Abstract
Foxp3+ CD4+ regulatory T (Treg) cells, recognized to be one of the most important defences of the human body against an inappropriate immune response, have recently gained attention from those outside immunology thanks to the compelling evidence for their capability to exert non-canonical immune functions in a variety of tissues in health and disease. The recent discovery of the differences between tissue-resident Treg cells and those derived from lymphoid organs is affecting the mindset of many investigators now questioning the broad applicability of observations originally based on peripheral blood/lymphoid organ cells. So far, the best characterized ‘Treg flavour’ comes from studies focused on their role in suppressing adipose tissue inflammation and obesity-driven insulin resistance. Adipose tissue derived Treg cells are distinct from their counterparts in lymphoid organs based on their transcriptional profile, T-cell receptor repertoire, and cytokine and chemokine receptor expression pattern. These cells are abundant in visceral adipose tissue of lean mice but their number is greatly reduced in insulin-resistant animal models of obesity. Interestingly, peroxisome-proliferator-activated receptor γ expression by visceral adipose tissue Treg cells is crucial for their accumulation, phenotype and function in the fat and surprisingly necessary for complete restoration of insulin sensitivity in obese mice by the anti-diabetic drug Pioglitazone. This review surveys recent findings relating to the unique phenotype and function of adipose tissue-resident Treg cells, speculates on the nature of their dynamics in lean and obese mouse models, and analyses their potential therapeutic application in the treatment of type 2 diabetes.
Keywords: adipose tissue, obesity, peroxisome-proliferator-activated receptor γ, regulatory T cells, type 2 diabetes
Introduction
Over the last two decades, obesity, type 2 diabetes and other elements of the metabolic syndrome have increased dramatically. Accordingly to the latest estimate from the Centers for Disease Control, more than one-third of US adults (35·7%) are obese.1 At present, it is recognized that obese adipose tissue displays an inflammatory phenotype, which is in part responsible for the metabolic dysfunction and insulin resistance that leads to the development of type 2 diabetes.2–4 The overload of nutrients, and the subsequent increase in adipose mass, triggers hypoxia, oxidative stress, and endoplasmic reticulum stress, which ultimately results in adipocyte dysfunction and induction of pro-inflammatory mediators such as tumour necrosis factor-α, interleukin-6 (IL-6) and leptin.5–10 The initial finding demonstrating that adipose tissue functions as an endocrine organ and mediates insulin resistance by producing tumour necrosis factor-α was an inspiration for later studies highlighting the contribution of macrophage accumulation to the amplification of adipose tissue inflammation.6,11–14 However, such an adipo/macro-centric view has been challenged recently following four independent studies that unravelled the role of mast cells15 and three different populations of T cells [CD4, CD8 and regulatory T (Treg) cells]16–18 which reside in adipose tissue at the onset of insulin resistance secondary to obesity. These studies initiated a new field of study defined ‘Immunometabolism’, which has been rapidly enriched by many other reports dissecting the contribution of several other immune cell types (B cells, neutrophils, eosinophils, type 2 innate lymphoid cells, CD4− CD8− γδ T cells and natural killer T cells) to the establishment and perpetuation of adipose tissue inflammation.19,20,20–28 The resulting conceptual framework is intricate and not easy to consolidate in one picture but certainly offers a deeper representation of the complex biology sustaining adipose tissue inflammation.
Among these emerging players, adipose-tissue-resident Treg cells, represent an attractive target to modulate for therapeutic purposes. Treg cells, making up approximately 5–20% of the CD4+ T-cell compartment, have been defined as one of the immune system's main guardians against inappropriate and over-reactive responses. They are involved in the control of autoimmunity, allergic responses, inflammation, and responses to infections and tumors.29
The majority of the published Treg cell studies from the past decade described Treg cells residing in the spleen or lymph nodes. However, the discovery that Treg cells are functionally diverse because of their capability for adapting to the phenotype of the target cell type under regulation, expanded the focus of more recent studies beyond the ‘average’ lymphoid organ-derived Treg cells.30–34 The paradigm of Treg cell heterogeneity has been offered by recent reports investigating their phenotypic diversity in non-lymphoid tissues. It turns out that Treg cells, traditionally thought to exclusively regulate the activities of other T cells, can also exert their regulatory functions on innate immune system cells,35–37 and appear to play a functional role in the pathogenesis of cancer, in the normalization of obesity-induced insulin resistance and, very recently, in the regeneration of injured skeletal muscles.18,38–47
This review focuses on studies characterizing the phenotype and function of Treg cells residing in adipose tissue, and their dynamics in lean and obese animal models; it evaluates the therapeutic potential of targeting Treg cells in the treatment of type 2 diabetes.
