafoli E, Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem 255: 9325–9330, 1980 [PubMed] [Google Scholar]
- 278.Lukacs GL, Kapus A, Fonyo A. Parallel measurement of oxoglutarate dehydrogenase activity and matrix free Ca2+ in fura-2-loaded heart mitochondria. FEBS Lett 229: 219–223, 1988 [DOI] [PubMed] [Google Scholar]
- 279.Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, Abboud HE. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol 302: C597–C604, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol 50: 408–416, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Magnus G, Keizer J. Model of beta-cell mitochondrial calcium handling and electrical activity. II. Mitochondrial variables. Am J Physiol Cell Physiol 274: C1174–C1184, 1998 [DOI] [PubMed] [Google Scholar]
- 282.Maker HS, Weiss C, Silides DJ, Cohen G. Coupling of dopamine oxidation (monoamine oxidase activity) to glutathione oxidation via the generation of hydrogen peroxide in rat brain homogenates. J Neurochem 36: 589–593, 1981 [DOI] [PubMed] [Google Scholar]
- 283.Maklashina E, Sher Y, Zhou HZ, Gray MO, Karliner JS, Cecchini G. Effect of anoxia/reperfusion on the reversible active/de-active transition of NADH-ubiquinone oxidoreductase (complex I) in rat heart. Biochim Biophys Acta 1556: 6–12, 2002 [DOI] [PubMed] [Google Scholar]
- 284.Malkevitch NV, Dedukhova VI, Simonian RA, Skulachev VP, Starkov AA. Thyroxine induces cyclosporin A-insensitive, Ca2+-dependent reversible permeability transition pore in rat liver mitochondria. FEBS Lett 412: 173–178, 1997 [DOI] [PubMed] [Google Scholar]
- 285.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J 222: 649–655, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 15: 633–644, 1996 [DOI] [PubMed] [Google Scholar]
- 287.Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, Sullivan PA. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun 36: 891–897, 1969 [DOI] [PubMed] [Google Scholar]
- 288.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Frances B. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol 284: H1460–H1467, 2003 [DOI] [PubMed] [Google Scholar]
- 289.McGuire BJ, Secomb TW. A theoretical model for oxygen transport in skeletal muscle under conditions of high oxygen demand. J Appl Physiol 91: 2255–2265, 2001 [DOI] [PubMed] [Google Scholar]
- 290.Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech Ageing Dev 128: 286–292, 2007 [DOI] [PubMed] [Google Scholar]
- 291.Menini S, Iacobini C, Ricci C, Oddi G, Pesce C, Pugliese F, Block K, Abboud HE, Giorgio M, Migliaccio E, Pelicci PG, Pugliese G. Ablation of the gene encoding p66Shc protects mice against AGE-induced glomerulopathy by preventing oxidant-dependent tissue injury and further AGE accumulation. Diabetologia 50: 1997–2007, 2007 [DOI] [PubMed] [Google Scholar]
- 292.Mercer JR, Yu E, Figg N, Cheng KK, Prime TA, Griffin JL, Masoodi M, Vidal-Puig A, Murphy MP, Bennett MR. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free Radic Biol Med 52: 841–849, 2012 [DOI] [PubMed] [Google Scholar]
- 293.Messner KR, Imlay JA. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem 274: 10119–10128, 1999 [DOI] [PubMed] [Google Scholar]
- 294.Messner KR, Imlay JA. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J Biol Chem 277: 42563–42571, 2002 [DOI] [PubMed] [Google Scholar]
- 295.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 296.Minotti G, Recalcati S, Menna P, Salvatorelli E, Corna G, Cairo G. Doxorubicin cardiotoxicity and the control of iron metabolism: quinone-dependent and independent mechanisms. Methods Enzymol 378: 340–361, 2004 [DOI] [PubMed] [Google Scholar]
- 297.Mironov SL, Richter DW. Oscillations and hypoxic changes of mitochondrial variables in neurons of the brainstem respiratory centre of mice. J Physiol 533: 227–236, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc 41: 445–502, 1966 [DOI] [PubMed] [Google Scholar]
- 299.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191: 144–148, 1961 [DOI] [PubMed] [Google Scholar]
- 300.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol Heart Circ Physiol 261: H1123–H1134, 1991 [DOI] [PubMed] [Google Scholar]
- 301.Modica-Napolitano JS, Brunelli BT, Koya K, Chen LB. Photoactivation enhances the mitochondrial toxicity of the cationic rhodacyanine MKT-077. Cancer Res 58: 71–75, 1998 [PubMed] [Google Scholar]
