Abstract
Objectives
We describe the first use of caval-aortic access and closure to enable transcatheter aortic valve replacement (TAVR) in patients who lacked other access options. Caval-aortic access refers to percutaneous entry into the abdominal aorta from the femoral vein through the adjoining inferior vena cava.
Background
TAVR is attractive in high risk or inoperable patients with severe aortic stenosis. Available transcatheter valves require large introducer sheaths, which risk major vascular complications or preclude TAVR altogether. Caval-aortic access has been successful in animals.
Methods
We performed a single center retrospective review of procedural and 30-day outcomes of prohibitive-risk patients undergoing TAVR via caval-aortic access.
Results
Between July 2013 and January 2014, 19 patients underwent TAVR via caval-aortic access. 79% were women. Caval-aortic access and tract closure was successful in all 19; TAVR was successful in 17. Six patients suffered modified VARC-2 major vascular complications, two (11%) of whom required intervention. Most (79%) required blood transfusion. There were no deaths attributable to caval-aortic access. Through 111 (39–229) days of follow up, there were no post-discharge complications related to tract creation or closure. All patients had persistent aorto-caval flow immediately post procedure. Of 16 who underwent repeat imaging after the first week, 15 (94%) had complete closure of the residual aorto-caval tract.
Conclusions
Percutaneous transcaval venous access to the aorta allows TAVR in otherwise ineligible patients, and may offer a new access strategy for other applications requiring large transcatheter implants.
Keywords: Caval-aortic, transcaval, transcatheter aortic valve replacement, extra-anatomic procedures
Introduction
Transcatheter aortic valve replacement (TAVR) is an effective treatment for patients with symptomatic severe aortic stenosis and high or prohibitive surgical risk (1,2). Transcatheter valves commercially available in the United States currently require large 18–24F inner diameter sheaths. This precludes TAVR in as many as one quarter of patients, particularly women with smaller iliofemoral arteries and those with peripheral artery disease (3,4). Large sheaths also risk major vascular complications including rupture, hemorrhage and death (5,6). Hybrid surgical and other alternative approaches are associated with significant morbidity and mortality and are contraindicated in many due to unfavorable anatomy or comorbidity (7).
Caval-aortic access entails delivering large vascular sheaths into the abdominal aorta via the femoral vein through the inferior vena cava. It has been demonstrated in pigs (8). The caval-aortic tract can be closed using nitinol occluder devices and, counter-intuitively, is well tolerated even when not repaired. We describe the first use of this technique in humans undergoing TAVR who were felt not to have other access options.
Methods
Case Selection
Patients were selected from the high risk structural heart disease program at Henry Ford Hospital, Detroit, MI. All had severe symptomatic aortic valvular heart disease deemed high or prohibitive surgical risk. The multidisciplinary team of surgeons, cardiologists and anesthesiologists concurred they would likely benefit from TAVR but were not suitable for femoral arterial or transapical delivery of the transcatheter valve. For the first 11 patients, transaortic surgical access was not an option at our institution; subsequent patients were also deemed ineligible for transaortic delivery (severe lung disease and morbid obesity, n=2; porcelain aorta, n=2; frailty and poor rehabilitation potential, n=3; prior chest irradiation, n=1). All underwent TAVR under general anesthesia using Sapien transcatheter heart valves (Edwards, Irvine, CA). Patients consented to clinical treatment despite explicitly high risk. The institutional review board of Henry Ford Hospital approved this analysis and report.
Technique of Caval-Aortic Access during TAVR
Contrast-enhanced CT was used to select a caval-aortic crossing trajectory with the least-calcified aortic wall and no interposed structures, to determine suitable angiographic projection angles, and to determine fluoroscopic landmarks in relation to lumbar vertebrae. After simultaneous aortography and venography, and heparin administration, a gooseneck snare was positioned to “receive” a crossing guidewire in orthogonal fluoroscopic projections (Figure 1). A coaxial crossing system (Figure 2) consisting of a stiff 0.014” guidewire (Asahi ConfianzaPro12, Abbott Vascular, Santa Clara, CA) inside a 0.035” wire convertor (Piggyback, Vascular Solutions, Minneapolis, MN) inside a support catheter (Navicross, Terumo, Somerset, NJ) was inserted into a guiding catheter (RDC or RDC1) selected based on caval diameter. The crossing system was directed from the cava towards the aortic snare, which served as a target. The proximal guidewire end was connected to a unipolar electrosurgery pencil (Valleylab, Covidien, Mansfield, MA) using forceps and the patient was connected to a ground pad. The distal crossing tip of the guidewire was extended 2–5mm beyond the wire convertor and energized in “cutting mode” at 50–70W to vaporize target tissue during 2–3 second bursts. After the first 9 patients, we amputated the distal 1cm of the guidewire to ease crossing. The snare confirmed intraluminal wire position and provided counter-traction to advance the crossing system into the aorta (Video Supplement). The crossing devices were replaced with a rigid guidewire (0.035” Lunderquist, Cook, Bloomington, IN). The appropriate sized 35cm long Edwards TAVR introducer sheath (Retroflex 3 models 9120S23 (22F) or 9120S26 (24F) was delivered from the femoral vein into the IVC, through the caval-aortic tract and into the abdominal aorta in a single step without progressive dilatation. Aortography was performed immediately after sheath placement to assure hemostasis. TAVR was then performed in the usual manner.
