Abstract
Pneumonia is one of the main causes of morbidity and mortality in the elderly. The elderly population has exponentially increased in the last decades and the current epidemiological trends indicate that it is expected to further increase. Therefore, recognizing the special needs of older people is of paramount importance. In this review we address the main differences between elderly and adult patients with pneumonia. We focus on several aspects, including the atypical clinical presentation of pneumonia in the elderly, the methods to assess severity of illness, the appropriate setting of care, and the management of comorbidities. We also discuss how to approach the common complications of severe pneumonia, including acute respiratory failure and severe sepsis. Moreover, we debate whether or not elderly patients are at higher risk of infection due to multi-drug resistant pathogens and which risk factors should be considered when choosing the antibiotic therapy. We highlight the differences in the definition of clinical stability and treatment failure between adults and elderly patients. Finally, we review the main outcomes, preventive and supportive measures to be considered in elderly patients with pneumonia.
Keywords: Community-acquired pneumonia, Elderly, Severity, Acute respiratory failure, Antibiotic treatment, Functional status
1. Introduction
A 79-year-old male with past medical history of hypertension, chronic stable angina, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD) class C GOLD, and prostate cancer was presented to the Emergency Department (ED) with acute respiratory symptoms. He complained of poor appetite and dyspnea for three days prior to presentation. The patient was able to walk with aids during the previous months and he had moderate cognitive impairment (Mini-Mental State Examination, MMSE: 18/30) [1]. His wife reported that he was more confused and drowsy than usual, and that he had gait and balance impairment in the previous 4–5 days. The patient denied any recent hospitalization or changes in his chronic sputum production, but reported two COPD exacerbations in the past year and recent contact with his niece who had flu-like symptoms. He was up-to-date with his annual flu-vaccine, but he had never been vaccinated against Streptococcus pneumoniae. Vital signs on arrival to the ED were: blood pressure 100/65 mm Hg, heart rate 110 bpm and respiratory rate 26 bpm, oxygen saturation 89% on room air and temperature of 36.8 °C. The patient was dehydrated and delirious (according to DSM IV-TR criteria) [2]. Chest auscultation revealed fine crackles in the right lung base and diffuse mild wheezing. Chest X-ray (CXR) on admission showed a right lower lobe consolidation. Laboratory analyses demonstrated normal white blood cell (WBC) count, blood glucose of 250 mg/dL, C-reactive protein (CRP) of 14 mg/dL (normal value: <0.5 mg/dL), procalcitonin (PCT) of 2.5 ng/mL (normal value: <0.5 ng/mL), serum lactate of 3 mmol/L, serum creatinine 1.5 mg/dL and urea nitrogen (BUN) 40 mg/dL. Arterial blood gas showed a mild hypoxemic respiratory failure (pH 7.38, PaCO2 43 mm Hg, PaO2 57 mm Hg on room air). The patient was subsequently admitted to the ward and was started on ceftriaxone and azithromycin for a presumptive diagnosis of community-acquired pneumonia (CAP). Supportive therapy consisted of supplemental oxygen, intravenous fluid replacement, prophylactic unfractionated heparin and a personalized diet. On day 4 of hospitalization, CRP and PCT began to trend down and the patient returned to his baseline mental status. However, he was still dyspneic and began to complain of palpitations. An electrocardiogram was performed and demonstrated new atrial fibrillation. The patient was started on an amiodarone drip while continuing prophylactic heparin. Sinus rhythm was achieved within a few hours after initiation of treatment. Finally, on day 7 of hospitalization, PCT was negative and the patient reached clinical stability, therefore antibiotic therapy was discontinued and the patient was discharged home. CXR at 1 month showed complete resolution of the consolidation, but the patient died two months following hospital discharge due to a presumptive cardiovascular event.
This case report includes some of the most common features presented by elderly patients with CAP.
The incidence of CAP in elderly patients is estimated to be 25–44 cases per 1000 persons [3], but it can be as high as 52 cases per 1000 persons in those aged 85 years or more [4].
In this review we address the main differences to consider when approaching elderly patients with pneumonia compared to adults.
2. Is the older patient a frail patient?
Our patient not only had multiple comorbidities, but he also presented with hypokinetic delirium superimposed on dementia and a sudden change in functional status (gait and balance impairment). Furthermore, he developed a series of adverse clinical events both during and after hospitalization. These characteristics are typical of frail subjects, who require a specific assessment and accurate identification in order to meet their complex healthcare needs. However, the recognition of a “frail” or “vulnerable” patient (e.g. a patient who is at increased risk of adverse events) is often suboptimal, since many physicians interchangeably use the terms multiple comorbidities, disability (or functional dependence) and frailty [5]. Recent studies suggest that comorbidities, disability and frailty, although interrelated, are distinct clinical entities, which may have different management and outcomes [5,6].
Several methods have been proposed to assess frail patients, but, since they may be challenging to use, frailty continues to be undetected in clinical practice [6–10]. However, gait speed and walking abilities may be used as clinical indicators of frailty in older subjects [11,12].
Acute mobility impairment and delirium are two typical geriatric syndromes [13,14]. Both these conditions are associated with negative clinical outcomes and can be triggered by an acute illness, such as pneumonia [15–18]. Functional status is the ability to manage daily routines; its deterioration may represent both a marker of frailty and a risk factor for infectious diseases [3,19].
Functional status can also predict clinical outcomes (e.g. clinical recovery, re-hospitalization and mortality), and is considered a clinical outcome itself. Bo and coworkers conducted a study on 659 older patients admitted to the intensive care unit (ICU) for any acute condition including pneumonia [20]. They found that in-hospital mortality depended not only on the severity of the acute illness and age, but also on preexisting conditions, such as loss of functional independence, severe and moderate cognitive impairment and low body mass index (BMI). Therefore, we suggest screening all elderly patients for the presence of delirium and acute mobility impairment both on admission and systematically during the hospitalization.
3. Is pneumonia presentation different in the elderly in comparison to adults?
Our patient presented to the ED with both typical (shortness of breath) and atypical (confusion and unsteadiness) symptoms of pneumonia. He was afebrile and his WBC count was normal, but both CRP and PCT were elevated. The diagnosis of pneumonia in elderly patients can be challenging because its clinical presentation may be different from younger adults. Klapdor and colleagues suggested that CAP could be a different entity in the elderly because of an atypical clinical presentation, more severe symptoms and higher long-term mortality in comparison to younger patients [21]. Several studies have further confirmed the atypical presentation of pneumonia in the elderly (Table 1) [15,22–27]. Therefore, we recommend clinicians to suspect pneumonia in older patients who have an atypical presentation (e.g. absence of radiological and laboratory abnormalities), to reduce the complications associated with delayed treatment.