- 302.Moreno-Sanchez R, Hansford RG. Dependence of cardiac mitochondrial pyruvate dehydrogenase activity on intramitochondrial free Ca2+ concentration. Biochem J 256: 403–412, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Moreno G, Poussin K, Ricchelli F, Salet C. The effects of singlet oxygen produced by photodynamic action on the mitochondrial permeability transition differ in accordance with the localization of the sensitizer. Arch Biochem Biophys 386: 243–250, 2001 [DOI] [PubMed] [Google Scholar]
- 304.Mracek T, Pecinova A, Vrbacky M, Drahota Z, Houstek J. High efficiency of ROS production by glycerophosphate dehydrogenase in mammalian mitochondria. Arch Biochem Biophys 481: 30–36, 2009 [DOI] [PubMed] [Google Scholar]
- 305.Muller U. Pathological mechanisms and parent-of-origin effects in hereditary paraganglioma/pheochromocytoma (PGL/PCC). Neurogenetics 12: 175–181, 2011 [DOI] [PubMed] [Google Scholar]
- 306.Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA 93: 9893–9898, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Murphy MP. Development of lipophilic cations as therapies for disorders due to mitochondrial dysfunction. Expert Opin Biol Ther 1: 753–764, 2001 [DOI] [PubMed] [Google Scholar]
- 308.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal 16: 476–495, 2012 [DOI] [PubMed] [Google Scholar]
- 310.Murphy MP, Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity 38: 201–202, 2013 [DOI] [PubMed] [Google Scholar]
- 311.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 312.Mustafa MG, Utsumi K, Packer L. Damped oscillatory control of mitochondrial respiration and volume. Biochem Biophys Res Commun 24: 381–385, 1966 [DOI] [PubMed] [Google Scholar]
- 313.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 314.Napankangas JP, Liimatta EV, Joensuu P, Bergmann U, Ylitalo K, Hassinen IE. Superoxide production during ischemia-reperfusion in the perfused rat heart: a comparison of two methods of measurement. J Mol Cell Cardiol 53: 906–915, 2012 [DOI] [PubMed] [Google Scholar]
- 315.Naviaux RK. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther 342: 608–618, 2012 [DOI] [PubMed] [Google Scholar]
- 316.Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 11: 809–815, 1997 [PubMed] [Google Scholar]
- 317.Nelson JS, Liaw LH, Berns MW. Tumor destruction in photodynamic therapy. Photochem Photobiol 46: 829–835, 1987 [DOI] [PubMed] [Google Scholar]
- 318.Neupert W. Protein import into mitochondria. Annu Rev Biochem 66: 863–917, 1997 [DOI] [PubMed] [Google Scholar]
- 319.Nosoh Y, Kajioka J, Itoh M. Effect of menadione on the electron transport pathway of yeast mitochondria. Arch Biochem Biophys 127: 1–6, 1968 [DOI] [PubMed] [Google Scholar]
- 320.Novgorodov SA, Gudz TI, Kushnareva YE, Zorov DB, Kudrjashov YB. Effect of ADP/ATP antiporter conformational state on the suppression of the nonspecific permeability of the inner mitochondrial membrane by cyclosporine A. FEBS Lett 277: 123–126, 1990 [DOI] [PubMed] [Google Scholar]
- 321.Novgorodov SA, Gudz TI, Kushnareva YE, Zorov DB, Kudrjashov YB. Effect of cyclosporine A and oligomycin on non-specific permeability of the inner mitochondrial membrane. FEBS Lett 270: 108–110, 1990 [DOI] [PubMed] [Google Scholar]
- 322.Novgorodov SA, Gudz TI, Mohr Yu E, Goncharenko EN, Yaguzhinsky LS. ATP-synthase complex: the mechanism of control of ion fluxes induced by cumene hydroperoxide in mitochondria. FEBS Lett 247: 255–258, 1989 [DOI] [PubMed] [Google Scholar]
- 323.O'Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J Physiol 529: 23–36, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265: 962–966, 1994 [DOI] [PubMed] [Google Scholar]
- 325.Ohnishi T. Iron-sulfur clusters/semiquinones in complex I. Biochim Biophys Acta 1364: 186–206, 1998 [DOI] [PubMed] [Google Scholar]
- 326.Olsen RK, Olpin SE, Andresen BS, Miedzybrodzka ZH, Pourfarzam M, Merinero B, Frerman FE, Beresford MW, Dean JC, Cornelius N, Andersen O, Oldfors A, Holme E, Gregersen N, Turnbull DM, Morris AA. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain 130: 2045–2054, 2007 [DOI] [PubMed] [Google Scholar]
- 327.Orlowski S, Nowak W. Topology and thermodynamics of gaseous ligands diffusion paths in human neuroglobin. Biosystems 94: 263–266, 2008 [DOI] [PubMed] [Google Scholar]
- 328.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem 279: 25689–25695, 2004 [DOI] [PubMed] [Google Scholar]
- 329.Otani H, Tanaka H, Inoue T, Umemoto M, Omoto K, Tanaka K, Sato T, Osako T, Masuda A, Nonoyama A. In vitro study on contribution of oxidative metabolism of isolated rabbit heart mitochondria to myocardial reperfusion injury. Circ Res 55: 168–175, 1984 [DOI] [PubMed] [Google Scholar]
- 330.Packer L, Utsumi R, Mustafa MG. Oscillatory states of mitochondria. 1. Electron and energy transfer pathways. Arch Biochem Biophys 117: 381–393, 1966 [DOI] [PubMed] [Google Scholar]
