Abstract
Lysosomal storage diseases are a heterogeneous group of hereditary disorders characterized by a deficiency in lysosomal function. Although these disorders differ in their etiology and phenotype those that affect the nervous system generally manifest as a profound deterioration in neurologic function with age. Over the past several decades implementation of various treatment regimens including bone marrow and cord blood cell transplantation, enzyme replacement, and substrate reduction therapy have proved effective for managing some clinical manifestations of these diseases but their ability to ameliorate neurologic complications remains unclear. Consequently, there exists a need to develop alternative therapies that more effectively target the central nervous system. Recently, direct intracranial transplantation of tissue-specific stem and progenitor cells has been explored as a means to reconstitute metabolic deficiencies in the CNS. In this chapter we discuss the merits of bone marrow-derived mesenchymal stem cells (MSCs) for this purpose. Originally identified as progenitors of connective tissue cell lineages, recent findings have revealed several novel aspects of MSC biology that make them attractive as therapeutic agents in the CNS. We relate these advances in MSC biology to their utility as cellular vectors for treating neurologic sequelae associated with pediatric neurologic disorders.
Keywords: Mesenchymal stem cells, marrow stromal cells, lysosomal storage diseases, pediatric, central nervous system, cell therapy, migration, paracrine signaling
1. Introduction
Children suffer from a variety of hereditary disorders that manifest as a profound deterioration in neurological function with age. Among these disorders the lysosomal storage diseases (LSDs) are most common. LSDs represent over 40 genetic disorders that result from defects in lysosomal function, which leads to accumulation of glycosaminoglycans, glycoproteins, or sphingolipids in organs throughout the body. Although rare, collectively these diseases have an incidence of approximately 1 in 7000–8000 live births (Winchester et al., 2000). Depending upon the specific enzyme deficiency, distinct patterns of substrate accumulation occurs in organs resulting in a wide spectrum of clinical symptoms (Moses, 1990). Additionally, the time of onset to disease, which ranges from infancy to adulthood, as well as the degree of clinical involvement is influenced both by the specific inherited genetic mutation and the level of enzyme deficiency. In some LSDs abnormal accumulation of storage material occurs within cells of the brain and spinal cord, making neuro-degeneration a prominent feature of these disorders. Biochemical and pathological studies indicate that specific neural cell types possess different sensitivities to accumulated storage material, making distinct brain regions susceptible to disease. For example in Gaucher disease, which is caused by a deficiency of glucocerebrosidase, significant neuronal losses have been observed within the basal ganglia, nuclei of the midbrain, cerebellum, dentate nucleus, and hypothalamus (Espinas and Faris, 1969; Kaye et al., 1986). A recent analysis of autopsy samples from patients with all three forms of Gaucher disease indicated that neuronal loss predominated in type 2 and 3 patients but patients with type 1 disease presented with astrogliosis (Wong et al., 2004). In contrast, patients with Niemann-Pick type C (NPC) typically exhibit widespread neuronal atrophy at early stages of the disease but at later stages Purkinje neurons in the cerebellum become uniquely sensitive to degeneration (Walkley and Suzuki, 2004). Alternatively, Sandhoff and Tay-Sachs patients exhibit widespread apoptosis throughout the cerebral cortex, cerebellum, and brain stem that affects neurons, oligodendrocytes, astrocytes, Purkinje cells, micro-glia, and vascular pericytes (Huang et al., 1997).
A subset of LSDs, the leukodystrophies, manifest as a profound degeneration of white matter due to defects in myelin metabolism. For example, metachromatic leukodystrophy (MLD), one of the most common leukodystrophies, results from the inability to degrade sulfated glycolipids due to a deficiency of the lysosomal enzyme arylsulfatase A (Gieselmann, 2008). Some MLD patients have normal arylsulfatase A activity but lack an activator protein that is involved in sulfatide degradation (Kolter and Sandhoff, 2005). Both defects result in the intra-lysosomal accumulation of sulfatide compounds in neural and non-neural tissues. Pathological features include diffuse demyelination and metachromatic-staining granules in glial cells and macrophages. Central and peripheral myelination is abnormal with widespread loss of myelinated oligodendroglia in the CNS and segmental demyelination of peripheral nerves (Gieselmann, 2008; Gieselmann and Krageloh-Mann, 2010). Symptoms typically manifest during peak periods of myelin formation in post-natal development resulting in progressive loss of both motor and cognitive functions followed by death in approximately five years. However, the onset of the disease may be delayed until adolescence or adulthood depending on the degree of enzyme deficiency. Recently, several new causative mutations have been identified for this disorder (Cesani et al., 2009; Galla et al., 2013; Luzi et al., 2013).
In summary, many LSDs present with neurological sequelae but the cause and extent of neuro-degeneration are dictated by the nature of the accumulated storage material and its relative toxicity to different cell types. Consequently, developing a single therapeutic approach to treat neurologic sequelae associated with LSDs is difficult since the therapy must reduce accumulated storage material in a variety of cell types localized within different anatomical regions of the brain. Presently, hematopoietic stem cell transplantation (HSCT) and umbilical cord blood transplantation (UCBT) attempt to achieve this result by providing the CNS with a continuous supply of micro-glial cells with normal lysosomal functions via the circulation. However, since the overall efficacy of these approaches remains uncertain, alternative treatment strategies designed to more efficiently target the CNS are under development.
2. Cell-Based Therapies for Lysosomal Storage Diseases
Over the past 25 years HSCT has been used to treat many types of sphingolipidosis and mucopolysaccharidosis (Krivit, 2002; Lund, 2013). This procedure provides hematopoietic cells from an unaffected donor to replace those of the host loaded with non-metabolized storage material. Moreover, enzyme produced by donor-derived cells or released during their turnover can accumulate in adjacent host cells or in plasma, reducing the amount of storage material both locally and systemically. HSCT has shown a measurable clinical benefit for the treatment of Hurler syndrome (Boelens et al., 2013; Guffon et al., 1998; Krivit et al., 1995; Peters et al., 1996; Sauer et al., 2009; Souillet et al., 2003), MLD (Biffi et al., 2008; Kidd et al., 1998a; Krageloh-Mann et al., 2013; Krivit et al., 1990; Malm et al., 1996), and Krabbe disease (Duffner et al., 2009; Gelinas et al., 2012; Krivit et al., 1998; McGraw et al., 2005; Pastores, 2009; Siddiqi et al., 2006). However, overall results in these patients are mixed as outcomes often exhibit marked between-subject variability. Moreover, some published case histories have reported no benefit to patients despite evidence of stable donor cell engraftment in bone marrow (Hoogerbrugge et al., 1995; Malm et al., 1996). Nevertheless, long-term follow up of Hurlers syndrome patients treated with HSCT indicated that the treatment does provide long-term, slow improvement in adaptive behaviors and psychomotor skills in children (Bjoraker et al., 2006; Lucke et al., 2007) albeit their continued development occurs at a slower than average rate and never accelerates to a normal range as compared to age-matched peers. As anticipated, children with good cognitive levels prior to HSCT and continued growth of cognition after HSCT also exhibited good adaptive functions.
Collectively, these studies reveal a clear benefit for early identification and treatment of patients with infantile forms of rapidly progressive neurodegenerative diseases. Consequently, UCB cells have gained prominence for the treatment of LSDs due to their rapid availability, enhanced engraftment potential, and reduced risk of graft versus host disease (GvHD) (Jaing, 2007; Mogul, 2000). UCBT has shown great benefit in delaying the onset of neurologic dysfunction when applied to storage disease patients who are asymptomatic at the time of the transplant (Martin et al., 2013; Prasad and Kurtzberg, 2010). Moreover, UCBT results in lower rates of graft failure, a higher incidence of full donor chimerism, normalization of serum enzyme levels, and a low rate of GvHD. Nevertheless, despite encouraging data regarding the effectiveness of both HSCT and UCBT (Boelens et al., 2013) both procedures suffer from several inherent complications associated with pre-conditioning, graft rejection, and development of acute and chronic GvHD that pose significant risks to the patient (Grewal et al., 2003; Martin et al., 2006; Ozen et al., 2007; Prasad and Kurtzberg, 2010). Recent studies also indicate that long-term mortality rates remain significantly higher after HSCT in patients with inborn errors of metabolism (Eapen et al., 2012). Debate lingers regarding the role of the blood brain barrier in limiting access of proteinaceous or cellular therapeutics to the CNS, as well (Begley et al., 2008). Consequently, more research is needed to develop standardized criteria for patient selection, treatment regimens, post-treatment supportive care, and short and long-term outcome analyses such that the overall therapeutic benefit of HSCT and UCBT can be better evaluated (Abdelhalim et al., 2014; Escolar et al., 2006). Continued long-term analysis of functional outcomes in patients is particularly important owing to the fact that anecdotal reports have surfaced suggesting that many pre-symptomatic children treated with UCB transplants have developed motor and language deterioration (Duffner et al., 2009).
3. Intracranial Stem Cell Transplantation
Rodent, feline, canine, and non-human primate LSD models have been described and the neuropathology exhibited in these animal models recapitulates to a large degree that of human patients. Consequently, they have proved valuable for evaluating novel cell-based therapies. For example, Synder et al. (Snyder et al., 1995) and Meng et al. (Meng et al., 2003) first reported that neuro-progenitor cell lines engineered to express β-glucuronidase (GUSB) showed widespread engraftment when injected intra-cranially into a mouse model of mucopolysaccharidoses type VII (MPS VII), leading to a decrease in storage material throughout the brain. Similarly, Fukuhara et al. (Fukuhara et al., 2006) reported that intracranial injection of fetal neural stem cells into MPS II mice increased brain GUSB activity to ~ 6% of wild type levels by 3 weeks post-transplant resulting in reduced levels of storage material in the brain and improved non-spatial hippocampus-dependent memory. More recent studies have confirmed that neural progenitor administration can ameliorate disease in animal models of LSD (Givogri et al., 2008; Shihabuddin and Cheng, 2011; Strazza et al., 2009). Others have reported positive effects following intracranial transplantation of engineered amniotic epithelial cells (Kosuga et al., 2001) and neuro-progenitor cells (Lacorazza et al., 1996) in animal models of MPS VII or Tay-Sachs disease, respectively.
