Abstract
Rho GTPases play a key role in the regulation of multiple essential cellular processes, including actin dynamics, gene transcription and cell cycle progression. Aberrant activation of Rac1, a member of Rho family of small GTPases, is associated with tumorigenesis, cancer progression, invasion and metastasis. Particularly, Rac1 is overexpressed and hyperactivated in highly aggressive breast cancer. Thus, Rac1 appears to be a promising and relevant target for the development of novel anticancer drugs. We identified the novel Rac1 inhibitor ZINC69391 through a docking-based virtual library screening targeting Rac1 activation by GEFs. This compound was able to block Rac1 interaction with its GEF Tiam1, prevented EGF-induced Rac1 activation and inhibited cell proliferation, cell migration and cell cycle progression in highly aggressive breast cancer cell lines. Moreover, ZINC69391 showed an in vivo antimetastatic effect in a syngeneic animal model. We further developed the novel analog 1A-116 by rational design and showed to be specific and more potent than the parental compound in vitro and interfered Rac1-P-Rex1 interaction. We also showed an enhanced in vivo potency of 1A-116 analog. These results show that we have developed novel Rac1 inhibitors that may be used as a novel anticancer therapy.
Keywords: Breast cancer, docking, Rac1 inhibitor, rational design, virtual screening.
INTRODUCTION
Rho-GTPases are molecular switches that cycle between two conformational states: an inactive GDP-bound form and an active GTP-bound form. This cycle is highly regulated by guanine nucleotide exchange factors (GEFs) that catalyze nucleotide exchange and mediate activation, and GTPase-activating proteins (GAPs), that stimulate GTP hydrolysis and inactivate the GTPase [1]. The active GTP-bound state binds preferentially to downstream effector proteins and actively transduces signals [2].
Rac1 is one of the most studied members of Rho-GTPases family and controls fundamental cellular processes. Rac1 is a major regulator in actin cytoskeleton reorganization; affecting endocytosis and trafficking, cell cycle progression, cell adhesion and migration [3].
Accumulating evidence indicates that Rac1 is overexpressed and/or hyperactivated in a wide range of tumors, including breast, colorectal, gastric, testicular, lung and brain cancer [4-7]. Several in vitro and in vivo studies indicate that Rac1 deregulation contributes to transformation and tumor progression. Moreover, the regulatory functions of Rac1 on cytoskeleton remodeling influence key processes such as invasion, migration and metastasis of cancer cells [8].
Importantly, in vivo analyses indicate that Rac1 is overexpressed in the early course of transformation and is hyperactivated in patients with very aggressive breast cancers [9]. In addition, Rac1 plays an essential role in Ras malignant trans-formation, and overexpression of a constitutively activated Rac1 causes malignant transformation in fibroblasts [10]. Considering the accumulating evidence implicating Rac1 in various cancer-promoting processes, this GTPase may be considered as a promising target for the development of novel anticancer drugs [11]. In terms of druggability, strategies such as ATP-competitive inhibitors developed to inhibit protein kinases are not applicable for Rho GTPases due to the low picomolar binding affinity of small GTPases for GTP and the millimolar cellular concentrations of GTP [12].
Recently it has been described a gain-of-function mutation in rac1 gene in sun-exposed melanomas, although Rac1 has been rarely found mutated in other human cancers [13, 14]. Rac1 upregulation is mostly due to alterations of its regulatory proteins. Interestingly, GEF activation is the most common mechanism for signal-mediated GTPase activation. This activation is commonly driven by aberrant signaling from growth factor receptors and upregulation or mutation of GEFs [12,15]. In this regard, many GEFs present a relevant role in cancer like Ect2, Tiam, Vav, P-Rex1, among others [15-17].
Two structurally unrelated families of GEFs have been described so far: the classical Dbl and the atypical Dock180-related families. The mechanism by which different Dbl-GEFs bind and activate Rac1 has been described in detail [18, 19]. Particularly, Trp56 residue of Rac1 was identified as a critical determinant of GEF specificity by Rac1-GEFs such as Tiam1 and Trio. Recently, it has been described that the unconventional Rac1-specific GEF Dock180, also shares Trp56 of Rac1 as a determinant for specific recognition [20]. This supports the idea of using the Rac1 surface containing Trp56 as a target to develop novel Rac1 inhibitors.
Computational methodologies have become a crucial component of drug discovery. Virtual screening is widely used to predict the binding of a large database of ligands to a particular target, with the goal of identifying the most promising compounds from the database. Hundreds of thousands of compounds may be evaluated in a virtual screen for further study [21, 22]. In order to identify novel Rac1 small molecule inhibitors targeting its surface containing Trp56, we conducted a docking-based virtual screening of more than 200.000 drug-like compounds obtained from ZINC database [23, 24]. Here, we report the identification of the lead Rac1 inhibitor ZINC69391. Importantly, ZINC69391 affected cell proliferation, cell cycle progression and migration of highly aggressive breast cancer cell lines. Moreover, ZINC69391 inhibited lung metastasis in vivo. We also show a more potent new analog derived by rational design, with higher docking scores than the lead molecule. In vitro and in vivo studies confirmed 1A-116 analog as a more potent inhibitor.
MATERIALS AND METHODS
Virtual Screening
To identify potential inhibitors of Rac1, the crystal structure of the protein at 1.38-Å was retrieved from the Protein Data Bank (PDB ID code 1MH1). The database used in our virtual screening was the public available ZINC database [23, 24]. We screened about 200.000 compounds of the drug-like subset. In this study the Rac1 surface containing Trp56 was the target. eHITS (SimBioSys Inc) [25] was used as docking based virtual screening software. The chemical compounds displaying the highest docking scores in the calculations were obtained from commercial vendors.
Docking of ZINC69391 and 1A-116
Docking simulations were carried out using the program Autodock4 [26] in the crystal structure of Rac1 (PDBID: 1MH1). We used the Lamarckian genetic algorithm for the conformational searches. The docking was centered in the region of the key residue Trp56, the grid size was define in order to include the residues present in the binding cleft and to allow enough place to the inhibitors to move during the docking process. The following parameters were used for all the simulations: a population size of 300 individuals (the population size parameter is related to the number of conformers that are used to start the simulation), 7.5 million energy evaluations, mutation rates of 0.02, crossover rate of 0.8 and an elitism value of 1. For each ligand, 250 independent docking runs were performed and results differing by less than 0.5 Å were clustered together.
Cell Lines
The mammary carcinoma cell line F3II is a highly aggressive and metastatic variant, established from a clonal subpopulation of a spontaneous hormone-independent Balb/c mouse mammary tumor [27]. Human breast cancer cell lines MDA-MB-231 and MCF7, and human embryonic kidney cell line HEK293T were purchased from American Type Culture Collection. Cells were grown in Dulbecco´s modified Eagle medium (DMEM) (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2mM glutamine and 80 μg/ml gentamicin at 37°C in 5% CO2 atmosphere. Cell cultures were routinely subcultured twice a week by tripsinization using standard procedures.
Compounds
NSC23766 and ZINC69391 were purchased from Chembridge (San Diego, CA). 1A-116 was synthesized as described in Supplemental Data. ZINC69391 and 1A-116 analogs were evaluated in the optimal dissolution conditions: DMSO and aqueous solution pH 5.5 respectively.
Cell Proliferation Assays
2 x 104 cells were plated in 96-well plates and then treated with different compounds for 72 hrs. Cell growth was measured by colorimetric MTT assay (Sigma). The concentration producing 50% inhibition (IC50) was determined by non-linear regression function of GraphPad Prism5®. Results shown correspond to the average of three separate experiments.
MDA-MB-231 were transiently transfected using a Rac1-G12V construct (cdna.org) or empty vector (pcDNA3) using Lipofectamine 2000 (Life Technologies) according to manufacturer instructions. This constitutively active Rac1 form was previously described [28]. 2 x 104 transfected cells were plated and treated with different concentrations of 1A-116 for 48 hs. Cell proliferation was measured using the MTT assay.
Affinity Precipitation of Rac1-GEFs
For Rac-Tiam1 precipitation, Log phase growing HEK293T cells were transfected using FuGENE HD (Roche Applied Science) with a HA epitope-tagged Tiam1(C1199) construct kindly provided by Alfredo Cáceres (INIMEC-CONICET, Córdoba, Argentina), which has been characterized previously [29, 30]. After 48 hs, cells were lysed in lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1% NP-40 and 10% glycerol) supplemented with protease inhibitor cocktail (Sigma). For Rac1-P-Rex1 precipitation, MCF7 cells that express high endogenous levels of P-Rex1 [31]. were lysed in EDTA Buffer (25 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 5mM β-glycerophosphate) supplemented with protease inhibitor cocktail. In both cases, the lysates were cleared and 300 to 400 μg of total protein was used per condition. Glutation Sepharose 4B beads (GE Healthcare) coupled with bacterially expressed GST-Rac1 were preincubated with different concentrations of ZINC69391 or 1A-116 for 30 or 15 min at 4°C and then added to each protein extract in the presence or absence of ZINC69391 or 1A-116, using vehicle as control. The beads were washed twice with lysis buffer, resuspended in sample buffer and boiled. The samples were analyzed by immunoblotting using mouse monoclonal anti-HA antibody (Millipore) for Tiam1 detection or rabbit polyclonal anti-P-Rex1 antibody (Abcam). The integrity of the purified fusion proteins was checked by SDS-PAGE and Coomassie Blue staining prior to use in the assays described below.
