Abstract
In childhood, excess adiposity and low fitness are linked to poor academic performance, lower cognitive function, and differences in brain structure. Identifying ways to mitigate obesity-related alterations is of current clinical importance. This study examined the effects of an 8-month exercise intervention on the uncinate fasciculus, a white matter fiber tract connecting frontal and temporal lobes. Participants consisted of 18 unfit, overweight 8–11 year-old children (94% Black) who were randomly assigned to either an aerobic exercise (n=10) or a sedentary control group (n=8). Before and after the intervention, all subjects participated in a diffusion tensor MRI scan. Tractography was conducted to isolate the uncinate fasciculus. The exercise group showed improved white matter integrity as compared to the control group. These findings are consistent with an emerging literature suggesting beneficial effects of exercise on white matter integrity.
Keywords: Children, Diffusion tensor imaging, Exercise, Obesity, Uncinate fasciculus
Childhood obesity has become a common condition, as it affects more than one third of children and adolescents (Ogden, Carroll, Kit, & Flegal, 2012). Excess adiposity in children is linked to lower cognitive function (Davis & Cooper, 2011), and elevated body mass index (BMI) is associated with poor academic performance, as well as differences in brain structure and function (Carnell, Gibson, Benson, Ochner, & Geliebter, 2012; Datar & Sturm, 2006; Verstynen et al., 2012). As such, there is a need to develop effective clinical strategies to prevent childhood obesity and the concomitant effects on brain structure and function. Recent work in children and adults suggests that participating in aerobic exercise plays a causal role in altering brain structure and function (Davis et al., 2011; Erickson et al., 2011; Krafft, Pierce, et al., 2014; Krafft, Schaeffer, et al., 2014; Krafft, Schwarz, et al., 2014; Voss et al., 2013). Some benefits of aerobic exercise have been demonstrated in older adults, but fewer studies report the effects of exercise on brain structure in children. In the current study, we tested the effects of an 8-month randomized controlled exercise intervention on a fronto-temporal white matter fiber tract (uncinate fasciculus) in overweight and obese children.
Obesity is a risk factor for cognitive compromise during childhood (Datar & Sturm, 2006; Maayan, Hoogendoorn, Sweat, & Convit, 2011; Wong, Boh, Wang, & Chia, 2007). There is a growing body of evidence that obesity-induced cognitive deficits are apparent at the level of white matter connections (Stanek et al., 2011; Verstynen et al., 2013; Yau, Castro, Tagani, Tsui, & Convit, 2012). One such connection, the uncinate fasciculus, is a major fronto-temporal white matter fiber tract with projections between the hippocampal area and dorsolateral prefrontal cortex (Schmahmann et al., 2007). Structural integrity of the uncinate fasciculus correlates with verbal memory proficiency during childhood (Mabbott, Rovet, Noseworthy, Smith, & Rockel, 2009). Because obese children show lower verbal memory proficiency than their normal weight peers (Wong et al., 2007), the uncinate fasciculus is an excellent candidate for informing cognitive interactions related to obesity.
During childhood, the structure of the uncinate fasciculus matures linearly (Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008), which supports increases in fronto-temporal communication (as measured by functional connectivity) corresponding to improvements in memory proficiency (Mabbott et al., 2009; Menon, Boyett-Anderson, & Reiss, 2005). Because rapid changes in synaptic connectivity and myelination occur during this period, the circuitry may be more vulnerable to obesity related alterations. This assertion remains to be demonstrated in children; however, higher BMI is linked to lower white matter structural integrity and lower cognitive function across the lifespan (Davis & Cooper, 2011; Gunstad et al., 2007; Stanek et al., 2011; Verstynen et al., 2013).
In contrast to the effects of obesity, the effects of aerobic exercise are beneficial for brain structure and function, as it is implicated in altering fronto-temporal circuitry, which leads to better memory performance (Erickson et al., 2011; Voss et al., 2013). In children, aerobic fitness is related to fronto-temporal circuitry development, with higher- and lower-fit children showing differential patterns of brain activation during a verbal memory task (Herting & Nagel, 2013). In a cross-sectional study, aerobic fitness is linked to greater integrity of uncinate fasciculus (Marks et al., 2007).
