In mammals, Wnt/β-catenin signaling features prominently in stem cells and cancers, but how and for what purposes have been matters of debate. In this review, Lien and Fuchs summarize the current knowledge of Wnt/β-catenin signaling and its downstream transcriptional regulators in normal and malignant stem cells, with particular emphasis on embryonic stem cells, hair follicle stem cells, and intestinal epithelial stem cells.
Keywords: LEF/TCF, stem cells, Wnt signaling, β-catenin
Abstract
In mammals, Wnt/β-catenin signaling features prominently in stem cells and cancers, but how and for what purposes have been matters of much debate. In this review, we summarize our current knowledge of Wnt/β-catenin signaling and its downstream transcriptional regulators in normal and malignant stem cells. We centered this review largely on three types of stem cells—embryonic stem cells, hair follicle stem cells, and intestinal epithelial stem cells—in which the roles of Wnt/β-catenin have been extensively studied. Using these models, we unravel how many controversial issues surrounding Wnt signaling have been resolved by dissecting the diversity of its downstream circuitry and effectors, often leading to opposite outcomes of Wnt/β-catenin-mediated regulation and differences rooted in stage- and context-dependent effects.
Wnt proteins are secreted glycoproteins that interact with seven-pass transmembrane receptors of the Frizzled (Fzd) family and/or single-pass transmembrane coreceptors, such as lipoprotein receptor-related protein 5/6 (Lrp5/6), Ror2, and Ryk (Vinson et al. 1989; Tamai et al. 2000; Wehrli et al. 2000; Liu et al. 2008). The interactions between Wnt ligands and their receptors result in the activation of various intracellular signaling cascades that can be cross-connected or act independently. Depending on the pathway activated, Wnt signaling can regulate a variety of diverse processes, including cell proliferation, differentiation, migration, and polarity and asymmetric cell division (Clevers and Nusse 2012). Not surprisingly, deregulation of Wnt signaling has been linked to a number of human diseases, including cancers (Holland et al. 2013).
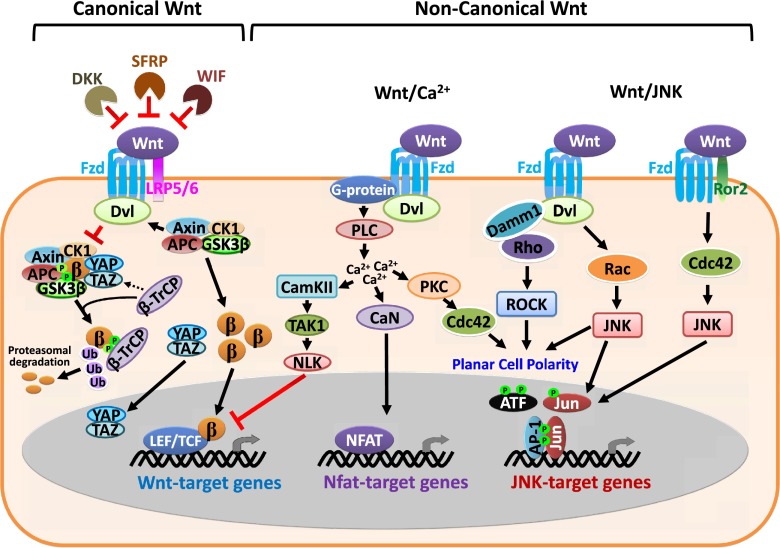
Wnt pathways fall into two general categories: canonical and noncanonical Wnt signaling. Canonical Wnt signaling is often referred to as the Wnt/β-catenin pathway, as it happens when Wnt-stimulated signals trigger β-catenin-dependent transcriptional activation. In contrast, noncanonical Wnt pathways, including the Wnt/Ca2+ (calcium) and Wnt/JNK (c-Jun N-terminal kinase) pathways, are β-catenin-independent and usually trigger a variety of different intracellular signaling cascades (Fig. 1; Kuhl et al. 2000, 2001; Oishi et al. 2003; Schambony and Wedlich 2007; Liu et al. 2008).
Figure 1.
Overview of Wnt signaling pathways. This schematic diagram displays simplified canonical (β-catenin-dependent) and noncanonical (β-catenin-independent) Wnt signaling pathways. In the absence of Wnt, β-catenin is targeted by a destructive complex that phosphorylates β-catenin for its degradation. This complex is composed of the core proteins Axin, CK1α, APC, and GSK3β. Like β-catenin, YAP/TAZ can also associate with this complex and in fact is essential for its recruitment of the β-TrCP E3 ubiquitin ligase, which ubiquinates and targets β-catenin for its degradation (Azzolin et al. 2014). Binding of Wnt to Fzd and LRP5/6 activates the cytosolic protein Dvl, leading to the inhibition of the complex. Accumulation of stabilized β-catenin in the presence of LEF/TCF transcription factors results in their translocation into the nucleus to activate Wnt-responsive genes. This activation can be suppressed by TAK1–NLN, which is activated through noncanonical Wnt pathways. Delineated here are also Wnt/Ca2+ and Wnt/JNK pathways, both of which are β-catenin-independent. Binding of Wnt isoforms to either Fzd or other tyrosine kinase-like receptors (e.g., Ror2) can trigger multiple signaling cascades. Some of them result in activation of small GTPase Rho, Rac, and Cdc42 that regulate cytoskeleton rearrangement and planar cell polarity (PCP); some of cascades trigger transcriptional events by activating transcription factors (e.g., NFAT or AP-1). The mechanisms underlying β-catenin-independent Wnt signaling are also likely to be determined by cellular context and remain elusive. See the text for references.
Canonical and noncanonical signaling are generally antagonistic and, consequently, mutually exclusive. However, they often reside at the intersection of critical cell fate choices. Indeed, many embryonic progenitors employ Wnt signaling in making fate choices during tissue morphogenesis. As developing tissues set aside long-lived stem cells to replenish dying or differentiating cells in homeostasis and repair damaged tissue following injury, these stem cells continue to use Wnt signaling to regulate their behavior and lineage programs (Holland et al. 2013). However, despite a growing body of research, it has remained ambiguous as to whether Wnt signaling pathways are essential for maintaining the fundamental properties common to all stem cells, such as long-term self-renewal, and, if so, how. In this review, we highlight recent studies that begin to shed light on these important issues. Although drawing on other models and pathways as needed, we focus primarily on the fascinating biology of canonical Wnt signaling in vertebrate stem cells and its complex regulation through the interactions of β-catenin with transcriptional regulators.
Overview of Wnt signaling pathways
Detailed descriptions of each of the classical Wnt signaling pathways have been extensively covered elsewhere (Nelson and Nusse 2004; Clevers and Nusse 2012; Holland et al. 2013), and hence we only briefly summarize them here. At the heart of the canonical Wnt signaling pathway is the silencing of glycogen synthase kinase 3β (GSK3β), which, if active, phosphorylates the N terminus of any cytoplasmic β-catenin not used in cell–cell adhesion (Hart et al. 1999; Liu et al. 1999a). GSK3β forms a complex with Axin, a priming kinase for β-catenin called casein kinase 1α (CK1α), adenomatous polyposis coli (APC), and YAP/TAZ (Liu et al. 2002; Lee et al. 2003; Azzolin et al. 2012). Also present in this so-called destructive complex is the β-transducin repeat-containing protein (β-TrCP) E3 ligase, which ubiquitinates phosphorylated β-catenin and targets it for proteasomal degradation (Fig. 1).
Upon the interaction of canonical Wnt ligands to its receptors, Fzd, and coreceptor, LRP5/6, the Dishevelled (Dvl) protein is recruited, the destruction complex is inhibited, and β-catenin is stabilized in the cytoplasm (Klingensmith et al. 1994; Noordermeer et al. 1994; Axelrod et al. 1998; Tamai et al. 2000; Wehrli et al. 2000). Although β-catenin itself has no DNA-binding domain, it can directly impact gene expression if it interacts with a transcriptional cofactor and translocates to the nucleus (Behrens et al. 1996).
The activation of canonical Wnt signaling can be blocked by extracellular proteins, such as Dickkopf (DKK), secreted Fizzled-related protein (SFRP), and Wnt inhibitory factor (WIF), all of which inhibit Wnt ligand–receptor interactions (Rattner et al. 1997; Glinka et al. 1998; Hsieh et al. 1999). Downstream pathway activation can also be suppressed, as exemplified by the antagonistic actions of intracellular kinase protein TGFβ-activated kinase 1 (TAK1)-activated Nemo-like kinase (NLK), which blocks β-catenin-induced transcriptional activity. Notably, the activation of TAK1–NLK may be mediated by one of the noncanonical Wnt pathways described below (Ishitani et al. 2003), further underscoring the opposing actions of these pathways.
Noncanonical Wnt pathways are more diverse and less well studied, and most of their attention comes from their ability to interfere with canonical Wnt/β-catenin signaling. Based on the intracellular mediators used, the noncanonical Wnt pathways can be subdivided into two general categories: the Wnt/Ca2+ and JNK pathways (Fig. 1). In the Wnt/Ca2+ pathway, the interaction of noncanonical Wnt ligands and receptors recruits Dvl and G protein and leads to the activation of phospholipase C (PLC), thereby triggering intracellular calcium release. Induced calcium ion flux can activate second messengers such as protein kinase C (PKC), calcium–calmodulin-dependent kinase II (CamKII), or the calcium-dependent phosphatase calcineurin (CaN) (Kuhl et al. 2000; Ahumada et al. 2002; Sheldahl et al. 2003; Kohn and Moon 2005; Ma and Wang 2007). In addition to TAK1/NLK, CamKII can also antagonize the Wnt/β-catenin pathway (Ishitani et al. 2003), while activated CaN can dephosphorylate nuclear factor of activated T-cell (NFAT) transcription factors, which can then enter the nucleus and activate their target genes (Murphy and Hughes 2002; Mikels and Nusse 2006).
