Abstract
B cell memory to T cell–dependent (TD) Ags are considered to largely reside in class-switched CD27+ cells. However, we previously observed that anti-RhD (D) Igs cloned from two donors, hyperimmunized with D+ erythrocytes, were predominantly of the IgM isotype. We therefore analyzed in this study the phenotype and frequency of D- and tetanus toxoid–specific B cells by culturing B cells in limiting dilution upon irradiated CD40L-expressing EL4.B5 cells and testing the culture supernatant. Most Ag-specific B cells for both TD Ags were found to reside in the IgM-expressing B cells, including CD27− B cells, in both hyperimmunized donors and nonhyperimmunized volunteers. Only shortly after immunization a sharp increase in Ag-specific CD27+IgG+ B cells was observed. Next, B cells were enriched with D+ erythrocyte ghosts and sorted as single cells. Sequencing of IGHV, IGLV, IGKV, and BCL6 genes from these D-specific B cell clones demonstrated that both CD27−IgM+ and CD27+IgM+ B cells harbored somatic mutations, documenting their Ag-selected nature. Furthermore, sequencing revealed a clonal relationship between the CD27−IgM+, CD27+IgM+, and CD27+IgG+ B cell subsets. These data strongly support the recently described multiple layers of memory B cells to TD Ags in mice, where IgM+ B cells represent a memory reservoir which can re-enter the germinal center and ensure replenishment of class-switched memory CD27+ B cells from Ag-experienced precursors.
Introduction
There are two main B cell compartments in blood, a naive CD27− population expressing IgM and IgD, carrying unmutated Ig genes and accounting for 60–70% of total B cells, and a memory CD27+ population, largely expressing isotype-switched Ig (IgG, IgA, or IgE) with somatic hypermutations (1). In agreement, CD27+ B cells are found in substantially higher concentrations in adult peripheral blood (PB) (2) than in cord blood (3). However, IgM memory cells also exist (4). In mice, long-lived IgM-expressing B cells are induced upon exposure to T cell-independent (TI) Ags and are referred to as memory cells because they can transfer immunity (5, 6). A similar subset of IgM-expressing memory B cells responding to TI Ags exists in human (7). Recently, two studies in mice further challenged the classical view on B cell memory by showing that long-term memory also for T cell–dependent (TD) Ags can reside in an IgM-expressing B cell pool (8, 9). By an elegant AID-mediated labeling method, it was demonstrated that the progeny of germinal center (GC) B cells are not only class switched but are also IgM-expressing memory cells (8). Upon booster, these IgM+ cells re-entered a GC where they underwent mutation and class switching, whereas the IgG+ cells mainly gave rise to IgG-secreting cells. Similarly, others showed by adoptive transfer experiments that IgM-expressing memory B cells are formed upon exposure to TD Ags (9) and, in addition to the study of Dogan et al. (8), these IgM-expressing B cells were found to return to the GC only when no Ag-specific IgG was present in serum. These studies show the existence of multiple layers of B cell memory in mice, although the precise role of these different subsets is not yet fully clear. It was proposed that IgM memory cells are a reservoir for class-switched memory B cells, whereas IgG memory B cells are the frontline responders by directly differentiating into Ab-secreting cells.
Whether similar layers of memory B cells exist in humans is not known. Tangye and Good (7) suggested that part of the CD27+IgM+ B cells might represent B cells that arise in early stages of a GC and exit before undergoing class switching. The relatively diminished replication history of IgM memory cells is in line with this hypothesis (10). However, the presence of CD27+IgM+ B cells producing Abs against bacterial polysaccharides in patients unable to mount a GC reaction suggests that at least part of these IgM+ B cells are actually circulating marginal zone B cells involved in responses to TI Ags (11).
Previously we studied the Ig gene repertoire of anti–RhD (D)-specific B cells in the PB of two volunteer hyperimmunized donors with high anti-D IgG titers. Surprisingly, 8 of 11 anti-D–specific CD19+ B cells, isolated using a CD40/CD40L culture system, produced IgM (12). Now we applied the same culture system to characterize D-specific and tetanus toxoid (TT)–specific B cells in normal and hyperimmunized donors in more detail. Both RhD and TT are nonglycosylated TD Ags; RhD is a highly immunogenic erythrocyte multispanning transmembrane protein and belongs to the Rh blood group, which is one of the most complex blood groups known in humans; TT is a soluble protein (13).
In the present study, we investigated whether IgM-expressing B cells also play an important role in the memory to TD Ags in humans.
Materials and Methods
Donors
Leukapheresis products or EDTA blood samples were collected from eight hyperimmunized anti-D and four anti-TT donors with high titers at Sanquin with informed consent, and the study was approved by the Ethics Advisory Board of our institute. Donors were hyperimmunized for at least 2 y, except for the youngest donor (RhD1), who was hyperimmunized for 10 mo. The proportions of the different B cell subsets of the hyperimmunized donors and their titers at time of collection are listed in Table I. Moreover, four nonhyperimmunized donors (controls) were included in the study. They all were immunized against TT at least four times in the early period since birth. Three of these donors received their last TT booster at least 10 y before this study and one 5 y earlier. Two of them were also examined 14 d after an additional TT booster.
Table I. B cell characteristics and immunization data for all donors.
| Donor |
Age (y) |
Proportion of CD19+ Cells (%) |
B Cell Subset Sizes (%) of Total Peripheral CD19+ B Cells | Last Booster (d) |
Titera |
Hyperimmunization (mo) |
||
|---|---|---|---|---|---|---|---|---|
| CD27−IgM+ |
CD27+IgM+ |
CD27+IgG+ |
||||||
| Anti-D Rh1 | 51 | 5.5 | 72.8b | 27.0c | 131 | 512 | 10 | |
| Anti-D Rh2 | 58 | 7.8 | 80.1b | 19.7c | 174 | 512 | 154 | |
| Anti-D Rh3 | 64 | 7.1 | 56.5b | 43.4c | 1181 | 8000 | 131 | |
| Anti-D Rh4 | 64 | 6.6 | 82.6 | 13.7 | 3.7 | 55 | 1000 | 225 |
| Anti-D Rh5 | 49 | 10.0 | 61.1 | 35.4 | 3.4 | 406 | 4000 | 42 |
| Anti-D Rh6 | 49 | 10.5 | 66.9 | 28.0 | 5.0 | 44 | 1000 | 45 |
| Anti-D Rh7 | 52 | 5.1 | 84.9 | 11.7 | 3.3 | 290 | 1000 | 34 |
| Anti-D Rh8 | 62 | 4.4 | 87.1 | 10.2 | 2.6 | 130 | 1000 | 214 |
| Anti-TT 1 | 55 | 4.5 | 56.1b | 43.8c | 116 | 12 | 46 | |
| Anti-TT 2 | 58 | 5.8 | 77.7b | 22.2c | 63 | 11 | 62 | |
| Anti-TT 3 | 36 | 9.9 | 84.7 | 5.9 | 9.4 | 570 | 9.1 | 75 |
| Anti-TT 4 | 48 | 12.0 | 87.3 | 4.8 | 8.0 | 169 | 8.4 | 86 |
| Ctrl 1 before | 35 | 17.0 | 72.1d | 27.8e | >10 y | < 1 | — | |
| Ctrl 2 before | 30 | 4.2 | 66.9d | 32.4e | >10 y | < 1 | — | |
| Ctrl 3 before | 29 | 8.8 | 59.6 | 17.9 | 5.4 | >5 y | 1.8 | — |
| Ctrl 4 before | 24 | 10.1 | 65.8 | 12.9 | 4.2 | >10 y | < 1 | — |
| Ctrl 1 after | 35 | 20.0 | 77.5d | 22.6e | 14 | 17.6 | — | |
| Ctrl 2 after | 30 | 18.1 | 67.4d | 32.5e | 14 | 6.0 | — | |
For anti-D, titer is measured based on agglutination, for TT by IgG ELISA.
IgM+ B cell subset size as percentage of sorted CD19+ B cells.
IgM− B cell subset size as percentage of sorted CD19+ B cells.
CD27− B cell subset size as percentage of sorted CD19+ B cells.