These and other studies prompted a phase I clinical trial to assess the safety of normal human central nervous system stem cells (HuCNS-SC) injected intra-cranially for treatment of Batten disease, a common form of a group of disorders referred to as ceroid lipofuscinosis, neuronal (CLN). At least ten genetically distinct forms of CLN exist, which have been associated with mutations in specific genes encoding soluble (CLN1, CLN2, CLN5, and CLN10) and trans-membrane (CLN3, CLN6, CLN7, and CLN8) proteins (Warrier et al., 2013). Defects in the activity of these proteins lead to excessive accumulation of lipo-pigments in cells of the brain, eyes, and other organs. For example, CLN1 results from a deficiency in the activity of palmitoyl protein thioesterase (PPT), a lysosomal enzyme that removes fatty acyl groups from cysteine residues on fatty acid modified proteins. CLN2 patients are deficient in tripeptidyl peptidase 1 (TTP1), a pepstatin-insensitive lysosomal peptidase that removes tri-peptides from the N-terminal of polypeptides (Bennett and Hofmann, 1999). All forms of CLN invariably prove fatal after a prolonged period of disability. Moreover, neurological sequelae associated with Batten disease have proven refractory to bone marrow transplants. For example, Lonnqvist et al. (Lonnqvist et al., 2001) reported that three infants who received bone marrow transplants, two of whom were asymptomatic at the time of treatment, developed disease by 2 to 3 years of age despite the fact that PPT1 enzyme activity was normalized in peripheral leukocytes. Other studies have documented a complete lack of enzyme transfer between donor and patient lymphocytes in vitro, which may account in part for the ineffectiveness of bone marrow transplantation in patients (Lake et al., 1995). Results from human clinical trials are anticipated based on animal studies, which also demonstrated no efficacy of bone marrow transplantation in canine (Deeg et al., 1990) and sheep (Westlake et al., 1995) models of the disease.
The aforementioned clinical trial, conducted at the Oregon Health and Science University and sponsored by StemCells Inc., targeted both infantile and late infantile forms of CLN1 and CLN2. A total of six children were treated by direct intracranial injection of allogeneic HuCNS-SC. In January of 2008 a 9 year old girl who received bilateral stem cell injections succumb to the disease. Post-mortem examination of the patient’s brain did not reveal a tumor but there was marked brain atrophy and neurons contained high levels of undigested lipofuscin consistent with advance stages of disease progression. Nevertheless, long-term (one year) survival of the transplanted donor cells was evident in tissues samples taken at autopsy. The five remaining patients were enrolled in a four-year long-term observational study, with three of the five surviving to the end of the four-year study. Assessment of the patients' cognitive and neurological function revealed stable scores in some tests, but the clinical outcomes were generally consistent with the expected course of impairment associated with the disease. For example, magnetic resonance imaging scans of the brain showed progressive atrophy consistent with the patient's neuropsychological performance but quality-of-life measures remained stable across all three surviving patients. While no specific conclusions about impacting the disease course could be made in the open-label trial, no safety concerns attributed to the HuCNS-SC cell transplantation were noted (Selden et al., 2013). HuCNS-SC cell transplantation was also recently evaluated in a 1-year, open-label phase-1 study for the treatment of Pelizeaus-Merzbacher Disease (PMD), a fatal myelination disorder that afflicts male children (Gupta et al., 2012). Herein, allogeneic HuCNS-SCs were surgically implanted into the frontal lobe white matter in four male subjects with an early-onset severe form of PMD. Once again the HuCNS-SC transplantation was well tolerated and modest gains in neurological function were observed in three of the four subjects. Magnetic resonance imaging revealed increased myelination in the region of transplantation compared to control white matter regions remote to the transplant sites indicative of durable cell engraftment within the brain.
The use of HuCNS-SCs for the treatment of Batten’s disease and PMD is advantageous for several reasons. First, HuCNS-SCs retain the ability to differentiate into various neural cell types, and therefore may potentially replace host brain cells lost to disease. Second, transplantation of the cells into their native tissue environment typically yields reliable and widespread engraftment. However, these advantages are offset by the fact that all transplants are allogeneic in nature and therefore immuno-suppression is required to achieve durable, long-term engraftment in vivo. Additionally, some Parkinson’s patients who received grafts of fetal neural tissue developed adverse side effects in the form of various movement disorders due to aberrant integration of the donor neural cells into the host CNS circuitry (Freed et al., 2001). Therefore, benefits afforded by restoration of deficient enzymatic activity may be offset by morbidities associated with aberrant integration of donor-derived neurons into the established brain circuitry.
3.1. MSCs as Vectors for Neurological Diseases
MSCs derived from adult bone marrow have also demonstrated efficacy in treating various neurological disorders. Based on early studies demonstrating that they regulate hematopoiesis and differentiate into connective tissue lineages, MSC-based therapies were initially developed to treat osteogenesis imperfecta (Horwitz et al., 1999; Horwitz et al., 2002) and to speed recovery from bone marrow transplantation (Koc et al., 2000). More recently, MSCs have been shown to secrete a broad array of powerful paracrine-acting factors that promote tissue repair by providing trophic support, suppressing inflammation, and modulating immune cell function (Caplan and Dennis, 2006; Caplan and Correa, 2011; Nauta and Fibbe, 2007; Phinney, 2007) (Figure 1). Consequently, MSC-based therapies are now being evaluated in human clinical trials for treatment of a broad array of disorders including myocardial infarction (Hare et al., 2009), GvHD (Ball et al., 2013; Le Blanc et al., 2008), severe systemic lupus erythematosus (Wang et al., 2012), and complex peri-anal fistulas (Yong Lee et al., 2013).
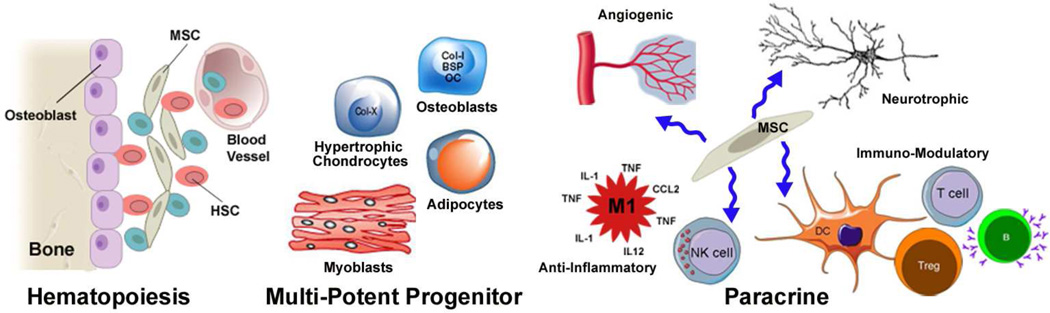
Figure 1. Biological functions of MSCs.
MSCs contribute to the hematopoietic stem cell niche in bone marrow by promoting retention of quiescent HSCs within the endosteal niche. HSCs can be recruited from the niche to divide and yield progeny that enter the vasculature. MSCs also regulate tissue homeostasis by serving as a source of progenitor cells that differentiate into connective tissue lineages including adipocytes, chondrocytes, myoblasts, and osteoblasts. MSCs and their progeny also secrete a plethora of paracrine acting factors that exhibit angiogenic, neurotrophic, anti-inflammatory, and immuno-modulatory activity. In the latter case these factors act on cell types that comprise both the innate and adaptive immune system.
Our laboratory was the first to demonstrate that bone marrow-derived MSCs injected directly into the CNS of newborn mice persistently engraft, migrate throughout a large volume of the brain, and at a low frequency acquire characteristics of neural cells (Kopen et al., 1999). Based on these and other related studies, MSCs were quickly exploited as cellular vectors to deliver deficient enzymes to the CNS in animal models of LSD. For example, intra-cranial injection of plastic adherent cells from murine bone marrow engineered to over express acid sphingomyelinase (ASM) into ASM-deficient mice were shown to exhibit widespread engraftment throughout the CNS, reducing brain levels of sphingomyelin, retard death of Purkinje cells, and prolonged animal survival (Jin et al., 2002). Combination therapy employing systemic administration of ASM-transduced bone marrow cells together with intra-cerebral MSC injection was also shown to restore ASM activity to near normal levels in most tissues including brain (Jin and Schuchman, 2003). This resulted in a marked reduction in sphingomyelin levels and preservation of greater than 80% of all Purkinje cells in the cerebellum. Sakurai et al. (Sakurai et al., 2004) further demonstrated that intracranial transplantation of MSCs engineered to express GUSB produced elevated enzyme levels in the olfactory bulb, striatum, and cerebral cortex, significantly reducing glycosaminoglycan levels and improving the cognitive function of MPS VIII mice. MSCs have also been shown to promote recovery in neurological function in experimental animal models of Parkinson’s disease, Huntington’s disease, Tourette syndrome, stroke, traumatic brain injury, and other disorders (Joyce et al., 2010; Phinney and Isakova, 2005; Teixeira et al., 2013). Collectively, these data demonstrate that MSCs are well-suited as vectors to treat neurologic sequelae associated with LSDs. Nevertheless, few if any studies have examined the molecular mechanisms that mediate MSC engraftment, survival, and migration in the CNS despite that fact that a better understanding of these processes may facilitate improved treatment regimens to increase efficacy.
3.2. MSC Engraftment in the Rodent and Non-Human Primate Brain
To analyze the engraftment kinetics and map the anatomical distribution of MSCs transplanted directly into the CNS our laboratory designed PCR primers and a Taqman® probe that specifically target the mouse Y chromosome (McBride et al., 2003). We then used quantitative real-time PCR to analyze brain tissue of female mice injected with primary MSCs enriched from the bone marrow of male mice. These analyses revealed that MSC engraftment levels were significantly greater in neonatal vs. adult transplant recipients despite the fact that only a small percentage of injected cells survived in vivo. Analysis of coronal brain slices from transplant recipients further demonstrated that male DNA was distributed along the brain neuraxis in both neonatal and adult transplant recipients, with the highest engraftment levels localized near the site of injection (bregma) in forebrain as well as in the cerebellum. These results were validated by visualization of male MSCs in brain tissue sections via fluorescent in situ hybridization using a Y chromosome probe. This analysis further revealed that MSCs localized to the striatum, cerebral cortex, granular layers of the hippocampus and cerebellum, and white matter tracts in forebrain (McBride et al., 2003; Phinney et al., 2006).