In parallel, PKC members can activate the small GTPase Cdc42, which can in turn funnel into the planar cell polarity (PCP) pathway (Fig. 1). PCP can also be coregulated by Rho and Rac GTPases, which are activated in Wnt/JNK noncanonical signaling. In contrast to calcium-regulated noncanonical signaling, Wnt/JNK signaling uses Ror2-dependent circuitry to activate downstream effectors of the activating protein-1 (AP-1) family of transcription factors (Fig. 1; Oishi et al. 2003; Schambony and Wedlich 2007). In intestinal homeostasis and cancer development, JNK/AP-1 has been shown to cross-interact with the Wnt/β-catenin pathway via an interaction between c-JUN and TCF4 (Nateri et al. 2005). Genome-wide chromatin immunoprecipitation (ChIP) analyses for β-catenin with human colon cancer cells further reveal that β-catenin-enriched regions contain both AP-1 and TCF4 consensus motifs (Bottomly et al. 2010), underscoring the cross-talk between these two Wnt pathways. These facets begin to illuminate the complexities involved in Wnt pathway activation and the potentially intersecting signaling cascades that can be triggered. In this review, we focus on canonical Wnt signaling and its downstream transcriptional effectors in mammalian embryonic and adult stem cells and their lineages.
Transcriptional regulators in the Wnt/β-catenin signaling pathway
Wnt/β-catenin signaling directs cell fate and proliferation in a variety of cell types (Blanpain and Fuchs 2009; Clevers and Nusse 2012). The core of the pathway is the stability of β-catenin, a protein that plays a dual role in intercellular junction formation and transcriptional regulation (Hulsken et al. 1994; Behrens et al. 1996; Nelson and Nusse 2004). Indeed, β-catenin was first characterized as an adherens junction protein, which, through its Armadillo repeats, binds to the core transmembrane adhesion protein E-cadherin and, through its N-terminal domain, associates with α-catenin, a protein that binds actin and other actin-regulators.
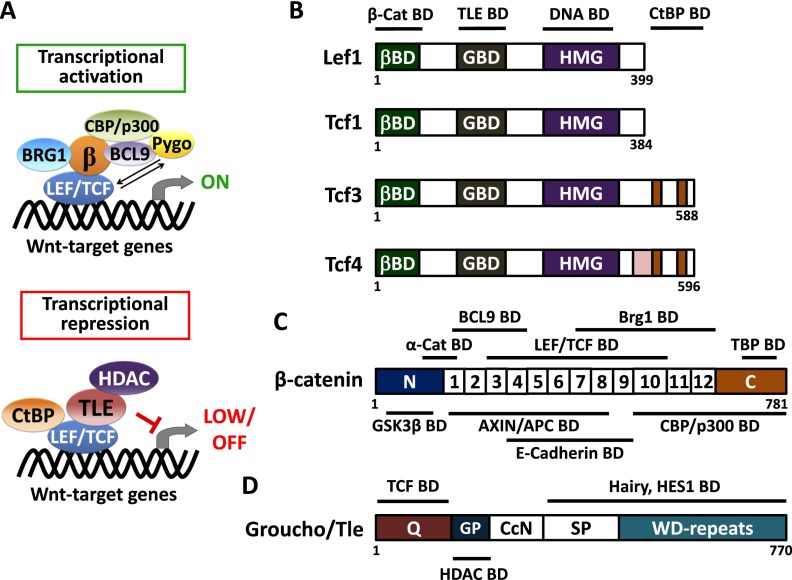
The discovery that β-catenin can also bind to members of the LEF/TCF family of DNA-binding proteins solved a long-standing Drosophila genetics puzzle as to how as the downstream effector of Wnt signaling, stabilized β-catenin, can enter the nucleus and influence the transcription of genes. Simultaneously, it shed light on a paradox in the mammalian transcriptional field as to how the group of LEF/TCF DNA-binding proteins can transactivate their targets (Behrens et al. 1996; Huber et al. 1996; Molenaar et al. 1996; Brunner et al. 1997; Korinek et al. 1997; van de Wetering et al. 1997; Hsu et al. 1998). Like other high-mobility group (HMG) box-containing proteins, LEF/TCF proteins possess minimal transcriptional activity on their own and must affect transcription by recruiting various binding cofactors, which in turn recruit chromatin modifiers to suppress or activate their target genes (Fig. 2A).
Figure 2.
Transcriptional regulation and structural organization of canonical Wnt regulators. (A) Schematic depicts a transcriptional activation or repression complex of LEF/TCF on Wnt target genes. In the activation mode, β-catenin interacts with a member of the LEF/TCF family of DNA-binding proteins. This conformation is thought to recruit histone modifiers CBP/p300 and BRG1 to yield an active chromatin structure for its target genes (Hecht et al. 2000; Takemaru and Moon 2000; Barker et al. 2001). Also participatory in this chromatin activation step is the H3K79 methyltransferase Dot1l, which, in the intestinal crypt, has been shown to be recruited to Wnt target genes in a β-catenin-dependent manner, thereby orchestrating broader chromatin remodeling and transcriptional elongation (Mahmoudi et al. 2010). Recruitment of BCL9 and Pygo are known to enhance β-catenin transactivator activity, although the mechanisms are still unfolding (Kramps et al. 2002; Thompson et al. 2002; Hoffmans and Basler 2004; Townsley et al. 2004; Li et al. 2007). Conversely, when nuclear β-catenin is absent, TCF3 and/or TCF4 proteins interact with transcriptional repressor Groucho/TLEs and in turn recruit histone deacetylase (HDAC) to yield an inactive chromatin state for the target genes. Another repressor, CtBP, has also been reported to interact with TCF4 for gene silencing (Cavallo et al. 1998; Brantjes et al. 2001; Valenta et al. 2003; Cuilliere-Dartigues et al. 2006; Arce et al. 2009; Cadigan and Waterman 2012). In general, whether LEF/TCF proteins act to activate or repress genes is determined by their binding partners and is cell context-dependent. (B–D) Structural organization of LEF/TCF member proteins, β-catenin, and Groucho/TLE protein. Line diagrams shown above and below the structures display binding domains for the indicated proteins. (B) LEF/TCF member proteins share a conserved structural organization, which consists of β-catenin-binding domain (βBD), putative Groucho/TLE-binding domain (GBD), and HMG DNA-binding domain. The CtBP-binding domain is seen for only TCF3 and TCF4 proteins. (C) The diagram displays the coactivator, β-catenin, which comprises 12 Armadillo repeats in the center of the protein structure. These repeats mediate most of the interactions between β-catenin and its binding partners, including the destruction complex components AXIN/APC, intercellular molecule E-cadherin, LEF/TCF transcription factors, histone modifier Brg1, and cofactor BCL9. The N-terminal region of β-catenin contains conserved phosphorylation residues for GSK3β for subsequent proteolytic degradation, and this region is also recognized by junctional protein α-catenin (Nelson and Nusse 2004). The C-terminal domain of β-catenin includes potent transcriptional transactivation elements that recruit coactivators TBP and CBP/p300. (D) The diagram depicts a model for the Groucho/TLE proteins (Chodaparambil et al. 2014). Most of them share a conserved structural organization, which consists of a glutamine-rich (Q) domain followed by a glycine/proline-rich (GP) domain, CcN domain, serine/proline-rich (SP) domain, and WD repeat domain. Recently, it was shown that TCF3 and TCF4 can bind to the N-terminal region of TLE1, while HDACs are thought to be recruited by TLEs through their GP domain. TCF1 and LEF1 appear to have weaker binding to TLEs, which may account for their more typical behavior as coactivators rather than repressors for Wnt signaling (Chodaparambil et al. 2014). Other than TCFs, Groucho/TLEs also interact with other transcriptional factors (e.g., HES1) through their C-terminal region (Grbavec et al. 1998).
The mammalian LEF/TCF family encompasses LEF1, TCF1 (encoded by Tcf7), TCF3 (encoded by Tcf7l1), and TCF4 (encoded by Tcf7l2) (Fig. 2B). In vitro studies with recombinant proteins revealed that these monomers recognize a core consensus sequence, the LEF/TCF DNA-binding motif (Giese et al. 1991; Hurlstone and Clevers 2002). Like E-cadherin, LEF/TCFs contain a domain that can interact with Armadillo repeats, which serve as the platform for β-catenin binding (Hecht et al. 2000; Barker et al. 2001). β-Catenin binds LEF/TCFs through Armadillo repeats 3–10 and then uses its C terminus to interact with other cofactors, including the chromatin modifiers CBP/p300 and Brg1, which ensure the efficient transcription of its target genes (Fig. 2C).
Among the many coactivators identified in Wnt-dependent transcription, the Drosophila Pygopus (Pygo) protein is particularly interesting. It was identified through its association with BCL9/Legless, which binds to β-catenin (Fig. 2A; Kramps et al. 2002; Thompson et al. 2002; Hoffmans and Basler 2004; Townsley et al. 2004; Li et al. 2007). That said, Pygo can also directly interact with TCFs in a Wnt-independent manner, where it appears to serve as a histone methylation reader and context-dependent LEF/TCF anti-repressor to facilitate subsequent Wnt-dependent transcription (de la Roche and Bienz 2007; Mieszczanek et al. 2008; Gu et al. 2013). Interactions between chromatin remodeling factors and β-catenin have been reviewed elsewhere (Mosimann et al. 2009).
The ability of LEF/TCF to repress genes has been attributed to transducin-like Enhancer of split (TLE) proteins, which are mammalian homologs of the Drosophila Groucho transcriptional corepressor (Roose et al. 1998). Although not exclusive to the Wnt pathway, TLE proteins regulate canonical Wnt transcription by binding to LEF/TCF family members and acting as adapters to recruit negative chromatin modifiers (Fig. 2A; Cavallo et al. 1998; Brantjes et al. 2001; Arce et al. 2009; Cadigan and Waterman 2012). It is known that in the absence of Wnt signaling, TCFs interact with a TLE tetramer (Brantjes et al. 2001). In turn, this complex has been shown to recruit histone deacetylases (HDACs) to form a specialized repressive chromatin structure that prevents the inappropriate activation of TCF target genes (Fig. 2D; Chen et al. 1999; Arce et al. 2009).
