SUMMARY
Protein modification by ubiquitin (UB) controls diverse cellular processes. UB is conjugated to cellular proteins by sequential transfer through an E1-E2-E3 enzymatic cascade. The cross-activities of 2 E1s, 50 E2s and thousands of E3s encoded by the human genome make it difficult to identify the substrate proteins of a specific E3 enzyme in the cell. One way to solve this problem is to engineer an orthogonal UB transfer (OUT) cascade in which the engineered UB (xUB) is relayed by engineered E1, E2 and E3 enzymes (xE1, xE2, xE3) to modify the substrate proteins of a specific E3. Here, we use phage display and mutagenesis to construct xUB-xE1 and xE1-xE2 pairs that are orthogonal to the native E1 and E2 enzymes. Our work on engineering the UB transfer cascades will enable us to use OUT to map the signal transduction networks mediated by protein ubiquitination.
INTRODUCTION
Ubiquitin (UB) is transferred through an E1-E2-E3 enzymatic cascade to the substrate proteins to regulate their intracellular stability and biological functions (Hershko and Ciechanover, 1998). E1, the UB activating enzyme, activates UB by a two step process whereby UB-AMP conjugate is formed via the condensation reaction between the UB C-terminal carboxylate and a molecule of ATP. Subsequently, the catalytic Cys residue of E1 captures the activated UB with the formation of a thioester bond between the thiol group of Cys and the C-terminal Gly residue of UB. UB in the UB~E1 conjugate (“~” designates the thioester linkage) is then transferred to a catalytic Cys residue of an E2, also known as UB-conjugating enzyme, to form an UB~E2 conjugate. Finally E3 UB ligases of HECT, RING or U-box types bridge the interaction between UB~E2 and substrate proteins in the cell to facilitate UB transfer to the substrate proteins (Figure 1A and S1A) (Pickart, 2001). So far, 2 E1s, 50 E2s and more than 1,000 E3s have been identified in the human genome (Deshaies and Joazeiro, 2009; Wenzel, et al., 2011). It has been shown that the two human E1 enzymes, Ube1 and Uba6, have overlapping yet distinct activities in transferring UB to various E2s (Jin, et al., 2007). Each E2 interacts with multiple E3s and each E3 can recognize multiple substrate proteins for UB modification (Deshaies and Joazeiro, 2009; Wenzel, et al., 2011). Furthermore multiple E2s can pair with the same E3 and different E2-E3 pairs may attach UB chains of different lengths and topologies to the substrate proteins. Together the E1, E2 and E3 enzymes assemble a complex network of UB transfer pathways for the modification of cellular proteins (Figure S1A).
Figure 1.
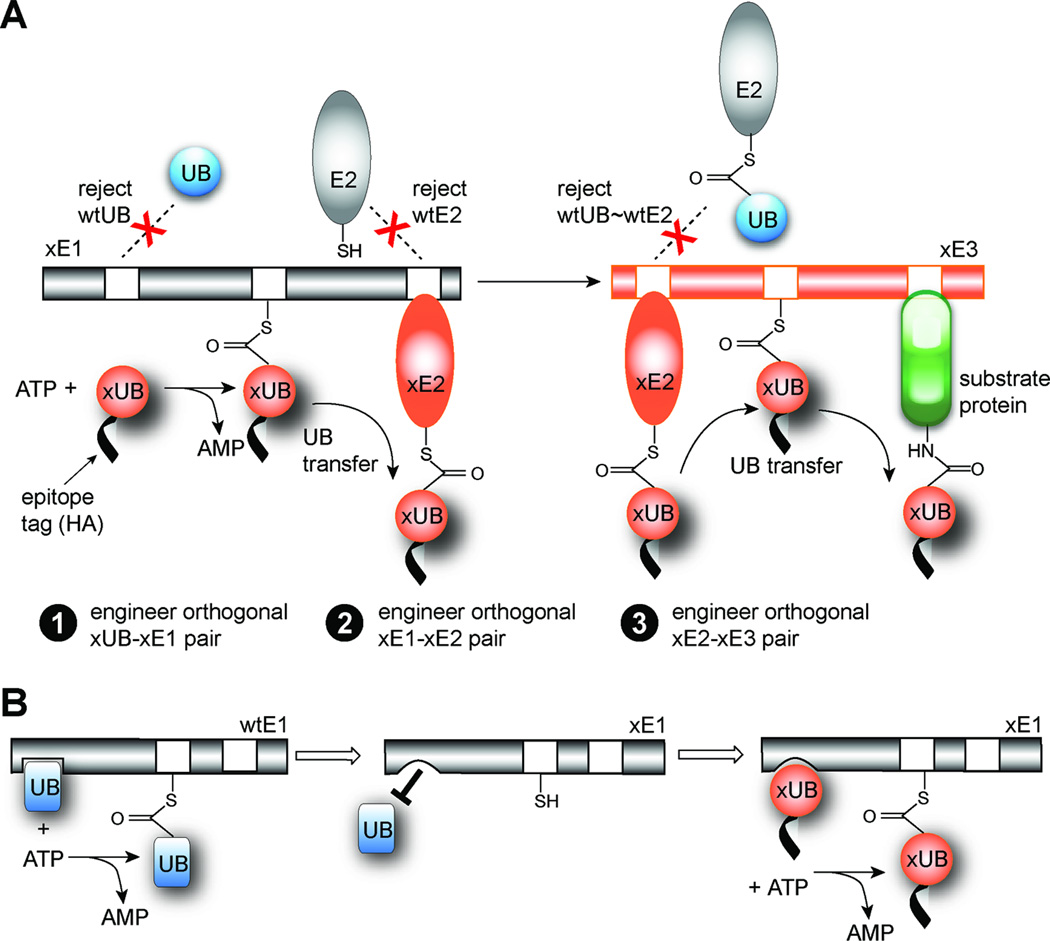
The orthogonal UB transfer (OUT) cascade. (A) Engineering xUB-xE1, xE1-xE2 and xE2-xE3 pairs for OUT. (B) Bump-and-hole strategy to create specific interactions between xUB and xE1.
Biochemical mechanisms of UB activation by E1 and transfer through the E1-E2-E3 cascade have been relatively well established (Pickart, 2001). However, interactions between E2 and E3 enzymes, and between E3 and their substrate proteins have been more difficult to study given the substantial cross-reactivities among them. Therefore, the biological functions of individual E2 and E3 enzymes in regulating protein ubiquitination and degradation are yet to be clearly defined.
We propose to untangle the complexity of protein ubiquitination networks in the cell by creating an orthogonal UB transfer pathway that is composed of engineered E1, E2, E3 enzymes (xE1, xE2 and xE3) (Figure 1A and S1B). The xE1-xE2-xE3 cascade would share no cross-reactivity with the native enzymes and enable the transfer of an affinity-tagged UB mutant (xUB) to engineered xE2 and xE3 enzymes and eventually to the substrate proteins of xE3. By identifying proteins conjugated to xUB, the modification targets of a specific xE3 in the cell can be elucidated. We refer to this method as “orthogonal UB transfer (OUT)”.
Engineering the OUT cascade requires three steps (Figure 1A). The first step requires the generation of an xUB-xE1 pair that can only activate xUB to form the xUB~xE1 conjugate and allow xUB entry into the OUT cascade without activating wild type UB (wtUB). In the second step, an xE1-xE2 pair that exclusively transfers xUB from xE1 to an engineered xE2 without transferring xUB to any of the wtE2s in the cell, is needed. The third and final step uses an xE2-xE3 pair to transfer xUB to the substrate proteins of xE3, using xE2 that only binds to the engineered xE3 but not to any wtE3 to ensure the exclusive transfer of xUB to the substrate proteins of xE3 in the cell (Figure 1A). Of a final note, since xUB is fused to an affinity tag such as HA or FLAG, the ubiquitination targets of the xE3 enzymes can be enriched by affinity purification and then identified by mass spectrometry.
Here we report that we have successfully engineered specific xUB-xE1 and xE1-xE2 pairs as the first two steps to implement the OUT cascade. We first generated E1 mutants with mutations in the adenylation domain (xE1(A)) that do not bind wtUB. We then used a combination of phage selection and site-directed mutagenesis to introduce complementary mutations into UB to restore the binding between xUB and xE1(A). This enables the activation of xUB by xE1(A) for the transfer of xUB through the OUT cascade, and results in xUB-xE1(A) pair fully orthogonal to the native UB-E1 pair.
In the next step, we engineered specific xE1-xE2 pairs using a similar strategy. We first prepared xE1(UFD) by introducing mutations into the ubiquitin fold domain (UFD) domain of E1 (Lee and Schindelin, 2008) to block its interaction with the native E2s, followed by phage selection to identify compatible mutations in E2 that restore interaction with xE1(UFD). We also combined the mutations in xE1(A) and xE1(UFD) to create an xE1 that can transfer xUB to a specific xE2 but not to native E2s. Additionally, xE2 only takes xUB from the xE1 but does not accept wtUB from a wtE1, ensuring exclusive transfer of xUB in the xE1-xE2 relay (Figure 1A). We also identified key sites in the H1 helix motif of E2 that are important for E1 recognition and showed that we can assemble xE1-xE2 pairs with different E2s by replacing the H1 helix in the native E2 with an H1 helix mutant. The creation of orthogonal UB transfer pathways through engineered xE1 and xE2 enzymes paves the way for us to implement an OUT cascade in the cell to profile the substrate specificities of E3s.
RESULTS
Structure – based design of the UB-E1 interface
Following the “bump-and-hole” strategy (Alaimo, et al., 2001; Hwang and Miller, 1987) to engineer UB-E1 interface, we first introduced mutations into the adenylation domain of E1 to create a mutant that does not bind and activate wtUB (Figure 1B). Our design of E1 mutations was based on the crystal structure of Uba1, the yeast E1 enzyme, with an UB molecule bound noncovalently to the adenylation domain of Uba1 (Figure S2A) (Lee and Schindelin, 2008). The crystal structure suggests that the C-terminal residues of UB with the sequence 71LRLRGG76 make extensive contacts with the Uba1 active site. Among them Arg72 plays a key role in binding to E1 since its side chain docks in a negatively charged pocket in the Uba1 adenylation domain (Figure S2B). The binding pocket is composed of the side chains of Gln576, Asp591 and the backbone carbonyl groups of Tyr586 and Ser589 of Uba1. Besides Arg72, Arg74 is another positively charged residue at the UB C-terminus and it makes electrostatic interactions with Glu594 of Uba1.