Rac1 Pull Down Assay
Two different protocols were used. One consisted in plating tumor cells at a density 2.5x105 cells/well in 6-well tissue plates and these cells were grown to 80% confluence and starved for 48 h. The cells were then treated for 1 h with the compounds and stimulated for 15 minutes with Epidermal Growth Factor (EGF) 100ng/ml (Life Technologies). The other experimental setting consisted in plating the cells and treating them for 24 hs in full growth medium conditions. Monolayers were washed with PBS and lysed in 150-GPLB Buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 0.5% NP40, 10% glycerol, pH 7.4) supplemented with a protease inhibitor cocktail (Sigma). Lysates were clarified and the protein concentrations were normalized. An aliquot was removed for determination of total Rac and the rest was incubated with Glutathione Sepharose 4B Beads (GE Healthcare) coupled with bacterially expressed GST-Pak. Bound complexes were washed with lysis buffer, resuspended in protein sample buffer, boiled and loaded onto a 12% SDS-PAGE gel. Proteins were transferred and blotted with mouse monoclonal antibody against Rac1 (Sigma). The same protocol was used for Cdc42 pull down assays using a monoclonal antibody anti-Cdc42 (Santa Cruz Biotechnology).
Cell Cycle Analysis
For cell cycle analysis by flow cytometry, cells were washed and incubated in serum-free D-MEM for synchronization for 24 h. Cells were then treated for 48 h with different concentrations of ZINC69391 in D-MEM supplemented with 10% FBS and collected by trypsinization. Cells were fixed in 70% Methanol in PBS and stained with propidium bromide (1mg/ml) (Life Technologies). Cell cycle progression was analyzed in a BD FACSCaliburTM (BD Biosciences) flow cytometer. Before recording 10000 events, the verification of the doublet discrimination function of the flow cytometer was performed with DNA QC Particles kit (BD Biosciences).
Actin Staining
Cells grown in glass coverslips were incubated overnight (16 h) in serum free DMEM, treated for 1h with ZINC69391 and then stimulated with EGF (100 ng/ml) (Life Technologies) for 15 minutes. Cells were fixed in 4% formaldehyde in PBS and stained with AlexaFluor555 conjugated phalloidin (Molecular Probes, Life Technologies) following the manufacturer's instructions. Images were recorded in an inverted fluorescence microscope (Nikon Eclipse T2000).
Wound Healing Migration Assay
Cell migration was measured using an in vitro wound healing assay as described [32]. Briefly, in vitro “scratch” wounds were created by scraping confluent F3II or MDA-MB-231 monolayers with a sterile pipette tip. After 16 hour incubation in DMEM with 10% FBS in the presence or absence of ZINC69391, cells were fixed and stained. Ten random micrographs per well were obtained and migration area was quantified using NISElements 3.0 (Nikon) software. Wound closure measurements were normalized to the maximum scratch area.
Experimental Lung Metastases
All animal protocols were approved by the Universidad Nacional de Quilmes institutional Animal Care Committee that follow the procedures in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals. Specific pathogen-free female BALB/c inbred mice from UNLP (Buenos Aires, Argentina), with an age of 8-10 weeks and an average weight of 20 g, were used. They were housed in plastic cages under standard conditions and had access to rodent chow and water ad libitum. On day 0, 2x105 viable F3II cells in 0.3 ml DMEM were injected into the lateral tail vein. Mice were injected i.p at daily doses of 25 mg/kg body weight ZINC69391 or vehicle. This dose was established using IC50 value in vitro as a reference [33]. Treatment was carried out from day 0 to day 21. On day 21 mice were sacrificed and lungs were excised and immediately fixed in Bouin´s solution. Superficial lung nodules were counted under dissection microscope. The same protocol was carried out to evaluate 1A-116 analog in vivo, but the treatment consisted in daily doses of 3 mg/kg bodyweight.
RESULTS
Identification of ZINC69391 Through a Docking-Based Virtual Library Screening
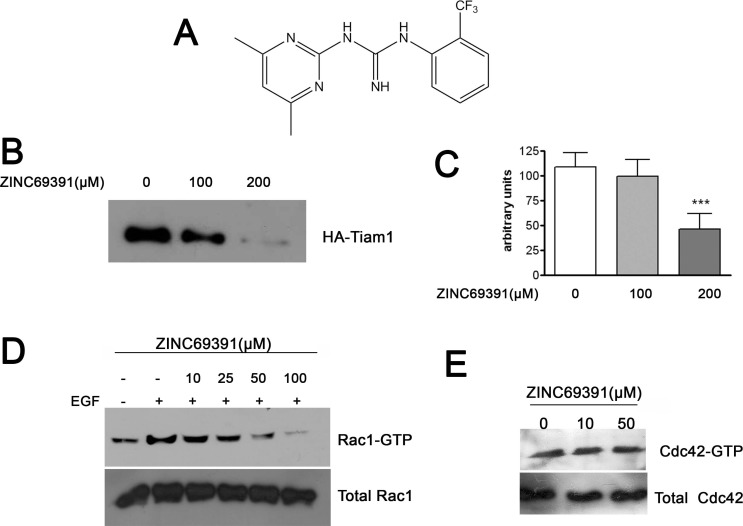
The Rac1 protein structure was extracted from PDB ID code 1MH1, and docking-based virtual screening was done considering the Rac1 surface area containing a critical tryptophane (Trp56) as the target. We explored about 200.000 compounds of the drug-like subset of ZINC database. Visual inspection of the top 100 hits was used to select 12 compounds for further in vitro characterization. From this small set of chemicals with high docking affinities the compound ZINC69391 showed significant antiproliferative effect on different cancer cell lines. Therefore, we continued our in vitro characterization with this compound. Its chemical structure is shown in Fig. (1A).
Fig. (1).
ZINC69391 is able to block Rac1 activation in breast cancer cells by interference of Rac1-Tiam1 interaction. A, Chemical structure of ZINC69391 (C14H15F3N5; molecular weight, 310.303) B, Concentration-dependent blockade of Rac1-Tiam1 interaction. Constitutive active Tiam1-HA tagged expressed in HEK-293T cells was affinity-precipitated with bacterially expressed Rac1 immobilized in Glutathione Agarose Beads in the presence of varying concentrations (100 and 200μM) of ZINC69391. Western blot analysis was carried out with antibodies for HA tag. The experiment was repeated 3 times. C, Densitometric analysis on the Western blot showed in B ***, p<0.001 determined by ANOVA cont. Dunnett´s Multiple Comparison Test. Each bar represents the media ± SD considering the results of three independent experiments D, Concentration-dependent Rac1 inhibition by ZINC69391 in F3II cells. Serum-starved F3II cells were treated for 1 hour at different ZINC69391 concentrations and stimulated with EGF (100ng/ml) for 15 minutes. E, Cdc42 was not affected by ZINC69391 treatment. F3II cells were treated in full growth media conditions.
ZINC69391 Decreases Rac1 Activation by Blocking Rac1-GEF Interaction in vitro
ZINC69391 showed high docking scores for Rac1-GEF interface, so we evaluated whether ZINC69391 was able to interfere the Rac1-GEF interaction in vitro. We performed an affinity precipitation assay using lysates from HEK293T cells overexpressing a constitutively activated mutant of Tiam1. We tested Tiam1(C1199) ability to bind to Rac1 immobilized on glutathione agarose beads in the presence of ZINC69391. As shown in Fig. (1B and C), Tiam1 interacted effectively with Rac1 and ZINC69391 was able to significantly interfere this association in a concentration-dependent manner.
ZINC69391 Interferes Rac1 Activation by EGF
EGF is one of the most described Rac1 activators in different cell types [34] and this activation can be mediated by one or more Rac1-GEFs, such as Tiam1 [35]. To evaluate the ability of ZINC69391 to interfere the Rac1 activation by EGF, pull down assays were carried out in serum-starved F3II cells after the stimulation with EGF. Treatment with ZINC69391, prior to EGF stimuli, dramatically impaired Rac1 pathway activation by this growth factor in a concentration dependent manner; whereas total Rac1 levels remained unchanged. Remarkably, high concentrations of ZINC69391 reduced Rac1 activation below non-stimulated basal levels (Fig. 1D).
To determine the specificity of ZINC69391 we tested the activity of this compound on the closely related Cdc42 GTPase activation. As shown in Fig. (1E), ZINC69391 had no effect on Cdc42-GTP levels even at 50 µM concentration in full growth media condition.
