Significance
Fibrillin microfibrils are extracellular matrix assemblies that provide the connective tissues of metazoan species with many of their biomechanical properties. They are also involved in regulating the production of extracellular matrix through their interactions with growth factors such as transforming growth factor-β. The process of microfibril assembly and its regulation are poorly understood. We have investigated the role of the conserved C-terminal propeptide of fibrillin-1 using an in vitro microfibril assay in which HEK293T cells, transiently expressing a GFP-tagged variant of fibrillin-1, are cocultured with fibroblasts to produce a recombinant microfibril network. Our data show that the C-terminal propeptide plays a crucial role in preventing premature intracellular microfibril assembly.
Abstract
Fibrillin microfibrils are 10–12 nm diameter, extracellular matrix assemblies that provide dynamic tissues of metazoan species with many of their biomechanical properties as well as sequestering growth factors and cytokines. Assembly of fibrillin monomers into microfibrils is thought to occur at the cell surface, with initial steps including proprotein processing, multimerization driven by the C terminus, and the head-to-tail alignment of adjacent molecules. At present the mechanisms that regulate microfibril assembly are still to be elucidated. We have used structure-informed protein engineering to create a recombinant, GFP-tagged version of fibrillin-1 (GFP-Fbn) to study this process. Using HEK293T cells transiently transfected with GFP-Fbn constructs, we show that (i) the C-terminal propeptide is an essential requirement for the secretion of full-length fibrillin-1 from cells; (ii) failure to cleave off the C-terminal propeptide blocks the assembly of fibrillin-1 into microfibrils produced by dermal fibroblasts; and (iii) the requirement of the propeptide for secretion is linked to the presence of domains cbEGF41-43, because either deletion or exchange of domains in this region leads to cellular retention. Collectively, these data suggest a mechanism in which the propeptide blocks a key site at the C terminus to prevent premature microfibril assembly.
The 10- to 12-nm-diameter microfibrils of the extracellular matrix (ECM) provide the tissues of metazoan species with many of their biomechanical properties. They also provide a scaffold for elastin deposition during elastogenesis and play a role in matrix regulation by sequestering growth factors such as transforming growth factor-β and the bone morphogenetic proteins. Fibrillins are a group of large (∼350 kDa), calcium-binding glycoproteins that are the major constituents of the microfibrils. Three fibrillin isoforms, fibrillin-1, -2, and -3, exist in humans. Mutations in the gene encoding fibrillin-1 (FBN1) lead to a range of inherited connective tissue disorders such as Marfan syndrome (MFS) and a group of disorders, including Weill–Marchesani syndrome, geleophysic dysplasia, and acromicric dysplasia, that are collectively referred to as acromelic dysplasias (1).
Despite progress in understanding the molecular and supramolecular organization of fibrillin and microfibrils, microfibril assembly and its regulation remain poorly understood. This information is fundamental to understanding the molecular pathogenesis of gain of function and loss of function diseases associated with FBN1 mutations. The assembly of fibrillin monomers into microfibrils occurs at the cell surface (2). Just before or after secretion, the propeptide sequences from the N and C termini are cleaved by furin (Fig. 1A) (3–6) at sites that have been conserved through evolution (7, 8). A regulatory role for the C-terminal propeptide was suggested by a study of a MFS-associated R2726W substitution in fibrillin-1 (9), which resulted in a loss of mutant C-terminal processing and a lack of incorporation of the unprocessed material into the insoluble matrix of cultured dermal fibroblasts. Inhibition of furin, using the inhibitor DecRVKR-CMK, also reduces microfibril assembly in fibroblast cultures (6), indicating that proprotein processing is a requirement for fibrillin incorporation into the matrix.
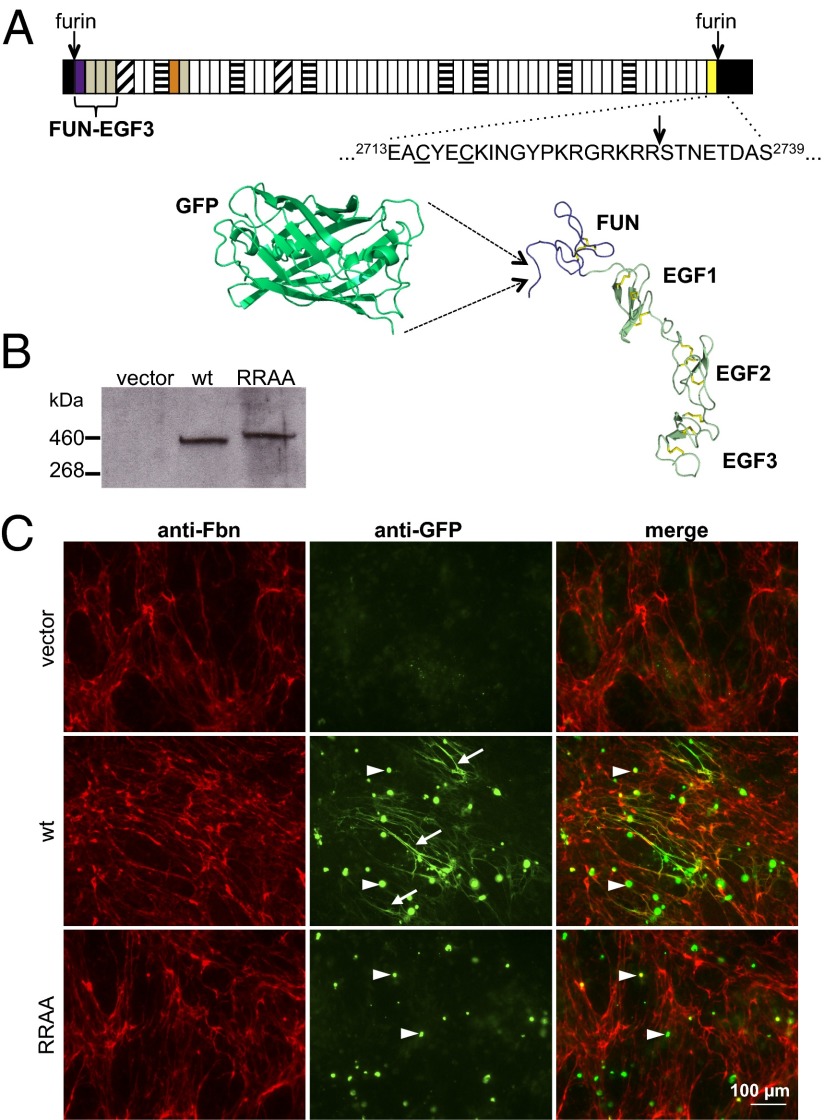
Fig. 1.
