Abstract
A panel of novel D2 and D3 dopamine receptor selective antagonists, partial agonists and full agonists have been evaluated for the ability to attenuate L-dopa associated abnormal involuntary movements (AIMs) in 6-hydroxydopamine (6-OHDA) unilaterally lesioned male Sprague Dawley rats, which is an animal model of L-dopa-induced dyskinesia (LID). LID is often observed in patients with Parkinson’s Disease following chronic treatment with L-dopa. The intrinsic activity of these dopaminergic compounds was determined using a forskolin-dependent adenylyl cyclase inhibition assay with transfected HEK 293 cells expressing either the human D2Long or D3 dopamine receptor subtype. For the initial experiments the 5-HT1A receptor selective partial agonist buspirone was used to verify our ability to quantitate changes in total AIMs and AIMs minus locomotor scores. Two D2 dopamine receptor selective antagonists, SV156 and SV293, were evaluated and found to minimally attenuate AIM scores in these animals. Four members of our WC series of D3 dopamine receptor selective compounds of varying intrinsic activity at the D3 dopamine receptor subtype, WC 10, WC 21, WC 26 and WC 44, were also evaluated and found to attenuate AIM scores in a dose dependent manner. The in vivo efficacy of the compounds increased when they were administered simultaneously with L-dopa, as compared to when the compounds were administered 60 minutes prior to the L-dopa/benserazide. It was also found that the D3 receptor antagonist WC 10 could inhibit the involuntary movements after they had achieved maximum intensity. Unlike the D1-like dopamine receptor selective agonist SKF 81297 and the D2-like dopamine receptor agonist bromocriptine which can precipitate abnormal involuntary movements in these unilaterally lesioned animals, abnormal involuntary movements were not observed after administration of our D3 receptor selective agonist WC 44. In addition, we evaluated the effect of these four D3 dopamine receptor selective compounds for their effect on a) spontaneous locomotion and b) coordination and agility using a rotarod apparatus. We also used a cylinder test to assess the effect of L-dopa on spontaneous and independent use of each of the rat’s forelimbs in the presence or absence of test compound. The results of these studies suggest that substituted phenylpiperazine D3 dopamine receptor selective compounds are potential pharmacotherapeutic agents for the treatment of L-dopa-associated dyskinesia in patients with Parkinson’s Disease.
Keywords: Parkinson’s Disease, dyskinesia, L-dopa, dopamine receptors, D3 dopamine receptors
Introduction
Parkinson’s Disease (PD) is a progressive, neurodegenerative disease characterized by the loss of midbrain dopamine neurons of the nigrostriatal pathway resulting in resting tremor, rigidity, bradykinesia and postural instability (Deumens et al., 2002). PD is generally diagnosed after substantial (70 to 80%) loss of dopaminergic neurons in the nigrostriatal pathway has occurred. Since deficits of >50% in the nigrostriatal pathway can be accommodated without the manifestation of symptoms of PD, there must be compensatory responses by the surviving dopaminergic neurons and other striatal cells to mitigate the effects of the progressive loss of dopaminergic innervations. Compensatory responses may include changes in postsynaptic dopamine receptor density and/or sensitivity of D1-like and D2-like dopamine receptors in PD patients. Animal studies suggest that this compensatory response might include denervation supersensitivity (Lee et al., 1978).
Although L-dopa treatment is the most effective therapy for PD, a common motor side effect in humans is the progressive development of involuntary movements, known as L-dopa-induced dyskinesia (LID) (Bezard et al., 2001). Since the prevalence of LID increases with the duration of treatment, which puts early onset PD patients at increased risk.
The discovery of multiple subtypes of D1-like (D1a and D1b) and D2-like (D2, D3 and D4) dopamine receptor subtypes necessitates a re-evaluation of the role of dopamine receptor subtype selective agonists, partial agonists and antagonists as therapeutic agents for the treatment and management of neurodegenerative disorders (Hermanowicz, 2007, Luedtke and Mach 2003). Several studies have suggested a role for the dopamine D3 receptor subtype in LID (Bezard et al., 2003; Hsu et al., 2004; Guigoni et al., 2005; Bordet et al., 1997). While it is generally accepted that the highest levels of D3 dopamine receptor subtype expression is in the limbic areas, the neuroanatomical distribution of D3 receptor mRNA expression in the human brain using in situ hybridization indicates a heterogeneous expression of D3 receptor mRNA throughout the human brain (Bouthenet et al., 1991). Thus, the D3 dopamine receptor may play a role in pyramidal motor functions (Suzuki et al., 1998).
While previous research has suggested that there are differences in the neuroanatomical distribution and pharmacological properties of the D3 dopamine receptor system in rodents and primates, there are also fundamental similarities (Sánchez-Peraute et al., 2007). Therefore, efforts have been made to develop non-primate animal models of PD to a) provide insights into the possible pathological mechanisms of the disease and b) assist in the screening/development of new therapeutic strategies for the treatment of PD. Despite differences in the dopaminergic pathways of primates and rodents, rat models provide an economical model system to investigate the mechanisms that lead to Parkinsonian-like pathologies and a mode for testing new therapeutic strategies for the treatment of this disorder (Deumens et al., 2002; Metz et al., 2005). The most common experimental strategy has been the use of the catecholamine neurotoxin 6-hydroxydopamine (6-OHDA), which is transported into both dopaminergic and noradrenergic neurons leading to neurodegeneraton of the nerve terminals and cells bodies. The most commonly used model involves a unilateral lesion of the medial forebrain bundle (MFB) which results in a) the destruction of A9 and A10 cell groups, b) depletion of dopamine in the ipsilateral caudate-putamen and c) denervation supersensitivity of the postsynaptic dopamine receptors in the ipsilateral caudate-putamen. It also results in a characteristic turning behavior following the administration of amphetamine or apomorphine (Ungerstedt 1968; Ungerstedt 1976).
A model of LID has been described for the L-dopa-dependent abnormal involuntary movements (AIMs) found in rats with unilateral 6-OHDA lesions. Following chronic administration of L-dopa, rats exhibit abnormal movements and postures reminiscent of LID in primates (Schallart et al., 2000). The rat AIMs predominantly affect the side of the body contralateral to the lesion. The severity of rat AIMs can be quantified using a rating scale somewhat analogous to that used for clinical studies. L-dopa-induced axial, limb and orolingual AIM scores can be significantly reduced by the acute administration of antidyskinetic drugs that are used in PD patients and/or nonhuman primates (Carta et al., 2006; Lundblad et al., 2002). Further validation of the rat dyskinetic model as an authentic model for identifying and evaluating candidate antidyskinetic compounds has been provided by the studies of Dekundy and colleagues (2007). These studies demonstrate the potential use of this rat model for the screening and discovery of novel anti-dyskinetic agents.
Because of the high degree of homology between the binding sites of the D2 and D3 dopamine receptor subtypes, it has been difficult to develop bona fide D2 or D3 receptor subtype selective compounds (Boundy et al., 1993; Luedtke and Mach, 2003). We reported the synthesis and pharmacological characterization of a panel of 2-methoxy substituted phenyl-piperazine compounds with high affinity (nM) and > 30-fold binding selectivity at the D3 dopamine receptor compared to the D2 receptor subtype (Chu et al., 2005) with varying intrinsic activity. In addition, we recently published a description of the first panel of antagonists that bind selectively (up to 100-fold) at D2 receptors compared to the D3 receptor subtype (Vangveravong et al., 2006).
In this communication we report our finding on the ability of our D2 and D3 dopamine receptor subtype selective compounds to modulate AIMs in unilaterally lesioned rats. Our results suggest that while our D2 receptor selective antagonists did not effectively attenuate AIMs score in unilaterally lesioned rats, our novel D3 receptor selective compounds may be candidates for the development of pharmacotherapeutic agents that can be used for the treatment of human LID.
Methods and Materials
Animals
This study was performed using male Sprague–Dawley rats that were unilaterally lesioned in the MFB by injection of 6-hydroxydopamine (Charles River) as previously described (Kumar et al., 2009). Upon arrival, animals were housed under a 12 hr light:12hr dark cycle with free access to water. The animals’ food was restricted to maintain their weights at approximately 350 grams. The treatment of the animals and the experimental procedures were approved by the appropriate university Institutional Animal Care and Use Committee. Animal care and housing were in adherence with the conditions set forth in the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996).
Test drugs and treatment regimens
The evaluation of test drugs was performed as previously described (Kumar et al., 2009). Briefly, 8 mg/kg L-dopa with 8 mg/kg benserazide in sterile saline (9g NaCl/liter) was administered to each rat as a daily intraperitoneal (i.p.) injection for 21 consecutive days to induce the stable development of L-dopa-induced AIMs. The test compounds were prepared at a concentration of 10 mg/ml in either sterile saline or water containing 5% dimethylsulfoxide (DMSO: Sigma). The injection volume was generally 0.3 ml. For the rotarod experiment haloperidol was dissolved (5 mg/ml) in an aqueous solvent system that contained 25% DMSO with 0.024 N HCl.
Test compounds and buspirone were evaluated using an observer blind, randomized design, whereby half of the rats were administered test drug or vehicle followed by L-dopa (8 mg/kg combined with 8 mg/kg benserazide). Throughout the course of these studies each animal received a minimum of two doses of L-dopa/benserazide (8 mg/kg each) per week to maintain the dyskinetic-like behaviors.
AIMs ratings
AIMs ratings were quantified as described previously (Kumar et al., 2009). Briefly, AIMs scored were determined by observing the animals in their home cages until the AIMs subsided. AIMs were scored in four categories, as discussed by Dekundy and colleagues (2007): 1) axial AIMs, 2) limb AIMs, 3) orolingual AIMs and 4) locomotive AIMs. Each of the four categories were scored on a severity scale from 0 to 4.
Adenylyl cyclase whole cell assay
Whole cell cyclic AMP accumulation was measured by an adaptation of the original method of Shimizu, Daly and Creverling (1969) (Su et al., 1976; Luedtke et al., 2000). Transfected HEK-293 cells expressing either the human D2L or D3 dopamine receptor were treated with serum-free medium containing 2,8-3H-adenine (Perkin-Elmer) (37 °C for 75 minutes). The media was then replaced with serum-free media containing 0.1 mM 3-isobutyl-1-methylxanthine (Sigma) and drugs for a total of 500 μl and incubated at 37 °C for 20 min. The reaction was stopped by addition of 500 μl of 10% trichloroacetic acid and 1 mM cyclic AMP. After centrifugation, the supernatants were fractionated using Dowex AG50W-X4 and neutral alumina to separate the 3H-ATP and the 3H-cyclic AMP (Saloman et al., 1974). Individual samples were corrected for column recovery spectrophotometrically (OD 259 nm).
Rotarod Test
A rotarod test was used to assess the effect of test drug on motor performance and coordination (Lundblad et al., 2003; Cenci and Lundblad 2005; Dekundy et al., 2007). The rotarod test was performed as previously described (Kumar et al., 2009; Cenci et al., 2005) using a gradually accelerating rotarod apparatus (AccuScan Instruments Inc., Columbus, OH) set to accelerate from 0 to 40 rpms over 90 seconds. Two training sessions were performed per day. On the fourth, sixth, eighth and tenth days rats were administered test drug i.p. at a dose equal to the IC50 valued for the inhibition of AIM scores and then evaluated at 30 and 60 minutes post drug administration. On the fifth, seventh, ninth and eleventh days rats were tested after administration with a similar volume of the matched vehicle. On the twelfth day animals were administered haloperidol at a dose of 5 mg/kg and on the following day animals were administered an analogous volume of vehicle. The data from the rotarod experiments is expressed as the mean number of seconds the lesioned animals were able to remain on the rotarod before falling (latency to fall).
