Abstract
The function of membrane-bound transporters is commonly affected by the milieu of the hydrophobic, membrane-spanning part of the transmembrane protein. Consequently, functional studies of these proteins often involve incorporation into a native-like bilayer where the lipid components of the membrane can be controlled. The classical approach is to reconstitute the purified protein into liposomes. Even though the use of such liposomes is essential for studies of transmembrane transport processes in general, functional studies of the transporters themselves in liposomes suffer from several disadvantages. For example, transmembrane proteins can adopt two different orientations when reconstituted into liposomes, and one of these populations may be inaccessible to ligands, to changes in pH or ion concentration in the external solution. Furthermore, optical studies of proteins reconstituted in liposomes suffer from significant light scattering, which diminishes the signal-to-noise value of the measurements. One attractive approach to circumvent these problems is to use nanodiscs, which are phospholipid bilayers encircled by a stabilizing amphipathic helical membrane scaffold protein. These membrane nanodiscs are stable, soluble in aqueous solution without detergent and do not scatter light significantly. In the present study, we have developed a protocol for reconstitution of the aa3- and ba3-type cytochrome c oxidases into nanodiscs. Furthermore, we studied proton-coupled electron-transfer reactions in these enzymes with microsecond time resolution. The data show that the nanodisc membrane environment accelerates proton uptake in both oxidases.
1. Introduction
One role of biological membranes is to define the boundary of cells and organelles. Intrinsic membrane proteins that span across these membranes are involved in fundamental processes such as signaling, energy conversion, motility and transport. A challenge associated with studies of these membrane proteins is to mimic the membrane environment, which is necessary to make the proteins soluble in water. The choice of this membrane mimetic is an important parameter because the structure and function of membrane proteins is often dependent on specific interactions between the membrane lipids and the protein [1-3]. Approaches to study purified membrane proteins in aqueous solutions include, for example, the use of detergents [4]. However, many membrane proteins are unstable and/or lose their function in detergent solution. In these cases, it may be more attractive to use liposomes, composed of different, well defined lipids, both native and synthetic, simulating a natural environment of the protein. Because such liposomes have an inner compartment that is sealed from the surrounding outside bulk solution, they allow studies of ion or molecule transport across the membrane. However, in many cases, studies of proteoliposomes suffer from significant drawbacks. For example, liposomes scatter light, which diminishes the signal-to-noise value in optical measurements. Furthermore, commonly, transmembrane proteins reconstituted in liposomes are only partially oriented such that there are two protein populations sensing different solvent phases. Similarly, a non-uniform enzyme orientation may influence studies in which non membrane-permeable interaction partners are added. In many cases, functional studies of membrane-bound proteins (including those that are involved in transmembrane transport) do not require sealed liposomes, an open system where both sides of the membrane protein are exposed to identical solvent environments (cf. pH, ionic strength etc.) is an advantage. A recently developed alternative to using liposomes in such cases is the so-called nanodisc technology [5,6]. These discs are planar phospholipid bilayers surrounded by two membrane-scaffold proteins (MSPs), whose amphiphilic helical structure covers the exposed hydrophobic edges of the lipid bilayer. Thus, the membrane proteins can be studied while soluble in aqueous solutions, yet residing in a native-like membrane bilayer environment of well-defined composition. The nanodiscs are more stable than liposomes and their size (Ø ffi 10–17 nm) and lipid content (120–330 lipids, for empty discs, depending on lipids [6]) renders dramatically less light scattering compared to liposomes (Ø ≈ 30–200 nm, 4–180 × 103 lipids), which makes the nanodisc technology ideal for studies of membrane proteins using optical techniques. In addition, the lower lipid-protein ratio in nanodiscs as compared to liposomes is of significant advantage in studies of proton uptake/release reactions because of the buffering capacity of phospholipid head groups.
