Abstract
Kaposi’s sarcoma associated herpesvirus (KSHV; also known as human herpesvirus 8) is the etiological agent of Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. These cancers often occur in the context of immunosuppression, which has made KSHV-associated malignancies an increasing global health concern with the persistence of the AIDS epidemic. KSHV has also been linked to several acute inflammatory diseases. KSHV exists between a lytic and latent lifecycle which allow the virus to transition between active replication and quiescent infection. KSHV encodes a number of proteins and small RNAs that are thought to inadvertently transform host cells while performing their functions of helping the virus persist in the infected host. KSHV also has an arsenal of components that aid the virus in evading the host immune response, which help the virus establish a successful lifelong infection. In this comprehensive review, we will discuss the diseases associated with KSHV infection, the biology of latent and lytic infection, and individual proteins and microRNAs that are known to contribute to host cell transformation and immune evasion.
Keywords: KSHV, HHV8, Kaposi’s sarcoma, primary effusion lymphoma, multicentric Castleman’s disease, latency locus, lytic replication, viral oncogenes, viral immune evasion
Malignancies and Syndromes Linked with KSHV Infection
KSHV infection is associated with three human malignancies: Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman’s disease (MCD) (Cesarman, Chang, Moore, Said, & Knowles, 1995; Chang et al., 1994; Gessain et al., 1996; Soulier et al., 1995). KS tumors are comprised of KSHV-infected cells of endothelial origin, whereas PEL and MCD are of B cell origin. KSHV is also associated with several acute inflammatory syndromes. In this section, we will discuss these KSHV-associated diseases, and the characteristics of KSHV-associated malignancies are summarized in Table 1.
Table 1.
Characteristics of KSHV-associated malignancies
| Disease | Presentation | Lineage & Primary Tumor Cell |
Clonality | KSHV Genomes |
|---|---|---|---|---|
| Kaposi’s sarcoma (KS) | Highly angiogenic. Lesions can be found on skin, visceral organs, or mucosal surfaces | Endothelial cell origin; tumor cells are spindle cells with mixed blood and lymphatic endothelial cell markers | Oligoclonal lesions | >99% of tumor cells contain KSHV genomes |
| Primary Effusion Lymphoma (PEL) | Non-Hodgkin lymphoma; B cell expansion in body cavity | B cell; CD20-; markers resemble partially differentiated plasma cell | Monoclonal | Each tumor cell has 50–100 copies of the KSHV genome |
| MulticentricCastleman’s Disease (MCD) | Plasmablastic variant of MCD | B cell; IgM λ-restricted plasmablasts | Typically polyclonal | Unknown |
Kaposi’s Sarcoma
The classical form of Kaposi’s sarcoma was first described in 1872 as a pigmented sarcoma of the skin by the Hungarian dermatologist, Moritz Kaposi (Kaposi, 1872). KS incidence rates started to increase dramatically with the onset of the AIDS epidemic in the 1980’s (Beral, Peterman, Berkelman, & Jaffe, 1990). The correlation between HIV-infected individuals and KS suggested an infectious agent was involved. About a decade later, representational difference analysis used by Chang and Moore identified novel gammaherpesvirus DNA sequences in KS lesion biopsies (Chang et al., 1994). In the years following the discovery of KSHV, PEL and MCD were also found to be causally linked to this human herpesvirus (Cesarman et al., 1995; Gessain et al., 1996; Soulier et al., 1995).
There are four forms of KS that have been described (Antman & Chang, 2000; Friedman-Kien & Saltzman, 1990; Wahman, Melnick, Rhame, & Potter, 1991). Classic KS, which was first identified by Moritz Kaposi, is found in elderly men of Mediterranean and eastern European descent (DiGiovanna & Safai, 1981). This form of KS is characterized by benign lesions on the upper and lower extremities, and rarely progresses to more aggressive disease. The second type of KS is African endemic KS, which occurs in eastern and central African countries (Friedman-Kien & Saltzman, 1990; Stein et al., 1994). The lymphadenopathic form of endemic KS is found almost exclusively in young African children and cause significant mortality (Dutz & Stout, 1960; Taylor, Templeton, Vogel, Ziegler, & Kyalwazi, 1971). AIDS-associated, or epidemic KS has become the most common type of KS in the past three decades, and is the most aggressive form of the disease (Beral & Newton, 1998; Beral et al., 1990; Biggar & Rabkin, 1996). AIDS-associated KS is considered an AIDS-defining illness (Mbulaiteye, Biggar, Goedert, & Engels, 2003). KS is currently the most common malignancy associated with HIV infection, and therefore is the most frequent cancer in many Sub-Saharan countries (Casper & Wald, 2007; Engels et al., 2006; Parkin, Wabinga, Nambooze, & Wabwire-Mangen, 1999; Parkin, 2006; Thomas, 2001). The fourth type of KS is iatrogenic/post-transplant KS, which is associated with the use of immune suppressive therapy for the prevention of organ transplant rejection (Andreoni et al., 2001; Marcelin, Calvez, & Dussaix, 2007; Siegel, Alper, Schutte, Robbins, & Blaufox, 1969). Interestingly, it was found that this type of KS occurs more often in KSHV-infected recipients rather than KSHV-negative recipients that receive an organ from a KSHV-positive donor (Barozzi et al., 2003; Francès et al., 2009).
KS lesions typically occur cutaneously on upper and lower extremities or on mucosal surfaces; however, lesions may also involve lymph nodes or may occur on visceral organs such as lung and spleen (Siegel et al., 1969; Tamburro et al., 2012). The immune status of the host and lymph node involvement are important factors in patient prognosis. KS disease progresses through six stages called the patch, plaque, nodular, lymphadenopathic, infiltrative, and florid stage (Kyalwazi, 1981; Taylor et al., 1971). KS lesions are highly angiogenic and as a result are usually red, purple, or brown in color. Additionally, the lesion vasculature is leaky, which allows for extravasation of erythrocytes and infiltration of inflammatory cells (Hussein, 2008). KS tumor cells are of endothelial cell origin, and the primary KSHV-infected cells found in the lesion are highly proliferative spindle-shaped cells (Boshoff et al., 1995; Staskus et al., 1999; Staskus et al., 1997). Over 95% of KS lesions contain KSHV DNA (Dupin et al., 1999), and most of the infected cells harbor the virus latently. Interestingly, KSHV infection of blood endothelial cells (BEC) can induce expression of lymphatic endothelial cell (LEC) markers and vice versa. This transcriptional reprogramming results in poorly differentiated endothelial cells that express mixed lineage markers such as CXCR4, CD34, VEGFR3, LYVE1, and PROX1 (Hansen et al., 2010; Hong et al., 2004; Morris, Punjabi, & Lagunoff, 2008). As opposed to a metastatic dissemination, KS lesions typically arise independently of one another; however this oligoclonality is not universal, and situations of monoclonal KS have also been reported (Duprez et al., 2007; Judde et al., 2000).
Primary Effusion Lymphoma
Shortly after its association with KS, KSHV was identified as the etiological agent of PEL (Cesarman et al., 1995). PEL is a non-Hodgkin lymphoma comprised of malignant, latently-infected B cells that expand within the pericardial, pleural, and peritoneal body cavities (Nador et al., 1996). Unlike KS, PEL is a monoclonal population of B cells as evidenced by clonal immunoglobin gene rearrangements, and each tumor cell has a high KSHV copy number ranging from 50–100 genomes per cell (Renne, Lagunoff, Zhong, & Ganem, 1996). Morphologically, PEL share features of both immunoblastic and anaplastic large-cell lymphomas (Carbone & Gloghini, 2008). Most PEL express CD45 and activation markers including CD30, CD38, and CD7, and epithelial membrane antigen (EMA) (Cesarman & Knowles, 1999). Interestingly, PEL expresses plasma cell markers such as CD138, VS38c, and MUM-1/IRF4, but has relatively low expression of B-cell associated antigens, suggesting that PEL resembles partially differentiated plasma cells rather than mature B cells (Carbone et al., 2001; Cesarman & Knowles, 1999; Jenner et al., 2003). Unlike other NHLs, PEL typically does not exhibit c-myc rearrangements or mutations in the ras, bcl2, or p53 genes (Cesarman & Knowles, 1999; Nador et al., 1996). PEL is frequently coinfected with the Epstein Barr virus (EBV) (Cesarman & Knowles, 1999).
Although PEL is characterized by a malignant serous effusion lacking a solid tumor mass, cases of solid PEL have also been reported (Carbone et al., 2005). These tumors typically present as an extracavitary lymphoma in extranodal or lymph node tissue and are composed of immunoblastic-like cells. These solid PEL are also KSHV positive, and have similar morphology, immunophenotype, immunoglobulin gene rearrangements to classical PEL (Carbone & Gloghini, 2008).
PEL is a very aggressive lymphoma, and the average survival time is about 6 months from diagnosis (Boulanger et al., 2005). The main prognostic factors that have been identified are the presence of highly active antiretroviral therapy (HAART) in HIV-positive patients before PEL diagnosis and the performance status of the patient prior to PEL diagnosis (Boulanger et al., 2005); however, it has also been suggested that the KSHV viral load may also be an accurate predictor of clinical outcome of PEL patients (Simonelli et al., 2006). The level of immune suppression and the amount of circulating CD4+ lymphocytes may also contribute to the aggressiveness of PEL.
Multicentric Castleman’s Disease
Around the same time as PEL, the plasmablastic variant of MCD was also found to be associated with KSHV infection (Gessain et al., 1996; Soulier et al., 1995). MCD also exists in a hyaline variant that is not associated with KSHV (Waterston & Bower, 2004). MCD is an uncommon disseminated lymphadenopathy characterized by an abnormal proliferation of IgM λ-restricted plasmablasts within the mantle zone of B cell follicles (Du et al., 2001; Soulier et al., 1995). MCD is considered non-neoplastic since the plasmablasts are typically polyclonal, however monoclonal B cell expansions have been observed (Radaszkiewicz, Hansmann, & Lennert, 1989). The plasmablasts are large with a vesicular nucleus containing one or more nucleoli (Dupin et al., 2000). Systemic symptoms and inflammation as well as involvement of multiple organs often accompany MCD diagnosis (Nishimoto et al., 2005; Waterston & Bower, 2004).
KSHV coinfection is observed in almost all HIV-positive MCD, although only a small proportion of cells in affected lymph nodes typically harbor the virus. Interestingly, KSHV infection in MCD is quite lytic as compared to KS and PEL (Chadburn et al., 2008; Polizzotto, Uldrick, Hu, & Yarchoan, 2012). KSHV is detected in less than 40% of HIV-negative MCD cases (Nishimoto et al., 2005; Parravicini et al., 1997; Soulier et al., 1995; Waterston & Bower, 2004); however, in patients coinfected with HIV and KSHV, MCD tends to be very aggressive with rapid disease progression. One cause of the high fatality among these cases is that other KSHV-associated malignancies including KS and PEL are frequently observed with HIV-associated MCD (Oksenhendler et al., 2002; Oksenhendler et al., 1996). MCD progression is thought to be driven by dysregulated cytokine levels, including IL-6, IL-10, and vascular endothelial growth factor (VEGF) (Nishi et al., 1999; Nishimoto et al., 2005; Oksenhendler et al., 2000; Yoshizaki et al., 1989). In KSHV+ MCD, expression of the virally-encoded IL6 (vIL6) cytokine likely exacerbates inflammation and disease progression. vIL6 can enhance cytokine signaling and further increase human IL6 and VEGF expression (Aoki et al., 1999; Aoki, Tosato, Fonville, & Pittaluga, 2001; Boulanger et al., 2004; Osborne, Moore, & Chang, 1999). A cohort of plasmablastic MCD patients with detectable vIL6 expression were found to have a rapidly fatal clinical course as compared to vIL6-negative MCD patients, suggesting the importance of cytokine signaling in MCD progression (Parravicini et al., 1997).
KSHV-associated Inflammatory Cytokine Syndrome
In the past few years, several studies have reported patients that present with MCD-like inflammatory symptoms but lack lymphadenopathy or other pathological evidence of true MCD (Uldrick et al., 2010). These patients typically have elevated cellular and viral cytokine levels, including human IL6, IL10, C-reactive protein, and the viral cytokine vIL6 (Uldrick et al., 2010). As compared to KS patients, high KSHV viral loads are also observed, indicative of a lytic or reactivated KSHV infection (Tamburro et al., 2012; Uldrick et al., 2010). Concurrent KS is frequently observed in these patients as well. Because of the systemic inflammatory symptoms, the proposed name for this disease is KSHV inflammatory cytokine syndrome or KICS. It differs from the chronic immune activation disease sometimes seen in HIV patients because two requirements for a KICS diagnosis include detection of high KSHV viral load and vIL6 cytokine levels (Polizzotto et al., 2012).
There has been some controversy as to whether KICS is truly a distinct syndrome, since its diagnosis is typically made by exclusion of an MCD diagnosis. It has been proposed that KICS is a heterogeneous condition or a “prodrome” that eventually evolves into KSHV+ MCD, although some patients never progress to this point (Polizzotto et al., 2012). Recently, a group investigated whether polymorphisms in the KSHV-encoded microRNAs (miRNA) could be correlated with the development of KICS (Ray et al., 2012). They found that a higher percentage KSHV+ MCD and KICS patients had single nucleotide polymorphisms (SNPs) in the KSHV miRNA loci than KS patients or KSHV+/KS-negative control patients. They also utilized classification tree analysis to determine combinations of SNPs that may predict development of KSHV+ MCD and KICS. Another recent case study identified a KICS patient with high viral loads of both KSHV and the ubiquitous human herpesvirus 6A, suggesting a possible role for other pathogens in development of KICS (Tamburro et al., 2012).
KSHV Immune Reconstitution Inflammatory Syndrome
A small percentage of patients that begin HAART to treat advanced HIV infection exhibit a rapid deterioration of their clinical status. This phenomenon is known as immune reconstitution inflammatory syndrome (IRIS). It is proposed that following immune reconstitution, the increase in functional CD4+ T cell populations causes an immune recognition and response to autoantigens or pathogens that were previously present but asymptomatic. Cases of IRIS have been reported against KSHV and other pathogens such as Mycobacterium tuberculosis, Mycobacterium avium, Cryptococcus neoformans, and human cytomegalovirus (CMV) (Shelburne et al., 2002). In many instances, treatment of the offending pathogen or use of anti-inflammatory drugs can improve prognosis. High morbidity is observed in patients experiencing KS flares following initiation of HAART (IRIS-KS), although administration of systemic chemotherapy can control flares and cause tumor regression (Leidner & Aboulafia, 2005; Letang et al., 2013). Interestingly, one study determined that IRIS-KS patients had a significantly higher CD4+ count at KS diagnosis following HAART initiation than patients who did not develop IRIS, and that the mean time to KS diagnosis following HAART was less than 2 months (Bower et al., 2005). They also found that patients receiving more potent HAART regimens were more prone to IRIS-KS development. Beginning HAART treatment prior to advanced HIV infection or diagnosis of AIDS-KS decreases the chance of IRIS-KS (Letang et al., 2013).
KSHV Biology: Virion, Transmission, and Viral Lifecycle
The Herpesviridae are a large family of double stranded DNA viruses that have broad species tropism. There are eight known human herpesviruses that fall into three subgroups: the α-, β-, and γ-herpesviruses. The α-herpesviruses include herpes simplex 1 (HHV1) and 2 (HHV2) as well as varicella zoster virus (VZV, HHV3), which is the causative agent of chicken pox. The β-herpesviruses include CMV (HHV5) and human herpesviruses 6 and 7. The γ-herpesviruses have transforming capabilities, and this subgroup includes KSHV (HHV8) as well as EBV (HHV4), which causes mononucleosis and several human malignancies (Cesarman, 2011). The gammaherpesvirus group is also divided into the γ-1 lymphocryptoviruses which includes EBV and the γ-2 rhadinoviruses which includes KSHV. Although some herpesviruses, such as EBV and CMV, are ubiquitous in the human population, others like KSHV have varying infection rates depending on geographic location (Uldrick & Whitby, 2011).
Similar to all herpesviruses, the KSHV virion is surrounded by a lipid bilayer envelope studded with the virally-encoded glycoproteins gB, gH, gM, gL, gN, ORF68, and K8.1 (Bechtel, Winant, & Ganem, 2005; Zhu, Chong, Wu, & Yuan, 2005). A proteinaceous tegument exists between the envelope and the viral capsid. The tegument contains viral proteins including ORFs 21, 33, 45, 63, 64, and 75 (J. T. Bechtel et al., 2005; Zhu et al., 2005) as well as 11 viral RNA transcripts (Bechtel, Grundhoff, & Ganem, 2005). KSHV has an icosahedral capsid that is made up of repeating patterns of five viral proteins including the major capsid protein (ORF25), ORF62, ORF26, ORF 17.5, and the small capsid protein (ORF65) (Nealon et al., 2001; Wu et al., 2000). The viral genome is made up of linear double stranded DNA that circularizes during latent infection. The genome contains approximately 140 kb of unique coding sequence that is flanked by 25–30 kb of repetitive terminal repeats (Renne et al., 1996). The KSHV open reading frames (ORFs) are numbered from ORF1 on the left end of the genome to ORF75 on the right end of the genome. ORFs that are unique to KSHV carry a “K” designation, such as ORF K1. KSHV also encodes microRNAs and other non-coding RNAs (Cai et al., 2005; Pfeffer et al., 2005; Samols, Hu, Skalsky, & Renne, 2005; Sun, Lin, Gradoville, & Miller, 1996).
It appears that KSHV is mainly transmitted by saliva (Cattani et al., 1999; de França, de Araújo, Ribeiro, & Leao, 2011), although there is potential for transmission by blood or blood products (Hladik et al., 2006), solid organ donation (Francès et al., 2009), or sexual contact (de Sanjose et al., 2009). In vivo, KSHV has been detected in endothelial cells, epithelial cells, B cells, and monocytes (Ambroziak et al., 1995; Blasig et al., 1997; Dupin et al., 1999; Pauk et al., 2000), but in culture, the virus can infect a wider variety of cells including fibroblasts, keratinocytes, B lymphocytes, monocytes, plasmacytoid dendritic cells (pDCs), endothelial cells, and epithelial cells (Akula et al., 2003; Kaleeba & Berger, 2006a, 2006b; Lagunoff et al., 2002; Raghu, Sharma-Walia, Veettil, Sadagopan, & Chandran, 2009; Rappocciolo et al., 2008; Rappocciolo et al., 2006; Renne, Blackbourn, Whitby, Levy, & Ganem, 1998; West, Gregory, Sivaraman, Su, & Damania, 2011).
The KSHV glycoproteins mediate fusion between the virus and the target cell (Pertel, 2002). gB, gH, ORF4, and gpK8.1A bind heparin sulfate which may aid the virus in interacting with cellular receptors (Akula, Wang, Vieira, & Chandran, 2001; Birkmann et al., 2001). gB contains an RGD integrin-binding motif and αVβ3 and αVβ5 integrins have been shown to play a role in viral entry (Akula, Pramod, Wang, & Chandran, 2002; Garrigues, Rubinchikova, DiPersio, & Rose, 2008; Wang, Akula, Sharma-Walia, Zeng, & Chandran, 2003). The dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing non-integrin (DC-SIGN) expressed on activated B cells, DCs, and macrophages can also bind KSHV (Rappocciolo et al., 2008; Rappocciolo et al., 2006). Furthermore, the 12-transmembrane glutamate/cysteine exchange transporter protein xCT can also serve as a receptor for KSHV (Kaleeba & Berger, 2006b). Once bound to a receptor, KSHV mainly enters the cell via clathrin-mediated endocytosis or macropinocytosis (Akula et al., 2003; Raghu et al., 2009).
