Abstract
Rationale
Progesterone and its metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP), have actions in the ventral tegmental area (VTA) that are required for lordosis, a characteristic mating posture of female rodents. 17β-estradiol (estradiol) co-varies with progestogens over natural cycles, enhances production of 3α,5α-THP, is required for successful reproductive behavior.
Objectives
A question of interest is the role of pregnane xenobiotic receptor (PXR), a nuclear receptor that regulates enzymes needed for the production of 3α,5α-THP, for estradiol-mediated lordosis. The hypothesis tested was that if PXR is involved in estradiol-mediated biosynthesis of 3α,5α-THP and reproductive behavior, knocking down expression of PXR in the VTA of estradiol-primed, but not vehicle-primed, rats should decrease lordosis and midbrain 3α,5α-THP; effects may be attenuated by 3α,5α-THP administered to the VTA.
Methods
Ovariectomized rats were administered subcutaneous injections of oil vehicle or estradiol. Rats were then administered PXR antisense oligonucleotides (PXR AS-ODNs; which are expected to locally knock down expression of PXR), or control (saline), infusions to the VTA. Rats were administered 3α,5α-THP or vehicle via infusions to the VTA. Reproductive behavior (paced mating task) of rats was determined in addition to exploratory (open field), affective (elevated plus maze), pro-social (social interaction task) behavior.
Results
Reproductive behavior (i.e. increased lordosis) was enhanced with estradiol-priming and infusions of 3α,5α-THP to the VTA. Infusions of PXR AS-ODNs to the VTA attenuated responses in estradiol-, but not vehicle-, primed rats, compared to control infusions.
Conclusions
PXR may be involved in a neuroregulatory response involving biosynthesis of 3α,5α-THP in the midbrain VTA of estradiol-primed rats.
Keywords: pregnane xenobiotic receptor (PXR); midbrain ventral tegmental area; 3α,5α,-THP
Introduction
One approach that has been taken to further understand the mechanisms of progestogens for complex behaviors is to use lordosis, a hormone-dependent mating posture of female rodents, as a bioassay. Progestogens amplify estradiol-initiated increases in reproductive behavior, in part through actions in brain regions, such as the ventromedial hypothalamus (Ahdieh et al. 1986; Christensen et al. 2011; Debold et al. 1982; Mani and Blaustein 2012; Micevych and Christensen 2012; Pleim et al. 1989; Rubin and Barfield 1983a,b) and the midbrain ventral tegmental area (VTA; Debold and Malsbury 1989; Frye 2011; Lisciotto et al. 1991; Pleim et al. 1990; 1991). Analyses of lordosis responding has been utilized to elucidate steroid receptor as well as novel neurotransmitter targets of progestogens in brain regions, such as the ventromedial hypothalamus (Balasubramanian et al., 2008a,b; García-Juárez et al., 2011; Georgescu and Pfaus 2006a,b; González-Mariscal et al., 1989; Etgen et al., 2006), and the midbrain VTA (reviewed in Frye 2011). Thus, there are novel mechanisms of action in brain circuits supporting lordosis.
A related question to understanding the novel targets of progestogens is elucidating the sources of progestogens in the midbrain. In this region, some of progesterone’s effects occur through actions of 5α-pregnan-3α-ol-20-one (3α,5α-THP). Ovarian progesterone is readily metabolized to dihydroprogesterone by 5α-reductase and then to 3α,5α-THP by 3α-hydroxysteroid dehydrogenase in the VTA; blocking any of these enzymes attenuates progesterone’s facilitating effects on lordosis and other socially-relevant behaviors, and 3α,5α-THP-replacement can reverse these effects (Beyer et al. 1999; Frye 2011; Mòdol et al. 2011). However, progesterone’s and 3α,5α-THP’s effects to prevent restraint stress-induced reductions in lordosis of female rats are not mitigated by administration of metabolism inhibitors, suggesting other sources of progestogens for effects on lordosis (Miryala et al. 2011). 3α,5α-THP is a neurosteroid produced de novo in the brain (Baulieu 1991). This de novo synthesis of 3α,5α-THP in the brain was noted by Purdy and colleagues decades ago following exposure to acute stressors (Barbaccia et al. 1996; Purdy et al. 1991; Vallée et al., 2000). In the midbrain VTA, de novo synthesis of 3α,5α-THP has been noted following social challenges, such as mating (reviewed in Frye 2011). Inhibitors of rate-limiting factors (e.g. 18-kDa translocator protein, steroidogenic acute regulatory protein, cytochrome P450-dependent side chain cleavage enzymes) involved in cholesterol metabolism, and, thereby, production of 3α,5α-THP attenuate lordosis (reviewed in Frye 2011; King et al. 2002; Mellon and Deschepper 1993; Papadopoulos et al., 2006). An important question is what other upstream factors may be important for regulating 3α,5α-THP biosynthesis.
Pregnane Xenobiotic Receptor (PXR) may be involved in 3α,5α-THP’s actions in the VTA. PXR acts as a transcription factor for cytochrome P450 enzymes involved in drug and steroid metabolism (Harmsen et al. 2007; Ma et al. 2008; Xu et al. 2005; Zhang et al. 2008). PXR has been referred to as the “master regulator” of xenobiotic clearance for its role in metabolism and efflux of these factors (Dussault and Forman 2002; Francis et al. 2003; Geick et al. 2001; Kliewer et al. 2002). PXR is most well-known for these effects in the liver, and other excretory and barrier tissues, such as the kidneys and intestines, and has received less focus on its potential in the central nervous system until more recently. It had been thought that PXR was not expressed in the central nervous system, but PXR has been identified in the central nervous system in many mammals, including humans, pigs, rabbits, and rodents (Bauer et al. 2004; Frye 2011; Frye et al. 2011; Lamba et al. 2004; Marini et al., 2007; Mellon et al. 2008). A role of hormonal milieu for PXR expression is supported by the observation that there is greater expression of PXR protein in the midbrain of proestrous rats compared to diestrous rats or male rats (Frye 2011). Activating PXR in the midbrain VTA with PXR ligands, including 3α,5α-THP, increases lordosis of estradiol-primed rats (Frye 2011), whereas blocking PXR in the midbrain VTA with antisense oligodeoxynucleotides (AS-ODN) infusions to this region reduced expression of PXR and 3α,5α-THP levels in the midbrain and lordosis responding of proestrous rats (Frye et al. 2013). Whether replacement of 3α,5α-THP following knock down of PXR in the VTA can reverse these effects is of interest.
