Abstract
We recently showed that Bendavia, a novel mitochondria-targeting peptide, reduced infarction and no-reflow across several experimental models. The purpose of this study was to determine the therapeutic timing and mechanism of action that underlie Bendavia’s cytoprotective property. In rabbits exposed to in vivo ischemia/reperfusion (30/180 min), Bendavia administered 20 min prior to reperfusion (0.05mg/kg/hr, i.v.) reduced myocardial infarct size by ~50% when administered for either 1 or 3 hours of reperfusion. However, when Bendavia perfusion began just 10 min after the onset of reperfusion, the protection against infarction and no–reflow was completely lost, indicating that the mechanism of protection is occurring early in reperfusion. Experiments in isolated mouse liver mitochondria found no discernible effect of Bendavia on blocking the permeability transition pore, and studies in isolated heart mitochondria showed no effect of Bendavia on respiratory rates. As Bendavia significantly lowered reactive oxygen species (ROS) levels in isolated heart mitochondria, the ROS-scavenging capacity of Bendavia was compared to well-known ROS scavengers using in vitro (cell-free) systems that enzymatically generate ROS. Across doses ranging from 1nM to 1mM, Bendavia showed no discernible ROS-scavenging properties, clearly differentiating itself from prototypical scavengers. In conclusion, Bendavia is a promising candidate to reduce cardiac injury when present at onset of reperfusion, but not after reperfusion has already commenced. Given that both infarction and no-reflow are related to increased cellular ROS, Bendavia’s protective mechanism of action likely involves reduced ROS generation (as opposed to augmented scavenging) by endothelial and myocyte mitochondria.
Keywords: mitochondria, reactive oxygen species, reperfusion, heart, peptide
Introduction
Early and successful reperfusion of the acutely ischemic myocardium is the most effective salvage against irreversible ischemic injury (infarction), but can also cause reperfusion injury. Among the cellular culprits that contribute to reperfusion injury, bursts of reactive oxygen species (ROS) occurring during early reperfusion have been known to occur since the late 1980’s1, 2. Transient production of ROS contributes to electromechanical dysfunction, infarction, and the coronary no-reflow phenomenon (reviewed in3, 4). Despite a clear understanding of the role of ROS in reperfusion injury, there are no adjuvant treatments currently administered in routine clinical practice to protect the heart against reperfusion injury.
The myocardial milieu is a hostile environment in early reperfusion. Increased ROS levels, aberrant cellular calcium handling, and restoration of cytosolic pH activate/open mitochondrial pores and decrease bioenergetic capacity5–9, often inducing necrotic and apoptotic cell death10. Scores of strategies have attempted to mitigate injury by targeting ROS and/or by blocking mitochondrial pores, but few have translated into clinical practice11–13. The National Institutes of Health has recently convened workshops seeking suggestions to improve the translation of basic science findings to the clinic. Several of these workshops have indicated the need for multi-center approaches in pre-clinical studies so that the efficacy of interventions can be tested across platforms14–16.
Accordingly, our groups have formed a consortium to investigate the pre-clinical efficacy of the mitochondria-targeting peptide, Bendavia. Bendavia (SS-31; MTP-131) is a tetra-peptide that has shown cytoprotective efficacy in a number of models (reviewed in17). Our groups previously found that post-ischemic administration of Bendavia reduced several indices of reperfusion injury across experimental models18, and Bendavia is now in clinical trials for acute coronary syndromes19. Administration of Bendavia before the onset of reperfusion reduced the extent of myocardial infarction and coronary no-reflow in intact animal models, and reduced cell death in isolated primary myocytes exposed to hypoxia/reoxygenation. The mechanism of protection involved reducing the extent of ROS-dependent cell death.
While these results are promising, several issues regarding Bendavia’s mechanism of action remain to be resolved. First, the timing of Bendavia administration that is required for cardioprotection is not clear. As an adjuvant therapy, the effective treatment window must be clearly defined to maximize benefits to patients, especially since the onset and etiology of myocardial injury varies as a function time20, 21. Incomplete understanding of the timing of myocyte death during/after ischemic injury remains a major hurdle for effective treatments, and is an aspect of cardioprotection that has been identified by experts in the field necessitating further study14, 16. Second, the protective mechanism(s) for Bendavia is still not fully known. The magnitude of protection provided by Bendavia is consistent with direct blockers of the mitochondrial permeability transition pore (PTP)18, 22–24, but whether Bendavia directly blocks PTP opening warrants in-depth investigation. Further, although Bendavia clearly reduces intracellular ROS levels in tissues exposed to oxidative challenge (reviewed in17), whether this is due to a ROS-scavenging effect or lowered ROS production is not known. This is an important issue to address regarding the mechanism of action, as previously tested ROS scavengers have shown equivocal results in clinical trials25, 26.
The present study represents a collaboration spanning 4 distinct research centers. Several experimental models of acute myocardial infarction were utilized to test the hypothesis that Bendavia’s effective treatment window is occurring early in the reperfusion period. The capacity of Bendavia to alter mitochondrial respiration, directly block the PTP, and scavenge ROS was also tested and compared to several positive controls.
Methods
All animal experiments were conducted with prior approval from the Institutional Animal Care and Use Committee at each institution (Good Samaritan Hospital, University of Southern California, QTest Labs, East Carolina University, and the University of Missouri at Columbia). Rabbit studies were conducted at the Heart Institute at the Good Samaritan Hospital, University of Southern California, and at QTest Labs in Columbus, Ohio. Guinea pig studies were done at East Carolina University, and studies using mouse mitochondria were done at the University of Missouri at Columbia. The animals used in these studies were maintained in accordance with the policies and guidelines of the Position of the American Heart Association on research animal use (American Heart Association, 1985) and the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (Department of Health, Education and Welfare Publication No. 85-230).
Rabbit Ischemia/Reperfusion and Coronary No-Reflow
Rabbit ischemia/reperfusion experiments were conducted in 2 study arms: 1. Administration of Bendavia during the ischemic period (studies conducted at QTest Labs), and 2. Administration of Bendavia after the onset of reperfusion (studies conducted at Good Samaritan Hospital).
For the administration of Bendavia during the ischemic period (Study Arm 1 above), anesthesia was induced intramuscularly with ketamine (35 mg/kg) and xylazine (5 mg/kg) in male New Zealand White rabbits. A catheter was placed in the ear vein and anesthesia was maintained by continuous infusion of propofol (approximately 60 mg/kg/hr). A solid-state catheter was inserted into the aortic root to measure heart rate and blood pressure at baseline and at 10 minutes of coronary artery occlusion. An additional catheter was placed into an ear vein to administer drug treatment. Body temperature was monitored and maintained within the physiological range using temperature-controlled water filled blankets and lamps. The thorax was opened and the heart suspended in a pericardial cradle. Sutures were placed around the left circumflex artery at a point between its origin and the base of the heart and around the left anterior descending artery or one of its branches in order to produce a risk zone comprising 50–60% of the left ventricle. The ends of the suture were threaded through a piece of tubing, forming a snare that was tightened to occlude the arteries. Following a stabilization period (~ 30 min), the rabbits were subjected to 30 minutes of coronary artery occlusion followed by 3 hours of reperfusion. At 10 minutes after the onset of ischemia, rabbits received an infusion of Bendavia (0.05 mg/kg/hr) or a similar volume of vehicle. Treatment was maintained for 60 minutes (n = 8) or for 180 minutes (n = 7). A separate group of rabbits received (prior to reperfusion) 25 mg/kg/hr of the permeability transition pore blocker cyclosporin-A (n = 6), following a treatment protocol previously shown to be cardioprotective in this model23, 24, 27. At the end of the reperfusion period, the ischemic risk area was assessed by re-tightening the coronary artery sutures and injecting Evans blue dye intravenously. The heart was removed and sliced, and the slices were incubated in triphenyltetrazolium chloride to delineate the areas of necrosis in the heart slices. Each slice was measured with a micrometer, photographed and scanned. Areas of the risk region and of necrosis were measured using Image J and expressed as a percentage of the total LV area in each slice. The sum of the areas of the slices was used to quantify the risk region and necrotic region in each heart. Risk and necrotic areas were expressed as a percentage of the area of the left ventricle below the occlusion site. Infarct size was expressed as a percentage of the risk region.
For the administration of Bendavia after reperfusion (Study Arm 2), the methods used for the rabbit model of acute myocardial infarction in the Kloner laboratory have been described previously18, 28. Briefly, ketamine (75 mg/kg) plus xylazine (10 mg/kg) anesthetized, open-chest male New Zealand White rabbits were used. Fluid-filled catheters were inserted into a carotid artery to measure hemodynamics and into the jugular veins to administer supplemental anesthesia (pentobarbital, ~ 50 mg/kg/hr) and to infuse the drug treatment. Body temperatures were monitored and maintained at approximately 38° C using a heating pad. The circumflex coronary artery or one of its major branches was isolated with a suture in order to produce a risk zone of approximately 30% of the left ventricle. The rabbits were subjected to 30 minutes of coronary artery occlusion (CAO) followed by 3 hours of reperfusion. After initiation of reperfusion, the rabbits were randomized to one of two groups: Group 1 received 0.05 mg/kg/hr of Bendavia, by intravenous infusion starting at 10 minutes after coronary artery reperfusion and continuing throughout reperfusion; Group 2 received an equivalent volume of saline starting at the same time point and continuing throughout reperfusion. The total volume infused was < 6 mLs. Heart rate and blood pressure were measured throughout the protocol.
