Abstract
Aims: Pancreatic cancer (PC) is the most aggressive malignant disease, ranking as the fourth most leading cause of cancer-related death among men and women in the United States. In this study, we provide evidence of chemotherapeutic effects of α-mangostin, a dietary antioxidant isolated from the pericarp of Garcinia mangostana L. against human PC. Results: The chemotherapeutic effect of α-mangostin was determined using four human PC cells (PL-45, PANC1, BxPC3, and ASPC1). α-Mangostin resulted in a significant inhibition of PC cells viability without having any effects on normal human pancreatic duct epithelial cells. α-Mangostin showed a dose-dependent increase of apoptosis in PC cells. Also, α-mangostin inhibited the expression levels of pNF-κB/p65Ser552, pStat3Ser727, and pStat3Tyr705. α-Mangostin inhibited DNA binding activity of nuclear factor kappa B (NF-κB) and signal transducer and activator 3 (Stat3). α-Mangostin inhibited the expression levels of matrix metallopeptidase 9 (MMP9), cyclin D1, and gp130; however, increased expression of tissue inhibitor of metalloproteinase 1 (TIMP1) was observed in PC cells. In addition, i.p. administration of α-mangostin (6 mg/kg body weight, 5 days a week) resulted in a significant inhibition of both primary (PL-45) and secondary (ASPC1) human PC cell-derived orthotopic and ectopic xenograft tumors in athymic nude mice. No sign of toxicity was observed in any of the mice administered with α-mangostin. α-Mangostin treatment inhibited the biomarkers of cell proliferation (Ki-67 and proliferating cell nuclear antigen [PCNA]) in the xenograft tumor tissues. Innovation: We present, for the first time, that dietary antioxidant α-mangostin inhibits the growth of PC cells in vitro and in vivo. Conclusion: These results suggest the potential therapeutic efficacy of α-mangostin against human PC. Antioxid. Redox Signal. 21, 682–699.
Introduction
Pancreatic cancer (PC) is one of the most fatal of all cancers and is ranked as the fourth most common cause of cancer-related deaths among both men and women in the United States (49). Human PC has the highest mortality rate among all cancers. For example, 94% of patients will die within 5 years of diagnosis and 74% of patients with PC will die within the first year of diagnosis (49). Incidence of human PC has been continuing to increase by 1.5% per year, whereas most other cancers have been declining (49). Despite these alarming statistics and the increasing PC incidence over the past several decades, the molecular and biochemical determinants of the disease remain poorly understood and no effective therapeutic regimen exists to significantly ameliorate the clinical course or prognosis of this disease (49). Gemcitabine is the only U.S. Food and Drug Administration (FDA)-approved chemotherapeutic drug for the treatment of PC, which confers a median survival advantage of only 6 months, an improvement of only 1 month over its predecessor (5-fluorouracil [5-FU]) (44). Addition of erlotinib, a tyrosine kinase inhibitor recently approved by FDA, includes only two more weeks to the average overall survival time (40). Therefore, it is necessary to intensify our efforts for the development of novel therapeutic strategies and agents for the prevention and treatment of PC. One approach to control this malignancy is to slow its progression through the use of nontoxic bioactive dietary agents or nutraceuticals consumed by humans. A comprehensive analysis provides a strong correlation between vegetables and fruit consumption and reduced risk of PC (33). Various studies, including ours, have shown anticancer activity of various natural agents against PC (7, 14, 27, 41, 46), which suggest that vegetables and fruits may impart some protection against the risk of PC.
Innovation.
Current conventional therapeutics, including chemotherapy and radiation, against pancreatic cancer (PC) has limited success with severe toxic side effects. In this communication, we present for the first time that α-mangostin, a dietary antioxidant derived from the pericarp of Garcinia Mangostana L., inhibits pancreatic tumor growth possibly via the inhibition of the signal transducer and activator 3 (Stat3) and nuclear factor kappa B (NF-κB) activation and their downstream target genes linked to cell proliferation, apoptosis, and metastasis. These findings suggest that α-mangostin could be developed as an agent against human PC.
α-Mangostin (Fig. 1A) is one of the dietary antioxidants found in the mangosteen fruit (Garcinia mangostana L.). The mangosteen fruit is native to Southeast Asia, where it has been used in traditional systems of medicine against various types of ailments for hundreds of years (43). The exocarp (i.e., outermost layer) of the mangosteen fruit is a rich source of polyphenolic substances, including tannins and xanthones. Recently, consumption of mangosteen products in the form of juices (e.g., Xango®) and dietary supplements (e.g., Nature Made® 50 mg mangostin/capsule) has increased in the United States without any observed toxicity. α-Mangostin has been receiving greater attention for its possible health promoting benefits that include antibacterial (47), anti-inflammatory (14), cardioprotective (16), antioxidant (53), and anticancer activities (2, 12, 17, 31, 35, 37, 42, 47a, 48). α-Mangostin has been shown to induce cell cycle arrest and apoptosis in various types of cancer cells (1, 36, 39). A study has shown that α-mangostin treatment of chondrosarcoma cells induces apoptosis via targeting MAPK and AKT signaling pathways (35). Studies have also shown anticancer effects of α-mangostin against highly metastatic human breast cancer cells in vitro (37) and in vivo (17). α-Mangostin has been shown to inhibit ectopic tumor growth of prostate cancer cells in athymic nude mice via targeting cyclin-dependent kinases (32). In addition, α-mangostin has been shown to induce autophagic death of glioblastoma cells and inhibit ectopic growth of glioblastoma cells xenograft tumors in athymic nude mice (12). A recent study has suggested antitumor activity of α-mangostin against colon cancer (1). However, no study has shown either in vitro or in vivo anticancer activity of α-mangostin against PC. In this study, we report for the first time that α-mangostin induces apoptosis and inhibits the growth of human PC cells both in vitro and in vivo. α-Mangostin-induced both apoptosis and the inhibition of human PC cells proliferation accompanied by the inhibition of nuclear factor kappa B (NF-κB), signal transducer and activator 3 (Stat3), and matrix metallopeptidase 9 (MMP9) signaling pathways.
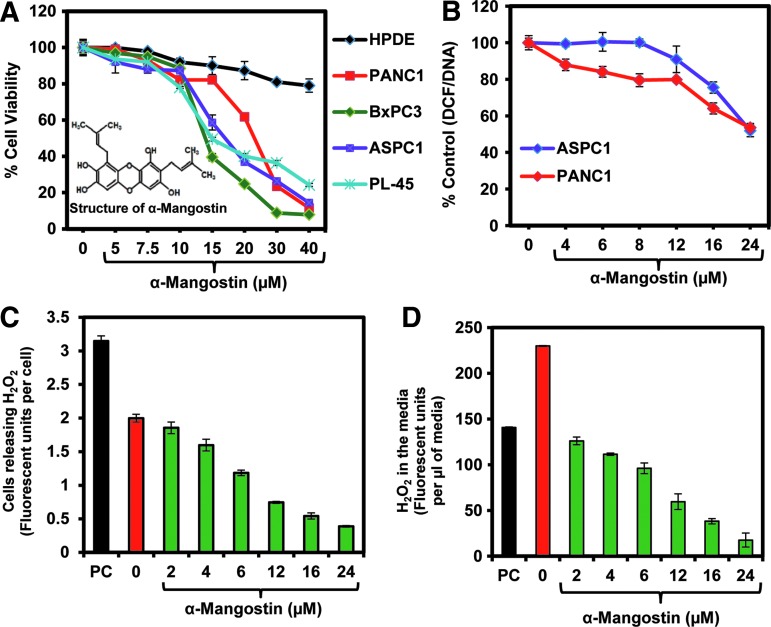
FIG. 1.
Effect of α-mangostin on cell viability of pancreatic cancer (PC) cells. (A) Cell viability. PC (PANC1, BxPC3, and ASPC1) and nontumorigenic immortalized HPDE cells were treated with α-mangostin at the indicated concentrations for 24 h, and cell viability was determined by the MTT assay. The values represent as percent viable cells compared to the vehicle-treated cells. Each value is the mean±SE of triplicate wells of each group. (B) Effect of α-mangostin on antioxidant activity as assessed by DCF/DNA assay in PC cells. Line graph is showing decrease in the DCF activity in ASPC1 and PANC1 cells with the treatment of α-mangostin normalized with total DNA contents. Values represent mean±SE of triplicate wells of each group. (C, D) Effect of α-mangostin on H2O2 levels in ASPC1 cells. In brief, ASPC1 cells were treated with specified concentrations of α-mangostin for 12 h. About 1.5×104 cells were taken for the assay to measure the intracellular H2O2 levels. (C) Values in the bar graph are shown fluorescent units/cells calculated from total fluorescent units, which is correlated with the amount of H2O2 levels. (D) Values in the bar graph are shown as fluorescent units/μl cell culture supernatant, which was calculated from the amount of total cell culture media taken for the assay. PC in panels (C) and (D) denotes positive control of H2O2. HPDE, human pancreatic duct epithelial; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide.
Results
α-Mangostin inhibits viability of human PC cells without affecting normal human pancreatic duct epithelial cells
We first performed a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide (MTT) assay to assess the effects of α-mangostin on cell viability of human PC cells (PANC1, BxPC3, and ASPC1) and nontumorigenic human pancreatic duct epithelial (HPDE) cells. α-Mangostin treatment inhibited a dose-dependent decrease in cell viability of PC cells (Fig. 1A). IC50 doses of α-mangostin for the tested PC cells (PL-45, PANC1, BxPC3, and ASPC1) varied from 13 to 17 μM (Fig. 1A). Interestingly, α-mangostin treatment, at a dose as high as 40 μM, did not significantly inhibit the viability of nontumorigenic HPDE cells (Fig. 1A).
α-Mangostin decreases reactive oxygen species levels in human PC cells
In this experiment (Fig. 1B), ASPC1 and PANC1 cells were treated with vehicle or α-mangostin (2–24 μM) for 12 h. 2′7′-Dichlorofluorescein diacetate (DCFH-DA)/DNA assay was performed to measure intracellular reactive oxygen species (ROS)/reactive nitrogen species (RNS) generation. α-Mangostin treatment resulted in a dose-dependent decrease in DCFH-DA/DNA content in both ASPC1 and PANC1 cells (Fig. 1B). These results indicate a decrease in intracellular ROS levels in α-mangostin-treated PC cells. We further confirmed the ROS scavenging activity of α-mangostin by measuring H2O2 level in ASPC1 cells and culture media. α-Mangostin treatment inhibited both intracellular generation (Fig. 1C) and a reduction of extracellular (Fig. 1D) H2O2 levels in ASPC1 cells.
