Abstract
Voltage-gated ion channels are important determinants of cellular excitability. The Hyperpolarization-activated Cyclic Nucleotide-gated (HCN) and KV7 (M-) channels are voltage-gated ion channels. Both channels are activated at sub-threshold potentials and have biophysical properties that mirror each other. KV7 channels inhibit neuronal excitability. Thus, mutations in KV7 channels that are associated with Benign Familial Neonatal Convulsions (BFNC) are likely to be epileptogenic. Mutations in HCN channels have also been associated with idiopathic epilepsies such as GEFS+. In addition, HCN channel expression and function are modulated during symptomatic epilepsies such as temporal lobe epilepsy. It is, though, unclear as to whether the changes in HCN channel expression and function associated with the various forms of epilepsy promote epileptogenesis or are adaptive. In this review, we discuss this as well as the potential for KV7 and HCN channels as drug targets for the treatment of epilepsy.
Voltage gated ion channels critically determine the intrinsic excitability of neurons as well as synaptic release. The distribution and properties of ion channels often differs between neurons in different brain regions (Nusser 2009). In addition, the location and biophysical properties of ion channels also varies within subcellular compartments (axons, dendrites and soma) of neurons. This variation in ion channel expression across cellular subtypes as well as within specific neuronal subcellular compartments is crucial for information processing and maintaining normal neural network excitability (Nusser 2009).
Alterations in voltage-gated ion channel expression and function have been associated with many different types of epilepsies (Steinlein and Noebels 2000; Lerche, Jurkat-Rott et al. 2001; Weber and Lerche 2008). Ion channels are also common targets for epilepsy treatment. Recent evidence suggests that the Hyperpolarization-activated Cyclic Nucleotide gated (HCN) and KV7 (M- or KCNQ) channels are likely to be new exciting therapeutic targets for epilepsies. In this review, we will discuss the reasons for this.
1. Biophysical properties, subcellular localisation and function
HCN and KV7 channels are subthreshold voltage-gated ion channels that activate and de-activate slowly with time constants in the order of 100 ms (Fig 1; (Biel, Wahl-Schott et al. 2009; Brown and Passmore 2009). HCN channels, as their name suggest, turn on at potentials below −50 mV (Fig 1). In contrast, KV7 channels, open at potentials more positive to −80 mV (Fig 1). Thus, both channels form a non-inactivating current at normal neuronal resting membrane potential (RMP). However, HCN channels are permeable to both Na+ and K+and the current, Ih, is therefore, inward at rest and depolarizes the RMP (Pape 1996; Robinson and Siegelbaum 2003; Biel, Wahl-Schott et al. 2009; Shah, Hammond et al. 2010). The KV7 or M- current, on the other hand, is outward at rest and hyperpolarizes the RMP (Shah, Migliore et al. 2008; Brown and Passmore 2009). Although HCN and KV7 currents have counteractive effects on the RMP, they both reduce the membrane resistance (Robinson and Siegelbaum 2003; Hu, Vervaeke et al. 2007; Shah, Migliore et al. 2008; Biel, Wahl-Schott et al. 2009; Brown and Passmore 2009). Hence their effects on intrinsic excitability are complex.
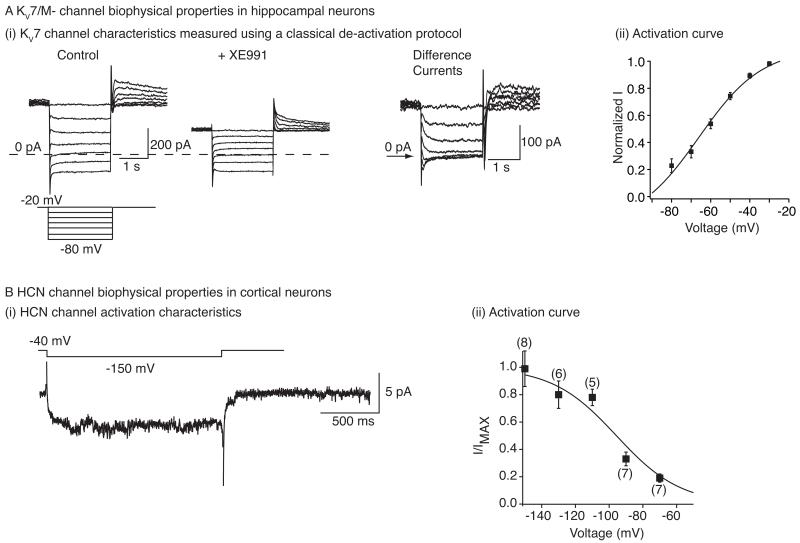
Fig 1. HCN and KV7/M- channel biophysical properties.
A(i) Example recordings of M-current de-activation in hippocampal pyramidal in response to hyperpolarizing potentials from a holding potential of −20 mV. Since there are a number of other conductances also present in these neurons, the recordings were obtained in the absence and presence of the KV7/M- channel inhibitor, XE991 (3 μM). The ‘difference’ current represents the M-current de-activation. (ii) The apparent activation curve of KV7 current produced from experimentally obtained conduction values as detailed in Shah et al. (2008). B(i) Example trace of the HCN channel current recorded from an entorhinal cortical dendrite when it was hyperpolarized to −150 mV from a holding potential of −40 mV. The recording was made in a cell-attached mode. B(ii) The activation curve of Ih obtained using cell-attached recordings made from entorhinal cortical neuron dendrites. A(i) and A(ii) have been adapted from Shah et al. (2008) whereas B(i) and B(ii) have been adapted from Shah et al. (2004).