CD27+ B cell subset size as percentage of sorted CD19+ B cells.
Production of fluorescent erythrocyte ghosts
Erythrocyte ghosts were produced from 25 ml 3% homozygous for the D Ag and negative for the C Ag of the Rh system, because those RBCs express the highest number of D Ag sites per RBC. The erythrocytes were diluted in PBS and centrifuged for 5 min at 2400 × g at room temperature. After decanting the supernatant, the pellet was resuspended in 25 ml ice-cold 5 mM sodium biphosphate solution (pH 8.2) and then centrifuged again for 30 min at 21,000 × g at 4°C. After washing, the purified ghosts were resuspended in the same sodium biphosphate solution and incubated with either a PKH26 (Sigma-Aldrich, St. Louis, MO) probe on ice for 5 min or with a CFSE (Life Technologies, Carlsbad, CA) probe at 37°C for 15 min. After adding 1% of human serum albumin (200 g/l; Sanquin, Amsterdam, the Netherlands) the ghosts were washed and resuspended in 300 μl IMDM (Lonza, Basel, Switzerland) with 10% FCS (Bodinco, Alkmaar, the Netherlands).
Labeling and sorting of B cells
B cells were purified from mononuclear cells by magnetic separation with CD19 beads (Miltenyi Biotec, Leiden, the Netherlands). B cells were labeled with CD27-allophycocyanin (BD Biosciences, Franklin Lakes, NJ), goat anti-human IgG and IgM (SouthernBiotech, Birmingham, AL) coupled with Alexa Fluor 405 and Alexa Fluor 700 dyes, respectively (Life Technologies). For Ag-specific B cell frequency studies, B cell subsets were sorted by FACSAria II cell sorter (BD Biosciences, San Jose, CA) in 1, 10, 33, 100, 330, 1000, and 3300 cells/well (for gate settings, see Supplemental Fig. 1). To enrich for anti-D–specific B cells, 300 μl PKH26-labeled ghosts and 300 μl CFSE-labeled ghosts were added to a suspension of 60 × 106 CD19+ B cells and incubated for 60 min at 4°C. Anti-D–specific B cells were sorted as single double-positive (PKH26+CFSE+) B cells per well (Supplemental Fig. 2).
EL4.B5 culture system
The B cells were plated in 96-well flat-bottom plates (Nunc, Roskilde, Denmark). These plates had been preseeded with CD40L-expressing EL4.B5 cells (50 Gy irradiated, 1 × 105/well, provided by Dr. R. Zubler, Geneva, Switzerland) in IMDM (Lonza) with 10% FCS (Bodinco), 0.1% human IgG-free apotransferrin (Sigma-Aldrich), and 2.5% T cell supernatant, produced by culturing T cells (50 × 106/ml derived from the buffy coat of a D+ donor) for 36 h in the presence of 5 μg/ml PHA-L (Murex, Dartford, U.K.) and 10 ng/ml PMA (Sigma-Aldrich). The plates were incubated at 37°C in a 5% CO2 humidified atmosphere for 9–10 d.
Testing of supernatants
Supernatants (200 μl) were harvested on day 9 or 10 and 50 μl was added to 50 μl 1% bromelain-treated D+ erythrocytes (ccDDEE) in 96-well round-bottom plates. Agglutination was scored after incubation at room temperature and at 37°C. Agglutinating supernatants were also tested for lack of agglutination with D− erythrocytes (ccddee).
ELISA plates (Nunc-Immuno MaxiSorp from Sigma-Aldrich) were coated overnight with goat anti-human IgM (1.8 μg/ml), goat anti-human IgG (1.3 μg/ml) (both from Jackson ImmunoResearch Laboratories, West Grove, PA) or with TT (2.3 μg/ml) (Statens Serum Institute, Copenhagen, Denmark) diluted in coating buffer (0.1 M Na2CO3, 0.1 M NaHCO3, pH 9.6). Culture supernatant was diluted with 2% milk (Campina)/PBS/0.025% Tween and incubated for 1 h at room temperature. Ab binding was detected with biotinylated goat F(ab′)2 anti-human IgM or goat F(ab′)2 anti-human IgG (0.5 μg/ml) (both from BioSource International, Camarillo, CA) and streptavidin-HRP (Thermo Scientific, Waltham, MA).
Calculation of B cell outgrowth
Wells producing Ag-specific Ig were scored and frequencies were calculated with L-Calc software (StemCell Technologies, Grenoble, France). To calculate Ag-specific B cell frequencies per subset, these frequencies were corrected for the number of viable B cells that were found to actually produce IgM or IgG, as determined from 24 wells sorted with single B cells of the different subsets. To estimate the distribution of the Ag-specific B cells among the subsets, we used the Ag-specific B cell frequencies (in relation to Ig production) and the size of each subset (Table I).
BCL6 mutation and Ig analysis
The progeny of single cell–sorted B cells that produced anti-D or for the control non–anti-D Ig were collected after 8 d and digested in 25 μl lysis buffer (1× GoTaq Flexi buffer; Promega, Madison, WI) and 0.1 mg/ml proteinase K (Roche). A 10th of the lysate was used for BCL6 mutation analysis by nested PCR (see Supplemental Table I) using a GenAmp PCR system 9700 (Applied Biosystems). PCR products were cleaned up (ExoSAP-IT; USB, Cleveland, OH) and sequenced with both nested primers and an extra reverse primer (BCL6-S) (ABI Prism 377 sequencer from Applied Biosystems, Foster City, CA). From the remainder RNA was extracted by TRIzol (Invitrogen, Carlsbad, CA) and reverse-transcribed using SMARTer RACE cDNA amplification kit (Clontech, Palo Alto, CA) with the 5′-RACE CDS primer A oligonucleotide. cDNA was amplified using universal primer A mix and four reverse primers (R1 primers in Supplemental Table I) specific to the IGHG, IGHM, IGLK, or IGLL C region, using the Advantage 2 PCR enzyme system (Clontech). The first PCR product (1.5 μl) was amplified by nested universal primer A and four reverse primers (R2 primers in Supplemental Table I). Both PCR products were cleaned up and sequenced with the appropriate nested R2-C primers. Sequences were aligned to their closest germline gene segment using IMGT/V-QUEST (http://imgt.org/IMGT_vquest).
Statistical analysis
Statistical analysis was performed using Prism (GraphPad Software, San Diego, CA).