To create unfavorable interactions between mutant E1 and wtUB, we generated three Uba1 mutants, A1, A2 and A3 (Table 1). A1 and A2 are triple mutants with Gln576 and Glu594 both mutated to Arg, and Asp591 mutated to either Arg or Asn. The A3 mutant leaves Gln576 intact but it has Asp591 mutated to Asn and Glu594 mutated to Arg. We found by ATP-PPi exchange assay (Haas and Rose, 1982) that neither A1 nor A2 is able to activate wtUB (Figure S3A). However, A3 can still activate wtUB at a rate slightly lower than wtUba1. We attribute the difference in activity to the strong repulsive interaction between Arg72 of wtUB and the Arg residue replacing Gln576 in A1 and A2, which is absent from A3 (Figure S2B). We also tested the formation of UB~E1 thioester conjugates by Western blot. We found that wtUB with an N-terminal HA tag (HA-UB) cannot form UB~E1 conjugates with A1 or A2, while it can with A3 (Figure S3B). Based on the orthogonality of A1 with wtUB, we decided to use the A1 mutant to select for compatible mutations in UB to engineer the xUB-xE1 pair.
Table 1.
Mutants of Uba1 and UB in this study.
| UB mutants | |
| UB8 | R72E |
| UB9 | R42D, R72E |
| UB10 (xUB) | R42E, R72E |
| E1 (Uba1) mutants | |
| xE1 (A) with mutations in the adenylation (A) domain | |
| A1 | Q576R, D591R, E594R |
| A2 | Q576R, D591N, E594R |
| A3 | D591N, E594R |
| A4 | Q576R, S589R |
| A5 | Q576R, S589R, D591R |
| A6 | Q576R, D591R |
| xE1 (UFD) with mutations in the ubiquitin fold (UFD) domain | |
| A7 | E1004K, D1014K, E1016K |
| xE1 with combined mutations in the A and UFD domains | |
| A8 | Q576R, S589R, E1004K, D1014K, E1016K |
| A9 | Q576R, S589R, D591R, E1004K, D1014K, E1016K |
| A10 | Q576R, D591R, E1004K, D1014K, E1016K |
| E2 mutants with swapped H1 helix with Ubc1 mutants | |
| C1-UbcH5a | 1MSRAEDIMEQIL12 replacing H1 helix of UbcH5a |
| C9-UbcH5a | 1MSRADEIMEQIL12 replacing H1 helix of UbcH5a |
| C2-UbcH7 | 1MSRADDIMDQIH12 replacing H1 helix of UbcH7 |
| C9-UbcH7 | 1MSRADEIMEQIL12 replacing H1 helix of UbcH7 |
Phage display to engineer UB-E1 interaction
To create UB mutants with restored binding to A1, we constructed a library of UB variants with randomized C-terminal sequences and used phage display to select for UB mutants that are reactive with A1 (Figure S4A). To construct the UB library for phage selection, we randomized the C-terminal 71LRLRG75 sequence of UB since these residues may interact with the mutated Gln576, Asp591 and Glu594 side chains in A1 (Figure S2B). We amplified the UB gene with primers Jun13 and Bo74 by the polymerase chain reaction (PCR) and cloned the amplified PCR fragments into the pJF3H phagemid (Figure S4B) (Barbas, et al., 2000; Crameri and Suter, 1993). The Bo74 primer had NNK codons (N, any of A, T, G or C; and K, either G or T) encoding residues 71–75 of UB (Table S1). The final diversity of the library was approximately 1 × 108, large enough to cover all the UB mutants with five randomized residues at their C-termini. We confirmed the display of UB on phage surface with an anti-HA antibody in ELISA and Western blot assays (Kay, et al., 1996) (Figure S4C and S4D).
To carry out phage selection, we first expressed the Uba1-A1 mutant as a fusion to the peptidyl carrier protein (PCP) (Figure S4A) (Yin, et al., 2006). We then used the Sfp phosphopantetheinyl transferase to catalyze the transfer of a biotin phosphopantetheinyl (Ppant) group from biotin CoA to a Ser residue in PCP. Biotin conjugated PCP-A1 fusion was then immobilized on a plate coated with streptavidin. We added the phage displayed UB library and ATP to the plate to initiate UB activation by A1. Once UB variants displayed on the phage surface were activated and formed a thioester linkage with A1, the corresponding phage particles were covalently bound to the streptavidin surface. After washing the plate, the bound phage were eluted with dithiothreitol (DTT) that cleaved the thioester bond in the UB~A1 complex. We set up model selections to show that one round of phage selection enriched UB phage from a mixture of phage displaying a SV5V virus protein (Ulane and Horvath, 2002) by more than 500-fold (Figure S4E–G). These results demonstrated that phage display can be used to select for A1-reactive UB mutants with high efficiency.
Phage selection of the UB library
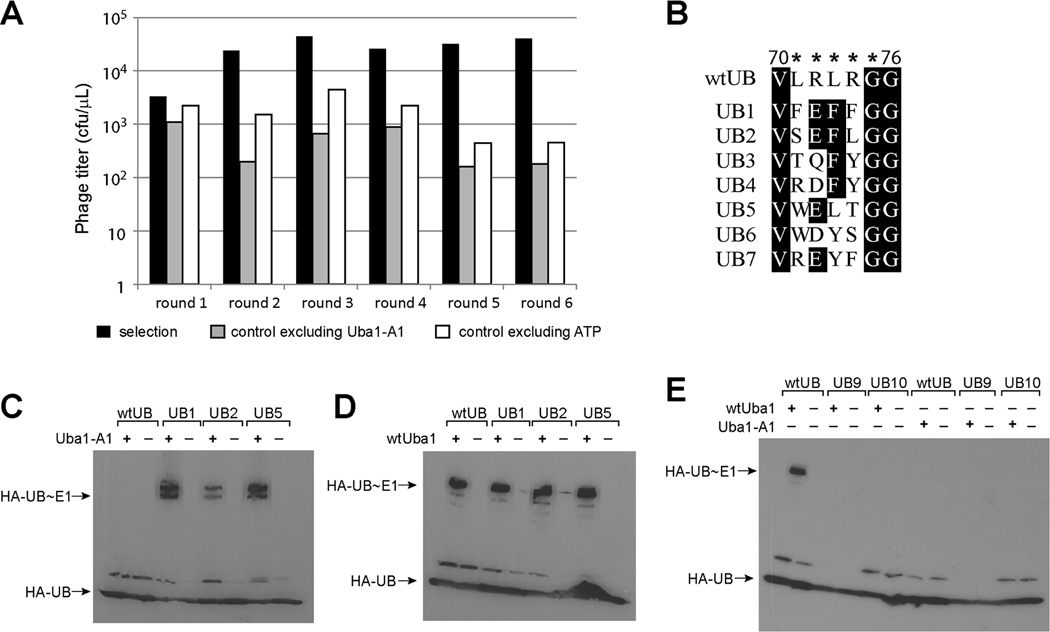
We then carried out iterative rounds of phage selection of the UB library. In each round, the number of phage particles used in the selection, the amount of PCP-A1 fusion coated on the streptavidin plate and the reaction time were decreased to make the selection more stringent. In parallel with the selection, we also set up controls in which either ATP or PCP-A1 was excluded from the selection reaction. We observed a steady increase of the phage enrichment from the selection reaction over the controls (Figure 2A). After the sixth round of selection we saw a greater than 500-fold enrichment of the UB displayed phage over the controls in which no PCP-A1 was bound to the plate for UB conjugation. This is a strong indication that phage were selected to bind to the streptavidin plate based on the catalytic formation of UB~A1 thioester conjugates. We sequenced 40 phage clones from the sixth round of selection. The alignment of the C-terminal sequences of the selected phage clones is shown in Figure 2B, with 71FEFFGG76 sequence found in UB1 clone being the most dominant in the selected pool. UB1 appeared 14 times out of 40 sequenced clones and others, such as UB2 and UB5, also appeared multiple times.
Figure 2.
Phage selection of catalytically active UB mutants with A1. (A) Phage titer of each round of selection. Phage selection of the UB library was performed in parallel to the controls in which either A1 or ATP was excluded from the reaction. Numbers of the eluted phage from the streptavidin plate for the selection and control reactions are plotted to a logarithmic scale. cfu, colony forming unit. (B) Alignment of the C-terminal sequences of the selected UB mutants after the sixth round of selection. Stars denote the C-terminal sequence of UB (71LRLRG75) that was randomized in the phage library. Gly76 is the last residue at the UB C-terminus. (C)-(D), Western blot analysis of the reactivities of UB mutants with A1 and wtUba1. (C) A1 cannot activate wtUB but can activate phage selected UB mutants UB1, UB2 and UB5 to form thioester conjugates. (D) wtUba1 is reactive with wtUB, UB1, UB2 and UB5. (E) UB mutants UB9 and UB10 are not reactive with either wtUba1 or Uba1-A1.
Alignment of the selected clones reveals a clear pattern of the C-terminal sequences that can be activated by A1. Most clones have a negatively charged residue at position 72 replacing the wt Arg, likely due to the need to compensate for Gln576Arg mutation in A1. To our surprise, positions 71, 73 and 74 of the UB mutants prefer bulky aromatic side chains such as Phe, Tyr or Trp, quite different from the Leu or Arg residues found in wtUB (Figure 3B). To examine this further, we use a model of UB1 binding to A1 based on the crystal structure of the UB-Uba1 complex (Lee and Schindelin, 2008) and show that the mutated residues in UB1 and A1 can be accommodated without significant adjustments of the peptide back bones of either UB or Uba1 (Figure S2C). In the resulting model, Glu72 in UB1 is engaged in electrostatic interactions with the side chains of Arg576 and Arg594 that were introduced into A1 by mutagenesis. The three Phe residues flanking Glu72 in UB1, though larger in size then the Leu or Arg residues in wtUB, do not exhibit any steric clashes with the residues of A1 at the UB binding site. Instead the modeled complex suggested that the Arg74Phe mutation of UB1 may be a good match with the Glu594Arg mutation in A1 (Figure S2C). As shown in the modeled structure, the Arg74Phe mutation in UB1 not only abolishes the electrostatic repulsion between Arg74 of wtUB and Arg594 of A1, but it also positions a Phe residue at the UB C-terminus that could potentially stabilize the positively charged Arg594 residue of A1 by cation-π interactions.