Following entry, virion proteins modulate cellular signaling pathways to alter the cytoskeleton to allow the capsid to be delivered to the nucleus where it uncoats and deposits the viral genome (Naranatt, Akula, Zien, Krishnan, & Chandran, 2003; Naranatt, Krishnan, Smith, & Chandran, 2005; Raghu et al., 2007; Sharma-Walia et al., 2005). Latency is typically established upon infection, in which a very limited repertoire of viral genes are expressed, including the latency-associated nuclear antigen (LANA), vCyclin, vFLIP, vIRF4 (LANA2), kaposin, and the viral microRNAs. LANA binds both of the terminal repeat regions to circularize the viral genome and tether it to the host chromosome. Conversely, lytic replication occurs infrequently after de novo infection or when the virus undergoes reactivation from latency. Reactivation is thought to be caused by a variety of cell stresses including cytokine signaling, cell differentiation, reactive oxygen species, or innate immune signaling by toll-like receptors (TLRs) (Chang, Renne, Dittmer, & Ganem, 2000; Gregory et al., 2009; Ye et al., 2011; Yu et al., 2007). In culture, histone deacetylase inhibitors and phorbol esters can also reactivate the virus (Yu et al., 1999). It was recently shown that depletion of cellular tousled like kinases (TLKs) can also contribute to reactivation of KSHV from latency (Dillon et al., 2013). The KSHV lytic transactivator, RTA, initiates a complex transcriptional program that results in the expression of all viral genes, replication of the viral genome, and the subsequent assembly, egress, and release of progeny virions (Lukac, Kirshner, & Ganem, 1999; Sun et al., 1998). Spontaneous lytic replication is also seen at varying levels in each of the KSHV-associated malignancies; however, the majority of the infected cells remain latent, suggesting a large role for the latent viral proteins in KSHV pathogenesis.
Viral Latency & Associated Proteins
Latency is the default lifecycle for KSHV following infection of a host cell. During latency, LANA circularizes and tethers the viral genome to the host chromosomes by simultaneously binding both the terminal repeats and host histones H2A and H2B (Barbera, Ballestas, & Kaye, 2004; Barbera et al., 2006; Cotter & Robertson, 1999). The viral genome is replicated by host machinery with each cell division, and therefore persists as it is passed to each daughter cell (Hu, Garber, & Renne, 2002; Verma, Choudhuri, Kaul, & Robertson, 2006). As mentioned, only a small portion of the viral genome is actively transcribed during latency, and this region is known as the latency locus. This locus includes the viral genes LANA, vFLIP, vCyclin, kaposin, and the viral microRNAs (Dittmer et al., 1998; Sin & Dittmer, 2013). The LANA promoter controls expression of LANA, vCyclin, and vFLIP while the kaposin promoter drives expression of three kaposin transcripts, a bicistronic transcript for vCyclin and vFLIP, and the twelve viral pre-miRNAs (Pearce, Matsumura, & Wilson, 2005; Talbot, Weiss, Kellam, & Boshoff, 1999). Additionally, PEL express vIRF3 (LANA2) during latency (Rivas, Thlick, Parravicini, Moore, & Chang, 2001). Transgenic mice expressing some or all of the KSHV latency locus have phenotypes characteristic of KSHV malignancies (Fakhari, Jeong, Kanan, & Dittmer, 2006; Sin & Dittmer, 2013). The viral latent genes and microRNAs have been investigated in depth to understand the mechanism by which KSHV causes disease. In this section, we will discuss each of the elements of the latency locus.
LANA
LANA is encoded by ORF73 and is KSHV’s major latency protein. It is responsible for tethering the viral episome to the host genome via the terminal repeats and histone interactions (Barbera et al., 2006; Cotter & Robertson, 1999), which allows host machinery to replicate and distribute the latent genome to daughter cells (Barbera et al., 2004; Hu et al., 2002; Verma et al., 2006). The phosphorylated DNA-damage response protein γH2AX and the cellular replication fork factors Timeless and Tipin are some of the many known cellular proteins that assist LANA in maintaining KSHV episomes (Ballestas & Kaye, 2011; Dheekollu, Chen, Kaye, & Lieberman, 2013; Jha et al., 2013). LANA has also been shown to positively and negatively affect the transcription of a number of host genes (An et al., 2005; Schwam, Luciano, Mahajan, Wong, & Wilson, 2000). This is likely mediated through LANA’s interaction with many transcription factors (Ballestas & Kaye, 2011). LANA can also autoregulate its expression by inducing transcription from the LANA promoter (Jeong et al., 2004). Furthermore, LANA and the LANA homologue in rhesus rhadinovirus (RRV) can repress transcription of the viral lytic transactivator, RTA (ORF50) to help maintain latency (DeWire & Damania, 2005; Lan, Kuppers, & Robertson, 2005; Lan, Kuppers, Verma, & Robertson, 2004).
LANA has several mechanisms by which it can promote host cell survival and proliferation. LANA can bind and inhibit p53 to reduce activation of p53-dependent reporter genes and cause chromosomal instability (Friborg, Kong, Hottiger, & Nabel, 1999; Si & Robertson, 2006). LANA can also bind and inactivate the tumor suppressor Rb leading to increased E2F-dependent reporter gene activation (Radkov, Kellam, & Boshoff, 2000). Furthermore, LANA induces cytoplasmic β-catenin accumulation by binding and sequestering GSK-3β in the nucleus (Fujimuro et al., 2003), thus allowing upregulation of the pro-growth proteins cyclin D and c-myc by the transcription factor LEF. LANA can also stabilize c-myc protein levels (Bubman, Guasparri, & Cesarman, 2007; Liu, Martin, Liao, & Hayward, 2007). LANA has been shown to increase telomerase expression, which increases the lifespan of infected cells (Verma, Borah, & Robertson, 2004). Finally, B cell-specific expression of LANA in a transgenic mouse model led to follicular hyperplasia, increased germinal center formation, and lymphomas, implicating LANA as a key player in KSHV-associated lymphomagenesis (Fakhari et al., 2006).
vCyclin
vCyclin is another latently-expressed protein and is encoded by ORF72. vCyclin shares sequence and functional homology with cellular cyclin D2 and can bind and activate the cyclin-dependent kinase cdk6 (Chang et al., 1996; Li et al., 1997). When in complex with cdk6, vCyclin can phosphorylate and inactivate the tumor suppressor Rb, the cdk inhibitor p27 (Kip), and the anti-apoptotic protein Bcl-2, collectively leading to cell cycle deregulation (Ellis et al., 1999; Godden-Kent et al., 1997; Ojala et al., 2000). vCyclin-cdk6 can also phosphorylate histone H1 and cdc25a (Godden-Kent et al., 1997). Interestingly, vCyclin transgenic mice develop lymphomas deficient in p53 (Verschuren et al., 2004). This is likely because vCyclin can also bind cdk9 which induces p53 phosphorylation and cell cycle arrest, so only cells which have lost p53 can continue to divide and expand into a lymphoma (Chang & Li, 2008).
vFLIP
KSHV K13 encodes vFLIP, which is a viral homolog of cellular FLIP (FLICE [protein FADD-like interleukin-1 beta-converting enzyme, now called caspase-8] inhibitory protein). vFLIP is expressed during latency, and contains two death effector domains that can associate with FADD and prevent the CD95 death receptor from activating the apoptosis-inducing protease caspase 8 (FLICE) (Thome et al., 1997). It was subsequently shown that vFLIP can bind procaspase 8 directly to prevent its cleavage into active caspase 8 (Bélanger et al., 2001). Furthermore, vFLIP persistently activates nuclear factor kappa B (NFκB) signaling through binding to IKKα, IKKβ, RIP, and the NEMO complex (Liu et al., 2002; Matta, Sun, Moses, & Chaudhary, 2003). This signaling contributes to the transforming potential of vFLIP (Sun, Zachariah, & Chaudhary, 2003). In vivo studies demonstrate that vFLIP transgenic mice can develop lymphomas and B cell-derived tumors (Ahmad et al., 2010; Ballon, Chen, Perez, Tam, & Cesarman, 2011; Chugh et al., 2005).
The Kaposins
ORF K12 encodes three transcripts that yield kaposin A, B, and C (Sadler et al., 1999). Kaposin is highly abundant in PEL, and is transforming in cell culture-based assays (Muralidhar et al., 1998). Kaposin A was shown to interact with the ARF guanine nucleotide exchange factor cytohesin-1 to mediate cellular transformation and activation of the ERK/MAPK pathway (Kliche et al., 2001). Kaposin B plays a role in preventing the decay of cytokine mRNAs by binding and activating the p38/MAPK target kinase MK2 (McCormick & Ganem, 2005, 2006). MK2 can inhibit the decay of mRNAs that contain AU-rich elements, which include cytokine mRNAs and the mRNA for PROX1. KSHV induces reprogramming of blood vascular endothelial cells towards a lymphatic lineage through upregulation of PROX1, and the ability of kaposin B to stabilize PROX1 mRNA is critical for this process (Yoo et al., 2010).
KSHV microRNAs
Similar to other members of the herpesvirus family, KSHV encodes 12 viral pre-microRNAs (pre-miRNAs) that are processed by the host proteins Drosha and Dicer to generate mature miRNAs. The KSHV pre-miRNAs are transcribed from the latent Kaposin/K12 promoter. While 10 of the pre-miRNAs are located in a Kaposin intron, the remaining 2 are located in the Kaposin protein-coding region and the Kaposin 3’ UTR (Cai et al., 2005; Pfeffer et al., 2005; Samols et al., 2005) . The 12 viral pre-miRNAs generate 24 mature miRNAs that have all been detected in KSHV-infected cells. Furthermore, RNA editing of the 5’ end of pre-miR-K12-10 can yield multiple mature miR-K12-10 species (Umbach & Cullen, 2010). Despite having a common promoter, the mature KSHV miRNAs each exist at high but variable levels in latent PEL cell lines, and miR-K12-10 and miR-K12-12 levels are further increased during lytic replication (Lin et al., 2010; Samols et al., 2005; Umbach & Cullen, 2010). In PEL cell lines, over 90% of the expressed mature miRNAs are KSHV miRNAs. Several studies comparing clinical samples of KS biopsies and PEL to cultured PEL cell lines report that the KSHV pre-miRNAs are expressed at even higher levels in vivo and that their sequences are highly conserved between patients (Marshall et al., 2007; O'Hara et al., 2009). Functional KSHV miRNAs are also found in the virion, along with mRNAs, cellular miRNAs, and other small RNA species (Lin, Li, Liang, & Lan, 2012).
A number of validated host and viral mRNA targets of the KSHV miRNAs have been identified. These targets are involved in a variety of viral and cellular processes including maintenance of viral latency, immune evasion, cell cycle regulation, cell survival and proliferation, and apoptosis. miR-K9* has been shown to directly target the 3’ UTR of the ORF50/RTA mRNA to prevent lytic reactivation (Bellare & Ganem, 2009), and miR-K12-5 may also indirectly suppress the RTA transcript (Lu, Stedman, Yousef, Renne, & Lieberman, 2010). Furthermore, miR-K12-4 can target the DNA methyltransferase repressor Rbl2 to epigenetically maintain latency (Lu et al., 2010), and miR-K12-1 targets the 3’ UTR of the NFκB repressor IκBα to enhance NFκB signaling and promote latency (Lei et al., 2010). Two components of the TLR/IL-1R signaling cascade, IRAK1 and MyD88, were identified as targets of miR-K12-9 and miR-K12-5, respectively, which results in reduced IL6 and IL8 inflammatory cytokine production (Abend et al., 2012). Furthermore, miR-K12-7 can directly bind the 3’ UTR of the mRNA of MICB, the stress-induced natural killer (NK) cell ligand, to repress MICB translation and promote viral immune evasion by diminishing NK cell function (Nachmani, Stern-Ginossar, Sarid, & Mandelboim, 2009). KSHV miR-K12-1 is able to prevent cell cycle arrest by targeting the CDK inhibitor p21 to promote cell division (Gottwein & Cullen, 2010). KSHV infection may prime B cells for transformation by expression of miR-K12-11, which is an ortholog of cellular miR-155 (Boss et al., 2011; Gottwein et al., 2007; Skalsky et al., 2007). miR-K12-11 targets the host protein Jarid2 and both miR-155 and miR-K12-11 can induce expansion of splenic CD19+ B cells in vivo (Boss et al., 2011; Dahlke et al., 2012). Additionally, miR-K12-11 and -1 can induce MAPK signaling, promigration factors, and cell invasiveness through indirectly suppressing the MAPK phosphatase DUSP1 (Qin et al., 2013).
The KSHV miRNAs also have several mechanisms of preventing apoptosis of host cells: miR-K12-10 variants inhibit TGF-β signaling by targeting the 3’ UTR of the TGF-β type II receptor (TβRII) (Lei et al., 2012) and miR-K12-10a suppresses the tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) receptor (TWEAKER) to prevent TWEAK-mediated caspase activation, apoptosis, and proinflammatory cytokine production (Abend, Uldrick, & Ziegelbauer, 2010). Furthermore, it was demonstrated that miR-K12-1, -3, and 4-3p can target the 3’ UTR of caspase 3 to downregulate this host protein and inhibit apoptosis (Suffert et al., 2011). The KSHV miRNAs have a variety of cellular and viral targets, only a handful of which have been discussed here. Collectively, the miRNAs work to drive KSHV pathogenesis by promoting latency, providing favorable growth conditions, and preventing apoptosis of infected cells.
In addition to the genes described above, some reports have also shown that viral genes such as K1, vIL6, and K15 are expressed at low levels during latency but are highly upregulated during lytic replication (Chandriani & Ganem, 2010; Chen, Choi, Sandford, & Nicholas, 2009; Sharp et al., 2002). These viral proteins are discussed in the section on lytic pro-growth proteins.
KSHV Lytic Cycle
The KSHV lytic cycle can ensue following primary infection or when a latently infected cell undergoes lytic reactivation. During the lytic cycle, a temporal transcriptional cascade begins that results in expression of viral immediate early, delayed early, and late genes followed by the subsequent assembly and egress of progeny virions (Sun et al., 1999). As discussed earlier, a variety of cell stresses can induce reactivation (Chang et al., 2000; Gregory et al., 2009; Ye et al., 2011; Yu et al., 2007; Yu et al., 1999). Ultimately, expression of the lytic transactivator, ORF50/RTA (replication and transcription activator), is required to initiate this complex stage of the viral lifecycle. RTA expression alone is sufficient to drive lytic replication, and suppression of RTA prevents reactivation (Lukac et al., 1999; Lukac, Renne, Kirshner, & Ganem, 1998; Sun et al., 1998; Xu et al., 2005). RTA is an immediate-early gene, and it is part of a polycistronic transcript that also encodes K8 and K8.1. Other immediate early genes include ORF45 and K4.2 (Zhu, Cusano, & Yuan, 1999). RTA has an activation domain and a DNA-binding domain on opposite ends of the protein. The DNA-binding domain allows RTA to directly bind and activate numerous viral promoters and the two KSHV origins of lytic replication, OriLyt-L and OriLyt-R (Chen, Ye, Xie, Kuhne, & Gao, 2009; Ziegelbauer, Grundhoff, & Ganem, 2006). The activation domain allows RTA to interact with cellular transcription factors and chromatin modification complexes to promote viral gene transcription (Guito & Lukac, 2012).
Delayed early genes are sensitive to cyclohexamide treatment because in order to be transcribed they require the function of the proteins encoded by immediate early genes. The delayed early genes include the viral DNA polymerase and viral proteins required for viral DNA synthesis, as well as the viral thymidine kinase, nucleotide reductase, ORF57, the signal transduction proteins K1, K15, and vGPCR, and the immune evasion proteins K3 (MIR1) and K5 (MIR2) (Sun et al., 1999). Following expression of the delayed early genes, viral DNA replication begins from OriLyt-L and OriLyt-R (AuCoin et al., 2004; Lin et al., 2003). The viral replication machinery includes the viral DNA polymerase, helicase, polymerase processivity factor, primase, primase-associated factor, and single strand binding protein (Wu et al., 2001) and replication is thought to occur by a rolling circle mechanism similar to other herpesviruses. Viral DNA replication stimulates expression of the KSHV late genes, which mainly encode structural proteins such as the viral capsid and envelope proteins (Lu et al., 2004). Linear genomes are packaged into the newly forming capsids. KSHV ORF67 and ORF69 assist in nuclear egress (Desai, Pryce, Henson, Luitweiler, & Cothran, 2012; Luitweiler et al., 2013), and KSHV glycoprotein B is thought to play a role in viral maturation and egress from the host cell (Krishnan, Sharma-Walia, Zeng, Gao, & Chandran, 2005; Subramanian, Sehgal, D'Auvergne, & Kousoulas, 2010).
Lytic KSHV Proteins Involved in Cell Growth and Survival
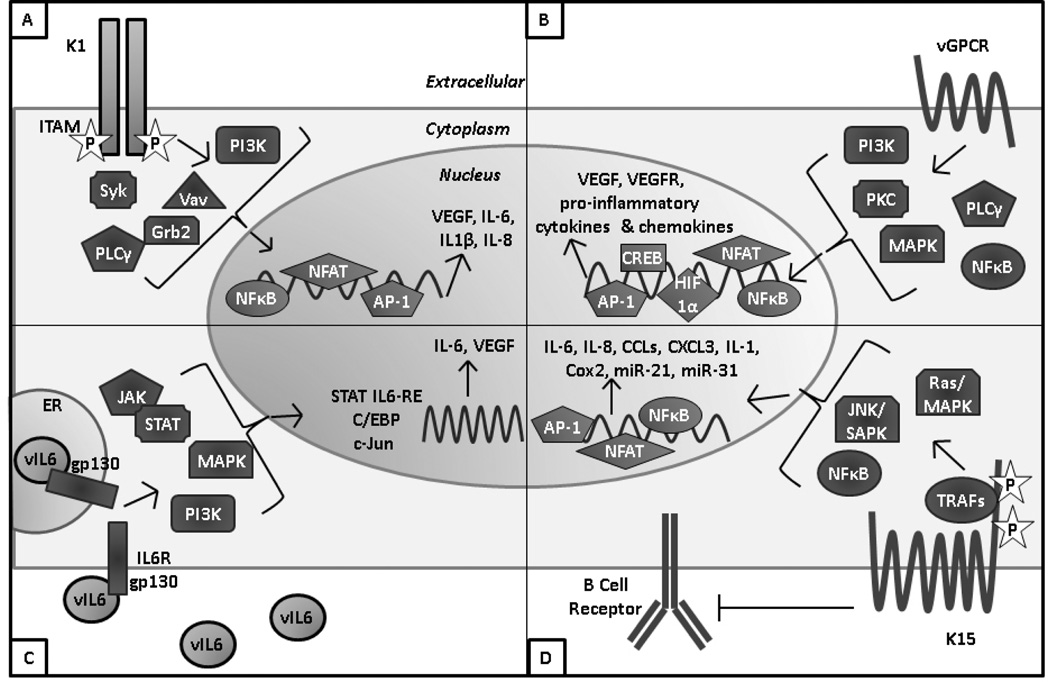
Lytic reactivation results in expression of all KSHV genes. As described earlier, several of the proteins encoded by the KSHV latency locus can drive cellular transformation. A number of proteins encoded by KSHV lytic genes also have pro-growth or transforming qualities, which are discussed in this section and summarized in Figure 1.