Given the link between aerobic exercise and increased uncinate fasciculus connectivity, we investigated white matter microstructure of the uncinate fasciculus using diffusion tensor imaging (DTI). DTI characterizes the degree of white matter coherence by measuring the anisotropy (directional dependence) of water diffusion within the tissue. Higher levels of anisotropic diffusion correspond to a greater extent of microstructural integrity of white matter (i.e., axonal membrane structure and myelination; Beaulieu, 2002) and are often indexed by fractional anisotropy (FA), a scalar measure from 0–1 with 1 indicating fully anisotropic diffusion. Another measure of white matter structural integrity is radial diffusivity (RD); lower values of RD primarily reflect a greater degree of myelination (Beaulieu, 2009; Song et al., 2002).
To evaluate whether exercise alters white matter integrity in uncinate fasciculus, we conducted an 8-month randomized controlled exercise intervention in which overweight or obese children were randomly assigned to either a 5 day/week exercise group or sedentary control group. Based on evidence that aerobic exercise is related to altered fronto-temporal structure and function (Erickson et al., 2011; Herting & Nagel, 2013; Marks et al., 2007; Voss et al., 2013), we hypothesized that the exercise group would show a greater increase in white matter integrity in the uncinate fasciculus than an attention control group who participated in sedentary activities.
Methods
Participants
Participants were a subset of children in a larger randomized trial (Krafft, Schwarz, et al., 2014), who were recruited from public schools around Augusta, Georgia, U.S.A. and were eligible if they were 8–11 years old, overweight (BMI ≥ 85th percentile; Ogden et al., 2002), and inactive (no regular physical activity program ≥ 1 hr/week). Participants with neurological or psychiatric disorders were excluded. Each child’s parent or guardian reported the child’s age, sex, race, and health status. The study took place at the Georgia Prevention Center at Georgia Regents University. The present study included 18 children who provided usable DTI data at baseline and post-test (10 in the exercise group and 8 in the control group; 17 Black and 1 White; see Table 1 for participant characteristics). Children and parents completed written informed assent and consent in accordance with the Human Assurance Committee of Georgia Regents University.
Table 1.
Baseline characteristics of participants
| Exercise Group | Control Group | |
|---|---|---|
| n | 10 | 8 |
| Age (years) | 9.9 (0.6) | 9.4 (0.8) |
| Black | 10 | 7 |
| White | 0 | 1 |
| Left-handed | 1 | 1 |
| Body mass index | 25.6 (3.7) | 27.2 (10.4) |
| Body fat | 36% | 34% |
| VO2 peak (ml/kg/min) | 28.4 (6.2) | 29.7 (5.8) |
Intervention
After baseline measures were completed, participants were randomly assigned by the study statistician to one of two groups: aerobic exercise or sedentary attention control. Groups were balanced by race and sex. Both groups were offered an after school program every school day for approximately 8 months. All participants were offered daily bus transportation after school to the Georgia Prevention Center where they spent half an hour on supervised homework time and were given a snack. Lead instructors rotated between the two groups every two weeks and assistants rotated between the two groups every week. Both groups earned points for performing desired behaviors that were redeemed for small prizes weekly. The reward schedule was periodically calibrated to keep the rewards offered to the groups similar.
For the exercise group, children participated for 60–75 minutes each day, including time to dress and transition, and 40 minutes of instructor led aerobic activities (e.g., tag or jump rope). Each child wore a heart rate monitor during every training session (S610i; Polar Electro, Oy, Finland) and the child’s average heart rate during the session was recorded every day. A child’s average heart rate during the intervention was calculated as the mean of these daily values, as described previously (Davis et al., 2012). Points were awarded daily if the child’s mean heart rate that day was above 140 beats per minute, with more points earned for higher average heart rates. Intensity and energy expenditure were estimated as described previously (Davis et al., 2012). In the attention control group, instructors led sedentary activities (e.g., art and board games) for 60–75 minutes daily. Heart rates were not monitored in the control group. Points were earned in the control group for participation and good behavior.
