Abstract
The vertebrate lens evolved to collect light and focus it onto the retina. In development, the lens grows through massive elongation of epithelial cells possibly recapitulating the evolutionary origins of the lens. The refractive index of the lens is largely dependent on high concentrations of soluble proteins called crystallins. All vertebrate lenses share a common set of crystallins from two superfamilies (although other lineage specific crystallins exist). The α-crystallins are small heat shock proteins while the β- and γ-crystallins belong to a superfamily that contains structural proteins of uncertain function. The crystallins are expressed at very high levels in lens but are also found at lower levels in other cells, particularly in retina and brain. All these proteins have plausible connections to maintenance of cytoplasmic order and chaperoning of the complex molecular machines involved in the architecture and function of cells, particularly elongated and post-mitotic cells. They may represent a suite of proteins that help maintain homeostasis in such cells that are at risk from stress or from the accumulated insults of aging.
Keywords: crystallins, chaperone, lens, retina, epithelial cell, stress
1: Introduction. Evolution of Lens and Crystallins: an elongation connection?
The vertebrate lens is an avascular cellular structure composed mainly of highly elongated, terminally differentiated fiber cells, most of which lack all organelles (Bassnett et al., 2011; Kuszak et al., 2004). In many species it survives and functions for decades, maintaining transparency and a gradient of refractive index (RI) to produce clear focused images on the light sensitive cells of the retina. Optical adaptation for a particular habitat is generally achieved by the shape of the gradient in ways that reduce spherical aberration (Kroger et al., 1994) or optimize chromatic aberration (Gustafsson et al., 2008; Kroger and Fernald, 1994). The RI of the lens is largely due to high concentrations of soluble proteins generically known as crystallins (Bloemendal et al., 2004; Slingsby et al., 2013; Wistow, 2012). The lens and its contents evolved at some early point in the history of vertebrates, but long after the evolution of light sensitive cells, the photoreceptors, that perform the key step in vision of transducing photons into neural impulses.
Although fossils are lacking, there are many examples of existing simple eyes, particularly in invertebrates, that have photosensitive patches or organized retinas with no lens (Ayala, 2007; Land and Fernald, 1992; Land and Nilsson, 2002; Shimeld et al., 2005). From the ontogeny of the eye and from the parallel example of existing simple eyes a simple, staged process can be imagined that led to the evolution of the modern lens in discrete steps that all produced a useful, evolutionary selected structure. Indeed, modelling has suggested that such an evolutionary process could be relatively rapid (Nilsson and Pelger, 1994). A layer of cells overlying the retinal anlagen gives rise to a lens in both vertebrates and cephalopods (Harris, 1997). Elongation of these cells, which is an early step in the formation of lens placode in mammals and continues throughout development (Fig 1), gives rise to a structure capable of gathering light. In a remarkable parallel example in vertebrates the parietal or median eye found in reptiles and amphibians acquires a “lens” of a single layer of elongated transparent cells (Fig 1) by a completely different mechanism from that in the familiar lateral eyes (Eakin, 1973; McDevitt, 1972), emphasizing again the connection between cell elongation and lens formation. (In mammals, the ancestral parietal eye is represented only by light sensitive structures in the pineal gland).
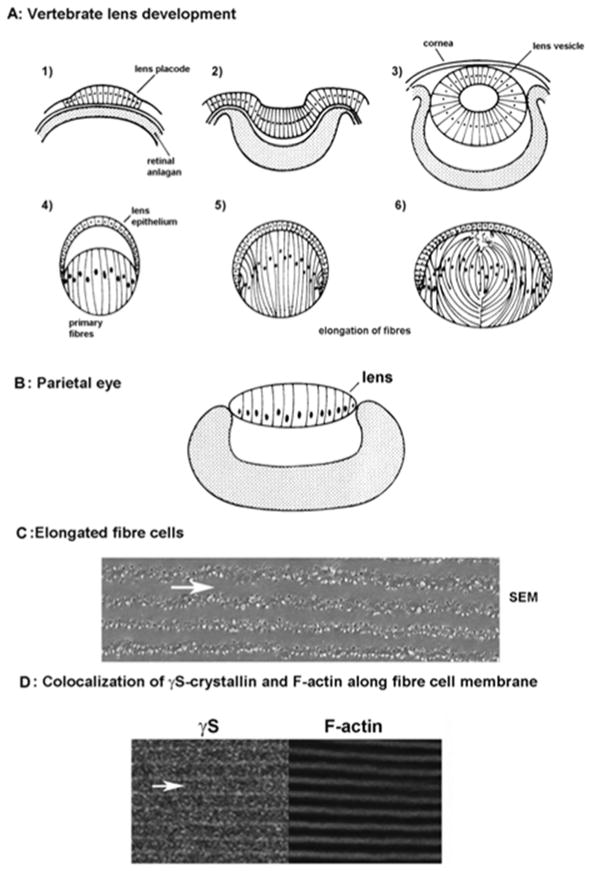
Figure 1. Lens formation is associated with cell elongation.
- Epithelial cells overlying the retinal anlagen elongate to form the lens placode
- Elongation continues as the optic vesicle invaginates.
- Formation of the lens vesicle and cornea
- Within the lens vesicle, primary fiber cells elongate
- Fibres fill the lens
- New layers of secondary fibres form throughout life through differentiation and elongation of equatorial epithelial cells.
B: Schematic structure of the lizard parietal eye (adapted from (Eakin, 1973)). A monolayer of elongated cells forms the simple cellular lens.
C: Elongation and intercalation of mature fiber cells. Scanning electron micrograph of a fiber cells from adult mouse lens. Arrow indicates the longitudinal axis of a fiber cell. Extensive cell-cell contacts are formed through intercalation of complex junctions. (For methods, see (Fan et al., 2012))
D: Co-localization of γS-crystallin and F-actin along the plasma membranes of mature mouse fiber cells (adapted from (Fan et al., 2012)).
Elongation of cells to produce a convex surface is a common feature of cellular lenses, but this alone would not produce an effective lens. The lens needs to be transparent, but also needs an increased refractive index (RI) to diffract and concentrate incoming light. It seems likely that during evolution this was achieved by upregulation of expression of some proteins that were already expressed in the ancestral epithelium and which could maintain elevated cytoplasmic concentrations while remaining transparent. These proteins may have had roles in maintaining the organization of the architecture and homeostasis of the elongating cells, particularly in regard to the enormously extended networks of cytoskeleton and membrane-bound junctional complexes that characterize the fiber cells of the vertebrate lens. In the vertebrate lens the fiber cells are extremely elongated, with the bulk of the plasma membrane derived from the adhesive sides that are enriched in proteins that link to the cytoskeleton. Highly prominent members of the lens membrane proteome are connexins, neural cadherin and lens specialized forms of aquaporin and claudin (Bassnett et al., 2009). In other cells, plasma membrane adhesion molecules link to the cytoskeleton and to organelles via giant linker molecules such as plectins, plakins and nesprins that contain multiple spectrin and plakin repeats and coiled-coils (Wilhelmsen et al., 2005). The α-, β-, and γ-crystallins which are represented in all vertebrate lineages may have evolved from proteins that support this kind of filament system and this may shed light on the functions of these proteins in lens and non-lens cells.
Particularly for image formation in more sophisticated eyes, the lens evolved mechanisms not only for creating increased RI but for regulating RI along the optical axis of the lens from the outer to the innermost layers (Pierscionek and Regini, 2012; Slingsby et al., 2013). This was achieved, at least in part, by increasing the diversity of crystallins through gene duplication and specialization and through regulated expression of crystallins in different layers of the lens to produce and fine-tune a gradient of RI to optimize optical performance.
Later in the evolution of vertebrates, particularly those whose ancestors emerged from the water to live on land, the optics of the lens adapted to requirements of the environment through changes in gene expression; further duplications of crystallin genes; deletion of crystallin genes; and even by de novo recruitment (through increased gene expression) of other proteins already expressed in the lens, often metabolic enzymes, as highly expressed crystallins (Wistow, 1993; Wistow and Piatigorsky, 1988).
Although crystallins are predominantly expressed in the lens, they necessarily arose from ancestors which had functions in non-lens cells. The nature of those functions may relate to the maintenance mechanisms of the lens and may also explain the expression of many crystallins outside the lens, particularly in other eye tissues.
The functional roles of the α-crystallins are clearly related to their membership in the chaperone-like small heat-shock proteins family (Clark et al., 2012). sHSPs are considered to bind exposed polypeptides, such as those in stressed, unfolding proteins, thereby blocking or retarding formation of aggregates, including fibrils, that can have toxic effects on the cell. One of the two α-crystallins found in most vertebrates, αB, is stress-inducible, widely expressed in other cells and is particularly associated with protein deposition diseases in neurological and other tissues.
The roles of the βγ-crystallins are not as clear. These proteins belong to a superfamily that includes members in microbes and sponges, yet appear to have been lost in most other metazoan phyla except chordates (Slingsby et al., 2013). An ancestral form of the βγ-crystallins is found in the calcium-enriched otolith, one of the paired pigmented sister cells that function in guidance in the larval stage of tunicates, a urochordate (Shimeld et al., 2005). Recent genome sequence for the phylum Ctenophora (comb jellies, whose few species, characterized by their glass-clear transparency, are common ocean surface swimmers around the world) (Ryan et al., 2013) also predicts the presence of proteins with βγ-crystallin domains. The tunicate and ctenophoran proteins and several microbial βγ-crystallin superfamily members have conserved calcium-binding sites that are lost in the lens crystallins (Kappe et al., 2010; Shimeld et al., 2005). Some of these proteins are involved in specialized calcium-dependent cellular roles associated with formation or maintenance of cellular structures such as spore coats or cysts (Wistow, 1990; Wistow et al., 1985).
Some members of the superfamily in vertebrates also appear to associate with components of cellular architecture such as the cytoskeleton, this is at least implied by the plasma membrane localization of Ep37/EDSP in the embryonic newt Cynops, and the rounded shape of melanoma cells which lack the protein AIM1 (Liu et al., 2008; Ray et al., 1996; Ray et al., 1997; Takabatake et al., 1992; Wistow et al., 1995). This might indicate another chaperone-like role, distinct from that of the sHSPs, in preventing formation of aggregates through “shepherding” other protein complexes (Fan et al., 2012).
The association of microbial superfamily βγ-crystallin members (and some sHsps) with structures such as spore coats also hints at a possible role in maintaining a low water-content, an attribute that could certainly fit some βγ-crystallins for a role in the high protein concentration lens (Chen et al., 2013; Zhao et al., 2013). However this role would seem to be dependent on the presence of high enough concentrations of βγ-crystallins to be able to influence overall water content. For the lower expression of these proteins seen outside the lens, this may not seem likely, however the shepherding role could still be effective at lower than lens concentrations if the crystallins are specifically targeted, perhaps to cytoskeletal filaments. In this scenario, their role in retina, brain and elsewhere could be in preventing tangling and aggregation of filaments, such as in neuronal cell processes thereby acting to maintain transport functions. In retinal cells that lie along the light path to the photoreceptors this could also help to keep the cell cytoplasm transparent by inhibiting formation of light scattering centers. This is a function that might be particularly important in very elongated cells, such as lens fiber cells, or in cell extensions such as axons.
There is another feature that may connect cells that are major sites of crystallin expression. Lens fiber cells, many neuronal cell types and retinal pigment epithelial cells are either post-mitotic or have low rates of turnover and replacement (Anderson et al., 1981; Frederikse et al., 2012). Very long lived cells may suffer stress from the gradual accumulation of deposits, such as amyloid-like fibrils, that are resistant to clearance by normal housekeeping mechanisms (Chiti and Dobson, 2009). Proteins such as α- and βγ-crystallins, which appear to have roles in retarding aggregation or perhaps in reducing tangling or clumping of complex macromolecules, could have evolved to delay some of the effects of aging in cells.
2: Structure of α-Crystallins
In common with most other small heat shock proteins (sHsps), lens α-crystallin is a highly polydisperse assembly. There are no methods for preparing α-crystallin in homogeneous form, and most biophysical techniques indicate an assembly size of around 800 kDa with newly synthesized lens α-crystallin being composed of an approximately 3:1 ratio of αA/αB chains (Horwitz, 2009). Nanoelectrospray mass spectrometry of unfolded/refolded or recombinant αB-crystallin indicates an ensemble of interconverting oligomeric forms smaller than lens alpha-crystallin, ranging from 10 to 40 subunits, centering around 28 subunits, yet including odd and even numbered oligomers (Aquilina et al., 2003). There is no precedent for a soluble, globular assembly with the characteristics of α-crystallin, as most globular protein assemblies have a discrete size in which protomers are arranged around one or more axes of rotational symmetry that form one of the four point groups: cyclic, dihedral, cubic (tetrahedral 23 or octahedral 432), icosahedral. This structural and conformational dynamism is considered central to the role of α-crystallin both as a lens protein and as a small heat shock protein chaperone.