Here, we have developed a protocol for reconstitution of the membrane-bound proton pump cytochrome c oxidase (CytcO) into nanodiscs composed of soybean lipids using the scaffold protein MSP1E3D1, which yields nanodiscs with a diameter of ~12 nm [7]. Two different CytcOs were studied, the aa3-type CytcO from Rhodobacter sphaeroides [8-10] and the ba3-type CytcO from Thermus thermophilus [11-13]. These CytcOs catalyze the reduction of O2 to H2O: O2 + 4H+ + 4e- → 2H2O (for review, see [14-19]). Electrons are delivered by a water-soluble cytochrome c, which docks at the more positive (p) side of the membrane. The primary elec-tron acceptor is a copper center, CuA, from which electrons are transferred to a heme group (heme a or b) and then to the catalytic site composed of heme a3 and CuB, where O2 binds and becomes reduced. Protons are taken up from the more negative (n) side of the membrane and are transferred through specific pathways. The aa3 oxidase has been shown to use two such pathways denoted by letters D and K, respectively, while the ba3 CytcO uses only one pathway that overlaps in space with the K pathway in the aa3 CytcO [20,21]. Results from earlier experiments with the aa3 CytcO have shown that internal electron transfer to or from the catalytic site, linked to proton uptake or release through the K pathway is sensitive to the membrane environment of the CytcO [22] (see also [23]). Using the nanodisc technology, we have studied the kinetics, with microsecond time resolution, of these internal electron and proton-transfer reactions.
2. Materials and methods
2.1. Growing and purification of cytochrome c oxidase
The aa3- and ba3-CytcOs were expressed in R. sphaeroides and T. thermophilus, respectively, and the His-tagged protein was purified using Ni-affinity chromatography as described earlier [24,25].
2.2. Reconstitution of CytcO into nanodiscs
The soybean phosphatidyl choline lipids (type II, Sigma) were washed by diethyl ether extraction and then precipitation using acetone. The lipids were then extensively dried to remove all traces of organic solvents. The washed lipids (final concentration of 40 mg/ml) were then resuspended in 50 mM Tris–HCl pH 7.5 containing 120 mM sodium cholate hydrate (Fluka/Sigma). The sample was sonicated to complete dissolution of the lipids using a tip sonicator (Ultra sonic VCX 130, Chemical Instruments AB, Sweden) for two minutes per ml sample in cycles of 30 s on and 30 s off. Particles from the sonicator tip were removed by centrifugation at 4500_g for 20 min. The lipid-cholate mixture was mixed with the MSP1E3D1 protein and aa3- or ba3-CytcO at an approximate molar ratio of 500:10:1 (Lipid:MSP:CytcO). Buffer was added to keep the cholate and lipid concentrations at 25 mM and 6 mg/ml, respectively. The sample mixture was incubated for one hour at room temperature with occasional gentle shaking. The detergent was then removed using a PD-10 column (GE Healthcare) pre-equilibrated with 50 mM Tris–HCl pH 7.5 and the eluate containing color was collected (the sample was loaded in several fractions, each having a volume of 500–750 ll). For nanodiscs containing fluorescent lipids, soybean lipids were first dissolved in chloroform and mixed with Texas Red (TR) DHPE (Invitrogen) to yield on aver-age one fluorescent lipid per 10 nanodiscs and the solvent was evaporated under a stream of nitrogen before the dry lipid film was dissolved using the same procedure as described above.
2.3. Analysis of CytcO-containing nanodiscs
Samples were concentrated using a protein concentrator tube (100 kDa, Millipore) and centrifuged at 10000_g for 30 min before injection of a volume of 100 μl onto a Superdex 200 10/300 GL Column (GE Healthcare) equilibrated with 50 mM phosphate buffer at pH 7.5 and 100 mM KCl. After injection, 1 ml fractions were col-lected at a flow rate 0.25 ml/min. Absorbance spectra at 240– 700 nm were recorded for each fraction using a dip probe (Cary 50, Varian). Fractions containing protein were concentrated by a factor of 10 and used for SDS–PAGE (NuPAGE 4–12% Bis–Tris, Invitrogen). The samples containing fluorescent lipids were analyzed in a 96 well plate reader (SpectraMax Gemini EM microplate spectrofluorometer Molecular Devices).
2.4. Preparation of the two-electron reduced aa3 CytcO and measurements of PCET
For aa3-CytcO, the nanodisc sample buffer was exchanged for 100 mM KCl on a PD-10 column and the pH was adjusted to 9.0 be-fore transferring it to an anaerobic cuvette. First, the atmosphere in the cuvette was exchanged to N2 and subsequently to CO. The reduction state of the enzyme was monitored using a spectropho-tometer (Cary 400 Varian) to reach the two-electron reduced state. The cuvette was then transferred to the flash photolysis apparatus, where the CO ligand was dissociated by applying a short laser flash (10 ns, 200 mJ, 532 nm, Nd:YAG Brilliant B, Quantel) and electron transfer was monitored at different single wavelengths. In samples containing detergent-solubilized enzyme solution, n-dodecyl β-D-maltoside (DDM) (Glycon) at a concentration of 0.05% was used. Measurements with the samples in detergent solution and in liposomes were carried out in a buffer composed of a mixture of 50 mM bis–tris propane and 50 mM CAPS at pH 9.