Figure 3.
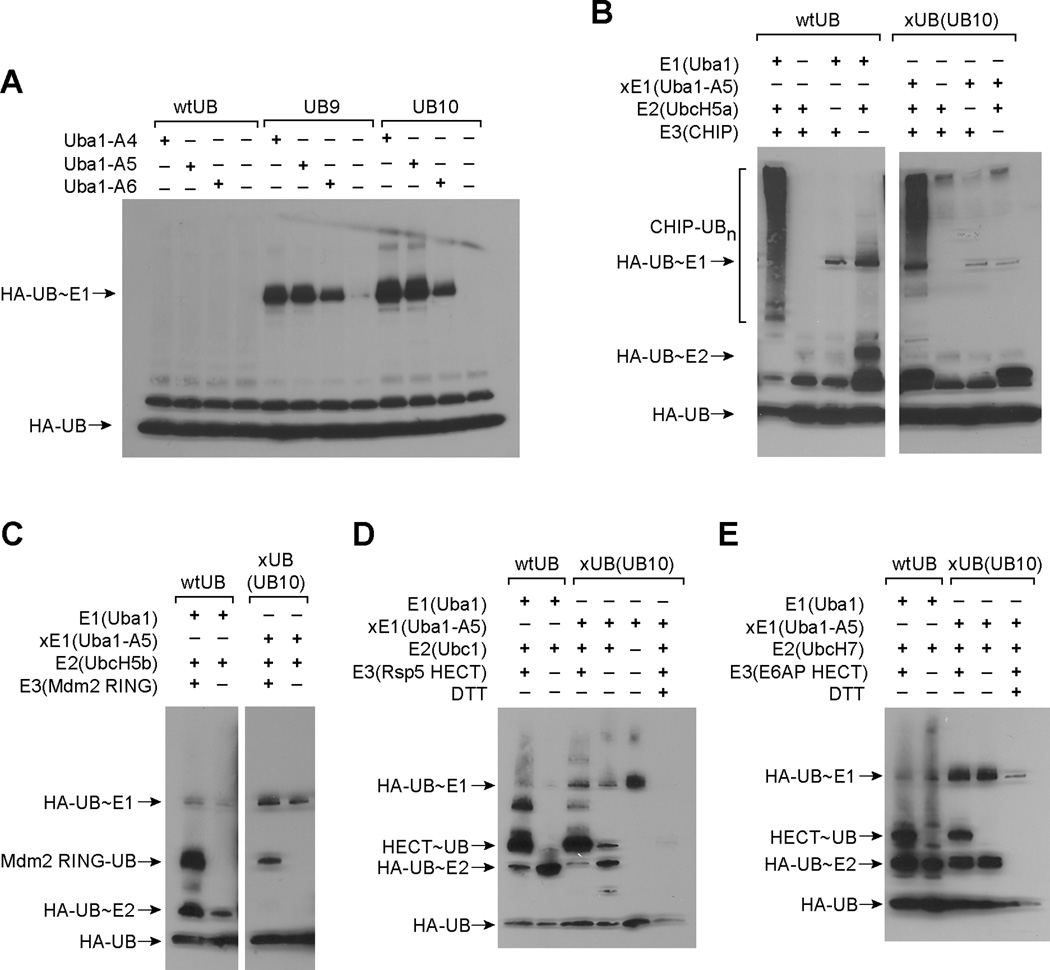
Reactivity of the xUB-xE1(A) pair assembled by UB mutant UB10 and Uba1 mutant A5. (A) UB mutants UB9 and UB10 can be activated by Uba1 mutants A4, A5 and A6 to form UB~E1 conjugates. (B) Polyubiquitination of CHIP using UB10 as the xUB, Uba1-A5 as xE1 and UbcH5a as E2. wtUB transfer to CHIP by wtUba1 and UbcH5a was used as a control. (C) xUB (UB10) transfer to the RING domain of Mdm2 catalyzed by xE1 (Uba1-A5) and UbcH5b. (D) Formation of UB~HECT thioester conjugate between xUB (UB10) and the HECT domain of Rsp5. DTT at 150 mM was added to the xUB transfer reaction in the last lane to cleave the thioester bond of the UB~HECT conjugate. (E) Formation of UB~HECT thioester conjugate between xUB (UB10) and the HECT domain of E6AP. DTT at 150 mM was also added to the xUB transfer reaction in the last lane.
We expressed UB mutants corresponding to the selected phage clones UB1, UB2 and UB5. We found that the UB mutants can all be efficiently activated by A1 as shown by the ATP-PPi exchange assay (Figure S5A). The same mutants can also be activated by the wtUba1 based on ATP-PPi exchange although at a rate about half of the wtUB (Figure S5B). We also found by Western blot that the UB clones UB1, UB2 and UB5 can form thioester conjugates with both A1 and wtUba1 (Figure 2C and 2D). In contrast wtUB cannot form covalent intermediates with A1 (Figure 2C). These results suggest that phage selection did enrich UB mutants that are reactive with A1. However these UB mutants still have residual activity with wtUba1. This notion is confirmed by characterizing the kinetics of UB1 activation by A1 and wtUba1 based on ATP-PPi exchange (Haas and Rose, 1982) (Table 2). We found that the catalytic efficiency (kcat/K1/2) of the UB1-A1 pair (19 µM−1min−1) is similar to that of the wtUB-wtUba1 pair (35 µM−1min−1). However, the kcat/K1/2 of UB1 with wtUba1 (1.9 µM−1min−1) is about one tenth of the UB1-A1 pair largely due to the increase in K1/2 of the UB1 mutant with wtUba1. Still based on the ATP-PPi exchange data, the UB1 mutant can be efficiently activated by the wtUba1. To test the specific role of Arg72Glu mutation, we generated an Arg72Glu UB mutant (UB8) and tested its activity. We found that UB8 is activated by both wtUba1 and A1 at high efficiency to form UB~E1 conjugates (Table 1, Figure S5C and S5D). Thus additional mutations are needed to completely eliminate UB8 interaction with wtUba1 in order to generate an xUB-xE1 pair that is orthogonal to the native UB transfer cascades.
Table 2.
Kinetics of UB activation by wtUba1 and Uba1 mutants measured by ATP-PPi exchange assay.
| K1/2 (µM) |
kcat (min−1) |
kcat/K1/2 (µM−1 min−1) |
K1/2 (µM) |
kcat (min−1) |
kcat/K1/2 (µM−1 min−1) |
||
|---|---|---|---|---|---|---|---|
| UB1 – wtUba1 | 8.2 ± 0.2 | 15 ± 3 | 1.9 | UB1 – Uab1-A1 | 2.0 ± 0.4 | 38 ± 7 | 19 |
| UB8 – wtUba1 | – | – | 0.075 | UB8 – Uba1-A5 | 1.4 ± 0.3 | 31 ± 2 | 22 |
| Cross reactivity of UB10 – Uba1-A5 with wtUB-wtUba1 pair | |||||||
| UB10 – wtUba1 | – | – | 0.012 | UB10 – Uba1-A5 | 4.4 ± 0.5 | 70 ± 9 | 16 |
| wtUB – wtUba1 | 1.4 ± 0.1 | 50 ± 5 | 35 | wtUB – Uba1-A5 | – | – | 0.011 |
Site-directed mutagenesis to create an xUB-xE1 pair that is orthogonal to the native UB-E1 pair
We identified another critical binding interface between UB and Uba1 that involves hydrogen bonding and electrostatic interactions between Arg42 of UB and Ser589 and Asp591 of Uba1 (Figure S2B). We thus introduced additional mutations to UB8 by changing Arg42 to Asp or Glu (mutants UB9 and UB10, Table 1). The ATP-PPi exchange assay showed that they cannot be activated by wtUba1 to any significant level (Figure S5E), and neither can they form thioester conjugates with wtUba1 (Figure 2E). Thus, charge reversed double mutations of UB at the sites of Arg42 and Arg72 disturb the interactions between UB and wtUba1 so that wtUba1 can no longer activate the UB mutants. We also found the A1 cannot activate UB9 and UB10 to form UB~A1 conjugates (Figure 2E). We thus needed to introduce complementary mutations into Uba1 to restore its interaction with the UB mutants. Based on the crystal structure of UB~Uba1 complex, we decided to test two double mutants, A4 (Gln576Arg/Ser589Arg), and A6 (Gln576Arg/Asp591Arg), and a triple mutant A5 (Gln576Arg/Ser589Arg/Asp591Arg) (Table 1).