- 331.Palmer JW, Pfeiffer DR. The control of Ca2+ release from heart mitochondria. J Biol Chem 256: 6742–6750, 1981 [PubMed] [Google Scholar]
- 332.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 107: 436–441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr 40: 171–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Patole MS, Swaroop A, Ramasarma T. Generation of H2O2 in brain mitochondria. J Neurochem 47: 1–8, 1986 [DOI] [PubMed] [Google Scholar]
- 335.Penna C, Perrelli MG, Pagliaro P. Mitochondrial pathways, permeability transition pore, and redox signaling in cardioprotection: therapeutic implications. Antioxid Redox Signal 18: 556–599, 2013 [DOI] [PubMed] [Google Scholar]
- 336.Perry TL, Godin DV, Hansen S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neurosci Lett 33: 305–310, 1982 [DOI] [PubMed] [Google Scholar]
- 337.Petlicki J, van de Ven TGM. The equilibrium between the oxidation of hydrogen peroxide by oxygen and the dismutation of peroxyl or superoxide radicals in aqeous solutions in contact with oxygen. J Chem Soc Faraday Trans 94: 2763–2767, 1998 [Google Scholar]
- 338.Pfanner N, Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem 59: 331–353, 1990 [DOI] [PubMed] [Google Scholar]
- 339.Pfeiffer DR, Kauffman RF, Lardy HA. Effects of N-ethylmaleimide on the limited uptake of Ca2+, Mn2+, and Sr2+ by rat liver mitochondria. J Biol Chem 253: 4165–4171, 1978 [PubMed] [Google Scholar]
- 340.Picard M, Taivassalo T, Gouspillou G, Hepple RT. Mitochondria: isolation, structure and function. J Physiol 589: 4413–4421, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315: 659–663, 2007 [DOI] [PubMed] [Google Scholar]
- 342.Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int 72: 1493–1502, 2007 [DOI] [PubMed] [Google Scholar]
- 343.Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Manskikh VN, Pulkova NV, Galkina SI, Skulachev VP, Zorov DB. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc Natl Acad Sci USA 110: E3100–3108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Popova EN, Pletjushkina OY, Dugina VB, Domnina LV, Ivanova OY, Izyumov DS, Skulachev VP, Chernyak BV. Scavenging of reactive oxygen species in mitochondria induces myofibroblast differentiation. Antioxid Redox Signal 13: 1297–1307, 2010 [DOI] [PubMed] [Google Scholar]
- 345.Pryde KR, Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem 286: 18056–18065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Pushpakiran G, Mahalakshmi K, Anuradha CV. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids 27: 91–96, 2004 [DOI] [PubMed] [Google Scholar]
- 347.Raha S, Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Am J Med Genet 106: 62–70, 2001 [DOI] [PubMed] [Google Scholar]
- 348.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25: 502–508, 2000 [DOI] [PubMed] [Google Scholar]
- 349.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 130: 427–439, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ramsay RR, Singer TP. Relation of superoxide generation and lipid peroxidation to the inhibition of NADH-Q oxidoreductase by rotenone, piericidin A, and MPP+. Biochem Biophys Res Commun 189: 47–52, 1992 [DOI] [PubMed] [Google Scholar]
- 351.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium 50: 222–233, 2011 [DOI] [PubMed] [Google Scholar]
- 352.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med 35: 626–635, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal 13: 1087–1123, 2010 [DOI] [PubMed] [Google Scholar]
- 355.Rieske JS, Baum H, Stoner CD, Lipton SH. On the antimycin-sensitive cleavage of complex 3 of the mitochondrial respiratory chain. J Biol Chem 242: 4854–4866, 1967 [PubMed] [Google Scholar]
- 356.Rivera J, Sobey CG, Walduck AK, Drummond GR. Nox isoforms in vascular pathophysiology: insights from transgenic and knockout mouse models. Redox Rep 15: 50–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358: 325–327, 1992 [DOI] [PubMed] [Google Scholar]
- 358.Robinson BH. Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. Biochim Biophys Acta 1364: 271–286, 1998 [DOI] [PubMed] [Google Scholar]
- 359.Rodrigo GC, Lawrence CL, Standen NB. Dinitrophenol pretreatment of rat ventricular myocytes protects against damage by metabolic inhibition and reperfusion. J Mol Cell Cardiol 34: 555–569, 2002 [DOI] [PubMed] [Google Scholar]
- 360.Romashko DN, Marban E, O'Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci USA 95: 1618–1623, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Rotig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 17: 215–217, 1997 [DOI] [PubMed] [Google Scholar]