During proestrus, there are sequential increases in estradiol followed by elevations in progestogen levels, such that our previous studies on the role of PXR for mating was not able to fully address whether these effects of PXR manipulations were estradiol- and/or progestogen-dependent. Estradiol can enhance neurosteroidogenic enzyme activity and, subsequently, 3α,5α-THP levels (Cheng and Karavolas 1975; Frye 2011). As well, estradiol-priming is required for 3α,5α-THP to facilitate the consummatory aspects of mating behavior of ovariectomized female rats (Frye et al. 2008). How these distinct effects of estradiol may involve PXR has not been systematically investigated. We hypothesized that if PXR is involved as an important factor in estradiol-mediated biosynthesis of 3α,5α,-THP in the midbrain and lordosis, infusions of PXR AS-ODNs to the VTA of estradiol-primed, but not vehicle-primed, rats should decrease reproductive behavior and midbrain 3α,5α-THP levels. Further, these effects may be attenuated by replacement of 3α,5α-THP to the midbrain VTA. Because reproduction of rats involves lordosis as well as changes in exploration, anxiety and social behaviors, and mating-induced biosynthesis of 3α,5α-THP occurs in corticolimbic structures (reviewed in Frye 2011), behavior of rats in the open field, elevated plus maze, and social interaction test was assessed with standard measures of reproduction (lordosis, proceptivity, and aggression/rejection).
Methods and Materials
Principles of laboratory animal care, “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003), as well as United States laws governing use of animal subjects in research were followed in completing the experiments described herein. These methods involving animal subjects in the experiments described in this report were pre-approved by the Institutional Animal Care and Use Committee at the institution where the experiment was conducted (The University at Albany-SUNY).
Animal Housing
Long-Evans female rats (N=158), approximately 55–60 days old, were obtained from the breeding colony in the Life Sciences Laboratory Animal Core Facility at The University at Albany-SUNY (original stock obtained from Taconic, Germantown, NY, USA), or were purchased from Taconic. All experimental rats were group-housed in polycarbonate cages (45 cm long × 24 cm wide × 21 cm high) with autoclaved woodchip bedding, and continuous access to Purina Rat Chow and tap water in their home cages. The housing room was maintained at 21 ± 1 °C and on a 12:12 h reversed light cycle (lights off at 08:00 h), such that rats were assessed during their dark/active phase. Breeder male rats from the colony (N=15) were utilized in the paced mating task, and were group-housed with other breeder males in the same room as the females, as described above. Age-, strain-, and weight-matched ovariectomized female rats (n=3, “stimulus rats”) were utilized in the social interaction task. These female stimulus rats were used for all the social interaction testing as they were habituated in the task, which reduces social interaction that they initiate with the experimental rat.
General procedure
Rats were included in this experiment in several small cohorts (10–25 rats/cohort) that were run consecutively so that the age of rats and the timing of recovery from surgery to when rats were manipulated and assessed remained consistent across cohorts (7 days).Cohorts were counterbalanced so that at least 1 animal was assigned each treatment condition in each cohort run. Adult female rats were ovariectomized and surgically implanted with guide cannulae aimed at the VTA and allowed to recover for one week. Starting 44 hours before behavioral testing, rats were primed with estradiol or vehicle via subcutaneous injections and received their first intra-VTA infusions of PXR AS-ODNs or saline (control). At hour 24 before behavioral testing, rats received a second subcutaneous injection or estradiol or vehicle and another dosing of intra-VTA infusions of PXR AS-ODNs or saline (control). One half-hour before behavioral testing (hour 43.5), rats received their last intra-VTA infusions of PXR AS-ODNs or saline (control) and then 3α,5α,-THP or β-cyclodextrin. Rats were tested at hour 44 in a single battery of consecutive tasks. Following β-cyclodextrin or 3α,5α-THP infusions, rats were tested in a single battery of tasks (consecutive assessments in the open field, elevated plus maze, social interaction, and paced mating tasks) to assess exploration/anxiety, social, and reproductive behaviors. Assessments of behaviors beyond lordosis is important because mating-induced biosynthesis of 3α,5α-THP has been shown in forebrain regions involved in motivated and affective processes, such as the striatum, hippocampus, and cortex in addition to the midbrain (Frye 2011). Immediately after testing, rats were euthanized and had tissues (brain and trunk blood) collected immediately after the last task in the battery (paced mating). Estradiol was measured in plasma and progestogens were measured in brain. Knock down of PXR in midbrain was confirmed with PXR qPCR.
Surgical Protocol
Experimental rats were ovariectomized via bilateral flank incisions and stereotaxically-implanted with bilateral guide cannulae aimed at the VTA during one surgical session. These surgeries were completed under general anesthesia from administration of xylazine (12 mg/kg) and ketamine (80 mg/kg). Stereotaxic coordinates from bregma for the midbrain VTA were 5.3 mm posterior, 0.4 mm lateral on the right and left, and 7.0 mm ventral (Paxinos and Watson, 1986). Guide cannulae consisted of 23-gauge stainless steel needles with 30-gauge removable inserts made from dental needles with sharp tips removed and sanded smooth. After surgery, rats were neurologically evaluated daily for their ability to right themselves, cage-climb, and show proper muscle tone and reflexive responses to hind limb extension. Rats were also evaluated on weight gain after surgery. All rats passed neurological evaluations and gained weight following surgery and were included in the experiment. Manipulations began after 7 days of recovery from surgery.
Estradiol priming
Rats were randomly-assigned to be administered subcutaneous estradiol (10 μg total, from two injections of 5 μg estradiol in 0.1 cc Wesson vegetable oil; ConAgra Foods, Omaha, NE) or vehicle (Wesson vegetable oil; two injections of 0.1 cc) at 44 and 24 hours before behavioral testing. Subcutaneous injections were to the scruff of the rats’ necks. Because rats were ovariectomized and either vehicle- or estradiol-primed, it was deemed important to verify the estradiol levels produced by the treatment during behavioral assessments. As such, levels in plasma (from trunk blood collected immediately after testing) were measured using estradiol radioimmunoassay methods that are described below in detail. Ovariectomized, estradiol-primed rats had higher estradiol levels in plasma (24.0+2.7 pg/ml) than did vehicle-administered rats (8.6±0.6 pg/ml).