Results
Most Ag-specific B cells are found in the IgM+ B cell subset
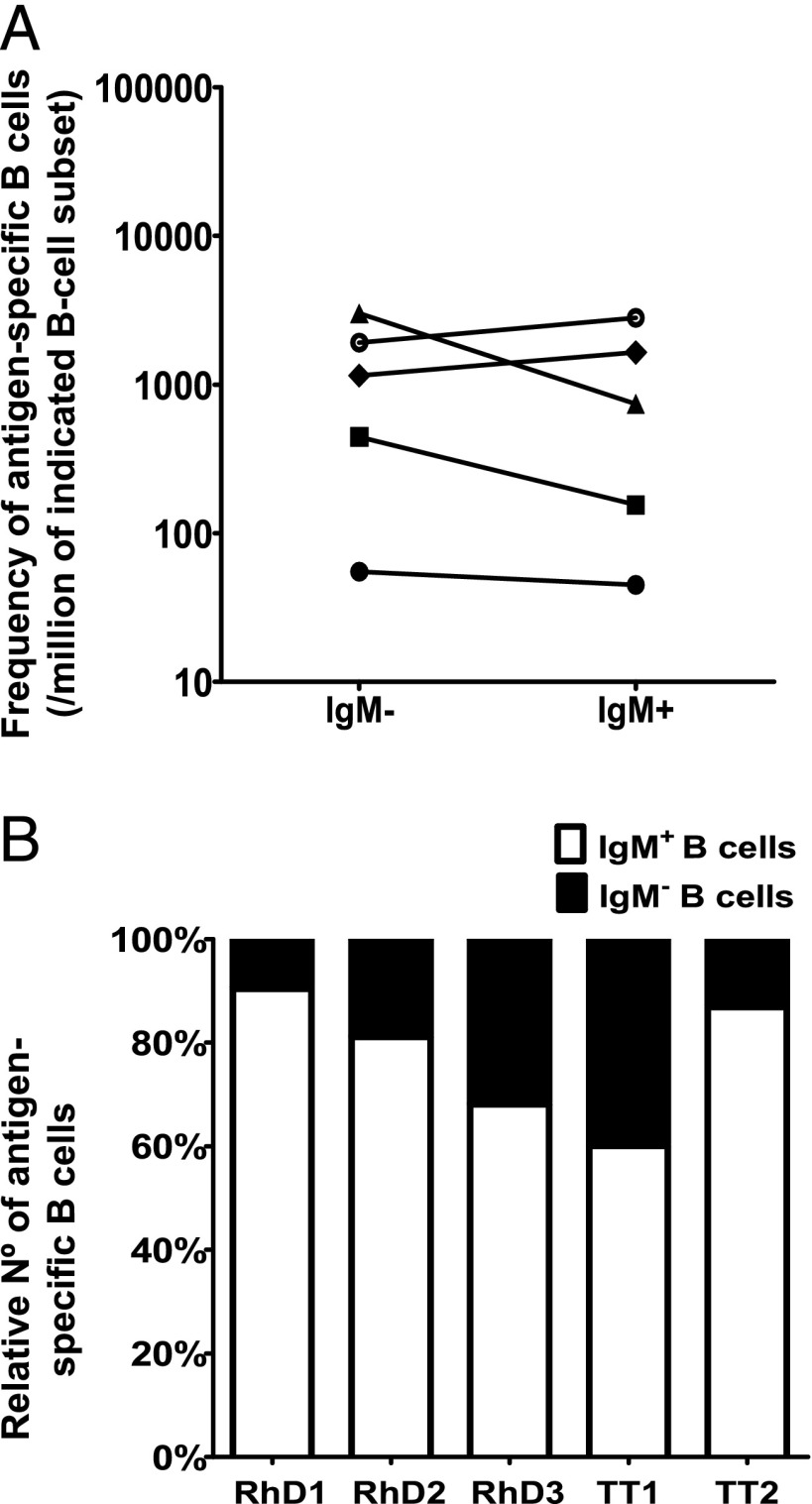
Because we previously found that most anti-D Ig clones from two hyperimmunized donors were IgM (12), we now sorted IgM+ and IgM− B cells in limiting dilution from three other anti-D and two anti-TT hyperimmunized donors. After culture for 10 d, Ag-specific Igs were identified by agglutination or TT-specific ELISA. The frequency of Ag-specific B cells was highest within the IgM− B cell subset in three donors (two anti-D and one anti-TT) and within the IgM+ B cell subset in the other two donors (Fig. 1A). To determine absolute numbers of IgM+ and IgM− Ag-specific B cells, the actual size of these B cell subsets was taken into account (Table I). The percentages found in this small group of donors are comparable to data observed in the literature (14–17), although the percentage of CD27+IgG+ B cells is somewhat lower. Because the IgM+ B cell subset was always the largest, the number of IgM+ Ag-specific B cells exceeded that of class-switched IgM− cells in all five analyzed hyperimmunized donors (Fig. 1B; mean, 77% of Ag-specific B cells are IgM-expressing B cells).
FIGURE 1.
Frequency of Ag-specific B cells is similar in IgM+ and class-switched IgM− B cells, but IgM+ exceeds class-switched B cells in absolute numbers. B cells were sorted based on their IgM+ or IgM− surface phenotype. The production of Ag-specific Abs was assessed after 10 d of culture with irradiated EL4B5 cells by either erythrocyte agglutination (rhesus D Ag) or ELISA (TT) in three anti-D and two anti-TT hyperimmunized donors. (A) The frequency of Ag-specific B cells was estimated by limiting dilution analysis and is expressed as a number of Ag-specific B cells per million of total PB B cells of that given subset. Each symbol represents a donor. (B) The relative number of IgM+ and IgM− B cells within the Ag-specific B cells was calculated for each donor taking the frequency and subset size of each B cell population into account.
Ag-specific IgM+ B cells reside in both CD27+ and CD27− B cell populations
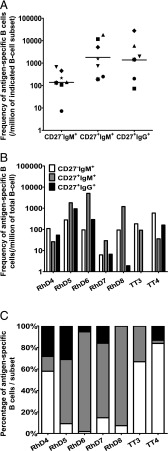
Further frequency analyses were performed on five other anti-D and two anti-TT hyperimmunized donors, but now also distinguishing IgM+ B cell subsets by CD27. The frequency of Ag-specific B cells was higher within the CD27+ populations (mean of 1 Ag-specific B cell of 185 and 161 sorted cells in CD27+IgM+ and CD27+IgG+ subsets, respectively, whereas in the CD27−IgM+ subset a mean of 1 Ag-specific B cell of 4000 cells was found) (Fig. 2A). However, again the highest absolute number of Ag-specific B cells per total B cells was found in the CD27−IgM+ subset in three donors and in the CD27+IgM+ subset in four donors (Fig. 2B). Moreover, together these IgM+ subsets make up most Ag-specific B cells in all donors (Fig. 2C).
FIGURE 2.
The largest proportion of Ag-specific B cells resides in IgM-expressing CD27− and CD27+ B cell populations. The frequency of Ag-specific B cells in five anti-D and two anti-TT hyperimmunized donors was determined by limiting dilution after sorting CD27−IgM+, CD27+IgM+, and CD27+IgG+ B cell subsets onto EL4B5 cells, allowing them to grow for 10 d before testing supernatants for specific Abs as described in Fig. 1. (A) Frequency of Ag-specific B cells given as a number of Ig-producing Ag-specific B cells per million cells within subset. Black lines indicate geometric means. (B) The frequencies reported are per million total B cells or (C) as relative number of cells from the different subsets. Note that no IgG+ anti-TT B cells were identified from the anti-TT3 donor.
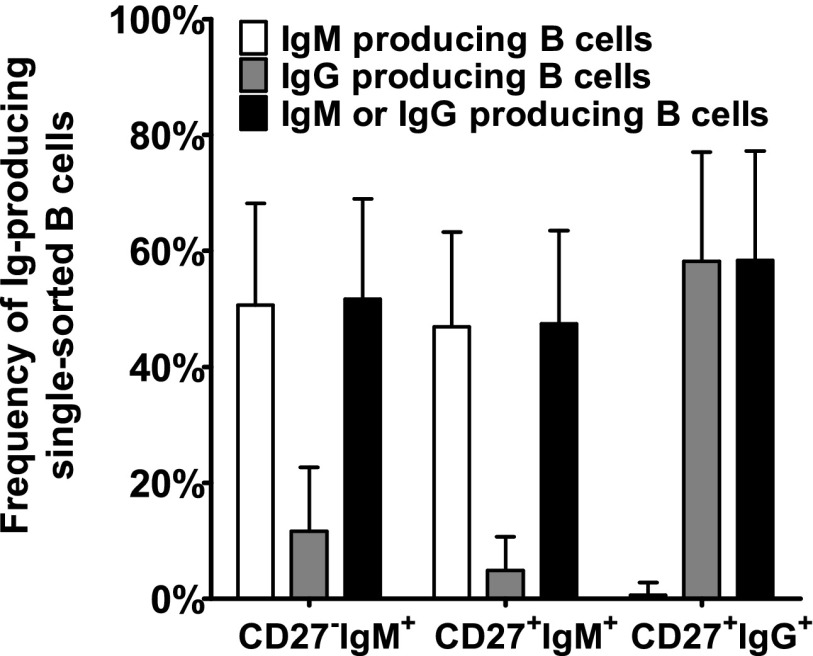
Different B cell subsets grow equally well
Because the growth of naive and memory B cells might be different (18, 19), we analyzed the capacity of the different B cell subsets to differentiate into Ig-producing clones under our conditions by sorting single cells of each B cell subset (Fig. 3). In 5–12% of wells in which single IgM+ B cells were sorted, also IgG was produced, probably due to class switching. However, the low number of IgM-producing cells in the CD27+IgG+ subset (mean, 1 of 156 B cells) may reflect casual contamination with IgM+ cells. On average, 1 of 2 wells in which CD27−IgM+ or CD27+IgM+ cells were sorted produced IgM, and 1 of 1.7 sorted CD27+IgG+ cells produced IgG.