α-Mangostin induces apoptosis in human PC cells
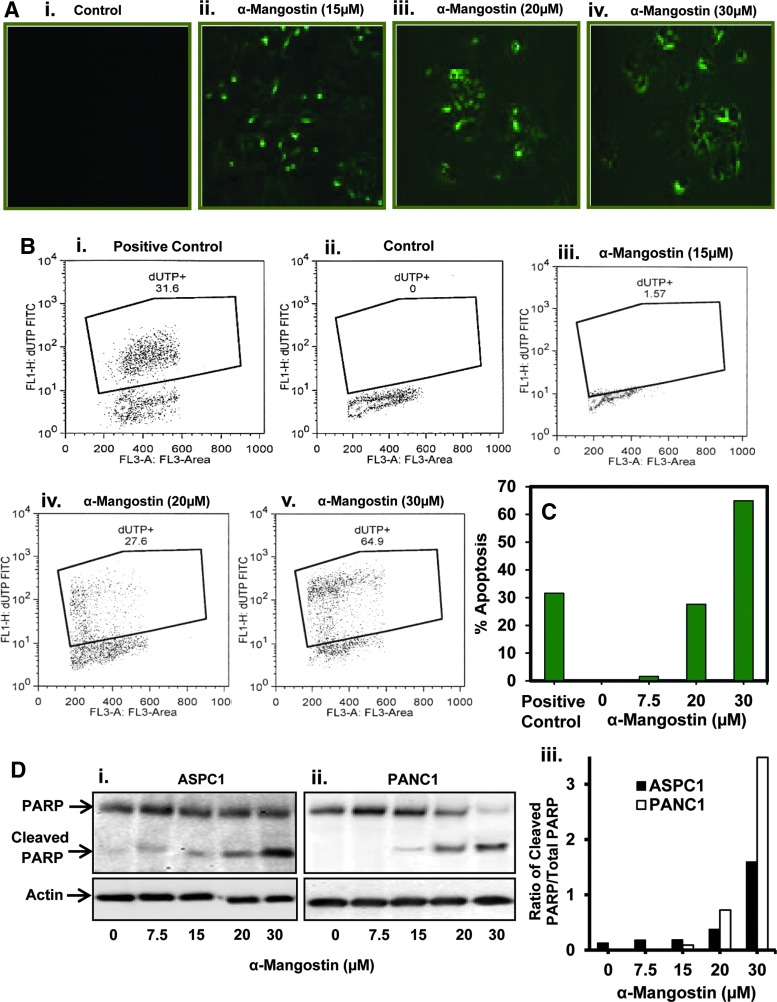
A possibility was explored whether decrease in PC cells viability by α-mangostin is due to the induction of apoptosis. We first analyzed apoptotic PC cells by Annexin V staining. In this experiment (Fig. 2A), cells were serum-starved overnight and treated with α-mangostin for 24 h. Cells were washed with phosphate-buffered saline (PBS) (1×) and then stained with Annexin V. Annexin V-positive cells were examined by fluorescent microscope. We observed that α-mangostin treatment (15–30 μM) resulted in a dose-dependent increase in apoptosis of PANC1 cells (Fig. 2Aii–iv). We also quantified the apoptosis-inducing effects of α-mangostin in PC cells by flow cytometry analysis. α-Mangostin treatment (30 μM) elicited a 64.9% increase in apoptosis of ASPC1 cells at 24 h post-treatment (Fig. 2C). We further examined the effects of α-mangostin on poly B-(ADP-ribose) polymerase (PARP) protein by western blot analysis. Cleavage of PARP protein is considered one of the biomarkers of apoptosis. α-Mangostin treatment showed a dose-dependent increase in the expression of cleaved PARP protein and a decrease expression of total PARP protein in ASPC1 (Fig. 2Di) and PANC1 (Fig. 2Dii) cells. Quantitative analysis of these results showed an increase in the ratio of cleaved PARP and total PARP proteins in α-mangostin-treated PC cells (Fig. 2Diii).
FIG. 2.
Effect of α-mangostin on apoptosis of PC cells. (A) Effect of α-mangostin on apoptosis induction in PC cells as determined by Annexin V staining. Cells were serum starved for overnight and treated with α-mangostin at indicated concentrations for 24 h. α-Mangostin-treated PANC1 cells showing apoptosis as observed by enhanced Annexin V staining (green) by fluorescent microscope. (B) Effect of α-mangostin on apoptosis of ASPC1 cells as assessed by flow cytometry analysis. Cells were serum starved for 24 h and treated with specified concentrations of α-mangostin for 24 h. Induction of apoptosis was quantified by flow cytometry by using APO-DIRECT apoptosis Kit. Representative flow images showing fluorescein-tagged dUTP-nucleotide-labeled cells in positive control cells provided by the kit described previously (Bi), vehicle control (Bii), and α-mangostin-treated (Biii-v) cells. (C) Bar graph represents % apoptotic cells in each group. (D) Effect of α-mangostin on cleaved and total PARP protein levels in PC cells as determined by western blot analysis. Cells were serum starved for 24 h and then treated with the indicated concentrations of α-mangostin for 24 h. Whole cell lysates were prepared for the analyses of total PARP and cleavage of PARP protein levels (Di, ii). Quantitative analysis of total and cleaved PARP proteins (Diii). Bar graph indicates the ratio of total PARP and cleaved PARP proteins. PARP, poly (ADP-ribose) polymerase.
α-Mangostin inhibits the activation of NF-κB signaling in PC cells
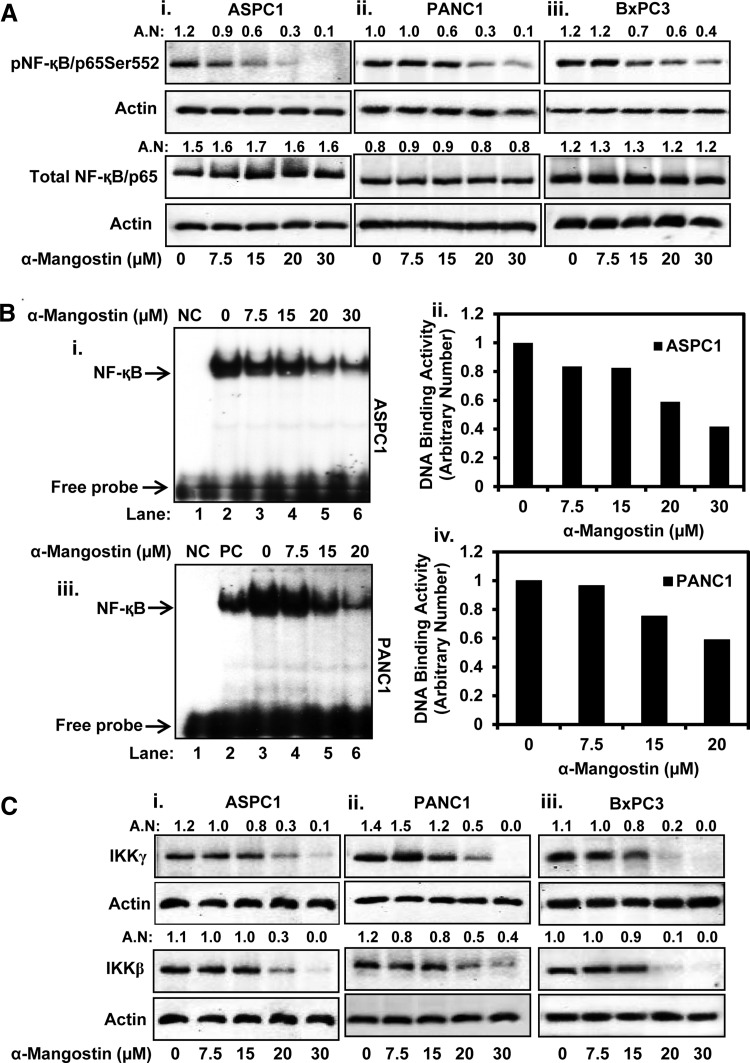
To define the molecular mechanisms of how α-mangostin inhibits PC cells growth and induces apoptosis, we evaluated the effects of α-mangostin on various signaling pathways involved in cell proliferation, chemotherapeutic drug resistance, and inhibition of apoptosis in PC cells. Numerous studies indicate that NF-κB is constitutively activated in most of the human PC (11, 51), which plays a critical role in chemoresistance (4, 18). We observed that α-mangostin dose-dependently inhibited pNF-κB/p65Ser552 in ASPC1 (Fig. 3Ai), PANC1 (Fig. 3Aii), and BxPC3 (Fig. 3Aiii) cells. However, no effect was observed on the expression of total NF-κB/p65 (Fig. 3Ai–iii). α-Mangostin treatment also inhibited NF-κB DNA binding activity in ASPC1 cells (Fig. 3Bi, ii). A similar effect was observed in PANC1 cells (Fig. 3Biii, iv). We further analyzed the effect of α-mangostin on the expression of IKKγ (NEMO) and IKKβ. We observed that α-mangostin inhibited protein levels of IKKγ and IKKβ in ASPC1 (Fig. 3Ci), PANC1 (Fig. 3Cii), and BxPC3 (Fig. 3Ciii) cells.
FIG. 3.
Effect of α-mangostin on NF-κB activation in PC cells. (A) Expression levels of NF-κB/p65Ser552, and total NF-κB/p65 in ASPC1 (Ai), PANC1 (Aii), and BxPC3 (Aiii) cells were determined by the western blot analysis. (B) Effect of α-mangostin on NF-κB DNA binding activity in ASPC1 (Bi, ii) and PANC1 (Biii, iv) cells as determined by the EMSA assays. Lane 1 (NC) of (Bi, iii) EMSA blots represents NC where mutant consensus sequence of NF-κB was used. Lane 2 in blot (Biii) denotes to positive control (PC). Bar graphs shown as (Bii, iv) are the quantitation of (Bi, ii) EMSA blots. (C) Effect of α-mangostin on upstream kinases of NF-κB (IKKβ and IKKγ) in ASPC1 (Ci), PANC1 (Cii), and BxPC3 (Ciii) cells as determined by the western blot analysis. AN denotes arbitrary number, which represents quantitation of the western blots. In (Ci), same actin blot was used to normalize the expression of IKKβ and IKKγ. EMSA, electrophoretic mobility shift assay; NF-κB, nuclear factor kappa B; NC, negative control.
