Abstract
Background
Data continues to emerge on the relative merits of different treatment modalities for prostate cancer. The purpose of this study is to compare patient-reported quality-of-life outcomes (QOL) after proton therapy (PT) and intensity-modulated radiation therapy (IMRT) for prostate cancer.
Methods
A comparison was performed of prospectively collected QOL data using the expanded prostate cancer index (EPIC) questionnaire. QOL data was collected during the first 2 years following treatment for men treated with PT and IMRT. PT was delivered to 1,243 men at a single center to 76-82Gy. IMRT was delivered to 204 men included in the Prostate Cancer Quality Assurance Study (PROSTQA) in doses of 75.6-79.4Gy.The Wilcoxon rank sum test was used to compare EPIC outcomes by modality using baseline-adjusted scores at different time points. Individual questions were assessed by converting to binary outcomes and testing with generalized estimating equations.
Results
No differences in changes in summary scores for bowel, urinary incontinence, urinary irritative/obstructive, and sexual domains were seen between the two cohorts. However, more men treated with IMRT reported moderate/big problems with rectal urgency (p=0.02) and frequent bowel movements (p=0.05) than men treated with PT.
Conclusions
There were no differences in QOL summary scores between the IMRT and PT cohorts during early follow-up up to 2-years. Response to individual questions suggests possible differences in specific bowel symptoms between the two cohorts. These outcomes highlight the need for further comparative studies of PT and IMRT.
Keywords: proton therapy, IMRT, prostate cancer, outcomes, genitourinary
Introduction
Conformal radiotherapy (RT) techniques for prostate cancer are expected to reduce urinary and rectal toxicity (1) and improve disease control through facilitation of dose escalation (2). The increased costs associated with these techniques (3) have led payors and insurers to demand clinical data demonstrating improved disease control and/or less toxicity.
Several comparative effectiveness studies of conventional radiation therapy, 3-dimensional conformal radiation therapy (3DCRT), IMRT, and PT have been reported. These studies have in common a reliance on Medicare claims as surrogates for actual clinical outcomes, but they differ somewhat in their findings (4-7). The use of Medicare claims rather than medical records may be a weakness, as medical claims codes identify interventions which may not reflect the relevant endpoints of disease control, specific treatment related toxicity, or patient-reported quality of life (QOL). Some of the reports have attracted considerable criticism (8, 9), and the authors of one study acknowledge the limitations of the Medicare database and the need for patient-reported QOL outcomes (7). A randomized trial comparing PT and intensity-modulated radiotherapy (IMRT) has been opened, but the comparative impact on late effects will not be known for some years (NCT01617161). We compared prospectively collected QOL outcomes from >1,400 men from two databases who were treated with PT or IMRT.
Materials, Patients, and Methods
The EPIC-26 questionnaire is a validated instrument that has 5 domains, including urinary incontinence (UI; 4 questions), urinary irritative/obstructive (UO, 4 questions), bowel function (BS, 6 questions), sexual function (SS, 6 questions), and hormonal function (HF, 5 questions), with each subscale is scored from 0 to 100, where 100 represents no problems and 0 represents substantial and significant problems with the specific subscale (10). Prospectively collected data from EPIC questionnaires from two patient cohorts, treated with PT and IMRT respectively, were compared.
The University of Florida (UF) institutional review board (IRB)-approved the study that included 1,482 men with localized prostate cancer who were treated at UF with passively-scattered PT treated between 2006 and 2010. Patients were excluded if they failed to complete treatment (n=6), did not consent to study inclusion (n=19), or received hypofractionated PT at 2.5 CGE per fraction or weekly docetaxel on treatment protocols (n=71), or pelvic nodal irradiation (n=45), leaving a total of 1,243 men in the PT cohort.
The EPIC questionnaire were collected on paper forms (prior to March 2009) or by a secure online medical records portal accessed over the Internet (after March 2009) at 6 months, 1 year, then annually.
Specific details of the PT simulation and treatment have been previously reported (11). Patients were treated at 1.8-2Gy per fraction with the majority (99%, n=1226) receiving between 78 and 82Gy (RBE) at 2Gy (RBE) per fraction.