4 HCN (HCN1-4) and 5 KV7 (KV7.1-KV7.5) channel subunits have been cloned. Both HCN and KV7 channels exist as tetramers of their respective subunits and can form homomers. HCN channels are likely to exist as heteromers too (Biel, Wahl-Schott et al. 2009). In addition, KV7.3 can form heteromers with KV7.2 and KV7.5 (Jentsch 2000; Delmas and Brown 2005; Brown and Passmore 2009). The gating and expression of KV7 and HCN channels in neurons is significantly modulated by intracellular signalling molecules. HCN channels, as their name suggests, are affected by cyclic nucleotides such as cyclic adenosine 3′,5′ monophosphate (cAMP; (DiFrancesco and Tortora 1991; Wainger, DeGennaro et al. 2001; Wang, Chen et al. 2002; Wang, Ramos et al. 2007). The effect of cyclic nucleotides is subunit dependent with the activation curve of HCN2 and HCN4 being shifted much more to the right than that of HCN1 channels in heterologous systems (for an in depth review, see (Biel, Wahl-Schott et al. 2009)). Moreover, HCN and KV7 channel activity is altered by phosphoinositides as well as kinases such as calcineurin and calcium/calmodulin dependent protein kinase II (for in depth reviews on the effects of signalling molecules on HCN channels and KV7 channels see (Biel, Wahl-Schott et al. 2009) and (Delmas and Brown 2005) respectively).
HCN1, HCN2 KV7.2, KV7.3 and KV7.5 expression is abundant in the hippocampus and cortex, areas of the brain known to be involved in idiopathic epilepsies as well as some types of acquired epilepsies (such as temporal lobe epilepsy; (Pape 1996; Jentsch 2000; Robinson and Siegelbaum 2003; Delmas and Brown 2005; Biel, Wahl-Schott et al. 2009; Brown and Passmore 2009). Interestingly, whilst the HCN subunits are highly localised to hippocampal and cortical pyramidal cell dendrites (Shah, Hammond et al. 2010), KV7.2 and KV7.3 are preferentially targeted to axons in these neurons (Devaux, Kleopa et al. 2004; Chung, Jan et al. 2006; Pan, Kao et al. 2006; Rasmussen, Frokjaer-Jensen et al. 2007; Shah, Migliore et al. 2008). This differential localisation in these neurons is important for understanding how these channels compute information. Consequently, whilst inhibition of HCN channels results in enhanced dendritic input resistance and lowering of RMP (Shah, Hammond et al. 2010), pharmacological block of KV7 channels has little effect on dendritic input resistance (Hu, Vervaeke et al. 2007; Shah, Migliore et al. 2008; Shah, Migliore et al. 2011). Thus, HCN, but not KV7, channel inhibitors increase dendritic excitatory synaptic potential (EPSP) amplitude and prolong EPSP decay rate, thereby boosting EPSP summation (Fig 2; (Magee 1998; Stuart and Spruston 1998; Magee 1999; Magee 2000; Williams and Stuart 2000; Berger, Larkum et al. 2001; Poolos, Migliore et al. 2002; Shah, Anderson et al. 2004; Huang, Walker et al. 2009; Shah, Hammond et al. 2010; Shah, Migliore et al. 2011). Hence, HCN channels exert a local effect on dendritic excitability. KV7, by being present at the axon initial segment, play a crucial role in regulating the action potential threshold (Shah, Migliore et al. 2008; Shah, Migliore et al. 2011). KV7 channel inhibition results in a lower action potential threshold and a greater propensity for EPSP-spike coupling (Shah, Migliore et al. 2008; Shah, Migliore et al. 2011). As action potentials can back-propagate into dendrites, this may indirectly result in altered dendritic excitability (Shah, Migliore et al. 2008).
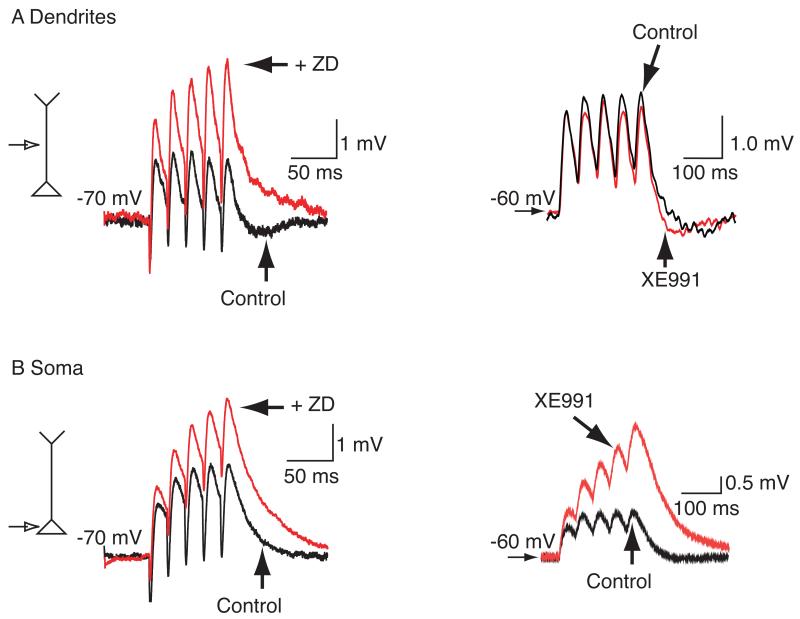
Fig 2. Modulation of EPSP integration by HCN and KV7/M- channel inhibitors.