2.5. Experiments with fully reduced ba3 CytcO in nanodiscs
Either a sample containing detergent-solubilized ba3 oxidase (~5 μM enzyme in 10 mM Hepes, pH 7.5, 0.05% DDM, 0.5 μM PMS) or nanodisc-reconstituted ba3 CytcO (~5 μM enzyme in 10 mM Hepes, pH 7.5, 0.5 μM PMS) was transferred to a Thunberg cuvette and an amount of Na-ascorbate corresponding to a final concentration of 2 mM was placed in the sidearm of the cuvette. The atmosphere was exchanged for N2 on a vacuum line and the CytcO was reduced upon addition of the ascorbate to the enzyme solution. Upon full reduction (as determined from the optical absorption spectrum) of the CytcO, the atmosphere was exchanged for CO and the sample was allowed to incubate for at least 30 min. Measurements of the reaction of the reduced enzyme with oxygen were performed using a locally modified flow-flash apparatus (Applied Photophysics) as described in [26]. Briefly, the CytcO nanodisc preparation (10 mM Hepes, pH 7.5) was rapidly mixed at a ratio of 1:2 with an O2-saturated solution (100 mM Hepes, pH 7.5) and the reaction was initiated by light-induced displacement of the CO ligand ~30 ms after mixing (10 ns, 200 mJ, 532 nm, Nd:YAG Brilliant B, Quantel). Electron and proton-transfer reactions were monitored at different wavelengths on an oscilloscope. Measurements with the detergent solubilized ba3 oxidase were performed identically, except that the O2-saturated solution contained additionally 0.05% DDM. For proton uptake measurements, buffer was removed from the protein samples using a PD-10 column pre-equilibrated with 150 mM KCl, pH ~7.5 (and 0.05% DDM in case of detergent solubilized enzyme). Measurements of proton uptake from solution during oxidation of the ba3 CytcO were performed using the pH sensitive dye phenol red as described in [21].
3. Results and discussion
Fig. 1A shows a size-exclusion chromatogram at 280 nm, reporting the total protein content (i.e., both MSP and CytcO) for nanodiscs composed of soybean lipids containing aa3 CytcO (de-noted “crude aa3-nanodiscs”). These CytcO-containing nanodiscs were obtained using a lipid:MSP:CytcO molecular ratio of approximately 500:10:1. As seen in the Figure, we observed mainly a single peak with a small contribution of larger fragments (see left-hand side of the peak). The presence of a single peak indicates that the main fraction of the nanodisc preparation was uniform having a well-defined size. The probability of complete reconstitution of all CytcOs into nanodiscs increases statistically with an increasing MSP:CytcO ratio. However, an excess MSP also results in formation of empty nanodiscs. The MSP:CytcO ratio of 10:1 is similar to that used in an earlier study of an ABC transporter with MSP1E3D1 [27] and corresponds to an excess MSP by a factor of 5 because two MSPs are present in each nanodisc.
Fig. 1.

Reconstitution of aa3 R. sphaeroides CytcO in soybean lipid nanodiscs. (a) Size-exclusion chromatogram at 280 nm for CytcO nanodiscs. (b) Analysis of 1 ml fractions from the gel filtration of CytcO nanodiscs supplemented with fluorescent lipids at a ratio of one fluorescent lipid per 10 nanodiscs. Detection of the total protein content at 280 nm (black squares), absorbance of oxidized CytcO at 420 nm (red circles) and fluorescence at 610 nm (fractions containing fluorescent lipids, excitation at 584 nm) (green bars). (c) SDS–PAGE where the lanes correspond to: 2 CytcO (0.1% DDM), 3 MSP, 4 and 12 CytcO nanodiscs before gel filtration and 5–11 fractions 8–14 (see panel b). (d) Absorption spectra of oxidized (dark colored) and reduced (light colored) CytcO in nanodiscs before gel filtration (red) and in fraction 11 (green, see panel b), all scaled to 1 lM CytcO.