Antisense Oligonucleotide Infusions
Rats were administered 1 μl control or PXR AS-ODN (5′ CTTGCGGAAGGGGCACCTCA 3′; 100 ng) infusions 0.5, 24 and 44 hours before behavioral testing (purchased from Invitrogen Life Technologies, Carlsbad, CA; Frye et al. 2013). The vehicle for PXR AS-ODN infusions was sterile saline. The “control” infusions were sterile saline as we have previously demonstrated no behavioral or endocrine differences between rats administered the control conditions of saline or scrambled ODN infusions to the VTA (Frye et al., 2013). Rats were administered three infusions of AS-ODN or the control condition because of the potential for AS-ODNs to degrade and become ineffective. The timing of these infusions was as per previous experiments and aimed to knock down PXR throughout the rise in estradiol levels post-priming as well as behavioral assessments.
3α,5α-THP infusions
Rats were either infused with β-cyclodextrin vehicle or 3α,5α,-THP 30 minutes before testing (hour 43.5). 3α,5α-THP (purchased from the late Dr. Robert Purdy, Scripps Research Institute, CA, whom I have enjoyed fruitful conversations and collaborations with for decades) was prepared to a concentration of 100 ng/1μl in β-cyclodextrin (Frye and Rhodes 2006).
Behavioral Testing
Rats were tested sequentially in the following battery of tasks in one testing session without time in between tasks in the order listed: open field, elevated plus maze, social interaction, and paced mating task. Behavioral assessments and corticosterone measurements in plasma in this and/or previous studies (e.g. Frye et al., 2013 and unpublished results) using the exact testing protocol or those in which shorter batteries (open field, elevated plus maze, and social interaction alone, or after paced mating, or paced mating alone) do not suggest a robust stress response among rats when tested in this battery, or carry-over behavioral effects due to stress from completing previous behavioral procedures. However, the potential for those effects need to be considered in interpreting the data. To be able to assess reproductive behavior in the context of exploration, anti-anxiety, and pro-social behavior, as well as interpret results in the context of previous findings, we have used this short battery of tasks with tissue collection immediately after. Behavioral data were simultaneously collected by using the Any-maze behavioral assessment computer program (Stoelting Inc., Wood Lawn, IL; for open field, elevated plus maze, and social interaction), or a digital video camera (for paced mating), and trained experimenters.
Open Field
Rats were assessed in this task, using methods as first described by Hall and Ballachey (1932), and recently in our laboratory (Frye et al. 2013), where central or inner 8 square entries are used as an index of anti-anxiety behavior and the total number of entries is used as an index of general motor/exploratory behavior.
Elevated Plus Maze
Rats were assessed in this task as first described by Handley and Mithani (1984) and recently reported by our laboratory (Frye et al. 2013). The time spent in the open arms (as compared to closed arms(was utilized as an index of anti-anxiety-like behavior because there were no differences between groups for the number of closed, open, or total arm entries, and the average percentage of open arm to total arm entries was low (~13% across groups).
Social Interaction
Rats were assessed in this task using methods first described by File and Hyde (1978), and recently implemented in our laboratory (Frye et al., 2013), where the total time spent by each experimental rat with one the stimulus rat engaging in any behaviors considered social interaction (crawling over and under partner, sniffing of partner, following with contact, anogenital investigation, tumbling, and grooming) were recorded. Separate measurements of these different types of social interactions were not determined.
Paced Mating
Reproductive responses of rats in the paced mating task, which uses a chamber with a partition that allows the female to spend time in close proximity to the male or not, were assessed (Erskine, 1985; Frye et al., 2013). Standard measures of mating behavior (lordosis quotients, lordosis ratings, proceptivity quotients, and aggression quotients) were recorded.
Tissue Collection and preparation for RIA and QPCR
Rats were euthanized by rapid decapitation immediately after testing. Whole brain and trunk blood were collected to measure steroid hormone levels in brain and plasma. Brains were immediately placed on dry ice and then stored at −80 until dissections. For dissections, punches from the midbrain, around the VTA, were taken from frozen slices for qPCR. At this time, whether cannulae/infusion tracks were aimed at the VTA was noted. There were 17 rats that had placement outside of the VTA (see Figure 1 for a representative picture of a “miss” to the VTA); their data was excluded from analyses of the data from rats with placement to the VTA. Although a method to determine spread of infusion (i.e. infusions of thionine or cresyl violet in the same volume as infusions of drug conditions) was not utilized here, we have previously reported that such infusions to the VTA spread approximately 1 mm in all directions and do not extend beyond the VTA to impinge on other nearby midbrain regions (Frye and Rhodes, 2006). The pattern of responses of rats with missed site placement of cannulae for behavioral measures and central steroid levels was unlike that observed in rats with infusions to the VTA. Following this punching out of the VTA, the rest of the midbrain that was remaining was then dissected out and placed in a chilled test tube and homogenized in 1 ml ddH2O to be used for steroid measurement by radioimmunoassay. The hippocampus, hypothalamus, and prefrontal cortex were dissected out from the thawed whole brain in a similar fashion.
Figure 1.
