Summary
Members of the NAC transcription factor family in barley appear to be highly involved in initiation and progression of leaf senescence via regulation of target genes having palindromic NAC-binding sequences in their promoters.
Key words: Barley, cereals, DNA microarray, gene expression, leaf senescence, NAC transcription factor, nutrient remobilization.
Abstract
The senescence process of plants is important for the completion of their life cycle, particularly for crop plants, it is essential for efficient nutrient remobilization during seed filling. It is a highly regulated process, and in order to address the regulatory aspect, the role of genes in the NAC transcription factor family during senescence of barley flag leaves was studied. Several members of the NAC transcription factor gene family were up-regulated during senescence in a microarray experiment, together with a large range of senescence-associated genes, reflecting the coordinated activation of degradation processes in senescing barley leaf tissues. This picture was confirmed in a detailed quantitative reverse transcription–PCR (qRT–PCR) experiment, which also showed distinct gene expression patterns for different members of the NAC gene family, suggesting a group of ~15 out of the 47 studied NAC genes to be important for signalling processes and for the execution of degradation processes during leaf senescence in barley. Seven models for DNA-binding motifs for NAC transcription factors were designed based on published motifs, and available promoter sequences of barley genes were screened for the motifs. Genes up-regulated during senescence showed a significant over-representation of the motifs, suggesting regulation by the NAC transcription factors. Furthermore, co-regulation studies showed that genes possessing the motifs in the promoter in general were highly co-expressed with members of the NAC gene family. In conclusion, a list of up to 15 NAC genes from barley that are strong candidates for being regulatory factors of importance for senescence and biotic stress-related traits affecting the productivity of cereal crop plants has been generated. Furthermore, a list of 71 senescence-associated genes that are potential target genes for these NAC transcription factors is presented.
Introduction
Plant nutrients are to a very large extent taken up and stored temporarily in the vegetative parts of cereal crop plants, either as structural components or as part of compounds in metabolic fluxes, until the seed filling and maturation stage, where they are remobilized to varying extents and translocated to the developing seeds. This developmental process is of the utmost importance for the productivity of crop plants, and a crucial part of it is the terminal process described as leaf senescence, in which a highly regulated degradation of the components of leaf tissues takes place (for reviews, see Buchanan-Wollaston et al., 2003; Gregersen et al., 2008; Gregersen, 2011). Senescence of plants is initiated in individual distal or lower organs and tissue parts, successively building up to whole-plant senescence (Noodén, 1988) which only specialized tissues in the reproductive organs survive. The monocarpic cereal crop plant constitutes a typical example of this process for which the only surviving tissues are the embryo and aleurone layer that are necessary for the seeds to germinate and develop into a new plant. A hallmark of the senescence process is the dismantling of the photosynthetic apparatus of the leaf chloroplasts (Krupinska et al., 2013), by which the plastidic proteins are degraded and turned into compounds amenable for transport from the leaf to other organs of the plants, eventually to the grain during the maturation stage.
The genetic regulation of the complex senescence process is executed via the spatial and temporary patterns of gene expression during the developmental process of the plant. Hence, the temporal regulation of senescence-associated genes (SAGs) has been intensively studied over the last two decades (e.g. Lohman et al., 1994). At the regulatory level, the changes in these gene expression patterns are governed by a network of gene transcription factors, which again, with respect to senescence, are regulated by a combination of time (i.e. ageing) and environmental cues. Presumably, the latter take place via the combined effects of plant hormones that channel the impacts from the environment into changes in gene expression patterns (Schippers et al., 2007). There are strong indications that members of the NAC transcription factor family are particularly involved in the signalling pathways that regulate senescence (Breeze et al., 2011). A number of studies on specific NAC genes show that several members of this transcription factor family also have impacts on the regulation and execution of the senescence process in cereals. Uauy et al. (2006) showed that accelerated senescence in wheat caused by the introgression of a trait for high grain protein content was in fact due to the presence of a NAC transcription factor gene, and a recent report (Zhou et al., 2013) demonstrated the regulation of senescence in rice by OsNAP/ONAC058. In addition, several individual NAC genes in Arabidopsis have been shown to regulate senescence, for example AtNAP/ANAC029 (Guo et al., 2006), ORE1/ANAC092 (Kim et al., 2009), ORS1/ANAC059 (Balazadeh et al., 2011), JUB1/ANAC042 (Wu et al., 2012), and NTL4/ANAC053 (Lee et al., 2012).
In the present work, the changes in gene expression taking place during the senescence process in barley (Hordeum vulgare) were studied in order to substantiate the understanding of the senescence process in a typical cereal plant. It is demonstrated that the generic senescence programme, studied mainly in Arabidopsis (e.g. Breeze et al., 2011), in terms of patterns of gene expression, is also operating in the barley crop plant. Selected results from a microarray experiment were confirmed by quantitative reverse transcription–PCR (qRT-PCR) and details on the co-expression of members of the NAC transcription factor gene family with a number of SAGs are shown. With the aim to elucidate further the importance of NAC transcription factors in the regulatory networks governing the senescence process, biologically relevant palindromic NAC binding sites (NACBSs) were searched for in the promoter regions of a large number of genes. A significant enrichment of NACBSs was found in the promoters of the differentially up-regulated genes during senescence. Together, these findings strongly support the notion of NAC transcription factors being involved in the regulation of the senescence process and, hence, to be important for regulation of the maturation processes taking place in cereal crop plants.
Material and methods
Plant material
For the microarray experiment, barley plants (H. vulgare, cv. Golden Promise) were grown in the greenhouse in pots containing a 50:50 peat–perlite mixture. A standard nutrient solution was supplied via the irrigation system. Artificial illumination was used for supplementation of sunlight and for ensuring a day/night cycle of 16/8h. Sampling of leaves was done between 12:00h and 14:00h. Non-senescing flag leaves were harvested ~5 d before anthesis, medium-senescing leaves at ~15 d post-anthesis, and senescing leaves at ~30 d post-anthesis, selecting leaves with ~50% green leaf area. Frozen homogenized leaf samples were used for RNA isolation as described previously (Christiansen et al., 2011)
For the qRT–PCR experiments, barley plants, cv. Golden Promise, were grown in greenhouse soil plots with weekly sowings. Artificial illumination was used for supplementation and for ensuring a day/night cycle of 16/8h. Single flag leaves were harvested across the soil plot at different developmental stages, ranging from young leaves at the pre-anthesis stage to senescing leaves close to the grain maturity stage where the leaves were completely chlorotic, but still turgid. Frozen homogenized leaf samples were used for RNA isolation as described previously (Christiansen et al., 2011) and for determination of total chlorophyll content, following extraction in 96% ethanol and UV measurements according to Lichtenthaler (1987). In the analyses of gene expression patterns, the single flag leaf samples were ordered according to decreasing chlorophyll content.
Microarray experiment
The Agilent 4×44 Barley Gene Expression Microarray (Agilent, http://www.genomics.agilent.com, last accessed 12 February 2014) was used for the microarray experiments. Sample quality control, sample labelling, hybridization, scanning, and data extraction were performed by imaGenes (Berlin, Germany), according to the complete integrated Agilent Workflow Solution certified by Agilent (www.imagenes-bio.de, last accessed 12 February 2014). In brief, the isolated RNA was reversed transcribed into cDNA, followed by an in vitro transcription reaction during which Cy3 labels were incorporated into cRNA, which was subsequently hybridized to the microarray chip in single channel hybridizations. Data acquisition was performed with an Agilent DNA microarray scanner with Feature Extraction Software. The quality filtering and statistical analysis of the raw data obtained was performed with the Agi4×44PreProcess (Lopez-Romero, 2011) and Limma R-packages (Smyth, 2005).
The Agilent barley microarray used contained redundant sequences at two levels. (i) Several target sequences, defined by the same primary accession number, were represented by from two to several probes in the chip. (ii) Since many of the target sequences were partial expressed sequence tags (ESTs), several of them in fact represent the same gene sequence. To cope with the redundancy, only one probe for each target sequence was used in the analysis, selected via the following procedure: the probe with the highest or second highest signal closest to the 3′ end of the target sequence was selected as a representative probe. The distal 3′ probes were favoured since the labelling of cRNA for the chip hybridization was performed from the poly(A) tail. Furthermore, the redundancy was alleviated by making blastN searches of the Agilent Chip probes against recently released full-length cDNA sequences of barley (Sato et al., 2009; Matsumoto et al., 2011). Up to three mismatches to the 60 nucleotide long probes were allowed. Subsequently, redundant probes representing the same sequence were removed as described above.