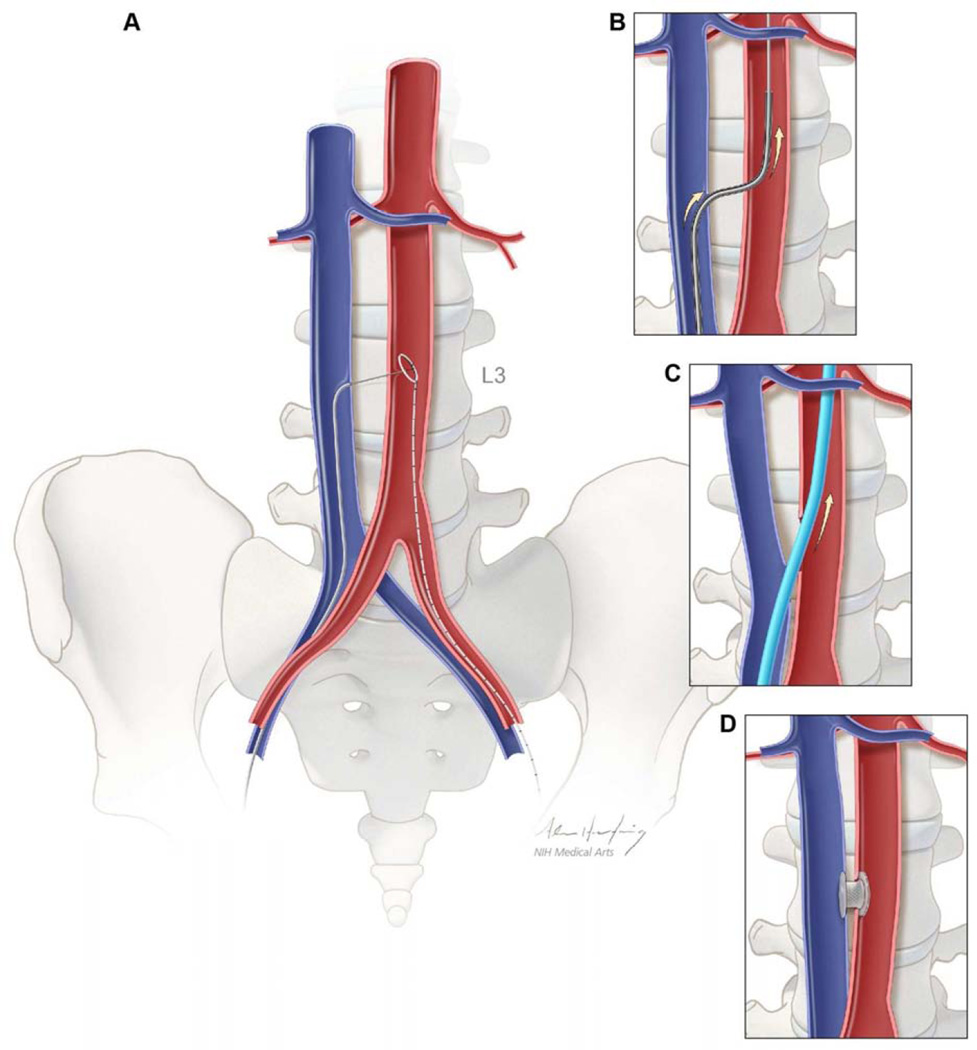
Figure 1. Schematic depiction of caval-aortic access.
(A) A catheter directs a transfemoral vein guidewire from the inferior vena cava towards a snare target positioned in the adjoining abdominal aorta. (B) A catheter is advanced over the guidewire into the aorta and used to introduce a more rigid guidewire. (C) The valve introducer sheath is advanced from the vena cava into the aorta. (D) After completion of TAVR, the aorto-caval access tract is closed with a nitinol occluder.
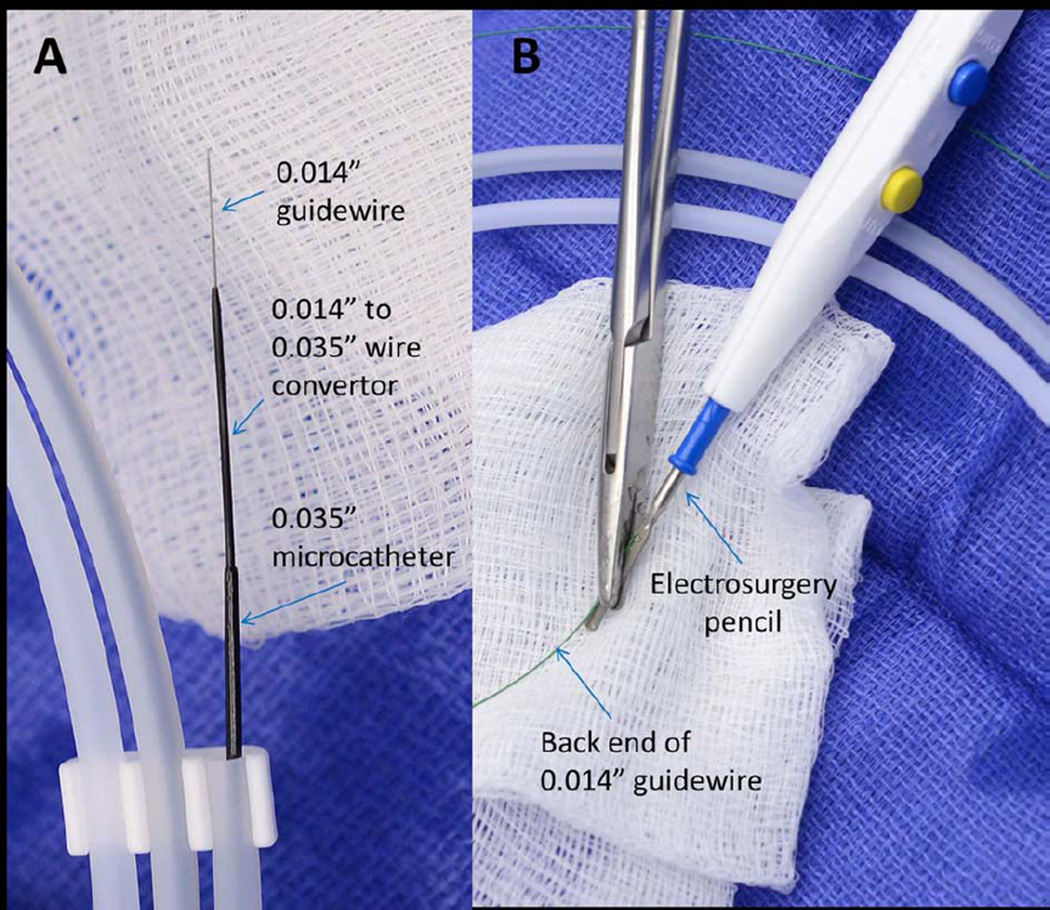
Figure 2. Crossing apparatus.
(A) A 0.014” guidewire is mounted coaxially inside a 0.014”-to-0.0135” wire convertor, inside a 0.035” inner-diameter microcatheter. (B). The back end of the guidewire is connected to an electrosurgery pencil.