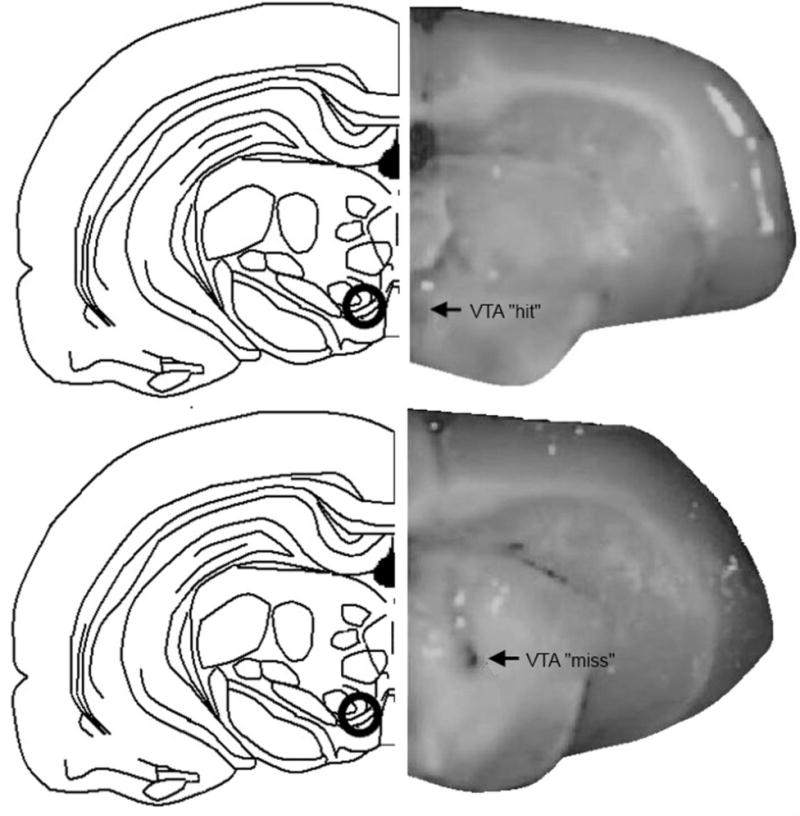
Depiction of midbrain ventral tegmental area (VTA) targeted with infusions (black circle) in drawings on the left (Top and Bottom panel). Pictures on the right show correct placement of VTA (“hit”; Top panel) or placement outside of the VTA (“miss”; Bottom panel), as determined by visual inspection during brain dissections.
qPCR methods
Standard qPCR methods were utilized on tissues that had been preserved in RNA later (Qiagen) immediately following dissection as described in detail in a previous report (Frye et al. 2013). Data were analyzed using the comparative cycle time (DeltaDeltaCT) method. The fold change in comparison to vehicle controls of the delta CT values of PXR versus actin are depicted (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
Radioimmunoassay for Steroid Hormones
Standard steroid extraction and radioimmunoassay techniques used by our laboratory were employed to measure plasma estradiol levels and brain progesterone, dihydroprogesterone, and 3α,5α-THP levels (Frye et al. 2013).
Statistical Analyses
Three-way analyses of variances (ANOVAs) were used to examine effects of hormone condition (vehicle, estradiol), PXR AS-ODN condition (control, PXR AS-ODN), and 3α,5α,-THP condition (β-cyclodextrin, 3α,5α,-THP) on behavioral and endocrine measures. The α level for statistical significance was p ≤ 0.05 for main effects and interactions. Fisher’s Least Significant Differences post hoc tests were used to determine treatment differences from vehicle controls at each independent variable level.
Results
PXR expression in the midbrain (Figure 1)
There was a significant main effect of PXR AS-ODN condition [F 1, 129 = 17.1, P < 0.01] to influence fold changes in PXR expression. Rats infused with PXR AS-ODN had lower PXR expression in the midbrain VTA compared to control infusions. There were no main effects for estradiol or 3α,5α,-THP condition, or interactions between these variables or with the AS-ODN condition, for PXR expression.
Behavioral measures (Table 1 and Figure 2)
Table 1.
Indicated in the tables are the number of observations per condition and the behavioral testing measures (means ± s.e.m. for each) of rats that had infusions of antisense oligodeoxynucleotides (AS-ODNs) or vehicle (control) to the midbrain ventral tegmental area (VTA).
| Hormone Condition | Condition | |||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | Estradiol | |||||||
| Intra-VTA AS-ODN | Control | PXR AS-ODN | Control | PXR AS-ODN | ||||
| Intra-VTA infusion | β-cyclodextrin | 3α,5α-THP | β-cyclodextrin | 3α,5α-THP | β-cyclodextrin | 3α,5α-THP | β-cyclodextrin | 3α,5α-THP |
| N | 20 | 20 | 13 | 18 | 15 | 19 | 16 | 20 |
| Total Open Field Entries | 246.9 ± 19.5 | 261.5 ± 32.8 | 263.8 ± 37.5 | 238.0 ± 17.2 | 219.1 ± 22.9 | 218.5 ± 18.9 | 254.5 ± 24.3 | 251.1 ± 31.6 |
| Central Open Field Entries | 105.6 ± 15.9 | 106.1 ± 23.8 | 118.1 ± 26.8 | 86.5 ± 15.8 | 65.2 ± 17.9 | 64.0 ± 16.5 | 89.3 ± 18.0 | 97.4 ± 22.4 |
| Inner 8 Open Field Entries | 31.5 ± 6.2 | 28.2 ± 8.1 | 35.7 ± 9.9 | 18.2 ± 4.0 | 17.3 ± 6.8 | 17.6 ± 6.4 | 31.6 ± 7.1 | 25.7 ± 8.1 |
| Open Arm Time (secs) | 20.1 ± 5.4 | 17.2 ± 5.8 | 11.4 ± 3.5 | 22.4 ± 6.8 | 31.5 ± 15.1 | 7.4 ± 2.6 | 20.1 ± 4.8 | 26.2 ± 7.7 |
| Total time Spent Interacting (secs) | 80.6 ± 8.5 | 80.7 ± 4.9 | 73.8 ± 6.7 | 75.2 ± 5.2 | 68.9 ± 8.7 | 76.8 ± 6.7 | 73.6 ± 8.9 | 78.7 ± 9.6 |
| Lordosis Ratings (scale 0–3) | 0.01 ± 0.01 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.6 ± 0.2^ | 1.0 ± 0.1^+ | 0.2 ± 0.5^ | 0.9 ± 0.2+ |
| Proceptivity Quotients (%) | 0.0 ± 0.0 | 0.09 ± 0.01 | 0.0 ± 0.0 | 0.0 ± 0.0 | 24.3 ± 7.9^ | 32.6 ± 8.7^ | 4.7 ± 2.3^* | 22.7 ± 6.7^* |
| Aggression Quotients (%) | 31.1 ± 5.8 | 16.6 ± 4.1 | 31.7 ± 5.8* | 28.9 ± 6.1* | 23.4 ± 7.2 | 13.3 ± 4.2 | 30.1 ± 7.8* | 26.5 ± 6.1* |
indicates a main effect of estradiol condition (P<0.05). + indicates a main effect of 3α,5α-THP condition (P<0.05).
indicates a main effect of PXR AS-ODN condition (P<0.05).