Figure 1. KSHV encodes a number of proteins that contribute to cell growth and transformation.
A) K1 is a transmembrane protein with a constitutively active immunoreceptor tyrosine activation motif (ITAM) that activates signaling through SH2-containing proteins. K1 expression results in production of VEGF and pro-inflammatory cytokines. B) vGPCR is a constitutively active homolog of the IL8 receptor that results in activation of numerous cell signaling pathways and transcription factors to increase production of VEGF, VEGFR, and proinflammatory cytokines and chemokines. C) vIL6 is a functional homolog of human IL6 that can signal through shared IL6 pathways including JAK/STAT, MAPK, and PI3K. This results in activation of multiple IL6 response elements and production of human IL6 and VEGF. D) K15 is a transmembrane protein with several tyrosine residues and SH2 and SH3 domains and in its cytoplasmic tail that are critical for K15’s interaction with cellular TRAFs and signaling through the MAPK and NFκB pathways. K15 signaling results in activation of numerous transcription factors and expression of pro-inflammatory cytokines and chemokines and several human miRNAs that are involved in cell motility.
K1
K1 is a single-pass transmembrane glycoprotein encoded by the first open reading frame of KSHV (Figure 1A). This protein is expressed on the cell and ER membranes and can be internalized to endosomes (Brinkmann & Schulz, 2006; Tomlinson & Damania, 2008). K1 is constitutively active and has a highly conserved intracellular immunoreceptor tyrosine-based activation motif (ITAM) on its C terminus (Lagunoff, Majeti, Weiss, & Ganem, 1999; Lee, Connole, Tang, Harris, & Jung, 2003; Lee, Guo, et al., 1998). Upon K1 oligomerization, the ITAM becomes autophosphorylated and can activate various Src homology 2 (SH2)-containing signaling proteins including PI3K (p85)/Akt, PLCγ, Vav, Syk, Lyn, RasGAP, and Grb2 (Lee et al., 2005; Prakash et al., 2005; Tomlinson & Damania, 2004). Additionally, ITAM signaling results in activation of NFκB, nuclear factor of activated T cells (NFAT), Oct-2 and AP-1(Prakash et al., 2005; Prakash et al., 2002).
Endothelial cells expressing K1 become immortalized in culture and primary marmoset T lymphocytes infected with a K1-expressing herpesvirus saimiri became immortalized to IL2-independent growth (Lee, Veazey, et al., 1998; Wang, Dittmer, Tomlinson, Fakhari, & Damania, 2006). K1 can also induce focus formation in rat fibroblasts (Lee, Veazey, et al., 1998). In vivo, K1 transgenic mice display constitutively active NFκB and Src family tyrosine kinase signaling and a fraction of the mice develop tumors (Prakash et al., 2002) There are several aspects of K1 signaling that likely contribute to its transforming function. K1’s activation of Akt results in inactivation of the proapoptotic forkhead (FKHR) transcription factor family which protects cells from FKHR- and Fas-mediated apoptosis (Tomlinson & Damania, 2004). Heat shock protein-90 and -40 (hsp90 and hsp40) were identified as K1 binding partners that are critical for both K1 expression and K1’s anti-apoptotic function (Wen & Damania, 2010). K1 also induces angiogenesis and VEGF production in primary human endothelial cells and cells derived from K1 transgenic animals (Prakash et al., 2005; Wang et al., 2006; Wang et al., 2004). Furthermore, K1 signaling can induce secretion of inflammatory cytokines that are implicated in KS lesion development, including IL6, GM-CSF, IL-1β, IL8, and IL10 (Lee et al., 2005; Prakash et al., 2002). A unique mechanism that K1 utilizes to prolong the life of B cells is to downregulate surface expression of the B cell receptor (BCR) by binding the µ chain of the BCR to retain the complex in the ER (Lee, Alvarez, Ishido, Lackner, & Jung, 2000). Overall, K1 is a multifunctional protein that can constitutively activate multiple pro-growth signaling pathways in KSHV infected cells.
Viral G protein Coupled Receptor (vGPCR)
KSHV ORF 74 encodes the viral G protein coupled receptor (vGPCR), which is a seven-pass transmembrane protein that shares homology with the human IL8 receptor (Figure 1B) (Cesarman et al., 1996). This lytic protein has been detected at low levels in cultured reactivated PEL and in KS, PEL, and MCD clinical specimens (Chiou et al., 2002). Conflicting reports have demonstrated that vGPCR has the ability to both sustain (Bottero et al., 2009) and repress (Cannon, Cesarman, & Boshoff, 2006) RTA expression and lytic replication. Although vGPCR can bind the CXC and CC families of chemokines, it is constitutively active even in the absence of ligand (Arvanitakis, Geras-Raaka, Varma, Gershengorn, & Cesarman, 1997; Bais et al., 1998; Gershengorn, Geras-Raaka, Varma, & Clark-Lewis, 1998). vGPCR activates a number of important signaling pathways, including PLC, PKC, MAPK, PI3K/Akt/mTOR, and NFκB (Montaner, 2007). Downstream signaling from these pathways activates the AP1, NFAT, NF-κB, HIF-1α, and CREB transcription factors which results in vGPCR-mediated production of VEGF, VEGF receptor (VEGFR), and proinflammatory cytokines and chemokines (Montaner, 2007). Through these signaling pathways, vGPCR can immortalize and promote the growth of endothelial cells in culture (Bais et al., 2003; Montaner, Sodhi, Pece, Mesri, & Gutkind, 2001; Sodhi et al., 2006). It was also demonstrated that endothelial cell-specific expression of vGPCR can cause formation of KS-like angioproliferative lesions in mice (Montaner et al., 2003). Similar to K1, vGPCR expression can also transform NIH3T3 fibroblasts, as well as rat kidney cells, which are then able to form tumors in nude mice (Bais et al., 1998). A line of transgenic mice expressing vGPCR in hematopoietic cells developed angioproliferative lesions resembling KS at multiple organ sites (Yang et al., 2000). However, another study with a line of transgenic mice with vGPCR expressed ubiquitously from an SV40 promoter found that lesions mainly occurred on the tail and/or legs and that only a small fraction of tumor cells actually expressed vGPCR (Guo et al., 2003). This work and others suggests a model of paracrine neoplasia by which vGPCR drives transformation of cells by inducing paracrine secretion of proinflammatory cytokines and angiogenic growth factors which can then work in concert with KSHV latent proteins to promote tumorigenesis.
Viral Interleukin-6 (vIL-6)
KSHV ORF K2 encodes the viral interleukin-6 (vIL6) cytokine (Figure 1C). vIL6 is induced upon lytic replication, but it is also expressed at low levels during latency. Although vIL6 has been detected in KSHV-associated malignancies (Aoki, Tosato, et al., 2001; Aoki, Yarchoan, et al., 2001), levels are highest in MCD lesions and patient sera (Parravicini et al., 1997). This protein shares about 25% amino acid identity and 63% similarity with human IL6 (hIL6) (Moore, Boshoff, Weiss, & Chang, 1996; Neipel et al., 1997; Nicholas et al., 1997). Additionally, vIL6 shares many functional characteristics with hIL6, and as a result the viral cytokine can activate gp130 (the IL6 receptor) and downstream signaling pathways, including the JAK/STAT, MAPK, and PI3K/Akt pathways (Hideshima et al., 2000; Molden, Chang, You, Moore, & Goldsmith, 1997). These pathways induce a variety of transcription factors and response elements (RE) such as the STAT1/3 and STAT5 IL6 RE, C/EBP, and c-jun promoter IL6 RE (JRE-IL-6) (Osborne et al., 1999). Activation of these pathways leads to expression of hIL6 (Mori et al., 2000) and VEGF (Aoki et al., 1999). However, vIL6 differs from the human cytokine in several regards. vIL6 does not require the gp80 subunit of the IL6 receptor to induce an intracellular signal, whereas hIL6 does (Aoki, Narazaki, Kishimoto, & Tosato, 2001; Chow, He, Snow, Rose-John, & Garcia, 2001; Wan, Wang, & Nicholas, 1999); however, gp80 can still bind to vIL6 and enhance signaling (Boulanger et al., 2004; Hu & Nicholas, 2006; Li, Wang, & Nicholas, 2001). Additionally, hIL6 is secreted much more efficiently than vIL6, and a large portion of expressed vIL6 is actually retained in the endoplasmic reticulum (ER) (Chen, Sandford, & Nicholas, 2009; Meads & Medveczky, 2004). In the ER, vIL6 interacts with the ER chaperone calnexin which impacts vIL6 localization and intracellular retention (D. Chen, Y. B. Choi, et al., 2009). Furthermore, vIL6 undergoes N-linked glycosylation which is required for its signaling activities (Dela Cruz et al., 2004).
vIL6 expression transforms NIH3T3 fibroblasts and these cells form tumors in nude mice (Aoki et al., 1999). vIL6 expression can also induce growth in mouse hybridoma (Hideshima et al., 2000), PEL (Chatterjee, Osborne, Bestetti, Chang, & Moore, 2002; Jones et al., 1999), BAF (Hu & Nicholas, 2006), and Hep3B hepatoma (Nicholas et al., 1997) cell lines. In endothelial cells, vIL6 expression induces proliferation, tubule formation, and neoangiogenesis (Zhou et al., 2013; Zhu et al., 2013). Additionally, vIL6 can help cells escape interferon (IFN)-induced growth arrest (Chatterjee et al., 2002). Furthermore, transgenic mice expressing vIL6 under the MHC class I promoter develop plasmablastic MCD-like disease, which is abrogated in the absence of endogenous IL6 (Suthaus et al., 2012).
K15
KSHV K15 is encoded by the rightmost open reading frame of the virus (Figure 1D). K15 has two highly divergent alleles called the predominant (P) and minor (M) forms, and these are present in different strains of KSHV (Poole et al., 1999). K15 is expressed at low levels in latent PEL, but is robustly induced following lytic reactivation. The transcript is spliced to yield multiple K15 proteins with 4-12 transmembrane domains that localize to lipid rafts (Choi, Lee, Shim, Li, & Jung, 2000; Glenn, Rainbow, Auradé, Davison, & Schulz, 1999). The short K15 cytoplasmic tail contains SH3 and SH2 signaling motifs and binding sites for TRAFs 1, 2, and 3 (Brinkmann, Pietrek, Dittrich-Breiholz, Kracht, & Schulz, 2007; Glenn et al., 1999). Several critical tyrosine residues within these motifs are constitutively phosphorylated by cellular Src family tyrosine kinases, which mediate activation of downstream signaling pathways. Pathways activated by K15 signaling include the Ras/MAPK, JNK/SAPK, and NFκB pathways as well as the NFAT/AP1 transcription factors (Brinkmann et al., 2003; Brinkmann et al., 2007; Cho, Choi, & Choi, 2008). This signaling activates transcription of a number of cellular cytokines and chemokines including IL6, IL8, CCL20, CCL2, CXCL3, IL-1α/β, and Cox2 (Brinkmann et al., 2007; Wang et al., 2007). K15 can also downregulate signal transduction and intracellular calcium mobilization induced by the BCR, which may help the virus maintain latency (Choi et al., 2000). A potential mechanism by which K15 accomplishes this may be through K15’s interaction with the tyrosine kinase Lyn, which plays a role in the regulation of BCR signaling (Cho et al., 2008). Additionally, the K15 M allele induces cell motility, and this is dependent on K15-mediated upregulation of the human miRNAs miR-21 and -31 (Tsai et al., 2009). K15 may contribute to KSHV-induced tumorigenesis through its ability to activate pro-growth signaling pathways, promote latency, and induce cell motility.
KSHV’s Activation and Evasion of the Host Immune Response
The human immune system is designed to recognize invading pathogens in order to launch an innate and adaptive response to eliminate infection. KSHV utilizes a number of mechanisms to dampen the immune response so that it can persist for the lifetime of the host. In this section, we will discuss aspects of the innate and adaptive immune response that are activated by KSHV infection as well as aspects that are suppressed by viral immune evasion techniques.
Immune Activation
Toll-like receptors (TLR) are innate pattern recognition receptors that recognize pathogen-associated molecular patterns (PAMPs) and induce NFκB signaling and production of type I IFN and proinflammatory cytokines (Kawai & Akira, 2010). KSHV can activate TLR3 during infection of primary human monocytes, and this upregulates TLR3 expression and the production of IFNβ and CXCL10 (Gregory & Damania, 2009; West & Damania, 2008) which are then downregulated as latency is established (Jacobs et al., 2013). Although KSHV can reduce TLR4 activity in endothelial cells, TLR4 activation is still capable of inhibiting KSHV infection because cells lacking this receptor are more susceptible to infection (Lagos et al., 2008). Thus, there is an initial TLR-mediated innate immune response to KSHV primary infection, but in many cases this response is subsequently downregulated by the virus. KSHV is also sensed by IFN gamma-inducible factor IFI-16, which triggers inflammasome formation and subsequent production of IL-1β (Kerur et al., 2011; Singh et al., 2013). Additionally, KSHV infection can activate plasmacytoid dendritic cells (pDCs) which results in TLR9-mediated production of IFNα (West et al., 2011).
KSHV-associated diseases typically occur in immune compromised patients, and it has been demonstrated that reconstitution of the immune system can result in KSHV-associated tumor regression (Bihl et al., 2007). This suggests a role for the adaptive immune response, particularly the CD8+ T cell response, in controlling KSHV infection and pathogenesis (Hislop & Sabbah, 2008; Lambert et al., 2006; Wang et al., 2001). CD4+ T cells can weakly recognize KSHV latent antigens such as LANA (Sabbah et al., 2012). Additionally, it was found that CD4+ and CD8+ T cells from KSHV-seropositive patients frequently recognize select groups of early lytic and late lytic KSHV genes (Robey et al., 2009). In a cohort of seven KSHV+/HIV+ KS patients on HAART, KSHV-specific immune responses were detected in six of the seven patients (Bihl et al., 2009). Interestingly, 100% of the non-progressor patients had KSHV-specific CD8+ cytotoxic T lymphocytes (CTLs) that simultaneously secreted IFNγ and TNFα in response to KSHV antigen whereas only 60% of the patients with progressive disease had a CD8+ CTL response (Bihl et al., 2009). Although most studies of the adaptive immune response to KSHV have investigated the T cell response, KSHV infection also generates a humoral response to a variety of viral antigens (Zheng et al., 2011).
Evasion of the Adaptive Immune Response
KSHV employs a variety of mechanisms to evade KSHV-specific adaptive immune responses (reviewed in (Aresté & Blackbourn, 2009)). These techniques mainly involve repressing viral antigen presentation, T cell activation, B cell receptor (BCR)-mediated B cell activation, and B cell differentiation.
KSHV infection of B cells, dendritic cells (DCs), macrophages, and endothelial cells results in decreased expression of the major histocompatibility complex class I (MHC-I) (Rappocciolo et al., 2006; Tomescu, Law, & Kedes, 2003). MHC-I is critical for the presentation of viral antigens to the T cell receptor (TCR) of CD8+ T cells. KSHV K3 and K5 (also called modulator of immune recognition (MIR) 1 and 2, respectively) are capable of ubiquitinating the MHC-I cytoplasmic tail to trigger endocytosis and proteasomal degradation of the complex (Coscoy, Sanchez, & Ganem, 2001; Ishido, Wang, Lee, Cohen, & Jung, 2000). vIRF1, vFLIP, and the virally-encoded shutoff and exonuclease protein (KSHV SOX) can also cause downregulation of MHC-I (Lagos et al., 2007; Zuo et al., 2008). vIRF3 and cellular suppressor of cytokine signaling 3 (SOCS3) were recently found to inhibit antigen presentation by the MHC-II complex by reducing the level of MHC-II transcripts (Butler et al., 2012; Zuo, Hislop, Leung, Sabbah, & Rowe, 2013). Additionally, vIRF3 expression renders PEL resistant to recognition by KSHV-specific CD4+ T cells. LANA, which is expressed in all KSHV-infected cells, has an acidic central repeat domain that prevents its antigenic processing to further hinder this process (Kwun et al., 2007; Zaldumbide, Ossevoort, Wiertz, & Hoeben, 2007). In addition to repressing antigen presentation, KSHV infection also causes downregulation of the costimulatory molecules CD80, CD86, CD1a, and CD83 on antigen presenting cells (APCs) (Gregory, Wang, West, Dittmer, & Damania, 2012). K5 likely plays a role in this, because it has been shown to downregulate CD86 and ICAM-1 (Coscoy & Ganem, 2001). These costimulatory molecules are required for TCR-mediated activation of CTLs, so the downregulation of these proteins is a mechanism by which KSHV infection inhibits the adaptive T cell immune response.
As discussed previously, B cells are one of the main target cells of KSHV infection. B cells are a critical part of the adaptive immune response, and following binding of antigen to the B cell receptor (BCR), these cells proliferate and differentiate into antibody-producing plasma cells or memory B cells (Shapiro-Shelef & Calame, 2005). Antibodies eliminate infection by binding to antigen that is either in the extracellular space or presented on the surface of infected cells. Antibody binding generally results in neutralization or phagocytosis of the pathogen or infected cell. If a B cell is unable to be activated through its BCR or unable to differentiate into a plasma cell, antibody production will not occur. One hypothesis is that KSHV targets these two aspects of B cell biology as a mechanism of adaptive immune evasion. The KSHV K5 protein can utilize its ubiquitin ligase activity to downregulate bone marrow stromal antigen 2 (BST-2, also called tetherin) which is an IFN-inducible protein that plays a role in B cell differentiation (Bartee, McCormack, & Früh, 2006). As mentioned earlier, the KSHV K1 signaling protein plays a role in downregulation of the BCR on the cell surface (Lee et al., 2000). Furthermore, KSHV K15 is capable of disrupting signaling from the BCR and possibly accelerating BCR internalization to further reduce BCR-mediated B cell activation (Choi et al., 2000; Lim et al., 2007). Collectively, this inhibition of both B cell differentiation and BCR signaling may help KSHV evade the B cell immune response.
Evasion of the Innate Immune Response
A large portion of the KSHV genome is devoted to evading the innate immune response of the host. The innate immune functions targeted by viral proteins include interferon production, interferon regulatory factor (IRF) activation, natural killer (NK) cell activity, complement activation, inflammasome activation, and chemokine activity. In this section we will discuss the strategies utilized by KSHV to hinder the innate immune response to allow the virus to persist for the lifetime of the host.