The lipid:MSP ratio of 100:2 used here is the same as found to be optimal for reconstitution of an ABC transporter [27] and similar to that used for reconstitution of bacteriorhodopsin [28]. A lower lipid:MSP ratio of 70:2 resulted in three peaks in the size-exclusion chromatogram (not shown) reflecting a non-uniform preparation. Previous studies have shown that on average one aa3-CytcO from R. sphaeroides is reconstituted per liposome [29] and there are no indications that this bacterial CytcO would require multimerization for function. Consequently, one CytcO per nanodisc/liposome is sufficient for function.
The collected fractions from gel-filtration experiment were analyzed by measuring the absorbance at 280 nm (total protein con-tent) and at 420 nm (oxidized CytcO). As shown in Fig. 1B, the elution patterns at these two wavelengths are very similar, indicating co-elution (and thus complex formation) of CytcO and total protein (i.e. both the MSP and CytcO) in fractions ~10–13. Furthermore, we prepared a sample containing a fluorescently labeled lipid (TR-DHPE) at a concentration corresponding to one fluorescent lipid molecule per 10 nanodiscs. An analysis of this sample using a fluorescence plate reader showed that the fluorescence signal overlaps with the total protein (A280) as well as the CytcO spectrum (A420) ( Fig. 1B), which further supports our conclusion that the aforementioned fractions contain CytcO within nanodiscs. It should be noted that the fluorescently-labeled lipids were only used in this experiment to detect co-localization of the nanodisc lipids and CytcO. The spectroscopic experiments discussed below were performed in the absence of TR-DHPE. The protein composition in the different fractions of the main peak was also verified using SDS PAGE electrophoresis ( Fig. 1C). Furthermore, the absorption spectra of the nanodisc-reconstituted CytcO before gel filtration were compared with the spectra of the protein in the fraction with the highest CytcO content, number 11, ( Fig. 1D). There are no differences, suggesting that the CytcO-nanodisc preparation prior to gel filtration is substantially homogeneous and can be used for functional studies without the gel filtration step.
We also reconstituted ba3 CytcO from T. thermophilus in nanodiscs using the same procedure and compared its optical properties with preparations of the same enzyme in detergent solution and reconstituted into liposomes ( Fig. 2). As seen in this figure, the spectra of the nanodisc-reconstituted and detergent-solubilized CytcOs were approximately the same. In contrast, the spectrum of the liposome preparation is heavily impacted by light scattering (cf. increasing absorbance with decreasing wavelength), illustrating the great advantage of using the nanodiscs over liposomes for optical studies.
Fig. 2.

Absorbance spectra of fully reduced ba3 CytcO. Shown are the absorption spectra of ba3 CytcO (in an anaerobic cuvette with 2 mM ascorbate and 0.5 lM PMS) solubilized in detergent solution (DDM), reconstituted in ~50 nm liposomes (SUV) and ~12 nm nanodiscs (ND). Protein concentrations used were ~5 μM, ~4 μM and ~5 μM for DDM, SUV and ND sample, respectively. The amplitudes of the spectra were then normalized to ease comparison.
The preparation containing aa3-CytcO reconstituted in nanodiscs was used for functional studies. Using a flash photolysis setup allowing microsecond time resolution [26], we followed internal electron transfer, coupled to proton transfer through the K proton pathway [22]. First, the CytcO was incubated in CO atmosphere under anaerobic conditions. This procedure renders the catalytic site (heme a3 and CuB) reduced with CO bound to heme a3, while heme a and CuA remain oxidized. Because the CO ligand stabilizes the reduced state of heme a3, laser flash-induced dissociation of the CO ligand results in internal electron transfer from heme a3 to heme a over a microsecond time scale [30,31]. This electron transfer is followed in time by an additional, slower electron transfer from heme a3 to heme a, coupled to proton transfer from the catalytic site, from a H2O molecule [32], through the K pathway with a time constant of 730 μs in detergent solution at pH 9 (cf. [22]). Results from earlier studies showed that the rate of this proton-coupled electron transfer (PCET) accelerates (τ ≈ 130 μs) upon incorporation of CytcO into soybean lipid liposomes. Furthermore, our studies using structural variants of CytcO in which K pathway residues were modified, indicate that this effect is due to specific interactions between amino acid residues at the CytcO–membrane interface near the K pathway orifice [22].
