Abstract
Objective
To determine optimal infertility therapy in women at the end of their reproductive potential.
Design
Randomized clinical trial.
Setting
Academic medical centers and private infertility center in a state with mandated insurance coverage.
Patients
Couples with ≥ 6 months of unexplained infertility; female partner aged 38–42.
Interventions
Randomized to treatment with 2 cycles of clomiphene citrate (CC) and intrauterine insemination (IUI), follicle stimulating hormone (FSH)/IUI, or immediate IVF, followed by IVF if not pregnant.
Main Outcome Measures
Proportion with a clinically recognized pregnancy, number of treatment cycles, and time to conception after 2 treatment cycles and at the end of treatment.
Results
154 couples were randomized to receive CC/IUI (N=51), FSH/IUI (N=52), or immediate IVF (N=51); 140 (90.9%) couples initiated treatment. Cumulative clinical pregnancy rates per couple after the first 2 cycles of CC/IUI, FSH/IUI, or immediate IVF were 21.6%, 17.3%, and 49.0%, respectively. After all treatment, 71.4% (110/154) of couples conceived a clinically recognized pregnancy and 46.1% delivered at least one live-born baby. 84.2% of all live born infants resulting from treatment were achieved from IVF. There were 36% fewer treatment cycles in the IVF arm compared to either COH/IUI arm and couples conceived a pregnancy leading to a live birth after fewer treatment cycles.
Conclusions
An RCT to compare treatment initiated with 2 cycles of COH/IUI to immediate IVF in older women with unexplained infertility demonstrated superior pregnancy rates with fewer treatment cycles in the immediate IVF group.
Keywords: Unexplained infertility, FORT-T Trial, in vitro fertilization, controlled ovarian hyperstimulation, clomiphene citrate, follicle stimulating hormone, intrauterine insemination (IUI), advanced reproductive age
INTRODUCTION
During the early years of assisted reproduction, treatment for unexplained infertility usually began with controlled ovarian hyperstimulation (COH) using clomiphene citrate (CC) and intrauterine insemination (IUI) (1). If pregnancy was not achieved, couples proceeded in a stepwise fashion to gonadotropin (FSH)/IUI treatment and then, if not pregnant, on to in vitro fertilization (IVF) (1). In 1999, a randomized clinical trial compared FSH/IUI with IUI alone, FSH alone, and intracervical insemination alone, and reported that the most successful treatment arm was FSH/IUI (2). One-third of the participating couples conceived after four treatment cycles. However, the success rate per cycle was only 9% and approximately one-third of the pregnancies were multiple births, including triplets and quadruplets. At that time the results supported the use of FSH/IUI over the other treatments studied. However, its high cost for a low success rate and high risk of multiple births, especially high order multiples, suggested that it might be more effective to move directly to IVF (3–5).
Recently, we reported in the Fast Track and Standard Treatment (FASTT) trial that FSH/IUI was of no added value in the treatment of younger couples with unexplained infertility (5). Results of the FASTT trial raised questions about optimal treatment strategies for couples with unexplained infertility who are at the end of the woman’s reproductive potential when there is a shortened time frame for conceiving. There has been the belief that, if using COH/IUI, it is best to bypass CC/IUI and begin with FSH/IUI. Supporting this approach is the decrease in the incidence of adverse events, such as ovarian hyperstimulation syndrome (OHSS) and multiple births, in this age group. On the other hand, gonadotropin costs increase as the dose of medication needed for COH in older women rises. Because both CC/IUI and FSH/IUI were commonly used in this population of women at the time the study was designed and the per cycle pregnancy rates for CC/IUI and FSH/IUI were similar in the FASTT trial data, we felt that equipoise existed and that it was important to compare all three treatments in this trial.
In this paper we report the results of a randomized clinical trial (RCT) to identify an effective treatment strategy for couples with unexplained infertility who presented for care at the end of their reproductive years. The trial was designed to compare efficacy after the first two treatment cycles of CC/IUI, FSH/IUI, or IVF, and at the end of all treatment. The hypothesis tested was that immediate IVF is a more effective treatment strategy for reproductively older women who demonstrate a reasonable chance for success than treatments that begin with two cycles of COH/IUI.
MATERIALS AND METHODS
We conducted a three-arm RCT to evaluate treatment strategies for older infertile couples. Treatment began with two cycles of one of the following regimens: CC/IUI, FSH/IUI, or immediate IVF. Couples who did not become pregnant were treated with IVF, up to a study maximum of six IVF cycles. The protocol was approved by the participating institutions’ institutional review boards. An independent Data and Safety Monitoring Board (DSMB) met annually.