Spontaneous locomotor activity
Spontaneous locomotion was examined at concentrations of 3.0 and 10 mg/kg of test compounds (i.p.) using an injection volume of 1.0 ml/kg using nonlesioned animals. Different cohorts of animals were used to test each compound. Within each cohort, rats were randomly assigned to groups that received either 10 mg/kg of test compound or vehicle (n = 6 to 10). Immediately after the injection the rats were placed into Plexiglas cages (44x24x20 cm for 1 hour) equipped with two sets of photocells and light sources located 21 cm apart and 4 cm above the floor of the cage. A computer-automated system recorded the number of times the photobeams were broken consecutively by the animal movement (crossovers). After a minimum of one week, the animals that had been treated previously with vehicle now received 3 mg/kg of the test compound and those that had previously been given the test compound now received vehicle and were immediately evaluated for locomotor activity (Khroyan et al., 1998; Khroyan et al., 1999).
Cylinder Test
A cylinder test was used to assess the spontaneous and independent use of each of the rat’s forelimbs in the context of an instinctive rearing behavior, with the rat standing on its hind legs and leaning on its forelimbs on the enclosing walls (Schallert and Tillerson, 2000; Cenci et al., 2005) as previously described (Kumar et al., 2009) using an open-ended Plexiglas cylinder (21x34 cm). The animal’s performance was based on the first forelimb to make contact (left, right or both) with the Plexiglas walls. Asymmetrical forelimb usage was calculated as the percentage of total performance, over a 5 minute time interval.
Statistical Analysis
SPSS 16 software was used for statistical analysis of the AIM scores and crossovers. ANOVA was used to determine the level of significant difference between the test drug treated and vehicle treated groups. In addition, the change in crossovers from controls was calculated for each test compound dosage group by subtracting the average number of crossovers of the respective vehicle group from the number of crossovers of individual subjects in the test compound dosage groups. The latter measure is graphed to illustrate the effects of the compounds on locomotion. Main effects and interactions were analyzed using tests of simple main effects and pair-wise Newman-Keuls tests. In the figures, the data points are generally presented as the mean values ± S.E.M. that was obtained for each treatment group and data is often expressed as a normalized (to 100) value.
For the rotarod experiment an ANOVA analysis with repeated measures was performed using Systat (Systat Software, Inc., San Jose, California). A probability value of p ≤ 0.05 was the minimum value considered to indicate significance.
Results
AIMs test
We examined conditions that might influence the expression of AIMs. First, during the consecutive 21 day administration of L-dopa with benserazide it was noticed that a) all animals exhibited some form of AIMs by the fourth day of L-dopa administration and b) at some time points, after day seven and through day twenty one, any given animal might not exhibit AIMS, even though the majority of animals exhibited intense involuntary movements (data not shown). Based upon that observation, any data obtained from an animal injected with vehicle and L-dopa/benserazide that did not exhibit AIMs was eliminated from the data set. Data from any animal injected with test drug that did not exhibit any AIMs was eliminated only if that animal seemed to be unique in the data set. However, results from test drugs administered at lowest doses for the dose response curves were retained.
Second, we observed that feeding the animals prior to the administration of L-dopa/benserazide (both at 8 mg/kg) resulted in a reduction in the observed involuntary behaviors. Figure 1 shows a scatter gram comparison of the total AIMs score for rats that had received rat chow the previous day (hungry animals) and for the same animals that were fed with approximately 30–40 grams of rat chow one hour prior to L-dopa administration (fed animals). The mean total AIMs score for the fed animals was 7.8 ± 4.3, while the mean total AIMs score for the hungry animals was 43.9 ± 1.6 (n = 9). Based upon those results, we initiated experiments in the morning and animals were fed in the afternoon of the previous day (always after L-dopa administration).
Figure 1. Comparison of AIM Scores Before and Sixty Minutes After Feeding Animals.
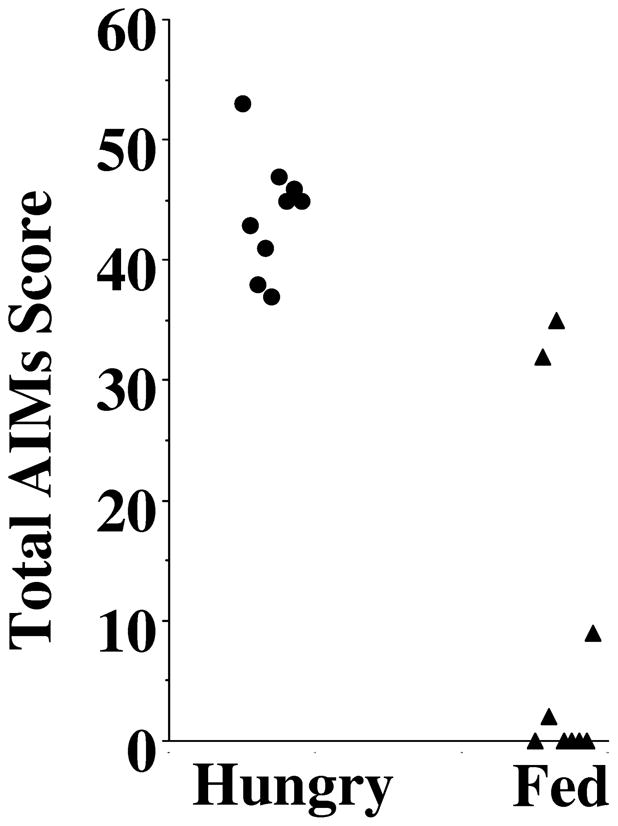
This figure shows a comparison of the effect feeding on the AIM scores of unilaterally lesioned rats administered L-dopa. Animals were administered a mixture of L-dopa (8 mg/kg) and benserazide (8 mg/kg) in sterile saline (9 g/L NaCl in water) by i.p. injection. Rats were either fed approximately 24 hours prior to L-dopa administration (Hungry, ●) or fed (approximately 30–40 grams of rat chow pellets) 1 hour prior to L-dopa administration (Fed, ▲).
We selected a dosing regimen of 10 mg/kg injected i.p. 60 minutes prior to L-dopa/benserazide administration for our preliminary studies for comparing the ability of our D2 and D3 dopamine receptor selective compounds to attenuate the involuntary movements in our unilaterally lesioned animals (Kumar et al., 2009).
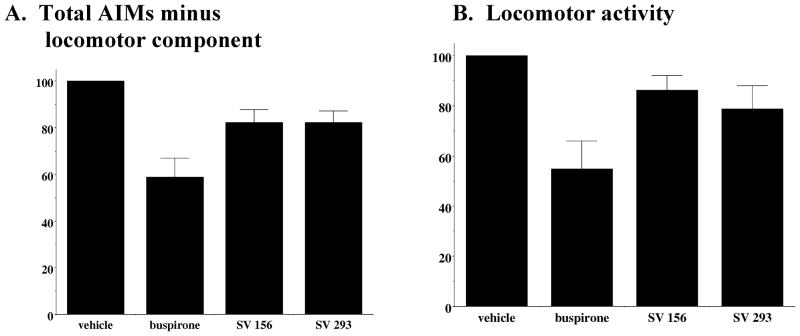
Dekundy and colleagues (2007) reported that for unilaterally lesioned dyskinetic rats the administration of buspirone (4 mg/kg) i.p. 30 minutes prior to L-dopa/benserazide (6 mg/kg and 12–15 mg/kg) led to an 83% decrease in axial, limb and orolingual AIM scores, without a decrease in L-dopa induced locomotor scores. For the animals used for this set of experiments we found that administration of buspirone (4 mg/kg) by i.p. injection 60 minutes prior to L-dopa/benserazide (8 mg/kg each) resulted in a 42.1 ± 8.7% reduction in the total AIM scores and a 41.1 ± 8.1% reduction in AIM scores minus the locomotor component. We also administered buspirone (4 mg/kg) by i.p. injection 30 minutes prior to L-dopa/benserazide (8 mg/kg each) and found that it resulted in a 52.1 ± 28.9% reduction in the total AIMs score and a 53.1 ± 6.9% reduction in AIMs score minus the locomotor component. Therefore, although we confirmed that buspirone could attenuate AIM scores, we were not able to achieve the magnitude of attenuation previously reported (Figure 3).
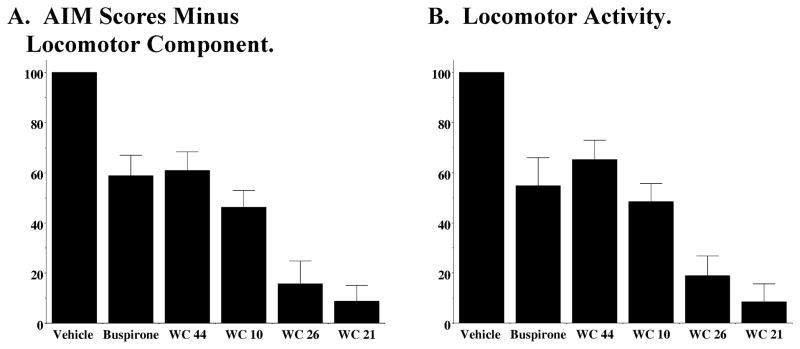
Figure 3. Effect of D2 Dopamine Receptor Subtype Selective Antagonists on L-Dopa-Dependent Abnormal Involuntary Movements.
The effect of buspirone (4 mg/kg) and the novel D2 dopamine receptor selective antagonists SV 293 and SV 156 (10 mg/kg) on (A) AIMs minus locomotor score and (B) locomotor score is shown. Each of the test drugs were administered i.p. 60 minutes prior to L-dopa administration. For this experiment L-dopa and benserazide were used at a dose of 8 mg/kg each. Values are the normalized mean scores ± S.E.M. (n ≥ 8). For buspirone the significance level for all three values is p ≤ 0.002. For the SV compounds the significance level is p > 0.01 for the total AIMs score, p > 0.01 for AIMs minus locomotor activity and p > 0.07 for the locomotor score. Data is presented as the summation of the total AIM scores for eight (n = 8) animals as a function of time.
It has been suggested that locomotive AIMs may not provide a specific measure of dyskinesia. Therefore, for this communication we provide our initial data for each drug as the a) AIMs score minus the locomotor component and b) locomotor component for our compounds. However, in general the magnitude of attenuation of scores by test drugs and the overall shape of our temporal plots were essentially identical if we plotted total AIMs or AIMs minus locomotor scores.
