Abstract
Sex differences in emotional memory have received increasing interest over the past decade. However, to date, no work has explored how a post-learning stressor might modulate the influence of sex hormone status on memory for gist and peripheral detail in an emotional versus neutral context. Here, we tested three predictions. First, compared to naturally cycling women (NC women) in the luteal phase, women on hormonal contraception (HC women) would have significantly blunted HPA reactivity to physical stress. Second, post-learning stress would enhance detail and gist memory from an emotional story in NC women, and finally, post-learning stress would not affect emotional memory for details or gist in HC women. Healthy NC and HC women viewed a brief, narrated story containing neutral or emotionally arousing elements. Immediately after, Cold Pressor Stress (CPS) or a control procedure was administered. One week later, participants received a surprise free recall test for story elements. NC women exhibited significantly greater cortisol increases to CPS compared to HC women. NC women who viewed the emotional story and were administered CPS recalled the most peripheral details overall and more gist from the emotional compared to the neutral story. In HC women, however, the post-learning cortisol release did not affect memory for gist or peripheral details from the emotional or neutral story in any way. Additionally, NC and HC women performed similarly on measures of attention and arousal. These findings suggest that in women, post-learning stress differentially affects memory for emotional information depending on their hormonal contraceptive status.
Keywords: hormonal contraception, emotional memory, stress, cortisol, pupil dilation
Introduction
Substantial evidence now documents sex differences in the neurobiology of emotional memory, and a subset of this research has explored the interactions between ovarian sex hormones and stress hormones and subsequent effects on emotional memory. The hypothalamic-pituitary-adrenal (HPA) axis and hypothalamic-pituitary-gonadal (HPG) axis have been shown to have bidirectional effects (Turner, Keating, & Tilbrook, 2012), and several studies have explored the relationship between these axes by testing women on hormonal contraceptives.
Hormonal contraceptives have a profound influence on HPG axis activity (Lobo & Stanczyk, 1994). These studies typically include women using combined formulations of hormonal contraception, which have both a synthetic estrogen (ethinyl estradiol) and synthetic progestin component. To prevent ovulation, these formulations use negative feedback to the hypothalamus to inhibit the mid-cycle peak of gonadotropin-releasing hormone (GnRH; Lobo & Stanczyk, 1994). In addition, these synthetic hormones suppress the release of lutenizing hormone (LH) and follicle stimulating hormone (FSH) from the pituitary to inhibit the gonadal production of endogenous estrogen, progesterone, and testosterone. By suppressing the production of endogenous sex steroid hormones, hormonal contraception likely alters not only stress hormone activity (Kudielka & Kirschbaum, 2005), but also sex/stress hormone interactions, and subsequently, memory for emotionally arousing stimuli and events.
Evidence for the relationship between sex and stress hormone activity in humans comes from studies demonstrating that menstrual cycle phase and hormonal contraceptive use have profound effects on stress hormone reactivity (Kirschbaum et al., 1999; Otterstetter et al., 1999; Nielsen et al., 2013; Bentz et al., 2013). Sex hormone influences on HPA and sympathetic nervous system (SNS) activity are observable when menstrual cycle phase and oral contraceptive use are accounted for in women; both circulating levels of endogenous and exogenous sex hormones appear to play a key role in modulating HPA and SNS function (Kudielka & Kirschbaum, 2005; Fujimoto et al., 1986; Kirschbaum et al., 1999; Kumsta et al., 2007).
Since studies from the human literature also demonstrate that sex/stress hormone interactions modulate emotional memory, it is likely that under stress, women on and off hormonal contraception will show different memory patters for emotionally arousing stimuli (Andreano & Cahill, 2006; Jackson, Payne, Nadel, & Jacobs, 2006; Andreano, Arjomandi, & Cahill, 2008; Felmingham, Tran, Phong, & Bryant, 2012). However, in spite of the evidence demonstrating that hormonal contraceptive use blunts stress hormone reactivity, there are limited studies on how hormonal contraception use affects learning and memory for emotional stimuli.
Some studies have utilized pharmacological manipulations to explore the relationship between hormonal contraceptive use, stress hormones, and memory. For example, Kuhlmann and Wolf (2005) tested how cortisol affected verbal retrieval in oral contraceptive users and non-users; results showed that exogenous cortisol administration impaired verbal retrieval in naturally cycling women, but not in those using oral contraception (Kuhlmann & Wolf, 2005). More recent studies with exogenous cortisol administration found that cortisol enhanced fear learning in oral contraceptive users compared to men and naturally cycling women (Merz, Tabbert, Schwechendiek, Klucken, Vaitl, Stark, & Wolf, 2012).
Other studies have used classical conditioning paradigms to explore learning and memory for arousing stimuli in hormonal contraceptive users. Several of these studies found that hormonal contraceptive use was associated with facilitated acquisition of classical conditioning responses (Beck et al., 2008; Holloway, Beck, & Servatius, 2011), and this facilitated learning persisted even under stress conditions (Merz, Wolf, Schweckendiek, Klucken, Vaitl, & Stark, 2013). Neuroimaging work with a classical fear conditioning paradigm revealed that in oral contraceptive users and men, there was a positive correlation between basal cortisol levels and differential activation in the amygdala; this correlation was not identified in luteal women (Merz, Stark, Vaitl, Tabbert, & Wolf, 2013). Together, these studies suggest that fear learning in women, even under conditions of stress, is affected by the use of hormonal contraception.
Finally, other work has explored emotional memory in hormonal contraceptive users using long-term memory paradigms. A recent study from our lab showed that women differentially recalled information from an emotional story depending on their hormonal contraceptive status (Nielsen, Ertman, Lakhani, & Cahill, 2011). Hormonal contraceptive users exhibited enhanced memory for gist, but not details of an emotional story whereas naturally cycling women exhibited enhanced emotional memory for details, but not gist (Nielsen et al., 2011). A subsequent study from the lab showed that a post-learning glucocorticoid release modulated emotional memory for positive images in women using hormonal contraception, but not naturally cycling women (Nielsen et al., 2013).
Together, the aforementioned studies indicate that compared to naturally cycling women, hormonal contraception users exhibit blunted HPA reactivity to stress and modified learning and memory for emotional stimuli. However, no studies to date have tested the effects of post-learning stress on long-term emotional memory for gist and detail in women on and off hormonal contraception. Since our previous work identified different retention patterns for emotional information in the aforementioned groups of women, the present study aimed to build on these findings. We tested a series of predictions about HPA reactivity and effects of post-learning stress on memory for emotional information. First, with respect to hormone responses to the physical stressor (Cold Pressor Stress Test; CPS), we predicted that women on hormonal contraception would have significantly blunted cortisol responses compared to naturally cycling women (luteal phase) (Kirschbaum et al., 1999; Nielsen et al., 2013). Second, we predicted naturally cycling women in the stress condition would exhibit enhanced memory for total details of an emotional story as well as gist and details from the most emotional phase, “phase 2” (Nielsen, Ahmed, & Cahill, 2013). Finally, we predicted that a post-learning release of glucocorticoids might not affect memory for gist or detail in hormonal contraceptive users since previous work has shown no effect of cortisol on memory for verbal information (Kuhlmann & Wolf, 2005) or negative stimuli (Nielsen et al., 2013) in these women.