After TAVR, the tract was closed with a nitinol occluder device marketed to close ductus arteriosus (Amplatzer Duct Occluder (ADO), St Jude Medical, St. Paul, MN) or intracardiac defects (Amplatzer muscular VSD occluder (MVSDO)) using the accompanying delivery system inside the TAVR sheath. Devices were selected to approach or exceed the outer diameter of the sheath (8.2 and 9.3 mm for Edwards 22 and 24Fr sheaths, respectively), and the distance between the aorta and cava. The occluders were deployed by exposing the distal disk in the aorta, retracting to appose the aortic wall, and then deploying the proximal device near or inside the cava. Aortography was performed immediately before and after device release to assure no retroperitoneal accumulation of contrast. The device was recaptured and repositioned if necessary, or replaced after re-advancing the sheath over a previously placed 0.014” “buddy” guidewire. All received protamine to reverse heparin anticoagulation. The femoral vein access site was closed using two prepositioned sutures (Perclose ProGlide, Abbott Vascular).
Patients underwent usual post-TAVR care. The first eight patients underwent systematic early CT. With further experience, this exam was performed before discharge unless contraindicated, or performed sooner if bleeding was suspected. In-hospital and 30-day outcomes were ascertained during clinical and imaging encounters. Patients with patent caval-aortic tracts at time of discharge were advised to undergo contrast enhanced CT at 30 days.
Analysis
Data are presented as mean ± standard deviation or median (range). Continuous variables were compared using a Student t-test. Crossing time is recorded as the interval between when the caval catheter is first directed at the aorta until the time the introducer sheath is placed in the aorta, usually including aortic root angiography. Closure time is the interval between first advancement of a nitinol occluder device until completion aortography. Major vascular complications and bleeding are classified according to VARC-2 (9), modified to disregard aortocaval fistulas.
Angiographic appearance of the caval-aortic tract after closure device placement (Figure 3) was graded as 0: complete hemostasis with occluded aorto-caval fistula; 1: patent aorto-caval fistula without contrast outside the tract; 2: patent aorto-caval fistula with persistent cruciformpattern contrast outside the aorto-caval fistula; and 3: free extravasation outside the aorto-caval fistula. CT retroperitoneal bleeding after TAVR (Figure 4) was graded as 0: absent; 1: blood evident without contrast extravasation; 2: blood evident with contrast extravasation; or 3: blood evident with organ displacement or with sentinel clot sign.
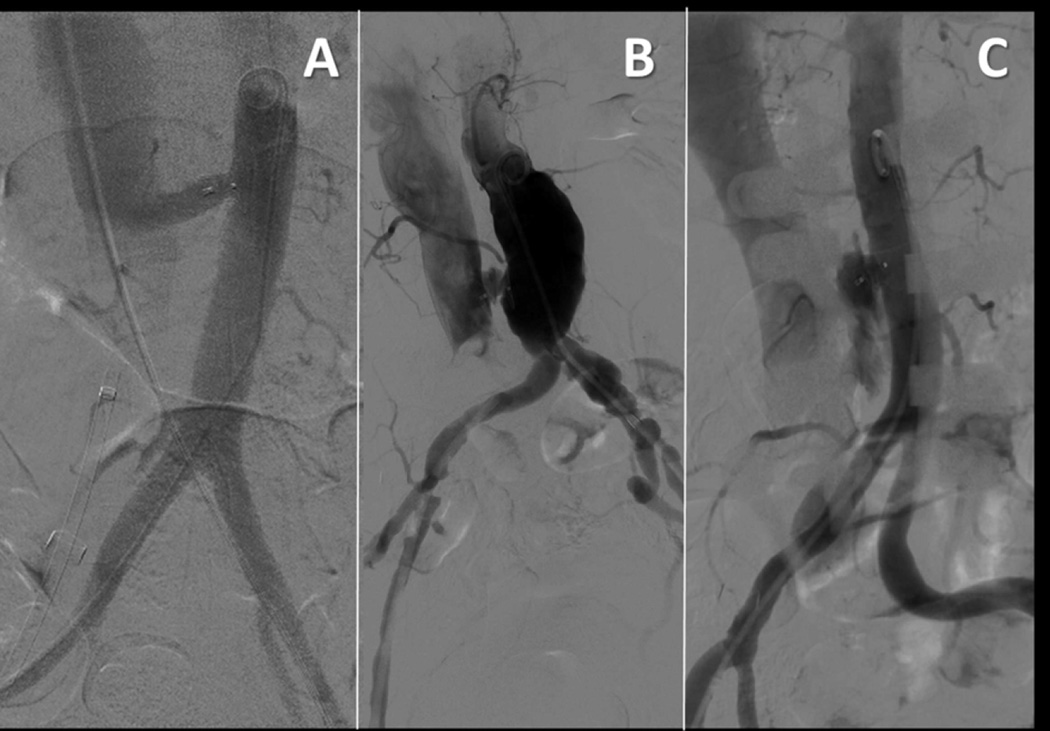
Figure 3. Typical angiographic patterns after caval-aortic TAVR.
(A) Patent aorto-caval fistula despite closure device (patient #16). (B) Patent aorto-caval fistula with persistent “cruciform” extra-aortic contrast (patient #13). (C) Contrast extravasation (patient #14).
