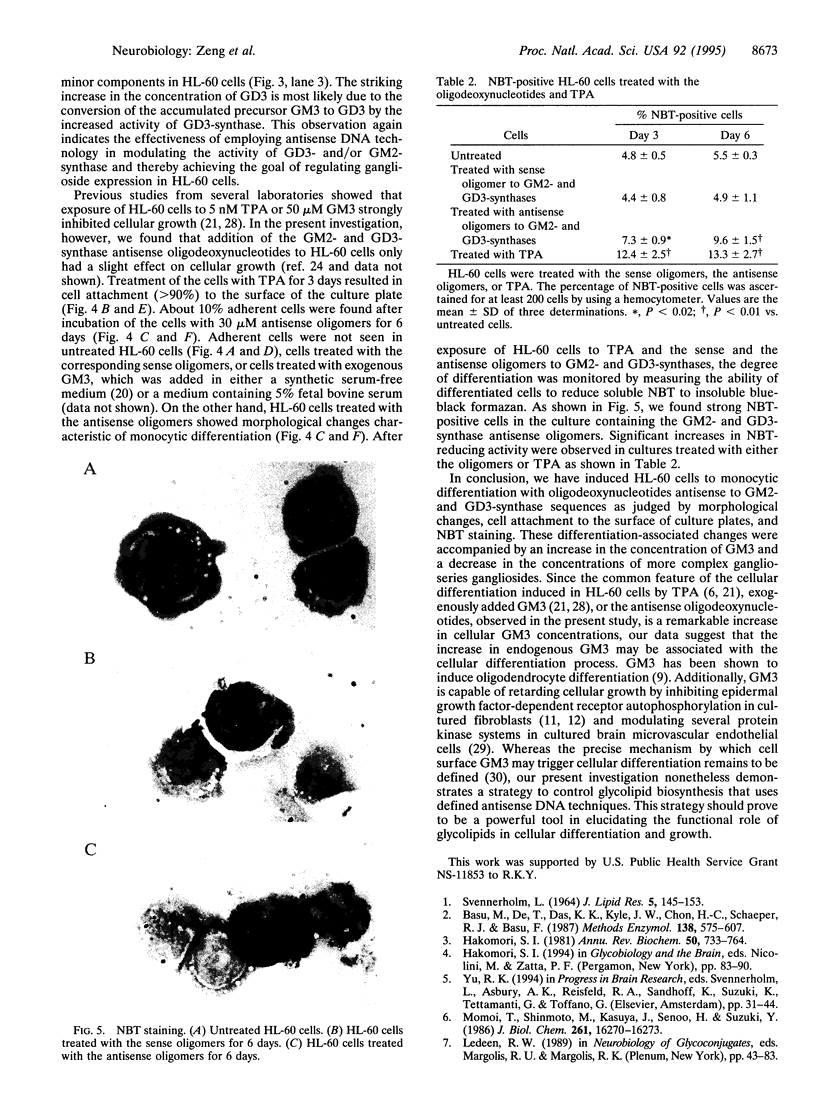
Abstract
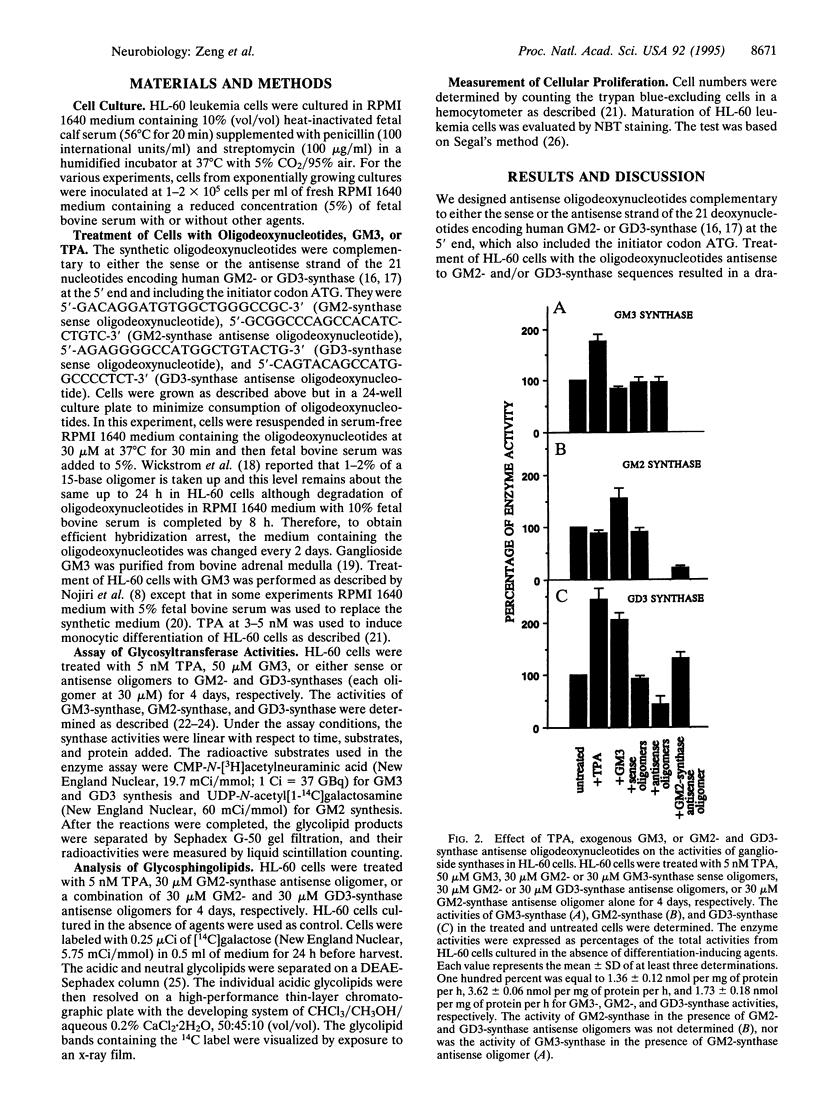
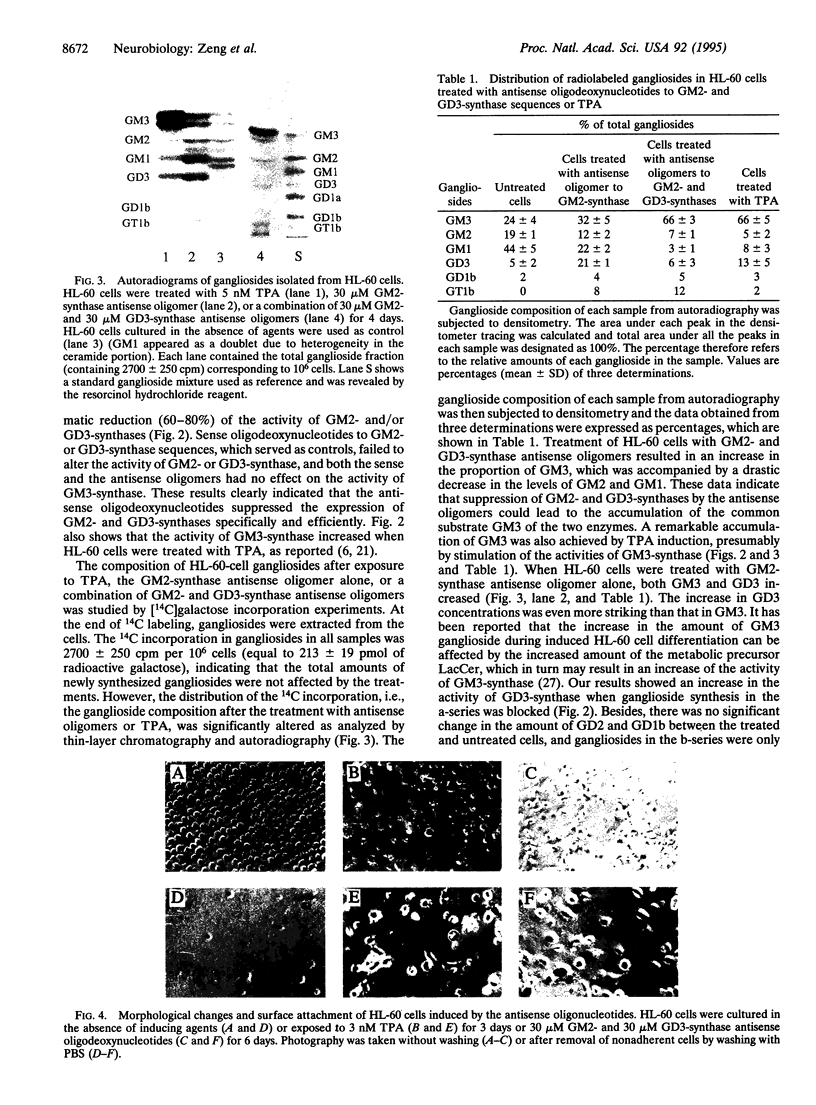
Treatment of the human promyelocytic leukemia cell line HL-60 with antisense oligodeoxynucleotides to UDP-N-acetylgalactosamine:beta-1,4-N-acetylgalactosaminyl-transferase (GM2-synthase; EC 2.4.1.92) and CMP-sialic acid:alpha-2,8-sialyltransferase (GD3-synthase; EC 2.4.99.8) sequences effectively down-regulated the synthesis of more complex gangliosides in the ganglioside synthetic pathways after GM3, resulting in a remarkable increase in endogenous GM3 with concomitant decreases in more complex gangliosides. The treated cells underwent monocytic differentiation as judged by morphological changes, adherent ability, and nitroblue tetrazolium staining. These data provide evidence that the increased endogenous ganglioside GM3 may play an important role in regulating cellular differentiation and that the antisense DNA technique proves to be a powerful tool in manipulating glycolipid synthesis in the cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu M., De T., Das K. K., Kyle J. W., Chon H. C., Schaeper R. J., Basu S. Glycolipids. Methods Enzymol. 1987;138:575–607. doi: 10.1016/0076-6879(87)38053-x. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E. G., Schlessinger J., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986 Feb 15;261(5):2434–2440. [PubMed] [Google Scholar]