The sequences of sHsps contain a central, conserved “α-crystallin domain” (ACD), flanked by a long variable N-terminal extension and a shorter better conserved C-terminal extension (Hilton et al., 2013). Because most sHsps are not amenable to crystallography, the compromise in understanding their quaternary structures at atomic resolution has been to solve 3D structures of any monodisperse assembly that can be identified from across the Kingdoms of Life, and look for common structural principles. The first crystal structure of a sHsp assembly came from an archaeal thermophile (MjHsp16.5) and showed how a 24-mer could be built by the assembly of symmetric dimers (pdb 1shs). The structure showed how the fold of the ACD dimerized by strand-exchange and assembled into an octahedral shape with point group 432 symmetry (Kim et al., 1998). Higher assembly in this example was driven only by a C-terminal β-strand extension, which differs from most polydisperse sHsps in which the N-terminal extension also plays a role (McHaourab et al., 2009). Crystal structures of complete sHsp assemblies from yeast and wheat show how, by relaxing the symmetry of the dimer, different orientations of the C-extension created by conformational flexibility of the linker between domain and extension, allow assemblies of (lower) dihedral symmetry, namely a 16-mer with 422 symmetry (pdb 3w1z, (Hanazono et al., 2013), and a 12-mer with 32 symmetry (pdb 1gme, (van Montfort et al., 2001)). The key assembly contact in all three of these sHsps is a sequence motif I-X-I/V in the C-terminal extension in one chain filling pockets in the ACD of another chain. The role of the flexible linker in sHsp assembly was reinforced when modification of this region in MjHsp16.5 (pdb 4eld) by insertion of a segment of human Hsp27 caused a switch in oligomer size from 24-mer to 48-mer, whilst maintaining assembly interactions as well as the point group symmetry (McHaourab et al., 2012). In the eukaryotic structures, relaxed dimer symmetry permits regions of some N-terminal extensions to be resolved as α-helical in conformation and located towards the interior of the assemblies (pdb 1gme; 3w1z).
Sequence analyses of sHsps indicated that the higher assembly components, the I-X-I/V motif and ACD pockets, were conserved, whereas the dimer interface regions diverged, making modelling unreliable. The lack of a monodisperse vertebrate sHsp assembly meant that only smaller assemblies, formed from truncated α-crystallin chains, could be imaged by crystallography. The human αB ACD, stripped of both extensions, formed a different kind of dimer (Bagneris et al., 2009) consistent with the sequence distinction between metazoan and non-metazoan sHsp sequences. Vertebrate α-crystallin ACD dimer structures have an extended anti-parallel β-sheet dimer interface that is conserved in constructs that included C-terminal extensions, although a register shift is permissible (Laganowsky et al., 2010; Laganowsky and Eisenberg, 2010). The docking of the I-X-I/V sequence motif into partner ACD pockets is conserved, though a change in strand direction is allowed, consistent with the palindromic sequence of the motif region. An sHsp structure (pdb 2bol) from tapeworm that has two consecutive ACDs but lacks a C-terminal sequence motif, showed that I-X-P sequences from other regions of the sequence could dock instead into ACD pockets (Stamler et al., 2005). These high resolution snapshots of subassembly species are consistent with solid state NMR spectroscopy of full-length reassociated αB assemblies (Jehle et al., 2010), indicating a conserved role for the I-X-I/V sequence motif in alpha-crystallin assembly. What was missing was the structure of the N-terminal region, although solid state NMR indicated it was likely flexible. The X-ray snapshots showed a deep groove at the anti-parallel dimer interface, into which we have speculated the N-terminal extensions might bind in a dynamic way (Clark et al., 2011; Slingsby et al., 2013). The idea is that under stress conditions, sHsp sequence extensions compete with unstructured regions of substrate proteins for binding to pockets and groove, in other words the dynamic assembly mechanism provides the basis of chaperone function.
This picture of an interaction between an N-terminal extension and a dimer groove is not in agreement with all the biophysical findings. The shape of the extended beta sheet across the anti-parallel interface is flat in the X-ray crystal structures whereas in the solid state NMR coordinates (pdb 2klr) it is twisted in a way that blocks the groove (Clark et al., 2011; Jehle et al., 2010). Statistical analysis of single particle images taken by electron microscopy can be used to reconstruct 3D electron density maps of large assemblies so long as different views of the object and their symmetric relationships can be distinguished. Early cryoEM studies indicated α-crystallins were too polydisperse to reconstruct with any symmetry, and hence the map showed few features other than a hollow shell (Haley et al., 2000). More recently there have been claims of a more homogeneous αB sample allowing the reconstruction of density maps from negative stain images consistent with a model of a 24-mer with 23 symmetry (Peschek et al., 2009). The structure of the αB ACD dimer coordinates (pdb 2klr) were fitted to this EM map as a trimer of dimers around the 3-fold axes in an arrangement similar to that seen around the 3-fold axes of the MjHsp16.5 and wheat X-ray structures (Jehle et al., 2010; Jehle et al., 2011) and to the negative stain EM structure of the Mycobacterium tuberculosis Acr1 12-mer with 23 symmetry (pdb 2byu) (Kennaway et al., 2005). A larger cryoEM dataset subsequently did reveal size polydispersity, but with a significant population of particles that could be classified as 24-mers with 23 symmetry (Braun et al., 2011). A new model was fitted to the map in which a trimer of dimers was modelled into a large anti-parallel beta barrel with the 3-fold axis passing through the middle of the barrel and through density modelled as the self-associating N-terminal extensions (pdb 2ygd) (Braun et al., 2011). When the three N-terminal serines known to be phosphorylated in vivo are mutated to three glutamates, several techniques indicated a redistribution towards smaller oligomers, with cryoEM reconstructions showing maintenance of the 23 (tetrahedral) 24-mer model along with 3D reconstructions of 12-mers and 6-mers that now comprise a larger proportion of the sample (Peschek et al., 2013). Other authors have argued that the level of size polydispersity of the αB-crystallin, as measured by gas phase IM-nanoMS is better modeled by an assembly mechanism based on interconverting asymmetric polyhedral models (Baldwin et al., 2011).
The 3D structure of the N-terminal domain of αB-crystallin is unknown which makes the unambiguous fitting of ACD dimer coordinates into EM maps difficult, especially when samples are polydisperse. The two EM based models have N-terminal extensions in completely different locations (Delbecq and Klevit, 2013), however they are each consistent with I-X-I/V motifs fitting in to ACD pockets. Both solid-state and solution NMR spectroscopy support significant occupation of the ACD pockets with I-X-I/V motifs (Delbecq et al., 2012; Jehle et al., 2010), although other NMR methods indicate that the motif is highly flexible at ambient temperatures and hovers over rather than binds into the pockets (Baldwin et al., 2012). A study using a combination of nanoMS and IMS to measure the impact of neighboring residues of the I-X-I sequence on the strength of its binding with an ACD pocket shows that in the context of a full assembly, increasing the binding of an interdimer contact (motif into pocket) destabilizes the extended anti-parallel beta sheet interface within a dimer (Hilton et al., 2013). These authors argue for a major role of the C-terminal region in regulating quaternary dynamics and hence access to substrate binding sites (see also Benesch review). This idea also fits with the notion that the extended anti-parallel interface groove, which crystallography has shown is blocked in the R120G human disease mutant, forms a regulated peptide binding site (Clark et al., 2011).
An unexpected view on the function of αB comes from studies showing that the peptide sequence DRFSVNLDVKHFSPEELKVK (residues 73–92) from human αB-crystallin can prevent protein aggregation in vitro, leading to its designation as a “minichaperone” (Bhattacharyya et al., 2006). Remarkably, following uptake by different mechanisms, exogenous administration of this peptide was found to protect fetal retinal pigment epithelial (RPE) cells from oxidative stress-induced apoptosis (Sreekumar et al., 2013). In the 3D structure this peptide comprises the B3 strand of one sheet of the alpha-crystallin domain and the edge B4 strand of the other sheet along with the arching loop that connects these strands. The seven hydrophobic side chains are buried between the sheets of this strand, although the B3-strand hydrophobic side chains have some solvent exposure depending on the presence of a B2 strand (Clark et al., 2011). The B3 strand occupies a strategic position as it forms the sides of the shared groove of the α-crystallin domain dimer, and Val 91 lines one of the B4/B8 pockets, both candidate substrate-binding regions. However, the peptide on its own would be expected to be dominated by its hydrophobic character and, assuming that αB-crystallin remains largely folded in its biologically active form, it is difficult to reconcile how the peptide can mimic the true function of αB. This suggests that the effect of the “minichaperone” may be through a different mechanism from the native sHsp.
Although the structural studies provide conflicting models of vertebrate sHsps, they underscore the dynamic nature of the system. The sHsps may have been selected for a role in the eye lens because they are large and polydisperse, and hence unlikely to crystallize or phase separate in the concentrated cytoplasm of the lens. We have outlined potential protein binding sites in the ACD that also function in (quasi)-assembly. We have suggested that the ability to form extended lattices between assemblies through their I-X-I/V motifs might be the basis of the water-insoluble alpha-crystallin which is characteristic of high refractive index regions of mature lenses without compromising transparency (Slingsby et al., 2013). Or they may have been recruited because of their role as stress proteins, with their main function in the lens being to bind other disordered proteins that cannot survive for a lifetime without the energy-hungry machines that provide quality control.
3: The roles of α-crystallins outside the lens
αB-crystallin is expressed in many tissues, notably in the retina, muscle and brain where it is often a biomarker of pathology (Andley, 2007). The two genes (CRYAA, CRYAB) that encode the lens α-crystallin A and B polypeptide chains, belong to the small heat shock protein (sHsp) family, whose ten members (HSPB1-B10) have distinctive tissue distributions (Carra et al., 2013; Kappe et al., 2003). Current understanding is that heat shock proteins play roles in cellular proteostasis where they support various stress mechanisms (Balch et al., 2008; Tyedmers et al., 2010; Vembar and Brodsky, 2008) with a particular importance in retinal degeneration (Athanasiou et al., 2013). In the cytosol the Heat Shock Response (HSR), under the control of a heat shock transcription factor (HSF), leads to upregulation of several classes of chaperone proteins including some sHsps and ATP-driven chaperones including Hsp70s for protein binding and refolding, while the Ubiquitin-Proteosomal system (UPS) ensures terminally misfolded proteins are tagged and trafficked to proteasomes for degradation (Basha et al., 2012). Quality control within the ER maintains the integrity of membrane and secreted proteins using several chaperones, predominantly Hsp70 family member HSPA5, also known as ER lumenal Ca(2+)-binding glucose-regulated protein 78 (GRP78), or BiP, which has an ER localization signal sequence. If the ER becomes overloaded, the Unfolded Protein Response (UPR) surveillance system is activated and sends defective proteins back to the cytosol for degradation (Walter and Ron, 2011). Larger protein aggregates can be trafficked to lysosomes for catabolism and recycling. If these mechanisms are overwhelmed by protein aggregation, the cells die by apoptosis or necrosis. As the exogenous expression of sHsps is strongly cytoprotective in many cell types they have become attractive as potential neuroprotective agents (Arrigo, 2013; Stetler et al., 2010).
However, the exact role of sHsps in protein homeostasis is unclear. Some members, including αB and αA, can prevent denaturation of model stressed proteins in vitro (Horwitz et al., 1998). Mutations in sHsp family members, particularly those that are upregulated by HSF, may lead to self-aggregation that can overwhelm the degradation pathways directly (Clark et al., 2012), or indirectly by a loss of other (largely unknown) functions in cellular homeostasis pathways. The activity of small heat shock proteins is considered to be networked to other chaperones, which use the energy of ATP to drive conformational changes needed for unfolding/refolding of damaged proteins in readiness for recycling or destruction (Buchberger et al., 2010). For those sHsps that behave as ATP-independent chaperones, it is possible that cytoprotection stems entirely from an ability to bind non-native proteins, so keeping them in a soluble state.
This ancestral ability of sHsps to bind non-native, probably unstructured, polypeptide chains may have been exploited for other cytoplasmic roles, such as an adaptor/scaffold/tether, for incorporation into other pathways involved in proteostasis, autophagy or cell death. An example is binding of the small heat shock protein HSPB8 to Bag3. Structural biology provides clear evidence that the Bag domain acts as a regulator of Hsp70 (HSPA8) ATPase activity by acting as a nucleotide exchange factor, and that the interactive surface residues are conserved in the Bag domain of human Bag3 (Sondermann et al., 2001). Outside the Bag domain, I-P-V sequences in Bag3 can bind HSPB8, most likely by insertion into B4/B8 pockets of the alpha-crystallin domain (Fuchs et al., 2010), in the same way that these sequence motifs have been observed to facilitate assembly of several crystallized sHsps (Slingsby et al., 2013). This interaction provides a link between a sHsp and a motor chaperone through a single adaptor/tether.
There are many studies reporting binding of αB to several different components of the apoptosis pathway (Acunzo et al., 2012). Interestingly, cytoprotection activity from ischemic brain injury in mice by overexpression of Hsp27 (HspB1) was chased back from cytochrome c towards the upstream activated kinase ASK-1, thus blocking cell death signaling (Stetler et al., 2008). The presence of classic phosphorylation sites in N-terminal extensions of the α-crystallins and other sHsps has long been held to denote a role in signaling following cytoskeletal stress (Launay et al., 2006). In the case of heat-induced necrotic cell death, a study in C elegans showed that preconditioning with heat resulted in localization of Hsp16.1 to the medial Golgi where it maintained calcium-homeostasis through interaction with a transmembrane cation transporter, ATPase PMR-1 (Kourtis et al., 2012). Hsp16.1 may stabilize the PMR-1 pump to allow clearance of calcium from the cytoplasm under stress. Human PMR-1 contains an I-P-V motif in the N-terminal cytoplasmic region, suggesting it too may be a target for sHsp binding. Recent evidence has suggested that αB does support the folding of multispan transmembrane proteins and be involved in their transport from the cytosol to the lumen of the ER (D’Agostino et al., 2013).