As part of a pre-clinical safety trial, we also evaluated the engraftment of MSCs transplanted to the CNS of infant and young adult rhesus macaques (Isakova et al., 2006; Isakova et al., 2007). Herein, unmatched MSCs from a universal male donor were delivered via stereo-tactic injection into the caudate putamen of female recipients and at various times post-transplant affects on neural development, behavior, motor performance, and cognition were evaluated. After sacrifice at 3 or 6 months post-transplant, coronal brain slices from each transplant recipient were subdivided into specimens of comparable size and anatomical location, analyzed by RT-PCR using a Macaca sp. Y chromosome-specific probe (Isakova et al., 2006), and the data transposed onto a physical map of the macaque brain. These analyses revealed that overall MSC engraftment levels were on average 17.8-fold higher (p<0.05) in infant vs. young adult transplant recipients with a maximal observed difference of 180-fold. Furthermore, male DNA was found to be widely distributed throughout both brain hemispheres, the anatomical location of which overlapped significantly between transplant recipients in both age groups. Infant macaques that received MSCs exhibited the highest engraftment levels in tissue specimens encompassing the somato-sensory, primary motor, and auditory cortex, caudate putamen, striatum, and hippocampus. Moreover, engraftment levels averaged over all infant transplant recipients were shown to vary significantly between different anatomical locations in the brain and as a function of time post-transplant. Therefore, MSCs preferentially localized to specific but overlapping anatomical regions and redistributed along the brain neuraxis as a function of time post-transplantation (Figure 2). This result is in stark contrast to that reported for fibroblasts, which have demonstrated a very limited capacity to migrate beyond the site of injection in brain (Kawaja et al., 1991; Senut et al., 1995; Taylor and Wolfe, 1997). Transplant recipients were also subject to a battery of age- and species-appropriate tests, which failed to reveal any adverse affects of MSC transplantation on cognition, fine and course motor function, behavior, or development. This outcome was obtained despite the fact that transplant recipients were monitored throughout a large proportion of their first year of life, during which social behavior, motor skills and cognitive abilities are rapidly developing. Collectively, these data indicate that MSCs disseminate non-randomly within the brain and generally exhibit low overall engraftment levels. However, significantly higher engraftment levels are achieved in infant vs. adult transplant recipients. This outcome is highly favorable with regard to therapy since LSDs with neurologic complications typically exhibit an early age of onset and early intervention is critical toward retarding or reversing disease progression.
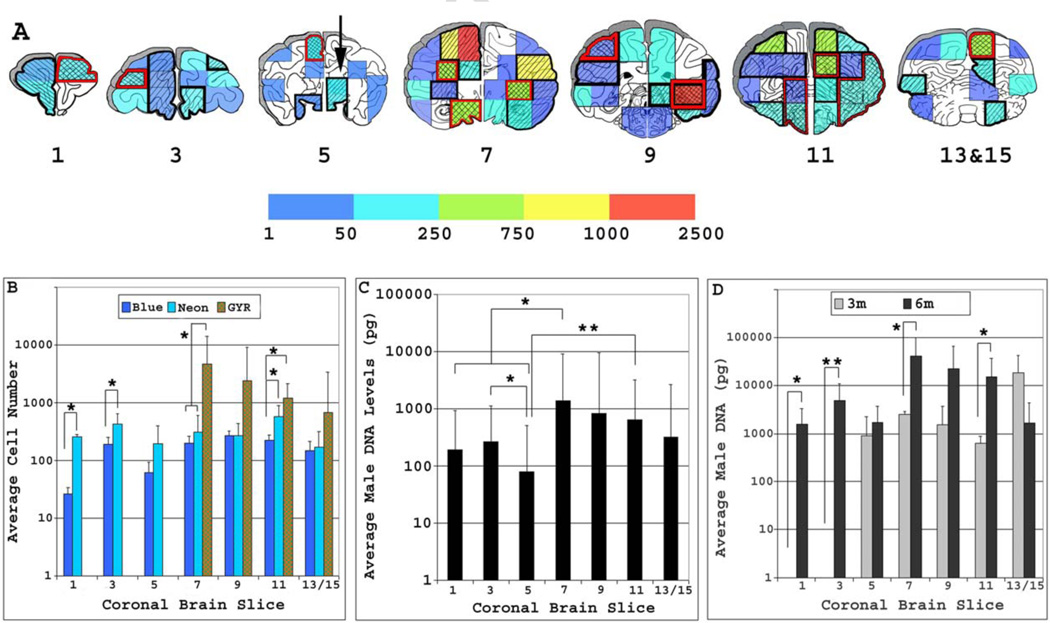
Figure 2. Anatomical distribution of MSCs engrafted in the infant macaque brain.
A) Schematic showing the average number of male MSCs, which ranged between 1 and 2500 cells (colored bar) contained within equivalent brain specimens harvested from each transplant recipient. Brain specimens with overlapping engraftment between 2 (diagonal lines bordered in black) or more then 3 (hatched lines bordered in red) transplant recipients are denoted. Regions in white contained no detectable male DNA. B) Plotted is the average (mean ± SD) number of MSCs contained within different brain specimens from the same coronal slice. Significant differences in overall engraftment levels were evident between brain specimens containing between 1–50 (blue), 51–250 (neon) or 251–2500 (Green –Yellow-Red, hatched boxes) MSCs, *; p<0.05. C) Plotted is the average (mean ± SD) male DNA levels contained within each respective 3 mm coronal brain from all infant transplant recipients, *; p<0.05, **; p<0.01. D) Plotted is the average (mean ± SD) male DNA levels contained within each respective 3 mm coronal brain slice from all infant transplant recipients sacrificed at 3 or 6 months post-injection, *; p<0.05, **; p<0.001. Coronal brain slices are numbered 1–13 in a rostral-to-caudal orientation. Reprinted with permission from (Isakova et al., 2007).
4. Cell-Matrix Interactions Important for MSC Survival
An important aspect of the aforementioned studies is the striking similarity in engraftment kinetics and anatomical distribution of MSCs when transplanted to the CNS of mice and non-human primates. These results suggest that engraftment and cell migration may be regulated by a conserved process in the brain. MSCs are adherent cells and therefore must bind specific extracellular matrix proteins such as fibronectin, laminin, and collagens via receptor mediated processes to ensure survival (Meredith et al., 1993; Song et al., 2007; Song et al., 2010). Attachment to a given substrate invokes the formation of stress fibers, which activates specific cytoskeletal signaling complexes to repress apoptosis (Ingber, 2002). For example, activation of the PI3K/Akt pathway following integrin receptor engagement induces expression of the anti-apoptotic protein Bcl-2 thereby enhancing cell survival (Matter and Ruoslahti, 2001). Integrin-mediated attachment to fibronectin in cooperation with growth factors also stimulates the mitogen activated protein kinase cascade, which regulates key intracellular enzymes and transcription factors that promote cell growth (Miyamoto et al., 1995; Miyamoto et al., 1996; Renshaw et al., 1997; Short et al., 1998). These effects are exemplified by the fact that over expression of the fibronectin receptor (α5β1 integrin) in cells leads to decreased cell proliferation, but this inhibition is overcome by attachment to fibronectin (Varner et al., 1995). In bone marrow, integrins and their receptors are known to play important roles in regulating tissue homeostasis. For example, integrins α4β1 (VLA-4) and α5β1 (VLA-5) make up the main adhesion receptors used by hematopoietic stem/progenitor cells to adhere to stroma (Teixido et al., 1992). These integrins bind to different regions of fibronectin (FN), VLA-4 and VCAM-1, which is expressed by marrow stromal cells. In addition to regulating cell migration, blocking antibodies to VLA-4 have also been shown to disrupt lymphopoiesis, myelopoiesis, and erythropoiesis in vitro as well as affect cell cycle progression in hematopoietic stem cells (Coulombel et al., 1997; Oostendorp and Dormer, 1997). Integrins and other cell adhesion molecules also play important roles in regulating osteoblast survival and differentiation (Bennett et al., 2001).
5. Neural Cell Adhesion Molecules and Cell Migration
Although MSCs express receptors for extracellular matrix proteins common to connective tissues including fibronectin, osteopontin, laminin, and collagens these proteins are not abundantly expressed within the CNS. Laminin-1, for example, is expressed during CNS development but exists predominantly in vessel basement membranes and in reactive glia in the adult brain (Hagg et al., 1989; Zhou, 1990). Laminin α-2 immuno-reactivity is evident in dendrites and dendritic spines in selected areas of the adult brain, predominately in the hippocampus and other limbic structures, which suggests a role in synaptic function and plasticity (Tian et al., 1997). Similarly, fibronectin is expressed mainly in association with blood vessels (Milner and Campbell, 2002) but is also up-regulated in glial cells in response to seizures (Hoffman et al., 1998) and focal brain injury (Tate et al., 2007). Limited expression of these matrix proteins in the brain may account for the poor survival of MSCs following direct intracranial injection.
In contrast, various neural cell adhesion molecules, such as L1, N-CAM, and cadherin 2 (CDH2) are expressed in many regions of the mouse (Bartsch et al., 1989; Miragall and Dermietzel, 1992), rat (Wagner et al., 1992), and human brain (Navratil et al., 1997) during development and in adulthood. These adhesion molecules play important roles in structural development and cell migration. In the latter case, the polysialylated neural cell-adhesion molecule (PSA-NCAM) has been shown to be essential for migration of neuroblasts from the sub ventricular zone to the olfactory bulb (Ono et al., 1994). Mice lacking NCAM exhibit a dramatic reduction in the size of the olfactory bulb due to accumulation of neural precursors along the rostral migratory stream (RMS) (Cremer et al., 1994). More recent studies indicate that NCAM functions as an alternative signaling receptor for glial-derived neurotrophic factor, which is produced in the OB, distributed along the RMS, and functions as a chemo-attractant for migrating neuroblasts (Paratcha et al., 2006). Similarly, CDH2 has been shown to regulate migration of precerebellar neurons in the developing hindbrain (Taniguchi et al., 2006) and post-mitotic neuroblasts in the subgranular zone of the dentate granular cell layer (Seki et al., 2007). Conditional knockout of CDH2 in mice also results in nearly complete randomization of intra-cortical structures, indicating that this adhesion molecule plays an important role in sorting of cells between boundary layers in the CNS during development (Kadowaki et al., 2007).
5.1. Tangential Migration of Interneurons
Alternatively, a large number of interneurons migrate tangentially throughout the brain in response to guidance cues that function over long distances. These guidance cues include the netrin, semaphorin, and slit family of proteins. Briefly, netrins are adhesion molecules with similarity to laminin that bind to deleted in colon cancer (DCC), neogenin 1 (NEO1) or Unc5H family members (de Castro, 2003). Netrins also bind extracellular matrix components via a basic domain at their carboxy terminus, which modifies their ability to diffuse in the brain. The ability of netrins to repel or attract neurons (or axons) is dependent upon specific receptor/ligand interactions. For example, neurons that express DCC or NEO1 are attracted to netrins while those that express Unc5H family members are repelled by them. Netrins have been reported to attract tangentially migrating neurons that will form the inferior oliva and repel migrating hypothalamic neurons and cerebellar granule cells (Causeret et al., 2002; Marin and Rubenstein, 2001; Marin, 2013). Other studies have indicated that netrins may also regulate the migration of glial cells (Tsai and Miller, 2002).