α-Mangostin inhibits constitutive activation of Stat3 in PC cells
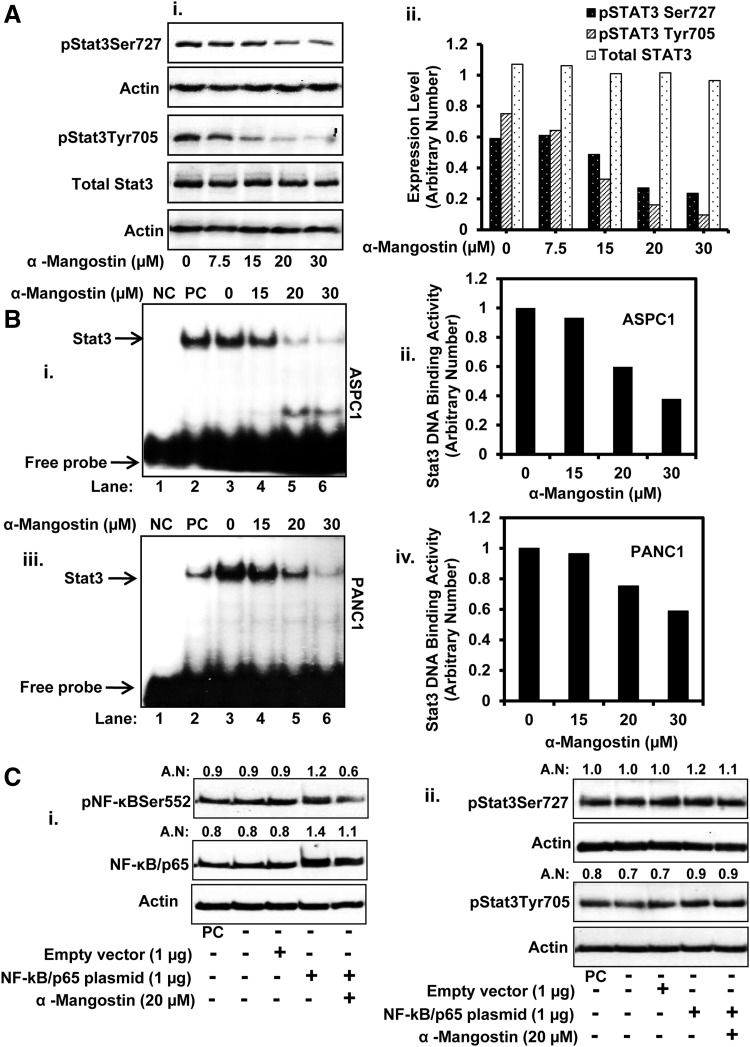
It has been shown that Stat3 activation is involved in PC development and metastasis through the induction of various genes responsible for tumor cell proliferation, cell survival, and carcinogenesis (15, 21, 22). Maximum activation of Stat3 requires phosphorylation at two amino acid residues (Tyr705 and Ser727). Stat3 phosphorylation at Tyr705 residue induces its dimerization through phosphotyrosine-SH-2 domain interaction (55), whereas Stat3 transcriptional activity and DNA binding activity is further enhanced through its phosphorylation at Ser727 residue (52). Therefore, we evaluated the effects of α-mangostin on Stat3 activation. α-Mangostin treatment inhibited protein expression of both pStat3Ser727 and pStat3Tyr705 (Fig. 4Ai, ii), but no effect was observed on total Stat3 protein (Fig. 4Ai, ii). We further assessed the effect of α-mangostin on DNA binding activity of Stat3. α-Mangostin treatment dose-dependently inhibited Stat3 DNA binding activity in ASPC1 (Fig. 4Bi, ii) and PANC1 (Fig. 4Biii, iv) cells. We next examined whether α-mangostin inhibits constitutive activation of Stat3 through NF-κB. ASPC1 cells were transiently transfected with NF-κB/p65 expressing plasmid for 48 h followed by α-mangostin (20 μM) treatment for 12 h. Western blot analysis was performed to determine the expression of pNF-κB/p65Ser552, total NF-κB, pStat3Ser727, and pStat3Tyr705. The overexpression of NF-κB/p65 in ASPC1 cells resulted in an increased expression of both pNF-κB/p65Ser552 (Fig. 4Ci) and total NF-κB (Fig. 4Ci), but with slight increase in Stat3 phosphorylation (Fig. 4Cii). Results also demonstrated that overexpression of NF-κB/p65 in ASPC1 cells abrogated the effects of α-mangostin on constitutive activation of Stat3 (Fig. 4Cii).
FIG. 4.
Effect of α-mangostin on the expression of pStat3Tyr705, pStat3Ser725, and Stat3 DNA binding in PC cells. (A) Effect of α-mangostin on protein level of pStat3Ser727 and pStat3Tyr705 in PANC1 cells (Ai) as determined by the western blot analysis. Bar graph (Aii) represents the quantitative analysis of the western blots. (B) Effect of α-mangostin on Stat3 DNA binding activity in ASPC1 (Bi, ii) and PANC1 (Biii, iv) cells as determined by EMSA. Lane 1 NC in the blots (Bi, Bii) represents the mutant consensus sequence of Stat3. Lane 2 in blot (Bi, ii) denotes positive control (PC). Bar graphs (Bii, iv) are the quantitative analysis of EMSA blots of (Bi, ii). (C) Effect of α-mangostin on NF-κB/p65-induced constitutive activation of Stat3 in PC cells. In brief, 80% confluent ASPC1 cells were transiently transfected with NF-κB/p65 overexpressing plasmids. In a parallel set, cells were also transfected with empty vector. After 48 h transfection, cells were treated with vehicle or α-mangostin for 12 h. Whole cell lysates were prepared and western blots were performed to examine the expression of pNF-κB/p65 (Ci), total NF-κB (Ci), pStat3Ser725 (Cii), and pStat3Tyr705 (Cii). PC in the blots denotes to positive control. All blots were quantified and normalized by actin antibody. AN denotes arbitrary number, which represents quantitation of the western blots. Stat3, signal transducer and activator 3.
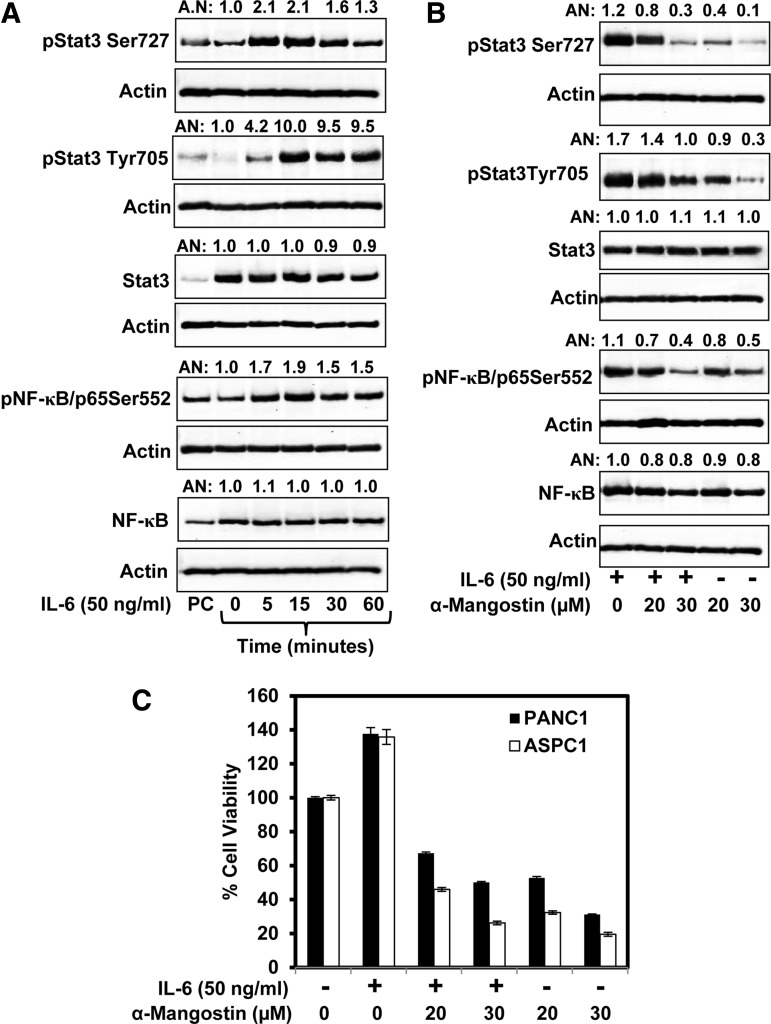
α-Mangostin inhibits interleukin-6-induced activation of Stat3 and NF-κB in PC cells
Interleukin-6 (IL-6) is a proinflammatory cytokine that activates Stat3 in PC cells (11, 12). We explored whether α-mangostin treatment inhibits IL-6-induced activation of Stat3 and NF-κB. We first standardized the IL-6 concentration and time for maximum activation of NF-κB and Stat3 in ASPC1 cells. In this experiment, 70% confluent ASPC1 cells were serum starved for 24 h and then treated with IL-6 (50 ng/ml) for 5, 15, 30, and 60 min. We observed maximum phosphorylation of Stat3 and NF-κB at 15 min post-treatment of IL-6 (Fig. 5A). However, IL-6 treatment showed no change in the expression of total Stat3 and NF-κB (Fig. 5A). To investigate the effect of α-mangostin on IL-6-induced phosphorylation of Stat3 and NF-κB, 70% confluent ASPC cells were treated with IL-6 (50 ng/ml) for 15 min, followed by α-mangostin treatment (20–30 μM) for 12 h. Western blot analysis results demonstrated that α-mangostin treatment inhibited IL-6-induced phosphorylation of both Stat3 and NF-κB (Fig. 5B). However, no change was observed in the expression of total Stat3 and NF-κB (Fig. 5B).
FIG. 5.
Effect of α-mangostin on IL-6 induced expressions of pStat3Tyr705, pStat3Ser725, pNF-κB Ser552, and proliferation of PC cells. (A) Effect of IL-6 treatment on the expression of pStat3Tyr705, pStat3Ser725, pNF-κB Ser552, total Stat3, and total NF-κB as determined by western blot analysis. (B) Effect α-mangostin on IL-6 induced expression of pStat3Tyr705, pStat3Ser725, and pNF-κB Ser552 in ASPC1 cells as determined by western blot analysis. In brief, cells were serum starved for 24 h and treated with IL-6 (50 ng/ml) for 15 min followed by α-mangostin treatment. Whole cell lysates were prepared for western blot analysis as described in the Materials and Methods section. (C) Effect of α-mangostin on IL-6-induced growth of ASPC1 and PANC1 cells as determined by MTT assay. IL-6, interleukin-6.
α-Mangostin inhibits IL-6-induced proliferation of PC cells
We explored whether IL-6 treatment reverses the effect of α-mangostin on PC cells proliferation. MTT assay was performed in ASPC1 and PANC1 cells treated with either IL-6 alone or in combination with α-mangostin at 20 and 30 μM concentrations for 24 h. Results illustrated that IL-6 treatment induced proliferation of both ASPC1 and PANC1 cells, which was significantly reduced with the treatment of α-mangostin at 20 and 30 μM concentrations (Fig. 5C).