D2 dopamine receptor selective compounds
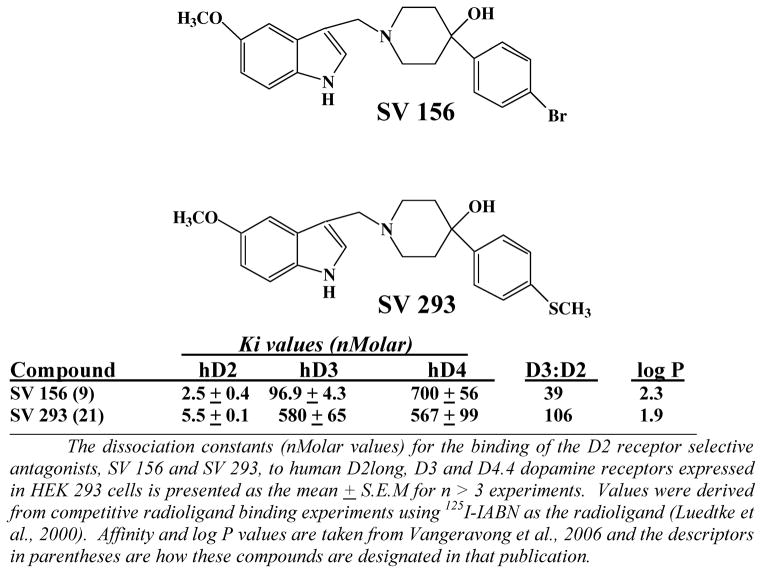
Figure 2 shows the structure and pharmacological profile at D2-like dopamine receptors of two of our novel D2 receptor selective antagonists, SV 156 and SV 293. We have evaluated several examples of this class of compounds, including SV 293, and none of them exhibit any intrinsic activity (agonist, partial agonist or inverse agonist activity) at D2 receptors. Therefore, we classify them as neutral antagonists at D2 receptors. SV 156 has approximately 40-fold binding selectivity for D2 dopamine receptors compared to the D3 receptor subtype, while SV 293 is approximately 100-fold selective. These compounds a) bind with low affinity to human D4 dopamine receptors and b) have log P values < 2.5, which indicates that they likely readily cross the blood brain barrier (Vangveravong et al., 2006).
Figure 2. Structure and Pharmacological Profile of SV Series D2 Receptor Subtype Selective Compounds.
The dissociation constants (nMolar values) for the binding of the D2 receptor selective antagonists, SV 156 and SV 293, to human D2long, D3 and D4.4 dopamine receptors expressed in HEK 293 cells is presented as the mean ± S.E.M. for n > 3 experiments. Values were derived from competitive radioligand binding experiments using 125I-IABN as the radioligand. Affinity and log P values are taken from Vangeravong et al., 2006 and the descriptors in parentheses are how these compounds are designated in that publication.
Figure 3 shows a comparison of the effect of SV 156 and SV 293 (10 mg/kg) to buspirone (4 mg/kg, 60 minute pre-administration) on L-dopa-dependent a) AIMs score minus the locomotor component (Figure 3A) and b) locomotor component (Figure 3B). Our D2 receptor selective compounds showed only a marginal ability to attenuate the L-dopa-dependent AIMs in our lesioned animals (15% attenuation). At this dose of SV 293 some of the animals exhibited neuroleptic-like effects, in so much as animals were less mobile than their vehicle control counterparts, while able to respond to external stimuli (moving or tapping on the plastic cage). Some, but not all, of the animals exhibited a flat posture-like behavior. We noticed that these adverse effects appeared maximally at 20 to 40 minutes after the administration of the test drug. Based upon these results we did not feel that our novel D2 selective antagonists represented viable candidates for further evaluation as candidate anti-dyskinetic compounds.
D3 dopamine receptor selective compounds
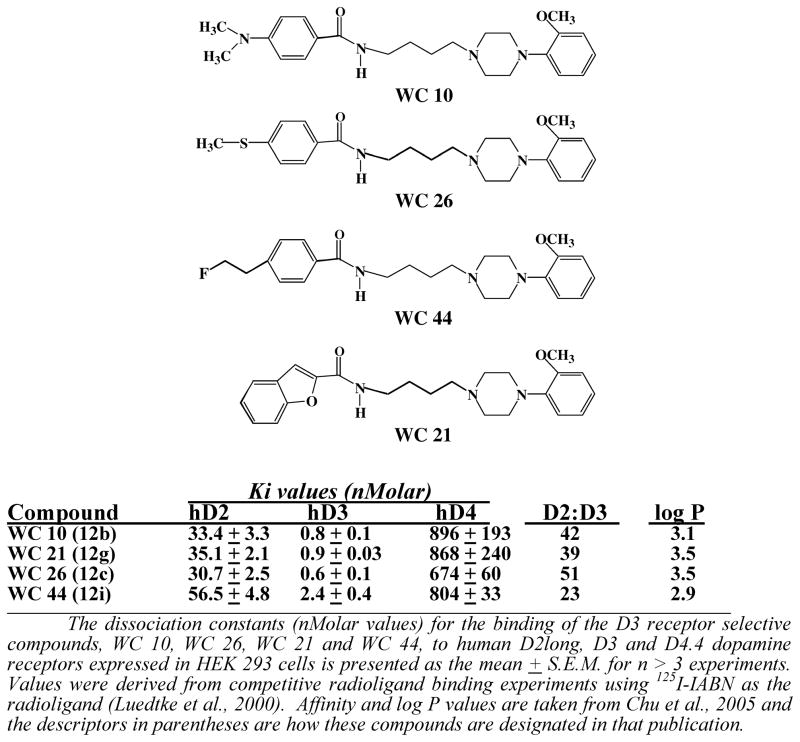
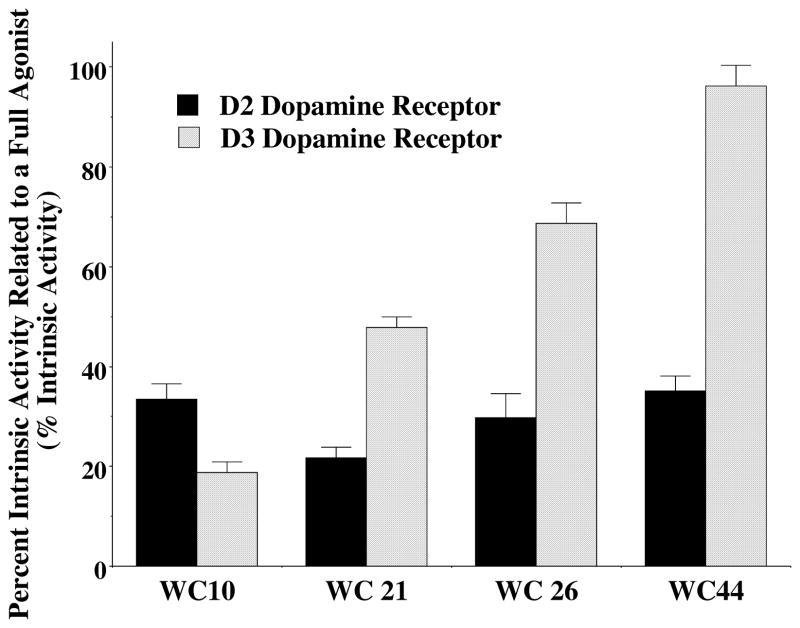
The structure and affinity profile of four of our D3 receptor selective compounds of varying intrinsic activity is shown in Figure 4 (Chu et al., 2005). These compounds exhibit >20-fold binding selectivity for the D3 dopamine receptor compared to the D2 receptor subtype. The intrinsic activity of the compounds at human D2 and D3 dopamine receptors using an adenylyl cyclase inhibition assay with stably transfected HEK 293 cells is shown in Figure 5. The intrinsic activity was measure relative to the full D2-like receptor agonist quinpirole. The ability of these compounds to influence the expression of L-dopa dependent AIMs is shown in Figure 6 using 10 mg/kg of compound administered i.p. 60 minutes prior to the administration of L-dopa. The following is a summary of our observations:
Figure 4. Structure and Pharmacological Profile of the WC Series D3 Receptor Subtype Selective Compounds.
The dissociation constants (nMolar values) for the binding of the D3 receptor selective compounds, WC 10, WC 21, WC 26 and WC 44, to human D2long, D3 and D4.4 dopamine receptors expressed in HEK 293 cells is presented as the mean ± S.E.M. for n ≥ 3 experiments. Values were derived from competitive radioligand binding experiments using 125I-IABN as the radioligand (Luedtke et al., 2000). Affinity and log P values are taken from Chu et al., 2005 and the descriptors in parentheses are how these compounds are designated in that publication.
Figure 5. Intrinsic Activity of D3 Receptor Selective Compounds Using an Inhibition of Forskolin-Dependent Stimulation Adenylyl Cyclase Assay.
The intrinsic activity of the D3 dopamine receptor compounds at both human D2 (solid black) and D3 (hatched) receptors is shown as the mean values ± S.E.M (n ≥ 3). Values were obtained using stably transfected HEK 293 expressing either the human D2 or D3 dopamine receptor subtype. All values were compared to the observed effect using the D2-like dopamine receptor full agonist quinpirole at a concentration ≥ 10x the Ki value obtained using radioligand binding studies. A whole cell adenylyl cyclase assay was used in which quinpirole and the tested compounds were evaluated for the ability to inhibit forskolin-dependent stimulation of adenylyl cyclase activity.
Figure 6. Effect of D3 Dopamine Receptor Subtype Selective Compounds on L-Dopa-Dependent Involuntary Movements with a 60 Minute Pretreatment Time.
The effect of buspirone (4 mg/kg) and the novel D3 dopamine receptor selective compounds, WC 10, WC 21, WC 26 and WC 44 (10 mg/kg) on (A) AIMs minus locomotor score and (B) locomotor score is shown. Each of the test drugs were administered i.p. 60 minutes prior to L-dopa/benserazide (8 mg/kg each) administration. Values are the normalized mean scores ± S.E.M. The number of independent experiments used to calculate the mean values is n > 8 for each compound except WC 21 (n = 5). For WC 44 the significance level for the total AIMs and the AIMs minus locomotor activity is p ≤ 0.001 and for the locomotor activity it is p < 0.01. For WC 10, WC 21 and WC 26 the significance level for each of the scores is p < 0.0001.
WC 10
WC 10 exhibits higher intrinsic activity at D2 receptors (33.3 ± 4.5% of maximum stimulation) compared to D3 receptors (19.5 ± 2.4%). We classify this compound as an antagonist/weak partial agonist at the D3 dopamine receptor subtype. It is approximately 40-fold selective for the binding of D3 receptors compared to the D2 receptor subtype (Figure 4). We find a 50% reduction in the AIMs and locomotor scores (Figure 6). No abnormal behavior was observed in the test animals during the 60 minute pretreatment period.
WC 44
We classify WC 44 as a full agonist at D3 receptors and a partial agonist at D2 receptors in our adenylyl cyclase inhibition assays. WC 44 is 23-fold selective for the binding of D3 receptors compared to the D2 receptor subtype (Figure 4). We find a 40% reduction in the AIMs and locomotor scores (Figure 6). It is worth noting that the administration of this full agonist at D3 dopamine receptors did not trigger any involuntary AIM-like movements in the test animals during the 60 minute pretreatment period.