STUDY POPULATION
Couples in which the woman was 38–42 years of age who sought care for unexplained infertility from August 2004–November 2009 at Boston IVF and November 2008–November 2009 at Brigham and Women’s Hospital were screened. Eligibility criteria included 6 months of attempted conception; at least one ovary and ipsilateral patent fallopian tube confirmed by hysterosalpingogram or laparoscopy; regular menstrual cycles of 21–45 days; and no pelvic pathology, ectopic pregnancy, or previous infertility treatment (except up to three cycles of clomiphene without IUI). Acceptable ovarian reserve was demonstrated by a clomiphene challenge test (100 mg clomiphene on cycle days 5–9; cycle day 3 and 10 FSH values < 15 mIU/mL and cycle day 3 estradiol value of < 100 pg/mL). Normal prolactin and thyroid-stimulating hormone levels and BMI ≤ 38 in the woman and a sperm concentration of ≥15 million total motile sperm or ≥ 5 million total motile sperm at reflex IUI preparation in her partner were required. Randomization was performed using permuted blocks of varying sizes, stratified by the woman’s age (38–41 vs. 42–43rd birthday). The allocation sequence was generated by an independent biostatistician and implemented by an epidemiologist. Randomization was never conducted by clinical staff and all clinical investigators were blinded to the outcome determinations.
TREATMENT PROTOCOL
Couples were treated with a standardized protocol agreed upon by all participating physicians. CC treatment was 100 mg orally daily for 5 days starting between cycle days 3–5 with serial ultrasound monitoring beginning between cycle days 10–12 and luteinizing hormone (LH) home monitoring beginning on cycle day 11. One IUI was performed either the day after the LH surge was detected or 36–40 hours after SC/IM administration of 10,000 IU hCG when the lead follicle was ≥ 18mm, whichever came first. If pregnancy was not achieved after two treatment cycles, patients proceeded to IVF.
Gonadotropin therapy was initiated on cycle day 3 with 300 IU of recombinant FSH SC for 3 days; the dose was adjusted as indicated by age (38–40 year olds could be given 150–300 IU FSH), pelvic ultrasound, and serum estradiol assessment until a lead follicle ≥ 17 mm or 2–3 follicles ≥ 15 mm in size were detected. A single IUI was performed the second morning after SC/IM administration of 10,000 IU of hCG. The protocol was repeated for a second FSH cycle unless the cycle was cancelled due to poor ovarian response or there were more than 6 follicles > 14 mm. Patients who demonstrated poor ovarian response in 1 cycle were treated with a low responder protocol consisting of an oral contraceptive followed by microdose leuprolide acetate. Patients with two cycles hindered by poor ovarian response were withdrawn unless they conceived and miscarried, in which case they were offered a third FSH cycle. Patients with adequate response who completed two cycles of FSH/IUI proceeded to IVF if not pregnant.
Patients randomized to the immediate IVF arm initiated therapy with an IVF protocol consisting of 21 days of an oral contraceptive followed by a microdose leuprolide acetate protocol (40 μg SC twice/day until the hCG injection) with a starting dose of twice daily gonadotropins (300 IU FSH in the morning and 150 IU HMG in the afternoon) for 3 days beginning on day 3 or 4 of leuprolide acetate. Adjustments to gonadotropin dosage were determined by estradiol monitoring and ultrasound; 10,000 IU hCG was given SC or IM when the lead follicle was ≥ 17 mm and at least three follicles were ≥ 15 mm in size. Oocyte retrieval was performed 36 hours after hCG and embryos were routinely transferred on day 3. The number of embryos transferred was based on ASRM guidelines for day 3 embryo transfers.6 Standardized cancellation criteria and low response protocols were used. Intracytoplasmic sperm injection (ICSI) was used only after failed fertilization or when < 10 million total motile sperm were available at IVF. Preimplantation genetic diagnosis (3.6% of cycles) and assisted embryo hatching (one-third of cycles) were performed when considered necessary.
Patients in all arms who did not become clinically pregnant after two treatment cycles continued with the IVF protocol, up to a maximum of 6 IVF cycles, usually 4 fresh and 2 thaw cycles, if available.
STUDY OUTCOMES
Patients were enrolled until they completed the treatment protocol, discontinued treatment due to poor response, took a hiatus from treatment of ≥ one year, or conceived an ongoing pregnancy of ≥ 20 weeks gestation. Couples were followed until discharge from the hospital of both mother and baby(ies), if pregnant, or until one year after completing the treatment protocol. Hiatus from treatment occurred for various medical and personal reasons. The closing date of the study for endpoint events was September 15, 2011.