At the end of the reperfusion period, 1 mL/kg of a 4% solution of thioflavin S was injected into the heart via a catheter placed into the left atrial appendage in order to define the region of no-reflow. Thioflavin S, a fluorescent green-yellow dye, stains endothelium, serves as a marker of perfusion, and is used as a standard marker for identifying zones of no-reflow. Viewed under ultraviolet light regions of the heart that are perfused fluoresce brightly while the no-reflow zone appears as a non-fluorescent, dark area. The coronary artery was then re-occluded and the ischemic risk region was delineated with 4 ml of a 50% solution of Unisperse blue dye (Ciba-Geigy, Hawthorne, NY) injected into the left atrium. KCl (12 mEq) was then injected into the left atrium, and the heart was excised, sliced and photographed. The heart slices were then incubated in triphenyltetrazolium chloride to delineate areas of necrosis. Measurements of risk zone, no-reflow zone and infarct size were calculated as previously described18, 28. Twenty rabbits were randomized to the study. One rabbit randomized to the vehicle group was excluded: the epicardial surface of the heart in this rabbit displayed pink blushing (rather than cyanosis) when examined at 10 minutes after coronary artery occlusion. The clamp occluding the artery had in all probability loosened allowing reperfusion. This heart had nearly no necrosis (< 2% of the left ventricle) when examined at the end of the protocol. Final data are reported in 10 rabbits in the Bendavia group and 9 rabbits in the vehicle group.
Guinea Pig Ischemia/Reperfusion
Isolated guinea pig hearts were exposed to ischemia/reperfusion using protocols well-established by the Brown Laboratory29–31. Male guinea pigs (n = 13 total) were anesthetized using a pentobarbital cocktail (35mg/kg; i.p. injection), and hearts were excised after the absence of animal reflexes. Hearts were retrograde perfused with modified Krebs-Henseleit buffer on a Langendorff apparatus (perfusion pressure 75mmHg), and instrumented for the simultaneous measurement of left ventricular function, volume-conducted electrocardiogram, and coronary flow. After a 20 minute stabilization period, hearts were exposed to 20 minutes of global ischemia followed by 2 hours of reperfusion. Hearts were randomly assigned to receive either no drug (control group, n = 7), or Bendavia (1nM, n = 6) beginning at the onset of reperfusion and continuing for the duration of the reperfusion period. At the end of reperfusion, hearts were dissected and stained for infarction using tetrazolium salt staining.
Mitochondrial Permeability Transition Pore Assays
Mouse liver mitochondria were isolated and assayed for PTP opening under energized and de-energized conditions using Dr. Baines’ established protocols32. Briefly, 0.25mg/mL mitochondria were placed in swelling buffer containing (in mM) 120 KCl, 10 Tris/HCl, and 5 KH2PO4 (pH 7.4), with (energized) or without (de-energized) 5 succinate (separate pilot experiments with glutamate/malate yielded similar results). Mitochondrial PTP opening was observed by a decrease in absorbance at 520nm after the addition of 25µM (energized conditions) or 250µM (de-energized conditions) CaCl2. The effects of Bendavia were compared to several positive controls known to influence PTP opening. Prior to calcium pulses, mitochondria were treated with 100µM Bendavia, 1mM of the radical scavenger mercaptopropionylglycine (MPG), 10µM of the superoxide dismutase mimetic manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP), or 10µM of the PTP blocker cyclosporin-A. Absorbance data were collected every 10 seconds, normalized to baseline absorbance (absorbance change from 520nm; ΔA520), and data are expressed as area above the curve. To confirm that PTP opening was evoked by the calcium pulse, and not by the experimental reagents, separate experiments were conducted using a water vehicle control instead of CaCl2. There was no discernible effect of any of the treatments on PTP opening in the absence of calcium (data not shown).
Isolated Mitochondria Experiments
In separate studies, rat heart mitochondria were isolated and rates of respiration and H2O2 production assessed using our established protocols22, 33. 200 µg of mitochondria were suspended in assay buffer containing (in mM): 110 K-MES, 35 KCl, 1 EGTA, 5 K2HPO4, 3 MgCl2, 5 mg/ml BSA, and 10 succinate (pH 7.4). State II (no ADP) and state III (2.5mM ADP) respiration (normalized to protein content) were assayed in a Oxygraph O2K high resolution respirometer (Oroboros Insruments, Austria). Rates of respiration were assessed with substrate only (reverse electron flow), or with 2 µM rotenone to block complex I (promoting forward electron flow). Mitochondrial H2O2 production was determined in assay buffer (no rotenone) containing 10µM Amplex Ultra Red (Invitrogen), 1U/mL horseradish peroxidase, and 10mM succinate. H2O2 fluorescence was converted to H2O2 concentration using a standard curve. Rates of respiration and H2O2 emission were tested in the presence or absence of 1nM Bendavia or 400U/mL catalase.
In Vitro Reactive Oxygen Species Scavenging Experiments
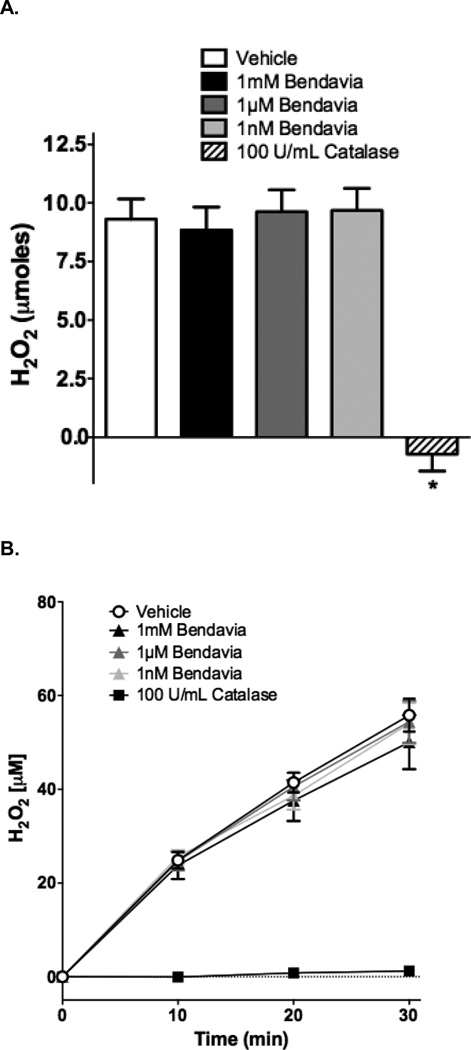
The ROS-scavenging capacity of Bendavia was determined with novel static and dynamic biochemical systems coupled with fluorometric measurements to measure ROS scavenging in cell-free systems. These enzymatic systems generate H2O2 and/or superoxide anion as reaction products34, 35. Because the overwhelming majority of ROS during conditions of oxidative stress are likely non-radical oxidants36, the effect of Bendavia on scavenging H2O2 in cell-free systems was examined first. For static H2O2 scavenging studies, 2mL potassium phosphate reaction buffer was placed into 12-well plates, and 100µM H2O2 was added for 10 minutes. Each well contained either Bendavia (final concentrations of 1nM, 1µM, and 1mM), or the positive control H2O2-scavenger catalase (100U/mL final concentration). H2O2 levels were detected using 10µM Amplex Ultra Red (Invitrogen) and 1U/mL horseradish peroxidase (485 nm excitation/590nm emission wavelengths). Raw fluorescence intensities were converted to nmol of H2O2 using a standard curve.
The H2O2-scavenging capacity of Bendavia was also assessed using a glucose-glucose oxidase reaction system that dynamically generates H2O2. 10mM glucose was dissolved in reaction buffer, and 100mU/mL of glucose oxidase were added to the wells to initiate the ROS-generating reaction. Each well contained either Bendavia (concentrations of 1nM, 1µM, and 1mM), or 100U/mL catalase as a positive control. The reaction was allowed to run for 30 min, and at minutes 10, 20, and 30 samples were taken from the wells and incubated with AmplexRed/horseradish peroxidase. In separate experiments, inclusion of the superoxide dismutase (SOD) mimetic TEMPO (2,2,6,6-tetramethylpyperidine-1-oxyl; 1mM final concentration) or SOD (25U/mL final concentration) in the reaction had no effect on the generation of H2O2, confirming that the dominant ROS produced by this reaction couple is H2O234, 35.