Recent in vitro structural analyses further show that the TLE tetramer functions in chromatin repression through binding to K20 methylated histone H4 tails, which in turn more readily form repressive complexes with TCF3 and TCF4 than with TCF1 and LEF1 (Chodaparambil et al. 2014). These findings agree well with recent in vivo ChIP and Illumina deep sequencing (ChIP-seq) and RNA sequencing (RNA-seq) on purified quiescent hair follicle stem cells (HFSCs), which show that TCF3, TCF4, and TLEs bind to common chromatin sites in the absence of Wnt signaling (Lien et al. 2014). These TCF3/TCF4/TLE-bound genes include chromatin-repressed genes that must be derepressed by canonical Wnt signaling in order to activate hair follicle fate specification (Lien et al. 2014). Although it was initially surmised that nuclear β-catenin directly binds LEF/TCF and displaces Groucho/TLE repressors (Daniels and Weis 2005), derepression may not necessarily involve a competitive mechanism (Chodaparambil et al. 2014).
In addition to TLEs, in vitro studies have shown that C-terminal-binding protein (CtBP) can bind to TCF4, repress Wnt-responsive reporter activity, and reduce expression of an endogenous Wnt target gene, Axin2 (Valenta et al. 2003; Cuilliere-Dartigues et al. 2006). Whether this interaction occurs and is relevant to TCF-mediated chromatin repression in vivo remains unknown; notably, however, CtBP-binding sites appear to be exclusive to TCF3 and TCF4. The preferential binding of these corepressors, TLE and CtBP, to TCF3 and TCF4 is interesting in light of the long-standing observation that in the hair follicle, TCF3 and TCF4 are present in quiescent stem cells, where Wnt reporter activity is silent. In contrast, LEF1 is present under conditions where Wnt reporter activity is active—initially in hair germ (HG) progenitors upon their activation and then at greatly amplified levels in the transit-amplifying matrix cells as they become fated to differentiate and make the hair shaft (DasGupta and Fuchs 1999; Merrill et al. 2001; Nguyen et al. 2006, 2009; Lien et al. 2014). Indeed, TCF3 and TCF4 often appear to function to lower transcriptional levels of their targets, while TCF1 and LEF1 are more typically viewed as transcriptional enhancers of Wnt signaling (Merrill et al. 2004; Yi et al. 2011; Wu et al. 2012). These opposing behaviors can be observed even when the proteins coexist under conditions that favor Wnt signaling. We return to the differential roles in transcriptional regulation of LEF/TCF regulators in the following section.
How receiving stem cells perceive external Wnt cues
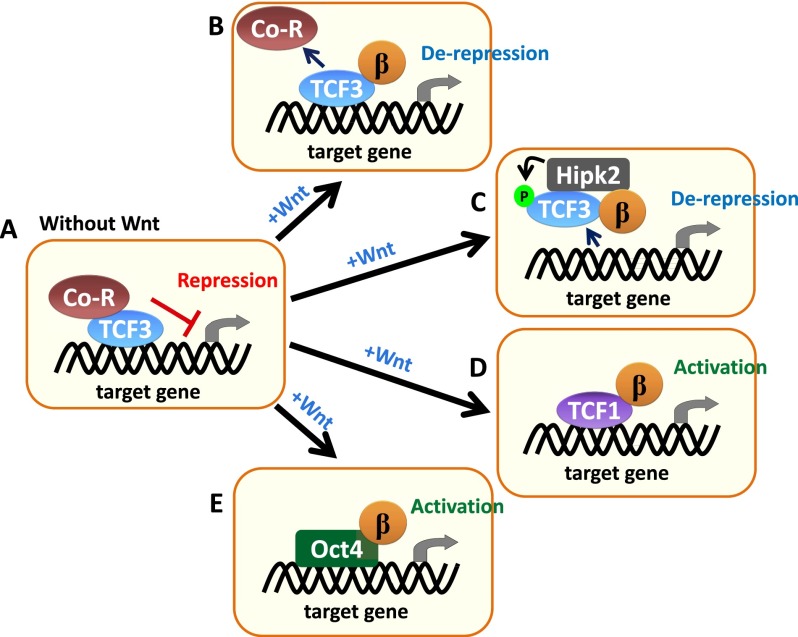
The first genome-wide mapping of TCF3 was performed on cultured murine embryonic stem (ES) cells, where it was shown that TCF3 binds in close proximity to the chromatin-bound sites of the core pluripotency transcription factors Oct4, Nanog, and Sox2 (Cole et al. 2008). In the absence of Wnt, expression of pluripotency factors are negatively regulated by TLE/Groucho or CtBP proteins (Fig. 3A; Pereira et al. 2006; Tam et al. 2008), and when Wnt signaling is activated or TCF3 is absent, core pluripotency factors are up-regulated (Cole et al. 2008; Yi et al. 2011). When combined with the recent findings of Chodaparambil et al. (2014) and the findings delineated for the hair follicle above (Merrill et al. 2001; Nguyen et al. 2006, 2009; Greco et al. 2009; Hsu et al. 2014; Lien et al. 2014), these findings suggest that binding of β-catenin to TCF3 alters the TCF3-bound repressive chromatin state, in turn activating target genes (Fig. 3B).
Figure 3.
Proposed mechanisms of Wnt/β-catenin signaling in ES cells. (A) In the absence of Wnt stimulation, β-catenin is degraded, and TCF3 interacts with corepressors to prohibit target genes from activation. Upon Wnt activation, stabilized β-catenin can either replace the corepressors, resulting in derepression of target genes (B), or mediate the interaction of Hipk2 with TCF3 (C) (Hikasa et al. 2010; Hikasa and Sokol 2011). Hipk2–TCF3 interaction causes phosphorylation of TCF3, and this modification leads to removal of TCF3 from target promoters, resulting in transcription derepression. (D) Furthermore, it has also been proposed that Hipk2-mediated release of the TCF3 repressor can lead to the replacement of the TCF1 activator working with β-catenin for target gene activation (Hikasa and Sokol 2011; Yi et al. 2011). (E) Another model proposed in ES cells is a TCF-independent regulation of β-catenin via interacting with ES cell core factor Oct4 to transactivate target genes (Pardo et al. 2010; van den Berg et al. 2010). These alternative models have been proposed to maintain the pluripotency of ES cells.
In Xenopus, the activation of TCF3-repressed target genes has been shown to occur by a different mechanism. In this case, β-catenin appears to stimulate the homeodomain-interacting protein kinase 2 (Hipk2), which in turn can phosphorylate TCF3 (Hikasa et al. 2010; Hikasa and Sokol 2011; Sokol 2011). Phosphorylated TCF3 exhibits a reduced DNA-binding affinity, suggesting a model in which Wnt-induced Hipk2 phosphorylation results in a depression of TCF3 target genes (Fig. 3C).
Whether this mechanism is operative in mammalian cells is not yet clear. However, HipK2 is expressed in mammalian cells, and it was recently reported that it can act to interfere with TLE/Hes-mediated inhibition of neuronal differentiation (Ciarapica et al. 2014). Additional intrigue stems from the finding that in Xenopus, TCF3 binding to its targets can be replaced by TCF1 following Hipk2-mediated removal of TCF3 (Hikasa and Sokol 2011). Notably, this kind of exchange between TCF3-mediated gene repression and TCF1-mediated gene activation of Wnt targets has also been identified in murine ES cells (Fig. 3D; Yi et al. 2011). That said, in ES cells, the switch from TCF3 to TCF1 upon Wnt-mediated activation of differentiation is achieved not by TCF3 phosphorylation but rather by β-catenin-dependent targeting of TCF3 for proteasomal degradation (Shy et al. 2013). This interesting facet opens the door for yet another dimension by which perceived canonical Wnt signaling can change within different cell types.
Although LEF/TCF member proteins have long been considered β-catenin’s primary interacting partners, several reports suggest that there are other means for canonical Wnt signaling to influence gene expression. For nearly a decade, evidence has been accumulating for a role for β-catenin in mediating gene regulation by steroid hormone receptors, including androgen and vitamin D receptors (Song et al. 2003; Shah et al. 2006; Palmer et al. 2008; Lee et al. 2013; Liu et al. 2013). Additionally, it has been suggested that β-catenin can interact with ES cell regulator Oct4 and in turn up-regulate Oct4 target genes (Fig. 3E; Pardo et al. 2010; van den Berg et al. 2010). These interactions seem to be dependent on the stabilization of β-catenin through canonical Wnt signaling. In general, however, these nonclassical models of Wnt/β-catenin-mediated gene regulation have been difficult to track, since many of these transcription factors have activities that are Wnt/β-catenin-independent and that can, in turn, impact on canonical Wnt signaling.
Wnt/β-catenin signaling and stem cell self-renewal: to β or not to β?
Stem cells have the ability to self-renew; i.e., to proliferate and remain in an undifferentiated state. They also have the capacity to differentiate upon signal stimulation. ES cells are characterized by their ability to generate all cell lineages of an organism, while adult stem cells exhibit a limited repertoire of differentiation pathways that are tailored to suit the particular needs of their host tissue.
ES cells exist in a naïve state, displaying a particular open chromatin structure with fewer epigenetic marks than adult stem cells (Nichols and Smith 2009). The decision of an ES cell to either proliferate and maintain this naïve state or commit to a particular differentiation program can be manipulated by changing culture conditions. Various signaling molecules, including Wnts, can modulate decisions of ES cells.