Interferon and IRF Inhibition by KSHV
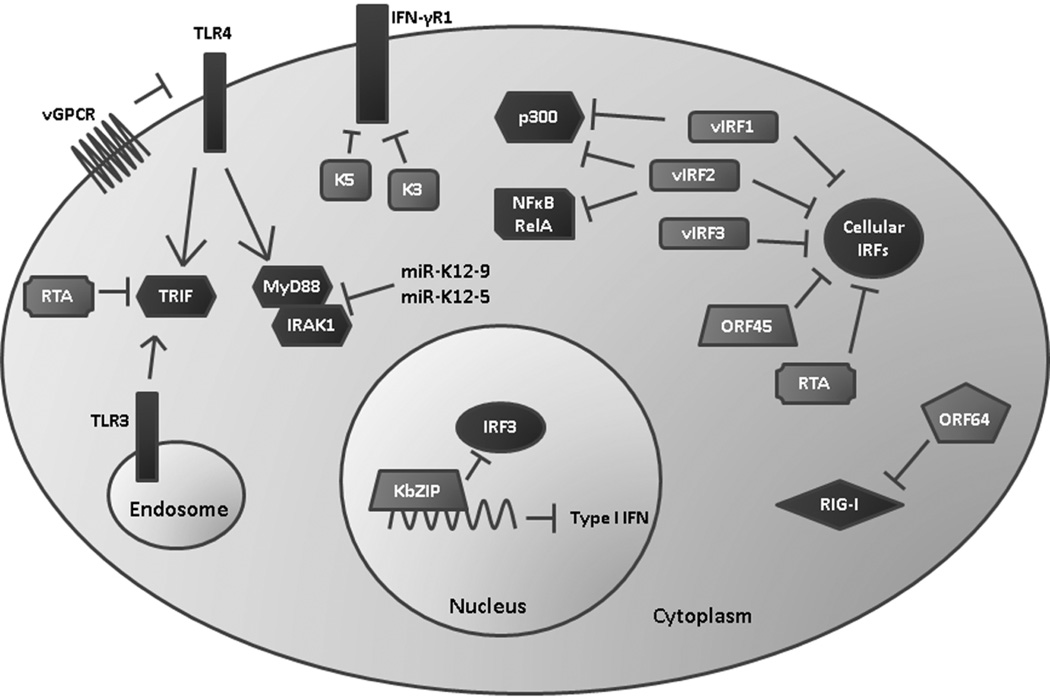
KSHV employs multiple mechanisms of inhibiting both IFN production and signaling since IFN is a potent antiviral defense that is detrimental to KSHV persistence (Figure 2) (Monini et al., 1999). The virus encodes four homologs of the cellular IRFs called vIRF 1-4 (Jacobs & Damania, 2011). The cellular IRFs are a large family of transcription factors that drive expression of type I IFN (IFNα and β) and a variety of cytokines and chemokines. Of the four KSHV-encoded vIRFs, only vIRFs 1, 2, and 3 have been shown to impact IFN signaling. vIRF1 can bind to and inhibit the transcriptional activities of IRF1, IRF3, and IRF7 (Ladislav Burysek et al., 1999; Lin et al., 2001). Additionally, vIRF1 can bind and sequester the transcriptional coactivator p300 that is required for IRF1- and IRF3-mediated transcription of type I IFN (Ladislav Burysek et al., 1999; Lin et al., 2001). vIRF2 is able to bind to cellular IRF1, 2, and 8 as well as NFκB RelA and p300 (Burysek, Yeow, & Pitha, 1999). vIRF2 is able to block type I IFN signaling and IFNα-, IFNλ-, and IRF1-dependent transactivation of the IFN stimulated response element (ISRE) promoter (Fuld, Cunningham, Klucher, Davison, & Blackbourn, 2006). More recently, IRF3 was identified as a binding partner of vIRF2, and it was shown that this interaction both suppresses IRF3-mediated transcription of IFNβ and enhances caspase-3-dependent degradation of IRF3 (Aresté, Mutocheluh, & Blackbourn, 2009). vIRF3 can interact with cellular IRFs 3 and 7 which diminishes the DNA-binding abilities of IRF7 (Joo et al., 2007). vIRF3 can also interact with IRF5 to inhibit IRF5-mediated IFN promoter activation and production of type I IFN (Barnes, Bi, Mancl, & Yang, 2011; Wies et al., 2009). Recently, it was shown that vIRFs 1 and 2, but not vIRF3, are capable of suppressing endogenous IFNβ message and protein expression following activation of TLR3 (Jacobs et al., 2013). Because KSHV can activate and upregulate the TLR3 pathway (West & Damania, 2008), this suggests that the vIRFs have a crucial function in evading the innate type I IFN response to KSHV infection. The vIRFs also have the ability to promote cell growth and prevent apoptosis (reviewed in (Jacobs & Damania, 2011)). Therefore, the vIRFs may have a twofold function in infected cells: first, to inhibit IFN to create a safe environment for KSHV, and second, to promote cell survival to allow for persistence of the virus in the host.
Figure 2. KSHV evasion of the host interferon response.
KSHV encodes viral interferon regulatory factors (vIRF 1-3) that antagonize the function of cellular IRFs, p300, and NFκB to suppress production of type I IFN. ORF45, RTA, and KbZIP have also been shown to interfere with IRF signaling. K3 and K5 are able to degrade the IFN3γR1 to reduce antiviral IFNγ signaling through the JAK/STAT pathway. Viral infection and expression of vGPCR reduces TLR4 expression, and RTA can induce degradation of the TLR3 and TLR4 mediator TRIF. miR-K12-9 and 5 downregulate IRAK1 and MYD88 which are also components of TLR signaling pathways. Reduction of TLR signaling results in reduced expression of type I IFN. Finally, ORF64 is able to deubiquitinate RIG-I which suppresses RIG-I mediated production of IFNβ.
There are several other KSHV-encoded proteins that can reduce the IFN response. It was demonstrated that KSHV ORF45 can interact with the inhibitory domain of cellular IRF7 (Sathish, Zhu, Golub, Liang, & Yuan, 2011). This interaction prevents IRF7’s phosphorylation and nuclear accumulation, which are both necessary for IRF7-mediated transcription of type I IFN (Zhu, King, Smith, Levy, & Yuan, 2002). ORF45 also competes with IRF7 for phosphorylation by IKKε and TBK1 which reduces overall levels of IRF7 phosphorylation (Liang et al., 2012). Infection of cells with an ORF45-null virus triggered a strong IRF7-dependent type I IFN response that rendered them resistant to subsequent vesicular stomatitis virus (VSV) infection (Zhu, Sathish, & Yuan, 2010). Interestingly, ORF45 is contained within the KSHV virion, which allows the virus to dampen the IFN response immediately upon infection (Zhu & Yuan, 2003). KSHV RTA can also act as an E3 ubiquitin ligase that induces the ubiquitination and degradation of IRF7 to reduce transcription of type I IFN genes (Yu, Wang, & Hayward, 2005). KSHV ORFK8 encodes a transcription factor KbZIP that can bind to the positive regulatory domain (PRD) I/III region of the IFNβ promoter to block IRF3-mediated IFNβ transcription (Lefort, Soucy-Faulkner, Grandvaux, & Flamand, 2007).
In addition to inhibition of type I IFN, KSHV can also repress signaling by IFNγ. The K3 and K5 proteins are able to induce degradation of the IFN-γ receptor 1 (IFN-γR1) which normally triggers IFNγ-mediated activation of the JAK/STAT pathway (Li, Means, Lang, & Jung, 2007). Signaling through this pathway induces expression of a wide variety of antiviral genes, which is suppressed following reduction of IFN-γR1 expression by K3 and K5 (Li et al., 2007). Between the vIRFs, ORF45, RTA, KbZIP, K3, and K5, KSHV utilizes a variety of mechanisms to evade IFN activation, suggesting the importance of avoiding this antiviral response in order for KSHV to persist in the host.
Evasion of Pattern Recognition Receptors
As mentioned earlier, the TLRs are pattern recognition receptors (PRR) that can be activated by invading pathogens. TLR activation triggers the production of antimicrobial cytokines and chemokines such as IFN, CCLs, and CXCLs through a variety of signaling proteins including NFκB, IRFs, and TRAFs. KSHV infection is able to downregulate TLR4 expression partly through the actions of vGPCR and vIRF1, and this subsequently suppresses expression of TNF-α, IL1-β, IL6, and IFNβ (Lagos et al., 2008). Furthermore, it was recently discovered that the ubiquitin ligase activity of KSHV RTA may cause the degradation of TRIF (Toll-IL-1 receptor (TIR) domain-containing adaptor-inducing β-IFN), which is a critical mediator of TLR3- and TLR4-induced type I IFN production (Ahmad et al., 2011). As mentioned previously, the KSHV-encoded miRNAs miR-K12-9 and miR-K12-5 target IRAK1 and MYD88, which are both essential components of TLR and IL-1 receptor signaling pathways (Abend et al., 2012) .
In addition to the TLRs, which are membrane-bound PRRs, host cells also express cytosolic receptors. These cytosolic PRRs include the RNA helicases RIG-I and MDA5, and the NLR (nucleotide-binding and oligomerization, leucine-rich repeat containing) protein family. RIG-I detects viral RNA and becomes ubiquitinated by TRIM25, which allows it to interact with the downstream signaling complex MAVS/IPS-1 (Gack et al., 2007; Kawai et al., 2005). Activation of this complex leads to the induction of type I IFN. KSHV encodes a deubiquitinase (DUB), ORF64, which is capable of deubiquitinating RIG-I to suppress RIG-I-mediated activation of the IFNβ promoter (Inn et al., 2011). NLRs sense a variety of microbial ligands, and their activation results in the assembly of an inflammasome complex which activates caspase-1 to generate mature IL-1β and IL18 (Martinon, Burns, & Tschopp, 2002). Production of IL-1β and IL18 in response to infection can lead to hyperinflammatory caspase 1-mediated cell death, called pyroptosis. KSHV ORF63 has homology to parts of cellular NLRP1, but lacks the effector caspase activation and recruitment (CARD) domain that is critical for inflammasome formation and function. ORF63 is able to interact with NLRP1 to prevent formation of both the NLRP1 and NLRP3 inflammasome and subsequent activation of caspase 1 (Gregory et al., 2011). ORF63’s function appears to be important for supporting viral gene expression and genome replication as well as suppressing IL-1β production.
Inhibition of Chemokine Signaling and Complement
KSHV encodes three homologs of cellular chemokines: viral CC-chemokine ligand 1 (vCCL1, also called vMIP1), vCCL2 (vMIP2), and vCCL3 (vMIP3) (Nicholas et al., 1997). vCCL1 is a ligand and agonist of CCR8 (Endres, Garlisi, Xiao, Shan, & Hedrick, 1999), whereas vCCL2 is a ligand that actually blocks signaling through multiple chemokine receptors including CCR-1, -2, -5, and -8 and CXCR-1, -2, and -4 (Dairaghi, Fan, McMaster, Hanley, & Schall, 1999). vCCL3 is an agonist for CCR4 (Stine et al., 2000). Collectively, binding of the viral chemokines to their respective cellular chemokine receptors is able to elicit a Th2-polarized response that is less cytotoxic to KSHV-infected cells than a Th1-polarized response (Stine et al., 2000; Weber et al., 2001).
The complement pathway acts as a bridge between the innate and adaptive immune system, since activation of complement can occur in an antibody-dependent or independent mechanism. Furthermore, phagocytosis of complement-bound pathogens or infected cells (opsonization) generates pathogen-derived antigens required to prime the adaptive immune system. Complement activation can occur through the classical, lectin, or alternative pathways which all result in the cleavage of complement component C3 into C3a and C3b by the C3 convertase (Zipfel & Skerka, 2009). C3b can then be deposited onto the surface of pathogens or infected cells to facilitate lysis, neutralization, or phagocytosis. Since complement activation occurs through an amplifying cascade of proteolytic events, cellular regulators of complement activation (RCA) proteins keep this pathway in check to avoid hyperinflammatory responses (Zipfel & Skerka, 2009). KSHV ORF4 encodes a structural and functional homolog to cellular RCA proteins called the KSHV complement control protein (KCP) (Mark et al., 2004; Mullick, Bernet, Singh, Lambris, & Sahu, 2003). KCP is able to prevent cleavage of C3 through accelerating the decay of the C3 convertase, by acting as an inhibitory cofactor to inactivate C3b and downstream complement molecules, and by preventing deposition of C3b onto target surfaces (Mark, Proctor, Blackbourn, Blom, & Spiller, 2008; Spiller, Blackbourn, Mark, Proctor, & Blom, 2003). By evading the complement pathway, the virus is able to avoid neutralization of extracellular virions by complement deposition, decrease the elimination of infected cells, and reduce the acquisition of viral antigens by phagocytes and APCs to inhibit the adaptive immune response.
Evasion of Natural Killer Cells
As discussed in the adaptive immune evasion section, KSHV downregulates MHC-I expression on APCs. NK cells are designed to sense and kill cells displaying abnormal MHC-I levels through their leukocyte Ig-like receptor 1 (LIR1) and killer inhibitory receptor (KIR), which recognize endogenous MHC-I molecules on cells. To prevent the elimination of infected cells with reduced MHC-I, KSHV utilizes multiple mechanisms to inhibit NK cell function. In addition to downregulating MHC-I, KSHV K5 also downregulates surface expression of ICAM-1 and B7-2 (CD86) to avoid NK-mediated cell cytotoxicity (Coscoy & Ganem, 2001; Ishido et al., 2000; Tomescu et al., 2003). NK cell killing requires activation of the NKG2D and NKp80 receptors. As mentioned earlier, the KSHV miRNA miR-K12-7 targets the NKG2D ligand MHC class I-related chain B (MICB) 3’UTR. This results in decreased expression of this NKG2D ligand and effectively reduces NK cell killing ability (Nachmani et al., 2009). K5 also decreases the surface expression MICB and another NKG2D ligand, MICA, as well as the NKp80 ligand activation-induced C type lectin (AICL) (Thomas et al., 2008). In these ways, KSHV has cleverly devised mechanisms to not only reduce activation of the adaptive immune system by downregulating MHC-I, but also to avoid the detrimental side effects of abnormal MHC-I levels on infected cells.
Conclusions
KSHV expresses a diverse repertoire of proteins and small RNAs that aid the virus in establishing a lifelong infection in the host. Many of these viral components are linked to transformation of host cells, linking KSHV with the development of several human malignancies. These cancers pose a large threat to global public health, particularly in areas that are still struggling with limited treatment options for HIV infection. Two decades of KSHV research has elucidated many of the mechanisms by which KSHV is able to establish and maintain infection in the host and initiate tumorigenesis; however, despite this extensive research, there are still aspects of viral infection and transformation that are not well understood. Further elucidating the unique mechanisms that KSHV uses to persist so successfully in the host will hopefully uncover novel therapeutic targets for the treatment of KSHV disease.
Acknowledgements
We thank the Damania lab members for helpful discussions. BD is supported by CA096500, DE018281 and CA019014. LG was supported by training grant, T32CA071341. BD is a Leukemia & Lymphoma Society Scholar and a Burroughs Wellcome Fund Investigator in Infectious Disease.