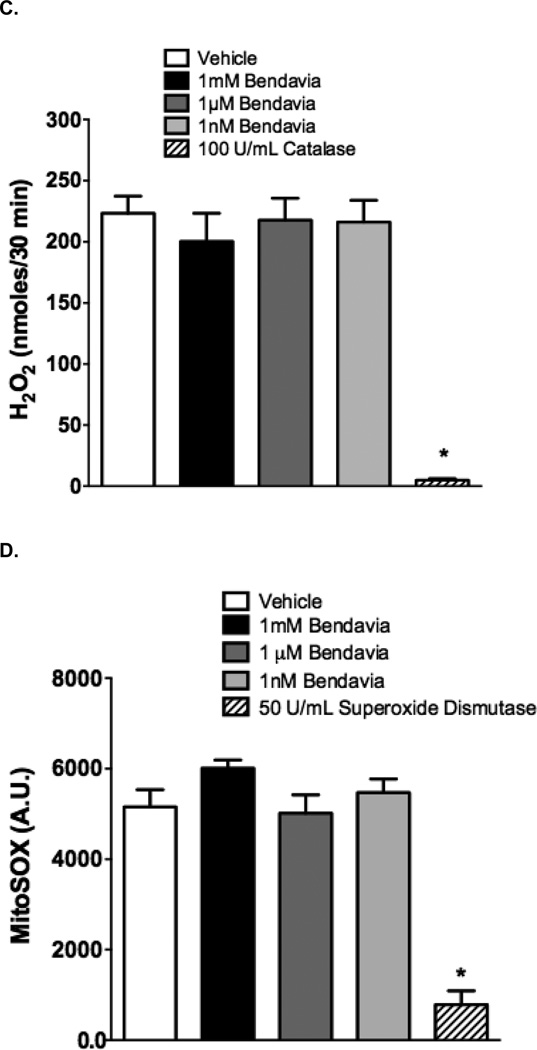
Finally, the ability of Bendavia to scavenge superoxide anion was determined using a xanthine/xanthine oxidase system. Superoxide is an early product of the incomplete reduction of oxygen to water, and although it is likely short-lived in living systems36, it is believed to be a major contributor to reperfusion injury10. This reaction generates both superoxide and H2O237, and we isolated the superoxide component by scavenging H2O2 throughout with catalase. For dynamic superoxide scavenging studies, 100mU/mL of xanthine oxidase were added to potassium phosphate reaction buffer containing 50U/mL catalase and 5µM of the superoxide sensor mitoSOX. Each well contained Bendavia at concentrations of 0mM (control), 1nM, 1µM, and 1mM, or 50U/mL of the positive control superoxide dismutase. After a 6-minute period to obtain background fluorescence, 100µM of xanthine was added to initiate the reaction for 10 minutes, with the average peak fluorescence taken over the last 4 minutes. MitoSOX fluorescence was obtained by determining the peak fluorescence minus background, and data are presented as arbitrary fluorescence units.
Statistical Analysis
In studies with two groups (Rabbit Study Arm 2 and guinea pig infarct size study), infarct sizes, areas at risk and areas of no-reflow were compared using Student’s t test. In rabbit studies where Bendavia duration was altered (Rabbit Study Arm 1, data in Figure 1), as well as isolated mitochondria studies, data were analyzed with an ANOVA, followed by Tukey’s multiple comparison post-hoc test. Changes in hemodynamic variables over time were analyzed by analysis of variance (repeated measures). Analysis of covariance (ANCOVA) was used to test for a group effect on the regression model of the no-reflow zone versus the risk zone. Data are expressed as mean ± SEM throughout the manuscript, and significance was declared when P<0.05.
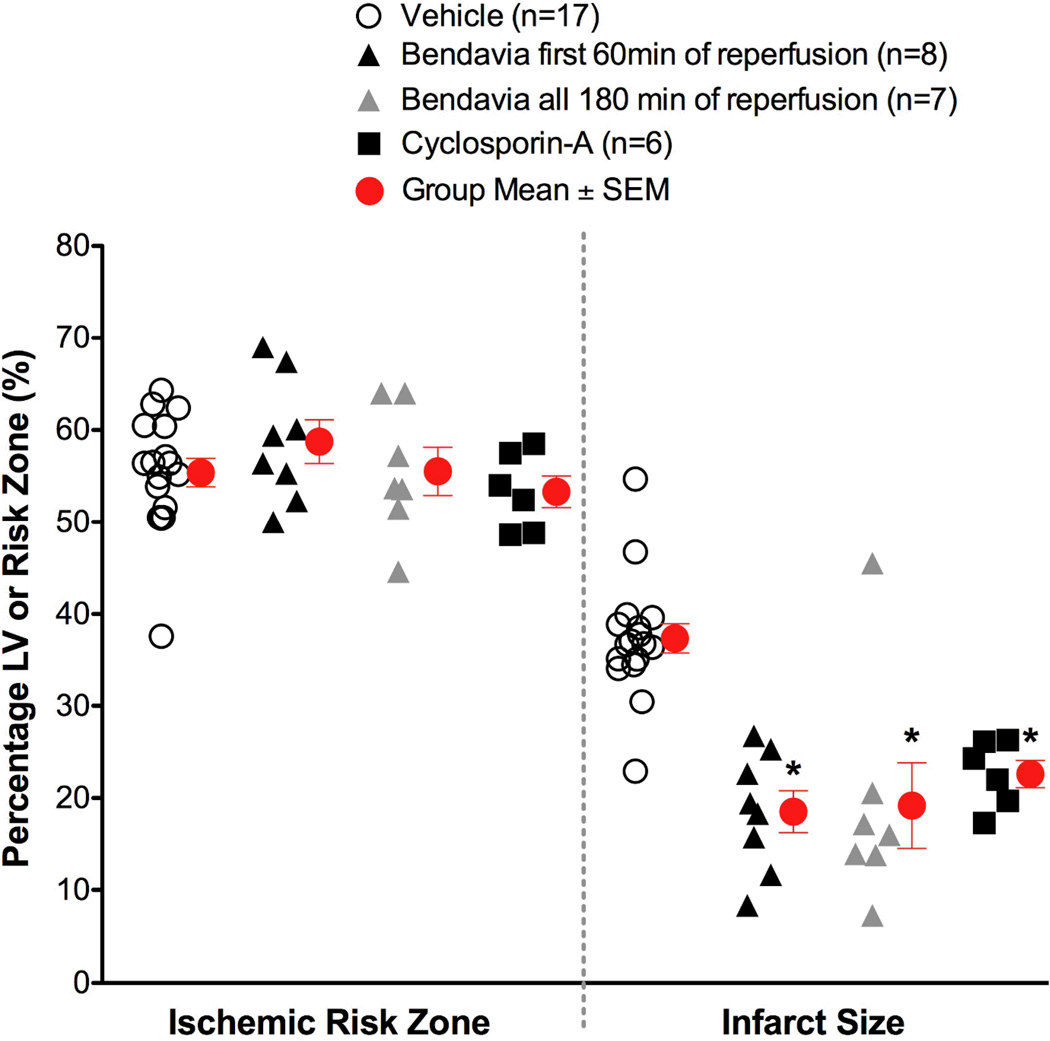
Figure 1.
Scatter plots for ischemic risk zone (expressed as a % of the LV) and infarct size (expressed as a % of the risk zone) from rabbits exposed to in vivo ischemia/reperfusion (30 min/3h, respectively). Bendavia infusion (0.05 mg/kg/hr) via a marginal ear vein began 20 minutes prior to reperfusion, and continued for either the first hour of reperfusion or all 3 hours of reperfusion. *, P<0.05 compared to Vehicle (ANOVA).
Results
Rabbit Ischemia/Reperfusion
There were no differences in the size of the zones at risk among experimental groups. Post-ischemic administration of Bendavia significantly reduced infarct size in rabbits (Figure 1). Bendavia treatment protected the heart equally well whether Bendavia was administered for the entire 3-hour reperfusion period, or just for the first hour of reperfusion (P<0.05 for both treatments versus control). Bendavia was equally effective as the permeability transition pore blocker, cyclosporin-A.