Western blot of the UB conjugation reaction with the Uba1 mutants showed that A4, A5 and A6 can catalyze the formation of UB~E1 conjugates with UB9 and UB10 (Figure 3A). Based on the Western blot, A4 and A5 catalyze the formation of UB~E1 conjugates more efficiently than A6. Since both A4 and A5 contain Ser589Arg mutation, but A6 does not, it seems that an Arg at position 589 is the key to establish interactions with an Asp or Glu side chain in the UB mutants. None of A4, A5 or A6 can activate wtUB for the formation of UB~E1 conjugates (Figure 3A). We also used UB mutant UB10 and Uba1 mutant A5 as a specific pair to characterize the kinetics of UB activation by ATP-PPi exchange. As shown in Table 2, UB10 is activated by A5 with a kcat/K1/2 of 16 µM−1min−1, matching the corresponding values of the wtUB-wtE1 pair with a kcat/K1/2 of 35 µM−1min−1. In contrast, the crossover pairs UB10-wtE1 and wtUB-A5 have very low activity of ATP-PPi exchange. These crossover pairs have a kcat/K1/2 more than 1,000-fold lower than that of the wt pair or the engineered pair (Table 2). These results clearly demonstrate the orthogonality between the UB10-A5 pair and the wtUB-wtUba1 pair. We also measured the activation kinetics of UB8 by A5 and wtUba1. Based on ATP-PPi exchange, we found that UB8 can be activated by A5 at a kcat/K1/2 of 22 µM−1min−1, approaching that of the wtUB-wtUba1 pair (35 µM−1min−1) (Table 2). The kcat/K1/2 of UB8 with the wtUba1 is 0.075 µM−1min−1, much smaller than that of UB8 activation by A5. The drastically different reactivity of UB8 with wtUba1 and A5 suggests that the Arg72Glu mutation in UB8 disrupts the binding of UB8 with wtUba1 but this mutation is compensated by the Gln576Arg mutation in A5 to afford high activity of UB8 with A5. Interestingly the kcat/K1/2 of the UB8 – wtUba1 pair (0.075 µM−1min−1) is 6-fold higher than the UB10 – wtUba1 pair (0.012 µM−1min−1) (Table 2). This suggests that the additional Arg42Glu mutation in UB10 further decreased the activity of the UB mutant with wtUba1.
We then used UB10 and A5 to assemble an xUB-xE1 pair and assay if they can transfer xUB to E2 and E3 enzymes such as UbcH5a and CHIP. CHIP is an U-box dependent E3 enzyme (Jiang, et al., 2001) that can be auto-ubiquitinated by uptaking UB from E2 enzyme UbcH5a. We tested if the UB10-A5 pair can support CHIP auto-ubiquitination by transferring UB10 to UbcH5a and then to CHIP. As shown in Figure 3B, when CHIP was incubated with HA-UB10, A5 and UbcH5a, intense ubiquitination of CHIP was detected on the Western blot with an anti-HA antibody. The smear of HA-UB10 conjugated species on the Western blot suggests the modification of CHIP with multiple copies of UB10. The pattern of CHIP polyubiquitination with UB10 and A5 is similar to CHIP polyubiquitination with wtUB and wtE1 (Figure 3B). This demonstrates that the UB10-A5 pair can function as well as the wtUB-E1 pair in supporting UB transfer to E2 and to E3 enzymes. We also obtained similar results when UB10 and A5 were used with UbcH5b as E2 and the RING domain of Mdm2 as E3 (Figure 3C). To test the transfer of UB10 to the HECT E3s, the HECT domains of Rsp5 and E6AP (Huang, et al., 1999; Huibregtse, et al., 1997) were reacted with UB10, A5 and E2 enzymes Ubc1 or UbcH7 for the formation of UB10~HECT thioester conjugates (Figure 3D and 3E). We found that UB10 can be efficiently transferred to the HECT domains through the relay of A5 and the E2s just as the transfer of wtUB mediated by wtUba1 and the E2s. We further confirmed the formation of thioester linkage in the UB10~HECT conjugate by treating the reaction mixture with 150 mM DTT prior to SDS polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. As expected, the UB transfer reactions with DTT treatment showed no UB~HECT conjugates on the Western blot. This suggests that UB10 was transferred by A5 and the E2 enzymes to the HECT domains of Rsp5 and E6AP to form thioester conjugates. We have thus demonstrated that UB10 and A5 can be used as an xUB-xE1(A) pair for the construction of the OUT cascade.
Structure-based design of the E1-E2 interface for the creation of an xE1-xE2 pair
Following our success to engineer UB binding site of Uba1 to generate orthogonal xUB-xE1 pairs, we continued to engineer the E2 binding site of Uba1 to create orthogonal xE1-xE2 pairs. Again we relied on structural information to guide the design of xE1-xE2 interface (Figure S2A and S2D). There is no crystal structure of an UB E1 in complex with an E2 enzyme, however, the crystal structure of the Nedd8 activating E1 (NAE) has been solved in a complex with an E2 enzyme Ubc12 and the Nedd8 protein (Huang, et al., 2007) and given the similarities between Nedd8 and NAE with UB and Uba1 respectively, the NAE-Ubc12-Nedd8 complex structure can be used to guide our design. In NAE-Ubc12-Nedd8 complex Ubc12 binds to Uba3 through direct interactions between the N-terminal H1 helix of Ubc12 and the C-terminal UFD domain of Uba3 (Huang, et al., 2007). Given the structural homology between NAE and Uba1 (Schulman and Harper, 2009) and the modeling study on Uba1 binding to a yeast E2 Ubc1 (Lee and Schindelin, 2008), we expected H1 helix of E2 to be a key contributor to E1 binding.
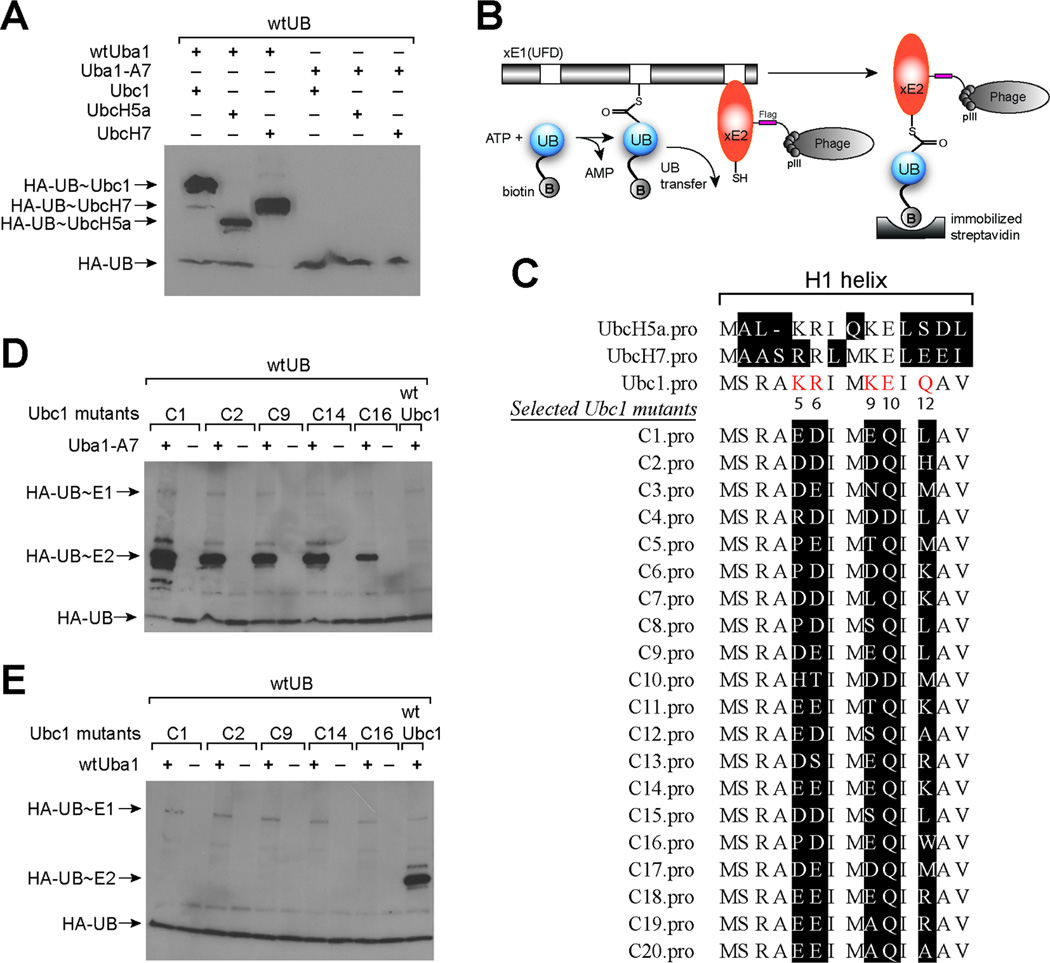
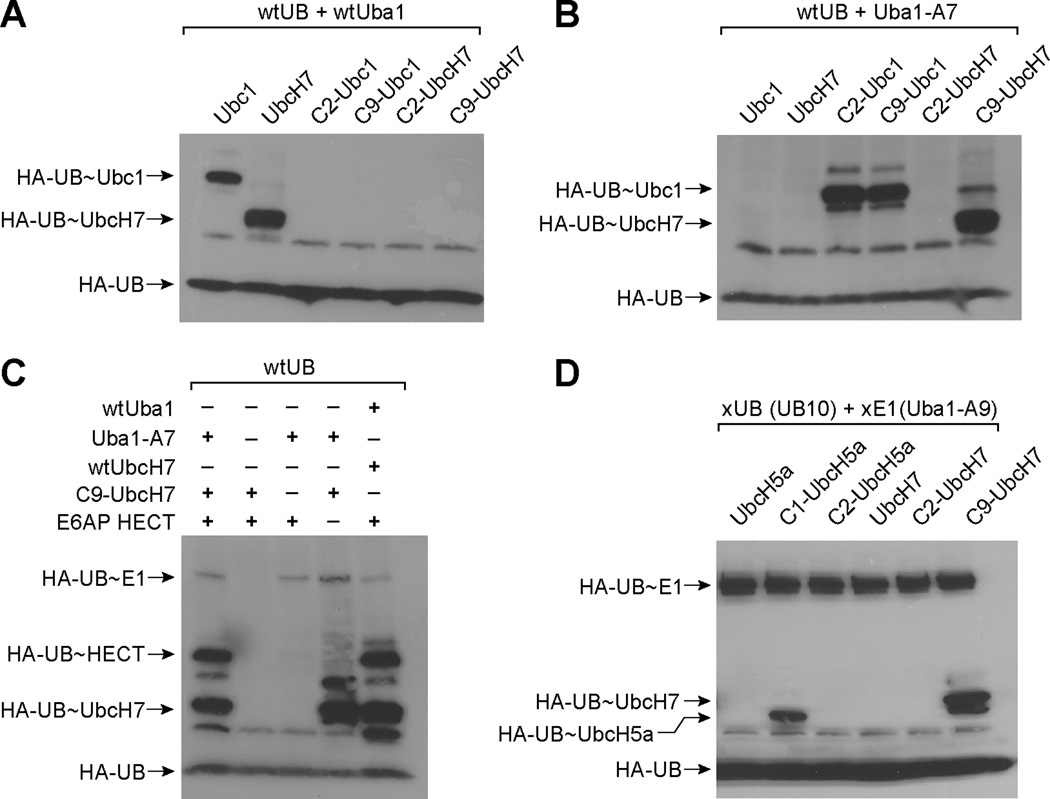
Previous work has also established that Uba1 and Ubc1 bind to each other through electrostatic interactions between three acidic residues (Glu1004, Asp1014 and Glu1016) of the UFD domain of Uba1 and a number of basic residues (Lys5, Arg6, Lys9, and Glu10) in the H1 helix of Ubc1 (Figure S2D). Mutations at these residues that reversed the charges of the side chains (Lys to Glu, or Glu and Asp to Lys) significantly reduced UB transfer from Uba1 to Ubc1 (Lee and Schindelin, 2008). The double mutations Lys5Glu and Lys9Glu in the H1 helix of Ubc1 were shown to block the formation of UB~E2 conjugates (Lee and Schindelin, 2008). These studies identified a “hotspot” on Uba1 that is composed of Glu1004, Asp1014 and Glu1016 for E2 binding. To acquire an Uba1 mutant that rejects native E2s, we constructed an A7 mutant of Uba1 with Glu1004, Asp1014 and Glu1016 of Uba1 replaced by Lys following the previous report (Lee and Schindelin, 2008) (Table 1). We found that A7 can no longer load UB onto native E2s such as Ubc1, UbcH5a and UbcH7 (Figure 4A). Since the mutations of A7 are in the UFD domain of Uba1, quite distant from the UB binding site of the E1 enzyme, we confirmed by ATP-PPi exchange and Western blot that A7 functions as wtUba1 in UB activation and formation of UB~E1 conjugate. We thus decided to use A7 as the xE1(UFD) for the engineering of the xE1-xE2 pair.