Materials and methods
Participants
Sixty-three naturally cycling (NC) and 55 hormonal contraceptive using (HC women) female undergraduates from the University of California, Irvine between the ages of 18–33 participated in this study, which was approved by the university’s Institutional Review Board. The participants received course credit or payment for their participation in the study. Participants were asked to refrain from alcohol, caffeine, and cardiovascular exercise for twenty-four hours prior to each experimental session to control for outside influences that could affect baseline stress hormone levels. To avoid contamination of salivary samples, participants were asked to fast one hour prior to each experimental session as well as refrain from brushing teeth within the hour before their appointment. Their compliance with this criteria was verified upon their arrival.
Of the participants, three naturally cycling women were excluded due to failure to return for the second experimental session (1) and having a cortisol response to the control condition (2). Six HC women were excluded based on their failure to return for the second experimental session (3), using a progesterone-only contraception (1), having salivary progesterone and 17β-estradiol levels more than 4 standard deviations above the mean (1), and exhibiting a cortisol response to the control condition (1). Of the participants included in the final analyses, 14 participants reported using one prescription medication (8 NC women, 6 HC women). The final analyses included data from 60 NC women and 49 HC women. The NC women were all in the “luteal” phase of the menstrual cycle (15–30 days from the start of menstruation; Azziz, Hincapie, Knochenhauer, Dewailly et al., 1999; Franklin, Ehrman, Lynch, Harper, Sciortino, O’Brien, & Childress, 2008; Sakaki & Mather, 2012; De Bondt, Van Hecke, Veraart, Leemans, Sijbers, et al., 2013). We used a forward day count from the first day of menstruation to determine menstrual cycle position. Of the HC women, all participants were on combined contraceptive formulations that had both ethinyl estradiol and a synthetic progestin; 8 HC women reported using triphasic formulations and 41 used monophasic formulations.
Procedures
All experimental sessions were conducted between the hours of 12:00 and 18:00 to control for the effects of circadian rhythm on cortisol levels. During the first experimental session, participants filled out a screening questionnaire and three cognitive assessments including the BEM Sex Roles Inventory (BEM; Bem, 1981), the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988), and the Mehrabian test (Mehrabian, 1994). The BEM was implemented to assess masculine and feminine influences/traits within each individual participant, whereas the PANAS was given to measure the participants’ affect at the time of testing. The Mehrabian was implemented to assess levels of trait anxiety (Mehrabian, 1994). These questionnaires were implemented to standardize the activities between each participant’s arrival and their baseline sample; scores from these questionnaires were not analyzed with respect to memory.
Fifteen minutes after their arrival, participants provided a 1-mL saliva sample using the “passive drool” collection method. Following the baseline saliva sample, participants underwent a 5-pt. calibration on the iView X RED eye-tracking system (SensoMotoric Instruments).
Participants then viewed either a brief, narrated story containing emotionally arousing elements or one containing only neutral elements. The story has been used in emotional memory research (Cahill, Prins, Weber, & McGaugh, 1994), and more specifically in studies exploring sex differences in emotional memory (Cahill & van Stegeren, 2003; Nielsen et al., 2011; Nielsen, Ahmed, & Cahill, 2013). The story was modified slightly in 2008; the re-attached leg slide (slide 8) was exchanged for a more modern looking image of two legs on a white hospital bed. All other images have remained the same since Cahill et al. (1994). In addition, all recent studies from our lab pertaining to sex hormone influences on gist and detail memory have used this more recent version. Each version of the story was composed of 11 slides, and the images on the slides were identical between the two versions of the story. The stories were also identical in the narratives associated with slides 1–4 or “phase 1” (i.e. an introduction to mother and son) and similar in those associated with slides 9–11 or “phase 3” (i.e. mother leaving a hospital to pick up her other child) of the slideshow. However, the stories were quite different in the narratives associated with slides 5 – 8 or “phase 2;” in this part of the story, the emotional version contained the most emotionally arousing elements (i.e. son is hit by a runaway car and is rushed to the hospital for surgery) unlike the neutral version of the story (i.e. mother and son see an accident and participate in a drill at the hospital). Immediately after the slide show, participants were asked to rate the story on a 1 – 9 scale of emotional arousal; 1 = “not at all emotionally arousing” and 9 = “extremely emotionally arousing.” Participants were free to rate the story using any of the numbers between 1 and 9.
NC women were randomly assigned to a CPS (N = 30) or control (N = 30) condition, and HC women (who represent a different population) were randomly assigned to either a CPS (N = 25) or control (N = 24). Those assigned to the CPS condition immersed their right hand in ice water (1 – 4°C) for up to three minutes, whereas participants in the control condition immersed their right hand in warm water (37°C) for the same length of time. Participants in both conditions were informed prior to the test that they could remove their hand from the water at any time without penalty. Of the participants included in the final analyses, 30 women removed their hand before the end of the three minute CPS session (14 NC, 16 HC). The data obtained from these women were included in the final analyses because they completed at least 15 seconds of the CPS task. Upon completion of CPS or control condition, participants provided a third 1-mL saliva sample. All participants were instructed to refrain from any stressful activities for the remainder of the session. Additional samples were collected 15 and 25 minutes after the CPS or control condition.
One week later, participants returned and provided one 1-mL saliva sample after a fifteen minute acclimation period. This sample was taken to maintain consistency between the experimental sessions and was not analyzed for cortisol, 17β-estradiol, progesterone, or testosterone levels. A surprise free recall test for slide recall and associated story elements was administered shortly after the saliva sample. During the test, participants were asked to write a brief phrase identifying each slide they remembered as well any elements of the story they could recall that were associated with each remembered slide. After completing the test, participants were debriefed and compensated with course credit.
Scoring of Recall Performance
Correct recall of a slide was credited if the identifying phrase used by the participant could unambiguously be attributed to a specific slide. Slide descriptions not clearly linked to a picture in the slide show were not counted. The vast majority of responses unambiguously identified a particular slide. A scoring template derived from our previous work with these stories (Nielsen et al., 2011; Nielsen, Ahmed, & Cahill, 2013; Cahill & van Stegeren, 2003; Cahill, Gorski, Belcher, & Huynh, 2004) was used to score recalled story elements as concerning either the “gist” or “details” of the story. In these previous studies, “gist” was defined by a consensus of ¾ independent judges as “any story element that could not be changed or altered without changing the fundamental story line” (Cahill & van Stegeren, 2003). In the scoring template used here and elsewhere (Nielsen et al., 2011; Nielsen, Ahmed, & Cahill, 2013), scored gist items reflect those determined to be “gist” by the independent judges in previous work (Cahill & van Stegeren, 2003). Gist items were derived from both the narrative and slides and the number that could be recalled varied by slide. Examples of gist items from the scoring template for phase 2 of the emotional version include “boy hit by a runaway car,” “boy critically injured,” and “the boy is taken to a nearby hospital.”