The second cohort included 204 men from the previously reported PROSTQA study treated at 9 university-affiliated hospitals who were treated with IMRT and had completed EPIC-26 prior to treatment and then at 2, 6, 12, and 24 months after treatment. These patients were treated between March 2003 and March 2006, according to individual institutional policies, with IMRT to the prostate, with or without seminal vesicles, without pelvic RT, to doses of 75.6 to 79.2Gy at 1.8-2Gy per fraction (12). Data on actual doses delivered to the IMRT patients was not available, but minimum and maximum doses to the planning target volume (PTV) was available for comparison with the same dose parameters in the PT cohort.
Due to variability in hormonal use between the cohorts, differences in hormonal function or hormonal questions were not investigated.
Statistics
SAS and JMP software were utilized for all statistical analyses (SAS Institute, Cary, NC). Differences between the two cohorts of patients in pretreatment patient-, disease-, and treatment-specific characteristics were assessed by Fisher's exact test for categorical variables and Wilcoxon's rank sum test the continuous variables (Table 1). Scores for EPIC were calculated as previously described (13, 14). The 6-month, 1-year, and 2-year post-treatment scores for each modality were compared to the baseline data for that modality by the Wilcoxon signed-rank sum test, a nonparametric analog to a paired t test. Differences from pretreatment values >50% of the standard deviation (15) at any point in time were considered to represent the minimally detectable difference. Differences in pretreatment scores for the various subscales between the two cohorts were assessed by the Wilcoxon rank sum test. The same method was used to compare baseline-adjusted outcomes between the two modalities at 6 months, 1 year, and 2 years after treatment; baseline adjustment for each patient and each domain was accomplished by subtracting the baseline score from the 6-month, 1-year, and 2-year scores. Patients without a baseline score were excluded from analysis. Since multiple domains were assessed for each patient, a post hoc Bonferroni adjustment was applied to the resulting p-values (Tables 2 and 3). An adjusted p-value of <0.05 was considered statistically significant.
Table 1.
Patient-, Cancer-, and Treatment-Specific Characteristics
| Characteristic | IMRT (n=204) | PT (n=1243) | P-Value |
|---|---|---|---|
| Median Age in Years (range) | 69 (46 - 84) | 66 (40 - >89) | <0.001 |
| Mean Body-mass Index (SD) | 28.6 (5.5) | 28.2 (4.3) | 0.64 |
| Mean Prostate Size in ml (SD) | 49.5 (27.2) | 41.5 (20.7) | 0.001 |
| No. of Patients (%) | No. of Patients (%) | ||
| Race | <0.001 | ||
| White | 166 (81%) | 1132 (91%) | |
| Black | 34 (17%) | 77 (6%) | |
| Other | 4 (2%) | 34 (3%) | |
| PSA | 0.12 | ||
| <4 ng/ml | 37 (17%) | 205 (17%) | |
| 4-10 ng/ml | 128 (63%) | 862 (69%) | |
| >10 ng/ml | 39 (19%) | 176 (14%) | |
| Gleason Score | 0.28 | ||
| <7 | 104 (51%) | 659 (53%) | |
| 7 | 86 (42%) | 466 (37%) | |
| >7 | 14 (7%) | 118 (10%) | |
| Clinical Stagea | 0.61 | ||
| T1 | 148 (73%) | 922 (74%) | |
| T2 | 56 (27%) | 317 (26%) | |
| T3 | 0 (0%) | 3 (<1%) | |
| Overall Risk level | 0.41 | ||
| Low | 83 (41%) | 567 (46%) | |
| Intermediate | 94 (46%) | 532 (43%) | |
| High | 27 (13%) | 143 (11%) | |
| ADT | 49 (24%) | 181 (15%) | 0.001 |
| Median PTV min (range) | 70.9 (40.7 - 90.2) | 74.1 (40.0 - 80.7) | <0.001 |
| Median PTVmax (range) | 81.5 (45.0 - 107.0) | 83.2 (60.5 - 93.3) | <0.001 |
PSA, prostate-specific antigen; IMRT, intensity modulated radiotherapy; PT, proton therapy; ADT, androgen deprivation therapy; PTV, planned target volume; SD, standard deviation
One proton therapy patient had no T stage
Table 2.