Annotation of differentially expressed genes during senescence was done using the information for the Agilent 4×44 Barley chip available at http://mapman.gabipd.org (last accessed 12 February 2014), which also formed the basis for the MapMan categorization of the target genes into functional bins, supplemented by manual annotation of a limited number of genes. The MapMan bins (Usadel et al., 2009) were used to analyse and visualize selected aspects of the differential gene expression during senescence.
qRT–PCR experiments
Procedures for RNA isolation, cDNA synthesis, primer design, primer efficiency testing, and quantitative real-time PCR were performed as described previously (Christiansen et al., 2011). Primers for 48 NAC genes were the same as used by Christiansen et al. (2011). Primers for qRT–PCR representing other genes in this study are listed in Supplementary Table S6 available at JXB online. Ct values exported from the ABI Prism 7900HT SDS software were used as raw data for the analysis of qRT–PCR data. The R software (R Development Core Team, 2005) and the add-on packages HTqPCR (Dvinge et al., 2009) and Limma (Smyth, 2005) were used for the manipulation and analysis of the raw Ct values. qRT–PCR runs showing high variation among the three technical replicates were manually inspected, and clear outliers and runs with aberrant dissociation curves were excluded from the analysis. Several possible reference genes [18S rRNA (AK251731), actin (AY145451), α-tubulin (X99623), EF1-α (Z50789), G6PDH (AM398980), HP68 protein (AK251800), HSP70 (AK248694), and Splicing factor 2 (AK249101) (Faccioli et al., 2007; Gregersen and Holm, 2007)] were tested for their stability across the range of tested samples using the tools in the R package SLqPCR (Kohl, 2007). Based on this analysis, the barley 18S rRNA gene was selected as the most stable reference gene to be used in the normalization of gene expression. The Splicing factor 2 (SP2) gene also showed high stability and was included in the presentation of the data as a control gene.
In silico co-expression analysis
Genes in barley co-expressed with barley NAC genes were investigated in silico using the resources from http://coexpression.psc.riken.jp/barley/search.pl, last accessed 12 February 2014 (Mochida et al. 2011). Only genes included in the Affymetrix Barley1 Chip are available in these resources, and hence only 33 out of the 48 NAC genes from Christiansen et al. (2011) could be analysed. The rankings in the co-expression list for each of the 33 NAC genes were compared with the gene expression data from the senescence microarray and qRT–PCR results, in order to substantiate the co-regulations observed there.
Promoter analysis
Putative barley promoter sequences (1000 bases upstream of ATG) and their corresponding coding sequences (CDS) were obtained from http://plants.ensembl.org/Hordeum_vulgare, last accessed 12 February 2014 (International Barley Genome Sequencing Consortium, 2012). For detection of putative NACBSs in the promoter sequences, the FIMO tool available at http://meme.nbcr.net/meme/cgi-bin/fimo.cgi (last accessed 12 February 2014) was used (Grant et al., 2011).
Results
Agilent 4×44K barley microarray experiment on flag leaf senescence
In order to study the general changes in gene expression taking place during senescence of the barley flag leaf, a microarray experiment was performed on three stages of senescence of the flag leaf of greenhouse-grown barley plants (cv. Golden promise). Sampling was done from young, just fully developed barley flag leaves (~5 d before anthesis); from medium-senescing flag leaves (~15 d post-anthesis); and from strongly senescing flag leaves (green leaf area ~50%; ~30 d post-anthesis). Three samples, each comprising 3–5 leaves, were used for the first and last harvest time points, and two samples for the medium stage. This provided RNA samples for, in total, eight hybridizations distributed over two Agilent 4×44 Barley Microarray chips. The hybridizations were performed by imaGenes (Berlin, Germany), from whom the extracted raw data were received. These raw data are available from ArrayExpress (http://www.ebi.ac.uk/arrayexpress/, last accessed 12 February 2014; accession no. E-MTAB-2133). The data were analysed using the Agi4×44PreProcess and Limma R-packages. Herein, mainly the data from the contrast between non-senescing and strongly senescing samples will be presented and discussed. In a standard hierarchical clustering analysis of the samples, one of the senescing samples, Sen3, appeared as an outlier with considerably lower signal values than the other two senescence samples. Accordingly, the Sen3 sample was omitted in the final data analysis, which, however, only marginally affected the ordering of differentially expressed genes.
During pre-processing of the data in the Agi4×44PreProcess software, background correction was performed with the method ‘half’, and normalization across the eight hybridizations with the method ‘quantile’. Low quality features, in particular those with signals close to or below background levels, were filtered out, according to default settings of the Agi4×44PreProcess software. The pre-processing resulted in 24 646 features with acceptable signal values. The statistical analysis of these filtered data using the Limma software resulted in ordered lists of gene probes according to the probability of their differential expression across the different contrasts in the experiment (Supplementary Table S1 available at JXB online). According to the adjusted P-values of the Limma tests for differential expression, a large number of genes appeared as differentially expressed in the contrast between strongly senescing and non-senescing flag leaves, presumably reflecting the immense physiological changes taking place in the leaves during senescence. Since a decision on a precise threshold between significant and non-significant differential gene expression in studies like this is difficult (Smyth et al., 2003), the top 5000 ranked probes (~20% of the filtered probes) were arbitrarily chosen for further analysis. The cut-off was at an adjusted P-value of ~0.00035 (see Supplementary Table S1 available at JXB online). The Agilent barley chip contained a high number of redundant sequences, which were handled according to the approach described in the Materials and methods. This resulted in a list of 3867 non-redundant differentially expressed genes that were subject to detailed analysis.
The MapMan tool was used to analyse the functional structure of the selected differentially expressed genes. This tool attempts to classify genes into functional bins at different levels of detail and allows versatile visualization of the relative expression levels of selected genes (Usadel et al., 2009). The top-bins describe rather broad groups of gene functions, such as protein or lipid metabolism. Supplementary Fig. S1 and Table S2 available at JXB online show the distribution of the 3867 selected probes across the top-bins, illustrating that there were many both up- and down-regulated genes in senescing leaves in most categories. The exceptions were, in particular, categories involved in photosynthesis [bins for photosystems (1) and tetrapyrrole biosynthesis (19)], which showed predominantly down-regulation of genes. A number of the up-regulated genes in the photosynthesis category are related to the photorespiration process, and their encoded functions in the context of senescence might be related to the turnover of glycine also originating from sources other than the photorespiration cycle (Bauwe et al., 2003). Top-bin 17, hormone metabolism, and top-bin 26, with miscellaneous functions, both showed a clear bias towards more up-regulated than down-regulated genes at this general functional level.
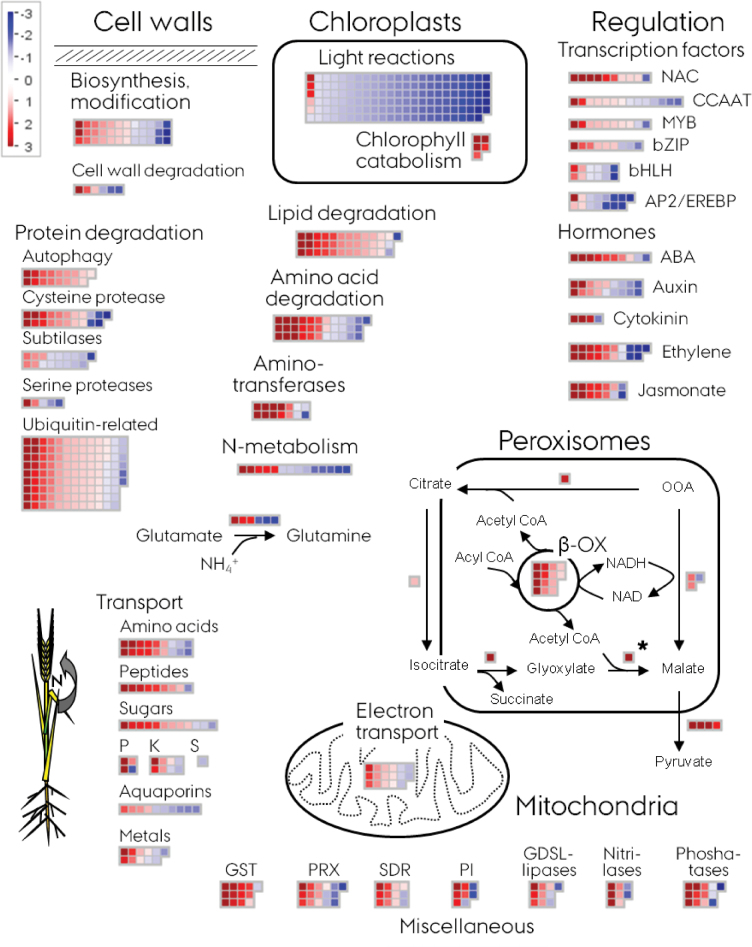
In order to study in more detail the gene expression during senescence in barley, a number of subcategories were selected from the MapMan annotation that, based on the senescence literature, are believed to be involved in senescence-associated processes. To visualize this analysis, the ‘senescence pathway’ map in Fig. 1 was designed, using the MapMan tools for generating novel maps. Figure 1 shows the up-regulation of a large number of genes in the selected categories. The accession numbers and relative expression values for the different categories are listed in Supplementary Table S3 available at JXB online.
Fig. 1.
MapMan ‘senescence pathway’: the distribution of gene expression in senescing compared with non-senescing barley flag leaves across selected MapMan sub-bins, with function suggested to be involved in senescence processes. Blue indicates down-regulation, and red up-regulation of gene expression. Details on the individual genes are provided in Supplementary Table S2 available at JXB online. *The malate synthase gene expression is from a qRT–PCR experiment, since this gene (accession no. AK357164) was not represented in the Agilent chip.