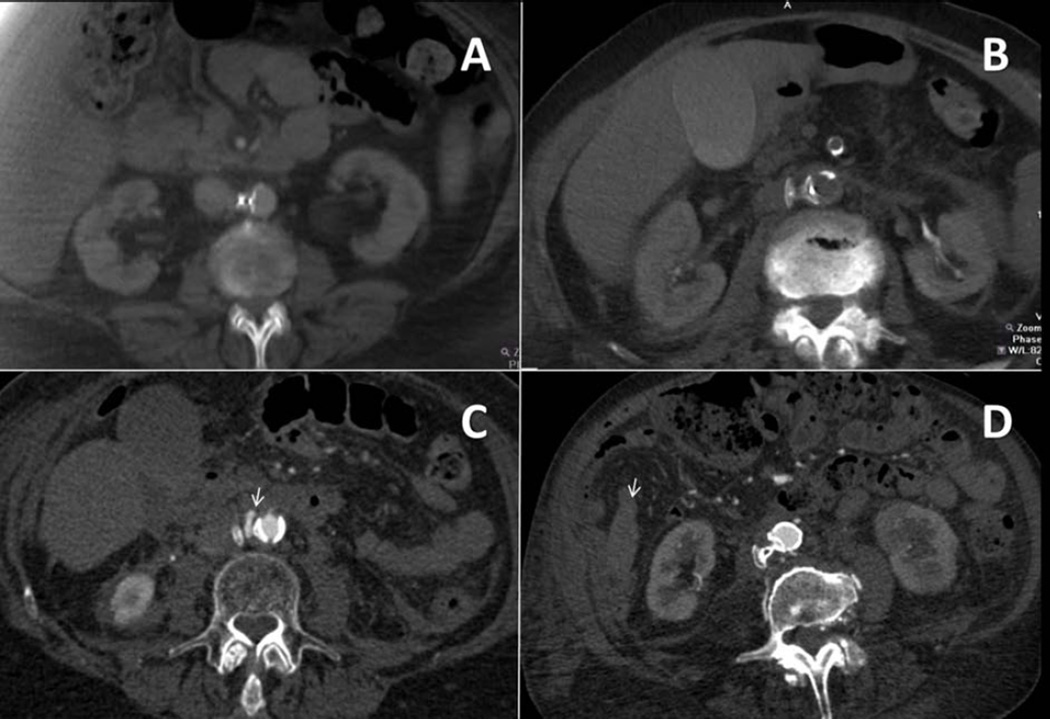
Figure 4. Typical CT patterns after caval-aortic TAVR.
(A) No evident bleeding (patient #16, day 4). (B) Mild retroperitoneal blood accumulation without contrast extravasation (patient #04, day 1). (C) Blood present with contrast extravasation (arrowhead, patient #03, day 1). (D) Large retroperitoneal blood accumulation or organ displacement (#09, day 0). In this patient, pararenal hematoma (arrow) is evident.
Results
Patients
Nineteen patients with symptomatic severe aortic valvular heart disease underwent TAVR using caval-aortic access between July 2013 and January 2014. Baseline demographics are displayed in Table 1. Fifteen were women. Table 2 enumerates contraindications to conventional TAVR. Two patients had prior aortic root surgery including one with a stentless bioprosthetic valve. One had aborted surgical AVR for porcelain aorta and another aborted transapical TAVR due to friable myocardium. Two had unsuccessful prior attempts to deliver femoral artery introducer sheaths for TAVR. Standard arterial access was not possible because of peripheral artery disease (37%) or inadequate iliofemoral artery caliber for the planned valve. The larger (“best”) iliofemoral artery minimum diameter was 5.7±1.0mm.
Table 1.
Baseline Demographics
| Characteristic | |
|---|---|
| Age (years), mean±SD | 80.7±8.3 |
| Female, n (%) | 15 (79) |
| BSA (m2), mean±SD | 1.79±0.23 |
| Prior cardiac surgery, n (%) | 7 (37) |
| Peripheral artery disease, n (%) | 7 (37) |
| eGFR, mL/min/1.73m2, mean±SD | 50.8±21.8 |
| Baseline hemoglobin (g/dL), mean±SD | 10.9±1.4 |
| NYHA class III or IV heart failure, n (%) | 16 (84) |
| Atrial fibrillation, n (%) | 10 (53) |
| STS predicted mortality (%), mean±SD | 7.8±3.8 |
| Euroscore II (%), mean±SD | 7.9±6.2 |
| Moderate-severe mitral regurgitation, n (%) | 7 (37) |
| Aortic valve area, cm2, mean±SD | 0.68±0.16 |
SD=standard deviation; BSA=body surface area; eGFR=estimated glomerular filtration rate; NYHA=New York heart association; STS=society thoracic surgery.
Table 2.
Caval-Aortic TAVR Details
| # | Sex | Reason for inoperability |
Best ilio- femora l MLD (mm) |
Sheath size (Fr) |
Crossing attempts (#) |
Crossing time (min) |
Closure device |
Device repositioned during closure |
Closure time (min) |
Hypotension during tract closure |
Angiographic pattern after closure |
Transfusion during TAVR |
CTRPH Score |
In- hospital complication |
Length of stay (days) |
Tract patency |
30-day MACE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Prior SAVR & root repair; Failed prior TA-TAVR; eGFR<30; STS >10 |
4.8 | 22 | 3 | 75 | 12/10mm ADO |
— | 17 | — | 1 | — | 1 | T, V | 15 | O, day 44 |
None |
| 2 | F | Frailty; Immunocompromized |
5.1 | 24 | 1 | 23 | 10/8mm ADO |
— | 11 | — | 1 | + | 0 | — | 9 | P, day 36 |
R |
| 3 | F | Porcelain aorta; Failed SAVR; Immunocompromised |
6.5 | 22 | 3 | 67 | 8mm MVSDO |
+ | 48 | + | 2 | + | 2 | T, V | 11 | O, day 7 |
None |
| 4 | F | Severe COPD; Frailty |
6.6 | 24 | 3 | 17 | 10mm MVSDO |
— | 22 | — | 2 | — | 1 | — | 6 | O, day 189 |
None |
| 5 | F | Pulmonary artery hypertension Failed TF-TAVR |
7.4 | 22 | 3 | 17 | 8mm MVSDO |
— | 7 | — | 1 | — | 1 | T | 8 | O, day 42 |
R |
| 6 | M | Frailty; Prior CABG; Low EF |
4.9 | 24 | 2 | 16 | 10/8mm ADO |
— | 13 | + | 1 | + | — | Death | NA | NA | NA |
| 7 | F | Frailty; Prior CABG; |
6.7 | 22 | 3 | 75 | 6mm MVSDO |
— | 7 | — | 1 | — | 0 | T | 4 | O, day 36 |
None |
| 8 | M | Severe COPD | 4.5 | 24 | 1 | 28 | 8/6mm ADO |
— | 8 | — | 1 | — | 1 | — | 2 | O, day 86 |
None |
| 9 | F | Prior aortic root repair; Frailty; Failed TF-TAVR; STS >10 |