Table 1.
Signs and symptoms associated with pneumonia in elderly patients.
| More common | Less common |
|---|---|
| Falls | Pleuritic chest pain |
| Acute change in functional status | Cough |
| Decreased appetite | Shortness of breath |
| Urinary incontinence | Fever |
| Delirium/acute confusional status | Leukocytosis |
4. How to evaluate the severity of the disease in elderly patients and how to choose the appropriate site of care?
Our patient presented with several clinical problems that must be considered when deciding the optimal setting of care: delirium, hypoxic respiratory failure and severe sepsis. Severity assessment tools can help in determining the optimal setting in which care should be provided. The Pneumonia Severity of Illness (PSI) score and the CURB-65 (stands for Confusion, blood Urea nitrogen, Respiratory rate, systolic or diastolic Blood pressure, and Age >65) are the most extensively studied scores assessing patients with CAP [28]. However, these scores present biases in the elderly population, particularly in assessing very elderly patients. Furthermore, studies from different countries showed that they are infrequently used by physicians in clinical practice, mainly because of the high number of variables needed to calculate each score [29,30].
Therefore, an accurate evaluation of the appropriate site of care for elderly patients cannot rely only on severity assessment scores and other factors should also be considered [31]. The Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) 2007 guidelines [32] recommend evaluating subjective factors when deciding the setting of care: patients’ ability to take oral medications, availability of outpatient support resources and caregivers in case of dependent patients, other medical or psycho-social needs (such as homelessness and poor functional status), and lack of response to previous adequate empiric antibiotic therapy [33]. However, clinicians’ experience and judgment in assessing patient’s severity is still the cornerstone of clinical management. Three possible scenarios must be taken into account when evaluating the severity of a patient with pneumonia: 1) onset of severe sepsis; 2) onset of acute respiratory failure; and 3) presence of decompensated comorbidities [34].
5. How to manage severe sepsis and acute respiratory failure in elderly patients?
Early recognition of sepsis in older compromised patients can be challenging. The classical criteria to define the systemic inflammatory response syndrome (SIRS: fever or hypothermia, tachycardia, tachypnea, or abnormal WBC count) can be absent in anergic patients. Therefore, the clinical diagnosis of sepsis can be delayed until the moment when multi-organ failure (MOF) and septic shock develop. In this scenario use of biomarkers can be helpful in early recognition. Serum lactate level is the most used biomarker to identify sepsis [19]. However, since lactate elevation corresponds to inadequate oxygen delivery/utilization, other comorbidities, such as anemia and severe dehydration, can cause its increase. Therefore, evaluation of serum lactate level could be considered in elderly patients with pneumonia at presentation and in cases where there is a prolonged time to reach clinical stability.
Acute respiratory failure can be treated with different levels of intensity of care, mainly depending on the etiology, type and severity of respiratory failure, including non-invasive ventilation (NIV) and mechanical ventilation (MV). However, despite the lack of evidence that these mechanisms of support are less effective in elderly patients, the elderly are often undertreated in clinical practice. Brandberg et al. reported that patients ≥80 years admitted to the ICU received significantly less and shorter MV support and had higher in-hospital mortality (33.7% vs. 22.8%) compared to younger patients [35]. Older patients had more limitations in care even after adjustment for severity score and comorbidities. Ely and colleagues evaluated whether age had an independent effect on the outcomes of patients admitted to the ICU who required MV [36]. Elderly patients (>75 years) had a similar duration of MV, ICU and hospital stay, and in-hospital mortality (38% vs. 31%), but lower cost of care when compared to younger patients. Therefore, it does not seem appropriate to restrict intensive care and ventilatory support only on the basis of chronologic age.
NIV has been used in the treatment of acute respiratory failure due to pneumonia [37,38]. Nava et al. performed a randomized controlled trial (RCT) to assess the effectiveness of NIV vs. standard medical therapy in very old patients (>75 years) with hypercapnic acute respiratory failure [39]. They showed that NIV decreased the need of intubation and the mortality rate, and improved arterial blood gases and dyspnea significantly faster than standard therapy alone. The authors concluded that NIV could be an alternative treatment for elderly patients considered poor candidates for intubation and those with a ‘do not intubate’ (DNI) order. In elderly patients at the end of their life (e.g. end-stage chronic diseases), pneumonia frequently represents the precipitating event leading to the patients’ death. In this scenario, palliative care is usually the treatment of choice and one of the main issues to face is patients’ worsening dyspnea. In a recent study, Nava et al. proved that NIV may be more effective than oxygen therapy in reducing dyspnea in this group of patients [40]. Therefore, NIV might play a role in the treatment of moderate–severe acute respiratory failure due to pneumonia, and in palliative care or patients with a DNI status.
6. What antibiotic treatment should be chosen in an elderly patient with CAP?
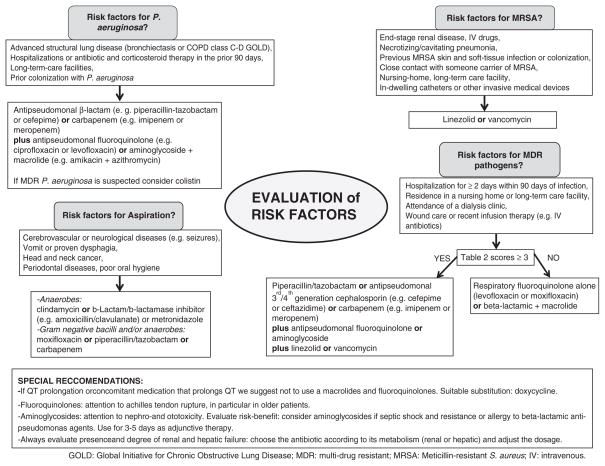
Several characteristics regarding microbiology patterns among elderly patients differ from adults with CAP, including the higher rates of pneumococcal and influenza virus pneumonia and the lower rates of atypical pathogens [23]. These considerations are important in the selection of empiric antimicrobial agents following the recommendations from the most recent clinical practice guidelines that focused on the multi-drug resistant risk factors, the severity of diseases and local patterns of antimicrobial resistance (Fig. 1) [32,41]. For outpatients and in-patients non-ICU a respiratory fluoroquinolone alone (levofloxacin or moxifloxacin) or a beta-lactamic plus a macrolide are appropriate choices. In ICU patients a beta-lactamic plus either a fluoroquinolone or a macrolide should be considered.