The senescence process, visually observed as yellowing of the leaves at the late sampling time point, was clearly reflected in the down-regulation of genes involved in the photosynthesis reactions taking place in the chloroplasts. In parallel, there was an up-regulation in the chloroplast of five genes involved in chlorophyll breakdown. Several different subcategories involved in protein and lipid degradation and carboxylic acid metabolism showed clear up-regulation. For protein degradation, the autophagy- and ubiquitin-related pathways, along with the cysteine peptidases, appeared as the predominantly up-regulated categories. The up-regulation of the lipid degradation pathways in the peroxisomes during senescence is well known (Yang et al., 2009), supported here by the strong up-regulation of a number of genes involved in the β-oxidation of fatty acids taking place in peroxisomes. This was accompanied by an up-regulation of genes encoding enzymes usually described to be involved in the glyoxylate cycle: citrate synthase, aconitase, and isocitrate lyase. There was unfortunately no probe in the chip representing the malate synthase step of the glyoxylate cycle. However, supplementary qRT–PCR results showed that this gene (accession no. AK357164) was strongly up-regulated (log ratio 9.8) during leaf senescence in barley. This value is inserted in Fig. 1. The strong up-regulation of genes leading to formation of malate was also accompanied by strong up-regulation of a number of genes encoding malic enzymes.
Up-regulated genes associated with mitochondrial functions were mainly related to the electron transport chain, indicating up-regulation of respiratory processes via, for example, the alternative oxidase. There was no clear indication, from top-bin 8 for tricarboxylic acid (TCA) metabolism, of a general up-regulation of the TCA cycle of the mitochondria.
In accordance with the presumed translocation of compounds and mineral nutrients from senescing leaves, a number of transporter gene transcripts were up-regulated during senescence, mainly comprising transporters for amino acids, peptides, and sugars, but there was also a clear indication for up-regulation of transporters involved in transport of minerals.
Top-bin 26, with miscellaneous functions, which showed an overall bias towards up-regulated genes in senescing leaves (Supplementary Fig. S1 available at JXB online), was analysed for sub-bins that in particular represented this up-regulation, and the seven sub-bins shown in Fig. 1 were pinpointed. They appeared to represent categories of functions involved in redox and catabolic processes, such as peroxidases and phosphatases.
With respect to regulation of the senescence process, several genes encoding transcription factors or genes involved in hormone regulation were up-regulated in senescing leaves. For the hormones, several genes involved in the biosynthesis, degradation, or responses to hormones were up-regulated, supporting the notion that hormones play a role in the regulation of senescence (Schippers et al., 2007). Regarding transcription factors, the NAC transcription factor family in particular had several members that were up-regulated during senescence. However, members of other transcription factor families were also transcriptionally up-regulated: bZIP, MYB, bHLH, AP2/EREBP, and CCAAT transcription factors.
With the aim to study further the association of NAC transcription factors with the senescence process, a more detailed analyses of the expression levels of these genes was carried out. Table 1 shows the comparison of the microarray results with the qRT–PCR results from Christiansen et al. (2011) whose gene expression experiments for the NAC genes were performed on similar, but not identical, senescing barley leaf material to that in the present study. Of the 48 barley NAC gene sequences described by Christiansen et al. (2011), only 27 were represented by probes in the Agilent chip. Seven of these probes had low expression levels that caused them to be filtered out during the pre-processing of the microarray data. Of the remaining 20 probes, 10 were among the selected differentially expressed genes at the late senescing time point as illustrated in Fig. 1. Only one, representing the HvNAC046 gene, showed down-regulated expression in senescing leaves. To illustrate the reproducibility of the results from the Agilent chip by qRT–PCR, Supplementary Fig. S2A available at JXB online shows the strong correlation between the senescence-associated expression levels of the barley NAC genes from the two experimental systems. There was a clear overlap between the NAC genes that showed significant differential expression in the two experimental approaches.
Table 1.
Barley NAC genes used in this study, with GenBank accession numbers, corresponding names of contigs in the Barley1 chip, and probe names for the Agilent microarrayGene expression fold changes (FC, log2 scale) in the microarray experiments are given for leaves at intermediate (M15D) and late (SEN) senescence stages (NS, non-senescing flag leaf). The qPCR data give the FC of expression in senescing compared with non-senescing flag leaves from Christiansen et al. (2012). Mean intensity and mean Ct values indicate the relative gene expression levels in the microarray and the qPCR experiments, respectively.
| Name | Accession no. | Barley1 chip | Agilent microarray | qPCR | ||||
|---|---|---|---|---|---|---|---|---|
| Log2 FC | ||||||||
| Contig name | Probe_name | M15D versus NS | SEN versus NS | Mean intensity (log2) | Log2 FC | Mean Ct | ||
| HvNAC001 | AK250475 | HD05L07r_at | A_13_p078941 | 2.27 | 4.05 | 9.01 | 3.73 | 22.62 |
| HvNAC002 | AK249396 | Contig5723_at | A_13_p017131 | 0.08 | 0.20 | 11.46 | –1.12 | 17.58 |
| HvNAC003 | AK249102 | Contig3361_at | A_13_p421415 | 0.61 | 1.47 | 13.52 | 2.78 | 16.84 |
| HvNAC004 | AM500853 | Contig3362_at | 1.57 | 19.64 | ||||
| HvNAC005 | AK251058 | Contig14026_at | A_13_p016456 | 2.47 | 3.95 | 9.07 | 5.39 | 21.29 |
| HvNAC006 | AM500854 | Contig6233_at | 3.30 | 17.46 | ||||
| HvNAC007 | AK249749 | Contig10340_at | A_13_p015456 | 0.36 | 0.94 | 12.16 | 1.29 | 19.98 |
| HvNAC008 | FR821737 | Contig9031_at | A_13_p150635 | 0.80 | 2.05 | 13.42 | 3.36 | 19.56 |
| HvNAC009 | FR819761 | Contig17688_at | –0.34 | 15.79 | ||||
| HvNAC010 | FR821754 | Contig13345_at | A_13_P093105 | 1.31 | 2.25 | 13.86 | 2.32 | 17.62 |
| HvNAC011 | AK251493 | HVSMEa0011M12r2_at | 4.02 | 23.5 | ||||
| HvNAC012 | FR819762 | –3.20 | 21.89 | |||||
| HvNAC013 | AK376297 | Contig11098_at | A_13_P553194 | 1.27 | 4.43 | 10.93 | 5.19 | 18.54 |
| HvNAC014 | FR821738 | Contig9757_at | 2.81 | 22.25 | ||||
| HvNAC015 | FR821739 | Contig6484_at | A_13_P068461 | 0.03 | 0.06 | 6.67 | –0.21 | 21.20 |
| HvNAC016 | AK366470 | Contig5241_at | A_13_p136620 | 0.21 | 0.98 | 12.61 | 1.54 | 15.57 |
| HvNAC017 | FR821740 | Contig8993_at | A_13_p576969 | a | a | 4.04 | 22.73 | |
| HvNAC018 | FR821741 | Contig9284_at | 3.97 | 23.47 | ||||
| HvNAC019 | FR819764 | 3.67 | 21.99 | |||||
| HvNAC020 | FR821742 | Contig10172_at | A_13_p094660 | 0.03 | 0.56 | 5.90 | 2.94 | 19.56 |
| HvNAC021 | AK370287 | Contig15867_at | A_13_p002701 | a | a | 3.92 | 22.61 | |
| HvNAC022 | AK365398 | 2.72 | 21.72 | |||||
| HvNAC023 | FR821745 | Contig13658_at | 2.25 | 22.58 | ||||
| HvNAC024 | FR821746 | Contig11340_at | A_13_p341882 | a | a | 3.81 | 22.80 | |
| HvNAC025 | AK364002 | A_13_P180884 | –0.01 | 0.76 | 5.95 | 3.73 | 22.97 | |
| HvNAC026 | FR819767 | A_13_P554129 | a | a | 2.94 | 21.43 | ||
| HvNAC027 | AK368213 | HM07L17r_at | A_13_P042621 | 2.39 | 5.29 | 8.36 | 6.43 | 19.90 |
| HvNAC028 | AB362161 | HVSMEk0006G11r2_s_at | A_13_p085361 | 0.07 | 0.16 | 5.94 | 0.38 | 17.74 |
| HvNAC029 | EU908210 | 3.29 | 19.79 | |||||
| HvNAC030 | DQ869679 | 4.24 | 24.64 | |||||
| HvNAC031 | AY672069 | A_13_p080271 | –0.43 | –0.46 | 9.26 | –0.53 | 18.75 | |
| HvNAC032 | AK248480 | A_13_p100800 | a | a | 3.42 | 23.81 | ||
| HvNAC033 | AK248449 | Contig19673_at | 3.10 | 25.66 | ||||
| HvNAC034 | AK249120 | A_13_p100355 | –0.27 | 0.13 | 7.62 | 2.00 | 22.56 | |
| HvNAC035 | FR821748 | Contig5740_at | A_13_p098010 | –0.09 | 0.12 | 9.06 | 1.37 | 18.31 |
| HvNAC036 | AL505464 | 4.18 | 21.53 | |||||
| HvNAC037 | AK371156 | 6.06 | 19.51 | |||||
| HvNAC038 | BY847894 | 2.74 | 22.04 | |||||
| HvNAC039 | AK370035 | Contig11856_at | 3.62 | 21.88 | ||||
| HvNAC040 | AK361879 | Contig15251_at | 2.36 | 20.96 | ||||
| HvNAC041 | FR821751 | Contig7432_at | A_13_P133280 | 0.36 | 0.85 | 12.72 | –0.76 | 16.84 |
| HvNAC042 | AK361273 | Contig17088_at | A_13_P069701 | a | a | –1.58 | 20.63 | |
| HvNAC043 | GH216054 | 2.12 | 22.81 | |||||
| HvNAC044 | AK364683 | Contig19862_at | 1.24 | 20.85 | ||||
| HvNAC045 | BF259201 | A_13_P292932 | a | a | 2.96 | 24.4 | ||
| HvNAC046 | AK252960 | Contig12579_at | A_13_P246531 | –1.19 | –1.74 | 8.88 | –2.01 | 18.59 |
| HvNAC048 | AK355552 | Contig13898_at | A_13_P157980 | 0.16 | –0.03 | 11.29 | 0.82 | 18.97 |
a Probes were filtered out during pre-processing of microarray data, due to low intensity levels.
