Abstract
Previously we identified a novel mutation (F71L) in the αA-crystallin gene associated with early onset of age-related cataract. However, it is not known how the missense substitution translates into reduced chaperone-like activity (CLA), and how the structural and functional changes lead to early onset of the disease. Herein, we show that under native conditions the F71L-mutant is not significantly different from wild-type with regard to secondary and tertiary structural organization, hydrophobicity and the apparent molecular mass of oligomer but has substantial differences in structural and functional properties following a heat treatment. Wild-type αA-crystallin demonstrated increased CLA, whereas the F71L-mutant substantially lost its CLA upon heat treatment. Further, unlike the wild-type αA-subunit, F71L-subunit did not protect the αB-subunit in hetero-oligomeric complex from heat-induced aggregation. Moreover, hetero-oligomer containing F71L and αB in 3:1 ratio had significantly lower CLA upon thermal treatment compared to its unheated control. These results indicate that α-crystallin complexes containing F71L-αA subunits are less stable and have reduced CLA. Therefore, F71L may lead to earlier onset of cataract due to interaction with several environmental factors (e.g., temperature in this case) along with the aging process.
Keywords: Age-related cataract, F71L-mutation, αA-crystallin, Chaperone-like activity, Thermal stability
1. Introduction
Crystallins are the major structural proteins of the eye lens and whose short ordered arrangement provides the physical basis for the lens transparency [1,2]. Impaired lens function, due to partial or complete loss of transparency is called cataract, a leading cause of blindness worldwide [3]. α-Crystallin, a small heat-shock protein (sHSP1) characterized by the presence of a conserved ‘α-crystallin domain’ at its C-terminal region, is one of the three major crystallins and constitutes about 40% of the total soluble proteins of the vertebrate eye lens [1,4]. Eye lens α-crystallin is composed of two subunits, αA and αB, encoded by CRYAA and CRYAB genes, respectively. Chaperone-like activity (CLA) of α-crystallin is considered to be critical for the maintenance of eye lens transparency (reviewed in [1,4,5]). Studies with single and double knockout animals of αA and αB have highlighted the importance of α-crystallin in lens transparency [6–8]. Further, certain point mutations in αA- and αB-crystallin genes are linked with non-syndromic, hereditary human cataracts [reviewed in 1,4]. However, many of these early onset cataracts are inherited by autosomal dominant mechanism that result in early onset either congenitally or relatively early in life.
Despite the availability of effective surgical treatment, cataracts still comprise a significant risk for visual impairment all over the world, particularly in older individuals. Cataract accounts for an estimated 16 million cases of blindness worldwide, with approximately half of all cases originating from Africa and Asia [3]. Age-related cataract (ARC) is generally considered as a multifactorial disease. While, epidemiological research has been focused mostly on the role of environmental risk factors, recent studies indicated a contribution of genetic factors in the pathogenesis of ARC [9,10]. Genetic predisposition in association with other etiological factors may contribute to ARC. Although, evidence for the genetic component to the development of ARC is increasing, genetic variations associated with ARC are very few compared to those for congenital and hereditary cataracts. Recently we identified a novel point mutation (F71L) in exon-2 of CRYAA (αA-crystallin) gene associated with early onset of age-related cataracts [11]. The age-at-onset of nuclear cataract in F71L patients was about 10 years earlier (50 years) when compared to the mean age-at-onset of nuclear cataract cases (60 years) in individuals without the mutation.
The effect of missense mutations on α-crystallin structure and function have been extensively studied [12–17] In general, point mutations in αA-crystallin result in altered structure, loss of chaperone- like activity, and appear to lead to cataract formation and abnormal lens development [13–17]. While the F71L-αA-crystallin generally displayed a loss of CLA that varied (10–90%) depending upon the client proteins and assay conditions the mutation did not significantly affect the apparent molecular mass, secondary and tertiary structure and hydrophobicity of αA-crystallin [11]. Sharma et al. [18] using a synthetic peptide (mini-αA-crystallin), have demonstrated the importance of sequence 70–88 in the chaperone- like function of αA-crystallin. A later study demonstrated that Phe-71 is critical for CLA [19]. It is intriguing to note that the missense mutation (F71L) in αA-crystallin did not cause major structural changes, but substantially lowered CLA [11]. Hence, the early age-at-onset of cataract in the individuals carrying F71L mutation in αA-crystallin might be due to a partial loss of in vivo CLA or enhanced susceptibility to structural alterations induced by deleterious environmental factors along with aging. Structural reorganization of α-crystallin due to thermotrophic and other factors is known to modulate the CLA [1]. In this study we report temperature-dependent structural and functional alterations in F71L-αA-crystallin and the thermal sensitivity of the mutant protein that may provide a molecular basis for the early on-set of cataract due to the F71L mutation.
2. Materials and methods
2.1. Expression and purification of recombinant wild-type and F71L mutant αA-crystallins
The recombinant wild-type and F71L mutant αA-crystallins were overexpressed in Escherichia coli and the proteins were purified to homogeneity according to previously reported methods [11,20]. The concentration of wild-type and F71L mutant αA-crystallin proteins was estimated by Lowry method.