As already mentioned above, results from earlier studies have shown that in the aa3 oxidases proton, transfer through the K-pathway accelerates upon reconstitution into a lipid membrane. The experiments outlined above, where the PCET is investigated in the absence of O2, make it possible to study this proton transfer time resolved. When a similar two electron reduced sample was prepared with the ba3 enzyme, no internal electron and proton transfer was observed upon CO dissociation as observed in the aa3 enzyme. Nonetheless, in the ba3 CytcO, proton transfer during reaction of the reduced CytcO with O2 occurs through a pathway that is spatially analogous to the K pathway [20]. In the R. sphaeroides aa3 CytcO, proton transfer during this reaction takes place through the other, D-pathway. Consequently, to compare effects of membrane reconstitution on proton transfer through the analogous pathway (the K pathway) in the aa3 and ba3 CytcOs, we studied different types of reactions in these systems.
The reaction of the reduced nanodisc-reconstituted (using MSP1E3D1, Ø ≈ 12 nm) ba3 CytcO with O2 was investigated and the data were compared to those obtained with ba3 CytcO in detergent-solution. The CytcO was first fully reduced in the presence of CO, which results in formation of the four-electron reduced CytcO with CO bound to heme a3. The sample was transferred to one of the syringes of a modified stopped-flow apparatus, while the other syringe was loaded with an O2-saturated buffer. The two solutions were then rapidly mixed, and after ~30 ms the blocking CO ligand was removed by a laser flash, which allowed O2 to bind to the reduced heme a3. Fig. 4 shows absorbance changes at 430 nm during this reaction. These changes are associated with oxidation/reduction of the heme groups, with a major contribution from heme b. Following photolysis to remove the CO-ligand (time = 0), the subsequent absorbance changes reflect the oxidation of the enzyme. The data show that for both CytcO in detergent solution and in nanodiscs, the enzyme became fully oxidized over a time scale of about 2 ms. Yet, significant differences between the data obtained under these different conditions were observed. As seen in Fig. 4A, the amplitude of the kinetic phase with a time constant of ~20 μs, associated with the initial oxidation of heme b was apparently larger for the nanodisc-reconstituted CytcO (qualitatively the same behavior was observed for ba3 CytcO in liposomes, data not shown). With the ba3 CytcO in detergent solution, this kinetic phase is associated with nearly 100% heme b oxidation, which is followed in time by nearly 100% heme b re-reduction from CuA [21,25,33,34]. Because the 20 μs decrease in absorbance is followed in time by an increase in absorbance (re-reduction of heme b) with a time constant of ~80 μs (~60 μs in nanodiscs), the observed amplitude of the absorbance decrease is diminished. In the nanodisc-reconstituted ba3 CytcO, the first electron transfer from heme b to the catalytic site occurred at the same rate, but the CuA-heme b electron-transfer equilibrium was significantly shifted away from heme b (i.e. the heme was not re-reduced to the same extent as in detergent solution) and, therefore, the amplitude of the absorbance decrease was significantly larger. The final oxidation of the CytcO displayed a time constant of ~1.2 ms (both in nanodiscs and in detergent solution).
Fig. 4.
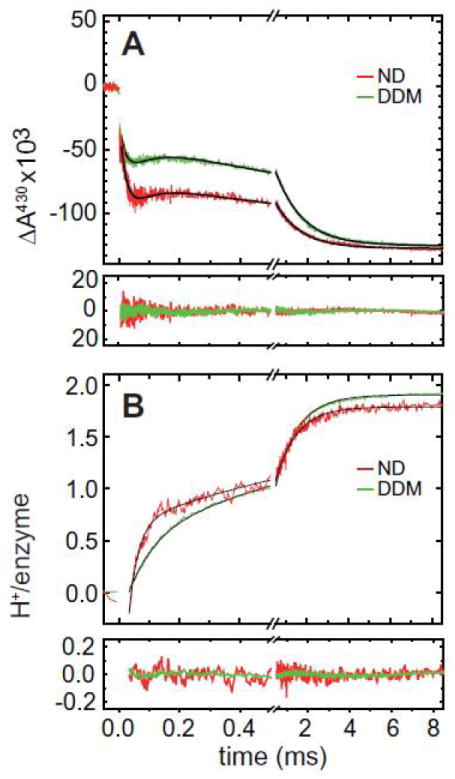
Reaction of the fully reduced ba3 CytcO with oxygen. Shown are the absorbance changes upon photo-dissociation of CO by a laser flash (at t = 0) and subsequent reaction of the reduced enzyme with oxygen (DDM, in detergent solution; ND, in nanodiscs). (a)Absorbance changes at 430 nm represent oxidation and reduction of the hemes (with a major contribution of heme b). (b) Absorbance changes at 572 nm, of the pH sensitive dye phenol red, associated with pH changes in the surrounding medium caused by proton uptake during oxidation of the CytcO. The process was biphasic where the rate constant of the faster phase was slower in detergent solution (τ ≈ 85 μs) than in nanodiscs (τ ≈ 35 μs). The rate of the slower component did not change significantly upon nanodisc reconstitution (τ ≈ 1.2 ms). The 572 nm absorbance changes were normalized by HCl titration and are shown converted to protons/enzyme. The traces are differences between those obtained in the absence and presence of buffer (to eliminate contribution from other events then proton uptake at 572 nm). The black lines represent fits of the data with a sum of exponential functions with time constant given in the text. The residuals are shown under each of the graphs.