GFP-Fbn fusion design and microfibril binding assay. (A) The structure of fibrillin-1 is dominated by calcium-binding EGF-like domains (white) interspersed with transforming growth factor β-binding protein-like (horizontal stripes) and hybrid (diagonal stripes) domains. Other regions include the fibrillin unique N-terminal (FUN; purple) and non-calcium-binding EGF-like (gray) domains, a proline-rich region (orange) and a conserved 2Cys domain (yellow). N- and C-terminal propeptides (black) are processed at furin cleavage sites (arrows) before microfibril assembly. The sequence around the C-terminal furin cleavage site is shown in relation to the cysteines (underlined) of the 2Cys domain. A model of GFP (pdb 2YOG), drawn to scale next to the fibrillin N-terminal domains (FUN-EGF3; pdb 2M74), is provided to show the size of the tag relative to the fibrillin-1 N terminus. (B) Anti-GFP Western blot of medium samples from cultures of HEK293T cells transiently transfected with empty vector or constructs encoding either GFP-Fbn or the GFP-FbnRRAA mutant (RRAA). The RRAA variant is secreted and has the expected shift to a higher molecular weight, confirming a lack of C-terminal processing. (C) FS2 fibroblasts cocultured with HEK293T cells transiently transfected with either empty vector (pcDNA), pcDNA-GFPFbn (GFP-Fbn) or pcDNA-GFPFbnRRAA (RRAA). Cells were stained for fibrillin (red) and GFP (green). GFP-Fbn cocultures showed extensive GFP-positive fibrillar networks (arrows). Although the RRAA mutant was secreted into the medium at levels comparable to wild-type GFP-Fbn (B), no GFP-positive fibrillar network was observed in RRAA cocultures. Intracellular GFP fluorescence (arrowheads) suggested similar transfection efficiencies in the GFP-Fbn and RRAA experiments. These cells were not stained with anti-fibrillin antibody under the conditions used, suggesting that the green fluorescence was due to intracellular accumulations of recombinant protein. This was verified by comparing the anti-fibrillin staining of transiently transfected HEK293T cells in single culture with or without permeabilization (Fig. S1).
Following processing, the secreted molecule undergoes a multimerization step regulated by the last four domains, including domains cbEGF41-43 (10). Although these domains direct this process, they do not form part of the multimerization domain, which is yet to be identified. Multimerized C-terminal fragments of fibrillin-1 have a high affinity for fibronectin, the presence of which is essential for microfibril assembly in higher metazoans (11). Multimerization also increases the apparent affinity of the fibrillin-1 C terminus for N-terminal fragments (10) and interactions between these regions lead to the head-to-tail arrangement of fibrillin monomers within the microfibril (12–14). Heparan sulfate proteoglycans play an essential role in microfibril assembly (15) most likely by localizing fibrillin to the cell surface and enhancing the avidity of interactions between the N and C termini (13). Recently, a heparin sulfate binding site was localized to the first four domains of fibrillin-1, with contributions from Arg62 in the fibrillin unique N-terminal domain (16).
Using data obtained from the determination of the structure and dynamics of the first four fibrillin-1 N-terminal domains (16, 17), we have developed a variant of fibrillin-1, GFP-Fbn, in which a GFP tag is positioned within a series of unstructured glycine residues at the N terminus of the processed protein. Using cocultures of human dermal fibroblasts with HEK293T cells transiently transfected to express GFP-Fbn, we studied how wild-type and mutant fibrillin-1 polypeptides are incorporated into microfibrils. Using a mutant form of fibrillin-1 in which the C-terminal propeptide cleavage site is inactivated, we show directly that unprocessed fibrillin-1 is secreted but prevented from incorporating into microfibrils. A further series of mutant constructs show that both the propeptide and domains cbEGF41-43 are required for the normal secretion of full-length fibrillin-1 from cells and that deletion of the C-terminal cbEGF domains results in defective microfibril binding. These data indicate an interdependence of the fibrillin C-terminal propeptide and domains cbEGF41-43, and suggest a model in which the C-terminal propeptide acts intramolecularly to prevent premature intracellular association.
Results
GFP-Fbn Secreted by HEK293T Cells Incorporates into Fibroblast-Derived Microfibrils in the ECM.
We have previously shown that the N terminus of the mature fibrillin-1 polypeptide, starting at Arg45 after furin cleavage, is highly flexible and unstructured (17). To create GFP-Fbn, the sequence encoding GFP was inserted within this flexible region, between Gly47 and Gly48 (Fig. 1A). The GFP tag is also flanked on its C-terminal side by six glycine residues (Materials and Methods), providing a flexible linker to minimize interference with the known intermolecular interactions of the N-terminal fibrillin-1 domain. The resulting fusion protein undergoes secretion and furin cleavage as expected for native fibrillin-1 (Fig. 1B).
Previous work (2, 18, 19) showed that fibrillin-1 produced by epithelial and HEK293 cells does not assemble, but could be incorporated into a microfibril network produced by fibroblasts. We therefore established a system in which HEK293T cells, which produce low levels of endogenous fibrillin-1 and are readily transfectable, are transiently transfected and cocultured with human skin fibroblasts, allowing the incorporation of the recombinant GFP-Fbn into the fibroblast-derived microfibril network. We used an anti-GFP antibody to show that the recombinant GFP-Fbn protein was deposited into the extracellular matrix in a network that was continuous with the network produced by the fibroblasts in the coculture (Fig. 1C). Direct visualization of autofluorescent GFP-Fbn was not possible in the ECM, most likely due to the relatively low concentrations of recombinant protein diffusing to the sites of assembly on the fibroblasts. HEK293T cells expressing GFP-Fbn were not able to produce a microfibril network in the absence of fibroblasts (Fig. S2). GFP-positive network formation was inhibited by heparin (Fig. S3), demonstrating that it is dependent on fibroblast-derived microfibril assembly. Fibronectin staining of GFP-Fbn cocultures was unaffected by heparin. Thus, this transient system recapitulates the features of microfibril assembly exhibited by fibroblast cultures alone.
Fibrillin-1 C-Terminal Propeptide Blocks GFP-Fbn Incorporation into Microfibrils.