2.2. Preheat treatment
Structural perturbation in case of α-crystallin generally refers (but not exclusive) to treatment of mild (low) concentrations of denaturants or heating to high temperatures followed by cooling it to ambient temperature (preheated). To understand thermotrophic mediated structural and functional properties, the wild-type and F71L αA-crystallins (1 mg/ml) were heated at 65 °C for 15 min in a water bath and allowed to cool-down to room temperature and referred henceforth as preheated wild-type αA- and preheated αA-F71L-αA-crystallin.
2.3. Size-exclusion chromatography
The apparent molecular mass of wild-type and F71L mutant αA-crystallins under normal conditions and after preheating was determined by size-exclusion chromatography on a 600 × 7.5 mm TSK-3000 SW column (Tosoh Co., Japan) using a Shimadzu HPLC system [21]. The column was equilibrated with 0.1 M sodium phosphate buffer, pH 6.9 containing 0.1 M sodium sulfate at a flow rate of 1 ml/min. Column was calibrated using standard molecular weight markers (thyroglobulin-669, γ-globulin-160, BSA-67, ovalbumin- 45 kDa).
2.4. ANS fluorescence studies
The surface hydrophobicity of normal and preheated αA-crystallins was measured using a hydrophobic probe, 8-anilino-1-naphthalene sulfonic acid (ANS). 50 μM ANS solution in 10 mM of sodium phosphate buffer was added to protein samples of 0.1 mg/ml and incubated in the dark at room temperature for 30 min. The samples were excited at 385 nm and the emission spectra were recorded between 400 and 600 nm using a Jasco-FP-6500 spectrofluorometer [11].
2.5. Circular dichroism studie
Secondary and tertiary structural changes of normal and preheated αA-crystallins were investigated by far- and near-UV CD spectra in a Jasco-810 spectropolarimeter [11,21,22]. Protein concentrations of 0.1 mg/ml and 1 mg/ml were used for recording the far- and near-UV CD spectra respectively. The reported CD spectra are the average of five accumulations.
2.6. Chaperone-like assays
The ability of normal and preheated αA-crystallins to prevent protein aggregation was assessed by using different target proteins under different assay conditions. Heat-induced aggregation of βL-and γ-crystallins at 60 °C and DTT-induced aggregation of insulin at 37 °C were monitored by measuring light scattering as a function of time at 360 nm using a Lamda-35 spectrophotometer (Perkin– Elmer) according to previously described methods [20,22].
3. Results
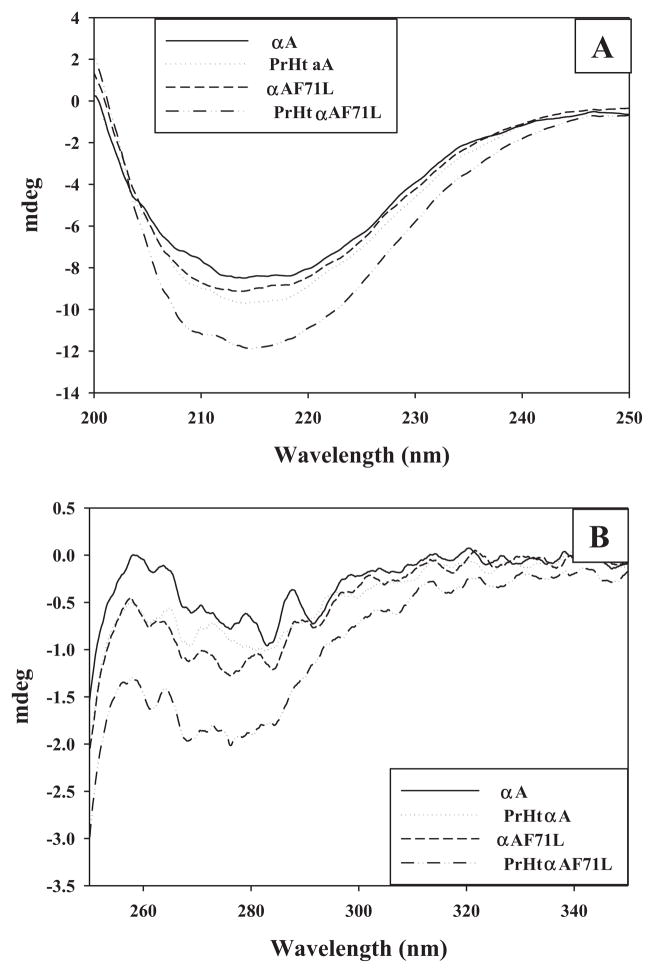
The secondary and tertiary structures of wild-type and F71L mutant αA-crystallins were determined under normal conditions and upon preheat treatment by far- and near-UV CD spectral analysis. The far-UV CD spectra of wild-type and F71L-αA-crystallins were similar suggesting no major differences in secondary structure (Fig. 1A). While the secondary structure of preheated wild-type αA-crystallin is essentially unchanged, a significant alteration in secondary structure was observed with preheated F71L αA-crystallin as evidenced by an increase in negative ellipticity in the region 210–220 nm indicating formation of beta-sheet rich structure (Fig. 1A). Although, the near-UV CD spectra for the two proteins were similar under normal conditions the near-UV CD spectra of preheated wild-type αA-crystallin revealed alteration at tertiary levels as reported previously [22]. However, compared to preheated wild-type αA-crystallin, preheated F71L-αA-crystallin showed dramatic changes in near-UV region, particularly loss of intensity between 270 and 290 nm region (Fig. 1B) which indicates a change in the chiral environment of tryptophan residues.