WC 26
We classify WC 26 as a partial agonist (68.7 ± 4.1% of maximum stimulation) at D3 receptors and a partial agonist at D2 receptors (29.8 ± 6.8%) for adenylyl cyclase inhibition (Figure 5). It is approximately 50-fold selective for the binding of D3 receptors compared to the D2 receptor subtype and exhibits an 80% reduction in the AIM scores. It also attenuates the locomotor scores by approximately 80% (Figure 6). Several of the animals exhibited neuroleptic-like effects that we observed for the D2 receptor selective compounds, which included reduced mobility. Several of the animals also exhibited a slit eye (ptosis) appearance, in contrast to the roundness observed in the control animals. However, animals remained conscious and responded to our tapping on the side of the plastic cage. WC 26 did not trigger any involuntary AIMs-like movements.
WC 21
We classify WC 21 as a partial agonist at D3 receptors (47.2 ± 5.6% of maximum stimulation) and a weak partial agonist at D2 receptors (21.8 ± 2.9%) for adenylyl cyclase inhibition (Figure 5). It is 40-fold selective for the binding of D3 receptors compared to the D2 receptor subtype. We found this compound exhibits the greatest attenuation of the AIM scores (approximately 90% reduction), however, it also attenuates the locomotor scores (Figure 6). WC 21 altered mobility and several of the animals also exhibited ptosis. These effects appeared to be more intense than those observed for WC 26 at the same dosage (10 mg/kg). WC 21 did not trigger involuntary AIMs-like movements in the test animals.
The effect of these four D3 receptor selective compounds is shown using a dose of 10 mg/kg with a 60 minute pretreatment on L-dopa dependent total AIM scores minus the locomotor component (Figure 6A) and locomotion (Figure 6B). No preferential attenuation of the AIM components (forelimb movement, torso twisting, orolingual or locomotion) by the four different D3 receptor selective compounds was observed.
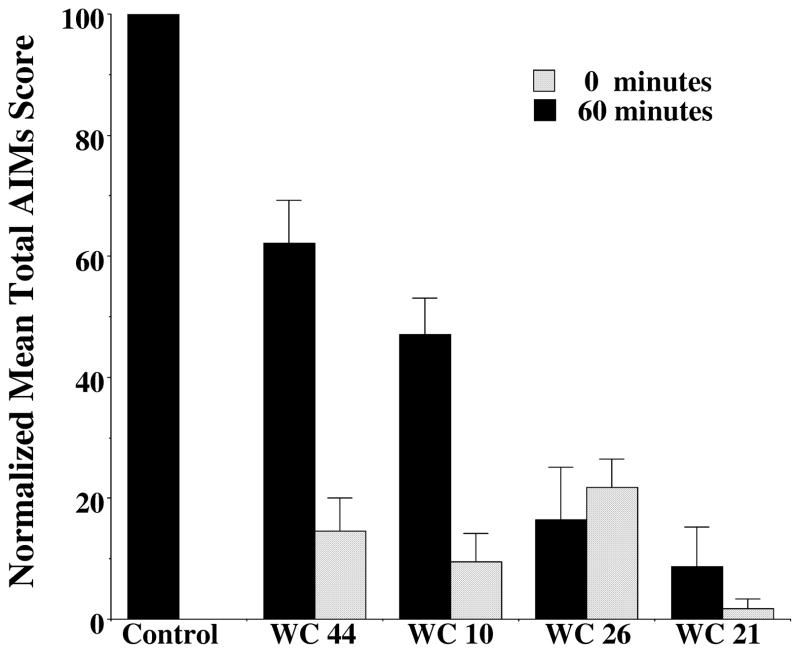
The magnitude of the AIMs score attenuation increased if test drug was administered simultaneously with the L-dopa, compared to administering test drug 60 minutes prior to L-dopa administration (Figure 7) for all compounds except WC 26. Therefore, subsequent dose response curves were carried out using a 0 minute pretreatment regimen.
Figure 7. Comparison of the Effect of Zero and Sixty Minute Pretreatment Time for WC 44, WC 10, WC 26 and WC 21 on the Attenuation of AIM Scores.
The effect of novel D3 dopamine receptor selective compounds, WC 44, WC 10, WC 26 and WC 21 (10 mg/kg, i.p.) on total AIMs score is shown. Each of the test drugs were administered i.p. at either 60 minutes prior (solid bars) or simultaneously (zero minutes: hatched bars) to L-dopa/benserazide i.p. administration (8 mg/kg each). Values are the normalized mean scores ± S.E.M relative to vehicle controls (Control) normalized to 100. The number of independent experiments used to calculate the mean values is n > 8 for each condition except for WC 26 at 60 minutes (n = 5). There is a significance level between the vehicle control values and the 60 minute pretreatment is p ≤ 0.01 and the level of significance between the control values and the 0 minute pretreatment time is p ≤ 0.0001.
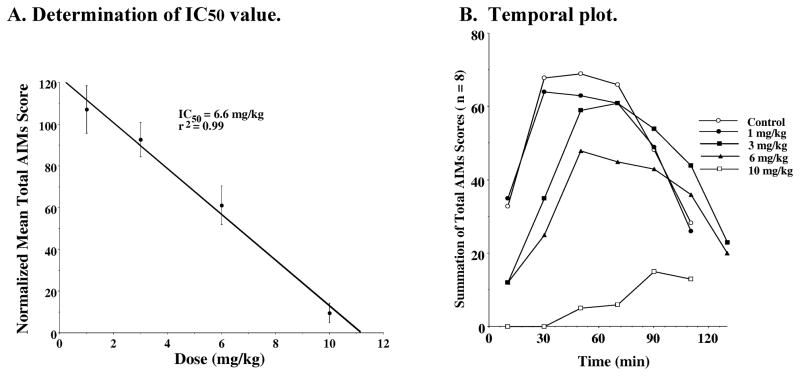
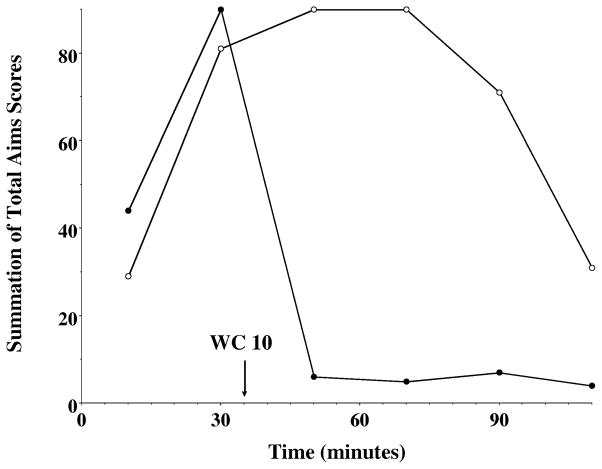
The data for the dose response curves for WC 10 fit well to a straight line with an IC50 value of 6.6 mg/kg (Figure 8A). When the data was plotted as a temporal plot we found that increasing doses of WC 10 attenuated the magnitude of the AIMs score but at doses of 3 and 6 mg/kg there appeared to be a slight increased in the duration of the AIMs (Figure 8B). We then asked whether WC 10 could stop the involuntary movements once they had reached their greatest intensity. For this experiment WC 10 was administered 35 minutes after the administration of L-dopa/benserazide. A dramatic and rapid reduction of involuntary movements was observed (Figure 9).
Figure 8. Dose Response Dependence of WC 10 for the Attenuation of Total AIM Scores.
A. Determination of IC50 value: Unilaterally lesioned rats were injected (i.p.) with varying doses of WC 10 (0 to 10 mg/kg) followed immediately with a constant dose of L-dopa and benserazide (8 mg/kg each). The percent of the normalized mean total AIMs score ± S.E.M. (n ≥ 8) relative to vehicle controls as a function of the dose of WC 10 is shown. The mean values ± S.E.M. for the normalized total AIMs values as a function of WC 10 concentrations are as follows: a) 1 mg/kg, 107 ± 11.4, b) 3.0 mg/kg, 92.6 ± 8.2, c) 6 mg/kg, 61.3 ± 9.2 and d) 10 mg/kg, 9.5 ± 4.7. A graph of the percent change in the AIMs score minus the locomotor component as a function of the dose of WC 10 is essentially identical to the one shown in this figure. The calculated IC50 value for this data was found to be 6.6 mg/kg using a linear regression analysis (r2 = 0.99). B. Temporal plot: The variation in the total AIMs score as a function of time is shown using zero time pretreatment with an i.p. injection of WC 10 at 10 mg/kg (□), 6 mg/kg (▲), 3 mg/kg (■), 1 mg/kg (●) or vehicle control (❍). Animals were also injected (i.p.) with 8 mg/kg L-dopa and benserazide. Each point is the summation of total AIMs scores for a total of 8 animals at each observation time point for each dose of WC 10. However, the temporal plot for the vehicle control curve is the mean value for all four experiments.
Figure 9. Ability of WC 10 to Reverse the L-Dopa Associated Abnormal Involuntary Movements in Unilaterally Lesioned Rats.
The temporal variation in the total AIMs score as a function of time is shown using 10 mg/kg WC 10 (●) or vehicle control (❍) thirty five minutes after l-dopa/benserazide (8 mg/kg each) administration. Each point is the summation of total AIMs score for a total of 10 animals at each observation time point. The time (minutes) denotes the amount of time after the administration of the L-dopa/benserazide and the arrow marks the time of the administration of WC 10.
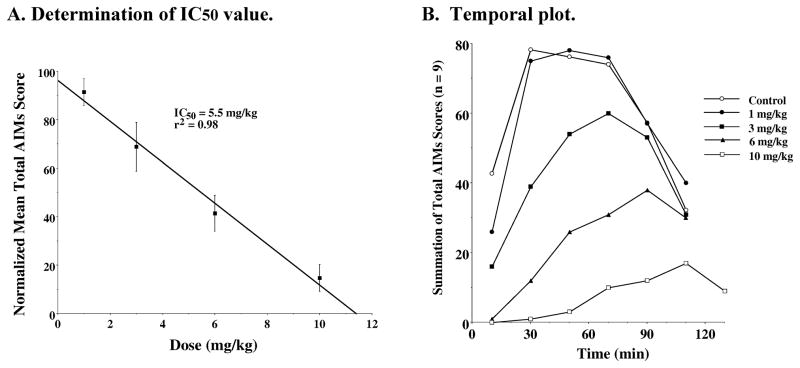
A dose response curve for the D3 receptor agonist WC 44 was performed and the data fit to a straight line (IC50 = 5.5 mg/kg) (Figure 10A). The temporal plots for WC 44 (Figure 10B) provided no evidence of a prolongation of the involuntary movements, that was observed for WC 10. Since, theoretically, an antagonist would be expected to inhibit the activity of an agonist, the in vivo effect of combining WC 10 and WC 44 was examined. When WC 10 and WC 44 were administered simultaneously an additive effect on L-dopa-dependent AIMs was observed (Figure 11).
Figure 10. Dose Response Dependence of WC 44 for the Attenuation of Total AIM scores.