The primary endpoint was clinical pregnancy rate after two cycles of treatment. The primary comparisons were of clomiphene/IUI vs. immediate IVF and FSH/IUI vs. immediate IVF, compared using Fisher’s exact test with a two sided α = 0.025. Although the goal was a live born baby, we used clinical pregnancy rate as the primary outcome because we believe it best reflects implantation and thus treatment efficacy. Additional analyses evaluated live birth rates after two cycles of treatment and clinical pregnancy and live birth rates after all treatments, as well as time to conception. Clinical pregnancy was defined as a fetal sac visible on ultrasound. Time to conception was defined as the length of time from the date of randomization to the date a pregnancy that resulted in a live birth was established (TTP). For those who did not conceive, time was censored either at the last menstrual period plus 28 days for those who completed the protocol, dropped out of the study after initiation of treatment, or were disenrolled for poor ovarian response; or date of disenrollment for those who went on hiatus for > one year (1 year plus 1 day) or those who dropped out of the study (date of drop out) prior to initiation of treatment.
STATISTICAL ANALYSIS
Analyses were by intention to treat and included all couples who were randomized. Per protocol analyses are also presented to evaluate outcomes for those couples who initiated at least one cycle of treatment. Exact binomial 97.5% confidence intervals were calculated. TTP was analyzed using a Cox proportional hazards model; hazard ratios and their 95% confidence limits are presented. Cumulative incidence of time to conception was plotted as one minus Kaplan-Meier estimates. Statistical significance was defined as P < 0.025 for the primary comparison and P < 0.05 elsewhere (all two-sided). Data analyses were carried out using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC).
Original power estimates indicated that we would have 85% power to detect a difference in clinical pregnancy rates between each IUI arm and the immediate IVF arm with a sample size of 150 couples per arm. After several years of recruitment it was apparent that a smaller sample size would achieve sufficient power due to the rising success rates of IVF. At an unplanned interim analysis, the DSMB determined that clinical pregnancy rates for each arm were sufficiently different from the assumptions used in the original power calculations to show that we would have adequate power to achieve a statistically significant result for the primary outcome with approximately 60 patients per arm. Given these estimates along with evolving clinical practice that threatened equipoise and a slower than anticipated rate of recruitment, we were advised by the DSMB to conclude recruitment at 150 couples.
RESULTS
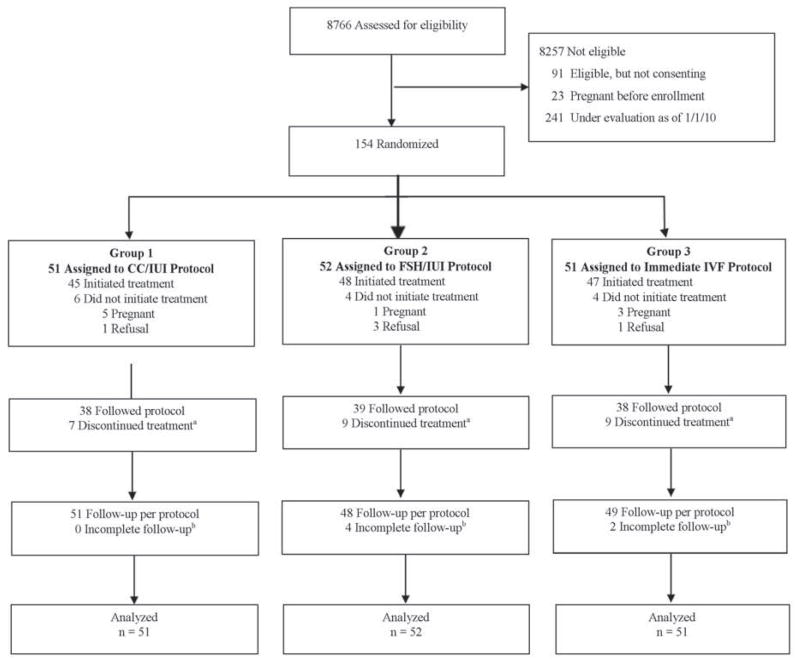
The study population comprised 154 couples with unexplained infertility who were randomized to either the CC/IUI arm (N=51), FSH/IUI arm (N=52), or immediate IVF arm (N=51) (Figure 1). The most common reasons for ineligibility were age outside the range, prior infertility treatment or not a candidate for study treatments, or not covered by a participating insurer. Fourteen couples (9.1%) did not initiate treatment, of these 9 conceived before starting and 5 deferred treatment. Of the 140 who initiated treatment, 115 (82.1%) completed the treatment protocol and 25 discontinued treatment after an average of 3.9 (IQR 3.0–5.0) treatment cycles over 12.8 months (IQR 8.8–17.4). There were 6 couples for whom follow-up after treatment was incomplete; their median follow-up time was 18.3 (IQR 12.0–21.4) months compared to 17.5 (IQR 12.3–21.9) months for the 148 couples who completed follow-up.
Figure 1. Screening, Randomization, and Enrollment of Study Couples.
When we considered only the first 2 cycles of treatment, the results were identical except for two couples in the IVF arm who discontinued treatment after the first IVF cycle.
a 25 couples chose to discontinue treatment after a median of 4.0 cycles (interquartile range, 3 to 5 cycles) over a median duration of 12.2 months (interquartile range, 8.8 to 17.4 months).
b The median duration of follow-up for these couples whom we were unable to contact was 18.3 months (interquartile range, 12.0 to 21.4 months) and was 17.5 months (interquartile range 12.3 to 21.9 months) among couples who completed follow-up.