Regulatory T cells: phenotypic and functional specialization
A decade has passed since the discovery of the forkhead transcription factor Foxp3 as a master regulator of CD4+ CD25+ Treg cell differentiation.48 While Foxp3 is a necessary orchestrator of the Treg cell phenotype, alone, it is not sufficient to recapitulate either their canonical gene signature49–51 or their functional specializations.30–32
The existence of a specialized population of Treg cells was documented for the first time in 2009 by parallel studies describing subtypes of Treg cells capable of further differentiation in response to distinct inflammatory signals.30–32 It has been proposed that Treg cells control polarized environments by up-regulating specific transcription factors previously associated with effector T-cell lineages. That is to say, depending on the character of the inflammation they respond to, the Treg cells have been found to adopt specific phenotypes related to that particular inflammatory environment. For example, Treg cells, are capable of expressing interferon regulatory factor 4, a transcription factor that is crucial for T helper type 2 (Th2) differentiation and function in sites of inflammation promoted by Th2 cells; meanwhile, they express T-box 21 and chemokine C-X-C motif receptor 3 (CXCR3) to control type 1 inflammation, or up-regulate signal transducer and activator of transcription 3 in settings where Th17 cells are the target of regulation.30–32 These findings initiated the idea that the regulators arm themselves with features similar to their targets to favour their homing and survival in specific locations.
The concept of specialized Treg cells was further established by later reports describing distinct Treg populations in non-lymphoid sites such as adipose tissue, atherosclerotic plaques, intestinal mucosa, placenta, skin, lung, liver, tumours, infected tissues and injured muscles.18,38–47,51–57 Moreover, Treg numbers and frequency in tissues have been reported to change considerably between healthy and diseased tissues. Under normal conditions, the tissue-Treg percentage is usually < 15% of the CD4 fraction.18 However, in certain tumours, half of infiltrating CD4+ T cells may be Treg cells.45 A similar scenario has played out in other disease states such as muscle injury, skin inflammation or infection.47,53,58–60 On the other hand, the dramatic decrease of Treg cells in an obese animal model as well as in atherosclerotic plaques serves as a reminder that there is not one common Treg cell dynamic in health versus disease. For the majority of these tissues, microarray or RNAseq-based gene-expression profiling of resident Treg cells is still lacking and the diversity of tissue versus lymphoid-derived Treg cells has been mainly described only on the basis of increased expression of markers such as cytotoxic T-lymphocyte antigen 4, CD103, glucocorticoid-induced tumour necrosis factor receptor family-related gene, IL-10, transforming growth factor-β and a combination of chemokine receptors.61 Because of the different methods used to purify Treg cells from tissues (single versus double cell sorting, different enzymatic or mechanical tissue digestion protocols) and to perform phenotypic analysis (gene expression profiling, flow cytometry, immunohistochemistry), it is difficult to understand to which extent they share similar features beside a common activated/memory phenotype.62–64
‘Fat’ regulatory T cells
The deepest knowledge of the dynamics, phenotype and function of tissue-resident Treg cells has been offered by several reports discussing the unique properties of Treg cells in adipose tissue. This is based on the analysis of specific gene expression signatures, transcription factors, distinct chemokine receptor patterns, T-cell receptor (TCR) repertoire, and ‘unconventional’ mechanism of action and cellular targets.
Regulatory T cells resident in visceral adipose tissue, also known as ‘Fat Tregs’, have been suggested to be involved in controlling metabolic parameters in disorders such as atherosclerosis, obesity and type 2 diabetes.18,40–42,46,65 They have been found to accumulate in visceral fat, but not in spleen, of male mice between 5 and 25 weeks of age (ref. 18 and D. Cipolletta, C. Benoist and D. Mathis, unpublished results). In normal adult male mice, visceral adipose tissue (VAT) -resident Treg cells account for a much larger fraction (50–70%) of CD4+ T cells, compared with their counterparts in the spleen, lymph nodes, other fat depots (subcutaneous and peri-renal) and non-lymphoid tissues, such as lung, liver, skin and muscle.18,40 However, it has been observed that VAT, but not spleen, Treg representation decreases in mice older than 40 weeks, which are affected by a decline in insulin sensitivity (D. Cipolletta, C. Benoist and D. Mathis, unpublished results). Interestingly, visceral adipose inflammation and insulin resistance have been associated with a dramatic reduction of VAT Treg cells in several animal models of obesity such as leptin-deficient mice (Lepob/ob), mice heterozygous for the yellow spontaneous mutation and male mice chronically fed a high-fat diet (HFD).18,40,66–68 In contrast, in female mice, which are protected from HFD-induced metabolic disorders, the expanded VAT displays a non-inflammatory nature that positively correlates with an increase in VAT Treg cells in response to the HFD feeding.69 Although the cause of gender differences in the susceptibility of type 2 diabetes are not clear, it has been speculated that oestrogens play a protective role in the development of the metabolic syndrome as suggested by increased cases of diabetes seen in postmenopausal women.70