Fig. 1.
Secondary and tertiary structure. Far-UV (A) and near-UV CD spectra (B) of normal and preheated wild-type and F71L-αA-crystallin. Data are representative of three independent experiments.
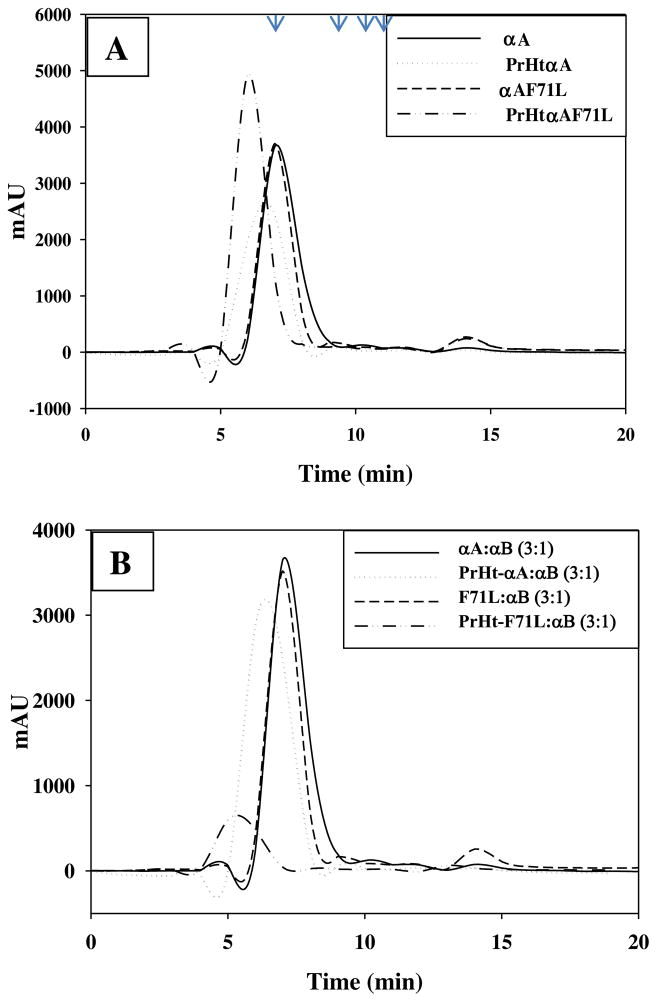
As reported previously elution profile of wild-type and F71L-αA-crystallin was found to be similar with an approximate oligomeric mass of 650 kDa (Fig. 2A). The preheated wild-type αA-crystallin eluted just before its respective unheated control indicating an increase in the aggregate molecular mass (approx. 850 kDa) upon structural perturbation. However, F71L-αA-crystallin eluted even before the preheated wild-type αA-crystallin (Fig. 2A). This indicates that preheat treatment of F71L-αA-crystallin led to formation of a predominantly large soluble aggregate of molecular mass >1000 kDa and suggests that temperature disrupts the integrity of the oligomeric assembly of the F71L mutant and thereby affects its stability. These observations are consistent with the previous studies that show thermally disintegration of the oligomeric structure with diminished chaperone activity for R116C and R116H αA-crystallin mutants [23–25]. We have also shown earlier that αB-homo-oligomer but not the hetero-oligomer with (3:1) αA to αB precipitated upon preheating indicating that αA imparts thermal stability to the hetero-oligomer by preventing the aggregation of αB-crystallin at higher temperature [21]. Therefore, we determined the mass of oligomeric complexes of normal and preheated hetero-oligomer with (3:1) αA to αB or αA-F71L to αB. Consistent with reported studies, preheating the hetero-oligomer of αA and αB prevented the aggregation of αB-crystallin and eluted before its respective unheated heteropolymer (Fig. 2B). However, in contrast to wild-type αA, αA-F71L was not only able to protect αB upon preheating of the hetero-oligomer of αA-F71L and αB, but it too precipitated along with αB substantiating the role of αA moiety in stabilizing the αB-crystallin (Fig. 2B).
Fig. 2.