FC values in bold indicates NAC genes that are among the genes selected as differentially expressed in senescing leaves.
qRT–PCR experiments
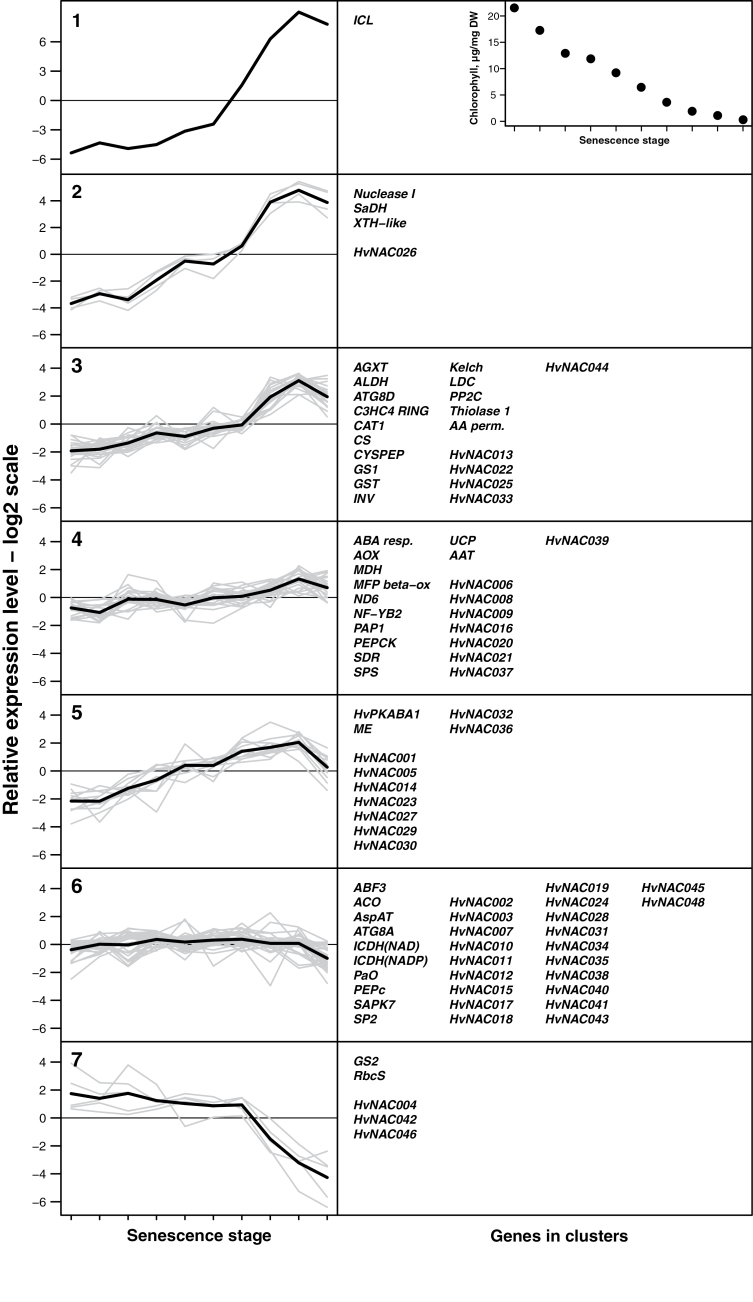
In order to study in more detail the regulation patterns of SAGs and the NAC genes during the progress of senescence, 44 SAGs were selected for detailed qRT–PCR studies across the different functional categories shown in the MapMan ‘senescence pathway’ in Fig. 1. Primarily genes encoding well-described functions were selected. The list of selected SAGs is given in Table 2 along with their expression levels in the microarray experiment. As input material for the qRT–PCR experiment, single barley flag leaves were harvested from plants at different stages of development from the pre-anthesis stage to completely senescing leaves. The chlorophyll content was determined and used as a measure of senescence progress, and expression levels for the selected genes and 47 barley NAC genes (Christiansen et al., 2011) were determined by qRT–PCR. The 18S RNA-normalized gene expression patterns were clustered by K-means clustering as implemented in the R software (R Development Core Team, 2005). Following a number of test clusterings and supported by a rule of thumb of √(n/2) clusters for k-means clustering, seven clusters were chosen as an optimal cluster number that divided the genes into distinct groups. Figure 2 shows the resulting clusters for 10 samples across the span of chlorophyll contents. The strong correlation between results from the Agilent chip and the qRT–PCR experiment for the selected SAGs are shown in Supplementary Fig. S2B available at JXB online. The difference in expression level between sample 9 and 1 from the qRT–PCR experiment was used as the senescence-associated expression that could be compared with the microarray expression results.
Table 2.
Barley senescence-associated genes used for the qRT–PCR experiment in Fig. 2, with probe names for the Agilent microarray, acession numbers (GenBank or http://plantta.jcvi.org/, last accessed 12 February 2014), blastX hits, abbreviations used in Fig. 2, and expression data from the microarray experimentThe genes were selected across different categories of gene functions shown in Fig. 1.
| Category | Probe name | Accession number | Putative function (BlastX hit) | Abbreviation in Fig. 2 | Agilent chip | ||
|---|---|---|---|---|---|---|---|
| Fold change (log2) | Average expression level | Adjusted P-value | |||||
| Carboxylic acid metabolism | |||||||
| A_13_P479945 | AK354442 | Aconitate hydratase, cytoplasmic | ACO | 0.99 | 11.4 | 5.55E-05 | |
| A_13_P529474 | AK361069 | Alanine-glyoxylate aminotransferase | AGXT | 4.50 | 9.6 | 0.000103 | |
| A_13_P114535 | AK364160 | Aspartate aminotransferase | AspAT | 1.92 | 10.2 | 9.14E-05 | |
| A_13_P518849 | AK252959 | Citrate synthase, peroxisomal | CS | 2.82 | 13.4 | 6.47E-06 | |
| A_13_P008951 | AK252731 | Glutamate decarboxylase | GAD | 0.84 | 13.9 | 0.000145 | |
| A_13_P113710 | TA28480_4513 | Glutamine synthetase 1 | GS1 | 2.94 | 13.2 | 1.67E-05 | |
| A_13_P125240 | AK364289 | Glutamine synthetase 2 | GS2 | –2.88 | 10.2 | 2.51E-05 | |
| A_13_P014696 | AK252018 | Isocitrate lyase | ICL | 11.60 | 8.2 | 2.40E-06 | |
| A_13_P135625 | AK368534 | Malate dehydrogenase, peroxisomal | MDH | 1.84 | 8.3 | 2.09E-05 | |
| A_13_P130815 | AK360118 | NAD-dependent isocitrate dehydrogenase | ICDH (NAD) | 0.85 | 11.8 | 0.001031 | |
| A_13_P134780 | TA34549_4513 | NADP malic enzyme, cytosolic | ME-2 | 5.92 | 8.1 | 2.51E-05 | |
| A_13_P094210 | AK252839 | NADP-dependent isocitrate dehydrogenase | ICDH (NADP) | 1.00 | 14.1 | 0.002635 | |
| A_13_P037031 | AK250093 | NADP-dependent malic enzyme | ME-1 | 3.65 | 12.9 | 3.48E-06 | |
| A_13_P140540 | TA36323_4513 | Phosphoenolpyruvate carboxykinase | PEPCK | 3.46 | 8.0 | 1.67E-05 | |
| A_13_P242685 | TA35051_4513 | Phosphoenolpyruvate carboxylase | PEPc | –1.33 | 9.5 | 0.000139 | |
| Fatty acid metabolism | |||||||
| A_13_P355167 | BG418084 | 3-Ketoacyl-CoA thiolase 2, peroxisomal | Thiolase 1 | 3.14 | 12.9 | 3.30E-06 | |
| A_13_P008556 | AK251077 | MFP glyoxysomal fatty acid beta-oxidation | MFP beta-ox | 1.91 | 13.7 | 1.23E-05 | |
| A_13_P540524 | AK372098 | Short-chain dehydrogenase/reductase (SDR) | SDR | 1.88 | 11.1 | 1.56E-05 | |
| Carbohydrate metabolism | |||||||
| A_13_P067481 | AK365330 | Endotransglucosylase/hydrolase | XTH-like | 9.62 | 11.1 | 6.47E-06 | |
| A_13_P583324 | AK367512 | Extracellular invertase | INV | 3.23 | 14.1 | 5.09E-05 | |
| A_13_P157610 | TA41920_4513 | Sucrose-phosphate synthase | SPS | 2.55 | 10.4 | 1.55E-05 | |
| Protein metabolism | |||||||
| A_13_P177459 | AK376180 | Lysine decarboxylase-like | LDC | 5.11 | 11.2 | 6.75E-06 | |
| A_13_P157210 | AK358908 | Papain-like cysteine peptidase | CYSPEP | 3.31 | 11.3 | 5.68E-06 | |
| A_13_P142895 | TA37051_4513 | Saccharopin dehydrogenase-like | SaDH | 8.46 | 9.9 | 2.90E-07 | |
| Autophagy | |||||||
| A_13_P361137 | BI947475 | Autophagy-related protein 8A | ATG8A | 1.97 | 14.6 | 5.30E-05 | |
| A_13_P515061 | AK250515 | Autophagy-related protein 8D | ATG8D | 3.70 | 10.7 | 4.99E-07 | |
| Nucleic acid metabolism | |||||||
| A_13_P000061 | AB028448 | Nuclease I | Nuclease I | 8.06 | 11.2 | 3.90E-06 | |
| Kinases/phosphatases | |||||||
| A_13_P077736 | AK372880 | Protein kinase HvPKABA1 | HvPKABA1 | 6.01 | 9.9 | 1.07E-05 | |
| A_13_P062196 | AK356066 | Protein phosphatase 2C | PP2C | 3.41 | 9.6 | 2.33E-05 | |
| A_13_P144685 | AK250358 | Serine/threonine-protein kinase SAPK7 | SAPK7 | 1.82 | 10.5 | 8.88E-06 | |
| Electron transport chain | |||||||
| A_13_P085996 | AK363239 | Alternative oxidase | AOX | 2.33 | 12.1 | 0.00016 | |
| A_13_P011006 | AK251682 | Mitochondrial uncoupling protein | UCP | 1.87 | 10.0 | 2.16E-05 | |
| A_13_P189899 | TA53161_4513 | NADH-ubiquinone oxidoreductase chain 6 | ND6 | 3.80 | 7.9 | 0.000152 | |
| A_13_P470383 | AK376855 | Succinate dehydrogenase | SuDH-2 | 2.06 | 9.2 | 2.24E-05 | |
| Miscellaneous | |||||||
| A_13_P008261 | AK370899 | ABA responsive element binding factor 3 | ABF3 | -0.16 | 10.1 | 0.265021 | |
| A_13_P421145 | AK363564 | Aba-responsive protein | ABA resp. | 3.60 | 12.3 | 5.04E-06 | |
| A_13_P002226 | AK252220 | Aldehyde dehydrogenase, cytosolic | ALDH | 4.07 | 11.8 | 1.80E-06 | |
| A_13_P460818 | AK353635 | Amino acid permease | AA perm. | 5.53 | 9.7 | 2.40E-06 | |
| A_13_P137455 | AK361903 | Amino acid transporter | AAT | 4.42 | 7.5 | 4.52E-06 | |
| A_13_P542217 | DN186744 | Catalase 1 | CAT1 | 3.57 | 7.1 | 1.26E-05 | |
