Abstract
Background
Patients with localized esophageal and esophagogastric junction cancer (EAC) receive chemoradiation then surgery (trimodality; TMT) or definitive chemoradiation (bimodality; BMT). Since distant metastases (DMs) are common but the details of the DM distribution and timing in a large cohort have not been described.
Methods
629 patients with localized EAC who had TMT or BMT were analyzed. Standard statistical methods were used to define the endpoints.
Results
The median follow-up time was 37.2 months (interquartile range: 17.8–65.0). Among 356 TMT patients, 33% (119) developed DM as their first relapse and among 273 patients with BMT, 40% (109) developed DM. 91% (TMT) and 96% (BMT) of DMs were diagnosed within 2 years of local therapy. The most common sites of DMs were: lung, distant nodes, liver, peritoneal cavity, bone, brain, and pleura in the order of frequency. The median overall survival of TMT patients with DM was 10.2 months (95% CI: 7.8–12.7) and that for BMT patients with DM was 7.8 months (95% CI: 5.7–9.9).
Conclusions
Following TMT or BMT, ≥33% of patients developed DMs and most DM occurred within 2 years (>90%) of local therapy. A clinical model that highly associates with high-risk for DM in TMT-eligible patients, prior to surgery, is desirable.
Keywords: Risk of metastases, Adenocarcinoma, Chemoradiation, Chemotherapy, Esophageal cancer, gastroesophageal cancer, Metastasis
Introduction
Patients with esophageal and/or esophagogastric junction cancer (EAC) often have poor prognosis even when EAC is localized. The incidence continues to rise in USA with an estimated 17790 persons to be diagnosed with EAC and 15210 deaths in 2013.1 When EAC is localized, patients are treated with preoperative chemoradiation (trimodality therapy [TMT]) or definitive chemoradiation (bimodality therapy [BMT]).2–4 We reported that distant metastases (DMs) are fairly frequent after TMT and BMT.5–7 Detail information on the timing and exact frequency of DM in this patient population has not been fully reported. Here we report the frequency, sites, and timing of DM in a large cohort of patients. Our ultimate goal is to develop a clinical model that will associate clinical parameters with high likelihood of DM, either prior to local therapy or prior to surgery. If such a clinical model is established, it may provide an opportunity to properly select therapies for the high-risk subset. Currently, no such model exists.
Method
Patients
The study cohort was identified from a prospectively maintained database in the Department of GI medical oncology at The University of Texas MD Anderson Cancer Center between 2002 and 2013. We included all patients with localized EAC who received TMT or BMT with a curative intent. All patients were staged by imaging study and upper endoscopic ultrasound. Therapy decision (TMT or BMT) was made at the multidisciplinary conference. The American Joint Committee on Cancer (AJCC) 6th edition was used for clinical Staging. No other selection criteria were applied. The Institutional Review Board approved this analysis.
Treatment
Chemotherapy with radiation included a fluoropyrimidine (intravenous or oral) and either a platinum compound or a taxane. Radiation with a total of 45 – 50.4 Gy was delivered in 1.8 Gy per fraction by one of the conformal techniques. Platinum or taxene were given weekly × 5 and fluoropyrimidine was given 5 days/week × 5. In TMT-eligible patients, an esophagectomy was performed after 6–8 weeks after the completion of chemoradiation. The primary surgeon selected the surgical technique (transthoracic [Ivor-Lewis], transhiatal, total [three-field technique], or minimally invasive esophagectomy with lymph node dissection).
Surveillance
Imaging studies and endoscopy were performed upon completion of local therapy. Additional follow-up data were obtained from our institution’s tumor registry and the hospital records or Social Security database.
Statistical Analysis
Time to DM was defined as the time from the end of local therapy. Overall survival (OS) for patients who developed DM was defined from the diagnosis of DM to death or last follow-up. The rate of distribution and timing of relapse were tabulated by frequency and percentage. OS values were obtained by the Kaplan-Meier method. Statistical analyses were performed using SPSS software (IBM SPSS statistics 21.Ink).