In order to unravel the role of sHsps in cell homeostasis it is instructive to look at the impact of knockouts. αA(HSPB4) is massively expressed in the eye lens, whereas αB(HSPB5) in addition to high expression in the lens is highly expressed in striated muscle, and is also found in many other cell types, particularly the neuroglia. This tissue distribution is broadly in keeping with current understanding of gene regulatory regions in the two α-crystallin encoding genes, and with the phenotypes of the homozygous mouse knockouts of cryaa (which caused early cataract) and cryab (which caused muscular degeneration without cataract), although the latter also knocked out a second muscle sHsps, HspB2 (Brady et al., 1997; Brady et al., 2001). An essential role for αB in human skeletal muscle was indicated when severe infantile myofibrillar myopathy was found to be caused by either a homozygous frameshift in exon 1 of CRYAB or a homozygous frameshift in exon 3 within the ACD, in different families (Forrest et al., 2011; Selcen, 2011). It was suggested that “the normal process of translocation of αB-crystallin to the Z-disk following muscle contraction is disrupted with consequent failure of the normal cellular mechanism for repair and maintenance of the functioning contractile apparatus” (Forrest et al., 2011). Myofibrillar myopathies are associated with disintegration of the Z-disks (specialization of muscle sarcomere for attachment of plus ends of actin filaments), with most myopathy mutations mapping to Z-disc components: desmin, αB-crystallin, myotilin, ZASP, filamin C and Bag3 (Selcen, 2011).
Some αB particles can be seen by electron microscopy to interact directly with desmin filaments (Houck et al., 2011). There is conflicting evidence concerning direct interaction between small heat shock proteins and with actin or tubulin filaments, leaving their role in cytoskeletal dynamics elusive (Mymrikov et al., 2011). In a recent phylogenomic analysis of muscle components, of the 28 components of Z-discs, four are unique to vertebrates (synaptopodin-2; telethonin; myozenin-1; small ankyrin-1, isoform 5) (Steinmetz et al., 2012). One possibility is that αB, which arose with the vertebrates, has co-evolved a cytoskeletal function with one of these Z-components in vertebrate striated muscle. Alternatively, high levels of cytoskeletal and membrane junction components containing I-X-I/V sequence motifs may require the support of substantial levels of αB.
A more subtle retinal vasculature phenotype has been described in cryab knockout mice in which retinal vessel density in the inner plexiform layer was reduced (Kase et al., 2010). Ocular angiogenesis is regulated by secretion of VEGF-A with increased ocular neovascularisation correlating with increased levels of VEGF-A. This has led to the idea that the level of VEGF-A protein expression is post-translationally regulated by αB, and that lowering levels of αB might be a useful therapy for many stress-induced ocular disorders (Kerr and Byzova, 2010; Liu et al., 2013). The finding that αB-crystallin and VEGF-A are colocalized in the endoplasmic reticulum in stressed RPE cells (Kase et al., 2010), most likely represents αB doing its cytosolic job under stress: binding the cytoplasmic regions of membrane proteins ready for trafficking through the ER-Golgi. VEGF-A contains a signal peptide sequence followed by a PDGF domain that has a cystine knot consisting of three intertwined disulfide bridges, and will likely need to be supported by the redox chaperones in the ER for their correct assembly. If endoplasmic-reticulum-associated protein degradation (ERAD) is activated and oxidized VEGF-A is sent back to the reducing cytosol, it could bind cytosolic αB in a manner similar to the in vitro insulin chaperone assay for the α-crystallins, possibly to regions suggested by protein array experiments (Ghosh et al., 2007b). Retinal proteome analysis in a mouse model of oxygen-induced retinopathy showed that knockdown of ORP-150 (an Hsp70 family member with an ER localization signal peptide) decreased the level of secreted VEGF in RPE and decreased neovascularization (Kim et al., 2012). Overall it seems that when cells are subjected to high levels of stress, several arms of the stress response are activated to prolong the life of cells and that this may include a role for αB that remains to be clarified.
Several functions have been ascribed to α-crystallin in the retina, including roles involved in cytoprotection, cell survival, inflammation, and autophagy (Kannan et al., 2012), although they may all stem from roles in homeostasis and the stress response. A large number of potential interaction partners have been described (Kannan et al., 2012), although there are few examples of direct interactions. All these observations suggest that αB (and perhaps also αA) has a broad range of housekeeping duties helping to organize and protect complex cytoplasmic and membrane bound proteins to maintain homeostasis in many cell types. In the lens, this contributes to preserving the order and stability of the hugely elongated fiber cells, with extensive junctional and cytoskeletal complexes and a protein dense cytoplasm.
4: Structure of β- and γ-crystallins
The two domains
β- and γ-crystallin gene families have evolved through the duplication and diversification of proteins based on a common 3D architecture (Slingsby et al., 2013; Wistow, 2012). These modular proteins have been examined in detail as they provide insights into the evolution of protein assemblies (Figure 2). Their recent rapid expansion in vertebrates by gene duplication has resulted in an extensive family of two-domain proteins, oligomeric β-crystallins and monomeric γ-crystallins, that constitute around 50% of the cytoplasmic lens proteins in mammals. Each β- and γ-crystallin chain folds into four Greek key motifs that are characterized by an unusual folded β-hairpin. Each domain is formed from two Greek key motifs that intercalate to form a β-sandwich with each pair of motifs arranged about an approximate dyad. The consecutive domains on each chain are connected by a short linker. The second motif in each lens βγ-crystallin domain is more conserved than the first. These conserved motifs have characteristic tyrosine and tryptophan corner residues buried in the core of the domain that underpins the β-sandwich fold, while a set of conserved hydrophobic and polar residues face outwards and are essential for higher domain assembly.
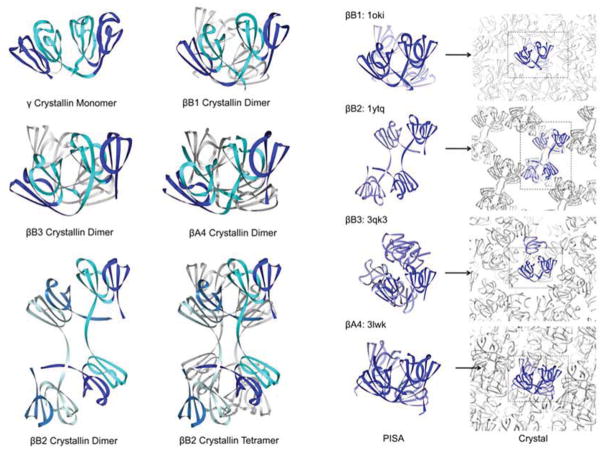
Figure 2. Modular assembly of β- and γ-crystallins from Greek key motifs and a “paired domain” interface.
The two columns on the left show structures of the β- and γ-crystallins currently in the PDB Protein Data Bank. The two columns on the right show calculated dimer structures of the β-crystallins, with their PDB identifiers, along with their arrangements within the crystal lattices. A γ-crystallin monomer has four Greek key motifs that form two similar domains organized about a pseudo twofold axis. The first motif of each domain is shown in dark blue and the second motif is in cyan. The cyan motifs provide the conserved tyrosine and tryptophan corners, as well as hydrophobic residues that form the “paired domain” interface as well as some conserved side chains including glutamines. γ-crystallin is monomeric because the “paired domain” interface is intra-chain. The same interface is present in all the resolved β-crystallins. βB2-crystallin is different as the linker is extended so the “paired domain” interface forms between two different chains which creates a domain swapped dimer. In βB1, βB3 and βA4-crystallin, the subunit domains are paired as in γ-crystallins, and so these dimers need an additional interface. This new interface is the same as that observed in the βB2 tetramer formed in the crystal lattice. The third column shows the calculated biological assemblies as calculated by PISA (Krissinel and Henrick, 2007). The fourth column shows the location in the crystal lattice of the conserved dimers highlighted in blue from the surrounding grey crystal lattice. In the case of βB3-crystallin, the calculated assembly unit is a trimer, but the conserved dimer interface is formed in the crystal lattice. This figure is adapted from Figure 5 and SFigure 1 from Slingsby et al (Slingsby et al., 2013).
A version of the presumed ancestral single domain of the vertebrate proteins can be seen in urochordate Ciona intestinalis ciona-crystallin (pdb 2bv2) which binds two calcium atoms. Very similar calcium-binding is seen in the 3D structures of several bacterial, archaeal, and mold βγ-crystallin-like domain structures (Slingsby et al., 2013). The binding sites can be detected in the sequences by the presence of the fingerprint D/N-X-X-S on each Greek key motif of a βγ-domain, with each calcium site requiring one conserved side chain from two motifs. Confidence in assigning one or two calcium binding sites in cephalochordate (amphioxus) βγ-domain sequences (Kappe et al., 2010) is bolstered by the single calcium binding site observed in the crystal structure of a βγ-domain protein from the sponge Geodia cydonium (pdb 4iau, (Vergara et al., 2013)). The recent genomic sequence determination for the Ctenophore (comb jelly) Mnemiopsis leidyi, a representative of another nonbilaterian metazoan lineage (Ryan et al., 2013), predicts the presence of several βγ-domain proteins, apparently from intron-less genes, some of which also contain the calcium binding site fingerprint. Conservation of calcium binding sites across so many lineages strengthens the idea that these nonbilaterian, archaeal and possibly bacterial βγ-domains are ancestrally related and that they are therefore likely related to the chordate βγ-crystallins too. However, this means that βγ-crystallin-like domains were frequently lost in many animal lineages (such as arthropods) and that the ancestral calcium-binding sites were themselves lost from lens crystallins for reasons unknown.
In terms of domain structure, the urochordate Ciona calcium-bound single domain (pdb 2bv2) which lacks the full set of domain pairing residues, does not domain pair in the crystal lattice and is a monomer in solution. While the monomeric structure could be ancestral in this lineage, it is also possible that the simple ocellus in Ciona represents a secondary loss of a more complex eye, and that in the same way ciona-crystallin may have lost its full set of domain-pairing hydrophobic residues. Indeed, although the Geodia sponge sequence comprises two βγ-crystallin-domains, the 3D structure shows that domain duplication is on a different evolutionary pathway from the chordates as the domain pairing interface is unrelated (Vergara et al., 2013). The ancestral relationship between lens crystallins and a βγ-domain (D3) from mammalian brain CRYBG3 (a relative of AIM1(Wistow, 2012)) is observed in the conserved domain pairing hydrophobic residues in the second Greek key motif (pdb 4fd9); the isolated domain forms a low stability monomer in solution but the domains pair like βγ-crystallins within the crystal lattice (Rajanikanth et al., 2012). Given the strong similarity in gene structure between β-crystallins and the AIM1 family (Ray et al., 1997), this was perhaps to be expected.
Missense mutations or post-translational modifications that alter highly conserved residues of the βγ-crystallin domain are likely to perturb the fold with an impact that will depend on the overall thermodynamic and kinetic stability of the protein. One of the most conserved residues in the βγ-crystallin Greek key motifs is a glycine which allows a sharp turn in the characteristic folded β-hairpin. A cataract-causing mutation, G18V, is located in the first folded hairpin in human γS-crystallin (Ma et al., 2009; Sun et al., 2005); the branched hydrophobic residue cannot take up the twisted backbone conformation available to glycine. The NMR solution structure of mutant γS-crystallin G18V under conditions where it is monomeric (pdb 2m3u) (Kingsley et al., 2013) shows the mutation causes local changes to the backbone and side chains compared with wildtype (pdb 2m3t). At neutral pH, under solution conditions where the mutant forms multimers that interact strongly with αB-crystallin, solution NMR has shown αB-crystallin binding to the N-terminal domain of γS-crystallin G18V (Kingsley et al., 2013).
The corner tryptophan in each of the lens βγ-crystallin domains, buried between β-sheets, is highly fluorescent in the absence of a second tryptophan contributed by a 310 helix, which acts as a quencher in each domain (Chen et al., 2009). These four tryptophans are conserved in all human lens βγ-crystallin domains except for the quenching tryptophan in the second domain of βB2 and βA2, while in fish γM-crystallins, the pair can be absent from one or both domains (Chen et al., 2013; Zhao et al., 2013). The Ciona calcium-bound domain has both corner and quenching tryptophans, suggesting that the pair may have an ancestral function, while in βγ-domains within AIM1 and CRYBG3, the quenching tryptophan is only moderately conserved, and it is absent in the sponge domains. As γ-crystallins have evolved into very stable proteins, particularly in mammals, the fold has become very tolerant of mutations, even in the core. For example, in human γD-crystallin a point mutation (W42R) to the corner tryptophan in the first domain causes nuclear congenital cataract, yet the solved structure (pdb 4gr7) shows only minor changes (Ji et al., 2013). However, the protein was less soluble, less stable, and more susceptible to tryptic digestion.
γ-crystallins
In γ-crystallins the inter-domain linker is bent and domain pairing results in monomer formation (Figure 2). In solution, all γ-crystallins exhibit low frictional ratios and partial specific volumes compared with other proteins (Chen et al., 2013). A survey of the amino acid composition of all proteins found that γ-crystallins have unusually high contents of residues with higher refractive index increments which may make a significant contribution to increased refractive power of the lens (Zhao et al., 2011). Some of these residues may also have important roles in protein interactions and the role of γ-crystallins in the low hydration environment of the lens.
A key sequence difference that correlates with presence in the higher refractive index lens core region is the Arg/Lys ratio. γS-crystallin, the single most abundant γ-crystallin in the human lens (Lampi et al., 1997; Wistow et al., 2002), is the most conserved family member and it is the major γ-crystallin of the more hydrated, cortical (outer) region of the lens. In human γS-crystallin sequence the Arg/Lys ratio is 13/10, while in orthologs in the zebrafish, Danio rerio, it is 16/8 (γS1) and 15/8 (γS2). In contrast, the major human γ-crystallins of the denser lens core, γC- and γD-crystallins, have Arg/Lys ratios of 20/3 and 21/1, respectively, while in Danio rerio γM2b- and γM7-crystallins the ratios are 21/3 and 20/2 respectively. The extremely high RI in the core of fish lenses may also benefit from γM-crystallins having very large numbers of methionines on the surface (Chen et al., 2013; Mahler et al., 2013; Zhao et al., 2013). The presence of these residues is associated with extremes in the hydrodynamic properties of compactness and low frictional ratio which are common to all γ-crystallins but particularly prominent in γM-crystallins of fish lenses.
