Abstract
Background
Recent evidence suggests that restricted and repetitive behaviors may differentiate children who develop autism spectrum disorder (ASD) by late infancy. How these core symptoms manifest early in life, particularly among infants at high-risk for the disorder, is not well characterized.
Methods
Prospective, longitudinal parent-report data (Repetitive Behavior Scales-Revised) were collected for 190 high-risk toddlers and 60 low-risk controls from 12 to 24 months age. Forty-one high-risk children were classified with ASD at age 2. Profiles of repetitive behavior were compared between groups using generalized estimating equations.
Results
Longitudinal profiles for children diagnosed with ASD differed significantly from high- and low-risk children without the disorder on all measures of repetitive behavior. High-risk toddlers without ASD were intermediate to low-risk and ASD positive counterparts. Toddlers with ASD showed significantly higher rates of repetitive behavior across subtypes at the 12 month time point. Repetitive behaviors were significantly correlated with adaptive behavior and socialization scores among children with ASD at 24 months-age but were largely unrelated to measures of general cognitive ability.
Conclusions
These findings suggest that as early as 12 months age, a broad range of repetitive behaviors are highly elevated in children who go on to develop ASD. While some degree of repetitive behavior is elemental to typical early development, the extent of these behaviors among children who develop ASD appears highly atypical.
Keywords: autism, repetitive behavior, high-risk siblings
Introduction
In his initial description of what would come to be known as autism, Kanner (1943) noted that the behavior of affected children could be globally characterized as “monotonously repetitious”. His astute account of what was then considered a relatively rare disorder included a number of repetitive behavior symptoms which now comprise current diagnostic criteria for autism spectrum disorder (ASD), including stereotyped movements, adherence to routines, resistance to change, and intense preoccupations (American Psychiatric Association, 2013). Although restricted, repetitive behaviors (RRBs) are quintessential to past and present clinical definitions of ASD, relatively little is known about the nature and course of these behaviors prior to diagnosis. Once thought to emerge after core social symptoms, recent findings raise the possibility that RRBs may be among the earliest behavioral manifestations of ASD (Kim & Lord, 2010; Ozonoff et al., 2008; Watson et al., 2007; Werner, Dawson, Munson, & Osterling, 2005). However, teasing apart aberrant behavior from that which is typical complicates attempts to identify whether and how RRBs portend the emergence of the disorder.
The repetitive behavior domain includes topographies ranging from motor stereotypies to circumscribed preoccupations with highly idiosyncratic interests. While the presence of such behaviors in older individuals may stand out as clearly indicative of pathology, this distinction is more ambiguous in young children for whom repetitive behaviors are both common and developmentally appropriate. In typically developing infants, for example, motor stereotypies occur frequently and in progressive fashion over the first year of life, after which time they give way to more variable and goal-directed behaviors (Thelen, 1979). Similarly, toddlers and preschool aged children commonly engage in fixed patterns of ritualistic behavior and adherence to routines evocative of ASD (Arnott et al., 2010; Evans et al., 1997; Leekam et al., 2007). These repetitive behaviors are likely constructive elements of early development which engender behavioral precision, efficiency, and adaptive outcomes (Ejiri & Masataka, 2001; Evans et al., 1997; Thelen, 1995; Wolff, 1967).
There is increasing evidence that repetitive behaviors manifest prior to age 2 in children who develop ASD at levels above and beyond those associated with typical or delayed development. Based on retrospective parent report data, significantly elevated RRBs have been described relative to typically developing (TD) children as early as 10 months of age (Werner et al., 2005) and to children who were TD or developmentally delayed (DD) at 12 months age (Watson et al., 2007). In a prospective study of 12-month-olds, children who developed ASD exhibited higher rates of repetitive and atypical object exploration in an experimental task (Ozonoff et al., 2008). Morgan and colleagues (Morgan, Wetherby, & Barber, 2008; Watt, Wetherby, Barber, & Morgan, 2008) identified increased rates and inventories of RRBs involving body and objects in 18–24 month old children with ASD in comparison to TD and DD controls using the Repetitive and Stereotyped Movement Scales (RSMS; Wetherby & Morgan, 2007). These findings were recently extended in a follow-up study of toddlers with ASD or TD closely matched on nonverbal developmental level (Barber, Wetherby, & Chambers, 2012). In a longitudinal study of Autism Diagnostic Observation Schedule (ADOS) assessment data, children with ASD were characterized by significantly elevated trajectories of RRBs relative to children who were TD or DD from 12–56 months age (Kim & Lord, 2010). This particular finding is striking considering the limited opportunity to observe RRBs in the context of an ADOS administration.
Given the familial liability associated with ASD, prospective sibling studies have not only afforded the opportunity to chart the early developmental origins ASD, but to investigate genetically informative phenotypes as well (Szatmari et al., 2007). In this vein, RRBs among unaffected siblings of children with ASD, who are at elevated risk for developing the disorder, have been directly examined in a handful of studies. Toth and colleagues (2007) found that high-risk siblings negative for ASD (HR-Neg) and TD children exhibited comparable levels of RRBs up until 2 years of age, at which time HR-Neg siblings were characterized by significantly less repetitive behavior than TD controls. Conversely, HR-Neg siblings have been shown to engage in significantly more repetitive play than TD counterparts at 18 months age during a brief sampling of free play (Christensen et al., 2010). Using the RSMS, Damiano et al. (2013) found that while 15-month old children who developed ASD displayed more topographies of RRBs than HR-Neg counterparts, overall rate of behavior did not significantly differ between groups. In a small pilot study, Loh and colleagues (2007) found that 12- and 18-month old children who later met ASD criteria differed from HR-Neg children and TD controls on only 2 of 9 forms of motor stereotypies. Given these inconsistent results and a rather lean body of work, it is difficult to discern how RRBs develop among HR-Neg children relative to low-risk or ASD-positive children.