FIGURE 3.
Ig production from single B cells is equally good in CD27− and CD27+ B cells. B cells were sorted as one cell per well and cultured as described in the legend to Fig. 1. Supernatants were tested for IgM and IgG production by ELISA, and the number of Ab-producing B cells are given as a mean percentage (bars), together with SDs from 14 different experiments.
The comparable outgrowth of the three B cell subsets shows that our observation that most Ag-specific B cells express IgM is not due to preferential stimulation of IgM+ cells.
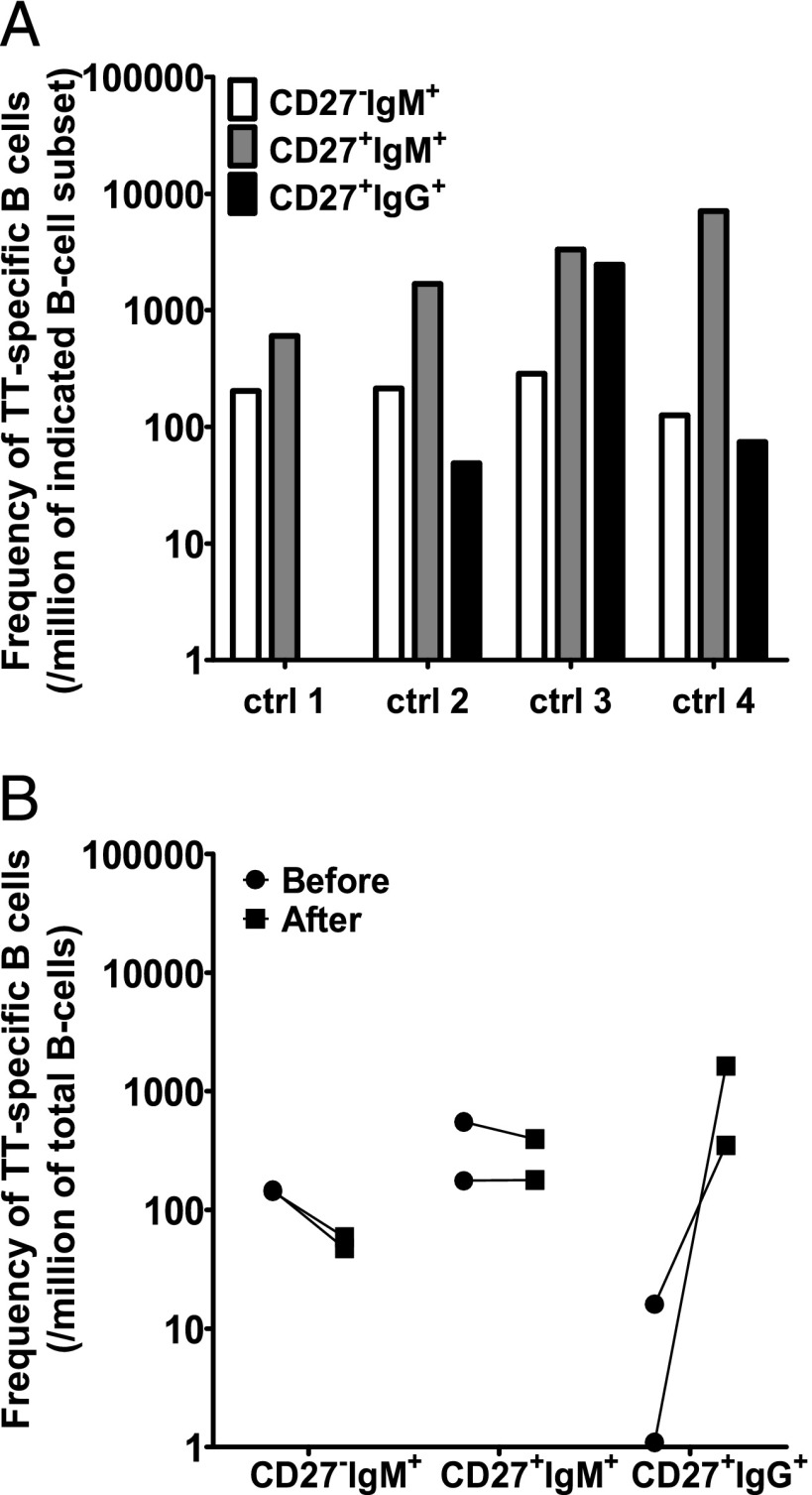
RhD- and TT-specific B cells in nonimmunized donors and cord blood
To exclude that these CD27−IgM+ B cells were naive B cells, we tested the presence of D-specific B cells in two D− nonimmunized donors, one D+ donor, and the presence of TT-specific cells in three cord blood samples. Neither IgM nor IgG Ag-specific B cells were found in any of these cases (data not shown), whereas the outgrowth of B cells was normal. To determine whether IgM+ Ag-specific B cells were related to hyperimmunization, TT-specific B cells were characterized in four nonhyperimmunized donors. All showed the highest frequency of TT-specific B cells in the CD27+IgM+ B cell subset (Fig. 4A), and again most Ag-specific B cells resided in the IgM+ B cell subset (88 ± 19%, mean ± SD). In one donor no TT-specific IgG-expressing B cells were found, but unfortunately only 10,000 IgG+ B cells were sorted in this donor. Two donors were also analyzed 14 d after a TT booster (Fig. 4B). The frequencies of TT-specific B cells in the CD27+IgM+ B cell subset did not change after the booster and were ∼1 of 5000. After booster a sharp augmentation of the number of TT-specific CD27+IgG+ cells was observed, from none or only 1 of 60,000 total B cells to 1 of 615 and 1 of 3,000. This increase was accompanied in both donors with a slight decline of TT-specific CD27−IgM+ cells (1 of 7,000 in steady-state to 1 of 20,000 after booster) (Fig. 4B). This increase in frequency of TT-specific CD27+IgG+ cells was paralleled with a rise in anti-TT IgG titer (Table I).
FIGURE 4.
IgM-expressing TT-specific B cells are present also in nonhyperimmunized donors. (A) The frequency of TT-specific B cells, in four nonhyperimmunized donors who had not received a TT booster for at least 10 y, was estimated by limiting dilution, as in Figs. 1–3. The frequency is given as the number of B cells per million within a subset. (B) Frequency of TT-specific B cells per million peripheral B cells in two control donors before and 14 d after receiving TT booster immunization.
Mutational status of the BCL6 and Ig genes in D-specific B cells
To investigate whether the Ag-specific IgM-expressing cells, especially the CD27− B cells, are bona fide memory B cells, we studied the mutational status of the Ig and BCL6 genes in D-specific B cells of an anti-D hyperimmunized donor (20, 21). CD27−IgM+, CD27+IgM+, and CD27+IgG+ cells that bound D+ ghosts were sorted as single cells. DNA and RNA were isolated from agglutinating wells. As control, randomly selected B cells producing IgM or IgG were analyzed.