ZINC69391 Inhibits Proliferative Ability of Cancer Cells by Arresting Cell Cycle in G1 Phase
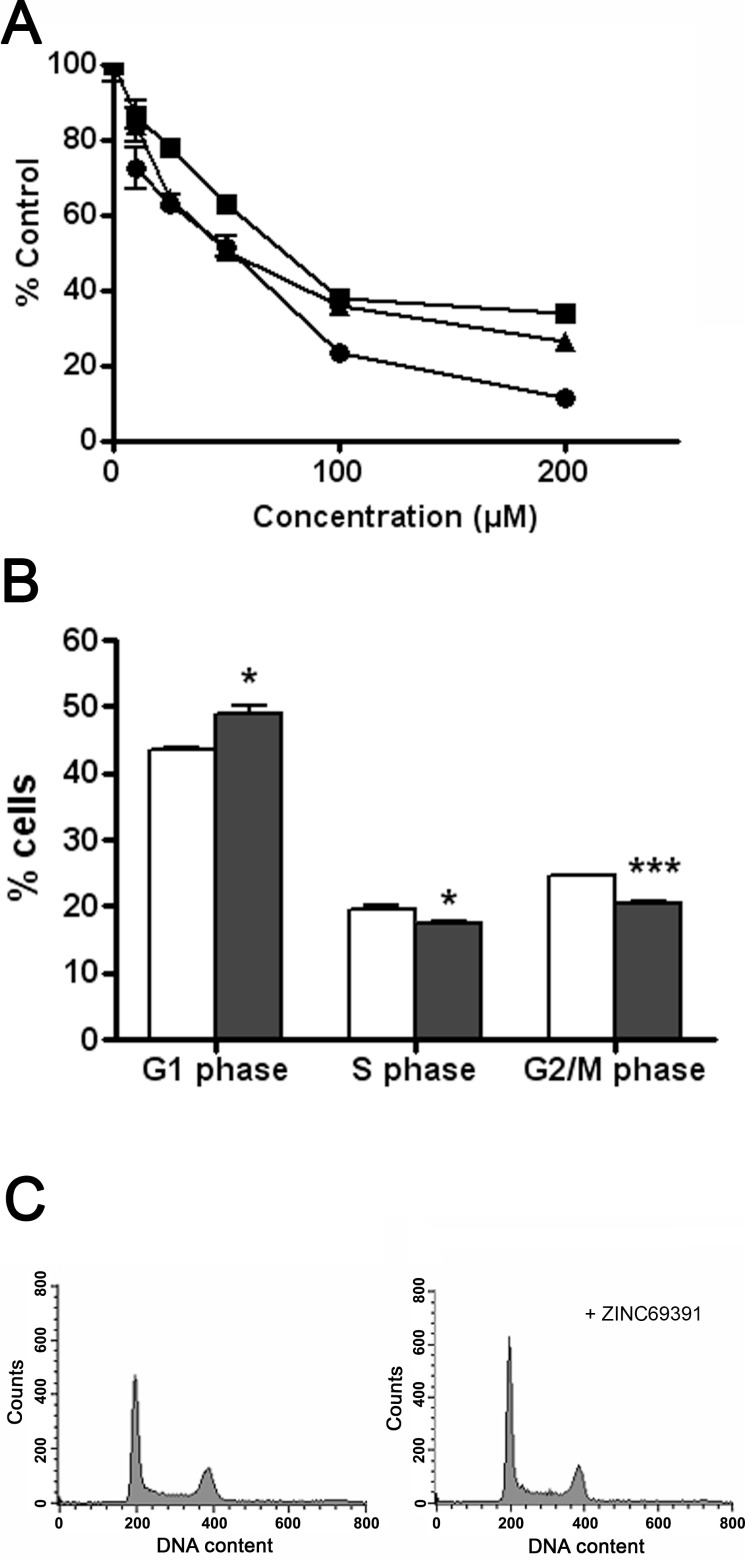
The aberrant Rac1 activity has been correlated with several aspects of malignancy in human cancers [8]. So we tested the effect of ZINC69391 on a panel of breast cancer cell lines, where Rac1 is important for transformation [36-38]. After 72 hours treatment, cell viability was measured using the MTT assay. As shown in Fig. (2A), ZINC69391 was able to inhibit the cell proliferation capacity on a panel of breast cancer cell lines in a concentration-dependent manner. ZINC69391 showed IC50 of 48 µM for MDA-MB-231, 61 µM for F3II and 31 µM for MCF7 cells. Similar results were obtained using trypan blue exclusion test of cell viability. We also compared the antiproliferative effect of ZINC69391 with the existing Rac1 inhibitor NSC23766 on F3II cells. NSC23766 inhibitor showed IC50 value of about 140 µM for F3II cells. This value is in line with previously reported values [39], showing a moderate antiproliferative activity in a highly aggressive tumor cell line.
Fig. (2).
ZINC69391 affects cell viability and cell cycle progression of aggressive breast cancer cells. A, ZINC69391 inhibits cell proliferation. Breast cancer cell lines F3II (●), MDA-MB-231 (■) and MCF7 (▲) were treated for 72 hours with different concentrations of ZINC69391. Cell viability was measured using MTT assay. B and C, ZINC69391 arrest cell cycle progression in G1 phase. MDA-MB-231 cells were synchronized and treated for 48 hours with ZINC69391 10μM. Cells were fixed, stained with propidium iodide and analyzed by FACS to estimate the percentage of cells in G1 phase, S phase and G2/M phase. Columns, mean of a representative experiment (n=3) of three independent experiments; bars, SD. *p<0.05. ***,p<0.001 determined by Student's t test versus control in each phase.
Because inhibition of Rac1 in breast cancer cell lines leads to G1 arrest, we next assessed the effect of ZINC69391 on cell cycle progression [40]. Log-phase growing MDA-MB-231 cells were synchronized and treated for 48 hs with 10µM ZINC69391 in the presence of FBS. As shown in Fig. (2B and 2C), ZINC69391 showed a significant increase of cells in G1 phase and a significant decrease of cells in S and G2/M phase.
ZINC69391 Affected Actin Reorganization and Inhibited Cell Migration
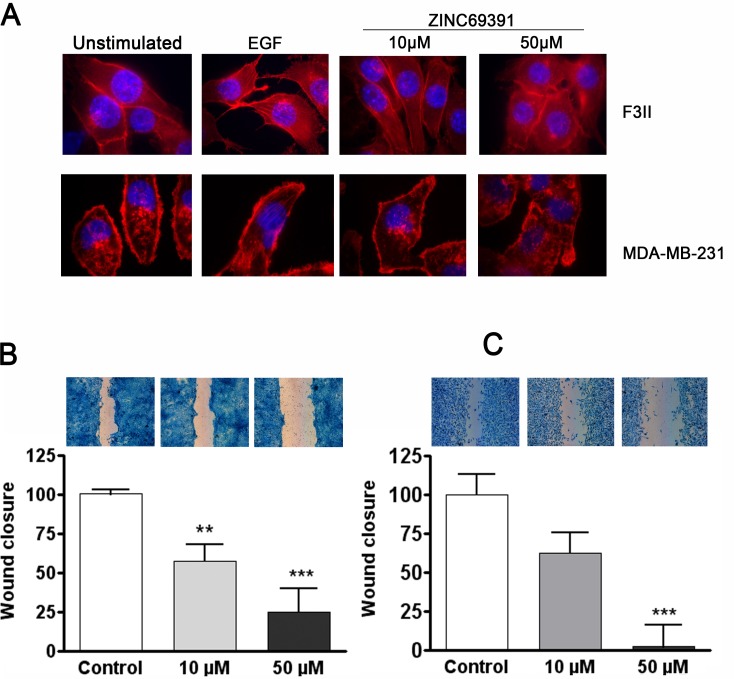
Cell migration and invasion are key processes in many important pathological situations and are fundamental aspects of the metastatic process. Members of the Rho family of small GTPases are key regulators of cell movement and actin cytoskeleton reorganization, particularly Rac1 drives cell motility by promoting lamellipodia formation [41, 42]. Importantly, it is well established that EGF induces cytoskeleton reorganization and cell migration through Rho-GTPases signaling pathway [43]. To evaluate whether ZINC69391 could interfere EGF-induced actin polymerization, we treated serum-starved MDA-MB-231 and F3II cells with ZINC69391 and then stimulated the cells for 15 minutes with EGF. As shown in Fig. 3A, EGF efficiently induced actin polymerization in the two cell lines. However, in the presence of ZINC693291, EGF-induced actin reorganization was significantly affected. This effect was shown at 10 µM but was much more evident at a concentration of 50 µM.
Fig. (3).
ZINC69391 blocks actin cytoskeleton reorganization and inhibits cell migration. A, Representative micrographs taken at 1000X showing inhibition of EGF-induced actin reorganization by ZINC69391 in F3II and MDA-MB-231 cells. Cells were grown on coverslips, serum-starved for 16 hs (untreated panel) and treated for 1 hour with ZINC69391 10 μM and 50 μM. After 15 minutes stimulation with EGF (100 ng/ml) (EGF control), cells were fixed and actin filaments were visualized with AlexaFluor555-phalloidin. B, Confluent monolayers of F3II cells were scratched and treated with ZINC69391 10 μM and 50 μM in presence of FBS. After 16 hours incubation, cells were fixed and stained. Wound closure was analyzed using NISElement 3.0. Experiments were carried out in triplicate plates. Results are expressed as percentage of wound closure, expressed as mean (n = 10). Bars, S.E.M. ***, p<0.001. ANOVA cont. Dunnett´s Multiple comparison test C, The same experimental design was carried out with MDA-MB-231 cells. Results are expressed as percentage of wound closure, and expressed as mean (n = 10), bars S.E.M. **, p<0.01; ***, p<0.001. ANOVA cont. Dunnett´s Multiple Comparison Test.