- 362.Rugarli EI, Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31: 1336–1349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Ruzicka FJ, Beinert H. A new iron-sulfur flavoprotein of the respiratory chain. A component of the fatty acid beta oxidation pathway. J Biol Chem 252: 8440–8445, 1977 [PubMed] [Google Scholar]
- 364.Sakai C, Tomitsuka E, Esumi H, Harada S, Kita K. Mitochondrial fumarate reductase as a target of chemotherapy: From parasites to cancer cells. Biochim Biophys Acta 1820: 643–651, 2012 [DOI] [PubMed] [Google Scholar]
- 365.Saks V, Dzeja PP, Guzun R, Aliev MK, Vndeli M, Terzic A, Wallimann T. System Analysis of Cardiac Energetics-Excitation-Contraction Coupling. Integration of Mitochondrial Respiration. Darmstadt: Wiley-VCH Verlag, 2007, p. 367–405 [Google Scholar]
- 366.Salet C, Moreno G. Photosensitization of mitochondria. Molecular and cellular aspects. J Photochem Photobiol B 5: 133–150, 1990 [DOI] [PubMed] [Google Scholar]
- 367.Salet C, Moreno G, Ricchelli F, Bernardi P. Singlet oxygen produced by photodynamic action causes inactivation of the mitochondrial permeability transition pore. J Biol Chem 272: 21938–21943, 1997 [DOI] [PubMed] [Google Scholar]
- 368.Samartsev VN, Smirnov AV, Zeldi IP, Markova OV, Mokhova EN, Skulachev VP. Involvement of aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria. Biochim Biophys Acta 1319: 251–257, 1997 [DOI] [PubMed] [Google Scholar]
- 369.Samec S, Seydoux J, Dulloo AG. Role of UCP homologues in skeletal muscles and brown adipose tissue: mediators of thermogenesis or regulators of lipids as fuel substrate? FASEB J 12: 715–724, 1998 [DOI] [PubMed] [Google Scholar]
- 370.Sandri G, Panfili E, Ernster L. Hydrogen peroxide production by monoamine oxidase in isolated rat-brain mitochondria: its effect on glutathione levels and Ca2+ efflux. Biochim Biophys Acta 1035: 300–305, 1990 [DOI] [PubMed] [Google Scholar]
- 371.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 9: 651–658, 1995 [DOI] [PubMed] [Google Scholar]
- 372.Scarpa A, Azzone GF. The mechanism of ion translocation in mitochondria. 4. Coupling of K+ efflux with Ca2+ uptake. Eur J Biochem 12: 328–335, 1970 [DOI] [PubMed] [Google Scholar]
- 373.Scholz W, Schutze K, Kunz W, Schwarz M. Phenobarbital enhances the formation of reactive oxygen in neoplastic rat liver nodules. Cancer Res 50: 7015–7022, 1990 [PubMed] [Google Scholar]
- 374.Schumacker PT. Hypoxia, anoxia, and O2 sensing: the search continues. Am J Physiol Lung Cell Mol Physiol 283: L918–L921, 2002 [DOI] [PubMed] [Google Scholar]
- 375.Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 124: 1172–1179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Schwarzlander M, Logan DC, Fricker MD, Sweetlove LJ. The circularly permuted yellow fluorescent protein cpYFP that has been used as a superoxide probe is highly responsive to pH but not superoxide in mitochondria: implications for the existence of superoxide ”flashes.“ Biochem J 437: 381–387, 2011 [DOI] [PubMed] [Google Scholar]
- 377.Schwarzlander M, Murphy MP, Duchen MR, Logan DC, Fricker MD, Halestrap AP, Muller FL, Rizzuto R, Dick TP, Meyer AJ, Sweetlove LJ. Mitochondrial ”flashes“: a radical concept repHined. Trends Cell Biol 22: 503–508, 2012 [DOI] [PubMed] [Google Scholar]
- 378.Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun 204: 169–175, 1994 [DOI] [PubMed] [Google Scholar]
- 379.Segretain D, Rambourg A, Clermont Y. Three dimensional arrangement of mitochondria and endoplasmic reticulum in the heart muscle fiber of the rat. Anat Rec 200: 139–151, 1981 [DOI] [PubMed] [Google Scholar]
- 380.Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem 285: 5748–5758, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Selivanov VA, Zeak JA, Roca J, Cascante M, Trucco M, Votyakova TV. The role of external and matrix pH in mitochondrial reactive oxygen species generation. J Biol Chem 283: 29292–29300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38: 225–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Severs NJ, Slade AM, Powell T, Twist VW, Warren RL. Correlation of ultrastructure and function in calcium-tolerant myocytes isolated from the adult rat heart. J Ultrastruct Res 81: 222–239, 1982 [DOI] [PubMed] [Google Scholar]
- 385.Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol 289: H237–H242, 2005 [DOI] [PubMed] [Google Scholar]