Semaphorins constitute a large family (~30 members) of secreted or trans-membrane proteins. The class III family of semaphorins binds to members of the neuropilin (NRP) family of receptors, NRP1 and NRP2, which are expressed in overlapping but distinct populations of neurons in the CNS (Kolodkin et al., 1997). Differences in the affinity of neuropilins for distinct semaphorins play a role in sorting of migrating interneurons in the brain. For example, Marin et al. (Marin et al., 2001) demonstrated that migrating cortical interneurons avoid entering the striatum because of a chemo-repulsive signal composed of semaphorin 3A and 3F secreted by striatal cells. Interneurons expressing neuropilins are directed to the cortex while those that do not are directed to the striatum. As anticipated, loss of NRP1 or NRP2 function results in an increased number of interneurons invading the striatum with concomitant alterations in the number and distribution of interneurons in the cortex.
Slit proteins also regulate interneuron migration during development. Three human homologs of the Drosophila Slit gene have been identified (Slit1–3) and these are secreted proteins that contain conserved protein-protein interaction domains including leucine-rich repeats and epidermal growth factor-like motifs (Itoh et al., 1998). Slit proteins bind to the roundabout homology (ROBO) family of receptors, which are members of the neural cell adhesion molecule family (Kidd et al., 1998b). Four ROBO family members have been identified in vertebrates to date (Huminiecki et al., 2002; Kidd et al., 1998b; Yuan et al., 1999) and these proteins have been shown to mediate homophilic as well as heterophilic adhesions (Hivert et al., 2002). During development, Slit expression is maintained in the ventricular zone of the lateral germinal eminence, where it functions to repel interneurons from the subventricular zone and direct them toward the cortex (Zhu et al., 1999). ROBO1 and ROBO2 show complimentary patterns of expression with respect to Slit, consistent with the fact that these molecules function to direct cortical interneurons along their appropriate tangential routes (Andrews et al., 2007). Interestingly, loss of ROBO1 function has also been reported to result in an increase in the number of interneurons entering the striatum during development (Andrews et al., 2006), suggesting that both ROB-Slit and NRP-Sema interactions play central roles in interneuron migration during development. A number of these mechanisms important for neural cell migration persist in the adult brain, albeit in a greatly diminished capacity (Ghashghaei et al., 2007; Goldman and Luskin, 1998). For example, Slit proteins have been shown to repel neuronal precursors migrating from the anterior subventricular zone to the olfactory bulb (Ghashghaei et al., 2007; Wu et al., 1999) and inhibit migration of inferior olivary neurons by silencing the attractive effects of netrin 1 (Causeret et al., 2002).
5.2. MSCs Express Neural Cell Adhesion Molecules and Guidance Receptors
We previously reported that MSCs express many of the aforementioned adhesion and receptor proteins important for cell migration in the CNS. Specifically, interrogation of the MSC transcriptome revealed expressed mRNAs encoding the neural adhesion molecules ninjurin 1 and CDH2 and the receptors NEO1, NRP1, NRP2, ROBO1 and ROBO4 (Phinney et al., 2006). The expression of these proteins was found to be conserved in mouse, non-human primate, and human MSCs. Moreover, immuno-fluorescent staining and FACS analysis revealed that expression of each protein was restricted to specific subpopulations of MSCs. Functional studies in vitro further revealed that binding of MSCs to CDH2 or netrin 1 (NTRN1) could be saturated in a dose-dependent manner. Moreover, binding to CDH2 or NTRN1 was inhibited to greater than 95% by pre-incubating cells with soluble CDH2 or a neutralizing antibody against NEO1, respectively. Therefore, CDH2 and NTRN1 function as homo- and heterophilic adhesion molecules, respectively, in MSCs. MSC migration was also shown to be significantly stimulated by exposure of cells to semaphorin 3A, repulsive guidance molecule A and NTRN1. This response could also be inhibited by pre-treating MSCs with neutralizing antibodies to the NEO1 and NRP1 receptors, demonstrating specificity of these ligand/receptor interactions.
Many of the adhesion molecules and guidance receptors we’ve identified within MSC subpopulations have specified functions in bone and marrow. For example, CDH2 is known to be expressed by osteoblasts and to regulate their function by modulating cell-to-cell adhesion (Cheng et al., 2000). Similarly, NRP2, which also binds to specific isoforms of VEGF (Neufeld et al., 2002), is expressed in osteosarcomas and promotes increased tumor vascularity resulting in a poor prognosis for survival (Handa et al., 2000). Additionally, ROBO1 expressed on leukocytes has been shown to regulate chemotaxis in response to secreted Slit protein (Wu et al., 2001). This protein is also expressed during development in muscle and cartilage and during mouse limb morphogenesis (Vargesson et al., 2001). Most recently, Slit2 was reported to be expressed on MSC-like populations within the bone marrow niche (Smith-Berdan et al., 2012). Expression of NEO1 has not been described in MSCs or stromal cells to our knowledge but this protein is known to have a broad tissue distribution (Meyerhardt et al., 1997). Consequently, while these proteins likely contribute to the function of MSCs in marrow, they may fortuitously impart MSCs with the capacity to engraft and migrate within the CNS. This hypothesis is consistent with the observation that MSCs transplanted to the CNS localize to anatomical regions that express the aforementioned adhesion molecules and guidance cues/receptors, such as the striatum, cerebral cortex, hippocampus and cerebellum (Goldman and Luskin, 1998; Marillat et al., 2002; Watakabe et al., 2006). Continued analysis of the role played by these proteins is anticipated to reveal novel insight into the mechanisms regulating MSC engraftment and migration in brain, thereby aiding in the development of improved cellular vectors that can be targeted to specific brain regions.
6. Recent Advances in MSC Biology and Consequence for Neurodegenerative Disorders
In recent years the field of MSC research has undergone a major paradigm shift. Initially, MSCs were thought to promote tissue regeneration via direct cell replacement, which reflected their capacity to differentiate into a variety of mesenchymal cell types. Over the past decade, accumulating evidence indicates that MSCs achieve a therapeutic effect largely via drug-like action by secreting a diverse array of paracrine acting factors. The later include proteins that exhibit anti-apoptotic, angiogenic, anti-inflammatory, neuro-regulatory, immunomodulatory and trophic activity.
6.1. Anti-Inflammatory and Immuno-Modulatory Activity
Recently, a growing number of laboratories have documented that MSCs possess immuno-modulatory properties important in regulating immune cell activity. Several recent reviews have been published that cover this topic (English, 2013; Le Blanc and Mougiakakos, 2012; Tolar et al., 2010). MSCs have also been shown to ameliorate injury-induced lung inflammation by suppressing expression of pro-inflammatory cytokines in vivo (Gupta et al., 2007; Ortiz et al., 2003; Ortiz et al., 2007). The anti-inflammatory and immuno-modulatory affects of MSCs may be advantageous in the treatment of LSDs since inflammation is a recognized aspect of disease that likely contributes to pathogenesis. For example, progressive, widespread glial cell activation has been reported in the brains of pre-symptomatic sheep afflicted with CLN (Oswald et al., 2005) and in a mouse model of this disease (Pontikis et al., 2004). Inflammation within the cerebral cortex is also evident by 1 month of age in mice afflicted with MPS I and IIB (Ohmi et al., 2003) and elevated levels of pro-inflammatory cytokines have also been detected in the brains of fetal mice afflicted with Gaucher disease (Hong et al., 2006). Progressive CNS inflammation also coincides with the onset of clinical symptoms in a mouse model of Tay-Sachs disease (Jeyakumar et al., 2003). In most cases, microglia activation is thought to occur in response to aberrant neural cell function or as part of a wider stress response in the brain and typically precedes neuronal cell loss. Several studies suggest that a vigorous inflammatory response contributes to disease pathogenesis. For example, bone marrow transplantation has been shown to suppress microglial activation and inhibit neuronal cell death in the absence of any detectable increase in neuronal GM2 ganglioside storage in a mouse model of Sandhoff disease (Wada et al., 2000). Treatment of Sandhoff mice with non-steroidal anti-inflammatory drugs has also been shown to result in a significant increase in life-span (Jeyakumar et al., 2004). In addition, animals lacking expression of the leukocyte chemokine MIP-1 alpha exhibit substantial decreases in the number of infiltrating macrophages into the CNS together with improved neurologic status and longer lifespan (Wu and Proia, 2004). Consistent with these findings, inflammation has also been reported as a prominent feature in other neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (Das and Basu, 2008).
Consistent with their role in immune-modulation, injection of unmodified MSCs into the cerebellum was shown to markedly reduce the extent of microglial and astrocyte activation and reduce levels of macrophage colony stimulating factor, a microglial activator, in a mouse model of NP-C disease (Bae et al., 2005). Interestingly, neural stem cells have also been shown to inhibit inflammation when transplanted to the brains of Sandhoff mice (Lee et al., 2007). MSCs also exhibit neuro-protective effects through anti-inflammatory action in models of Parkinson’s disease (Chao et al., 2009), Alzheimer’s disease (Lee et al., 2012) and traumatic brain injury (Zhang et al., 2013). Therefore, stem cell transplants may have the added benefit of reducing inflammation associated with LSDs.
6.2. Paracrine Signaling
Genomics-based studies including work from our own lab have shown that MSCs secrete various neurotrophins and other proteins that promote neural cell survival and neurite outgrowth under stressful conditions and following injury in vivo (Crigler et al., 2006; Joyce et al., 2010; Pisati et al., 2007; Qu et al., 2007). This capacity likely is related to the fact that bone and marrow are innervated by nervous tissue and different stromal subtypes respond to sympathetic efferent input to directly modulate hematopoiesis (Phinney, 2002). Indeed, several studies indicate that paracrine signaling contributes to the therapeutic effect of MSC-based therapies for the treatment of storage disease. For example, a recent study demonstrated that MSCs transplanted into the hippocampus of NP-C mice at an early stage of disease progression resulted in recovery of motor function due to enhanced neuronal cell survival and proliferation (Seo et al., 2011). Herein, MSC administration suppressed cellular apoptosis, resulted in increased PI3K/AKT and JAK2/STAT3 signaling, and promoted restoration of neurotransmitter homeostasis within the brain. A related study reported that embryonic NSCs from NP-C mice, which exhibited impaired self-renewal and decreased rates of neuronal differentiation, recovered these activities to a large extent when co-cultured with marrow-derived MSCs (Lee et al., 2013). Moreover, intra-cerebral transplantation of MSCs to NP-C mice augmented proliferation and neuronal differentiation of NSCs within the subventricular zone via release of chemokine (C-C motif) ligand 2 (CCL2). Other studies have shown that co-culture of immortalized marrow-derived MSCs with Schwann cells derived from Twitcher mice induced proliferation and neurite outgrowth grown in the presence of psychosine, the toxic substrate that accumulates in this disease and that the neuritogenic effect of MSCs was ameliorated by addition of neutralizing anti-BDNF antibodies (Miranda et al., 2011).