Thus, we next attempted to examine the effect of ZINC69391 on cell migration using a wound healing assay. In this assay, cells sense the free space left by the scratch and migrate together as a sheet. Rac1 is essential for forward movement in fibroblasts and astrocytes [1]. We evaluated the effect of ZINC69391 on wound closure with two highly invasive breast cancer cell lines. As shown in Fig. 3B MDA-MB-231 cells treated with ZINC69391 50 µM and 10 µM significantly reduced cell migration by 100% and nearly 40% respectively compared to control. A similar effect was observed on F3II cell line, where ZINC69391 50 µM inhibited 80% and 10 µM inhibited 50% wound closure compared to control (Fig. 3C).
Thus, non toxic concentrations of ZINC69391 effectively inhibited actin reorganization and cell migration of MDA-MB-231 and F3II cells.
Rac1 Inhibition by ZINC69391 Impairs Metastatic Lung Colonization in a Syngenic Animal Model
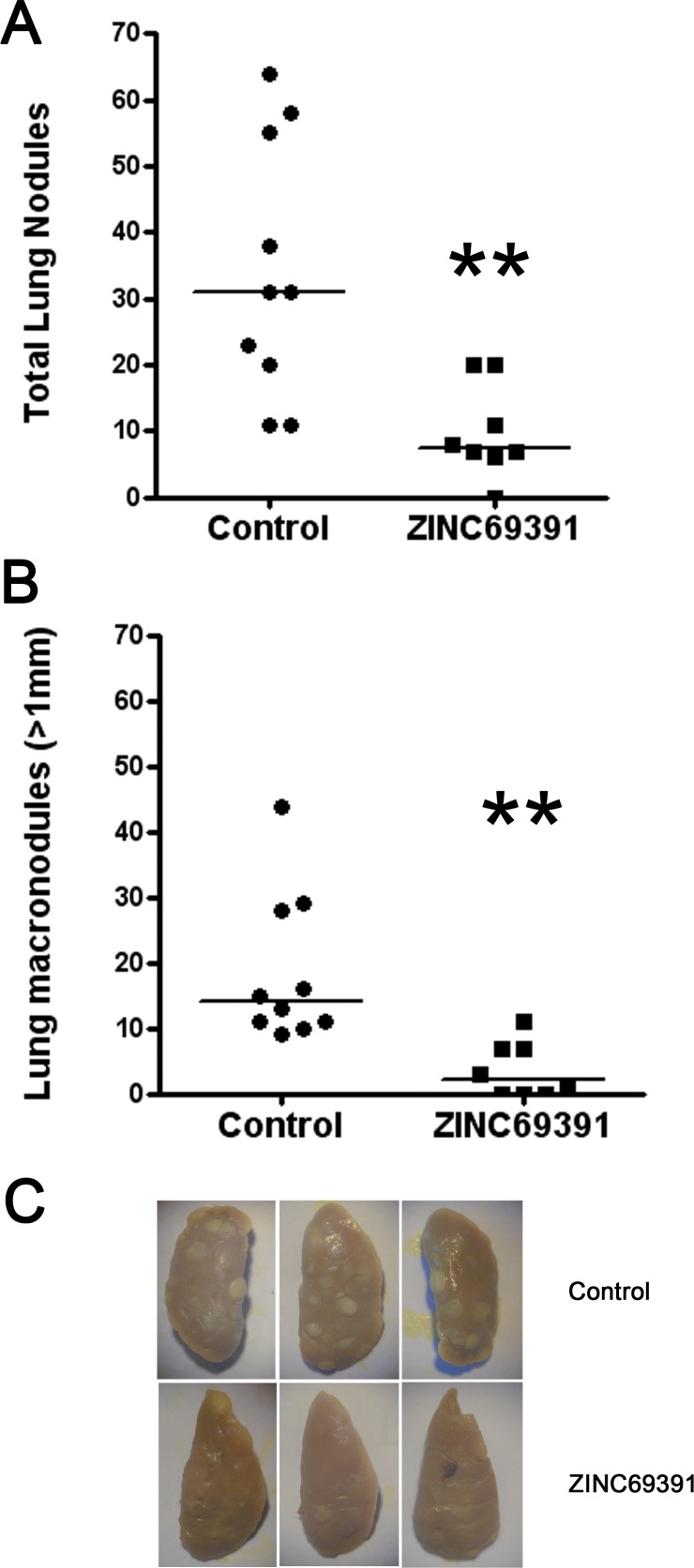
To extent our in vitro findings, we tested ZINC69391 effect in an experimental metastasis animal model of breast cancer. On the designated day 0 of the experiment 2x105 viable F3II cells were administered i.v to BALB/c mice and then injected i.p at daily doses of 25 mg/kg body weight from day 0 to 21. At day 21, mice were sacrificed and superficial lung metastases were counted under a dissecting microscope.
Daily treatment of mice with compound ZINC69391 at 25mg/kg/day significantly reduced by about 60% the formation of total metastatic lung colonies, as shown in Fig. 4A. The same results were observed when we consider tumor metastases larger than 1 mm diameter (Fig. 4B). As expected, compound ZINC69391 was well tolerated in adult female BALB/c mice. In all cases, treatment caused no significant changes in animal weight when compared to the control group (data not shown).
Fig. (4).
Anti-metastatic effect of ZINC69391 on F3II cells. A, 2x105 viable F3II cells were injected into the lateral tail vein and the mice were treated i.p with a daily dose of 25 mg/kg. On day 21 mice were sacrificed and superficial lung nodules were counted. Each data point represents the number of total lung nodules per mouse in each treatment group (n=10 control group, n=8 treated group) **, p < 0.01. Mann-Whitney test. B, Experimental formation of lung macronodules (>1 mm in diameter). **p < 0.01 Mann-Whitney Test C, Representative left lung lobes were photographed.
Rational Design of ZINC69391-Derived Novel Analog 1A-116
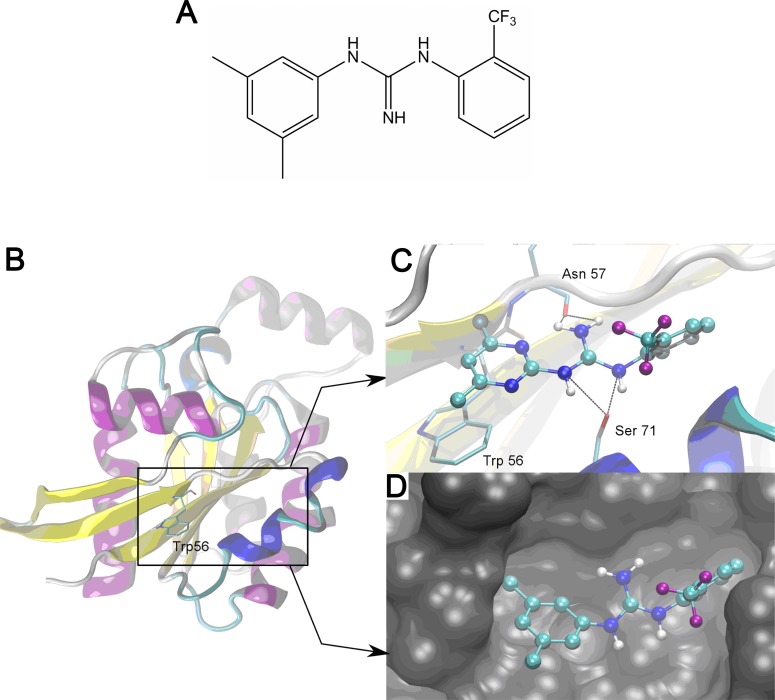
Employing ZINC69391 as lead structure, a series of derivatives were designed varying the structure of the pyrimidine ring that is believed to be crucial in the interaction with Rac1 (data not shown). From this group, analogue 1A-116 (Fig. 5A) was selected for further studies due to its enhanced predicted binding free energy (-6.77 Kcal/mol for 1A-116 vs -5.86 Kcal/mol for ZINC69391). As it can be seen in Fig. (5C and 5D), both compounds share the same pose and key interactions. A detailed analysis of the protein-drug interaction shows that in both cases several hydrogen bonds are established between the guanidine nitrogen atoms and residues Asp57 and Ser71. Moreover, pi-stacking interactions are observed between the methylated aromatic ring of the drugs and the aromatic ring of Trp56. Probably, the increase in the predicted binding affinity and experimental inhibitory potency of 1A-116 can be attributed to differences in water solvation and in the interaction with Trp56. We expect a decrease on the free energy of water solvation (ΔGsw) for 1A-116. Whereas ZINC69391 has a relatively polar pyrimidine ring that can perform hydrogen bond with a protic solvent like water, 1A-116 has a more hydrophobic benzene ring. Indeed, the predicted Log P [44] is greater for 1A-116 than ZINC69391 (4.41 vs. 3.85). This will indicate that the penalty to become bound is greater for ZINC69391 as compared to 1A-116. Finally, pi-stacking interactions with Trp56 are expected to be stronger because of the replacement of the pyrimidine ring with a benzenic one.
Fig. (5).
Best docking poses of ZINC69391 and its derived novel analog 1A-116 over Rac1. A Chemical structure of 1A-116 (C16H16F3N3; molecular weight, 307.31) B, Cartoon representation of the structure of Rac1 showing the binding site defined by Trp56. C, The binding pocket of ZINC69391 (Drawn with ball and sticks representation) depicting key interactions with Rac1 (Structure drawn in cartoon and residues drawn in Licorice representation). D, Rac1 surface with 1A-116 docking pose (Drawn in ball and sticks).