- Gu X. B., Gu T. J., Yu R. K. Purification to homogeneity of GD3 synthase and partial purification of GM3 synthase from rat brain. Biochem Biophys Res Commun. 1990 Jan 15;166(1):387–393. doi: 10.1016/0006-291x(90)91957-t. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Kanda T., Ariga T., Yamawaki M., Pal S., Katoh-Semba R., Yu R. K. Effect of nerve growth factor and forskolin on glycosyltransferase activities and expression of a globo-series glycosphingolipid in PC12D pheochromocytoma cells. J Neurochem. 1995 Feb;64(2):810–817. doi: 10.1046/j.1471-4159.1995.64020810.x. [DOI] [PubMed] [Google Scholar]
- Kanda T., Ariga T., Yamawaki M., Yu R. K. GM3 regulates protein kinase systems in cultured brain microvascular endothelial cells. J Neurochem. 1993 Nov;61(5):1969–1972. doi: 10.1111/j.1471-4159.1993.tb09842.x. [DOI] [PubMed] [Google Scholar]
- Kiguchi K., Henning-Chubb C., Huberman E. Glycosphingolipid patterns in human promyelocytic HL-60 leukemia cells susceptible or resistant to differentiation induction by phorbol 12-myristate 13-acetate. Biochim Biophys Acta. 1993 Mar 10;1176(1-2):27–36. doi: 10.1016/0167-4889(93)90173-m. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Momoi T., Shinmoto M., Kasuya J., Senoo H., Suzuki Y. Activation of CMP-N-acetylneuraminic acid:lactosylceramide sialyltransferase during the differentiation of HL-60 cells induced by 12-O-tetradecanoylphorbol-13-acetate. J Biol Chem. 1986 Dec 5;261(34):16270–16273. [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1992 Jun 15;267(17):12082–12089. [PubMed] [Google Scholar]