| A_13_P234439 | AK357085 | Ferritin | Ferritin | 3.71 | 15.1 | 2.40E-05 | |
| A_13_P135220 | AK371360 | Glutathione transferase | GST | 2.59 | 11.4 | 3.66E-05 | |
| A_13_P139245 | TA35901_4513 | Kelch repeat-containing F-box-like | FBK | 4.17 | 10.3 | 4.99E-07 | |
| A_13_P278734 | AK353593 | Pheophorbide a oxygenase, chloroplastic | PaO | 2.25 | 13.4 | 1.28E-05 | |
| A_13_P132770 | AK375026 | Purple acid phosphatase 1 | PAP1 | 1.69 | 12.1 | 2.45E-05 | |
| A_13_P081911 | AK252711 | RUBISCO small subunit | RUBISCO | –2.53 | 14.9 | 0.000144 | |
| A_13_P399036 | AK249101 | Splicing factor 2 | SP2 | –0.29 | 6.2 | 0.289038 | |
| A_13_P153380 | AK370727 | Transcription factor subunit NF-YB2 | TF NF-YB2 | 2.05 | 10.2 | 1.08E-05 | |
| A_13_P171920 | AK368972 | Zinc finger (C3HC4-type RING finger)-like protein | C3HC4 RING | 3.91 | 10.4 | 2.62E-06 | |
Fig. 2.
K-means clustering of gene expression patterns for 44 selected senescence-associated genes and 47 NAC genes, as measured by relative qRT–PCR across 10 samples of barley flag leaves at progressive stages of senescence. The samples were ordered according to the chlorophyll content, shown in the insert at the upper right corner. Names of genes in the individual clusters are given in the right column of boxes. Abbreviations of gene names are shown in Table 2.
Since mostly up-regulated SAGs were selected, most of the clusters in Fig. 2 also showed up-regulation of gene expression, or only slight changes in expression, during the progress of senescence; however, with distinct patterns for the different clusters. Cluster 7 was the only cluster that showed clear down-regulation at the later stages of senescence. Genes encoding Rubisco small subunit and glutamate synthase 2 were selected as known markers of senescence, and three NAC genes showed a similar down-regulation to that of these marker genes. Cluster 6 comprised a large group of genes, amongst them almost half of the 47 NAC genes, which seemed only marginally affected by the progress of senescence. Accordingly, the apparent up-regulation of many of these genes in the microarray experiment, for example PaO, ACO, or ATG8A, was not reflected in the expression profile of this cluster, apart from a slight transient up-regulation at the mid-stages. The NAC genes HvNAC003, -7, and -10 were included in cluster 6, and the apparent up-regulation in the microarray of these three NAC genes was hence not confirmed.
Clusters 1–5 all showed up-regulation of gene expression towards the later stages of senescence, however, with different patterns. Cluster 1 showed the extreme up-regulation of an isocitrate lyase gene (ICL), which confirmed the strong up-regulation also observed in the microarray experiment. None of the other selected SAGs had a similar expression pattern and hence the ICL gene was the only gene in cluster 1. In the microarray experiment, the ICL probe represented by far the most strongly up-regulated gene on the chip, with a log2-fold change of 11.6. Cluster 2 also showed strong, ~8-fold (log2) up-regulation of three SAGs, encoding nuclease I, saccharopine dehydrogenase, and a xyloglucan endo-transglycosylases/hydrolase (XTH). Again, this confirmed the strong up-regulation also observed in the microarray data of these genes. Cluster 2 also showed the strong up-regulation of one NAC gene, HvNAC026, encoding a close homologue of AtXND1, a putative negative regulator of xylem development in A. thaliana (Zhao et al., 2008). This gene was represented in the Agilent chip, but due to very low signal levels in the non-senescing samples, it was filtered out during the pre-processing of the microarray data. In the qRT–PCR experiment, the expression level in non-senescing tissue was very low as well, which then gave rise to a strong relative up-regulation at the later stages of senescence.
Clusters 3, 4, and 5 all showed a clear increase in transcript levels over the developmental panel of flag leaves, however, with some noticeable differences. Cluster 3 showed a relatively strong peak towards the end of senescence development. Compared with that, cluster 5 showed an increase in expression levels at earlier senescence stages. Cluster 4 showed an intermediate pattern between cluster 3 and 5. Cluster 3 contained a number of genes encoding products involved in turnover of proteins, lipids, and carboxylic/amino acids, for example a papain-like cysteine peptidase, a thiolase, and a glutamine synthase. Four NAC genes were present in cluster 3, three of which (HvNAC013, -22, and -25) belonged to the NAC-d group (Christiansen et al., 2011). Clusters 4 and 5 differed mainly in the amplitude of transcript accumulation. Whereas cluster 5 showed around a 4-fold (log2) difference between the early and late stages of senescence, cluster 4 only showed an ~2-fold (log2) difference. Cluster 5 also showed a tendency to level off towards the later stages of senescence. This cluster contained nine of the up-regulated NAC genes, and, noticeably, five of these (HvNAC005, -23, -27, -29, and -30) belong to the same phylogenetic group, the NAC-a group (Christiansen et al., 2011).
NACBSs in barley gene promoters
Several studies of the DNA binding activity of NAC domain proteins have determined a number of DNA sequence motifs (NACBSs) with high affinity for binding to NAC domains. They all contain a CGT core sequence with varying number of surrounding consensus nucleotides, which is thought to facilitate binding of the NAC transcription factors of promoter elements (see Table 3 for references). Hence, the core CGT sequence appears important for the DNA binding of many NAC transcription factors. However, with such a short motif, it is difficult to determine target genes based on promoter sequence analysis, and, when looking outside the core sequence, the consensus is not strong, making it difficult even with longer motifs to determine target genes. From the published consensus sequences, there are, however, in addition to the occurrence of the CGT core sequence, two other striking features: (i) the core sequence seems to occur in repeats, either direct or, most often, inverted repeats (palindromes), with varying number of spanning nucleotides; and (ii) in several cases the core sequence is preceded by T in the –2 and/or –3 positions. Preliminary data are available from studies on barley NAC domain proteins (HvNAC005 and -013; Søren Lindemose, personal communication), indicating that the consensus NACBSs in barley obey similar rules.
Table 3.