5.6 | 22 | 2 | 34 | 8mm MVSDO |
+ | 37 | + | 2 | + | 1 | T, V | 15 | O, day 15 |
None |
| 10 | F | COPD; porcelain aorta |
5.0 | 22 | 1 | 14 | 6mm MVSDO |
— | 8 | 2 | + | 0 | MI | 8 | O, day 8 |
None | |
| 11 | M | Low EF, prior CABG; eGFR<30; STS >10; |
6.0 | 24 | 1 | 14 | 8mm MVSDO |
+ | 21 | — | 2 | — | — | — | 8 | P, day 1 |
None |
| 12 | M | Frailty, prior CABG |
6.1 | 24 | 1 | 10 | 8mm MVSDO |
— | 18 | + | 2 | + | 2 | S, CIN | 34 | P, day 1 |
None |
| 13 | F | Age >90; Frailty; Porcelain aorta; STS >10 |
4.0 | 22 | 1 | 24 | 6mm MVSDO |
— | 3 | — | 2 | — | 0 | T | 8 | O, day 120 |
None |
| 14 | F | Low EF; Prior CABG; STS >10; |
7.6 | 24 | 1 | 14 | 8mm MVSDO |
+ | 15 | + | 3 | — | 1 | T, V | 13 | O, day 7 |
None |
| 15 | F | Age >90; Frailty; COPD; STS >10; |
5.6 | 22 | 1 | 20 | 6mm MVSDO |
— | 3 | — | 2 | + | — | — | 2 | O, day 9 |
None |
| 16 | F | COPD, Morbid obesity |
5.3 | 24 | 1 | 34 | 10/8mm ADO |
+ | 12 | — | 1 | — | 0 | T | 5 | O, day 56 |
None |
| 17 | F | Immunocompromised, porcelain aorta, religious beliefs |
5.0 | 22 | 1 | 30 | 6mm MVSDO |
— | 7 | — | 2 | — | 0 | V | 8 | O, day 54 |
None |
| 18 | F | Frailty, severe COPD |
5.0 | 24 | 1 | 15 | 8mm MVSDO |
— | 11 | — | 2 | — | 1 | T, V, GIB |
14 | O, day 57 |
None |
| 19 | F | Age, frailty, prior radiation/lymph node dissection |
6.5 | 24 | 1 | 10 | 8mm MVSDO |
— | 4 | — | 2 | — | 1 | T, GIB, CIN |
12 | O, day 36 |
None |
MLD=minimal luminal diameter; SAVR=surgical aortic valve replacement; TA-TAVR=transapical TAVR; eGFR=estimated glomerular filtration rate; STS=Society thoracic surgery predicted mortality score; COPD=chronic obstructive pulmonary disease; TF-=transfemoral; CABG=coronary artery bypass grafting; EF=ejection fraction; ADO=Amplatzer duct occluder; MVSDO= Amplatzer muscular ventricular septal defect occluder; RPH=Retroperitoneal hemorrhage; R=readmission; T=transfusion; V=vascular complication; MI=myocardial; infarction; S=Stroke; CIN=contrast induced nephropathy requiring hemodialysis; GIB=gastrointestinal bleed; P=patent; O=Occluded;.
Procedural outcomes
A representative procedure is shown in Figure 5 and the Video Supplement. Caval-aortic access was successful in all 19 patients. It required 1.4±0.8 puncture attempts and on average 20 (range 10–75) minutes. Crossings were midway (0.5±0.2) between the right renal artery and the aortic bifurcation, typically over the third lumbar vertebral body (±0.5 vertebrae). At this position, the caval-aortic interluminal distance was 6±3mm (range 3–12mm), and the caval and aortic diameters were 21±3mm and 16±4mm, respectively. There were no hemodynamic changes during puncture or sheath placement, and the sheath was confirmed hemostatic by immediate abdominal aortography in all.
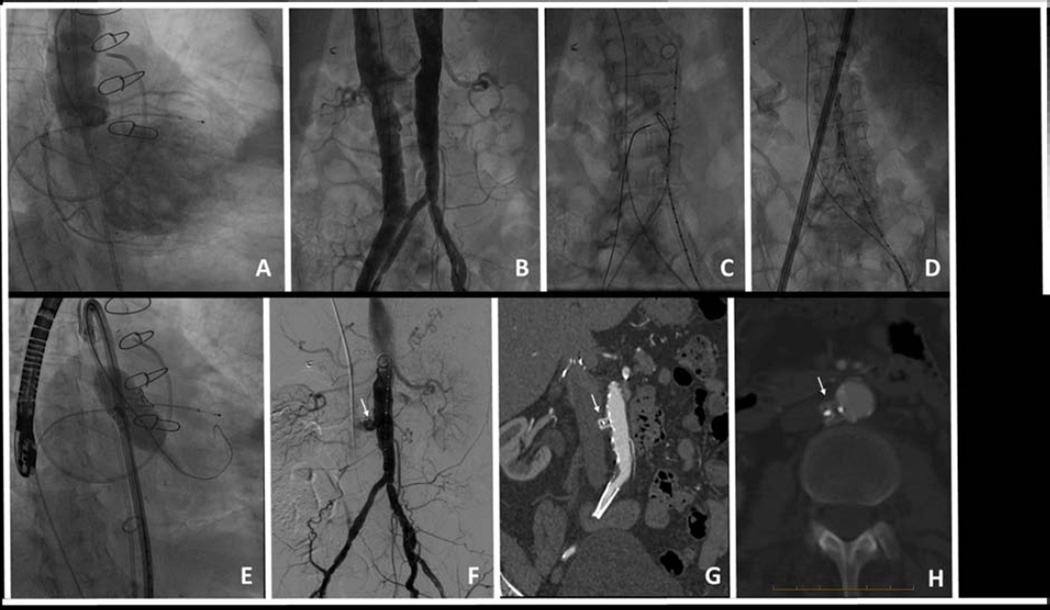
Figure 5. Planning and technique of caval-aortic access.
Caval-aortic access for TAVR in patient #1. (A) Aortography shows severe regurgitation of a bioprosthetic aortic valve causing left ventricular dilation and intractable heart failure. (B) Simultaneous caval and aortic angiograms. (C) A guidewire is directed from the cava and energized to cross into a prepositioned aortic snare. (D) A 8.2 mm diameter sheath is advanced along this guidewire tract from the femoral vein and cava into the aorta. (E) TAVR is performed using a 23mm balloon-expandable valve. (F) The caval-aortic tract is closed with a nitinol duct occluder (arrow). Completion aortography shows mild residual aorto-caval shunt across the access tract but no contrast extravasation. (G, H) A contrast-enhanced CT performed 42 days later shows complete occlusion of the tract.