A Example recordings showing the effects of the HCN channel inhibitor, ZD7288 (ZD, 15 μM) and the KV7/M- channel blocker, XE991 (3 μM) on entorhinal cortical (EC) dendritic αEPSP summation and hippocampal dendritic αEPSP integration respectively. αEPSPs were obtained by current injection of the alpha waveform (for methods see (Shah, Anderson et al. 2004). B Traces demonstrating the effects of ZD7288 and XE991 on αEPSP summation in EC and hippocampal soma respectively. The figure has been adapted from (Shah, Anderson et al. 2004) and (Shah, Migliore et al. 2011).
At the soma of cortical and hippocampal pyramidal neurons, though, where both channels are present, albeit at lower densities, treatment with either HCN or KV7 channel inhibitors augments the input resistance, leading to enhanced EPSP amplitudes and slower decay. Consequentially, EPSP summation and EPSP-spike coupling is increased (Fig 2; (Magee 1998; Hu, Vervaeke et al. 2007; George, Abbott et al. 2009; Huang, Walker et al. 2009; Shah, Migliore et al. 2011). Since both HCN and KV7 channels are voltage-dependent channels with different activation curves, the proportion of HCN and KV7 channels open at a given potential is likely to vary. Hence, their combined effect on somatic EPSP kinetics and integration will depend on this factor (George, Abbott et al. 2009; Shah, Migliore et al. 2011). Therefore, their effects on somatic cell excitability are likely to be complex and reliant on whether both channels are modulated and whether the modulation results in up- or downregulation of their activity.
Besides pyramidal neuron dendrites, HCN and KV7 channels are present in certain cortical synaptic terminals and interneurons too (Biel, Wahl-Schott et al. 2009; Brown and Passmore 2009). KV7 channels have been postulated to reduce synaptic transmission and lower interneuron excitability (Martire, Castaldo et al. 2004; Lawrence, Saraga et al. 2006; Peretz, Sheinin et al. 2007; Luisi, Panza et al. 2009). On the other hand, depending on the other channels that are present, HCN channels can inhibit (Aponte, Lien et al. 2006) or enhance synaptic release (Bender, Soleymani et al. 2003; Huang, Lujan et al. 2011). HCN channels also have a varied effect on interneuron excitability. For example HCN channel blockers reduce the activity of CA1 oriens-lacunosum moleculare interneurons (Matt, Michalakis et al. 2011) but have little effect on the action potential firing frequency of fast-spiking interneurons in the dentate gyrus (Aponte, Lien et al. 2006). In contrast, a reduction in HCN channels following induction of temporal lobe epilepsy leads to an increase in the excitability of CA3 oriens-lacunosum moleculare interneurons (Dugladze, Vida et al. 2007). Hence, the overall effect on cortical and hippocampal neural network excitability caused by modulation of KV7 and HCN channels is likely to depend on the change in the balance between excitation and inhibition.
In addition to the hippocampus and cortex, the thalamus also plays a critical role in certain epilepsies (e.g. absence epilepsy). All HCN subunits are expressed in the thalamus, though the cell-type localisation varies. Thus, HCN2, HCN4 and HCN1 are expressed in the adult thalamic dorsolateral geniculate nucleus and the thalamic ventrobasal complex whereas HCN3 is predominantly present in the intergeniculate leaflet (Roeloffs, Wickenden et al. 2008; Rogawski and Bazil 2008; Wickenden, Krajewski et al. 2008; Czuczwar, Wojtak et al. 2010). The thalamic relay neurons are the principle neurons in the thalamus (Pape 1996). These are pacemaker neurons. The HCN current, Ih, together with the low threshold T-type Ca2+ channels is involved in generating this rhythmogenicity. Ih induces a slow depolarization that results in activation of T-type Ca2+ channels and action potentials (Pape 1996). This can enhance recurrent network activity in the thalamus. Indeed, Ih potentiation was found to enhance and accelerate oscillations within the thalamus (Yue and Huguenard 2001). Incomplete inhibition of Ih, on the other hand, led to slowing down of the oscillations and more robust thalamic responses, indicating that partial reduction in Ih may lead to epileptogenesis within the thalamus (Yue and Huguenard 2001).
HCN2 channels are present in reticular thalamic neurons too, which are GABAergic neurons (Luszczki, Wu et al. 2009). Here, these are localized to dendritic spines and reduce excitatory synaptic summation in a similar manner to that in hippocampal and cortical pyramidal neurons (Luszczki, Wu et al. 2009). Thus, inhibition of Ih led to enhanced inhibitory post-synaptic potential (IPSP) frequency in thalamic neurons (Luszczki, Wu et al. 2009). Interestingly, these neurons also express KV7/M-channels (Geiger, Weber et al. 2006). However, how these channels may affect neuronal firing or neural network excitability remains to be explored. Nevertheless, it is quite clear that there are a number of cells in different brain regions in which both HCN and KV7 channels are present. In the next part of this review, we explore whether one or both channels are affected in various epilepsies and whether they can be useful targets for treatments.