DNA-binding motifs for NAC domain proteins with the CGT sequence as an essential core of the NAC-binding sequence (NACBS)
| Reference | Plant species | Methodology | NAC protein | Suggested NACBS |
|---|---|---|---|---|
| Xie et al. (2002) | A. thaliana | Test of putative NACBS from 35S promoter | NAC1 (ANAC021) | CTGACGTAAGGGATGACGCAC |
| Olsen et al. (2005) | A. thaliana | CASTing to select binding oligonulceotides | ANAC019 ANAC092 |
[TA][TG][TAGC]CGT[GA] T[TAG][GA]CGT[GA][TCA][TAG] |
| Xue et al. (2006) | T. aestivum | CELD-fusion method | TaNAC69 | GAGATCCGT
GCACAGTACGTAACTGTTACA GAGGTGTTTAATGTTTAC ACG TCTCTAGTG |
| Zhang and Gan (2012) | A. thaliana | EMSA on SAG113 promoter sequence | AtNAP (ANAC029) | CACGTAAGT |
| Balazadeh et al. (2011) | A. thaliana | CELD-fusion method | ORS1 (ANAC059) | [AG]CGT[AG] (4–5n)[AG][CT]ACGCAA |
| Wu et al. (2012) | A. thaliana | CELD-fusion method | JUB1 (ANAC042) | TGCCGT(7n)ACG
TGCCGT(7n)CCGC |
| Welner et al. (2012) | A. thaliana | DNase I footprinting | TTGCGTG(6n)CACGCAA |
In order to investigate the possible regulation of SAGs by NAC transcription factors in barley, seven model NACBS motifs (NACBS-1, -5, -6, -7, -8, -9, and -10; see Supplementary Table S4 available at JXB online for details) were designed based on the information in Table 3, with one repeat of the core sequence as a non-variable part, and with a 5–10 nucleotide span to an inverted repeat. Minor weights were given to less conserved nucleotides outside the core, based on the suggested NACBSs in Table 3. Only the NACBS-1 model did not have an inverted repeat, but instead had a strong conservation of T in the –2 and –3 positions relative to the CGT core. The model NACBS motifs were described in letter probability matrices and used in FIMO searches at http://meme.nbcr.net/meme/cgi-bin/fimo.cgi (last accessed 12 February 2014) against available promoter sequences of genes represented in the Agilent barley chip. The Agilent chip probe sequences were assigned to promoter sequences via blastN searches against the downloaded CDS sequences. This process presumably again reflected the sequence redundancy in the Agilent chip, inasmuch as only 12 643 unique promoter sequences were found for the 24 646 filtered probes from the analysis of the microarray experiment. However, the relatively low number might also reflect the provisional status of the gene models in the current barley genome sequences.
FIMO searches for the individual model motifs were performed on 2435 promoters found among the 3867 differentially up- or down-regulated genes, and on all 12 643 promoters. The results (Table 4) show that there was a significant over-representation of the model NACBSs in promoters from up-regulated genes, but not in promoters of down-regulated genes. A threshold level of P=1.0E-4 was used in the FIMO analysis (Grant et al., 2011). There were a total of 532 hits across the eight NACBS models for the up-regulated genes, but since some of them targeted the same promoter sequence, 333 non-redundant promoter sequences contained at least one of the putative NACBS motifs.
Table 4.
Occurrence of putative binding sites for NAC transcription factors (NACBSs) in the promoters of senescence-associated barley genesPromoter sequences (1000 bases upstream of ATG) were obtained from http://plants.ensembl.org/Hordeum_vulgare (last accessed 12 February 2014), and searched with the model NACBSs using the FIMO tool at http://meme.nbcr.net/meme/cgi-bin/fimo.cgi (last accessed 12 February 2014).
| Name of NACBS | Model NACBS motifa | All genes (12 643) | Down-regulated genes (1329) | Up-regulated genes (1106) |
|---|---|---|---|---|
| NACBS-1 | nttnCGTgnnn | 817 | 84NS | 108** |
| NACBS-5 | ttnCGT(n)5ACGnnn | 619 | 68 NS | 80** |
| NACBS-6 | ttnCGT(n)6ACGnnn | 511 | 68* | 56* |
| NACBS-7 | ttnCGT(n)7ACGnnn | 556 | 51NS | 69** |
| NACBS-8 | ttnCGT(n)8ACGnnn | 747 | 74NS | 93** |
| NACBS-9 | ttnCGT(n)9ACGnnn | 459 | 54NS | 64** |
| NACBS-10 | ttnCGT(n)10ACGnnn | 529 | 73** | 62** |
| Total, non-redundant | 2813 | 321NS | 333** | |
| Per 1000 genes | 222 | 242 | 301 |
Asterisks indicate statistical significance at the *P≤0.05 and**P≤0.01 level, using a hypergeometric test. NS, not significant.
aSee Supplementary Table S4 available at JXB online for details on the weight for each nucleotide base.
In silico co-expression studies
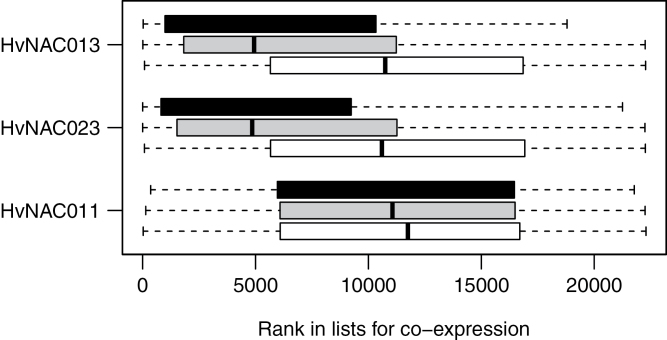
In order to validate the results on putative NACBSs amongst the up-regulated genes during senescence, the overall gene co-expression with NAC genes was investigated in the barley co-expression database available at http://coexpression.psc.riken.jp/barley/search.pl, last accessed 12 February 2014 (Mochida et al., 2011). Since this database is built on data from the Affymetrix Barley1 microarray chip, only genes represented by probes in this chip could be studied. The co-expression lists for the 32 NAC genes that were represented in the Barley1 chip (Table 1) were downloaded. The distribution of the up-regulated genes containing a putative NACBS in the lists ranked for co-expression with NAC genes were extracted. Indeed, the NACBS- containing genes overall showed tendencies of co-regulation with a number of the NAC genes, interpreted from a distribution in the co-expression lists biased towards the top (data not shown). Looking in more detail, it was evident that this distribution was most prominent for NACBS-8 with a span of eight nucleotides between the two inverted repeats of the core motif. The analysis was confined to the 71 up-regulated genes having the NACBS-8 motif in the promoter (see Supplementary Table S5 available at JXB online for details on these genes) and distributions in the co-expression lists for the 32 NAC genes were obtained as illustrated in Supplementary Fig. S3 available at JXB online. A number of NAC genes clearly showed a higher degree of general co-expression with the 71 genes having the NACBS-8 in the promoter than with up-regulated genes without any NACBS motif in the promoter. This was particularly prominent for HvNAC008, -13, -23, and -44 that were all located in clusters in Fig. 2 with considerable up-regulation during senescence. As examples, distributions of genes with or without NACBS-8 in the co-regulation lists for HvNAC013 and -23 (both with high co-regulation) and for HvNAC011 (low co-regulation) are shown in Fig. 3. It is not surprising that genes without any NACBS in the promoter also show tendencies of co-regulation with NAC genes, since indirect effects via regulatory networks and other regulatory pathways are also involved in the regulation of senescence. Furthermore, some of the NAC genes (e.g. HvNAC013) themselves contain NACBSs in their promoter, and may, hence, be part of gene activation cascades. In conclusion, the co-expression data support the notion that the occurrence of NAC-binding sites in the promoters of SAGs is of importance for the up-regulation of these genes during senescence in barley.
Fig. 3.
The distribution in co-expression lists for three NAC genes (HvNAC013, -23, and -11) of the 71 senescence-up-regulated genes having NACBS-8 in their promoter (dark grey), 1181 up-regulated genes without any NACBS (light grey), and 500 randomly selected genes (white). The lower the rank, the higher the co-expression with the indicated NAC genes (Mochida et al., 2011).
Discussion
When the microarray experiment described in this work was conceived, the barley Agilent 4×44 Barley Microarray was the platform that had the best representation of the barley gene transcriptome. During the course of the experiment and the analysis of the results, new data sources for barley genomics information have become available, in particular the release of 24 000 full-length barley cDNA sequences (Sato et al., 2009; Matsumoto et al., 2011) and the recent release of a draft barley genome (International Barley Genome Sequencing Consortium, 2012), which provide valuable new information for genomics research in barley. An attempt was made to update relevant information for the Agilent microarray content according to these new data sources, for example in relation to redundancy in the probe representation of genes and in the analysis of promoter elements.
The rationale for carrying out an analysis of the senescence process in barley, considering that a number of comprehensive studies on senescence in Arabidopsis have been performed (Buchanan-Wollaston et al., 2003,2005; Breeze et al. 2011) is that it is important to understand the senescence process in more detail in cereal plants, since this process is part of the maturation of cereal crop plants and hence of importance for the determination of crop yield and grain quality. Hence, this study adds to results from previous studies in cereals with more limited or different scopes (Gregersen and Holm., 2007; Jukanti et al., 2008).