TAVR was successful in 17 patients. One (#6) required emergency surgery to retrieve an embolized transcatheter valve into the left ventricle after low deployment in too large an annulus. Another (#12) had valve embolization into the aorta due to failure of rapid pacing during deployment.
All patients underwent successful closure device implantation into the caval-aortic tract, including the one proceeding to surgery (Table 2). Closure required 11 minutes (range 3–37) and 1.3±0.7 device deployment attempts. In five cases, the device was recaptured and replaced because of initial malposition or contrast extravasation before final positioning. In five (26%), there was transient hypotension during tract closure, including three requiring device repositioning, while two others requiring repositioning did not. There were two other patients who experienced hypotension during otherwise uncomplicated closure device placement.
At the conclusion of the procedure, there was residual aorto-caval flow through the device tract in all patients (Table 2, Figure 3). In twelve (63%) there was persistent extra-aortic contrast during completion aortography (cruciform appearance, n=11; frank extravasation, n=1). In only 5 of these, the device was repositioned. Only 4 of 12 exhibited hypotension.
In-hospital and 30-day Outcomes
There was 1 TAVR-related death during attempted surgical retrieval of an embolized transcatheter valve (Table 3). All 18 survivors were either extubated immediately after TAVR (n=15) or the following morning (n=3).
Table 3.
In-hospital and 30-day outcomes.
| Outcome, n=19 | In-hospital | 30-day | Narrative |
|---|---|---|---|
| Death (from any cause), n | 1 | 0 | During surgery for embolized transcatheter valve |
| Death (access-related), n | 0 | 0 | |
| Vascular complication – arterial, n | 6 | 0 | Three had large retroperitoneal hematomas, One had small aortic pseudoaneurysm Two had focal aortic dissection |
| Requiring intervention, n | 2 | 0 | Two endografts; one for retroperitoneal bleeding 24 hours post, one for retroperitoneal bleeding with hypotension 6 hours post |
| Vascular complication – venous, n | 1 | 0 | One deep vein thrombosis at the access site, treated with anticoagulation |
| Requiring intervention, n | 0 | 0 | |
| Stroke, n | 1 | 0 | |
| New onset claudication, n | 0 | 0 | |
| New onset CHF, n | 0 | 0 | |
| New onset GI symptoms, n | 1 | 0 | Nausea |
Vascular complications requiring intervention
Six patients experienced modified VARC-2 major vascular complications, including two who were treated with percutaneous aortic stent-grafts. Patient #3 had mild abdominal pain and some peri-aortic blood evident on CT; although hemodynamically stable she underwent transfemoral aortic tube stent-graft placement one day after TAVR. Patient #14 had cruciform-pattern extra-aortic contrast after device repositioning, and had hypotension requiring vasopressors. Six hours afterwards she underwent aortic stent-graft treatment and no longer required vasopressors. Patient #9 had a retroperitoneal hematoma and transient hypotension that was managed conservatively. She had hypotension during closure device repositioning, and required one unit of blood and transient vasopressors after the procedure
Vascular complications not requiring intervention
Patient #1 had a small retro-aortic pseudoaneurysm detected on CT, which resolved on follow-up CT with conservative management. Patients #17 and #18 had focal self-contained aortic dissections in the vicinity of the caval-aortic closure device. The dissections were partially healed on CT two months after TAVR.
CT evaluation
When obtained during hospitalization (n=16), CT revealed blood in the retroperitoneal space in nine (56%, mean retroperitoneal bleeding score 0.9±0.9), graded as mild in 6 (Table 2). In one (#9 as described above), the retroperitoneal bleeding was noteworthy (Figure 4).
Bleeding and transfusions
The baseline hemoglobin was 10.9±1.4 g/dL. Fifteen (79%) required blood transfusions (mean total 3±4 units), seven during TAVR, and ten after TAVR. These include two suffering major gastrointestinal hemorrhage, one requiring cardiac surgery, two requiring aortic endografts, and one (patient #9) with retroperitoneal hemorrhage requiring overnight vasopressors. The most serious bleeding was observed in the three patients with elevated INR during TAVR, including the two requiring endografts and the one requiring vasopressors (INR 2.3±0.3 vs. 1.4±0.3 without bleeding, p=0.0002). Overall these are classified as VARC-2 life-threatening bleeding (16%), major bleeding (32%), minor bleeding (47%), and none (5%).
No patient had ischemic or embolic complications related to caval-aortic access. One had deep vein thrombosis of the femoral vein used for caval-aortic access, which was treated with anticoagulation. The patient with valve embolization into the aorta suffered an ischemic stroke. Two patients developed contrast-induced nephropathy requiring temporary hemodialysis. Nine (50%) exhibited mild transient thrombocytopenia (50–100,000 cells/µL). One more experienced profound asymptomatic thrombocytopenia (<50,000 cells/µL), not attributable to heparin, which resolved after 6 weeks.
Mean length of stay after TAVR was 8±8 days (range 2–37 days).
Follow up through 111±57 (range 36–229) days on the 18 survivors revealed no post-discharge access-related adverse events. One patient was readmitted for chest pain and another was readmitted for diastolic heart failure. Of 16 who underwent repeat imaging (CT, n=14; angiography, n=2) after the first week, 15 (94%) exhibited complete closure of the caval-aortic tract (83% overall closure rate) by 42±50 (range 7–189) days after TAVR. Follow up imaging was not performed on two patients due to renal insufficiency
Discussion
We describe a novel technique enabling TAVR using 8–9mm outer diameter sheaths in patients otherwise ineligible. Introducer sheaths were delivered to the aorta via a tract from the IVC created by radiofrequency perforation with subsequent closure using commercial nitinol occluder devices.
Caval-aortic access and tract closure was successful in all 19 patients. One fatality was unrelated to caval-aortic access. All patients tolerated the technique, but most required blood transfusion. Most had a residual aorto-caval shunt upon discharge, which was not hemodynamically significant, and which occluded in 15 of 18 survivors by 42 (7–189) days.