Using a pH-sensitive dye in an unbuffered solution, we studied proton-uptake reactions from the aqueous environment during oxidation of the ba3 oxidase. These proton-uptake reactions are difficult to measure using liposomes for several reasons. On one hand, having two populations with differently oriented enzymes severely complicates the discrimination between the protons that are used as substrate for O2 reduction and those that are pumped across the membrane. While cumbersome, this drawback might be partially circumvented by using a high concentration of protonophores, rendering the pumped protons invisible. On the other hand, the much higher lipid-to-protein ratio in liposomes drastically increases the buffer capacity of the solution (due to presence of phospholipid head groups) and thus results in a decreased signal in measurements of proton uptake/release reactions. As shown in Fig. 4B, proton uptake through the K pathway analogue could readily be detected in nanodisc-reconstituted enzyme. As with the detergent-solubilized ba3 CytcO, two kinetic phases were observed associated with proton uptake from solution. The rate constant of the faster of these processes accelerated from ~85 μs in detergent solution to ~35 μs in nanodiscs. The slower component displayed similar rates for CytcO in detergent solution and in nanodiscs (~1.2 ms).
As already mentioned above, the nanodisc technology combines the advantage of having the protein in a native-like environment, yet yielding samples that are as optically clear as when having the protein in detergent solution. In recent years, nanodiscs have successfully been used to incorporate and study a range of membrane proteins such as, for example, G protein-coupled receptors [35], rhodopsins [36], ABC transporters [27], FoF1-ATP synthase [37] and bacteriorhodopsin [38], opening up new possibilities to study these systems in a membrane environment. In the present study, we have shown that CytcO from bacterial sources can be reconstituted into nanodiscs. The improved optical properties of such CytcO-containing nanodiscs, compared to CytcO-containing liposomes is obvious from Fig. 2. The observed preservation of specific functional properties, which are unique to the presence of the membrane environment of the aa3 CytcO reinforces the usefulness of nanodiscs as membrane mimetics. Here, the rate of a PCET through the K-proton pathway, which ends near the protein-membrane interface was significantly accelerated. Furthermore, our study with the ba3 CytcO shows that a similar acceleration of proton transport through the K-pathway analogue is observed during the O2 reaction. In conclusion, the CytcO-nanodisc system displays the same experimental behavior as CytcO reconstituted in liposomes and it offers a unique system for optical studies in general and for investigation of effects of interaction partners, which react with the CytcO surfaces that are exposed to the solvent.
Fig. 3.

Proton-coupled electron transfer in aa3 CytcO. Electron transfer from heme a3 to heme a is detected as an absorbance increase at 598 nm after flash photolysis of the CO ligand from the two-electron reduced CytcO in 0.05% DDM, soybean liposomes or soybean nanodiscs (ND) at pH 8.9–9. All traces scaled to 1 lM reacting enzyme
Acknowledgments
These studies were supported by a grant from the Swedish Research Council (to P.B.) and, by grants HL 16101 (to R.B.G) and GM 33775 (to S.G.S.) from the National Institutes of Health. C.v.B. is supported by a fellowship from the Swiss National Science Foundation (SNF).