References
- Abend JR, Ramalingam D, Kieffer-Kwon P, Uldrick TS, Yarchoan R, Ziegelbauer JM. Kaposi's Sarcoma-Associated Herpesvirus MicroRNAs Target IRAK1 and MYD88, Two Components of the Toll-Like Receptor/Interleukin-1R Signaling Cascade, To Reduce Inflammatory-Cytokine Expression. Journal of Virology. 2012;86(21):11663–11674. doi: 10.1128/JVI.01147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend JR, Uldrick T, Ziegelbauer JM. Regulation of Tumor Necrosis Factor-Like Weak Inducer of Apoptosis Receptor Protein (TWEAKR) Expression by Kaposi's Sarcoma-Associated Herpesvirus MicroRNA Prevents TWEAK-Induced Apoptosis and Inflammatory Cytokine Expression. Journal of Virology. 2010;84(23):12139–12151. doi: 10.1128/JVI.00884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Groshong JS, Matta H, Schamus S, Punj V, Robinson LJ, Chaudhary PM. Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 cooperates with Myc to promote lymphoma in mice. Cancer Biology & Therapy. 2010;10(10):1033–1040. doi: 10.4161/cbt.10.10.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad H, Gubbels R, Ehlers E, Meyer F, Waterbury T, Lin R, Zhang L. Kaposi Sarcoma-associated Herpesvirus Degrades Cellular Toll-Interleukin-1 Receptor Domain-containing Adaptor-inducing β-Interferon (TRIF) Journal of Biological Chemistry. 2011;286(10):7865–7872. doi: 10.1074/jbc.M110.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula SM, Naranatt PP, Walia N-S, Wang F-Z, Fegley B, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus (Human Herpesvirus 8) Infection of Human Fibroblast Cells Occurs through Endocytosis. Journal of Virology. 2003;77(14):7978–7990. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula SM, Pramod NP, Wang F-Z, Chandran B. Integrin α3β1 (CD 49c/29) Is a Cellular Receptor for Kaposi's Sarcoma-Associated Herpesvirus (KSHV/HHV-8) Entry into the Target Cells. Cell. 2002;108(3):407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- Akula SM, Wang F-Z, Vieira J, Chandran B. Human Herpesvirus 8 Interaction with Target Cells Involves Heparan Sulfate. Virology. 2001;282(2):245–255. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- Ambroziak JA, Blackbourn DJ, Herndier BG, Glogau RG, Gullett JH, McDonald AR, Levy JA. Herpes-Like Sequences in HIV-Infected and Uninfected Kaposi's Sarcoma Patients. Science. 1995;268(5210):582–583. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- An F-Q, Compitello N, Horwitz E, Sramkoski M, Knudsen ES, Renne R. The Latency-associated Nuclear Antigen of Kaposi's Sarcoma-associated Herpesvirus Modulates Cellular Gene Expression and Protects Lymphoid Cells from p16 INK4A-induced Cell Cycle Arrest. Journal of Biological Chemistry. 2005;280(5):3862–3874. doi: 10.1074/jbc.M407435200. [DOI] [PubMed] [Google Scholar]
- Andreoni M, Goletti D, Pezzotti P, Pozzetto A, Monini P, Sarmati L, Rezza G. Prevalence, Incidence and Correlates of HHV-8/KSHV Infection and Kaposi's Sarcoma in Renal and Liver Transplant Recipients. Journal of Infection. 2001;43(3):195–199. doi: 10.1053/jinf.2001.0899. [DOI] [PubMed] [Google Scholar]
- Antman K, Chang Y. Kaposi's Sarcoma. New England Journal of Medicine. 2000;342(14):1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Jaffe E, Chang Y, Jones K, Teruya-Feldstein J, Moore P, Tosato G. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93(12):4034–4043. [PubMed] [Google Scholar]
- Aoki Y, Narazaki M, Kishimoto T, Tosato G. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma–associated herpesvirus: Presented in part at the 42nd annual meeting of the American Society of Hematology, December 4, 2000, in San Francisco, CA. Blood. 2001;98(10):3042–3049. doi: 10.1182/blood.v98.10.3042. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Tosato G, Fonville TW, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood. 2001;97(8):2526–2527. doi: 10.1182/blood.v97.8.2526. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Yarchoan R, Wyvill K, Okamoto S-i, Little RF, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma–associated herpesvirus–linked disorders. Blood. 2001;97(7):2173–2176. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- Aresté C, Blackbourn DJ. Modulation of the immune system by Kaposi's sarcoma-associated herpesvirus. Trends in Microbiology. 2009;17(3):119–129. doi: 10.1016/j.tim.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Aresté C, Mutocheluh M, Blackbourn DJ. Identification of Caspase-mediated Decay of Interferon Regulatory Factor-3, Exploited by a Kaposi Sarcoma-associated Herpesvirus Immunoregulatory Protein. Journal of Biological Chemistry. 2009;284(35):23272–23285. doi: 10.1074/jbc.M109.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385(6614):347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- AuCoin DP, Colletti KS, Cei SA, Papousková I, Tarrant M, Pari GS. Amplification of the Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP) Virology. 2004;318(2):542–555. doi: 10.1016/j.virol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Mesri EA. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391(6662):86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, Dias S, Mesri EA. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell. 2003;3(2):131–143. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- Ballestas ME, Kaye KM. The latency-associated nuclear antigen, a multifunctional protein central to Kaposi’s sarcoma-associated herpesvirus latency. Future Microbiology. 2011;6(12):1399–1413. doi: 10.2217/fmb.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon G, Chen K, Perez R, Tam W, Cesarman E. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice. The Journal of clinical investigation. 2011;121(3):1141–1153. doi: 10.1172/JCI44417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera AJ, Ballestas ME, Kaye KM. The Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen 1 N Terminus Is Essential for Chromosome Association, DNA Replication, and Episome Persistence. Journal of Virology. 2004;78(1):294–301. doi: 10.1128/JVI.78.1.294-301.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera AJ, Chodaparambil JV, Kelley-Clarke B, Joukov V, Walter JC, Luger K, Kaye KM. The Nucleosomal Surface as a Docking Station for Kaposi's Sarcoma Herpesvirus LANA. Science. 2006;311(5762):856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- Barnes BJ, Bi X, Mancl ME, Yang L. Modulation of interferon regulatory factor 5 activities by the Kaposi sarcoma-associated herpesvirus-encoded viral interferon regulatory factor 3 contributes to immune evasion and lytic induction. Journal of Interferon & Cytokine Research. 2011;31(4) doi: 10.1089/jir.2010.0084. 373+ [DOI] [PubMed] [Google Scholar]
- Barozzi P, Luppi M, Facchetti F, Mecucci C, Alu M, Sarid R, Torelli G. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat Med. 2003;9(5):554–561. doi: 10.1038/nm862. [DOI] [PubMed] [Google Scholar]
- Bartee E, McCormack A, Früh K. Quantitative Membrane Proteomics Reveals New Cellular Targets of Viral Immune Modulators. PLoS Pathog. 2006;2(10):e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel J, Grundhoff A, Ganem D. RNAs in the Virion of Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2005;79(16):10138–10146. doi: 10.1128/JVI.79.16.10138-10146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel JT, Winant RC, Ganem D. Host and Viral Proteins in the Virion of Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2005;79(8):4952–4964. doi: 10.1128/JVI.79.8.4952-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger C, Gravel A, Tomoiu A, Janelle ME, Gosselin J, Tremblay MJ, Flamand L. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. Journal of human virology. 2001;4(2):62–73. [PubMed] [Google Scholar]
- Bellare P, Ganem D. Regulation of KSHV Lytic Switch Protein Expression by a Virus-Encoded MicroRNA: An Evolutionary Adaptation that Fine-Tunes Lytic Reactivation. Cell Host & Microbe. 2009;6(6):570–575. doi: 10.1016/j.chom.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V, Newton R. Overview of the Epidemiology of Immunodeficiency-Associated Cancers. JNCI Monographs. 1998;1998(23):1–6. doi: 10.1093/oxfordjournals.jncimonographs.a024164. [DOI] [PubMed] [Google Scholar]
- Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? The Lancet. 1990;335(8682):123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- Biggar R, Rabkin C. The epidemiology of AIDS-related neoplasms. Hematol. Oncol. Clin. North Am. 1996;10(5):997–1010. doi: 10.1016/s0889-8588(05)70380-4. [DOI] [PubMed] [Google Scholar]
- Bihl F, Berger C, Chisholm J, Henry L, Bertisch B, Trojan A, Mueller N. Cellular immune responses and disease control in acute AIDS-associated Kaposi's sarcoma. AIDS (London) 2009;23(14):1918–1922. doi: 10.1097/QAD.0b013e3283300a91. [DOI] [PubMed] [Google Scholar]
- Bihl F, Mosam A, Henry L, Chisholm J, Dollard S, Gumbi P, Brander C. Kaposi's sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi's sarcoma. AIDS (London) 2007;21(10):1245–1252. doi: 10.1097/QAD.0b013e328182df03. [DOI] [PubMed] [Google Scholar]
- Birkmann A, Mahr K, Ensser A, Yağuboğlu S, Titgemeyer F, Fleckenstein B, Neipel F. Cell Surface Heparan Sulfate Is a Receptor for Human Herpesvirus 8 and Interacts with Envelope Glycoprotein K8.1. Journal of Virology. 2001;75(23):11583–11593. doi: 10.1128/JVI.75.23.11583-11593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer NH, Stürzl M. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. Journal of Virology. 1997;71(10):7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, Thomas A, O'Leary JJ. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1(12):1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- Boss IW, Nadeau PE, Abbott JR, Yang Y, Mergia A, Renne R. A Kaposi's Sarcoma-Associated Herpesvirus-Encoded Ortholog of MicroRNA miR-155 Induces Human Splenic B-Cell Expansion in NOD/LtSz-scid IL2Rγnull Mice. Journal of Virology. 2011;85(19):9877–9886. doi: 10.1128/JVI.05558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero V, Sharma-Walia N, Kerur N, Paul AG, Sadagopan S, Cannon M, Chandran B. Kaposi Sarcoma-associated herpes virus (KSHV) G protein-coupled receptor (vGPCR) activates the ORF50 lytic switch promoter: A potential positive feedback loop for sustained ORF50 gene expression. Virology. 2009;392(1):34–51. doi: 10.1016/j.virol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger E, Gérard L, Gabarre J, Molina J-M, Rapp C, Abino J-F, Oksenhendler E. Prognostic Factors and Outcome of Human Herpesvirus 8–Associated Primary Effusion Lymphoma in Patients With AIDS. Journal of Clinical Oncology. 2005;23(19):4372–4380. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- Boulanger MJ, Chow D-c, Brevnova E, Martick M, Sandford G, Nicholas J, Garcia KC. Molecular Mechanisms for Viral Mimicry of a Human Cytokine: Activation of gp130 by HHV-8 Interleukin-6. Journal of Molecular Biology. 2004;335(2):641–654. doi: 10.1016/j.jmb.2003.10.070. [DOI] [PubMed] [Google Scholar]
- Bower M, Nelson M, Young AM, Thirlwell C, Newsom-Davis T, Mandalia S, Stebbing J. Immune Reconstitution Inflammatory Syndrome Associated With Kaposi's Sarcoma. Journal of Clinical Oncology. 2005;23(22):5224–5228. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- Brinkmann MM, Glenn M, Rainbow L, Kieser A, Henke-Gendo C, Schulz TF. Activation of Mitogen-Activated Protein Kinase and NF-κB Pathways by a Kaposi's Sarcoma-Associated Herpesvirus K15 Membrane Protein. Journal of Virology. 2003;77(17):9346–9358. doi: 10.1128/JVI.77.17.9346-9358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann MM, Pietrek M, Dittrich-Breiholz O, Kracht M, Schulz TF. Modulation of Host Gene Expression by the K15 Protein of Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2007;81(1):42–58. doi: 10.1128/JVI.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann MM, Schulz TF. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. Journal of general virology. 2006;87(5):1047–1074. doi: 10.1099/vir.0.81598-0. [DOI] [PubMed] [Google Scholar]
- Bubman D, Guasparri I, Cesarman E. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene. 2007;26(34):4979–4986. doi: 10.1038/sj.onc.1210299. [DOI] [PubMed] [Google Scholar]
- Burysek L, Yeow W-S, Lubyova B, Kellum M, Schafer SL, Huang YQ, Pitha PM. Functional Analysis of Human Herpesvirus 8-Encoded Viral Interferon Regulatory Factor 1 and Its Association with Cellular Interferon Regulatory Factors and p300. Journal of Virology. 1999;73(9):7334–7342. doi: 10.1128/jvi.73.9.7334-7342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burysek L, Yeow W, Pitha P. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) Journal of human virology. 1999;2(1):19–32. [PubMed] [Google Scholar]
- Butler LM, Jeffery HC, Wheat RL, Long HM, Rae PC, Nash GB, Blackbourn DJ. Kaposi's Sarcoma-Associated Herpesvirus Inhibits Expression and Function of Endothelial Cell Major Histocompatibility Complex Class II via Suppressor of Cytokine Signaling 3. Journal of Virology. 2012;86(13):7158–7166. doi: 10.1128/JVI.06908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(15):5570–5575. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Cesarman E, Boshoff C. KSHV G protein-coupled receptor inhibits lytic gene transcription in primary-effusion lymphoma cells via p21-mediated inhibition of Cdk2. Blood. 2006;107(1):277–284. doi: 10.1182/blood-2005-06-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Gloghini A. KSHV/HHV8-associated lymphomas. British Journal of Haematology. 2008;140(1):13–24. doi: 10.1111/j.1365-2141.2007.06879.x. [DOI] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Larocca LM, Capello D, Pierconti F, Canzonieri V, Gaidano G. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus–related lymphomas. Blood. 2001;97(3):744–751. doi: 10.1182/blood.v97.3.744. [DOI] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Vaccher E, Cerri M, Gaidano G, Dalla-Favera R, Tirelli U. Kaposi's Sarcoma-Associated Herpesvirus/Human Herpesvirus Type 8-Positive Solid Lymphomas: A Tissue-Based Variant of Primary Effusion Lymphoma. The Journal of Molecular Diagnostics. 2005;7(1):17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper C, Wald A. The use of antiviral drugs in the prevention and treatment of Kaposi sarcoma, multicentric Castleman disease and primary effusion lymphoma. Current topics in microbiology and immunology. 2007;312:289–307. doi: 10.1007/978-3-540-34344-8_11. [DOI] [PubMed] [Google Scholar]
- Cattani P, Capuano M, Cerimele F, La Parola IL, Santangelo R, Masini C, Fadda G. Human Herpesvirus 8 Seroprevalence and Evaluation of Nonsexual Transmission Routes by Detection of DNA in Clinical Specimens from Human Immunodeficiency Virus-Seronegative Patients from Central and Southern Italy, with and without Kaposi’s Sarcoma. Journal of Clinical Microbiology. 1999;37(4):1150–1153. doi: 10.1128/jcm.37.4.1150-1153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Letters. 2011;305(2):163–174. doi: 10.1016/j.canlet.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's Sarcoma–Associated Herpesvirus-Like DNA Sequences in AIDS-Related Body-Cavity–Based Lymphomas. New England Journal of Medicine. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Knowles DM. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Seminars in Cancer Biology. 1999;9(3):165–174. doi: 10.1006/scbi.1998.0118. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Nador RG, Bai F, Bohenzky RA, Russo JJ, Moore PS, Knowles DM. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. Journal of Virology. 1996;70(11):8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadburn A, Hyjek EM, Tam W, Liu Y, Rengifo T, Cesarman E, Knowles DM. Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD) Histopathology. 2008;53(5):513–524. doi: 10.1111/j.1365-2559.2008.03144.x. [DOI] [PubMed] [Google Scholar]
- Chandriani S, Ganem D. Array-Based Transcript Profiling and Limiting-Dilution Reverse Transcription-PCR Analysis Identify Additional Latent Genes in Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2010;84(11):5565–5573. doi: 10.1128/JVI.02723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Renne R, Dittmer D, Ganem D. Inflammatory Cytokines and the Reactivation of Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication. Virology. 2000;266(1):17–25. doi: 10.1006/viro.1999.0077. [DOI] [PubMed] [Google Scholar]
- Chang P-C, Li M. Kaposi's Sarcoma-Associated Herpesvirus K-Cyclin Interacts with Cdk9 and Stimulates Cdk9-Mediated Phosphorylation of p53 Tumor Suppressor. Journal of Virology. 2008;82(1):278–290. doi: 10.1128/JVI.01552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of Herpesvirus-Like DNA Sequences in AIDS-Associated Kaposi's Sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Chang Y, Moore PS, Talbot SJ, Boshoff CH, Zarkowska T, Godden-Kent D, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382(6590):410–410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-Induced Cell Proliferation and Immune Evasion of Interferon Activity. Science. 2002;298(5597):1432–1435. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- Chen D, Choi YB, Sandford G, Nicholas J. Determinants of Secretion and Intracellular Localization of Human Herpesvirus 8 Interleukin-6. Journal of Virology. 2009;83(13):6874–6882. doi: 10.1128/JVI.02625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Sandford G, Nicholas J. Intracellular Signaling Mechanisms and Activities of Human Herpesvirus 8 Interleukin-6. Journal of Virology. 2009;83(2):722–733. doi: 10.1128/JVI.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ye F, Xie J, Kuhne K, Gao S-J. Genome-wide identification of binding sites for Kaposi's sarcoma-associated herpesvirus lytic switch protein, RTA. Virology. 2009;386(2):290–302. doi: 10.1016/j.virol.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou C-J, Poole LJ, Kim PS, Ciufo DM, Cannon JS, ap Rhys CM, Hayward GS. Patterns of Gene Expression and a Transactivation Function Exhibited by the vGCR (ORF74) Chemokine Receptor Protein of Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2002;76(7):3421–3439. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N-H, Choi Y-K, Choi J-K. Multi-transmembrane protein K15 of Kaposi's sarcoma-associated herpesvirus targets Lyn kinase in the membrane raft and induces NFAT/AP1 activities. Exp Mol Med. 2008;40:565–573. doi: 10.3858/emm.2008.40.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-K, Lee B-S, Shim SN, Li M, Jung JU. Identification of the Novel K15 Gene at the Rightmost End of the Kaposi's Sarcoma-Associated Herpesvirus Genome. Journal of Virology. 2000;74(1):436–446. [PMC free article] [PubMed] [Google Scholar]
- Chow D-c, He X-l, Snow AL, Rose-John S, Garcia KC. Structure of an Extracellular gp130 Cytokine Receptor Signaling Complex. Science. 2001;291(5511):2150–2155. doi: 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- Chugh P, Matta H, Schamus S, Zachariah S, Kumar A, Richardson JA, Chaudhary PM. Constitutive NF-κB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(36):12885–12890. doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. The Journal of clinical investigation. 2001;107(12):1599–1606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. The Journal of Cell Biology. 2001;155(7):1265–1274. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter MA, Robertson ES. The Latency-Associated Nuclear Antigen Tethers the Kaposi's Sarcoma-Associated Herpesvirus Genome to Host Chromosomes in Body Cavity-Based Lymphoma Cells. Virology. 1999;264(2):254–264. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- Dahlke C, Maul K, Christalla T, Walz N, Schult P, Stocking C, Grundhoff A. A microRNA Encoded by Kaposi Sarcoma-Associated Herpesvirus Promotes B-Cell Expansion In Vivo . PLoS ONE. 2012;7(11):e49435. doi: 10.1371/journal.pone.0049435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairaghi DJ, Fan RA, McMaster BE, Hanley MR, Schall TJ. HHV8-encoded vMIP-I Selectively Engages Chemokine Receptor CCR8: AGONIST AND ANTAGONIST PROFILES OF VIRAL CHEMOKINES. Journal of Biological Chemistry. 1999;274(31):21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- de França TRT, de Araújo RA, Ribeiro CMB, Leao JC. Salivary shedding of HHV-8 in people infected or not by human immunodeficiency virus 1. Journal of Oral Pathology & Medicine. 2011;40(1):97–102. doi: 10.1111/j.1600-0714.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Mbisa G, Perez-Alvarez S, Benavente Y, Sukvirach S, Hieu NT, Whitby D. Geographic Variation in the Prevalence of Kaposi Sarcoma–Associated Herpesvirus and Risk Factors for Transmission. Journal of Infectious Diseases. 2009;199(10):1449–1456. doi: 10.1086/598523. [DOI] [PubMed] [Google Scholar]