A more direct indication of a negative correlation between VAT Treg abundance and insulin resistance comes from Treg gain- and loss-of-function studies. In vivo induction of Treg cells by using IL-2/anti-IL-2 complexes has been found to significantly improve insulin sensitivity in obese mice.18,71 Similarly, adoptive transfer of CD4+ T cells expressing GATA binding protein 3 (GATA3) has been demonstrated to normalize insulin resistance, which might be an effect entirely due to the Treg cell fraction because they are the only CD4 subset expressing GATA3 in VAT (refs 16,40 and D. Cipolletta, C. Benoist and D. Mathis, unpublished results). Conversely, Treg depletion by diphtheria toxin in a mouse model where Foxp3 promoter/enhancer elements diphtheria toxin receptor72 leads to spontaneous impairment of insulin signalling in adipose tissue, muscle and liver.18
Interestingly, microarray-based gene expression profiling revealed that VAT Treg cells are the epitome of specialized Treg cells. While maintaining approximately 60% of the canonical Treg signature, VAT Treg cells differentially express many genes in comparison with their counterpart Treg cells in lymphoid organs. The differentially expressed genes are mainly associated with lymphocyte migration, extravasation and lipid metabolism.18,40 Of note, the VAT Treg gene signature is less represented in the few VAT Treg cells extracted from old (> 40 weeks) mice fed normal chow and obese individuals (refs 18,40 and D. Cipolletta, C. Benoist and D. Mathis, unpublished results). Although these data are only correlative and not capable of clearly demonstrating whether the loss of the lean signature is responsible for the dynamics of VAT Treg cells in aging or obesity, it represents another case of Treg cell plasticity in response to diverse environmental cues, in health and disease.
To date, the origin of VAT Treg cells, as well as the nature of their population fluctuations in lean (increased) and in obese (decreased) states has not been completely addressed. Several distinct mechanisms might explain their dynamics in the VAT: response to adipokines, VAT-restricted antigen(s), conversion from CD4+ conventional T cells, recruitment and/or retention via chemokine/chemokine receptors, response to an unfavourable environment (death, inhibited influx, or premature efflux of T cells from adipose tissue), or expression of specific transcription factors.
VAT Treg cells: thymic or peripherally induced?
Regulatory T cells can have a dual origin. Natural Treg cells migrate from the thymus to the periphery after positive selection by high-avidity interactions with self antigens.73 Alternatively, upon antigen stimulation and in the presence of transforming growth factor-β,74,75 IL-276 or retinoic acid,77 conventional CD4+ T cells can acquire Foxp3 expression in the periphery, becoming peripheral Treg cells, which (in mouse, but not in human78) retain suppressive functions. Alternatively, migration of Treg cell precursors in tissues could occur during fetal life, in a similar way to what has been described for macrophages, although this remains controversial.79
It has also been proposed that the Treg TCR repertoire is shaped toward the recognition of self antigens,29 a feature that in theory would promote their localization in non-lymphoid tissues to keep autoimmune and inflammatory responses in check. On the other hand, the specificity of antigen recognition by the TCR might result not only in lineage commitment but potentially in the activation and retention of Treg cells at peripheral tissue sites. The analysis of the TCR repertoire has been used by Feuerer et al.18 to understand whether VAT T cells are similar to lymphoid organ T cells or if they are in situ expanded cells, or conventional T cells cytokine-converted into Treg cells. This analysis revealed that there is very little overlap between the TCR repertoire of VAT Treg cells and the one displayed by lymphoid-organ Treg cells, suggesting that the former might not derive from their circulating counterparts. Furthermore, the VAT-derived Treg cell and conventional T cell TCR repertoires are markedly distinct, making it very unlikely that the accumulation of VAT Treg cells results from a local conversion of conventional T cells.18 Rather, the presence of repeated VAT Treg TCR clones suggests the existence of specific antigen(s) that might be responsible for their accumulation in adipose tissue.18 To date, the VAT-restricted antigens for VAT Treg cell recognition, accumulation and retention remain undiscovered. Although challenging, it might be necessary to first confirm the published TCR sequencing performed on the ‘Limited’ mouse line,80 wherein the restricted TCR diversity is confined to the complementarity-determining region (CDR) 3α, on a wild-type mouse to exclude the loss of any fat-specific TCR recombination due to a restricted repertoire. It will also be useful to extend this analysis on VAT Treg cells derived from mice at different ages and on different diets (normal chow versus HFD) to verify any correlation between the TCR bias and VAT Treg cell dynamics and the disease. Lastly, because VAT Treg cells are enriched in VAT but not in subcutaneous and peri-renal fat, the analysis of their TCR repertoires might help in understanding the origin of their divergent distribution between fat depots.
Chemokine receptor-mediated Treg cell recruitment in adipose tissue
It is feasible that the recruitment and retention of Treg cells in adipose tissue is mediated by a combination of VAT-specific antigen(s) recognition and expression of specific chemokine receptors.