- 386.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 22: 197–217, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci 123: 894–902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 388.Silachev DN, Isaev NK, Pevzner IB, Zorova LD, Stelmashook EV, Novikova SV, Plotnikov EY, Skulachev VP, Zorov DB. The mitochondria-targeted antioxidants and remote kidney preconditioning ameliorate brain damage through kidney-to-brain cross-talk. PLoS One 7: e51553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Simonson SG, Zhang J, Canada AT, Jr, Su YF, Benveniste H, Piantadosi CA. Hydrogen peroxide production by monoamine oxidase during ischemia-reperfusion in the rat brain. J Cereb Blood Flow Metab 13: 125–134, 1993 [DOI] [PubMed] [Google Scholar]
- 390.Singla M, Guzman G, Griffin AJ, Bharati S. Cardiomyopathy in multiple Acyl-CoA dehydrogenase deficiency: a clinico-pathological correlation and review of literature. Pediatr Cardiol 29: 446–451, 2008 [DOI] [PubMed] [Google Scholar]
- 391.Skulachev MV, Antonenko YN, Anisimov VN, Chernyak BV, Cherepanov DA, Chistyakov VA, Egorov MV, Kolosova NG, Korshunova GA, Lyamzaev KG, Plotnikov EY, Roginsky VA, Savchenko AY, Severina II, Severin FF, Shkurat TP, Tashlitsky VN, Shidlovsky KM, Vyssokikh MY, Zamyatnin AA, Jr, Zorov DB, Skulachev VP. Mitochondrial-targeted plastoquinone derivatives Effect on senescence and acute age-related pathologies. Curr Drug Targets 12: 800–826, 2011 [DOI] [PubMed] [Google Scholar]
- 392.Skulachev V. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem Biophys Res Commun 441: 275–279, 2013 [DOI] [PubMed] [Google Scholar]
- 393.Skulachev VP. A biochemical approach to the problem of aging: ”megaproject“ on membrane-penetrating ions. The first results and prospects. Biochemistry 72: 1385–1396, 2007 [DOI] [PubMed] [Google Scholar]
- 394.Skulachev VP. Energy transformation in the respiratory chain. Curr Top Bioenerg 4: 127, 1971 [Google Scholar]
- 395.Skulachev VP. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 26: 23–29, 2001 [DOI] [PubMed] [Google Scholar]
- 396.Skulachev VP. Power transmission along biological membranes. J Membr Biol 114: 97–112, 1990 [DOI] [PubMed] [Google Scholar]
- 397.Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys 29: 169–202, 1996 [DOI] [PubMed] [Google Scholar]
- 398.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta 1363: 100–124, 1998 [DOI] [PubMed] [Google Scholar]
- 399.Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, Kopnin BP, Korshunova GA, Lichinitser MR, Obukhova LA, Pasyukova EG, Pisarenko OI, Roginsky VA, Ruuge EK, Senin II, Severina II, Skulachev MV, Spivak IM, Tashlitsky VN, Tkachuk VA, Vyssokikh MY, Yaguzhinsky LS, Zorov DB. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta 1787: 437–461, 2009 [DOI] [PubMed] [Google Scholar]
- 400.Skulachev VP, Bogachev AV, Kasparinsky FO. Principles of Bioenergetics. Berlin: Springer-Verlag, 2013, p. 436 [Google Scholar]
- 401.Slodzinski MK, Aon MA, O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J Mol Cell Cardiol 45: 650–660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 2: 342–352, 2001 [DOI] [PubMed] [Google Scholar]
- 403.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA 88: 3671–3675, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Smith RA, Adlam VJ, Blaikie FH, Manas AR, Porteous CM, James AM, Ross MF, Logan A, Cocheme HM, Trnka J, Prime TA, Abakumova I, Jones BA, Filipovska A, Murphy MP. Mitochondria-targeted antioxidants in the treatment of disease. Ann NY Acad Sci 1147: 105–111, 2008 [DOI] [PubMed] [Google Scholar]
- 405.Smith RA, Hartley RC, Cocheme HM, Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci 33: 341–352, 2012 [DOI] [PubMed] [Google Scholar]
- 406.Smith RA, Murphy MP. Mitochondria-targeted antioxidants as therapies. Discov Med 11: 106–114, 2011 [PubMed] [Google Scholar]
- 407.Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem 263: 709–716, 1999 [DOI] [PubMed] [Google Scholar]
- 408.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA 100: 5407–5412, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Sokolove PM, Kester MB, Haynes J. Interaction of adriamycin aglycones with isolated mitochondria. Effect of selenium deficiency. Biochem Pharmacol 46: 691–697, 1993 [DOI] [PubMed] [Google Scholar]
- 410.Sorgato MC, Keller BU, Stuhmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature 330: 498–500, 1987 [DOI] [PubMed] [Google Scholar]
- 411.Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem 71: 2112–2122, 1998 [DOI] [PubMed] [Google Scholar]
- 412.Srere PA. Protein crystals as a model for mitochondrial matrix proteins. Trends Biochem Sci 6: 4–7, 1981 [Google Scholar]
- 413.Srere PA. The structure of the mitochondrial inner membrane - matrix compartment. Trends Biochem Sci 7: 377–378, 1982 [Google Scholar]