“Details” were defined as all other recalled elements and the number of details that could be recalled differed by slide (Cahill & van Stegeren, 2003). Examples of detail items from the scoring template for phase 2 of the emotional story include “hospital – light brown,” “parked car in background,” and “boy post-surgery.”
Of the slides that were correctly recalled, the associated story elements were scored as either a “gist” or “detail” if the story element corresponded to a “gist” or “detail” item on the scoring template. Most of the story elements listed by the participants (83.4%) were classifiable by this method as either “gist” or “detail.” Recall performance was scored by two independent judges. Agreement between the two judges was 97%. The relatively few cases of disagreement were decided by a third independent judge.
Eye Movements and Pupil Dilation
Fixation duration and pupil dilation were measured using the iView X RED eye-tracking software at a sampling rate of 120 Hz. We selected these measures for analysis because fixation time has been used as an index of attention and visual processing (Dalton et al., 2005), and pupil dilation is considered a reliable measure of arousal (Gilzenrat, Nieuwenhuis, Jepma, & Cohen, 2010; Einhauser, Stout, Koch, & Carter, 2008). Standard analysis procedures were used (Gilzenrat, Nieuwenhuis, Jepma, & Cohen, 2010; Einhauser, Stout, Koch, & Carter, 2008). Fixation and pupil dilation data for each participant were exported using the eye-tracking analysis software program BeGaze 2 (SensoMotoric Instruments). Eye movement events (fixations, saccades, blinks) for the duration of each slide (approximately 15 seconds) were exported. Fixation time % was determined by adding the time of each fixation event within a slide and dividing the total fixation time by the total time of the slide; this was determined for each slide (data not shown) and each phase.
In order to examine whether NC and HC women visually explored the slides differently, we conducted an area of interest (AOI) analysis on two of the most emotional and visually complex slides of phase 2 of the emotional story (Poole & Ball, 2005). We selected four AOIs between the two slides. Within each AOI, we examined the number of glances (entries) the participant made into each area.
In between each slide, a grayscale image matched for luminance appeared for 3 s to control the level of illumination prior to picture onset (Bradley et al., 2008). Average pupil dilation was calculated for each image using methods adopted from Bradley et al. (2008); the average baseline diameter in the 3 s before the image presentation was subtracted from the average pupil diameter in the 3 s following the offset of the narration. This approach was necessary because in this paradigm, the visual images in both the emotional and neutral stories were identical (Cahill, Prins, Weber, & McGaugh, 1994). The emotional response to each slide is determined by the narrative in the slide. Thus, the most appropriate method for assessing an arousal response to each slide is to assess pupil dilation before and after the slide’s narration.
Saliva Samples
Saliva samples were immediately frozen for a minimum of twenty-four hours to allow mucins to precipitate. Prior to the assays, they were thawed and centrifuged at 2,080 x g for 15 minutes to extract particulates from saliva. Samples were then centrifuged a second time at 2,080 x g for 10 minutes to extract any additional particulates from saliva. Clear supernatant was decanted into microtubes.
Salivary Measurement of Sex Steroid Hormones
Salivary levels of 17β-estradiol, progesterone, and testosterone were measured using Salimetrics (State College, PA) ELISA kits and measured optically using BioTek Instruments, Inc. ELx808 Absorbance Microplate Reader (Winooski, VT). We assayed two saliva samples for 17β-estradiol, progesterone, and testosterone; from these samples, we determined the average levels of these hormones. We used the assays to determine overall group differences between NC and HC women, and mean values of 17β-estradiol and progesterone were within the expected ranges of the used assays (2.56 ± 0.84 pg/mL and 131.00 ± 54.5 pg/mL, respectively) for luteal women (Salimetrics, State College, PA). The observed ranges of the 17β-estradiol (3.02 ± 0.98 pg/mL) and progesterone (111.25 ± 87.34pg/mL) from the assay were also similar to the expected ranges. The inter- and intra-assay variations for 17β-estradiol (7.86%; 8.34%), progesterone (6.97%; 14.99%), and testosterone (8.85%; 11.48%) were within the expected ranges from our lab.
Salivary Measurement of Cortisol
Salivary cortisol levels were measured using Salimetrics (State College, PA) cortisol ELISA kits and measured optically using BioTek Instruments, Inc. ELx808 Absorbance Microplate Reader (Winooski, VT). The cortisol response was computed by subtracting the amount of salivary cortisol present in the “pre-CPS” baseline sample from the amount in the “15 min post-CPS” sample, which was taken approximately 15 minutes after termination of CPS (Nielsen et al., 2013). This sample was indicative of the time point when cortisol levels were expected to peak.
Participants who exhibited an increase of at least 0.05μg/dL from the “pre-CPS” to the “15 min post-CPS sample” were classified as “CPS Cortisol Responders.” This criterion was established by analyzing cortisol responses to CPS or the control procedure in over 300 participants from studies in our lab and used in our previous study of cortisol responses to stress in NC and HC women (Nielsen et al., 2013). We established this criterion since other cortisol responder criteria used (e.g. Miller et al., 2013) was based on the Trier Social Stress Test, a very potent psychosocial stressor. Based on our criterion for the average cortisol response to a physical stressor, any control participant exhibiting an increase 0.05μg/dL was excluded from the experiment prior to the final analyses. Inter- and intra-assay variations for cortisol were 5.31% and 10.67%, respectively.
Statistical Analysis
We used one-way ANOVAs to analyze differences in cortisol responses and PANAS, BEM, and Mehrabian test scores between HC and NC women. We also used a 2 × 2 × 2 ANOVA, 2 × 2 ANOVAs and one-way ANOVAs to assess differences in slide recall and gist and detail recall in the emotional and neutral stories in two groups of participants.
Results
Participants
NC women (M = 20.1, SD = 1.9) and HC women (M = 20.5, SD = 1.8) did not differ significantly in age. A chi-square test of independence also determined that the two groups did not differ significantly in ethnicity, X2(4, n = 109) = 3.6, n.s.
Sex Hormone Levels and Menstrual Cycle Position
Menstrual cycle position in naturally cycling women was determined by self-report and verified using salivary hormonal assays. NC women had significantly higher levels of salivary progesterone (F(1, 107) = 12.3, p < .001), 17β-estradiol (F(1, 107) = 16.1, p < .001), and testosterone (F(1, 107) = 18.9, p < .0001) compared to HC women.
Emotional Arousal Ratings and Cognitive Questionnaires
There were no significant differences in arousal ratings for the emotional (F(1,53) = .55, p ≫ 0.1) or neutral (F(1, 52) = .02, p ≫ 0.1) stories between the two groups. NC women (F(1, 58) = 43.1, p < .0001) and HC women (F(1, 47) = 58.3, p < .0001) both rated the emotional story as significantly more emotionally arousing than the neutral story.
Participants were given three cognitive questionnaires prior to the start of the experiment. NC and HC women scored did not score significantly differently on any of the cognitive questionnaires (PANAS, BEM, or Mehrabian).