Fig. 1.
Antimicrobial treatment options for patients with community-acquired pneumonia according to the risk factors.
6.1. Are elderly patients at higher risk for multi-drug resistant (MDR) pathogens?
Elderly patients may have multiple risk factors for acquiring MDR pathogen infections. Frequent contact with the health system is one of the most important risk factor and includes prior hospitalizations, long-term facilities or nursing homes, home healthcare programs (home intravenous therapy and wound care), hemodialysis, and antibiotic treatment in the previous 3 months. In the attempt to optimize treatments for patients presenting with the previous risk factors, the terms healthcare-associated pneumonia (HCAP) and nursing home-acquired pneumonia (NHAP) were created. The causative agents of both HCAP and NHAP may resemble those isolated in hospital-acquired pneumonia (HAP) rather than CAP (e. g. Pseudomonas aeruginosa and Enterobacteriaceae) [42]. However, the real need for broad-spectrum antimicrobial coverage in hospitalized patients with HCAP and NHAP is still matter of significant controversy [43,44]. Furthermore, to consider all HCAP patients at higher risk of infection with MDR pathogens may be an overestimation of the real risk [45–47]. Therefore, different investigators developed scoring systems to better predict the presence of MDR pathogens (Table 2) [48,49]. These scores can help to identify patients that will really benefit from a broad-spectrum antibiotic course, although their validation in large cohorts of patients is needed.
Table 2.
Scoring systems to assess the multi-drug resistant pathogens in patients with pneumonia.
| Shorr
|
Aliberti
|
||
|---|---|---|---|
| Score | Variable | Score | Variable |
| 1 | Admitted to ICU within 24 h from admission | 0 | No risk factors for MDR pathogens |
| 0.5 | ≥1 of the following: CVD, diabetes, COPD, antimicrobial therapy in the prior 90 days, immunosuppression, home wound care or IV therapy | ||
| 2 | Chronic hemodialysis | ||
| 3 | Long-term facility resident | ||
| 3 | Long-term care facility resident | ||
| 4 | Hospitalization in the prior 90 days | 4 | Hospitalization in the prior 90 days |
| 5 | Chronic renal failure | ||
| Maximum score 10 | Maximum score 12.5 | ||
| <3 pts | prevalence MDR < 20% | ≤0.5 pts | prevalence MDR = 8% |
| 3–5 pts | prevalence MDR = 55% | ≥3 pts | prevalence MDR = 38% |
| >5 pts | prevalence MDR > 75% | ||
MDR: multi-drug resistant; CVD: cardiovascular diseases; COPD: chronic obstructive pulmonary disease; IV: intra venous; ICU: intensive care unit; pts: points.
6.2. Aspiration
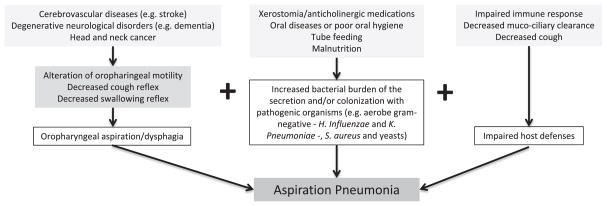
Elderly patients present more risk factors for developing aspiration pneumonia than younger patients (Fig. 2). Aging itself can alter the swallowing mechanism and host defenses, but comorbidities, cognitive impairment and disability are the main reasons why aspiration pneumonia is more common in elderly patients [50]. Moreover, aspiration is a risk factor for severe pneumonia and carries a high mortality rate [51,52].
Fig. 2.
Etiopathology of aspiration pneumonia.
According to a recent meta-analysis [53], there are several conditions associated with increased risk of aspiration in the elderly, including age, male gender, lung diseases, dysphagia, diabetes mellitus, severe dementia, angiotensin I-converting enzyme deletion genotype, poor oral health, malnutrition, Parkinson’s disease, use of antipsychotic drugs, proton pump inhibitors and angiotensin-converting enzyme inhibitors. These conditions need to be carefully assessed in all elderly patients at risk.
Diagnosing aspiration pneumonia can be challenging, particularly when the episode is not witnessed. It can be suspected if risk factors are present or there is evidence of infiltrates in gravity-dependent segments. A good diagnostic tool is the fibreoptic endoscopic evaluation of swallowing (FEES), which detects aspiration of secretions and can be performed at the bedside [50].
The most common pathogens isolated are oropharyngeal flora, including anaerobes, Gram-positive cocci and Gram-negative bacilli. Antibiotics with specific anaerobic activity are strongly recommended in patients with periodontal disease, those expectorating putrid sputum or those with necrotizing pneumonia or lung abscess on chest radiograph [54].
6.3. Immunosuppression
Epidemiological studies suggest an increased predisposition of the elderly to infections [55]. This finding has led to question whether elderly patients have a higher degree of immunosuppression compared to younger patients, due to the interaction of the aging of the immune system, comorbidities and medications.
According to recent literature, there is a remodeling of the immune system during senescence: a reduced production of B and T cells in the bone marrow and thymus and diminished function of mature lymphocytes [55,56]. Meyer et al. in a comprehensive review listed several comorbidities that alter the immune system and predispose elderly patients to pneumonia, including: diabetes, chronic renal or hepatic failure, congestive heart failure, malignancy, asplenia, immunosuppressive therapy (such as corticosteroids), alcoholism and malnutrition [57,58]. Moreover, comorbidities, such as COPD and bronchiectasis, can alter the defensive mechanisms of the lung, diminishing muco-ciliary clearance. In a recent study, Sousa and colleagues compared the causative agent of pneumonia in 115 immunocompromised vs. non-immunocompromised elderly patients [59]. S. pneumoniae was the most common causative agent in both groups, but Gram-negative bacilli were more frequent among immunocompromised patients, particularly, P. aeruginosa and Nocardia spp.