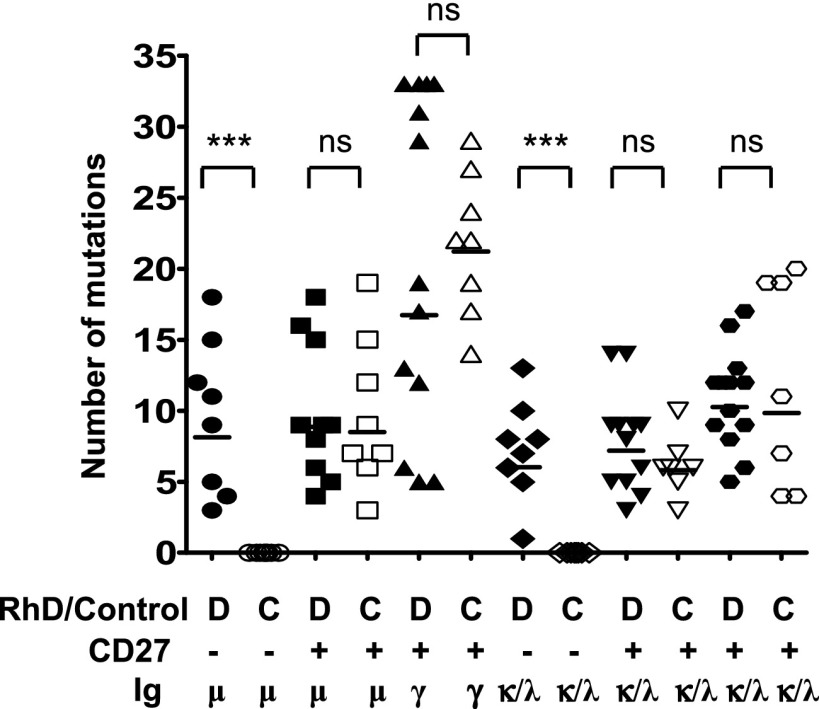
Not only CD27+ subsets, but also anti-D–specific CD27−IgM+ subsets carried mutations in the BCL6 gene, indicating their GC experience (22–24) (Table II), whereas in control wells no mutations were found in CD27−IgM+ cells (Table II). Additionally, somatic mutations in both IGHV and IGLV/IGKV were found only in anti-D–specific CD27−IgM+ cells and not in non–anti-D–specific CD27−IgM+ cells (Fig. 5). As expected, mutations were present in all CD27+ subsets, with CD27+IgG+ cells showing the highest number. Collectively, these data clearly prove AID exposure of IgM+ D-specific B cells, including CD27− B cells.
Table II. Comparative analysis of mutations within the 5′-noncoding region of the BCL6 gene in single D-specific B cells isolated from a hyperimmunized donor versus non–D-specific B cells from two nonhyperimmunized donors.
| Donor | B Cell Subset | PCR Efficiencya | One Allele/Two Alleles Amplifiedb | Mutated Cells (%) | No. Mutations for Each Sequencec | Mutation Frequencyd (%) |
|---|---|---|---|---|---|---|
| Anti-D | CD27+IgM+ | 8/8 | 0/8 | 3 (37.5) | 1 × 2, 1× 1, 1× 1 | 0.0384 |
| CD27+IgM+ | 13/14 | 0/13 | 2 (15.3) | 1 × 1, 1 × 1 | 0.0118 | |
| CD27+IgG+ | 11/15 | 1/10 | 4 (36.4) | 1 × 1, 1 × 1, 1 × 1, 1 × 1 | 0.0293 | |
| Controls | CD27+IgM+ | 8/8 | 0/8 | 0 (0) | 0 | 0 |
| CD27+IgM+ | 8/8 | 0/8 | 2 (25.0) | 1 × 1, 1 × 2 | 0.028846 | |
| CD27+IgG+ | 8/8 | 0/8 | 1 (12.5) | 1 × 3 | 0.028846 |
Numbers of positive PCRs versus single cells analyzed.
Number of PCR products containing one allele could be confirmed in one case only (one sequence of the anti-D hyperimmunized donor harbored a mutation that made clear that only one allele was amplified). For all the other unmutated PCR products, we assumed that both alleles were amplified.
In A × B, A denotes the number of sequences with B mutations.
The derived percentage of mutations in a total of 650 bp of the Bcl6-MMC considered from each sequence as described by Seifert and Küppers (25).
FIGURE 5.
Both D-specific CD27−IgM+ and CD27+IgM+ B cells contain somatic mutations, indicative of a post-GC origin. The number of mutations per Ab for the variable H chain (VH, μ or γ) and variable L chain (VL, κ or λ) are grouped for their either anti-D specificity (D) or control (C), as well as their CD27 expression. ***p < 0.0001 when comparing anti-D–specific and control B cells within B cell subsets using a one-tailed Student t test.
Anti-D–specific B cells from different subsets are clonally related
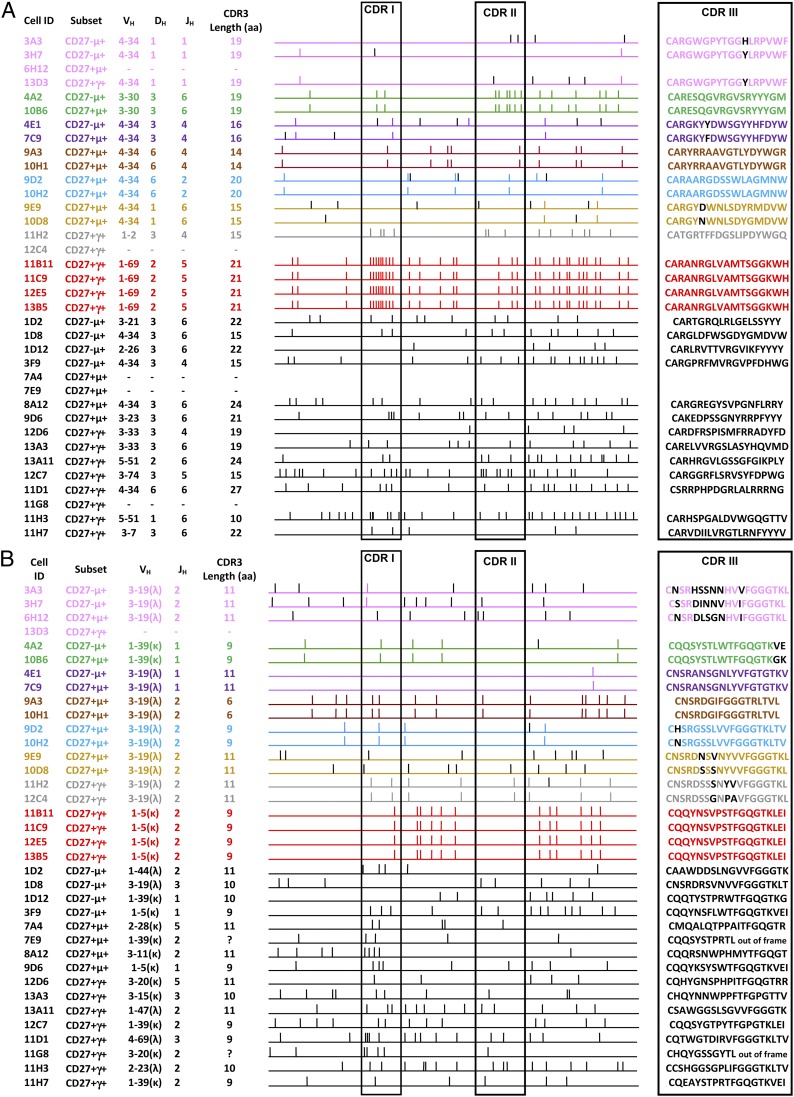
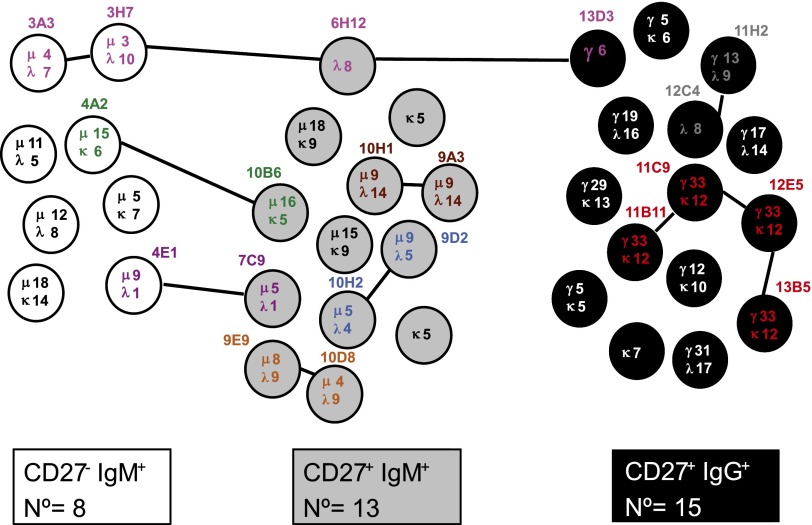
The results of sequence analysis of rearranged Ig genes from 36 d-specific B cell clones of donor RhD4 are summarized in Fig. 6. The lengths of the CDR3 regions were similar for the three B cell subsets. For 20 of 36 sequences, a related clone was found either within the same subset or between subsets. Mutations found in the BCL6 gene were identical in the CD27−IgM+ clone 4A2 and the CD27+IgM+ clone 10B6, as well as for the related clones 13B5 and 12E5. In Fig. 7 the clonal relationship is displayed. Six of 13 CD27+IgM+ cells (46%) and 6 of 15 CD27+IgG+ cells (40%) showed clonal relatedness, whereas only 2 of 8 CD27−IgM+ cells (25%) were related. Some anti-D–specific B cell clones were clonally related among the three different B cell subsets. In particular, 4 of 8 CD27−IgM+ cells were related to three CD27+IgM+ clones, whereas only 1 of 15 CD27+IgG+ cells were clonally related with cells of the other two subsets. One clone was even present in all three subsets.