A. Determination of IC50 value: Unilaterally lesioned rats were injected (i.p.) with varying doses of WC 44 (0 to 10 mg/kg) followed immediately with a constant dose of L-dopa and benserazide (8 mg/kg each). The percent of the mean total AIMs score ± S.E.M. (n ≥ 9) relative to vehicle controls as a function the dose of WC 44 is shown. The mean values ± S.E.M. for the normalized total AIMs values as a function of WC 44 concentrations are as follows: a) 1 mg/kg, 91.4 ± 5.5, b) 3.0 mg/kg, 68.8 ± 10.1, c) 6 mg/kg, 41.4 ± 7.4 and d) 10 mg/kg, 14.6 ± 5.5. The calculated IC50 value for this data was found to be 5.5 mg/kg using a linear regression analysis (r2 = 0.98). B. Temporal plot: The variation in the total AIMs score as a function of time is shown using zero time pretreatment with an i.p. injection of WC 44 at 10 mg/kg (□), 6 mg/kg (▲), 3 mg/kg (■), 1 mg/kg (●) or vehicle control (❍). Animals were also injected (i.p.) with 8 mg/kg L-dopa and benserazide. Each point is the summation of total AIMs score for a total of 9 animals at each observation time point for each dose of WC 44. The temporal plot for the vehicle control curve is the mean value for all four experiments.
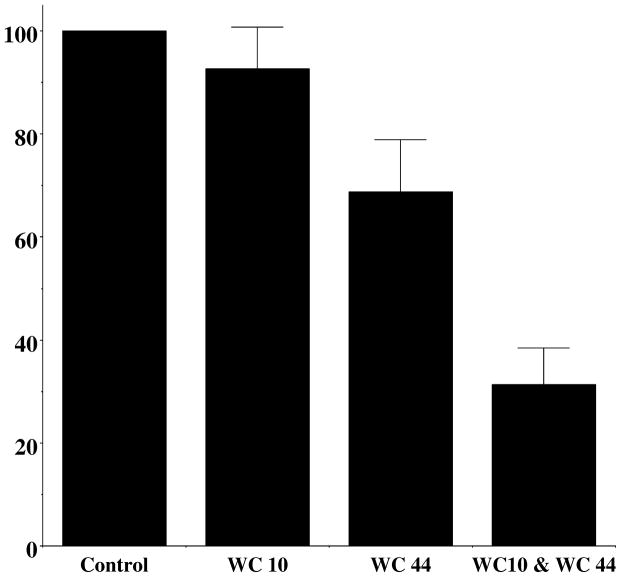
Figure 11. The Effect of Co-Administration of WC 10 and WC 44 on Total AIM Scores.
A comparison of the effect of combining WC 10 and WC 44 in the total AIMs score is shown relative to the administration of each compound alone. Each drug was administered at a dose of 3 mg/kg i.p. L-dopa/benserazide was then immediately administered at a dose of 8 mg/kg, each. The normalized AIM scores ± S.E.M. were a) WC 10, 92.6 ± 8.2 (n = 10; p = 0.6), b) WC 44, 68.8 ± 10.1 (n = 10; p = 0.02) and c) WC 10 plus WC 44, 31.4 ± 7.0 (n = 9; p ≤ 0.0001).
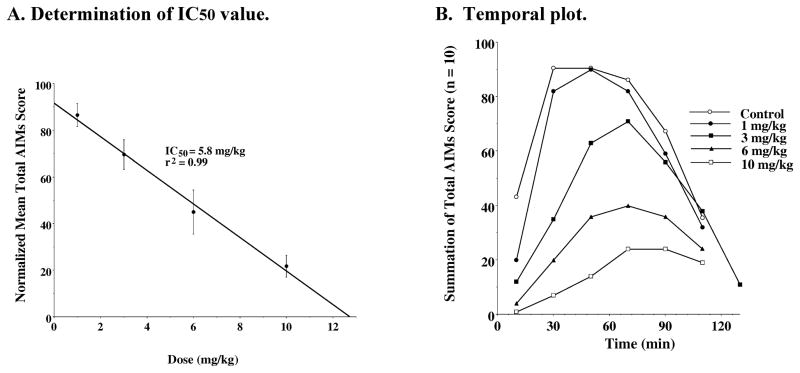
Concurrently, the in vivo activity of our partial agonists WC 21 and WC 26 were evaluated by performing dose response curves (Figure 12 and Figure 13). As with WC 10 and WC 44, the dose response data for the two partial agonists fit reasonably well to a straight line. The IC50 values for WC 21 and WC 26 were calculated to be 3.8 mg/kg and 5.8 mg/kg, respectively (Figure 12A and Figure 13A). We observed less of the neuroleptic-like side effects when WC 21 or WC 26 were administered at doses of 3 mg/kg or 6 mg/kg, compared to when these compounds were administered at a dose of 10 mg/kg (Figure 6). Although WC 21 was our most potent compound for inhibiting the AIMs, at a dose of 10 mg/kg with a 0 minute pretreatment, it decreased locomotor activity and had sedative-like properties.
Figure 12. Dose Response Dependence of WC 21 for the Attenuation of Total AIM Scores.
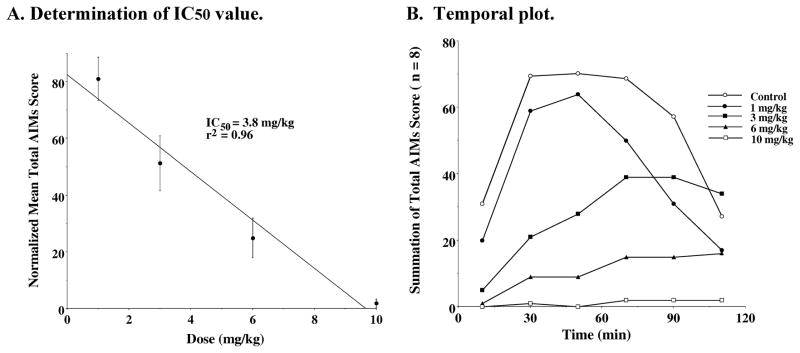
A. Determination of IC50 value: Unilaterally lesioned rats were injected (i.p.) with varying doses of WC 21 (0 to 10 mg/kg) followed immediately with a constant dose of L-dopa and benserazide (8 mg/kg each). The percent of the mean total AIMs score (n ≥ 8) relative to vehicle controls as a function of the dose of WC 21 is shown. The mean values ± S.E.M. for the normalized total AIMs values as a function of WC 21 concentrations are as follows: a) 1 mg/kg, 81.0 ± 7.7, b) 3.0 mg/kg, 51.2 ± 9.7, c) 6 mg/kg, 24.9 ± 7.0 and d) 10 mg/kg, 1.8 ± 1.5. The calculated IC50 value for this data was found to be 3.8 mg/kg using a linear regression analysis (r2 = 0.96). B. Temporal plot: The variation in the total AIMs score as a function of time is shown using zero time pretreatment with i.p. injection of WC 21 at 10 mg/kg (□), 6 mg/kg (▲), 3 mg/kg (■), 1 mg/kg (●) or vehicle control (❍). Animals were also injected (i.p.) with 8 mg/kg L-dopa and benserazide. Each point is the summation of total AIMs score for a total of 8 animals at each observation time point for each dose of WC 21. The temporal plot for the vehicle control curve is the mean value for all four experiments.
Figure 13. Dose Response Dependence of WC 26 for the Attenuation of Total AIM Scores.
A. Determination of IC50 value: Unilaterally lesioned rats were injected (i.p.) with varying doses of WC 26 (0 to 10 mg/kg) followed immediately with a constant dose of L-dopa and benserazide (8 mg/kg each). The percent of the mean total AIM score (n ≥ 10) relative to vehicle controls as a function of the dose of WC 26 is shown. The mean values ± S.E.M. for the normalized total AIMs values as a function of WC 26 concentrations are as follows: a) 1 mg/kg, 86.5 ± 4.9, b) 3.0 mg/kg, 69.7 ± 6.3, c) 6 mg/kg, 45.0 ± 9.5 and d) 10 mg/kg 21.8 ± 4.7. The calculated IC50 value for this data was found to be 5.8 mg/kg using a linear regression analysis (r2 = 0.99). B. Temporal plot: The variation in the total AIMs score as a function of time is shown using zero time pretreatment with an i.p. injection of WC 26 at 10 mg/kg (□), 6 mg/kg (▲), 3 mg/kg (■), 1 mg/kg (●) or vehicle control (❍). Animals were also injected (i.p.) with 8 mg/kg L-dopa and benserazide. Each point is the summation of total AIMs score for a total of 10 animals at each observation time point for each dose of WC 26. The temporal plot for the vehicle control curve is the mean value for all four experiments.
Spontaneous locomotor activity
To further investigate possible motor side effects of our compounds, the effect of test drug on spontaneous locomotor activity was evaluated as the number of crossovers from the one end of the locomotor activity chambers to the other end after the administration of either 3 mg/kg or 10 mg/kg of our WC series compounds in non-lesioned rats (Figure 14). The antagonist WC 10 was the most potent inhibitor of spontaneous locomotor activity since both doses produced a time-dependent decrease in crossovers relative to controls (p < 0.05). This decrease was most evident during the first 10 minutes of testing at the 3 mg/kg dose of WC10 and during the first 40 min at the 10 mg/kg dose. At a dose of 10 mg/kg the agonist WC 44 produced a time-dependent decrease in crossovers relative to controls (p < 0.005) and to the 3 mg/kg dose (p < 0.001). The time course of the decrease in crossovers appeared biphasic with a smaller decrease during the first 20 minutes of testing relative to WC 10. This effect was followed by a) a return to control levels during the next 10 minutes of testing, b) another decrease in crossovers observed during the next 20 minutes of testing and c) a return to control levels during the last 10 minutes of the session.
Figure 14. Effects of WC 10, WC 44, WC 26 and WC 21 on spontaneous locomotor activity of non-lesioned rats.
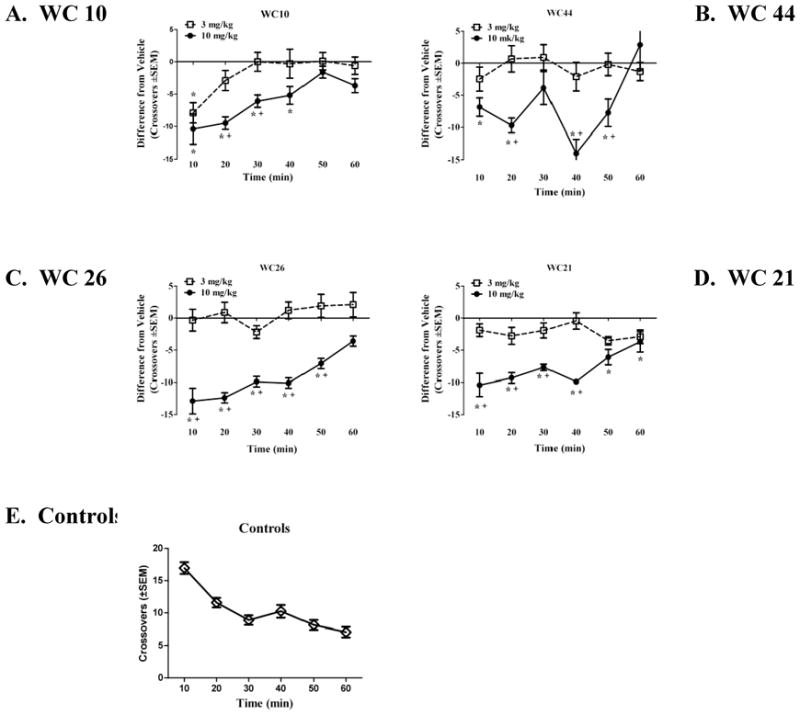
To investigate the effect of the compounds on spontaneous locomotor activity, the mean difference in crossovers (± SEM) from the vehicle-pretreated controls is illustrated. This difference value was calculated by subtracting the average number of crossovers of the respective vehicle group from the number of crossovers of individual subjects in the WC10 (A), WC44 (B), WC26 (C) and WC21 (D) dosage groups. The horizontal line provides a reference point for controls relative to the drug dosage groups. Doses of 3 (□) and 10 (●) mg/kg, IP were examined for each compound. For a more direct comparison to controls, the average number of crossovers of the combined control groups (◇)is shown across time in 10-min periods (E). Asterisks (*) designate a difference from vehicle controls and a plus sign (+) designates a difference from the 3 mg/kg dosage group (p < 0.05).