In the present study, we sought to clarify the development of repetitive behavior from 12 to 24 months among TD children and high-risk siblings who did and did not meet criteria for ASD at 2 years age. We were particularly interested in examining how subtypes of RRBs manifest during this early interval. To this end, we examined longitudinal parent-reported repetitive behaviors in siblings of children with ASD, who are at high risk for the disorder, and low risk control children (LR) at 12 and 24 months age. Previous work has suggested that subtypes of RRBs are differentially associated with cognitive and adaptive behavior measures among individuals with ASD (Bishop, Richler, & Lord, 2006; Bodfish, Symons, Parker, & Lewis, 2000; Lam & Aman, 2007). Among very young children, however, this relationship is less certain (Bishop et al., 2006; Kim & Lord, 2010; Morgan et al., 2008; Ozonoff et al., 2008). As such, we explored the relationship between select cognitive and adaptive behavior measures among toddlers with ASD at age 2 years.
Methods
Participants
Participants were part of the Infant Brain Imaging Study, an ongoing longitudinal study and Autism Center of Excellence Network. Infants were recruited, screened, and assessed at one of four sites: the University of North Carolina, the University of Washington, the Children’s Hospital of Philadelphia, and Washington University in St. Louis. Initial exclusion criteria included: (1) evidence of a genetic condition or syndromes; (2) significant medical or neurological condition affecting development; (3) significant vision or hearing impairment; (4) children with birth weight < 2000 g or gestational age < 36 wks; (5) significant perinatal adversity or prenatal exposure to neurotoxins, (6) contraindication for MRI, (7) predominant home language other than English, (8) children who were adopted or half siblings, (9) 1st degree relative with psychosis, schizophrenia, or bipolar disorder, and (10) twins.
Infants at high-risk were defined as such if they had an older sibling with a diagnosis of ASD who also met criteria for the disorder on the Social Communication Questionnaire (SCQ; Rutter, Bailey, Lord, & Berument, 2003) and Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Couteur, 1994). Infants at low-risk were defined as such if they had TD siblings who screened negative for ASD on the SCQ and had no first degree relatives with a neurodevelopmental disability. All study procedures were approved by the institutional review boards at each site, and informed, written consent was obtained from all participating families.
The present study included 253 children with 12- and/or 24-four-month parent rated repetitive behavior data and complete behavioral assessments at age 24 months. Participants were grouped according to risk status (low- or high-risk sibling) and diagnostic outcome based on clinical best-estimate made by experienced, licensed clinicians using the DSM-IV checklist and supported by all available assessment data, including the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). Children who scored in the range of “autism” or “ASD” on the ADOS, but who were determined to be ASD-negative by clinical best-estimate were included in the HR-Neg group (n = 6). Three LR children meeting criteria for ASD were excluded to maintain the structure of the HR sibling design. This approach to categorization yielded 250 total participants: 60 low-risk (LR) controls, 149 children classified as high-risk ASD negative (HR-Neg), and 41 classified as high-risk ASD positive (HR-ASD). All participants in the present study had complete repetitive behavior data for at least one time point, and 78% had data for both. Descriptive and demographic data for study participants are presented in Table 1.
TABLE 1.
Study sample characteristics
| HR-ASD | HR-Neg | LR-Neg | p1 | |
|---|---|---|---|---|
| Number of subjects | 41 | 149 | 60 | |
| ADOS RRB2 | 2.7 (1.8) | 0.7 (1.0) | 0.3 (0.6) | <.001 |
| ADOS severity | 5.7 (1.8) | 1.7 (1.3) | 1.3 (0.6) | <.001 |
| ADOS social-affective | 10.7 (3.6) | 2.5 (2.9) | 1.8 (1.8) | <.001 |
| Race/Ethnicity (%) | .691 | |||
| Asian | 2.4 | 1.3 | 3.3 | |
| Black | 2.4 | 1.3 | 5.0 | |
| Hispanic | 2.4 | 4.7 | 5.0 | |
| White (non-Hispanic) | 85.4 | 84.6 | 85.0 | |
| Other | 7.3 | 8.1 | 1.7 | |
| SES3 | 4.1 (1.4) | 4.5 (1.2) | 4.7 (1.2) | .159 |
| Time 1 | ||||
| n | 36 | 140 | 53 | |
| Age (months) | 12.6 | 12.5 | 12.7 | .460 |
| Sex (% male) | 78 | 57 | 59 | .071 |
| Mullen ELC4 | 90.3 (15.4) | 99.4 (12.4) | 108.3 (11.2) | <.001 |
| Mullen NVDQ5 | 105.9 (16.2) | 111.5 (12.9) | 119.9 (10.7) | <.001 |