If risk factors for immunosuppression are present, it is important to keep a high level of awareness for P. aeruginosa and other opportunistic pathogens.
6.4. Chronic obstructive pulmonary disease
Advanced stage COPD is common in elderly patients. Patients with advanced COPD with frequent exacerbations are at higher risk of pneumonia and also of P. aeruginosa colonization [26,27]. In these patients P. aeruginosa may be considered as the potential pathogen causing pneumonia and a combination therapy should be started to maximize bacterial coverage and exerts a lower resistance selection pressure (Fig. 1) [28]. Furthermore, recent observations associated the use of inhaled corticosteroids to an increased risk of pneumonia in patients with COPD [60,61]. However, further studies are warranted to better investigated this possible association.
7. How do I evaluate clinical stability in the elderly?
Clinical stability should be evaluated as an early outcome in patients with pneumonia. The most common criteria to define clinical stability are recommended by clinical practice guidelines published by the ATS 2001 and the ATS/IDSA 2007 (Table 3). The former is based on both patient’s symptoms and clinical/laboratory data, the latter on objective clinical measures. A recent study performed by Aliberti et al. on a population of 500 adults hospitalized with CAP showed that the criteria recommended by the ATS 2001 identified clinical stability significantly earlier than those recommended by the ATS/IDSA 2007 [62]. However, the two sets of criteria were clinically equivalent to determine clinical failure or death within 30-days of clinical presentation. The use of inflammatory biomarkers may also assist clinicians in the evaluation of response to antibiotic therapy in elderly patients. Menendez et al. found that low levels of CRP and PCT, in addition to clinical criteria, might improve the prediction of absence of severe complications in hospitalized patients with CAP [63]. However, the effect of immune deficit on PCT levels is still a subject of controversy [64,65]. Therefore, the interpretation of PCT results in the elderly requires special attention because of the higher incidence of immunosuppression in this population.
Table 3.
Criteria for clinical stability described in clinical practice guidelines.
| ATS 2001 criteria | ATS/IDSA 2007 criteria |
|---|---|
| Improved symptoms of pneumonia such as cough and shortness of breath | Temperature ≤ 37.8 °C Heart rate ≤ 100 beats/min |
| Lack of fever for at least 8 h | Respiratory rate ≤ 24 breaths/min |
| Improving leukocytosis (↓ at least 10% from the prior day) | Systolic blood pressure ≥ 90 mm Hg SatO2 ≥ 90% or PaO2 ≥60 mm Hg on room air Normal mental status |
SatO2: oxygen saturation; PaO2: partial pressure of oxygen in arterial blood gas.
The challenge in the elderly remains the atypical clinical presentation, the absence of fever and leukocytosis, which makes the ATS/IDSA 2007 criteria a more suitable option to assess clinical stability in older adults. Another personalized method to assess clinical stability is to follow the parameters (symptoms, laboratory exams or vital signs) that were altered on admission, and the acute changes in cognitive and functional status, especially in frail subjects. Bellelli and colleagues showed that in elderly patients with delirium at presentation, its resolution may represent a clinical marker of improvement [15]. Furthermore, time to achieve clinical stability may differ between adult and elderly patients. It usually takes 3 to 7 days in adult patients without major co-morbidities, but it may require longer time in frail elderly patients.
8. How do I manage treatment failure and which complications should I expect in an elderly patient with pneumonia?
Treatment failure is the most common reason for not achieving clinical stability and its incidence ranges between 6% and 15% [66]. Our patient had atrial fibrillation on day 4 and probably this is the reason for a delay in clinical stability. Different options to manage treatment failure are summarized in Table 4.
Table 4.
Management of treatment failure in elderly patients with CAP.
| More extensive diagnostic work-up |
| E.g. bronchoscopic evaluation, ultrasonography, and/or CT scan |
| Expansion of antimicrobial coverage |
| E.g. clinical suspicion for MDR or less common pathogens |
| Evaluation of other infectious sources and/or nosocomial superinfections |
| E.g. UTI due to urinary catheter |
| Rule out non-infectious process |
| E.g. pulmonary embolism and CHF |
| Reassessment of duration, doses, route, drug interactions of the selected antimicrobial agents |
| E.g. adjust antibiotic dosage according to creatinine clearance; proarrhytmic effect of macrolides and fluoroquinolones due to possible QT prolongation |
| Optimization of adjuvant therapies |
| E.g. nutritional assessment, hydration and oxygen support |
| Stabilization and treatment of medical comorbidities |
| E.g. concomitant CHF and COPD exacerbations |
CT: computerized tomography; UTI: urinary tract infections; CHF: congestive heart failure.
COPD: chronic obstructive pulmonary disease.
A study by Aliberti et al. retrospectively evaluated 500 patients hospitalized for CAP, 67 (13%) of them experienced clinical failure. The most common causes of clinical failure were severe sepsis, myocardial infarction (MI), progressive pneumonia and HAP [67]. Several authors have recently reported an increased risk of cardiovascular events in hospitalized patients with CAP [68,69]. A meta-analysis by Corrales-Medina et al. highlighted how common cardiovascular events are among patients with pneumonia, with an overall cardiac events rate of 18% in the 17 studies evaluated [70]. Another manuscript by Corrales-Medina and colleagues performed on 2287 adult patients with CAP found that older age, nursing home residence, preexisting cardiovascular disease and pneumonia severity were associated with cardiac complications [71]. The most common events were worsening CHF (67%), new or worsening arrhythmias (22%) and MI (4%). The development of cardiac complications was associated with a 60% increase in the risk of 30-day mortality. A recent review tried to explore the causal association between pneumonia and cardiovascular events to improve preventive intervention [72].
9. Are elderly patients at higher risk of re-hospitalization or increased long-term mortality?
Our patient died 2 months after the hospitalization, thought to be due to a cardiovascular event, despite a complete resolution of pneumonia. We are used to think about pneumonia as an acute event. However, pneumonia may be a life-changing event with long-term sequelae, particularly in elderly patients [58]. Up to 60% of elderly patients hospitalized for acute illnesses risk a loss of independent physical function [73,74]. The mechanisms are still unclear, but inflammation could play a role in patients with pneumonia. Cohen et al. described an association between increased pro-inflammatory cytokines and functional disability [75]. Furthermore, in the frail elderly, an acute disease can cause loss of physiologic reserve, which results in an incomplete or prolonged recovery.