Cortisol Responses to CPS
Cortisol reactivity
Of the CPS participants, 24 (80%) NC women and 16 (64%) HC women displayed a cortisol increase of 0.05 μg/dL in response to the physical stressor (i.e. from cortisol at baseline (“pre-CPS”) to the 15 minutes post-CPS sample); the number of CPS Cortisol Responders in each group did not differ significantly, X2(2, N = 109) = 1.8, p ≫ 0.1.
Both NC (F(1, 58) = 48.6, p < .0001) and HC (F(1, 47) = 15.2, p < .001) women exhibited a significantly larger cortisol response to CPS compared to the control. However, when the magnitude of the cortisol response to CPS was assessed, NC women had a significantly larger cortisol response to CPS compared to HC women (F(1, 53) = 5.01, p < .05, η2 = .07; see Fig. 1a).
Fig. 1.
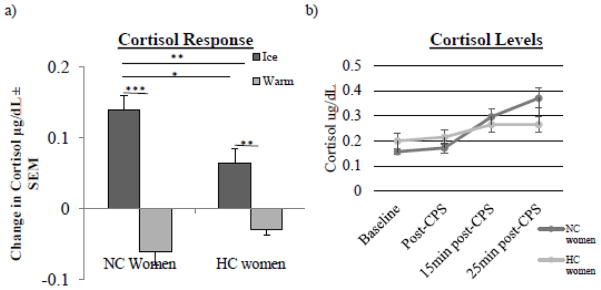
Cortisol response in NC and HC women. a, Cortisol response to ice and warm conditions in NC and HC women. A 2 × 2 ANOVA revealed a significant interaction between contraceptive status (NC v. HC) and stress condition (Ice v. Warm) for cortisol response (two asterisks, p < .01). NC women in Ice (n = 30) exhibited larger cortisol responses compared to HC women in Ice (n = 25; one asterisk, p < .05) and NC women in Warm (n = 30; three asterisks, p < .001). HC women in Ice (n = 25) exhibited larger cortisol responses than HC women in Warm (n = 24; two asterisks, p < .01). b, Cortisol levels in ice condition. In the ice condition, a series of t-tests revealed that NC (n = 30) and HC (n = 25) women did not differ in their cortisol levels at any time point. Values are means ± s.e.m.
Since the cortisol response is a change between pre-CPS cortisol and 15 min post-CPS cortisol, we examined whether cortisol levels in NC and HC women differed pre-CPS and/or 15 min post-CPS; analyses showed that levels of cortisol in NC and HC women did not differ at either time point in the stress condition (Fig. 1b).
We also examined whether the cortisol increase to CPS varied with the preceding emotional story condition. Both NC (F(1, 28) = 0.18, p = 0.68) and HC (F(1, 23) = 0.55, p = 0.47) women displayed equivalent cortisol increases to CPS in the emotional and neutral story conditions.
Memory findings
Slide Recall
We first considered the total recall of all slides from the emotional and neutral stories and whether the slide recall depended on contraceptive status or stress condition. An overall 2 × 2 × 2 ANOVA for total slide recall with contraceptive status (NC v. HC), emotional story content (Emo v. Neu), and stress condition (Ice v. Warm) as independent factors revealed a main effect of emotion on slide recall (F(1, 107) = 13.7, p < .001, η2 = .12). The other main effects and interactions were non-significant. We next used a 2 × 2 ANOVA with emotional story content and stress condition as independent factors to explore total slide recall separately in NC and HC women. Both NC women (F(1, 58) = 10.5, p < .01) and HC women (F(1, 47) = 4.2, p < .05) exhibited a significant main effect of emotion on total slide recall, indicating that they both recalled more slides from the emotional compared to the neutral story.
We next examined the recall of slides in each phase of the story. An overall 2 × 2 × 2 ANOVA for phase 1 slide recall with contraceptive status (NC v. HC), emotional story content (Emo v. Neu), and stress condition (Ice v. Warm) as independent factors revealed a main effect of emotion on slide recall (F(1, 107) = 7.1, p < .01, η2 = .07). The other main effects and interactions were non-significant. When we used separate 2 × 2 ANOVAs for phase 1 slide recall with emotional story content and stress condition as independent factors, only NC women exhibited an effect of emotion for phase 1 slide recall (F(1, 58) = 7.3, p < .01, η2 = .12), suggesting that the main effect of emotion in the 2 × 2 × 2 ANOVA for phase 1 slide recall was driven only by NC women.
An overall 2 × 2 × 2 ANOVA for phase 2 slide recall with contraceptive status (NC v. HC), emotional story content (Emo v. Neu), and stress condition (Ice v. Warm) as independent factors revealed a main effect of emotion on slide recall (F(1, 107) = 10.6, p < .01, η2 = .10) and a significant interaction effect of contraceptive status x emotion x stress condition (F(3, 105) = 5.9, p < .05, η2 = .06). The other main effects and interactions were non-significant. A series of separate 2 × 2 ANOVAs for phase 2 slide recall with emotional story content and stress condition as independent factors revealed that only NC women exhibited an effect of emotion on phase 2 slide recall (F(1, 58) = 13.2, p < .001, η2 = .19); indicating that the effects observed in the overall 2 × 2 × 2 ANOVA for phase 2 slide recall were driven by recall in NC women.
An overall 2 × 2 × 2 ANOVA for phase 3 slide recall with contraceptive status (NC v. HC), emotional story content (Emo v. Neu), and stress condition (Ice v. Warm) as independent factors revealed no significant main effects or interactions between any of the factors. Therefore, we did not explore these results in further analyses.
Recall of Gist and Detail
In analyzing gist and detail, we first considered total gist and detail retention. An overall 2 × 2 × 2 ANOVA for total gist recall with contraceptive status (NC v. HC), emotional story content (Emo v. Neu), and stress condition (Ice v. Warm) as independent factors revealed main effects of emotion (F(1, 107) = 9.9, p < .01, η2 = .09) and contraceptive status (F(1, 107) = 4.04, p < .05, η2 = .04) (Fig. 2a). All other main effects and interactions were non-significant.
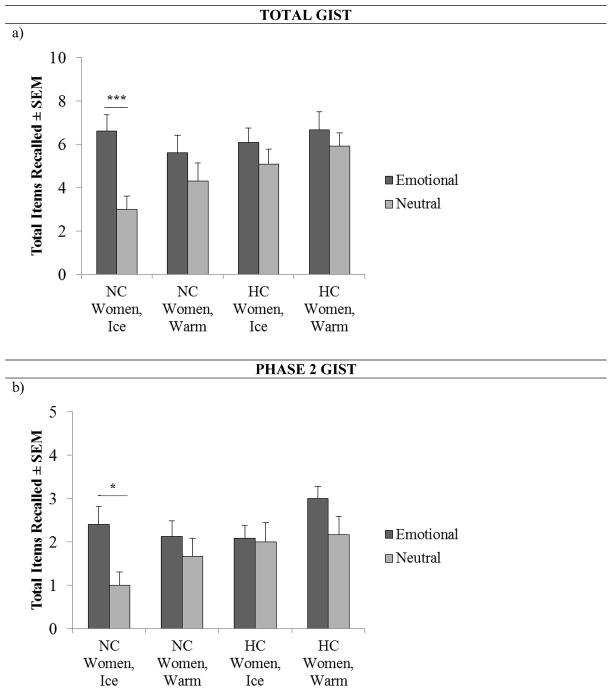
Fig. 2.