Several studies have demonstrated that different profiles of chemokine receptors can determine selective Treg cell accumulation in tissue.81 For example, in non-inflamed skin, lung and liver there is an enrichment of Treg cells that are chemokine C-C motif receptor (CCR) 4+ CD103+ whereas inflamed human liver is populated by CXCR3+ CCR10+ Treg cells.38,81,82 However, the expression of specific patterns of chemokine receptors might be influenced by the type of T-cell response (Th1, Th2 and Th17) within the tissue in question.81 VAT Treg cells offer a great example of this concept.
Regulatory T cells residing in adipose tissue display a distinct chemokine receptor pattern that might be responsible for their specific accumulation in lean VAT: CCR1, CCR2, CCR3, CCR5, CCR9 and CXCR6 are over-expressed while CCR6, CCR7 and CXCR3, are under-represented in VAT Treg cells.18 Interestingly, in obese adipose tissue, Treg cells show a decrease in CCR1, CCR2 and CXCR6 expression and conversely an up-regulation of CXCR3, as expected during an ongoing Th1 immune response (D. Cipolletta, C. Benoist and D. Mathis, unpublished results). However, the acquisition of different chemokine receptors offers an important alternative interpretation of the VAT Treg dynamics in obesity: does the lack of the ‘lean’ chemokine receptor pattern compromise Treg accumulation and retention in adipose tissue? Additional studies focused on the identification of chemokine receptors retained by Treg cells resident in adipose tissue other than visceral adipose tissue would be useful to understand their net contribution to Treg cell dynamics in the fat.
Adipokine-mediated Treg cell modulation in adipose tissue
An interesting model explaining the dramatic reduction in VAT Treg cells in obese states has been proposed by Matarese et al.,83 who described the inhibitory effect of leptin on Treg cell proliferation.84 The elevated levels of leptin, which increase with obesity, could impair Treg cell proliferation and perhaps explain their decreased numbers. However, this speculation is difficult to reconcile with the striking loss of VAT Treg cells in the leptin-deficient mouse model.18 In fact, in Lepob/ob mice, the VAT Treg cell percentages and numbers, after an initial expansion, significantly decrease at 14 weeks. The observed VAT Treg cell kinetics and the strong representation of the VAT Treg-cell-specific gene signatures in cells extracted from 3- to 5-week-old Lepob/ob mice (before any development of severe insulin resistance) serve as further confirmation that leptin deficiency does not impact the VAT Treg cell phenotype or their accumulation in the fat (D. Cipolletta, C. Benoist and D. Mathis, unpublished results).
In contrast to leptin, adiponectin, an anti-inflammatory adipokine, retains insulin-sensitizing properties and negatively correlates with body mass index while positively correlating with Treg cell representation in VAT.85 Although adiponectin's direct effect on VAT Treg cells has not been demonstrated, it has been reported to induce the synthesis of the anti-inflammatory cytokine IL-10 in macrophages in an in vitro setting.86 Interestingly, Treg cells in VAT express a much higher level of IL-10 (136-fold augmentation of IL-10 transcripts) in comparison with lymph node Treg cells.18 Interleukin-10 represents one of the main cytokines produced by Treg cells to exert their regulatory function on effector cells.87 However, in an adipocyte model that recapitulates induction of insulin resistance by treatment with tumour necrosis factor-α, IL-10 could also act directly on adipocytes by suppressing markers of inflammation and restoring the translocation of the membrane transporter for glucose, GLUT4.18 In addition, it has been demonstrated that adiponectin-treated dendritic cells can promote Treg cell expansion via the programmed death-1/programmed death-1 ligand pathway.88 The effect of adiponectin and other adipokines on immune cells is worth exploring to understand whether their differential expression in diverse fat depots might contribute to the accumulation of Treg cells or other immune cells preferentially in VAT.
Peroxisome-proliferator-activated receptor γ contribution to the generation and maintenance of VAT Treg phenotype
Peroxisome-proliferator-activated receptor γ (PPARγ) is a nuclear receptor superfamily member generally accepted to be the master regulator of adipocyte differentiation and function. It is also known for its anti-inflammatory properties, mediated by its direct interaction with nuclear factor-κB.4,89 PPARγ has been recently described as the major orchestrator of VAT Treg cell accumulation, phenotypes and function.40 The specific and enriched expression of PPARγ in VAT Treg cells was identified by comparing the gene expression profiles of visceral fat and lymphoid organ T-cell subsets.40 Interestingly, PPARγ positively and negatively correlates with the most strongly up- or down-regulated genes, in the comparison of VAT Treg cells with lymphoid organ Treg cells, respectively. This was also directly demonstrated by the induction of a VAT Treg cell profile via ectopic co-expression of FOXP3 and PPARγ in conventional T cells.40 Specifically, VAT Treg cells express both PPARγ isoforms 1 and 2 with preference for the former. The biological relevance of these two isoforms in VAT Treg cells, and in adipocytes, is still not clear, although it has been reported that both are capable, in in vitro experiments, of interacting with FOXP3 to induce most of the VAT Treg over-expressed genes. However, a distinction between the two protein variants must exist because only the PPARγ isoform 1 induces repression of genes that are under-represented in the VAT Treg cells.40 Furthermore, PPARγ expression in VAT Treg cells is crucial for VAT Treg cell accumulation in adipose tissue. Mice lacking PPARγ specifically in Treg cells have very few Treg cells in the visceral adipose tissue while maintaining normal numbers in other lymphoid and non-lymphoid organs. Notably, PPARγ-deficient Treg cells down-regulate the specific VAT Treg gene expression signature.40
Peroxisome-proliferator-activated receptor γ is also important for the maintenance of the unique VAT Treg phenotype, as demonstrated by the effect of the PPARγ inhibitor, GW9662. Treatment of wild-type mice with GW9662 down-regulates GATA3 expression in VAT Treg cells, resembling the Treg phenotype of the PPARγ mutant mouse.40 Another unique feature of VAT Treg cells, depending on PPARγ-induced CD36 expression, is their capacity to uptake lipids,40 a feature that is not present in the PPARγ mutant VAT Treg cells.