- Nakamura M., Ogino H., Nojiri H., Kitagawa S., Saito M. Characteristic incorporation of ganglioside GM3, which induces monocytic differentiation in human myelogenous leukemia HL-60 cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):782–789. doi: 10.1016/0006-291x(89)92668-5. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Tsunoda A., Sakoe K., Gu J., Nishikawa A., Taniguchi N., Saito M. Total metabolic flow of glycosphingolipid biosynthesis is regulated by UDP-GlcNAc:lactosylceramide beta 1-->3N-acetylglucosaminyltransferase and CMP-NeuAc:lactosylceramide alpha 2-->3 sialyltransferase in human hematopoietic cell line HL-60 during differentiation. J Biol Chem. 1992 Nov 25;267(33):23507–23514. [PubMed] [Google Scholar]
- Nojiri H., Takaku F., Terui Y., Miura Y., Saito M. Ganglioside GM3: an acidic membrane component that increases during macrophage-like cell differentiation can induce monocytic differentiation of human myeloid and monocytoid leukemic cell lines HL-60 and U937. Proc Natl Acad Sci U S A. 1986 Feb;83(3):782–786. doi: 10.1073/pnas.83.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss U., Gu X., Gu T., Yu R. K. Purification and characterization of CMP-N-acetylneuraminic acid:lactosylceramide (alpha 2-3) sialyltransferase (GM3-synthase) from rat brain. J Biol Chem. 1993 Dec 15;268(35):26273–26278. [PubMed] [Google Scholar]
- SVENNERHOLM L. THE GANGLIOSIDES. J Lipid Res. 1964 Apr;5:145–155. [PubMed] [Google Scholar]
- Sasaki K., Kurata K., Kojima N., Kurosawa N., Ohta S., Hanai N., Tsuji S., Nishi T. Expression cloning of a GM3-specific alpha-2,8-sialyltransferase (GD3 synthase). J Biol Chem. 1994 Jun 3;269(22):15950–15956. [PubMed] [Google Scholar]
- Segal A. W. Nitroblue-tetrazolium tests. Lancet. 1974 Nov 23;2(7891):1248–1252. doi: 10.1016/s0140-6736(74)90758-2. [DOI] [PubMed] [Google Scholar]