Consistent with these studies, several reports have shown that the bone marrow microenvironment is altered in patients with storage disease, affecting both hematopoiesis and MSC function. For example, a prospective analysis of MSCs from 10 patients with type 1 Gaucher disease (GD) revealed defects in cell growth, cell cycle abnormalities, and decreased capacities to differentiate into osteoblasts (Lecourt et al., 2013). GD-MSCs also secreted soluble factors that stimulated osteoclasts resorbing activities and exhibited a reduced capacity to support hematopoiesis in vitro. A separate study found that MSCs from Gaucher patients also exhibited a marked increase in COX-2, prostaglandin E2, interleukin-8, and CCL2 production compared with normal controls and that treatment of normal MSCs with the glucocerebrosidase inhibitor conduritol B epoxide also induced expression of CCL2 (Campeau et al., 2009). These results suggest that the altered secretome displayed by GD-MSCs may contribute to skeletal and immune disease manifestations in these patients. Patients with Hurler’s syndrome also exhibit skeletal defects and examination of MSCs from these patients revealed an increased capacity to support osteoclastogenesis as compared to MSCs from unaffected controls (Gatto et al., 2012). The later was correlated with up regulation of the RANKL/RANK/OPG pathway in Hurler MSCs. Therefore, paracrine activities that contribute to the therapeutic activity of MSCs may by dysregulated in LSD patients and contribute to disease pathophysiology.
6.3. Not all MSCs are Created Equal
Although initially isolated from bone marrow, MSCs have been identified in a variety of other tissues and organs. The ubiquitous distribution of MSCs in most tissues is attributed to their similarity to peri-vascular cells in vivo. This concept originated from studies demonstrating that bone marrow-derived MSCs express antigens common to endothelial cells and pericytes, such as STRO1 (Gronthos et al., 1994), CD146 and 3G5 (Shi and Gronthos, 2003) and conversely that post-capillary venule pericytes from bone marrow and peri-vascular cells in most blood vessels exhibit MSC-like characteristics (Brachvogel et al., 2005; Crisan et al., 2008; Shi and Gronthos, 2003). Several studies have shown that peri-vascular cells, pericytes and fibroblasts from different tissues closely resemble the surface phenotype of MSCs, exhibit similar genome wide expression profiles, and share similar functional properties based on qualitative in vitro assays (Covas et al., 2008; Crisan et al., 2008). Close examination of these data, however, reveal clear differences in expressed levels of lineage restricted mRNAs between pericytes and MSCs (Covas et al., 2008) and functional differences between cell types can be readily discriminated using rigorous in vivo assays (da Silva Meirelles et al., 2006). For example, in one study ectopic transplantation of bone marrow-derived MSCs yielded hetero-topic bone tissue whereas dental pulp-derived MSCs produced dentin and pulp tissue (Batouli et al., 2003). Similarly, the capacity to generate bone and cartilage is weaker for placental and adipose-derived MSCs as compared to those from bone marrow, and the contribution to muscle fiber formation in vivo is greater with post-natal skeletal muscle pericytes than bone marrow-derived MSCs (Dellavalle et al., 2011). Therefore, while MSCs from different tissues share similarities in phenotypes and gene expression profiles, differences in function may be distinguished experimentally provided the assays are sufficiently rigorous. Consequently, not all MSCs are equivalent and their unique tissue-specific attributes must be carefully evaluated prior to use in clinical therapy.
MSCs also exhibit profound species-specific differences in their biological properties. Nevertheless, these differences are rarely discussed with respect to outcomes obtained from translational studies. Species-specific differences in activity are particularly relevant in LSD research as several well-established mouse models exist that are widely employed in pre-clinical studies. Two prominent differences that exist between human and rodent MSCs are sensitivity to atmospheric oxygen and immunogenicity. In the former case, human MSCs exhibit robust growth in culture and are amenable to large scale expansion (Digirolamo et al., 1999; Sotiropoulou et al., 2006). However, development of methods to procure and expand primary mouse MSCs has been significantly more challenging due to the overall poor growth of these cells in vitro (Baddoo et al., 2003). Consequently a growing number of laboratories have adopted purification schemes that select for rapidly proliferating subpopulations which emerge from plastic adherent marrow cultures following long-term culture expansion (Al-Khaldi et al., 2003; Li et al., 2008; Meirelles Lda and Nardi, 2003; Peister et al., 2004; Sun et al., 2003). These subpopulations typically survive in culture for over 50 passages in vitro and as such resemble immortalized cell lines. Indeed, cell immortalization occurs at a much higher frequency in rodent vs. human populations due to differences in checkpoint control mechanisms (Prowse and Greider, 1995; Wadhwa et al., 2004). For example, growth restrictive conditions have been shown to select for cells with inactivating mutations in p53, a protein mutated in the vast majority of immortalized rodent cell lines (Harvey and Levine, 1991; Sherr and DePinho, 2000). Consistent with these findings, we recently demonstrated that the poor growth of primary mouse MSCs is due to oxidative stress induced by exposure to atmospheric oxygen and that oxygen-induced growth inhibition is p53 dependent (Boregowda et al., 2012). Consequently, long-term exposure of mouse MSCs to atmospheric oxygen selects for clones with reduced or absent p53 function, which allows escape from oxygen-induced growth inhibition. Therefore, many studies claiming to use primary mouse MSCs in reality employ clonally selected immortalized cell lines. This is problematic for two reasons. First, clonally selected populations do not recapitulate all of biological properties inherent to the parent population. Second, MSCs that lack a functional p53 protein are insensitive to oxidative stress. Therefore, these cells are anticipated to exhibit enhanced engraftment and survival in vivo as compared to primary cells, particularly when implanted into inflamed or diseased tissue. Therefore, outcomes from these studies require careful evaluation especially when extrapolating results to the human condition.
As noted above, a growing body of literature exists that MSCs possess immuno-suppressive activity (English, 2013; Le Blanc and Mougiakakos, 2012), which has spurred the use of allogeneic human MSCs in clinical therapies. However, studies conducted in experimental animals indicate that allogeneic MSCs trigger donor-specific cellular and humeral immune responses in vivo. For example, pre-clinical studies conducted in rodents (Camp et al., 2009; Eliopoulos et al., 2005; Rossignol et al., 2009; Sudres et al., 2006), swine (Poncelet et al., 2007), equine (Pigott et al., 2013) and non-human primates (Isakova et al., 2010; Isakova et al., 2014) demonstrate that allogeneic MSCs induce measurable anti-donor T and B cell mediated responses. Indeed, the detection of donor-specific antibodies in the serum of transplant recipients provides clear evidence of allo-antigen recognition by B cells. Species specific differences in MSC allo-reactivity may result from the fact that allograft responses in humans are typically evaluated using mixed lymphocyte reactions in vitro, which is not a reliable predictor of their suppressor activity in human patients (von Bahr et al., 2012). Recent studies indicate that immuno-modulatory activity of MSCs may be determined by a balance between inhibitory and stimulatory factors (Zhou et al., 2013). Therefore, experimental conditions particularly with regard to in vitro studies may yield disparate outcomes. Therefore, caution is needed when extrapolating results obtained with allogeneic MSCs in translational models to clinical therapy in LSD patients.
7. Summary
MSCs posses a number of unique biological attributes that make them well suited as cellular vectors to treat neurologic disorders. They show durable and widespread engraftment in the CNS, which may be mediated by expressed neural adhesion molecules and guidance receptor proteins. They also express anti-inflammatory and immuno-modulatory factors as well as a host of neural regulatory proteins. Nevertheless, continued research is necessary to better define these unique aspects of MSC biology, determine how tissue and species-specific differences in biology affect therapeutic potency, and to develop methodologies to exploit these attributes in ways that yield maximal benefit to patients.
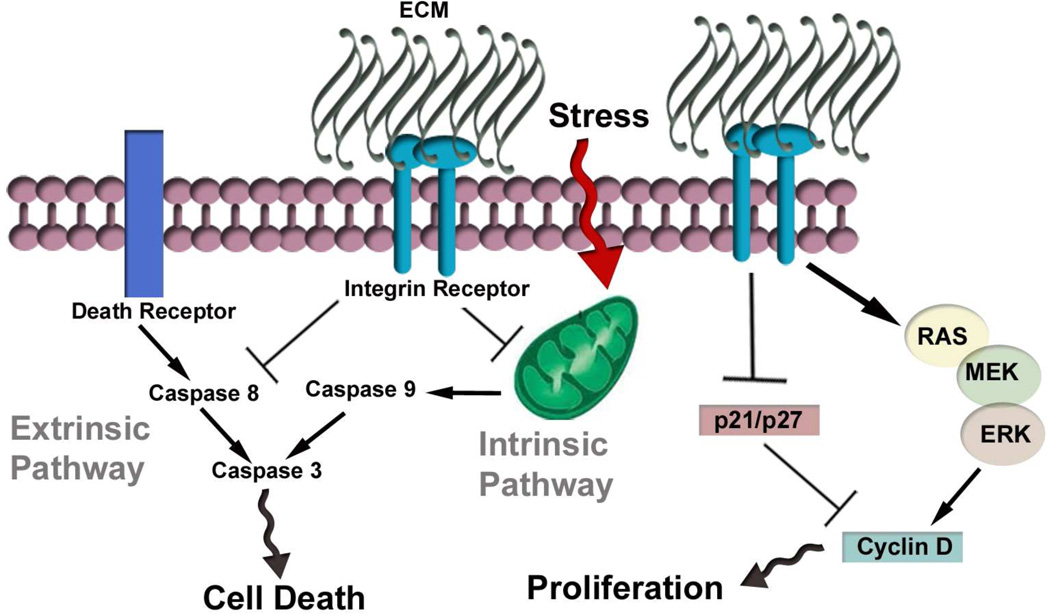
Figure 3. Integrin signaling regulates cell proliferation and survival.
Growth factor signaling (not shown) and cell adhesion are required for transmitting signals to the Ras/Mek/Erk signaling pathway, which then stimulates cell proliferation via increasing cyclin D1 transcription and degrading the cyclin dependent kinase inhibitors p21 and p27. Signals from integrin receptors also repress the intrinsic and extrinsic cell death pathways. When both ECM and serum survival signals are absent activation of p53 dependent pathways induce cellular apoptosis.