Results
Patient of Characteristics
We studied a total of 629 consecutive patients (356 patients who had TMT and 273 patients who had BMT) between 2002 and 2013. The median age was 63 years (range, 20 – 91 years). Most of the patients were men (88.1%) and white (89.3%). Most primary site and histology were EGJ (84.9%) and adenocarcinoma (87.4%). The clinical characteristics of these patients are summarized in Table 1. The median follow-up time was 37.2 months (interquartile range, 17.8 to 65 months) for patients who remain alive.
Table 1.
Pretreatment characteristics
| Covariate | No. of eligible patients (n=629) | % of total |
|---|---|---|
| Age, years | ||
| Median | 63 | |
| Minimum-maximam | 20–91 | |
| Gender | ||
| Males | 554 | 88.1% |
| Females | 75 | 11.9% |
| Race | ||
| White | 562 | 89.3% |
| Others | 67 | 10.7% |
| Primary site | ||
| Esophagus | 95 | 15.1% |
| EGJ | 534 | 84.9% |
| Histology | ||
| Adenocarcinoma | 550 | 87.4% |
| Squamous cell carcinoma | 79 | 12.6% |
| Histologic Grade | ||
| Well-Moderate | 298 | 47.4% |
| Poorly | 331 | 52.6% |
| Baseline T Stage | ||
| T1 | 11 | 1.7% |
| T2 | 64 | 10.2% |
| T3 | 537 | 85.4% |
| T4 | 17 | 2.7% |
| Baseline N Stage | ||
| N0 | 221 | 35.1% |
| N1 | 408 | 64.9% |
| Baseline M Stage | ||
| M0 | 614 | 97.6% |
| M1a | 15 | 2.4% |
| Baseline Stage | ||
| Stage I | 10 | 1.6% |
| Stage II | 236 | 37.5% |
| Stage III | 368 | 58.5% |
| Stage IVA | 15 | 2.4% |
Abbreviation: EGJ, esophagogastric junction
Pattern of Failure
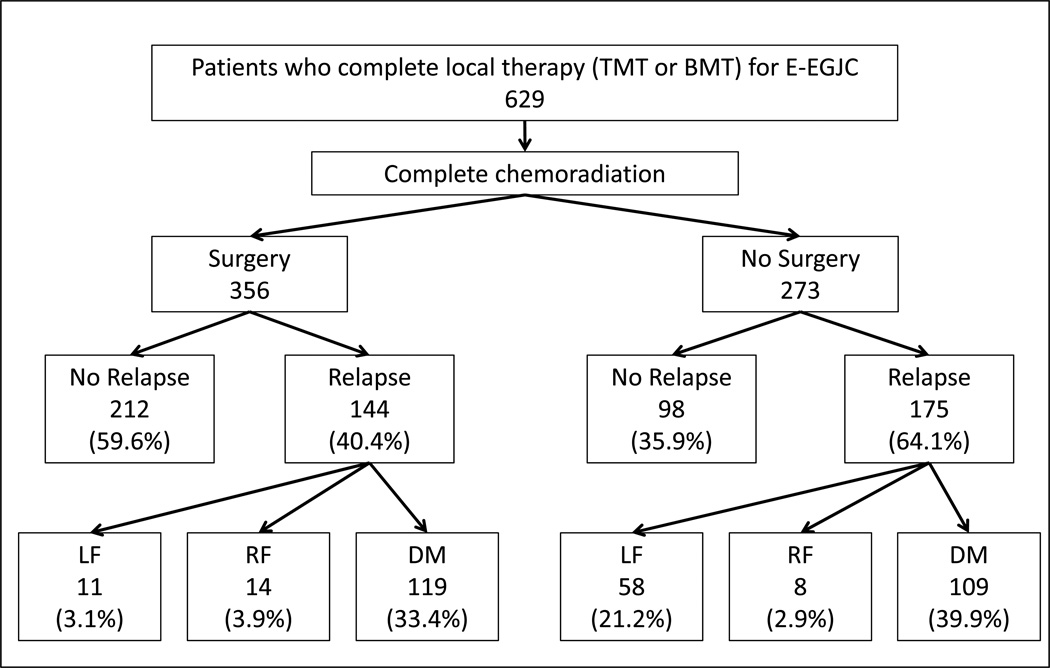
Patterns of failure are summarized in Figure 1. Forty percent (144 of 356 patients) who had TMT, 64% (175 of 273 patients) who had BMT had a relapse. Of 144 relapses after TMT, 83% were DM and 62% of 175 relapses after BMT were DM.