FIGURE 6.
Analysis of anti-D–specific B cell IGVH and IGVL sequences. The schematic diagram illustrates the clonal relationship in the H (A) and L (B) chains of anti-D–specific B cells isolated from the RhD4 hyperimmunized donor. Each small vertical stick represents a point mutation, and each horizontal line indicates a unique B cell sequence, whereas the clonally related B cells and shared mutations are grouped in different colors. Each cell ID and the B cell subset are given. Additionally, the V(D)J region usage and the CDR3 amino acidic length are also displayed to point out the closeness of the different B cell clones. VH and VK/VL genes were sequenced for all 36 cells, except for four VH sequences and one VK/VL sequence that could not be amplified. VH gene usage was in 15 cases restricted to use VH4–34, with 13 residing in both IgM-expressing B cell subsets and of which 11 showed clonal relatedness. These 11 sequences also restrictedly used the same VL1–39 gene. Two B cell clones were found to be clonally related by their VL3–19 gene sequence only, because in one clone the Ig VH sequence was not amplified.
FIGURE 7.
Clonal relationship of anti-D–specific B cells. VH and VL chain genes were sequenced for all, except for four VH sequences and one Vκ/λ sequence that could not be amplified. A schematic representation is shown of the IGVH and IGVL sequence relationship among D-specific B cell clones belonging to the CD27−IgM+ (n = 8), CD27+IgM+ (n = 13), and CD27+IgG+ (n = 15) subsets derived from the RhD4 hyperimmunized donor. Lines connect the B cell clones whose Ig VH and/or VL regions showed the same sequence, harboring the same or a different number of somatic point mutations as indicated by the numbers within the circle. Colors match the scheme used in Fig. 6.
Discussion
In this study, we show that most circulating Ag-specific memory B cells in anti-D and anti-TT hyperimmunized individuals are IgM+ B cells, in some donors even CD27−IgM+ B cells, and that these IgM+ B cell are bona fide memory cells. We have studied two strong TD Ags, a particulate (RhD) as well as a soluble (TT) Ag, and they showed comparable results. Our findings in humans support previous data observed in mice suggesting the existence of multiple layers of B cell memory with different effector functions (8).
Using a CD40/CD40L culture system, the frequency of Ag-specific B cells in PB was analyzed in anti-D and anti-TT hyperimmunized donors in steady-state. In line with our previous findings (12), we demonstrate that the most in number of either anti-D– or anti-TT–specific B cells expressed IgM, although the highest frequency of Ag-specific B cells was found in most donors within the CD27+IgG+ subset. It was excluded that this was an artifact of our CD40L culture approach, because both IgM- and IgG-secreting B cells were equally stimulated in this system in contrast to studies showing discrepancies between naive and memory cells using a similar method (18, 19). The different B cell source (tonsils and spleen versus PB) likely containing more activated B cells compared with PB, the absence of a more complex cytokine environment, and a reduced number of CD40L feeder cells per culture could be responsible for these disparities.
D-specific B cells were only found in alloimmunized individuals and TT-specific B cells were absent in cord blood, indicating that they are elicited upon immunization. A key characteristic of memory B cells is the presence of somatic hypermutations within Ig genes. The same mutation machinery affects off-target genes (23, 26, 27) where mutations occur at a lower frequency (27). Although the classical idea was that most IgM+ memory B cells are produced in a GC-independent fashion (11, 29, 30), we found that all anti-D–specific CD27−IgM+ and CD27+IgM+ B cells harbored mutations in their Ig genes, whereas in non–anti-D–producing cells, mutations were found only in the CD27+ B cell subsets. Additionally, several anti-D CD27−IgM+ and CD27+IgM+ clones contained mutations in BCL6. Somatic mutations in IgM+ cells have been found before (25, 31–33) in CD27+IgM+ B cells (25) as well as in CD27−IgM+ B cells (34). However, to our knowledge we are the first to describe these mutations in TD Ag-specific CD27−IgM+ B cells. The anti-D–specific IgM+ populations (both CD27− and CD27+) showed fewer mutations in their Ig compared with CD27+IgG+ cells, indicating that these cells undergo less affinity maturation. Because we did not stain for IgD, we cannot conclude whether the CD27+IgM+ cells are natural effector cells (IgM+IgD+) or IgM-only cells (35)
In recent years, many studies have discussed the origin and role of IgM memory B cells both in mice and humans. At least part of these memory cells are thought to be marginal zone B cells residing in a specialized area of the spleen where they have unique access to blood-borne Ags and pathogens. For this reason they might represent the first line of defense against bacterial infections responding to T cell–independent Ags (36–38) and developing in absence of GCs (39, 40). Alternatively, this IgM memory B cell pool also contained cells sharing characteristics with IgG+ memory B cells: somatic hypermutations in their Ig genes, upregulation of activation markers, and replication history, although limited (35, 41, 42). We now definitively show that IgM+ B cells are present within the memory pool specific for the TD Ags RhD (13) and TT, and that they have features demonstrating GC experience (43, 44).
Another general assumption is that memory B cells express CD27 (1). In all donors we found Ag-specific B cells in the CD27−IgM+ population, and in three of seven hyperimmunized donors this population even represented most Ag-specific B cells. We found these cells years after the last booster and in individuals without a measurable anti-TT titer. This suggests that CD27 expression is not expressed on all IgM+ memory B cells. Dogan et al. (8) observed that peanut agglutinin, the classical marker for GC B cells in mice, also did not stain most Ag-specific IgM+ B cells detected after a prolonged time from the last booster. Also, Wirths and Lanzavecchia (45) identified a minor population of CD27− PB B cells that behaved similar to memory cells and were identified based on their inability to extrude rhodamine. However, this CD27− memory population consisted mainly of IgG+ and IgA+ cells, supporting the findings of another group that showed the presence of PB memory CD27−IgG+ B cells (14, 35). Ehrhardt et al. (46) described CD27− memory B cells in tonsils expressing FcRH4, but these CD27− memory cells seem to be confined to the tonsils and were found to be absent from PB (data not shown) (47). CD27− memory B cells have been described in patients with chronic immune activation such as systemic lupus erythematosus (48) and HIV-1 (49). Because hyperimmunization might cause chronic immune activation, we analyzed TT-specific B cells in healthy control donors with a very low TT titer. Although most TT-specific B cells were found in the CD27+IgM+ subset in steady-state, a considerable percentage of the TT-specific B cells were CD27−IgM+ in all four donors, and in two donors the frequency of TT-specific CD27−IgM+ B cells exceeded the frequency of TT-specific CD27+IgG+ B cells. Therefore, we conclude that the presence of CD27−IgM+ and CD27+IgM+ Ag-specific cells is not linked to chronic immune activation, although the loss of CD27 might be more pronounced after repeated immunizations, as CD27 can be shed from activated B and T lymphocytes (50). Alternatively, the lack of CD27 might represent the inability to gain sustained support from CD70-expressing activated T cells to complete the GC reaction in an early CD40L-mediated TD manner. This may thereby explain why CD27− B cells harbor a lower rate of mutations than do their CD27+ counterpart (51–54).