At a dose of 10 mg/kg the partial agonist WC 26, decreased crossovers relative to controls (p < 0.005) and relative to the 3 mg/kg dose (p < 0.05) throughout the first 50 min of testing. A similar pattern was observed with 10 mg/kg of the partial agonist WC 21 relative to controls (p < 0.05). However, relative to the 3 mg/kg dose a decrease was only observed during the first 40 min (p < 0.001). There were no significant differences among the controls and the animals that were administered 3 mg/kg of WC 21 or WC 26.
Rotarod evaluation
A rotarod evaluation of our lesioned animals in the presence or absence of test drug was conducted to determine the effect of test drug on motor performance and coordination of the unilaterally lesioned animals (Carta et al., 2006). Each test drug was administered at a dose equal to its in vivo IC50 value. After six training sessions performed over three days, lesioned animals were administered test compounds in a sequential manner (day 4, WC 44; day 6, WC 26; day 8, WC 21; day 10, WC 10) based upon decreasing intrinsic activity at D3 dopamine receptors. Rotarod performance was evaluated at both 30 and 60 minutes post injection. On the intervening days (days 5, 7, 9 and 11) animals were administered vehicle that was used on the previous day (Figure 15). First, we found no statistical difference (p > 0.05) between the 30 minute and the 60 minute time points for the test compounds. Second, we found no significant difference (p > 0.05) when we compared the amount of time that animals were able to stay on the rotarod when injected with vehicle compared to the training session. Therefore, vehicle administration did not appear to be a factor in the latency to fall. Third, we found no significant difference in the mean latency to fall times for WC 44, WC 26 and WC 21 compared to vehicle or to the training session. Finally, there seemed to be a decrease in latency to fall when animals were administered WC 10 (6.6 mg/kg) compared to its vehicle (a 25% reduction), which is due in part to the fact that there was a 15% to 20% increase in the latency to fall for this vehicle compared to the a) last two days of the training session or b) the previous three vehicles (days 5, 7 and 9) that were administered. However, no significant difference (p > 0.05) criteria of significance) was observed when WC 10 was compared to the training session or all the test drug vehicles. On the final two days of the experiment the effect of the administration of haloperidol (5.0 mg/kg) was evaluated. A >50% reduction in the latency to fall compared to its matched vehicle was observed, which was significantly different from both the a) matched vehicle control, b) training session and c) mean values for all the vehicle controls.
Figure 15. Rotarod evaluation of WC Series Compounds.
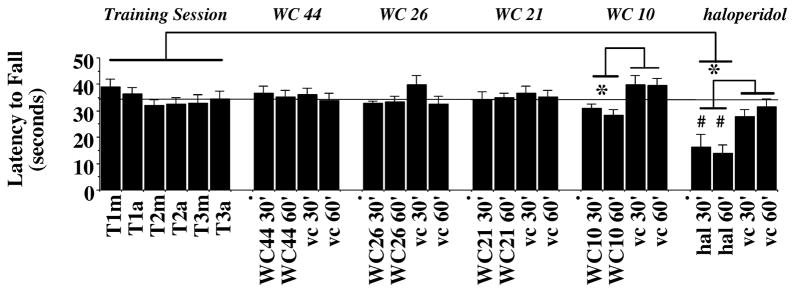
A rotarod apparatus was used to assess the effect of the WC series compounds on motor coordination and agility of unilaterally lesioned rats in the presence or absence of test compound. The data is presented as the mean amount of time the animal remained on the rotarod before falling (Latency to Fall; in seconds) ± S.E.M. The acceleration conditions for the rotarod test were 0 to 44 rpms in 90 seconds. This experiment was performed on 13 consecutive days. Days 1 through 3 were training sessions that were conducted either in the morning or afternoon (T1m through T3a, respectively). After the training session was completed, lesioned rats were administered a test compound (i.p.) at a dose equal to the IC50 value for the inhibition of total AIMs score and evaluated at 30 minutes (30′) or 60 minutes (60′) post drug administration: a) agonist WC 44, 5.5 mg/kg, day 4 (WC44 30′ and WC 44 60′), b) partial agonist WC 26, 5.8 mg/kg, day 6 (WC26 30′ and WC26 60′), c) partial agonist WC 21, 3.8 mg/kg, day 8 (WC21 30′ and WC21 60′) and d) antagonist WC 10, 6.6 mg/kg, day 10 (WC10 30′ and WC10 60′). Haloperidol (hal) was included as a positive control (5 mg/kg, day 12 (hal 30′ and hal 60′). On the days following the administration of test compound (days 5, 7, 9, 11 and 13), the appropriate matched vehicle (vc) was administered and animals were evaluated at 30 minutes (30′) or 60 minutes (60′) post vehicle administration (vc 30′ and vc 60′). An ANOVA with repeated measures was used for the statistical evaluation of the data using Systat software. An asterisk (*) indicates significance (p ≤ 0.05) when comparing the effect of the mean values for the test drug to either a) its matched vehicle control or b) the mean of the training sessions. A number symbol (#) signifies significance (p < 0.001) for the comparison of the values for the test drug (here haloperidol) compared to values for the matched vehicle control. The dotted line parallel to the x-axis is the mean value (34.60 seconds) for the training session.
Cylinder test
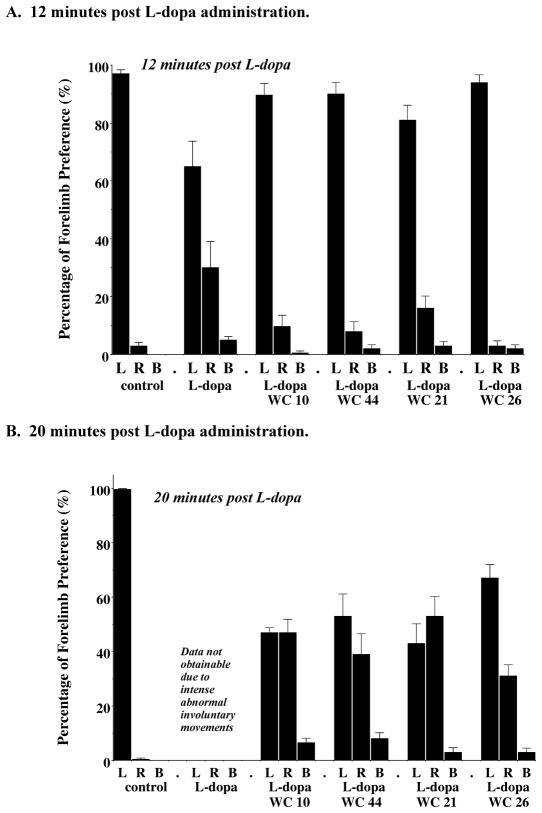
Test drugs were administered at a dose equal to the calculated IC50 value. Animals were evaluated at a 12 and 20 minute time point post drug injection with either a) vehicle or b) L-dopa/benserazide (8 mg/kg) in the presence or absence of test drug. For this group of animals, administration of vehicle resulted in a marked preferential use of the ipsilateral, uncompromised (left) forelimb (>95% preference). At the 12 minute time point post L-dopa administration, animals increased their use of their compromised forelimb. Animals injected with L-dopa and test drug (WC 10, WC 21, WC 26 or WC 44) exhibited forelimb usage similar to that observed for the vehicle control animals (Figure 16A). At the 20 minute time point, animals that were administered both L-dopa and test drug exhibited nonpreferential use of their forelimbs (Figure 16B). It was not possible to measure forelimb usage at the 20 minute time point when animals were administered L-dopa/benserazide in the absence of test drug due to the intensity of the AIMs.
Figure 16. Cylinder Test Evaluation of the Effect of the WC Series compounds at a dose equal to the IC50 Values.
The cylinder test was performed to assess the independent use of each forelimb in the context of the rat’s natural rearing/exploratory behavior. The data shown is the mean percent preferential usage of either the left (L), right (R) or both (B) forelimbs ± S.E.M. Unilaterally lesioned animals were administered either a) saline (control), b) L-dopa and benserazide (L-dopa) at a dose of 8 mg/kg each, c) L-dopa and benserazide with the antagonist WC 10 (L-dopa/WC 10) at a dose equal to the calculated IC50 value (6.6 mg/kg), d) L-dopa and benserazide with the agonist WC 44 (L-dopa/WC 44) at a dose equal to the calculated IC50 value (5.5 mg/kg), e) L-dopa and benserazide with the partial agonist WC 21 (L-dopa/WC 21) at a dose equal to the calculated IC50 value (3.8 mg/kg) or f) L-dopa and benserazide with the partial agonist WC 26 (L-dopa/WC 26) at a dose equal to the calculated IC50 value (5.8 mg/kg). Animals were evaluated at (A) 12 minutes and (B) 20 minutes post injection. At 20 minutes post L-dopa/benserazide administration data on forelimb usage was not obtainable due to the intensity of the L-dopa-dependent abnormal involuntary movements. At the 12 minute time point, animals were evaluated for a total of 5 minutes and then returned to their home cage for 4 minutes. At the 20 minute time point animals were further evaluated for 5 minutes. The number of independent experiments is twelve (n = 12) for all drugs tested, except for WC 21 where n = 11.
Discussion
There are several pathways within the basal ganglia that likely contribute to the expression of LID in humans. Following neurodegeneration there is an imbalance between direct and indirect neuronal circuitry in the basal ganglia (Bezard et al., 2001; Brotchie, 2005; Dekundy et al., 2007). An over-activity of the direct striatal output pathway, providing direct GABAergic connection, may lead to a striatal inhibition of the output of the basal ganglia, internal globus pallidus and substantia nigra pars reticulata (Fabbrini et al., 2007). In addition, there is probably involvement of glutamatergic transmission, since the majority of excitatory pathways in the basal ganglia utilize glutamate. There is also evidence for N-methyl-D-aspartate (NMDA) receptor involvement since NMDA antagonists have been shown to reduce motor complications associated with L-dopa (Bibbiani et al., 2005). In addition, there is evidence that dopamine can be synthesized and released from surviving serotonergic neurons causing an unregulated release of dopamine when an insufficient number of dopaminergic neurons are available (Bibbiani et al., 2001; Carta et al., 2007; Oh et al., 2002).
The observation that LID develops gradually during the course of L-dopa therapy suggests a possible denervation supersensitivity of the D1-like and/or D2-like dopamine receptors that might contribute to the development of L-dopa-dependent dyskinetic movements (Sánchez-Pernaute et al., 2007). Studies by Sokoloff and co-workers indicate that D3 dopamine receptor expression may play a role in the development of LID because L-dopa administration was reported to up-regulate D3 receptors in the striatum of 6-OHDA lesioned animals (Bordet et al., 1997; Bordet et al., 2000). There is also experimental evidence to suggest that there might be an interplay between the D1 and D3 dopamine receptor subtypes when they are co-expressed (Schwartz et al., 1998a; Schwartz et al., 1998b; Sánchez-Pernaute et al., 2007).