Figure 2.
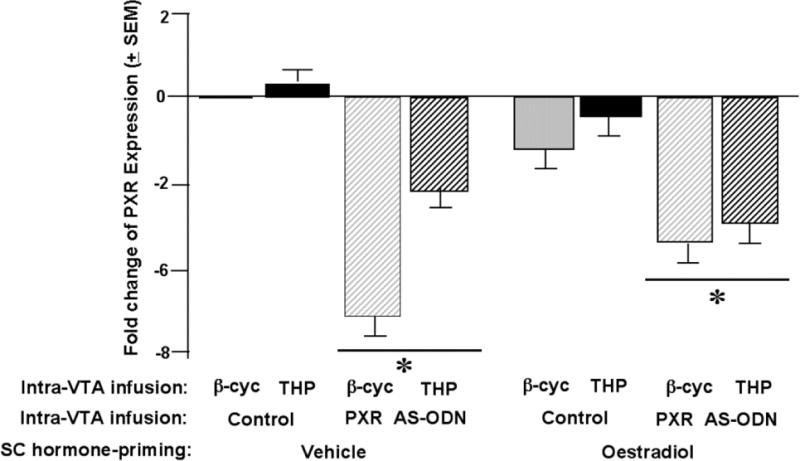
Bars depicts the mean fold change of pregnane xenobiotic receptor (PXR) expression (fold-change from control) in the midbrain ventral tegmental area (VTA). * indicates a main effect of PXR antisense oligodeoxynucleotide (AS-ODN) condition (P<0.05).
There were no statistically significant main effects, or interactions between variables, for any measures collected in the open field, elevated plus maze, or social interaction tasks (Table 1). There were significant differences between groups for reproductive measures (described as follows). See Figure 2 for lordosis quotients data and Table 1 for lordosis ratings, proceptivity quotients, and aggression quotients data.
There was significant interaction between estradiol condition and PXR AS-ODN condition for lordosis quotients [F 1, 133 = 9.5, P< 0.01], and proceptivity quotients [F 1, 133 = 3.860, P = 0.05], but not lordosis ratings or aggression quotients. Estradiol-primed rats infused with PXR-AS-ODN had lower lordosis quotients and proceptivity quotients than did those infused with saline (control condition) to the VTA.
There was a significant interaction between estradiol condition and 3α,5α-THP condition for lordosis quotients [F 1, 133 = 4.5, P < 0.03], lordosis ratings [F 1, 133 = 6.759, P < 0.01], and proceptivity quotients [F 1, 133 = 3.711, P = 0.05], but not aggression quotients. Estradiol-primed rats infused with 3α,5α-THP had higher lordosis quotients and ratings and proceptivity quotients compared to controls. There were no interactions between estradiol condition, PXR-AS-ODN condition and 3α,5α-THP condition for lordosis quotients or ratings, or proceptivity or aggression quotients.
There was a main effect for estradiol condition for lordosis quotients [F 1, 133 = 138.4, P<0.01], lordosis ratings [F 1, 133 = 51.995, P < 0.01], and proceptivity quotients [F 1, 133 = 33.770, P < 0.01], but not aggression quotients. Rats administered estradiol had increased lordosis quotients, lordosis ratings, and proceptivity quotients compared to vehicle-administered rats.
There was a significant main effect of PXR AS-ODN condition for lordosis quotients [F 1, 133 = 11.5, P < 0.01], proceptivity quotients [F 1, 133 = 4.591, P = 0.03], and aggression quotients [F 1, 133 = 3.705, P = 0.05], but not lordosis ratings. Rats infused with PXR AS-ODN, compared to control infusions, had decreased lordosis quotients, proceptivity quotients and increased aggression quotients.
There was a significant main effect for 3α,5α-THP condition for lordosis quotients [F 1,133 = 15.900, P< 0.01] and ratings [F 1, 133 = 10.436, P< 0.01], but not proceptivity quotients or aggression quotients. Rats infused with 3α,5α-THP had increased lordosis quotients and ratings compared to rats infused with β-cyclodextrin.
3α,5α-THP levels in the midbrain (Figure 2)
There was significant interaction between estradiol condition and AS-ODN condition [F 1, 133 = 6.3, P < 0.01] for 3α,5α-THP levels in the midbrain VTA, such that estradiol-primed, but not vehicle-primed, rats had higher levels of 3α,5α-THP levels in the midbrain with control compared to PXR AS-ODN infusions. There were no interactions between estradiol condition, PXR AS-ODN condition and 3α,5α-THP condition for 3α,5α-THP levels in the midbrain.
There was a main effect for estradiol condition [F 1, 133 = 4.4, P = 0.03] for 3α,5α-THP levels in midbrain VTA. Rats administered estradiol had higher levels of midbrain 3α,5α-THP compared to rats administered vehicle. There was a significant main effect of PXR AS-ODN condition [F 1, 133 = 13.2, P < 0.01] for 3α,5α-THP levels in the midbrain. Rats infused with PXR AS-ODN had significantly lower 3α,5α-THP in the midbrain compared to those infused with control condition. There was a main effect for 3α,5α-THP condition [F 1, 133 = 3.7, P = 0.05] for 3α,5α-THP levels, with 3α,5α-THP infusions increasing 3α,5α-THP levels in midbrain compared to β-cyclodextrin.
Progesterone, dihydroprogesterone, and 3α,5α-THP levels (Table 2)
Table 2.