Raw Expanded Prostate Cancer Index Composite (EPIC) Scores with Adjusted P-values (Absolute Shift Compared to Baseline)
| Proton Therapy | IMRT | ||||||
|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | P-value | |
| Bowel Summary (6 mo) | 0 | −83 | 46 | 0 | −63 | 58 | 0.17 |
| Bowel Summary (1 yr) | −4 | −83 | 46 | 0 | −71 | 58 | 0.92 |
| Bowel Summary (2 yr) | −4 | −71 | 29 | 0 | −79 | 67 | 0.99 |
| Urinary Incontinence (6 mo) | 0 | −67 | 60 | 0 | −71 | 46 | 0.31 |
| Urinary Incontinence (1 yr) | 0 | −100 | 52 | 0 | −71 | 34 | 0.99 |
| Urinary Incontinence (2 yr) | 0 | −100 | 56 | 0 | −56 | 44 | 0.99 |
| Urinary Irritative/Obstructive (6 mo) | 0 | −88 | 56 | 0 | −94 | 38 | 0.99 |
| Urinary Irritative/Obstructive (1 yr) | 0 | −75 | 50 | 0 | −63 | 50 | 0.27 |
| Urinary Irritative/Obstructive (2 yr) | 0 | −75 | 50 | 0 | −50 | 38 | 0.99 |
| Sexual Summary (6 mo)a | 0 | −100 | 100 | 0 | −94 | 58 | 0.99 |
| Sexual Summary (1 yr)a | 0 | −100 | 92 | 0 | −96 | 58 | 0.99 |
| Sexual Summary (2 yr)a | 0 | −100 | 100 | 0 | −83 | 71 | 0.99 |
Sexual summary score was calculated for men who did not receive androgen deprivation therapy
mo, month; yr, years; min, minimum; max, maximum; p-value- Wilcoxon's rank sum test with Bonferonni adjustment
Table 3.
Percent of Men with Minimally Detectable Differences from their Baseline Expanded Prostate Cancer Index Composite (EPIC) Scores*
| EPIC Domain at Follow-up Periods | Proton Therapy | IMRT | P value† |
|---|---|---|---|
| Bowel Summery | |||
| 6 months | 25% | 39% | 0.002 |
| 1 year | 41% | 37% | 0.99 |
| 2 years | 37% | 38% | 0.99 |
| Urinary Incontinence | |||
| 6 months | 22% | 28% | 0.36 |
| 1 year | 31% | 29% | 0.99 |
| 2 years | 32% | 34% | 0.99 |
| Urinary Irritative/Obstructive | |||
| 6 months | 18% | 25% | 0.99 |
| 1 year | 23% | 20% | 0.99 |
| 2 year s | 17% | 18% | 0.99 |
| Sexual Summery | |||
| 6 months | 27% | 31% | 0.99 |
| 1 year | 36% | 36% | 0.99 |
| 2 years | 40% | 41% | 0.99 |
Great than 50% decline from baseline score
p value, Wilcoxon's rank sum test with Bonferonni adjustment
As previously reported (16), two approaches were used to analyze dichotomized responses to each question covering urinary, bowel, and sexual function. Baseline differences in individual question responses between the two modalities were assessed with Fisher's exact test. Six-month, 1-year, and 2-year responses were assessed simultaneously with repeated-measures generalized estimating equations with unstructured correlation via PROC GENMOD in SAS (Table 4). The primary prognostic factor in each model was treatment modality, but baseline response, use of androgen deprivation therapy (ADT), age (<65 years vs. ≥65 years) and prostate size were entered into the models as covariates to control. A post hoc Bonferroni adjustment was also included to adjust for the 21 questions evaluated (excluding the hormone function questions, which were not utilized).
Table 4.