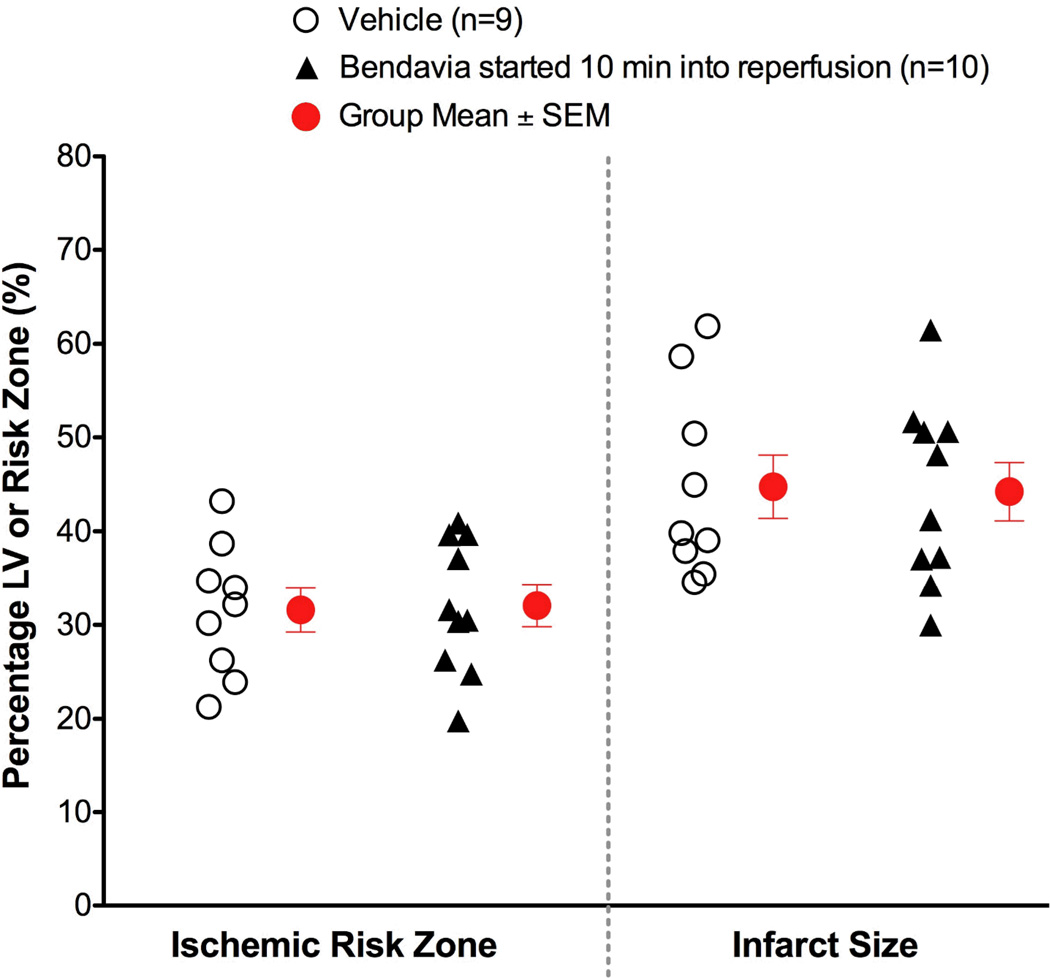
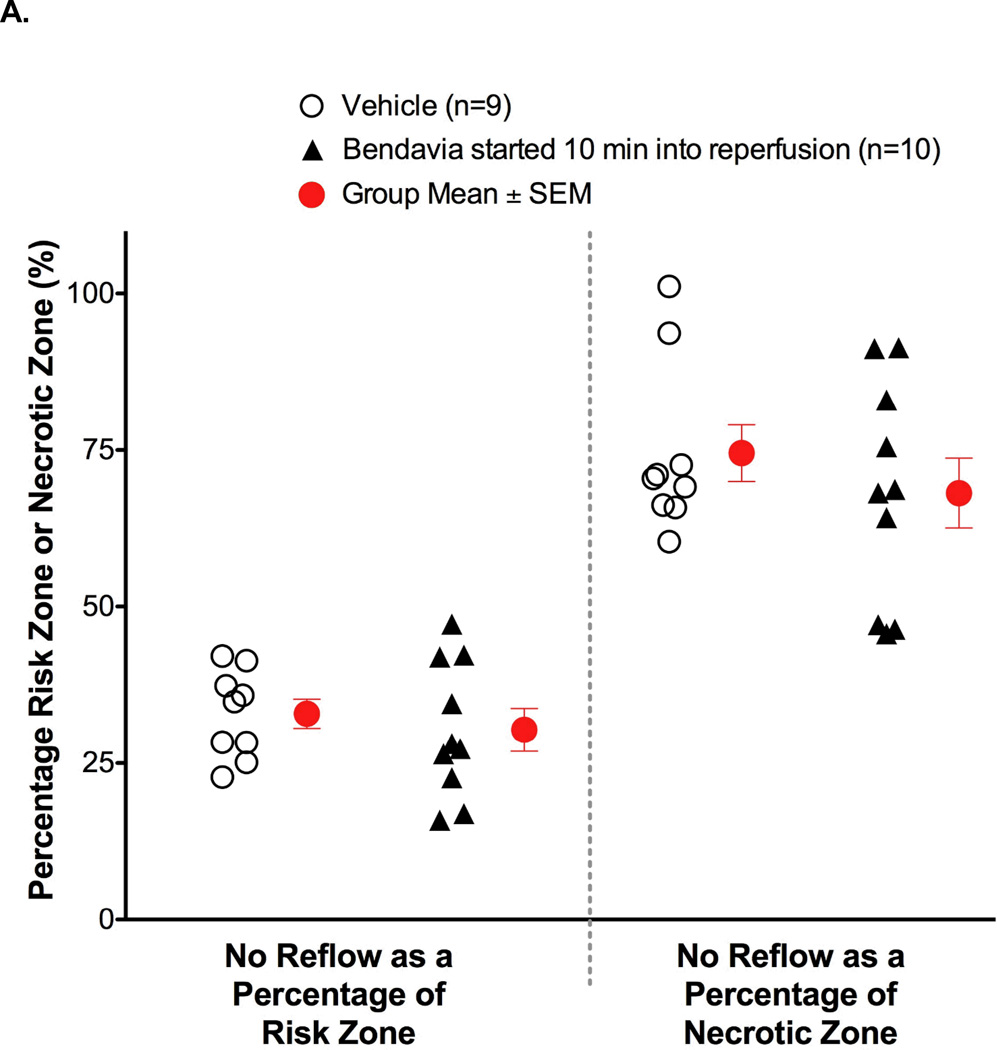
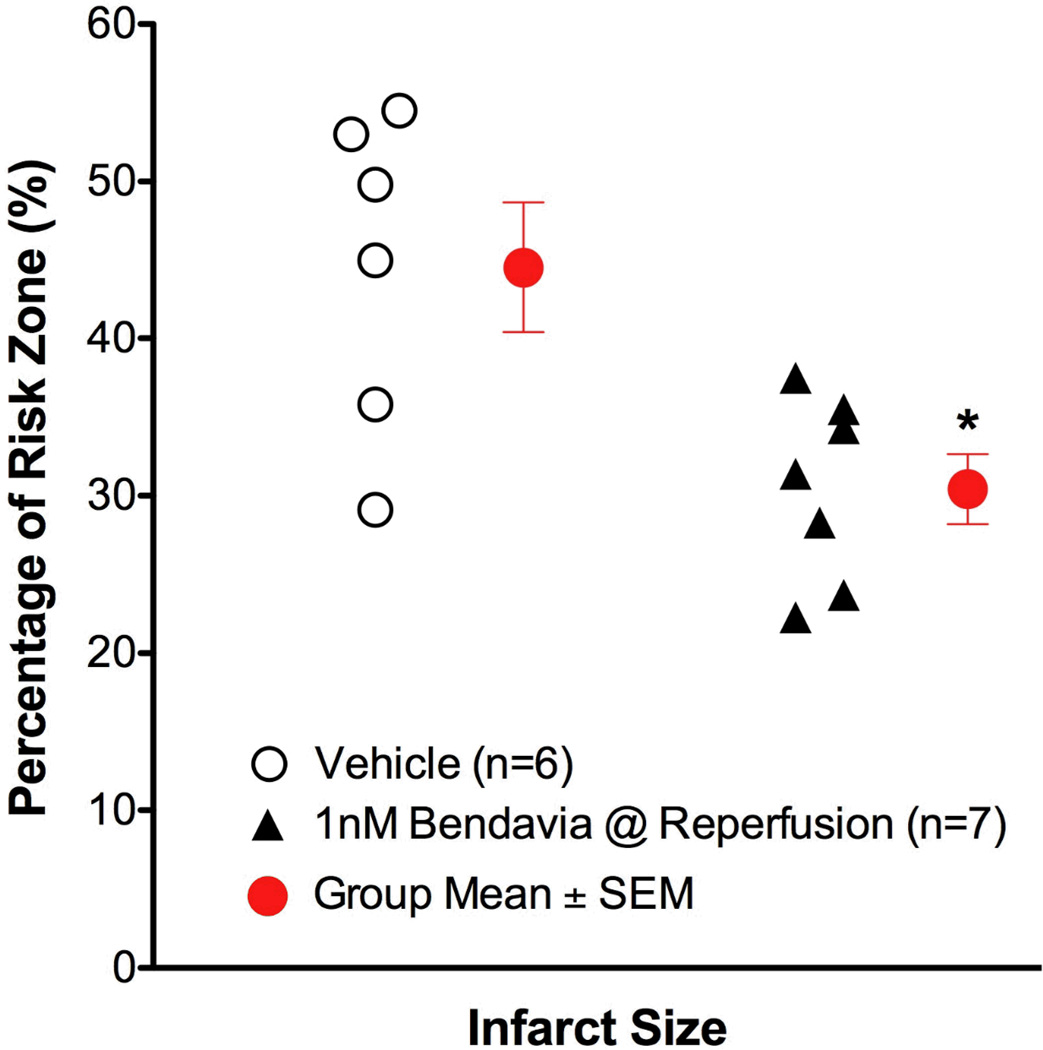
When Bendavia treatment was administered beginning 10 minutes into reperfusion, the cardioprotection was no longer observed. Infarct size was not different between placebo and Bendavia-treated rabbits (Figure 2). Further, the no-reflow defect expressed either as a fraction of the risk zone or as a fraction of the necrotic zone, was of similar size between groups. There was no beneficial effect on the reduction of anatomic no-reflow when Bendavia treatment began 10 minutes after the onset of reperfusion (Figure 3).