Indicated in the table are the number of observations per condition and the mean (ng/mg ± s.e.m.) steroid levels of progesterone and dihydroprogesterone and 3α,5α-THP in the midbrain, cortex, hippocampus, and hypothalamus, among rats that had antisense oligodeoxynucleotides (AS-ODNs) or vehicle (control) infusions to the midbrain ventral tegmental area (VTA).
| Hormone condition | Condition | |||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | Estradiol | |||||||
| Intra-VTA AS-ODN | Control | PXR AS-ODN | Control | AS-ODN | ||||
| Intra-VTA infusion | β-cyclodextrin | 3α,5α-THP | β-cyclodextrin | 3α,5α-THP | β-cyclodextrin | 3α,5α-THP | β-cyclodextrin | 3α,5α-THP |
| n | 20 | 20 | 13 | 18 | 15 | 19 | 16 | 20 |
| Midbrain | ||||||||
| Progesterone | 2.5 ± 0.4 | 1.8 ± 0.5 | 3.8 ± 1.4 | 1.9 ± 0.4 | 4.1 ± 1.6 | 4.4 ± 2.3 | 3.7 ± 1.1 | 2.2 ± 0.7 |
| Dihydroprogesterone | 15.5 ± 2.7 | 17.0 ± 3.2 | 24.2 ± 7.2 | 14.6 ± 3.6 | 18.1 ± 3.1 | 14.1 ± 2.2 | 12.7 ± 3.2 | 9.8 ± 1.9 |
| 3α,5α-THP | See Figure 2 | |||||||
| Cortex | ||||||||
| Progesterone | 2.8 ± 0.8 | 2.1 ± 0.6 | 3.3 ± 1.2 | 1.3 ± 0.4 | 3.2 ± 0.9 | 2.3 ± 1.0 | 2.5 ± 1.1 | 2.6 ± 1.1 |
| Dihydroprogesterone | 13.3 ± 2.4 | 10.8 ± 1.8 | 12.1 ± 6.0 | 7.8 ± 1.3 | 7.4 ± 1.4 | 11.0 ± 3.4 | 9.4 ± 2.7 | 9.8 ± 2.2 |
| 3α,5α-THP | 6.8 ± 1.5 | 11.1 ± 1.8 | 9.7 ± 4.5 | 7.8 ± 1.2 | 9.9 ± 2.0 | 4.9 ± 0.7 | 5.7 ± 0.7 | 6.3 ± 0.8 |
| Hippocampus | ||||||||
| Progesterone | 4.2 ± 1.8 | 2.4 ± 0.8 | 1.9 ± 0.5 | 1.2 ± 0.4 | 2.1 ± 0.4 | 1.5 ± 0.4 | 2.1 ± 0.7 | 1.5 ± 0.4 |
| Dihydroprogesterone | 13.5 ± 1.6 | 11.6 ± 1.4 | 8.3 ± 2.6 | 8.6 ± 1.8 | 6.4 ± 1.2 | 7.5 ± 1.3 | 11.2 ± 2.3 | 9.2 ± 1.7 |
| 3α,5α-THP | 8.3 ± 1.4 | 8.2 ± 1.4 | 8.5 ± 2.1 | 10.0 ± 1.8 | 9.4 ± 2.0 | 6.6 ± 1.1 | 5.1 ± 1.0 | 4.6 ± 0.7 |
| Hypothalamus | ||||||||
| Progesterone | 3.9 ± 0.9 | 2.2 ± 1.0 | 3.7 ± 1.1 | 3.0 ± 0.9 | 3.2 ± 0.6 | 2.0 ± 0.5 | 2.9 ± 0.9 | 2.1 ± 0.7 |
| Dihydroprogesterone | 17.6 ± 2.8 | 19.2 ± 2.6 | 20.7 ± 4.1 | 14.5 ± 3.2 | 20.1 ± 12.4 | 12.4 ± 2.0 | 11.5 ± 2.4 | 11.6 ± 2.3 |
| 3α,5α-THP | 9.0 ± 12.8 | 12.8 ± 1.4 | 10.4 ± 15.2 | 15.2 ± 3.2 | 19.0 ± 10.8 | 10.9 ± 1.4 | 13.3 ± 3.4 | 8.4 ± 1.2 |
There were no statistically significant differences in progesterone or dihydroprogesterone levels in the midbrain or cortex, hippocampus and hypothalamus; Despite differences in midbrain 3α,5α-THP levels, there were no statistically significant differences in 3α,5α-THP levels measured in the cortex, hippocampus and hypothalamus.
Discussion
Results partially supported our hypothesis that PXR may be involved in production of 3α,5α-THP in the midbrain VTA of estradiol-primed rats, a necessary condition for 3α,5α-THP facilitation of lordosis and other reproductively-relevant behaviors (exploration, anti-anxiety, social). Although there were no effects of hormone and PXR manipulations for exploration and anti-anxiety-like behavior (i.e. total and central entries in the open field; open arm time in the plus maze) or pro-social (i.e. time spent by experimental rat engaging in social interaction with a conspecific) behavior, there were robust differences among estradiol-primed rats for reproductive behavior (i.e. lordosis quotients and ratings, proceptivity quotients). Lordosis and proceptivity of ovariectomized rats was enhanced with estradiol-priming and infusions of 3α,5α,-THP to the midbrain VTA, but attenuated with infusions of PXR AS-ODNs to the VTA coincident with reductions in PXR expression and 3α,5α-THP levels in the midbrain. These data suggest that PXR may be involved in the biosynthesis of 3α,5α,-THP in the midbrain VTA of estradiol-primed rats and subsequent effects for reproductive responding.
The present study confirms and extends the role of estradiol for 3α,5α-THP-facilitated lordosis in the midbrain VTA. Here, 3α,5α-THP’s effects to increase lordosis, an effect blocked by PXR AS-ODNs, was only observed in rats that were estradiol-primed. Moreover, the effect of manipulating PXR may be specific to 3α,5α-THP in the midbrain. This is supported by infusions of PXR AS-ODNs reducing 3α,5α-THP, but not altering other progestogens, in the midbrain, and only reducing lordosis to levels that are typical in rats primed with estradiol alone. However, alternative interpretations of our findings need to be explored. Although 3α,5α-THP levels in the estradiol-primed animals are comparable to those of vehicle treated 3α,5α-THP levels, behavior of the estradiol-primed animals are more robust than any of the vehicle groups. Combined with the fact that there is also no additive effect of 3α,5α-THP, these results suggest that it is possible that another unrelated pathway is involved in estradiol-induced lordosis, with 3α,5α-THP having a permissive role with estradiol-priming. In addition, 3α,5α-THP is able to induce lordosis behavior in the vehicle treated (non-estradiol primed) PXR AS-ODN group to the same level as controls. For both lordosis quotients and midbrain 3α,5α-THP levels, there are significant interactions, with the most robust effects of 3α,5α-THP infusions to increase, and PXR AS-ODN infusions to decrease, lordosis and midbrain 3α,5α-THP in estradiol-, rather than vehicle-, primed rats. Experiments are ongoing to further understand the role of PXR as a homeostatic regulator in this system.