Outcomes by Specific Expanded Prostate Cancer Index Composite (EPIC) Question
| Baseline | 6 months | 1 year | 2 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Question | IMRT (%) | P-valuea | IMRT (%) | IMRT (%) | IMRT (%) | P-valueb | P-valuec | ||||
| No. of Patients Answering | |||||||||||
| EPICd | 204 | 1212 | 192 | 1115 | 191 | 1093 | 177 | 963 | |||
| Urinary Irritation/obstruction | |||||||||||
| Dysuria | 1 | 0 | 0.35 | 6 | 3 | 2 | 5 | 1 | 2 | 0.50 | .99 |
| Hematuria | 1 | 0 | 0.04 | 2 | 1 | 1 | 1 | 2 | 1 | 0.33 | .99 |
| Weak stream | 15 | 9 | 0.03 | 12 | 7 | 15 | 11 | 11 | 8 | 0.07 | .99 |
| Frequency | 15 | 13 | 0.38 | 17 | 12 | 15 | 14 | 13 | 11 | 0.48 | .99 |
| Urinary Incontinence | |||||||||||
| Leaking > Daily | 6 | 3 | 0.02 | 10 | 3 | 9 | 5 | 7 | 5 | 0.008 | .16 |
| Frequent Dribbling | 2 | 2 | 0.72 | 2 | 3 | 4 | 4 | 3 | 4 | 0.25 | .99 |
| Any Pad Use | 1 | 2 | 0.5 | 4 | 3 | 3 | 4 | 5 | 4 | 0.58 | .99 |
| Leaking Problem | 2 | 1 | 0.33 | 5 | 2 | 5 | 3 | 5 | 4 | 0.06 | .99 |
| Overall Urinary Problem | 10 | 8 | 0.32 | 11 | 8 | 13 | 11 | 11 | 10 | 0.68 | .99 |
| Bowel Function | |||||||||||
| Urgency | 3 | 2 | 0.45 | 9 | 5 | 13 | 8 | 15 | 7 | 0.001 | .02 |
| Frequency | 2 | 1 | 0.39 | 8 | 3 | 8 | 6 | 10 | 4 | 0.003 | .05 |
| Fecal Incontinence | 1 | 1 | 0.98 | 4 | 2 | 3 | 3 | 3 | 3 | 0.28 | .99 |
| Bloody Stools | 2 | 0 | 0.04 | 2 | 2 | 6 | 8 | 7 | 8 | 0.06 | .99 |
| Rectal Pain | 3 | 1 | 0.02 | 5 | 2 | 3 | 3 | 6 | 2 | 0.19 | .99 |
| Overall Bowel Problem | 3 | 2 | 0.09 | 8 | 4 | 10 | 9 | 11 | 7 | 0.21 | .99 |
| Sexual Functione | |||||||||||
| Poor Erections | 36 | 25 | 0.003 | 46 | 33 | 49 | 41 | 53 | 42 | 0.24 | .99 |
| Difficulty with Orgasm | 31 | 21 | 0.003 | 40 | 27 | 45 | 32 | 42 | 32 | 0.06 | .99 |
| Erection not Firm | 47 | 33 | <0.001 | 56 | 39 | 59 | 47 | 59 | 49 | 0.21 | .99 |
| Erections not Reliable | 45 | 34 | 0.01 | 51 | 41 | 58 | 47 | 60 | 48 | 0.19 | .99 |
| Poor Sexual Function | 34 | 29 | 0.18 | 43 | 35 | 46 | 41 | 47 | 44 | 0.71 | .99 |
| Overall Sexuality Problem | 17 | 21 | 0.29 | 29 | 29 | 30 | 36 | 33 | 35 | 0.36 | .99 |
Fisher's exact test
Generalized estimating equation p-value adjusted for baseline difference, ADT, age, and prostate size
Generalized estimating equation p-value adjusted for baseline difference, ADT, age, and prostate size but with Bonferroni adjustment for 21 questions
Recorded in absolute number
sexual function for patients who did not receive ADT
IMRT, intensity-modulated radiotherapy; PT, proton therapy
Results
Patient- and treatment-specific characteristics are illustrated in Table 1. IMRT patients treated were older (median age, 69 vs. 66 years; p<0.001), had larger prostate volumes (mean, 49.5 vs. 41.5 grams; p=0.0014), were less likely to be white (81% vs. 91% white; p<0.001), were more likely to be treated with ADT (24% vs. 15%; p=.00013), and received both a lower minimum dose to the PTV (median, 70.9 vs. 74.1Gy; p<0.001) and a lower maximum PTV dose (median, 81.5 vs. 83.2Gy; p<0.001).