Figure 4.
Engineering UB transfer from E1 to E2. (A) UB transfer from wtUba1 and A7 to wild type Ubc1, UbcH5a and UbcH7. (B) The phage selection scheme for the engineering of orthogonal xE1-xE2 pairs. UB is labeled with biotin and it can be transferred to E2 variants displayed on phage surface by the E1 mutant xE1(UFD). Phage displaying E2 variants conjugated to biotin-UB are selected by binding to the streptavidin surface. (C) Alignment of phage selected Ubc1 mutants with wt Ubc1, UbcH5a and UbcH7. Randomized residues in the H1 helix of the Ubc1 library are colored in red. (D) UB transfer from A7 to the Ubc1 mutants C1, C2, C9, C14 and C16 from phage selection. (E) UB transfer from wtUba1 to the Ubc1 mutants from phage selection.
Phage selection of catalytically active E1-E2 pairs
Next we engineered Ubc1 with complementary mutations so that it can specifically interact with A7. For this purpose we constructed an Ubc1 library with randomized residues at Lys5, Arg6, Lys9, Glu10 and Gln12, which are all on the same side of H1 helix, facing the UFD domain of A7. We then used phage display to select Ubc1 mutants with restored binding and reactivity with A7.
We developed a phage display method to engineer an E2 enzyme based on the formation of the UB~E2 thioester conjugate catalyzed by E1 (Figure 4B). In this method we displayed the E2 enzyme on phage with the pCom3H phagemid (Barbas, et al., 2000). We also prepared biotin conjugated UB by expressing UB with a 11-residue ybbR tag fused to its N-terminus (Yin, et al., 2006). We then used the Sfp enzyme to covalently attach biotin to a Ser residue in the ybbR tag through a phosphopantetheinyl linker. To use phage display to select for E2 variants that could uptake UB from A7, we combined ATP, biotin conjugated UB (biotin-UB), and A7 with phage displayed E2 library. In the reaction mixture, biotin-UB was first activated by A7 to form a biotin-UB ~A7 thioester (Figure 4B). The activated biotin-UB was then transferred to phage displayed E2 variants that could bind to A7. Through this process, phage particles displaying E2 variants that were reactive with A7 were labeled with biotin due to the formation of biotin-UB~E2 conjugates. These phage particles were selected by binding to a streptavidin surface. After washing to remove phage not labeled with biotin, the bound phage were eluted from the streptavidin surface with 10 mM dithiothreitol (DTT) that cleaved the thioester bond between biotin-UB and E2. Eluted phage were amplified for the subsequent round of reaction with biotin-UB and A7. By model selection, we confirmed that one round of selection was able to enrich Ubc1 phage from a mixture with phage displaying the EntE protein (Ehmann, et al., 2000) by more than 100-fold (Figure S6C). We then carried out iterative rounds of selection of the E2 library to identify variants that could pair with A7.
Phage selection of an Ubc1 library to identify xE2
Through rounds of selection, we observed a steady increase in phage enrichment comparing to the controls in which either A7 or biotin-UB was excluded from the reaction (Figure S6D). At the end of the eighth round of selection, the phage enrichment of the selection reaction over the controls was more than 1,000 fold. This was a strong indication that the library had converged to a pool of E2 clones that are catalytically active with A7. We sequenced phage clones after the eighth round of selection. The alignment of the H1 helix region of the selected Ubc1 clones is shown in Figure 4C. The most evident feature of the selected clones is that most clones have negatively charged Asp or Glu residues replacing positively charged Lys5, Arg6 and Lys9 in wtUbc1. Furthermore almost all selected Ubc1 clones have a neutral side chain (Gln residue) instead of Glu9 in wtUbc1. The residues at Gln12 is less converged but still shows a bias for either hydrophobic residues (Leu or Met), or positively charged residues such as Lys and Arg.
Phage selected Ubc1 mutants can function as xE2 to pair with A7
To verify the reactivity of phage selected Ubc1 mutants with A7, we expressed the Uba1 mutants C1, C2, C9, C14 and C16 that appeared multiple times among the clones being sequenced and assayed if they could form E2~UB conjugates catalyzed by A7. In the assay we reacted HA tagged UB (HA-UB) and A7 with Ubc1 mutants in the presence of ATP. The reaction mixtures were analyzed by SDS-PAGE under non-reducing conditions to preserve the thioester linkage between UB and the E1 and E2 enzymes. We then probed the Western blot of the PAGE gel with a mouse anti-HA antibody and a goat anti-mouse antibody conjugated to horseradish peroxidase (HRP). As shown in Figure 4D, HA-UB can be transferred by A7 to all the Ubc1 mutants selected by phage display as demonstrated by the formation of UB~Ubc1 conjugates. In contrast, A7 cannot transfer biotin-UB to wtUbc1 at a noticeable level. We also tested the reactivity of wtUba1 with the Ubc1 mutants and found that wtUba1 cannot load HA-UB onto the selected Ubc1 mutants (Figure 4E). These results demonstrate that UB can be exclusively transferred by A7 to the Ubc1 mutants. Thus, the Ubc1 mutants identified in phage selection can be used as xE2s to pair with A7 for the assembly of xE1-xE2 pairs.
The A7-xE2 pair can transfer wtUB to E3 enzymes
Next, we tested if Ubc1 mutants from phage selection can transfer UB to a target E3, the HECT domain of E6AP, in the presence of A7. From the crystal structure of the E2 enzyme UbcH7 in complex with the HECT domain of E6AP (Huang, et al., 1999), and published mutational analysis (Eletr and Kuhlman, 2007; Huang, et al., 1999), it can be seen that the N-terminal H1 helix of UbcH7, and especially residues Arg5, Arg6 and Lys9 that correspond to Lys5, Arg6 and Lys9 in the wtUbc1, contribute to UbcH7-HECT interaction (Figure S7A). Given that in the Ubc1 mutants from phage selection these residues are most often replaced by Asp and Glu, we were interested in assaying if the mutant Ubc1 selected for interacting with A7 could still support UB transfer to the HECT domain of E6AP. Additionally, UbcH7 and Ubc1 share some additional features, like Phe63, which are thought to be important for HECT domain binding and thus, wtUbc1 should be able to interact with E6AP and support UB transfer. Indeed we found that wtUba1-wtUbc1 pair can transfer UB to the HECT domain of E6AP and the efficiency of UB transfer is similar to that of the wtUba1-wtUbcH7 pair (Figure S7B). We then tested the transfer of wtUB from A7 to the E6AP HECT domain through Ubc1 mutants from phage selection. We observed significant transfer of wtUB to the E6AP HECT domain through A7 and phage selected Ubc1 mutants C2, C9 and C14 as shown by the formation of wtUB~HECT conjugates (Figure S7C). This suggests that the Ubc1 mutants with charge-reversed mutations in the H1 helix can still interact with the HECT domain of an E3 enzyme to support UB transfer in the E1-E2-E3 cascade. Interestingly when we used the mismatched wtE1-mutant Ubc1 pair to transfer UB to the E6AP HECT, we could not see any formation of the UB~HECT conjugate (Figure S7D). These results demonstrate that the Ubc1 mutants from the phage selection are orthogonal to the native E1 but they can transfer UB to the downstream E3 enzymes by taking up UB from A7.
The wtUba1-wtUbc1 pair has previously been found to transfer UB to the HECT domain of Rsp5 (Huibregtse, et al., 1997). We used the C9 mutant of Ubc1 as xE2 to test the transfer of wtUB through the A7-xE2 pair to the HECT domain of Rsp5. As shown in Figure S7E, the A7-xE2 pair can transfer UB to the HECT domain of Rsp5 as demonstrated by the formation of UB~HECT conjugate. When either A7 or xE2 was excluded, UB attachment to the HECT domain was not seen. Western blot analysis also showed that the A7-xE2 pair transfers wtUB to the Rsp5 HECT at a lower efficiency than the wtUba1-wtUbc1 pair. These results suggest that the A7-xE2 pair is functional in transferring wtUB to the HECT domains of E3 enzymes. However the mutations in the H1 helix of xE2 may interfere with the binding of xE2 to the native E3 enzymes. So further engineering work need to be done to create specific xE2-xE3 pairs.