2. KV7 and HCN channel mutations associated with epilepsy
The underlying cause of epileptic seizures or syndromes can be symptomatic (including brain tumors, brain injury or stroke) or idiopathic (Steinlein and Noebels 2000; Lerche, Weber et al. 2005; Weber and Lerche 2008). Most idiopathic epilepsies are genetic in origin, with many of the epilepsy-associated genes encoding ion channels. Several mutations in KV7/KCNQ genes have been associated with Benign Familial Neonatal Convulsion (BFNC), a syndrome characterized by the occurrence of seizures in the first few days of infancy and remitting within a few weeks (Biervert, Schroeder et al. 1998; Charlier, Singh et al. 1998; Singh, Charlier et al. 1998; Lerche, Biervert et al. 1999; Jentsch 2000; Coppola, Castaldo et al. 2003). These KV7 mutations result in a decreased current when expressed in heterologous systems (Schroeder, Kubisch et al. 1998; Lerche, Biervert et al. 1999; Coppola, Castaldo et al. 2003). Interestingly, some of the mutations also disrupt KV7 channel trafficking to axons in cultured hippocampal neurons (Chung, Jan et al. 2006). Altogether, these results suggest that these mutations are likely to enhance neuronal excitability by increasing EPSP-spike coupling and enhancing action potential firing frequency leading to augmented neurotransmitter release (Vervaeke, Gu et al. 2006; Yue and Yaari 2006; Hu, Vervaeke et al. 2007; Shah, Migliore et al. 2008). Consistent with this, mice that lack KV7 subunit expression and mice in which mutated KV7.2 subunits are knocked in, are epileptic (Peters, Hu et al. 2005; Otto, Yang et al. 2006; Singh, Otto et al. 2008). Unlike transgenic mice, though, the probability of recurrence of seizures in adulthood in humans is between 15-16% (Ronen, Rosales et al. 1993; Weber and Lerche 2008). It is unclear whether this discrepancy is due to species differences or other reasons such as the expression of different KV7/KCNQ splice variants in the neonatal brain (Weber and Lerche 2008).
In contrast to KV7 channels, relatively little is known about the association between HCN channel mutations in humans and idiopathic epilepsies. Mutations in HCN1 and HCN2 subunits have been discovered in patients with idiopathic generalised epilepsy (Tang, Sander et al. 2008). One such mutation, a recessive loss-of-function mutation in HCN2 that has been associated with idiopathic generalized epilepsy, has been shown to increase the excitability of rat cortical neurons (DiFrancesco, Barbuti et al. 2011). Interestingly, a gain of function mutation in HCN2 has been described in a small population of patients with febrile seizures and genetic epilepsy with febrile seizures plus (GEFS+; (Dibbens, Reid et al. 2010). This is consistent with the increase in hippocampal HCN2 channel expression that has been reported in animal models of febrile seizures (Brewster, Bender et al. 2002). The significance of this for epileptogenesis, though, remains to be explored as mice lacking HCN2 channels have absence epilepsy (Ludwig, Budde et al. 2003). In addition, in a rat model of spontaneous absence epilepsy, cortical Ih is reduced, which may at least partially contribute to increased cortical neuron excitability (Strauss, Kole et al. 2004; Kole, Brauer et al. 2007). Ih in thalamic relay neurons, though, is unaltered in pre-epileptic WAG/Rij or GAERS rats, which are recognized genetic models of absence epilepsy (Budde, Caputi et al. 2005; Kuisle, Wanaverbecq et al. 2006). There is, though, altered HCN isoform expression such that HCN1 expression is enhanced in the thalamus of these rodents (Budde, Caputi et al. 2005; Kuisle, Wanaverbecq et al. 2006; Brodie 2010). As HCN1 is less sensitive to cAMP compared with HCN2 and HCN4, the sensitivity of Ih to cAMP is reduced in thalamic neurons in the WAG/Rij and GAERS rats, which may facilitate burst discharges in thalamic neurons, leading to enhanced spontaneous synchronization of thalamic and reticular neurons (Budde, Caputi et al. 2005; Kuisle, Wanaverbecq et al. 2006). It remains to be determined if this may lead to spike-wave-discharges that are characteristic of absence epilepsy.
3. Seizure-induced alterations in HCN/KV7 channel function
There is considerable evidence in animal models and humans to suggest that HCN channel function is altered as a consequence of seizure-activity. This is perhaps not surprising as HCN channels are highly susceptible to plasticity (Biel, Wahl-Schott et al. 2009). Seizure-induced plasticity of HCN channels was first detected in a model of febrile seizures (Chen, Wang et al. 2001). Ih was found to be augmented both at the soma and dendrites of hippocampal CA1 pyramidal neurons obtained from adults that had experience febrile seizures several weeks prior (Chen, Wang et al. 2001; Dyhrfjeld-Johnsen, Morgan et al. 2008). In addition, there was a reduction in hippocampal HCN1 subunit expression and an increase in HCN2 subunits (Brewster, Bender et al. 2002). Interestingly, a decrease in dendritic K+ channel function was detected too (Dyhrfjeld-Johnsen, Morgan et al. 2008). Hence, though the cells were hyperexcitable, the underlying mechanism(s) responsible for this are unclear. Further, whether Ih is epileptogenic under these conditions remains to be evaluated as only a third of the rodents subjected to febrile seizures develop chronic epilepsy (Walker and Kullmann 1999; Dube, Brewster et al. 2009)
Seizure-induced alterations in Ih and HCN protein levels have also been found in animal models of temporal lobe epilepsy (TLE) as well as cortical tissue obtained from TLE patients. TLE is a common, form of adult epilepsy, which is initiated by a brain insult or trauma (e.g. status epilepticus or brain tumors; (Engel 1996). A characteristic feature of this disorder is a seizure-free period, the so-called latent period, between the precipitating factor and the onset of chronic epilepsy (spontaneous behavioural seizures; (Engel 1996). Many of the clinical symptoms of this disorder can be mimicked in rodents by the administration of chemoconvulsants such as kainic acid (White 2002). Using the kainic acid model, it was shown that Ih and HCN protein levels were reduced in the entorhinal cortex (EC) within 24 hr of the first seizure (Shah, Anderson et al. 2004). This decrease in Ih was persistent for at least one week. In addition, EC layer III pyramidal soma and dendrites were hyperexcitable. The dendritic hyperexcitability, in particular, could be mimicked in wildtypes using the Ih inhibitor, ZD7288, suggesting that the reduction in Ih could at least partly contribute to this. Similar results have been found in hippocampal CA1 pyramids and O-LM interneurons in animal models at the acute and chronic stages of the condition (Dugladze, Vida et al. 2007; Jung, Jones et al. 2007; Shin, Brager et al. 2008; Marcelin, Chauviere et al. 2009; Jung, Warner et al. 2011). In addition, a loss in Ih function has been found in cortical neurons present in chronically epileptic tissue obtained from patients (Wierschke, Lehmann et al. 2010). The changes in HCN protein levels observed in all animal models as well as humans are likely to be due to transcriptional as well as post-translational changes (Bender, Soleymani et al. 2003; Powell, Ng et al. 2008; Jung, Bullis et al. 2010; McClelland, Flynn et al. 2011). Further, seizure induced changes in activity-dependent trafficking may also be a contributing factor (Shin, Brager et al. 2008).