Compound 1A-116 was synthesized (see supplementary material) and experimentally evaluated. Interestingly, in agreement with predicted properties, we found that 1A-116 is increased compared to ZINC69391 in the experimental inhibitory potency as described in the following sections.
1A-116 Inhibited Rac1-Mediated Tumoral Cell Proliferation
To evaluate the effect of 1A-116 on cell proliferation of F3II, we measured cell viability by the MTT metabolic assay. 1A-116 inhibited cell proliferation in a concentration-dependent manner and showed a more potent antiproliferative activity than the parental compound. As shown in Table 1, 1A-116 showed a significant increase in antiproliferative activity compared to ZINC69391 on F3II cells, showing an IC50 value of 4 µM, a 15-fold reduction compared to the parental ZINC69391 compound and a significantly lower value compared to the Rac1 inhibitor NSC23766. In line with this evidence, 1A116 also showed to be more potent on MDA-MB-231 cells. On these cells, 1A-116 showed an IC50 value of 21 µM, while ZINC69391 showed a IC50 value of 48 µM.
Table 1.
IC50 values of the different compounds on F3II cells.
| NSC27366 (Reference) | ZINC69391 (Parental) | 1A-116 (Analog) |
|---|---|---|
| 140 μM | 61 μM | 4 μM |
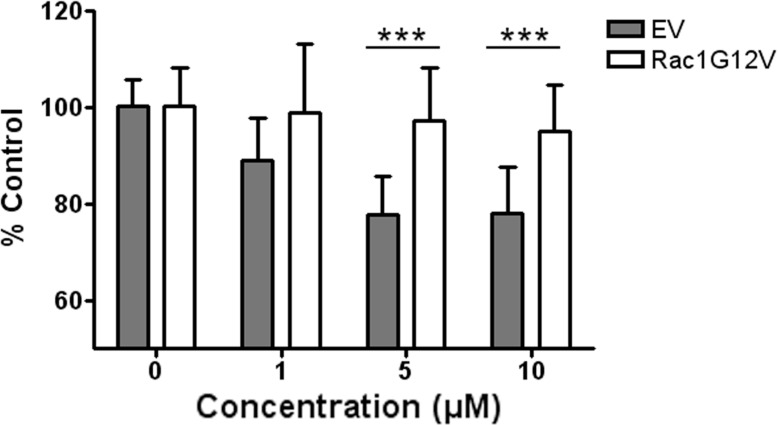
To further demonstrate that the in vitro antitumoral activity of 1A-116 depends of Rac1 signaling inhibition, we evaluated the effect of 1A-116 on MDA-MB-231 breast cancer cells transiently overexpressing the constitutively active variant Rac1-G12V. As show in Fig. (6) overexpression of Rac1-G12V significantly attenuated the inhibitory effects of 1A-116 on cell proliferation compared to empty-vector transfected cells. This same effect was observed in F3II mammary carcinoma cells overexpressing Rac1-G12V (data not shown).
Fig. (6).
Expression of Rac1-G12V constitutively active variant attenuates the effect of 1A-116. MDA-MB-231 were transfected with Rac1-G12V variant or empty vector (EV). These cells were treated for 48 hs with different concentrations of 1A-116 analog. The constitutively active Rac1 variant confers GEF independence, showing an attenuated effect of 1A116 analog compared to the control cells. Bars SD, ***p<0.001. EV vs. Rac1 G12V at each concentration. Two way ANOVA cont. Bonferroni Test.
1A-116 is Able to Interfere Rac1-P-Rex1 Interaction and Reduce Rac1 Activation at Lower Doses Compared to the Parental Compound
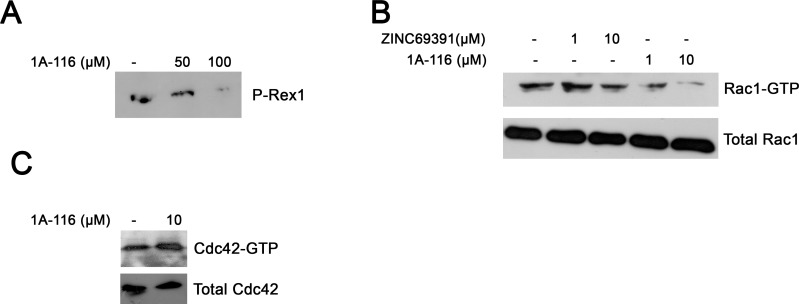
In the last years compelling evidence indicated that P-Rex1 is a Rac1-GEF relevant in breast cancer [31,45]. For this reason we evaluated weather 1A-116 was able to intefere this Rac-GEF interaction, using a affinity precipitation assay. As shown in Fig. (7A), 1A-116 was able to block Rac1-P-Rex1 interaction in vitro.
Fig. (7).
1A-116 interferes P-Rex1-Rac1 interaction, being a more potent Rac1 inhibitor than ZINC69391. A, Affinity precipitation showing that 1A-116 is capable of blocking P-Rex1-Rac1 interaction. B, F3II cells were treated for 12 hour with ZINC69391 and 1A-116 in full growth conditions. Pull down assays were carried out and then were analyzed by Western blot. 1A-116 markedly reduced Rac1-GTP levels compared to ZINC69391 C, 1A-116 had no effect on Cdc42 activation.
We next assessed weather 1A-116 was able to inhibit Rac1 activation more effectively. To this end, we determined intracellular Rac1-GTP levels in the highly aggressive breast cancer cell line F3II by pull down assay in full growth medium. As shown in Fig. (7B), 1A-116 was able to reduce Rac1-GTP intracellular levels in a concentration-dependent manner. Moreover, 1A-116 dramatically impaired Rac1 activation at low micromolar range (1 µM). In contrast, ZINC69391 showed no effect in 1 µM and a modest effect in 10 µM under these experimental situations. Total Rac1 levels remained unchanged in all conditions. Thus, 1A-116 is a more potent Rac1 inhibitor in vitro than the parental compound, particularly at low concentrations.
We also evaluated the specificity of 1A-116 analog. We determined the effect of this compound on the closely related Cdc42 GTPase activation. As shown in Fig. (6B), 1A-116 analog had no effect on Cdc42-GTP levels even at 10 µM, concentration where Rac1 is dramatically affected (Fig. 7C).
1A-116 Analog Showed Improved Antimetastatic Activity in vivo
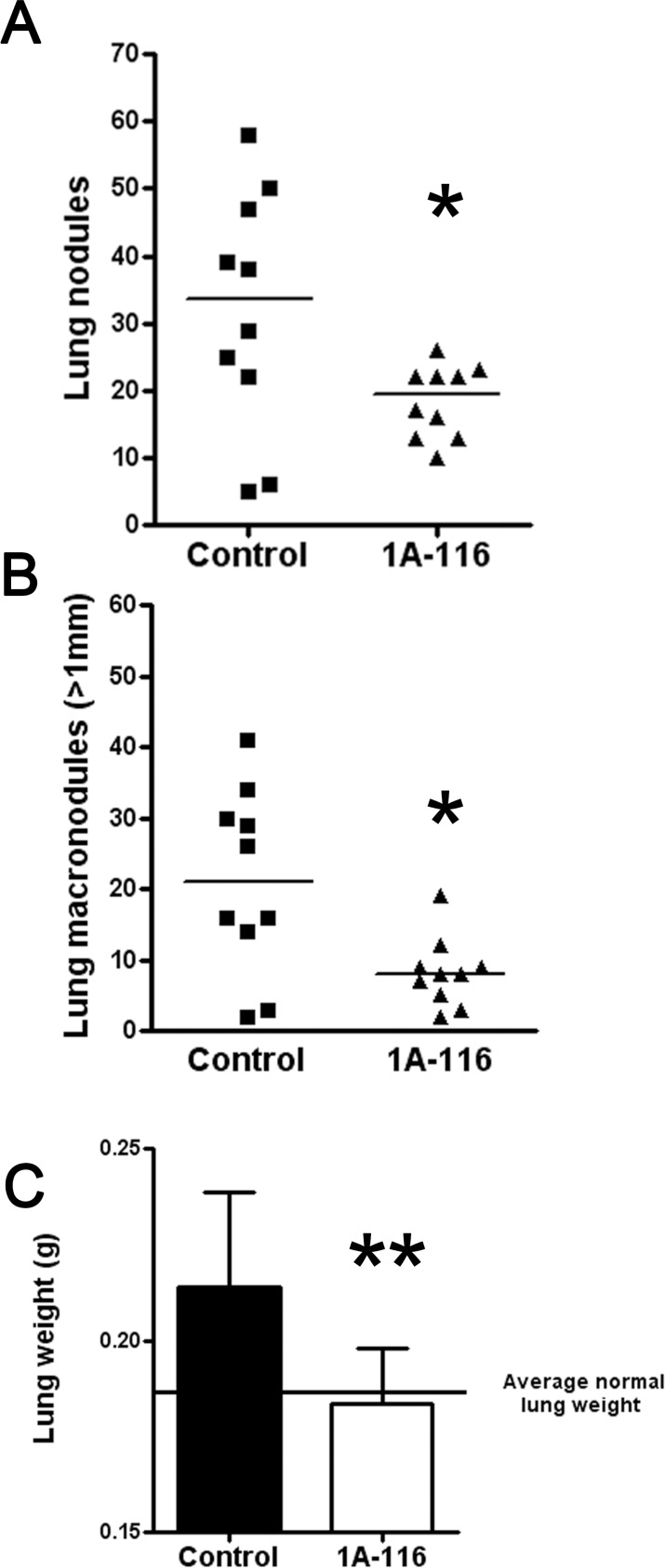
We tested 1A-116 effect on in vivo lung colonization of F3II cells by carrying out an experimental metastasis protocol as previously described. Daily treatment of mice with compound 1A-116 at 3mg/kg body weight/day reduced about 60% the formation of total metastatic lung colonies, as shown in Fig. 8A. A significant antitumor activity was obtained for macronodules (more than 1 mm in diameter) by treatment with 1A-116 in this highly aggressive breast cancer model (Fig. 8B). We also weighted lungs corresponding to both groups. The treatment with 1A-116 reduced the total lung weight compared to the control group, leading to a total weight similar to the average pulmonary weight of Balb/c mice [46].