Further demonstration of the crucial role of PPARγ in the biology of VAT Treg cells in mice, has been provided by their modulation upon treatment with pioglitazone, an anti-diabetic drug of the class of thiazolidinediones. An impressive enrichment of Treg cells was observed only in adipose tissue from Pioglitazone-treated mice, whether on a normal chow or HFD regimen, and in the latter, it positively correlated with an improvement of insulin resistance.40
When and how Treg cells up-regulate PPARγ is still a matter of discussion, which may require the engineering of a lineage traceable PPARγ mouse model to address this question. However, considering that PPARγ is activated by free fatty acids and their metabolites, it is possible that non-fat-derived Treg cells adopt their ‘Fat phenotype’ by sensing them and, as a result, migrating to the VAT. Alternatively, Treg cells might be first recruited to the fat by specific antigen recognition and/or chemokine attraction, and then express PPARγ in response to local cues. Another open question is why Treg cells need PPARγ to survive in VAT. A plausible explanation comes from the theory that Treg cells acquire the phenotype of the cells that they want to control. According to this notion, Treg cells resident in adipose tissue might express PPARγ to match their phenotype with resident monocytes, macrophages and/or adipocytes. The observation that Pioglitazone treatment of obese Treg-PPARγ mutants is less effective in reducing the infiltration/conversion of pro-inflammatory monocytes and macrophages, and that lean Treg-PPARγ mutants shows increased macrophage accumulation in VAT, clearly support this theory.
The relationship between adiposity and Treg cells is, however, controversial, because only one report claims a significantly decreased adipocyte size following adoptive Treg cell transfer in mouse models of obesity,67 while several other studies never reported any impact of the increase in VAT Treg cell representation on body weight and/or adipocyte numbers and sizes.16,18,40,66
PPARγ post-transcriptional modulation in VAT Treg cells
The specific VAT Treg phenotype (i.e. GATA3+, CCR2+, KLRG1+, CD103−) is under-represented in obese mice (D. Cipolletta, C. Benoist and D. Mathis, unpublished results). Surprisingly, this was not associated with a reduced expression of PPARγ or with impaired function, because treatment with Pioglitazone can rescue the ‘lean’ signature in VAT Treg cells.40 The discovery that anti-diabetic PPARγ ligands inhibit the obesity-induced phosphorylation of PPARγ on serine 273, and this modification leads to dysregulation of many PPARγ target genes,90,91 may offer an intriguing clue to this apparent paradox. PPARγ phosphorylation results from the activation of the cyclin-dependent kinase 5, consequent to obesity-driven pro-inflammatory cytokine induction from both adipocytes and immune cells residing in adipose tissue.92 Therefore, it is likely that obesity-induced post-transcriptional modifications of PPARγ affect the VAT Treg phenotype and, consequently, accumulation. Future studies are needed to confirm or identify PPARγ modifications and underlying mechanisms leading to decreases in VAT Treg numbers.
The therapeutic potential of modulating VAT Treg cells in metabolic disease
The current approaches for the treatment of type 2 diabetes have been focused on sulphonylureas, biguanides and thiazolidinediones. Recently, however, researchers have started exploring the effects of these drugs on immune cells and their contribution to therapeutic outcome. For example, Pioglitazone has been found to boost the accumulation of Treg cells in VAT, but not other tissues, in lean and obese mice.40 Strikingly, lean mice with a specific deficiency of PPARγ in Treg cells did not accumulate VAT Treg cells whereas obese individuals, carrying the same mutation, respond only partially to Pioglitazone treatment.40 This unexpected finding suggests that the restoration of metabolic indices induced by Pioglitazone occurs in part by acting on VAT Treg cells, and provides proof of principle that this subset of immune cells is capable of impacting metabolic parameters.40 Similarly, Metformin, belonging to the class of biguanides, increases the number and fraction of VAT Treg cells in obese mice fed an HFD.93 However, the authors of this work do not address whether metformin acts on Treg cells exclusively resident in VAT or whether other molecular mechanisms mediate the effect93 (Fig. 1).