Although a reduction in Ih enhances pyramidal cell activity, the effects on neural network excitability may be more complex as HCN channels are expressed post-synaptically in interneurons as well as pre-synaptically in interneurons and principle neurons (Biel, Wahl-Schott et al. 2009). It is not known if seizure activity affects HCN channel expression in all cell types and neuronal subcellular compartments in a similar manner. Therefore, does a reduction in Ih following the induction of TLE facilitate the process of epileptogenesis? HCN1 null mice have been used to address this question as HCN2 null mice have absence epilepsy (Ludwig, Budde et al. 2003). HCN1 null mice do not have spontaneous seizures and the EEG also appears to be normal (Nolan, Malleret et al. 2003; Nolan, Malleret et al. 2004; Huang, Walker et al. 2009; Santoro, Lee et al. 2010). The mice though were more susceptible to chemoconvulsants such as kainic acid and pilocarpine as well as kindling (Fig 3; (Huang, Walker et al. 2009; Santoro, Lee et al. 2010). In addition, the latent period was significantly shorter (Fig 3; (Huang, Walker et al. 2009). Moreover, although the EEG appeared to be similar in wildtypes and HCN1 null mice, the balance between excitatory and inhibitory inputs was altered in the entorhinal cortex such that the frequency of excitatory inputs far exceeded the inhibitory inputs in HCN1 null tissue whereas the converse occurred in wildtype tissue (Huang, Walker et al. 2009). It is likely that this is not the case in all parts of the cortex or in other brain regions. Indeed, in the neocortex, tonic GABA currents have been shown to be upregulated in HCN1 null mice (Chen, Shu et al. 2010). This might thus explain why these mice are not epileptic. Nonetheless, there is substantial evidence to suggest that a decrease in HCN channel function may aid the process of epileptogenesis in some forms of epilepsy.
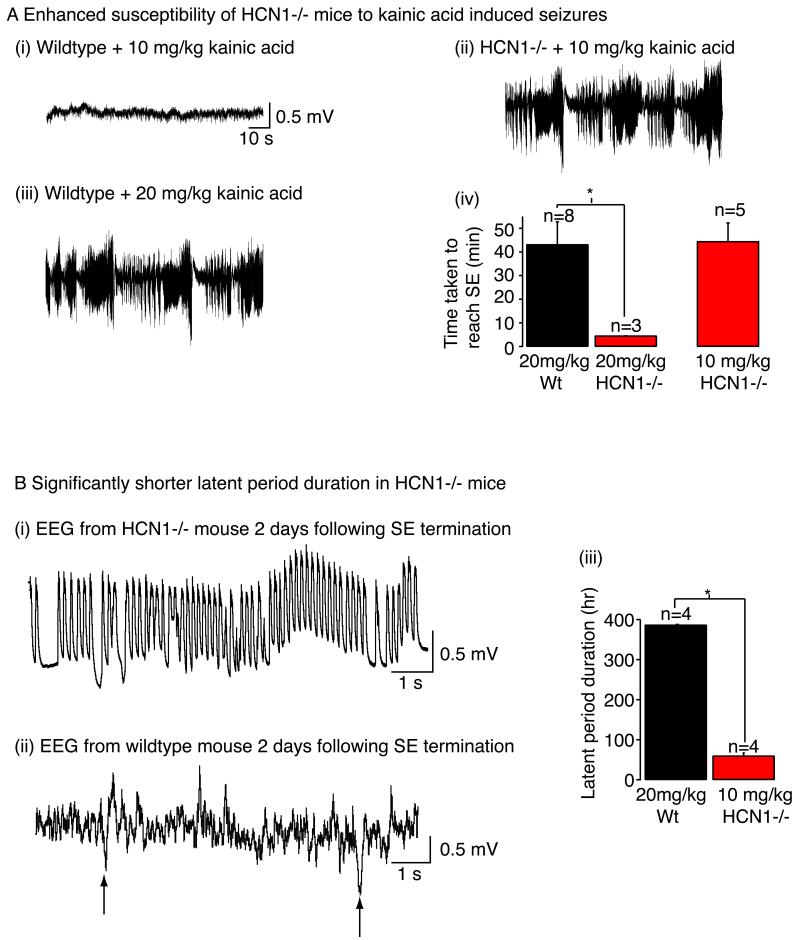
Fig 3. Seizure susceptibility of HCN1 null mice.