| Vineland ABC6 | 89.3 (18.5) | 93.9 (20.4) | 101.7 (10.2) | .004 |
| Time 2 | ||||
| n | 36 | 125 | 50 | |
| Age (months) | 24.8 (1.2) | 24.7 (0.9) | 24.8 (1.0) | .639 |
| Sex (% male) | 80 | 59 | 56 | .040 |
| Mullen ELC4 | 84.3 (21.9) | 102.8 (17.1) | 112.7 (15.6) | <.001 |
| Mullen NVDQ5 | 91.4 (14.6) | 102.0 (14.5) | 110.8 (14.4) | <.001 |
| Vineland ABC6 | 90.2 (11.2) | 101.6 (8.4) | 104.2 (8.2) | <.001 |
Omnibus ANOVA (Age, Mullen, SES, Vineland) and Fisher’s exact tests (Race/Ethnicity, Sex)
Autism Diagnostic Observation Schedule, Restricted and Repetitive Behavior, age 24 months
Available for 92% of total sample, socioeconomic status composite score reflects household income and parent(s) educational level, scale: 1–7
Early Learning Composite score
Nonverbal developmental quotient
Adaptive Behavior Composite score
Measures
Repetitive Behavior Scales – Revised (RBS-R; Bodfish, Symons, Parker, & Lewis, 2000)
The RBS-R is a parent/caregiver rated measure covering a broad range of repetitive behaviors consisting of 43 items across 6 subscales. Each item represents a discrete and observable behavioral topography. The RBS-R has been independently validated for use among individuals with ASD including toddlers and pre-school age children (Lam & Aman, 2007; Mirenda et al., 2010). Subscales include stereotypical, self-injurious, compulsive, ritualistic, sameness, and restricted behaviors. Parents based ratings upon observations of their child’s behavior over the previous month. The RBS-R provides total and subscale scores using two scales: an inventory, or items endorsed score, and a weighted score which reflects degree of severity (mild, moderate, or severe). We reasoned that the more clinically focused severity scores may be developmentally inappropriate, less valid, and more prone to rater bias. For example, it is unclear how a parent would judge repetitive hand flapping to be a mild versus moderate “problem” in the context of a 12-month-old child. Rather, our interest was in whether or not repetitive behaviors were or were not present in a given child’s behavioral repertoire. As such, we elected to focus our analysis on counts of items endorsed by parents. Group distributions for unadjusted RBS-R scores are presented in Figure 1.
Figure 1.
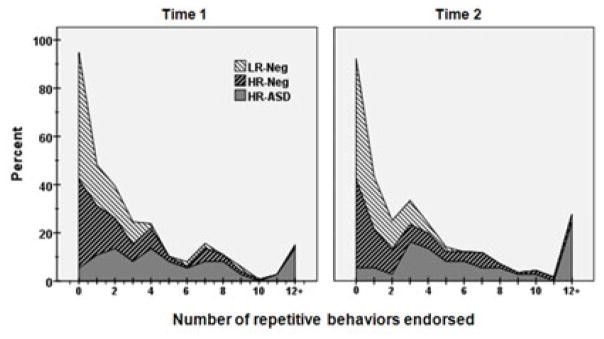
Distributions by group of unadjusted total items endorsed from the Repetitive Behavior Scales, Revised.
Mullen Scales of Early Learning (Mullen, 1995)
The Mullen is a standardized developmental assessment suitable for children aged 0–68 months. It provides an Early Learning Composite (ELC) score, which indexes overall cognitive and motor development. We also derived a nonverbal developmental quotient (NVDQ) from Mullen fine motor and visual reception age-equivalent scores. The Mullen was administered by trained research staff, with reliability initially established and maintained across clinical sites.
Vineland Adaptive Behavior Scales-II (Vineland II; Sparrow, Balla, & Cicchetti, 2005)
The Vineland-II is a standardized, semi-structured and norm-referenced functional skills assessment of adaptive behavior administered in this study as a parent interview. The measure is designed to characterize a child’s day-to-day adaptive behavior. It provides an Adaptive Behavior Composite (ABC) score as well as indexes of adaptive function in each of four subdomains, including socialization, which includes measures of interpersonal relationships and play and leisure skills. Seven participants were missing data at either 12 or 24 months, including 2 participants from the HR-ASD group.
Statistical Analysis
Longitudinal response profiles from ages 12 to 24 months for RBS-R items endorsed were analyzed by generalized estimating equations fit for a loglinear Poisson distribution and unstructured correlation matrix. In-line with previous factor analytic studies (Lam & Aman, 2007; Mirenda et al., 2010), we collapsed ritual and sameness subscales into a single factor. Dependent measures included total RBS-R, and stereotypical, self-injurious, compulsive, ritual and sameness (combined), and restricted behavior subscales.
Subject data was treated as a repeated variable with time point fit as the within-subject structure. Model predictors included Group, Time, and Group X Time. As expected, sex ratio differed between groups and was included in the model to control for potential behavioral differences attributable to this variable. Estimated marginal means for 12 and 24 month time points were generated and tested for cross-sectional group differences from the model. Bonferroni corrected pair-wise tests were performed following significant omnibus test results.