Baseline demographic and reproductive characteristics of the couples in the three arms were similar (Table 1). No significant differences were noted for age at randomization, race, smoking history, female BMI, parity, or semen analysis. Statistically significant differences were observed for gravidity (higher percent of those in the immediate IVF arm had never conceived) and cycle day 3 FSH values (slightly higher mean value for the FSH arm).
Table 1.
Demographic and reproductive characteristics of couples (N = 154), by treatment group.
| Characteristic | Mean ± SD or N (%) | |||||
|---|---|---|---|---|---|---|
| Clomiphene/IUI (N = 51) | Gonadotropin/IUI (N = 52) | Immediate IVF (N = 51) | ||||
| Female | Male | Female | Male | Female | Male | |
| Age at randomization | 40.3 ± 1.3 | 39.6 ± 4.4 | 40.6 ± 1.2 | 40.0 ± 4.6 | 39.9 ± 1.4 | 40.1 ± 5.3 |
| Caucasian | 40 (78.4) | 42 (82.4) | 43 (84.3) | 47 (90.4) | 44 (88.0) | 42 (87.5) |
| Hispanic | 1 (2.1) | 1 (2.0) | 3 (6.0) | 1 (2.0) | 0 (0) | 0 (0) |
| Current or past cigarette smoking | 14 (27.5) | 17 (33.3) | 13 (25.0) | 13 (25.0) | 15 (30.6) | 21 (45.7) |
| Household income: | ||||||
| < $60,000 | 3 (5.9) | 3 (5.8) | 3 (5.9) | |||
| $60,000–99,000 | 11 (21.5) | 10 (19.2) | 3 (5.9) | |||
| $100,000–139,000 | 13 (25.5) | 10 (19.2) | 12 (23.5) | |||
| ≥ $140,000 | 23 (45.1) | 29 (55.8) | 32 (62.7) | |||
| Unknown | 1 (2.0) | 0 (0) | 1 (2.0) | |||
| Female body mass index (kg/m2) | 24.9 ± 4.8 | 24.9 ± 4.2 | 23.7 ± 4.5 | |||
| Female reproductive history: | ||||||
| Prior use of oral contraceptives | 43 (84.3) | 43 (82.7) | 40 (80.0) | |||
| Prior laparoscopy (< 1 year) | 0 (0) | 0 (0) | 0 (0) | |||
| Absence of prior pregnancies | 29 (58.0)a | 21 (40.4)a | 35 (68.6)a | |||
| Absence of prior live births | 15 (30.0) | 11 (21.2) | 16 (31.4) | |||
| Cycle day 3 FSH (mIU/ml) | 7.3 ± 2.2b | 8.8 ± 2.7b | 7.8 ± 2.4b | |||
| Cycle day 3 Estradiol (pg/ml) | 46.7 ± 17.7 | 39.8 ± 14.3 | 44.7 ± 17.8 | |||
| Male semen analysis: | ||||||
| Sperm concentration (M/ml) | ||||||
| Mean ± SD | 72.5 ± 81.5 | 72.4 ± 57.0 | 78.4 ± 57.2 | |||
| 25th – 50th – 75th percentiles | 36.0 – 55.5 – 87.0 | 33.0 – 54.0 – 101.0 | 41.0 – 76.0 – 104.0 | |||
| Sperm motility (%) | ||||||
| Mean ± SD | 64.1 ± 14.5 | 62.3 ± 12.0 | 64.5 ± 14.5 | |||
| 25th – 50th – 75th percentiles | 57.0 – 63.5 – 74.0 | 54.0 – 64.0 – 71.0 | 55.0 – 67.0 – 76.0 | |||
| Study site: | ||||||
| Boston IVF | 48 (94.1) | 47 (90.4) | 46 (90.2) | |||
| Brigham and Women’s Hospital | 3 (5.9) | 5 (9.6) | 5 (9.8) | |||
| Insurance company at randomization: | ||||||
| Blue Cross/Blue Shield of MA | 28 (54.9) | 39 (75.0) | 32 (62.8) | |||
| Harvard Pilgrim Health Care | 13 (25.5) | 9 (17.3) | 8 (15.7) | |||
| Tufts Health Plan | 10 (19.6) | 4 (7.7) | 11 (21.6) | |||
P = 0.01
P = 0.009
PREGNANCY RATES
Statistically significant differences in the clinical pregnancy rates per couple were observed after the first two cycles of treatment: 21.6%, 17.3%, and 49.0 %, for CC/IUI, FSH/IUI, and immediate IVF, respectively (P = 0.0067/P = 0.0007) (Table 2). The number of initiated cycles was 263 and eleven of the 45 clinical pregnancies were treatment-independent. The live birth rates per couple were 15.7%, 13.5%, and 31.4%, respectively (P = 0.101/P = 0.035). When comparing couples who initiated treatment in the COH groups (CC/IUI and FSH/IUI) combined to the immediate IVF group after two treatment cycles, there were also statistically significant differences in clinical pregnancy rates per cycle: 7.3% (95% CI 3.9–13.2) vs. 24.7 (95% CI 16.6–35.1), P = 0.0003, and per couple: 14.0 (95% CI 7.7–22.7) vs. 44.7 (95% CI 30.2–60.0), P = 0.0001, and live birth rates per cycle: 5.1% (95% CI 2.2–11.0) vs. 15.3% (95% CI 8.3–26.4), P = 0.008, and per couple: 9.7% (95% CI 4.5–17.6) vs. 27.7% (95% CI 15.6–42.6), P = 0.01.