Molecular mass. Size-exclusion chromatography profiles of normal and preheated wild-type and F71L mutant αA-crystallin on a 600 × 7.5 mm TSK-3000 SW column (A). Positions of molecular weight markers is shown in top with arrows; thyroglobulin-669, γ-globulin-160, BSA-67, ovalbumin-45 kDa. Data are representative of three independent experiments. Size-exclusion chromatography profiles of normal and preheated heteropolymer of α-crystallin with (3:1) αA to αB or F71L-αA- to αB (B). Data are representative of three experiments.
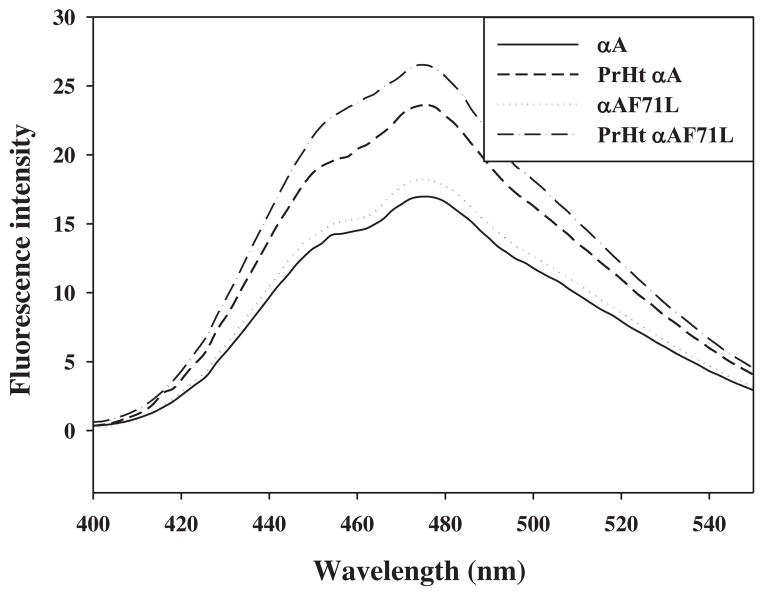
Consistent with the previous studies, the intensity of ANS fluorescence emission of preheated wild-type αA-crystallin was higher than its corresponding unheated variant (Fig. 3). However, the ANS-binding to preheated F71L αA-crystallin increased significantly than the preheated wild-type αA-crystallin (Fig. 3) implying that the available hydrophobic surface is increased appreciably. Notably, this increase in hydrophobicity did not result in a corresponding increase in CLA. Instead, heat-treatment caused a loss of CLA, suggesting that at higher temperatures the structural stability of F71L is altered due to unfolding and exposure of hydrophobic sites.
Fig. 3.
Hydrophobicity. ANS-fluorescence spectra of normal and preheated wild-type and F71L-αA-crystallin. Data are representative of three independent experiments.
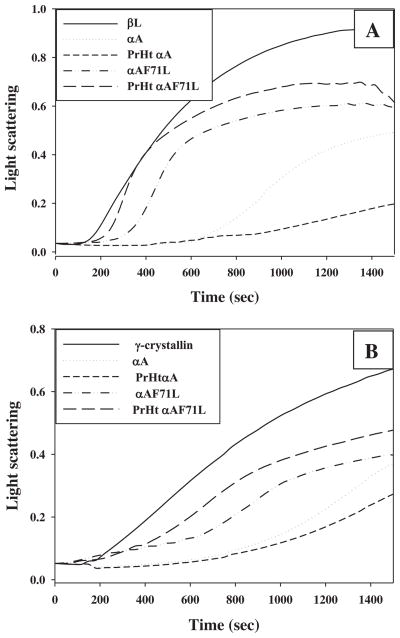
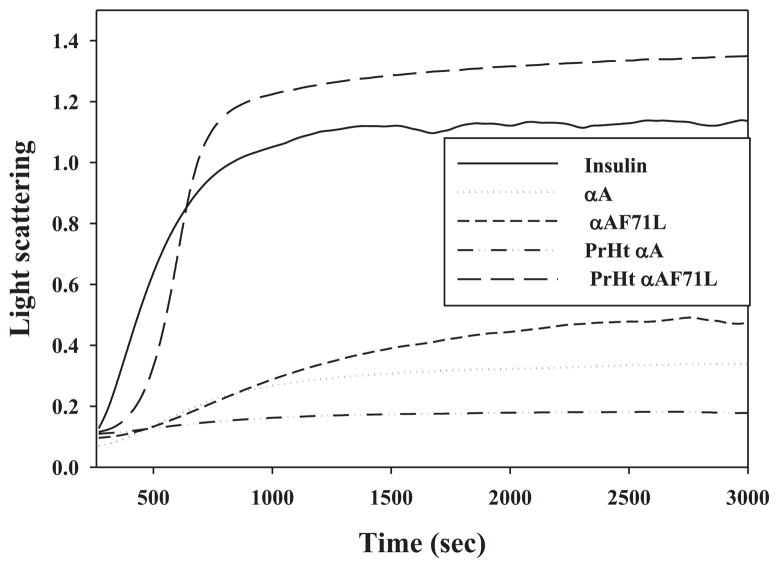
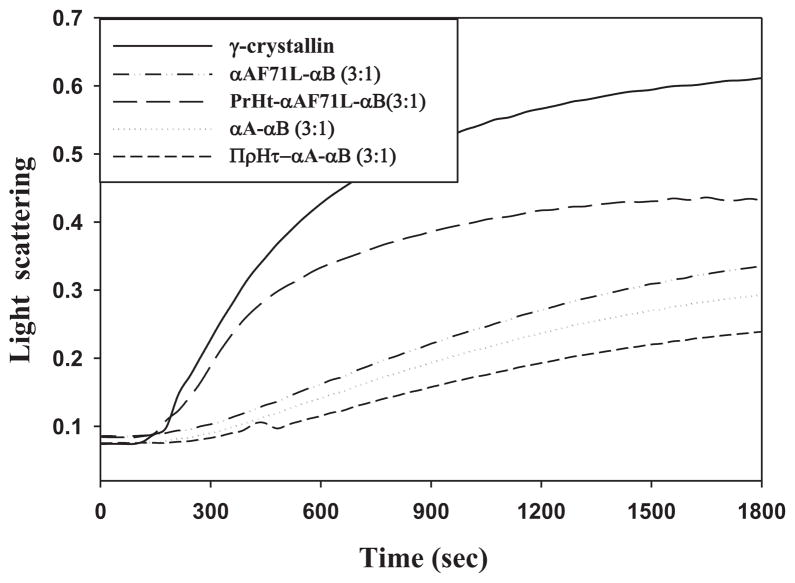
Previously we reported a conditional loss of CLA for F71L compared to αA-crystallin, where the degree of loss was dependent on the aggregation assay conditions. We observed particularly greater losses of CLA when measurements were carried out using thermal aggregation assays [11]. It is therefore, important to study the CLA of preheated versions in the context of temperature-induced conformational changes of wild-type and F71L-mutant. Thus, the CLA of preheated wild-type and F71L-αA-crystallin was investigated by a battery of thermal and chemical aggregation assays. As reported previously by many studies, in the heat-induced aggregation of βL- and γ-crystallin, the preheated wild-type αA-crystallin showed enhanced protection compared to normal wild-type αA-crystallin (Fig. 4). In contrast, the preheated F71L-mutant showed a decreased CLA in suppressing the heat-induced aggregation of βL- and γ-crystallin compared to normal