Abstract
During childhood, verbal learning and memory are important for academic performance. Recent fMRI studies have reported on the functional correlates of verbal memory proficiency, but few have reported the underlying structural correlates. The present study sought to test the relationship between fronto-temporal white matter integrity and verbal memory proficiency in children. Diffusion weighted images were collected from 17 Black children (age 8–11 years) who also completed the California Verbal Learning Test. To index white matter integrity, fractional anisotropy values were calculated for bilateral uncinate fasciculus. The results revealed that low anisotropy values corresponded to poor verbal memory, whereas high anisotropy values corresponded to significantly better verbal memory scores. These findings suggest that a greater degree of myelination and cohesiveness of axonal fibers in uncinate fasciculus underlie better verbal memory proficiency in children.
Keywords: Children, Uncinate Fasciculus, Verbal Memory, Diffusion Tensor Imaging
Introduction
The ability to encode and recall verbal information is important for academic performance in childhood. Retaining a string of words in verbal short-term memory, for example, is related to reading efficiency [1] and scores on tests of working memory are related to standardized test scores [2]. As such, proficiency at tasks involving verbal memory is important for positive educational outcomes. In recent years, functional neuroimaging studies have reported on the neural correlates of proficient verbal memory function in children. Functional magnetic resonance imaging (fMRI) results confirm suggestions by human lesion cases [3] that the ability to encode verbal information is supported by the medial temporal lobes [4]. Furthermore, functional connectivity between medial temporal lobes and dorsolateral prefrontal cortex may support memory encoding [5] that strengthens with development into adulthood [6]. Although the functional correlates of verbal memory proficiency in children have been reported, few studies have examined underlying white matter structural connections [7].
It is well known that memory performance depends upon the functional integrity of the hippocampus and dorsolateral prefrontal cortex [8]. The uncinate fasciculus (UF) is a major white matter fiber tract with projections between the hippocampal area and dorsolateral prefrontal cortex [9], making it a strong candidate for supporting the interaction between these two regions. To measure the structural integrity of this tract, diffusion tensor imaging can be used to characterize the degree of white matter coherence by measuring the anisotropy (directional dependence) of water diffusion within the tissue. Higher levels of anisotropic diffusion ostensibly correspond to a greater extent of microstructural integrity of white matter (i.e., axonal membrane structure and myelination) [10] and are often indexed by fractional anisotropy (FA), a scalar measure from 0–1 with 1 indicating fully anisotropic diffusion.
To our knowledge, only one study has reported on the relationship between white matter integrity in the UF and verbal memory proficiency in children [7]; that study, however, differed in analysis from the present study, utilizing seed-point probabilistic tractography at the individual level rather than the group based voxel-wise analysis used in this study. The approach described here, Tract-Based Spatial Statistics (TBSS) [11], is focused on reducing cross-subject variability in voxels contributing to FA values of a fiber tract and is thus more replicable than individual level tractography. We utilized TBSS to test the association between fronto-temporal white matter integrity and verbal memory proficiency in a sample of 8–11 year old children. Participants underwent a diffusion tensor imaging scan to assess white matter integrity of the UF. Additionally, the children completed the California Verbal Learning Test – Children’s Version (CVLT-C) as a test of their verbal memory proficiency. Given the evidence that improved fronto-temporal connectivity supports better memory [6; 7], we hypothesized that greater white matter integrity in UF would be associated with higher verbal recall scores.
Methods
Participants
Participants were a subset of 8–11 year old children in a randomized trial [12], who were recruited from public schools around Augusta, Georgia, U.S.A. Participants with neurological or psychiatric disorders were excluded. Each child’s parent or guardian reported the child’s age, sex, and race. The study took place at the Georgia Prevention Center at Georgia Regents University. The present study included 17 children who provided usable diffusion tensor imaging data at baseline (mean age = 9.5 years, SD = 0.7; 52% female; 100% Black). Children and parents completed written informed assent and consent in accordance with the Human Assurance Committee of Georgia Regents University.
Memory measure
Verbal learning and memory was assessed using the California Verbal Learning Test–Children’s Version (CVLT-C) [13]. The CVLT-C measures recall of a shopping list of 15 items (e.g., grapes, blocks, belt), which is read aloud five times to the child. The CVLT-C includes conditions to test immediate recall (i.e., at the end of the list), short-delay free and cued recall (i.e., following a 15 word distractor list), and long-delay free and cued recall (i.e., after 20 min).