New xE1-xE2 pairs can be constructed with other E2s by swapping the H1 helix from phage selected Ubc1 mutant
Our phage selection results demonstrate that the H1 helices of E2 enzymes play a dominant role in bridging the interactions between E1 and E2. A sequence alignment of E2 enzymes involved in UB transfer also reveals a highly conserved H1 helix that is heavily populated by positively charged residues (Figure 4C). The sequence homology of the H1 helix reflects the structural requirement imposed on various E2 enzymes to bind to the same UFD domain of the E1 for UB transfer (Lee and Schindelin, 2008). We thus thought that replacing the H1 helix in a different E2 subtype with the mutated H1 helix of an Ubc1 clone might yield a “hybrid E2” unable to bindwtE1 but able to interact with A7 for UB transfer. In this way we could construct new xE1-xE2 pairs with various E2 subtypes simply by substituting the native H1 helix with the mutated H1 helix of Ubc1. To test this idea we replaced the first 12 residues of UbcH7 (MAASRRLMKELE) constituting the H1 helix with the corresponding sequences of Ubc1 mutants C2 (MSRADDIMDQIH) and C9 (MSRADEIMEQIL) (Figure 4C and Table 1). We refer to the H1-swapped forms of UbcH7 as C2-UbcH7 and C9-UbcH7, respectively. We then tested the ability of C2-UbcH7 and C9-UbcH7 to take up UB from wtE1 and A7. A Western blot of the UB transfer reaction showed that while neither hybrid can be loaded with UB by the wtE1 (Figure 5A), C9-UbcH7 can efficiently uptake UB from A7 (Figure 5B). In contrast, the C2-UbcH7 cannot form UB~E2 conjugates catalyzed by A7, suggesting that a subtle difference in the H1 sequence may strongly affect the efficiency of UB transfer from E1 to E2. Since wtUbcH7 cannot be loaded with UB by A7 (Figure 5B), the replacement of the native H1 helix in UbcH7 with the H1 helix in C9 mutant restores the binding and reactivity of the hybrid UbcH7 with A7. We further confirmed that the C9-UbcH7 hybrid can transfer UB to the HECT domain of E6AP (Figure 5C). The formation of an UB~HECT conjugate is dependent on the presence of both A7 and the C9-UbcH7 in the reaction mixture. Overall these results suggest that the native H1 helix of E2 enzyme can be swapped with the mutated H1 helix from phage selection for the construction of xE1-xE2 pairs that are orthogonal to the native enzymes.
Figure 5.
Reactivity of xE1 with hybrid UbcH7 and UbcH5a. (A) wtUB transfer to the hybrid UbcH7 from wtUba1. (B) wtUB transfer to the hybrid UbcH7 from Uba1-A7. (C) wtUB transfer to the HECT domain of E6AP from A7 and hybrid C9-UbcH7. (D) xUB (UB10) transfer by xE1 (Uba1-A9) with combined A domain and UFD domain mutations to the hybrid E2s in which the N-terminal H1 helixes of native UbcH5a and UbcH7 were replaced with the sequences of Ubc1 mutants from phage selection.
An xE1-xE2 cascade that is orthogonal to the native E1 and E2 enzymes for xUB transfer
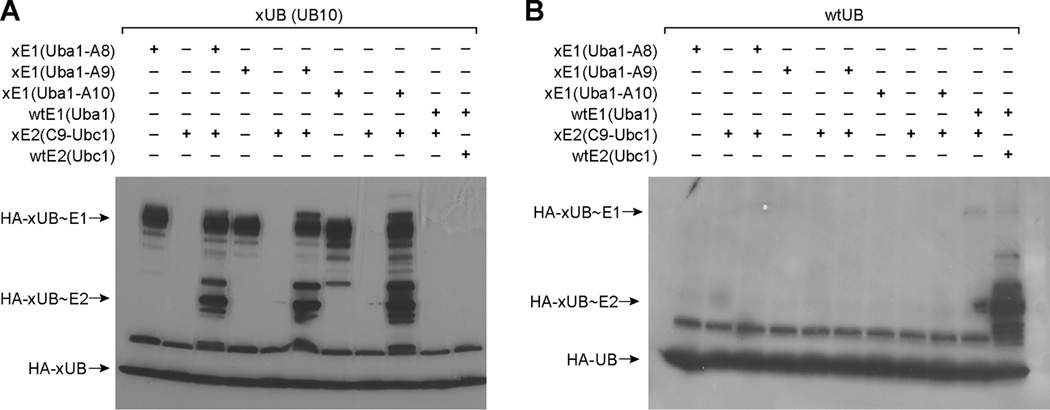
We next tested if the xUB-xE1(A) pair we engineered could relay with the A7-xE2 pairs to assemble an UB transfer cascade from xE1 to xE2 that is orthogonal to the native E1 and E2 enzymes. We combine the mutations in the adenylation domain of A4, A5 and A6, demonstrated to function as xE1(A), with the mutations in the UFD domain of A7 to generate Uba1 mutants A8, A9 and A10 (Table 1). The xE1s with combined mutations in the A and UFD domains should only recognize xUB and xE2 and transfer xUB to xE2. They should neither activate wtUB to transfer it to xE2 nor should they activate xUB and transfer it to native E2 enzymes. To test the reactivity of the xE1-xE2 cascade for xUB transfer, we used a combination of xE1 and xE2 to synthesize xUB~xE2 conjugates. We also cross reacted xUB with wtE1 and wtE2, or wtUB with xE1 and xE2 to test the orthogonality of the xE1-xE2 cascade with the wtE1-wtE2 cascade. As shown in Figure 6A, various xE1-xE2 combinations can activate xUB and transfer xUB to xE2 for the formation of xUB~xE2 conjugates. In contrast when xE1 or both xE1 and xE2 were replaced by the wtE1 or wtE2 enzymes, no xUB transfer to the wtE1 or wtE2 enzyme was observed. Furthermore wtUB cannot be transferred by the xE1-xE2 cascade for the formation of wtUB~xE2 conjugates (Figure 6B). These results prove that the xE1-xE2 pair we engineered is indeed an orthogonal pathway for the transfer of xUB to a specific xE2 enzyme.
Figure 6.
Orthogonal transfer of xUB from xE1 to xE2. (A) Uba1 mutants A8, A9 and A10 can function as xE1 to transfer xUB (UB10) to the C9 mutant of Ubc1 (xE2). (B) wtUB cannot be transferred by the xE1s to xE2.
We also found that hybrid E2s generated by replacing the N-terminal H1 helix of the wtE2 with the phage selected H1 sequence can pair with xE1 with combined mutations to support the transfer of xUB. When A9 was used as an xE1 to activate xUB, we observed that C9-UbcH7 can be loaded with xUB catalyzed by xE1 (Figure 5D). Similarly we generated C1-UbcH5a (Figure 4C and Table 1) and found that C1-UbcH5a can be loaded with xUB by A9 (Figure 5D). We also observed that C9-UbcH5a and C2-UbcH7 were not able to interact properly with A9 and be loaded with xUB further supporting the view that the sequence of the H1 helix may significantly affect xUB transfer. Overall these results prove that we can engineer E1 interfaces with UB and E2 to specifically transfer xUB to an engineered xE2 enzyme and construct an xE1-xE2 cascade that is orthogonal to native E1 and E2 enzymes.
DISCUSSION
Engineering protein posttranslational modification enzymes with orthogonal reactivity for the elucidation of cellular signaling pathways
Protein ubiquitination in the cell is complicated by the cross-reactivities among E1, E2 and E3 enzymes (Figure S1A). These enzymes assemble overlapping and intersecting networks for UB transfer to the cellular target proteins. The complexity of the E1-E2-E3 cascades makes it a significant challenge to identify the ubiquitination substrates of a specific E3 enzyme and elucidate the protein ubiquitination pathways. Many enzymes responsible for protein posttranslational modification (PTM) share the same complexity as the E1-E2-E3 cascade (Walsh, 2005). Typically they exist in the cell in many subtypes and cross-react to modify substrate proteins. Since various subtypes of the same class of PTM enzymes attach the same modification group on the cellular proteins, it has been difficult to identify the direct substrates of a specific PTM enzyme in the cell.
One strategy to elucidate the complex protein modification pathways is to engineer the PTM enzymes so that they can have orthogonal catalytic activities with the native enzymes. One successful example is to engineer kinases to use an ATP analog for protein phosphorylation. The ATP analog is not recognized as a substrate by 500 or so native kinases (Manning, et al., 2002). When the ATP analog – engineered kinase pair is applied to cellular proteins, only the targets of the engineered kinase are phosphorylated by the radioactive ATP analog. The identification of radiolabeled proteins in the cell leads to the elucidation of the substrate proteins of the kinase. The engineering of specific ATP analog – kinase pair removes the cross-reactivity of ATP with the native kinases (Liu, et al., 1998; Shah, et al., 1997). It creates a separate path for the transfer of phosphate to the modification targets of a specific kinase and greatly facilitates the elucidation of the substrate proteins of individual kinases (Bishop, et al., 2000).
Adopting a similar strategy, we used protein engineering to create xUB-xE1 and xE1-xE2 pairs that are orthogonal to their native enzyme counterparts. Through the relay of the engineered xE1 and xE2, an affinity tagged xUB can be exclusively transferred to a designated xE2. We have thus created a separate path to synthesize xUB~xE2 thioesters without cross reactivities with the native UB transfer pathways. Potentially xUB can be further transferred to the partner E3s of xE2 and to the substrates of the E3 enzymes. So far about 50 E2s have been identified in the human genome. They are loaded with UB by the two E1 enzymes that show overlapping yet distinct specificities for various E2s (Jin, et al., 2007). Each E2 can also interact with multiple E3 enzymes and deliver UB to their substrate proteins. Because of the complex cross reactivities of E2s with E1 and E3 enzymes, the biological functions of individual E2s are hard to define. We envision that we can use the xE1-xE2 pair to synthesize a specific xUB~xE2 conjugate in the presence of the native E2s in the cell. By identifying the cellular proteins modified with xUB, the UB transfer targets of xE2 and the corresponding native E2 can be revealed. This will help to elucidate the biological functions of individual E2 enzymes. The creation of the xE1-xE2 pair in this study also brings us one step closer to the construction of an OUT system that transfers xUB through the xE1-xE2-xE3 cascade to the substrate proteins of xE3 (Figure 1A). We are currently engineering specific xE2-xE3 pairs with HECT and RING types of E3s.