Pulse-chase studies have shown that the furin inhibitor, DecRVKR-CMK inhibits incorporation of fibrillin-1 into the ECM, indicating that processing of fibrillin-1 is a requirement for microfibril assembly (6). To specifically investigate the role of C-terminal propeptide processing, we substituted residues R2730 and R2731 (Fig. 1A) at the C-terminal furin cleavage site with alanines to create GFP-FbnRRAA. This construct was soluble and secreted into the medium at a similar concentration to GFP-Fbn (Fig. 1B), and the observed increase in mass of the GFP-FbnRRAA protein relative to GFP-Fbn confirms the lack of cleavage of the C-terminal propeptide. Despite its secretion into the culture medium at levels comparable to those seen for GFP-Fbn, no GFP-labeling of microfibrils in cocultures of fibroblasts with HEK293T cells expressing GFP-FbnRRAA was observed (Fig. 1C) in multiple (n > 10) independent experimental repeats. This result shows directly that covalent attachment of the C-terminal propeptide blocks incorporation of fibrillin-1 into microfibrils.
C-terminal Propeptide Is Required for Secretion of Full-Length Fibrillin-1 from Cells.
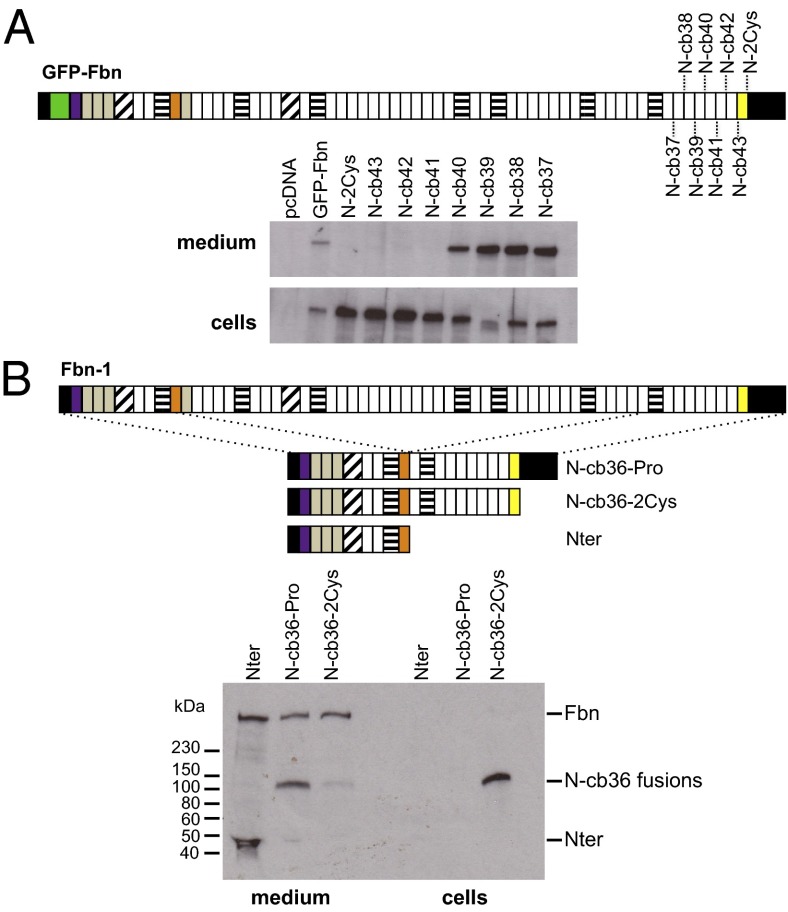
The effect of removing the C-terminal propeptide on fibrillin-1 secretion and microfibril assembly was investigated by replacing the codon for residue S2732 (Fig. 1A), immediately downstream of the C-terminal furin cleavage site, with a stop codon (N-2Cys). Western blots of cell lysates and medium of cells transfected with this construct showed that it was retained in cells (Fig. 2A), indicating that the propeptide was essential for the secretion of full-length fibrillin-1. Sequential removal of domains from the C terminus showed that constructs truncated at domains cbEGF41, cbEGF42, or cbEGF43 are retained in cells; however, secretion could be rescued by creating shorter constructs truncated at domains cbEGF40 (N-cb40), cbEGF39 (N-cb39), cbEGF38 (N-cb38), and cbEGF37 (N-cb37) (Fig. 2A). In all cases where a lack of secretion was observed, RT-PCR was used to show that the effect was not due to decreased RNA expression levels (Fig. S4).
Fig. 2.
Secretion profiles of GFP-Fbn C-terminal truncations. (A) A series of constructs were created with the sequential loss of the C-terminal domains. The N-2Cys construct corresponds to the region from the N terminus to the 2Cys domain (Fig. 1A). The “N-cb” constructs correspond to truncations encompassing the N terminus to the C-terminal cbEGF domains. Constructs truncated at the 2Cys domain or at domains cbEGF41, cbEGF42 or cbEGF43 were retained in cells whereas constructs truncated at domains cbEGF40, cbEGF39, cbEGF38 or cbEGF37 were secreted. (B) MSU-1.1 fibroblasts were stably transfected with empty pKG52(polyA) vector (39, 43), which expresses the Nter construct, or plasmids expressing the fusion proteins N-cb36-Pro or N-cb36-2Cys. Fusion proteins expressed by pools of clones were detected using an antibody raised against the fibrillin-1 Prorich domain (orange). The N-cb36 fusions appeared as bands at ∼100 kDa and the Nter control fragment as a band at ∼44 kDa. N-cb36-Pro was secreted into the medium while N-cb36-2Cys was retained in cells, indicating the role of the C-terminal propeptide in the secretion of fibrillin from microfibril-assembling cells. Endogenous fibrillin-1 expressed by the fibroblasts (Fbn) is seen in the medium of all pools, showing that retention of the truncated forms of recombinant fibrillin-1 do not affect secretion of the full-length protein.
To determine the effect of intracellularly retained, truncated fibrillin-1 on endogenous wild-type fibrillin-1 secretion, pools of stably transfected MSU-1.1 fibroblast clones were established that express fibrillin-1 minigenes in which the N-terminal domains were fused to C-terminal domains with (N-cb36-Pro) or without (N-cb36-2Cys) the propeptide (Fig. 2B). N-cb36-Pro was secreted efficiently, whereas the truncated N-cb36-2Cys protein was largely retained within cells, consistent with the HEK293T data. RT-PCR showed that this was not due to differences in RNA expression levels (Fig. S5). Importantly, the levels of endogenous fibrillin-1 secreted by the recombinant pools were similar, showing that the retained N-cb36-2Cys construct does not interact with endogenous fibrillin-1 to inhibit its secretion.