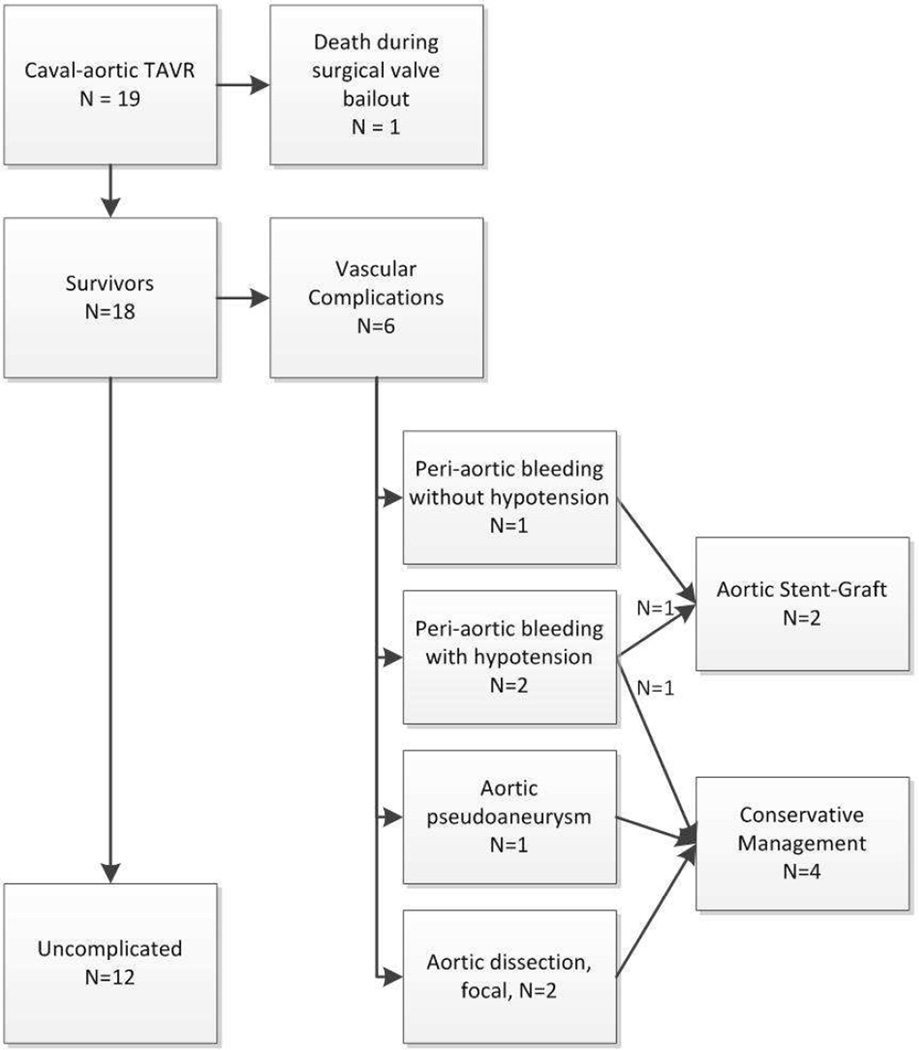
These patients were very ill and were judged to be at extreme risk, often out of proportion to the STS predicted mortality score of 7.8±3.8%, evidenced by the reasons they were ineligible for surgical AVR (Table 2). TAVR in this early series was successful in 17/19 (89%), compared with 92% in the post-market STS/ACC transcatheter valve therapy registry (10). Vascular complications were common in this early experience with patients having no attractive options. All had heavily calcified and atherosclerotic aortas; three had access into abdominal aortic aneurysm. Of nineteen, six (31%) had vascular complications classified as VARC-2 major: three had retroperitoneal bleeding, one had a small aortic pseudoaneurysm and two had focal aortic dissection. Two patients with bleeding were managed with stent-grafts and the others were managed conservatively (Figure 6). From our perspective, endograft therapy and blood transfusion are the important complications.
Figure 6. Outcomes of caval-aortic access.
Death and major vascular complications are depicted.
One-quarter of patients in this series had transient hypotension during the procedure, which responded to crystalloid or vasopressors. One of these had low baseline filling pressures, and the others presumably had bleeding during (re)positioning of the closure device. Early in the experience we obtained immediate post-procedure CT systematically; later we obtained immediate CT only if we suspected active bleeding. Patient #3 underwent aortic stent graft even though she was hemodynamically stable, because she had mild abdominal pain and CT evidence of more-than-mild retroperitoneal blood. In retrospect we believe we should have managed this patient conservatively. However, two patients had significant retroperitoneal bleeding, manifest as persistent hypotension, that required clinical intervention. One of these two required low dose vasopressors for <12 hours, and another required endograft therapy to achieve hemodynamic stability.
Retroperitoneal hematoma was expected and common because our technique uses permeable closure devices and pressurization of the retroperitoneal space with subsequent venous outflow. Unlike other patients with spontaneous, traumatic, or iatrogenic retroperitoneal arterial hemorrhage, none of ours developed abdominal compartment syndrome or required surgical evacuation. In our series, baseline hemoglobin was low, and substantial hemoglobin decline and transfusion were common. In two patients, blood loss was attributed to major gastrointestinal hemorrhage. We note that in this early experience patients were volume-expanded and transfused aggressively out of caution. Overall we consider these complications acceptable in light of the paucity of therapeutic options available to these patients. We expect bleeding risk to be reduced by purpose-built crossing and closure devices.
The rationale for caval-aortic access is that iliofemoral veins are larger and more compliant than corresponding arteries, the IVC is close to the abdominal aorta usually without interposed structures, and traumatic or aneurysmal aorto-caval fistulas do not necessarily cause immediate hemodynamic compromise (8). We speculate that a patent caval fenestration allows immediate decompression of aortic hemorrhage because of the relatively higher pressure of the retroperitoneal interstitial space. The retroperitoneum behaves as a relatively confined space that retains insufflation gas or saline during laparoscopic surgery, and that is pressurized 5–13mm Hg after 1L fluid infusion in cadavers (11,12). In animals, intentional failure to close the aorto-caval fistula is well tolerated, and free of retroperitoneal bleeding (8). Consistent with these considerations, our first patient became hypotensive when we inadvertently withdrew the sheath tip just outside the aorta yet still occluding the cava and the pressure returned to normal immediately after we withdrew the sheath farther to allow blood to reenter the IVC. Five other patients tolerated 5–7 minute intervals between removal and replacement of the closure device when there was an unconstrained aorto-caval fistula, which is in sharp contrast to the immediate hemodynamic collapse typically seen shortly after iliac artery perforation.