Figure 2.
Scatter plots for ischemic risk zone (expressed as a % of the LV) and infarct size (expressed as a % of the risk zone) from rabbits exposed to in vivo ischemia/reperfusion (30 min/3h, respectively). Bendavia infusion (0.05 mg/kg/hr) began 10 minutes after the onset of reperfusion. There were no differences in infarct size or area at risk between control and Bendavia-treated groups.
Figure 3.
Anatomic no-reflow in hearts given 0.05 mg/kg/hr Bendavia beginning 10 minutes after the onset of reperfusion. (A). No-reflow, expressed as either a percentage of the risk zone, or a percentage of the necrotic zone, was no different between control and Bendavia-treated rabbits (P>0.05 for both comparisons). (B). Relationship between no-reflow zone and the ischemic risk zone. There was no significant group effect when Bendavia administration began 10 minutes after the onset of reperfusion (P > 0.05, ANCOVA).
Bendavia treatment had no effect on heart rate or mean arterial blood pressure in either of the rabbit protocols. Similarly, there were no significant group differences in systolic or diastolic blood pressure (data not shown).
Guinea Pig Ischemia/Reperfusion
Administration of Bendavia at the onset of reperfusion significantly reduced the extent of infarction (P<0.05; Figure 4). There were no differences in the extent of left ventricular developed pressure recovery, ventricular arrhythmia, rates of contraction or relaxation, or coronary flow in any of the treatment groups (data not shown). The extent of protection in these studies is similar to the cardioprotection we previously observed in guinea pig hearts18, with one key difference. The anesthetic used in these studies was pentobarbital, whereas before we used a ketamine/xylazine cocktail. Given that the anesthetic regimen can significantly influence infarct size in cardioprotection studies31, 38–40, the consistency across these studies with Bendavia treatment is encouraging.
Figure 4.
Infarct sizes in isolated guinea pig hearts exposed to 20 min global ischemia and 2 hours of reperfusion. Bendavia treatment (1nM, dissolved in Krebs-Henseleit buffer) began at the onset of reperfusion and continued throughout the protocol. Since this is a global ischemia model, the risk zone is by definition 100% of the LV. *, P < 0.05 versus control (Student’s t-test).
Isolated Mitochondria Experiments
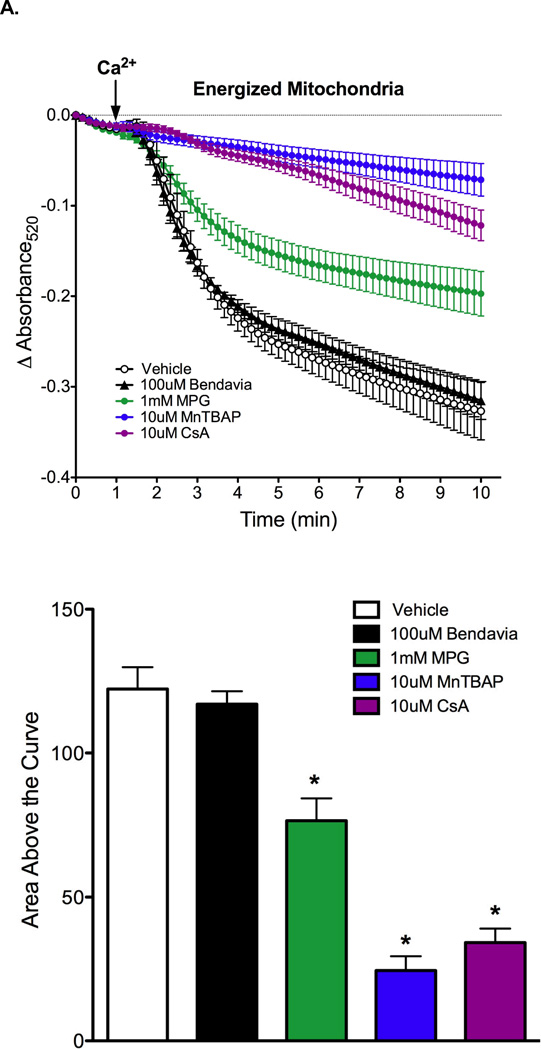
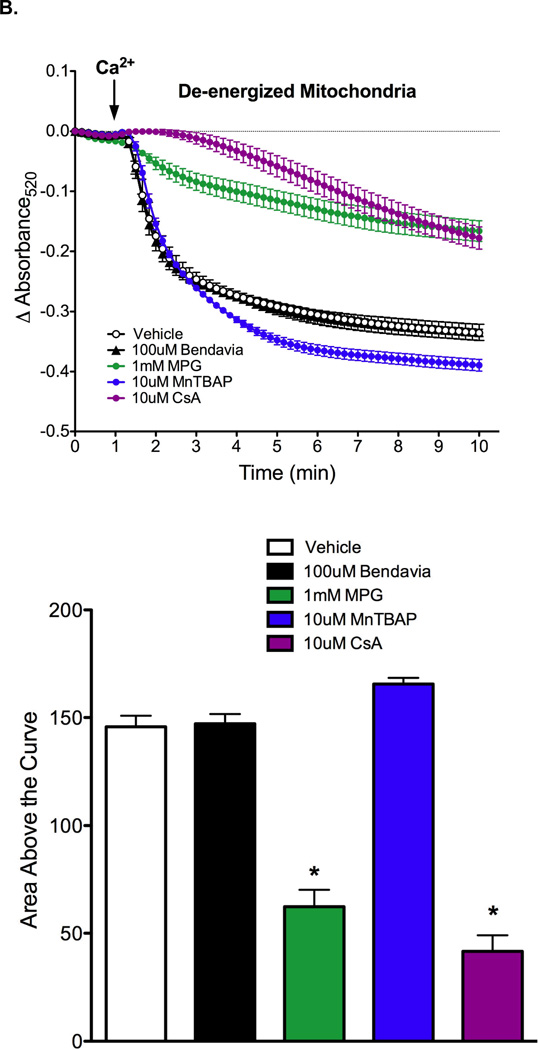
Results on the effect of Bendavia on PTP opening in energized and de-energized mitochondria are presented in Figures 5A and 5B, respectively. PTP opening was evoked by calcium pulses, and is reflected by a decrease in absorbance at 520nm. Regardless of the energetic status of the mitochondria, Bendavia had no effect on PTP opening, in clear contrast to the radical scavenger MPG, the manganese superoxide dismutase mimetic MnTBAP, and the direct PTP blocker cyclosporin-A (CsA). MnTBAP delayed PTP opening in energized, but not de-energized mitochondria.
Figure 5.
Lack of influence of Bendavia on permeability transition pore (PTP) opening in isolated mitochondria subjected to a calcium pulse. A. Mitochondria were energized with glutamate/malate. The positive controls MPG (a radical scavenger), MnTBAP (a manganese superoxide dismutase mimetic), and CsA (cyclosporin-A, a direct PTP blocker) were utilized for comparison with Bendavia. B. Experiments were repeated under de-energized conditions (no glutamate/malate). PTP opening is depicted by mitochondrial swelling, which is reflected by a decrease in the absorbance of light at 520nm. *, P<0.05 (ANOVA).