In evolutionary terms, the ancestral γ-crystallin would most likely have resembled fish γS-crystallin more than the specialized core γ-crystallins. γS-crystallin domains have the same paired fluorescing and quenching tryptophan arrangement as ciona-crystallin, whereas the urochordate sequence has no arginines and no methionines. Thus the adaptation to a role for a high refractive index medium in the vertebrate lens appears to correlate with substitution of arginine for lysine along with increased content of methionine. Arginine is distinctive in having some aromatic character with the positive charge delocalized around the three terminal nitrogen atoms making it versatile in its ability to form fluctuating ion pairs (as glimpsed in the disorder in the very high resolution human γD-crystallin crystal structure, pdb 1hk0, (Basak et al., 2003), or interactions with other aromatics side chains; put another way it promotes protein-protein interactions, rather than interacting with high levels of solvent water. In an analogous way, the relatively polarizable electrons of the sulfur atom in the methionine side chain may also contribute to interactions with aromatic side chains (Valley et al., 2012).
Being monomeric, the entire surface of γ-crystallins is accessible to solvent. Their surfaces have extensive networks of polar interactions, particularly rich in arginine-containing ion pairs, while in fish, γM-crystallins are also rich in surface methionines. These features produce surfaces with a low potential for tight binding of surface water while allowing complex interactions with both water and other proteins. This may allow γ-crystallins to be highly soluble while binding relatively little water and perhaps to interact with and stabilize other proteins without tight binding and aggregation.
Lens formation requires a protein in solution to be concentrated almost to the solid state. Several human congenital cataracts are associated with catastrophic loss of protein solubility caused by mutations of arginines (Clark et al., 2012; Wistow, 2012) which could be due to perturbation of the low macroscopic dipole moment of γ-crystallins (Purkiss et al., 2007). The lens core γ-crystallins have been characterized as having attractive interactions that enable concentrated solutions to exist close to fluid-fluid phase separation (Bloemendal et al., 2004; Wang et al., 2010), an attribute likely correlated with their function as dense packing proteins. The balance of forces that enable a protein with a low overall charge yet high solubility to exist close to a critical point whilst maintaining short-range order is likely delicate. The optical perfection of eye lenses is testimony of the power of natural selection to find the right “surface solution” for protein packing density appropriate to a given position along the optical refractive index gradient.
β-crystallins
The two classes of β-crystallins, the basic (βB1, βB2 and βB3) and acidic (βA3/1, βA2 and βA4), all have N-terminal sequence extensions, compared to γ-crystallins, while the basic ones also have C-terminal extensions. βB2-crystallin dimerizes by domain swapping (pdb 2bb2; 1ytq) whereby the domain pairing of two domains in γ-crystallins is recapitulated except that the “paired domain” interaction is intermolecular and the connecting peptide extended (Figure 2) (Bax et al., 1990). By contrast, in homo-oligomeric, N-terminally truncated human βB1-crystallin [pdb 1oki] domain pairing is intrachain as in the γ-crystallins, with the monomer-monomer (dimer) interface recapitulating the domain swapped dimer-dimer interface found in the crystal lattices of bovine and human βB2-crystallin (Figure 2) (Van Montfort et al., 2003). Homo-oligomers of βB3 and βA4-crystallins (solved by high throughput structural genomics consortia) show intrachain domain pairing like that in γ-crystallin and truncated βB1-crystallin (Figure 2). In the human βB3-crystallin structure [pdb 3qk3] the calculated biological assembly is a trimer, although exploration of the crystal lattice also reveals a dimer contact similar to that in βB1-crystallin (Figure 2 (Downard et al., 2011). In human βA4-crystallin [3lwk] the calculated biological assembly has the same dimer arrangement as βB1-crystallin (Figure 2). Only rudimentary stumps of the sequence extensions can be seen in these β-crystallin crystal structures. βB2-crystallin is generally the predominant β-crystallin in the lens and forms dimers and hetero-oligomers with all the β-crystallin chains, but there are no 3D structures of the heterodimers or higher-order oligomers. The domain swapped dimeric βB2-crystallin is thermodynamically the least stable of all crystallins, is involved in dynamic subunit exchange with other β-crystallins in vitro (Bateman et al., 2003; Dolinska et al., 2009; MacDonald et al., 2005) and can act as a “chaperone” to stabilize/co-assemble other β-crystallins in cellulo (Marin-Vinader et al., 2006). βB2- and βA3-crystallin homodimers undergo subunit exchange to form a mixture of homo- and heterodimers (Hejtmancik et al., 1997; Takata et al., 2009). If βA3 homodimers associate like βA4 (Figure 2), then βB2- and βA3-crystallin homodimers would have their own distinctive mode of domain pairing, so heterodimerization would involve linker remodeling by one of the chains. Homodimers of mouse βB1-crystallin and human βA3-crystallin form a dimer-tetramer equilibrium (Chan et al., 2008). When most of the βB1 N-terminal extension was removed such that it resembled an in vivo processed chain, heterotetramer formation was enhanced, whereas the βA3-crystallin N-terminal extension was dispensable (Dolinska et al., 2009). The truncated βB1 construct resembles that used to solve the crystal structure of βB1-crystallin (pdb 1oki). Again, assuming the βA3-crystallin dimer resembles the structure of βA4, there are two possible scenarios for higher assembly. If, on mixing, the dimers underwent subunit-exchange and domain swapping to form heterodimers, they could then form a heterodimer-heterodimer using the released interface, to form a heterotetramer like the βB2-crystallin lattice tetramer (Figure 2). Or, if they both remain domain paired as in the homodimers, a new interface could be used to form the higher assembly. In this case the assembly could be accomplished in a similar manner to interface 1 in the lattice of truncated βB1-crystallin whereby the stumps of the sequence extensions of one dimer patch a domain surface on another dimer (see Figure 4a in reference (Van Montfort et al., 2003).
When post-translational modifications such as deamidation, or cataract causing point mutations, occur on the assembly interfaces, they have an impact on quaternary structure. For example, with age in human lens, significant levels of chemical modification occur to a pair of glutamines conserved in β-crystallins that are close to the hydrophobic patch involved in domain pairing in nearly all vertebrate βγ-crystallins (Lampi et al., 2001). The geometry of the paired domain arrangement places each glutamine close to the approximate dyad where it lies alongside its partner glutamine from a second domain (Figure 3). If one of these glutamines becomes deamidated in βB2-crystallin, where the paired domains come from different chains, one negative charge will be added to each of the paired domains at the dimer interface. If a single glutamine is deamidated in βB1-crystallin, then one negative charge is added to each of the intramolecular paired domain interfaces. Congenital cataract with microcornea is caused by S129R mutation in βB1-crystallin which occurs at the dimer interface, close to the 2-fold axis (Figure 3) (Wang et al., 2013). The altered side chain, in this case the replacement of small neutral serine for bulky positively charged arginine, results in two close positive charges at the dimer interface, in line with the experimental finding that the homodimer shifted to monomer.
Figure 3. Changes to side chains that perturb assembly interactions.
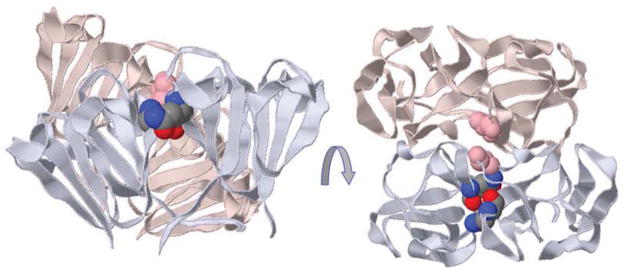
Four domains are shown from βB2 tetramer, with the “paired domains” painted in uniform colors. In a domain swapped dimer, the “paired domains” come from different chains, but are from the same chain in other β-crystallin homodimers and in γ-crystallins. In βB2 tetramer (shown) the four domains at the dimer-dimer interface are also from different chains, whereas in other β-crystallin homodimers, they come from two chains. Two glutamines colored in CPK are appended to the paired N- and C-terminal lavender domains showing their side-by-side location close to the local 2-fold between the domains. The side chain topologically equivalent to Ser129 in βB1 is shown (misty rose) appended to both C-terminal domains showing that a single mutation will clash with itself at this interface in a homo-oligomer.
The evolution of this complex mixture of dynamic interacting β-crystallins has involved the addition of sequence extensions to a conserved “domain-pair”. To what extent these extensions play roles in oligomer assembly, or, by acting as spacers between assemblies, contribute to setting up the RI gradient, is unclear. In terms of the structural biology, what is lacking is the definition at the atomic level of the role of the intact extensions in hetero-oligomer assembly. A strong case can be made for oligomer polydispersity in lens α- and β-crystallins being advantageous for transparency (Slingsby et al., 2013). To what extent the expansion of the β-crystallin family in vertebrates has benefited other vertebrate-specific functions is less clear. Evolution may have selected for properties specifically in the lens that were nevertheless useful in non-lens cell types. Furthermore, as the gene expression patterns and native structures of β-crystallins evolved co-dependently for lens, this may have favored expression of the whole suite of proteins, rather than individual members, outside the lens when needed.
5: Non-lens Expression of β- and γ-crystallins
Although crystallins were originally thought to be specific to the lens, it gradually became clear that they could also be found at much lower levels in other tissues. This was first observed for δ-crystallin of the chicken lens which was immunochemically detected in retina (Thomson et al., 1978; Thomson et al., 1981). Later it was discovered that many “taxon-specific” crystallins were identical to metabolic enzymes which retained their ancestral roles in many cell types while serving as recruited crystallins (Wistow and Piatigorsky, 1987; Wistow et al., 1987; Wistow and Piatigorsky, 1988). It was also discovered that the α-crystallins were related to sHSPs (Ingolia and Craig, 1982) and eventually it was shown that αB is indeed a functional sHSP that is stress inducible in many tissues (Bhat and Nagineni, 1989; Clark et al., 2012). However, while the non-lens expression of αB-crystallin is conceptually straightforward - it is a bona fide sHSP with stress response roles in several tissues - the expression of the lens-specialized αA outside the lens is not so easy to rationalize from a biological point of view. The same is true for the β- and γ-crystallins. Like αA, they have most often been detected in other parts of the eye, particularly the retina.
The evolutionary history of lens places it in the ontological context of the rest of the eye (Grainger, 1992; Piatigorsky, 1981). As proteins were recruited to serve as crystallins, they were probably selected from among those that were already part of the repertoire of the cells of eye cup and overlying tissues. The genes for these proteins would only have needed some limited modification in their promoter sequences to achieve higher level expression; no major chromatin remodeling or wholesale promoter engineering would have been needed. As such, it is to be expected that the crystallins (or their ancestors) had useful roles in non-lens tissues of the eye. Such roles could still exist in the modern vertebrate eye. However, since the shared ontology of different eye tissues is reflected in overlap in transcriptional mechanisms for genes in different parts of the eye (cell types of related developmental lineages are likely to have many transcription factors in common), expression of crystallins outside the lens, particularly in the retina and brain, could also be the result of transcriptional “leakiness”.
Among the β- and γ-crystallins, some genes are highly conserved with orthologs throughout the vertebrates. These include all the β-crystallins (βA1, 2, 4; βB1, 2, 3) and two γ-crystallins (γS, N) (Wistow et al., 2005). Their ancient origins make these candidates for proteins with ancient, conserved, non-lens functions. Even so, some of them are apparently dispensable even in the lens and have been lost or suppressed in some species (Wistow, 2012), suggesting that they lack an important conserved, non-lens role. For example, in primates CRYGN appears to be a pseudogene (Wistow et al., 2005) while CRYBA2 is expressed in lens but produces very little protein (Lapko et al., 2003; Wistow et al., 2002), probably through a block in translation. Many γ-crystallins have also been lost during evolution. The large class of γM-crystallins in fish seems to have been completely eliminated in terrestrial species. Their replacements in mammals, γA-F-crystallins, also seem to be dispensable, with some members eliminated in primates and even in the guinea pig (Simpanya et al., 2008). In birds, no genes for either the γM or γA-F classes exist. Genes coding for γS and γN are present in bird genomes but their level of expression is low and it is not clear that they produce functional proteins.
Non-lens ocular expression of βγ-crystallins
The first reports of crystallins, including β-crystallins, in the retina came from work on chicken embryos (Clayton et al., 1985; Thomson et al., 1978; Thomson et al., 1981). This work particularly associated crystallin expression with the phenomenon of transdifferentiation of chick neural retina or retinal pigment epithelium (RPE) in culture into “lentoid” bodies (Clayton et al., 1986), underscoring the close developmental linkage of lens with other tissues of the eye cup. The first description of a β-crystallin in mammalian retina was for βB2-crystallin in mouse and cat neural and pigmented retinas and in cat iris (Head et al., 1995). The first detection of non-lens expression of a γ-crystallin was for γS-crystallin which was detected in retina and cornea in bovine eye (Jaworski and Wistow, 1996) and in mouse retina (Sinha et al., 1998). Later γA-F-crystallins were detected in mouse retina (Jones et al., 1999).
All six γ-crystallins, along with αA, αB and γS-crystallins were detected in rat retina by mass spectroscopy and they were found to be upregulated upon exposure to intense light, suggesting a protective role (Sakaguchi et al., 2003). Later work provided evidence that distribution of αB and γ-crystallins in photoreceptors was under circadian regulation (Organisciak et al., 2011). α- and β-crystallins were also found to be upregulated in the degenerating retina of the rd1 mouse (Cavusoglu et al., 2003). All mouse crystallins were detected in retina using RTPCR (Xi et al., 2003). Microarray analysis of rat retina injured by mechanical tearing showed upregulation of αA, αB, βA1/3, βA4, βB2, βB3, γA, γC, γE (Vazquez-Chona et al., 2004). This study also found upregulation of highly lens-specific components of differentiated lens fiber cells, including BFSP1 (an intermediate filament protein) and Lim2 (a claudin) in retina, raising the question of possible contamination with RNA from lens itself. Similarly, another study using RNAseq described upregulation of crystallins and several fiber cell specific genes in a mouse model of diabetes (Kandpal et al., 2012). Again the possibility of contamination or leakage of RNA from lens, whether by damage during dissection or through effects of the disease model needs to be considered.