Catalysis-based phage selection to engineer specific xUB-xE1 and xE1-xE2 pairs
In this study we developed phage selection methods based on E1 catalyzed UB transfer to engineer specific interactions between xUB and xE1, and xE1 and xE2. To select for specific UB-E1 pairs, we displayed an UB library on the phage with randomized C-terminal residues. UB clones that were catalytically active with the E1 mutant xE1(A) would be covalently bound to E1 by thioester linkages and be immobilized on the streptavidin plate coated with the E1 enzyme (Figure S4A). To select for specific E1-E2 pairs, we displayed an E2 library with randomized H1 helix on phage. We then used the E1 mutant xE1(UFD) to transfer biotin-UB to the E2 variants displayed on phage surface. If the E2 variants are recognized by xE1(UFD) to relay UB transfer, the E2 clone would form thioester conjugates with biotin-UB and be selected by binding to the streptavidin plate (Figure 4B). The catalysis-based phage selection methods are more preferable than binding-based selection since the binding affinity of the noncovalent UB-E1 and E1-E2 complex are weak with Kd’s in the range of 0.3–5 µM (Burch and Haas, 1994; Eletr, et al., 2005; Huang, et al., 2008). Various UBL proteins such as Nedd8 (Hori, et al., 1999), SUMO (Johnson, 2004), and ISG15 (Loeb and Haas, 1992) adopt similar cascade reactions with their own sets of E1 and E2 enzymes for protein modification. We expect that the phage selection methods we developed to engineer UB transfer pathways can also be used to manipulate the reactivities of UBLs with the cognate E1 and E2 enzymes.
Engineering key interactions between UB and E1
Based on the results of phage selection, we generated Arg42Glu and Arg72Glu mutants of UB and identified mutations in Uba1 that complement the mutations in UB for the assembly of a specific xUB-xE1 pair. The importance of Arg42 and Arg72 of UB in E1 binding is well established in the literature. Previous mutagenesis studies showed that both Arg72Leu and Arg42Leu mutations lead to higher Kd for E1 binding when compared to wt (Burch and Haas, 1994). In another study, alkylation of Arg42 and Arg72 of UB was found to attenuate the activity of UB to stimulate ATP-PPi exchange with E1 by more than 85% (Duerksen-Hughes, et al., 1987). Arg72 of UB was also found to be a gatekeeping residue to prevent UB from reacting with the E1 enzyme of the UBL protein Nedd8 (Souphron, et al., 2008). UB and the Nedd8 share 58% sequence identify and they have almost the same C-terminal sequence except that Nedd8 has an Ala residue replacing Arg72 of UB. Despite their high degree of homology, UB and Nedd8 each have their own E1s to activate their transfer to the target proteins (Schulman and Harper, 2009). When Arg72 of UB was mutated to Ala or Leu, it was found that the UB mutant can be activated by the E1 of Nedd8 (Bohnsack and Haas, 2003; Souphron, et al., 2008; Walden, et al., 2003). This reveals that a key function of the Arg72 residue of UB is to ensure the specific recognition of UB by its own E1. The importance of Arg42 and Arg72 for the biological activities of UB has also been demonstrated in yeast complementation studies. It was shown that when Arg42 or Arg72 of UB was mutated to Ala, the mutant UB was not able to complement the growth of a yeast strain in which all endogenous UB genes were deleted (Sloper-Mould, et al., 2001).
Our studies verify the key roles of Arg42 and Arg72 in E1 recognition. We found that Arg42Glu and Arg72Glu mutations in UB block the activation of the UB mutant (xUB) by E1. To engineer an E1 mutant (xE1) that can bind and activate xUB, we mutated residues Gln576, Ser589 and Asp591 in Uba1 to Arg so that the newly introduced Arg residues in E1 can pair with the Glu residues at positions 42 and 72 of UB to restore UB-E1 interaction (Figure S2B and S2C). This resulted in successful construction of a specific xUB-xE1 pair for xUB activation.
Using the H1 helix of E2 to engineer an E1-E2 interaction
The modeled structure of Uba1-Ubc1 complex suggests electrostatic interactions between the UFD domain of Uba1 and the H1 helix of Ubc1 play important roles in E1-E2 interaction (Lee and Schindelin, 2008) (Figure S2A and S2D). We mutated Glu1004, Asp1014 and Glu1016 of Uba1 to positively charged Lys residues. As the result of these charge-reversal mutations, the A7 mutant of Uba1 that we refer to as xE1(UFD), can no longer bind to native E2s. We carried out phage selection on an Ubc1 library with randomized residues in the H1 helix to identify complementary mutations that restore the E1-E2 interaction. Phage selection enriched negatively charged residues such as Asp and Glu at positions 5, 6 and 9 of Ubc1, matching well with the counter charges introduced by the Lys mutations in A7 (Figure 4C). Interestingly a Glu10Gln mutation in the H1 helix seems to dominate the Ubc1 library after phage selection suggesting the importance of the mutation to A7-xE2 interaction. It is not evident from the modeled structure of Uba1-Ubc1 complex (Lee and Schindelin, 2008) why a neutral Gln residue replacing the negatively charged Glu in the H1 helix could lead to a better binding between mutant Ubc1 (xE2) and A7 (Figure S2D). The unexpected selection of the Glu10Gln mutation in Ubc1 library demonstrates the effectiveness of phage display in optimizing protein-protein interactions between E1 and E2.
Our study suggests that the H1 helix of E2 can be engineered to create specific xE1-xE2 pairs. Previous mutagenesis studies showed that the H1 helix of E2s plays an important role to bridge the E1-E2 interaction. Mutations in the H1 helix of E2s such as Ubc1, Ubc4 and Cdc34 have been found to impair the formation of UB~E2 conjugates catalyzed by E1 (Lee and Schindelin, 2008; Pitluk, et al., 1995; Sullivan and Vierstra, 1991). Here we used phage display to redesign the sequence of the H1 helix in xE2 to remove its binding with the wtE1 and to generate specific interactions between xE2 and xE1. We also showed that the H1 helix from phage selection is portable to other E2s such as UbcH5a and UbcH7 for the construction of xE1-xE2 pairs. These pairs create a separate path to transfer xUB to a specific E2 enzyme.
SIGNIFICANCE
The human genome encodes 2 E1s, 50 E2s and more than 1,000 E3s. Together they assemble the complex network of UB transfer in the cell. Because of the complex cross-reactivities among E1, E2 and E3 enzymes, it has been a challenge to individually assign the biological function of E2 and E3 enzymes. We plan to identify the substrate proteins of a specific E3 enzyme by constructing an “orthogonal UB transfer (OUT)” cascade that is composed of engineered E1, E2 and E3 enzymes (xE1, xE2 and xE3). Through this cascade, an affinity tagged UB mutant (xUB) can be activated and transferred to the substrate proteins of a specific E3 in order to elucidate the cellular targets of individual E3 enzymes. To construct the OUT cascade, we used a combination of catalysis-based phage selection and site-directed mutagenesis to engineer xUB-xE1 and xE1-xE2 pairs are free of cross reactivity with their native enzyme counterparts. We further combined the engineered xUB-xE1 and xE1-xE2 pairs to implement an orthogonal pathway to activate xUB and transfer it a specific E2 for protein ubiquitination. We have thus accomplished the first two steps of a three-step protein engineering sequence for the construction of an OUT cascade.
EXPERIMENTAL PROCEDURES
Phage selection of the UB library
Phage library selection followed the same procedure as the model selection. To initiate the reaction between phage displayed UB and the A1 mutant of Uba1, 100 µL of phage displaying the UB library was added to each well of the streptavidin plate coated with the PCP fusion of Uba1-A1. For the first round of selection, 1 × 1011 UB library phage in 100 µL reaction buffer was added to each well that was coated with the A1 mutant at a level of 125 pmol per well. The reaction was allowed to proceed for 1 hour at room temperature. The supernatant was discarded and the plate was washed 30 times with TBS-T and 30 times with TBS, each time with 200 µL of solution per well. After washing, phage bound to the streptavidin surface were eluted by adding 100 µL 10 mM dithiothreitol (DTT) in TBS to each well with 10-minute incubation. The eluted phage were combined, added to 10 mL of log phase E. coli XL1-Blue cells and shaken at 37°C for one hour to infect the cells. The cells were then plated on LB agar plates supplemented with 2% (w/v) glucose and 100 µg/mL ampicillin. After overnight incubation at 37°C, colonies on the plates were scratched and the phagemid DNA was extracted with a QIAprep plasmid miniprep kit (Qiagen). The phagemid DNA was then used for the next round of phage production and selection. During phage selection, control reactions were also set up in which ATP was eliminated from the reaction or plain streptavidin coated well with no PCP-A1 binding was used for selection. Phage particles eluted from the selection or the control reactions were titered. After the first round of selection, the number of the input phage particles, the amount of PCP-A1 on the plate, and the reaction time were decreased in the subsequent selection rounds to make the selection more stringent. Eventually, 1010 phage particles were incubated in each well with 5 pmol of PCP-A1 for 10 minutes at room temperature during the sixth round of selection. After six rounds of selection, phage clones were sequenced with the Jun13 primer.