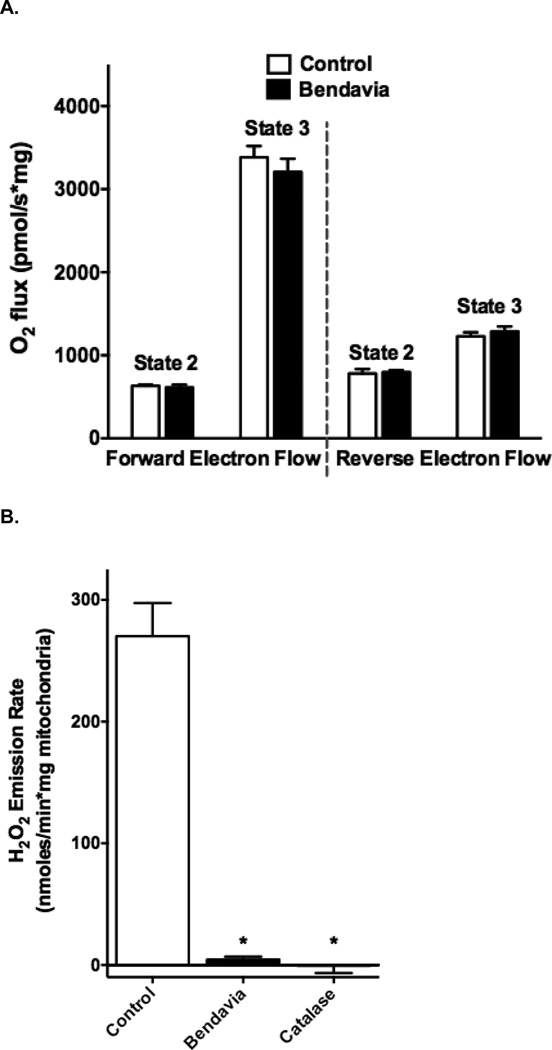
Mitochondrial respiration and H2O2 emission data are presented in Figure 6. Rates of state 2 and 3 respiration in heart mitochondria were not altered in the presence of Bendavia (Figure 6A) whether mitochondria were respiring in the presence of rotenone (forward electron flow) or not (reverse electron flow). Mitochondrial H2O2 emission was significantly reduced with both Bendavia and catalase (Figure 6B).
Figure 6.
Effects of Bendavia on heart mitochondrial respiration and H2O2 emission. A. State 2 and 3 respiration in the presence (forward electron flow) or absence (reverse electron flow) of rotenone. B. H2O2 emission in mitochondria was significantly decreased with Bendavia or catalase. *, P<0.05 (ANOVA).
In Vitro Reactive Oxygen Species Scavenging Capacity
Our studies examining the scavenging capacity of Bendavia are presented in Figure 7. The protective effects of Bendavia have been attributed to mitochondrial targeted ROS scavenging by the peptide17, 33. To directly test this hypothesis, ROS production was measured in cell-free ROS-generating systems in the presence or absense of Bendavia. In both a static H2O2 incubation and a dynamic H2O2 generating system, Bendavia concentrations ranging from 1 nM to 1 mM had no effect on H2O2 levels, in clear contrast to the H2O2 reductions evoked by the positive control catalase (Figure 7A–C). Likewise, Bendavia over the same concentration range failed to alter the rate of superoxide generation in a cell-free superoxide generating system, again in direct contrast to a positive control (superoxide dismutase; Figure 7D).
Figure 7.
Lack of ROS-scavenging capacity of Bendavia in in vitro, cell-free systems. The ability of Bendavia to scavenge ROS (specifically H2O2) was tested with either a static H2O2 incubation (A), or a dynamic glucose-glucose oxidase reaction, which generates H2O2 as a reaction product (B). The net amount of H2O2 produced under each condition in B was calculated after 30 minutes of the glucose-glucose oxidase protocol, and are presented in (C). H2O2 levels were assessed by Amplex Red fluorescence. Bendavia did not influence H2O2 levels across a wide range of concentrations tested, in clear contrast to the positive control catalase. D. Additionally, the superoxide (O2•−) scavenging capacity of Bendaiva was tested using a xanthine-xanthine oxidase reaction. Bendavia also showed no observable scavenging capacity for O2•−, in contrast to the positive control superoxide dismutase (SOD). *, P<0.05 (ANOVA).
Discussion
In this study, our groups tested the hypothesis that Bendavia, a novel mitochondria-targeting peptide, would reduce reperfusion injury in a specific time window. To the best of our knowledge, several aspects of this study represent novel findings. First, we show for the first time that administration of Bendavia is equally effective when given for either 1 hour or 3 hours of reperfusion. Second, treatment with Bendavia beginning just 10 minutes into the reperfusion period had absolutely no effect on the extent of myocardial infarction or the development of coronary no-reflow, indicating that the effective window for treatment occurs early in reperfusion. Third, Bendavia had no direct effect on blocking the permeability transition pore in either energized or de-energized mitochondria. Fourth, the protection elicited by Bendavia is independent of a direct ROS-scavenging mechanism. Taken together, this study enhances the understanding of how and when this cell-permeable, mitochondria-targeting peptide protects heart tissue from reperfusion injury.
Early Reperfusion: A Case for Targeting Mitochondria
While prompt reperfusion remains the most effective therapy for acute myocardial infarction, reperfusion injury is a significant problem and can influence both short- and long-term survival after an infarction41. Reperfusion injury is multi-factorial at the cellular level, and varies as a function of time. Accordingly, therapies designed to reduce the burden of reperfusion injury must be given at the proper time to be effective. In general, early reperfusion is characterized by a burst in cellular (mitochondrial) ROS, calcium overload, and a restoration of intracellular pH, all of which promote the opening of mitochondrial pores (such as the permeability transition pore, reviewed in21, 42, 43). In the later phases of reperfusion injury, apoptotic cell death predominates, and the infiltration of inflammatory cells begins to remodel the scar in the weeks (and perhaps months) after the insult.
Bursts of reactive oxygen injury were first described in the setting of acute myocardial infarction in the late 1980’s. Both Bolli et al. and Garlick et al. used free radical spin traps testing the coronary effluent and observed a drastic (and transient) increase in ROS within the first 20 minutes of reperfusion, peaking within the first 5 minutes1, 2, 44. Subsequent studies have confirmed that mitochondria are a significant source of these ROS bursts, with the majority of ROS likely existing as non-radical oxidants (since free radicals are notoriously short-lived and rapidly dismutated to non-radical oxidant species)36. Early attempts to reduce the mitochondrial ROS bursts included superoxide mimetics and glutathione precursors such as N-acetylcytsteine. Although there were early promising results in animal models45–47, these approaches are plagued with problems including permeability issues, and side-effects secondary to very high (millimolar) concentrations of the compounds48–52. More recent strategies have employed anti-oxidants conjugated to mitochondria-targeting lipophilic cations (reviewed in53). These approaches appear promising, but require polarized mitochondria for effective targeting, which means that the compound may only be helpful to mitochondria that are adequately recovering from ischemia. Furthermore, as these compounds may depolarize mitochondria in high doses, these approaches may be self-limiting, with a narrow therapeutic window17.
Cardioprotection with the Mitochondria-Targeting Peptide Bendavia
The reduction in myocardial infarct size observed herein is consistent with previous studies using Bendavia (SS-31; MTP-131)18, 22, 54. Central to the development of Bendavia into a clinical therapy is identification of the mechanism of action. A wide variety of models have previously suggested that Bendavia reduces intracellular ROS levels, most commonly assessed by fluorescence monitoring of H2O2 with fluorophores (summarized in17, 55, 56). Our data in isolated heart mitochondria (Figure 6) also show a clear effect in keeping ROS emission low, and are consistent with previous studies using mouse liver mitochondria57. These studies are promising, yet the findings mostly represent lowered ROS ‘levels’. As ROS levels represent a balance between production and scavenging, previous studies have not typically differentiated between improved ROS-scavenging versus lower overall ROS-production with Bendavia. Herein, a novel series of in vitro, cell-free reaction systems reveal that Bendavia does not appear to function as a direct ROS scavenger. These findings suggest that Bendavia is targeting mitochondria and reducing ROS production by a mechanism distinct from electron scavenging, most likely through an as yet undefined interaction with the electron transport system. Very recent studies suggest that Bendavia may be targeting the mitochondrial phospholipid cardiolipin58. The activity of each of the mitochondrial proteins associated with electron transport depends cardiolipin59, and Bendavia’s protective effect may involve optimizing cardiolipin microdomains to reduce electron leak from the inner membrane (recently reviewed in60).
Our results in this study are consistent with the idea that strategies aimed at reducing the ROS burden will be most effective early in reperfusion. In a recent study, Kloner, Brown and colleagues showed that ROS-dependent cell death in primary cardiac myocytes exposed to hypoxia/reoxygenation was significantly blunted with Bendavia, resulting in substantially higher cell survival during early reperfusion (cell death in Bendavia-treated cells was similar to untreated cells during the hypoxia period)18. The data presented herein underscore the importance of therapeutic timing in early reperfusion. In intact rabbits exposed to ischemia/reperfusion, our groups previously observed that Bendavia was effective at reducing infarction and the extent of coronary no-reflow if started in the minutes prior to reperfusion18, but not if started 10 minutes into reperfusion (current study). These findings are consistent with other models of cardioprotection such as activation of the RISK pathway61 and “ischemic post-conditioning”62, which also lose their effectiveness if not begun immediately at reperfusion. While we cannot completely rule out damage prevented during ischemia that is attenuated with Bendavia, we previously found that Bendavia was equally effective whether given 10 minutes into ischemia or 1 minute prior to reperfusion18. These data suggest that the early reperfusion window appears to be the crucial therapeutic window for cardioprotection with Bendavia.