- Dela Cruz CS, Lee Y, Viswanathan SR, El-Guindy AS, Gerlach J, Nikiforow S, Miller G. N-linked Glycosylation Is Required for Optimal Function of Kaposi's Sarcoma Herpesvirus–encoded, but Not Cellular, Interleukin 6. The Journal of Experimental Medicine. 2004;199(4):503–514. doi: 10.1084/jem.20031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PJ, Pryce EN, Henson BW, Luitweiler EM, Cothran J. Reconstitution of the Kaposi's Sarcoma-Associated Herpesvirus Nuclear Egress Complex and Formation of Nuclear Membrane Vesicles by Coexpression of ORF67 and ORF69 Gene Products. Journal of Virology. 2012;86(1):594–598. doi: 10.1128/JVI.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Damania B. The Latency-Associated Nuclear Antigen of Rhesus Monkey Rhadinovirus Inhibits Viral Replication through Repression of Orf50/Rta Transcriptional Activation. Journal of Virology. 2005;79(5):3127–3138. doi: 10.1128/JVI.79.5.3127-3138.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheekollu J, Chen H-S, Kaye KM, Lieberman PM. Timeless-dependent DNA Replication-coupled Recombination Promote KSHV Episome Maintenance and Terminal Repeat Stability. Journal of Virology. 2013 doi: 10.1128/JVI.02211-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanna J, Safai B. Kaposi's sarcoma. Retrospective study of 90 cases with particular emphasis on the familial occurrence, ethnic background and prevalence of other diseases. American Journal of Medicine. 1981;71(5):779–783. doi: 10.1016/0002-9343(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Dillon PJ, Gregory SM, Tamburro K, Sanders MK, Johnson GL, Raab-Traub N, Damania B. Tousled-like Kinases Modulate Reactivation of Gammaherpesviruses from Latency. Cell Host & Microbe. 2013;13(2):204–214. doi: 10.1016/j.chom.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A Cluster of Latently Expressed Genes in Kaposi’s Sarcoma-Associated Herpesvirus. Journal of Virology. 1998;72(10):8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M-Q, Liu H, Diss TC, Ye H, Hamoudi RA, Dupin N, Isaacson PG. Kaposi sarcoma–associated herpesvirus infects monotypic (IgMλ) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97(7):2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- Dupin N, Diss TL, Kellam P, Tulliez M, Du M-Q, Sicard D, Boshoff C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8–positive plasmablastic lymphoma. Blood. 2000;95(4):1406–1412. [PubMed] [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proceedings of the National Academy of Sciences. 1999;96(8):4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez R, Lacoste V, Brière J, Couppié P, Frances C, Sainte-Marie D, Gessain A. Evidence for a Multiclonal Origin of Multicentric Advanced Lesions of Kaposi Sarcoma. Journal of the National Cancer Institute. 2007;99(14):1086–1094. doi: 10.1093/jnci/djm045. [DOI] [PubMed] [Google Scholar]
- Dutz W, Stout A. Kaposi's sarcoma in infants and children. Cancer. 1960;13:684–694. doi: 10.1002/1097-0142(196007/08)13:4<684::aid-cncr2820130408>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ellis M, Peng Chew Y, Fallis L, Freddersdorf S, Boshoff C, Weiss RA, Mittnacht S. Degradation of p27Kip cdk inhibitor triggered by Kaposi's sarcoma virus cyclin-cdk6 complex. EMBO J. 1999;18(3):644–653. doi: 10.1093/emboj/18.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres MJ, Garlisi CG, Xiao H, Shan L, Hedrick JA. The Kaposi's Sarcoma–related Herpesvirus (KSHV)-encoded Chemokine vMIP-I is a Specific Agonist for the CC Chemokine Receptor (CCR)8. The Journal of Experimental Medicine. 1999;189(12):1993–1998. doi: 10.1084/jem.189.12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels E, Pfeiffer R, Goedert J, Virgo P, McNeel T, Scoppa S, Biggar R. Trends in cancer risk among people with AIDS in the United States. AIDS. 2006;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. The Journal of clinical investigation. 2006;116(3):735–742. doi: 10.1172/JCI26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francès C, Marcelin AG, Legendre C, Chevret S, Dussaix E, Lejeune J, Lebbé C. The Impact of Preexisting or Acquired Kaposi Sarcoma Herpesvirus Infection in Kidney Transplant Recipients on Morbidity and Survival. American Journal of Transplantation. 2009;9(11):2580–2586. doi: 10.1111/j.1600-6143.2009.02816.x. [DOI] [PubMed] [Google Scholar]
- Friborg J, Kong W-p, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402(6764):889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi's sarcoma. Journal of the American Academy of Dermatology. 1990;22(6, Part 2):1237–1250. doi: 10.1016/0190-9622(90)70169-i. [DOI] [PubMed] [Google Scholar]
- Fujimuro M, Wu FY, ApRhys C, Kajumbula H, Young DB, Hayward GS, Hayward SD. A novel viral mechanism for dysregulation of [beta]-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat Med. 2003;9(3):300–306. doi: 10.1038/nm829. [DOI] [PubMed] [Google Scholar]
- Fuld S, Cunningham C, Klucher K, Davison AJ, Blackbourn DJ. Inhibition of Interferon Signaling by the Kaposi's Sarcoma-Associated Herpesvirus Full-Length Viral Interferon Regulatory Factor 2 Protein. Journal of Virology. 2006;80(6):3092–3097. doi: 10.1128/JVI.80.6.3092-3097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo C-H, Urano T, Liang C, Sun L, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Garrigues HJ, Rubinchikova YE, DiPersio CM, Rose TM. Integrin αVβ3 Binds to the RGD Motif of Glycoprotein B of Kaposi's Sarcoma-Associated Herpesvirus and Functions as an RGD-Dependent Entry Receptor. Journal of Virology. 2008;82(3):1570–1580. doi: 10.1128/JVI.01673-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn MC, Geras-Raaka E, Varma A, Clark-Lewis I. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. The Journal of clinical investigation. 1998;102(8):1469–1472. doi: 10.1172/JCI4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A, Sudaka A, Briere J, Fouchard N, Nicola M, Rio B, Diebold J. Kaposi sarcoma-associated herpes-like virus (human herpesvirus type 8) DNA sequences in multicentric Castleman's disease: is there any relevant association in non-human immunodeficiency virus-infected patients? [letter; comment] Blood. 1996;87(1):414–416. [PubMed] [Google Scholar]
- Glenn M, Rainbow L, Auradé F, Davison A, Schulz TF. Identification of a Spliced Gene from Kaposi’s Sarcoma-Associated Herpesvirus Encoding a Protein with Similarities to Latent Membrane Proteins 1 and 2A of Epstein-Barr Virus. Journal of Virology. 1999;73(8):6953–6963. doi: 10.1128/jvi.73.8.6953-6963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, Weiss RA, Mittnacht S. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. Journal of Virology. 1997;71(6):4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Cullen BR. A Human Herpesvirus MicroRNA Inhibits p21 Expression and Attenuates p21-Mediated Cell Cycle Arrest. Journal of Virology. 2010;84(10):5229–5237. doi: 10.1128/JVI.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi J-TA, Cullen BR. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450(7172):1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SM, Damania B. KSHV and the toll of innate immune activation. Cell Cycle. 2009;8(20):3246–3247. doi: 10.4161/cc.8.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S-i, Reed JC, Damania B. Discovery of a Viral NLR Homolog that Inhibits the Inflammasome. Science. 2011;331(6015):330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SM, Wang L, West JA, Dittmer DP, Damania B. Latent Kaposi's Sarcoma-Associated Herpesvirus Infection of Monocytes Downregulates Expression of Adaptive Immune Response Costimulatory Receptors and Proinflammatory Cytokines. Journal of Virology. 2012;86(7):3916–3923. doi: 10.1128/JVI.06437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. Toll-like receptor signaling controls reactivation of KSHV from latency. Proceedings of the National Academy of Sciences. 2009;106(28):11725–11730. doi: 10.1073/pnas.0905316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guito J, Lukac DM. KSHV Rta promoter specification and viral reactivation. Frontiers in Microbiology. 2012;3 doi: 10.3389/fmicb.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. Kaposi's Sarcoma-Like Tumors in a Human Herpesvirus 8 ORF74 Transgenic Mouse. Journal of Virology. 2003;77(4):2631–2639. doi: 10.1128/JVI.77.4.2631-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Henderson S, Lagos D, Nikitenko L, Coulter E, Roberts S, Boshoff C. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes & Development. 2010;24(2):195–205. doi: 10.1101/gad.553410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Teoh G, Raje N, Treon SP, Tai Y-T, Anderson KC. Characterization of Signaling Cascades Triggered by Human Interleukin-6 versus Kaposi’s Sarcoma-associated Herpes Virus-encoded Viral Interleukin 6. Clinical Cancer Research. 2000;6(3):1180–1189. [PubMed] [Google Scholar]
- Hislop AD, Sabbah S. CD8+ T cell immunity to Epstein-Barr virus and Kaposi’s sarcoma-associated herpes virus. Seminars in Cancer Biology. 2008;18(6):416–422. doi: 10.1016/j.semcancer.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Hladik W, Dollard SC, Mermin J, Fowlkes AL, Downing R, Amin MM, Lackritz EM. Transmission of Human Herpesvirus 8 by Blood Transfusion. New England Journal of Medicine. 2006;355(13):1331–1338. doi: 10.1056/NEJMoa055009. [DOI] [PubMed] [Google Scholar]
- Hong Y-K, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36(7):683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- Hu F, Nicholas J. Signal Transduction by Human Herpesvirus 8 Viral Interleukin-6 (vIL-6) Is Modulated by the Nonsignaling gp80 Subunit of the IL-6 Receptor Complex and Is Distinct from Signaling Induced by Human IL-6. Journal of Virology. 2006;80(21):10874–10878. doi: 10.1128/JVI.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Garber AC, Renne R. The Latency-Associated Nuclear Antigen of Kaposi's Sarcoma-Associated Herpesvirus Supports Latent DNA Replication in Dividing Cells. Journal of Virology. 2002;76(22):11677–11687. doi: 10.1128/JVI.76.22.11677-11687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M. Immunohistological evaluation of immune cell infiltrate in cutaneous Kaposi's sarcoma. Cell Biology International. 2008;32:157–162. doi: 10.1016/j.cellbi.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Inn K-S, Lee S-H, Rathbun JY, Wong L-Y, Toth Z, Machida K, Jung JU. Inhibition of RIG-I-Mediated Signaling by Kaposi's Sarcoma-Associated Herpesvirus-Encoded Deubiquitinase ORF64. Journal of Virology. 2011;85(20):10899–10904. doi: 10.1128/JVI.00690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishido S, Wang C, Lee B-S, Cohen GB, Jung JU. Downregulation of Major Histocompatibility Complex Class I Molecules by Kaposi's Sarcoma-Associated Herpesvirus K3 and K5 Proteins. Journal of Virology. 2000;74(11):5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Damania B. The Viral Interferon Regulatory Factors of KSHV: Immunosuppressors or Oncogenes? Frontiers in immunology. 2011;2 doi: 10.3389/fimmu.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Gregory SM, West JA, Wollish AC, Bennett CL, Blackbourn DJ, Damania B. The Viral Interferon Regulatory Factors of Kaposi's Sarcoma-Associated Herpesvirus Differ in Their Inhibition of Interferon Activation Mediated by Toll-Like Receptor 3. Journal of Virology. 2013;87(2):798–806. doi: 10.1128/JVI.01851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner RG, Maillard K, Cattini N, Weiss RA, Boshoff C, Wooster R, Kellam P. Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphoma has a plasma cell gene expression profile. Proceedings of the National Academy of Sciences. 2003;100(18):10399–10404. doi: 10.1073/pnas.1630810100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Orvis J, Kim JW, McMurtrey CP, Renne R, Dittmer DP. Regulation and Autoregulation of the Promoter for the Latency-associated Nuclear Antigen of Kaposi's Sarcoma-associated Herpesvirus. Journal of Biological Chemistry. 2004;279(16):16822–16831. doi: 10.1074/jbc.M312801200. [DOI] [PubMed] [Google Scholar]
- Jha HC, Upadhyay SK, Aj MP, Lu J, Cai Q, Saha A, Robertson ES. H2AX phosphorylation is important for LANA mediated KSHV episome persistence. Journal of Virology. 2013 doi: 10.1128/JVI.03575-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of Interleukin-10 (IL-10) and Viral IL-6 in the Spontaneous Growth of Kaposi’s Sarcoma Herpesvirus-Associated Infected Primary Effusion Lymphoma Cells. Blood. 1999;94(8):2871–2879. [PubMed] [Google Scholar]
- Joo CH, Shin YC, Gack M, Wu L, Levy D, Jung JU. Inhibition of Interferon Regulatory Factor 7 (IRF7)-Mediated Interferon Signal Transduction by the Kaposi's Sarcoma-Associated Herpesvirus Viral IRF Homolog vIRF3. Journal of Virology. 2007;81(15):8282–8292. doi: 10.1128/JVI.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judde J-G, Lacoste V, Brière J, Kassa-Kelembho E, Clyti E, Couppié P, Gessain A. Monoclonality or Oligoclonality of Human Herpesvirus 8 Terminal Repeat Sequences in Kaposi's Sarcoma and Other Diseases. Journal of the National Cancer Institute. 2000;92(9):729–736. doi: 10.1093/jnci/92.9.729. [DOI] [PubMed] [Google Scholar]
- Kaleeba JAR, Berger EA. Broad target cell selectivity of Kaposi's sarcoma-associated herpesvirus glycoprotein-mediated cell fusion and virion entry. Virology. 2006a;354(1):7–14. doi: 10.1016/j.virol.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kaleeba JAR, Berger EA. Kaposi's Sarcoma-Associated Herpesvirus Fusion-Entry Receptor: Cystine Transporter xCT. Science. 2006b;311(5769):1921–1924. doi: 10.1126/science.1120878. [DOI] [PubMed] [Google Scholar]
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syph. 1872;4:265–273. [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6(10):981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. IFI16 Acts as a Nuclear Pathogen Sensor to Induce the Inflammasome in Response to Kaposi Sarcoma-Associated Herpesvirus Infection. Cell Host & Microbe. 2011;9(5):363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliche S, Nagel W, Kremmer E, Atzler C, Ege A, Knorr T, Haas J. Signaling by Human Herpesvirus 8 kaposin A through Direct Membrane Recruitment of cytohesin-1. Molecular Cell. 2001;7(4):833–843. doi: 10.1016/s1097-2765(01)00227-1. [DOI] [PubMed] [Google Scholar]
- Krishnan HH, Sharma-Walia N, Zeng L, Gao S-J, Chandran B. Envelope Glycoprotein gB of Kaposi's Sarcoma-Associated Herpesvirus Is Essential for Egress from Infected Cells. Journal of Virology. 2005;79(17):10952–10967. doi: 10.1128/JVI.79.17.10952-10967.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwun HJ, da Silva SR, Shah IM, Blake N, Moore PS, Chang Y. Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen 1 Mimics Epstein-Barr Virus EBNA1 Immune Evasion through Central Repeat Domain Effects on Protein Processing. Journal of Virology. 2007;81(15):8225–8235. doi: 10.1128/JVI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyalwazi SK. Kaposi's sarcoma: clinical features, experience in Uganda. Antibiotics and chemotherapy. 1981;29:59–69. doi: 10.1159/000397440. [DOI] [PubMed] [Google Scholar]
- Lagos D, Trotter MWB, Vart RJ, Wang H-W, Matthews NC, Hansen A, Boshoff C. Kaposi sarcoma herpesvirus–encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood. 2007;109(4):1550–1558. doi: 10.1182/blood-2006-05-024034. [DOI] [PubMed] [Google Scholar]
- Lagos D, Vart RJ, Gratrix F, Westrop SJ, Emuss V, Wong P-P, Boshoff C. Toll-like Receptor 4 Mediates Innate Immunity to Kaposi Sarcoma Herpesvirus. Cell Host & Microbe. 2008;4(5):470–483. doi: 10.1016/j.chom.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff M, Bechtel J, Venetsanakos E, Roy A-M, Abbey N, Herndier B, Ganem D. De Novo Infection and Serial Transmission of Kaposi's Sarcoma-Associated Herpesvirus in Cultured Endothelial Cells. Journal of Virology. 2002;76(5):2440–2448. doi: 10.1128/jvi.76.5.2440-2448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi’s sarcoma-associated herpesvirus. Proceedings of the National Academy of Sciences. 1999;96(10):5704–5709. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M, Gannagé M, Karras A, Abel M, Legendre C, Kerob D, Caillat-Zucman S. Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood. 2006;108(12):3871–3880. doi: 10.1182/blood-2006-03-014225. [DOI] [PubMed] [Google Scholar]
- Lan K, Kuppers DA, Robertson ES. Kaposi's Sarcoma-Associated Herpesvirus Reactivation Is Regulated by Interaction of Latency-Associated Nuclear Antigen with Recombination Signal Sequence-Binding Protein Jκ, the Major Downstream Effector of the Notch Signaling Pathway. Journal of Virology. 2005;79(6):3468–3478. doi: 10.1128/JVI.79.6.3468-3478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan K, Kuppers DA, Verma SC, Robertson ES. Kaposi's Sarcoma-Associated Herpesvirus-Encoded Latency-Associated Nuclear Antigen Inhibits Lytic Replication by Targeting Rta: a Potential Mechanism for Virus-Mediated Control of Latency. Journal of Virology. 2004;78(12):6585–6594. doi: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-S, Alvarez X, Ishido S, Lackner AA, Jung JU. Inhibition of Intracellular Transport of B Cell Antigen Receptor Complexes by Kaposi's Sarcoma–Associated Herpesvirus K1. The Journal of Experimental Medicine. 2000;192(1):11–22. doi: 10.1084/jem.192.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-S, Connole M, Tang Z, Harris NL, Jung JU. Structural Analysis of the Kaposi's Sarcoma-Associated Herpesvirus K1 Protein. Journal of Virology. 2003;77(14):8072–8086. doi: 10.1128/JVI.77.14.8072-8086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-S, Lee S-H, Feng P, Chang H, Cho N-H, Jung JU. Characterization of the Kaposi's Sarcoma-Associated Herpesvirus K1 Signalosome. Journal of Virology. 2005;79(19):12173–12184. doi: 10.1128/JVI.79.19.12173-12184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Guo J, Li M, Choi J-K, DeMaria M, Rosenzweig M, Jung JU. Identification of an Immunoreceptor Tyrosine-Based Activation Motif of K1 Transforming Protein of Kaposi’s Sarcoma-Associated Herpesvirus. Molecular and Cellular Biology. 1998;18(9):5219–5228. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Jung JU. Deregulation of cell growth by the K1 gene of Karposi's sarcoma-associated herpesvirus. Nat Med. 1998;4(4):435–440. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- Lefort S, Soucy-Faulkner A, Grandvaux N, Flamand L. Binding of Kaposi's Sarcoma-Associated Herpesvirus K-bZIP to Interferon-Responsive Factor 3 Elements Modulates Antiviral Gene Expression. Journal of Virology. 2007;81(20):10950–10960. doi: 10.1128/JVI.00183-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Bai Z, Ye F, Xie J, Kim C-G, Huang Y, Gao S-J. Regulation of NF-[kappa]B inhibitor I[kappa]B[alpha] and viral replication by a KSHV microRNA. Nat Cell Biol. 2010;12(2):193–199. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhu Y, Jones T, Bai Z, Huang Y, Gao S-J. A Kaposi's Sarcoma-Associated Herpesvirus MicroRNA and Its Variants Target the Transforming Growth Factor β Pathway To Promote Cell Survival. Journal of Virology. 2012;86(21):11698–11711. doi: 10.1128/JVI.06855-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidner RS, Aboulafia D. Recrudescent Kaposi's sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS patient care and STDs. 2005;19(10):635–644. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- Letang E, Lewis J, Bower M, Mosam A, Borok M, Campbell T, Easterbrook P. Immune reconstitution inflammatory syndrome associated with kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS (London) 2013:1. doi: 10.1097/QAD.0b013e328360a5a1. [DOI] [PubMed] [Google Scholar]