In the analysis of the microarray data, a MapMan ‘pathway’ map for the senescence process in barley was constructed, as shown in Fig. 1, in order to place a visual structure on top of the gene expression results. The map illustrates the coordinated up-regulation of degradation processes, along with transport and regulatory components. The categories of genes emerging from the analysis are in good alignment with previous gene expression analyses of the senescence process both in dicots (Breeze et al., 2011) and in monocots (Gregersen and Holm, 2007; Jukanti et al., 2008). This comprised categories with a large number of genes involved in protein degradation, in particular the categories of autophagy, cysteine proteases, and ubiquitin-related protein turnover. There was, however, only a partial overlap between the present results and those of Jukanti et al. (2008) when looking at the detailed probe level. Of the 3867 differentially regulated genes in the present study, only ~700 were found among the 2927 genes listed in the comparison between leaves of a stay-green barley cultivar and an early senescing cultivar in the work of Jukanti et al. (2008). This discrepancy presumably reflects different experimental set-ups, in addition to considerable noise in the analysis of microarray data for both studies.
The inter-related up-regulation of amino acid and lipid metabolism seems central to the senescence process, and here peroxisomal processes appear highly involved. In the end, this complex of up-regulated processes is believed to work as a way of rescuing nitrogen released during protein degradation for translocation in the form of amino acids to other plant parts, in particular to the developing grain (Feller et al., 1994). In the peroxisome, a glyoxylate cycle-like process going from isocitrate to succinate and malate appears to be up-regulated, with the isocitrate lyase gene being the strongest up-regulated gene in the present study. The supplementary qRT–PCR experiment with malate synthase also showed the encoding gene to be strongly up-regulated. However, during senescence the glyoxylate pathway is probably not—as in a textbook description of it—connected to gluconeogenesis. More probably, it is part of anaplerotic pathways that, through the actions of the up-regulated amino acid transferases, could convert products from the fatty acid breakdown via malate/pyruvate into carbon skeletons (Brown et al., 2010). These can be utilized in the re-assimilation of nitrogen via the glutamine synthetase reaction. This latter reaction stands as the central hub for the presumed translocation of nitrogen from senescing cereal leaves (Kichey et al., 2005), and accordingly the two probes representing cytosolic glutamine synthetase (GS1) showed clear up-regulation. In contrast, three probes showed strong down-regulation of the chloroplastic GS2 gene. There was no indication of up-regulation of the mitochondrial TCA cycle for the generation of NADP(H) reducing agents or for anaplerotic purposes. The possible needs for these agents for the cells were presumably met via the massive up-regulation of lipid and protein degradation processes. Mitochondrial functions that appeared to be up-regulated were mainly related to the electron transport chain, reflecting possible needs for ATP generation or for channelling of energy into dissipating processes, for example via the alternative oxidase.
At the regulatory level, the up-regulation of a number of genes encoding transcription factors was evident, involving genes encoding NAC, bZIP, MYB, bHLH, AP2/EREBP, and CCAAT transcription factors. In their comprehensive gene expression time course study, Breeze et al. (2011) showed these same families to contain the most strongly up-regulated transcription factor genes during senescence in Arabidopsis. Hence, there seems to be a conservation of the senescence process even at the complex regulatory level across the plant kingdom. The family of NAC transcription factors increasingly stands out as a key regulatory family for the senescence process and for abiotic stress responses (Nakashima et al., 2012). The microarray data strongly supported that genes of this gene family in barley in particular are highly associated with the senescence process. This was previously indicated by studies with other species as well, in particular in Arabidopsis (Balazadeh et al., 2008; Breeze et al., 2011), but also in wheat (Gregersen and Holm, 2007). Individual NAC genes have also been shown to regulate directly various parts of the senescence network (e.g. Guo et al., 2006; Kim et al., 2009; Balazadeh et al., 2011; Wu et al., 2012); however, the combined role of this gene family in regulating the complex senescence process has yet to be investigated in more detail. This is particularly pertinent since a high degree of redundancy in their role and functions might be expected with the occurrence of the high number of closely related genes in the genome.
In this study, the up-regulation of NAC genes during senescence was confined to a limited number of genes. Not all the NAC genes were represented in the microarray, and hence the qRT–PCR extended the number of studied barley NAC genes to 47 well-characterized genes (Christiansen et al., 2011). The qRT–PCR experiment (and results from Christiansen et al., 2011) confirmed the microarray results on differential regulation during senescence of seven out of 10 NAC genes. Furthermore, the qRT–PCR experiment (supported by unpublished data) showed up-regulation of an additional 8–10 NAC genes. Hence, in order to summarize the findings on NAC genes and regulation of senescence from this work, ~15 genes that are up-regulated during senescence can be listed, with some of the genes having strong and reproducible expression patterns whereas others are more variable across experiments. Based in particular on their different expression patterns, they can be divided into three groups: (1) HvNAC026 stands out on its own as a very strongly up-regulated gene with almost no expression in non-senescing leaves. The most closely related gene in Arabidopsis, AtXND1, is not among the SAGs selected by Breeze et al. (2011); however, in the gene expression atlas presented by Schmid et al. (2005), AtXND1 is clearly up-regulated in senescing Arabidopsis leaves. Nevertheless, the present report is the first one to place a focus on this gene as senescence associated. (2) A group of genes with a relatively early up-regulation that tend to level off towards the later stages of senescence, exemplified by the closely related NAC-a genes HvNAC005, -23, -27, -29, and -30. (3) Genes up-regulated during the late burst of degradation processes in the leaf tissues. This is exemplified by the NAC-d genes HvNAC013, -22, and -25. Further studies are needed in order to fine-tune this classification. However, a preliminary hypothesis would be that the group 2 genes, with their early induction, are involved in signalling processes, inasmuch as this group of genes seems to be associated with abscisic acid (ABA) processes. Most of them are up-regulated following ABA treatment (Christiansen et al., 2011) and the closest homologue of HvNAC005 and -23 in Arabidopsis is the AtNAP gene, a known regulator of ABA signalling (Zhang et al., 2012). The group 3 genes might be involved in regulation of the final execution of the degradation process by direct regulation of genes encoding degradation enzymes. Evidence supporting this is that one of the strongest co-regulated SAG genes with the group 3 NAC genes, the cysteine protease gene AK358908, does indeed have an NACBS in its promoter (data not shown). Exactly which NAC transcription factor binds to this site still needs experimental testing.
One of the ways to study the direct regulation of target genes of transcription factors is to screen for the putative binding sites in promoter sequences of possible target genes. Thus, Breeze et al. (2011) suggested the NAC transcription factors to play a key role in senescence regulation, based on the discovery of an over-representation of putative NACBSs in the promoters of certain clusters of SAGs. An attempt was made to use a similar approach in this study in barley, for which the recently published draft genome (International Barley Genome Sequencing Consortium, 2012) provides new opportunities to perform genome-wide studies, even though the gene models are still preliminary. The analysis of NACBSs was extended to the more complex situation of palindromic occurrences of NACBSs. The difficulty in searching for NACBSs in promoter sequences on the basis of consensus sequences (see Table 3) is mainly due to the rather short CGT core sequence with only weak conservation of the surrounding sequences. In order to address this, seven models of palindromic sequences for the core sequences were established (Table 4; Supplementary Table S4 available at JXB online) with varying span widths and with varying weights on nucleotides in surrounding sequences based on the suggested NACBSs in Table 3. An over-representation of these model NACBSs could indeed be detected in 333 out of 1106 available promoter sequences of up-regulated genes during senescence, suggesting that they play a role in the regulation.
In order to substantiate the role of the putative NACBS for regulation by NAC transcription factors, the general co-expression of SAGs having an NACBS in their promoter sequence with the NAC genes was investigated. The analysis clearly showed that 71 up-regulated SAGs that had an NACBS in the promoter, compared with those that did not, exhibited strong general co-expression with senescence-associated NAC genes. Hence, not only during senescence, but in general, the occurrence of the NACBSs seems to favour co-expression of SAGs with some of the NAC transcription factor genes, which implies the importance of the NACBSs for the regulation of target genes by the NAC transcription factors. The 71 up-regulated SAGs (Supplementary Table S4 available at JXB online) constitute a list of possible target genes, for which further results from testing of direct interaction between promoter elements and NAC transcription factors are pending.
In conclusion, considering the large degree of conservation observed between the well-characterized Arabidopsis senescence processes and the results from barley presented here, the visual senescence pathway map and expression data are likely to be representative for other cereals as well. Furthermore, additional evidence of the importance of not only single NAC transcription factors but multiple members of the family for a concerted regulation of the complex senescence processes was provided. A list of up to15 barley NAC genes that are particularly interesting for this regulation process is presented. Finally, a list of 71 novel putative target genes of the NAC transcription factors is presented.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Distribution of 3867 differentially expressed probes from the Agilent microarray experiment across the 35 top-bins of the MapMan annotations.
Figure S2. Correlation of gene expression levels in the Agilent microarray experiment and in qRT–PCR experiments.
Figure S3. Distributions in co-expression lists for 32 NAC genes.
Table S1. Result table after Limma analysis of microarray data.
Table S2. Annotations of genes in the MapMan top-bins of Supplementary Fig. S1.
Table S3. Annotations of genes in MapMan pathway of Supplementary Fig. 1.