Total gist and phase 2 gist recall in NC and HC women. a, NC women in Ice recalled significantly more total gist in the emotional (n = 15) compared to the neutral (n = 15) condition (three asterisks, P < .001, one-way ANOVA). b, NC women in Ice recalled significantly more phase 2 gist in the emotional (n = 15) compared to the neutral (n = 15) condition (one asterisk, p < .05, one-way ANOVA) Values are means ± s.e.m.
We also assessed total detail memory using an overall 2 × 2 × 2 ANOVA with contraceptive status (NC v. HC), emotional story content (Emo v. Neu), and stress condition (Ice v. Warm) as independent factors. The ANOVA revealed a main effect of emotion (F(1, 107) = 6.6, p = .01, η2 = .06) and a near significant interaction effect of contraceptive status x emotion x stress condition (F(3, 105) = 2.9, p = .09, η2 = .03; Fig. 3a). All other main effects and interactions were non-significant.
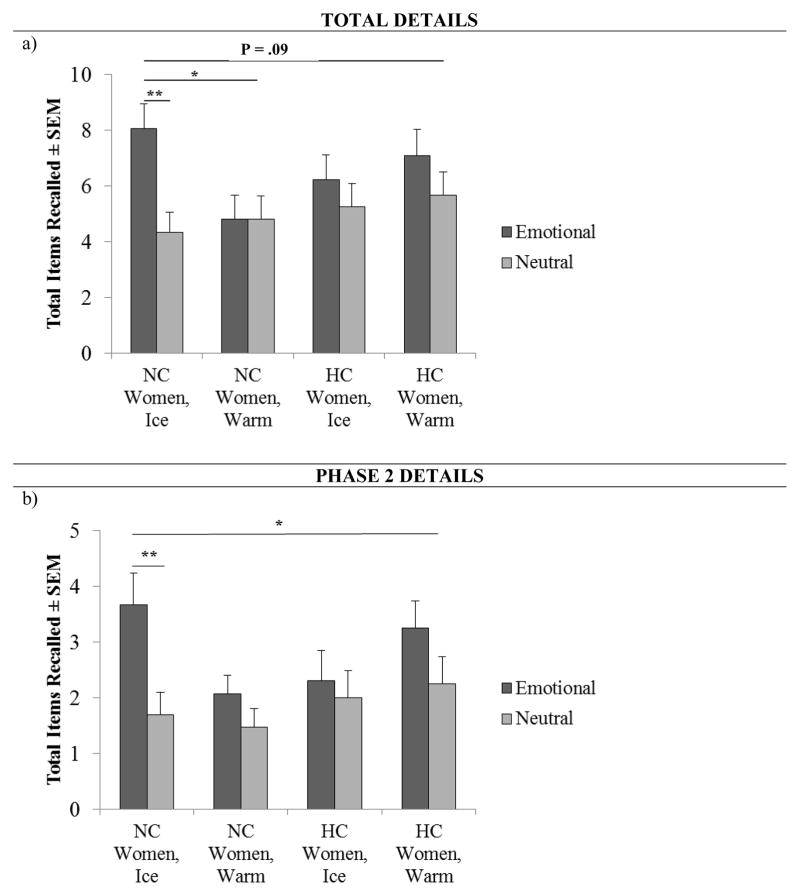
Fig. 3.
Total detail and phase 2 detail recall in NC and HC women. a, A 2 × 2 × 2 ANOVA revealed a trend toward a significant interaction between contraceptive status (NC v. HC), story condition (Emo v. Neu) and stress condition (Ice v. Warm) for total detail recall (p = .09). In NC Women, a 2 × 2 ANOVA revealed a significant interaction between story condition and stress condition for total detail recall (one asterisk, p < .05). NC women in Ice recalled significantly more total details in the emotional (n = 15) compared to the neutral (n = 15) condition (two asterisks, P < .01, one-way ANOVA). b, A 2 × 2 × 2 ANOVA revealed a significant interaction between contraceptive status (NC v. HC), story condition (Emo v. Neu) and stress condition (Ice v. Warm) for phase 2 detail recall (one asterisk, p < .05). NC women in Ice recalled significantly more phase 2 details in the emotional (n = 15) compared to the neutral (n = 15) condition (two asterisks, P < .01, one-way ANOVA). Values are means ± s.e.m.
Given that there was an effect of emotion on total gist and detail recall in the overall ANOVAs, we further examined total gist and detail memory in both groups of women using a series of 2 × 2 ANOVAs with emotional story content (Emo v. Neu) and stress condition (Ice v. Warm) as independent factors. Although both groups displayed an effect of emotion for total slide recall, they exhibited different memory for gist and detail from the emotional story. For NC women, a 2 × 2 ANOVA for total gist memory revealed a main effect of emotion (F(1, 58) = 10.5, p < .01, η2 = .16), but all other main effects and interactions were non-significant. A Tukey HSD analysis revealed that this main effect of emotion was driven by a significant difference in total gist recall between NC women administered CPS; in this case, there was significantly more gist recalled from the emotional compared to the neutral story when NC women encountered post-learning stress (p < .05; Fig. 2a).
However, a 2 × 2 ANOVA for total detail memory revealed that NC women exhibited a main effect of emotion (F(1, 58) = 5.3, p < .05, η2 = .09; Fig. 3a) and a significant interaction effect between emotion x stress condition (F(2, 57) = 4.2, p < .05, η2 = .08; Fig. 3a). Post-hoc Tukey HSD analyses revealed that NC women who saw the emotional story and were administered CPS recalled significantly more details than any other group (p < .05; Fig. 3a).
For HC women, however, the pattern of retention for gist and detail was quite different. A 2 × 2 ANOVA for total gist memory revealed no main effects or significant interactions, and a 2 × 2 ANOVA for total detail memory revealed that HC women exhibited no main effects or significant interactions.
Recall of Gist and Detail by Story Phase
We next examined which story phase was driving the emotional memory enhancements for gist and detail in NC and HC women. First, we assessed gist and detail memory from the emotional story using separate overall 2 × 3 × 2 ANOVAs with contraceptive status (NC v. HC), story phase (1 v. 2 v. 3), and stress condition (Ice v. Warm) content as independent factors. For gist memory, the overall 2 × 3 × 2 ANOVA revealed significant main effects of story phase (F(2, 163) = 18.7, p < .0001). No other main effects or interactions were significant. For detail memory, the overall 2 × 3 × 2 ANOVA revealed a significant main effects of story phase (F(2, 163) = 20.3, p < .0001) and a significant interaction effect between contraceptive status x stress condition (F(2, 163) = 6.8, p < .01). Post-hoc, Tukey HSD analyses revealed that NC women administered CPS recalled significantly more details than NC women in the control condition (p < .05). No other main effects or interactions were significant.