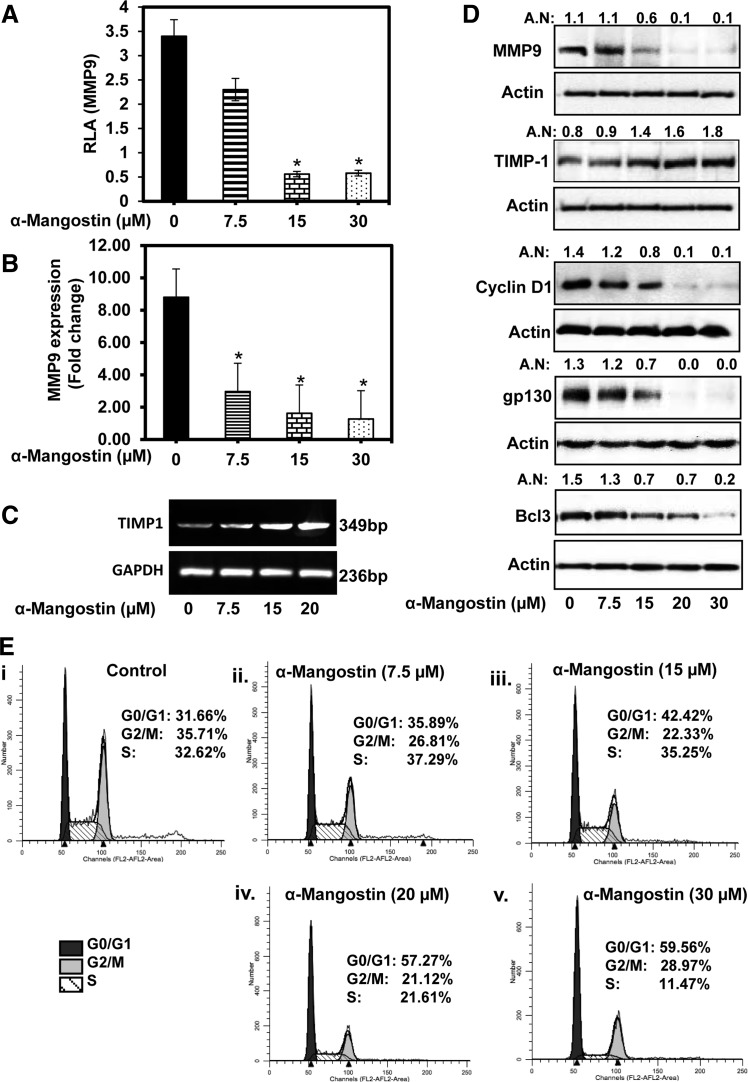
α-Mangostin inhibits the expression of MMP9, cyclin D1, gp130, and Bcl3, and increases TIMP1 expression
α-Mangostin treatment resulted in a significant (p<0.01) decrease in MMP9 promotor activity (Fig. 6A), MMP9 mRNA expression (Fig. 6B), and MMP9 protein levels (Fig. 6D) in PC cells. Also, α-mangostin treatment of PANC1 cells resulted in an increase in tissue inhibitor of metalloproteinase 1 (TIMP1) mRNA (Fig. 6C) and TIMP1 protein expression (Fig. 6D) levels. Treatment with α-mangostin elicited the inhibition of protein levels of cyclin D1 and interleukin-6 receptor (gp130), the downstream target genes of Stat3 (Fig. 6D) in PC cells. Bcl3 is another downstream target gene of Stat3, which regulates the expression of TIMP1 (49). Interestingly, α-mangostin treatment of PC cells resulted in the inhibition of Bcl3 protein levels (Fig. 6D).
FIG. 6.
Effect of α-mangostin on the expressions of MMP9, TIMP1, cyclin D1, gp130, and Bcl3 in PC cells. (A) The effects of α-mangostin on MMP9 promoter activity. Briefly, cells were transiently cotransfected with MMP9 (1 μg) and Renilla luciferase (50 ng) plasmids in a 24-well plate. At 48 h post-transfection, cells were treated with α-mangostin for 12 h. Cells were lysed, and luciferase activity was recorded using plate reader. Luciferase value of MMP-9 promoter was normalized by the value of Renilla luciferase. RLV of MMP9 promoter shown in the bar graph are mean±SE of three wells of each group. (B) Effect of α-mangostin on mRNA expression of MMP9 as analyzed by qRT-PCR. Bar graph shows the fold change in MMP9 mRNA expression normalized to GAPDH. (C) Effect of α-mangostin on mRNA expression of TIMP1 as analyzed by RT-PCR. (D) Effect of α-mangostin on protein expression of MMP9, TIMP1, cyclinD1, gp130, and Bcl3 as analyzed by western blot analysis. (E) Effect of α-mangostin on cell cycle analysis by flow cytometry. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP9, matrix metallopeptidase 9; RLV, relative luciferase values; TIMP1, tissue inhibitor of metalloproteinase 1; qRT-PCR, quantitative real-time polymerase chain reaction.
α-Mangostin arrests cell cycle in G0/G1 phase of cell cycle
In this experiment (Fig. 6E), 70% confluent cells were serum starved for 24 h and then treated with α-mangostin (7.5–30 μM) for 24 h. Cell cycle analyses were performed by flow cytometry. α-Mangostin treatment resulted in an increase arrest of cell cycle in G0/G1 phase of the cell cycle. Results showed 35.89%, 42.42%, 57.27%, and 59.58% cell cycle arrest in G0/G1 phase of cell cycle at 7.5, 15, 20, and 30 μM doses of α-mangostin, respectively, compared to vehicle-treated cells (31.66%) (Fig. 6ii–v). α-Mangostin treatment resulted in a dose-dependent decrease of cells in S phase of the cell cycle compared to vehicle treatment.
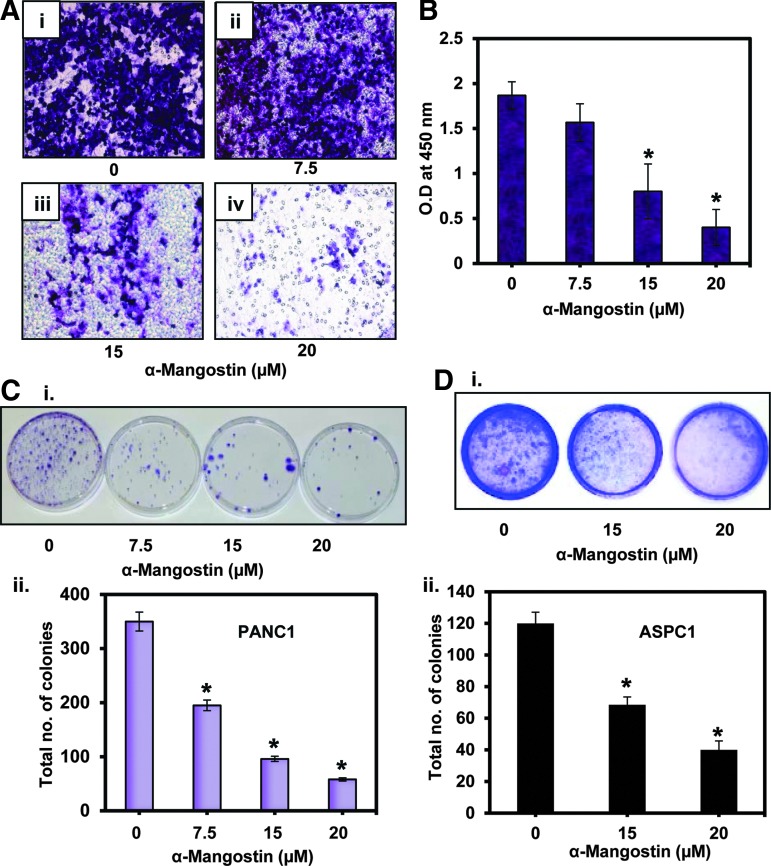
α-Mangostin inhibits the cell invasion and colony formation of PC cells
In this experiment (Fig. 7), cells were treated with increasing doses of α-mangostin for 12 h. Cell viability was determined by the trypan blue exclusion method, and viable cells were taken for the in vitro chemoinvasion assay through an artificial matrix membrane (Millipore, Billerica, MA). We found that α-mangostin treatment significantly inhibited the invasion of ASPC1 cells (Fig. 7A, B). We further determined the effects of α-mangostin on cell proliferation of PC cells. Results demonstrated a significant (p<0.01) decrease in colony formation in PANC1 (Fig. 7Ci, ii) and ASPC1 (Fig. 7Di, ii) cells.
FIG. 7.
Effect of α-mangostin on cell invasion and colony formation of PC cells. (A) Effect of α-mangostin on cell invasion. PANC1 cells were treated with the indicated concentrations of α-mangostin for 24 h. Viable cells, as determined by the trypan blue exclusion method, were used for the invasion assay. Representative photographs of the invading cells through the artificial matrix of control (Ai) and α-mangostin-treated cells (Aii–iv). (B) Bar graph represents quantification of the invaded cells as determined by colorimetric measurement at 490 nm. Values in the graph are the mean±SE of three separate wells of each group. (C) Effect of α-mangostin on colony formation. PANC1 cells (70% confluent) were treated with the indicated concentrations of α-mangostin for 24 h. Media was replaced at every alternate day for 3 weeks. Colonies formed in plates were stained with 0.005% crystal violet and photographed. Shown are the representative pictures of control and α-mangostin-treated group (Ci). Bar graph represents % colonies formation in each group (Cii). (D) Effect of α-mangostin on anchorage-independent growth of ASPC1 cells as assessed by soft agar colony formation. Shown are representative soft agar colony plates of the control and α-mangostin groups (Di). Bar graph represents mean±SE of colonies from four plates from each group (Dii).
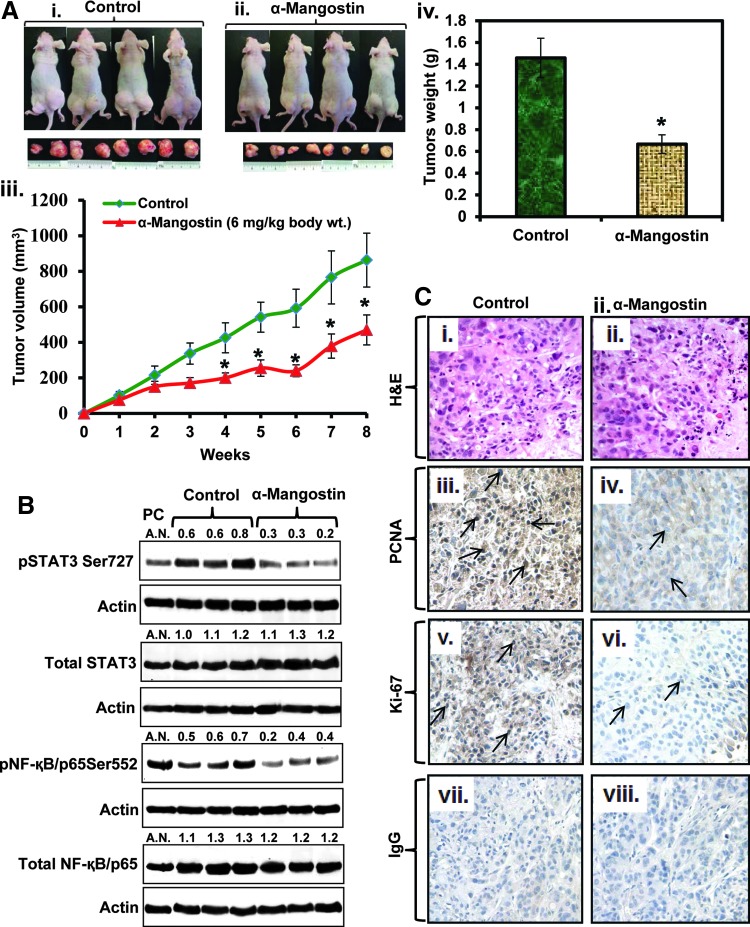
α-Mangostin administration inhibits the growth of ASPC1 cells xenograft tumors in athymic nude mice
In this experiment, highly aggressive ASPC1 cells were ectopically xenografted in athymic nude mice, and 3 days post-xenograft, α-mangostin was administered (6 mg/kg, body weight, i.p. 5 days a week) for 8 weeks. α-Mangostin treatment inhibited the growth of ASPC1 cells xenograft tumors in athymic nude mice as determined by a significant (p=0.0033) difference in tumor volume (Fig. 8Aii, iii) and tumor weight (Fig. 8Aiv) compared to control mice. The average volume of tumors in control mice reached the targeted volume of 1000 mm3 after 8 weeks. At this time, the average tumor volume of α-mangostin-treated mice was only 500 mm3 (Fig. 8Aiii). There was a significant interaction between treatment and time, so differences were tested over time. The observed differences in tumor development were statistically significant (p<0.05) starting at week 4 and continuing until week 8. α-Mangostin (6 mg/kg body weight, i.p.) administration inhibited phosphorylation of both Stat3 and NF-κB in excised xenograft tumor tissues compared with vehicle-treated mice xenograft tumor tissues (Fig. 8B). However, the expression of total Stat3 and NF-κB levels remained unchanged compared to the vehicle-treated group (Fig. 8B). Immunohistochemistry results exhibited a decreased expression of proliferating cell nuclear antigen (PCNA) (Fig. 8Ciii, iv) and Ki-67 (Fig. 8Cv, vi) in excised tumors of α-mangostin-treated mice compared to the tumors of control mice.