Table 2.
Clinical pregnancy and live birth rates per couple, by randomization assignment for the first two treatment cycles and at the end of all treatment. Includes treatment-independent pregnancies.
| 1st two treatment cycles | Duration of study | ||||
|---|---|---|---|---|---|
| Randomized treatment arm | No. couples (%) | No. clinical pregnanciesa, b (%, 97.5% CI) | No. live birthsc (%, 97.5% CI) | No. clinical pregnanciesd (%, 97.5% CI) | No. live birthsd (%, 97.5% CI) |
| Clomiphene citrate (CC)/IUI | 51 (33.1) | 11 (21.6, 10.2–37.3) | 8 (15.7, 6.2–30.5) | 38 (74.5, 58.4–86.9) | 25 (49.0, 32.9–65.2) |
| Gonadotropin (FSH)/IUI) | 52 (33.8) | 9 (17.3, 7.3–32.2) | 7 (13.5, 4.9–27.6) | 34 (65.4, 49.0–79.5) | 22 (42.3, 27.1–58.7) |
| Immediate IVF | 51 (33.1) | 25 (49.0, 32.9–65.2) | 16 (31.4, 17.7–47.9) | 38 (74.5, 58.4–86.9) | 24 (47.1, 31.2–63.4) |
| Totale | 154 | 45 (29.2, 21.3–38.2) | 31 (20.1, 13.4–28.4) | 110 (71.4, 62.5–79.3) | 71 (46.1, 37.0–55.4) |
Abbreviations: IUI = Intrauterine insemination; IVF = In vitro fertilization.
Number of clinical pregnancies includes all ultrasound confirmed pregnancies, including pregnancy losses.
For clinical pregnancy rate after first two treatment cycles: P = 0.0067 for comparison between CC/IUI and immediate IVF; P = 0.0007 for comparison between FSH/IUI and immediate IVF.
For live birth rate after first two treatment cycles: P = 0.101 for comparison between CC/IUI and immediate IVF; P = 0.035 for comparison between FSH/IUI and immediate IVF.
For clinical pregnancy and live birth rates after all treatment there are no statistically significant differences, reflecting subsequent IVF treatment in all arms.
Of these, there were 5, 2, and 4 clinical pregnancies and 5, 1, and 3 live births in the CC/IUI, FSH/IUI and immediate IVF arms, respectively, that occurred before treatment was initiated or between treatment cycles one and two. Over the duration of the study there were 11, 3, and 9 clinical pregnancies and 7, 1, and 6 live births in the CC/IUI, FSH/IUI, and immediate IVF arms, respectively, that occurred outside of treatment cycles.
The 140 couples who initiated treatment underwent a total of 487 treatment cycles (Table 3). Couples in the immediate IVF arm completed 36% fewer cycles than those in either one of the COH/IUI arms. 39/45 (86.7%) couples in the CC/IUI arm and 37/48 (77.1%) couples in the FSH/IUI arm did not conceive after two cycles of treatment compared to 23/47 (48.9%) couples in the IVF arm (P = 0.0004). All unsuccessful couples moved on to IVF, where 35/99 couples (35.4%) conceived a pregnancy leading to a live birth. For all randomized couples, at the end of treatment, 71.4% (110/154) conceived a clinically recognized pregnancy and 46.1% (71/154) delivered at least one live-born baby (Table 2). Twenty-three (14.9%) of the clinical pregnancies and 14 (9.1%) of the live births were treatment cycle-independent, occurring either before initiation or between study treatment cycles. Excluding these independent pregnancies, 84.2% (48/57) of all live births were conceived through IVF. Of all couples who delivered a live birth, the pregnancy resulted from IVF for 83.3% (15/18) of those starting treatment with CC/IUI and 71.4% (15/21) of those starting with FSH/IUI.
Table 3.