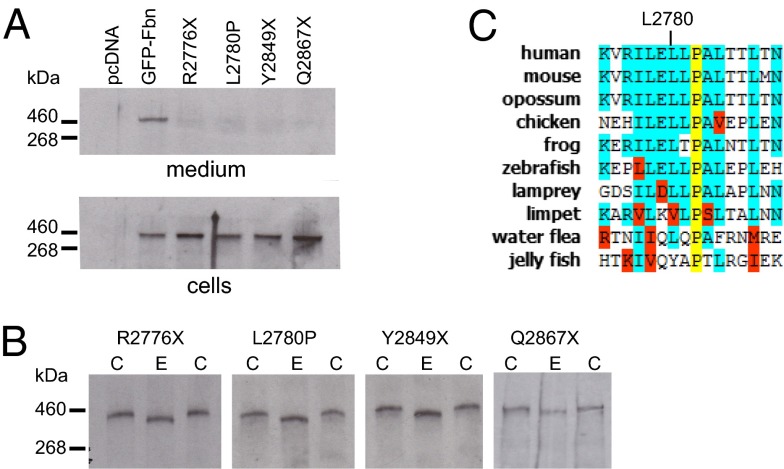
Several MFS-causing mutations result in substitutions or truncations of the C-terminal propeptide (20–22). To investigate the effects of these mutations, we introduced mutations into GFP-Fbn that resulted in either premature termination codons [R2776X (21), Y2489X (22), and Q2867X (23)] or an amino acid substitution (L2780P; ref. 20). Because these mutations affect the final exon of FBN1, the truncated transcripts escape nonsense-mediated decay in vivo (24). Expression of all four MFS-associated variants of GFP-Fbn resulted in intracellular retention (Fig. 3A). Digestion of cell lysates with endoglycosidase H (Fig. 3B) showed that the MFS-associated variants were only modified by simple sugars, indicating that they are retained in the endoplasmic reticulum, which is consistent with protein misfolding and aggregation (Fig. 3B). Residue L2780 is of particular interest because it is in a region of relatively high sequence conservation (Fig. 3C), and its substitution with a proline may lead to structural changes in the propeptide. These data emphasize a requirement for native propeptide structure for the secretion of fibrillin-1 from the cell.
Fig. 3.
Secretion profiles of MFS-associated variants of GFP-Fbn. (A) HEK293T cells were transfected with empty vector, pcDNA-GFPFbn, or constructs encoding the MFS-associated mutants (R2776X, L2780P, Y2849X, and Q2867X). Western blots were developed with an anti-GFP antibody. All MFS-associated variants were retained in the cells. (B) Western blots of cell fractions incubated with EndoH (E) showed a reduction in mass compared with untreated controls (C), indicating modification with simple sugars consistent with retention in the endoplasmic reticulum. (C) L2780 is in a region of high sequence conservation in the C-terminal propeptide. Species listed: human (Homo sapiens), mouse (Mus musculus), opossum (Monodelphis domestica), chicken (Gallus gallus), frog (Xenopus tropicalis), zebrafish (Danio rerio), lamprey (Petromyzon marinus), limpet (Lottia gigantea) water flea (Daphnia pulex) and jelly fish (Clytia hemisphaerica).
Functional Interactions Between the C-Terminal Propeptide and Domains cbEGF41-43.
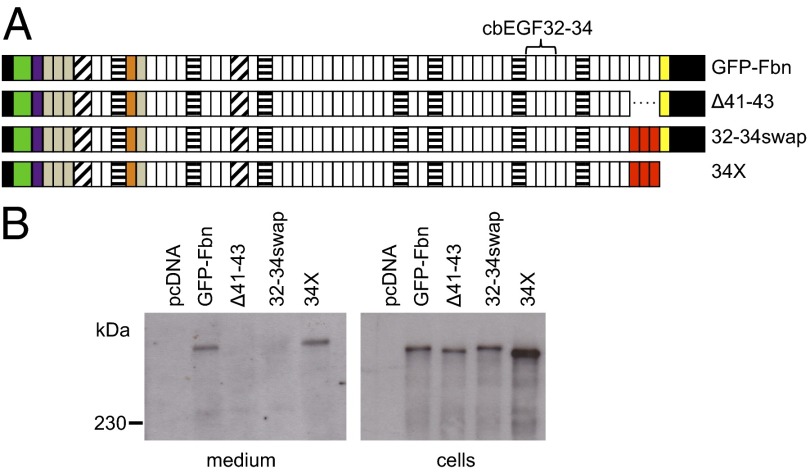
Domains cbEGF41-43 have previously been shown to play a role in microfibril assembly through their specific interaction with the fibrillin-1 N terminus of an adjacent molecule, and their role in regulating C-terminal multimerization (10). To determine whether the deletion of these domains has an effect on the binding of GFP-Fbn to microfibrils, we created the construct Δ41–43 (Fig. 4A). Despite the presence of the propeptide, Δ41–43 was not secreted from transiently transfected HEK293T cells (Fig. 4B). This result indicates that this region, in addition to the propeptide, is essential for the secretion of full-length fibrillin-1 and suggests that removal of domains cbEGF41-43 may lead to misfolding or aggregation of the C-terminal region (25). Replacement of cbEGF41-43 with cbEGF32-34 (32-34swap), which is known to fold independently (26), did not rescue secretion. This finding indicates that the lack of secretion is due to the disruption of a specific interaction rather than a general spatial requirement for the presence of three cbEGF domains. The insertion of a stop codon after the swapped-in cbEGF32-34 region (34X), thus decoupling the C terminus from the propeptide, salvaged secretion (Fig. 4B). These data suggest that the intracellular retention seen in the Δ41–43 and 32–34swap constructs results from the loss of a critical interaction that stabilizes the C-terminal domains.
Fig. 4.
Secretion profiles of C-terminal domain swap constructs. (A) Constructs used to investigate the roles of the C-terminal cbEGF domains in fibrillin-1 secretion and microfibril binding. Domains cbEGF32-34, which lack the ability to interact with the fibrillin-1 N-terminal domains and fold autonomously (16), are highlighted in red where they have replaced cbEGF41-43. (B) Deletion of domains cbEGF41-43 (Δ41–43) results in intracellular retention of fibrillin-1. This could not be salvaged by replacing cbEGF41-43 with cbEGF32-34 (32-34-swap). Deleting the last two domains from 32-34swap (34X) rescued secretion.
Domains cbEGF41-2Cys Are Required for Efficient Binding of GFP-Fbn to Microfibrils.