Since the overall ANOVAs had significant main effects, we further investigated gist and detail memory in NC and HC women in the different story phases. We next assessed gist and detail memory in each phase of the story using a series of 2 × 2 ANOVAs with emotional story content (Emo v. Neu) and stress condition (Ice v. Warm) as independent factors. In NC women, for phase 3, there were no main effects of emotional story content or stress condition, nor an interaction effect between these factors, on memory for gist or detail. For phase 1, however, we observed a main effect of emotion for gist memory (F(1, 58) = 7.6, p < .01, η2 = .12), but no main effects or significant interactions for detail memory. We also observed a main effect of emotion for gist memory in phase 2 (F(1, 58) = 6.3, p < .05; Fig. 2b, η2 = .10); no other main effects or significant interactions were significant. For phase 2 detail memory, we found main effects of emotion (F(1, 58) = 9.2, p < .01, η2 = .14) and stress condition (F(1, 58) = 5.0, p < .05, η2 = .08); there were no significant interactions between emotion x stress condition (F(2, 57) = 2.5, p = .12) (see Fig. 3b). Interestingly in HC women, we observed no significant main effects or interaction effects between emotion x stress condition on memory for gist and detail in any of the story phases.
Since the 2 × 2 ANOVAs revealed significant main effects, we used a series of one-way ANOVAs to reveal that in NC women in the Ice condition, detail memory was enhanced in phase 1 (F(1, 28) = 6.0, p < .05, η2 = .17) and phase 2 (F(1, 28) = 7.8, p < .01, η2 = .22). A separate series of one-way ANOVAs showed that in NC women in Ice condition, gist memory was enhanced in phase 1 (F(1, 28) = 8.2, p < .01, η2 = .23), phase 2 (F(1, 28) = 7.4, p = .01, η2 = .21), and phase 3 (F(1, 28) = 6.3, p < .05). To be consistent with each group of women, we also used one-way ANOVAs revealed there to be no emotional memory enhancements for gist or detail in any story phase, regardless of stress condition in HC women.
Cortisol and Memory for Gist and Detail
To assess the relationship between post-learning cortisol and memory for gist and detail, we ran several regression analyses for emotional memory in NC and HC women. Regressions for cortisol x total gist memory, cortisol x total detail memory, cortisol x phase 2 gist memory, and cortisol x phase 2 detail memory were not significant for NC or HC women.
Attention and Arousal
As an index of attention, we determined the average fixation time percentage for the four slides within phase 2, the most emotional phase of the emotional story. NC and HC women who were administered CPS spent equivalent percentages of time fixated on the slides in phase 2 of the emotional story (F(1, 26) = 1.6, p > 0.1; Fig. 4a). In addition to assessing attention in phase 2, we also examined whether participants explored the slides similarly in the emotional story as indicated by the number of glances made in the specified AOIs (Poole & Ball, 2005; Nielsen et al., 2011; Nielsen, Ahmed, & Cahill, 2013). There were no significant differences between NC and HC women in the number of glances into any of the AOIs selected (data not shown).
Fig. 4.
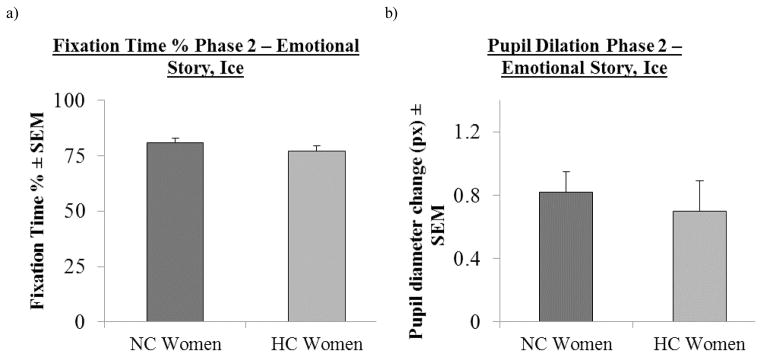
Attention and arousal in Phase 2 of the emotional story for NC and HC women in Ice. a, No significant differences in average fixation time between NC and HC women (n = 15 and n = 13, respectively). Values ± s.e.m. b, No significant differences in pupil dilation change between NC and HC women (n = 15 and n = 13, respectively).Values ± s.e.m
Pupil diameter changes indicated arousal to the emotional component (phase 2) of the emotional story (Koss, 1986; Rajkowski, Kubiak, & Aston-Jones, 1993; Aston-Jones & Cohen, 2005; Nielsen et al., 2011; Nielsen, Ahmed, & Cahill, 2013). NC and HC women all exhibited equivalent average pupil diameter changes in response to phase 2 of the emotional story (F(1, 26) = .28, p ≫ 0.1; Fig. 4b). Additionally, no differences in average pupil dilation were observed in phase 1 (F(1, 26) = .89, p ≫ 0.1) or phase 3 (F(1, 26) = 2.95, p = 0.1).
Discussion
The present findings are the first to demonstrate that a post-learning stressor affects memory for gist and detail from an emotional story, and that the relationship between post-learning stress and retention of emotional information differs in women on and off hormonal contraception. These results support our predictions that compared to naturally cycling women in the luteal phase, women on hormonal contraception exhibit significantly blunted stress hormone responses and altered retention of information from an emotional event.
This study revealed several key findings related to stress hormone responses and sex/stress hormone influences on emotional memory for gist and detail. With respect to cortisol responses to the stressor (CPS), NC women exhibited significantly larger cortisol increases compared to HC women; these results support previous findings from our lab (Nielsen et al., 2013) and are consistent with other work (Kirschbaum et al., 1999). With respect to memory, retention of the emotional story varied with both hormonal contraceptive status and stress condition. Only NC women in the stress condition had enhanced recall for overall gist and detail, and phase 2 gist and detail, from the emotional compared to the neutral story. HC women exhibited no emotional memory enhancements for gist or detail from the emotional story, regardless of stress condition.
From Nielsen et al. (2011) and Nielsen, Ahmed, and Cahill (2013), we hypothesized that sex/stress hormone interactions at encoding have a critical influence on emotional memory for different types of information (e.g., Andreano & Cahill, 2009). Results from the present study are consistent with this possibility and suggest that endogenous sex hormone levels at encoding can interact with a post-learning cortisol release to modulate emotional memory for gist and detail in women. The present data are also consistent with Nielsen et al. (2011) in that we again found enhanced recall of emotional details in NC women, but not HC women. Additionally, these differences do not appear to be the result of differences in attention or arousal at encoding given our eye-tracking results. These memory differences between the two groups of women may result from differences in mechanisms underlying glucocorticoid modulation of emotional memory; since HC women have significantly blunted cortisol responses to CPS, there may not be a sufficient release of glucocortioids to modulate memory for emotional material. Additionally, since HPG and HPA axes have bidirectional effects (Turner, Keating, & Tilbrook, 2012), the effects of cortisol on memory consolidation in NC and HC women could differ due to their vastly different HPG axis activity. Based on the results from this study, we cannot determine which mechanism underlies the observed effects; however, future work should further explore these potential explanations for the findings.