Several studies have identified that advanced age is associated with higher risk of long term-mortality in CAP [76,77]. Kaplan defined pneumonia as “the old man’s friend”, after finding that almost half of the older patients hospitalized with CAP died in the subsequent year [78]. The mortality was considerably higher than that of either the general population or control patients hospitalized for reasons other than CAP. Bruns and colleagues showed that causes of death in the years following an episode of CAP were mainly related to comorbidities (malignancy 27%, respiratory diseases 27% [COPD 19%], and vascular diseases 16%), and not attributable to recurrent pneumonia [79].
Two recent manuscripts addressed the topic of 30-day hospital readmission after hospitalization for pneumonia in elderly patients [80,81]. Lindenauer et al. evaluated 226,545 hospitalizations and calculated a 30-day readmission rate of 17.4% [80]. Dharmarajan et al. described a 30-day readmission rate of 18.3%; 22.4% of the readmissions were caused by a new pneumonia [81]. A review by Calvillo-King et al. examined 20 studies regarding readmission and mortality after pneumonia [82]. Most of them evaluated age, and found that older age was associated with worse outcomes (age ≥65 OR = 2.7 [0.3–21.6]). However, most of these studies were not adjusted for variables such as frailty, and cognitive and functional status. Therefore, it is likely that age represents a surrogate indicator of disability rather than a risk factor per se.
10. Are there other outcomes we should consider in the elderly with pneumonia?
Our patient had several conditions, including physical, cognitive and nutritional impairment, which might be affected by an acute event, such as pneumonia. A recent paper by Davydow et al. showed how hospitalization for pneumonia in adults >50 years was associated with subsequent functional decline, cognitive impairment and depressive symptoms [83]. Moreover, the degree of functional decline after an infectious disease seems to occur in a dose–effect manner (the greater the infectious insult, the higher the risk of functional decline) [33]. A similar dose–response effect was found in long-term care residents, in which the number of infectious episodes was associated with functional decline [84].
Age-related muscle atrophy due to prolonged immobilization, pneumonia-related hypoxia and systemic inflammation have been proposed to be the main pathogenic mechanisms. A study by Mundy et al. showed how early mobilization of hospitalized pneumonia patients was associated with a reduction in the overall hospital length of stay without increasing the risk of adverse outcomes [85].
Physical rehabilitation is often used after hospitalizations for cardiovascular and cerebrovascular events or for chronic diseases, such as COPD. Increased utilization of rehabilitation after a CAP event in selected categories of elder patients could prevent or ameliorate subsequent physical dysfunction.
Another factor that seems to impact mortality is the patient’s nutritional status. Low albumin level and low BMI were found to be risk factors associated with long-term mortality in patients with CAP [20,58]. Furthermore, a recent study by King et al. examined a cohort of 18,746 veterans hospitalized for CAP and stratified for BMI class [86]. Underweight patients had increased 90-day mortality (OR 1.40, 95% CI 1.14–1.73), while obesity seemed to play a protective effect (OR 0.86, 95% CI 0.74–0.99). Furthermore, a poor nutritional status can be the first sign of other illnesses, such as dysphagia, dementia and malignancies.
Despite their importance, a recent audit by Lindhard et al. highlighted that nutritional status and mobilization are rarely assessed in hospitalized patients with CAP [30].
11. What are the most relevant preventive measures in elderly patients with pneumonia?
Our patient was up-to-date with the flu-vaccination, but he had never been vaccinated for S. pneumoniae. S. pneumoniae is the most common etiologic pathogen both in elderly and younger adult patients with pneumonia. At present, two pneumococcal vaccines are available for use in adults: the 23-valent pneumococcal polysaccharide vaccine (PPSV) and the 13-valent protein-polysaccharide conjugate vaccine (PCV13). According to the CDC guidelines [www.cdc.org], PPSV is recommended for use in all adults older than 65 years of age and for persons who are 2 years and older and at high risk for pneumococcal disease. PCV13 is recommended for children and has been recently approved for use in people over 50 years old [87]. The main disadvantage of PPSV is that it may be less effective than PCV13, because of poorer immunogenicity. Nevertheless, it may present some advantages as it provides protection against 10 additional serotypes [87]. Moreover, patients who received PPSV and developed pneumonia seem to have a lower risk of bacteremia, decreased length of stay and lower rate of mortality or ICU admission [88–90]. PPSV seems effective in preventing invasive pneumococcal disease (mainly bacteremic pneumococcal pneumonia), but its effectiveness in preventing non-invasive pneumococcal infections is less certain. Recent systematic reviews comparing immunogenicity of the two vaccines in adults have concluded that PCV13 is at least as effective as PPSV for the strains that both vaccines cover, but a definitive advantage of PCV13 has not been demonstrated [91,92]. Large RCTs are currently evaluating the efficacy of PVC13 in older adults [93].
Large cohort studies have shown that influenza vaccination significantly reduces the risk of influenza infections and mortality in elderly patients, although this efficacy differs according to co-morbid conditions and demographic factors [94,95]. However, despite the persistent concerns regarding the vaccine’s protection and safety in immunocompromised patients or immediately after recovery from pneumonia, there is consensus that both vaccines should be recommended.
Learning points.
In elderly patients with acute illnesses, such as CAP, it is recommended that a plan be established on admission regarding the aggressiveness of treatment (high/medium intensity – full code- or supportive/palliative care –DNI or ‘do not resuscitate’). This plan should take into account patients’ wishes (living will), functional and cognitive status, and severity of comorbidities, but not age alone.
Elderly patients often have a significant number of risk factors associated with higher risk of MDR pathogens.
Systematic evaluation of cognitive, nutritional (hydration included) and functional status must become an integral part of the clinical examination of elderly patients with CAP in order to recognize early impairment and establish supportive measures.
Long-term mortality rate after CAP hospitalization is unacceptably high, particularly in the elderly population. Effective preventive measures are desirable and immunization compliance must be improved. Close follow-up both by general practitioners and specialists is also recommended. The aim should be early recognition of new infectious events and decompensation of chronic comorbidities.