EPIC summary scores at baseline, 6 months, 1year, and 2 years following treatment are depicted in Figures 1A-D for PT and IMRT. Following treatment the only changes in summary scores from baseline that met the minimally detectable difference were observed for bowel summary at 6 months, 1 year, and 2 years for IMRT and in bowel summary at 1 year and 2 years for PT (Figures 1A-D). Both groups showed decline in bowel summary scores, but there were no statistically significant differences in QOL changes between groups for BS, UI, UO or SS (only among men who did not receive ADT) at any time (Table 2). When looking at the percent of men with a minimally detectable difference at the various time points for the different summary scores, the only remarkable difference between the IMRT and PT cohorts was for the bowel summary component at the 6-month follow-up (Table 3).
Figure 1.
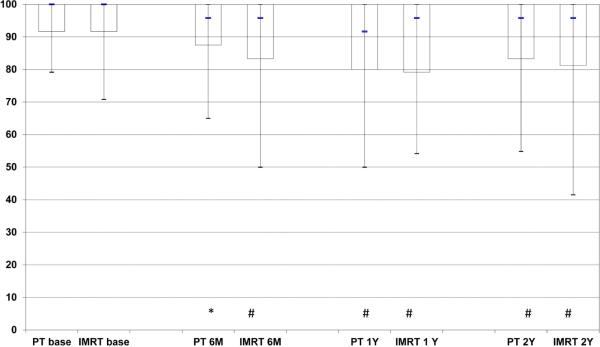
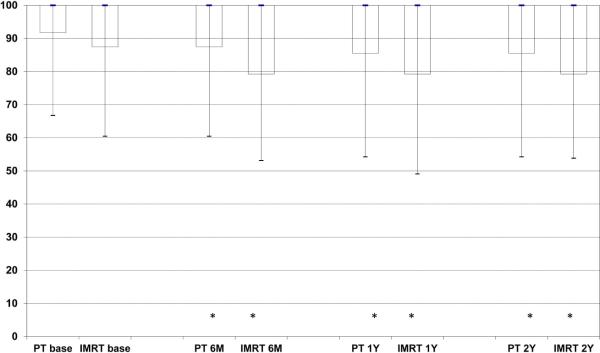
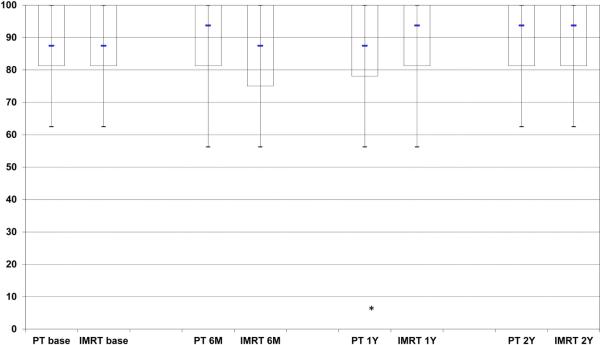
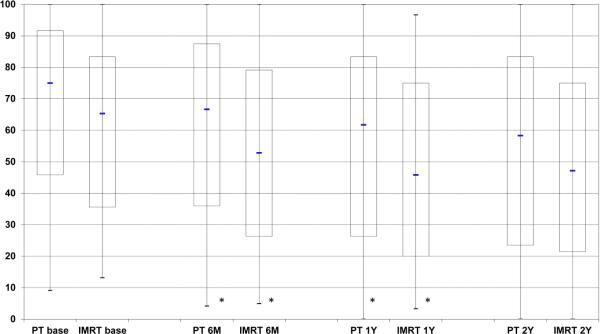
Expanded Prostate Cancer Index Composite (EPIC) summary scores over time for men treated with intensity-modulated radiotherapy or proton therapy for prostate cancer. Bar and whisker graphs at baseline and 6 months, 1 year, and 2 years after proton therapy or intensity-modulated radiotherapy for A) bowel summary score, B) urinary incontinence score, C) urinary irritative/obstructive score, and D) sexual summary score (no androgen deprivation therapy). The bottom whisker represents the cut-off for the score of the lowest 5%, the bottom bar represents the cut-off score for the lowest quartile, the blue line represents the median score, the top of the bar represents the cut-off for the top quartile, and the top of the whisker represents the cut-off for the score of the top 5%. At the bottom of the graph the asterisk (*) represents a statistically significant change from baseline score for each treatment modality and time point, while a pound sign (#) represents a statistically significant and minimally detectable (>50% of the baseline standard deviation) change from the baseline score.