Diffusion MRI data acquisition
Images were acquired at Georgia Regents University on a 3T GE Signa Excite HDx MRI system (General Electric Medical Systems, Milwaukee, WI). During scanning, head positions were stabilized with a vacuum pillow and/or foam padding. Diffusion images were acquired using an echo planar imaging (EPI) sequence (acquisition matrix = 128 × 128, 60 interleaved slices, voxel size = 1 × 1 × 2.4 mm, FOV = 256 × 256 mm, TR = 15500 ms, TE = min-full, 3 B0 images, 30 diffusion weighted images, b = 1000 s/mm2).
Diffusion MRI data analysis
Raw diffusion images were converted from GE DICOM format to NIFTI format using the dcm2nii tool [14]. For each subject, volumes were visually inspected for motion artifacts; volumes distorted by motion were removed from the image series and b value/vector tables (< 9% of total volumes removed; average number of volumes/subject removed = 4). Diffusion tensor image analysis was conducted using the FMRIB Software Library [15]. Diffusion images were registered to the first B0 image and corrected for eddy-current induced distortions. Non-brain tissue was removed using the Brain Extraction Tool [16]. FA images were created by fitting diffusion tensors to each voxel using the Diffusion Toolbox. Voxelwise analysis of FA data was carried out using TBSS [11]. FA images were aligned and transformed into standard space (MNI152; Montreal Neurological Institute, McGill, Canada). Individual FA images contributed to a cross-subject mean FA image which was used to generate a FA skeleton representing the center of large fiber tracts common across the group.
To isolate the UF, a series of binary masks were applied to each subject (see Figure 1). First, a mask based on each subject’s FA image was generated to include FA values ≥ .2 [7]. Second, the Johns Hopkins University white-matter tractography atlas [17] was used to create binary masks corresponding to left and right UF based on probability values (i.e., the probability that a voxel contains the UF); UF values ≥ 5% were included in the mask [18]. Third, a TBSS FA skeleton mask (based on the group FA image) was applied to each individual to include only voxels containing white matter which were common to the group. After applying the masks, a mean FA value was calculated for each subject’s left and right UF. Left and right mean FA values were correlated with verbal memory scores using SPSS Version 17 (IBM, Armonk, NY). One short-delay free recall score was identified as an influential outlier and therefore not included in the left or right UF FA correlation analyses (pairwise deletion). Following the hypothesis-based correlation analysis, an exploratory k means clustering analysis was conducted to test the possibility that data-driven group assignment could be used to predict differences in verbal memory scores (k = 2, input: left and right UF FA values; ≤ 10 iterations).
Figure 1.
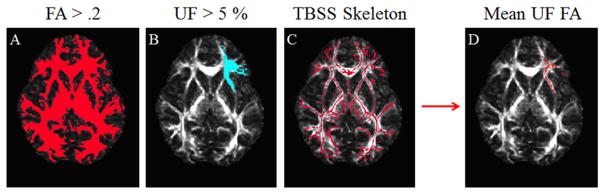
Binary masks overlaid on axial slice of FA values in MNI space (z = 2mm); overlay is colored in red or blue, indicating which voxels to include in the final analysis. Panel A shows a binary mask based on FA values. Panel B shows a binary mask of uncinate fasciculus created from the Johns Hopkins University white matter tractography atlas. Panel C shows a binary mask created from the Tract-Based Spatial Statistics skeleton. Panel D shows the multiplication of masks A, B, and C. Voxels in the resultant mask (D) contributed to a mean for the final analyses. Images displayed in radiological convention.