In addition to expression in the neural retina, αB, βA1/3, βA4, βB2 and γS-crystallins were identified by mass spectroscopy in drusen, deposits basal to the RPE that may be associated with age-related macular degeneration (AMD), in humans, while βB2 and γS-crystallins were similarly detected in monkey drusen (Crabb et al., 2002; Umeda et al., 2005). In a mouse model for RPE damage induced by dietary D-galactose, after 20 weeks exposure upregulation of expression of γS, βA1/3, βA4, βB2 and αA in RPE was detected (Tian et al., 2005), again suggesting a stress-related or protective role. Like lens fiber cells and many neuronal cell types, RPE cell types have little or no mitotic activity (Anderson et al., 1981; Dunn et al., 1996). Unlike lens cells, they are highly metabolically active and mediate transport between the vasculature and the photoreceptors. As RPE cells age, pigmented deposits accumulate while basal deposition of proteins and lipids is associated with AMD (Curcio et al., 2011; Gehrs et al., 2006; Wyatt et al., 2013). Perhaps elevation of expression of crystallins is part of a late stage stress-response to preserve RPE, or at least its function as a component of the blood-retina barrier, as long as possible.
Recently, an examination of crystallin expression patterns in retina taken from large scale microarray analyses of multiple strains of laboratory mice showed extremely large variation in expression levels from strain to strain (Templeton et al., 2013). Further studies showed poor correlation of RNA and protein levels and also found large variation between eyes in the same animal (Templeton et al., 2013). Such variation calls into question the importance of non-lens expression. At the same time, variable degrees of contamination of retina samples by lens material during dissection could also contribute to higher levels of transcript in some samples. The authors pointed out that the crystallin genes, along with some others with similar expression patterns, share promoter elements, particularly Maf response elements, raising the issue of “leaky” expression in retina of genes that have been optimized for very high expression in lens.
With regard to non-lens expression of γ-crystallins, particularly in mouse retina, an interesting early experiment used the promoter of the mouse Cryge gene, encoding γE (then called γ2), to direct expression of diphtheria toxin in a transgenic mouse (Breitman et al., 1989). Lens fiber cells were eliminated as expected, however, although iris was absent, the sensory retina, although convoluted, was apparently intact in the microphthalmic transgenic eye. While this might argue against expression of the gene in the retina, it is also possible that the construct used lacked sequences required for retina expression or that only a limited number of cells were targeted. This might suggest a quite discrete expression pattern in particular cell subtypes.
Proposed functional ocular roles outside the lens
It seems clear that there is at least some level of expression of βγ-crystallins in various parts of the retina. But do the genes have functional roles in the retina? In the Nuc1 rat there is a mutation in βA1/3 which is associated with defects in normal development and regression of intraocular blood vessels (Zhang et al., 2005). β-crystallins and γ-crystallins were detected in astrocytes surrounding intraocular vessels in the normal developing rat eye and crystallins were found to be induced by hypoxia in cultured human astrocytes (Zhang et al., 2005). The authors initially suggested that βA1/3 was specific to astrocytes in the retina and showed that there were defects in intermediate filaments in Nuc1 astrocytes (Sinha et al., 2008). Subsequently, expression of βA1/3 was detected in astrocytes, ganglion cells and RPE (Parthasarathy et al., 2011). Later it was shown that βA1/3 was localized to lysosomes in the RPE and that phagocytosis of outer segments was defective in the Nuc1 rat (Zigler et al., 2011). Nuc1 astrocytes were also shown to be defective for “anoikis”, apoptosis related to defective cell-matrix contacts, and this was associated with problems in Golgi trafficking (Ma et al., 2011). Overexpression of the mutant allele under the control of the GFAP promoter in a transgenic mouse produced defective astrocytes and was associated with differences in retinal vasculature (Sinha et al., 2012). Astrocytes from Nuc1 eye were also shown to be defective in endolysosomal acidification and this was linked to defects in processing of Notch and inhibition of Notch target genes (Valapala et al., 2013).
The authors of the Nuc1 studies propose several essential roles for βA1/3 in astrocyte development and function, in filaments, lysosomes, endosomes, Golgi and in “life death decisions”. However, it seems at least plausible that in several of these phenomena the phenotype might be due to a gain-of-function of the misfolded mutant βA1/3 interfering with subcellular processes such as trafficking. Nevertheless, several cataractogenic mutations of βA1/3 are known in both human (Bateman et al., 2000; Kannabiran et al., 1998; Yang et al., 2011; Yang et al., 2012) and mouse (Graw et al., 1999). These involve exon skipping or loss of key residues that would certainly produce a misfolded, non-functional protein in astrocytes. However no ocular vascular phenotype has been noted for these mutations. This suggests that there may be a rather specific effect in Nuc1 rather than a general loss-of-function or gain-of-function effect for βA1/3.
In the lens, it seems that β-crystallins exist predominantly as hetero-oligomers (Slingsby et al., 2013). This raises the possibility that individual members of the family may not have specific roles as homo-oligomers. In lens and in other tissues, the deleterious effects of misfolded β-crystallins may be amplified by their missing or defective interaction with other, normal β-crystallins, perhaps recruiting them into misfolded, potentially toxic aggregates.
Another series of papers has focused on a proposed role for βγ-crystallins in the function of retinal ganglion cells (RGC) and optic nerve (Piri et al., 2013). It was observed that damage to the lens was associated with neuroprotective and regenerative effects on ganglion cells in a model of traumatic optic nerve damage in rats (Heiduschka et al., 2004). Later it was shown that βB2 was present in filopodial protrusions and axons of RGC and in the supernatant of retina primary cultures (Liedtke et al., 2007). Overexpression of βB2 in RGCs promoted axonogenesis which was blocked by antibody to βB2 and conditioned medium containing βB2 also promoted axon growth. Green fluorescent protein tagged βB2 fusion protein was shown to be taken up by cultured cells (Liedtke et al., 2007). The role of βB2 was expanded to RPE derived cells by showing that βB2 or neuronal progenitor cells (NPC) expressing βB2 could protect ARPE-19 cells in culture from UV damage and that the βB2 expressing NPCs injected into the vitreous could protect RPE in a rat model of optic nerve trauma (Bohm et al., 2013). An autocrine role for βB2 was proposed. In contrast, others also observed regenerative effects on RGCs associated with lens damage but have attributed this to macrophage activation rather than a direct role by crystallins (Leon et al., 2000; Yin et al., 2003).
The proposed role for βB2 in RGCs is quite surprising. The mechanism of secretion of βB2 into medium is unclear since βB2 lacks any sort of secretion signal. Furthermore, βγ-crystallins are intracellular proteins and contain relatively exposed cysteine and methionine residues that are vulnerable to oxidation. In the reducing environment of the cell they are protected but outside the cell oxidation, including disulfide crosslinking would be expected. Aside from their vulnerability to the extracellular environment, βγ-crystallins have no structural similarity to any known signaling proteins and there is no evidence for any receptor that might bind them. However the possibility remains that crystallins could be exported from one cell to another via exosomes, small membrane vesicles that have been proposed to act as transport systems for a number of factors in different cell types (Raposo and Stoorvogel, 2013). Whether such a mechanism could deliver biologically useful amounts of crystallins to a target cell is unclear.
Several experiments on β-crystallins in the RGC model used proteins purified from lens and these could contain low levels of other factors with biological activity. Other experiments used cells expressing βB2 that might also be secreting other factors. A knockout mouse for βB2 has been described (Zhang et al., 2008). Age-related cataract was observed but no retina effects have been reported, although it is quite possible that subtle phenotypes would not have been noticed. Similarly, several cataractogenic mutations of βB2 have been documented in humans (Wistow, 2012) and again no retina phenotype has been reported.
Expression of βγ-crystallins in brain and other tissues
Beyond expression in neural and other tissues of the eye, β-crystallins, most abundantly βB1 (http://mouse.brain-map.org/) and βB2, have also been detected in other tissues, particularly in the brain (Dirks et al., 1998; Head et al., 1991; Head et al., 1995; Magabo et al., 2000). Indeed, examination of the brain in the O377 mouse mutant (Ganguly et al., 2008) of βB2 have revealed reduced hippocampal size while behavioral and neuroanatomical tests revealed defects in sensorimotor gating and changes in neuronal activity in the dentate gyrus (Sun et al., 2013). Some alterations in gene expression, including genes for NMDA receptor subunits were also noted. The O377 mutant has a splice junction defect which leads to insertion of 19 residues in the middle of the C-terminal domain (Ganguly et al., 2008). This would certainly be expected to lead to misfolding and probably aggregation of the mutant protein. Again this raises the possibility of a gain-of-function effect from the misfolded protein in addition to any loss-of-function.
Other proposed functions
Within their shared structural fingerprint of repeated pairs of modified Greek-key motifs that form tightly folded domains, all known β-crystallins and many γ-crystallins share conserved pairs of tryptophan residues (Wistow et al., 2005). These make important contributions to the hydrophobic core of each domain and to domain stability (Mahler et al., 2013). Tryptophan side chains can absorb and emit photons in the UV range. This can make them susceptible to oxidative damage in the lens while transmitted UV could be harmful for the retina. In βγ-crystallins, these problems are obviated by a UV quenching mechanism that is based on an interplay of the Trp residues and the protein backbone that significantly reduces and red shifts the Trp emission spectrum in the folded protein (Chen et al., 2009; Kosinski-Collins et al., 2004). This mechanism can help protect both lens and retina from UV damage and would seem to be a useful role in both tissues.
While UV exposure might not be a major problem for modern aquatic species, it is thought that during the Cambrian period, when the precursor of the vertebrate lens/cornea began to evolve, UV levels were much higher so that a UV quenching mechanism could have been beneficial. Even in modern surface swimmers, such as zebrafish, a UV filter mechanism in the lens could contribute to the function and discrimination ability of the short wave cone pigments in the retina (Bowmaker and Hunt, 2006).
The βγ-crystallin superfamily contains many members expressed in diverse micro-organisms. From the first member noted, Protein S of Myxococcus xanthus (Wistow et al., 1985) it became apparent that many of these microbial proteins contain calcium binding sites which can be clearly visualized in structural studies (Bagby et al., 1994; Clout et al., 2001). However, the conserved fingerprint of the calcium binding site is not present in vertebrate βγ-crystallins (Suman et al., 2011) and although it has been suggested that they may retain some affinity for calcium, presumably through a different site, it is not clear that this is biologically significant. No bound calcium has ever been observed in any of the crystal structures for lens βγ-crystallins.
A more surprising idea is that human βA3-crystallin possesses autodegradative serine protease activity (Gupta et al., 2010). While there is evidence for degradation of the purified recombinant βA3 protein under some conditions, βγ-crystallins show no similarity to any known proteases (or indeed any other class of enzyme).
A common organizational role in lens and non-lens?
Is there a broad rationale for the role of βγ-crystallins in retina that might tie together some of these observations? In the lens, βγ-crystallins play a role in maintaining the short range order required for transparency (Bloemendal et al., 2004; Slingsby et al., 2013). They belong to a superfamily that contains members associated with cellular architecture in eukaryotes (Ray et al., 1996; Ray et al., 1997; Takabatake et al., 1992; Wistow et al., 1995) or structures including spores or cysts in microorganisms (Wistow, 1990; Wistow et al., 1985). In mammals there are three genes for large proteins that contain multiple βγ-crystallin superfamily domains; AIM1 (absent in melanoma 1) and its two relatives, AIMIL/CRYBG2 and CRYBG3 (Wistow, 2012). In addition to their β-crystallin-like domains, these proteins contain a C-terminal trefoil domain. Intriguingly, a non-lens βγ-crystallin relative identified in skin secretions of a frog (Bombina maxima) and designated βγ-CAT (Liu et al., 2008), has been shown to dimerize with a trefoil domain protein. Trefoil domains are widespread and may have many different functions, but some are known to be important in organization of actin filaments (Jansen et al., 2011). Recently, examination of a knockout mouse model for γS-crystallin has revealed a role for this highly conserved protein in the organization of actin filaments (Fan et al., 2012) (Figure 1), suggesting that the protein and its relatives can act to stabilize and prevent aggregation of large macromolecular complexes such as cytoskeletal filaments. In the lens this would help prevent the formation of light scattering centers. It could also help maintain normal trafficking networks that rely on such structures. Indeed, clearance of degraded organelles in the maturing lens fiber cells is also defective in the γS KO mouse (Fan et al., 2012). Such a role could be useful in retinal neurons, particularly in axons. These cells, which lie over the photoreceptors in the path of incoming light, need to be transparent and to scatter as little light as possible (indeed some retinal cells act as wave guides to enhance light transmission (Franze et al., 2007)). Perhaps crystallins (including α-crystallins (Ghosh et al., 2007a)) help to avoid tangling and clumping of intracellular components, including cytoskeleton and the large complexes involved in junctions and intracellular transport, in these cells. They might play a similar role in another transparent ocular tissue, the cornea (Jaworski and Wistow, 1996; Ren et al., 2010). Normal function in many neuronal and glial cells also requires efficient transport of many components along the axons and cell processes. Again, crystallins could act to stabilize filaments and tubules to promote and protect neuronal function and this might be particularly important under certain conditions of stress.
A generalized protective role in keeping complex cell components from aggregating and in maintenance of cytoarchitecture could also be important in long-lived, non-mitotic or post-mitotic cells such as lens fibre cells, neurons and RPE. A protective role for sHsps in protein deposition disease is already quite clearly implicated (Clark et al., 2012); perhaps increased levels of βγ-crystallins could add another level of protection in cells subject to such disease?