- 414.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 415.Starkov AA. “Mild” uncoupling of mitochondria. Biosci Rep 17: 273–279, 1997 [DOI] [PubMed] [Google Scholar]
- 416.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann NY Acad Sci 1147: 37–52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Starkov AA. An update on the role of mitochondrial alpha-ketoglutarate dehydrogenase in oxidative stress. Mol Cell Neurosci 55: 13–16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Starkov AA, Fiskum G. Myxothiazol induces H2O2 production from mitochondrial respiratory chain. Biochem Biophys Res Commun 281: 645–650, 2001 [DOI] [PubMed] [Google Scholar]
- 419.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 86: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 420.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24: 7779–7788, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Stelmashook EV, Isaev NK, Lozier ER, Goryacheva ES, Khaspekov LG. Role of glutamine in neuronal survival and death during brain ischemia and hypoglycemia. Int J Neurosci 121: 415–422, 2011 [DOI] [PubMed] [Google Scholar]
- 422.Stelmashook EV, Lozier ER, Goryacheva ES, Mergenthaler P, Novikova SV, Zorov DB, Isaev NK. Glutamine-mediated protection from neuronal cell death depends on mitochondrial activity. Neurosci Lett 482: 151–155, 2010 [DOI] [PubMed] [Google Scholar]
- 423.Stern MD, Cheng H. Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium 35: 591–601, 2004 [DOI] [PubMed] [Google Scholar]
- 424.Stock D, Gibbons C, Arechaga I, Leslie AG, Walker JE. The rotary mechanism of ATP synthase. Curr Opin Struct Biol 10: 672–679, 2000 [DOI] [PubMed] [Google Scholar]
- 425.Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res 55: 659–665, 1999 [DOI] [PubMed] [Google Scholar]
- 426.Stoner CD, Sirak HD. Adenine nucleotide-induced contraction of the inner mitochondrial membrane. I. General characterization. J Cell Biol 56: 51–64, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Stoner CD, Sirak HD. Adenine nucleotide-induced contraction on the inner mitochondrial membrane. II. Effect of bongkrekic acid. J Cell Biol 56: 65–73, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA 100: 3497–3500, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Szabo I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem 266: 3376–3379, 1991 [PubMed] [Google Scholar]
- 431.Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337: 473–475, 1989 [DOI] [PubMed] [Google Scholar]
- 432.Takeshige K, Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J 180: 129–135, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496: 238–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Tarasov AI, Griffiths EJ, Rutter GA. Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 52: 28–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0/F1-ATPase. Am J Physiol Cell Physiol 278: C423–C435, 2000 [DOI] [PubMed] [Google Scholar]
- 436.Tomitsuka E, Kita K, Esumi H. The NADH-fumarate reductase system, a novel mitochondrial energy metabolism, is a new target for anticancer therapy in tumor microenvironments. Ann NY Acad Sci 1201: 44–49, 2010 [DOI] [PubMed] [Google Scholar]
- 437.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14: 537–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci 24: 7771–7778, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P, Lanfrancone L, Pelicci PG. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene 21: 3872–3878, 2002 [DOI] [PubMed] [Google Scholar]
- 440.Trotter EW, Grant CM. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol Microbiol 46: 869–878, 2002 [DOI] [PubMed] [Google Scholar]
- 441.Trouillard M, Meunier B, Rappaport F. Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of the respiratory chain. Proc Natl Acad Sci USA 108: E1027–1034, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem 265: 11409–11412, 1990 [PubMed] [Google Scholar]
- 443.Turnbull DM, Bartlett K, Eyre JA, Gardner-Medwin D, Johnson MA, Fisher J, Watmough NJ. Lipid storage myopathy due to glutaric aciduria type II: treatment of a potentially fatal myopathy. Dev Med Child Neurol 30: 667–672, 1988 [DOI] [PubMed] [Google Scholar]
- 444.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Twig G, Graf SA, Wikstrom JD, Mohamed H, Haigh SE, Elorza A, Deutsch M, Zurgil N, Reynolds N, Shirihai OS. Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. Am J Physiol Cell Physiol 291: C176–C184, 2006 [DOI] [PubMed] [Google Scholar]
