Abstract
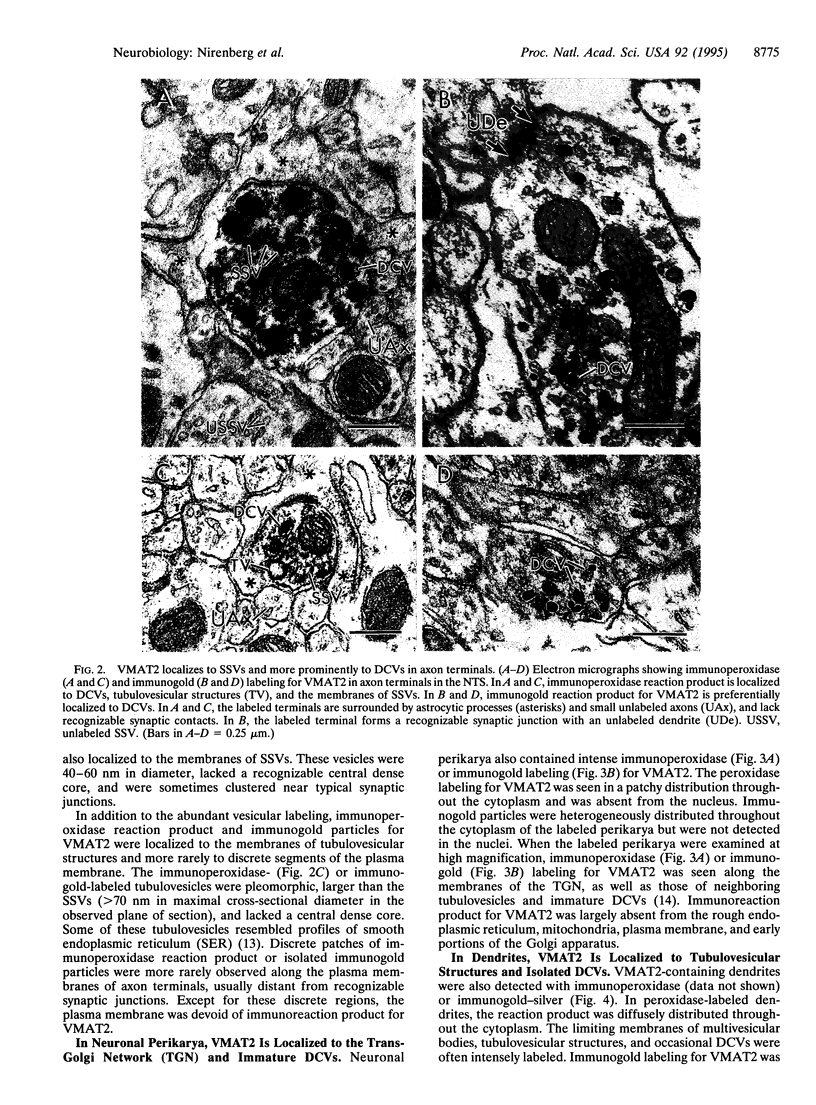
In central neurons, monamine neurotransmitters are taken up and stored within two distinct classes of regulated secretory vesicles: small synaptic vesicles and large dense core vesicles (DCVs). Biochemical and pharmacological evidence has shown that this uptake is mediated by specific vesicular monamine transporters (VMATs). Recent molecular cloning techniques have identified the vesicular monoamine transporter (VMAT2) that is expressed in brain. This transporter determines the sites of intracellular storage of monoamines and has been implicated in both the modulation of normal monoaminergic neurotransmission and the pathogenesis of related neuropsychiatric disease. We used an antiserum against VMAT2 to examine its ultrastructural distribution in rat solitary tract nuclei, a region that contains a dense and heterogeneous population of monoaminergic neurons. We find that both immunoperoxidase and immunogold labeling for VMAT2 localize to DCVs and small synaptic vesicles in axon terminals, the trans-Golgi network of neuronal perikarya, tubulovesicles of smooth endoplasmic reticulum, and potential sites of vesicular membrane recycling. In axon terminals, immunogold labeling for VMAT2 was preferentially associated with DCVs at sites distant from typical synaptic junctions. The results provide direct evidence that a single VMAT is expressed in two morphologically distinct types of regulated secretory vesicles in central monoaminergic neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki C. Beta-adrenergic receptors: astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J Neurosci. 1992 Mar;12(3):781–792. doi: 10.1523/JNEUROSCI.12-03-00781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet A., Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3(10):851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Chan J., Aoki C., Pickel V. M. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990 Aug;33(2-3):113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheramy A., Leviel V., Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981 Feb 12;289(5798):537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- De Potter W. P., Kurzawa R., Miserez B., Coen E. P. Evidence against differential release of noradrenaline, neuropeptide Y, and dopamine-beta-hydroxylase from adrenergic nerves in the isolated perfused sheep spleen. Synapse. 1995 Feb;19(2):67–76. doi: 10.1002/syn.890190202. [DOI] [PubMed] [Google Scholar]
- Edwards R. H. Neural degeneration and the transport of neurotransmitters. Ann Neurol. 1993 Nov;34(5):638–645. doi: 10.1002/ana.410340504. [DOI] [PubMed] [Google Scholar]