To explore the relationship of RBS-R measures to select cognitive and adaptive behavior outcomes among the HR-ASD group, nonparametric Spearman correlations were performed. Our primary interest was to characterize the relationship between cognitive measures and subtypes of RRBs in children with ASD at ages 12 and 24 months. Variables included in this analysis were Mullen ELC and NVDQ scores, as well as Vineland-II ABC and socialization standard scores. The socialization subscale was included to provide a preliminary investigation into the relationship between RRBs and social skill development. We hypothesized that repetitive behavior would be negatively associated with social skills in early childhood. Correlation analyses were uncorrected for multiple comparisons. All analyses were performed with SPSS 18 (Chicago, IL).
Results
Groups did not differ by age at either the 12- or 24-month assessments, F(2, 228) = .78, p = .460 and, F(2, 208) = .45, p = .639, respectively. Groups differed significantly by Mullen ELC scores at 12 months, F(2,228) = 22.15, p < .001 and 24 months, F(2,208) = 27.21, p < .001, with post-hoc pairwise comparisons indicating that each of the three groups differed significantly from one another. Groups differed in terms of sex ratio at both the 12- (Fisher’s exact test; p = .071) and 24-month visits (p = .035). Groups differed in terms of ADOS social affect total, F(2, 248) = 147.34, p < .001, ADOS restricted and repetitive behavior, F(2, 248) = 66.00, p < .001, and severity score, F(2, 248) = 175.10, p < .001. Pairwise, all groups differed from one another on ADOS restricted and repetitive behavior. For social affect total and severity score, HR-Neg and LR-Neg differed significantly from HR-ASD but not from each other, although this relationship was approaching significance for ADOS severity at p = .06.
Longitudinal patterns of repetitive behavior
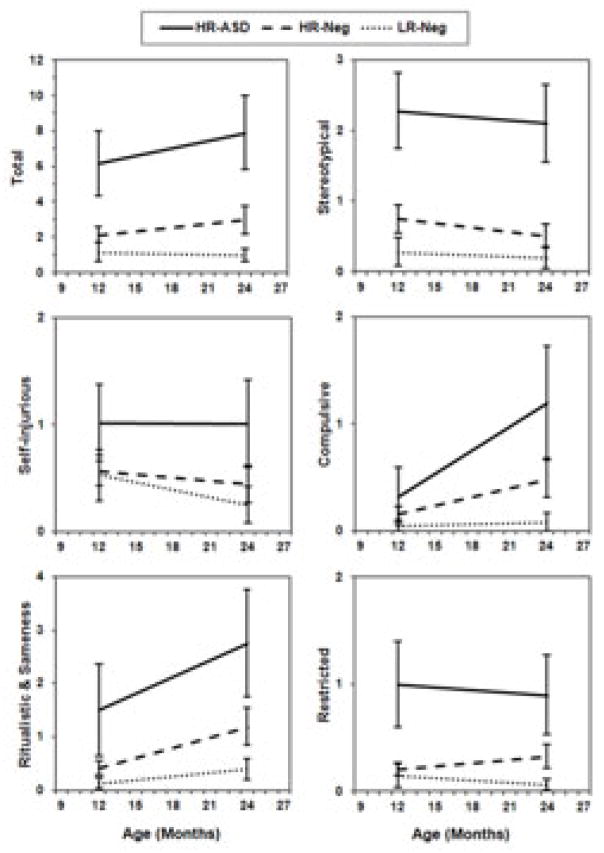
Longitudinal profiles for the main effect of Group significantly differed for total RBS-R and all subscales (Figure 2). This included total items endorsed, χ2 = 74.2, p < 0.0001; stereotypical behavior, χ2 = 97.3, p < 0.0001; self-injurious behavior, χ2 = 18.4, p = 0.0001; compulsive behavior, χ2 = 22.8, p < 0.0001; ritual-sameness behavior, χ2 = 46.0, p < 0.0001; and restricted behavior, χ2 = 48.8, p < 0.0001. Posthoc analysis of longitudinal profiles revealed that groups (LR, HR-Neg, & HR-ASD) significantly differed from one another on RBS-R composite score and stereotypical, ritual/sameness, and restricted subscales. HR-ASD and HR-Neg groups differed significantly from LR children but not each other on the compulsive subscale, while HR-Neg and LR children differed significantly from HR-ASD but not from each other on the self-injurious behavior subscale. There were significant effects for Time, characterized by an increase in reported behaviors from 12 to 24 months, in measures of compulsive (χ2 = 5.4, p < 0.02) and ritual-sameness behaviors (χ2 = 23.6, p < 0.0001). Results for the interaction of Group X Time did not reach the level of significance for any RBS-R measures.
Figure 2.
Estimated marginal means for Repetitive Behavior Scales-Revised total and subscale items endorsed for outcomes groups at 12 and 24 months age. Error bars represent 95% confidence intervals.
Cross-sectional patterns of repetitive behavior
Estimated marginal means and standard errors were generated from the longitudinal model and group means compared within this model at each of the 12 and 24 month time points. As with the longitudinal profiles, groups differed significantly on all RBS-R measures at the cross-sectional 12 and 24 month time points. Descriptive statistics and test results from these analyses, along with Bonferroni corrected pair-wise comparisons and effect sizes, are presented in Table 2. As a follow-up, we partially examined the validity of parent reported RRB by examining its relationship to repetitive behavior recorded during the ADOS administration. Total items endorsed on the RBS-R was significantly (albeit modestly) correlated with ADOS RRB algorithm scores at the 24 month time point, rs(211) = .28, p < .0001. We also examined whether findings for the HR-Neg group were driven by children who were subthreshold for ASD. This was done two ways. First, we reanalyzed the HR-Neg group removing the subset of children meeting ADOS criteria for an ASD (n = 6). This change had a negligible effect on the above described results. Second, we examined the correlation between 24 months ADOS social-affective algorithm scores and total RBS-R inventory scores at both 12 and 24 months age within the HR-Neg group. These associations were non-significant, p > .85.