References
- 1.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Lee AG. Lipid-protein interactions. Biochem Soc Trans. 2011;39:761–766. doi: 10.1042/BST0390761. [DOI] [PubMed] [Google Scholar]
- 3.Hunte C, Richers S. Lipids and membrane protein structures. Curr Opin Struct Biol. 2008;18:406–411. doi: 10.1016/j.sbi.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim Biophys Acta Biomembr. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Bayburt TH, Grinkova YV, Sligar SG. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2002;2:853–856. [Google Scholar]
- 6.Bayburt TH, Sligar SG. Membrane protein assembly into nanodiscs. FEBS Lett. 2010;584:1721–1727. doi: 10.1016/j.febslet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods in Enzymology. 2009;Chapter 11:211–231. doi: 10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin L, Hiser C, Mulichak A, Garavito RM, Ferguson-Miller S. Identification of conserved lipid/detergent-binding sites in a high-resolution structure of the membrane protein cytochrome c oxidase. Proc Natl Acad Sci USA. 2006;103:16117–16122. doi: 10.1073/pnas.0606149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson-Ek M, Abramson J, Larsson G, Törnroth S, Brzezinski P, Iwata S. The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J Mol Biol. 2002;321:329–339. doi: 10.1016/s0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Qin L, Ferguson-Miller S. Crystallographic and online spectral evidence for role of conformational change and conserved water in cytochrome oxidase proton pump. Proc Natl Acad Sci USA. 2011;108:1284–1289. doi: 10.1073/pnas.1012846108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soulimane T, Buse G, Bourenkov GP, Bartunik HD, Huber R, Than ME. Structure and mechanism of the aberrant ba3-cytochrome c oxidase from Thermus thermophilus. EMBO J. 2000;19:1766–1776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luna VM, Chen Y, Fee JA, Stout CD. Crystallographic studies of Xe and Kr binding within the large internal cavity of cytochrome ba3 from Thermus thermophilus: Structural analysis and role of oxygen transport channels in the heme-Cu oxidases. Biochemistry. 2008;47:4657–4665. doi: 10.1021/bi800045y. [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Chen Y, Doukov T, Soltis SM, Stout CD, Fee JA. Combined microspectrophotometric and crystallographic examination of chemically reduced and X-ray radiation-reduced forms of cytochrome ba3 oxidase from Thermus thermophilus: structure of the reduced form of the enzyme. Biochemistry. 2009;48:820–826. doi: 10.1021/bi801759a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosler JP, Ferguson-Miller S, Mills DA. Energy transduction: proton transfer through the respiratory complexes. Annu Rev Biochem. 2006;75:165–187. doi: 10.1146/annurev.biochem.75.062003.101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belevich I, Verkhovsky MI. Molecular mechanism of proton translocation by cytochrome c oxidase. Antioxid Redox Signal. 2008;10:1–29. doi: 10.1089/ars.2007.1705. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson-Miller S, Babcock GT. Heme/copper terminal oxidases. Chem Rev. 1996;96:2889–2907. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 17.Wikström M, Verkhovsky MI. Mechanism and energetics of proton translocation by the respiratory heme-copper oxidases. Biochim Biophys Acta (BBA) – Bioenerg. 2007;1767:1200–1214. doi: 10.1016/j.bbabio.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Brzezinski P, Gennis RB. Cytochrome c oxidase: exciting progress and remaining mysteries. J Bioenerg Biomembr. 2008;40:521–531. doi: 10.1007/s10863-008-9181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaila VRI, Verkhovsky MI, Wikström M. Proton-coupled electron transfer in cytochrome oxidase. Chem Rev. 2010;110:7062–7081. doi: 10.1021/cr1002003. [DOI] [PubMed] [Google Scholar]