Summary and Prospects.
The crystallins must have arisen from proteins with functions that existed before the evolution of the lens. They were recruited from among the transcriptome/proteome of cells from the same embryonic layers that give rise to brain and retina. Some of those proteins that were able to achieve high concentration, maintain optical clarity and also act to stabilize the structures of highly elongated cells became crystallins. It is possible that they retain their ancient roles in neuronal cells of retina and brain and in other long-lived post-mitotic cells such as retinal pigment epithelium. They may help to maintain functionality in these cells by stabilizing cytoskeleton and other complexes, and preventing tangling, aggregation and deposition.
Animal models should shed more light on functional roles of crystallins, but it should be recognized that in lens and in other cell types multiple crystallins are co-expressed. It may be that they exert their full effects as interacting complexes of crystallins rather than as individual proteins. This may confound interpretation of some single gene deletions in which phenotypes might either be masked by redundancy or exaggerated by gain-of-function defects in larger complexes missing one component. Better models might involve expression or deletion of groups of crystallins, rather than single genes, in specific cells. Indeed, it would be interesting to see whether expression of a suite of crystallins would have protective effects in a range of aging or stressed cells.
Although crystallins are expressed elsewhere, they are at their highest concentration in the lens and so too may be some of their important potential partners, such as proteins of the cytoskeleton and junctional complexes. Proteome analyses that search for interacting partners of crystallins in lens may give important clues to function.
Much is known about structural details of individual crystallins, but further insights into their functions probably require molecular analysis of full-length hetero-dimers and higher oligomers. Biophysical tools are emerging whereby spectroscopy can provide residue specific details of interaction between crystallins, such as between alpha-crystallin and destabilized mutant gamma-crystallins. Improved detectors for cryoEM of single particles extend the complementary use of this technique with protein crystallography, which is continually being improved through collaborative efforts such as the CCP4 (http://www.ccp4.ac.uk/). These imaging techniques can clarify the nature of interactions between crystallins and candidate cytoskeletal and junctional complexes, helping to distinguish those components making direct contact from those that are part of a much bigger complex, or merely a constituent of a highly complex, regulated biochemical process, such as cell death. Now that super-resolution fluorescence microscopy is closing the resolution gap between protein 3D structure and subcellular (filament) systems, the simple lens cell may be a window into unknown terrains of cell biology.
Acknowledgments
CS is grateful for the financial support of the Medical Research Council, UK (grant G0801846), and the synchrotron beamline staff at the European Synchrotron Radiation Facility (Grenoble, France) the Diamond Light Source (Oxfordshire, UK) and Soleil, France (partially funded through BIOSTRUCT-X). GW is supported by the Intramural program of the National Eye Institute, National Institutes of Health, USA. We thank Dr Alice Clark for many useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44:1622–31. doi: 10.1016/j.biocel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. The onset of pigment epithelial proliferation after retinal detachment. Invest Ophthalmol Vis Sci. 1981;21:10–6. [PubMed] [Google Scholar]
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, Bateman OA, Slingsby C, Robinson CV. Polydispersity of a mammalian chaperone: mass spectrometry reveals the population of oligomers in alphaB-crystallin. Proc Natl Acad Sci U S A. 2003;100:10611–6. doi: 10.1073/pnas.1932958100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP. Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett. 2013;587:1959–69. doi: 10.1016/j.febslet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Athanasiou D, Aguila M, Bevilacqua D, Novoselov SS, Parfitt DA, Cheetham ME. The cell stress machinery and retinal degeneration. FEBS Lett. 2013;587:2008–17. doi: 10.1016/j.febslet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala FJ. Darwin’s greatest discovery: design without designer. Proc Natl Acad Sci U S A. 2007;104(Suppl 1):8567–73. doi: 10.1073/pnas.0701072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby S, Harvey TS, Eagle SG, Inouye S, Ikura M. NMR-derived three-dimensional solution structure of protein S complexed with calcium. Structure. 1994;2:107–122. doi: 10.1016/s0969-2126(00)00013-7. [DOI] [PubMed] [Google Scholar]
- Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J Mol Biol. 2009;392:1242–52. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Lioe H, Hilton GR, Baker LA, Rubinstein JL, Kay LE, Benesch JL. The polydispersity of alphaB-crystallin is rationalized by an interconverting polyhedral architecture. Structure. 2011;19:1855–63. doi: 10.1016/j.str.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Walsh P, Hansen DF, Hilton GR, Benesch JL, Sharpe S, Kay LE. Probing dynamic conformations of the high-molecular-weight alphaB-crystallin heat shock protein ensemble by NMR spectroscopy. J Am Chem Soc. 2012;134:15343–50. doi: 10.1021/ja307874r. [DOI] [PubMed] [Google Scholar]
- Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human gammaD crystallin (1.25 A) and the R58H mutant (1.15 A) associated with aculeiform cataract. J Mol Biol. 2003;328:1137–47. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–17. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Shi Y, Vrensen GF. Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci. 2011;366:1250–64. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis. 2009;15:2448–63. [PMC free article] [PubMed] [Google Scholar]
- Bateman JB, Geyer DD, Flodman P, Johannes M, Sikela J, Walter N, Moreira AT, Clancy K, Spence MA. A new betaA1-crystallin splice junction mutation in autosomal dominant cataract. Invest Ophthalmol Vis Sci. 2000;41:3278–85. [PubMed] [Google Scholar]
- Bateman OA, Sarra R, van Genesen ST, Kappe G, Lubsen NH, Slingsby C. The stability of human acidic beta-crystallin oligomers and hetero-oligomers. Exp Eye Res. 2003;77:409–22. doi: 10.1016/s0014-4835(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Bax B, Lapatto R, Nalini V, Driessen H, Lindley PF, Mahadevan D, Blundell TL, Slingsby C. X-ray analysis of βB2-crystallin and evolution of oligomeric lens proteins. Nature. 1990;347:776–780. doi: 10.1038/347776a0. [DOI] [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochemistry and Biophysics Research Communications. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45:3069–76. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H, De Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–85. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Bohm MR, Melkonyan H, Oellers P, Thanos S. Effects of crystallin-beta-b2 on stressed RPE in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol. 2013;251:63–79. doi: 10.1007/s00417-012-2157-7. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, Hunt DM. Evolution of vertebrate visual pigments. Curr Biol. 2006;16:R484–9. doi: 10.1016/j.cub.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci U S A. 1997;94:884–9. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–34. [PubMed] [Google Scholar]
- Braun N, Zacharias M, Peschek J, Kastenmuller A, Zou J, Hanzlik M, Haslbeck M, Rappsilber J, Buchner J, Weinkauf S. Multiple molecular architectures of the eye lens chaperone alphaB-crystallin elucidated by a triple hybrid approach. Proc Natl Acad Sci U S A. 2011;108:20491–6. doi: 10.1073/pnas.1111014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman ML, Bryce DM, Giddens E, Clapoff S, Goring D, Tsui LC, Klintworth GK, Bernstein A. Analysis of lens cell fate and eye morphogenesis in transgenic mice ablated for cells of the lens lineage. Development. 1989;106:457–463. doi: 10.1242/dev.106.3.457. [DOI] [PubMed] [Google Scholar]
- Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–52. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Carra S, Rusmini P, Crippa V, Giorgetti E, Boncoraglio A, Cristofani R, Naujock M, Meister M, Minoia M, Kampinga HH, Poletti A. Different anti-aggregation and pro-degradative functions of the members of the mammalian sHSP family in neurological disorders. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110409. doi: 10.1098/rstb.2011.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavusoglu N, Thierse D, Mohand-Said S, Chalmel F, Poch O, Van-Dorsselaer A, Sahel JA, Leveillard T. Differential proteomic analysis of the mouse retina: the induction of crystallin proteins by retinal degeneration in the rd1 mouse. Mol Cell Proteomics. 2003;2:494–505. doi: 10.1074/mcp.M300029-MCP200. [DOI] [PubMed] [Google Scholar]
- Chan MP, Dolinska M, Sergeev YV, Wingfield PT, Hejtmancik JF. Association properties of betaB1- and betaA3-crystallins: ability to form heterotetramers. Biochemistry. 2008;47:11062–9. doi: 10.1021/bi8012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Callis PR, King J. Mechanism of the very efficient quenching of tryptophan fluorescence in human gamma D- and gamma S-crystallins: the gamma-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage. Biochemistry. 2009;48:3708–16. doi: 10.1021/bi802177g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao H, Schuck P, Wistow G. Solution properties of gamma-crystallins: Compact structure and low frictional ratio are conserved properties of diverse gamma-crystallins. Protein Sci. 2013 doi: 10.1002/pro.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Amyloid formation by globular proteins under native conditions. Nat Chem Biol. 2009;5:15–22. doi: 10.1038/nchembio.131. [DOI] [PubMed] [Google Scholar]
- Clark AR, Lubsen NH, Slingsby C. sHSP in the eye lens: Crystallin mutations, cataract and proteostasis. Int J Biochem Cell Biol. 2012;44:1687–97. doi: 10.1016/j.biocel.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Clark AR, Naylor CE, Bagneris C, Keep NH, Slingsby C. Crystal structure of R120G disease mutant of human alphaB-crystallin domain dimer shows closure of a groove. J Mol Biol. 2011;408:118–34. doi: 10.1016/j.jmb.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton RM, Errington LH, Jeanny JC, Bower JD, Cuthbert J, Duncan G. The Lens: Transparency and Cataract. Vol. 6. Eurage, Rijswijk; 1985. Crystallins in non transparent structures of the embryo; pp. 201–208. [Google Scholar]
- Clayton RM, Jeanny JC, Bower DJ, Errington LH. The presence of extralenticular crystallins and its relationship with transdifferentiation to lens. Curr Top Dev Biol. 1986;20:137–151. doi: 10.1016/s0070-2153(08)60660-2. [DOI] [PubMed] [Google Scholar]
- Clout NJ, Kretschmar M, Jaenicke R, Slingsby C. Crystal structure of the calcium-loaded spherulin 3a dimer sheds light on the evolution of the eye lens betagamma-crystallin domain fold. Structure (Camb) 2001;9:115–24. doi: 10.1016/s0969-2126(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–45. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino M, Lemma V, Chesi G, Stornaiuolo M, Cannata Serio M, D’Ambrosio C, Scaloni A, Polishchuk R, Bonatti S. The cytosolic chaperone alpha-crystallin B rescues folding and compartmentalization of misfolded multispan transmembrane proteins. J Cell Sci. 2013;126:4160–72. doi: 10.1242/jcs.125443. [DOI] [PubMed] [Google Scholar]
- Delbecq SP, Jehle S, Klevit R. Binding determinants of the small heat shock protein, alphaB-crystallin: recognition of the ‘IxI’ motif. Embo J. 2012;31:4587–94. doi: 10.1038/emboj.2012.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq SP, Klevit RE. One size does not fit all: the oligomeric states of alphaB crystallin. FEBS Lett. 2013;587:1073–80. doi: 10.1016/j.febslet.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks RP, Van Genesen ST, KrUse JJ, Jorissen L, Lubsen NH. Extralenticular expression of the rodent betaB2-crystallin gene. Exp Eye Res. 1998;66:267–9. doi: 10.1006/exer.1997.0439. [DOI] [PubMed] [Google Scholar]
- Dolinska MB, Sergeev YV, Chan MP, Palmer I, Wingfield PT. N-terminal extension of beta B1-crystallin: identification of a critical region that modulates protein interaction with beta A3-crystallin. Biochemistry. 2009;48:9684–95. doi: 10.1021/bi9013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downard KM, Kokabu Y, Ikeguchi M, Akashi S. Homology-modelled structure of the betaB2B3-crystallin heterodimer studied by ion mobility and radical probe MS. Febs J. 2011;278:4044–54. doi: 10.1111/j.1742-4658.2011.08309.x. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–69. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Eakin RM. The Third Eye. University of California Press; Berkeley and Los Angeles: 1973. p. 1. [Google Scholar]
- Fan J, Dong L, Mishra S, Chen Y, Fitzgerald P, Wistow G. A role for gammaS-crystallin in the organization of actin and fiber cell maturation in the mouse lens. FEBS J. 2012;279:2892–904. doi: 10.1111/j.1742-4658.2012.08669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest KM, Al-Sarraj S, Sewry C, Buk S, Tan SV, Pitt M, Durward A, McDougall M, Irving M, Hanna MG, Matthews E, Sarkozy A, Hudson J, Barresi R, Bushby K, Jungbluth H, Wraige E. Infantile onset myofibrillar myopathy due to recessive CRYAB mutations. Neuromuscul Disord. 2011;21:37–40. doi: 10.1016/j.nmd.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J. Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci U S A. 2007;104:8287–92. doi: 10.1073/pnas.0611180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederikse PH, Kasinathan C, Kleiman NJ. Parallels between neuron and lens fiber cell structure and molecular regulatory networks. Dev Biol. 2012;368:255–60. doi: 10.1016/j.ydbio.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Poirier DJ, Seguin SJ, Lambert H, Carra S, Charette SJ, Landry J. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J. 2010;425:245–55. doi: 10.1042/BJ20090907. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Favor J, Neuhauser-Klaus A, Sandulache R, Puk O, Beckers J, Horsch M, Schadler S, Vogt Weisenhorn D, Wurst W, Graw J. Novel allele of crybb2 in the mouse and its expression in the brain. Invest Ophthalmol Vis Sci. 2008;49:1533–41. doi: 10.1167/iovs.07-0788. [DOI] [PubMed] [Google Scholar]