An analysis of individual items comprising each domain was also planned and performed. At baseline the only differences between the two cohorts were the IMRT cohort were more likely to report baseline moderate/big problems with hematuria (p=0.04), daily urinary leakage (p=0.02), bloody stools (p=0.02), and rectal pain (p=0.04). The IMRT cohort also reported more moderate/big problems within the sexual domain, including poor erections, difficulty with orgasms, erections not sufficient for intercourse, and unreliable erections (all p<0.01; Table 4). When comparisons over time were controlled for differences between groups in age, prostate size, and ADT use as well as baseline QOL, there were no significant differences between the cohorts except for more frequent reports in the IMRT cohort of “moderate” or “big problems” with rectal urgency (p=0.02) and bowel frequency (p=0.05).
Discussion
The study reported herein compares patient-reported QOL outcomes in PT and IMRT cohorts using the EPIC questionnaire. No significant differences were observed in QOL EPIC summary scores for bowel, urinary, and sexual function between the IMRT and PT cohorts, despite higher minimum and maximum PTV doses in the PT cohort, a factor expected to be associated with higher toxicity.
EPIC urinary summary scores were similar between IMRT and PT cohorts in this study, concurring with similar urinary toxicity rates between IMRT and PT implied by surrogate data reported in the Medicare studies (5, 6, 17). However, the EPIC bowel outcomes in this study did not correlate with findings in two of the Medicare studies (5, 6), which implied worse bowel toxicity with PT than IMRT. The Medicare studies have been criticized for their reliance on surrogate rather than actual clinical data, such as using colonoscopy claims to infer rectal toxicity. Furthermore, absence of any clinical and treatment details in the Medicare studies may also have confounded conclusions since radiation dose, dose fractionation, and dose distribution, the most important predictive factors for gastrointestinal toxicity were not available and could have been significantly impacted by the dose-escalation studies being performed during the time period at the two proton centers (12, 18). In contrast to the Medicare studies, the current study is based on prospectively collected actual patient-reported clinical outcomes using the same QOL instrument and acquisition times between PT and IMRT cohorts, and, as such, should provide a more reliable comparison of functional outcomes.
In the present study, bowel summary scores were similar between the groups, correlating with the findings by Yu et al (7), who reported on more recent Medicare patient population that included patients treated at different proton centers. The results are also similar to those recently reported by Gray et al (19), who compared QOL outcomes of 95 men treated at Massachusetts General Hospital with protons using the Talcott prostate symptom index (20) with those treated with IMRT from the PROSTQA database using the EPIC questionnaire demonstrating little difference in bowel problems >6 months following treatment.
IMRT and PT both produce “high radiation dose” volumes that conform to the target; IMRT does so at the expense of exposing a larger volume of non-targeted tissue to “low and moderate radiation doses” (21, 22). Thus, toxicities and functional outcomes related to high radiation dose exposure, such as rectal bleeding, are expected to be similar between IMRT and PT if target doses and daily doses are similar (22, 23). On the other hand, the rate of toxicities related to larger volumes of non-targeted tissue receiving low- and moderate-dose radiation exposure might be expected to be higher with IMRT than PT. In the present study, the rate of rectal bleeding trended to being worse in the PT cohort, which might be explained by the higher prescription doses given to the PT cohort. Studies investigating rectal toxicities other than bleeding—such as, rectal syndrome, which includes rectal urgency, frequency, and incontinence—have demonstrated correlations with the volumes of the rectum receiving both high and low to moderate doses (18, 24, 25). These studies have focused on patients treated with 3DCRT rather than IMRT, but the dose-volume relationships serve to demonstrate the type of toxicity improvements that might be expected with reductions in the volume of rectum exposed to low to moderate radiation doses with PT compared with IMRT. Therefore, a potentially important finding of the present study is the analysis that identified significantly worse bowel urgency and bowel frequency in the IMRT group, a problem that affects QOL and can persist more than 10 years following radiation (26). Nevertheless, alternate explanations such as differing use of image-guided therapy, differing use of aspirin or other anti-coagulants, target margins, interobserver variability, older age, or larger prostate volumes in the IMRT cohort could also influence these results (27).