- 20.Chang HY, Hemp J, Chen Y, Fee JA, Gennis RB. The cytochrome ba3 oxygen reductase from Thermus thermophilus uses a single input channel for proton delivery to the active site and for proton pumping. Proc Natl Acad Sci USA. 2009;106:16169–16173. doi: 10.1073/pnas.0905264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smirnova I, Reimann J, Von Ballmoos C, Chang HY, Gennis RB, Fee JA, Brzezinski P, Ädelroth P. Functional role of Thr-312 and Thr-315 in the proton-transfer pathway in ba3 cytochrome c oxidase from Thermus thermophilus. Biochemistry. 2010;49:7033–7039. doi: 10.1021/bi100749p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Näsvik Öjemyr L, Lee HJ, Gennis RB, Brzezinski P. Functional interactions between membrane-bound transporters and membranes. Proc Natl Acad Sci USA. 2010;107:15763–15767. doi: 10.1073/pnas.1006109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin L, Mills DA, Buhrow L, Hiser C, Ferguson-Miller S. A conserved steroid binding site in cytochrome c oxidase. Biochemistry. 2008;47:9931–9933. doi: 10.1021/bi8013483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell DM, Gennis RB. Rapid purification of wildtype and mutant cytochrome c oxidase from Rhodobacter sphaeroides by Ni(2+)-NTA affinity chromatography. FEBS Lett. 1995;368:148–150. doi: 10.1016/0014-5793(95)00626-k. [DOI] [PubMed] [Google Scholar]
- 25.Von Ballmoos C, Gennis RB, Ädelroth P, Brzezinski P. Kinetic design of the respiratory oxidases. Proc Natl Acad Sci USA. 2011;108:11057–11062. doi: 10.1073/pnas.1104103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brändén M, Sigurdson H, Namslauer A, Gennis RB, Ädelroth P, Brzezinski P. On the role of the K-proton transfer pathway in cytochrome c oxidase. Proc Natl Acad Sci USA. 2001;98:5013–5018. doi: 10.1073/pnas.081088398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez FJD, Orelle C, Davidson AL. Functional reconstitution of an ABC transporter in nanodiscs for use in electron paramagnetic resonance spectroscopy. J Am Chem Soc. 2010;132:9513–9515. doi: 10.1021/ja104047c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayburt TH, Grinkova YV, Sligar SG. Assembly of single bacteriorhodopsin trimers in bilayer nanodiscs. Arch Biochem Biophys. 2006;450:215–222. doi: 10.1016/j.abb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Cvetkov TL, Prochaska LJ. Biophysical and biochemical characterization of reconstituted and purified Rhodobacter sphaeroides cytochrome c oxidase in phospholipid vesicles sheds insight into its functional oligomeric structure. Protein Expr Purif. 2007;56:189–196. doi: 10.1016/j.pep.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Verkhovsky MI, Jasaitis A, Wikström M. Ultrafast haem-haem electron transfer in cytochrome c oxidase. Biochim Biophys Acta – Bioenerg. 2001;1506:143–146. doi: 10.1016/s0005-2728(01)00220-1. [DOI] [PubMed] [Google Scholar]
- 31.Ädelroth P, Brzezinski P, Malmström BG. Internal electron transfer in cytochrome c oxidase from Rhodobacter sphaeroides. Biochemistry. 1995;34:2844–2849. doi: 10.1021/bi00009a014. [DOI] [PubMed] [Google Scholar]
- 32.Brändén M, Namslauer A, Hansson Ö, Aasa R, Brzezinski P. Water-hydroxide exchange reactions at the catalytic site of heme-copper oxidases. Biochemistry. 2003;42:13178–13184. doi: 10.1021/bi0347407. [DOI] [PubMed] [Google Scholar]
- 33.Siletsky SA, Belevich I, Jasaitis A, Konstantinov AA, Wikström M, Soulimane T, Verkhovsky MI. Time-resolved single-turnover of ba3 oxidase from Thermus thermophilus. Biochim Biophys Acta. 2007;1767:1383–1392. doi: 10.1016/j.bbabio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Smirnova IA, Zaslavsky D, Fee JA, Gennis RB, Brzezinski P. Electron and proton transfer in the ba3 oxidase from Thermus thermophilus. J Bioenerg Biomembr. 2008;1:7. doi: 10.1007/s10863-008-9157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leitz AJ, Bayburt TH, Barnakov AN, Springer BA, Sligar SG. Functional reconstitution of b2-adrenergic receptors utilizing self-assembling Nanodisc technology. BioTechniques. 2006;40:601–612. doi: 10.2144/000112169. [DOI] [PubMed] [Google Scholar]
- 36.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 37.Ishmukhametov R, Hornung T, Spetzler D, Frasch WD. Direct observation of stepped proteolipid ring rotation in E. coli Fo F1 -ATP synthase. EMBO Journal. 2010;29:3911–3923. doi: 10.1038/emboj.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayburt TH, Sligar SG. Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Sci. 2003;12:2476–2481. doi: 10.1110/ps.03267503. [DOI] [PMC free article] [PubMed] [Google Scholar]