The studies reported in this communication were designed to investigate the potential of D2 or D3 receptor selective compounds as antidyskinetic drugs for the treatment of LID in patients with Parkinson’s Disease. In vitro and in vivo pharmacological studies were undertaken to determine if there is a correlation between the dopamine receptor subtype selectivity or intrinsic activity with the ability to attenuate AIM scores in rats.
For these studies we used an established rat model of LID, which uses unilateral 6-OHDA) lesions (Schallert et al., 2000; Deumens et al., 2002; Metz et al., 2005). Further validation of the rat 6-OHDA unilateral lesion model as an authentic animal model for identifying and evaluating potential antidyskinetic compounds has been provided by Dekundy and colleagues (2007).
Before initiating our studies on the effect of our novel D2 or D3 receptor selective compounds on AIM scores, we noticed that the interval between feeding and L-dopa/benserazide administration was an important factor in the manifestation of abnormal movements. Animals fed 1–2 hours prior to L-dopa administration appeared to have reduced AIM scores compared to animals fed 12 to 18 hours prior. This observation is consistent with several studies in Parkinson’s patients indicating that consumption of neutral amino acids, such as phenylalanine, leucine or isoleucine, can interfere with the transport of L-dopa from the plasma to the brain (Nutt et al., 1984; Eriksson et al., 1986; Stout et al., 1998; Carter et al., 1989; Pincus and Barry, 1987). It is also consistent with the observation of Mizuta and Kuno (1993) that pretreatment of unilaterally lesioned rats with high doses of amino acids reduces L-dopa induced rotations. Therefore, for these studies animals were always fed >12 hours prior to testing.
Initial studies with our D2 dopamine receptor selective antagonists, SV 293 and SV 156 (Vangveravong et al., 2006), showed minimal effects on L-dopa-dependent abnormal movements. At a dose of 10 mg/kg, reduced locomotor effects and a slit eye appearance were observed 20 to 40 minutes after the administration of the test drugs. Based upon these observations, we discontinued further in vivo testing of these compounds.
Concurrently, we evaluated the effect of our novel D3 receptor selective substituted phenylpiperazine compounds (Chu et al., 2005). This panel of compounds exhibited varying levels of intrinsic activity at D3 dopamine receptors when evaluated in vitro using a forskolin-dependent inhibition of adenylyl cyclase assay. The dissociation constants for these compounds at D3 receptors ranged from 0.6 to 2.4 nM and the D3 versus D2 receptor selectivity ranged from 23- to 53-fold.
Each of the novel D3 dopamine receptor selective compounds was capable of attenuating the AIM scores in our unilaterally lesioned rats. With the possible exception of WC 26, each of the compounds appeared to be more effective if administered simultaneously with L-dopa, when compared with a 60 minute pretreatment regimen. IC50 values for these compounds ranged from approximately 4 to 7 mg/kg. There was no obvious correlation between the in vivo IC50 values and a) D3 receptor affinity, b) D2 to D3 receptor selectivity or c) intrinsic activity for adenylyl cyclase inhibition at D3 receptors. The magnitude of the attenuation was essentially the same for both total AIMs and AIMs minus locomotor scores for all of the substituted phenylpiperazines that were tested. These two methods of scoring the abnormal movements were examined because it has been suggested that the locomotor component may not be an appropriate correlate of the dyskinesia (Cenci and Lundblad, 2005; Lundblad et al., 2002; Lundblad et al., 2005).
Using WC 10, we found that the dyskinetic-like movements of our 6-OHDA lesioned animals could not only be prevented, but could also be rapidly and dramatically reversed by the administration of our D3 receptor selective antagonist. This observation is noteworthy because it indicates a dynamic and active process is occurring which can be halted even when it has reached its maximum intensity.
It was pharmacologically perplexing to find that each of our four D3 receptor selective compounds were able to attenuate AIM scores despite that fact that they exhibited variations in the intrinsic activity in an adenylyl cyclase assay. Despite this variation in intrinsic activity each of the compounds have IC50 values within the same order of magnitude. We had anticipated that in vitro intrinsic activity at adenylyl cyclase might be an indicator of in vivo efficacy. Clearly that was not the case.
It is known that D2-like dopamine receptors are linked to several different second messenger systems besides the inhibition of adenylyl cyclase (Chio et al., 1994; MacKenzie et al., 1994; Asghari et al., 1995; Watts & Neve, 1997; Robinson & Caron 1997), including mitogenesis (Pilon et al., 1994; Griffon et al., 1997; Schwartz et al., 1998a), activation of G-protein-coupled inward rectifier potassium (GIRK) channels (Kuzhikandathil & Oxford, 2000; Kuzhikandathil et al., 1998; Kuzhikandathil et al., 2004) and activation of the phospholipase D (PLD) enzyme (Everett and Senogles, 2004; Senogles, 2003; Senogles, 2000). Classical receptor theory has generally assumed that a compound has essentially the same efficacy for all effector pathways linked to a receptor. However, there is now evidence that ligands can have differing efficacy and/or potency for different effector pathways. This revision of classic receptor theory is termed functional selectivity (Gay et al., 2004; Kenakin 2002; Clarke 2005). Therefore, a compound, such as WC 44, that is classified as a partial agonist at D2 receptors maybe be a full agonist at the D3 receptor subtype. In addition, a compound that is an antagonist for one receptor linked second messenger system may demonstrate intrinsic activity for a different pathway.
One possible reason that both our D3 antagonist, WC 10, and our agonist, WC 44, are both capable of attenuating the AIM scores in the unilaterally 6-OHDA lesioned animals is that their intrinsic activity has thus far only been defined using an adenylyl cyclase assay but their in vivo efficacy for this experimental behavior system may not directly involve the modulation of adenylyl cyclase activity. The in vivo co-administration of WC 10 and WC 44 was found to be additive in the attenuation of the AIMs score, rather than being competitive or synergistic. This result suggests a common mechanism of action for the two compounds in this behavioral paradigm.
It has been previously reported that the nonselective dopaminergic agonist apomorphine, the D1-like dopamine receptor selective agonist SKF 81297 and the D2-like dopamine receptor selective agonist bromocriptine can precipitate AIMs in these unilaterally lesioned animals. It is worth noting that although we found that WC 44 was a full agonist for the adenylyl cyclase inhibition, there was no indication that this compound could precipitate abnormal involuntary movements when administered alone. In addition, neither of the two partial agonists, WC 21 and WC 26, induced involuntary movements in our 6-OHDA unilaterally lesioned animals. These observations were in contrast to our preliminary studies indicating that subcutaneous (s.c.) administration of the putative D3 dopamine receptor selective agonist (+)-PD-128907 (1 and 3 mg/kg) can precipitate AIMs in lesioned animals, although those scores were predominated by increased locomotor activity with mild forelimb dystonic movements.
To characterize potential motor side effects of our compounds we tested their effects in a) nonlesioned rats using an automated locomotor activity chambers and b) unilaterally lesioned rats using a rotarod apparatus. Compounds were tested for effects on spontaneous locomotion at doses of 3.0 mg/kg and 10.0 mg/kg. At the higher dose we found that each of the compounds appeared to attenuate spontaneous locomotion, especially within the first 20 minutes of the 60 minute session. However, at the lower dose spontaneous locomotion approached normal values. Similarly, when WC series compounds were tested at doses equal to their AIMs IC50 values there was little to no difference observed in rotarod performance, with the possible exception of compound WC 10.
A cylinder test was used to investigate if our D3 dopamine receptor selective compounds might interfere with the beneficial effects of L-dopa in our unilaterally lesioned animals. The results of the cylinder test suggest that while the WC series compounds may have delayed the onset of the beneficial effects of L-dopa on forelimb preferential usage (at 12 minutes post L-dopa administration), by 20 minutes the animals were utilizing their forelimbs in a manner similar to that observed previously in unlesioned animals.
Other potential side effects were observed in the present study with both D2 and D3 receptor selective compounds, including reduced locomotion, flat posture and ocular ptosis. Flat postures were observed in some, but not all, animals, suggesting a possible pharmacogenetic effect. Ocular ptosis and reduced locomotion were prominent when the D2 receptor selective compounds were administered, suggesting that co-blockade of D2 receptors in vivo by our D3 receptor subtypes selective WC series compounds may be responsible for these adverse side effects that were primarily observed at the highest dose tested (10 mg/kg). These observations suggest that adverse effects may be minimized if compounds with increased D2:D3 affinity ratios were used in future studies.
WC 44, WC 26 and WC 21 had little to no effect on rotarod performance when administered at a concentration corresponding to their IC50 values. The administration of WC 10 resulted in a 15–25% reduction of rotarod performance, depending on which controls were used for the comparative analysis. There appeared to be no significant difference when the results from WC 10 were compared to a) baseline training session values, b) the mean of the matched vehicle controls or c) the mean of all of the vehicle controls. However, significance was achieved when the WC 10-dependent values were compared to its matched vehicle control group that had slightly elevated latencies compared to all other control values.
In conclusion, we evaluated a panel of novel D2 receptor antagonists and D3 dopamine receptor selective antagonists, partial agonists and full agonists for the ability to attenuate L-dopa associated AIMs in unilaterally lesioned male Sprague Dawley rats. Two D2 dopamine receptor selective antagonists were found to minimally attenuate AIM scores. Four D3 dopamine receptor selective compounds were found to attenuate AIM scores in a dose dependent manner. The in vivo efficacy of the compounds increased when they were administered simultaneously with L-dopa, as compared to when the compounds were administered 60 minutes prior to L-dopa/benserazide. It was also found that the D3 receptor antagonist WC 10 could inhibit the involuntary movements after they had achieved maximum intensity. AIMs were not observed after administration of our D3 receptor selective agonist (WC 44) or partial agonists (WC 21 and WC 26) alone. Although varying degrees of locomotor side effects were observed with these D3 receptor selective compounds at high doses, these studies suggest that the development of D3 dopamine receptor selective compounds may represent a viable strategy for the identification of pharmacotherapeutic agents that can be used to treat/manage L-dopa-associated dyskinesia in patients with Parkinson’s Disease.
Acknowledgments
The authors would like to thank Dr. Steven Gold and Mr. Brian Potts for their insightful discussions on the AIMs scoring system. We would like to thank Drs. Eunson Jung, Michael Forster and Nathalie Sumien for their assistance with the statistical analysis and helpful discussions. We would also like to thank Felicia Duke for her excellent technical assistance. This research was supported by a Community Fast Track 2006 from the Michael J. Fox Foundation for Parkinson’s Research and an award from NIH/NIDA (R01 DA23957-01).