TABLE 2.
Longitudinal profiles and estimated marginal means by group for Repetitive Behavior Scales-Revised (RBS-R).
| Repetitive behavior | HR-ASD (a) |
HR-Neg (b) |
LR-Neg (c) |
Omnibus1 | Post-hoc2 | IRR3
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EMM | SE | EMM | SE | EMM | SE | χ2 | p | (a) vs. (b) | (a) vs. (c) | ||
| Time 1 (12 months) | |||||||||||
| RBS-R Total Endorsed | 6.35 | 0.98 | 2.16 | 0.23 | 1.22 | 0.26 | 48.9 | <0.0001 | a>b,c | 2.9 | 5.2 |
| Stereotypical | 2.29 | 0.28 | 0.75 | 0.10 | 0.29 | 0.10 | 57.3 | <0.0001 | a>b>c | 3.1 | 8.0 |
| Self-injurious | 1.04 | 0.19 | 0.57 | 0.08 | 0.54 | 0.12 | 8.5 | 0.014 | 1.8 | 1.9 | |
| Compulsive | 0.43 | 0.18 | 0.20 | 0.05 | 0.08 | 0.04 | 7.5 | 0.024 | 2.2 | 5.6 | |
| Ritual/Sameness | 1.63 | 0.45 | 0.43 | 0.07 | 0.15 | 0.06 | 28.5 | <0.0001 | a,b>c | 3.8 | 10.6 |
| Restricted | 0.99 | 0.19 | 0.21 | 0.04 | 0.15 | 0.07 | 35.7 | <0.0001 | a>b,c | 4.7 | 6.5 |
| Time 2 (24 months) | |||||||||||
| RBS-R Total Endorsed | 8.14 | 1.19 | 2.97 | 0.40 | 1.03 | 0.19 | 77.8 | <0.0001 | a>b>c | 2.7 | 7.9 |
| Stereotypical | 2.13 | 0.28 | 0.50 | 0.09 | 0.20 | 0.08 | 64.0 | <0.0001 | a>b,c | 4.2 | 10.5 |
| Self-injurious | 1.05 | 0.23 | 0.45 | 0.09 | 0.26 | 0.09 | 15.3 | 0.001 | a>c | 2.3 | 4.0 |
| Compulsive | 1.19 | 0.27 | 0.48 | 0.09 | 0.08 | 0.05 | 23.1 | 0.0001 | a>c | 2.5 | 14.9 |
| Ritual/Sameness | 2.89 | 0.57 | 1.20 | 0.18 | 0.42 | 0.10 | 36.7 | <0.0001 | a>b>c | 2.4 | 6.9 |
| Restricted | 0.88 | 0.19 | 0.33 | 0.06 | 0.06 | 0.03 | 25.8 | <0.0001 | a,b>c | 2.6 | 14.3 |
Generalized estimating equations. Two-sided significance level of .05.
Bonferroni corrected.
Incident rate ratio
Relationship to cognitive development and adaptive behavior in ASD
At 12 months age, total RBS-R inventory scores for HR-ASD children were not significantly correlated with either Mullen ELC or NVDQ scores, rs(36) = −.20, p = .25 and rs (36) = −.13, p = −.45, respectively. These variables were not associated with any RBS-R subscales (p > .35) with the exception of the restricted subscale, with which both ELC [rs (34) = −.37, p = .03] and NVDQ [rs (36) = −.36, p = .03] were significantly correlated. Vineland measures for Adaptive Behavior Composite and socialization were not significantly correlated with any RBS-R measure at age 12 months.
At 24 months age, total RBS-R item endorsed scores were not significantly correlated with either Mullen ELC or NVDQ scores, rs (36) = .11, p = .52 and rs (36) = .03, p = .88, respectively. Neither ELC nor NVDQ were significantly associated with any RBS-R subscale, p > .20. Vineland ABC scores were significantly negatively correlated with total RBS-R [rs (34) = −.38, p = .03], stereotypical behavior [rs (34) = −.43, p = .01], compulsive behavior [rs (31) = −.43, p = .01], and restricted behavior [rs (34) = −.44, p = .01]. Self-injurious behavior and ritual-sameness behavior were not significantly correlated with Vineland-II ABC scores, p > .50. Vineland socialization scores were significantly negatively correlated with total RBS-R [rs (34) = −.43, p = .01], stereotypical behavior [rs (34) = −.55, p = .001], and restricted behavior [rs (34) = −.51, p = .002]. Self-injurious behavior, compulsive behavior, and ritual-sameness behavior were not significantly correlated with socialization scores.