Health Measures
BMI was measured using a Cardinal/Detecto Model CN20 scale (Webb City, MO) and a Seca 216 stadiometer (Hamburg, Germany). Body fat was measured with a dual-energy x-ray absorptiometry scan (Discovery W, Hologic Inc., Bedford, MA). VO2 peak was measured with an aerobic fitness treadmill test (Modified Balke Protocol for Poorly Fit Children, American College of Sports Medicine, 2000). The test used a Cardiac Science TM65 Treadmill (Bothell, Washington) and a Parvo Medics TrueOne 2400 Metabolic Measuring System (Sandy, Utah). Statistical analyses of health measures were conducted in SPSS version 17 (IBM, Armonk, NY).
MR Image Acquisition
Images were acquired at Georgia Regents University on a 3T GE Signa Excite HDx MRI system (General Electric Medical Systems, Milwaukee, WI). During scanning, head position was stabilized with a vacuum pillow and/or foam padding. Diffusion images were acquired using an echo planar imaging sequence (acquisition matrix = 128 × 128, 60 interleaved slices, voxel size = 1 × 1 × 2.4 mm, FOV = 256 × 256 mm, TR = 15500 ms, TE = min-full, 3 B0 images, 30 diffusion weighted images, b = 1000 s/mm2).
Image Analysis
Raw diffusion images were converted from GE DICOM format to NIFTI format using the dcm2nii tool (Rorden, 2007). For each subject, volumes were visually inspected for motion artifacts; volumes distorted by motion were removed from the image series and b value/vector tables (volumes removed M = 2, SD = 2.7). To test for inhomogeneity of gradient application due to volume removal, volume gradient vectors were plotted on a sphere after motion volumes were removed. If any surface of the sphere had a gap greater than the free surface area of 6 non-collinear directions, the participant was excluded from analysis. Diffusion tensor image analysis was conducted using the FMRIB Software Library (Smith et al., 2004). Diffusion images were registered to the first B0 image and corrected for eddy-current induced distortions. Non-brain tissue was removed using the Brain Extraction Tool (Smith, 2002).
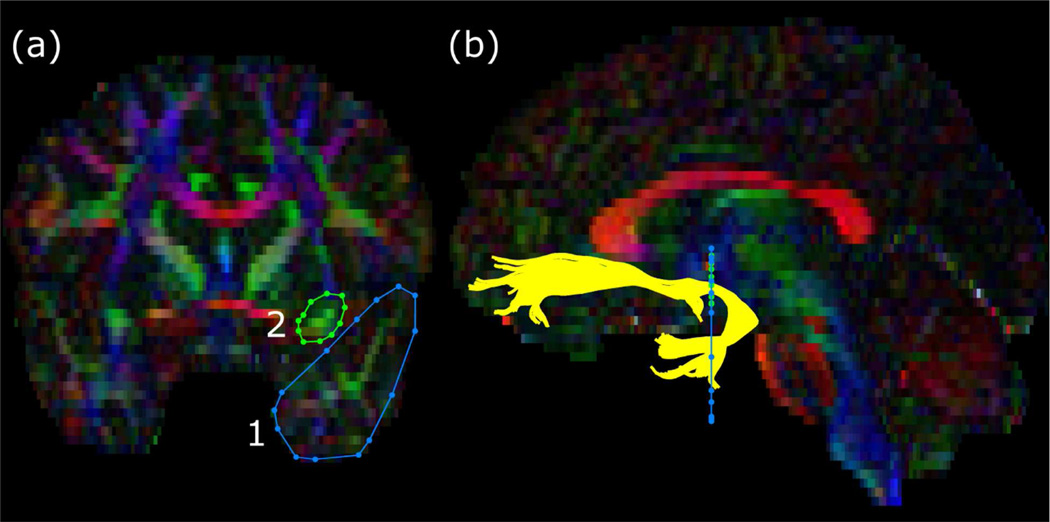
Tractography was conducted using the ExploreDTI software package (Leemans, Jeurissen, Sijbers, & Jones, 2009) with whole brain tensors in individual space. To isolate uncinate fasciculus, regions of interest were drawn (see Figure 1) following established anatomical markers (Wakana et al., 2007). Average diffusivity values (i.e., FA and RD) were extracted for each subject’s left and right uncinate fasciculus for baseline and post-test images. Statistical analyses of diffusivity measures were conducted in SPSS version 17 (IBM, Armonk, NY). The effects of the intervention were calculated by subtracting baseline values from posttest values (controlled for race and sex), then comparing the difference (i.e., change from baseline to post-test) using independent sample t-tests.