Figure 1.
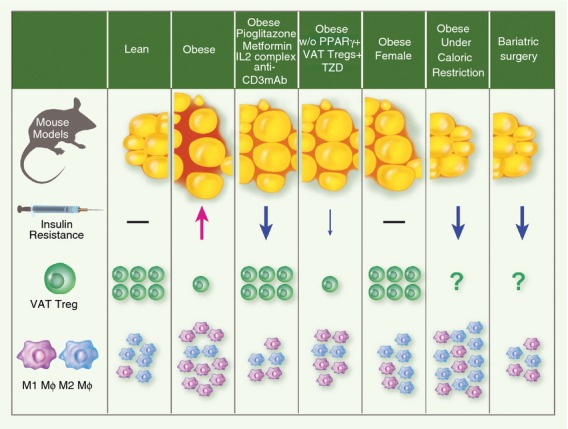
Cellular and metabolic balance in adipose tissue. In lean mice, visceral adipose tissue (VAT) is enriched by anti-inflammatory macrophages (M2 MΦ), and regulatory T (Treg) cells. In contrast, in obese mice, there is a switch in cellular equilibrium: fewer Treg cells and a predominance of pro-inflammatory macrophages (M1 MΦ). The anti-diabetic drug, Pioglitazone, which is a synthetic Peroxisome-proliferator-activated receptor γ (PPARγ) ligand, and anti-CD3-monoclonal antibody treatment boost the accumulation of Treg cells and reduces the infiltration of M1 MΦ in VAT of obese mice. Similarly, obese mouse treatment with either Metformin, or interleukin-2 (IL-2) complexes, increases VAT Treg cells although their effect on the modulation of M1 MΦ remains uncharacterized. Strikingly, obese mice, carrying the specific deficiency of PPARγ in Treg cells do not accumulate VAT Treg cells and consequently respond only partially to Pioglitazone treatment. Obese female mice, which are historically known to be protected from high-fat diet-induced insulin resistance, are enriched by M2 MΦ and Treg cells. Early stages of weight loss and fasting are marked by a rapid recruitment of anti-inflammatory macrophages to VAT in response to lipolysis.100 Macrophage infiltration is instead reduced in VAT of patients which have undergone gastric bypass surgery for weight loss. However, this observation was made 3 months after bariatric surgery and provides only a late snapshot of MΦ phenotypes in VAT.100 The effect of caloric restriction and/or bariatric surgery on Treg dynamics in adipose tissue remains to be addressed and represents an interesting opportunity to verify the inverse correlation between Treg cell numbers and insulin resistance.
Moreover, treatment with an anti-CD3 monoclonal antibody (mAb), which has been shown to globally deplete effector T cells and concomitantly enhance the representation of Treg cells, has been evaluated in the context of immunotherapy of type 2 diabetes by two different groups. By administering the non-mitogenic F(ab’)2 fragment of anti-CD3 for only 5 days, Winer et al. achieved a long-term normalizing effect on insulin resistance and glucose tolerance in HFD-fed mice.16 They documented a correlation between the improved metabolic indices and the increase in numbers of adipose tissue Treg cells and anti-inflammatory macrophages16 (Fig. 1). In contrast, a study conducted by Ilan et al.66, explored the effect of oral administration of an anti-CD3 mAb in combination with β-glucosylceramide, an intermediate of glycosphingolipid metabolism reported to ameliorate the metabolic syndrome in Lepob/ob mice by reducing the numbers of hepatic natural killer T cells. This combinatorial approach was tested on Lepob/ob mice and was found to induce CD4+ transforming growth factor-β1 latency-associated peptide (LAP) T cells, and concomitantly reduce natural killer T cells in the mesenteric lymph nodes, blood and spleen.66 Surprisingly, only in adipose tissue, did Treg cells, and not CD4+ LAP+ T cells, increase during the combination treatment. However, this result and none of the effects on the metabolic and pathological abnormalities could be recapitulated when Lepob/ob mice were treated with either anti-CD3 or β-glucosylceramide alone.66 The contradictory outcomes between the two different anti-CD3 mAb used may be easily reconcilable if the latter study was supported by commonly accepted measures of insulin resistance, such as insulin blood levels, glucose and insulin tolerance test, HOMA-IR (Homeostatic Model Insulin Resistance), and evaluation of insulin receptor signalling.
Another intriguing way to increase the Treg cells is by administration of IL-2 and an IL-2-specific mAb complex.71,94 Indeed, intraperitoneal injection of this complex for 6 days improved glucose tolerance and insulin sensitivity in HFD-fed mice by increasing the fraction of Treg cells in the spleen and abdominal fat18 (Fig. 1).