Footnotes
Conflict of interests
The authors have no conflicts of interests to declare related to this manuscript. Dr Restrepo time is partially protected by Award Number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The funding agencies had no role in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the author and do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Trull TJ, Vergés A, Wood PK, Jahng S, Sher KJ. The structure of Diagnostic and Statistical Manual of Mental Disorders (4th edition, text revision) personality disorder symptoms in a large national sample. Pers Disord. 2012;3:355–69. doi: 10.1037/a0027766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssens J-P, Krause K-H. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–24. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 4.Jackson ML, Neuzil KM, Thompson WW, Shay DK, Yu O, Hanson CA, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–50. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–9. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: a new measure to predict mortality in institutionalized older people. J Am Geriatr Soc. 2003;51:96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- 10.Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–6. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 11.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE. Mobility performance: a high-tech test for geriatricians. J Gerontol A Biol Sci Med Sci. 2003;58:712–4. doi: 10.1093/gerona/58.8.m712. [DOI] [PubMed] [Google Scholar]
- 14.Morley JE, Flaherty JH, Thomas DR. Geriatricians, continuous quality improvement, and improved care for older persons. J Gerontol A Biol Sci Med Sci. 2003;58:M809–12. doi: 10.1093/gerona/58.9.m809. [DOI] [PubMed] [Google Scholar]
- 15.Bellelli G, Guerini F, Cerri AP, Trabucchi M. A sudden decline in mobility status as an early sign of acute infection in elderly patients: evidence from three case reports. Aging Clin Exp Res. 2012;24:281–4. doi: 10.1007/BF03325259. [DOI] [PubMed] [Google Scholar]
- 16.Bellelli G, Speciale S, Morghen S, Torpilliesi T, Turco R, Trabucchi M. Are fluctuations in motor performance a diagnostic sign of delirium? J Am Med Dir Assoc. 2011;12:578–83. doi: 10.1016/j.jamda.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Khokhar SR, Stern Y, Bell K, Anderson K, Noe E, Mayeux R, et al. Persistent mobility deficit in the absence of deficits in activities of daily living: a risk factor for mortality. J Am Geriatr Soc. 2001;49:1539–43. doi: 10.1046/j.1532-5415.2001.4911251.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263–70. doi: 10.1111/j.1532-5415.2004.52354.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker CA, Griffith DM, Gray AJ, Datta D, Hay AW. Early lactate clearance in septic patients with elevated lactate levels admitted from the emergency department to intensive care: time to aim higher? J Crit Care. 2013;28(5):832–7. doi: 10.1016/j.jcrc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Bo M, Massaia M, Raspo S, Bosco F, Cena P, Molaschi M, et al. Predictive factors of inhospital mortality in older patients admitted to a medical intensive care unit. J Am Geriatr Soc. 2003;51:529–33. doi: 10.1046/j.1532-5415.2003.51163.x. [DOI] [PubMed] [Google Scholar]
- 21.Klapdor B, Ewig S, Pletz MW, Rohde G, Schütte H, Schaberg T, et al. Community-acquired pneumonia in younger patients is an entity on its own. Eur Respir J. 2012;39:1156–61. doi: 10.1183/09031936.00110911. [DOI] [PubMed] [Google Scholar]
- 22.Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J. 2008;101:1141–5. doi: 10.1097/SMJ.0b013e318181d5b5. [quiz 1132, 1179] [DOI] [PubMed] [Google Scholar]
- 23.Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother. 2010;8:47–62. doi: 10.1016/j.amjopharm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Kelly E, MacRedmond RE, Cullen G, Greene CM, McElvaney NG, O’Neill SJ. Community-acquired pneumonia in older patients: does age influence systemic cytokine levels in community-acquired pneumonia? Respirology. 2009;14:210–6. doi: 10.1111/j.1440-1843.2008.01423.x. [DOI] [PubMed] [Google Scholar]
- 25.Ahkee S, Srinath L, Ramirez J. Community-acquired pneumonia in the elderly: association of mortality with lack of fever and leukocytosis. South Med J. 1997;90:296–8. doi: 10.1097/00007611-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Metlay JP, Schulz R, Li YH, Singer DE, Marrie TJ, Coley CM, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453–9. [PubMed] [Google Scholar]
- 27.Fernández-Sabé N, Carratalà J, Rosón B, Dorca J, Verdaguer R, Manresa F, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore) 2003;82:159–69. doi: 10.1097/01.md.0000076005.64510.87. [DOI] [PubMed] [Google Scholar]
- 28.Stupka JE, Mortensen EM, Anzueto A, Restrepo MI. Community-acquired pneumonia in elderly patients. J Aging Health. 2009;5:763–74. doi: 10.2217/ahe.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serisier DJ, Williams S, Bowler SD. Australasian respiratory and emergency physicians do not use the pneumonia severity index in community-acquired pneumonia. Respirology. 2013;18:291–6. doi: 10.1111/j.1440-1843.2012.02275.x. [DOI] [PubMed] [Google Scholar]
- 30.Lindhardt T, Klausen HH, Christiansen C, Smith LL, Pedersen J, Andersen O. Elderly patients with community-acquired pneumonia are not treated according to current guidelines. Dan Med J. 2013;60:A4572. [PubMed] [Google Scholar]
- 31.Aliberti S, Faverio P, Blasi F. Hospital admission decision for patients with community-acquired pneumonia. Curr Infect Dis Rep. 2013;15:167–76. doi: 10.1007/s11908-013-0323-7. [DOI] [PubMed] [Google Scholar]
- 32.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres OH, Muñoz J, Ruiz D, Ris J, Gich I, Coma E, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603–9. doi: 10.1111/j.1532-5415.2004.52492.x. [DOI] [PubMed] [Google Scholar]
- 34.Ewig S, Woodhead M, Torres A. Towards a sensible comprehension of severe community-acquired pneumonia. Intensive Care Med. 2011;37:214–23. doi: 10.1007/s00134-010-2077-0. [DOI] [PubMed] [Google Scholar]