Figure 1.
Regions of interest for the uncinate fasciculus overlaid on a representative subject’s fractional anisotropy map. The colors of the map represent the principal direction of diffusion (red = left-right, green = anterior-posterior, blue = inferior-superior). The left panel (a) shows the locations of the regions of interest (1, temporal lobe; 2, frontal lobe) drawn on a coronal slice as per Wakana et al. (Wakana et al., 2007). The right panel (b) shows the resultant fibers of the uncinate fasciculus (yellow) traversing the regions of interest against a sagittal slice.
Results
Health Measures
The children included in this report who were assigned to the exercise group had a mean attendance of 60% (SD = 30%) and mean heart rate during exercise sessions of 161 (SD = 8) beats/min, with intensity mean of 6.3 (SD = 1.6) metabolic equivalents. The mean estimated daily and total energy expenditures for these children in the exercise group were 247 (SD = 40) kcal/d and 20,693 (SD = 10,613) kcal, respectively. Children assigned to the sedentary control group had a mean attendance of 72% (SD = 23%). The groups did not significantly differ in intervention attendance, t(16) = .94, p = .35. At baseline, the exercise group (n = 10) and attention control group (n = 8) did not differ significantly in age, gender, BMI, percent body fat, or VO2 peak. From baseline to post-test, the exercise group showed significantly greater loss of body fat (M = −3.5%, SD = 4.7) than the control group (M = 0.5%, SD = 2.4; t(16) = 2.19, p = .04, two-tailed). The groups did not significantly differ in BMI or VO2 peak at post-test.
White Matter Structure
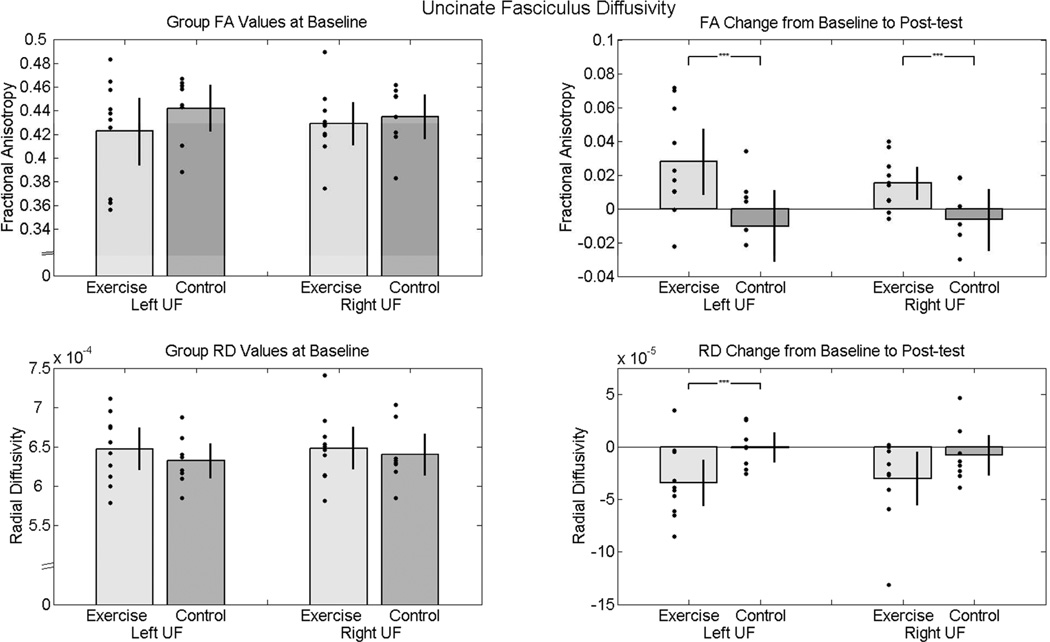
At baseline, the groups did not differ significantly in FA or RD values of left or right uncinate fasciculus. From baseline to post-test, the exercise group showed a significantly greater positive change in bilateral uncinate fasciculus FA when compared to the control group (left: t(16) = 2.56, p = .02, two-tailed; right: t(16) = 2.42, p = .02, two-tailed; see Figure 2). Additionally, the exercise group showed a significantly greater negative change in left uncinate fasciculus radial diffusivity (t(16) = 2.21, p = .04, two-tailed). Increases in FA and decreases in RD suggest an increase in white matter structural coherence and myelination in the exercise group. The exercise and control groups did not significantly differ in baseline to post-test changes of right uncinate fasciculus radial diffusivity (t(16) = 1.44, p = .16, two-tailed). No significant correlations were found between changes in measures of white matter integrity (FA and RD) and measures of fatness or fitness (BMI, percent body fat, and VO2 peak).