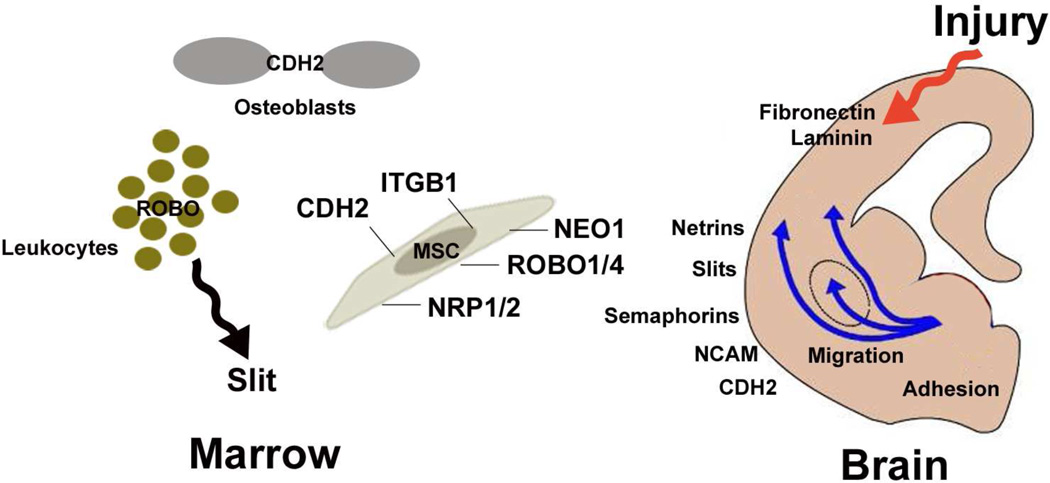
Figure 4. Proteins expressed by MSCs possess dual functionality in marrow and brain.
MSCs express a variety of adhesion molecules and receptor proteins that perform specific functions in bone marrow. For example, MSCs express a broad array of integrin proteins that function as receptors for fibronectin, collagen, and laminin, which are widely expressed in marrow. MSCs also express CDH2, which is known to promote cell adhesion and regulate differentiation of osteoblasts. In addition, SLIT/ROBO interactions are known to regulate cheomtaxis of various leukocyte populations in marrow. Finally, NRP receptors regulate vessel formation by binding vascular endothelial growth factors and play a role in bone homeostasis by regulating osteoblast and osteoclast differentiation. Netrins, slits, semaphorins, CDH2, and NCAM also perform well described roles in neural cell survival, adhesion, and migration (see text) and several studies indicate that injury induces expression of fibronectin and laminin in the brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelhalim AN, et al. Patterns of magnetic resonance imaging abnormalities in symptomatic patients with Krabbe disease correspond to phenotype. Pediatr Neurol. 2014;50:127–134. doi: 10.1016/j.pediatrneurol.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Al-Khaldi A, et al. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003;10:621–629. doi: 10.1038/sj.gt.3301934. [DOI] [PubMed] [Google Scholar]
- Andrews W, et al. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Andrews WD, Barber M, Parnavelas JG. Slit-Robo interactions during cortical development. J Anat. 2007;211:188–198. doi: 10.1111/j.1469-7580.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddoo M, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- Bae JS, et al. Neuroglial activation in Niemann-Pick Type C mice is suppressed by intracerebral transplantation of bone marrow-derived mesenchymal stem cells. Neurosci Lett. 2005;381:234–236. doi: 10.1016/j.neulet.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Ball LM, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- Bartsch U, Kirchhoff F, Schachner M. Immunohistological localization of the adhesion molecules L1, N-CAM, and MAG in the developing and adult optic nerve of mice. J Comp Neurol. 1989;284:451–462. doi: 10.1002/cne.902840310. [DOI] [PubMed] [Google Scholar]
- Batouli S, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976–981. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- Begley DJ, Pontikis CC, Scarpa M. Lysosomal storage diseases and the blood-brain barrier. Curr Pharm Des. 2008;14:1566–1580. doi: 10.2174/138161208784705504. [DOI] [PubMed] [Google Scholar]
- Bennett JH, Moffatt S, Horton M. Cell adhesion molecules in human osteoblasts: structure and function. Histol Histopathol. 2001;16:603–611. doi: 10.14670/HH-16.603. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Hofmann SL. The neuronal ceroid-lipofuscinoses (Batten disease): a new class of lysosomal storage diseases. J Inherit Metab Dis. 1999;22:535–544. doi: 10.1023/a:1005564509027. [DOI] [PubMed] [Google Scholar]
- Biffi A, et al. Metachromatic leukodystrophy: an overview of current and prospective treatments. Bone Marrow Transplant. 2008;42(Suppl 2):S2–S6. doi: 10.1038/bmt.2008.275. [DOI] [PubMed] [Google Scholar]
- Bjoraker KJ, et al. Long-term outcomes of adaptive functions for children with mucopolysaccharidosis I (Hurler syndrome) treated with hematopoietic stem cell transplantation. J Dev Behav Pediatr. 2006;27:290–296. doi: 10.1097/00004703-200608000-00002. [DOI] [PubMed] [Google Scholar]
- Boelens JJ, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–3987. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boregowda SV, et al. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: implications for long-term culture expansion. Stem Cells. 2012;30:975–987. doi: 10.1002/stem.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachvogel B, et al. Perivascular cells expressing annexin A5 define a novel mesenchymal stem celllike population with the capacity to differentiate into multiple mesenchymal lineages. Development. 2005;132:2657–2668. doi: 10.1242/dev.01846. [DOI] [PubMed] [Google Scholar]
- Camp DM, et al. Cellular immune response to intrastriatally implanted allogeneic bone marrow stromal cells in a rat model of Parkinson's disease. J Neuroinflammation. 2009;6:17. doi: 10.1186/1742-2094-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau PM, et al. Characterization of Gaucher disease bone marrow mesenchymal stromal cells reveals an altered inflammatory secretome. Blood. 2009;114:3181–3190. doi: 10.1182/blood-2009-02-205708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causeret F, et al. Slit antagonizes netrin-1 attractive effects during the migration of inferior olivary neurons. Dev Biol. 2002;246:429–440. doi: 10.1006/dbio.2002.0681. [DOI] [PubMed] [Google Scholar]
- Cesani M, et al. Characterization of new arylsulfatase A gene mutations reinforces genotype-phenotype correlation in metachromatic leukodystrophy. Hum Mutat. 2009;30:E936–E945. doi: 10.1002/humu.21093. [DOI] [PubMed] [Google Scholar]
- Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. J Neuroimmunol. 2009;216:39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Cheng SL, et al. A dominant negative cadherin inhibits osteoblast differentiation. J Bone Miner Res. 2000;15:2362–2370. doi: 10.1359/jbmr.2000.15.12.2362. [DOI] [PubMed] [Google Scholar]
- Coulombel L, et al. Expression and function of integrins on hematopoietic progenitor cells. Acta Haematol. 1997;97:13–21. doi: 10.1159/000203655. [DOI] [PubMed] [Google Scholar]
- Covas DT, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Cremer H, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Crigler L, et al. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- de Castro F. Chemotropic molecules: guides for axonal pathfinding and cell migration during CNS development. News Physiol Sci. 2003;18:130–136. doi: 10.1152/nips.01414.2002. [DOI] [PubMed] [Google Scholar]
- Deeg HJ, et al. Batten's disease: failure of allogeneic bone marrow transplantation to arrest disease progression in a canine model. Clin Genet. 1990;37:264–270. doi: 10.1111/j.1399-0004.1990.tb04188.x. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Digirolamo CM, et al. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- Duffner PK, et al. The long-term outcomes of presymptomatic infants transplanted for Krabbe disease: report of the workshop held on July 11 and 12, 2008, Holiday Valley, New York. Genet Med. 2009;11:450–454. doi: 10.1097/GIM.0b013e3181a16e04. [DOI] [PubMed] [Google Scholar]
- Eapen M, et al. Long-term survival and late deaths after hematopoietic cell transplantation for primary immunodeficiency diseases and inborn errors of metabolism. Biol Blood Marrow Transplant. 2012;18:1438–1445. doi: 10.1016/j.bbmt.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos N, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- Escolar ML, et al. A staging system for infantile Krabbe disease to predict outcome after unrelated umbilical cord blood transplantation. Pediatrics. 2006;118:e879–e889. doi: 10.1542/peds.2006-0747. [DOI] [PubMed] [Google Scholar]
- Espinas OE, Faris AA. Acute infantile Gaucher's disease in identical twins. An account of clinical and neuropathologic observations. Neurology. 1969;19:133–140. doi: 10.1212/wnl.19.2.133. [DOI] [PubMed] [Google Scholar]
- Freed CR, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Fukuhara Y, et al. Histopathological and behavioral improvement of murine mucopolysaccharidosis type VII by intracerebral transplantation of neural stem cells. Mol Ther. 2006;13:548–555. doi: 10.1016/j.ymthe.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Galla D, et al. An Italian cohort study identifies four new pathologic mutations in the ARSA gene. J Mol Neurosci. 2013;50:284–290. doi: 10.1007/s12031-013-0006-8. [DOI] [PubMed] [Google Scholar]
- Gatto F, et al. Hurler disease bone marrow stromal cells exhibit altered ability to support osteoclast formation. Stem Cells Dev. 2012;21:1466–1477. doi: 10.1089/scd.2011.0555. [DOI] [PubMed] [Google Scholar]
- Gelinas J, et al. Child Neurology: Krabbe disease: a potentially treatable white matter disorder. Neurology. 2012;79:e170–e172. doi: 10.1212/WNL.0b013e3182735c8b. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–151. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- Gieselmann V. Metachromatic leukodystrophy: genetics, pathogenesis and therapeutic options. Acta Paediatr. 2008;(Suppl. 97):15–21. doi: 10.1111/j.1651-2227.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Gieselmann V, Krageloh-Mann I. Metachromatic leukodystrophy--an update. Neuropediatrics. 2010;41:1–6. doi: 10.1055/s-0030-1253412. [DOI] [PubMed] [Google Scholar]
- Givogri MI, et al. Multipotential neural precursors transplanted into the metachromatic leukodystrophy brain fail to generate oligodendrocytes but contribute to limit brain dysfunction. Dev Neurosci. 2008;30:340–357. doi: 10.1159/000150127. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Luskin MB. Strategies utilized by migrating neurons of the postnatal vertebrate forebrain. Trends Neurosci. 1998;21:107–114. doi: 10.1016/s0166-2236(97)01191-0. [DOI] [PubMed] [Google Scholar]
- Grewal SS, et al. Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood. 2003;101:4233–4244. doi: 10.1182/blood-2002-08-2510. [DOI] [PubMed] [Google Scholar]
- Gronthos S, et al. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- Guffon N, et al. Follow-up of nine patients with Hurler syndrome after bone marrow transplantation. J Pediatr. 1998;133:119–125. doi: 10.1016/s0022-3476(98)70201-x. [DOI] [PubMed] [Google Scholar]
- Gupta N, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Gupta N, et al. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T, et al. Laminin-like antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment. Neuron. 1989;3:721–732. doi: 10.1016/0896-6273(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Handa A, et al. Neuropilin-2 expression affects the increased vascularization and is a prognostic factor in osteosarcoma. Int J Oncol. 2000;17:291–295. doi: 10.3892/ijo.17.2.291. [DOI] [PubMed] [Google Scholar]
- Hare JM, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Levine AJ. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Hivert B, et al. Robo1 and Robo2 are homophilic binding molecules that promote axonal growth. Mol Cell Neurosci. 2002;21:534–545. doi: 10.1006/mcne.2002.1193. [DOI] [PubMed] [Google Scholar]
- Hoffman KB, et al. Seizure induced synthesis of fibronectin is rapid and age dependent: implications for long-term potentiation and sprouting. Brain Res. 1998;812:209–215. doi: 10.1016/s0006-8993(98)00727-6. [DOI] [PubMed] [Google Scholar]