- 35.Brandberg C, Blomqvist H, Jirwe M. What is the importance of age on treatment of the elderly in the intensive care unit? Acta Anaesthesiol Scand. 2013;57(6):698–703. doi: 10.1111/aas.12073. [DOI] [PubMed] [Google Scholar]
- 36.Ely EW, Evans GW, Haponik EF. Mechanical ventilation in a cohort of elderly patients admitted to an intensive care unit. Ann Intern Med. 1999;131:96–104. doi: 10.7326/0003-4819-131-2-199907200-00004. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer M, Cosentini R, Nava S. The use of non-invasive ventilation during acute respiratory failure due to pneumonia. Eur J Intern Med. 2012;23:420–8. doi: 10.1016/j.ejim.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160:1585–91. doi: 10.1164/ajrccm.160.5.9903015. [DOI] [PubMed] [Google Scholar]
- 39.Nava S, Grassi M, Fanfulla F, Domenighetti G, Carlucci A, Perren A, et al. Non-invasive ventilation in elderly patients with acute hypercapnic respiratory failure: a randomised controlled trial. Age Ageing. 2011;40:444–50. doi: 10.1093/ageing/afr003. [DOI] [PubMed] [Google Scholar]
- 40.Nava S, Ferrer M, Esquinas A, Scala R, Groff P, Cosentini R, et al. Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol. 2013;14:219–27. doi: 10.1016/S1470-2045(13)70009-3. [DOI] [PubMed] [Google Scholar]
- 41.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64:iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 42.Chroneou A, Zias N, Beamis JF, Jr, Craven DE. Healthcare-associated pneumonia: principles and emerging concepts on management. Expert Opin Pharmacother. 2007;8:3117–31. doi: 10.1517/14656566.8.18.3117. [DOI] [PubMed] [Google Scholar]
- 43.El-Solh AA, Niederman MS, Drinka P. Nursing home-acquired pneumonia: a review of risk factors and therapeutic approaches. Curr Med Res Opin. 2010;26:2707–14. doi: 10.1185/03007995.2010.530154. [DOI] [PubMed] [Google Scholar]
- 44.Ma HM, Wah JLS, Woo J. Should nursing home-acquired pneumonia be treated as nosocomial pneumonia? J Am Med Dir Assoc. 2012;13:727–31. doi: 10.1016/j.jamda.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Kollef MH. Health care-associated pneumonia: perception versus reality. Clin Infect Dis. 2009;49:1875–7. doi: 10.1086/648430. [DOI] [PubMed] [Google Scholar]
- 46.Ewig S, Welte T, Torres A. Is healthcare-associated pneumonia a distinct entity needing specific therapy? Curr Opin Infect Dis. 2012;25:166–75. doi: 10.1097/QCO.0b013e32835023fb. [DOI] [PubMed] [Google Scholar]
- 47.Chalmers JD, Taylor JK, Singanayagam A, Fleming GB, Akram AR, Mandal P, et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis. 2011;53:107–13. doi: 10.1093/cid/cir274. [DOI] [PubMed] [Google Scholar]
- 48.Aliberti S, Di Pasquale M, Zanaboni AM, Cosentini R, Brambilla AM, Seghezzi S, et al. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis. 2012;54:470–8. doi: 10.1093/cid/cir840. [DOI] [PubMed] [Google Scholar]
- 49.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med. 2008;168:2205–10. doi: 10.1001/archinte.168.20.2205. [DOI] [PubMed] [Google Scholar]
- 50.Kikawada M, Iwamoto T, Takasaki M. Aspiration and infection in the elderly: epidemiology, diagnosis and management. Drugs Aging. 2005;22:115–30. doi: 10.2165/00002512-200522020-00003. [DOI] [PubMed] [Google Scholar]
- 51.Torres OH, Gil E, Pacho C, Ruiz D. Update of pneumonia in the elderly. Rev Esp Geriatr Gerontol. 2013;48:72–8. doi: 10.1016/j.regg.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Heppner HJ, Sehlhoff B, Niklaus D, Pientka L, Thiem U. Pneumonia Severity Index (PSI), CURB-65, and mortality in hospitalized elderly patients with aspiration pneumonia. Z Gerontol Geriatr. 2011;44:229–34. doi: 10.1007/s00391-011-0184-3. [DOI] [PubMed] [Google Scholar]
- 53.Van der Maarel-Wierink CD, Vanobbergen JNO, Bronkhorst EM, Schols JMGA, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12:344–54. doi: 10.1016/j.jamda.2010.12.099. [DOI] [PubMed] [Google Scholar]
- 54.Marik PE. Pulmonary aspiration syndromes. Curr Opin Pulm Med. 2011;17:148–54. doi: 10.1097/MCP.0b013e32834397d6. [DOI] [PubMed] [Google Scholar]
- 55.Dewan SK, Zheng S, Xia S, Bill K. Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin Med J (Engl) 2012;125:3325–31. [PubMed] [Google Scholar]
- 56.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–65. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer KC. The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly. Semin Respir Crit Care Med. 2010;31:561–74. doi: 10.1055/s-0030-1265897. [DOI] [PubMed] [Google Scholar]
- 58.Restrepo MI, Faverio P, Anzueto A. Long-term prognosis in community-acquired pneumonia. Curr Opin Infect Dis. 2013;26:151–8. doi: 10.1097/QCO.0b013e32835ebc6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sousa D, Justo I, Domínguez A, Manzur A, Izquierdo C, Ruiz L, et al. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin Microbiol Infect. 2013;19:187–92. doi: 10.1111/j.1469-0691.2012.03765.x. [DOI] [PubMed] [Google Scholar]
- 60.Eurich DT, Lee C, Marrie TJ, Majumdar SR. inhaled corticosteroids and risk of recurrent pneumonia: a population-based, nested case–control study. Clin Infect Dis. 2013;57(8):1138–44. doi: 10.1093/cid/cit472. [DOI] [PubMed] [Google Scholar]
- 61.Yawn BP, Li Y, Tian H, Zhang J, Arcona S, Kahler KH. Inhaled corticosteroid use in patients with chronic obstructive pulmonary disease and the risk of pneumonia: a retrospective claims data analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:295–304. doi: 10.2147/COPD.S42366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aliberti S, Zanaboni AM, Wiemken T, Nahas A, Uppatla S, Morlacchi LC, et al. Criteria for clinical stability in hospitalised patients with community-acquired pneumonia. Eur Respir J. 2012;42(3):742–9. doi: 10.1183/09031936.00100812. [DOI] [PubMed] [Google Scholar]
- 63.Menéndez R, Martinez R, Reyes S, Mensa J, Polverino E, Filella X, et al. Stability in community-acquired pneumonia: one step forward with markers? Thorax. 2009;64:987–92. doi: 10.1136/thx.2009.118612. [DOI] [PubMed] [Google Scholar]