F71L-αA-crystallin (Fig. 4). Similar results were found in the heat-induced aggregation of citrate synthase assay even at 60 °C (data not shown). In the DTT-induced aggregation assay of insulin, the unheated F71L-mutant showed a marginal (10%) decrease in CLA compared to that of unheated wild-type αA-crystallin, while the preheated wild-type protein exhibited enhanced CLA over its unheated variant (Fig. 5). However, the preheated F71L-mutant completely lost its chaperone-like activity and co-aggregated with the client protein in DTT-induced aggregation assay of insulin (Fig. 5). The increase in light scattering of preheated F71L-αA-crystallin due to co-aggregation in the insulin assay might be a result of non-specific hydrophobic interactions between F71L-mutant and reduced insulin B-chain. From the aggregation assays, it is apparent that the CLA of F71L-mutant is dependent on client protein but more so on temperature. Though F71L is a homozygous missense mutation [11], the in vitro studies described above were carried out on subunits of αA and αB separately. However, the situation in the eye lens is not same where the α-crystallin is known to exist as a heteropolymer with αA and αB in 3:1 ratio [21,22,26]. Further, we have shown earlier very clearly that αA subunit plays an important role in the heteropolymer in that it also provides stability to αB [21,27]. Therefore, we have combined F71L-αA with αB in 3:1 ratio and allowed subunits to exchange to equilibrium. Then, we investigated the effect of thermal stability on CLA. CLA of preheated (3:1) αA to αB was significantly increased compared to unheated (3:1) αA to αB in both γ-crystallin (Fig. 6) and β-crystallin assays (not shown). In contrast, CLA of preheated (3:1) F71L-αA to αB was significantly lower compared to unheated (3:1) F71L-αA to αB in both γ-crystallin (Fig. 6) and β-crystallin assays (not shown).
Fig. 4.
Chaperone-like activity. Chaperone-like activity of normal and preheated wild-type and F71L-αA-crystallin (0.05 mg/ml) in heat-induced aggregation of βL-crystallin (0.25 mg/ml) (A) and γ-crystallin (0.25 mg/ml) assay (B) at 60 °C. Data are representative of three independent experiments.
Fig. 5.
Chaperone-like activity. Chaperone-like activity of normal and preheated wild-type and F71L-αA-crystallin (0.4 mg/ml) in DTT-induced aggregation of insulin (0.3 mg/ml) assay at 37 °C. Data are representative of three independent experiments.
Fig. 6.