We found the overall procedural time related to caval aortic access and repair to be similar to that typically required for standard femoral artery access for TAVR, including pre-placement of vascular sutures, cross-over protection, and balloon inflation during vascular hemostasis. In addition, there appeared to be a “learning curve” of fewer puncture attempts and shorter crossing and closing times as experience accrued (Table 2).
One patient had non-antibody mediated, severe asymptomatic thrombocytopenia (nadir platelet count of 24,000 cells/µL) that resolved at approximately the same time the caval-aortic tract was found to be closed and 8 others experienced >50% decreases in platelet counts without evidence of other hemolysis. Isolated and profound platelet consumption has been described after device closure of ductus arteriosus, attributed to mechanical platelet injury. The thrombocytopenia seen in this series may reflect platelet consumption from bleeding or from residual aorto-caval shunting (13,14).
Fifteen of the 19 patients undergoing transcaval TAVR in this report were women. This likely reflects the smaller diameter of ilio-femoral vessels in women. Twelve (63%) would have had insufficient iliofemoral artery caliber (≤6.0mm) to allow TAVR using second generation 16–20Fr devices and 9 (47%) still would have had insufficient vessel size (≤5.5mm) currently required for third generation 14-F compatible devices both currently investigational in the US. Our technique may also enable non-surgical delivery of other large devices including thoracic aortic endografts, percutaneous left ventricular assist devices, and future larger valves for aortic insufficiency.
Limitations to the generalizability of our report include the small numbers of patients treated at a single center, and only short-term follow up. Conversely, we expect purpose-built access and closure devices should outperform the commercial devices we used off-label in this experience.
Conclusions
We have demonstrated feasibility of venous access to the aorta to allow TAVR in otherwise ineligible patients. This technique challenges conventional wisdom about intentionally violating the wall of the aorta. Caval-aortic access may prove useful for TAVR and other large-caliber device therapy even as future TAVR delivery devices diminish in size. Further clinical testing is warranted.
Supplementary Material
Acknowledgements
The authors thank the patients for their courage and their trust. Alan Hoofring of NIH Medical Arts provided illustrations. Drs Greenbaum and Lederman are inventors on patent applications for devices for caval-aortic access, that have been assigned to their employers, Henry Ford Hospital and the National Institutes of Health, respectively.
Supported by the Institute for Structural Heart Disease, Division of Cardiology, Henry Ford Health System (ABG, WWO, GP, MEG, JFW, RLC) and the Division of Intramural Research (Z01-HL006040), National Heart Lung and Blood Institute, National Institutes of Health (RJL).
Drs. Greenbaum and Lederman are inventors on patent applications for devices for caval-aortic access; these patent applications have been assigned to their employers, Henry Ford Hospital and the National Institutes of Health, respectively. The work was funded intramurally by the Center for structural heart disease, Henry Ford hospital, Detroit, MI (ABG, WWO) and by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (RJL). Thank you for considering this paper.
Abbreviations
- ADO
Amplatzer duct occluder
- AVR
Aortic valve replacement
- CT
Computed tomography
- IVC
Inferior vena cava
- MVSDO
Amplatzer muscular ventricular septal defect occluder
- TAVR
Transcatheter aortic valve replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. The New England journal of medicine. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Blanke P, Euringer W, Baumann T, et al. Combined assessment of aortic root anatomy and aortoiliac vasculature with dual-source CT as a screening tool in patients evaluated for transcatheter aortic valve implantation. AJR American journal of roentgenology. 2010;195:872–881. doi: 10.2214/AJR.10.4232. [DOI] [PubMed] [Google Scholar]
- 4.Babcock MJ, Lavine S, Strom JA, Bass TA, Guzman LA. Candidates for transcatheter aortic valve replacement: Fitting the PARTNERS criteria. Catheter Cardiovasc Interv. 2013;82:655–661. doi: 10.1002/ccd.24706. [DOI] [PubMed] [Google Scholar]
- 5.Genereux P, Webb JG, Svensson LG, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER trial. J Am Coll Cardiol. 2012;60:1043–1052. doi: 10.1016/j.jacc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Toggweiler S, Leipsic J, Binder RK, et al. Management of vascular access in transcatheter aortic valve replacement: part 2: vascular complications. JACC Cardiovasc Interv. 2013;6:767–776. doi: 10.1016/j.jcin.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Dewey TM, Bowers B, Thourani VH, et al. Transapical Aortic Valve Replacement for Severe Aortic Stenosis: Results From the Nonrandomized Continued Access Cohort of the PARTNER Trial. Ann Thorac Surg. 2013 doi: 10.1016/j.athoracsur.2013.05.093. [DOI] [PubMed] [Google Scholar]
- 8.Halabi M, Ratnayaka K, Faranesh AZ, Chen MY, Schenke WH, Lederman RJ. Aortic access from the vena cava for large caliber transcatheter cardiovascular interventions: pre-clinical validation. Journal of the American College of Cardiology. 2013;61:1745–1746. doi: 10.1016/j.jacc.2013.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA : the journal of the American Medical Association. 2013;310:2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 11.Grimm MR, Vrahas MS, Thomas KA. Pressure-volume characteristics of the intact and disrupted pelvic retroperitoneum. The Journal of trauma. 1998;44:454–459. doi: 10.1097/00005373-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kohler D, Sellei RM, Sop A, et al. Effects of pelvic volume changes on retroperitoneal and intra-abdominal pressure in the injured pelvic ring: a cadaveric model. The Journal of trauma. 2011;71:585–590. doi: 10.1097/TA.0b013e318224cd62. discussion 590. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Zhu XY. Severe thrombocytopenia complicating transcatheter occlusion of a patent ductus arteriosus. J Invasive Cardiol. 2013;25:E88–E92. [PubMed] [Google Scholar]
- 14.McCabe JM, Huang PH, Riedl LA, et al. Incidence and implications of idiopathic thrombocytopenia following transcatheter aortic valve replacement with the Edwards Sapien valves: A single center experience. Catheter Cardiovasc Interv. 2013 doi: 10.1002/ccd.25206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.