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Houck SA, Clark JI. Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol. 2007a;39:1804–15. doi: 10.1016/j.biocel.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Shenoy AK, Jr, Clark JI. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry. 2007b;46:6308–17. doi: 10.1021/bi700149h. [DOI] [PubMed] [Google Scholar]
- Grainger RM. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends in Genetics. 1992;8:349–355. doi: 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- Graw J, Jung M, Loster J, Klopp N, Soewarto D, Fella C, Fuchs H, Reis A, Wolf E, Balling R, Hrabe de Angelis M. Mutation in the betaA3/A1-crystallin encoding gene Cryba1 causes a dominant cataract in the mouse. Genomics. 1999;62:67–73. doi: 10.1006/geno.1999.5974. [DOI] [PubMed] [Google Scholar]
- Gupta R, Chen J, Srivastava OP. A serine-type protease activity of human lens betaA3-crystallin is responsible for its autodegradation. Mol Vis. 2010;16:2242–52. [PMC free article] [PubMed] [Google Scholar]
- Gustafsson OS, Collin SP, Kroger RH. Early evolution of multifocal optics for well-focused colour vision in vertebrates. J Exp Biol. 2008;211:1559–64. doi: 10.1242/jeb.016048. [DOI] [PubMed] [Google Scholar]
- Haley DA, Bova MP, Huang QL, McHaourab HS, Stewart PL. Small heat-shock protein structures reveal a continuum from symmetric to variable assemblies. J Mol Biol. 2000;298:261–72. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- Hanazono Y, Takeda K, Oka T, Abe T, Tomonari T, Akiyama N, Aikawa Y, Yohda M, Miki K. Nonequivalence observed for the 16-meric structure of a small heat shock protein, SpHsp16.0, from Schizosaccharomyces pombe. Structure. 2013;21:220–8. doi: 10.1016/j.str.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Harris WA. Pax-6: where to be conserved is not conservative. Proc Natl Acad Sci U S A. 1997;94:2098–100. doi: 10.1073/pnas.94.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head MW, Peter A, Clayton RM. Evidence for the extralenticular expression of members of the β-crystallin gene family in the chick and a comparison with δ-crystallin during differentiation and transdifferentiation. Differentiation. 1991;48:147–156. doi: 10.1111/j.1432-0436.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Head MW, Sedowofia K, Clayton RM. betaB2-crystallin in the mammalian retina. Experimental Eye Research. 1995;61:423–428. doi: 10.1016/s0014-4835(05)80137-x. [DOI] [PubMed] [Google Scholar]
- Heiduschka P, Fischer D, Thanos S. Neuroprotection and regeneration after traumatic lesion of the optic nerve. Klin Monbl Augenheilkd. 2004;221:684–701. doi: 10.1055/s-2004-813054. [DOI] [PubMed] [Google Scholar]
- Hejtmancik JF, Wingfield PT, Chambers C, Russell P, Chen HC, Sergeev YV, Hope JN. Association properties of betaB2- and betaA3-crystallin: ability to form dimers. Protein Eng. 1997;10:1347–52. doi: 10.1093/protein/10.11.1347. [DOI] [PubMed] [Google Scholar]
- Hilton GR, Hochberg GK, Laganowsky A, McGinnigle SI, Baldwin AJ, Benesch JL. C-terminal interactions mediate the quaternary dynamics of alphaB-crystallin. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110405. doi: 10.1098/rstb.2011.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha crystallin: the quest for a homogeneous quaternary structure. Exp Eye Res. 2009;88:190–4. doi: 10.1016/j.exer.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J, Huang QL, Ding L, Bova MP. Lens alpha-crystallin: chaperone-like properties. Methods Enzymol. 1998;290:365–83. doi: 10.1016/s0076-6879(98)90032-5. [DOI] [PubMed] [Google Scholar]
- Houck SA, Landsbury A, Clark JI, Quinlan RA. Multiple sites in alphaB-crystallin modulate its interactions with desmin filaments assembled in vitro. PLoS ONE. 2011;6:e25859. doi: 10.1371/journal.pone.0025859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Procedings of the National Academy of Sciences USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Collins A, Yang C, Rebowski G, Svitkina T, Dominguez R. Mechanism of actin filament bundling by fascin. J Biol Chem. 2011;286:30087–96. doi: 10.1074/jbc.M111.251439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski C, Wistow G. LP2, a differentiation-associated lipid-binding protein expressed in bovine lens. Biochem J. 1996;320 (Pt 1):49–54. doi: 10.1042/bj3200049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, Rajagopal P, Bardiaux B, Markovic S, Kuhne R, Stout JR, Higman VA, Klevit RE, van Rossum BJ, Oschkinat H. Solid-state NMR and SAXS studies provide a structural basis for the activation of alphaB-crystallin oligomers. Nat Struct Mol Biol. 2010;17:1037–42. doi: 10.1038/nsmb.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevit RE. N-terminal domain of alphaB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc Natl Acad Sci U S A. 2011;108:6409–14. doi: 10.1073/pnas.1014656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Jung J, Koharudin LM, Gronenborn AM. The human W42R gammaD-crystallin mutant structure provides a link between congenital and age-related cataracts. J Biol Chem. 2013;288:99–109. doi: 10.1074/jbc.M112.416354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Makwana J, Neal MJ. Retinal expression of gamma-crystallins in the mouse. Investigative Ophthalmology and Visual Science. 1999;40:3017–3020. [PubMed] [Google Scholar]
- Kandpal RP, Rajasimha HK, Brooks MJ, Nellissery J, Wan J, Qian J, Kern TS, Swaroop A. Transcriptome analysis using next generation sequencing reveals molecular signatures of diabetic retinopathy and efficacy of candidate drugs. Mol Vis. 2012;18:1123–46. [PMC free article] [PubMed] [Google Scholar]
- Kannabiran C, Rogan PK, Olmos L, Basti S, Rao GN, Kaiser-Kupfer M, Hejtmancik JF. Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol Vis. 1998;4:21. [PubMed] [Google Scholar]
- Kannan R, Sreekumar PG, Hinton DR. Novel roles for alpha-crystallins in retinal function and disease. Prog Retin Eye Res. 2012;31:576–604. doi: 10.1016/j.preteyeres.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Purkiss AG, van Genesen ST, Slingsby C, Lubsen NH. Explosive expansion of betagamma-crystallin genes in the ancestral vertebrate. J Mol Evol. 2010;71:219–30. doi: 10.1007/s00239-010-9379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway CK, Benesch JL, Gohlke U, Wang L, Robinson CV, Orlova EV, Saibil HR, Keep NH. Dodecameric structure of the small heat shock protein Acr1 from Mycobacterium tuberculosis. J Biol Chem. 2005;280:33419–25. doi: 10.1074/jbc.M504263200. [DOI] [PubMed] [Google Scholar]
- Kerr BA, Byzova TV. alphaB-crystallin: a novel VEGF chaperone. Blood. 2010;115:3181–3. doi: 10.1182/blood-2010-01-262766. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–9. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jin J, Kim YJ, Kim Y, Yu HG. Retinal proteome analysis in a mouse model of oxygen-induced retinopathy. J Proteome Res. 2012;11:5186–203. doi: 10.1021/pr300389r. [DOI] [PubMed] [Google Scholar]
- Kingsley CN, Brubaker WD, Markovic S, Diehl A, Brindley AJ, Oschkinat H, Martin RW. Preferential and Specific Binding of Human alphaB-Crystallin to a Cataract-Related Variant of gammaS-Crystallin. Structure. 2013;21:2221–7. doi: 10.1016/j.str.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski-Collins MS, Flaugh SL, King J. Probing folding and fluorescence quenching in human gammaD crystallin Greek key domains using triple tryptophan mutant proteins. Protein Sci. 2004;13:2223–35. doi: 10.1110/ps.04627004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis N, Nikoletopoulou V, Tavernarakis N. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature. 2012;490:213–8. doi: 10.1038/nature11417. [DOI] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–97. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Kroger RH, Campbell MC, Munger R, Fernald RD. Refractive index distribution and spherical aberration in the crystalline lens of the African cichlid fish Haplochromis burtoni. Vision Res. 1994;34:1815–22. doi: 10.1016/0042-6989(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Kroger RH, Fernald RD. Regulation of eye growth in the African cichlid fish Haplochromis burtoni. Vision Res. 1994;34:1807–14. doi: 10.1016/0042-6989(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res. 2004;78:673–87. doi: 10.1016/j.exer.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, Benesch JL, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19:1031–43. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganowsky A, Eisenberg D. Non-3D domain swapped crystal structure of truncated zebrafish alphaA crystallin. Protein Sci. 2010;19:1978–84. doi: 10.1002/pro.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Shih M, Shearer TR, Smith JB, Smith DL, David LL. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem. 1997;272:2268–75. doi: 10.1074/jbc.272.4.2268. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Oxford JT, Bachinger HP, Shearer TR, David LL, Kapfer DM. Deamidation of human beta B1 alters the elongated structure of the dimer. Exp Eye Res. 2001;72:279–88. doi: 10.1006/exer.2000.0950. [DOI] [PubMed] [Google Scholar]
- Land MF, Fernald RD. The evolution of eyes. Annu Rev Neurosci. 1992;15:1–29. doi: 10.1146/annurev.ne.15.030192.000245. [DOI] [PubMed] [Google Scholar]
- Land MF, Nilsson DE. Oxford Animal Biology Series. Oxford University Press; 2002. Animal Eyes. [Google Scholar]
- Lapko VN, Smith DL, Smith JB. Expression of betaA2-crystallin in human lenses. Exp Eye Res. 2003;77:383–5. doi: 10.1016/s0014-4835(03)00156-8. [DOI] [PubMed] [Google Scholar]
- Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A. Cell signaling pathways to alphaB-crystallin following stresses of the cytoskeleton. Exp Cell Res. 2006;312:3570–84. doi: 10.1016/j.yexcr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–26. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke T, Schwamborn JC, Schroer U, Thanos S. Elongation of axons during regeneration involves retinal crystallin beta b2 (crybb2) Mol Cell Proteomics. 2007;6:895–907. doi: 10.1074/mcp.M600245-MCP200. [DOI] [PubMed] [Google Scholar]
- Liu L, Qi X, Chen Z, Shaw L, Cai J, Smith LH, Grant MB, Boulton ME. Targeting the IRE1alpha/XBP1 and ATF6 arms of the unfolded protein response enhances VEGF blockade to prevent retinal and choroidal neovascularization. Am J Pathol. 2013;182:1412–24. doi: 10.1016/j.ajpath.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SB, He YY, Zhang Y, Lee WH, Qian JQ, Lai R, Jin Y. A novel non-lens betagamma-crystallin and trefoil factor complex from amphibian skin and its functional implications. PLoS ONE. 2008;3:e1770. doi: 10.1371/journal.pone.0001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Sen T, Asnaghi L, Valapala M, Yang F, Hose S, McLeod DS, Lu Y, Eberhart C, Zigler JS, Jr, Sinha D. betaA3/A1-Crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways through the PKD/Bit1-signaling axis. Cell Death Dis. 2011;2:e217. doi: 10.1038/cddis.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Piszczek G, Wingfield PT, Sergeev YV, Hejtmancik JF. The G18V CRYGS mutation associated with human cataracts increases gammaS-crystallin sensitivity to thermal and chemical stress. Biochemistry. 2009;48:7334–41. doi: 10.1021/bi900467a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JT, Purkiss AG, Smith MA, Evans P, Goodfellow JM, Slingsby C. Unfolding crystallins: the destabilizing role of a beta-hairpin cysteine in betaB2-crystallin by simulation and experiment. Protein Sci. 2005;14:1282–92. doi: 10.1110/ps.041227805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magabo KS, Horwitz J, Piatigorsky J, Kantorow M. Expression of betaB(2)-crystallin mRNA and protein in retina, brain, and testis. Invest Ophthalmol Vis Sci. 2000;41:3056–60. [PMC free article] [PubMed] [Google Scholar]
- Mahler B, Chen Y, Ford J, Thiel C, Wistow G, Wu Z. Structure and Dynamics of the Fish Eye Lens Protein, gammaM7-Crystallin. Biochemistry. 2013;50:3579–3587. doi: 10.1021/bi400151c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Vinader L, Onnekink C, van Genesen ST, Slingsby C, Lubsen NH. In vivo heteromer formation. Expression of soluble betaA4-crystallin requires coexpression of a heteromeric partner. Febs J. 2006;273:3172–82. doi: 10.1111/j.1742-4658.2006.05326.x. [DOI] [PubMed] [Google Scholar]
- McDevitt DS. Presence of lateral eye lens crystallins in the median eye of the american chameleon. Science. 1972;175:763–4. doi: 10.1126/science.175.4023.763. [DOI] [PubMed] [Google Scholar]
- McHaourab HS, Godar JA, Stewart PL. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry. 2009;48:3828–37. doi: 10.1021/bi900212j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaourab HS, Lin YL, Spiller BW. Crystal structure of an activated variant of small heat shock protein Hsp16.5. Biochemistry. 2012;51:5105–12. doi: 10.1021/bi300525x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–59. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- Nilsson DE, Pelger S. A pessimistic estimate of the time required for an eye to evolve. Proc R Soc Lond B Biol Sci. 1994;256:53–58. doi: 10.1098/rspb.1994.0048. [DOI] [PubMed] [Google Scholar]