The present findings do differ from Nielsen et al. (2011) in some respects. We expected there might be differences, given that the current study incorporated a post-learning stressor in the experimental design. However, we did not predict that naturally cycling women in the stress condition would exhibit enhanced recall of overall and phase 2 gist from the emotional compared to the neutral story. Our lab recently showed that women in the luteal phase exhibited enhanced emotional memory for details as well as gist from phase 2 (Nielsen, Ahmed, & Cahill, 2013). However, post-learning cortisol may be interacting with arousal at encoding to enhance memory for all types of information from the emotional compared to the neutral story. Previous research has shown that women in the luteal phase of the menstrual cycle do exhibit enhanced emotional memory under post-learning stress conditions (Andreano, Arjomandi, & Cahill, 2008; Felmingham, Fong, & Bryant, 2012). However, it is also plausible that post-learning stress had an impairing effect on the consolidation for neutral memories. For total gist recall, NC women in the stress condition showed lower neutral memory compared to the warm condition. Although our data supports the notion of a gist memory enhancement across the entire emotional story in NC women, it may be the case that the enhanced emotional gist memory was observed due to an impairment of the neutral memories across all three phases. Thus, future work should focus on the effects of post-learning stress on neutral memory in NC women to more fully understand the potential impairing effect of cortisol on memory consolidation.
This study is only the third to investigate the influence of hormonal contraception on long-term emotional memory (Nielsen et al., 2011; Nielsen et al., 2013), and the first to test whether a post-learning stressor differentially affects long-term emotional memory for gist and detail in hormonal contraception users and non-users. The findings strengthen the argument that failure to account for hormonal contraceptive status at encoding can lead to inaccurate conclusions about the influence of emotional arousal on recall of gist and detail.
The goal of this study was to better understand the relationship between sex hormone status at encoding and post-learning stress and their collective influence on memory for an emotional event. Although the results indicate that hormonal contraception use and stress condition play a role in modulation of memory for emotional gist and detail, these findings raise several questions that should be further explored in future work. For example, in this study, HC women were self-selected users, which may contribute to a potential “survivor effect” (Oinonen & Mazmanian, 2002); these women have tolerated hormonal contraception well enough to continue taking the medication, which may speak to differences in mood or cognition compared to women who discontinued hormonal contraception use. As a result, our findings may undermine any mood or cognitive differences between NC and HC women (Mordecai, Rubin, & Maki, 2008). While this is an important point to consider, it should be noted that there were no significant differences on the cognitive measures given in this study.
Another caveat to this study was that we included HC women who were tested in both the “on” and “off” active contraception weeks. This may have important implications as research has shown differential memory effects in contraceptive users that were in “on” v. “off” weeks (Mordecai, Rubin, & Maki, 2008). Active v. inactive pill use may also affect the magnitude of the cortisol response to different stressors. It has been hypothesized that the blunted cortisol responses in hormonal contraceptive users are driven by the cortisol binding globulin enhancing effect of ethinyl estradiol (Fujimoto et al., 1986; Kirschbaum et al., 1999; Kumsta et al., 2007). Thus, active v. inactive pill users may exhibit different cortisol responses to stressors since ethinyl estradiol is only present in the active contraception (Lobo & Stanczyk, 1994). Future work on hormonal contraception use, stress, and memory should account for this and explore possible differences between the active v. inactive groups.
A third caveat to the study is that we were unable to assess sex differences given the lack of a male control group. Although the present study was designed to examine the effects of post-learning stress on long-term emotional memory in women on and off hormonal contraception, we acknowledge that the discussion of sex/stress interactions and their subsequent effects on memory are somewhat limited by the lack of a male control group. Future work should test this paradigm in men, and also follicular women (low hormone phase of the menstrual cycle), to determine the emotional memory patterns of individuals with naturally low levels of endogenous estrogen and progesterone. These additional studies would provide a more complete picture of sex/stress hormone interactions and their modulation of emotional memory for gist and detail.
A final caveat to this study is that there were no observable emotional memory enhancements in the HC women, regardless of stress condition. We predicted that cortisol would not have enhancing effects on emotional memory for gist and detail based on previous work showing that cortisol did not affect retrieval memory (Kuhlmann & Wolf, 2005) or memory for negative stimuli (Nielsen et al., 2013) in hormonal contraceptive users. However, HC women in the control condition also showed no enhancement for gist memory in the emotional compared to the neutral story. A potential explanation for this could be that the four generations of synthetic progestins differentially modulate emotional memory (Mordecai, Rubin, & Maki, 2008). Previous studies with hormonal contraceptive users have included more participants in their cells (Nielsen et al., 2011; Nielsen et al., 2013; Merz et al., 2012; Merz et al., 2013), and these larger groups sizes may account for the differential effects of synthetic progestins. Newer formulations are less androgenic than previous generations (Lobo & Stanczyk, 1994; Sitruk-Ware, 2004), and no studies to date have assessed whether different progestins are associated with altered learning and memory processes in hormonal contraception users. It is entirely possible that a particular generation of progestin is driving the effects of hormonal contraception on learning and memory. Future work should explore whether different generations of synthetic progestins have different effects on stress responses, and subsequently, emotional memory.
Previous studies with the emotional story paradigm suggested that their findings might have important implications for understanding disorders of emotional memory that disproportionately affect women, such as post-traumatic stress disorder (PTSD), anxiety, depression (Breslau, Davis, Andreski, & Peterson, 1991; Breslau, Davis, Andreski, Peterson, & Schultz, 1997; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). However, none of these studies investigated the influence of cortisol released post-learning on emotional memory. Cortisol is known to be engaged by emotionally arousing events, has documented effects on emotional memory in animals and humans (McGaugh, 2000), and is altered in patients with PTSD (Yehuda, 2002). Cortisol response to stress has also been shown to be modulated by sex, menstrual cycle phase, and hormonal contraceptive use (Kirschbaum et al., 1999; Nielsen et al., 2013). Thus, the present study aimed to expand the emotional story paradigm to better simulate an emotionally arousing event in an individual’s life. Although our current study did not explicitly examine disorders of emotional memory, the findings support the notion that sex hormone levels at the time of trauma may be critical in modulating emotional memory for the event; the data also support findings from studies of PTSD in women in both laboratory models (Soni, Curran, and Kamboj, 2013) and clinical populations (Ferree et al., 2012).
Results from the present study strengthen the argument that sex hormones and contraceptive status at encoding interact with post-learning stress to modulate memory for emotional events. Thus, further investigation of how these factors influence memory for emotional events appears warranted for a full understanding of disorders related to emotional memory.
Acknowledgments
This work was supported by R01 MH057508 to LC. We would also like to thank Annie Hu for her assistance in data collection, and W. Jake Jacobs for insightful comments.