Figure 2.
Diffusivity values of uncinate fasciculus at baseline and post-test. The bar graphs show mean diffusivity values for each group (gray and dark gray bars) and standard error bars with individual data points (black dots) overlaid. The left panel shows that the two groups did not significantly differ in left or right uncinate fasciculus white matter integrity (FA and RD) at baseline. The right panel illustrates the increase from baseline to post-test in white matter integrity (increased FA and decreased RD) in the exercise group as compared to the control group. The black brackets with asterisks indicate a significant t test at alpha = .05 (controlled for race and gender).
Discussion
The focus of this study was to test the effects of an 8-month exercise intervention on fronto-temporal white matter integrity in overweight and obese children. We hypothesized that children who were randomly assigned to an exercise group would show a greater increase in white matter integrity in a fronto-temporal fiber tract (uncinate fasciculus) than children assigned to a sedentary attention control group. As expected, the exercise group showed significantly greater positive change in FA than the sedentary control group. The exercise group also showed a greater negative change in RD than the sedentary control group. Increases in FA are generally thought to be related to increases in the structural coherence and myelination of the fiber tract (Beaulieu, 2002). More specifically, analysis of eigenvalues from tractography data has demonstrated that as myelination increases, parallel water diffusivity (λ1; along the axon) stays relatively static while perpendicular water diffusivity (λ23; against the axon) decreases (Lebel et al., 2008; Song et al., 2002); these trends effectively drive the increases in FA and decreases in RD. In the present study, the results illustrate an exercise-induced increase in FA and decrease in RD, suggesting an increase in white matter structural coherence and myelination.
The present results are consistent with an emerging literature suggesting beneficial effects of aerobic training on white matter integrity across the lifespan (Krafft, Schaeffer, et al., 2014; Voss et al., 2013). Here, we found exercise-induced improvements in FA of uncinate fasciculus in children, an effect which has only previously been demonstrated in adults. In a one-year exercise intervention study,Voss et al. (2013) randomly assigned sedentary older adults to either an aerobic exercise or stretching (control) condition and found that increases in aerobic fitness were associated with greater changes in wide-spread prefrontal and temporal FA in the aerobic group. Although the direction of aerobic exercise-induced white matter change seems to be consistent across age groups, the magnitude of the effect may not be. White matter integrity in the uncinate fasciculus changes dynamically across the lifespan with increases during childhood, a plateau during adulthood, and then a decrease during older adulthood (Hasan et al., 2009). Accordingly, the magnitude of exercise-induced change of white matter integrity may differ across age groups. The benefits of exercise may be greater for children, who are developing brain networks, than for adults, who are in a state of dedifferentiation (Knudsen, 2004). Indeed, the superior longitudinal fasciculus also demonstrated exercise-induced improvement in this trial (Krafft, Schaeffer, et al., 2014).
Few studies have investigated exercise and white matter, particularly in children. The extant literature linking physical fitness and white matter integrity arises predominantly from small cross-sectional studies in adults (e.g., Johnson, Kim, Clasey, Bailey, & Gold, 2012; Marks, Katz, Styner, & Smith, 2011; Marks et al., 2007), with only a handful of experimental studies documenting causal evidence of fitness improvements and brain changes (Erickson et al., 2011; Voss et al., 2013). The current study adds to the experimental work of exercise intervention on white matter integrity (Krafft, Schaeffer, et al., 2014) in children. The specific mechanisms of exercise training-induced brain changes in humans, however, are not yet clear.