All of the studies mentioned here have generated a strong motivation and interest to verify whether these findings might be translatable to human biology. However, to date, the phenotype, dynamics and role of VAT Treg cells in human obesity and metabolic disorders is still unknown. Three independent studies reported a body mass index-dependent decreased FOXP3 expression in the omentum of obese humans;16,18,68 whereas a conflicting report from Zeyda et al.95 showed that FOXP3 transcripts were increased in VAT from obese humans. These contradictory results might be explained by the fact that in humans, FOXP3 might be transiently expressed by activated CD4+ effector T cells in addition to Treg cells.96 Alternatively, this discrepancy might be explained by the enrolment of more females than males (16 : 4) in this study, which would agree with the previous observation of increased Treg populations in adipose tissue of obese female, but not male, mice69 (Fig. 1).
Finally, several studies with contrasting findings have focused on the analysis of Treg representation in the peripheral blood of obese and lean donors. Whereas some reports claim a decrease in circulating Treg cells from obese donors,97,98 another study shows that morbidly obese subjects have selective increases in naive, memory, Treg and Th2 cells.99 Although we do not yet know whether the mouse biology of VAT Treg cells will translate to humans, it might be worthwhile to study the phenotype of omental Treg cells coming from lean and obese mice before assuming that circulating Treg cells could recapitulate and represent their biology. From the emerging evidence, it also seems necessary to keep the analysis of immune resident cells from female and male donors separate so as to identify the possible contribution of sex hormones to the immune and metabolic systems.
Concluding remarks and future prospects
The dramatic reduction in Treg cells in the adipose tissue of obese mice and their increased representation in lean rodents upon treatment with anti-diabetic drugs, indicates that these cells might influence metabolic indices (Fig. 1). The recent finding that PPARγ is a crucial molecular orchestrator of VAT Treg cell accumulation, phenotype and function and that its expression in these cells is necessary for complete restoration of insulin sensitivity in obese mice by the anti-diabetic drug Pioglitazone, has unravelled a previously unknown cellular mechanism involved in the pathogenesis of insulin resistance, and provided proof-of-principle that discrete populations of Treg cells with unique functions can be precisely targeted to therapeutic ends. Still, many questions remain to be addressed: What is the origin of VAT Treg cells? What are their antigen(s)? Why are they diminished from VAT during obesity? What are the differences between VAT Treg cells in female mice (protected from HFD-induced type 2 diabetes) and male mice (susceptible to type 2 diabetes) and what determines their increase in the former and/or decrease in the latter? Lastly, whereas in mice it has been demonstrated that Treg cells come in ‘different flavours’ based on their anatomic location and are able to exert ‘non-immunological functions’ (such as the control metabolic parameters), in humans, the study of immune responses has been confined to analyses of peripheral blood cells that might not have the same properties of the cellular pool in the tissues. Hence, can we find specialized, unique Treg cells in human adipose tissue and do they play a role in type 2 diabetes as well? What precise functions do they perform in the adipose tissue? Can they be specifically targeted? Does anti-diabetic drug treatment increase Treg cell representation in human fat? What is the effect of drug-free treatment of type 2 diabetes such as, caloric restriction and/or bariatric surgery on Treg cell dynamics in adipose tissue (Fig. 1)?100
Studies aimed to address these questions ultimately may result in the identification of novel targets based on the modulation of these immune cells in the context of obesity-driven type 2 diabetes.
Acknowledgments
I would like to thank Drs Diane Mathis and Christophe Benoist who were involved in the generation of the unpublished results herein. I would like to thank Drs Michael Terry Wilson, Pamela Holland, William Fanslow, Rick Kendall, Anthony Polverino and Marc Gavin, for critical review of the manuscript.
Disclosures
The author declares no conflict of interest.