Discussion
We examined longitudinal parent-reported patterns of repetitive behavior in typically developing toddlers and toddlers at familial high-risk for ASD, a subset of whom met criteria for the disorder, at ages 12 and 24 months age. We found significant group effects for all subtypes of RRBs over this age interval. The effect of Time was significant for compulsive and ritual/sameness behaviors from 12 to 24 months age, in-line with previous work finding that higher-order RRBs increase over time (Richler, Huerta, Bishop, & Lord, 2010). This age-related effect is likely related in part to a child’s increasing ability to perform complex behaviors. Perhaps most notably, we identified robust differences between toddlers who were HR-ASD and both LR- and HR-Neg comparison children at 12 months age. This adds to a growing body of work suggesting that repetitive behavior may be an early emerging symptom of ASD (Kim & Lord, 2010; Ozonoff et al., 2008; Watson et al., 2007; Werner et al., 2005) and converges with findings based on clinical coding performed by blind raters (Elison et al., under review). It further raises the possibility that a simple, inexpensive parent-report measure of repetitive behavior could predict risk by pulling for disorder-specific differences early in development. In interpreting the present results, readers should bear in mind that the RBS-R is not a direct observational measure, and it is possible that scores between risk groups reflect some degree of bias as parents of an older child with ASD are likely more knowledgeable of and attentive to atypical behavior than parents of TD children. The strength of prospective sibling studies, however, is that any such bias is well controlled in HR-Neg versus HR-ASD comparisons.
We also found that RRBs were present across a continuum of cognitive ability in toddlers with ASD, and not restricted to lower-functioning children. The sole exception to this was for restricted behavior, which was modestly negatively correlated with Mullen ELC and NVDQ at 12 months age. This finding is consistent with previous reports suggesting that the relationship between RRBs and cognitive measures develops over time (Bishop et al., 2006; Kim & Lord, 2010; Morgan et al., 2008; Ozonoff et al., 2008). While certain RRBs may be negatively associated with IQ in older children with ASD, these behaviors may be largely independent of cognitive ability early in development. We did, however, find that total RBS-R and most subscale scores were correlated with Vineland adaptive behavior and socialization scores at age 24 months. With regard to socialization, we hypothesized that higher rates of RRBs would be linked to fewer age-appropriate social skills. The present results suggest a possible trade-off between repetitive behavior and social skill acquisition during early development, wherein children who engage in more RRBs may have fewer opportunities to scaffold behaviors necessary for adaptive social interaction (Lam, Bodfish, & Piven, 2008; Mirenda et al., 2010; Ozonoff et al., 2008). Conversely, difficulties in socialization may reduce opportunities to develop age-appropriate functional play skills, contributing to a void in a child’s behavioral repertoire that becomes increasingly filled with restricted and repetitive behaviors.
Among HR-Neg children, RRBs were elevated relative to LR controls. This suggests a few possibilities. First, the presence of repetitive behavior may be a component phenotype of ASD (Szatmari et al., 2007). Shared familial liability for ASD may be globally expressed by differences in RRBs relative to LR children, a position supported by results pertaining to unaffected siblings (Christensen et al., 2010; Damiano et al., 2013) and parents of probands (Hollander, King, Delaney, Smith, & Silverman, 2003; Hurley, Losh, Parlier, Reznick, & Piven, 2007). By definition, those meeting criteria for the disorder fall on the more extreme end of a continuum of RRBs, with HR-Neg children intermediate to LR and HR-ASD counterparts. Second, the relatively higher rates of RRBs among the HR-Neg group may be the product of a subgroup of children who, while not yet meeting criteria for ASD, are nonetheless characterized by atypical development. Recent findings suggest that about 20% of HR-Neg children will follow an early atypical developmental trajectory characterized by developmental delay and subthreshhold autistic symptoms (Georgiades et al., 2013; Messinger et al., 2013). Many such children may later meet criteria for ASD or another neurodevelopmental disorder, such as specific learning disability or language impairment. Thus, elevated rates of RRBs among toddlers who are HR-Neg may be driven by a subgroup of toddlers who are developing atypically but have not yet reached an age at which a diagnostic classification may be made.
In the present sample, we partially explored the possibility that observed elevations among the HR-Neg group were due to the effect of children not yet meeting clinical criteria for ASD. This was done by removing the subset of HR-Neg children who met ADOS criteria for an ASD, a change which had no effect on longitudinal or cross-sectional group-differences. We also found no association between social-affective scores on the ADOS and RBS-R total inventory scores in this group. Together, these data suggests that in our sample, intermediate levels of RRB among HR-Neg children were not driven by a subset of children with subthreshold symptoms. However, this does not rule out the possibility that HR-Neg children with elevated RRB will later develop ASD.
For at-risk infants and toddlers, there may be a reciprocal relationship between repetitive behavior and social communication, whereby an overabundance of the former interferes with the acquisition of latter (Lee, Odom, & Loftin, 2007; Wolff & Piven, 2013). Stereotypical motor behavior, for example, requires minimal information processing, and with persistence, becomes less responsive to external stimuli (Mason, 1991). As such, relatively fixed patterns of behavior become increasingly entrenched and may preclude the acquisition of more dynamic action. In TD toddlers, most RRBs diminish by 12 months age as behavior becomes more variable, goal directed, and responsive to environmental demands (Thelen, 1979, 1995). For very young children at-risk for ASD, the persistence of invariant behavior may crowd out opportunities for more adaptive and increasingly complex responses to novel demands, such as those involving flexible attention to the fluid and socially communicative behavior of caregivers. In this regard, RRBs may be more than symptomatic of ASD – they may also contribute to its emergence in at-risk infants and toddlers by impeding developmental plasticity relevant to social cognition (Chawarska, Macari, & Shic, 2013; Elison, Paterson, et al., 2013; Elison, Wolff, et al., 2013). Alternative hypotheses include the possibility that social skill deficits provide continued opportunities for restricted patterns of behavior, or that these phenomena co-occur but are otherwise unrelated in terms of development. While the present findings can only indirectly speak to these possibilities, the negative association between RRBs and adaptive social behavior do lend support to the position that social deficits and RRBs are interrelated. Future work might more directly explore the links between repetitive behavior and early development among young children at-risk for ASD.