- Steigerwald J. C., Basu S., Kaufman B., Roseman S. Sialic acids. Enzymatic synthesis of Tay-Sachs ganglioside. J Biol Chem. 1975 Sep 10;250(17):6727–6734. [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X. J., Gu X. B., Sartorelli A. C., Yu R. K., Santorelli A. C. Effects of inducers of differentiation on protein kinase C and CMP-N-acetylneuraminic acid:lactosylceramide sialyltransferase activities of HL-60 leukemia cells. J Lipid Res. 1989 Feb;30(2):181–188. [PubMed] [Google Scholar]
- Yim S. H., Farrer R. G., Hammer J. A., Yavin E., Quarles R. H. Differentiation of oligodendrocytes cultured from developing rat brain is enhanced by exogenous GM3 ganglioside. J Neurosci Res. 1994 Jun 15;38(3):268–281. doi: 10.1002/jnr.490380305. [DOI] [PubMed] [Google Scholar]
- Yim S. H., Yavin E., Hammer J. A., Quarles R. H. Exogenous GM3 ganglioside stimulates process formation and glycoprotein release by cultured bovine oligodendrocytes. J Neurochem. 1991 Dec;57(6):2144–2147. doi: 10.1111/j.1471-4159.1991.tb06435.x. [DOI] [PubMed] [Google Scholar]
- Yu R. K., Macala L. J., Taki T., Weinfield H. M., Yu F. S. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988 Jun;50(6):1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Hakomori S., Kitamura K., Igarashi Y. GM3 directly inhibits tyrosine phosphorylation and de-N-acetyl-GM3 directly enhances serine phosphorylation of epidermal growth factor receptor, independently of receptor-receptor interaction. J Biol Chem. 1994 Jan 21;269(3):1959–1965. [PubMed] [Google Scholar]