Results
Correlation analyses
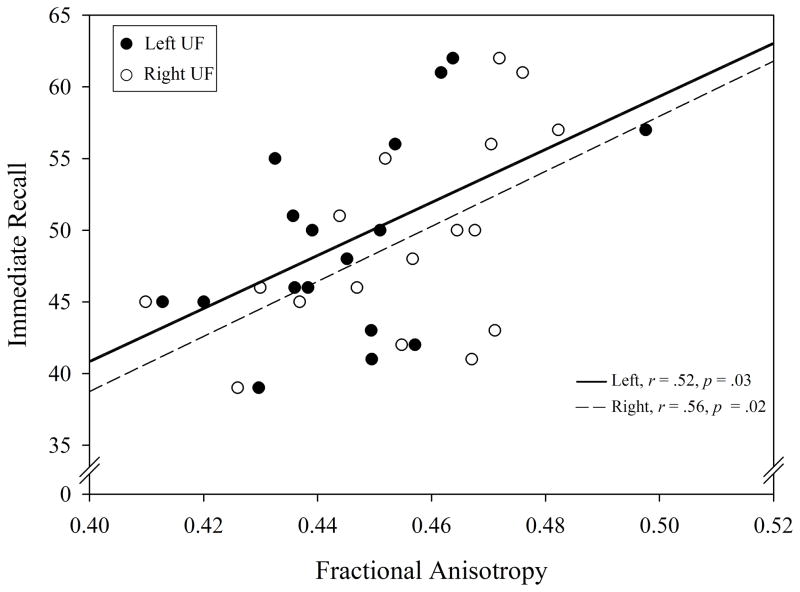
Positive linear relationships were found between FA values in left and right UF and CVLT-C scores. Left UF FA values (n = 17) significantly positively correlated with immediate recall scores (r(15) = .52, p = .03; see Figure 2). A sensitivity analysis omitting one outlier in left UF (FA = .49, immediate recall = 57) did not substantially affect the correlation (r(14) = .46, p = .07). Right UF FA (n = 17) values were significantly positively correlated with immediate recall scores, r(15) = .56, p = .02. No age- or sex-related effects were present for FA values in left or right UF. There were no significant relationships between FA values in left or right UF and short-delay free recall, short-delay cued recall, long-delay free recall, and long-delay cued recall scores.
Figure 2.
Immediate recall plotted as a function of fractional anisotropy values in left (filled circles) and right (unfilled circles) uncinate fasciculus. Regression lines plotted as solid and dashed lines indicating left and right UF, respectively.
K means clustering
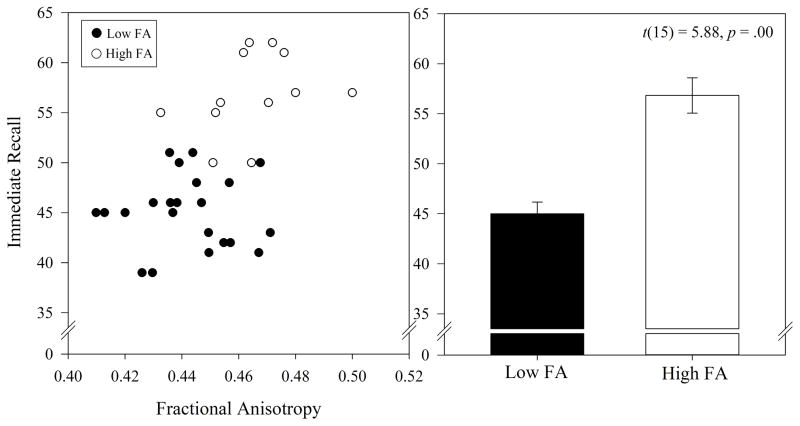
K means clustering was used to test the possibility that data-driven group assignment could be used to predict differences in verbal memory scores. As such, bilateral FA values were entered into a k means clustering analysis to classify participants as belonging to a low or a high FA group (k = 2), with the idea that participants in the low FA group would have less coherent white matter structure than participants in the high FA group. Between group memory scores were then assessed. Using k = 2 (i.e., low and high FA), the clustering analysis yielded 11 participants as low and 6 participants as high FA (see Figure 3, left). The low FA group showed significantly lower immediate recall scores than the high FA group (see Figure 3, right).
Figure 3.
Left: k means clustering of left and right uncinate fasciculus anisotropy values. Using k = 2, participants were categorized as having low or high fractional anisotropy values. Right: low and high FA groups significantly differed on number of items recalled. Bars represent mean FA values of low and high groups.
Discussion
The goal of this study was to test whether greater fronto-temporal white matter anisotropy was associated with higher verbal memory scores in children. Analyses demonstrated that FA values in left and right UF showed a positive linear relationship with immediate recall scores as measured by the CVLT-C; higher FA values were associated with better immediate recall scores. An exploratory k means cluster analysis was used to test the hypothesis that, when conditioning on low and high FA values (with high values indicating better white matter integrity), the two clusters would significantly differ on verbal memory scores. This method of clustering yielded support for the correlation analysis by illustrating a clear dichotomy of anisotropy values, with the low FA group showing significantly lower verbal memory scores than the high FA group.