Recent research has investigated the impact of different rectal complications on global QOL following radiation therapy for prostate cancer. Krol et al (28), evaluated anorectal function in 85 men at least 1 year after conventional dose prostate radiotherapy using the EPIC, the Fecal Incontinence QOL scale, and anal manometry. They found that fecal incontinence and rectal urgency most greatly influenced overall QOL along with impaired anal resting pressure. It was also demonstrated that urgency of defecation has a more severe impact upon patient-reported QOL than rectal bleeding, despite clinicians’ greater concern with rectal bleeding, which can be treated and resolved, compared to urgency, which can worsen over time and for which there is little treatment for its symptomology (29). Therefore, these differences in discreet rectal symptoms between PT and IMRT treatments as assessed from patient-reported outcomes are intriguing and warrant further evaluation.
The strengths of the current study are the prospective design for data collection, the use of PRO from contemporary IMRT and PT series, the use of a common QOL instrument, and the collaborative effort between institutions using IMRT and institutions using PT. Although patients were not randomized to RT modalities, baseline information on clinical factors, QOL, and RT details permitted some adjustments for differences between the cohorts. Nevertheless, no level of statistical manipulation can account for how PT patients may have sought out (and traveled) to receive treatment in expectation of fewer side effects and better QOL. This exact criticism, however, can be made for the Medicare studies, which were unable to statistically account for these differences either. Another potential weakness is that the patients receiving PT were treated consistently at a single academic center, while the IMRT patients were treated at 9 different academic centers; however, the same could be said with the Medicare studies, whereby the vast majority of the patients treated before 2008 would have been treated at one institution [18]. Additional weaknesses include that the comparative analysis plan was post hoc, although the QOL data were collected prospectively and the difference in EPIC data collection between the cohorts, with patients who received IMRT undergoing a phone-assisted interview while those who received PT read and completed their questionnaires without assistance, could potentially lead to bias in either direction.
The findings from this study provide evidence of excellent and comparable QOL outcomes for prostate cancer patients treated with either contemporary IMRT or PT. Although similar bowel, urinary, and sexual scores were observed with IMRT and PT, potential differences in specific functional outcomes, such as bleeding, rectal urgency and bowel frequency, were also observed and may reflect differences in radiation dose distributions between IMRT and PT, differences in patient characteristics, or both. Further investigation will be necessary to validate these findings and to identify the underlying mechanisms that account for them.
Supplementary Material
Acknowledgements
The PROSTQA Consortium includes contributions in cohort design, patient accrual and follow-up from the following investigators: Larry Hembroff (Michigan State University, East Lansing, MI); John T. Wei, and Laurel Northouse (University of Michigan, Ann Arbor, MI); Eric A Klein and Jay Ciezki (Cleveland Clinic, Cleveland, OH); Gerald Andriole (Washington University, St. Louis, MI); Mark Litwin and Chris Saigal (University of California—Los Angeles Medical Center, Los Angeles, CA); Thomas Greenfield, PhD (Berkeley, CA), Louis Pisters and Deborah Kuban (MD Anderson Cancer Center, Houston, TX); Jim Hu and Adam Kibel (Brigham and Women's Hospital, Boston, MA); Douglas Dahl and Anthony Zietman (Massachusetts General Hospital, Boston, MA); Peter Chang and Irving Kaplan (Beth Israel Deaconess Medical Center, Boston, MA).
We also acknowledge PROSTQA Data Coordinating Center Project Management by Jill Hardy, MS (Michigan State University, East Lansing, MI), Erin Najuch (Dana Farber Cancer Institute, Boston, MA), grant administration by Beth Doiron, BA (Beth Israel Deaconess Medical Center, Boston, MA), and technical support from coordinators at each clinical site.