In addition to these specific effects only being observed in estradiol-primed rats, there was behavioral and site-specificity observed. For example, no effects were noted in the open field, elevated plus maze, or social interaction tasks. This was somewhat unexpected, particularly in the elevated plus maze, given previous results showing clear effects of estradiol-priming to enhance these behaviors. In the present study, compared to these past studies, a pulsed dosing of estradiol was utilized with rats receiving 10 μg of estradiol over two injections administered 44 and 24 hours before behavioral testing (coincident with PXR AS-ODN or control infusions). This dosing regimen and/or the testing of rats in a brief consecutive battery of tasks as was utilized in the present experiment may have produced these attenuated responses of rats to estradiol in the elevated plus maze as well as the other tasks assessed here. As well, no differences were noted for progesterone or dihydroprogesterone levels in any of the brain regions where these progestogens were measured, or in 3α,5α-THP levels outside of the midbrain. Thus, the most robust effects herein were for 3α,5α-THP biosynthesis in the midbrain and changes in a motivated behavior, lordosis, mediated by this region.
We had anticipated that 3α,5α-THP-replacement in rats that received PXR AS-ODNs would have reversed these effects of PXR knockdown in the brain for lordosis and 3α,5α-THP, but this was not observed. PXR AS-ODNs reduced lordosis of estradiol-primed rats as well as levels of midbrain 3α,5α-THP, and abrogated effects of subsequent infusions of 3α,5α-THP to the VTA. Activated PXR regulates gene transcription in a ligand-dependent fashion, which promotes the production of a wide array of proteins, including cytochrome P450 enzymes, involved in drug metabolism and clearance as well as steroid metabolism from cholesterol (Harmsen et al. 2007; Ma et al. 2008; Xu et al. 2005; Zhang et al. 2008). Given that 3α,5α-THP is a ligand for PXR (Frye 2011; Kliewer et al. 2002; Lamba et al. 2004; Moore et al. 2000; Watkins et al. 2001), it may be that PXR requires activation by 3α,5α-THP to initiate effects on cholesterol metabolism involving CYP enzymes, which ultimately induce greater production of 3α,5α-THP. An idea is that mating-induced biosynthesis of 3α,5α-THP functions as a homeostatic response for the initiation and termination of mating behavior, both of which are necessary for successful reproductive outcomes, noted in different laboratory rodent species (Frye 2011). Thus, PXR may be another important neuroregulatory factor in the biosynthesis of 3α,5α-THP in the midbrain, and resulting actions on lordosis in the adult.
In summary, the present work extends previous studies on the functional role of PXR for cholesterol metabolism in the central nervous system. Here we found that lordosis was enhanced with estradiolsocial-priming and infusions of 3α,5α-THP to the VTA. Infusions of PXR AS-ODNs to the VTA attenuated responses in estradiol-, but not vehicle-, primed rats, compared to control infusions. Indeed, it may be that PXR is acting in the central nervous system, much like in the liver, to regulate metabolizing enzymes, receptors, and efflux transporters, to promote homeostasis.
Figure 3.
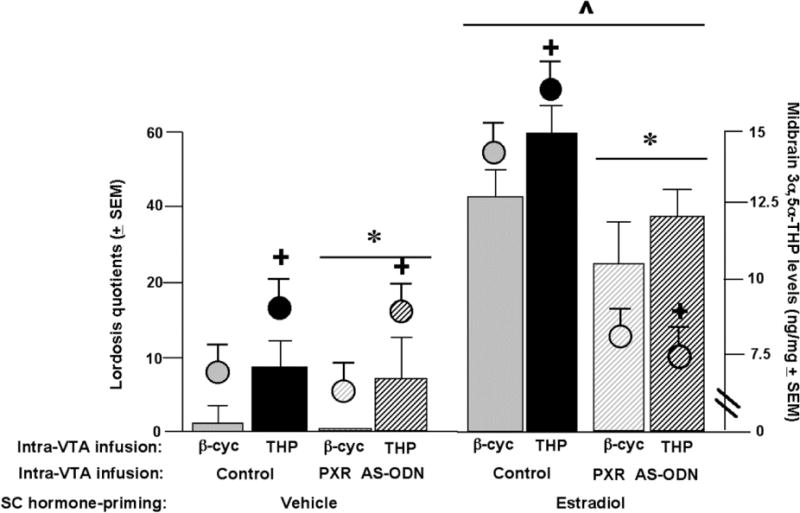
Circles indicate the mean (± s.e.m) 3α,5α-THP (ng/mg) levels in the midbrain and bars indicate the mean (± s.e.m.) lordosis quotient. ^ indicates a main effect of estradiol condition (P<0.05). + indicates a main effect of 3α,5α-THP condition (P<0.05). * indicates a main effect of PXR antisense oligodeoxynucleotide (AS-ODN) condition (P<0.05).
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (MH0676980; RMH067698B) as well as the National Institute of General Medical Sciences of the National Institutes of Health (P20GM103395). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Technical assistance, provided by Drs. Paris and Rusconi, and Dan DaCosta, Ryan Keller, Amy Kohtz, Danielle Llaneza, Jennifer Torgersen, Aaron Sheppard, and Zhenhong Zhao, is greatly appreciated. Experiments described herein comply with the current laws of the United States.
Footnotes
Conflict of Interest: All authors report that they have no conflicts of interest (financial or otherwise) that would bias them to the outcome of these experiments.