FIG. 8.
Effects of α-mangostin on ASPC1 cells xenograft tumors in athymic nude mice. (A) Effects of α-mangostin on ASPC1 cell-derived ectopic xenograft tumors in athymic nude mice. Briefly, ASPC1 cells (2.0×106) were suspended in 1:1 medium fixed with Matrigel and subcutaneously injected on both flanks of the mice. Three days later, the mice (n=8) were treated for 8 weeks with α-mangostin (6 mg/kg body weight, i.p. 5 days a week) in a 0.2-ml PBS containing 25% PEG. Control mice were administered with 0.2 ml of PBS containing 25% PEG. Representative photographs of control (Ai) and α-mangostin-treated (Aii) mice bearing xenograft tumors. (Aiii) Average tumor volume of control and α-mangostin-treated mice was plotted over the weeks. Each value in the tumor growth curve represents mean±SE of 16 tumors. (Aiv) Excised xenograft tumor weights of control and α-mangostin-treated mice after 8 weeks. Values in bar graph represent mean±SE of 16 tumors of each group. Asterisks represent the level of significance (p<0.001). (B) Effect of α-mangostin on the expression of pStat3Ser727, pNF-κB Ser552, NF-κB, and Stat3 in ASPC1 cells xenograft tumors as analyzed by western blot analysis. (C) Effect of α-mangostin on cell proliferation markers (PCNA and Ki-67) in excised xenograft tumors sections. Representative photographs of H&E sections of excised tumor obtained from control (Ci) and α-mangostin-treated (Cii) mice. Representative photographs show the expression levels of PCNA (Ciii, iv) and Ki-67 (Cv, vi) in control and α-mangostin-treated excised xenograft tumors. NC images of control (Cvii) and α-mangostin-treated (Cviii) excised tumor tissues section by using IgG antibody. H&E, hematoxylin and eosin; IgG, immunoglobulin G; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen; PEG, polyethylene glycol.
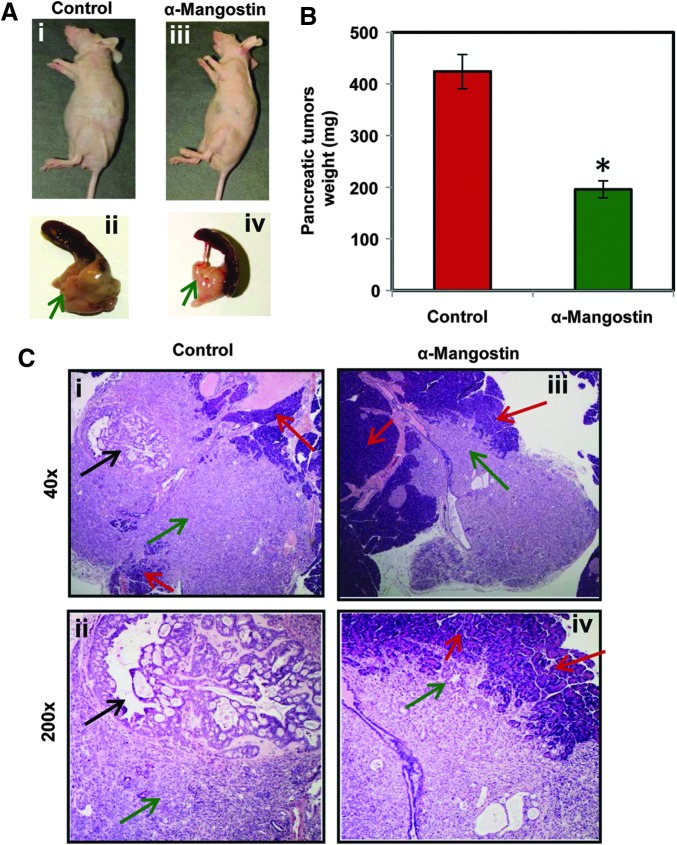
α-Mangostin administration inhibits the growth of primary human PC (PL-45) cell-derived orthotopic xenograft tumors in athymic nude mice
Because of heterogenecity of PC, we also evaluated therapeutic efficacy of α-mangostin in pancreatic orthotopic xenograft mouse model. In this experiment, a total of 20 athymic nude mice were used and 2×106 PL-45 cells xenografted into the pancreas. α-Mangostin treatment was started 3 weeks after cells implantation and continued till 9 weeks. α-Mangostin-treated mice showed the inhibition of orthotopic xenograft tumors, which was examined by weight and histopathology of xenograft tumors (Fig. 9A). We observed a significant (p<0.01) decrease of xenograft tumor weights in α-mangostin-treated mice compared to control (Fig. 9B). Histopathological analysis revealed that all the mice of control group developed poorly differentiated carcinoma in the pancreas, whereas α-mangostin-treated mice showed small and poorly nondifferentiated carcinoma (Fig. 9C). No metastasis was observed into the spleen, lungs, and liver of any mouse from both control and α-mangostin-treated groups, suggesting that PL-45 cells only form localized tumors.
FIG. 9.
Effects of α-mangostin on PL-45 cell-derived pancreatic orthotopic xenograft tumors in athymic nude mice. A total of 20 mice were used and divided into two groups control (n=10) and α-mangostin (n=10). About 2×106 primary human PC cells (PL-45) were implanted into the pancreas of each mouse. α-Mangostin treatment (6 mg/kg body weight i.p. 5 days a week) was started 3 weeks after cells implantation and continued till 9 weeks. Control group mice received 0.2 ml PBS containing 25% PEG. All the mice were sacrificed at 9 weeks. (A) Representative pictures of control (Ai, ii) and α-mangostin-treated (Aiii, iv) mice and their excised pancreatic tumors. (B) Bar graph illustrating pancreatic tumor weight of control and α-mangostin-treated mice. Values in graph represent mean±SE of 10 mice in each group. (C) Histopathological analysis of pancreatic orthotopic xenograft tumors of control and α-mangostin-treated mice. Representatives of H&E-stained slides showing orthotopic human pancreas cancer in mouse pancreas. Red arrow, mouse pancreas; green arrow, poorly differentiated cancer; black arrow, well-differentiated cancer with glandular structure formation.
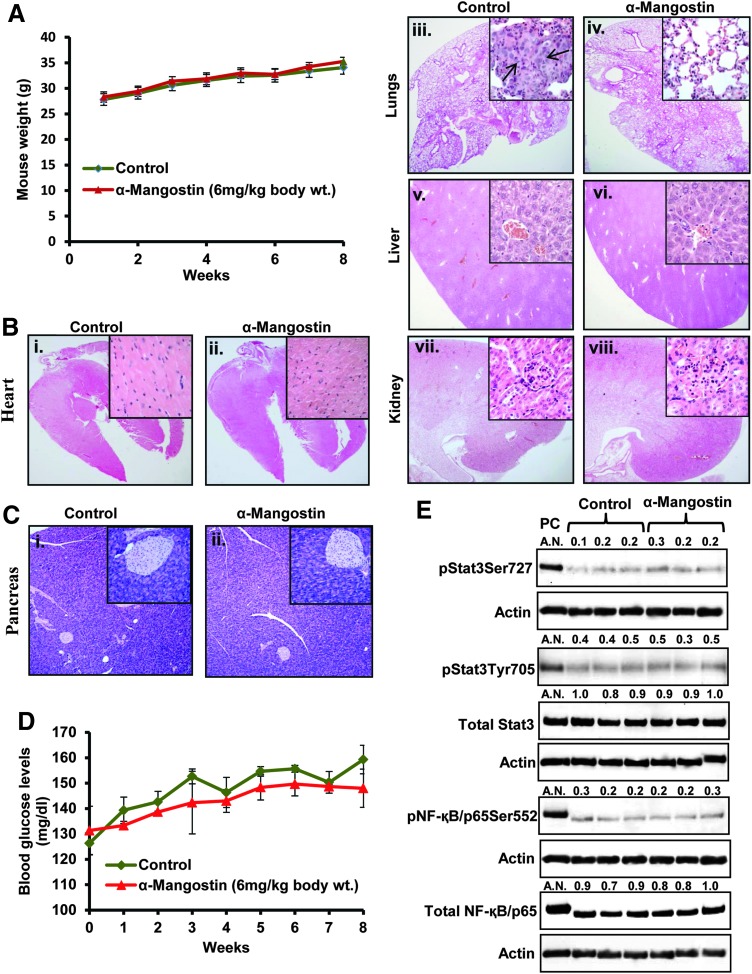
α-Mangostin administration has no systemic toxicity in mice
We evaluated the toxic effects of α-mangostin administration (6 mg/kg body weight, i.p.) in athymic nude mice. Body weight of both control and α-mangostin mice was recorded weekly. Both control and α-mangostin-treated mice showed normal weight gain (Fig. 10A). Histopathological examination revealed no toxic injury of α-mangostin administration in the heart (Fig. 10Bi, ii), lungs (Fig. 10Biii, iv), liver (Fig. 10Bv, vi), or kidneys (Fig. 10Bvii, viii) of any mice. Interestingly, one control mouse was found to have PC cells metastasis into the lungs (Fig. 10Biii). However, no metastasis was observed in any of the α-mangostin-administered mice. In another set of experiment, we evaluated the toxic effects of α-mangostin administration (6 mg/kg body weight i.p.) in pancreatic tissues of C57BL/6 wild-type mice. Results showed no toxic effects of α-mangostin administration in normal pancreatic tissues of wild-type mice as observed by morphological integrity of acinar and islet cells and structures (Fig. 10Ci, ii). We also examined the blood glucose levels weekly of each individual mouse of both groups. We did not observe any significant change in the blood glucose levels in α-mangostin-treated animals (Fig. 10D). However, a normal time-dependent slight increase of blood glucose levels was observed in mice of both α-mangostin- and vehicle-treated groups (Fig. 10D). Next, we determined the effect of α-mangostin administration on the expression of pStat3, pNF-κB/p65, total Stat3, and NF-κB/p65 in excised normal pancreatic tissues of both groups. We observed very low expression of pStat3Tyr705, pStat3Ser727, and pNF-κB/p65Ser552 in the pancreatic tissues. However, constitutive levels of total Stat3 and NF-κB were observed in pancreatic tissues (Fig. 10E). α-Mangostin administration showed no change in the levels of phosphorylation as well as total levels of Stat3 and NF-κB (Fig. 10E). These results suggest that α-mangostin has no effect on these signaling molecules in normal pancreatic tissues.
FIG. 10.