Pluripotency can be maintained as long as conditions favor expression of the core transcription factors Oct4, Sox2, and Nanog (Boyer et al. 2005). Indeed, pluripotency can be established from adult tissue cells with a slightly different cohort of factors: Oct4, Sox2, Klf4, and c-Myc (Takahashi and Yamanaka 2006; Takahashi et al. 2007). This reprogramming process, which generates so-called induced pluripotent stem cells (iPSCs), is robust and works with a wide range of differentiated cells. Additionally, myc is a Wnt/β-catenin signaling target gene, and Wnts, GSK3β inhibitors, and Tcf7l1 ablation enhance somatic cell reprogramming and iPSC formation (Lluis et al. 2008, 2011; Marson et al. 2008).
The efficiency of Wnt/β-catenin-stimulated reprograming appears to be stage-dependent, and, intriguingly, TCF3/4 and LEF1/TCF1 act temporally in this process (Ho et al. 2013). Moreover, the differential action of LEF/TCF members in iPSC reprogramming is the reverse of that seen in the progression of ES cells to differentiate (Yi et al. 2011). As discussed above, β-catenin’s potency on iPSC reprogramming stems in part from its direct interaction with reprogramming factors to enhance expression of pluripotency circuitry genes (Zhang et al. 2014).
Whether Wnt/β-catenin signaling is essential for the maintenance of ES cells has been controversial. While many reports indicated that the Wnt/β-catenin pathway is required for the establishment and self-renewal of ES cells (for example, Sato et al. 2004; ten Berge et al. 2011), others have found that its activation results in differentiation toward mesoderm and endoderm lineages (Lindsley et al. 2006; Bakre et al. 2007; Davidson et al. 2012). Although some of these seemingly opposing differences can be attributed to differences between human and mouse ES cells, relative differences in levels of Wnt signaling are well known to affect cell fate outcome and could be a contributing factor (for review, see Merrill 2012). In this regard, it was recently reported that within human ES cell (hESC) populations, some cells are more Wnt-sensitive than others, and, upon differentiation, the Wnt(high) hESCs predominantly form endodermal and cardiac cells, whereas the Wnt(low) hESCs generate primarily neuroectodermal cells (Blauwkamp et al. 2012). Similarly, short-term treatment of the GSK3β inhibitor BIO or soluble Wnt3a protein has been shown to stimulate ES cell self-renewal and maintain pluripotency, while long-term treatment of ES cells with Wnt3a reduces their self-renewing capacity and leads to differentiation. Conversely, blocking Wnt function or loss of β-catenin in ES cell cultures leads to a morphology and gene expression profile more similar to that of the epiblast, a later developmental stage in the embryo (ten Berge et al. 2011; Wray et al. 2011).
Although these data provide a compelling case that Wnt/β-catenin can influence the naïve pluripotent state, several findings are difficult to reconcile. During embryogenesis, even though TCF3 is expressed in the mouse epiblast, canonical Wnt signaling has not been detected until gastrulation, where Wnt reporters become active concomitant with LEF1 and TCF1 in the primitive streak (Liu et al. 1999b). Moreover, a pregastrulation phenotype arises either upon complete TCF3 loss or when a knock-in mutation is generated to produce endogenous TCF3 lacking its β-catenin-interacting domain (Merrill et al. 2004; Wu et al. 2012). These findings indicate that at least in mice, TCF3 is required for early embryonic development, while Wnt/β-catenin signaling functions later, in cell lineage specification. Further corroborating these results is the fact that cultured mouse ES cells targeted for β-catenin loss can self-renew but are defective in differentiation (Lyashenko et al. 2011).
Similar findings have been reported for adult HFSCs, which can self-renew and be passaged long-term in the absence of β-catenin when they are maintained in culture conditions that favor TCF3/4 repression (Lien et al. 2014). In contrast, in vivo, the TCF3/4-high stem cells can persist for months in the absence of β-catenin, but the cells are unable to induce hair lineage differentiation (Choi et al. 2013; Lien et al. 2014). Moreover, when stimulated by plucking the old hair formed prior to Ctnnb1 ablation, β-catenin-deficient HFSCs proliferate, move upward, and promote sebocyte differentiation, resulting in an enlarged sebaceous gland (Lien et al. 2014). These findings underscore HFSC roles for (1) β-catenin in directing fate choices and differentiation and (2) TCF3/4 in lowering transcriptional activity of its targets. They further demonstrate convincingly that canonical Wnt/β-catenin is not an obligatory requirement for stem cell viability.
Wnt signaling and asymmetric cell divisions
A priori, the seeming paradoxes surrounding canonical Wnt signaling’s role in stem cell self-renewal could be attributable to species-specific or cell type-specific differences or Wnt levels (Reya et al. 2003; Sato et al. 2004; ten Berge et al. 2011; Wray et al. 2011). Several tantalizing alternatives add to these possibilities. One is that Wnt/β-catenin may function specifically in asymmetric cell divisions rather than self-renewal per se. To maintain a balance of dividing and differentiating cells within a tissue, many progenitors divide asymmetrically, yielding one progenitor and one cell fated to differentiation (Neumuller and Knoblich 2009; Yamashita et al. 2010). Caenorhabditis elegans embryos show early signs of Wnt/β-catenin-dependent asymmetric cell divisions, which distinguish and specify the fates of early progenitors (Mizumoto and Sawa 2007; Owraghi et al. 2010; Ren and Zhang 2010). More recently, it was shown that if a bead of Wnt3a is applied to one side of a murine ES cell, β-catenin will asymmetrically distribute to its two daughter nuclei (Habib et al. 2013).
These findings suggest that polarized canonical Wnt signaling could be important in promoting asymmetric cell divisions, providing a putative explanation for how Wnts may function in both stem cells and promoting differentiation. Moreover, since many stem and progenitor cells can divide both asymmetrically and symmetrically (Lechler and Fuchs 2005; Poulson and Lechler 2010) and since this can shift to primarily symmetric divisions in cancers (Driessens et al. 2012; Mascre et al. 2012), it is tempting to speculate that when Wnt signaling is apolarized or too high, symmetrical divisions can arise, which may include fating both daughters to differentiate. If so, such shifts in Wnt levels, coupled with the ability of Wnts to influence asymmetric cell divisions, could explain many seemingly contradictory findings and shift the attention from species and cell type differences to ones rooted in polarization and the levels of Wnt signaling perceived internally by the receiving stem cell/progenitor.
YAP/TAZ and Wnt/β-catenin: juggling proliferation between two intersecting pathways
Another interesting facet in tissue-specific regulation by LEF/TCFs and β-catenin is that Wnt signaling cascades often intersect with other signaling pathways to display synergistic or antagonistic action on stem cell behavior. A particularly intriguing intersection is the pathway involving the YAP/TAZ transcriptional regulators, which govern proliferation in a variety of cells and tissues. YAP/TAZ can be regulated by mechanosensing, a feature that can block its inhibitory kinase, Hippo, and enable YAP/TAZ to translocate into the nucleus and function as transcriptional cofactors for the TEAD family of DNA-binding proteins (Varelas et al. 2010; Heallen et al. 2011; Azzolin et al. 2012; Imajo et al. 2012; Aragona et al. 2013). Hippo signaling can also regulate β-catenin, and, reciprocally, YAP/TAZ can inhibit Wnt/β-catenin signaling (Varelas et al. 2010; Heallen et al. 2011; Imajo et al. 2012).
Interestingly, Wnt signaling can also induce YAP/TAZ stabilization and nuclear translocation in a manner independent of Hippo signaling. Unexpectedly, YAP/TAZ turns out to be essential for the recruitment of β-TrCP into β-catenin’s destruction complex, which is active in the absence of Wnts (Fig. 1). Upon Wnt stimulation, YAP/TAZ is released from the destruction complex, and this in turn promotes β-catenin’s stabilization and activation as well as YAP’s nuclear translocation (Azzolin et al. 2014). Whether YAP/TAZ and β-catenin transcriptional programs coordinately govern stem cell behavior in homeostasis and wound repair and, if so, how are not yet clear. The fact that YAP/TAZ can be activated in both a Wnt-dependent and a Wnt-independent manner makes it a particularly intriguing topic for intense future research.