Because upon booster immunizations mainly IgG is produced and Ag-specific IgM is predominantly seen only during primary immune response, we applied our culture system also after recent booster. Indeed, a 25-fold rise in frequency of TT-specific CD27+IgG+ cells and a 2.5-fold decrease in TT-specific CD27−IgM+ cells were observed 14 d after immunization. Probably CD27+IgG+ B cells do not really recirculate in the PB but preferentially reside in clusters near the contracted GCs from where they can rapidly proliferate upon secondary challenge (55).
Although the role of IgM+ memory cells in a secondary response remains unclear, two studies described possible roles for IgM+ B cells in mice (8, 9). Dogan et al. (8) suggested that there is a memory compartment of long-lived and mutated IgM+ cells, which can reinitiate a GC round upon further antigenic challenge and partly switch to replenish the IgG+ memory pool. In their model, the IgG+ cells have an immediate effector and protective function by rapidly becoming IgG-secreting plasmocytes but display little ability to re-enter the GC. Pape et al. (9) also proposed a role for the Ag-specific Ig. IgG+ B cells were activated in the presence of high-affinity neutralizing serum whereas IgM+ memory B cells poorly respond until the IgG titers wane. They observed that the number of IgM+ memory B cells remained stable, up to 500 d after priming, whereas the IgG+ B cells declined with a half-life of ∼50 d (9). This discrepancy between the two murine studies may be related to the persistence of GC reactions in the response to particulate Ags: SRBCs in the study of Dogan et al. (8), and soluble Ags in the study of Pape et al. (9, 56). It is relevant that we obtained similar results using a soluble Ag (TT) and a particulate Ag (D+ RBCs), and that the kinetics of the memory subsets also resembled the observations of Pape et al. More recently, another group studied the response to a TD Ag infection in mice and defined a subset of IgM memory B cells expressing CD11c, CD73, and PD-L2 (57). The absence of this IgM memory subset was found to abrogate the onset of IgG recall responses following a specific Ag challenge, thereby (again) supporting the idea that IgM memory plays an important role in maintaining long-term immunity.
Our findings in humans give support to the above-mentioned results obtained in mice (8, 9, 57), suggesting that IgM+ memory represents a pool of long-lasting memory cells continuously replenishing the IgG+ memory pool after repeated exposure. This may be beneficial upon second encounter with a mutated form of the original pathogen, triggering their ability to re-enter the GC, switch, and acquire additional mutations, resulting in higher affinity to the mutated Ag, as also suggested in an elegant murine study describing GC-independent unmutated IgG+ memory B cells (58), which, similar to the IgM memory B cells characterized in this study, are implied to confer a great flexibility to the immune system by retaining germline sequences. Meanwhile, IgG+ memory B cells originating from the first encounter mainly form a rapid burst of plasma cells that boost the level of serum Ab, but with little capability of reinitiating a GC. Our findings on the clonal relationship between Ig and BCL6 mutations among the d-specific B cells isolated from all B cell subsets support this model of multiple layers of memory responses. Because anti-D–specific IgM+ memory populations (both CD27− and CD27+) harbored fewer mutations compared with their CD27+IgG+ counterparts, this suggests that they underwent fewer rounds of affinity maturation.
In conclusion, our findings show that both in hyperimmunized donors and control donors who received booster vaccinations in the distant past, most RhD- and TT Ag–specific B cells circulating in PB reside in the IgM-expressing B cell subset. These IgM+ B cells seem to provide a long-lasting memory. Their lower level of mutations make these cells more flexible in responding to variants of the same pathogen compared with IgG+ B cells carrying a highly specific BCR (59).
Supplementary Material
This work was supported by a grant from the Landsteiner Foundation for Blood Transfusion Research.
The online version of this article contains supplemental material.
- D
- RhD
- GC
- germinal center
- PB
- peripheral blood
- TD
- T cell–dependent
- TI
- T cell–independent
- TT
- tetanus toxoid.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Klein U., Rajewsky K., Küppers R.. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188: 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer D., Holter W., Majdic O., Fischer G. F., Knapp W.. 1990. CD27 expression by a distinct subpopulation of human B lymphocytes. Eur. J. Immunol. 20: 2679–2684 [DOI] [PubMed] [Google Scholar]
- 3.Scheeren F. A., Nagasawa M., Weijer K., Cupedo T., Kirberg J., Legrand N., Spits H.. 2008. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J. Exp. Med. 205: 2033–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Good-Jacobson K. L., Tarlinton D. M.. 2012. Multiple routes to B-cell memory. Int. Immunol. 24: 403–408 [DOI] [PubMed] [Google Scholar]
- 5.Alugupalli K. R., Leong J. M., Woodland R. T., Muramatsu M., Honjo T., Gerstein R. M.. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21: 379–390 [DOI] [PubMed] [Google Scholar]
- 6.Obukhanych T. V., Nussenzweig M. C.. 2006. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203: 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangye S. G., Good K. L.. 2007. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J. Immunol. 179: 13–19 [DOI] [PubMed] [Google Scholar]
- 8.Dogan I., Bertocci B., Vilmont V., Delbos F., Mégret J., Storck S., Reynaud C. A., Weill J. C.. 2009. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 10: 1292–1299 [DOI] [PubMed] [Google Scholar]
- 9.Pape K. A., Taylor J. J., Maul R. W., Gearhart P. J., Jenkins M. K.. 2011. Different B cell populations mediate early and late memory during an endogenous immune response. Science 331: 1203–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Zelm M. C., Szczepanski T., van der Burg M., van Dongen J. J.. 2007. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J. Exp. Med. 204: 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weller S., Braun M. C., Tan B. K., Rosenwald A., Cordier C., Conley M. E., Plebani A., Kumararatne D. S., Bonnet D., Tournilhac O., et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104: 3647–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohmen S. E., Mulder A., Verhagen O. J., Eijsink C., Franke-van Dijk M. E., van der Schoot C. E.. 2005. Production of recombinant Ig molecules from antigen-selected single B cells and restricted usage of Ig-gene segments by anti-D antibodies. J. Immunol. Methods 298: 9–20 [DOI] [PubMed] [Google Scholar]
- 13.Stott L. M., Barker R. N., Urbaniak S. J.. 2000. Identification of alloreactive T-cell epitopes on the rhesus D protein. Blood 96: 4011–4019 [PubMed] [Google Scholar]
- 14.Fecteau J. F., Côté G., Néron S.. 2006. A new memory CD27−IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J. Immunol. 177: 3728–3736 [DOI] [PubMed] [Google Scholar]
- 15.Morbach H., Eichhorn E. M., Liese J. G., Girschick H. J.. 2010. Reference values for B cell subpopulations from infancy to adulthood. Clin. Exp. Immunol. 162: 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y. C., Kipling D., Dunn-Walters D. K.. 2011. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front. Immunol. 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caraux A., Klein B., Paiva B., Bret C., Schmitz A., Fuhler G. M., Bos N. A., Johnsen H. E., Orfao A., Perez-Andres M., Myeloma Stem Cell Network . 2010. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138− and CD138+ plasma cells. Haematologica 95: 1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fecteau J. F., Néron S.. 2003. CD40 stimulation of human peripheral B lymphocytes: distinct response from naive and memory cells. J. Immunol. 171: 4621–4629 [DOI] [PubMed] [Google Scholar]
- 19.Tangye S. G., Avery D. T., Deenick E. K., Hodgkin P. D.. 2003. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 170: 686–694 [DOI] [PubMed] [Google Scholar]
- 20.Dent A. L., Shaffer A. L., Yu X., Allman D., Staudt L. M.. 1997. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276: 589–592 [DOI] [PubMed] [Google Scholar]