Chaperone-like activity. Chaperone-like activity of normal and preheated hetero-oligomer of α-crystallin with αA to αB or with F71L-αA-αB in 3:1 ratio (0.03 mg/ml) in heat-induced aggregation of γ-crystallin (0.25 mg/ml) assay at 60 °C. Data are representative of three independent experiments.
4. Discussion
We have identified a novel mutation (F71L) in the exon-2 of CRYAA gene associated early-onset of ARC and reported that despite insignificant structural changes, the F71L mutant αA-crystallin displayed significant loss of CLA, particularly in thermal aggregation assays performed at elevated temperatures [11]. Nevertheless, the reasons for the time lag shown by this mutation in the expression of a cataract phenotype around 50 years of age compared to 60 years without the mutation are not clear. It is plausible that some genes may be involved in adult cataract with late expression of phenotype due to interaction with several environmental factors during aging.
Eye lens α-crystallin is a hetero-oligomer composed of two similar subunits, αA and αB, present in a 3:1 molar ratio in most vertebrates [26]. Previously, we showed that despite high sequence similarity, there is a dichotomy in structure and function of αA-and αB-crystallins that is consistent with a molecular basis for the existence of α-crystallin hetero-oligomer with 3:1 αA to αB ratio [20–22,27]. Our studies showed that αA-crystallin is not only more stable but also imparts stability to the hetero-oligomer by preventing the aggregation of αB-crystallin at higher temperatures. Interestingly, α-crystallin complexes containing F71L subunits instead of wild-type αA subunits were not only able to protect αB-crystallin but also unable to suppress αB-mediated co-aggregation of lens proteins [27]. In fact, F71L mutant precipitated upon heating to high temperatures and contributed to increased light scattering at high temperature in these studies. Generally, upon heating, α-crystallin undergoes structural changes resulting in increased exposure of additional hydrophobic sites associated with increased CLA [1,20–22,28,29]. Once exposed to high temperatures, the protein upon cooling does not return to its original conformational state but adopts a conformation characterized by increased surface hydrophobicity and molecular mass (aggregate size) [1,29–31]. This increase in CLA of α-crystallin upon structural perturbation is mediated by αA-but not αB crystallin. Therefore, in the present study we investigated alterations in the molecular chaperone-like function of α-crystallin, and the associated structural changes, upon structural perturbation (preheat treatment at 65 °C for 15 min followed by cooling) of wild-type and F71L-αA-crystallins to understand the mechanism of early-onset cataract due to F71L mutation in αA-crystallins.
On comparison of human recombinant wild-type and F71L-αA-crystallin by size-exclusion chromatography, fluorescence and far-and near-UV CD studies it appears that αA-F71L missense mutation did not significantly affect the apparent molecular mass, secondary and tertiary structures or hydrophobicity of αA-crystallin under normal conditions. However, the F71L-αA-crystallin displayed significant loss of CLA in thermal treatment but little to no loss or insignificant loss in chemical aggregation assays [11]. These observations were consistent with the previous studies made on replacement of Phe-71 with Gly (F71G) which did not indicate structural changes, but CLA was severely impaired in αA-F71G crystallin [19]. It is reported that the Phe-71 contributes to the chaperone-like action of αA-crystallin and the 70–88-region in αA-crystallin is identified as the functional chaperone-like binding site in αA-crystallin [19]. However, temperature-dependent studies on both the wild-type and the F71L mutant revealed an increase in the oligomeric mass, shift in tryptophan fluorescence emission wavelength, alteration in secondary and tertiary structure, and further loss of CLA in F71L-mutant protein suggesting that the heat stability of the protein was affected by the mutation.
The preheated wild-type αA-crystallin exhibited a better protection in chaperone-like assays compared to wild-type αA-crystallin. These observations support the hypothesis that the regions of αA-crystallin become more exposed with temperature; a structural transition above 50 °C might therefore be important for its CLA [20,28–30]. In contrast, the preheated F71L mutant exhibited a decreased CLA in all the aggregation assays despite a slight increase in hydrophobicity. Based on our and others studies, it has been shown that hydrophobicity plays an important role but it is not the sole determinant of α-crystallin CLA [1,20,32]. Therefore, it is unlikely that all the exposed hydrophobic patches on F71L are involved in suppressing the substrate protein aggregation. The increase in ANS intensity might also be due to ANS-binding to residues other than those involved in chaperone-like activity [32,33]. Other factors like charge and structural integrity may influence the functional property to different extents. The preheated F71L mutant αA-crystallin showed almost complete loss of chaperone-like activity in all the aggregation assays and it co-aggregated along with insulin in DTT-induced aggregation. These observations support the idea that the chaperone-like activity of the mutant was dependent on client protein properties along with environmental factors that enhanced aggregation of substrate proteins might be directly related to the onset of cataract [34].