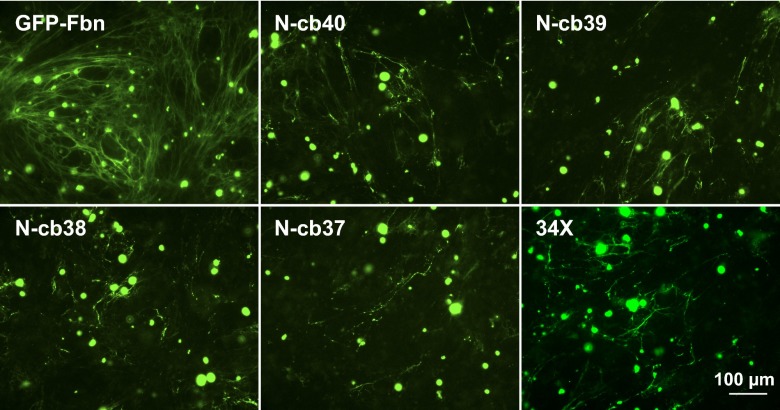
In cocultures using HEK293T cells expressing the secreted GFP-Fbn N-cb variants, incorporation of the N-cb constructs into microfibrils was fragmented and sporadic compared with wild-type GFP-Fbn (Fig. 5). In addition, the 34X construct, which is secreted into the medium (Fig. 4B), displayed results comparable to those seen for N-cb40. These experiments are by their nature only semiquantitative, but independent repetitions (≥3 for each construct) consistently showed defective incorporation of the N-cb mutants. Analysis of media samples (Fig. 2A) further showed that the reduction in incorporation was not due to a reduction in the level of secretion of these constructs. These data highlight the importance of the C-terminal region, from cbEGF41 to the 2Cys domain, for the efficient incorporation of fibrillin-1 into microfibrils, not just for secretion.
Fig. 5.
Microfibril binding by truncated versions of GFP-Fbn. FS2 fibroblasts were cocultured with HEK293T cells transiently transfected to express GFP-Fbn, the secreted N-cb truncations, or 34X. Cocultures were stained using an anti-GFP antibody. The N-cb40 truncation shows deficient microfibril incorporation relative to wild-type. Construct 34X showed no improvement in microfibril binding relative to N-cb40, suggesting that a specific interaction involving domains cbEGF41-2Cys is involved in the binding of GFP-Fbn to microfibrils.
Discussion
Various mouse models have been created to study the role of fibrillins in tissue homeostasis and disease pathology (27, 28), however whole organism models do not provide a molecular understanding of the microfibril assembly process. Additionally, some simpler model organisms such Drosophila melanogaster and Caenorhabditis elegans, do not have fibrillin homologs (8). To address this issue, we used recent structural data on the fibrillin-1 N terminus (16, 17) to develop a tissue culture-based microfibril incorporation assay using a GFP-tagged variant of fibrillin-1.
The choice of site for the introduction of an epitope tag into fibrillin-1 is complicated by the highly disulphide-bonded nature of the molecule and the lack of high resolution data on the molecular surfaces involved in intermolecular interactions. The determination of the structure of the fibrillin-1 N-terminal domains showed that the N terminus of the mature polypeptide is unstructured and highly flexible (17), providing an opportunity for the creation of a tagged version of human fibrillin-1. We placed the sequence for enhanced green fluorescent protein within a series of glycine residues immediately downstream of the N-terminal furin cleavage site. Using this construct, we developed a transient HEK293T/ fibroblast coculture microfibril binding assay. A similar assay system has recently been described using stably transfected HEK293 cells in coculture with mouse fibroblasts (19).
Processing of fibrillin by furin has been indicated as a prerequisite for microfibril incorporation by previous studies (6, 9); our assays using the GFP-FbnRRAA mutant now confirm these data. This unprocessed mutant is secreted and allowed us to show specifically and directly that blocking the cleavage of the C-terminal propeptide of full-length fibrillin-1 prevents binding to microfibrils. Deletion of the C-terminal propeptide, as in the construct N-2Cys, results in intracellular retention. Our data thus indicate that the presence of the propeptide is essential for the secretion of full-length fibrillin-1 and prevents premature assembly. The lack of an effect seen on the levels of secreted endogenous fibrillin-1 in fibroblasts stably expressing the C-terminally truncated N-cb36-2Cys construct indicates that the retained, presumably aggregated species do not interact with endogenous fibrillin-1 polypeptides to prevent their secretion. These recombinant data are consistent with previous data from MFS fibroblast lines that always show some full-length fibrillin-1 secretion, irrespective of the fibrillin-1 mutation. The retention of some of the N-cb constructs (Fig. 2A) was interesting considering that constructs spanning the fibrillin-1 C terminus without the propeptide have previously been purified from culture medium (10). The reason for this is unknown, but may relate to differences in recombinant protein concentration or the presence of the N terminus in our fragments.
To investigate the role of the propeptide further, we assessed the effects of disease-associated mutations on GFP-Fbn secretion. A previous study of a MFS-associated truncation in the C-terminal propeptide, W2756X, showed that loss of the bulk of the propeptide resulted in intracellular retention (29). The R2776X, Y2849X and Q2867X MFS-associated truncations (21–23) show the same effect in our GFP-Fbn system. The Q2867X truncation, which was associated with a case of classical MFS (23), lacks only 5 residues from the C terminus, demonstrating that essentially the entire propeptide is required for its function. In addition, the intracellular retention caused by the single amino acid L2780P substitution (20), which affects a highly conserved leucine, suggests that the function of the propeptide is dependent on its native structure and/or interactions. Recently a correlation has been established between a subset of mutations affecting the fibrillin-1 C terminus and a neonatal, progeroid form of MFS (30–34). All of these mutations result in a frameshift with the introduction of a stop codon before the sequence encoding the C-terminal furin cleavage site (33). The transcripts in these cases are unlikely to undergo nonsense-mediated decay (24) and the resulting polypeptides would be similar to the N-2Cys and N-cb43 variants described here. Further investigation is required to determine whether these mutant fibrillins are retained, like our truncated constructs, or if they are secreted and function in a dominant negative manner.
Hubmacher et al. (10) showed that the final four domains of processed fibrillin-1, including domains cbEGF41-43, regulate the multimerization of the C terminus, although they do not contain the actual multimerization site. This C-terminal assembly results in the formation of structures that are similar in appearance to the beaded filaments observed in preparations of isolated microfibrils when viewed by rotary shadowing (35) and increases the apparent affinity of this region for N-terminal domains. N- to C-terminal interactions between fibrillin molecules then mediate the head-to-tail alignment of fibrillin monomers early in microfibril assembly (12, 14). We have now shown that deletion of domains cbEGF41-43 from full-length fibrillin results in intracellular retention, and that replacing this region with domains cbEGF32-34 is not sufficient to salvage secretion. Removal of the 2Cys and C-terminal propeptide domains from the 32–34swap construct (34X) rescued secretion and showed that the intracellular retention observed for the 32–34swap construct was due to the presence of these C-terminal domains. Collectively, our data indicate a functional interplay between domains cbEGF41-2Cys and the propeptide. A folding dependence of cbEGF41-43 on the propeptide, or vice versa, with the latter potentially acting as an intramolecular chaperone, would prevent premature assembly in the cell and would be a reasonable explanation for our observed data. The architecture of the C-terminal/propeptide complex would then mask a site required to facilitate the C-terminal multimerization observed by Hubmacher et al. (10). Although we cannot exclude the possibility that the propeptide prevents the N- to C-terminal interactions that allow head-to-tail growth of microfibrils, this seems unlikely considering that intermolecular interactions between the N and C termini are relatively weak and dependent on the increased avidity observed on C-terminal multimerization.