References
- Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano J, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33(6):874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill LF. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17(6):466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning and Memory. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53:1286–193. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bem SL. Bem sex-role inventory. Redwood City, CA: Consulting Psychologists Press, Inc; 1981. [Google Scholar]
- Beck KD, McLaughlin J, Bergen MT, Cominski TP, Moldow RL, Servatius RJ. Facilitated acquisition of the classically conditioned eyeblink response in women takin oral contraceptives. Behavioral Pharmacology. 2008;19:821–828. doi: 10.1097/FBP.0b013e32831c3b82. [DOI] [PubMed] [Google Scholar]
- Bentz D, Michael T, Wilhelm FH, Hartmann FR, Kunz S, von Rohr IRR, de Quervain DJF. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Perry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. Journal of Experimental Psychology – Learning, Memory, and Cognition. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of General Psychiatry. 1991;48(3):216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Belcher A, Huynh Q. The influence of sex versus sex-related traits on long-term memory for gist and detail of an emotional story. Consciousness and Cognition. 2004;13(2):391–400. doi: 10.1016/j.concog.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Consciousness and Cognition. 1995;4:410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an fMRI investigation. Learning and Memory. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, van Stegeren A. Sex-related impairment of memory for emotional events with β-adrenergic blockade. Neurobiology of Learning and Memory. 2003;79(10):81–88. doi: 10.1016/s1074-7427(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhäuser W, Stout J, Koch C, Carter O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proceedings of the National Academy of Sciences. 2008;105(5):1704–1709. doi: 10.1073/pnas.0707727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertman N, Andreano JM, Cahill L. Progesterone at encoding predicts subsequent emotional memory. Learning and Memory. 2011;18:759–763. doi: 10.1101/lm.023267.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Tran TP, Fong WC, Bryant RA. Sex differences in emotional memory consolidation: the effect of stress-induced salivary alpha-amylase and cortisol. Biological Psychology. 2012;89(3):539–544. doi: 10.1016/j.biopsycho.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Ferree NK, Wheeler M, Cahill L. The influence of emergency contraception on post-traumatic stress symptoms following sexual assault. Journal of Forensic Nursing. 2012;8:122–130. doi: 10.1111/j.1939-3938.2012.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Dolan RJ. Hemispheric asymmetries in global/local processing are modulated by perceptual salience. Neuropsychologia. 1999;37:31–40. doi: 10.1016/s0028-3932(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Fujimoto NY, Willanueva AL, Hopper B, Moscinski M, Rebar RW. Increased adrenocortical responsiveness to exogenous ACTH in oral contraceptive users. Advances in Contraception. 1986;2:343–53. doi: 10.1007/BF02340051. [DOI] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognitive Affective and Behavioral Neuroscience. 2010;10(2):252–269. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway JL, Beck KD, Servatius RJ. Facilitated acquisition of the classically conditioned eyeblink response in females is augmented in those taking oral contraceptives. Behavioural Brain Research. 2011;216:301–307. doi: 10.1016/j.bbr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic Stress Disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Koss MC. Pupillary dilation as an index of central nervous system α2-adrenoreceptor activation. Journal of Pharmacological Methods. 1986;15:1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Cortisol and memory retrieval in women: influence of menstrual cycle and oral contraceptives. Psychopharmacology. 2005;183:65–71. doi: 10.1007/s00213-005-0143-z. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Hellhammer DH, Wüst S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32(8–10):1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Lobo RA, Stanczyk FZ. New knowledge in the physiology of hormonal contraceptives. American Journal of Obstetrics and Gynecology. 1994;170:1499–507. doi: 10.1016/s0002-9378(94)05011-8. [DOI] [PubMed] [Google Scholar]
- Mackiewicsz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of National Academy of Sciences. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: Interaction with other brain systems. Proceedings of the National Academy of Sciences USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Mehrabian A. Manual for the revised trait arousability (converse of stimulus screening) scale. University of California; Los Angeles: 1994. pp. 1–10. [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Neuronal correlates of extinction learning are modulated by sex hormones. Social Cognitive and Affective Neuroscience. 2012;7(7):819–830. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Stark R, Vaitl D, Tabbert K, Wolf OT. Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biological Psychology. 2013;92(1):82–89. doi: 10.1016/j.biopsycho.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Miller RM, Nat R, Plessow F, Nat R, Kirshbaum C, Nat R, Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosomatic Medicine. 2013;75(8):832–840. doi: 10.1097/PSY.0000000000000002. [DOI] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Hormones and Behavior. 2008;54:286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiology of Learning and Memory. 2013;106:56–65. doi: 10.1016/j.nlm.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage alters memory for an emotional story. Neurobiology of Learning and Memory. 2011;96(2):378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological Psychology. 2013;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? Journal of Affective Disorders. 2002;70:229–240. doi: 10.1016/s0165-0327(01)00356-1. [DOI] [PubMed] [Google Scholar]
- Otterstetter O, Szymanski LM, Kamimori GH, Kessler CM, Gold MR, Fernhall B. Hemeostatic responses to maximal exercise in oral contraceptive users. American Journal of Obstetrics and Gynecology. 1999;181(4):958–963. doi: 10.1016/s0002-9378(99)70332-7. [DOI] [PubMed] [Google Scholar]
- Poole A, Ball LJ. Eye tracking in human-computer interaction and usability research. In: Ghaoui C, editor. Encyclopedia of human interaction. 2005. pp. 211–219. [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Correlations between locus ceruleus (LC) neural activity, pupil diameter and behavior in monkey support a role on LC in attention. Society for Neuroscience Abstracts. 1993;19:974. [Google Scholar]
- Rapkin AJ, Morgan M, Sogliano C, Biggio G, Concas A. Decreased neuroactive steroids induced by combined oral contraceptive pills are not associated with mood changes. Fertility and Sterility. 2006;85(5):1371–1378. doi: 10.1016/j.fertnstert.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Cahill L, McGaugh JL. Interaction of emotionally activated neuromodulatory systems in regulating memory storage. In: Ishikawa K, McGaugh JL, Sakata H, editors. Brain Processes and Memory. Elsevier; Amsterdam: 1996b. pp. 39–54. [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory, and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Savic I, Lindstrom P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proceedings of the National Academy of Sciences. 2008;105:9403–9408. doi: 10.1073/pnas.0801566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlitz L, Diener E. Sex differences in the recall of affective experiences. Journal of Personality and Social Psychology. 1998;74(1):262–271. doi: 10.1037//0022-3514.74.1.262. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. New progestogens: a review of their effects in perimenopausal and postmenopausal women. Drugs Aging. 2004;13:865–883. doi: 10.2165/00002512-200421130-00004. [DOI] [PubMed] [Google Scholar]
- Soni M, Curran VH, Kamboj SK. Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiology of Learning and Memory. 2013 doi: 10.1016/j.nlm.2013.04.003. http://dx.doi.org/10.1016/j.nlm.2013.04.003. [DOI] [PubMed]
- Strange BA, Dolan RJ. β-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;31(101):11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AI, Keating CL, Tillbrook AJ. Sex steroids. Rjeka, Croatia: InTech; 2012. Sex differences and the role of sex steroids in sympatho-adrenal medullary system and hypothalamo-pituitary adrenal axis responses to stress; pp. 115–136. [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Molecular Psychiatry. 2008;13(3):325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Zylicz SA, Pieters S, Mattern C, Jan Verkes R, Buitelaar JK, Fernández G. Testosterone increases amygdala reactivity inmiddle-aged women to a young adulthood level. Neuropsychopharmacology. 2009;(34):539–547. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. New England Journal of Medicine. 2002;346(2):108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]