- Organisciak D, Darrow R, Barsalou L, Rapp C, McDonald B, Wong P. Light induced and circadian effects on retinal photoreceptor cell crystallins. Photochem Photobiol. 2011;87:151–9. doi: 10.1111/j.1751-1097.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy G, Ma B, Zhang C, Gongora C, Samuel Zigler J, Jr, Duncan MK, Sinha D. Expression of betaA3/A1-crystallin in the developing and adult rat eye. J Mol Histol. 2011;42:59–69. doi: 10.1007/s10735-010-9307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek J, Braun N, Franzmann TM, Georgalis Y, Haslbeck M, Weinkauf S, Buchner J. The eye lens chaperone alpha-crystallin forms defined globular assemblies. Proc Natl Acad Sci U S A. 2009;106:13272–7. doi: 10.1073/pnas.0902651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek J, Braun N, Rohrberg J, Back KC, Kriehuber T, Kastenmuller A, Weinkauf S, Buchner J. Regulated structural transitions unleash the chaperone activity of alphaB-crystallin. Proc Natl Acad Sci U S A. 2013;110:E3780–9. doi: 10.1073/pnas.1308898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Pierscionek BK, Regini JW. The gradient index lens of the eye: an opto-biological synchrony. Prog Retin Eye Res. 2012;31:332–49. doi: 10.1016/j.preteyeres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Piri N, Kwong JM, Caprioli J. Crystallins in retinal ganglion cell survival and regeneration. Mol Neurobiol. 2013;48:819–28. doi: 10.1007/s12035-013-8470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkiss AG, Bateman OA, Wyatt K, Wilmarth PA, David LL, Wistow GJ, Slingsby C. Biophysical properties of gammaC-crystallin in human and mouse eye lens: the role of molecular dipoles. J Mol Biol. 2007;372:205–22. doi: 10.1016/j.jmb.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanikanth V, Srivastava SS, Singh AK, Rajyalakshmi M, Chandra K, Aravind P, Sankaranarayanan R, Sharma Y. Aggregation-prone near-native intermediate formation during unfolding of a structurally similar nonlenticular betagamma-crystallin domain. Biochemistry. 2012;51:8502–13. doi: 10.1021/bi300844u. [DOI] [PubMed] [Google Scholar]
- Ray ME, Su YA, Meltzer PS, Trent JM. Isolation and characterization of genes associated with chromosome-6 mediated tumor suppression in human malignant melanoma. Oncogene. 1996;12:2527–33. [PubMed] [Google Scholar]
- Ray ME, Wistow G, Su YA, Meltzer PS, Trent JM. AIM1, a novel non-lens member of the betagamma-crystallin superfamily, is associated with the control of tumorigenicity in human malignant melanoma. Proc Natl Acad Sci U S A. 1997;94:3229–34. doi: 10.1073/pnas.94.7.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Liu T, Jia C, Qi X, Wang Y. Physiological expression of lens alpha-, beta-, and gamma-crystallins in murine and human corneas. Mol Vis. 2010;16:2745–52. [PMC free article] [PubMed] [Google Scholar]
- Ryan JF, Pang K, Schnitzler CE, Nguyen AD, Moreland RT, Simmons DK, Koch BJ, Francis WR, Havlak P, Smith SA, Putnam NH, Haddock SH, Dunn CW, Wolfsberg TG, Mullikin JC, Martindale MQ, Baxevanis AD. The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science. 2013;342:1242592. doi: 10.1126/science.1242592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Miyagi M, Darrow RM, Crabb JS, Hollyfield JG, Organisciak DT, Crabb JW. Intense light exposure changes the crystallin content in retina. Exp Eye Res. 2003;76:131–3. doi: 10.1016/s0014-4835(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Selcen D. Myofibrillar myopathies. Neuromuscul Disord. 2011;21:161–71. doi: 10.1016/j.nmd.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld SM, Purkiss AG, Dirks RP, Bateman OA, Slingsby C, Lubsen NH. Urochordate betagamma-crystallin and the evolutionary origin of the vertebrate eye lens. Curr Biol. 2005;15:1684–9. doi: 10.1016/j.cub.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Simpanya MF, Wistow G, Gao J, David LL, Giblin FJ, Mitton KP. Expressed sequence tag analysis of guinea pig (Cavia porcellus) eye tissues for NEIBank. Mol Vis. 2008;14:2413–27. [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Esumi N, Jaworski C, Kozak CA, Pierce E, Wistow G. Cloning and mapping the mouse Crygs gene and non-lens expression of [gamma]S-crystallin. Mol Vis. 1998;4:8. [PubMed] [Google Scholar]
- Sinha D, Klise A, Sergeev Y, Hose S, Bhutto IA, Hackler L, Jr, Malpic-Llanos T, Samtani S, Grebe R, Goldberg MF, Hejtmancik JF, Nath A, Zack DJ, Fariss RN, McLeod DS, Sundin O, Broman KW, Lutty GA, Zigler JS., Jr betaA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol Cell Neurosci. 2008;37:85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Valapala M, Bhutto I, Patek B, Zhang C, Hose S, Yang F, Cano M, Stark WJ, Lutty GA, Zigler JS, Wawrousek EF. betaA3/A1-crystallin is required for proper astrocyte template formation and vascular remodeling in the retina. Transgenic Res. 2012;21:1033–42. doi: 10.1007/s11248-012-9608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013;22:367–80. doi: 10.1002/pro.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 2001;291:1553–7. doi: 10.1126/science.1057268. [DOI] [PubMed] [Google Scholar]
- Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R, Hinton DR. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci. 2013;54:2787–98. doi: 10.1167/iovs.12-11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler R, Kappe G, Boelens W, Slingsby C. Wrapping the alpha-crystallin domain fold in a chaperone assembly. J Mol Biol. 2005;353:68–79. doi: 10.1016/j.jmb.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Steinmetz PR, Kraus JE, Larroux C, Hammel JU, Amon-Hassenzahl A, Houliston E, Worheide G, Nickel M, Degnan BM, Technau U. Independent evolution of striated muscles in cnidarians and bilaterians. Nature. 2012;487:231–4. doi: 10.1038/nature11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, Vosler P, Zhang L, Signore A, Graham SH, Chen J. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28:13038–55. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Gan Y, Zhang W, Liou AK, Gao Y, Cao G, Chen J. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman SK, Mishra A, Ravindra D, Yeramala L, Sharma Y. Evolutionary remodeling of betagamma-crystallins for domain stability at cost of Ca2+ binding. J Biol Chem. 2011;286:43891–901. doi: 10.1074/jbc.M111.247890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ma Z, Li Y, Liu B, Li Z, Ding X, Gao Y, Ma W, Tang X, Li X, Shen Y. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J Med Genet. 2005;42:706–10. doi: 10.1136/jmg.2004.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Holter SM, Stepan J, Garrett L, Genius J, Kremmer E, Hrabe de Angelis M, Wurst W, Lie DC, Bally-Cuif L, Eder M, Rujescu D, Graw J. Crybb2 coding for betaB2-crystallin affects sensorimotor gating and hippocampal function. Mamm Genome. 2013;24:333–48. doi: 10.1007/s00335-013-9478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabatake T, Takahashi TC, Takeshima K. Cloning of an epidermis-specific Cynops cDNA from a neurula library. Develop Growth Differ. 1992;34:277–283. doi: 10.1111/j.1440-169X.1992.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Takata T, Woodbury LG, Lampi KJ. Deamidation alters interactions of beta-crystallins in hetero-oligomers. Mol Vis. 2009;15:241–9. [PMC free article] [PubMed] [Google Scholar]
- Templeton JP, Wang X, Freeman NE, Ma Z, Lu A, Hejtmancik F, Geisert EE. A crystallin gene network in the mouse retina. Exp Eye Res. 2013;116C:129–140. doi: 10.1016/j.exer.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson I, Wilkinson CE, Jackson JF, De Pomerai DI, Clayton RM, Truman DES, Williamson R. Isolation and cell-free translation of chick lens crystallin mRNA during normal development and transdifferentiation of neural retina. Developmental Biology. 1978;65:372–382. doi: 10.1016/0012-1606(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Thomson I, Yasuda K, De Pomerai DI, Clayton RM, Okada T. The accumulation of lens-specific protein and mRNA in cultures of n retina from 3 1/2-day chick embryos. Experimental Cell Research. 1981;135:445–449. doi: 10.1016/0014-4827(81)90188-9. [DOI] [PubMed] [Google Scholar]
- Tian J, Ishibashi K, Reiser K, Grebe R, Biswal S, Gehlbach P, Handa JT. Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: a comprehensive transcriptional response. Proc Natl Acad Sci U S A. 2005;102:11846–51. doi: 10.1073/pnas.0504759102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–88. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis) Faseb J. 2005;19:1683–5. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- Valapala M, Hose S, Gongora C, Dong L, Wawrousek EF, Samuel Zigler J, Jr, Sinha D. Impaired endolysosomal function disrupts Notch signalling in optic nerve astrocytes. Nat Commun. 2013;4:1629. doi: 10.1038/ncomms2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley CC, Cembran A, Perlmutter JD, Lewis AK, Labello NP, Gao J, Sachs JN. The methionine-aromatic motif plays a unique role in stabilizing protein structure. J Biol Chem. 2012;287:34979–91. doi: 10.1074/jbc.M112.374504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8:1025–30. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Van Montfort RL, Bateman OA, Lubsen NH, Slingsby C. Crystal structure of truncated human betaB1-crystallin. Protein Sci. 2003;12:2606–12. doi: 10.1110/ps.03265903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Chona F, Song BK, Geisert EE., Jr Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45:2737–46. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–57. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara A, Grassi M, Sica F, Pizzo E, D’Alessio G, Mazzarella L, Merlino A. A novel interdomain interface in crystallins: structural characterization of the betagamma-crystallin from Geodia cydonium at 0.99 A resolution. Acta Crystallogr D Biol Crystallogr. 2013;69:960–7. doi: 10.1107/S0907444913003569. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhao WJ, Liu H, Gong H, Yan YB. Increasing betaB1-crystallin sensitivity to proteolysis caused by the congenital cataract-microcornea syndrome mutation S129R. Biochim Biophys Acta. 2013;1832:302–11. doi: 10.1016/j.bbadis.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lomakin A, McManus JJ, Ogun O, Benedek GB. Phase behavior of mixtures of human lens proteins Gamma D and Beta B1. Proc Natl Acad Sci U S A. 2010;107:13282–7. doi: 10.1073/pnas.1008353107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol. 1990;30:140–5. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- Wistow G. Lens crystallins: gene recruitment and evolutionary dynamism. Trends Biochem Sci. 1993;18:301–6. doi: 10.1016/0968-0004(93)90041-k. [DOI] [PubMed] [Google Scholar]
- Wistow G. The human crystallin gene families. Hum Genomics. 2012;6:26. doi: 10.1186/1479-7364-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G, Bernstein SL, Wyatt MK, Behal A, Touchman JW, Bouffard G, Smith D, Peterson K. Expressed sequence tag analysis of adult human lens for the NEIBank Project: over 2000 non-redundant transcripts, novel genes and splice variants. Mol Vis. 2002;8:171–84. [PubMed] [Google Scholar]
- Wistow G, Jaworski C, Rao PV. A non-lens member of the beta gamma-crystallin superfamily in a vertebrate, the amphibian Cynops. Exp Eye Res. 1995;61:637–9. doi: 10.1016/s0014-4835(05)80058-2. [DOI] [PubMed] [Google Scholar]
- Wistow G, Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987;236:1554–6. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- Wistow G, Summers L, Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens beta gamma-crystallins. Nature. 1985;315:771–3. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- Wistow G, Wyatt K, David L, Gao C, Bateman O, Bernstein S, Tomarev S, Segovia L, Slingsby C, Vihtelic T. gammaN-crystallin and the evolution of the betagamma-crystallin superfamily in vertebrates. FEBS J. 2005;272:2276–91. doi: 10.1111/j.1742-4658.2005.04655.x. [DOI] [PubMed] [Google Scholar]
- Wistow GJ, Mulders JW, de Jong WW. The enzyme lactate dehydrogenase as a structural protein in avian and crocodilian lenses. Nature. 1987;326:622–4. doi: 10.1038/326622a0. [DOI] [PubMed] [Google Scholar]
- Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wyatt MK, Tsai JY, Mishra S, Campos M, Jaworski C, Fariss RN, Bernstein SL, Wistow G. Interaction of complement factor h and fibulin3 in age-related macular degeneration. PLoS ONE. 2013;8:e68088. doi: 10.1371/journal.pone.0068088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol Vis. 2003;9:410–9. [PubMed] [Google Scholar]
- Yang Z, Li Q, Ma Z, Guo Y, Zhu S, Ma X. A G-->T splice site mutation of CRYBA1/A3 associated with autosomal dominant suture cataracts in a Chinese family. Mol Vis. 2011;17:2065–71. [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Su D, Li Q, Yang F, Ma Z, Zhu S, Ma X. A novel T-->G splice site mutation of CRYBA1/A3 associated with autosomal dominant nuclear cataracts in a Chinese family. Mol Vis. 2012;18:1283–8. [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–93. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Gehlbach P, Gongora C, Cano M, Fariss R, Hose S, Nath A, Green WR, Goldberg MF, Zigler JS, Jr, Sinha D. A potential role for beta- and gamma-crystallins in the vascular remodeling of the eye. Dev Dyn. 2005;234:36–47. doi: 10.1002/dvdy.20494. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li J, Huang C, Xue L, Peng Y, Fu Q, Gao L, Li W. Targeted knockout of the mouse betaB2-crystallin gene (Crybb2) induces age-related cataract. Invest Ophthalmol Vis Sci. 2008;49:5476–83. doi: 10.1167/iovs.08-2179. [DOI] [PubMed] [Google Scholar]
- Zhao H, Brown PH, Magone MT, Schuck P. The molecular refractive function of lens gamma-Crystallins. J Mol Biol. 2011;411:680–99. doi: 10.1016/j.jmb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chen Y, Rezabkova L, Wu Z, Wistow G, Schuck P. Solution properties of gamma-crystallins: Hydration of fish and mammal gamma-crystallins. Protein Sci. 2013 doi: 10.1002/pro.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler JS, Jr, Zhang C, Grebe R, Sehrawat G, Hackler L, Jr, Adhya S, Hose S, McLeod DS, Bhutto I, Barbour W, Parthasarathy G, Zack DJ, Sergeev Y, Lutty GA, Handa JT, Sinha D. Mutation in the betaA3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J Cell Sci. 2011;124:523–31. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]