- Erickson J. D., Eiden L. E., Hoffman B. J. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen L. B., Jessell T. M., Cuello A. C., Iversen L. L. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976 Mar 18;260(5548):258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Joh T. H., Shikimi T., Pickel V. M., Reis D. J. Brain tryptophan hydroxylase: purification of, production of antibodies to, and cellular and ultrastructural localization in serotonergic neurons of rat midbrain. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3575–3579. doi: 10.1073/pnas.72.9.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Storage and release of neurotransmitters. Cell. 1993 Jan;72 (Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Levitt P., Moore R. Y. Origin and organization of brainstem catecholamine innervation in the rat. J Comp Neurol. 1979 Aug 15;186(4):505–528. doi: 10.1002/cne.901860402. [DOI] [PubMed] [Google Scholar]
- Liu Y., Peter D., Roghani A., Schuldiner S., Privé G. G., Eisenberg D., Brecha N., Edwards R. H. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992 Aug 21;70(4):539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Liu Y., Roghani A., Edwards R. H. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Schweitzer E. S., Nirenberg M. J., Pickel V. M., Evans C. J., Edwards R. H. Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells. J Cell Biol. 1994 Dec;127(5):1419–1433. doi: 10.1083/jcb.127.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley B. E., Engle M. G., Humphreys S., Vascik D. A., Howes K. A., Newton B. W., Elde R. P. Monoamine synaptic structure and localization in the central nervous system. J Electron Microsc Tech. 1990 May;15(1):20–33. doi: 10.1002/jemt.1060150104. [DOI] [PubMed] [Google Scholar]
- Mercer L., del Fiacco M., Cuello A. C. The smooth endoplasmic reticulum as a possible storage site for dendritic dopamine in substantia nigra neurones. Experientia. 1979 Jan 15;35(1):101–103. doi: 10.1007/BF01917903. [DOI] [PubMed] [Google Scholar]
- Morris J. F., Pow D. V. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec. 1991 Dec;231(4):437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Simons K., Dotti C. G. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992 Oct;119(1):123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S. A molecular glimpse of vesicular monoamine transporters. J Neurochem. 1994 Jun;62(6):2067–2078. doi: 10.1046/j.1471-4159.1994.62062067.x. [DOI] [PubMed] [Google Scholar]
- Schwarzenbrunner U., Schmidle T., Obendorf D., Scherman D., Hook V., Fischer-Colbrie R., Winkler H. Sympathetic axons and nerve terminals: the protein composition of small and large dense-core and of a third type of vesicles. Neuroscience. 1990;37(3):819–827. doi: 10.1016/0306-4522(90)90111-g. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y., Keen J. N., Bejerano M., Steiner-Mordoch S., Wallach M., Findlay J. B., Schuldiner S. Homology of a vesicular amine transporter to a gene conferring resistance to 1-methyl-4-phenylpyridinium. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9730–9733. doi: 10.1073/pnas.89.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A. Biogenesis of secretory granules. Implications arising from the immature secretory granule in the regulated pathway of secretion. FEBS Lett. 1991 Jul 22;285(2):220–224. doi: 10.1016/0014-5793(91)80805-d. [DOI] [PubMed] [Google Scholar]
- Winkler H. The adrenal chromaffin granule: a model for large dense core vesicles of endocrine and nervous tissue. J Anat. 1993 Oct;183(Pt 2):237–252. [PMC free article] [PubMed] [Google Scholar]