Phage selection of the Ubc1 library
Library selection followed the same procedure as the model selection. In the first round of selection, 500 µL reactions were set up with 1 µM A7, 5 µM biotin-UB, 2.5 mM ATP, 50 mM MgCl2, and 7.5 × 1011 Ubc1 library phage in TBS buffer (pH 7.5). The reaction was allowed to proceed for 1 hour at room temperature before 3% BSA in TBS buffer (pH 7.5) was added to a final BSA concentration of 1%. The phage solution was then distributed into the wells of a streptavidin coated plate. The streptavidin plate was incubated at room temperature for 1 hour. The supernatant was discarded and the plate was washed 30 times with 0.05% (v/v) Tween 20, 0.05% (v/v) Triton X-100 in TBS and 30 times with TBS, each time with 200 µL of solution per well. After washing, phage bound to the streptavidin surface were eluted by adding 100 µL 10 mM dithiothreitol (DTT) in TBS to each well and incubating for 10 minutes. The eluted phage were combined, added to 10 mL of log phase E. coli XL1-Blue cells and shaken at 37°C for one hour to infect the cells. The cells were then plated on LB agar plates supplemented with 2% (w/v) glucose and 100 µg/mL ampicillin. After overnight incubation at 37°C, colonies on the plates were scratched and the phagemid DNA was extracted with a QIAprep Plasmid Miniprep kit (Qiagen). The phagemid DNA was then used for the next round of phage production and selection. Also, phage particles eluted from the selection or the control reactions were titered. After the first round of selection, the number of the input phage particles, the concentration of A7 and biotin-UB, and the reaction time were decreased in the subsequent selection rounds to make the selection more stringent. Eventually, 1010 phage particles were incubated with 0.1 µM biotin-UB, 0.05 µM A7 for 5 minutes at room temperature for the eighth round of selection. After the seventh and eighth round of selection, phage clones were sequenced with the Jun13 primer.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a lab startup grant from the University of Chicago, and a National Science Foundation CAREER award (1057092). This work was also funded in part by the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust. We thank Professors Carlos F. Barbas (Scripps), Bernard Roizman (U Chicago), Suzanne D. Conzen (U Chicago), Carl G. Maki (Rush U), Linda Hicke (Northwestern U), Jon M. Huibregtse (U Texes, Austin) and Cam Patterson (U North Carolina) for generously providing materials for this research. We thank Jeffrey Schneider and David Boon (U Chicago) for proofreading the manuscript.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes results of the phage model selection, UB transfer reaction, Western blot, figures, table of the PCR primers, and additional experimental procedures.
REFERENCES
- Alaimo PJ, Shogren-Knaak MA, Shokat KM. Chemical genetic approaches for the elucidation of signaling pathways. Curr Opin Chem Biol. 2001;5:360–367. doi: 10.1016/s1367-5931(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Barbas CF, 3rd, Burton DR, Scott JK, Silverman GJ. Phage Display, A laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Bishop A, Buzko O, Heyeck-Dumas S, Jung I, Kraybill B, Liu Y, Shah K, Ulrich S, Witucki L, Yang F, et al. Unnatural ligands for engineered proteins: new tools for chemical genetics. Annu Rev Biophys Biomol Struct. 2000;29:577–606. doi: 10.1146/annurev.biophys.29.1.577. [DOI] [PubMed] [Google Scholar]
- Bohnsack RN, Haas AL. Conservation in the mechanism of Nedd8 activation by the human AppBp1-Uba3 heterodimer. J Biol Chem. 2003;278:26823–26830. doi: 10.1074/jbc.M303177200. [DOI] [PubMed] [Google Scholar]
- Burch TJ, Haas AL. Site-directed mutagenesis of ubiquitin. Differential roles for arginine in the interaction with ubiquitin-activating enzyme. Biochemistry. 1994;33:7300–7308. doi: 10.1021/bi00189a035. [DOI] [PubMed] [Google Scholar]
- Christensen DE, Klevit RE. Dynamic interactions of proteins in complex networks: identifying the complete set of interacting E2s for functional investigation of E3-dependent protein ubiquitination. Febs J. 2009;276:5381–5389. doi: 10.1111/j.1742-4658.2009.07249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri R, Suter M. Display of biologically active proteins on the surface of filamentous phages: a cDNA cloning system for selection of functional gene products linked to the genetic information responsible for their production. Gene. 1993;137:69–75. doi: 10.1016/0378-1119(93)90253-y. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- Duerksen-Hughes PJ, Xu XX, Wilkinson KD. Structure and function of ubiquitin: evidence for differential interactions of arginine-74 with the activating enzyme and the proteases of ATP-dependent proteolysis. Biochemistry. 1987;26:6980–6987. doi: 10.1021/bi00396a019. [DOI] [PubMed] [Google Scholar]
- Ehmann DE, Shaw-Reid CA, Losey HC, Walsh CT. The EntF and EntE adenylation domains of Escherichia coli enterobactin synthetase: sequestration and selectivity in acyl-AMP transfers to thiolation domain cosubstrates. Proc Natl Acad Sci U S A. 2000;97:2509–2514. doi: 10.1073/pnas.040572897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletr ZM, Huang DT, Duda DM, Schulman BA, Kuhlman B. E2 conjugating enzymes must disengage from their E1 enzymes before E3-dependent ubiquitin and ubiquitin-like transfer. Nat Struct Mol Biol. 2005;12:933–934. doi: 10.1038/nsmb984. [DOI] [PubMed] [Google Scholar]
- Eletr ZM, Kuhlman B. Sequence determinants of E2-E6AP binding affinity and specificity. J Mol Biol. 2007;369:419–428. doi: 10.1016/j.jmb.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AL, Rose IA. The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J. Biol. Chem. 1982;257:10329–10337. [PubMed] [Google Scholar]
- Haas AL, Warms JV, Rose IA. Ubiquitin adenylate: structure and role in ubiquitin activation. Biochemistry. 1983;22:4388–4394. doi: 10.1021/bi00288a007. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hori T, Osaka F, Chiba T, Miyamoto C, Okabayashi K, Shimbara N, Kato S, Tanaka K. Covalent modification of all members of human cullin family proteins by NEDD8. Oncogene. 1999;18:6829–6834. doi: 10.1038/sj.onc.1203093. [DOI] [PubMed] [Google Scholar]
- Huang DT, Hunt HW, Zhuang M, Ohi MD, Holton JM, Schulman BA. Basis for a ubiquitin-like protein thioester switch toggling E1-E2 affinity. Nature. 2007;445:394–398. doi: 10.1038/nature05490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DT, Zhuang M, Ayrault O, Schulman BA. Identification of conjugation specificity determinants unmasks vestigial preference for ubiquitin within the NEDD8 E2. Nat Struct Mol Biol. 2008;15:280–287. doi: 10.1038/nsmb.1387. [DOI] [PubMed] [Google Scholar]
- Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, Pavletich NP. Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade. Science. 1999;286:1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- Huibregtse JM, Yang JC, Beaudenon SL. The large subunit of RNA polymerase II is a substrate of the Rsp5 ubiquitin-protein ligase. Proc Natl Acad Sci U S A. 1997;94:3656–3661. doi: 10.1073/pnas.94.8.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YW, Miller DL. A mutation that alters the nucleotide specificity of elongation factor Tu, a GTP regulatory protein. J. Biol. Chem. 1987;262:13081–13085. [PubMed] [Google Scholar]
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J. Biol. Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- Jin J, Li X, Gygi SP, Harper JW. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447:1135–1138. doi: 10.1038/nature05902. [DOI] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annu. Rev. Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Kay BK, Winter J, McCafferty J. Phage Display of Peptides and Proteins. Boston: Academic Press, Inc.; 1996. [Google Scholar]
- Lee I, Schindelin H. Structural insights into E1-catalyzed ubiquitin activation and transfer to conjugating enzymes. Cell. 2008;134:268–278. doi: 10.1016/j.cell.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shah K, Yang F, Witucki L, Shokat KM. Engineering Src family protein kinases with unnatural nucleotide specificity. Chem. Biol. 1998;5:91–101. doi: 10.1016/s1074-5521(98)90143-0. [DOI] [PubMed] [Google Scholar]
- Loeb KR, Haas AL. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J. Biol. Chem. 1992;267:7806–7813. [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Pitluk ZW, McDonough M, Sangan P, Gonda DK. Novel CDC34 (UBC3) ubiquitin-conjugating enzyme mutants obtained by charge-to-alanine scanning mutagenesis. Mol Cell Biol. 1995;15:1210–1219. doi: 10.1128/mcb.15.3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10:319–331. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Liu Y, Deirmengian C, Shokat KM. Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc Natl Acad Sci U S A. 1997;94:3565–3570. doi: 10.1073/pnas.94.8.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloper-Mould KE, Jemc JC, Pickart CM, Hicke L. Distinct functional surface regions on ubiquitin. J. Biol. Chem. 2001;276:30483–30489. doi: 10.1074/jbc.M103248200. [DOI] [PubMed] [Google Scholar]
- Souphron J, Waddell MB, Paydar A, Tokgoz-Gromley Z, Roussel MF, Schulman BA. Structural dissection of a gating mechanism preventing misactivation of ubiquitin by NEDD8's E1. Biochemistry. 2008;47:8961–8969. doi: 10.1021/bi800604c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan ML, Vierstra RD. Cloning of a 16-kDa ubiquitin carrier protein from wheat and Arabidopsis thaliana. Identification of functional domains by in vitro mutagenesis. J Biol Chem. 1991;266:23878–23885. [PubMed] [Google Scholar]
- Ulane CM, Horvath CM. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology. 2002;304:160–166. doi: 10.1006/viro.2002.1773. [DOI] [PubMed] [Google Scholar]
- Walden H, Podgorski MS, Huang DT, Miller DW, Howard RJ, Minor DL, Jr., Holton JM, Schulman BA. The structure of the APPBP1-UBA3-NEDD8-ATP complex reveals the basis for selective ubiquitin-like protein activation by an E1. Mol. Cell. 2003;12:1427–1437. doi: 10.1016/s1097-2765(03)00452-0. [DOI] [PubMed] [Google Scholar]
- Walsh CT. Posttranslational Modification of Proteins: Expanding Nature's Inventory. Englewood, Colorado, USA: Roberts & Co Press; 2005. [Google Scholar]
- Wenzel DM, Stoll KE, Klevit RE. E2s: structurally economical and functionally replete. Biochem J. 2011;433:31–42. doi: 10.1042/BJ20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Lin AJ, Golan DE, Walsh CT. Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nature Protocols. 2006;1:280–285. doi: 10.1038/nprot.2006.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.