- 446.Vacek TP, Vacek JC, Tyagi SC. Mitochondrial mitophagic mechanisms of myocardial matrix metabolism and remodelling. Arch Physiol Biochem 118: 31–42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266: 37–56, 2004 [DOI] [PubMed] [Google Scholar]
- 448.Van den Berg CJ, Garfinkel D. A stimulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem J 123: 211–218, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273: 18092–18098, 1998 [DOI] [PubMed] [Google Scholar]
- 450.Vasington FD, Gazzotti P, Tiozzo R, Carafoli E. The effect of ruthenium red on Ca2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta 256: 43–54, 1972 [DOI] [PubMed] [Google Scholar]
- 451.Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem 237: 2670–2677, 1962 [PubMed] [Google Scholar]
- 452.Vasquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J Biol Chem 275: 14064–14069, 2000 [DOI] [PubMed] [Google Scholar]
- 453.Verbon EH, Post JA, Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 511: 1–6, 2012 [DOI] [PubMed] [Google Scholar]
- 454.Vinogradov AD. Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim Biophys Acta 1364: 169–185, 1998 [DOI] [PubMed] [Google Scholar]
- 455.Vinogradov AD, Grivennikova VG. Generation of superoxide-radical by the NADH:ubiquinone oxidoreductase of heart mitochondria. Biochemistry 70: 120–127, 2005 [DOI] [PubMed] [Google Scholar]
- 456.Votyakova TV, Reynolds IJ. DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79: 266–277, 2001 [DOI] [PubMed] [Google Scholar]
- 457.Vyssokikh MY, Katz A, Rueck A, Wuensch C, Dorner A, Zorov DB, Brdiczka D. Adenine nucleotide translocator isoforms 1 and 2 are differently distributed in the mitochondrial inner membrane and have distinct affinities to cyclophilin D. Biochem J 358: 349–358, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Waggoner AS. Dye indicators of membrane potential. Annu Rev Biophys Bioeng 8: 47–68, 1979 [DOI] [PubMed] [Google Scholar]
- 459.Waggoner AS. The use of cyanine dyes for the determination of membrane potentials in cells, organelles, and vesicles. Methods Enzymol 55: 689–695, 1979 [DOI] [PubMed] [Google Scholar]
- 460.Wahllander A, Soboll S, Sies H, Linke I, Muller M. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett 97: 138–140, 1979 [DOI] [PubMed] [Google Scholar]
- 461.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell 134: 279–290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Waypa GB, Schumacker PT. O2 sensing in hypoxic pulmonary vasoconstriction: the mitochondrial door re-opens. Respir Physiol Neurobiol 132: 81–91, 2002 [DOI] [PubMed] [Google Scholar]
- 463.Weber NE, Blair PV. Ultrastructural studies of beef heart mitochondria. II. Adenine nucleotide induced modifications of mitochondrial morphology. Biochem Biophys Res Commun 41: 821–829, 1970 [DOI] [PubMed] [Google Scholar]
- 464.Weber NE, Blair PV. Ultrastuctural studies of beef heart mitochondria. I. Effects of adenosine diphosphate on mitochondrial morphology. Biochem Biophys Res Commun 36: 987–993, 1969 [DOI] [PubMed] [Google Scholar]
- 465.Wei AC, Liu T, Cortassa S, Winslow RL, O'Rourke B. Mitochondrial Ca2+ influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporine A. Biochim Biophys Acta 1813: 1373–1381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Wei L, Dirksen RT. Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondrial superoxide flashes: from discovery to new controversies. J Gen Physiol 139: 425–434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 107: 8788–8793, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem 248: 3582–3592, 1973 [PubMed] [Google Scholar]
- 469.Weisova P, Anilkumar U, Ryan C, Concannon CG, Prehn JH, Ward MW. “Mild mitochondrial uncoupling” induced protection against glutamate excitotoxicity in primary neurons requires AMPK activity. Biochim Biophys Acta 1817: 744–753, 2012 [DOI] [PubMed] [Google Scholar]
- 470.Whatley SA, Curti D, Das Gupta F, Ferrier IN, Jones S, Taylor C, Marchbanks RM. Superoxide, neuroleptics and the ubiquinone and cytochrome b5 reductases in brain and lymphocytes from normals and schizophrenic patients. Mol Psychiatry 3: 227–237, 1998 [DOI] [PubMed] [Google Scholar]
- 471.Wiesner RJ, Rosen P, Grieshaber MK. Pathways of succinate formation and their contribution to improvement of cardiac function in the hypoxic rat heart. Biochem Med Metab Biol 40: 19–34, 1988 [DOI] [PubMed] [Google Scholar]
- 472.Williams RJ. Chemical advances in evolution by and changes in use of space during time. J Theor Biol 268: 146–159, 2011 [DOI] [PubMed] [Google Scholar]
- 473.Williams RJ. Possible functions of chains of catalysts. J Theor Biol 1: 1–17, 1961 [DOI] [PubMed] [Google Scholar]