- Li H, Wang H, Nicholas J. Detection of Direct Binding of Human Herpesvirus 8-Encoded Interleukin-6 (vIL-6) to both gp130 and IL-6 Receptor (IL-6R) and Identification of Amino Acid Residues of vIL-6 Important for IL-6R-Dependent and -Independent Signaling. Journal of Virology. 2001;75(7):3325–3334. doi: 10.1128/JVI.75.7.3325-3334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, Neipel F, Jung JU. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. Journal of Virology. 1997;71(3):1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Means R, Lang S, Jung JU. Downregulation of Gamma Interferon Receptor 1 by Kaposi's Sarcoma-Associated Herpesvirus K3 and K5. Journal of Virology. 2007;81(5):2117–2127. doi: 10.1128/JVI.01961-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Fu B, Wu F, Li X, Yuan Y, Zhu F. ORF45 of Kaposi's Sarcoma-Associated Herpesvirus Inhibits Phosphorylation of Interferon Regulatory Factor 7 by IKKε and TBK1 as an Alternative Substrate. Journal of Virology. 2012;86(18):10162–10172. doi: 10.1128/JVI.05224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Seet BT, Ingham RJ, Gish G, Matskova L, Winberg G, Pawson T. The K15 Protein of Kaposi's Sarcoma-Associated Herpesvirus Recruits the Endocytic Regulator Intersectin 2 through a Selective SH3 Domain Interaction†. Biochemistry. 2007;46(35):9874–9885. doi: 10.1021/bi700357s. [DOI] [PubMed] [Google Scholar]
- Lin CL, Li H, Wang Y, Zhu FX, Kudchodkar S, Yuan Y. Kaposi's Sarcoma-Associated Herpesvirus Lytic Origin (ori-Lyt)-Dependent DNA Replication: Identification of the ori-Lyt and Association of K8 bZip Protein with the Origin. Journal of Virology. 2003;77(10):5578–5588. doi: 10.1128/JVI.77.10.5578-5588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Genin P, Mamane Y, Sgarbanti M, Battistini A, Harrington W, Hiscott J. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene. 2001;20(7):800–811. doi: 10.1038/sj.onc.1204163. [DOI] [PubMed] [Google Scholar]
- Lin X, Li X, Liang D, Lan K. MicroRNAs and Unusual Small RNAs Discovered in Kaposi's Sarcoma-Associated Herpesvirus Virions. Journal of Virology. 2012;86(23):12717–12730. doi: 10.1128/JVI.01473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-T, Kincaid RP, Arasappan D, Dowd SE, Hunicke-Smith SP, Sullivan CS. Small RNA profiling reveals antisense transcription throughout the KSHV genome and novel small RNAs. RNA. 2010;16(8):1540–1558. doi: 10.1261/rna.1967910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Martin HJ, Liao G, Hayward SD. The Kaposi's Sarcoma-Associated Herpesvirus LANA Protein Stabilizes and Activates c-Myc. Journal of Virology. 2007;81(19):10451–10459. doi: 10.1128/JVI.00804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. The Human Herpes Virus 8-encoded Viral FLICE Inhibitory Protein Physically Associates with and Persistently Activates the IκB Kinase Complex. Journal of Biological Chemistry. 2002;277(16):13745–13751. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic Regulation of Kaposi's Sarcoma-Associated Herpesvirus Latency by Virus-Encoded MicroRNAs That Target Rta and the Cellular Rbl2-DNMT Pathway. Journal of Virology. 2010;84(6):2697–2706. doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Suen J, Frias C, Pfeiffer R, Tsai M-H, Chuang E, Zeichner SL. Dissection of the Kaposi's Sarcoma-Associated Herpesvirus Gene Expression Program by Using the Viral DNA Replication Inhibitor Cidofovir. Journal of Virology. 2004;78(24):13637–13652. doi: 10.1128/JVI.78.24.13637-13652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luitweiler EM, Henson BW, Pryce EN, Patel V, Coombs G, McCaffery JM, Desai PJ. Interactions of the Kaposi's Sarcoma-Associated Herpesvirus Nuclear Egress Complex: ORF69 Is a Potent Factor for Remodeling Cellular Membranes. Journal of Virology. 2013;87(7):3915–3929. doi: 10.1128/JVI.03418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Kirshner JR, Ganem D. Transcriptional Activation by the Product of Open Reading Frame 50 of Kaposi’s Sarcoma-Associated Herpesvirus Is Required for Lytic Viral Reactivation in B Cells. Journal of Virology. 1999;73(11):9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Renne R, Kirshner JR, Ganem D. Reactivation of Kaposi's Sarcoma-Associated Herpesvirus Infection from Latency by Expression of the ORF 50 Transactivator, a Homolog of the EBV R Protein. Virology. 1998;252(2):304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- Marcelin A, Calvez V, Dussaix E. KSHV after an organ transplant: should we screen? Current topics in microbiology and immunology. 2007;312:245–262. doi: 10.1007/978-3-540-34344-8_9. [DOI] [PubMed] [Google Scholar]
- Mark L, Lee WH, Spiller OB, Proctor D, Blackbourn DJ, Villoutreix BO, Blom AM. The Kaposi's Sarcoma-associated Herpesvirus Complement Control Protein Mimics Human Molecular Mechanisms for Inhibition of the Complement System. Journal of Biological Chemistry. 2004;279(43):45093–45101. doi: 10.1074/jbc.M407558200. [DOI] [PubMed] [Google Scholar]
- Mark L, Proctor DG, Blackbourn DJ, Blom AM, Spiller OB. Separation of decay-accelerating and cofactor functional activities of Kaposi's sarcoma-associated herpesvirus complement control protein using monoclonal antibodies. Immunology. 2008;123(2):228–238. doi: 10.1111/j.1365-2567.2007.02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall V, Parks T, Bagni R, Wang CD, Samols MA, Hu J, Whitby D. Conservation of Virally Encoded MicroRNAs in Kaposi Sarcoma-Associated Herpesvirus in Primary Effusion Lymphoma Cell Lines and in Patients with Kaposi Sarcoma or Multicentric Castleman Disease. Journal of Infectious Diseases. 2007;195(5):645–659. doi: 10.1086/511434. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Molecular Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Matta H, Sun Q, Moses G, Chaudhary PM. Molecular Genetic Analysis of Human Herpes Virus 8-encoded Viral FLICE Inhibitory Protein-induced NF-κB Activation. Journal of Biological Chemistry. 2003;278(52):52406–52411. doi: 10.1074/jbc.M307308200. [DOI] [PubMed] [Google Scholar]
- Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune Deficiency and Risk for Malignancy Among Persons with AIDS. J. Acquired. Immun. Def. Synd. 2003;32(5):527–533. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- McCormick C, Ganem D. The Kaposin B Protein of KSHV Activates the p38/MK2 Pathway and Stabilizes Cytokine mRNAs. Science. 2005;307(5710):739–741. doi: 10.1126/science.1105779. [DOI] [PubMed] [Google Scholar]
- McCormick C, Ganem D. Phosphorylation and Function of the Kaposin B Direct Repeats of Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2006;80(12):6165–6170. doi: 10.1128/JVI.02331-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meads MB, Medveczky PG. Kaposi's Sarcoma-associated Herpesvirus-encoded Viral Interleukin-6 Is Secreted and Modified Differently Than Human Interleukin-6: EVIDENCE FOR A UNIQUE AUTOCRINE SIGNALING MECHANISM. Journal of Biological Chemistry. 2004;279(50):51793–51803. doi: 10.1074/jbc.M407382200. [DOI] [PubMed] [Google Scholar]
- Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi’s Sarcoma-associated Herpesvirus-encoded Cytokine Homolog (vIL-6) Activates Signaling through the Shared gp130 Receptor Subunit. Journal of Biological Chemistry. 1997;272(31):19625–19631. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- Monini P, Carlini F, Stürzl M, Rimessi P, Superti F, Franco M, Ensoli B. Alpha Interferon Inhibits Human Herpesvirus 8 (HHV-8) Reactivation in Primary Effusion Lymphoma Cells and Reduces HHV-8 Load in Cultured Peripheral Blood Mononuclear Cells. Journal of Virology. 1999;73(5):4029–4041. doi: 10.1128/jvi.73.5.4029-4041.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S. Akt/TSC/mTOR activation by the KSHV G protein-coupled receptor: emerging insights into the molecular oncogenesis and treatment of Kaposi's sarcoma. Cell Cycle. 2007;6(4):438–443. doi: 10.4161/cc.6.4.3843. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Gutkind JS. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3(1):23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi’s Sarcoma-associated Herpesvirus G Protein-coupled Receptor Promotes Endothelial Cell Survival through the Activation of Akt/Protein Kinase B. Cancer Research. 2001;61(6):2641–2648. [PubMed] [Google Scholar]
- Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular Mimicry of Human Cytokine and Cytokine Response Pathway Genes by KSHV. Science. 1996;274(5293):1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- Mori Y, Nishimoto N, Ohno M, Inagi R, Dhepakson P, Amou K, Yamanishi K. Human herpesvirus 8-encoded interleukin-6 homologue (viral IL-6) induces endogenous human IL-6 secretion. Journal of Medical Virology. 2000;61(3):332–335. doi: 10.1002/1096-9071(200007)61:3<332::aid-jmv8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Morris VA, Punjabi AS, Lagunoff M. Activation of Akt through gp130 receptor signaling is required for Kaposi's sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J Virol. 2008;82(17):8771–8779. doi: 10.1128/JVI.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick J, Bernet J, Singh AK, Lambris JD, Sahu A. Kaposi's Sarcoma-Associated Herpesvirus (Human Herpesvirus 8) Open Reading Frame 4 Protein (Kaposica) Is a Functional Homolog of Complement Control Proteins. Journal of Virology. 2003;77(6):3878–3881. doi: 10.1128/JVI.77.6.3878-3881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhar S, Pumfery AM, Hassani M, Sadaie MR, Azumi N, Kishishita M, Rosenthal LJ. Identification of Kaposin (Open Reading Frame K12) as a Human Herpesvirus 8 (Kaposi’s Sarcoma-Associated Herpesvirus) Transforming Gene. Journal of Virology. 1998;72(6):4980–4988. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse Herpesvirus MicroRNAs Target the Stress-Induced Immune Ligand MICB to Escape Recognition by Natural Killer Cells. Cell Host & Microbe. 2009;5(4):376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Nador R, Cesarman E, Chadburn A, Dawson D, Ansari M, Sald J, Knowles D. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88(2):645–656. [PubMed] [Google Scholar]
- Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus Induces the Phosphatidylinositol 3-Kinase-PKC-ζ-MEK-ERK Signaling Pathway in Target Cells Early during Infection: Implications for Infectivity. Journal of Virology. 2003;77(2):1524–1539. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus Modulates Microtubule Dynamics via RhoA-GTP-Diaphanous 2 Signaling and Utilizes the Dynein Motors To Deliver Its DNA to the Nucleus. Journal of Virology. 2005;79(2):1191–1206. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon K, Newcomb WW, Pray TR, Craik CS, Brown JC, Kedes DH. Lytic Replication of Kaposi's Sarcoma-Associated Herpesvirus Results in the Formation of Multiple Capsid Species: Isolation and Molecular Characterization of A, B, and C Capsids from a Gammaherpesvirus. Journal of Virology. 2001;75(6):2866–2878. doi: 10.1128/JVI.75.6.2866-2878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neipel F, Albrecht JC, Ensser A, Huang YQ, Li JJ, Friedman-Kien AE, Fleckenstein B. Human herpesvirus 8 encodes a homolog of interleukin-6. Journal of Virology. 1997;71(1):839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J, Ruvolo VR, Burns WH, Sandford G, Wan X, Ciufo D, Reixz MS. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3(3):287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- Nishi J-I, Arimura K, Utsunomiya A, Yonezawa S, Kawakami K, Maeno N, Maruyama I. Expression of vascular endothelial growth factor in sera and lymph nodes of the plasma cell type of Castleman's disease. British Journal of Haematology. 1999;104(3):482–485. doi: 10.1046/j.1365-2141.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, Kishimoto T. Humanized anti–interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- O'Hara AJ, Chugh P, Wang L, Netto EM, Luz E, Harrington WJ, Dittmer DP. Pre-Micro RNA Signatures Delineate Stages of Endothelial Cell Transformation in Kaposi Sarcoma. PLoS Pathog. 2009;5(4):e1000389. doi: 10.1371/journal.ppat.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol. 2000;2(11):819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- Oksenhendler E, Boulanger E, Galicier L, Du M, Dupin N, Diss T, Meignin V. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331–2336. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- Oksenhendler E, Carcelain G, Aoki Y, Boulanger E, Maillard A, Clauvel J, Agbalika F. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood. 2000;96(6):2069–2073. [PubMed] [Google Scholar]
- Oksenhendler E, Duarte M, Soulier J, Cacoub P, Welker Y, Cadranel J, Raphael M. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS (London) 1996;10(1):61–67. [PubMed] [Google Scholar]
- Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Human Immunology. 1999;60(10):921–927. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- Parkin D, Wabinga H, Nambooze S, Wabwire-Mangen F. AIDS-related cancers in Africa: maturation of the epidemic in Uganda. AIDS. 1999;13(18):2563–2570. doi: 10.1097/00002030-199912240-00010. [DOI] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. International Journal of Cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Parravicini C, Corbellino M, Paulli M, Magrini U, Lazzarino M, Moore P, Chang Y. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman's disease. The American journal of pathology. 1997;151(6):1517–1522. [PMC free article] [PubMed] [Google Scholar]
- Pauk J, Huang M-L, Brodie SJ, Wald A, Koelle DM, Schacker T, Corey L. Mucosal Shedding of Human Herpesvirus 8 in Men. New England Journal of Medicine. 2000;343(19):1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- Pearce M, Matsumura S, Wilson AC. Transcripts Encoding K12, v-FLIP, v-Cyclin, and the MicroRNA Cluster of Kaposi's Sarcoma-Associated Herpesvirus Originate from a Common Promoter. Journal of Virology. 2005;79(22):14457–14464. doi: 10.1128/JVI.79.22.14457-14464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel PE. Human Herpesvirus 8 Glycoprotein B (gB), gH, and gL Can Mediate Cell Fusion. Journal of Virology. 2002;76(9):4390–4400. doi: 10.1128/JVI.76.9.4390-4400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, Grasser FA, Tuschl T. Identification of microRNAs of the herpesvirus family. Nat Meth. 2005;2(4):269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- Polizzotto MN, Uldrick TS, Hu D, Yarchoan R. Clinical Manifestations of Kaposi Sarcoma Herpesvirus (KSHV) Lytic Activation: Multicentric Castleman Disease (KSHV-MCD) and the KSHV Inflammatory Cytokine Syndrome (KICS) Frontiers in Microbiology. 2012;3 doi: 10.3389/fmicb.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LJ, Zong J-C, Ciufo DM, Alcendor DJ, Cannon JS, Ambinder R, Hayward GS. Comparison of Genetic Variability at Multiple Loci across the Genomes of the Major Subtypes of Kaposi’s Sarcoma-Associated Herpesvirus Reveals Evidence for Recombination and for Two Distinct Types of Open Reading Frame K15 Alleles at the Right-Hand End. Journal of Virology. 1999;73(8):6646–6660. doi: 10.1128/jvi.73.8.6646-6660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O, Swamy OR, Peng X, Tang Z-Y, Li L, Larson JE, Samaniego F. Activation of Src kinase Lyn by the Kaposi sarcoma–associated herpesvirus K1 protein: implications for lymphomagenesis. Blood. 2005;105(10):3987–3994. doi: 10.1182/blood-2004-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O, Tang Z-Y, Peng X, Coleman R, Gill J, Farr G, Samaniego F. Tumorigenesis and Aberrant Signaling in Transgenic Mice Expressing the Human Herpesvirus-8 K1 Gene. Journal of the National Cancer Institute. 2002;94(12):926–935. doi: 10.1093/jnci/94.12.926. [DOI] [PubMed] [Google Scholar]
- Qin Z, Dai L, Defee M, Findlay VJ, Watson DK, Toole BP, Parsons C. Kaposi's Sarcoma-Associated Herpesvirus Suppression of DUSP1 Facilitates Cellular Pathogenesis following De Novo Infection. Journal of Virology. 2013;87(1):621–635. doi: 10.1128/JVI.01441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaszkiewicz T, Hansmann M, Lennert K. Monoclonality and polyclonality of plasma cells in Castleman's disease of the plasma cell variant. Histopathology. 1989;14(1):11–24. doi: 10.1111/j.1365-2559.1989.tb02110.x. [DOI] [PubMed] [Google Scholar]
- Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med. 2000;6(10):1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, Chandran B. Lipid Rafts of Primary Endothelial Cells Are Essential for Kaposi's Sarcoma-Associated Herpesvirus/Human Herpesvirus 8-Induced Phosphatidylinositol 3-Kinase and RhoA-GTPases Critical for Microtubule Dynamics and Nuclear Delivery of Viral DNA but Dispensable for Binding and Entry. Journal of Virology. 2007;81(15):7941–7959. doi: 10.1128/JVI.02848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus Utilizes an Actin Polymerization-Dependent Macropinocytic Pathway To Enter Human Dermal Microvascular Endothelial and Human Umbilical Vein Endothelial Cells. Journal of Virology. 2009;83(10):4895–4911. doi: 10.1128/JVI.02498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human Herpesvirus 8 Infects and Replicates in Primary Cultures of Activated B Lymphocytes through DC-SIGN. Journal of Virology. 2008;82(10):4793–4806. doi: 10.1128/JVI.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Rinaldo CR. DC-SIGN Is a Receptor for Human Herpesvirus 8 on Dendritic Cells and Macrophages. The Journal of Immunology. 2006;176(3):1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- Ray A, Marshall V, Uldrick T, Leighty R, Labo N, Wyvill K, Whitby D. Sequence Analysis of Kaposi Sarcoma–Associated Herpesvirus (KSHV) MicroRNAs in Patients with Multicentric Castleman Disease and KSHV-Associated Inflammatory Cytokine Syndrome. Journal of Infectious Diseases. 2012;205(11):1665–1676. doi: 10.1093/infdis/jis249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited Transmission of Kaposi’s Sarcoma-Associated Herpesvirus in Cultured Cells. Journal of Virology. 1998;72(6):5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. Journal of Virology. 1996;70(11):8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas C, Thlick A-E, Parravicini C, Moore PS, Chang Y. Kaposi's Sarcoma-Associated Herpesvirus LANA2 Is a B-Cell-Specific Latent Viral Protein That Inhibits p53. Journal of Virology. 2001;75(1):429–438. doi: 10.1128/JVI.75.1.429-438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RC, Lagos D, Gratrix F, Henderson S, Matthews NC, Vart RJ, Gotch FM. The CD8 and CD4 T-Cell Response against Kaposi's Sarcoma-Associated Herpesvirus Is Skewed Towards Early and Late Lytic Antigens. PLoS ONE. 2009;4(6):e5890. doi: 10.1371/journal.pone.0005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah S, Jagne YJ, Zuo J, de Silva T, Ahasan MM, Brander C, Hislop AD. T-cell immunity to Kaposi sarcoma–associated herpesvirus: recognition of primary effusion lymphoma by LANA-specific CD4+ T cells. Blood. 2012;119(9):2083–2092. doi: 10.1182/blood-2011-07-366476. [DOI] [PubMed] [Google Scholar]
- Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A Complex Translational Program Generates Multiple Novel Proteins from the Latently Expressed Kaposin (K12) Locus of Kaposi’s Sarcoma-Associated Herpesvirus. Journal of Virology. 1999;73(7):5722–5730. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols MA, Hu J, Skalsky R, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. Journal of Virology. 2005;79(14):9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish N, Zhu FX, Golub EE, Liang Q, Yuan Y. Mechanisms of Autoinhibition of IRF-7 and a Probable Model for Inactivation of IRF-7 by Kaposi's Sarcoma-associated Herpesvirus Protein ORF45. Journal of Biological Chemistry. 2011;286(1):746–756. doi: 10.1074/jbc.M110.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwam DR, Luciano RL, Mahajan SS, Wong L, Wilson AC. Carboxy Terminus of Human Herpesvirus 8 Latency-Associated Nuclear Antigen Mediates Dimerization, Transcriptional Repression, and Targeting to Nuclear Bodies. Journal of Virology. 2000;74(18):8532–8540. doi: 10.1128/jvi.74.18.8532-8540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5(3):230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 Induced by Kaposi's Sarcoma-Associated Herpesvirus (Human Herpesvirus 8) Early during Infection of Target Cells Are Essential for Expression of Viral Genes and for Establishment of Infection. Journal of Virology. 2005;79(16):10308–10329. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TV, Wang H-W, Koumi A, Hollyman D, Endo Y, Ye H, Boshoff C. K15 Protein of Kaposi’s Sarcoma-Associated Herpesvirus Is Latently Expressed and Binds to HAX-1, a Protein with Antiapoptotic Function. Journal of Virology. 2002;76(2):802–816. doi: 10.1128/JVI.76.2.802-816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, Hamill R, Rodriguez-Barradas M, Greenberg S, Atmar R, Musher D, Trautner B. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81(3):213–227. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Si H, Robertson ES. Kaposi's Sarcoma-Associated Herpesvirus-Encoded Latency-Associated Nuclear Antigen Induces Chromosomal Instability through Inhibition of p53 Function. Journal of Virology. 2006;80(2):697–709. doi: 10.1128/JVI.80.2.697-709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J, Alper J, Schutte J, Robbins H, Blaufox L. Disseminated visceral Kaposi's sarcoma: Appearance after human renal homograft operation. JAMA. 1969;207(8):1493–1496. [PubMed] [Google Scholar]
- Simonelli C, Tedeschi R, Gloghini A, Spina M, Talamini R, De Paoli P, Carbone A. Prognostic Factors in Human Herpesvirus 8–Related Lymphoproliferative Disorders Associated With HIV Infection. Journal of Clinical Oncology. 2006;24(1):209. doi: 10.1200/JCO.2005.04.1178. [DOI] [PubMed] [Google Scholar]
- Sin S-H, Dittmer DP. Viral latency locus augments B cell response in vivo to induce chronic marginal zone enlargement, plasma cell hyperplasia and lymphoma. Blood. 2013 doi: 10.1182/blood-2012-03-415620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus Latency in Endothelial and B Cells Activates Gamma Interferon-Inducible Protein 16-Mediated Inflammasomes. Journal of Virology. 2013;87(8):4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Renne R. Kaposi's Sarcoma-Associated Herpesvirus Encodes an Ortholog of miR-155. Journal of Virology. 2007;81(23):12836–12845. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, Montaner S. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10(2):133–143. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, Sigaux F. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease [see comments] Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- Spiller OB, Blackbourn DJ, Mark L, Proctor DG, Blom AM. Functional Activity of the Complement Regulator Encoded by Kaposi's Sarcoma-associated Herpesvirus. Journal of Biological Chemistry. 2003;278(11):9283–9289. doi: 10.1074/jbc.m211579200. [DOI] [PubMed] [Google Scholar]
- Staskus KA, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Haase AT. Cellular Tropism and Viral Interleukin-6 Expression Distinguish Human Herpesvirus 8 Involvement in Kaposi’s Sarcoma, Primary Effusion Lymphoma, and Multicentric Castleman’s Disease. Journal of Virology. 1999;73(5):4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Haase AT. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. Journal of Virology. 1997;71(1):715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Spencer D, Ruff P, Lakier R, MacPhail P, Bezwoda W. Endemic African Kaposi's sarcoma: clinical and therapeutic implications. 10-year experience in the Johannesburg Hospital (1980–1990) Oncology. 1994;51(1):63–69. doi: 10.1159/000227312. [DOI] [PubMed] [Google Scholar]
- Stine JT, Wood C, Hill M, Epp A, Raport CJ, Schweickart VL, Chantry D. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95(4):1151–1157. [PubMed] [Google Scholar]
- Subramanian R, Sehgal I, D'Auvergne O, Kousoulas KG. Kaposi's Sarcoma-Associated Herpesvirus Glycoproteins B and K8.1 Regulate Virion Egress and Synthesis of Vascular Endothelial Growth Factor and Viral Interleukin-6 in BCBL-1 Cells. Journal of Virology. 2010;84(4):1704–1714. doi: 10.1128/JVI.01889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffert G, Malterer G, Hausser J, Viiliäinen J, Fender A, Contrant M, Pfeffer S. Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis. PLoS Pathog. 2011;7(12):e1002405. doi: 10.1371/journal.ppat.1002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Zachariah S, Chaudhary PM. The Human Herpes Virus 8-Encoded Viral FLICE-inhibitory Protein Induces Cellular Transformation via NF-κB Activation. Journal of Biological Chemistry. 2003;278(52):52437–52445. doi: 10.1074/jbc.M304199200. [DOI] [PubMed] [Google Scholar]
- Sun R, Lin S-F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proceedings of the National Academy of Sciences. 1998;95(18):10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Lin S-F, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi’s Sarcoma-Associated Herpesvirus Gene Expression. Journal of Virology. 1999;73(3):2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Lin SF, Gradoville L, Miller G. Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proceedings of the National Academy of Sciences. 1996;93(21):11883–11888. doi: 10.1073/pnas.93.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthaus J, Stuhlmann-Laeisz C, Tompkins VS, Rosean TR, Klapper W, Tosato G, Rose-John S. HHV-8–encoded viral IL-6 collaborates with mouse IL-6 in the development of multicentric Castleman disease in mice. Blood. 2012;119(22):5173–5181. doi: 10.1182/blood-2011-09-377705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot SJ, Weiss RA, Kellam P, Boshoff C. Transcriptional Analysis of Human Herpesvirus-8 Open Reading Frames 71, 72, 73, K14, and 74 in a Primary Effusion Lymphoma Cell Line. Virology. 1999;257(1):84–94. doi: 10.1006/viro.1999.9672. [DOI] [PubMed] [Google Scholar]
- Tamburro KM, Yang D, Poisson J, Fedoriw Y, Roy D, Lucas A, Dittmer DP. Vironome of Kaposi sarcoma associated herpesvirus-inflammatory cytokine syndrome in an AIDS patient reveals co-infection of human herpesvirus 8 and human herpesvirus 6A. Virology. 2012;433(1):220–225. doi: 10.1016/j.virol.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Templeton A, Vogel C, Ziegler J, Kyalwazi S. Kaposi's sarcoma in Uganda: a clinico-pathological study. Int J Cancer. 1971;15(8):122–135. doi: 10.1002/ijc.2910080116. [DOI] [PubMed] [Google Scholar]
- Thomas JO. Acquired immunodeficiency syndrome-associated cancers in Sub-Saharan Africa. Seminars in Oncology. 2001;28(2):198–206. doi: 10.1016/s0093-7754(01)90092-2. [DOI] [PubMed] [Google Scholar]
- Thomas M, Boname JM, Field S, Nejentsev S, Salio M, Cerundolo V, Lehner PJ. Down-regulation of NKG2D and NKp80 ligands by Kaposi's sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proceedings of the National Academy of Sciences. 2008;105(5):1656–1661. doi: 10.1073/pnas.0707883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386(6624):517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- Tomescu C, Law WK, Kedes DH. Surface Downregulation of Major Histocompatibility Complex Class I, PE-CAM, and ICAM-1 following De Novo Infection of Endothelial Cells with Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2003;77(17):9669–9684. doi: 10.1128/JVI.77.17.9669-9684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CC, Damania B. The K1 Protein of Kaposi's Sarcoma-Associated Herpesvirus Activates the Akt Signaling Pathway. Journal of Virology. 2004;78(4):1918–1927. doi: 10.1128/JVI.78.4.1918-1927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CC, Damania B. Critical Role for Endocytosis in the Regulation of Signaling by the Kaposi's Sarcoma-Associated Herpesvirus K1 Protein. Journal of Virology. 2008;82(13):6514–6523. doi: 10.1128/JVI.02637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y-H, Wu M-F, Wu Y-H, Chang S-J, Lin S-F, Sharp TV, Wang H-W. The M Type K15 Protein of Kaposi's Sarcoma-Associated Herpesvirus Regulates MicroRNA Expression via Its SH2-Binding Motif To Induce Cell Migration and Invasion. Journal of Virology. 2009;83(2):622–632. doi: 10.1128/JVI.00869-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldrick TS, Wang V, O'Mahony D, Aleman K, Wyvill KM, Marshall V, Yarchoan R. An Interleukin-6-Related Systemic Inflammatory Syndrome in Patients Co-Infected with Kaposi Sarcoma-Associated Herpesvirus and HIV but without Multicentric Castleman Disease. Clinical Infectious Diseases. 2010;51(3):350–358. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi Sarcoma pathogenesis, and treatment of Kaposi Sarcoma. Cancer Letters. 2011;305(2):150–162. doi: 10.1016/j.canlet.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JL, Cullen BR. In-Depth Analysis of Kaposi's Sarcoma-Associated Herpesvirus MicroRNA Expression Provides Insights into the Mammalian MicroRNA-Processing Machinery. Journal of Virology. 2010;84(2):695–703. doi: 10.1128/JVI.02013-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC, Borah S, Robertson ES. Latency-Associated Nuclear Antigen of Kaposi's Sarcoma-Associated Herpesvirus Up-Regulates Transcription of Human Telomerase Reverse Transcriptase Promoter through Interaction with Transcription Factor Sp1. Journal of Virology. 2004;78(19):10348–10359. doi: 10.1128/JVI.78.19.10348-10359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC, Choudhuri T, Kaul R, Robertson ES. Latency-Associated Nuclear Antigen (LANA) of Kaposi's Sarcoma-Associated Herpesvirus Interacts with Origin Recognition Complexes at the LANA Binding Sequence within the Terminal Repeats. Journal of Virology. 2006;80(5):2243–2256. doi: 10.1128/JVI.80.5.2243-2256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschuren EW, Hodgson JG, Gray JW, Kogan S, Jones N, Evan GI. The Role of p53 in Suppression of KSHV Cyclin-induced Lymphomagenesis. Cancer Research. 2004;64(2):581–589. doi: 10.1158/0008-5472.can-03-1863. [DOI] [PubMed] [Google Scholar]
- Wahman A, Melnick SL, Rhame FS, Potter JD. The Epidemiology of Classic, African, and Immunosuppressed Kaposi's Sarcoma. Epidemiologic Reviews. 1991;13(1):178–199. doi: 10.1093/oxfordjournals.epirev.a036068. [DOI] [PubMed] [Google Scholar]
- Wan X, Wang H, Nicholas J. Human Herpesvirus 8 Interleukin-6 (vIL-6) Signals through gp130 but Has Structural and Receptor-Binding Properties Distinct from Those of Human IL-6. Journal of Virology. 1999;73(10):8268–8278. doi: 10.1128/jvi.73.10.8268-8278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F-Z, Akula SM, Sharma-Walia N, Zeng L, Chandran B. Human Herpesvirus 8 Envelope Glycoprotein B Mediates Cell Adhesion via Its RGD Sequence. Journal of Virology. 2003;77(5):3131–3147. doi: 10.1128/JVI.77.5.3131-3147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brinkmann MM, Pietrek M, Ottinger M, Dittrich-Breiholz O, Kracht M, Schulz TF. Functional characterization of the M-type K15-encoded membrane protein of Kaposi's sarcoma-associated herpesvirus. Journal of general virology. 2007;88(6):1698–1707. doi: 10.1099/vir.0.82807-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B. Immortalization of Primary Endothelial Cells by the K1 Protein of Kaposi's Sarcoma–Associated Herpesvirus. Cancer Research. 2006;66(7):3658–3666. doi: 10.1158/0008-5472.CAN-05-3680. [DOI] [PubMed] [Google Scholar]
- Wang L, Wakisaka N, Tomlinson CC, DeWire SM, Krall S, Pagano JS, Damania B. The Kaposi’s Sarcoma-Associated Herpesvirus (KSHV/HHV-8) K1 Protein Induces Expression of Angiogenic and Invasion Factors. Cancer Research. 2004;64(8):2774–2781. doi: 10.1158/0008-5472.can-03-3653. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Jenkins FJ, Jacobson LP, Kingsley LA, Day RD, Zhang Z-W, Rinaldo CR. Primary human herpesvirus 8 infection generates a broadly specific CD8+ T-cell response to viral lytic cycle proteins. Blood. 2001;97(8):2366–2373. doi: 10.1182/blood.v97.8.2366. [DOI] [PubMed] [Google Scholar]
- Waterston A, Bower M. Fifty years of multicentric Castleman's disease. Acta Oncologica. 2004;43(8):698–704. doi: 10.1080/02841860410002752. [DOI] [PubMed] [Google Scholar]
- Weber KSC, Gröne H-J, Röcken M, Klier C, Gu S, Wank R, Weber C. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. European Journal of Immunology. 2001;31(8):2458–2466. doi: 10.1002/1521-4141(200108)31:8<2458::aid-immu2458>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wen KW, Damania B. Hsp90 and Hsp40/Erdj3 are required for the expression and anti-apoptotic function of KSHV K1. Oncogene. 2010;29(24):3532–3544. doi: 10.1038/onc.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Damania B. Upregulation of the TLR3 Pathway by Kaposi's Sarcoma-Associated Herpesvirus during Primary Infection. Journal of Virology. 2008;82(11):5440–5449. doi: 10.1128/JVI.02590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JA, Gregory SM, Sivaraman V, Su L, Damania B. Activation of Plasmacytoid Dendritic Cells by Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2011;85(2):895–904. doi: 10.1128/JVI.01007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wies E, Hahn AS, Schmidt K, Viebahn C, Rohland N, Lux A, Neipel F. The Kaposi's Sarcoma-associated Herpesvirus-encoded vIRF-3 Inhibits Cellular IRF-5. Journal of Biological Chemistry. 2009;284(13):8525–8538. doi: 10.1074/jbc.M809252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FY, Ahn J-H, Alcendor DJ, Jang W-J, Xiao J, Hayward SD, Hayward GS. Origin-Independent Assembly of Kaposi's Sarcoma-Associated Herpesvirus DNA Replication Compartments in Transient Cotransfection Assays and Association with the ORF-K8 Protein and Cellular PML. Journal of Virology. 2001;75(3):1487–1506. doi: 10.1128/JVI.75.3.1487-1506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Lo P, Yu X, Stoops J, Forghani B, Zhou Z. Three-dimensional structure of the human herpesvirus 8 capsid. Journal of Virology. 2000;74(20):9646–9654. doi: 10.1128/jvi.74.20.9646-9654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, AuCoin DP, Huete AR, Cei SA, Hanson LJ, Pari GS. A Kaposi's Sarcoma-Associated Herpesvirus/Human Herpesvirus 8 ORF50 Deletion Mutant Is Defective for Reactivation of Latent Virus and DNA Replication. Journal of Virology. 2005;79(6):3479–3487. doi: 10.1128/JVI.79.6.3479-3487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T-Y, Chen S-C, Leach MW, Manfra D, Homey B, Wiekowski M, Lira SA. Transgenic Expression of the Chemokine Receptor Encoded by Human Herpesvirus 8 Induces an Angioproliferative Disease Resembling Kaposi's Sarcoma. The Journal of Experimental Medicine. 2000;191(3):445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Zhou F, Bedolla RG, Jones T, Lei X, Kang T, Gao S-J. Reactive Oxygen Species Hydrogen Peroxide Mediates Kaposi's Sarcoma-Associated Herpesvirus Reactivation from Latency. PLoS Pathog. 2011;7(5):e1002054. doi: 10.1371/journal.ppat.1002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Kang J, Lee HN, Aguilar B, Kafka D, Lee S, Hong Y-K. Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus. PLoS Pathog. 2010;6(8):e1001046. doi: 10.1371/journal.ppat.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, Komori T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74(4):1360–1367. [PubMed] [Google Scholar]
- Yu F, Feng J, Harada JN, Chanda SK, Kenney SC, Sun R. B cell terminal differentiation factor XBP-1 induces reactivation of Kaposi’s sarcoma-associated herpesvirus. FEBS Letters. 2007;581(18):3485–3488. doi: 10.1016/j.febslet.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Yu Y, Black JB, Goldsmith CS, Browning PJ, Bhalla K, Offermann MK. Induction of human herpesvirus-8 DNA replication and transcription by butyrate and TPA in BCBL-1 cells. Journal of general virology. 1999;80(1):83–90. doi: 10.1099/0022-1317-80-1-83. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang SE, Hayward GS. The KSHV Immediate-Early Transcription Factor RTA Encodes Ubiquitin E3 Ligase Activity that Targets IRF7 for Proteosome-Mediated Degradation. Immunity. 2005;22(1):59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zaldumbide A, Ossevoort M, Wiertz EJHJ, Hoeben RC. In cis inhibition of antigen processing by the latency-associated nuclear antigen I of Kaposi sarcoma Herpes virus. Molecular Immunology. 2007;44(6):1352–1360. doi: 10.1016/j.molimm.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zheng D, Wan J, Cho YG, Wang L, Chiou C-J, Pai S, Hayward SD. Comparison of Humoral Immune Responses to Epstein-Barr Virus and Kaposi’s Sarcoma–Associated Herpesvirus Using a Viral Proteome Microarray. Journal of Infectious Diseases. 2011;204(11):1683–1691. doi: 10.1093/infdis/jir645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Xue M, Qin D, Zhu X, Wang C, Zhu J, Lu C. HIV-1 Tat Promotes Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) vIL-6-Induced Angiogenesis and Tumorigenesis by Regulating PI3K/PTEN/AKT/GSK-3β Signaling Pathway. PLoS ONE. 2013;8(1):e53145. doi: 10.1371/journal.pone.0053145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FX, Chong JM, Wu L, Yuan Y. Virion Proteins of Kaposi's Sarcoma-Associated Herpesvirus. Journal of Virology. 2005;79(2):800–811. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FX, Cusano T, Yuan Y. Identification of the Immediate-Early Transcripts of Kaposi’s Sarcoma-Associated Herpesvirus. Journal of Virology. 1999;73(7):5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proceedings of the National Academy of Sciences. 2002;99(8):5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FX, Sathish N, Yuan Y. Antagonism of Host Antiviral Responses by Kaposi's Sarcoma-Associated Herpesvirus Tegument Protein ORF45. PLoS ONE. 2010;5(5):e10573. doi: 10.1371/journal.pone.0010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FX, Yuan Y. The ORF45 Protein of Kaposi's Sarcoma-Associated Herpesvirus Is Associated with Purified Virions. Journal of Virology. 2003;77(7):4221–4230. doi: 10.1128/JVI.77.7.4221-4230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Guo Y, Yao S, Yan Q, Xue M, Hao T, Lu C. Synergy between Kaposi's sarcoma-associated herpesvirus (KSHV) vIL-6 and HIV-1 Nef protein in promotion of angiogenesis and oncogenesis: role of the AKT signaling pathway. Oncogene. 2013 doi: 10.1038/onc.2013.136. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer J, Grundhoff A, Ganem D. Exploring the DNA Binding Interactions of the Kaposi's Sarcoma-Associated Herpesvirus Lytic Switch Protein by Selective Amplification of Bound Sequences In Vitro. Journal of Virology. 2006;80(6):2958–2967. doi: 10.1128/JVI.80.6.2958-2967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- Zuo J, Hislop AD, Leung C, Sabbah S, Rowe M. KSHV encoded vIRF3 modulates MHC-II antigen presentation through CIITA dependent and independent mechanisms: implications for oncogenesis. Journal of Virology. 2013 doi: 10.1128/JVI.00250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Thomas W, van Leeuwen D, Middeldorp JM, Wiertz EJHJ, Ressing ME, Rowe M. The DNase of Gammaherpesviruses Impairs Recognition by Virus-Specific CD8+ T Cells through an Additional Host Shutoff Function. Journal of Virology. 2008;82(5):2385–2393. doi: 10.1128/JVI.01946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]