Considering that the preheated F71L mutant has a larger hydrophobic exposure when compared to wild-type αA-crystallin protein, the increase in amount of aggregation might be caused due to non-specific hydrophobic interactions between F71L mutant and the substrate protein. These results suggests that the impaired CLA of preheated F71L mutant might be due to altered specificity towards potential substrate proteins, since there is an alteration in its structure as evidenced by larger oligomers and changed CD spectra. Other mutations are known to decrease the overall structural stability of αA-crystallin such as the G98R mutation [15] and other important proteins [34,35]. The temperature-induced conformational studies on F71L-αA-crystallin also indicated alterations in both structural stability and chaperone-like activity.
Heat stability studies corroborated the effect of temperature on the dynamic quaternary structure of the F71L mutant similar to that R116C mutant [36]. This study indicates that Phe-71 is not only essential for the chaperone-like activity, but is also important in maintaining the structural integrity of the protein at higher temperatures (it plays a role in thermal stability of the protein). These data suggest that the F71L mutant has a compromised structural stability, and in course of time (due to alterations in body temperatures and environmental factors) loses its native structural conformation, which effects its protein-protein interactions resulting in defective CLA and aggregation of potential client proteins. It might also be more susceptible to age-related modifications and contribute to the early-on-set-cataract formation in individuals carrying the mutation. Size-exclusion chromatography studies with preheated hetero-oligomer with αA-F71L to αB in 3:1 ratio indicate that αA-F71L precipitated along with αB instead of stabilizing it.
Together the data presented in this study indicate that the F71L mutant is somewhat unstable even under physiological conditions. Considering that the protein, under the best of conditions, should last several decades of time, it seems likely that the early onset cataract at 5 decades may be reflecting the slow but insidious loss of the αA subunit over time. Further, the presence of the mutant αA subunit destabilized the wild-type αB subunits, which resulted in a reduction in CLA and solubility following heat treatment. In vivo, this could be equivalent to an acceleration of α-crystallin loss over time, which would slowly predispose the lens to cataract formation. From the present study, it is apparent that the molecular basis for the development of cataract in the affected individuals might be loss of thermal stability of the protein due to substitution of Phe-71 which results in the formation of highly oligomerized αA-crystallin with altered structural stability and defective chaperone- like activity.
Acknowledgments
This work was supported by grants from the Department of Science and Technology and the Department of Biotechnology, Government of India to GBR. V.D. was supported by a Postdoctoral fellowship from the Department of Biotechnology, and V.S.R. was supported by a research fellowship from the University Grants Commission, Government of India.
Abbreviations
- ARC
age related cataract
- ANS
8-anilino-1-naphthalene sulfonic acid
- CLA
chaperone-like activity
- PrHt
preheated
- sHSP
small heat shock proteins
References
- 1.Reddy GB, Kumar PA, Kumar MS. Chaperone like activity and hydrophobicity of α-crystallin IUBMB Life. Crit Rev. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 2.Bleomendal H, de jong WW, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and Vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 5.Kumar PA, Reddy GB. Modulation of alpha-crystallin chaperone activity: a target to prevent or delay cataract. IUBMB Life. 2009;61:485–495. doi: 10.1002/iub.176. [DOI] [PubMed] [Google Scholar]
- 6.Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. αB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- 7.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse αA-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein αB-crystallin. Proc Natl Acad Sci USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle DL, Takemoto L, Brady JP, Wawrousek EF. Morphological characterization of the αA- and αB-crystallin double knockout mouse lens. BMC Ophthalmol. 2003;3:3. doi: 10.1186/1471-2415-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hejtmancik JF, Kantorow M. Molecular genetics of age related cataract. Exp Eye Res. 2004;79:3–9. doi: 10.1016/j.exer.2004.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: putting cataract on the map. Mol Vis. 2010;16:2007–2015. [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagyalaxmi SG, Srinivas P, Barton KA, Kumar KR, Vidyavathi M, Petrash JM, Bhanuprakash Reddy G, Padma T. A novel mutation (F71L) in alpha-crystallin with defective chaperone like function associated with age-related cataract. Biochim Biophys Acta. 2009;1792:974–981. doi: 10.1016/j.bbadis.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in αB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci USA. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu CD, Kymes S, Petrash JM. A transgenic mouse model for human autosomal dominant cataract. Invest Ophthalmol Vis Sci. 2006;47:2036– 2044. doi: 10.1167/iovs.05-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koteiche HA, McHaourab HS. Mechanism of a hereditary cataract phenotype Mutations in alpha-crystallin activate substrate binding. J Biol Chem. 2006;281:14273–14279. doi: 10.1074/jbc.M512938200. [DOI] [PubMed] [Google Scholar]