Effect of α-mangostin administration on body weight and systemic toxicity in athymic nude mice. (A) Effect of α-mangostin treatment on the body weight of athymic nude mice. Body weight of each mouse in control and α-mangostin-treated group was recorded weekly. Each value represents the mean±SE of eight mice in each group. (B) Representative photographs of H&E-stained sections of excised heart (Bi, ii), lungs (Biii, iv), liver (Bv, vi), and kidneys (Bvii, viii) of control and α-mangostin-treated mice. H&E-stained tissue sections of the lungs show two small foci of metastatic cancer in one of the control mice (Biii) indicated by black arrow, but no lung metastasis was observed in any of the α-mangostin-treated mice. Magnification: 20×and 400×(insets). (C) Representative photographs of H&E-stained sections of excised pancreas (Ci, ii) of control and α-mangostin-treated wild-type C57BL/6 mice. (D) Line bar graph representing the weekly blood glucose levels in control and α-mangostin-treated mice. Values in the graph represent mean±SE of four mice in each group. (E) Western blot analysis of pStat3Ser727, pNF-κB Ser552, NF-κB, and Stat3 in excised pancreatic tissues of control and α-mangostin-treated wild-type mice pancreatic tissues. Each lane represents individual mouse pancreatic tissue sample of control and α-mangostin-treated mice. ASPC1 cells lysates was used as positive control (PC). AN denotes arbitrary number, which represents the quantitation of the western blots.
Discussion
PC is a significant health problem worldwide, including the United States. Prevention and treatment of PC is a major challenge because of inadequate diagnostic and therapeutic strategies. Despite a greater understanding of the molecular pathways involved in PC development and metastasis, the use of individual targeted agents has failed to provide significant improvements in the survival of PC patients. Numerous studies have shown the involvement of multiple signaling pathways in the development, progression, and metastasis of PC (54). It is evident that natural products, individually or in combination with known chemotherapeutic drugs, have a role in the prevention of PC via targeting multiple signaling pathways (7, 14, 41, 46). In this study, we report that α-mangostin, a dietary agent derived from the pericarp of G. mangostana L, inhibits pancreatic tumor growth probably via inhibiting the activation of Stat3 and NF-κB and their downstream target genes linked to cell proliferation, apoptosis, and metastasis.
Cancer cells proliferate in an uncontrolled manner and are often resistant to apoptosis in response to various chemotherapeutic drugs. Our results indicated the ability of α-mangostin to sensitize PC cells toward apoptosis. α-Mangostin treatment did not show any toxicity to the normal HPDE cells. These results are consistent with previously published reports suggesting selective toxicity of α-mangostin against cancer cells (32).
The transcription factor NF-κB, a regulator of innate immunity and inflammation, represents a molecular bridge between chronic inflammation and development of various types of cancers, including PC (3, 8, 29). In addition, NF-κB is involved in proliferation, cell survival, and invasion of PC cells induced by inflammatory stimuli originated from the tumor microenvironment (34, 38). It has been shown that p65 subunit of NF-κB is constitutively expressed in 67% of human PC but not in normal pancreatic duct epithelial tissues (11, 51). Moreover, suppression of NF-κB activity either by pharmacological inhibitor (5) or by IκBα super-repressors (20) strongly enhanced the apoptosis-inducing potential of chemotherapeutic drugs in resistant PC cells. Therefore, targeting NF-κB is considered to be a very attractive therapeutic approach against PC. Our data illustrated that α-mangostin treatment inhibited NF-κB/p65 phosphorylation and its DNA binding activity in PC cells. α-Mangostin treatment also inhibited NF-κB upstream kinases IKKγ (NEMO) and IKKβ in PC cells, indicating that the inhibition of NF-κB might be due to inhibition of upstream kinases. Overall, these results provide strong evidence that NF-κB is one of the molecular targets of α-mangostin in PC cells.
It has been reported that functions mediated by NF-κB are at least partially performed in cooperation with Stat3 (24). This interaction occurs at several subcellular levels and in a highly context-dependent manner. NF-κB controls the expression of Stat3 induced by tumor microenvironment cytokines and growth factors, most notably the proinflammatory cytokine IL-6 (25, 56), which has been reported to be elevated in the serum of PC patients (10). A study has shown that IL-6/Stat3 signaling pathways promote pancreatic intraepithelial neoplasia (PanIN) progression and PC development (21). Taken together, these studies suggest co-existence and cross talk between NF-κB and Stat3 signaling pathways in PC. Our data are significant as we observed that α-mangostin treatment of PC cells inhibited Stat3 activation, which was assessed by decreased phosphorylation of both Tyr705 and Ser727 residues and inhibition of DNA binding activity of Stat3. These findings clearly demonstrate that Stat3 is another molecular target of α-mangostin in PC cells. However, further in-depth study is required to explore whether α-mangostin directly or in cooperation with NF-κB inhibits Stat3 signaling in PC cells. Our results also indicate the similar effects of α-mangostin administration on NF-κB and Stat3 phosphorylation in ASPC1 cells xenograft tumors tissues. α-Mangostin administration did not elicit the inhibition of NF-κB and Stat3 phosphorylation in normal pancreatic tissues of wild-type mice. Taken together, these results suggest that α-mangostin selectively inhibit Stat3 and NF-κB in PC cells without affecting normal pancreatic tissues. Also, overexpression of NF-κB/p65 in ASPC1 cells abrogated the effects of α-mangostin on constitutive activation of Stat3. These results provide evidence that α-mangostin targets Stat3 signaling partially through the NF-κB pathway.
MMPs are involved in proteolysis of extracellular matrix, forcing cancer cell invasion, establishing metastatic deposit, and in angiogenesis, which is required for tumor growth (23). A recent study has shown the correlation of MMP9 expression with the higher grade of human PC (19). Our data demonstrated a significant decrease in mRNA and protein expression of MMP9 in PC cells. These results are in accord with the previously published reports, where α-mangostin treatment resulted in the inhibition of MMPs activation in prostate cancer cells (37, 48). TIMPs occur naturally within the extracellular matrix, which inhibit both pro- and active-MMPs and provide a homeostatic environment in the matrix. Evidence indicate that overexpression of TIMP1 or TIMP2 in PC cells inhibits the invasion of PC cells in vitro and tumor growth in vivo (45). Our results indicated a significant increase of both mRNA and protein expression of TIMP1 in α-mangostin-treated PC cells. One may conclude that the inhibition of MMP9 expression by α-mangostin is the result of increased TIMP1 expression in PC cells. Moreover, in vitro chemoinvasion functional assay results also demonstrated the anti-invasive potential of α-mangostin against PC cells, which may be due in part to MMP9 inhibition and the increase of TIMP1 expression in PC cells.
Because induction of apoptosis, inhibition in growth, and invasion of PC cells were observed under in vitro conditions, we asked whether these findings could be translated into in vivo situations. An i.p. administration of α-mangostin (6 mg/kg body weight) to athymic nude mice showed significant inhibition of ASPC1 cell-derived ectopic xenograft tumors. In addition, α-mangostin also inhibited human primary PC (PL-45) cell-derived orthotopic xenograft tumors in athymic nude mice. These results in both the models suggest the therapeutic potential of α-mangostin against PC. We did not observe any metastasis in any of the mouse in both the groups suggesting that PL-45 cells form only localized tumors.
It is important in drug development that the therapeutic agent should be nontoxic or minimally toxic. Previous study has shown no toxicity in rats when α-mangostin was administered 200 mg/kg body weight by oral gavage for up to 8 days (16). Our results also suggested no toxicity of α-mangostin administration (6 mg/kg body weight i.p.) in the normal pancreatic tissues of C57BL/6 wild-type mice. Our data of histopathological analysis of excised organs (heart, lungs, liver, and kidneys) demonstrated no toxicity in any of the α-mangostin-treated mice, which indicates the use of α-mangostin as a safe therapeutic agent against PC.
In summary, PC is a devastating disease, which has no successful therapy. Thus, there is an urgent need to discover novel nontoxic agents, which could be used in the treatment of human PC. This study provides evidence that dietary antioxidant α-mangostin has potential anticancer activity against PC, which is, in part, due to the inhibition of NF-κB, Stat3, and MMP9 signaling pathways. We suggest that α-mangostin may be used for the prevention and/or treatment of human PC.
Materials and Methods
Cell lines
Human PC cell lines PANC1, BxPC3, and PL-45 (primary PC cell) were obtained from American Type Culture Collection (Manassas, VA). PANC1 and BxPC3 cells were cultured in the DMEM high-glucose or RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin and streptomycin), respectively. ASPC1 cells were a kind gift from Prof. Fazlul H. Sarkar (Wayne State University, Detroit, MI) and cultured in the same medium as PANC1 cells. Immortalized HPDE-HPV-16E6/E7 cells were purchased from Applied Biological Materials (ABM), Inc. (Richmond, Canada) and were cultured in the DMEM high-glucose medium containing 10% FBS and 1% antibiotics (penicillin and streptomycin).
Chemicals and antibodies
α-Mangostin >95% pure was purchased from ChromaDex (Irvin, CA). Actin, Bcl3, cyclin D1, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), total NF-κB/p65, MMP9, PCNA, Ki-67, and total Stat3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse and rabbit immunoglobulin G (IgG) were also procured from Santa Cruz Biotechnology. Antibodies specific to pStat3Tyr705 and pStat3Ser727 were obtained from BD Biosciences (San Jose, CA). Antibodies specific to cleaved PARP and total PARP, IKKγ, IKKβ, and pNF-κB/p65Ser536 were purchased from Cell Signaling Technology (Danvers, MA). NF-κB/p65 overexpressing plasmid was a kind gift from Prof. Shigeki Miyamoto, Department of Oncology, UW-Madison, WI. Recombinant IL-6 was purchased from Sigma-Aldrich (St. Louis, MO).
Cell viability
HPDE and PC cells (PANC1, BxPC3, and ASPC1) were cultured in 24-well plates. After 70% confluence, cells were treated with α-mangostin (5–20 μM) for 24 h. Cell viability was determined by MTT assay as described earlier (26). In a separate experiment, MTT assay was performed to determine the effects of α-mangostin on IL-6-induced proliferation of ASPC1 and PANC1 cells. Approximately 70% confluent cells were treated with IL-6 (50 ng/ml) alone or 20 and 30 μM concentrations of α-mangostin alone or in combination of IL-6 (50 ng/ml) and with α-mangostin (20 and 30 μM) for 24 h.
Intracellular ROS/RNA analysis using DCFH-DA
This assay measures various ROS/RNS, such as hydroxyl radical, H2O2, and peroxynitrite (28). Approximately 70% confluent ASPC1 and PANC1 cells in 96-well culture plates were treated with increasing concentrations of α-mangostin and assayed for the estimation of ROS levels in intact cells using the fluorescent DCF dye (Invitrogen Grand Island, NY). In brief, cell cultures were washed with 200 μl Kreb's Ringer buffer (20 mM HEPES, 10 mM dextrose, 127 mM NaCl, 5.5 mM KCl, 1 mM CaCl2, 2 mM MgSO4), prewarmed to 37°C and then incubated at 37°C in 100 μl Kreb's Ringer buffer containing 10 μg/ml DCFH-DA dye for 60 min. Each 96-well culture plate was scanned on a Biotek Synergy 4 Plate Scanner (Biotek Winooski, VT) using the 485/530 nm filter excitation and emission set.