Number (%) of couples initiating treatment cycles, total number of cycles initiated, and pregnancy rates by treatment group per initiated cycle and per couple who initiated treatment, separately for the first two cycles of treatment and subsequent IVF treatments.
| Randomized treatment arm and treatment type | No. couples who initiated treatment (%) | No. cycles initiated | Clinical pregnancy ratesa | Live birth rates | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. | Per cycle %, 97.5% CI | Per couple %, 97.5% CI | No. | Per cycle %, 97.5% CI | Per couple %, 97.5% CI | |||
| CC/IUI (n = 51) | ||||||||
| CC/IUI | 45 (88.2) | 87 | 6 | 6.9, 2.5–17.7b | 13.3, 4.3–28.8b | 3 | 3.4, 0.7–16.1b | 6.7, 1.1–20.1b |
| IVF | 39 (86.7) | 95 | 21 | 22.1, 13.0–35.0 | 53.8, 38.5–68.5 | 15 | 15.8, 7.9–29.1 | 38.5, 21.6–57.6 |
| Total | 182 | 27 | 14.8, 9.7–22.1 | 60.0, 42.3–76.0 | 18 | 9.9, 5.5–17.0 | 40.0, 24.0–57.7 | |
| FSH/IUI (n = 52) | ||||||||
| FSH/IUI | 48 (92.3) | 91 | 7 | 7.7, 2.8–19.6c | 14.6, 5.3–29.7c | 6 | 6.6, 2.1–18.9c | 12.5, 4.0–27.1c |
| IVF | 37 (77.1) | 96 | 24 | 25.0, 14.7–39.1 | 64.9, 45.2–81.5 | 15 | 15.6, 7.5–29.6 | 40.5, 24.8–57.9 |
| Total | 187 | 31 | 16.6, 11.0–24.3 | 64.6, 47.4–79.4 | 21 | 11.2, 6.3–19.1 | 43.8, 27.7–60.8 | |
| Immediate IVF (n = 51) | ||||||||
| 1st 2 IVF | 47 (92.2) | 85 | 21 | 24.7, 16.1–35.7 | 44.7, 28.4–61.9 | 13 | 15.3, 7.6–28.3 | 27.7, 14.3–44.7 |
| ≥ 3rd IVF | 23 (48.9) | 33 | 8 | 24.2, 10.5–46.6 | 34.8, 14.5–60.1 | 5 | 15.2, 4.5–40.4 | 21.7, 6.3–46.7 |
| Total | 118 | 29 | 24.6, 16.1–35.7 | 61.7, 44.4–77.1 | 18 | 15.3, 8.2–26.7 | 38.3, 22.9–55.7 | |
| All treatment initiated | 140 (90.9) | 487 | 87 | 17.9, 14.3–22.1 | 62.1, 52.4–71.3 | 57 | 11.7, 8.8–15.4 | 40.7, 31.8–50.2 |
Abbreviations: CC = Clomiphene citrate; FSH = Gonadotropins; IUI = Intrauterine insemination; IVF = In vitro fertilization.
Note: For IVF treatment cycles, 2 of the cycles in the CC/IUI arm, 2 of the cycles in the FSH/IUI arm, and 3 of the cycles in the immediate IVF arm used cryopreserved embryos that had been collected in an earlier cycle and frozen for use in subsequent cycles.
Number of clinical pregnancies includes all ultrasound confirmed pregnancies, including pregnancy losses.
P values for comparison between CC/IU vs. 1st 2 immediate IVF cycles: P = 0.0015 for clinical pregnancy rate per cycle; P=0.0012 for clinical pregnancy rate per couple; P=0.0085 for live birth rate per cycle; P=0.0119 for live birth rate per couple.
P values for comparison between FSH/IUI vs. 1st 2 immediate IVF cycles: P=0.0033 for clinical pregnancy rate per cycle; P=0.0016 for clinical pregnancy per couple; P=0.09 for live birth rate per cycle; P=0.08 for live birth rate per couple.
Among those who had a live birth, the average number of treatment cycles needed to establish the pregnancy was 3.4 (± 1.5) in the CC/IUI arm, 3.3 (± 1.9) in the FSH/IUI arm, and 1.9 (± 0.8) in the immediate IVF arm (P = 0.004).
TIME TO CONCEPTION
After 2 cycles of treatment, the comparison of the TTP between the COH and immediate IVF groups was statistically significantly different both when excluding (Hazard Ratio (HR) = 2.86, 95% CI 1.22–6.68, P = 0.02) (Supplemental Figure 1a) or including (HR = 2.24, 95% CI 1.17–4.32, P = 0.02) the treatment cycle-independent pregnancies (Supplemental Figure 1b). The mean TTP after two treatment cycles of CC/IUI was 2.1 ± 0.1 months, for FSH/IUI was 3.0 ± 0.1 months and for immediate IVF was 5.7 ± 0.2 months, reflecting the longer time it takes to prepare for and complete 1 IVF cycle. After completion of all treatment, however, the average times were 9.1 ± 0.6, 12.4 ± 1.0, and 8.7 ± 0.5 months for CC/IUI, FSH/IUI, and immediate IVF, respectively. For both COH arms combined vs. immediate IVF, the average times to conception were 12.2 ± 0.7 and 8.7 ± 0.5 months, indicating that those who conceived in the IVF arm did so on average 3.5 months faster.