Our data suggest an interaction between the last three cbEGF domains and unique C-terminal region of fibrillin-1 that is crucial for regulating fibrillin assembly. Several extracellular proteins have C-terminal propeptides that regulate their assembly into larger complexes. Collagen C-terminal propeptides are involved in trimerisation and chain selection within the cell, and in maintaining the solubility of the protein until secretion when C-terminal propeptide cleavage initiates fibril assembly (36). Similarly, C-terminal propeptides control the assembly of zona pellucida domain-containing proteins by blocking intracellular association (37, 38). We propose a mechanism in which the C-terminal propeptide of fibrillin-1 influences the interactions of its adjacent domains to prevent premature aggregation within cells (Fig. 6). Furin processing of the propeptide at the cell surface is then coupled to the multimerization step that initiates microfibril assembly.
Fig. 6.
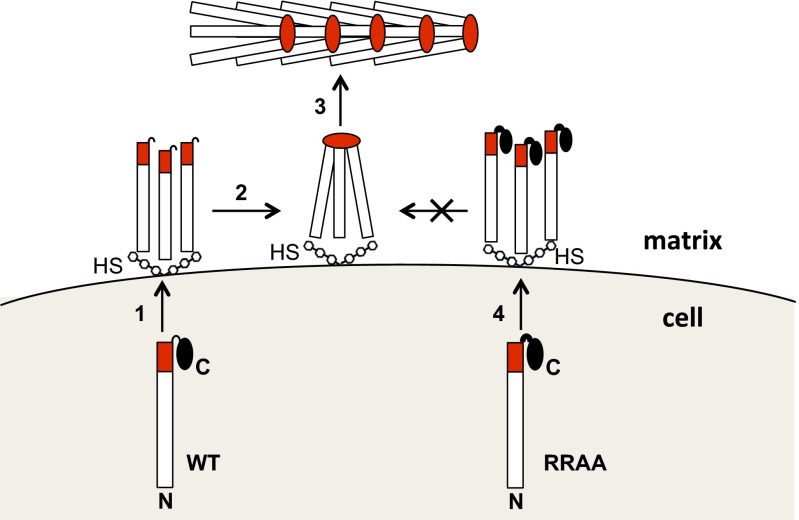
Model for the role of the fibrillin-1 C-terminal propeptide in microfibril assembly. In the secretory pathway, the C-terminal propeptide (black oval) interacts with the C terminus (red), masking a critical assembly site. It may also directly chaperone folding of this region. Propeptide truncations and missense mutations (N-2Cys and MFS mutations), or deletion of cbEGF41-43 (Δ41–43), lead to misfolding and intracellular retention. Just before or after secretion (1), the propeptide is cleaved by furin. At the cell surface, heparan sulfate proteoglycans (HS) bind fibrillin at multiple sites (only N-terminal binding is shown for clarity) and limit its diffusion into the matrix. After propeptide cleavage, the exposed C terminus multimerizes at a site N-terminal to cbEGF41-43 (2), which is an initial step in microfibril assembly (3). Failure to cleave the propeptide, as in the RRAA variant (4), allows secretion but blocks microfibril assembly.
Materials and Methods
Plasmid Construction and Mutagenesis.
The sequence encoding EGFP was initially inserted into a variant of the plasmid pKG52(polyA), which was modified to encode a fragment of fibrillin-1 from the N terminus to the proline-rich region (39), using an overlapping PCR method as described (40). The resulting plasmid, pKG-GFPNPro, has an EGFP DNA sequence positioned immediately downstream of the sequence encoding the fibrillin-1 N-terminal furin cleavage site. The N-terminal sequence of the processed, secreted polypeptide is RGGAAA-EGFP-AAAGGGGGGHDAL, where residues in bold correspond to residues from the fibrillin-1 unique N-terminal domain, EGFP refers to the enhanced green fluorescent protein sequence, and residues in plain text correspond to sequences resulting from the insertion of NotI restriction sites either side of the GFP sequence and a poly-glycine linker.
To create pcDNA-GFPFbn, the SalI–EcoRI fragment from the plasmid pFib (41) was subcloned into pUC18 to create pUC-FibSalEco. The SpeI–KpnI fragment of this plasmid was then replaced with the corresponding SpeI–KpnI fragment from pKG-GFPNPro to produce pUC-GFPFibSalEco. The SpeI–EcoRI fragment from this plasmid was used to replace the corresponding fragment in pFib to create pCR-GFPFbn, which encodes a full-length fibrillin-1 with an N-terminal GFP tag. The SalI fragment of pCR-GFPFbn was inserted into the XhoI site of pcDNA3.1/V5-HisA (Invitrogen) to create the expression construct, pcDNA-GFPFbn. Mutant versions of pcDNA-GFPFbn were created either by an overlapping PCR method (40), or with the QuikChange Lightning mutagenesis kit (Agilent).
Plasmids pKGNter-cb36-Pro and pKGNter-cb36-2Cys, encoding constructs of the fibrillin-1 N terminus up to the proline-rich region fused to either domains cbEGF36-propeptide (residues 2291–2871) or cbEGF36-2Cys (residues 2291–2731), respectively, were created using the pKG52(polyA) vector as described (39). Pools of stable, puromycin resistant cell lines expressing these constructs were created using MSU-1.1 fibroblasts (>80 clones per pool) to overcome the low transient transfection efficiency of fibroblasts and to average out the effects of variations in expression levels between individual clones.
Antibodies.
The rabbit anti–fibrillin-1 polyclonal antibody, raised against the proline-rich region, has been described (39). Chicken anti-GFP (ab13970) and goat anti-chicken HRP conjugate (ab6877) were from Abcam. Goat anti-chicken Alexa488 and goat anti-rabbit Alexa568 were from Invitrogen. Goat anti-rabbit HRP conjugate (A8275) was from Sigma.
Microfibril Incorporation Assay.