Limitations
As the RBS-R is not specific to toddlers and contains some items which may not be developmentally appropriate to this age group, e.g. compulsive item/object counting, a more focused measure might identify behaviors unique to early development. However, most RBS-R items do converge with similar measures of RRBs used in studies of young children (Evans et al., 1997; Thelen, 1979). This study followed participants through age 2 years and based diagnostic classification upon assessments made at that time. It is possible that this approach, which maximizes the specificity of an ASD classification, is less sensitive to later-emerging cases of ASD. Following children to later ages would offer the opportunity to characterize more stable outcomes and identify possible latent classes among children who are HR. Finally, the present data are limited to inventories of repetitive behavior. While this speaks to the repertoire of RRBs among participants, it does not capture differences in frequency.
Conclusion
Restricted and repetitive behaviors are associated with typical early development, occurring as a chain of developmental events which allow for more flexible and complex patterns of purposeful behavior. For young children who go on to develop ASD, this sequence of development may be substantially altered. Findings from the present study, along with those from previous work, strongly suggest that multiple topographies of RRBs are evident among young toddlers who go on to meet diagnostic criteria for ASD as early 12 months age. The timing and nature of these phenomena raise the possibility that RRBs may contribute to a chain of developmental events giving rise to the autism phenotype. At the least, early elevations in RRBs may signify increased risk for ASD and offer a target for intervention which capitalizes on the highly plastic developing brain (Restivo et al., 2005).
Key points.
Repetitive behaviors occur as part of typical early development and precede more adaptive and goal-directed forms of behavior.
Recent evidence suggests that restricted, repetitive behaviors associated with autism spectrum disorder emerge by early toddlerhood.
We found that longitudinal patterns of repetitive behavior were significantly higher from 12 to 24 months age in familial high-risk children who developed ASD in comparison to high- and low-risk children without ASD.
Substantial differences in parent-reported repetitive behavior at the 12 month time point suggest that these behaviors may be a readily observable early marker of ASD.
Acknowledgments
This study was supported by grants from NIH (R01-HD055741, HD055741-S1, MH101653, & P30-HD03110)), Autism Speaks, and the Simons Foundation. The authors thank ‘The Infant Brain Imaging Study (IBIS) Network’ families for participating in this research. IBIS Network is an NIH funded Autism Center of Excellence project and consists of a consortium of 7 universities in the U.S. and Canada. Clinical Sites: University of North Carolina: J. Piven (IBIS Network PI), H.C. Hazlett, J.C. Chappell; University of Washington: S. Dager, A. Estes, D. Shaw; Washington University: K. Botteron, R. McKinstry, J. Constantino, J. Pruett; Children’s Hospital of Philadelphia: R. Schultz, S. Paterson; University of Alberta: L. Zwaigenbaum; Data Coordinating Center: Montreal Neurological Institute: A.C. Evans, D.L. Collins, G.B. Pike, P. Kostopolous, S. Das; Image Processing Core: University of Utah: G. Gerig; University of North Carolina: M. Styner; Statistical Analysis Core: University of North Carolina: H. Gu; Genetics Analysis Core: University of North Carolina: P. Sullivan, F. Wright.
Footnotes
Conflicts of interest statement: No conflicts declared.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Arnott B, McConachie H, Meins E, Fernyhough C, Le Couteur A, Turner M, Leekam S. The frequency of restricted and repetitive behaviors in a community sample of 15-month-old infants. Journal of Developmental and Behavioral Pediatrics. 2010;31(3):223–9. doi: 10.1097/DBP.0b013e3181d5a2ad. [DOI] [PubMed] [Google Scholar]
- Barber AB, Wetherby AM, Chambers NW. Brief report: repetitive behaviors in young children with autism spectrum disorder and developmentally similar peers: a follow up to Watt et al. (2008) Journal of Autism and Developmental Disorders. 2012;42(9):2006–12. doi: 10.1007/s10803-011-1434-3. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychology. 2006;12(4–5):247–67. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–43. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry. 2013;74(3):195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L, Hutman T, Rozga A, Young GS, Ozonoff S, Rogers SJ, Sigman M. Play and developmental outcomes in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2010;40(8):946–57. doi: 10.1007/s10803-010-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Nahmias A, Hogan-Brown AL, Stone WL. What do repetitive and stereotyped movements mean for infant siblings of children with autism spectrum disorders? Journal of Autism and Developmental Disorders. 2013;43(6):1326–35. doi: 10.1007/s10803-012-1681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejiri K, Masataka N. Co-occurences of preverbal vocal behavior and motor action in early infancy. Developmental Science. 2001;4(1):40–48. [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Piven J. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. American Journal of Psychiatry. 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Heimer DC, Paterson SJ, Gu H, Hazlett HC, Piven J. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Developmental Science. 2013;16(2):186–97. doi: 10.1111/desc.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Reznick JS, Meadows A, Botteron KN, Estes AM, Piven J. Repetitive behavior in 12-month-olds later classified with ASD. n.d. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Leckman JF, Carter A, Reznick JS, Henshaw D, King RA, Pauls D. Ritual, habit, and perfectionism: the prevalence and development of compulsive-like behavior in normal young children. Child Development. 1997;68(1):58–68. [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, Zwaigenbaum L, Bryson S, Brian J, Roberts W, Garon N. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. JAMA Psychiatry. 2013;70(1):42–8. doi: 10.1001/2013.jamapsychiatry.1. [DOI] [PubMed] [Google Scholar]