- Hong YB, Kim EY, Jung SC. Upregulation of proinflammatory cytokines in the fetal brain of the Gaucher mouse. J Korean Med Sci. 2006;21:733–738. doi: 10.3346/jkms.2006.21.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerbrugge PM, et al. Allogeneic bone marrow transplantation for lysosomal storage diseases. The European Group for Bone Marrow Transplantation. Lancet. 1995;345:1398–1402. doi: 10.1016/s0140-6736(95)92597-x. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JQ, et al. Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases. Hum Mol Genet. 1997;6:1879–1885. doi: 10.1093/hmg/6.11.1879. [DOI] [PubMed] [Google Scholar]
- Huminiecki L, et al. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- Isakova IA, et al. Preclinical evaluation of adult stem cell engraftment and toxicity in the CNS of rhesus macaques. Mol Ther. 2006;13:1173–1184. doi: 10.1016/j.ymthe.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Isakova IA, et al. Age- and dose-related effects on MSC engraftment levels and anatomical distribution in the central nervous systems of nonhuman primates: identification of novel MSC subpopulations that respond to guidance cues in brain. Stem Cells. 2007;25:3261–3270. doi: 10.1634/stemcells.2007-0543. [DOI] [PubMed] [Google Scholar]
- Isakova IA, et al. Cell-dose-dependent increases in circulating levels of immune effector cells in rhesus macaques following intracranial injection of allogeneic MSCs. Exp Hematol. 2010;38:957–967. e1. doi: 10.1016/j.exphem.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakova IA, et al. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS One. 2014;9:e87238. doi: 10.1371/journal.pone.0087238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A, et al. Cloning and expressions of three mammalian homologues of Drosophila slit suggest possible roles for Slit in the formation and maintenance of the nervous system. Brain Res Mol Brain Res. 1998;62:175–186. doi: 10.1016/s0169-328x(98)00224-1. [DOI] [PubMed] [Google Scholar]
- Jaing TH. Umbilical cord blood transplantation: application in pediatric patients. Acta Paediatr Taiwan. 2007;48:107–111. [PubMed] [Google Scholar]
- Jeyakumar M, et al. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain. 2003;126:974–987. doi: 10.1093/brain/awg089. [DOI] [PubMed] [Google Scholar]
- Jeyakumar M, et al. NSAIDs increase survival in the Sandhoff disease mouse: synergy with N-butyldeoxynojirimycin. Ann Neurol. 2004;56:642–649. doi: 10.1002/ana.20242. [DOI] [PubMed] [Google Scholar]
- Jin HK, et al. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinasedeficient mice delays the onset of neurological abnormalities and extends their life span. J Clin Inves. 2002;109:1183–1191. doi: 10.1172/JCI14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HK, Schuchman EH, et al. Ex vivo gene therapy using bone marrow-derived cells: combined effects of intracerebral and intravenous transplantation in a mouse model of Niemann-Pick disease. Mol Ther. 2003;8:876–885. doi: 10.1016/j.ymthe.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Joyce N, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, et al. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, et al. Intracerebral grafting of cultured autologous skin fibroblasts into the rat striatum: an assessment of graft size and ultrastructure. J Comp Neurol. 1991;307:695–706. doi: 10.1002/cne.903070414. [DOI] [PubMed] [Google Scholar]
- Kaye EM, et al. Type 2 and type 3 Gaucher disease: a morphological and biochemical study. Ann Neurol. 1986;20:223–230. doi: 10.1002/ana.410200208. [DOI] [PubMed] [Google Scholar]
- Kidd D, et al. Long-term stabilization after bone marrow transplantation in juvenile metachromatic leukodystrophy. Arch Neurol. 1998a;55:98–99. doi: 10.1001/archneur.55.1.98. [DOI] [PubMed] [Google Scholar]
- Kidd T, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionary conserved guidance receptors. Cell. 1998b;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Koc ON, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuga M, et al. Engraftment of genetically engineered amniotic epithelial cells corrects lysosomal storage in multiple areas of the brain in mucopolysaccharidosis type VII mice. Mol Ther. 2001;3:139–148. doi: 10.1006/mthe.2000.0234. [DOI] [PubMed] [Google Scholar]
- Krageloh-Mann I, et al. Juvenile metachromatic leukodystrophy 10 years post transplant compared with a non-transplanted cohort. Bone Marrow Transplant. 2013;48:369–375. doi: 10.1038/bmt.2012.155. [DOI] [PubMed] [Google Scholar]
- Krivit W, et al. Treatment of late infantile metachromatic leukodystrophy by bone marrow transplantation. N Engl J Med. 1990;322:28–32. doi: 10.1056/NEJM199001043220106. [DOI] [PubMed] [Google Scholar]
- Krivit W, et al. Survival in Hurler's disease following bone marrow transplantation in 84 patients. Bone Marrow Transplant. 1995;15:S182–S185. [Google Scholar]
- Krivit W, et al. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med. 1998;338:1119–1126. doi: 10.1056/NEJM199804163381605. [DOI] [PubMed] [Google Scholar]
- Krivit W. Stem cell bone marrow transplantation in patients with metabolic storage diseases. Adv Pediatr. 2002;49:359–378. [PubMed] [Google Scholar]
- Lacorazza HD, et al. Expression of human beta-hexosaminidase alpha-subunit gene (the gene defect of Tay-Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nat Med. 1996;2:424–429. doi: 10.1038/nm0496-424. [DOI] [PubMed] [Google Scholar]
- Lake BD, et al. Bone marrow transplantation in Batten disease (neuronal ceroid-lipofuscinosis). Will it work? Preliminary studies on coculture experiments and on bone marrow transplant in late infantile Batten disease. Am J Med Genet. 1995;57:369–373. doi: 10.1002/ajmg.1320570253. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- Lecourt S, et al. A prospective study of bone marrow hematopoietic and mesenchymal stem cells in type 1 Gaucher disease patients. PLoS One. 2013;8:e69293. doi: 10.1371/journal.pone.0069293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, et al. Bone-marrow-derived mesenchymal stem cells promote proliferation and neuronal differentiation of Niemann-Pick type C mouse neural stem cells by upregulation and secretion of CCL2. Hum Gene Ther. 2013;24:655–669. doi: 10.1089/hum.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Lee JP, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Comparative study of mesenchymal stem cells from C57BL/10 and mdx mice. BMC Cell Biol. 2008;9:24. doi: 10.1186/1471-2121-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnqvist T, et al. Hematopoietic stem cell transplantation in infantile neuronal ceroid lipofuscinosis. Neurology. 2001;57:1411–1416. doi: 10.1212/wnl.57.8.1411. [DOI] [PubMed] [Google Scholar]
- Lucke T, et al. Developmental outcome in five children with Hurler syndrome after stem cell transplantation: a pilot study. Dev Med Child Neurol. 2007;49:693–696. doi: 10.1111/j.1469-8749.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- Lund TC. Hematopoietic stem cell transplant for lysosomal storage diseases. Pediatr Endocrinol Rev. 2013;11(Suppl 1):91–98. [PubMed] [Google Scholar]
- Luzi P, et al. Sixteen novel mutations in the arylsulfatase A gene causing metachromatic leukodystrophy. Gene. 2013;530:323–328. doi: 10.1016/j.gene.2013.08.065. [DOI] [PubMed] [Google Scholar]
- Malm G, et al. Clinical outcome in four children with metachromatic leukodystrophy treated by bone marrow transplantation. Bone Marrow Transplant. 1996;17:1003–1008. [PubMed] [Google Scholar]
- Marillat V, et al. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002;442:130–155. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Marin O, et al. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Marin O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur J Neurosci. 2013;38:2019–2029. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- Martin HR, et al. Neurodevelopmental outcomes of umbilical cord blood transplantation in metachromatic leukodystrophy. Biol Blood Marrow Transplant. 2013;19:616–624. doi: 10.1016/j.bbmt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Martin PL, et al. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12:184–194. doi: 10.1016/j.bbmt.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Matter ML, Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5:7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
- McGraw P, et al. Krabbe disease treated with hematopoietic stem cell transplantation: serial assessment of anisotropy measurements--initial experience. Radiology. 2005;236:221–230. doi: 10.1148/radiol.2353040716. [DOI] [PubMed] [Google Scholar]
- Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- Meng XL, et al. Brain transplantation of genetically engineered human neural stem cells globally corrects brain lesions in the mucopolysaccharidosis type VII mouse. J Neurosci Res. 2003;74:266–277. doi: 10.1002/jnr.10764. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Jr., Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, et al. Identification and characterization of neogenin, a DCC-related gene. Oncogene. 1997;14:1129–1136. doi: 10.1038/sj.onc.1200935. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Developmental regulation of beta1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- Miragall F, Dermietzel R. Immunocytochemical localization of cell adhesion molecules in the developing and mature olfactory system. Microsc Res Tech. 1992;23:157–172. doi: 10.1002/jemt.1070230206. [DOI] [PubMed] [Google Scholar]
- Miranda CO, et al. Systemic delivery of bone marrow-derived mesenchymal stromal cells diminishes neuropathology in a mouse model of Krabbe's disease. Stem Cells. 2011;29:1738–1751. doi: 10.1002/stem.724. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, et al. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, et al. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogul MJ. Unrelated cord blood transplantation vs matched unrelated donor bone marrow transplantation: the risks and benefits of each choice. Bone Marrow Transplant. 2000;25(Suppl 2):S58–S60. doi: 10.1038/sj.bmt.1702372. [DOI] [PubMed] [Google Scholar]
- Moses SW. Pathophysiology and dietary treatment of the glycogen storage diseases. J Pediatr Gastroenterol Nutr. 1990;11:155–174. doi: 10.1097/00005176-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Navratil E, et al. Expression of cell adhesion molecules by microvascular endothelial cells in the cortical and subcortical regions of the normal human brain: an immunohistochemical analysis. Neuropathol Appl Neurobiol. 1997;23:68–80. [PubMed] [Google Scholar]
- Neufeld G, Kessler O, Herzog Y. The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol. 2002;515:81–90. doi: 10.1007/978-1-4615-0119-0_7. [DOI] [PubMed] [Google Scholar]