Hoechst DNA assay
For DNA analysis, each culture plate from the DCF oxidation assay was equilibrated to room temperature in the dark. Without aspirating DCF solution, Hoechst dye (final concentration: 20 μg/ml) was added to each well in 200 μl of high-salt TNE buffer (10 mM Tris, 1 mM EDTA, 2 M NaCl [pH 7.4]). Plates were incubated in the dark at room temperature for 24 h and then scanned on a Biotek Synergy-4 Plate Scanner using the 360/460 nm filter excitation and emission set. The DCF fluorescence units were normalized to the Hoechst-DNA fluorescence units for each well and used as a measure of the level of ROS being generated.
Hydrogen peroxide assay
Cell releasing H2O2 and extracellular H2O2 levels in ASPC1 cells were measured using Invitrogen Amplex Red Hydrogen Peroxide Assay Kit. All the procedures were followed as per the manufacturer's protocol. In brief, ASPC1 cells were treated with various concentrations of α-mangostin for 12 h. Both cells culture media and total cells were collected. Cells were washed three times in PB (0.143 M sodium phosphate, 6.3 mM EDTA, pH: 7.5) and collected in PB. 1.5×104 cells were taken for the assay to measure cell releasing H2O2. Twenty microliters of cell culture media was used for the assay to measure the extracellular H2O2 levels. Obtained fluorescent units were calculated as per cell or per microliter cell culture media, which represent the amount of H2O2 produced inside the cells or in cell culture media.
Detection of apoptosis
Apoptosis-inducing effect of α-mangostin on PC cells was analyzed by Annexin-V-FLUOS Kit (Roche, Indianapolis, IN). In brief, PANC1 cells were cultured in two chamber slides up to 70% confluent and treated with α-mangostin (15–30 μM) for 24 h. Cells were washed with PBS (1×) and kept in the Annexin-V solution for 20 min. Photographs were captured under fluorescent microscope. For quantitative analysis of apoptosis, ASPC1 cells were cultured in a 100-mm Petri dish. Approximately 70% confluent cells were serum starved for 24 h and treated with α-mangostin (5–30 μM) for 24 h. Apoptosis was quantified by APO-DIRECT Kit (BD Pharmingen™, San Jose, CA).
NF-κB/p65 overexpression
Approximately 80% confluent ASPC1 cells were transiently transfected with 1 μg of NF-κB/p65 overexpressing plasmid by using transfecting agent (Lipofectamine 2000) (Invitrogen). In a parallel set, cells were also transfected with empty vector pcdna3.1 (Invitrogen). Forty-eight hours post-transfection, cells were treated with α-mangostin (20 μM) for 12 h. Whole cell lysates were prepared for western blot analysis.
Cell cycle analysis
Effect of α-mangostin on cell cycle distribution of ASPC1 cells was analyzed by flow cytometry as described (26). In brief, 70% confluent cells were serum starved for 24 h and then treated with α-mangostin (7.5–30 μM) for 24 h. Flow cytometry was performed with the FACScan (Becton Dickinson). A minimum of 10,000 cells per sample were counted and the DNA histograms were further analyzed using ModiFitLT software (Verity Software House) for cell cycle analysis.
Western blot analysis
ASPC1, PANC1, and BxPC3 cells were cultured with their respective media described previously. Approximately 70% confluent cells were treated with α-mangostin (7.5, 15, 20, and 30 μM) for 12 h. Control cells were treated with vehicle (1:10 μl ratio of DMSO and PBS). To determine the effects of α-mangostin on IL-6-induced activation of NF-κB and Stat3, ∼70% confluent ASPC1 cells were serum starved for 24 h. Cells were treated with IL-6 (50 ng/ml) for 15 min followed by α-mangostin (20 and 30 μM) treatment for 12 h. Total cell lysates were prepared for western blot analysis as described (27). All blots were developed using Amersham's enhanced chemiluminescence reagent using FOTO/Analyst Luminary Work Station (Fotodyne, Inc., Hartland, WI). Blots were quantitated by densitometric analysis using TotalLab Nonlinear Dynamic Image analysis software (Nonlinear USA, Inc., Durham, NC).
Electrophoretic mobility shift assay
PC cells (ASPC1 and PANC1) were treated with α-mangostin (7.5, 15, 20, and 30 μM) for 12 h. Control cells were treated with vehicle described previously (27). Nuclear extracts were prepared as described (27). Electrophoretic mobility shift assay (EMSA) was performed for DNA binding activity of Stat3 and NF-κB as described (27).
Luciferase assay
About 70%–80% confluent PANC1 cells were transiently transfected with human MMP9 luciferase reporter plasmid (pGL3-MMP9, 1 μg), a gift from Dr. Dougles D. Boyden (MD Anderson Cancer Center, Houston, TX) along with 50 ng of Renilla luciferase reporter plasmid pRL-TK (Promega, Madison, WI) using transfecting agent (Lipofectamine 2000) (Invitrogen). Forty-eight hours post-transfection, cells were treated with α-mangostin (7.5, 15, and 30 μM) for 12 h. Dual Luciferase Assay Reagent Kit was procured from Promega. Cells were harvested using 100 μl of the lysis buffer (1×) provided along with the assay kit. Luciferase activity was measured on plate reader (BioTek Synergy-4). Results are expressed as relative luciferase activity of MMP9.
Quantitative real-time polymerase chain reaction
About 70%–80% confluent PC cells were treated with α-mangostin (7.5–30 μM) for 12 h. Cells were harvested for RNA preparation. The cDNA was synthesized from 1.0 μg RNA using SuperScript First-Strand Synthesis Kit (Invitrogen). All the instructions were followed as per kit protocol. The following primer sets for MMP9 and GAPDH were used for the assay. MMP9 forward primer: 5′-gcggagattgggaaccagctgta-3′, reverse primer: 5′-gacgcgcctgtgtacacccaca-3′ and GAPDH forward primer: 5′-gtctcctctgacttcaacagcg-3′, reverse primer: 5′-accaccctgttgctgtagccaa-3′. Briefly, a total of 50 μl reaction mixture consisted of 25 μl of 2×FastStart Universal SYBR Green master mix, 30.0 pmol (2 μl) of forward and reverse primers, polymerase chain reaction (PCR) grade water, and 50–100 ng of cDNA (3–5 μl). The cDNA was amplified with an initial denaturation at 95°C for 3.0 min followed by sequential cycles of denaturation at 94°C for 10 s and annealing at 55°C for 30 s. A total of 40 cycles were run. Differences in Ct values of positive control (GAPDH) and α-mangostin-treated samples were used to determine the relative or fold change MMP9 expression.
Semiquantitative RT-PCR
PCR reactions were carried out using forward and reverse primer combinations for TIMP1 (forward, 5′-GACCTACACTGTTGGCTGTGAG-3′; reverse, 5′-AAGAAAGATGGGAGTGGGAACAGG-3′) and GAPDH (forward, 5′-aatcccatcaccatcttccaggag-3′; reverse, 5′gcattgctgatgatcttgaggctg-3′). PCR reaction standardization kits were obtained from Epicentre (Madison, WI). The cDNA was amplified with an initial denaturation at 94°C for 2 min followed by sequential cycles of denaturation at 94°C for 45 s, annealing at 59°C for 45 s, and extension at 72°C for 1 min for 30 cycles, with a final extension at 72°C for 7 min.
In vitro chemoinvasion assay
Approximately 70% confluent cells were treated with α-mangostin (5–30 μM) for 24 h. Equal numbers of viable cells from each group were taken for the assay. All the procedures were followed according to the manufacturer instructions (Chemicon International, Temecula, CA).
Colony formation
PANC1 cells (8×103) were seeded in tissue culture dishes and treated with desired concentrations of α-mangostin for 24 h. The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humid environment. Colonies that formed in 3 weeks were fixed with 10% buffered formalin, stained with 2% gentian violet (w/v methanol solution), washed with water, and air-dried.
In another experiment, anchorage-independent growth of ASPC1 cells was determined by soft agar colony formation assay. In brief, 70% confluent cells were treated with vehicle or α-mangostin (5–30 μM) for 24 h, and 100% viable cells were taken for the assay. Briefly, equal volumes of agar (1%, DNA grade) and DMEM (with 20% FBS) were mixed at 40°C to make 0.5% agar in 35-mm tissue culture plates (Corning, Tewksbury, MA) as a base agar. Cells (0.1 ml of 2.0×105/ml) were suspended in 3 ml of DMEM (with 20% FBS) mixed with 3 ml of 0.7% agar, and then, 1.5 ml of cell suspension was added to each well (as 0.35% top agar) with final concentration of 5000 cells/well. Top agar was covered with the culture medium and was incubated at 37°C and 5% CO2 in humidified incubator for 2 weeks. Colonies were counted and photographed after staining with 0.005% crystal violet.
Ectopic xenograft study
Six-week-old athymic nude mice were purchased from Harlan Laboratory (Madison, WI), housed under pathogen-free environment with a 12-h light/12-h dark schedule, and fed with an autoclaved diet and water ad libitum. To establish ectopic xenograft tumors in mice, ASPC1 cells (2.0×106) were suspended in 1:1 medium fixed with Matrigel and subcutaneously injected on both flanks of the mice. Three days later, the mice (n=8) were treated with an i.p. injection (5 days in a week) of α-mangostin (6 mg/kg body weight) in 0.2 ml of PBS containing 25% polyethylene glycol (PEG) for 8 weeks. Control group mice (n=8) were administered with 0.2 ml of PBS containing 25% PEG. Mice were weighed and tumors were measured weekly by a caliper. Tumor volumes were calculated by the formula 0.5238×L×W×H, where L is length, W is width, and H is the height of the tumor. All the mice were euthanized when tumors of the control group reached the targeted volume of 1000 mm3.
Orthotopic xenograft study
A total of 20 athymic nude mice were used for the study. To establish orthotopic xenograft tumors, we used human primary PC cells (PL-45). In brief, cells were harvested from subconfluent culture and washed once in the serum-free medium and suspended in HBSS. Only single cell suspension with >90% viability were used for the injection. The pancreas of anaesthetized mice was exposed through a midline laparotomy incision and by retraction of the spleen. Cells (2.0×106) in 50 μl of HBSS containing 1% (v/v) Matrigel were injected into the parenchyma of the pancreas with 27-gauge hypodermic needle and a Hamilton syringe. The abdominal wound was closed by suture followed by clipping. Two weeks later, the mice (n=10) were treated with an i.p. injection (5 days in a week) of α-mangostin (6 mg/kg body weight) in 0.2 ml of PBS containing 25% PEG for 8 weeks. Control group mice (n=10) were administered with 0.2 ml of PBS containing 25% PEG. All mice of both the groups were sacrificed at 9 weeks, and orthotopic tumors were excised for weight measurement and histopathological analysis.
Statistical analysis
A mixed repeated measures model was used to model log-transformed tumor volume using the GLIMMIX procedure, in SAS software, Version 9.2 of the SAS System for Unix, copyright © 2008 SAS Institute, Inc. The variance between the tumor volume measures on the right and left sides was incorporated as a subsampling error nested within the overall experimental error using a compound symmetry structure; the covariance chosen for the overall experimental error (mouse over time) was an autoregressive covariance structure.