- 21.Ye B. H., Cattoretti G., Shen Q., Zhang J., Hawe N., de Waard R., Leung C., Nouri-Shirazi M., Orazi A., Chaganti R. S., et al. 1997. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat. Genet. 16: 161–170 [DOI] [PubMed] [Google Scholar]
- 22.Shen H. M., Peters A., Baron B., Zhu X., Storb U.. 1998. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science 280: 1750–1752 [DOI] [PubMed] [Google Scholar]
- 23.Pasqualucci L., Migliazza A., Fracchiolla N., William C., Neri A., Baldini L., Chaganti R. S., Klein U., Küppers R., Rajewsky K., Dalla-Favera R.. 1998. BCL-6 mutations in normal germinal center B cells: evidence of somatic hypermutation acting outside Ig loci. Proc. Natl. Acad. Sci. USA 95: 11816–11821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng H. Z., Du M. Q., Koulis A., Aiello A., Dogan A., Pan L. X., Isaacson P. G.. 1999. Nonimmunoglobulin gene hypermutation in germinal center B cells. Blood 93: 2167–2172 [PubMed] [Google Scholar]
- 25.Seifert M., Küppers R.. 2009. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J. Exp. Med. 206: 2659–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon M. S., Kanegai C. M., Doerr J. R., Wall R.. 2003. Somatic hypermutation of the B cell receptor genes B29 (Igβ, CD79b) and mb1 (Igα, CD79a). Proc. Natl. Acad. Sci. USA 100: 4126–4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbiani D. F., Bunting S., Feldhahn N., Bothmer A., Camps J., Deroubaix S., McBride K. M., Klein I. A., Stone G., Eisenreich T. R., et al. 2009. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol. Cell 36: 631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M., Duke J. L., Richter D. J., Vinuesa C. G., Goodnow C. C., Kleinstein S. H., Schatz D. G.. 2008. Two levels of protection for the B cell genome during somatic hypermutation. Nature 451: 841–845 [DOI] [PubMed] [Google Scholar]
- 29.Taylor J. J., Pape K. A., Jenkins M. K.. 2012. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J. Exp. Med. 209: 597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruetzmann S., Rosado M. M., Weber H., Germing U., Tournilhac O., Peter H. H., Berner R., Peters A., Boehm T., Plebani A., et al. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197: 939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warsame A., Delabie J., Malecka A., Wang J., Trøen G., Tierens A.. 2012. Monocytoid B cells: an enigmatic B cell subset showing evidence of extrafollicular immunoglobulin gene somatic hypermutation. Scand. J. Immunol. 75: 500–509 [DOI] [PubMed] [Google Scholar]
- 32.Tierens A., Delabie J., Michiels L., Vandenberghe P., De Wolf-Peeters C.. 1999. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood 93: 226–234 [PubMed] [Google Scholar]
- 33.Stein K., Hummel M., Korbjuhn P., Foss H. D., Anagnostopoulos I., Marafioti T., Stein H.. 1999. Monocytoid B cells are distinct from splenic marginal zone cells and commonly derive from unmutated naive B cells and less frequently from postgerminal center B cells by polyclonal transformation. Blood 94: 2800–2808 [PubMed] [Google Scholar]
- 34.Weston-Bell N., Townsend M., Di Genova G., Forconi F., Sahota S. S.. 2009. Defining origins of malignant B cells: a new circulating normal human IgM+D+ B-cell subset lacking CD27 expression and displaying somatically mutated IGHV genes as a relevant memory population. Leukemia 23: 2075–2080 [DOI] [PubMed] [Google Scholar]
- 35.Berkowska M. A., Driessen G. J., Bikos V., Grosserichter-Wagener C., Stamatopoulos K., Cerutti A., He B., Biermann K., Lange J. F., van der Burg M., et al. 2011. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 118: 2150–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin F., Kearney J. F.. 2000. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol. Rev. 175: 70–79 [PubMed] [Google Scholar]
- 37.Martin F., Kearney J. F.. 2000. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12: 39–49 [DOI] [PubMed] [Google Scholar]
- 38.Kraal G. 1992. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 132: 31–74 [DOI] [PubMed] [Google Scholar]
- 39.Weller S., Faili A., Garcia C., Braun M. C., Le Deist F F., de Saint Basile G G., Hermine O., Fischer A., Reynaud C. A., Weill J. C.. 2001. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. USA 98: 1166–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weller S., Mamani-Matsuda M., Picard C., Cordier C., Lecoeuche D., Gauthier F., Weill J. C., Reynaud C. A.. 2008. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J. Exp. Med. 205: 1331–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein U., Küppers R., Rajewsky K.. 1997. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood 89: 1288–1298 [PubMed] [Google Scholar]
- 42.Good K. L., Avery D. T., Tangye S. G.. 2009. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J. Immunol. 182: 890–901 [DOI] [PubMed] [Google Scholar]
- 43.Muramatsu M., Sankaranand V. S., Anant S., Sugai M., Kinoshita K., Davidson N. O., Honjo T.. 1999. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol. Chem. 274: 18470–18476 [DOI] [PubMed] [Google Scholar]
- 44.Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T.. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102: 553–563 [DOI] [PubMed] [Google Scholar]
- 45.Wirths S., Lanzavecchia A.. 2005. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur. J. Immunol. 35: 3433–3441 [DOI] [PubMed] [Google Scholar]
- 46.Ehrhardt G. R., Hsu J. T., Gartland L., Leu C. M., Zhang S., Davis R. S., Cooper M. D.. 2005. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med. 202: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falini B., Tiacci E., Pucciarini A., Bigerna B., Kurth J., Hatzivassiliou G., Droetto S., Galletti B. V., Gambacorta M., Orazi A., et al. 2003. Expression of the IRTA1 receptor identifies intraepithelial and subepithelial marginal zone B cells of the mucosa-associated lymphoid tissue (MALT). Blood 102: 3684–3692 [DOI] [PubMed] [Google Scholar]
- 48.Wei C., Anolik J., Cappione A., Zheng B., Pugh-Bernard A., Brooks J., Lee E. H., Milner E. C., Sanz I.. 2007. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 178: 6624–6633 [DOI] [PubMed] [Google Scholar]
- 49.Cagigi A., Du L., Dang L. V., Grutzmeier S., Atlas A., Chiodi F., Pan-Hammarström Q., Nilsson A.. 2009. CD27− B-cells produce class switched and somatically hyper-mutated antibodies during chronic HIV-1 infection. PLoS ONE 4: e5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon H., Na H. Y., Chong K. H., Kim T. J.. 2006. P2X7 receptor-dependent ATP-induced shedding of CD27 in mouse lymphocytes. Immunol. Lett. 102: 98–105 [DOI] [PubMed] [Google Scholar]
- 51.Grewal I. S., Flavell R. A.. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16: 111–135 [DOI] [PubMed] [Google Scholar]
- 52.Jacquot S., Kobata T., Iwata S., Morimoto C., Schlossman S. F.. 1997. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J. Immunol. 159: 2652–2657 [PubMed] [Google Scholar]
- 53.Kobata T., Jacquot S., Kozlowski S., Agematsu K., Schlossman S. F., Morimoto C.. 1995. CD27-CD70 interactions regulate B-cell activation by T cells. Proc. Natl. Acad. Sci. USA 92: 11249–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toellner K. M., Jenkinson W. E., Taylor D. R., Khan M., Sze D. M., Sansom D. M., Vinuesa C. G., MacLennan I. C.. 2002. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J. Exp. Med. 195: 383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aiba Y., Kometani K., Hamadate M., Moriyama S., Sakaue-Sawano A., Tomura M., Luche H., Fehling H. J., Casellas R., Kanagawa O., et al. 2010. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc. Natl. Acad. Sci. USA 107: 12192–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weill J. C., Le Gallou S., Hao Y., Reynaud C. A.. 2013. Multiple players in mouse B cell memory. Curr. Opin. Immunol. 25: 334–338 [DOI] [PubMed] [Google Scholar]
- 57.Yates J. L., Racine R., McBride K. M., Winslow G. M.. 2013. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J. Immunol. 191: 1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takemori T., Kaji T., Takahashi Y., Shimoda M., Rajewsky K.. 2014. Generation of memory B cells inside and outside germinal centers. Eur. J. Immunol. 44: 1258–1264 [DOI] [PubMed] [Google Scholar]
- 59.Purtha W. E., Tedder T. F., Johnson S., Bhattacharya D., Diamond M. S.. 2011. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 208: 2599–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.