Fig. (8).
Effect of 1A-116 treatment on experimental lung tumor formation. F3II cells were injected intravenously in mice (n=10), as described in “Materials and Methods.” A, Total lung tumor nodules experimentally formed. The control group was treated with vehicle and the second group of animals was treated with 1A-116 analog with a 3 mg/kg body weight/day dose. *p < 0.05 Mann-Whitney Test. B, Experimental formation of lung macronodules (>1 mm in diameter). *p < 0.05 Mann-Whitney Test. C, Total weight of lungs corresponding to both groups. The continuous line represents the average pulmonary weight of Balb/c mice described by Han et al., [46]. **p<0.01 Mann-Whitney Test.
Remarkably, 1A-116 analog demonstrated a higher antimetastatic activity compared to the parental compound ZINC69391, showing similar lung colonization reduction using an 8-times lower dose.
No apparent toxicity was evidenced after this 21-day protocol. The body weight of all the living mice (n=10) were measured and there was no statistical difference between the groups (data not shown).
DISCUSSION
The Rho-GTPase Rac1 plays an important role in the regulation of fundamental cellular processes such as actin cytoskeleton reorganization, cell migration and proliferation [1]. This regulation is achieved through a variety of activators and inhibitors, such as GEFs and GAPs. It is well established that Rac1 aberrant regulation or expression is associated with oncogenic transformation and metastasis in various cell types, including breast cancer. Importantly, a part of this aberrant signaling is driven by alterations in its regulatory proteins [4]. Two lines of evidence support that Rac1 expression correlates with tumor histological grade but not to Her2 status. Fritz et al., [6] first showed this correlation in 50 breast cancer specimens, also Ma et al., [47] showed that high levels of Rac1/Cdc42 expression in 339 breast cancer specimens significantly correlated with breast cancer staging, proliferation index (Ki67), lymph node metastasis, tumor invasion and low ER expression.
Other studies show that Rac1 is found to be higher expressed in breast tumors than in the surrounding normal breast tissue, presenting a therapeutic opportunity for the development of novel therapies [9, 48].
Several studies have described that a Rac1 splice variant, designated Rac1b, is expressed in breast cancer specimens and cell lines [9, 49, 50]. Rac1b has shown to have a high intrinsic guanine nucleotide exchange activity and this activity was not further stimulated by Tiam1 and Vav2 [51].
To date, there are many therapeutic strategies based on signaling inhibitors in breast cancer. Targeting HER2/neu receptor by monoclonal antibodies and tyrosine kinase inhibitors has been one of the most successful strategies developed in the last 20 years [52]. There are also other molecular targets being evaluated in breast cancer such as mTOR and HSP90, among others [53, 54]. Molecular targeted therapies offer an interesting perspective in current breast cancer treatments, adding novel options to traditional therapeutics such as surgery, chemotherapy, radiotherapy and hormone-based therapies.
Numerous studies have provided compelling evidence in support of the critical role of Rac1 in many cancer-associated processes. We have previously described that the expression of the catalytic domain of beta2-chimaerin, a protein with Rac1-GAP activity, in F3II cells markedly affected proliferation, migration, invasion and metastasis. Thus, inactivation of the Rac1 pathway by beta2-chimaerin affects the dissemination of cancer cells in vivo [35].
In line with this idea, Rac1 is a validated and promising target for novel therapeutic approaches in cancer [55, 11]. Several strategies have been reported to target the Rac1 signaling pathway. One of the most studied strategies is to inhibit C-terminal isoprenylation of Rac1 by affecting different enzymes involved in the biosynthetic pathway: HMGCoA reductase inhibitors (statins) or geranylgeranyl-transferase I inhibitors [56]. Because this strategy focuses on isoprenylation of GTPases, these inhibitors are not specific to Rac1, affecting other GTPases such as Cdc42 and Rho. There has also been reported that low concentration of azathioprine-generated 6-Thio-GTPs inhibits the intracellular activation of Rac1 in T-lymphocytes and breast cancer cells, but this strategy seems to be nonspecific, especially at high concentrations [57-59]. More interestingly are the molecules described to inhibit specifically Rac1 signaling pathway by inhibiting Rac1-GEF interaction. At present we can mention Rac1 inhibitor NSC23766 and other analogs such as EHop-016 [38, 60-62]; compounds that inhibit GEF activity [63-65]; compounds that displace the guanine nucleotide such as EHT 1864 [66]; compounds that inhibit Rac1-effector interaction, such as Phox-I1 [67], and molecules that inhibit Rac1 effectors, such as the Pak inhibitor PF-3758309 [68].
In this study, we identified ZINC69391, a structurally simple dialkylated guanidine derivative Rac1 inhibitor, by docking-based virtual library screening. We screened 200.000 molecules of the drug-like subset of the public available ZINC database using e-HITS® software for the structure-based in silico screening. We sought compounds able to block Rac1 activation by different GEFs, including Dbl and Dock180 GEF families. We identified a new family of Rac1 inhibitors, introducing a novel chemical backbone as a lead compound for drug development. In this regard, we designed and synthesized a first group of analogs derived from the parental ZINC69391 compound. As a first lead-derived compound, we selected and evaluated 1A-116 analog showing enhanced antitumoral activity.
Data published by Schnelzer [9] and Singh [50], described Rac1b as a splice variant with a C-terminal 19-residue insertion, that confers a high exchange activity compared to Rac1 and is unaffected by Tiam1 and Vav2 interaction. In line with this evidence, we hypothesize that molecules with the ability to interfere Rac1-GEF interaction would have no effect on Rac1b variant activation in contrast to other inhibitors that present a mechanism that involves guanine nucleotide displacement, such as the EHT 1864 inhibitor [66].
Our results showed that ZINC69391 was capable to interfere in vitro Rac1 interaction with its GEF Tiam1 as predicted by docking scores. Since Trp56 is a key residue in Rac1 recognition by different GEFs, we expected this new family of compounds to interfere the interaction of Rac1 with other GEFs that share the same Rac1 activation mechanism. In this regard, P-Rex1 has been strongly associated with invasion and metastasis in breast cancer [45]. We show here that 1A-116 analog was able to interfere P-Rex1-Rac1 interaction, potentially affecting cancer-related cellular functions regulated by P-Rex1 signaling, such as proliferation, motility, and invasion. P-Rex expression has been correlated with patient outcome in breast cancers, and reduction of P-Rex expression has been shown to restrict proliferation and/or metastatic dissemination [31, 45].Therefore, targeting P-Rex proteins may be therapeutically relevant in neoplasias in which those proteins play an important role in regulating their growth or dissemination.
We showed that ZINC69391 was able to impair Rac1 activation by EGF, a described activator of Rac1 signaling pathway [34]. Notably, this Rac1 inhibitor is able also to reduce basal Rac1-GTP levels. This is particularly important in the context of tumor biology, where a targeted inhibitor is required to be effective in a constitutively hyperactivated signaling pathway.
Cdc42 and Rac1 share about 70% structure homology but they have different specific GEFs and effectors. It has also been described that Cdc42 presents different key residues involved in GEF interaction. Cdc42 presents a Phe in the crucial 56 position. [37]. Therefore, we expected ZINC69391 not to have any effect on Cdc42 activation, given the change in residue identity. Remarkably, ZINC69391 showed to be a Rac1 specific inhibitor, having no effect on the closely-related GTPase Cdc42 in full growth conditions.
The cancer metastatic process involves a series of coupled events. These include detachment of the cells of the primary tumor, migration of these cells and dissemination through blood vessels. Finally, the cells arrive to the secondary site, adhere and proliferate. Rac1 plays a key role in most of these biological processes. ZINC69391 showed antiproliferative activity in a concentration-dependent manner on different breast cancer cell lines, where Rac1 is relevant [36-38]. This antiproliferative effect was measured using the indirect MTT method and these results correlated with data obtained by the trypan blue exclusion assay. This effect was at least in part due to a significant G1 phase arrest, at a concentration lower than the IC50 value. In this regard, it was previously established that Rac1 inhibition by overexpression of ß2-chimaerin is related with cell cycle arrest. Particularly, Rac1 modulation affects cyclin D1 expression in breast cancer cell lines, reducing cell proliferation [38]. Coincident with these findings, we showed cell proliferation inhibition and cell cycle arrest with a novel pharmacological Rac1 inhibitor. Moreover, we sought for the effect of 1A-116 on Rac1-mediated cell proliferation. We transfected the constitutively active Rac1-G12V mutant in two breast cancer cell lines. Rac1-G12V is a GEF-independent Rac1 variant, that activates constitutively the Rac1 pathway. As expected, 1A-116 was unable to reduce cell proliferation on these GEF-independent breast cancer cells, evidencing the link of antiproliferative activity of the compound with the modulation of the intracelullar Rac1 signaling.