Wnt/β-catenin signaling in balancing growth and differentiation of tissues
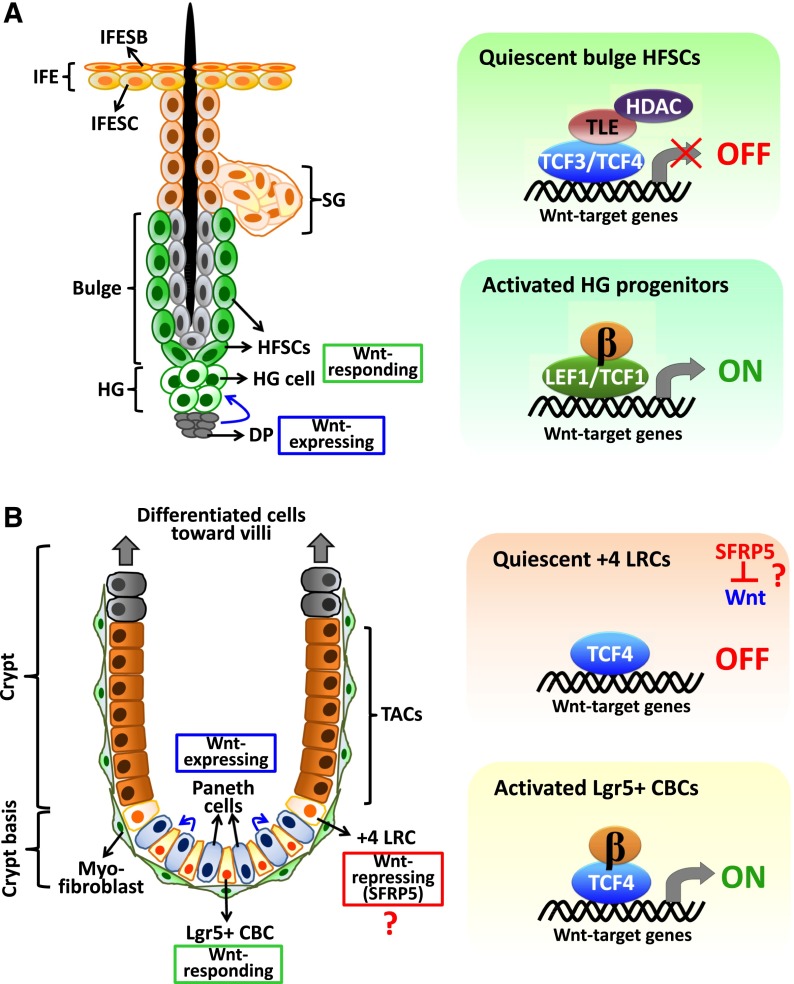
Many of the paradigms for canonical Wnt signaling in balancing homeostasis have been unearthed through the study of adult mammalian tissue stem cells, particularly the intestine and skin. The behaviors of adult stem cells are largely modulated by factors coming from their microenvironment, or niche. While canonical Wnt signaling affects the behavior of many adult stem cells, signaling can be high or low depending on the stem cells and their niche. In the quiescent HFSC niche, perceived Wnt signaling is low, corresponding to the high levels of TCF3, TCF4, and TLEs as well as BMP and calcium signaling, which leads to high levels of nuclear NFATc1, typically viewed as a noncanonical Wnt effector (DasGupta and Fuchs 1999; Merrill et al. 2001; Horsley et al. 2008; Keyes et al. 2013; Lien et al. 2014). Reductions in BMP signaling and elevation of canonical Wnt signaling are necessary to stabilize β-catenin, repress TCF3/4, and activate LEF1 to send the stem cells along the hair differentiation lineage (Fig. 4A; Greco et al. 2009; Hsu et al. 2014). It remains to be determined whether this switch involves Wnt/β-catenin-mediated targeting of TCF3/4 for proteasomal degradation, as happens in murine ES cells (Shy et al. 2013), or phosphorylation-dependent relief of TCF3/4 binding to its chromatin sites, as reported in Xenopus (Hikasa et al. 2010; Hikasa and Sokol 2011). Given the complexities unraveled thus far, it would not be surprising to see some hitherto as yet alternative pathway is at the root of the regulatory circuit.
Figure 4.
Wnt/β-catenin signaling in adult HFSCs and intestinal stem cells (ISCs). (A) Wnt/β-catenin signaling regulation in HFSCs, located primarily at the base of the bulge in a region referred to as the HG. This small cluster of HFSCs is abutted next to the dermal papilla and is the first to proliferate at the telogen-to-anagen transition. Our recent data have shown that TCF3/TCF4 act as transcriptional repressors by recruiting TLEs and HDACs to constrain Wnt target gene expression in quiescent HFSCs during telogen (Lien et al. 2014). Interestingly, at the start of each new hair cycle, TCF3 and TCF4 decline, while LEF1 and TCF1 levels are elevated following Wnt/β-catenin signaling and are concomitant with the emergence of the transit-amplifying pool of Shh-expressing matrix cells (Hsu et al. 2014). Thus, here we propose a LEF/TCF switch model from TCF3/4-mediated transcriptional repression in HFSCs to LEF1/TCF1-dependent gene activation in HG cells. (B) Wnt/β-catenin signaling regulation in ISCs. ISCs, including Lgr5+ CBCs and +4 LRCs, and their niche Paneth cells are located at the base of crypt (Barker et al. 2007; Sato et al. 2011; Tian et al. 2011; Yan et al. 2012). The transient amplifying cells (TACs) migrate upward and differentiate along the crypt–villus axis. The crypt cells are surrounded by myofibroblasts. At the crypt basis where Wnt levels are high, Paneth cells are indicated as the Wnt-secreting source to stimulate Wnt-responding CBCs (Sato et al. 2011), whereas the expression of Wnt inhibitor SFRP5 was detected in +4 LRCs (Gregorieff et al. 2005), and this might prevent them from activation in the Wnt-rich environment. Among LEF/TCF family proteins, TCF4 has been shown as a critical regulator in adult intestinal epithelial cells (van Es et al. 2012).
For the hair follicle, this change in signaling is achieved through prolonged transcriptional cross-talk occurring between the “primed” HG progenitors at the base of the niche and a cluster of underlying dermal papilla (DP) cells, which serve as a transient stimulus for initiation of stem cell activity. These primed HG progenitors have a lower threshold for activation when receiving stimulating cues from the niche (Greco et al. 2009). However, once these progenitors get activated and form the pool of transit-amplifying progeny, self-renewal of the HFSCs to replenish the niche is accomplished by Shh signaling emanating from the transient amplifying cells (TACs) (Hsu et al. 2014). This proliferation is brief: Soon, the TACs (and their Shh signal) and the DP (and their BMP inhibitory signals) move too far away from the stem cell niche, which then returns to quiescence (Hsu et al. 2011, 2014). Intriguingly, when β-catenin levels are artificially high in transgenic HFSCs, they stimulate the proliferation of neighboring HFSCs non-cell-autonomously (Deschene et al. 2014). Given that Shh activation is a downstream event of Wnt signaling in the hair follicle, it will be interesting to see whether this phenomena entails the same mechanism as described by Hsu et al. (2014).
In the mature hair follicle, sustained cross-talk between the LEF1-expressing TACs and the DP in the bulb leads to sustained canonical Wnt signaling, which drives hair keratin gene transcription and hair formation (Zhou et al. 1995; DasGupta and Fuchs 1999). As TACs halt proliferation and differentiation, hair growth stops, the follicle regresses upward, and the DP returns to its position below the stem cell niche. Interestingly, overexpression of a mutant form of LEF1 that is unable to bind β-catenin alters the fate choices of HFSCs and TACs toward sebocytes (Merrill et al. 2001; Niemann et al. 2002; Petersson et al. 2011). Similar differentiation defects were also found in β-catenin-null hair follicles and depilation-activated HFSCs that lack β-catenin (Huelsken et al. 2001; Lien et al. 2014). The synchrony of these cycles of stem cell activation and lineage differentiation (along with the switch from TCF3/4 to LEF1) and the changes in levels of perceived Wnt signaling have made the murine hair cycle an excellent model for dissecting these underlying signaling circuitries.
In contrast, the intestinal epithelium has served as an ideal system for examining the signaling pathways that drive continuous stem cell activity. Every 3–5 d, a transit-amplifying progeny of the intestinal stem cell (ISC) has moved outward and differentiated and is sloughed from the villus. The process is fueled by the near-continual cycling of the multipotent stem cells that reside within the crypt (Barker et al. 2007; Tian et al. 2011; Clevers and Nusse 2012; Yui et al. 2012). These ISCs are responsible for generating the villus cells, enteroendocrine cells, goblet cells, and Paneth cells. While transit-amplifying progenitors form the enteroendocrine, goblet, and villus cells, Paneth cells are considered as niche cells for ISCs and are the major source of Wnt signaling in the crypt (Fig. 4B; Sato et al. 2011; Yan et al. 2012).
The importance of Wnt/β-catenin signaling in the maintenance of the adult murine intestine was first demonstrated by the loss of intestinal crypts that arises from either β-catenin deficiency or ectopic expression of the Wnt inhibitor DKK1 (Pinto et al. 2003; Ireland et al. 2004). This corroborated earlier studies showing that failure to express TCF4 led also to a failure to form/maintain ISCs and intestinal crypts (Korinek et al. 1998). Conversely, ISCs can generate minigut organoid cultures and maintain them long term in vitro as long as R-spondin is present to stimulate canonical Wnt signaling (Sato et al. 2009). Intriguingly, optimal maintenance of ISCs in vitro is also achieved by inhibiting BMP signaling (He et al. 2004; Sato et al. 2009), suggesting that these two signals may go hand in hand in stimulating primed or active stem cells. In contrast, Shh signaling has not been described in the intestinal epithelium, indicating that downstream signaling by the TACs may be suited to the particular needs of each epithelium.
Another interesting parallel between HFSCs and ISCs is that they each exist in two discrete states based in part on their sensitivity to Wnt signaling: In both cases, the more quiescent stem cells (hair follicle bulge and crypt +4 ISCs) are in a more Wnt-restricted microenvironment, while the primed progenitors (HG) or active stem cells (0 to +3 ISCs) have a reduced threshold for activation upon canonical Wnt stimulation (Sangiorgi and Capecchi 2008; Greco et al. 2009; Tian et al. 2011; Buczacki et al. 2013; Ritsma et al. 2014). In both cases, Wnt/β-catenin activity is associated with proliferation and cell fate determination, and, as discussed above, these two features might be inseparable if Wnt/β-catenin turns out to function in these tissues at least in part to promote asymmetric cell divisions. Moreover, in a fashion strikingly similar to what was first described for the terminally differentiating cells of the hair follicle (Zhou et al. 1995; DasGupta and Fuchs 1999; Merrill et al. 2001), very high levels of Wnt signaling in the intestine are associated with terminal differentiation—in this case, the Paneth cell (Andreu et al. 2005, 2008).
While parallels between stem cell activation and fate specification in the intestine and hair follicle are quite striking, their paradigms have been technically more challenging to test in epidermis. In humans, the epidermis turns over every 4 wk. Its near continual state of homeostatic flux is attributable to stem cells that reside within the innermost, basal layer. These cells have elevated levels of integrins (Jones et al. 1995) and appear to exist in both quiescent and active states (Lavker and Sun 1982; Bickenbach and Holbrook 1987). In contrast, the interfollicular epidermis of hairy mammals is considerably thinner and less proliferative than in higher primates. Moreover and in contrast to human epidermal stem cells (Green 1991) and murine HFSCs (Blanpain et al. 2004), murine epidermal cells have thus far failed in typical assays used to measure stemness, such as long-term culture and engraftments. In vivo documentation of stemness by long-term lineage tracing is also fraught with the caveat that scratch-induced wounding can complicate interpretations, even for studies of the thicker epidermis of the mouse ear, tail, or hindlimb (Clayton et al. 2007; Mascre et al. 2012; Lim et al. 2013). Hence, even though it has long been established that basal epidermal cells generate columnar units of differentiating cells (Allen and Potten 1974; Vasioukhin et al. 1999), it still remains unresolved as to whether the murine basal layer harbors a discrete population of stem cells with longer-term potential than its neighbors.