There is evidence that motor training may affect white matter integrity in children. For example, children who practice piano extensively show higher FA in motor-related frontal fiber tracts than in non-musician controls (Bengtsson et al., 2005). In adults, a motor training (juggling) study demonstrated increases in FA following a 4-week training period (Scholz, Klein, Behrens, & Johansen-Berg, 2009). This increase was limited to posterior parietal cortex, but other motor training studies suggest more widespread structural changes, including regions of the prefrontal cortex. For example,Taubert et al. (2010) measured white and gray matter changes following six weeks of training on a whole-body dynamic balance task and found performance related changes in left prefrontal and right parietal regions. The authors attribute this change (decrease in FA) to learning-related increases in cell density or axonal arborization. Thus, the increases in prefrontal cortex white matter integrity reported here could be related to complex motor skill practice, instead of, or in addition to improvements in cardiovascular fitness.
The present results were based on a sample of unfit, overweight or obese children drawn from an understudied minority population (94% Black). Given this specific sampling pool, the results should be interpreted with the following caveats. First, the sample size is relatively small. Second, our sample was exclusively overweight children and results may not represent a neurotypical response to aerobic exercise intervention. Accordingly, in the context of white matter development, the exercise group could have increased to the level of what is developmentally typical. A direct comparison of white matter integrity in lean children to that in overweight children would clarify this issue. Third, the exercise group did not show a greater improvement in aerobic fitness than the control group in this study. This DTI study was not powered to detect such an effect; neuroscience measures may be more precise and sensitive to exercise intervention than fitness tests.
Strengths of this study include that it is among the first neuroimaging investigations to pair DTI and a long-term exercise intervention (8 months) in children to examine fronto-temporal white matter structure. Additionally, the present sample of children consisted of an understudied population as all but one participant was Black. Of importance, the comparison to a sedentary attention control group in this study demonstrates that the white matter changes were due to exercise, rather than other potentially beneficial effects of attending a supervised after school program.
The findings from the present study suggest that participation in an after school exercise program increases fronto-temporal white matter integrity in unfit children who are overweight or obese. Because increased white matter integrity is related to improved cognitive function in children (Mabbott et al., 2009), the present results might inform education policy by demonstrating physical activity effects on children’s brain structure. With childhood obesity prevalence at unprecedented levels (Ogden et al., 2012), there is a need to develop effective clinical strategies to prevent childhood obesity and the concomitant effects on brain structure and function. This study provides a timely contribution by adding experimental evidence in Black children, who are disproportionately affected by obesity and its sequelae (Liese et al., 2006; Wang, Gortmaker, & Taveras, 2011) to the growing body of literature suggesting that aerobic exercise not only improves cardiovascular health, but also has beneficial effects on brain structure.
Acknowledgments
DJS, CEK, CLD, and JEM conceived and carried out experiments and analyzed data. JDA, NEY, and TL conceived and carried out experiments. NFS, LC, JEP, and ALR carried out experiments. This work was supported by the National Institutes of Health (grant number R01 HL87923) and the National Science Foundation Graduate Research Fellowship Program.