References
- 1.Centers for Disease Control and Prevention. 2013 National Diabetes Fact Sheet. URL http://www.cdc.gov/obesity/data/facts.html [accessed on 1 December 2013] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 5.Bosello O, Zamboni M. Visceral obesity and metabolic syndrome. Obes Rev. 2000;1:47–56. doi: 10.1046/j.1467-789x.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 10.Minamino T, Orimo M, Shimizu I, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–7. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 11.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 14.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 18.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Schipper HS, Rakhshandehroo M, van de Graaf SF, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–54. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–59. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L, Parekh VV, Gabriel CL, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA. 2012;109:E1143–52. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch L, Nowak M, Varghese B, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–87. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:22617–22. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talukdar S, Oh da Y, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Chaudhry A, Kas A, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature. 2009;458:351–6. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–91. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–8. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet+ Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37:501–10. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–9. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy TJ, Choileain NN, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–63. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–20. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 38.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;10:1007–13. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–47. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23:431–7. doi: 10.1016/j.smim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–53. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 43.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 44.Xie JJ, Wang J, Tang TT, et al. The Th17/Treg functional imbalance during atherogenesis in ApoE–/– mice. Cytokine. 2010;49:185–93. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanchot C, Terme M, Pere H, et al. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron. 2013;6:147–57. doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 49.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919–24. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu W, Ergun A, Lu T, et al. A multiply redundant genetic switch ‘locks in’ the transcriptional signature of regulatory T cells. Nat Immunol. 2012;13:972–80. doi: 10.1038/ni.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–21. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–88. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radziewicz H, Dunham RM, Grakoui A. PD-1 tempers Tregs in chronic HCV infection. J Clin Invest. 2009;199:450–3. doi: 10.1172/JCI38661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claassen MA, de Knegt RJ, Janssen HL, Boonstra A. Retention of CD4+ CD25+ FoxP3+ regulatory T cells in the liver after therapy-induced hepatitis C virus eradication in humans. J Virol. 2011;85:5323–30. doi: 10.1128/JVI.02551-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez AM, Zhu J, Huang X, Yang Y. The development and function of memory regulatory T cells after acute viral infections. J Immunol. 2012;189:2805–14. doi: 10.4049/jimmunol.1200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brincks EL, Roberts AD, Cookenham T, et al. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J Immunol. 2013;190:3438–46. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–84. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehtimäki S, Lahesmaa R. Regulatory T cells control immune responses through their non-redundant tissue specific features. Front Immunol. 2013;4:294. doi: 10.3389/fimmu.2013.00294. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomura M, Honda T, Tanizaki H, et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest. 2010;120:883–93. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3+ effector regulatory T cells. Trends Immunol. 2013;34:74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Peters JH, Koenen HJ, Fasse E, Tijssen HJ, Ijzermans JN, Groenen PJ, Schaap NP, Kwekkeboom J, Joosten I. Human secondary lymphoid organs typically contain polyclonally-activated proliferating regulatory T cells. Blood. 2013;122:2213–23. doi: 10.1182/blood-2013-03-489443. [DOI] [PubMed] [Google Scholar]
- 65.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur Heart J. 2006;27:2530–7. doi: 10.1093/eurheartj/ehl222. [DOI] [PubMed] [Google Scholar]
- 66.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA. 2010;107:9765–70. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eller K, Kirsch A, Wolf AM, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–62. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deiuliis J, Shah Z, Shah N, et al. Visceral adipose inflammation in obesity is associated with critical alterations in Tregulatory cell numbers. PLoS One. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7:e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rettberg JR, Yao J, Brinton RD. A master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody–cytokine immune complexes. Science. 2006;311:1924–7. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 72.Thorburn J, Frankel AE, Thorburn A. Apoptosis by leukemia cell-targeted diphtheria toxin occurs via receptor-independent activation of Fas-associated death domain protein. Clin Cancer Res. 2003;9:861–5. [PubMed] [Google Scholar]
- 73.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–85. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–26. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–86. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive humanCD4+ FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-β dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yona S, Kim KW, Wolf Y. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Correia-Neves M, Waltzinger C, Mathis D, Benoist C. The shaping of the T cell repertoire. Immunity. 2001;14:21–32. doi: 10.1016/s1074-7613(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 81.Ding Y, Xu J, Bromberg JS. Regulatory T cell migration during an immune response. Trends Immunol. 2012;33:174–80. doi: 10.1016/j.it.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS, Adams DH. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J Immunol. 2006;177:593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]
- 83.Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol Med. 2010;16:247–56. doi: 10.1016/j.molmed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 84.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 85.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 87.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsang JY, Li D, Ho D, Peng J, Xu A, Lamb J, Chen Y, Tam PK. Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int Immunopharmacol. 2011;11:604–9. doi: 10.1016/j.intimp.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 89.Szanto A, Nagy L. The many faces of PPARγ: anti-inflammatory by any means? Immunobiology. 2008;213:789–803. doi: 10.1016/j.imbio.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 90.Choi JH, Banks AS, Estall JL, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature. 2010;466:451–6. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi JH, Banks AS, Kamenecka TM, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–81. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–59. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 93.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2013 doi: 10.1136/gutjnl-2012-303839. gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 94.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–97. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeyda M, Huber J, Prager G, Stulnig TM. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring) 2010;19:743–8. doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- 96.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 97.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, Nikolajczyk BS. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–72. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luczyński W, Wawrusiewicz-Kurylonek N, Iłendo E, Bossowski A, Głowińska-Olszewska B, Krętowski A, Stasiak-Barmuta A. Generation of functional T-regulatory cells in children with metabolic syndrome. Arch Immunol Ther Exp (Warsz) 2012;60:487–95. doi: 10.1007/s00005-012-0198-6. [DOI] [PubMed] [Google Scholar]
- 99.van der Weerd K, Dik WA, Schrijver B, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2010;61:401–8. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Red Eagle A, Chawla A. In obesity and weight loss, all roads lead to the mighty macrophage. J Clin Invest. 2010;120:3437–40. doi: 10.1172/JCI44721. [DOI] [PMC free article] [PubMed] [Google Scholar]