The positive relationship between fronto-temporal anisotropy and short-term verbal memory proficiency may reflect a greater degree of myelination and cohesiveness [10]. These structural properties are ostensibly related to conduction velocity and thus efficiency of communication of neural signals between regions connected by white matter fibers [19]. Increased neuron fiber diameter may lead to greater conductance along the axon, whereas myelin reduces intra- to extracellular capacitance and increases resistance [20]. Thus, within the context of typical brain development, increased signal transmission efficiency via improved white matter integrity could bolster verbal memory ability in children. Studies using fMRI measures of functional connectivity in adolescents provide support for this assertion such that greater fronto-temporal functional connectivity supports increased memory efficiency in adolescents [6], with greater activation in frontal and temporal regions supporting better memory performance [21].
In summary, the results from this study illustrate the positive linear relationship between UF anisotropy and short-term verbal memory proficiency in a sample of 8–11 year old Black children. This relationship is consistent with that found in older Canadian children and adolescents [7] using a probabilistic tractography approach. The present study utilized a group based voxel-wise approach, with additional support from data driven k means clustering which delineated clear low and high FA clusters corresponding to low and high verbal memory scores, respectively. Understanding the neural correlates of verbal memory proficiency in childhood could inform education policy – identifying ways to improve fronto-temporal white matter integrity could lead to better performance in the classroom.
Acknowledgments
DJS, CEK, CLD, and JEM conceived and carried out experiments and analyzed data. JDA, NEY, and TL conceived and carried out experiments. NFS, LC, ALR, and JEP carried out experiments. This work was supported by the National Institutes of Health (R01 HL87923) and the National Science Foundation Graduate Research Fellowship Program.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Liberman IY, Shankweiler D. Speech, the alphabet, and teaching to read. In: Resnick L, Weaver PA, editors. Theory and practice of early reading. Hillsdale, N.J: Erlbaum Associates; 1976. pp. 109–127. [Google Scholar]
- 2.Gathercole SE, Pickering SJ, Knight C, Stegmann Z. Working memory skills and educational attainment: evidence from national curriculum assessments at 7 and 14 years of age. Appl Cognitive Psych. 2004;18:1–16. [Google Scholar]
- 3.Salorio CF, Slomine BS, Grados MA, Vasa RA, Christensen JR, Gerring JP. Neuroanatomic correlates of CVLT–C performance following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2005;11:686–696. doi: 10.1017/S1355617705050885. [DOI] [PubMed] [Google Scholar]
- 4.Maril A, Davis PE, Koo JJ, Reggev N, Zuckerman M, Ehrenfeld L, et al. Developmental fMRI study of episodic verbal memory encoding in children. Neurology. 2010;75:2110–2116. doi: 10.1212/WNL.0b013e318201526e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grady CL, McIntosh AR, Craik FIM. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- 6.Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Cognitive Brain Res. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Mabbott DJ, Rovet J, Noseworthy MD, Smith ML, Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 9.Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuro Image. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Krafft CE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, Pierce JE, et al. An 8-month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity. 2014;22:232–242. doi: 10.1002/oby.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delis D, Kramer J, Kaplan E, Ober B. California verbal learning test children’s version. California: Psychological Assessment Resources, Inc; 1994. [Google Scholar]
- 14.Rorden C. DCM2NII (Version October 7) [Computer software] 2007. [Google Scholar]
- 15.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuro Image. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuro Image. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJW, et al. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuro Image. 2008;42:1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholz J, Tomassini V, Johansen-Berg H. Individual differences in white matter microstructure in the healthy brain. In: Johansen-Berg H, Behrens T, editors. Diffusion MRI: From quantitative measurement to in vivo neuroanatomy. Academic Press; 2009. pp. 237–349. [Google Scholar]
- 20.Hodgkin AL, Huxley AF. Propagation of electrical signals along giant nerve fibres. P Roy Soc Lond B Bio. 1952;140:177–183. doi: 10.1098/rspb.1952.0054. [DOI] [PubMed] [Google Scholar]
- 21.Herting MM, Nagel BJ. Differences in brain activity during a verbal associative memory encoding task in high-and low-fit adolescents. J Cognitive Neurosci. 2013;24:595–612. doi: 10.1162/jocn_a_00344. [DOI] [PMC free article] [PubMed] [Google Scholar]