Funding
This work was supported by the National Institute of Health (RO1 CA95662, 1RC1CA14596).
Disclosure: This work was financially supported by grants from the National Institute of Health (RO1 CA95662, 1RC1CA14596) and ACR-RTOG. Daniel A. Hamstra reports payment from Myriad Health and Bayer Health for consultant services and payment from Varian Health for lectures. Bradford Hoppe reports receiving an honorarium from Procure for lectures. Martin G. Sanda is a board member of Medicametrix, and he reports payment from Sanofi-Aventis for lectures. Howard M. Sandler is a board member of Eviti, and he reports payment from Millennium, Bayer, and Varian Health for consultant services.
Footnotes
This study was presented at the 55th Annual Meeting of the American Society for Radiation Oncology (ASTRO) in Atlanta, GA, USA on September 22–25, 2013.
References
- 1.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekelman JE, Mitra N, Efstathiou J, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Shen S, Moore DF, et al. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60:908–916. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deville C, Ben-Josef E, Vapiwala N. Radiation therapy modalities for prostate cancer. Jama. 308:451. doi: 10.1001/jama.2012.8110. author reply 451-452. [DOI] [PubMed] [Google Scholar]
- 9.Mendenhall NP, Schild S, Slater J. Radiation therapy modalities for prostate cancer. Jama. 308:450–451. doi: 10.1001/jama.2012.8112. author reply 451-452. [DOI] [PubMed] [Google Scholar]
- 10.Szymanski KM, Wei JT, Dunn RL, et al. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 76:1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendenhall NP, Li Z, Hoppe BS, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:213–221. doi: 10.1016/j.ijrobp.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Sandler HM, Liu PY, Dunn RL, et al. Reduction in patient-reported acute morbidity in prostate cancer patients treated with 81-Gy Intensity-modulated radiotherapy using reduced planning target volume margins and electromagnetic tracking: assessing the impact of margin reduction study. Urology. 2010;75:1004–1008. doi: 10.1016/j.urology.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 14.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 15.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 16.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 17.Hoppe BS, Nichols RC, Henderson RH, et al. Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger. Cancer. 2012;118:4619–4626. doi: 10.1002/cncr.27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorino C, Fellin G, Rancati T, et al. Clinical and dosimetric predictors of late rectal syndrome after 3D-CRT for localized prostate cancer: preliminary results of a multicenter prospective study. Int J Radiat Oncol Biol Phys. 2008;70:1130–1137. doi: 10.1016/j.ijrobp.2007.07.2354. [DOI] [PubMed] [Google Scholar]
- 19.Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer. 2013 doi: 10.1002/cncr.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark JA, Talcott JA. Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care. 2001;39:1118–1130. doi: 10.1097/00005650-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Vargas C, Fryer A, Mahajan C, et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:744–751. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- 22.Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker SL, Dong L, Michalski JM, et al. Do intermediate radiation doses contribute to late rectal toxicity? An analysis of data from radiation therapy oncology group protocol 94-06. Int J Radiat Oncol Biol Phys. 84:390–395. doi: 10.1016/j.ijrobp.2011.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151–1161. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 25.al-Abany M, Helgason AR, Cronqvist AK, et al. Toward a definition of a threshold for harmless doses to the anal-sphincter region and the rectum. Int J Radiat Oncol Biol Phys. 2005;61:1035–1044. doi: 10.1016/j.ijrobp.2004.07.706. [DOI] [PubMed] [Google Scholar]
- 26.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamstra DA, Stenmark M, Ritter T, et al. Age and Comorbid Illness Are Associated With Late Rectal Toxicity Following Dose-Escalated Radiation Therapy for Prostate Cancer. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 28.Krol R, Smeenk RJ, van Lin EN, et al. Impact of late anorectal dysfunction on quality of life after pelvic radiotherapy. Int J Colorectal Dis. 2013;28:519–526. doi: 10.1007/s00384-012-1593-5. [DOI] [PubMed] [Google Scholar]
- 29.Yeoh EK, Holloway RH, Fraser RJ, et al. Pathophysiology and natural history of anorectal sequelae following radiation therapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2012;84:e593–599. doi: 10.1016/j.ijrobp.2012.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