ADVERSE EVENTS
There were no significant differences in the numbers of adverse outcomes or multiple births between the groups (Supplemental Table 1). There were 12 sets of twins (16.9% of all live births) during the treatment cycles, one each from CC/IUI and FSH/IUI treatment and a treatment independent pregnancy and 9 from IVF cycles. Higher-order multiple births were limited to one set of triplets in the immediate IVF arm (IVF cycle 1). The most common protocol deviation was cancelled IVF cycles, which occurred in approximately 20% of all IVF cycles and did not differ significantly by treatment assignment.
DISCUSSION
In this RCT of reproductively older couples with unexplained infertility, the clinical pregnancy rates in the immediate IVF arm were statistically significantly higher after two cycles of treatment than in the treatment arms that initiated therapy with either CC/IUI or FSH/IUI. In addition, when comparing the number of treatment cycles per live birth for each of the arms, a statistically significant difference existed with fewer treatment cycles per live birth in the immediate IVF arm. At the conclusion of the trial, 46% of couples delivered a baby, the majority of whom were conceived using IVF.
We hypothesized that for older women treatment beginning with immediate IVF would be the most successful strategy so long as there was evidence of a reasonable chance for success before and during treatment. For this, we used a baseline clomiphene challenge test that required an acceptable ovarian response and established criteria to assess predicted success during the trial. Evidence has shown that the use of FSH/IUI does not increase pregnancy rates sufficiently over those of CC/IUI for younger women (5,7,8). Studies for these younger couples have demonstrated an increased risk of multiple births, especially high order multiples from gonadotropin therapy (9). Costs for gonadotropins used in IUI protocols are estimated to be two to five times higher than costs for clomiphene (1,10).
There are few randomized trials comparing treatment strategies for women at the end of their reproductive years. The lack of data for women 40 years or older has hampered clinical care. Historically, there has been the practice of beginning therapy with FSH/IUI, avoiding CC/IUI in reproductively older women, based on limited data. It is known, however, that older women require larger doses of gonadotropins with resulting higher economic cost. Given that success rates for COH/IUI reported from RCTs in younger women are mostly < 10% per cycle and similar when either CC or FSH is used, we predicted that these success rates would drop further as the female partner approached the end of reproduction. Success rates for CC/IUI and FSH/IUI in our trial were similar and minimally lower than reported rates for younger women (2,5,8,9). When combined, COH/IUI live birth rates in our trial were only 5.1% per cycle and COH/IUI was responsible for only 15.8% of all treatment-related live births.
We had a number of couples in this older group who were eligible but declined participation because of the concern of being randomized to immediate IVF. Thus, there are couples who may want to begin treatment with COH/IUI. The trial data support the use of CC/IUI for such treatment; it is similar in efficacy to FSH/IUI and easier and less costly for the patients.
A strength of FORT-T is the Massachusetts Infertility Mandate that requires insurers to pay for treatment. Generous insurance coverage allowed trial couples to complete therapy per protocol. The lack of comprehensive insurance coverage for infertility treatment is the reason why other infertility RCTs are only able to compare treatments over a limited number of cycles rather than compare entire treatment strategies. Comparison of treatment strategies makes it possible to determine the maximum success of tested treatment paradigms. Another strength was the fact that the trial was conducted at two large IVF centers allowing for standardization of protocols and a large volume of patients for recruitment.
Limitations, as with many RCTs, include the relatively small number of participants on which to base generalizations, but this does not impact the internal validity of the results. Secondly, by necessity, neither the patients nor their providers were blind to their treatment assignment. To prevent this knowledge from influencing treatment assignment, choice, or outcome determinations, standardized eligibility criteria at baseline and for continuing treatment, including formal continuation and stopping rules, were applied by the study investigators. Randomization was conducted off-site by the epidemiologist and transmitted to the couple after consent was obtained. Acceptable ovarian reserve was demonstrated by clomiphene challenge test, the standard practice during the time of the study. While there may be a shift toward the use of anti-Mullerian hormone (AMH) levels and antral follicle count today, these results remain generalizable to practice today given that the best assessment of ovarian reserve is response during treatment. Lastly, costs are not considered. We made the decision to focus on treatment efficacy after two cycles of treatment rather than cost effectiveness, noting that the high cost of adverse outcomes would be limited in this age group due to the relative infrequency of OHSS and high order multiple births and the need to understand the best treatment in the shortened time frame for success in these reproductively older women.