- 64.Mikuła T, Cianciara J, Wiercińska-Drapało A. Is there any influence of immune deficit on procalcitonin results? Hum Immunol. 2011;72:1194–7. doi: 10.1016/j.humimm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Bele N, Darmon M, Coquet I, Feugeas J-P, Legriel S, Adaoui N, et al. Diagnostic accuracy of procalcitonin in critically ill immunocompromised patients. BMC Infect Dis. 2011;11:224. doi: 10.1186/1471-2334-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sialer S, Liapikou A, Torres A. What is the best approach to the nonresponding patient with community-acquired pneumonia? Infect Dis Clin North Am. 2013;27:189–203. doi: 10.1016/j.idc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Aliberti S, Amir A, Peyrani P, Mirsaeidi M, Allen M, Moffett BK, et al. Incidence, etiology, timing, and risk factors for clinical failure in hospitalized patients with community-acquired pneumonia. Chest. 2008;134:955–62. doi: 10.1378/chest.08-0334. [DOI] [PubMed] [Google Scholar]
- 68.Peyrani P, Ramirez J. What is the association of cardiovascular events with clinical failure in patients with community-acquired pneumonia? Infect Dis Clin North Am. 2013;27:205–10. doi: 10.1016/j.idc.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Soto-Gomez N, Anzueto A, Waterer GW, Restrepo MI, Mortensen EM. Pneumonia: an arrhythmogenic disease? Am J Med. 2013;126:43–8. doi: 10.1016/j.amjmed.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125:773–81. doi: 10.1161/CIRCULATIONAHA.111.040766. [DOI] [PubMed] [Google Scholar]
- 72.Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 73.El Solh A, Pineda L, Bouquin P, Mankowski C. Determinants of short and long term functional recovery after hospitalization for community-acquired pneumonia in the elderly: role of inflammatory markers. BMC Geriatr. 2006;6:12. doi: 10.1186/1471-2318-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmer RM. Acute hospital care of the elderly: minimizing the risk of functional decline. Cleve Clin J Med. 1995;62:117–28. doi: 10.3949/ccjm.62.2.117. [DOI] [PubMed] [Google Scholar]
- 75.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–8. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 76.Mortensen EM, Kapoor WN, Chang C-CH, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37:1617–24. doi: 10.1086/379712. [DOI] [PubMed] [Google Scholar]
- 77.Johnstone J, Eurich DT, Majumdar SR, Jin Y, Marrie TJ. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine (Baltimore) 2008;87:329–34. doi: 10.1097/MD.0b013e318190f444. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan V, Clermont G, Grifdin MF, Kasal J, Watson RS, Linde-Zwirble WT, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163:317–23. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 79.Bruns AHW, Oosterheert JJ, Cucciolillo MC, El Moussaoui R, Groenwold RHH, Prins JM, et al. Cause-specific long-term mortality rates in patients recovered from community-acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect. 2011;17:763–8. doi: 10.1111/j.1469-0691.2010.03296.x. [DOI] [PubMed] [Google Scholar]
- 80.Lindenauer PK, Normand S-LT, Drye EE, Lin Z, Goodrich K, Desai MM, et al. Development, validation, and results of a measure of 30-day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6:142–50. doi: 10.1002/jhm.890. [DOI] [PubMed] [Google Scholar]
- 81.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–63. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calvillo-King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28:269–82. doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davydow DS, Hough CL, Levine DA, Langa KM, Iwashyna TJ. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med. 2013;126(7):615–24. doi: 10.1016/j.amjmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Büla CJ, Ghilardi G, Wietlisbach V, Petignat C, Francioli P. Infections and functional impairment in nursing home residents: a reciprocal relationship. J Am Geriatr Soc. 2004;52:700–6. doi: 10.1111/j.1532-5415.2004.52205.x. [DOI] [PubMed] [Google Scholar]
- 85.Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest. 2003;124:883–9. doi: 10.1378/chest.124.3.883. [DOI] [PubMed] [Google Scholar]
- 86.King P, Mortensen EM, Bollinger M, Restrepo MI, Copeland LA, Pugh MJV, et al. Impact of obesity on outcomes for patients hospitalised with pneumonia. Eur Respir J. 2013;41:929–34. doi: 10.1183/09031936.00185211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vila-Corcoles A, Ochoa-Gondar O. Preventing pneumococcal disease in the elderly: recent advances in vaccines and implications for clinical practice. Drugs Aging. 2013;30(5):263–76. doi: 10.1007/s40266-013-0060-5. [DOI] [PubMed] [Google Scholar]
- 88.Mykietiuk A, Carratalà J, Domínguez A, Manzur A, Fernández-Sabé N, Dorca J, et al. Effect of prior pneumococcal vaccination on clinical outcome of hospitalized adults with community-acquired pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis. 2006;25:457–62. doi: 10.1007/s10096-006-0161-8. [DOI] [PubMed] [Google Scholar]
- 89.Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis. 2006;42:1093–101. doi: 10.1086/501354. [DOI] [PubMed] [Google Scholar]
- 90.Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938–43. doi: 10.1001/archinte.167.18.1938. [DOI] [PubMed] [Google Scholar]
- 91.Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52:633–40. doi: 10.1093/cid/ciq207. [DOI] [PubMed] [Google Scholar]
- 92.Fedson DS, Nicolas-Spony L, Klemets P, van der Linden M, Marques A, Salleras L, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines. 2011;10:1143–67. doi: 10.1586/erv.11.99. [DOI] [PubMed] [Google Scholar]
- 93.Hak E, Grobbee DE, Sanders EAM, Verheij TJM, Bolkenbaas M, Huijts SM, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med. 2008;66:378–83. [PubMed] [Google Scholar]
- 94.Voordouw BCG, van der Linden PD, Simonian S, van der Lei J, Sturkenboom MCJM, Stricker BHC. Influenza vaccination in community-dwelling elderly: impact on mortality and influenza-associated morbidity. Arch Intern Med. 2003;163:1089–94. doi: 10.1001/archinte.163.9.1089. [DOI] [PubMed] [Google Scholar]
- 95.Ortqvist A, Granath F, Askling J, Hedlund J. Influenza vaccination and mortality: prospective cohort study of the elderly in a large geographical area. Eur Respir J. 2007;30:414–22. doi: 10.1183/09031936.00135306. [DOI] [PubMed] [Google Scholar]