Similar questions surround the role of canonical Wnt/β-catenin signaling in the interfollicular epidermis. When mice are targeted for loss of β-catenin (Ctnnb1) in their entire skin epithelium, hair follicles do not form, but mice are viable for at least a year and display a normal, even somewhat hyperproliferative, epidermis (Huelsken et al. 2001). Similarly, when the Wnt pathway is antagonized through ectopic expression of DKK1, only hair follicle formation is severely compromised (Andl et al. 2002).
The most compelling argument for an active role for Wnt signaling/β-catenin in epidermal stem cell/progenitor maintenance comes from recent lineage tracing studies with an inducible Cre recombinase gene driven by the sensitive Wnt promoter Axin2 (Lim et al. 2013). The lineage tracing data suggest that the sporadic basal cells marked by activated Axin2-CreER are long-term progenitors. When Axin2-CreER and Ctnnb1fl/fl mice are crossed, the epidermis exhibits severe hypoproliferative defects. Several explanations can account for the seemingly disparate results obtained with loss of β-catenin in all (K14-Cre) versus a subset (Axin2-CreER) of basal cells. The simplest is that one or more of the many nonepithelial Axin2-expressing cell types in both the skin and other organs indirectly affect epidermal progenitors in a manner opposite to cell-autonomous effects. Another possibility is that growth inhibitory signals from neighboring wild-type basal epidermal progenitors provide negative cues to adjacent Ctnnb1-null progenitors, which might restrict their otherwise hyperproliferative growth. Alternatively, there could be fundamental differences between nonhairy and hairy skin (Huelsken et al. 2001; Nguyen et al. 2009; Choi et al. 2013; Lim et al. 2013; Lien et al. 2014). When taken together with additional ambiguities surrounding the extent to which Axin2 and β-catenin levels are pure readouts for Wnt signaling as well as the caveats discussed above regarding adult mouse epidermis as a model for stem cell biology, it will not be a simple task to sift through the plethora of possible mechanisms underlying Wnt/β-catenin signaling in the epidermis.
Compounding the complexities underlying how Wnt signaling functions in regulating stem cell behavior are the strikingly different microenvironments of HFSCs, ISCs, and epidermal stem cells. Each of these stem cell niches displays unique heterologous components and distinctive architecture. Such differences are likely to profoundly impact the ways in which canonical Wnt signaling is perceived and used by tissue stem cells. In this regard, differences in the mechanical properties of these tissues might be particularly relevant, given that both mechanotransduction and canonical Wnt signaling can activate YAP/TAZ and in turn affect homoeostasis (Azzolin et al. 2014). Although our review concentrated primarily on the stem cells of the hair follicle, intestine, and epidermis, it is important to note that many of these general issues are likely to apply to other stem cells, including those of the mammary gland and the hematopoietic and nervous systems, where Wnt/β-catenin signaling has been shown to modulate their function (Holland et al. 2013; Ring et al. 2014; Van Camp et al. 2014). As our understanding of these complex interactions continues to unfold, so too will our knowledge of why Wnts are at the root of stem cell behavior and nevertheless are able to exert tissue-specific variations in their control.
Other interesting twists on how Wnt/β-catenin signaling impacts stem cells and tissue homeostasis emerge from studies on its deregulation, which has long been associated with cancers. The topic has been reviewed recently (Clevers 2011; Atlasi et al. 2014), and hence we cover it only briefly here, where for both intestinal and skin epithelia, constitutive activation of Wnt signaling has been linked genetically as an underlying root of malignancy. Thus, stabilizing mutations in β-catenin or loss of the β-catenin inhibitor APC promotes hyperproliferation and predisposes their tissues to cancer (Morin et al. 1997; Gat et al. 1998; Chan et al. 1999). Recent studies on APC-null intestinal cancers show that they are YAP/TAZ-dependent (Azzolin et al. 2014). Thus, when YAP/TAZ is ablated along with APC in intestinal epithelium, crypt architecture for its stem cell residents is maintained, as is their ability to undergo normal tissue homeostasis. These findings underscore the importance of cross-talk between these two pathways in governing stem cell behavior, whether normal or cancerous.
When β-catenin (Ctnnb1) is targeted in pre-established cutaneous papillomas, these benign tumors shrink and do not progress to SCCs, suggesting a key role for β-catenin as well in governing the process (Malanchi et al. 2008). An in vivo skin-specific genome-wide RNAi screen in mice provided further support for this notion, as β-catenin surfaced as a strong positive regulator of oncogenic growth (Beronja et al. 2013). Intriguingly, however, β-catenin also emerged in this screen as a negative regulator of normal embryonic epidermal growth (Beronja et al. 2013), consistent with the observations discussed above for K14-Cre-mediated Ctnnb1 ablation. Although possible differences in YAP/TAZ were not explored in this study, β-catenin deficiency in the epidermis caused mild perturbations in cell–cell adhesion, a feature also linked to cancers in humans. Together, these findings unveil yet another context-dependent means by which cell proliferation can be regulated by Wnt-dependent and Wnt-independent mechanisms.
Transcriptional network of Wnt/β-catenin signaling with other regulators
One of the intriguing questions regarding Wnt/β-catenin gene regulation is how LEF/TCF DNA-binding proteins and their cofactor, β-catenin, act to recognize and regulate their target genes. Chromatin profilings of LEF/TCF proteins in different cell lines and/or in vivo tissues point to the view that their target genes and cooperative regulators are dependent on cell type and context. This is perhaps best exemplified by the finding that following acute injury, Wnt-induced hematopoietic cells of the erythroid lineage guide nuclear TCF4 to regulatory sites of key blood genes that are already bound by GATA2 (Trompouki et al. 2011). In contrast, in neonatal and adult livers, TCF4 plays an essential role in regulating the constellation of genes that govern metabolism, and here the metabolic genes show co-occupancy of TCF4 and the liver transcription factor CDX2 (Verzi et al. 2010). Interestingly, adult liver-specific Tcf7l2-null mice display improved glucose homeostasis when maintained on a high-fat diet, suggesting the possibility that inhibition of Wnt signaling may be beneficial in metabolic disease (Boj et al. 2012).
Global chromatin mapping studies in proliferative murine ES cells show that TCF3 binds to not only active pluripotency genes but also repressed differentiation genes (Cole et al. 2008). A similar diversity exists for TCF3/4-bound target genes in quiescent HFSCs, where Wnt/β-catenin-regulated hair follicle fate genes are transcriptionally repressed, and TCF3/4-bound HFSC transcription factors are transcribed and govern stemness (Lien et al. 2014). In HFSCs, however, TCF3/4 are nuclear in the face of little or no β-catenin (Lien et al. 2014), indicating that β-catenin is not an obligatory partner for their nuclear translocation, as it seems to be for hematopoietic cells (Trompouki et al. 2011). Moreover, only one-third of TCF3-bound genes are shared between ES cells and HFSCs, reinforcing the view that DNA-bound TCFs are not merely awaiting the binding of their coactivators to turn on Wnt target genes but collaborate with their tissue-specific regulators to execute tissue-dependent regulation.
As noted above, in intestinal crypts, the highest level of Wnt activity takes place at the crypt base, with a diminishing gradient upward along the crypt–villus axis. In contrast, while BMP4 signaling is high in the surrounding mesenchymal cells of the crypt–villus, the BMP inhibitor Noggin is high at the crypt bottom, thereby reducing the effective levels of BMP signaling perceived by the ISCs (He et al. 2004). The inverse crossroads of Wnt and BMP signaling are especially felt by the +4 LRCs, where high BMP signaling and reduced Wnt/β-catenin activity compromises ISC proliferation in a fashion similar to what is seen in the quiescent bulge niche of the HFSCs (Greco et al. 2009; Hsu et al. 2011). How the antagonism is achieved at the transcriptional level remains to be addressed. However, in the hematopoietic system, BMPs and Wnt/β-catenin signaling work together to promote erythroid differentiation, and here the downstream transcriptional effector of BMP signaling, pSMAD1, binds to the same chromatin regions as TCF4 and GATA1 to govern erythroid gene expression (Trompouki et al. 2011). Moreover, when the myeloid lineage regulator C/EBPα is induced in erythroid cells, SMAD1 shifts to sites newly occupied by C/EBPα. Together, these findings provide tantalizing insights into how external signaling pathways and their downstream transcriptional effectors may synergize with master transcriptional regulators to impact the behavior and fate specification of stem cells. How these mechanisms differ under conditions where signaling pathways antagonize awaits further study, but given the dazzling array of transcriptional modifiers of LEF/TCFs, the diverse effectors of Wnt signaling suggest many tantalizing clues to pursue.
Concluding statements
In this review, we summarized our current knowledge of the Wnt/β-catenin signaling pathway and its core regulators in embryonic and adult stem cells. Although the roles of Wnt/β-catenin signaling in stem cell self-renewal, asymmetric cell division, fate specification, and terminal differentiation are varied and still unfolding, it is increasingly clear that a major component of this diversity is the plethora of stage- and context-dependent modifiers that intersect and influence the pathway. In addition, a growing body of evidence suggests that not all TCF-bound target genes are modulated upon Wnt/β-catenin activation (Cole et al. 2008; Lien et al. 2014). In this regard, it will be interesting to see the impact of master regulators in trumping the effects of TCF targets and, conversely, the effects of both TCF and Wnt/β-catenin levels on target gene expression. Given the recent findings of Trompouki et al. (2011), the influence of other signaling pathways seems likely to profoundly affect these decisions as well.