Cardioprotection from Bendavia: Indirectly Keeping Mitochondrial Pores Closed
During this early reperfusion window, mitochondria often become overwhelmed by ROS bursts, and display collapses/oscillations in mitochondrial membrane potential (ΔΨm). The loss of ΔΨm hampers the ability of mitochondria to maintain ATP production, and can lead to the onset of infarction, pump dysfunction, and arrhythmia (recently reviewed in7). Attempts to maintain ΔΨm often focus on keeping specific mitochondrial pores/channels closed, with the mitochondrial permeability transition pore (PTP) being the most common target examined (including in recent human clinical trials63). Consistent with the idea of maintaining bioenergetic integrity, Bendavia leads to sustainment of ΔΨm in cardiomyocytes during ischemia/reperfusion18, suggesting that Bendavia might act as a direct PTP blocker. Additional support for this notion came from studies where Bendavia was quantitatively as cardioprotective as strategies (such as cyclosporin-A or NIM811) that directly block the mitochondrial PTP (Figure 1 and18, 22).
The findings of the present study significantly extend the knowledge about Bendavia and the PTP, showing that the ability of Bendavia to sustain ΔΨm is probably indirect. Since mitochondria in the reperfused heart display very heterogeneous energetics (i.e. mitochondria can be polarized, depolarized, or oscillating between energetic states64, 65), it is imperative to investigate the effects of mitochondria-targeting compounds across mitochondrial energetic states. In this study, Bendavia had no direct PTP-blocking effects in isolated mitochondria regardless of the energetic status, in clear contrast to cyclosporin-A and well-known ROS scavengers. Since ROS production is known to promote PTP opening5, 6, it is tempting to speculate that Bendavia promotes tissue salvage/viability by lowering ROS production and indirectly keeping the PTP closed. We can also rule out mitochondrial uncoupling as a protective mechanism with Bendavia, as there was no effect of Bendavia on the rate of respiration regardless of energetic state (Figure 6).
In general, the magnitude of cardioprotection provided by Bendavia is proportional to the extent of injury. For example, in studies where the zone-at-risk comprises >50% of the left ventricle, the magnitude of protection by Bendavia is highest. The rabbit data in Figure 1 (zone at risk was >50% of the LV), and the guinea pig data in both Figure 5 and Kloner et al.18 (where 100% of the LV was ischemic) showed a 40–50% reduction in infarct size with Bendavia. In previous sheep and rabbit models18 where the risk zone comprised a smaller portion of the left ventricle (25–30% of the LV), the extent of protection with Bendavia was smaller, averaging a 10–20% reduction. These findings are consistent with a recently published clinical trial63, where cardioprotection with cyclosporin was the most prominent in patients with the largest areas-at-risk (also noted by Downey and Cohen12).
Several limitations to our study/interpretations are worth noting. First, we found that Bendavia did not block the PTP in isolated mitochondria, but these studies were performed in liver mitochondria. While the data are consistent with our previous work in heart mitochondria22, we did not investigate PTP opening in heart mitochondria herein. Second, we have shown that ΔΨm is better maintained in cells during hypoxia/reoxygenation with Bendavia18. We cannot rule out that Bendavia may have PTP-blocking effects in intact cells by interacting with endogenous regulators of PTP in isolated cells (which may be washed out in isolated mitochondrial experiments).
Another limitation in this study is that we did not determine the efficacy of Bendavia alongside current standard-of-care therapies for acute coronary syndromes patients. Specifically, very recent studies suggest that platelet inhibitors, which patients would normally receive, can themselves confer cardioprotection by activating protective signaling cascades66. Future studies determining if there are additive effects of Bendavia alongside platelet inhibitors will be an important advancement. Furthermore, as the rabbit experiments in Figures 1 and 2 were conducted at different academic institutions, the area at risk varied between models. This is likely due to different locations of coronary occlusion, and/or different strains of rabbit used across groups. Finally, the long-term efficacy of Bendavia in the context of ischemia/reperfusion injury must be determined. Given that chronic Bendavia treatment shows efficacy in preserving cardiac function in models of heart failure67, 68, the long-term benefit of post-ischemic Bendavia treatment is of significant interest.
In conclusion, our groups have taken a consortium approach to understand how and when this mitochondria-targeting peptide protects the heart. This study provides novel evidence that Bendavia remains an attractive candidate for the reduction of cardiac reperfusion injury if administered early in reperfusion, and that the mechanism of action appears to be distinct from direct PTP block, mitochondrial uncoupling, or ROS scavenging.
Acknowledgements
None.
Grants
This work was supported in part by the American Heart Association (Pre-Doctoral Fellowship to CR Frasier and DA Brown), NIH grant DK096906 (to PD Neufer), and Stealth Peptides.
Disclosures
These studies received partial funding from Stealth Peptides. DA Brown and PD Neufer have served as consultants for Stealth Peptides.
References
- 1.Bolli R, Jeroudi MO, Patel BS, DuBose CM, Lai EK, Roberts R, McCay PB. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:4695–4699. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garlick PB, Davies MJ, Hearse DJ, Slater TF. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circulation research. 1987;61:757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz BG, Kloner RA. Coronary no reflow. Journal of molecular and cellular cardiology. 2012;52:873–882. doi: 10.1016/j.yjmcc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Yellon DM, Dana A. The preconditioning phenomenon: A tool for the scientist or a clinical reality? Circulation research. 2000;87:543–550. doi: 10.1161/01.res.87.7.543. [DOI] [PubMed] [Google Scholar]
- 5.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovascular research. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 6.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic research in cardiology. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DA, O'Rourke B. Cardiac mitochondria and arrhythmias. Cardiovascular research. 2010;88:241–249. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters AM, Porter GA, Jr, Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circulation research. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: The holy grail of cardioprotection. Basic research in cardiology. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 10.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiological reviews. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludman AJ, Yellon DM, Hausenloy DJ. Cardiac preconditioning for ischaemia: Lost in translation. Dis Model Mech. 2010;3:35–38. doi: 10.1242/dmm.003855. [DOI] [PubMed] [Google Scholar]
- 12.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G. Cardioprotection: Chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 14.Kloner RA, Schwartz Longacre L. State of the science of cardioprotection: Challenges and opportunities--proceedings of the 2010 nhlbi workshop on cardioprotection. Journal of cardiovascular pharmacology and therapeutics. 2011;16:223–232. doi: 10.1177/1074248411402501. [DOI] [PubMed] [Google Scholar]
- 15.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: The need for translation into clinical therapy. Circulation research. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM. New horizons in cardioprotection: Recommendations from the 2010 national heart, lung, and blood institute workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxidants & redox signaling. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 18.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, 3rd, Gorman JH, Sloan RC, Frasier CR, Watson CA, Bostian PA, Kypson AP, Brown DA. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective peptide. J Am Heart Assoc. 2012;1:e001644. doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakrabarti AK, Feeney K, Abueg C, Brown DA, Czyz E, Tendera M, Janosi A, Giugliano RP, Kloner RA, Weaver WD, Bode C, Godlewski J, Merkely B, Gibson CM. Rationale and design of the embrace stemi study: A phase 2a, randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of intravenous bendavia on reperfusion injury in patients treated with standard therapy including primary percutaneous coronary intervention and stenting for st-segment elevation myocardial infarction. American heart journal. 2013;165:509–514. e507. doi: 10.1016/j.ahj.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatric cardiology. 2011;32:258–262. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- 21.Baines CP. How and when do myocytes die during ischemia and reperfusion: The late phase. Journal of cardiovascular pharmacology and therapeutics. 2011;16:239–243. doi: 10.1177/1074248411407769. [DOI] [PubMed] [Google Scholar]