Figure 1.
Distribution and Timing of DM
The 7 most common anatomic sites of DM, as the first exclusive event, were lung (20% of DM for TMT and 17% of DM for BMT), distant lymph nodes (13% and 20%), liver (14% and 11%), peritoneum (10% and 13%), bone (6% and 11%), brain (8% in each group) and pleura (7% and 5%; Table 2). The cumulative rates of DM were 90.8% after TMT, 96.3% after BMT within 2 years after the completion of treatment and 97.5% after TMT and 99.1% after BMT within 3 years.
Table 2.
Duration-specific rate of all of DM from completion of treatment Duration-specific rate of DM from completion of treatment (months)
| 0–12 | 13–24 | 25–36 | 37–48 | 48- | Total | |
|---|---|---|---|---|---|---|
| DM following 356 TMT | 87 | 21 | 8 | 2 | 1 | 119 |
| * | 24.4% | 5.9% | 2.2% | 0.6% | 0.3% | 33.4% |
| ** | 73.1% | 17.6% | 6.7% | 1.7% | 0.8% | 100.0% |
| *** | 73.1% | 90.8% | 97.5% | 99.2% | 100.0% | |
| DM following 273 BMT | 93 | 12 | 3 | 0 | 1 | 109 |
| * | 34.1% | 4.4% | 1.1% | 0.0% | 0.4% | 39.9% |
| ** | 85.3% | 11.0% | 2.8% | 0.0% | 0.9% | 100.0% |
| *** | 85.3% | 96.3% | 99.1% | 99.1% | 100.0% | |
Denominators are number of total patients with each treatment; 356 with TMT and 273 with BMT.
Denominators are number of distant metastasis in each treatment; 119 with TMT and 109 with BMT.
Cumulative % of failure
OS after DM
The median OS time of the total of DM was 10.2 months (95% confidence interval [CI]: 7.8–12.7) for TMT patients and 7.8 months (95CI: 5.7–9.9) for BMT patients. Those with pleural DM had the shortest OS; 1.5 months (95% CI: 1.0–2.1) for TMT and 3.5 months (95% CI: 3.3–3.6) for BMT.
Discussion
Our analysis that represents the largest cohort of patients demonstrates that DM as a first sign of relapse after local therapy of EAC patients is common. TMT patients, with relatively less advanced local EAC have a slightly lower frequency of DM vs. the BMT patients who often have more advanced local EAC. Additionally, it is often disappointing if a TMT patient develops DM (especially within 12 months of surgery). If we can identify patients who are destined to develop DM, we may be able to change the clinical algorithm (for example, delay surgery for certain period of time if the risk for DM is high), however, it is unclear what we could do for BMT patients who are destined to develop DM (prolonged induction systemic therapy?). Currently, there is no clinical or biomarker model associated with high likelihood of the development of DM. Such a model would be desirable to explore various clinical approaches.
This study is retrospective and has all the limitations of a retrospective analysis and the strength of study includes that it has the largest number of patients ever reported for characterizing DM.
In conclusion, our data show that DM is a common occurrence after local therapy of patients with EAC. A clinical variables’ model to predict DM would be desirable.
Acknowledgements
UT M. D. Anderson Cancer Center Multidisciplinary Research G rants, CA138671, CA172741, CA129926 from NCI (JAA) and Generous Donors.
Footnotes
Disclosure Statement
The authors have no financial or any other conflict of interest.
References
- 1.Institution NC. SEER Stat Fact Sheets: Esophagus. [accessed Sep. 25, 2013];2013 Available from URL: http://seer.cancer.gov/statfacts/html/esoph.html.
- 2.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. Jama. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Sudo K, Taketa T, Correa AM, et al. Locoregional Failure Rate After Preoperative Chemoradiation of Esophageal Adenocarcinoma and the Outcomes of Salvage Strategies. Journal of Clinical Oncology. 2013 doi: 10.1200/JCO.2013.51.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632–2640. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amini A, Ajani J, Komaki R, et al. Factors Associated with Local-Regional Failure After Definitive Chemoradiation for Locally Advanced Esophageal Cancer. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-3303-0. [DOI] [PubMed] [Google Scholar]