HEK293T cells grown in six-well plates were transfected with GFP-Fbn constructs using Lipofectamine 2000 at ∼90% confluence. Twenty-four h after transfection, cells were trypsinized, resuspended in 3 mL of Dulbecco's modified Eagle medium supplemented with 2 mM glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 10% (vol/vol) FBS (complete DMEM). Cocultures of human primary dermal fibroblasts (FS2 cells; ref. 42) and transfected HEK293T cells were established by combining 7.5 × 104 of each cell type in 400 μL of complete DMEM per well of an eight-well Lab Tek II chambered slide (Nunc). Cocultures were grown for 5 d, changing the medium daily.
Secretion Assays.
Two milliliters of the cell suspension remaining from the HEK293T transfections used to establish cocultures were transferred to 25-cm2 tissue culture flasks (Greiner) with an additional 3 mL of complete DMEM and grown for a further 3 d. Medium samples were harvested and centrifuged at 16,200 × g for 5 min. Aliquots (450 μL) were mixed with 180 μL of reducing SDS/PAGE loading buffer and boiled. Cell samples were prepared by washing off the cells from the 25 cm2 tissue culture flasks into 5 mL ice-cold PBS and combining 250 μL of cells with 250 μL of loading buffer before boiling. Forty microliters of medium samples and 30 μL of cell samples prepared as above were loaded onto 10-well gels (or 20 μL and 15 μL, respectively, for 15-well gels). Medium samples were also taken directly from cocultures to confirm expression of GFP fusions under these conditions. Immunoblotting was carried out as described (25, 39), using a chicken anti-GFP antibody followed by a goat anti-chicken HRP conjugate and enhanced chemiluminescent detection. Deglycosylation assays with endoglycosidase H were carried out as described (39).
Immunofluorescence Microscopy.
Cocultures of HEK293T and FS2 cells were washed twice with PBS and fixed with 4% (wt/vol) paraformaldehyde in PBS for 10 min at room temperature. After quenching with 50 mM NH4Cl in PBS for 3 min and rinsing with PBS, cells were blocked with 10% (vol/vol) FBS in PBS (PBS-F) for 15 min at room temperature. Cells were then incubated with primary antibodies against fibrillin (1:200) and GFP (1:1,000) diluted in PBS-F for 60 min without prior permeabilization. After washing five times with PBS-F, the slides were incubated with fluorescently labeled secondary antibodies diluted 1:200 in PBS-F for 60 min at room temperature. Slides were then washed three times with PBS-F, and nuclei were counterstained with 1 μg/mL DAPI in PBS for 5 min. After further washes with PBS and water, the slides were coverslipped with Vectashield (Vector Laboratories). Images were collected using a Zeiss Axioplan 2 microscope with AxioVision Rel. 4.8 software. Contrast was adjusted with ImageJ software. Direct visualization of GFP-Fbn fluorescence was not possible in the ECM (Fig. S2), most likely due to the low concentrations of recombinant protein diffusing to the sites of assembly on the fibroblasts. As the cells were not permeabilized before staining, intracellular GFP-Fbn fluorescence could be used to compare transfection efficiencies between constructs.
Supplementary Material
Acknowledgments
This work was supported by Arthritis Research UK Grant 19810.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401697111/-/DCSupplemental.
References
- 1.Le Goff C, Cormier-Daire V. From tall to short: The role of TGFβ signaling in growth and its disorders. Am J Med Genet C Semin Med Genet. 2012;160C(3):145–153. doi: 10.1002/ajmg.c.31337. [DOI] [PubMed] [Google Scholar]
- 2.Dzamba BJ, et al. Assembly of epithelial cell fibrillins. J Invest Dermatol. 2001;117(6):1612–1620. doi: 10.1046/j.0022-202x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- 3.Milewicz DM, Pyeritz RE, Crawford ES, Byers PH. Marfan syndrome: Defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992;89(1):79–86. doi: 10.1172/JCI115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallis DD, et al. Profibrillin-1 maturation by human dermal fibroblasts: Proteolytic processing and molecular chaperones. J Cell Biochem. 2003;90(3):641–652. doi: 10.1002/jcb.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lönnqvist L, Reinhardt D, Sakai L, Peltonen L. Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet. 1998;7(13):2039–2044. doi: 10.1093/hmg/7.13.2039. [DOI] [PubMed] [Google Scholar]
- 6.Raghunath M, et al. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci. 1999;112(Pt 7):1093–1100. doi: 10.1242/jcs.112.7.1093. [DOI] [PubMed] [Google Scholar]
- 7.Piha-Gossack A, Sossin W, Reinhardt DP. The evolution of extracellular fibrillins and their functional domains. PLoS ONE. 2012;7(3):e33560. doi: 10.1371/journal.pone.0033560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson I, Jensen S, Handford P. TB domain proteins: Evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem J. 2011;433(2):263–276. doi: 10.1042/BJ20101320. [DOI] [PubMed] [Google Scholar]
- 9.Milewicz DM, et al. A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest. 1995;95(5):2373–2378. doi: 10.1172/JCI117930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubmacher D, et al. Biogenesis of extracellular microfibrils: Multimerization of the fibrillin-1 C terminus into bead-like structures enables self-assembly. Proc Natl Acad Sci USA. 2008;105(18):6548–6553. doi: 10.1073/pnas.0706335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabatier L, et al. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20(3):846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin G, et al. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J Biol Chem. 2002;277(52):50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- 13.Cain SA, et al. Heparan sulfate regulates fibrillin-1 N- and C-terminal interactions. J Biol Chem. 2008;283(40):27017–27027. doi: 10.1074/jbc.M803373200. [DOI] [PubMed] [Google Scholar]
- 14.Marson A, et al. Homotypic fibrillin-1 interactions in microfibril assembly. J Biol Chem. 2005;280(6):5013–5021. doi: 10.1074/jbc.M409029200. [DOI] [PubMed] [Google Scholar]
- 15.Tiedemann K, Bätge B, Müller PK, Reinhardt DP. Interactions of fibrillin-1 with heparin/heparan sulfate, implications for microfibrillar assembly. J Biol Chem. 2001;276(38):36035–36042. doi: 10.1074/jbc.M104985200. [DOI] [PubMed] [Google Scholar]