- 474.Williamson JR, Corkey BE. Assay of citric acid cycle intermediates and related compounds–update with tissue metabolite levels and intracellular distribution. Methods Enzymol 55: 200–222, 1979 [DOI] [PubMed] [Google Scholar]
- 475.Wittenberg BA, Wittenberg JB. Oxygen pressure gradients in isolated cardiac myocytes. J Biol Chem 260: 6548–6554, 1985 [PubMed] [Google Scholar]
- 476.Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta 1183: 41–57, 1993 [DOI] [PubMed] [Google Scholar]
- 477.Wu CC, Liu YB, Lu LS, Lin CW. Imaging reactive oxygen species dynamics in living cells and tissues. Front Biosci 1: 39–44, 2009 [DOI] [PubMed] [Google Scholar]
- 478.Wu YN, Munhall AC, Johnson SW. Mitochondrial uncoupling agents antagonize rotenone actions in rat substantia nigra dopamine neurons. Brain Res 1395: 86–93, 2011 [DOI] [PubMed] [Google Scholar]
- 479.Yaniv Y, Juhaszova M, Nuss HB, Wang S, Zorov DB, Lakatta EG, Sollott SJ. Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives. Ann NY Acad Sci 1188: 133–142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Yaniv Y, Juhaszova M, Sollott SJ. Age-related changes in myocardical ATP supply and demand mechanisms. Trends in Endocinol Metab 24: 495–505, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299: 700–704, 2003 [DOI] [PubMed] [Google Scholar]
- 482.Zhang L, Yu L, Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem 273: 33972–33976, 1998 [DOI] [PubMed] [Google Scholar]
- 483.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Zhou L, Aon MA, Almas T, Cortassa S, Winslow RL, O'Rourke B. A reaction-diffusion model of ROS-induced ROS release in a mitochondrial network. PLoS Comput Biol 6: e1000657, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Zhou L, Aon MA, Liu T, O'Rourke B. Dynamic modulation of Ca2+ sparks by mitochondrial oscillations in isolated guinea pig cardiomyocytes under oxidative stress. J Mol Cell Cardiol 51: 632–639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Zhou L, O'Rourke B. Cardiac mitochondrial network excitability: insights from computational analysis. Am J Physiol Heart Circ Physiol 302: H2178–H2189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Zhu J, Rebecchi MJ, Glass PS, Brink PR, Liu L. Interactions of GSK-3beta with mitochondrial permeability transition pore modulators during preconditioning: age-associated differences. J Gerontol A Biol Sci Med Sci 68: 395–403, 2013 [DOI] [PubMed] [Google Scholar]
- 488.Zoccarato F, Toscano P, Alexandre A. Dopamine-derived dopaminochrome promotes H2O2 release at mitochondrial complex I: stimulation by rotenone, control by Ca2+, and relevance to Parkinson disease. J Biol Chem 280: 15587–15594, 2005 [DOI] [PubMed] [Google Scholar]
- 489.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta 1241: 139–176, 1995 [DOI] [PubMed] [Google Scholar]
- 490.Zorov DB, Bannikova SY, Belousov VV, Vyssokikh MY, Zorova LD, Isaev NK, Krasnikov BF, Plotnikov EY. Reactive oxygen and nitrogen species: friends or foes? Biochemistry 70: 215–221, 2005 [DOI] [PubMed] [Google Scholar]
- 491.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Zorov DB, Isaev NK, Plotnikov EY, Silachev DN, Zorova LD, Pevzner IB, Morosanova MA, Jankauskas SS, Zorov SD, Babenko VA. Perspectives of mitochondrial medicine. Biochemistry 78: 979–990, 2013 [DOI] [PubMed] [Google Scholar]
- 493.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757: 509–517, 2006 [DOI] [PubMed] [Google Scholar]
- 494.Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res 83: 213–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Zorov DB, Kinnally KW, Tedeschi H. Voltage activation of heart inner mitochondrial membrane channels. J Bioenerg Biomembr 24: 119–124, 1992 [DOI] [PubMed] [Google Scholar]
- 496.Zorov DB, Kobrinsky E, Juhaszova M, Sollott SJ. Examining intracellular organelle function using fluorescent probes: from animalcules to quantum dots. Circ Res 95: 239–252, 2004 [DOI] [PubMed] [Google Scholar]
- 497.Zorov DB, Krasnikov BF, Kuzminova AE, Vysokikh M, Zorova Mitochondria revisited LD. Alternative functions of mitochondria. Biosci Rep 17: 507–520, 1997 [DOI] [PubMed] [Google Scholar]
- 498.Zorov DB, Plotnikov EY, Jankauskas SS, Isaev NK, Silachev DN, Zorova LD, Pevzner IB, Pulkova NV, Zorov SD, Morosanova MA. The phenoptosis problem: what is causing the death of an organism? Biochemistry 77: 742–753, 2012 [DOI] [PubMed] [Google Scholar]
- 499.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem 263: 1353–1357, 1988 [PubMed] [Google Scholar]
- 500.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84: 1404–1407, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]