Rho GTPases and Rac1 in particular, have long been recognized as key molecules in actin cytoskeleton remodeling and cell migration regulation. ZINC69391 effectively inhibited actin reorganization and cell migration in highly aggressive breast cancer MDA-MB-231 and F3II cells. Since these biological events play a significant role in invasion and metastasis, we further investigated the activity of this new Rac1 inhibitor on an established highly aggressive experimental disease by intravenous lung colonization by breast cancer cells. Although the tail-vein cell injection experimental metastasis model does not measure the early steps of the cancer metastatic process, it is a validated method to study the capabilities of cancer cells to form secondary tumors [69]. Our results showed that a daily treatment with ZINC69391 after the i.v injection of cells significantly reduces lung colonization about 60%. Hence, we show for the first time that a small Rac1 inhibitor is able to impair significantly in vivo lung metastasis of highly aggressive breast cancer cells using a therapeutic treatment schedule. Although we showed that ZINC69391 inhibits metastasis by its inhibition of Rac1, we have not yet defined the Rac1 signature. Only microarray analysis can provide quantitative gene expression information allowing for the generation of a molecular signature. However, from previous works we can speculate what kind of genes will be affected. Rac1 activation promotes changes related to cytoskeleton reorganization and other responses through multiple mediators. Rac-GTP induces the activation of WAVE through either Irsp53 or Nap125-PIR121 complexes. WAVE activates Arp2/3, leading to changes in actin cytoskeleton. Rac also activates PAK, which exerts its effects through Arp2/3 and LIMK activation or MLCK inhibition. In addition, Rac forms part of the NADPH oxidase complex that generates reactive oxygen species. Rac/PAK activates MAPKs implicated in stress response, mitogenesis, and survival [70].
Employing ZINC69391 as a lead structure several analogues were designed and synthesized in order to optimize potency and establish structure-activity relationships. 1A-116 was selected from this group because of its better docking scores that correlated with better in vitro inhibitory activity when compared to parental compound ZINC69391. Indeed, 1A-116 proved to be a more potent Rac1 inhibitor in vitro, with enhanced antiproliferative activity on highly aggressive breast cancer cells. In this regard, we were able to reduce the IC50 values 15 times comparing to the parental compound, showing an inhibitory effect in the low micromolar range. The parental ZINC69391 compound and the 1A-116 analog were found to be much more potent than the previously reported Rac1 inhibitor NSC23766, showing an important difference in IC50 values.
Remarkably, low concentrations such as 1 and 10 micromolar of 1A-116 analog were able to reduce Rac1 activation in normal full growth media condition. Under these full growth conditions, ZINC69391 was also able to reduce modestly Rac1-GTP levels, assessing the effect of the parental compound on Rac1 activation, which was previously showed under an EGF-induction experimental setting. This improved potency did not affect Rac1 specificity, since Cdc42-GTP levels were not affected by 1A-116 treatment. To further investigate the improvement of 1A-116 activity, we tested the analog in vivo using a lower dose compared to the parental compound. We correlated the change in potency in vitro with the in vivo dose, using 3 mg/kg body weight/day. These experiments showed a similar reduction of lung colonization compared to the parental compound but using an 8 times lower dose. This tendency is showed in both total lung nodules and macronodules counts, confirming that 1A-116 is a more potent Rac1 inhibitor in vitro and in vivo.
CONCLUSIONS
In summary, we have identified ZINC69391, a novel Rac1 inhibitor, by docking-based virtual library screening. This compound affected Rac1 activation in vitro and many Rac1-driven cellular processes involved in cancer progression, such as proliferation, cell cycle progression and migration. ZINC69391 also showed a significant antimetastatic activity in vivo. We further designed and synthesized 1A-116, a more potent Rac1 inhibitor analog that showed an enhanced antiproliferative activity and elicits a similar in vivo response as its parental compound, using a significantly lower dose. Because Rac1 activity is central to cancer cell migration and invasion the novel, less toxic, and more specific Rac1 inhibitors presented here appear to be promising candidates for further development. Currently we are carrying out a more detailed characterization of them in order to proceed with preclinical studies in cancer models.
ACKNOWLEDGEMENTS
We thank Valeria Segatori for assistance with Flow Cytometry experiments and Juan Garona for assistance with in vivo experiments. We thank Dr. Alfredo Cáceres for providing us with the Tiam-C1199 plasmid.
CONFLICT OF INTEREST
The authors disclose no potential conflicts of interest. This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de Quilmes, Agencia de Promoción Científica y Tecnológica (ANPCyT), Instituto Nacional de Tecnología Industrial (INTI) and by a grant from Chemo-Romikin S.A. (Argentina). Comin M.J., Alonso D.F., Gomez D.E. and Lorenzano Menna P. have filled a patent regarding the findings described in this manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 2.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348(Pt 2):241–55. [PMC free article] [PubMed] [Google Scholar]
- 3.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a "Rac" of all trades. Cell Mol. Life Sci. 2009;66(3):370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellenbroek SI, Collard JG. Rho GTPases: Functions and association with cancer. Clin. Exp. Metastasis. 2007;24(8):657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 5.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 1999;81(5):682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: Expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer. 2002;87(6):635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, Yoshida K. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin. Cancer Res. 2004;10:4799–4805. doi: 10.1158/1078-0432.CCR-0436-03. [DOI] [PubMed] [Google Scholar]
- 8.Buongiorno P, Bapat B. Rho GTPases and cancer. Prog. Mol. Subcell. Biol. 2005;40:29–53. doi: 10.1007/3-540-27671-8_2. [DOI] [PubMed] [Google Scholar]
- 9.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer Overexpression mutation analysis and characterization of a new isoform, Rac1b. Oncogene. 2000;19(26):3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 10.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz G, Kaina B. Rho GTPases Promising cellular targets for novel anticancer drugs. Curr. Cancer Drug Targets. 2006;6(1):1–1. [PubMed] [Google Scholar]
- 12.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs validated and tractable targets for cancer therapy?. Nat Rev Cancer. 2010;10(12):842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, Ariyan S, Narayan D, Dutton-Regester K, Capatana A, Holman EC, Bosenberg M, Sznol M, Kluger HM, Brash DE, Stern DF, Materin MA, Lo RS, Mane S, Ma S, Kidd KK, Hayward NK, Lifton RP, Schlessinger J, Boggon TJ, Halaban R. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44(9):1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, Dicara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, Stemke-Hale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DS, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6(2):167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 16.Fields AP, Justilien V. The guanine nucleotide exchange factor (GEF) Ect2 is an oncogene in human cancer. Adv. Enzyme Regul. 2010;50(1):190–200. doi: 10.1016/j.advenzreg.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Leeuwen FN, Van der Kammen RA, Habets GG, Collard JG. Oncogenic activity of Tiam1 and Rac1 in NIH3T3 cells. Oncogene. 1995;11(11):2215–2221. [PubMed] [Google Scholar]
- 18.Gao Y, Xing J, Streuli M, Leto TL, Zheng Y. Trp(56) of rac1 specifies interaction with a subset of guanine nucleotide exchange factors. J. Biol. Chem. 2001;276(50):47530–47541. doi: 10.1074/jbc.M108865200. [DOI] [PubMed] [Google Scholar]
- 19.Karnoub AE, Worthylake DK, Rossman KL, Pruitt WM, Campbell SL, Sondek J, Der CJ. Molecular basis for Rac1 recognition by guanine nucleotide exchange factors. Nat. Struct. Biol. 2001;8(12):1037–1041. doi: 10.1038/nsb719. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Ramachandran S, Lin MC, Cerione RA, Erickson JW. A minimal Rac activation domain in the unconventional guanine nucleotide exchange factor Dock180. Biochemistry. 2011;50(6):1070–80. doi: 10.1021/bi100971y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoichet BK. Virtual screening of chemical libraries. Nature. 2004;432(7019):862–865. doi: 10.1038/nature03197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosconati S, Forli S, Perryman AL, Harris R, Goodsell DS, Olson AJ. Virtual screening with autodock: Theory and practice. Expert Opin. Drug Discov. 2010;5(6):597–607. doi: 10.1517/17460441.2010.484460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin JJ, Shoichet BK. ZINC--a free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 2005;45(1):177–182. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Coleman RG. ZINC: A free tool to discover chemistry for biology. J. Chem. Inf. Model. 2012;52(7):1757–1768. doi: 10.1021/ci3001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zsoldos Z, Reid D, Simon A, Sadjad SB, Johnson AP. eHiTS: A new fast, exhaustive flexible ligand docking system. J. Mol. Graph Model. 2007;26(1):198–212. doi: 10.1016/j.jmgm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Morris GM, Huey R, Olson AJ. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinformatics. 2008;Chapter 8:Unit 8.14. doi: 10.1002/0471250953.bi0814s24. [DOI] [PubMed] [Google Scholar]