- Hollander E, King A, Delaney K, Smith CJ, Silverman JM. Obsessive–compulsive behaviors in parents of multiplex autism families. Psychiatry Research. 2003;117(1):11–16. doi: 10.1016/s0165-1781(02)00304-9. [DOI] [PubMed] [Google Scholar]
- Hurley RSE, Losh M, Parlier M, Reznick JS, Piven J. The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders. 2007;37(9):1679–90. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kim SH, Lord C. Restricted and repetitive behaviors in toddlers and preschoolers with autism spectrum disorders based on the Autism Diagnostic Observation Schedule (ADOS) Autism Research. 2010;3(4):162–73. doi: 10.1002/aur.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KSL, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–66. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lam KSL, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. Journal of Child Psychology and Psychiatry. 2008;49(11):1193–200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Odom SL, Loftin R. Social engagement with peers and stereotypical behavior of chidlren with autism. Journal of Positive Behavior Interventions. 2007;9:67–79. [Google Scholar]
- Leekam S, Tandos J, McConachie H, Meins E, Parkinson K, Wright C, Le Couteur A. Repetitive behaviours in typically developing 2-year-olds. Journal of Child Psychology and Psychiatry. 2007;48(11):1131–8. doi: 10.1111/j.1469-7610.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- Loh A, Soman T, Brian J, Bryson SE, Roberts W, Szatmari P, Zwaigenbaum L. Stereotyped motor behaviors associated with autism in high-risk infants: a pilot videotape analysis of a sibling sample. Journal of Autism and Developmental Disorders. 2007;37(1):25–36. doi: 10.1007/s10803-006-0333-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23. [PubMed] [Google Scholar]
- Lord Catherine, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mason GJ. Stereotypies: a critical review. Animal Behaviour. 1991;41(6):1015–1037. [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, Sigman M. Beyond autism: a baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(3):300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenda P, Smith IM, Vaillancourt T, Georgiades S, Duku E, Szatmari P, Zwaigenbaum L. Validating the Repetitive Behavior Scale-revised in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2010;40(12):1521–30. doi: 10.1007/s10803-010-1012-0. [DOI] [PubMed] [Google Scholar]
- Morgan L, Wetherby AM, Barber A. Repetitive and stereotyped movements in children with autism spectrum disorders late in the second year of life. Journal of Child Psychology and Psychiatry. 2008;49(8):826–37. doi: 10.1111/j.1469-7610.2008.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: AGS Publishing; 1995. [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12(5):457–72. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proceedings of the National Academy of Sciences USA. 2005;102(32):11557–62. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Development and Psychopathology. 2010;22(1):55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C, Berument S. Social Communication Questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. Vineland adaptive behavior scales. 2. Shoreview, MN: AGS Publishing; 2005. [Google Scholar]
- Szatmari P, Maziade M, Zwaigenbaum L, Mérette C, Roy MA, Joober R, Palmour R. Informative phenotypes for genetic studies of psychiatric disorders. American Journal of Medical Genetics Part B. 2007;144B(5):581–8. doi: 10.1002/ajmg.b.30426. [DOI] [PubMed] [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Animal Behaviour. 1979;27(Pt 3):699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Thelen E. Motor development. A new synthesis. The American Psychologist. 1995;50(2):79–95. doi: 10.1037//0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37(1):145–57. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LR, Baranek GT, Crais ER, Steven Reznick J, Dykstra J, Perryman T. The first year inventory: retrospective parent responses to a questionnaire designed to identify one-year-olds at risk for autism. Journal of Autism and Developmental Disorders. 2007;37(1):49–61. doi: 10.1007/s10803-006-0334-4. [DOI] [PubMed] [Google Scholar]
- Watt N, Wetherby AM, Barber A, Morgan L. Repetitive and stereotyped behaviors in children with autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders. 2008;38(8):1518–33. doi: 10.1007/s10803-007-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, Dawson G, Munson J, Osterling J. Variation in early developmental course in autism and its relation with behavioral outcome at 3–4 years of age. Journal of Autism and Developmental Disorders. 2005;35(3):337–350. doi: 10.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Morgan L. Repetitive and Stereotyped Movement Scales: Companion to the CSBS. Florida State University; Tallahassee, FL: 2007. Unpublished manual. [Google Scholar]
- Wolff JJ, Piven J. On the emergence of autism: neuroimaging findings from birth to preschool. Neuropsychiatry. 2013;3(2):209–222. [Google Scholar]
- Wolff PH. The role of biological rhythms in early psychological development. Bulletin of the Menninger Clinic. 1967;31(4):197–218. [PubMed] [Google Scholar]