Table S4. Letter probability matrices used for FIMO analysis.
Table S5. List of 71 putative target genes of NAC transcription factors.
Table S6. List of primers used in qRT–PCR experiments
Acknowledgements
This work was funded by the Danish Research Council for Technology and Production Sciences.
Glossary
Abbreviations:
- CDS
coding sequence
- Ct
threshold cycle
- NAC
NAM, ATAF1, CUC
- NACBS
NAC binding sequence
- qRT–PCR
quantitative reverse transcription–PCR
- SAG
senescence-associated gene.
References
- Balazadeh S, Kwasniewski M, Caldana C, Mehrnia M, Zanor MaIs, Xue GP, Mueller-Roeber B. 2011. ORS1, an H2O2-responsive nac transcription factor, controls senescence in Arabidopsis thaliana. Molecular Plant 4, 346–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe H, Kolukisaoglu U. 2003. Genetic manipulation of glycine decarboxylation. Journal of Experimental Botany 54, 1523–1535 [DOI] [PubMed] [Google Scholar]
- Breeze E, Harrison E, McHattie S, et al. 2011. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. The Plant Cell 23, 873–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJ, Palmer BG, Stanley S, et al. 2010. C4 acid decarboxylases required for C4 photosynthesis are active in the mid-vein of the C3 species Arabidopsis thaliana, and are important in sugar and amino acid metabolism. The Plant Journal 61, 122–133 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D. 2003. The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnology Journal 1, 3–22 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, et al. 2005. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. The Plant Journal 42, 567–585 [DOI] [PubMed] [Google Scholar]
- Christiansen M, Holm P, Gregersen P. 2011. Characterization of barley (Hordeum vulgare L.) NAC transcription factors suggests conserved functions compared to both monocots and dicots. BMC Research Notes 4, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvinge H, Bertone P. 2009. HTqPCR: high-throughput analysis and visualization of quantitative real-time PCR data in R. Bioinformatics 25, 3325–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccioli P, Ciceri G, Provero P, Stanca A, Morcia C, Terzi V. 2007. A combined strategy of in silico transcriptome analysis and web search engine optimization allows an agile identification of reference genes suitable for normalization in gene expression studies. Plant Molecular Biology 63, 679–688 [DOI] [PubMed] [Google Scholar]
- Feller U, Fischer A. 1994. Nitrogen-metabolism in senescing leaves. Critical Reviews in Plant Sciences 13, 241–273 [Google Scholar]
- Grant CE, Bailey TL, Noble WS. 2011. FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen PL. 2011. Senescence and nutrient remobilization in crop plants. In: Hawkesford MJ, Barraclough PB, eds. The molecular and physiological basis of nutrient use efficiency in crops. Wiley-Blackwell, 83–102 [Google Scholar]
- Gregersen PL, Holm PB. 2007. Transcriptome analysis of senescence in the flag leaf of wheat (Triticum aestivum L.). Plant Biotechnology Journal 5, 192–206 [DOI] [PubMed] [Google Scholar]
- Gregersen PL, Holm PB, Krupinska K. 2008. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biology 10, 37–49 [DOI] [PubMed] [Google Scholar]
- Guo YF, Gan SS. 2006. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. The Plant Journal 46, 601–612 [DOI] [PubMed] [Google Scholar]
- International Barley Genome Sequencing Consortium. 2012. A physical, genetic and functional sequence assembly of the barley genome. Nature 491, 711–716 [DOI] [PubMed] [Google Scholar]
- Jukanti AK, Heidlebaugh NM, Parrott DL, Fischer IA, McInnerney K, Fischer AM. 2008. Comparative transcriptome profiling of near-isogenic barley (Hordeum vulgare) lines differing in the allelic state of a major grain protein content locus identifies genes with possible roles in leaf senescence and nitrogen reallocation. New Phytologist 177, 333–349 [DOI] [PubMed] [Google Scholar]
- Kichey T, Le Gouis J, Sangwan B, Hirel B, Dubois F. 2005. Changes in the cellular and subcellular localization of glutamine synthetase and glutamate dehydrogenase during flag leaf senescence in wheat (Triticum aestivum L.). Plant and Cell Physiology 46, 964–974 [DOI] [PubMed] [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG. 2009. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323, 1053–1057 [DOI] [PubMed] [Google Scholar]
- Kohl M. 2007. Bioconductor-package SLqPCR: functions for analysis of real-time quantitative PCR data at SIRS-Lab GmbH.
- Krupinska K, Biswal U, Biswal B. 2013. The dynamic role of chloroplasts in integrating plant growth and development. In: Biswal B, Krupinska K, Biswal UC, eds. Plastid development in leaves during growth and senescence. Dordrecht, The Netherlands: Springer, 3–16 [Google Scholar]
- Lee S, Seo PJ, Lee HJ, Park CM. 2012. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. The Plant Journal 70, 831–844 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids—pigments of photosynthetic biomembranes. Methods in Enzymology 148, 350–382 [Google Scholar]
- Lohman KN, Gan S, John MC, Amasino RM. 1994. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiologia Plantarum 92, 322–328 [Google Scholar]
- Lopez-Romero P. 2011. Agi4×44PreProcess: preprocessing of Agilent 4×44 array data. R package version 1.14.0.
- Matsumoto T, Tanaka T, Sakai H, et al. 2011. Comprehensive sequence analysis of 24,783 barley full-length cDNAs derived from 12 clone libraries. Plant Physiology 156, 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K, Uehara-Yamaguchi Y, Yoshida T, Sakurai T, Shinozaki K. 2011. Global landscape of a co-expressed gene network in barley and its application to gene discovery in Triticeae crops. Plant and Cell Physiology 52, 785–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. 2012. NAC transcription factors in plant abiotic stress responses. Biochimica et Biophysica Acta 1819, 97–103 [DOI] [PubMed] [Google Scholar]
- Noodén LD. 1988. Whole plant senescence. In: Noodén LD, Leopold AC, eds. Senescence and aging in plants. San Diego, CA: Academic Press, 392–439 [Google Scholar]
- Olsen AN, Ernst HA, Lo Leggio L, Skriver K. 2005. NAC transcription factors: structurally distinct, functionally diverse. Trends in Plant Science 10, 79–87 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2005. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org (last accessed 12 February 2014). [Google Scholar]
- Sato K, Shin I, Seki M, Shinozaki K, Yoshida H, Takeda K, Yamazaki Y, Conte M, Kohara Y. 2009. Development of 5006 full-length cDNAs in barley: a tool for accessing cereal genomics resources. DNA Research 16, 81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers JHM, Jing H-C, Hille J, Dijkwel PP. 2007. Developmental and hormonal control of leaf senescence. In: Gan S, ed. Senescence processes in plants. Oxford: Blackwell Publishing, 145–170 [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. 2005. A gene expression map of Arabidopsis thaliana development. Nature Genetics 37, 501–506 [DOI] [PubMed] [Google Scholar]
- Smyth GK. 2005. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, eds. Bioinformatics and computational biology solutions using R and Bioconductor. Springer: New York, 397–420. [Google Scholar]
- Smyth GK, Yang Y, Speed TP. 2003. Statistical issues in microarray data analysis. Methods in Molecular Biology 323, 111–136 [DOI] [PubMed] [Google Scholar]
- Uauy C, Brevis JC, Dubcovsky J. 2006. The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. Journal of Experimental Botany 57, 2785–2794 [DOI] [PubMed] [Google Scholar]
- Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M. 2009. A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, Maize. Plant, Cell and Environment 32, 1211–1229 [DOI] [PubMed] [Google Scholar]
- Welner DH, Lindemose S, Grossmann JG, Mollegaard NE, Olsen AN, Helgstrand C, Skriver K, Lo Leggio L. 2012. DNA binding by the plant-specific NAC transcription factors in crystal and solution: a firm link to WRKY and GCM transcription factors. Biochemical Journal 444, 395–404 [DOI] [PubMed] [Google Scholar]
- Wu A, Allu AD, Garapati P, et al. 2012. JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. The Plant Cell 24, 482–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Guo HS, Dallman G, Fang S, Weissman AM, Chua NH. 2002. SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 419, 167–170 [DOI] [PubMed] [Google Scholar]
- Xue G, Bower NI, McIntyre CL, Riding GA, Kazan K, Shorter R. 2006. TaNAC69 from the NAC superfamily of transcription factors is up-regulated by abiotic stresses in wheat and recognises two consensus DNA-binding sequences. Functional Plant Biology 33, 43–57 [DOI] [PubMed] [Google Scholar]
- Yang ZL, Ohlrogge JB. 2009. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and switchgrass and in Arabidopsis beta-oxidation mutants. Plant Physiology 150, 1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Gan SS. 2012. An abscisic acid–AtNAP transcription factor–SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiology 158, 961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KW, Xia XY, Zhang YY, Gan SS. 2012. An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. The Plant Journal 69, 667–678 [DOI] [PubMed] [Google Scholar]
- Zhao C, Avci U, Grant EH, Haigler CH, Beers EP. 2008. XND1, a member of the NAC domain family in Arabidopsis thaliana, negatively regulates lignocellulose synthesis and programmed cell death in xylem. The Plant Journal 53, 425–436 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang W, Liu L, Chen T, Zhou F, Lin Y. 2013. Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. BMC Plant Biology 13, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.