A(i), A(ii) and A(iii) Example traces showing the electroencephalography (EEG) recorded from HCN1 null mice and wildtype littermates when administered kainic acid either at concentrations of 10 mg/kg or 20 mg/kg. A(iv) Graph summarising the time taken to induce status epilepticus (SE) with the varying doses of kainic acid. NB Administration of 20 mg/kg kainic acid to HCN1 null mice was lethal whilst treatment with 10 mg/kg kainic acid had little effect on wildtypes for up to 4 hr. B(i) and B(ii) EEG recordings from HCN1 null and wildtype mice 2 days following termination of SE induced with 10 mg/kg and 20 mg/kg kainic acid respectively. The arrows in B(ii) mark the presence of interictal spikes B(iii) Graph showing the latent period duration (i.e. the time between termination of SE and detection of the first spontaneous seizure) in HCN1 null and wildtype mice when treated with kainic acid. The figure has been adapted from (Huang, Walker et al. 2009).
In contrast to HCN channels, there is very little evidence that KV7 channel expression and function are altered by seizure activity. Interestingly, in human tissue obtained from TLE patients, KV7.5 expression appeared to be not dissimilar to control tissue (Zolles, Wenzel et al. 2009). It is, though, possible that the function of the channels is impaired. Nevertheless, this indicates that KV7 channel modulators may be effective for treatment of patients with TLE. Indeed, the KV7 channel enhancers, retigabine and ICA-27243, have been shown to effectively suppress seizures in TLE models (Rostock, Tober et al. 1996; Tober, Rostock et al. 1996; DiFrancesco, Barbuti et al. 2011).
4. Therapeutic potential for HCN and KV7 channel modulators
Consistent with the role of KV7 channels in dampening neuronal excitability, the KV7 channel enhancer, retigabine (egozabine) is effective as an anticonvulsant in several animal models of epilepsy (Rogawski and Bazil 2008; Czuczwar, Wojtak et al. 2010). Phase III clinical trials showed that retigabine suppressed between 45-50% of baseline seizures in patients with partial onset seizures (Czuczwar, Wojtak et al. 2010). Therefore, it has recently received approval in Europe and USA as an adjunct for the treatment of partial epilepsy. Retigabine, however, at high concentrations can also act as a GABAA receptor agonist (Rogawski and Bazil 2008; Czuczwar, Wojtak et al. 2010). It’s effects though are likely to be via enhancing KV7 channel activity as another more selective KV7 channel enhancer, ICA-27243 is highly effective as a broad-spectrum anticonvulsant in animal models of epilepsy (Roeloffs, Wickenden et al. 2008).
In contrast to KV7 channels, the role of HCN channels in epilepsy is clearly more complex. In some forms of epilepsy such as TLE, enhancing HCN channel function may be beneficial. Some anticonvulsants such as lamotrigine and gabapentin have been suggested to increase HCN channel activity at rest (Poolos, Migliore et al. 2002; Surges, Freiman et al. 2003). However, these compounds do have other modes of action (Brodie 2010) and it is unclear if their effects on seizure activity are due to enhancing HCN channel function or due to their other effects. Can increasing HCN channel activity alter epileptogenesis? It has recently been demonstrated that increasing HCN channel expression by disrupting the interaction between the Neuron-Restrictive Silence Factor (NRSF) and HCN1 restores HCN channel function in CA1 pyramidal dendrites 3 days following status epilepticus and thereby delays the onset of spontaneous seizure activity (McClelland, Flynn et al. 2011). This did not, though, prevent spontaneous seizure activity as a number of other mechanisms such as altered phosphorylation signalling may contribute to the persistent downregulation of HCN channel function too. Certainly a recent study has shown that in chronically epileptic animals, calcineurin and p38 MAPK activity is enhanced and reduced respectively. Consequently, the activation curve of Ih in chronically ‘epileptic’ hippocampal CA1 neurons is shifted to the left, resulting in fewer HCN channels being available at rest (Jung, Bullis et al. 2010). Since HCN channel activity is altered in a significant number of different cell types following the induction of epilepsy, it is unclear whether these phosphorylation dependent changes occur in all of these cell types. An alternate approach may be to better understand the cellular mechanisms by which a reduction in Ih leads to enhanced epilepsy. Would targeting these be more beneficial for the treatment of certain forms of epilepsy? In addition, for other forms of epilepsy (e.g. febrile seizures) would inhibiting Ih suppress seizure activity? Undoubtedly, further work is required to evaluate the link between Ih and epilepsy.
Acknowledgements
This work was supported by a European Research Council Starter Independent Grant (ERC_2010_StG_20091118, MMS), an MRC New Investigator Award (G0700369, MMS) and a Wellcome Trust project grant (WT087363MA, MMS).