- 15.Singh D, Raman B, Ramakrishna T, Rao CM. Mixed oligomer formation between human αA-crystallin and its cataract-causing G98R mutant: structural, stability and functional differences. J Mol Biol. 2007;373:1293–1304. doi: 10.1016/j.jmb.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Xi J-h, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo – a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- 17.Bera S, Thampi P, Cho WJ, Abraham EC. A positive charge preservation at position 116 of αA-crystallin is critical for its structural and functional integrity. Biochemistry. 2002;41:12421–12426. doi: 10.1021/bi0204140. [DOI] [PubMed] [Google Scholar]
- 18.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alpha-A crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 19.Santhoshkumar P, Sharma KK. Phe71 is essential for chaperone-like function in alpha A-crystallin. J Biol Chem. 2001;276:47094– 47099. doi: 10.1074/jbc.M107737200. [DOI] [PubMed] [Google Scholar]
- 20.Reddy GB, Das KP, Petrash JM, Surewicz WK. Temperature-dependent chaperone activity and structural properties of human alphaA- and alphaB crystallins. J Biol Chem. 2000;275:4565–4570. doi: 10.1074/jbc.275.7.4565. [DOI] [PubMed] [Google Scholar]
- 21.Srinivas P, Narahari A, Petrash JM, Swamy MJ, Reddy GB. Importance of eye lens α-crystallin heteropolymer with 3:1 αA to αB ratio: stability, aggregation and modification. IUBMB Life. 2010;62:693–702. doi: 10.1002/iub.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivas PN, Reddy PY, Reddy GB. Significance of alpha-crystallin heteropolymer with a 3:1 alphaA/alphaB ratio: chaperone-like activity, structure and hydrophobicity. Biochem J. 2008;414:453–460. doi: 10.1042/BJ20080544. [DOI] [PubMed] [Google Scholar]
- 23.Kelley PB, Abraham EC. Thermally induced disintegration of the oligomeric structure of αB-crystallin mutant F28S is associated with diminished chaperone activity. Mol Cell Biochem. 2003;252:273–278. doi: 10.1023/a:1025568417000. [DOI] [PubMed] [Google Scholar]
- 24.Bera S, Abraham EC. The αA-crystallin R116C mutant has a higher affinity for forming heteroaggregates with αB-crystallin. Biochemistry. 2002;41:297–305. doi: 10.1021/bi011010v. [DOI] [PubMed] [Google Scholar]
- 25.Min Pang, Jing-Tan Su, Shan Feng, Zhi-Wei Tang, Feng Gu, Zhang Meng, Ma Xu, Yong-bin Yan. Effects of congenital cataract mutation R116H on αA-crystallin structure, function and stability. Biochim Biophys Acta (BBA)-Prot Proteom. 2010;1804:948–956. doi: 10.1016/j.bbapap.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 26.de Jong WW, Lubsen NH, Kraft HJ. Molecular evolution of the eye lens. Progr Ret Eye Res. 1994;13:391–442. [Google Scholar]
- 27.Srinivas PN, Patil MA, Reddy GB. Temperature-dependent coaggregation of eye lens αB- and β-crystallins. Biochem Biophys Res Commun. 2011;405:486–490. doi: 10.1016/j.bbrc.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 28.Das KP, Surewicz WK. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of alpha-crystallin. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- 29.Raman B, Rao CM. Chaperone-like activity and temperature-induced structural changes of alpha-crystallin. J Biol Chem. 1997;272:23559– 23564. doi: 10.1074/jbc.272.38.23559. [DOI] [PubMed] [Google Scholar]
- 30.Raman B, Rao CM. Chaperone-like activity and quaternary structure of alpha-crystallin. J Biol Chem. 1994;269:27264–27268. [PubMed] [Google Scholar]
- 31.Maulucci G, Papi M, Arcovito G, De Spirito M. The thermal structural transition of α-crystallin inhibits the heat induced self-aggregation. PLoS One. 2011;6:e18906. doi: 10.1371/journal.pone.0018906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar MS, Kapoor M, Sinha S, Reddy GB. Insights into hydrophobicity and the chaperone-like function of alphaA- and alphaB-crystallins: an isothermal titration calorimetric study. J Biol Chem. 2005;280:21726–21730. doi: 10.1074/jbc.M500405200. [DOI] [PubMed] [Google Scholar]
- 33.Sharma KK, Kumar GS, Murphy AS, Kester K. Identification of 1,1′-bi(4-anilino)naphthalene-5,5′-disulfonic acid binding sequences in alpha-crystallin. J Biol Chem. 1998;273:15474–15478. doi: 10.1074/jbc.273.25.15474. [DOI] [PubMed] [Google Scholar]
- 34.Murugesan R, Santhoshkumar P, Sharma KK. Cataract-causing alphaAG98R mutant shows substrate-dependent chaperone activity. Mol Vis. 2007;13:2301–2309. [PubMed] [Google Scholar]
- 35.Feng S, Zhao TJ, Zhou HM, Yan YB. Effects of the single point genetic mutation D54G on muscle creatine kinase activity, structure and stability. Int J Biochem Cell Biol. 2007;39:392–401. doi: 10.1016/j.biocel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Shroff NP, Cherian-Shaw M, Bera S, Abraham EC. Mutation of R116C results in highly oligomerized αA-crystallin with modified structure and defective chaperone-like function. Biochemistry. 2000;39:1420–1426. doi: 10.1021/bi991656b. [DOI] [PubMed] [Google Scholar]