Despite intensive studies on stem cells in the past years, the answers to several important questions regarding whether and how Wnts, β-catenin, and LEF/TCFs govern stem cell maintenance, proliferation, and/or fate selection remain elusive. It is still not clear how TCF3 can simultaneously have a negative influence on differentiation genes and a positive one on stemness genes in both ES cells and HFSCs. In addition, if hESCs and ISCs are in a Wnt-activated state, how can Wnt/β-catenin also influence fate decision? One potential explanation for this paradox is based on the recent observation that the directional application of a Wnt bead to one side of an ES cell can lead to preferential accumulation of β-catenin in the daughter nucleus of closest proximity (Habib et al. 2013). However, if Wnts control asymmetric cell divisions in mammalian stem cells as they do in C. elegans, what defines the polarity of the Wnt signal? This is particularly difficult to envision for cultured ES cells submerged in culture medium. Another critical question is whether the Wnt-independent functions of β-catenin and/or β-catenin-independent functions of Wnt signaling contribute to the complexities of stem cell regulation. Superimposed on these issues is the increasing evidence that noncanonical Wnt pathways can powerfully influence stem cell behavior ranging from the roles of PCP in symmetric cell divisions of muscle satellite stem cells (Le Grand et al. 2009) to roles for noncanonical Wnt signaling in the hematopoietic stem cell niche as well as its aging counterparts (Sugimura et al. 2012; Florian et al. 2013). Whether these pathways compete for shared components such as the Dvl proteins and whether there are feedback circuitries that affect the regulation of these Wnt pathways will no doubt provide plenty of fuel for future investigations.
Acknowledgments
We thank our many colleagues in canonical Wnt signaling for valuable discussions on this topic and for their provocative insights stimulated by their many fine contributions to this field of research. In this regard, we give special thanks to Stefano Piccolo for discussions and for providing his manuscript prior to publication. W.-H.L. was supported by the Jane Coffin Child Fellowship and is currently an independent investigator of the de Duve Institute, Belgium. E.F. is an investigator of the Howard Hughes Medical Institute. The research that has led up to this review was supported by a grant to W.-H.L. from Fonds de la Recherche Scientifique (FNRS; F.6002.14) and grants to E.F. from the National Institutes of Health (R01-AR31737).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.244772.114.
References
- Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY 2002. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science 298: 2006–2010 [DOI] [PubMed] [Google Scholar]
- Allen TD, Potten CS 1974. Fine-structural identification and organization of the epidermal proliferative unit. J Cell Sci 15: 291–319 [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE 2002. WNT signals are required for the initiation of hair follicle development. Dev Cell 2: 643–653 [DOI] [PubMed] [Google Scholar]
- Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, et al. 2005. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132: 1443–1451 [DOI] [PubMed] [Google Scholar]
- Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S, Lamarque D, Laurent-Puig P, Perret C, Romagnolo B 2008. A genetic study of the role of the Wnt/β-catenin signalling in Paneth cell differentiation. Dev Biol 324: 288–296 [DOI] [PubMed] [Google Scholar]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S 2013. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154: 1047–1059 [DOI] [PubMed] [Google Scholar]
- Arce L, Pate KT, Waterman ML 2009. Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression. BMC Cancer 9: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlasi Y, Looijenga L, Fodde R 2014. Cancer Stem cells, pluripotency, and cellular heterogeneity: a WNTer perspective. Curr Top Dev Biol 107: 373–404 [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev 12: 2610–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S 2012. Role of TAZ as mediator of Wnt signaling. Cell 151: 1443–1456 [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. 2014. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell doi: 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW 2007. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J Biol Chem 282: 31703–31712 [DOI] [PubMed] [Google Scholar]
- Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H 2001. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBO J 20: 4935–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382: 638–642 [DOI] [PubMed] [Google Scholar]
- Beronja S, Janki P, Heller E, Lien WH, Keyes BE, Oshimori N, Fuchs E 2013. RNAi screens in mice identify physiological regulators of oncogenic growth. Nature 501: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickenbach JR, Holbrook KA 1987. Label-retaining cells in human embryonic and fetal epidermis. J Invest Dermatol 88: 42–46 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E 2009. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10: 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648 [DOI] [PubMed] [Google Scholar]
- Blauwkamp TA, Nigam S, Ardehali R, Weissman IL, Nusse R 2012. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun 3: 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boj SF, van Es JH, Huch M, Li VS, Jose A, Hatzis P, Mokry M, Haegebarth A, van den Born M, Chambon P, et al. 2012. Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell 151: 1595–1607 [DOI] [PubMed] [Google Scholar]
- Bottomly D, Kyler SL, McWeeney SK, Yochum GS 2010. Identification of β-catenin binding regions in colon cancer cells using ChIP-Seq. Nucleic Acids Res 38: 5735–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. 2005. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122: 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantjes H, Roose J, van De Wetering M, Clevers H 2001. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res 29: 1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K 1997. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385: 829–833 [DOI] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ 2013. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69 [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Waterman ML 2012. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 4: a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A 1998. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395: 604–608 [DOI] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, Fuchs E 1999. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet 21: 410–413 [DOI] [PubMed] [Google Scholar]
- Chen G, Fernandez J, Mische S, Courey AJ 1999. A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev 13: 2218–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodaparambil JV, Pate KT, Hepler MR, Tsai BP, Muthurajan UM, Luger K, Waterman ML, Weis WI 2014. Molecular functions of the TLE tetramerization domain in Wnt target gene repression. EMBO J 33: 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, et al. 2013. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 13: 720–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarapica R, Methot L, Tang Y, Lo R, Dali R, Buscarlet M, Locatelli F, del Sal G, Rota R, Stifani S 2014. Prolyl isomerase Pin1 and protein kinase HIPK2 cooperate to promote cortical neurogenesis by suppressing Groucho/TLE:Hes1-mediated inhibition of neuronal differentiation. Cell Death Differ 21: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH 2007. A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189 [DOI] [PubMed] [Google Scholar]
- Clevers H 2011. The cancer stem cell: premises, promises and challenges. Nat Med 17: 313–319 [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R 2012. Wnt/β-catenin signaling and disease. Cell 149: 1192–1205 [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA 2008. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 22: 746–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuilliere-Dartigues P, El-Bchiri J, Krimi A, Buhard O, Fontanges P, Flejou JF, Hamelin R, Duval A 2006. TCF-4 isoforms absent in TCF-4 mutated MSI-H colorectal cancer cells colocalize with nuclear CtBP and repress TCF-4-mediated transcription. Oncogene 25: 4441–4448 [DOI] [PubMed] [Google Scholar]
- Daniels DL, Weis WI 2005. β-Catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol 12: 364–371 [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557–4568 [DOI] [PubMed] [Google Scholar]
- Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT 2012. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci 109: 4485–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche M, Bienz M 2007. Wingless-independent association of Pygopus with dTCF target genes. Curr Biol 17: 556–561 [DOI] [PubMed] [Google Scholar]
- Deschene ER, Myung P, Rompolas P, Zito G, Sun TY, Taketo MM, Saotome I, Greco V 2014. β-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science 343: 1353–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C 2012. Defining the mode of tumour growth by clonal analysis. Nature 488: 527–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian MC, Nattamai KJ, Dorr K, Marka G, Uberle B, Vas V, Eckl C, Andra I, Schiemann M, Oostendorp RA, et al. 2013. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 503: 392–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E 1998. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95: 605–614 [DOI] [PubMed] [Google Scholar]
- Giese K, Amsterdam A, Grosschedl R 1991. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev 5: 2567–2578 [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C 1998. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362 [DOI] [PubMed] [Google Scholar]
- Grbavec D, Lo R, Liu Y, Stifani S 1998. Transducin-like Enhancer of split 2, a mammalian homologue of Drosophila Groucho, acts as a transcriptional repressor, interacts with Hairy/Enhancer of split proteins, and is expressed during neuronal development. Eur J Biochem 258: 339–349 [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E 2009. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H 1991. Cultured cells for the treatment of disease. Sci Am 265: 96–102 [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H 2005. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129: 626–638 [DOI] [PubMed] [Google Scholar]
- Gu B, Watanabe K, Sun P, Fallahi M, Dai X 2013. Chromatin effector Pygo2 mediates Wnt-notch crosstalk to suppress luminal/alveolar potential of mammary stem and basal cells. Cell Stem Cell 13: 48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R 2013. A localized Wnt signal orients asymmetric stem cell division in vitro. Science 339: 1445–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R et al. 1999. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol 9: 207–210 [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. 2004. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat Genet 36: 1117–1121 [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF 2011. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332: 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R 2000. The p300/CBP acetyltransferases function as transcriptional coactivators of β-catenin in vertebrates. EMBO J 19: 1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY 2011. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J Biol Chem 286: 12093–12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY 2010. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell 19: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R, Papp B, Hoffman JA, Merrill BJ, Plath K 2013. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell Reports 3: 2113–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmans R, Basler K 2004. Identification and in vivo role of the Armadillo–Legless interaction. Development 131: 4393–4400 [DOI] [PubMed] [Google Scholar]
- Holland JD, Klaus A, Garratt AN, Birchmeier W 2013. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 25: 254–264 [DOI] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E 2008. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132: 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J 1999. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398: 431–436 [DOI] [PubMed] [Google Scholar]
- Hsu SC, Galceran J, Grosschedl R 1998. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol Cell Biol 18: 4807–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E 2011. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E 2014. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157: 935–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R 1996. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev 59: 3–10 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W 2001. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105: 533–545 [DOI] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J 1994. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol 127: 2061–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlstone A, Clevers H 2002. T-cell factors: turn-ons and turn-offs. EMBO J 21: 2303–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E 2012. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J 31: 1109–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ 2004. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of β-catenin. Gastroenterology 126: 1236–1246 [DOI] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K 2003. The TAK1–NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/β-catenin signaling. Mol Cell Biol 23: 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM 1995. Stem cell patterning and fate in human epidermis. Cell 80: 83–93 [DOI] [PubMed] [Google Scholar]