Histopathological examination
The heart, liver, lungs, kidneys, and pancreas from control and α-mangostin-treated mice were fixed in 10% neutral buffered formalin, transferred to PBS (pH 7.4) and then sectioned in 4 μm thickness. Slides were stained with hematoxylin and eosin (H&E) and examined by Dr. Weixiong Zhong, a certified pathologist in the Department of Pathology, University of Wisconsin-Madison. In other experiment, the effect of α-mangostin on normal pancreatic tissues was analyzed. Six-week-old eight wild-type mice (C57BL/6 back ground) were divided into two groups. Mice were treated with α-mangostin and vehicle for 8 weeks as described previously in the xenograft study. Mice of both groups were euthanized, and their pancreas was excised for tissue sectioning and whole tissue lysates preparation.
Blood glucose level
Blood was collected weekly from the tail of each mouse. Blood glucose levels were examined using the Contour Blood Glucose Monitoring System (Mishawaka, IN).
Immunohistochemistry
Part of the excised ASPC1 xenograft tumors from control and α-mangostin-treated mice was fixed in 10% neutral buffered formalin, transferred to PBS (pH 7.4), and then sectioned into 4 μm thickness. These section slides were used for immunohistochemistry of PCNA and Ki-67 as described previously (26).
Abbreviations Used
- DCFH-DA
2′7′-dichlorofluorescein diacetate
- EMSA
electrophoretic mobility shift assay
- FBS
fetal bovine serum
- FDA
Food and Drug Administration
- 5-FU
5-fluorouracil
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Gp130
interleukin-6 receptor
- H&E
hematoxylin and eosin
- HPDE
human pancreatic duct epithelial
- IgG
immunoglobulin G
- IκBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- IKKβ
inhibitor of nuclear factor kappa-B kinase subunit beta
- IKKγ
inhibitor of nuclear factor kappa-B kinase subunit gamma
- IL-6
interleukin-6
- i.p.
intraperitoneal
- MAPK
MAP kinase
- MMP
matrix metallopeptidase
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide
- NC
negative control
- NF-κB
nuclear factor kappa B
- PanIN
pancreatic intraepithelial neoplasia
- PARP
poly (ADP-ribose) polymerase
- PBS
phosphate-buffered saline
- PC
pancreatic cancer
- PCNA
proliferating cell nuclear antigen
- PEG
polyethylene glycol
- qRT-PCR
quantitative real-time polymerase chain reaction
- RLV
relative luciferase values
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SH-2
Src homology 2
- Stat3
signal transducer and activator 3
- TIMP1
tissue inhibitor of metalloproteinase 1
Acknowledgments
We acknowledge Department of Human Oncology Pilot Project grant. We are thankful to Nancy E. Dreckschmidt for technical support in EMSA. We are also thankful to Dr. Hirak Basu and Dr. Luksana Chaiswing for helping us in antioxidant and oxidative stress experiments.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aisha AF, Abu-Salah KM, Ismail Z, and Majid AM. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement Altern Med 12: 104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Akao Y, Nakagawa Y, Iinuma M, and Nozawa Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int J Mol Sci 9: 355–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algül H, Adler G, and Schmid RM. NF-kappaB/Rel transcriptional pathway: implications in pancreatic cancer. Int J Gastrointest Cancer 31: 71–78, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, and Schäfer H. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene 22: 3243–3251, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Arlt A, Vorndamm J, Breitenbroich M, Fölsch UR, Kalthoff H, Schmidt WE, and Schäfer H. Inhibition of NF-kappaB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene 20: 859–868, 2001; Erratum in: Oncogene 21:2611, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Aziz MH, Manoharan HT, and Verma AK. Protein kinase C epsilon, which sensitizes skin to sun's UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3. Cancer Res 67: 1385–1394, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Wang Z, Kong D, and Sarkar FH. 3,3′-diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res 69: 5592–5600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Billadeau DD. Primers on molecular pathways. The glycogen synthase kinase-3beta. Pancreatology 7: 398–402, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Block KM, Hanke NT, Maine EA, and Baker AF. IL-6 stimulates STAT3 and Pim-1 kinase in pancreatic cancer cell lines. Pancreas 41: 773–781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Yang G, Jiang T, Huang K, Cao J, and Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res 29: 51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler NM, Canete JJ, and Callery MP. Increased expression of NF-kappa B subunits in human pancreatic cancer cells. J Surg Res 118: 9–14, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chao AC, Hsu YL, Liu CK, and Kuo PL. α-Mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J Agric Food Chem 59: 2086–2096, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Chen LG, Yang LL, and Wang CC. Anti-inflammatory activity of Mangostins from Garcinia mangostana. Food Chem Toxicol 46: 688–693, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, and Saluja AK. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4: 156ra139, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darnell JE. Validating Stat3 in cancer therapy. Nat Med 11: 595–596, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Devi Sampath P. and Vijayaraghavan K. Cardioprotective effect of alpha-Mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol 21: 336–339, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Doi H, Shibata MA, Shibata E, Morimoto J, Akao Y, Iinuma M, Tanigawa N, and Otsuki Y. Panaxanthone isolated from pericarp of Garcinia mangostana L. suppresses tumor growth and metastasis of a mouse model of mammary cancer. Anticancer Res 29: 2485–2495, 2009 [PubMed] [Google Scholar]
- 18.Dong QG, Sclabas GM, Fujioka S, Schmidt C, Peng B, Wu T, Tsao MS, Evans DB, Abbruzzese JL, McDonnell TJ, and Chiao PJ. The function of multiple IkappaB NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene 21: 6510–6519, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Durlik M. and Gardian K. Metalloproteinase 2 and 9 activities in the development of pancreatic cancer. Pol Przegl Chir 84: 377–382, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Fujioka S, Sclabas GM, Schmidt C, Niu J, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, and Chiao PJ. Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene 22: 1365–1370, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Fukuda A, Wang SC, Morris JP, 4th, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, and Hebrok M. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 19: 441–455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germain D. and Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res 13: 5665–5669, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Gialeli C, Theocharis AD, and Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278: 16–27, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, and Schmid RM. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology 123: 2052–2063, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Grivennikov SI. and Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev 20: 65–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafeez BB, Imtiaz IA, Asim M, Malik A, Afaq F, Adhami VM, Saleem M, and Mukhtar H. Delphinidin, a major anthocyanidin present in pigmented fruits and vegetables inhibits the prostate cancer cells xenograft in athymic nude mice. Cancer Res 68: 8564–8572, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafeez BB, Jamal MS, Fischer JW, Mustafa A, and Verma AK. Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-κB signaling pathways. Int J Cancer 131: 2175–2186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliwell B. and Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142: 231–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holcomb B, Yip-Schneider M, and Schmidt CM. The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy. Pancreas 36: 225–235, 2008 [DOI] [PubMed] [Google Scholar]
- 30.This reference has been deleted.
- 31.Hung SH, Shen KH, Wu CH, Liu CL, and Shih YW. Alpha-mangostin suppresses PC-3 human prostate carcinoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen expression through the JNK signaling pathway. J Agric Food Chem 57: 1291–1298, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM, and Mukhtar H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 33: 413–419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Key TJ. Fruit and vegetables and cancer risk. Br J Cancer 104: 6–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong R, Sun B, Jiang H, Pan S, Chen H, Wang S, Krissansen GW, and Sun X. Downregulation of nuclear factor-kappaB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett 291: 90–98, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Krajarng A, Nakamura Y, Suksamrarn S, and Watanapokasin R. α-Mangostin induces apoptosis in human chondrosarcoma cells through downregulation of ERK/JNK and Akt signaling pathway. J Agric Food Chem 59: 5746–5754, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Kurose H, Shibata MA, Iinuma M, and Otsuki Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with α-mangostin extracted from mangosteen pericarp. J Biomed Biotechnol 2012: 672428, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YB, Ko KC, Shi MD, Liao YC, Chiang TA, Wu PF, Shih YX, and Shih YW. alpha-Mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the ERK signaling pathway in MCF-7 human breast adenocarcinoma cells. J Food Sci 75: H13–H23, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, and Wirth T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett 295: 214–228, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto K, Akao Y, Yi H, Ohguchi K, Ito T, Tanaka T, Kobayashi E, Iinuma M, and Nozawa Y. Preferential target is mitochondria in alpha-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorg Med Chem 12: 5799–5806, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, and Parulekar W. National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25: 1960–1966, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Murtaza I, Saleem M, Adhami VM, Hafeez BB, and Mukhtar H. Suppression of cFLIP by lupeol, a dietary triterpene, is sufficient to overcome resistance to TRAIL-mediated apoptosis in chemoresistant human pancreatic cancer cells. Cancer Res 69: 1156–1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nabandith V, Suzui M, Morioka T, Kaneshiro T, Kinjo T, Matsumoto K, Akao Y, Iinuma M, and Yoshimi N. Inhibitory effects of crude alpha-Mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1, 2-dimethylhydrazine in the rat. Asian Pac J Cancer Prev 5: 433–438, 2004 [PubMed] [Google Scholar]
- 43.Obolskiy D, Pischel I, Siriwatanametanon N, and Heinrich M. Garcinia mangostana L.: a phytochemical and pharmacological review. Phytother Res 23: 1047–1065, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Park JK, Ryu JK, Lee JK, Yoon WJ, Lee SH, Kim YT, and Yoon YB. Gemcitabine chemotherapy versus 5-fluorouracil-based concurrent chemoradiotherapy in locally advanced unresectable pancreatic cancer. Pancreas 33: 397–402, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Rigg AS. and Lemoine NR. Adenoviral delivery of TIMP1 or TIMP2 can modify the invasive behavior of pancreatic cancer and can have a significant antitumor effect in vivo. Cancer Gene Ther 8: 869–878, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Sahu RP. and Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst 101: 176–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakagami Y, Iinuma M, Piyasena KG, and Dharmaratne HR. Antibacterial activity of alpha-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine 12: 203–208, 2005 [DOI] [PubMed] [Google Scholar]
- 47a.Shibata M, Iinuma M, Morimoto J, Kurose H, Akamatsu K, Okuno Y, Akao Y, Otsuki Y. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 9: 69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shih YW, Chien ST, Chen PS, Lee JH, Wu SH, and Yin LT. Alpha-mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3 integrin/FAK/ERK and NF-kappaB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem Biophys 58: 31–44, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Siegel R, Naishadham D, and Jemal A. Cancer statistics, 2012. CA Cancer J Clin 62: 10–29, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Wakefield A, Soukupova J, Montagne A, Ranger J, French R, Muller WJ, and Clarkson RW. Bcl3 selectively promotes metastasis of ERBB2-driven mammary tumors. Cancer Res 73: 745–755, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Weichert W, Boehm M, Gekeler V, Bahra M, Langrehr J, Neuhaus P, Denkert C, Imre G, Weller C, Hofmann HP, Niesporek S, Jacob J, Dietel M, Scheidereit C, and Kristiansen G. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer 97: 523–530, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen Z, Zhong Z, and Darnell JE., Jr.Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82: 241–250, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Williams P, Ongsakul M, Proudfoot J, Croft K, and Beilin L. Mangostin inhibits the oxidative modification of human low density lipoprotein. Free Radic Res 23: 175–184, 1995 [DOI] [PubMed] [Google Scholar]
- 54.Wong HH. and Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol 6: 412–422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimura A, Naka T, and Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7: 454–465, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Yu H. and Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 4: 97–105, 2004 [DOI] [PubMed] [Google Scholar]