References
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- Beaulieu C. The Biological Basis of Diffusion Anisotropy. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI: From quantitative measurement to in vivo neuroanatomy. Academic Press; 2009. pp. 105–126. [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensivepianopracticinghasregionallyspecificeffectsonwhitematterdevelopment. Nature Neuroscience. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Carnell S, Gibson C, Benson L, Ochner C, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obesity Reviews. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datar A, Sturm R. Childhood overweight and elementary school outcomes. International Journal of Obesity. 2006;30(9):1449–1460. doi: 10.1038/sj.ijo.0803311. [DOI] [PubMed] [Google Scholar]
- Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R, Gower BA. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. Journal of the American Medical Association. 2012;308(11):1103–1112. doi: 10.1001/2012.jama.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Cooper S. Fitness, fatness, cognition, behavior, and academic achievement among overweight children: Do cross-sectional associations correspond to exercise trial outcomes? Preventive Medicine. 2011;52:S65–S69. doi: 10.1016/j.ypmed.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, Naglieri JA. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychology. 2011;30(1):91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley Ba. Ameta-regressiontoexaminetherelationshipbetweenaerobicfitnessandcognitiveperformance. Brain Research Reviews. 2006;52(1):119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gunstad JP, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Research. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M, Nagel B. Differences in brain activity during a verbal associative memory encoding task in high-and low-fit adolescents. Journal of Cognitive Neuroscience. 2013;24(4):595–612. doi: 10.1162/jocn_a_00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuro Image. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HK, Tuulari JJ, Hirvonen J, Lepomaki V, Parkkola R, Hiltunen J, Nummenmaa L. Obesity is associated with white matter atrophy: A combined diffusion tensor imaging and voxel-based morphometric study. Obesity (Silver Spring) 2013;21(12):2530–2537. doi: 10.1002/oby.20386. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Krafft CE, Pierce JE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, McDowell JE. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience. 2014;256:445–455. doi: 10.1016/j.neuroscience.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft CE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, Pierce JE, McDowell JE. An 8-month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity (Silver Spring) 2014;22(1):232–342. doi: 10.1002/oby.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft CE, Schaeffer DJ, Schwarz NF, Chi L, Weinberger A, Pierce JE, McDowell JE. Improved fronto-parietal white matter integrity is associated with attendance in an after-school exercise program. Developmental Neuroscience. 2014;36(1):1–9. doi: 10.1159/000356219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones D. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Paper presented at the 17th Annual Meeting of Intl Soc Mag Reson Med; Hawaii, USA. 2009. [Google Scholar]
- Liese AD, D’Agostino RB, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, Williams DE. TheburdenofdiabetesmellitusamongUSyouth:prevalenceestimatesfromtheSEARCHforDiabetesinYouthStudy. Pediatrics. 2006;118(4):1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring) 2011;19(7):1382–1387. doi: 10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott DJ, Rovet J, Noseworthy MD, Smith ML, Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Research. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity:relationship to cerebral white matter integrity in the brain of active and sedentary older adults. British Journal of Sports Medicine. 2011;45(15):1208–1215. doi: 10.1136/bjsm.2009.068114. [DOI] [PubMed] [Google Scholar]
- Marks BL, Madden DJ, Bucur B, Provenzale JM, White LE, Cabeza R, Huettel SA. Role of aerobic fitness and aging on cerebral white matter integrity. Annals of the New York Academy of Sciences. 2007;1097:171–174. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- Menon V, Boyett-Anderson J, Reiss A. Maturation of medial temporal lobe response and connectivity during memory encoding. Cognitive Brain Research. 2005;25(1):379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Monti J, Hillman C, Cohen N. Aerobicfitnessenhancesrelationalmemoryinpreadolescentchildren:TheFITKidsrandomizedcontroltrial. Hippocampus. 2012;22(9):1876–1882. doi: 10.1002/hipo.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Journal of the American Medical Association. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11773541. [DOI] [PubMed] [Google Scholar]
- Rorden C. DCM2NII (Version October 7) [Computer software] 2007 [Google Scholar]
- Schmahmann J, Pandya D, Wang R, Dai G, D’ Arceuil H, de Crespigny A, Wedeen V. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Traininginduceschangesinwhite-matterarchitecture. Nature Neuroscience. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring) 2011;19(3):500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P. Dynamicpropertiesofhumanbrainstructure:learning-relatedchangesincorticalareasandassociatedfiberconnections. Journal of Neuroscience. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein A, Erickson KI, Sheu LK, Marsland AL, Gianaros PJ. Competing physiological pathways link individual differences in weight and abdominal adiposity to white matter microstructure. NeuroImage. 2013;79:129–137. doi: 10.1016/j.neuroimage.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosomatic Medicine. 2012;74(7):682–690. doi: 10.1097/PSY.0b013e318261909c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Human Brain Mapping. 2013;34(11):2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Gortmaker SL, Taveras EM. Trendsandracial/ethnicdisparitiesinsevereobesityamongUSchildrenandadolescents, 1976–2006. International Journal of Pediatric Obesity. 2011;6(1):12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- Wong P, Boh G, Wang J, Chia M. Obesity affects verbal memory in adolescents among top academic achievers in Singapore. Asian Journal of Exercise & Sports Science. 2007;4(1):47–55. [Google Scholar]
- Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130(4):856–864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]