- Ohmi K, et al. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci U S A. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, et al. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994;13:595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Oostendorp RA, Dormer P. VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells. Leuk Lymphoma. 1997;24:423–435. doi: 10.3109/10428199709055581. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald MJ, et al. Glial activation spreads from specific cerebral foci and precedes neurodegeneration in presymptomatic ovine neuronal ceroid lipofuscinosis (CLN6) Neurobiol Dis. 2005;20:49–63. doi: 10.1016/j.nbd.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Ozen M, et al. Severe graft versus host disease in a patient with globoid cell leukodystrophy following umbilical cord blood transplantation: resemblance to the twitcher mouse model. Turk J Pediatr. 2007;49:304–306. [PubMed] [Google Scholar]
- Paratcha G, Ibanez CF, Ledda F. GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Mol Cell Neurosci. 2006;31:505–504. doi: 10.1016/j.mcn.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Pastores GM. Krabbe disease: an overview. Int J Clin Pharmacol Ther. 2009;47(Suppl 1):S75–S81. doi: 10.5414/cpp47075. [DOI] [PubMed] [Google Scholar]
- Peister A, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Peters C, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- Phinney DG. Building a consensus regarding the nature and origin of mesenchymal stem cells. J Cell Biochem. 2002;(Suppl. 38):7–12. doi: 10.1002/jcb.10084. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- Phinney DG, et al. Murine mesenchymal stem cells transplanted to the central nervous system of neonatal versus adult mice exhibit distinct engraftment kinetics and express receptors that guide neuronal cell migration. Stem Cells Dev. 2006;15:437–447. doi: 10.1089/scd.2006.15.437. [DOI] [PubMed] [Google Scholar]
- Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007;6:2884–2889. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- Pigott JH, et al. Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Comp Orthop Traumatol. 2013;26:453–460. doi: 10.3415/VCOT-13-01-0008. [DOI] [PubMed] [Google Scholar]
- Pisati F, et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007;16:41–55. doi: 10.3727/000000007783464443. [DOI] [PubMed] [Google Scholar]
- Poncelet AJ, et al. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783–790. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- Pontikis CC, et al. Late onset neurodegeneration in the Cln3‒/‒ mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 2004;1023:231–242. doi: 10.1016/j.brainres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions. Br J Haematol. 2010;148:356–372. doi: 10.1111/j.1365-2141.2009.07974.x. [DOI] [PubMed] [Google Scholar]
- Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu R, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. Embo j. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J, et al. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J Cell Mol Med. 2009;13:2547–2558. doi: 10.1111/j.1582-4934.2008.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, et al. Brain transplantation of genetically modified bone marrow stromal cells corrects CNS pathology and cognitive function in MPS VII mice. Gene Ther. 2004;11:1475–1481. doi: 10.1038/sj.gt.3302338. [DOI] [PubMed] [Google Scholar]
- Sauer M, et al. Allogeneic blood SCT for children with Hurler's syndrome: results from the German multicenter approach MPS-HCT 2005. Bone Marrow Transplant. 2009;43:375–3781. doi: 10.1038/bmt.2008.328. [DOI] [PubMed] [Google Scholar]
- Seki T, et al. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J Comp Neurol. 2007;502:275–290. doi: 10.1002/cne.21301. [DOI] [PubMed] [Google Scholar]
- Selden NR, et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J Neurosurg Pediatr. 2013;11:643–652. doi: 10.3171/2013.3.PEDS12397. [DOI] [PubMed] [Google Scholar]
- Senut MC, et al. Regional differences in responsiveness of adult CNS axons to grafts of cells expressing human neurotrophin 3. Exp Neurol. 1995;135:36–55. doi: 10.1006/exnr.1995.1064. [DOI] [PubMed] [Google Scholar]
- Seo Y, et al. Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transplant. 2011;20:1033–1047. doi: 10.3727/096368910X545086. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA, et al. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Cheng SH. Neural stem cell transplantation as a therapeutic approach for treating lysosomal storage diseases. Neurotherapeutics. 2011;8:659–667. doi: 10.1007/s13311-011-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SM, Talbott GA, Juliano RL. Integrin-mediated signaling events in human endothelial cells. Mol Biol Cell. 1998;9:1969–1980. doi: 10.1091/mbc.9.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi ZA, Sanders DB, Massey JM. Peripheral neuropathy in Krabbe disease: effect of hematopoietic stem cell transplantation. Neurology. 2006;67:268–272. doi: 10.1212/01.wnl.0000230156.01228.33. [DOI] [PubMed] [Google Scholar]
- Smith-Berdan S, et al. Dynamic expression of the Robo ligand Slit2 in bone marrow cell populations. Cell Cycle. 2012;11:675–682. doi: 10.4161/cc.11.4.19146. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Song H, et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrowderived mesenchymal stem cells. Stem Cells. 2007;25:1431–1438. doi: 10.1634/stemcells.2006-0467. [DOI] [PubMed] [Google Scholar]
- Song H, et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–563. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- Souillet G, et al. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- Strazza M, et al. Significant correction of pathology in brains of twitcher mice following injection of genetically modified mouse neural progenitor cells. Mol Genet Metab. 2009;97:27–34. doi: 10.1016/j.ymgme.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Sudres M, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- Sun S, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, et al. Classic cadherins regulate tangential migration of precerebellar neurons in the caudal hindbrain. Development. 2006;133:1923–1931. doi: 10.1242/dev.02354. [DOI] [PubMed] [Google Scholar]
- Tate CC, Tate MC, LaPlaca MC. Fibronectin and laminin increase in the mouse brain after controlled cortical impact injury. J Neurotrauma. 2007;24:226–230. doi: 10.1089/neu.2006.0043. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Wolfe JH. Decreased lysosomal storage in the adult MPS VII mouse brain in the vicinity of grafts of retroviral vector-corrected fibroblasts secreting high levels of beta-glucuronidase. Nat Med. 1997;3:771–774. doi: 10.1038/nm0797-771. [DOI] [PubMed] [Google Scholar]
- Teixeira FG, et al. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixido J, et al. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Invest. 1992;90:358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, et al. Laminin-alpha2 chain-like antigens in CNS dendritic spines. Brain Res. 1997;764:28–38. doi: 10.1016/s0006-8993(97)00420-4. [DOI] [PubMed] [Google Scholar]
- Tolar J, et al. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Miller RH. Glial cell migration directed by axon guidance cues. Trends Neurosci. 2002;25:173–175. doi: 10.1016/s0166-2236(00)02096-8. discussion 175–6. [DOI] [PubMed] [Google Scholar]
- Vargesson N, et al. Expression patterns of Slit and Robo family members during vertebrate limb development. Mech Dev. 2001;106:175–180. doi: 10.1016/s0925-4773(01)00430-0. [DOI] [PubMed] [Google Scholar]
- Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell. 1995;6:725–740. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bahr L, et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012;18:557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Wada R, Tifft CJ, Proia RL. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc Natl Acad Sci U S A. 2000;97:10954–10959. doi: 10.1073/pnas.97.20.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, et al. The ARF-p53 senescence pathway in mouse and human cells. Histol Histopathol. 2004;19:311–316. doi: 10.14670/HH-19.311. [DOI] [PubMed] [Google Scholar]
- Wagner AP, Beck KD, Reck G. Neural cell adhesion molecule (NCAM) and N-cadherin mRNA during development and aging: selective reduction in the 7.4-kb and 6.7-kb NCAM mRNA levels in the hippocampus of adult and old rats. Mech Ageing Dev. 1992;62:201–208. doi: 10.1016/0047-6374(92)90056-j. [DOI] [PubMed] [Google Scholar]
- Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Wang D, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years experience. Cell Transplant. 2012 doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier V, Vieira M, Mole SE. Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. Biochim Biophys Acta. 2013;1832:1827–1830. doi: 10.1016/j.bbadis.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Watakabe A, et al. Binding and complementary expression patterns of semaphorin 3E and plexin D1 in the mature neocortices of mice and monkeys. J Comp Neurol. 2006;499:258–273. doi: 10.1002/cne.21106. [DOI] [PubMed] [Google Scholar]
- Westlake VJ, et al. Hematopoietic cell transplantation in fetal lambs with ceroid-lipofuscinosis. Am J Med Genet. 1995;57:365–368. doi: 10.1002/ajmg.1320570252. [DOI] [PubMed] [Google Scholar]
- Winchester B, Vellodi A, Young E. The molecular basis of lysosomal storage diseases and their treatment. Biochem Soc Trans. 2000;28:150–154. doi: 10.1042/bst0280150. [DOI] [PubMed] [Google Scholar]
- Wong K, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Wu JY, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YP, Proia RL. Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proc Natl Acad Sci U S A. 2004;101:8425–8430. doi: 10.1073/pnas.0400625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Lee W, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for crohn's fistula. Stem Cells. 2013 doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- Yuan SS, et al. Cloning and functional studies of a novel gene aberrantly expressed in RB-deficient embryos. Dev Biol. 1999;207:62–75. doi: 10.1006/dbio.1998.9141. [DOI] [PubMed] [Google Scholar]
- Zhang R, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC. Four patterns of laminin-immunoreactive structure in developing rat brain. Brain Res Dev Brain Res. 1990;55:191–201. doi: 10.1016/0165-3806(90)90200-i. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. Mesenchymal stromal cells augment CD4+ and CD8+ T-cell proliferation through a CCL2 pathway. Cytotherapy. 2013;15:1195–1207. doi: 10.1016/j.jcyt.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Zhu Y, et al. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]