- Keyes BE, Segal JP, Heller E, Lien WH, Chang CY, Guo X, Oristian DS, Zheng D, Fuchs E 2013. Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci 110: E4950–E4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N 1994. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev 8: 118–130 [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT 2005. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium 38: 439–446 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H 1997. Constitutive transcriptional activation by a β-catenin–Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K 2002. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin–TCF complex. Cell 109: 47–60 [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT 2000. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 16: 279–283 [DOI] [PubMed] [Google Scholar]
- Kuhl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D 2001. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/β-catenin and Wnt/Ca2+ signaling. Mech Dev 106: 61–76 [DOI] [PubMed] [Google Scholar]
- Lavker RM, Sun TT 1982. Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science 215: 1239–1241 [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW 2003. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 1: E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Madar A, David G, Garabedian MJ, Dasgupta R, Logan SK 2013. Inhibition of androgen receptor and β-catenin activity in prostate cancer. Proc Natl Acad Sci 110: 15710–15715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA 2009. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Rheaume C, Teng A, Bilanchone V, Munguia JE, Hu M, Jessen S, Piccolo S, Waterman ML, Dai X 2007. Developmental phenotypes and reduced Wnt signaling in mice deficient for pygopus 2. Genesis 45: 318–325 [DOI] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E 2014. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol 16: 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R 2013. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 342: 1226–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM 2006. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development 133: 3787–3796 [DOI] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X 1999a. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci 96: 6273–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A 1999b. Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22: 361–365 [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Liu Y, Rubin B, Bodine PV, Billiard J 2008. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem 105: 497–502 [DOI] [PubMed] [Google Scholar]
- Liu XH, Wu Y, Yao S, Levine AC, Kirschenbaum A, Collier L, Bauman WA, Cardozo CP 2013. Androgens up-regulate transcription of the Notch inhibitor Numb in C2C12 myoblasts via Wnt/β-catenin signaling to T cell factor elements in the Numb promoter. J Biol Chem 288: 17990–17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis F, Pedone E, Pepe S, Cosma MP 2008. Periodic activation of Wnt/β-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell 3: 493–507 [DOI] [PubMed] [Google Scholar]
- Lluis F, Ombrato L, Pedone E, Pepe S, Merrill BJ, Cosma MP 2011. T-cell factor 3 (Tcf3) deletion increases somatic cell reprogramming by inducing epigenome modifications. Proc Natl Acad Sci 108: 11912–11917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C 2011. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol 13: 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang HY 2007. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J Biol Chem 282: 28980–28990 [DOI] [PubMed] [Google Scholar]
- Mahmoudi T, Boj SF, Hatzis P, Li VS, Taouatas N, Vries RG, Teunissen H, Begthel H, Korving J, Mohammed S, et al. 2010. The leukemia-associated Mllt10/Af10-Dot1l are Tcf4/β-catenin coactivators essential for intestinal homeostasis. PLoS Biol 8: e1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, et al. 2008. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature 452: 650–653 [DOI] [PubMed] [Google Scholar]
- Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R 2008. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 3: 132–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C 2012. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489: 257–262 [DOI] [PubMed] [Google Scholar]
- Merrill BJ 2012. Wnt pathway regulation of embryonic stem cell self-renewal. Cold Spring Harb Perspect Biol 4: a007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E 2001. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 15: 1688–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E 2004. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development 131: 263–274 [DOI] [PubMed] [Google Scholar]
- Mieszczanek J, de la Roche M, Bienz M 2008. A role of Pygopus as an anti-repressor in facilitating Wnt-dependent transcription. Proc Natl Acad Sci 105: 19324–19329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R 2006. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context. PLoS Biol 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K, Sawa H 2007. Cortical β-catenin and APC regulate asymmetric nuclear β-catenin localization during asymmetric cell division in C. elegans. Dev Cell 12: 287–299 [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW 1997. Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275: 1787–1790 [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K 2009. β-Catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol 10: 276–286 [DOI] [PubMed] [Google Scholar]
- Murphy LL, Hughes CC 2002. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3β pathway. J Immunol 169: 3717–3725 [DOI] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A 2005. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437: 281–285 [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R 2004. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303: 1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller RA, Knoblich JA 2009. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23: 2675–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E 2006. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127: 171–183 [DOI] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E 2009. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet 41: 1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Smith A 2009. Naive and primed pluripotent states. Cell Stem Cell 4: 487–492 [DOI] [PubMed] [Google Scholar]
- Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM 2002. Expression of ΔNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129: 95–109 [DOI] [PubMed] [Google Scholar]
- Noordermeer J, Klingensmith J, Perrimon N, Nusse R 1994. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 367: 80–83 [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et al. 2003. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8: 645–654 [DOI] [PubMed] [Google Scholar]
- Owraghi M, Broitman-Maduro G, Luu T, Roberson H, Maduro MF 2010. Roles of the Wnt effector POP-1/TCF in the C. elegans endomesoderm specification gene network. Dev Biol 340: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM 2008. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE 3: e1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M, Lang B, Yu L, Prosser H, Bradley A, Babu MM, Choudhary J 2010. An expanded Oct4 interaction network: implications for stem cell biology, development, and disease. Cell Stem Cell 6: 382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Yi F, Merrill BJ 2006. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol 26: 7479–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Brylka H, Kraus A, John S, Rappl G, Schettina P, Niemann C 2011. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J 30: 3004–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H 2003. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, Lechler T 2010. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol 191: 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J 1997. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci 94: 2859–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Zhang H 2010. Wnt signaling controls temporal identities of seam cells in Caenorhabditis elegans. Dev Biol 345: 144–155 [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423: 409–414 [DOI] [PubMed] [Google Scholar]
- Ring A, Kim YM, Kahn M 2014. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev doi: 10.1007/s12015-014-9515-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsma L, Ellenbroek SI, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J 2014. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507: 362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H 1998. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395: 608–612 [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR 2008. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH 2004. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10: 55–63 [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H 2011. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambony A, Wedlich D 2007. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 12: 779–792 [DOI] [PubMed] [Google Scholar]
- Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, et al. 2006. The molecular basis of vitamin D receptor and β-catenin crossregulation. Mol Cell 21: 799–809 [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT 2003. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 161: 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy BR, Wu CI, Khramtsova GF, Zhang JY, Olopade OI, Goss KH, Merrill BJ 2013. Regulation of Tcf7l1 DNA binding and protein stability as principal mechanisms of Wnt/β-catenin signaling. Cell Reports 4: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SY 2011. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 138: 4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP 2003. β-Catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol Cell Biol 23: 1674–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R, He XC, Venkatraman A, Arai F, Box A, Semerad C, Haug JS, Peng L, Zhong XB, Suda T, et al. 2012. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150: 351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT 2000. The transcriptional coactivator CBP interacts with β-catenin to activate gene expression. J Cell Biol 149: 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B 2008. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells 26: 2019–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407: 530–535 [DOI] [PubMed] [Google Scholar]
- ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R 2011. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 13: 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M 2002. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol 4: 367–373 [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ 2011. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Cliffe A, Bienz M 2004. Pygopus and Legless target Armadillo/β-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol 6: 626–633 [DOI] [PubMed] [Google Scholar]
- Trompouki E, Bowman TV, Lawton LN, Fan ZP, Wu DC, DiBiase A, Martin CS, Cech JN, Sessa AK, Leblanc JL, et al. 2011. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell 147: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Lukas J, Korinek V 2003. HMG box transcription factor TCF-4’s interaction with CtBP1 controls the expression of the Wnt target Axin2/Conductin in human embryonic kidney cells. Nucleic Acids Res 31: 2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp JK, Beckers S, Zegers D, Van Hul W 2014. Wnt signaling and the control of human stem cell fate. Stem Cell Rev 10: 207–229 [DOI] [PubMed] [Google Scholar]
- van den Berg DL, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA 2010. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell 6: 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799 [DOI] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, et al. 2012. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol 32: 1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, et al. 2010. The Hippo pathway regulates Wnt/β-catenin signaling. Dev Cell 18: 579–591 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E 1999. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci 96: 8551–8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi MP, Hatzis P, Sulahian R, Philips J, Schuijers J, Shin H, Freed E, Lynch JP, Dang DT, Brown M, et al. 2010. TCF4 and CDX2, major transcription factors for intestinal function, converge on the same cis-regulatory regions. Proc Natl Acad Sci 107: 15157–15162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN 1989. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature 338: 263–264 [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407: 527–530 [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A 2011. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 13: 838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CI, Hoffman JA, Shy BR, Ford EM, Fuchs E, Nguyen H, Merrill BJ 2012. Function of Wnt/β-catenin in counteracting Tcf3 repression through the Tcf3–β-catenin interaction. Development 139: 2118–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Yuan H, Cheng J, Hunt AJ 2010. Polarity in stem cell division: asymmetric stem cell division in tissue homeostasis. Cold Spring Harb Perspect Biol 2: a001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. 2012. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci 109: 466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ 2011. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 13: 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, et al. 2012. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18: 618–623 [DOI] [PubMed] [Google Scholar]
- Zhang P, Chang WH, Fong B, Gao F, Liu C, Al Alam D, Bellusci S, Lu W 2014. Regulation of iPS cell induction by Wnt/β-catenin signaling. J Biol Chem 289: 9221–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Byrne C, Jacobs J, Fuchs E 1995. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 9: 700–713 [DOI] [PubMed] [Google Scholar]