References
- Ahdieh HB, Brown TJ, Wade GN, Blaustein JD. Hypothalamic nuclear progestin receptors and the duration of sexual receptivity in ovariectomized and ovariectomized-hysterectomized rats. Physiol Behav. 1986;36:211–5. doi: 10.1016/0031-9384(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: I. Protein kinase C activation in the hypothalamus of female rats. Endocrinology. 2008a;149:5509–17. doi: 10.1210/en.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology. 2008b;149:5518–26. doi: 10.1210/en.2008-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–72. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–9. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- Beyer C, González-Flores O, Ramírez-Orduña JM, González-Mariscal G. Indomethacin inhibits lordosis induced by ring A-reduced progestins: possible role of 3α-oxoreduction in progestin-facilitated lordosis. Horm Behav. 1999;35:1–8. doi: 10.1006/hbeh.1998.1457. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Subcellular distribution and properties of progesterone (delta4- steroid) 5α-reductase in rat medial basal hypothalamus. J Biol Chem. 1975;250:7997–8003. [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–9. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBold JF, Malsbury CW. Facilitation of sexual receptivity by hypothalamic and midbrain implants of progesterone in female hamsters. Physiol Behav. 1989;46:655–60. doi: 10.1016/0031-9384(89)90347-8. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Malsbury CW, Harris VS, Malenka R. Sexual receptivity: brain sites of estrogen action in female hamsters. Physiol Behav. 1982;29:589–93. doi: 10.1016/0031-9384(82)90224-4. [DOI] [PubMed] [Google Scholar]
- Dussault I, Forman BM. The nuclear receptor PXR: a master regulator of "homeland" defense. Crit Rev Eukaryot Gene Expr. 2002;12:53–64. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.30. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized/adrenalectomized hormone-primed rats. J Comp Physiol Psychol. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- Etgen AM, González-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–75. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Novel substrates for, and sources of, progestogens for reproduction. J Neuroendocrinol. 2011;23:961–73. doi: 10.1111/j.1365-2826.2011.02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA, Rusconi JC. Motivated behaviors and levels of 3α,5α-THP in the midbrain are attenuated by knocking down expression of pregnane xenobiotic receptor in the midbrain ventral tegmental area of proestrous rats. J Med Sex. 2013;10:1692–706. doi: 10.1111/jsm.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Exploratory, anti-anxiety, social, and sexual behaviors of rats in behavioral estrus is attenuated with inhibition of 3α,5α-THP formation in the midbrain ventral tegmental area. Behav Brain Res. 2008;193:269–76. doi: 10.1016/j.bbr.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Walf AA, Rusconi JC. Effects and Mechanisms of 3α, 5α,-THP on Emotion, Motivation, and Reward Functions Involving Pregnane Xenobiotic Receptor. Front Neurosci. 2011;5:136. doi: 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- García-Juárez M, Beyer C, Soto-Sánchez A, Domínguez-Ordoñez R, Gómora-Arrati P, Lima-Hernández FJ, Eguibar JR, Etgen AM, González-Flores O. Leptin facilitates lordosis behavior through GnRH-1 and progestin receptors in estrogen-primed rats. Neuropeptides. 2011 Feb;45(1):63–7. doi: 10.1016/j.npep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- González-Mariscal G, González-Flores O, Beyer C. Intrahypothalamic injection of RU486 antagonizes the lordosis induced by ring A-reduced progestins. Physiol Behav. 1989;46:435–8. doi: 10.1016/0031-9384(89)90016-4. [DOI] [PubMed] [Google Scholar]
- Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–7. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors I. Behavioral effects of glutamate and its selective receptor agonists AMPA, NMDA and kainate. Pharmacol Biochem Behav. 2006a;83:322–32. doi: 10.1016/j.pbb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors. II. Behavioral effects of selective glutamate receptor antagonists AP-5, CNQX, and DNQX. Pharmacol Biochem Behav. 2006b;83:333–41. doi: 10.1016/j.pbb.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hall CS, Ballachey EL. A study of the rat’s behavior in a field: a contribution to method in comparative psychology. University of California Publications in Psychology. 1932;6:1–12. [Google Scholar]
- Handley SL, Mithani S. Effects of α-adrenoreceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn-Schmeideberg’s Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev. 2007;33:369–80. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22:10613–20. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine asPXR activators. Toxicol Appl Pharmacol. 2004;199:251–65. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Lisciotto CA, DeBold JF. Ventral tegmental lesions impair sexual receptivity in female hamsters. Brain Res Bull. 1991;26:877–83. doi: 10.1016/0361-9230(91)90252-f. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Gonzalez FJ. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD. Neural progestin receptors and female sexual behavior. Neuroendocrinology. 2012;96:152–61. doi: 10.1159/000338668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini S, Nannelli A, Sodini D, Dragoni S, Valoti M, Longo V, Gervasi PG. Expression, microsomal and mitochondrial activities of cytochrome P450 enzymes in brain regions from control and phenobarbital-treated rabbits. Life Sci. 2007;80(10):910–7. doi: 10.1016/j.lfs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–92. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–20. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol. 2012;33:331–41. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miryala CS, Hassell J, Adams S, Hiegel C, Uzor N, Uphouse L. Mechanisms responsible for progesterone’s protection against lordosis-inhibiting effects of restraint II. Role of progesterone metabolites. Horm Behav. 2011;60:226–32. doi: 10.1016/j.yhbeh.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mòdol L, Darbra S, Pallarès M. Neurosteroids infusion into the CA1 hippocampal region on exploration, anxiety-like behaviour and aversive learning. Behav Brain Res. 2011;222:223–9. doi: 10.1016/j.bbr.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 2000. 2000;275:15122–7. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington (DC): National Academies Press (US); 2003. [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–12. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Lisciotto CA, DeBold JF. Facilitation of sexual receptivity in hamsters by simultaneous progesterone implants into the VMH and ventral mesencephalon. Horm Behav. 1990;24:139–51. doi: 10.1016/0018-506x(90)90001-e. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. Academic Press; NY, NY: 1986. [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983a;37:218–24. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrinology. 1983b;113:797–804. doi: 10.1210/endo-113-2-797. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Vallée M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–66. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–33. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- Xu DX, Chen YH, Wang JP, Sun MF, Wang H, Wei LZ, Wei W. Perinatal lipopolysaccharide exposure downregulates pregnane X receptor and Cyp3a11 expression in fetal mouse liver. Toxicol Sci. 2005;87:38–45. doi: 10.1093/toxsci/kfi239. [DOI] [PubMed] [Google Scholar]
- Zhang B, Xie W, Krasowski MD. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics. 2008;9:1695–709. doi: 10.2217/14622416.9.11.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]