In conclusion, in one of the only RCTs to compare treatment strategies for older couples with unexplained infertility, the data show that for couples who present at the end of their reproductive years and who demonstrate a reasonable chance for continued success, the most successful treatment is immediate IVF. About half will have a live birth (more than 80% of these will be singletons). For couples who do not want immediate IVF, our data support the use of CC/IUI rather than FSH/IUI, given the comparable success rates. However, for couples initiating treatment with CC/IUI who then move on to IVF, the majority of their infants will be conceived by IVF and, on average, they will undergo more treatment cycles than patients who elect immediate IVF.
Supplementary Material
Acknowledgments
Supported by award R01HD44547 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Participating Physicians at Boston IVF: Michael M. Alper, Steven R. Bayer, Brian M. Berger, Merle J. Berger, Selwyn P. Oskowitz, Alan S. Penzias, David Ryley, Kim L. Thornton, and Alison Zimon; at Brigham and Women’s Hospital: Raymond Anchan, Rachel Ashby, Janis Fox, Antonio Gargiulo, Elizabeth Ginsburg, Mark D. Hornstein, Serene Srouji, Brian Walsh, and Elena Yanushpolsky.
Steering Committee: Dartmouth-Hitchcock Medical Center, Lebanon, NH: Marlene B. Goldman, Sc.D., Richard H. Reindollar, M.D.; Boston IVF, Waltham, MA: Kim L. Thornton, M.D., David Ryley, M.D., Alison Zimon, M.D.
Data and Safety Monitoring Board: Brigham and Women’s Hospital, Boston: Julie E. Buring, Sc.D., chair; Tufts-New England Medical Center, Boston: David P. Chelmow, M.D.; Dartmouth- Hitchcock Medical Center, Lebanon, NH: Paul Manganiello, M.D. (2003–2006); Mount Sinai School of Medicine and Maimonides Medical Center, Brooklyn: David B. Seifer, M.D. (2007–2009).
We are grateful to the couples who participated in the trial and to Colleen Sullivan, Rachel Neff, M.P.H., Rosanna Batista, M.P.H, Alicia Every, Ph.D., Betsy Broadman, Megan Daley, Alice Hensley, Hannah Richardson, Pamela Wang, Erica Wellington, and Eric Worthing.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or NIH.
(ClinicalTrials.gov number: NCT00246506.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guzick DS, Sullivan MW, Adamson GD, Cedars MI, Falk RJ, Peterson EP, et al. Efficacy of treatment for unexplained infertility. Fertil Steril. 1998;70:207–213. doi: 10.1016/s0015-0282(98)00177-0. [DOI] [PubMed] [Google Scholar]
- 2.Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor-Litvak P, Steinkampf MP, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. N Engl J Med. 1999;340:177–183. doi: 10.1056/NEJM199901213400302. [DOI] [PubMed] [Google Scholar]
- 3.Reindollar RH, Goldman MB. Gonadotropin therapy: a 20th century relic. Fertil Steril. 2012;97:813–818. doi: 10.1016/j.fertnstert.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClamrock HD, Jones HW, Adashi EY. Ovarian stimulation and intrauterine insemination at the quarter centennial: implications for the multiple births epidemic. Fertil Steril. 2012;97:802–809. doi: 10.1016/j.fertnstert.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 5.Reindollar RH, Regan MM, Neumann PJ, Levine B, Thornton KL, Alper MM, Goldman MB. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94:888–899. doi: 10.1016/j.fertnstert.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 6.The Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Criteria for number of embryos to transfer: a committee opinion. Fertil Steril. 2013;99:44–46. doi: 10.1016/j.fertnstert.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 7.Custers IM, van Rumste MME, van der Steeg JW, van Wely M, Hompes PGA, Bossuyt P, et al. Long-term outcome of couples with unexplained subfertility and an intermediate prognosis initially randomized between expectant management and immediate treatment. Hum Reprod. 2012;27:444–450. doi: 10.1093/humrep/der389. [DOI] [PubMed] [Google Scholar]
- 8.Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Relationship of follicle numbers and estradiol levels to multiple implantation in 3,608 intrauterine insemination cycles. Fertil Steril. 2001;75:69–78. doi: 10.1016/s0015-0282(00)01631-9. [DOI] [PubMed] [Google Scholar]
- 9.Gleicher N, Oleske DM, Tur-Kaspa I, Vidali A, Karande V. Reducing the risk of high-order multiple pregnancy after ovarian stimulation with gonadotropins. N Engl J Med. 2000;343:2–7. doi: 10.1056/NEJM200007063430101. [DOI] [PubMed] [Google Scholar]
- 10.Katz P, Showstack J, Smith JF, Natchtigall RD, Millstein SG, Wing H, et al. Costs of infertility treatment: results from an 18-month prospective cohort study. Fertil Steril. 2011;95:915–921. doi: 10.1016/j.fertnstert.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.