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AIM
abnormal involuntary movement
- DMSO
dimethylsulfoxide
- i.p
intraperitoneal
- LID
L-dopa-induced dyskinesia
- NMDA
N-methyl-D-aspartate
- MFB
medial forebrain bundle
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson’s Disease
- s.c
subcutaneous
- TMS
transmembrane spanning
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochemistry. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Bézard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nature Medicine. 2003;9:762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- Bézard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: Potential for new therapies. Nature Reviews/Neuroscience. 2001;2:577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Costantini LC, Patel R, Chase TN. Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Experimental Neurology. 2005;192:73–78. doi: 10.1016/j.expneurol.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology. 2001;57:1829–1834. doi: 10.1212/wnl.57.10.1829. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci USA. 1997;94:3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. European J Neuroscience. 2000;12:2117–2123. doi: 10.1046/j.1460-9568.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Luedtke RR, Gallitano AL, Smith JE, Filtz TM, Kallen RG, Molinoff PB. Expression and characterization of the rat D3 dopamine receptor: pharmacologic properties and development of antibodies. J Pharmacology and Experimental Therapeutics. 1993;264:1002–1011. [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Matres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Research. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Brotchie JM. Nondopaminergic mechanisms in levodopa-induced dyskinesia. Movement Disorders. 2005;20:919–931. doi: 10.1002/mds.20612. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. J Neurochemistry. 2006;96:1718–1727. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Carter JH, Nutt JG, Woodward WR, Hatcher LF, Trotman TL. Amount and distribution of dietary protein affects clinical response to levodopa in Parkinson’s disease. Neurology. 1989;39:552–556. doi: 10.1212/wnl.39.4.552. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Utility of 6-hydroxydopamine lesioned rats in the preclinical screening of novel treatments for Parkinson Disease. In: LeDoux M, editor. Animal Methods of Movement Disorders. Elsevier Academic Press; San Diego CA: 2005. pp. 193–208. [Google Scholar]
- Chio CL, Lajiness ME, Huff RM. Activation of heterologously expressed D3 dopamine receptors: comparison with D2 dopamine receptors. Molecular Pharmacology. 1994;45:51–60. [PubMed] [Google Scholar]
- Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D3 receptor ligands. Bioorganic & Medicinal Chemistry. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Clarke WP. What’s for lunch at the conformational cafeteria? Molecular Pharmacology. 2005;67:1819–1821. doi: 10.1124/mol.105.013060. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behavioral Brain Research. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s Disease in rats: An evaluation of 6-OHDA lesions of the nigrostraital pathway. Experimental Neurology. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Eriksson T, Wiesel K, Carlsson A. Rat brain concentration of administered amino acids: Dependence on time of day for administration. J Neural Transmission. 1986;65:83–91. doi: 10.1007/BF01249614. [DOI] [PubMed] [Google Scholar]
- Everett PB, Senogles SE. D3 dopamine receptor activates phospholipase D through a pertussis toxin-insensitive pathway. Neuroscience Letters. 2004;371:34–39. doi: 10.1016/j.neulet.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Geotz CG. Levodopa-induced dyskinesias. Motor Movements. 2007;22:1379–1389. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Molecular Pharmacology. 2004;66:97–105. doi: 10.1124/mol.66.1.97. [DOI] [PubMed] [Google Scholar]
- Griffon N, Pilon C, Sautel F, Schwartz JC, Sokoloff P. Two intracellular signaling pathways for the dopamine D3 receptor: opposite and synergistic interactions with cyclic AMP. J Neurochemistry. 1997;68:1–9. doi: 10.1046/j.1471-4159.1997.68010001.x. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Håkansson K, Bioulac BH, Gross CE, Sokoloff P, Fisone G, Gurevich EV, Bloch B, Bezard E. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism & Related Disorders. 2005;11(Suppl 1):S25–S29. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Hermanowicz N. Drug therapy for Parkinson’s Disease. Seminars in Neurology. 2007;27:97–105. doi: 10.1055/s-2007-971177. [DOI] [PubMed] [Google Scholar]
- Hsu A, Togasaki DM, Bezard E, Sokoloff P, Langston JW, Di Monte DA, Quik M. Effect of the D3 dopamine receptor partial agonist BP897 [N-[4-(4-(2-methoxyphenyl)piperazinyl)butyl]-2-naphthamide] on L-3,4-dihydroxyphenylalanine-induced dyskinesias and parkinsonism in squirrel monkeys. J Pharmacology and Experimental Therapeutics. 2004;311:770–777. doi: 10.1124/jpet.104.071142. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Efficacy at G-protein-coupled receptors. Nature Review Drug Discovery. 2002;1:103–110. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Baker DA, Fuchs RA, Manders N, Neisewander JL. Differential effects of 7-OH-DPAT on amphetamine-induced stereotypy and conditioned place preference. Psychopharmacology (Berl) 1998;139:332–341. doi: 10.1007/s002130050724. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Fuchs RA, Beck AM, Groff RS, Neisewander JL. Behavioral interactions produced by co-administration of 7-OH-DPAT with cocaine or apomorphine in the rat. Psychopharmacology (Berl) 1999;142:383–392. doi: 10.1007/s002130050903. [DOI] [PubMed] [Google Scholar]
- Kumar R, Riddle LR, Griffin SA, Chu W, Vangvervong S, Neissewander J, Mach RH, Luedtke RR. Evaluation of D2 and D3 Dopamine Receptor Selective Compounds on L-Dopa Dependent Abnormal Involuntary Movements in Rats. 2009. (see previous manuscript) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Oxford GS. Dominant-negative mutants identify a role for GIRK channels in D3 dopamine receptor-mediated regulation of spontaneous secretory activity. J General Physiology. 2000;115:697–706. doi: 10.1085/jgp.115.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Westrich L, Bakhos S, Pasuit J. Identification and characterization of novel properties of the human D3 dopamine receptor. Molecular & Cellular Neuroscience. 2004;26:144–155. doi: 10.1016/j.mcn.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Yu W, Oxford GS. Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines. Molecular & Cellular Neuroscience. 1998;12:390–402. doi: 10.1006/mcne.1998.0722. [DOI] [PubMed] [Google Scholar]
- Lee T, Seeman P, Rajput A, Farley J, Hornykiewicz O. Receptor basis for dopaminergic supersensitivity in Parkinson’s disease. Nature. 1978;273:59–61. doi: 10.1038/273059a0. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Freeman RA, Boundy VA, Martin MW, Huang Y, Mach RH. Characterization of (125)I-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38:438–449. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Mach RH. Progress in developing D3 dopamine receptor ligands as potential therapeutic agents for neurological and neuropsychiatric disorders. Current Pharmaceutical Design. 2003;9:643–671. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Anderson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioral measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. European J Neuroscience. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA. Pharmacological validation of a mouse model of L-DOPA-induced dyskinesia. Experimental Neurology. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Vaudano E, Cenci MA. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J Neurochemistry. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- MacKenzie RG, VanLeeuwen D, Pugsley TA, Shih YH, Demattos S, Tang L, Todd RD, O’Malley KL. Characterization of the human dopamine D3 receptor expressed in transfected cell lines. European J Pharmacology. 1994;266:79–85. doi: 10.1016/0922-4106(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Metz GA, Tse A, Ballermann M, Smith LK, Fouad K. The unilateral 6-OHDA rat model of Parkinson’s disease revisited: an electromyographic and behavioral analysis. European J Neuroscience. 2005;22:735–744. doi: 10.1111/j.1460-9568.2005.04238.x. [DOI] [PubMed] [Google Scholar]
- Mizuta E, Kuno S. Rotations induced by L-dopa in parkinsonian rats are reduced by an ingestion of amino acids. J Neural Transmission. 1993;6:1435–1463. doi: 10.1007/BF02260923. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Woodward WR, Hamerstad JP, Carter JH, Anderson JL. The “on-off” phenomenon in Parkinson’s disease. Relation to levadopa absorption and transport. New England J Medicine. 1984;310:483–488. doi: 10.1056/NEJM198402233100802. [DOI] [PubMed] [Google Scholar]
- Oh JD, Bibbiani F, Chase TN. Quetiapine attenuates levodopa-induced motor complications in rodent and primate parkinsonian models. Experimental Neurology. 2002;177:557–564. doi: 10.1006/exnr.2002.8009. [DOI] [PubMed] [Google Scholar]
- Pilon C, Lévesque D, Dimitriadou V, Griffon N, Martres MP, Schwartz JC, Sokoloff P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108–15 neuroblastoma-glioma hybrid cell line. European J Pharmacology. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Pincus JH, Barry KM. Plasma levels of amino acids correlate with motor fluctuations in parkinsonism. Archives Neurology. 1987;44:1006–1009. doi: 10.1001/archneur.1987.00520220012007. [DOI] [PubMed] [Google Scholar]
- Robinson SW, Caron MG. Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Molecular Pharmacology. 1997;52:508–514. doi: 10.1124/mol.52.3.508. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O. In vivo evidence of D(3) dopamine receptor sensitization in parkinsonian primates and rodents with l-dopa-induced dyskinesias. Neurobiology Disease. 2007;27:220–227. doi: 10.1016/j.nbd.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL III, Sandberg PR, editors. Central Nervous System Diseases: Innovative Models of CNS Diseases From Molecule to Therapy. Humana Press; Totowa, New Jersey: 2000. pp. 131–151. [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical abalation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P. Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Research Review. 1998a;26:236–242. doi: 10.1016/s0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Ridray S, Bordet R, Diaz J, Sokoloff P. D1/D3 receptor relationships in brain coexpression, coactivation, and coregulation. Advances Pharmacology. 1998b;42:408–411. doi: 10.1016/s1054-3589(08)60775-9. [DOI] [PubMed] [Google Scholar]
- Senogles SE. The dopamine receptor stimulates phospholipase D activity: a novel signaling pathway for dopamine. Molecular Pharmacology. 2000;2000:455–462. doi: 10.1124/mol.58.2.455. [DOI] [PubMed] [Google Scholar]
- Senogles SE. D2s dopamine receptor mediates phospholipase D and antiproliferation. Molecular and Cellular Endocrinology. 2003;209:61–69. doi: 10.1016/j.mce.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Daly JW, Creveling CR. A radioisotopic method for measuring the formation of adenosine 3′,5′-cyclic monophosphate in incubated slices of brain. J Neurochemistry. 1969;16:1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Saloman Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Analytical Biochemistry. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Stout DB, Huang SC, Melega WP, Releigh MJ, Phelps ME, Barrio JR. Effects of large neutral amino acid concentration on 6-[F-18] fluro-l-dopa kinetics. J Cerebral Blood Flow Metabolism. 1998;18:43–51. doi: 10.1097/00004647-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Su YF, Cubeddu L, Perkins JP. Regulation of adenosine 3′:5′-monophosphate content of human astrocytoma cells: desensitization to catecholamines and prostaglandins. J Cyclic Nucleotide Research. 1976;2:257–270. [PubMed] [Google Scholar]
- Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Research. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxydopamine-induced degereration of the nigrostriatal dopamine pathway: the turning syndrome. Pharmacology & Therapeutics Ther [B] 1976;2:37–40. doi: 10.1016/0306-039x(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European J Pharmacology. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Vangveravong S, McElveen E, Taylor M, Griffin SA, Luedtke RR, Mach RH. Identification and pharmacologic characterization of D2 dopamine receptor selective antagonists. Bioorganic & Medicinal Chemistry. 2006;14:815–825. doi: 10.1016/j.bmc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Neve KA. Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Molecular Pharmacology. 1997;52:181–186. doi: 10.1124/mol.52.2.181. [DOI] [PubMed] [Google Scholar]