- 22.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, Brown DA. Mitochondrial permeability transition in the diabetic heart: Contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. Journal of molecular and cellular cardiology. 2012;52:1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial stat3 and its role in myocardial ischemia/reperfusion. Basic research in cardiology. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skyschally A, Schulz R, Heusch G. Cyclosporine a at reperfusion reduces infarct size in pigs. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2010;24:85–87. doi: 10.1007/s10557-010-6219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N, et al. Recombinant human superoxide dismutase (h-sod) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- 26.Tsujita K, Shimomura H, Kawano H, Hokamaki J, Fukuda M, Yamashita T, Hida S, Nakamura Y, Nagayoshi Y, Sakamoto T, Yoshimura M, Arai H, Ogawa H. Effects of edaravone on reperfusion injury in patients with acute myocardial infarction. The American journal of cardiology. 2004;94:481–484. doi: 10.1016/j.amjcard.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Leshnower BG, Kanemoto S, Matsubara M, Sakamoto H, Hinmon R, Gorman JH, 3rd, Gorman RC. Cyclosporine preserves mitochondrial morphology after myocardial ischemia/reperfusion independent of calcineurin inhibition. The Annals of thoracic surgery. 2008;86:1286–1292. doi: 10.1016/j.athoracsur.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hale SL, Kloner RA. Cardioprotection with adenosine-regulating agent, gp531: Effects on no-reflow, infarct size, and blood flow following ischemia/ reperfusion in the rabbit. Journal of cardiovascular pharmacology and therapeutics. 2010;15:60–67. doi: 10.1177/1074248409357742. [DOI] [PubMed] [Google Scholar]
- 29.Frasier CR, Sloan RC, Bostian PA, Gonzon MD, Kurowicki J, Lopresto SJ, Anderson EJ, Brown DA. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol. 2011;111:1751–1759. doi: 10.1152/japplphysiol.01214.2010. [DOI] [PubMed] [Google Scholar]
- 30.Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O'Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. Journal of molecular and cellular cardiology. 2010;48:673–679. doi: 10.1016/j.yjmcc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan RC, Rosenbaum M, O'Rourke D, Oppelt K, Frasier CR, Waston CA, Allan AG, Brown DA. High doses of ketamine-xylazine anesthesia reduce cardiac ischemia-reperfusion injury in guinea pigs. J Am Assoc Lab Anim Sci. 2011;50:349–354. [PMC free article] [PubMed] [Google Scholar]
- 32.McGee AM, Baines CP. Phosphate is not an absolute requirement for the inhibitory effects of cyclosporin a or cyclophilin d deletion on mitochondrial permeability transition. The Biochemical journal. 2012;443:185–191. doi: 10.1042/BJ20111881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial h2o2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. The Journal of clinical investigation. 2009 doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson QH, Swoboda BE, Massey V. Kinetics and mechanism of action of glucose oxidase. The Journal of biological chemistry. 1964;239:3927–3934. [PubMed] [Google Scholar]
- 35.Tao Z, Raffel RA, Souid AK, Goodisman J. Kinetic studies on enzyme-catalyzed reactions: Oxidation of glucose, decomposition of hydrogen peroxide and their combination. Biophysical journal. 2009;96:2977–2988. doi: 10.1016/j.bpj.2008.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free radical biology & medicine. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Haessler R, Kuzume K, Chien GL, Wolff RA, Davis RF, Van Winkle DM. Anaesthetics alter the magnitude of infarct limitation by ischaemic preconditioning. Cardiovascular research. 1994;28:1574–1580. doi: 10.1093/cvr/28.10.1574. [DOI] [PubMed] [Google Scholar]
- 40.Walsh RS, Tsuchida A, Daly JJ, Thornton JD, Cohen MV, Downey JM. Ketamine-xylazine anaesthesia permits a katp channel antagonist to attenuate preconditioning in rabbit myocardium. Cardiovascular research. 1994;28:1337–1341. doi: 10.1093/cvr/28.9.1337. [DOI] [PubMed] [Google Scholar]
- 41.Miller TD, Christian TF, Hopfenspirger MR, Hodge DO, Gersh BJ, Gibbons RJ. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mtc sestamibi imaging predicts subsequent mortality. Circulation. 1995;92:334–341. doi: 10.1161/01.cir.92.3.334. [DOI] [PubMed] [Google Scholar]
- 42.Halestrap AP. What is the mitochondrial permeability transition pore? Journal of molecular and cellular cardiology. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochimica et biophysica acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Bolli R. Oxygen-derived free radicals and postischemic myocardial dysfunction ("stunned myocardium") Journal of the American College of Cardiology. 1988;12:239–249. doi: 10.1016/0735-1097(88)90381-6. [DOI] [PubMed] [Google Scholar]
- 45.Bognar Z, Kalai T, Palfi A, Hanto K, Bognar B, Mark L, Szabo Z, Tapodi A, Radnai B, Sarszegi Z, Szanto A, Gallyas F, Jr, Hideg K, Sumegi B, Varbiro G. A novel sod-mimetic permeability transition inhibitor agent protects ischemic heart by inhibiting both apoptotic and necrotic cell death. Free radical biology & medicine. 2006;41:835–848. doi: 10.1016/j.freeradbiomed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Chi LG, Tamura Y, Hoff PT, Macha M, Gallagher KP, Schork MA, Lucchesi BR. Effect of superoxide dismutase on myocardial infarct size in the canine heart after 6 hours of regional ischemia and reperfusion: A demonstration of myocardial salvage. Circulation research. 1989;64:665–675. doi: 10.1161/01.res.64.4.665. [DOI] [PubMed] [Google Scholar]
- 47.Kilgore KS, Friedrichs GS, Johnson CR, Schasteen CS, Riley DP, Weiss RH, Ryan U, Lucchesi BR. Protective effects of the sod-mimetic sc-52608 against ischemia/reperfusion damage in the rabbit isolated heart. Journal of molecular and cellular cardiology. 1994;26:995–1006. doi: 10.1006/jmcc.1994.1120. [DOI] [PubMed] [Google Scholar]
- 48.Abe M, Takiguchi Y, Ichimaru S, Tsuchiya K, Wada K. Comparison of the protective effect of n-acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. Journal of pharmacological sciences. 2008;106:571–577. doi: 10.1254/jphs.fp0071664. [DOI] [PubMed] [Google Scholar]
- 49.Alberola A, Such L, Gil F, Zaragoza R, Morcillo EJ. Protective effect of n-acetylcysteine on ischaemia-induced myocardial damage in canine heart. Naunyn-Schmiedeberg's archives of pharmacology. 1991;343:505–510. doi: 10.1007/BF00169553. [DOI] [PubMed] [Google Scholar]
- 50.Brunet J, Boily MJ, Cordeau S, Des Rosiers C. Effects of n-acetylcysteine in the rat heart reperfused after low-flow ischemia: Evidence for a direct scavenging of hydroxyl radicals and a nitric oxide-dependent increase in coronary flow. Free radical biology & medicine. 1995;19:627–638. doi: 10.1016/0891-5849(95)00077-b. [DOI] [PubMed] [Google Scholar]
- 51.Kingma JG, Jr, Rouleau JR. Effect of n-acetylcysteine on tissue necrosis during acute myocardial infarction in rabbits. The Canadian journal of cardiology. 1989;5:321–326. [PubMed] [Google Scholar]
- 52.Sochman J, Kolc J, Vrana M, Fabian J. Cardioprotective effects of n-acetylcysteine: The reduction in the extent of infarction and occurrence of reperfusion arrhythmias in the dog. International journal of cardiology. 1990;28:191–196. doi: 10.1016/0167-5273(90)90060-i. [DOI] [PubMed] [Google Scholar]
- 53.Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, Da Ros T, Hurd TR, Smith RA, Murphy MP. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 2005;70:222–230. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 54.Frasier CR, Moukdar F, Patel HD, Sloan RC, Stewart LM, Alleman RJ, La Favor JD, Brown DA. Redox-dependent increases in glutathione reductase and exercise preconditioning: Role of nadph oxidase and mitochondria. Cardiovascular research. 2013;98:47–55. doi: 10.1093/cvr/cvt009. [DOI] [PubMed] [Google Scholar]
- 55.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates atp recovery and reduces ischemic kidney injury. Journal of the American Society of Nephrology : JASN. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane-from discovery to clinical development. Pharmaceutical research. 2011 doi: 10.1007/s11095-011-0476-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. The Journal of biological chemistry. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 58.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound ss-31 re-energizes ischemic mitochondria by interacting with cardiolipin. Journal of the American Society of Nephrology : JASN. 2013 doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 60.Brown DA, Sabbah HN, Shaikh SR. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacology & therapeutics. 2013 doi: 10.1016/j.pharmthera.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. American journal of physiology. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 62.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovascular research. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. The New England journal of medicine. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto-Ida M, Akao M, Takeda T, Kato M, Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation. 2006;114:1497–1503. doi: 10.1161/CIRCULATIONAHA.106.628834. [DOI] [PubMed] [Google Scholar]
- 65.Slodzinski MK, Aon MA, O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. Journal of molecular and cellular cardiology. 2008;45:650–660. doi: 10.1016/j.yjmcc.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang XM, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV. Two classes of anti-platelet drugs reduce anatomical infarct size in monkey hearts. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2013;27:109–115. doi: 10.1007/s10557-012-6436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. Journal of the American College of Cardiology. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabbah HN, Gupta RC, Szekely K, Wang M, Zhang K, Rstogi S. Bendavia (mtp-131), a novel mitochondria-targeting peptide, improves activity and expression of cytochrome c oxidase and attenuates mediators of cardiomyocyte apoptosis in left ventricular myocardium of dogs with advanced heart failure. Journal of the American College of Cardiology. 2013;61 [Google Scholar]