References
- Aponte Y, Lien CC, et al. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol. 2006;574(Pt 1):229–243. doi: 10.1113/jphysiol.2005.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Soleymani SV, et al. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23(17):6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Larkum ME, et al. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85(2):855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, et al. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89(3):847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279(5349):403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Brewster A, Bender RA, et al. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22(11):4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MJ. Antiepileptic drug therapy the story so far. Seizure: the journal of the British Epilepsy Association. 2010;19(10):650–655. doi: 10.1016/j.seizure.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156(8):1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Caputi L, et al. Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J Neurosci. 2005;25(43):9871–9882. doi: 10.1523/JNEUROSCI.2590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C, Singh NA, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet. 1998;18(1):53–55. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, et al. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117(5):491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shu S, et al. Homeostatic regulation of synaptic excitability: tonic GABA(A) receptor currents replace I(h) in cortical pyramidal neurons of HCN1 knock-out mice. J Neurosci. 2010;30(7):2611–2622. doi: 10.1523/JNEUROSCI.3771-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Jan YN, et al. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci U S A. 2006;103(23):8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G, Castaldo P, et al. A novel KCNQ2 K+ channel mutation in benign neonatal convulsions and centrotemporal spikes. Neurology. 2003;61(1):131–134. doi: 10.1212/01.wnl.0000069465.53698.bd. [DOI] [PubMed] [Google Scholar]
- Czuczwar P, Wojtak A, et al. Retigabine: the newer potential antiepileptic drug. Pharmacological reports: PR. 2010;62(2):211–219. doi: 10.1016/s1734-1140(10)70260-7. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6(11):850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, et al. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24(5):1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Reid CA, et al. Augmented currents of an HCN2 variant in patients with febrile seizure syndromes. Ann Neurol. 2010;67(4):542–546. doi: 10.1002/ana.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco JC, Barbuti A, et al. Recessive loss-of-function mutation in the pacemaker HCN2 channel causing increased neuronal excitability in a patient with idiopathic generalized epilepsy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(48):17327–17337. doi: 10.1523/JNEUROSCI.3727-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube CM, Brewster AL, et al. Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev. 2009;31(5):366–371. doi: 10.1016/j.braindev.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugladze T, Vida I, et al. Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2007;104(44):17530–17535. doi: 10.1073/pnas.0708301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Morgan RJ, et al. Upregulated H-Current in Hyperexcitable CA1 Dendrites after Febrile Seizures. Front Cell Neurosci. 2008;2:2. doi: 10.3389/neuro.03.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JJ. Introduction to temporal lobe epilepsy. Epilepsy Research. 1996;26(1):141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- Geiger J, Weber YG, et al. Immunohistochemical analysis of KCNQ3 potassium channels in mouse brain. Neuroscience letters. 2006;400(1-2):101–104. doi: 10.1016/j.neulet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- George MS, Abbott LF, et al. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nat Neurosci. 2009;12(5):577–584. doi: 10.1038/nn.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, et al. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27(8):1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Lujan R, et al. Presynaptic HCN1 channels regulate Cav3.2 activity and neurotransmission at select cortical synapses. Nat Neurosci. 2011;14(4):478–486. doi: 10.1038/nn.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Walker MC, et al. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci. 2009;29(35):10979–10988. doi: 10.1523/JNEUROSCI.1531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1(1):21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Jung S, Bullis JB, et al. Downregulation of dendritic HCN channel gating in epilepsy is mediated by altered phosphorylation signaling. J Neurosci. 2010;30(19):6678–6688. doi: 10.1523/JNEUROSCI.1290-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Jones TD, et al. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27(47):13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Warner LN, et al. Rapid Loss of Dendritic HCN Channel Expression in Hippocampal Pyramidal Neurons following Status Epilepticus. J Neurosci. 2011;31(40):14291–14295. doi: 10.1523/JNEUROSCI.1148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Brauer AU, et al. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol. 2007;578(Pt 2):507–525. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuisle M, Wanaverbecq N, et al. Functional stabilization of weakened thalamic pacemaker channel regulation in rat absence epilepsy. J Physiol. 2006;575(Pt 1):83–100. doi: 10.1113/jphysiol.2006.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, Saraga F, et al. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci. 2006;26(47):12325–12338. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche H, Biervert C, et al. A reduced K+ current due to a novel mutation in KCNQ2 causes neonatal convulsions. Ann Neurol. 1999;46(3):305–312. doi: 10.1002/1531-8249(199909)46:3<305::aid-ana5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lerche H, Jurkat-Rott K, et al. Ion channels and epilepsy. Am J Med Genet. 2001;106(2):146–159. doi: 10.1002/ajmg.1582. [DOI] [PubMed] [Google Scholar]
- Lerche H, Weber YG, et al. Ion channel defects in idiopathic epilepsies. Curr Pharm Des. 2005;11(21):2737–2752. doi: 10.2174/1381612054546815. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. Embo J. 2003;22(2):216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi R, Panza E, et al. Activation of pre-synaptic M-type K+ channels inhibits [3H]D-aspartate release by reducing Ca2+ entry through P/Q-type voltage-gated Ca2+ channels. J Neurochem. 2009;109(1):168–181. doi: 10.1111/j.1471-4159.2009.05945.x. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Wu JZ, et al. Isobolographic characterization of interactions of retigabine with carbamazepine, lamotrigine, and valproate in the mouse maximal electroshock-induced seizure model. Naunyn-Schmiedeberg’s archives of pharmacology. 2009;379(2):163–179. doi: 10.1007/s00210-008-0349-9. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18(19):7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2(6):508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1(3):181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Marcelin B, Chauviere L, et al. h channel-dependent deficit of theta oscillation resonance and phase shift in temporal lobe epilepsy. Neurobiol Dis. 2009;33(3):436–447. doi: 10.1016/j.nbd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Martire M, Castaldo P, et al. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci. 2004;24(3):592–597. doi: 10.1523/JNEUROSCI.3143-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt L, Michalakis S, et al. HCN2 channels in local inhibitory interneurons constrain LTP in the hippocampal direct perforant path. Cell Mol Life Sci. 2011;68(1):125–137. doi: 10.1007/s00018-010-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S, Flynn C, et al. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011;70(3):454–464. doi: 10.1002/ana.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119(5):719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115(5):551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009 doi: 10.1016/j.tins.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Otto JF, Yang Y, et al. A spontaneous mutation involving Kcnq2 (Kv7.2) reduces M-current density and spike frequency adaptation in mouse CA1 neurons. J Neurosci. 2006;26(7):2053–2059. doi: 10.1523/JNEUROSCI.1575-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kao T, et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26(10):2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Peretz A, Sheinin A, et al. Pre- and postsynaptic activation of M-channels by a novel opener dampens neuronal firing and transmitter release. J Neurophysiol. 2007;97(1):283–295. doi: 10.1152/jn.00634.2006. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, et al. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8(1):51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, et al. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5(8):767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Powell KL, Ng C, et al. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia. 2008;49(10):1686–1695. doi: 10.1111/j.1528-1167.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen HB, Frokjaer-Jensen C, et al. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120(Pt 6):953–963. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Roeloffs R, Wickenden AD, et al. In vivo profile of ICA-27243 [N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide], a potent and selective KCNQ2/Q3 (Kv7.2/Kv7.3) activator in rodent anticonvulsant models. The Journal of pharmacology and experimental therapeutics. 2008;326(3):818–828. doi: 10.1124/jpet.108.137794. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Bazil CW. New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels. Current neurology and neuroscience reports. 2008;8(4):345–352. doi: 10.1007/s11910-008-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen GM, Rosales TO, et al. Seizure characteristics in chromosome 20 benign familial neonatal convulsions. Neurology. 1993;43(7):1355–1360. doi: 10.1212/wnl.43.7.1355. [DOI] [PubMed] [Google Scholar]
- Rostock A, Tober C, et al. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23(3):211–223. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Santoro B, Lee JY, et al. Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia. 2010;51(8):1624–1627. doi: 10.1111/j.1528-1167.2010.02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Kubisch C, et al. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396(6712):687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, et al. Seizure-Induced Plasticity of h Channels in Entorhinal Cortical Layer III Pyramidal Neurons. Neuron. 2004;44(3):495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Hammond RS, et al. Dendritic ion channel trafficking and plasticity. Trends Neurosci. 2010;33(7):307–316. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Migliore M, et al. Differential effects of Kv7 (M-) channels on synaptic integration in distinct subcellular compartments of rat hippocampal pyramidal neurons. J Physiol. 2011 doi: 10.1113/jphysiol.2011.220913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Migliore M, et al. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2008;105(22):7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Brager D, et al. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol Dis. 2008;32(1):26–36. doi: 10.1016/j.nbd.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Charlier C, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18(1):25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Singh NA, Otto JF, et al. Mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions show seizures and neuronal plasticity without synaptic reorganization. J Physiol. 2008 doi: 10.1113/jphysiol.2008.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein OK, Noebels JL. Ion channels and epilepsy in man and mouse. Curr Opin Genet Dev. 2000;10(3):286–291. doi: 10.1016/s0959-437x(00)00079-4. [DOI] [PubMed] [Google Scholar]
- Strauss U, Kole MH, et al. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19(11):3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci. 1998;18(10):3501–3510. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges R, Freiman TM, et al. Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. Epilepsia. 2003;44(2):150–156. doi: 10.1046/j.1528-1157.2003.36802.x. [DOI] [PubMed] [Google Scholar]
- Tang B, Sander T, et al. Mutation analysis of the hyperpolarization-activated cyclic nucleotide-gated channels HCN1 and HCN2 in idiopathic generalized epilepsy. Neurobiol Dis. 2008;29(1):59–70. doi: 10.1016/j.nbd.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober C, Rostock A, et al. D-23129: a potent anticonvulsant in the amygdala kindling model of complex partial seizures. Eur J Pharmacol. 1996;303(3):163–169. doi: 10.1016/0014-2999(96)00073-8. [DOI] [PubMed] [Google Scholar]
- Vervaeke K, Gu N, et al. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. J Physiol. 2006;576(Pt 1):235–256. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, et al. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411(6839):805–810. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- Walker MC, Kullmann DM. Febrile convulsions: a ‘benign’ condition? Nat Med. 1999;5(8):871–872. doi: 10.1038/11308. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen S, et al. Activity-dependent regulation of HCN pacemaker channels by cyclic AMP: signaling through dynamic allosteric coupling. Neuron. 2002;36(3):451–461. doi: 10.1016/s0896-6273(02)00968-6. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129(2):397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Weber YG, Lerche H. Genetic mechanisms in idiopathic epilepsies. Dev Med Child Neurol. 2008;50(9):648–654. doi: 10.1111/j.1469-8749.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- White HS. Animal models of epileptogenesis. Neurology. 2002;59(9 Suppl 5):S7–S14. doi: 10.1212/wnl.59.9_suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Krajewski JL, et al. N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): a novel, selective KCNQ2/Q3 potassium channel activator. Molecular pharmacology. 2008;73(3):977–986. doi: 10.1124/mol.107.043216. [DOI] [PubMed] [Google Scholar]
- Wierschke S, Lehmann TN, et al. Hyperpolarization-activated cation currents in human epileptogenic neocortex. Epilepsia. 2010;51(3):404–414. doi: 10.1111/j.1528-1167.2009.02275.x. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol. 2000;83(5):3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- Yue BW, Huguenard JR. The role of H-current in regulating strength and frequency of thalamic network oscillations. Thalamus & related systems. 2001;1(2):95–103. doi: 10.1016/S1472-9288(01)00009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006;95(6):3480–3495. doi: 10.1152/jn.01333.2005. [DOI] [PubMed] [Google Scholar]
- Zolles G, Wenzel D, et al. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron. 2009;62(6):814–825. doi: 10.1016/j.neuron.2009.05.008. [DOI] [PubMed] [Google Scholar]