- 27.Alonso DF, Farías EF, Urtreger A, Ladeda V, Vidal MC, Bal De Kier Joffé E. Characterization of F3II, a sarcomatoid mammary carcinoma cell line originated from a clonal subpopulation of a mouse adenocarcinoma. J. Surg. Oncol. 1996;62(4):288–297. doi: 10.1002/(SICI)1096-9098(199608)62:4<288::AID-JSO14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Best A, Ahmed S, Kozma R, Lim L. The Ras-related GTPase Rac1 binds tubulin. J. Biol. Chem. 1996;271(7):3756–3762. doi: 10.1074/jbc.271.7.3756. [DOI] [PubMed] [Google Scholar]
- 29.Habets GG, Scholtes EH, Zuydgeest D, Van Der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77(4):537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 30.Stam JC, Sander EE, Michiels F, Van Leeuwen FN, Kain HE, Van der Kammen RA, Collard JG. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J. Biol. Chem. 1997;272(45):28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- 31.Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, Luo J, MBenovic JL, Klein-Szanto A, Yagi H, Gutkind JS, Parsons RE, Kazanietz MG. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol. Cell. 2011;40:877. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segatori VI, Otero LL, Fernandez LE, Gomez DE, Alonso DF, Gabri MR. Antitumor protection by NGcGM3/VSSP vaccine against transfected B16 mouse melanoma cells overexpressing N-glycolylated gangliosides. In vivo. 2012;26(4):609–617. [PubMed] [Google Scholar]
- 33. National Institute of Health (NIH). Guidance Document on Using In Vitro Data to Estimate In Vivo Starting Doses for Acute Toxicity. NIH Publication No: 01-4500. 2001 [Google Scholar]
- 34.Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16(16):4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh RE, Kiyokawa E, Aoki K, Nishioka T, Akiyama T, Matsuda M. Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF. J. Cell Sci. 2008;121(Pt 16):2635–2642. doi: 10.1242/jcs.028647. [DOI] [PubMed] [Google Scholar]
- 36.Menna PL, Skilton G, Leskow FC, Alonso DF, Gomez DE, Kazanietz MG. Inhibition of aggressiveness of metastatic mouse mammary carcinoma cells by the beta2-chimaerin GAP domain. Cancer Res. 2003;63(9):2284–2291. [PubMed] [Google Scholar]
- 37.Snyder JT, Worthylake DK, Rossman KL, Betts L, Pruitt WM, Siderovski DP, Der CJ, Sondek J. Structural basis for the selective activation of Rho GTPases by Dbl exchange factors. Nat. Struct. Biol. 2002;9(6):468–475. doi: 10.1038/nsb796. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Liu Y, Leskow FC, Weaver VM, Kazanietz MG. Rac-GAP-dependent inhibition of breast cancer cell proliferation by {beta}2-chimerin. J. Biol. Chem. 2005;280(26):24363–24370. doi: 10.1074/jbc.M411629200. [DOI] [PubMed] [Google Scholar]
- 39.Montalvo-Ortiz BL, Castillo-Pichardo L, Hernández E, Humphries-Bickley T, De la Mota-Peynado A, Cubano LA, Vlaar CP, Dharmawardhane S. Characterization of EHop-016 novel small molecule inhibitor of Rac GTPase. J Biol Chem. 2012;287(16):13228–13238. doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, Zhang B. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1 survivin and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9(6):1657–1668. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 41.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 42.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114(Pt 15):2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Wang Q, Wang Y, Chen X, Wang Z. PLC-gamma1 and Rac1 coregulate EGF-induced cytoskeleton remodeling and cell migration. Mol Endocrinol. 2009;23(6):901–913. doi: 10.1210/me.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, Tanchuk VY, Prokopenko VV. Virtual computational chemistry laboratory design and description. J. Comput Aid Mol Des. 2005;19(6):453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- 45.Montero JC, Seoane S, Ocana A, Pandiella A. P-Rex1 participates in Neuregulin-ErbB signaltransduction and its expression correlates with patient outcome in breast cancer. Oncogene. 2011;30:1059–1071. doi: 10.1038/onc.2010.489. [DOI] [PubMed] [Google Scholar]
- 46.Han SSR, Cho CK, Lee YW, Yoo HS. Antimetastatic and immunomodulating effect of water extracts from various mushrooms. J Acupunct Meridian Stud. 2009;2(3):218–227. doi: 10.1016/S2005-2901(09)60058-3. [DOI] [PubMed] [Google Scholar]
- 47.Ma J, Xue Y, Liu W, Yue C, Bi F, Xu J, Zhang J, Li Y, Zhong C, Chen Y. Role of activated rac1/cdc42 in mediating endothelial cell proliferation and tumor angiogenesis in breast cancer. PLoS One. 2013;8(6):e66275. doi: 10.1371/journal.pone.0066275. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Katz E, Sims AH, Sproul D, Caldwell H, Dixon MJ, Meehan RR, Harrison DJ. Targeting of Rac GTPases blocks the spread of intact human breast cancer. Oncotarget. 2012;3(6):608–619. doi: 10.18632/oncotarget.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jordan P, Brazåo R, Boavida MG, Gespach C, Chastre E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene. 1999;18(48):6835–6839. doi: 10.1038/sj.onc.1203233. [DOI] [PubMed] [Google Scholar]
- 50.Radisky D, Levy D.D, Littlepage L.E., Liu H, Nelson C.M., Fata J.E, Leake D., Godden E.L., Albertson D.G, Nieto M.A, Werb, Z., Bissell M.J. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. . Nature. 2005;436(7047):123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ. Rac1b a tumor associated constitutively active Rac1 splice variant promotes cellular transformation. Oncogene. 2004;23(58):9369–9380. doi: 10.1038/sj.onc.1208182. [DOI] [PubMed] [Google Scholar]
- 52.Herter-Sprie GS, Greulich H, Wong KK. Activating mutations in ERBB2 and their impact on diagnostics and treatment. Front. Oncol. 2013;3:86. doi: 10.3389/fonc.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39(8):935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Jhaveri K, Modi S. HSP90 inhibitors for cancer therapy and overcoming drug resistance. Adv. Pharmacol. 2012;65:471–517. doi: 10.1016/B978-0-12-397927-8.00015-4. [DOI] [PubMed] [Google Scholar]
- 55.Aznar S, Fernández-Valerón P, Espina C, Lacal JC. Rho GTPases Potential candidates for anticancer therapy. Cancer Lett. 2004;206(2):181–191. doi: 10.1016/j.canlet.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 56.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy Molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, Hildner K, Bartsch B, Holtmann M, Blumberg R, Walczak H, Iven H, Galle PR, Ahmadian MR, Neurath MF. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Invest. 2003;111(8):1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poppe D, Tiede I, Fritz G, Becker C, Bartsch B, Wirtz S, Strand D, Tanaka S, Galle PR, Bustelo XR, Neurath MF. Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J. Immunol. 2006;176(1):640–651. doi: 10.4049/jimmunol.176.1.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menna PL, Parera RL, Cardama GA, Alonso DF, Gomez DE, Farina HG. Enhanced cytostatic activity of statins in mouse mammary carcinoma cells overexpressing ß2-chimaerin. Mol. Med. Report. 2009;2(1):97–102. doi: 10.3892/mmr_00000068. [DOI] [PubMed] [Google Scholar]
- 60.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA. 2004;101(20):7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferri N, Corsini A, Bottino P, Clerici F, Contini A. Virtual screening approach for the identification of new Rac1 inhibitors. J. Med. Chem. 2009;52(14):4087–4090. doi: 10.1021/jm8015987. [DOI] [PubMed] [Google Scholar]
- 62.Hernández E, De La Mota-Peynado A, Dharmawardhane S, Vlaar CP. Novel inhibitors of Rac1 in metastatic breast cancer. P R Health Sci. J. 2010;29(4):348–356. [PubMed] [Google Scholar]
- 63.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté JF, Blangy A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J. Bone Miner. Res. 2011;26(5):1099–1110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouquier N, Vignal E, Charrasse S, Weill M, Schmidt S, Léonetti JP, Blangy A, Fort P. A cell active chemical GEF inhibitor selectively targets the Trio/RhoG/Rac1 signaling pathway. Chem. Biol. 2009;16(6):657–666. doi: 10.1016/j.chembiol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Blangy A, Bouquier N, Gauthier-Rouvière C, Schmidt S, Debant A, Leonetti JP, Fort P. Identification of TRIO-GEFD1 chemical inhibitors using the yeast exchange assay. Biol. Cell. 2006;98(9):511–522. doi: 10.1042/BC20060023. [DOI] [PubMed] [Google Scholar]
- 66.Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 2007;282(49):35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- 67.Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, Meller J, Seibel W, Mizrahi A, Pick E, Filippi MD, Zheng Y. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chem. Biol. 2012;19(2):228–242. doi: 10.1016/j.chembiol.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, Kraynov E, Popoff I, Christensen JG, Martinez R, Kephart SE, Marakovits J, Karlicek S, Bergqvist S, Smeal T. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc. Natl. Acad. Sci. USA. 2010;107(20):9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pearson HB, Pouliot N, Rahul Jandial, Kent Hunter. Modeling Metastasis In Vivo.In Metastatic Cancer Integrated Organ System and Biological Approach. Landes Bioscience. 2012 [Google Scholar]
- 70.Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. Rac signaling in breast cancer A tale of GEFs and GAPs. Cell Signal. 2012;24(2):353–362. doi: 10.1016/j.cellsig.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.