- 16.Yadin DA, et al. Structure of the fibrillin-1 N-terminal domains suggests that heparan sulfate regulates the early stages of microfibril assembly. Structure. 2013;21(10):1743–1756. doi: 10.1016/j.str.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadin DA, Robertson IB, Jensen SA, Handford PA, Redfield C. (1)H, (13)C and (15)N assignments of the four N-terminal domains of human fibrillin-1. Biomol NMR Assign. 2012;8(1):75–80. doi: 10.1007/s12104-012-9456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charbonneau NL, et al. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J Biol Chem. 2003;278(4):2740–2749. doi: 10.1074/jbc.M209201200. [DOI] [PubMed] [Google Scholar]
- 19.Hubmacher D, Bergeron E, Fagotto-Kaufmann C, Sakai LY, Reinhardt DP. Early fibrillin-1 assembly monitored through a modifiable recombinant cell approach. Biomacromolecules. 2014;15(4):1456–1468. doi: 10.1021/bm5000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliday DJ, et al. Twelve novel FBN1 mutations in Marfan syndrome and Marfan related phenotypes test the feasibility of FBN1 mutation testing in clinical practice. J Med Genet. 2002;39(8):589–593. doi: 10.1136/jmg.39.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayward C, Porteous ME, Brock DJ. Identification of a novel nonsense mutation in the fibrillin gene (FBN1) using nonisotopic techniques. Hum Mutat. 1994;3(2):159–162. doi: 10.1002/humu.1380030212. [DOI] [PubMed] [Google Scholar]
- 22.Gao LG, et al. Identification of a novel lethal fibrillin-1 gene mutation in a Chinese Marfan family and correlation of 3′ fibrillin-1 gene mutations with phenotype. Chin Med J (Engl) 2010;123(20):2874–2878. [PubMed] [Google Scholar]
- 23.Stheneur C, et al. Identification of the minimal combination of clinical features in probands for efficient mutation detection in the FBN1 gene. Eur J Hum Genet. 2009;17(9):1121–1128. doi: 10.1038/ejhg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagy E, Maquat LE. A rule for termination-codon position within intron-containing genes: When nonsense affects RNA abundance. Trends Biochem Sci. 1998;23(6):198–199. doi: 10.1016/s0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- 25.Whiteman P, et al. Cellular and molecular studies of Marfan syndrome mutations identify co-operative protein folding in the cbEGF12-13 region of fibrillin-1. Hum Mol Genet. 2007;16(8):907–918. doi: 10.1093/hmg/ddm035. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson S, et al. Molecular effects of homocysteine on cbEGF domain structure: Insights into the pathogenesis of homocystinuria. J Mol Biol. 2005;346(3):833–844. doi: 10.1016/j.jmb.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Charbonneau NL, et al. In vivo studies of mutant fibrillin-1 microfibrils. J Biol Chem. 2010;285(32):24943–24955. doi: 10.1074/jbc.M110.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivieri J, Smaldone S, Ramirez F. Fibrillin assemblies: Extracellular determinants of tissue formation and fibrosis. Fibrogenesis Tissue Repair. 2010;3:24. doi: 10.1186/1755-1536-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raghunath M, Kielty CM, Steinmann B. Truncated profibrillin of a Marfan patient is of apparent similar size as fibrillin: Intracellular retention leads to over-N-glycosylation. J Mol Biol. 1995;248(5):901–909. doi: 10.1006/jmbi.1995.0270. [DOI] [PubMed] [Google Scholar]
- 30.Goldblatt J, Hyatt J, Edwards C, Walpole I. Further evidence for a marfanoid syndrome with neonatal progeroid features and severe generalized lipodystrophy due to frameshift mutations near the 3′ end of the FBN1 gene. Am J Med Genet A. 2011;155A(4):717–720. doi: 10.1002/ajmg.a.33906. [DOI] [PubMed] [Google Scholar]
- 31.Graul-Neumann LM, et al. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3′ terminus of the FBN1-gene. Am J Med Genet A. 2010;152A(11):2749–2755. doi: 10.1002/ajmg.a.33690. [DOI] [PubMed] [Google Scholar]
- 32.Horn D, Robinson PN. Progeroid facial features and lipodystrophy associated with a novel splice site mutation in the final intron of the FBN1 gene. Am J Med Genet A. 2011;155A(4):721–724. doi: 10.1002/ajmg.a.33905. [DOI] [PubMed] [Google Scholar]
- 33.Jacquinet A, et al. Neonatal progeroid variant of Marfan syndrome with congenital lipodystrophy results from mutations at the 3′ end of FBN1 gene. Eur J Med Genet. 2014;57(5):230–234. doi: 10.1016/j.ejmg.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Takenouchi T, et al. Severe congenital lipodystrophy and a progeroid appearance: Mutation in the penultimate exon of FBN1 causing a recognizable phenotype. Am J Med Genet A. 2013;161A(12):3057–3062. doi: 10.1002/ajmg.a.36157. [DOI] [PubMed] [Google Scholar]
- 35.Keene DR, Maddox BK, Kuo HJ, Sakai LY, Glanville RW. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrils. J Histochem Cytochem. 1991;39(4):441–449. doi: 10.1177/39.4.2005373. [DOI] [PubMed] [Google Scholar]
- 36.Bourhis JM, et al. Structural basis of fibrillar collagen trimerization and related genetic disorders. Nat Struct Mol Biol. 2012;19(10):1031–1036. doi: 10.1038/nsmb.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. A duplicated motif controls assembly of zona pellucida domain proteins. Proc Natl Acad Sci USA. 2004;101(16):5922–5927. doi: 10.1073/pnas.0401600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaeffer C, Santambrogio S, Perucca S, Casari G, Rampoldi L. Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol Biol Cell. 2009;20(2):589–599. doi: 10.1091/mbc.E08-08-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiteman P, Handford PA. Defective secretion of recombinant fragments of fibrillin-1: Implications of protein misfolding for the pathogenesis of Marfan syndrome and related disorders. Hum Mol Genet. 2003;12(7):727–737. doi: 10.1093/hmg/ddg081. [DOI] [PubMed] [Google Scholar]
- 40.Jensen SA, Corbett AR, Knott V, Redfield C, Handford PA. Ca2+-dependent interface formation in fibrillin-1. J Biol Chem. 2005;280(14):14076–14084. doi: 10.1074/jbc.M412832200. [DOI] [PubMed] [Google Scholar]
- 41.Kettle S, Card CM, Hutchinson S, Sykes B, Handford PA. Characterisation of fibrillin-1 cDNA clones in a human fibroblast cell line that assembles microfibrils. Int J Biochem Cell Biol. 2000;32(2):201–214. doi: 10.1016/s1357-2725(99)00120-x. [DOI] [PubMed] [Google Scholar]
- 42.Loeys BL, et al. Mutations in fibrillin-1 cause congenital scleroderma: Stiff skin syndrome. Sci Transl Med. 2010;2(23):23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider R, et al. Biophysical characterisation of fibulin-5 proteins associated with disease. J Mol Biol. 2010;401(4):605–617. doi: 10.1016/j.jmb.2010.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.