Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease in which abnormal immune responses are mediated by tissue-binding autoantibodies and immune complex deposition. Because most SLE patients are women of child-bearing age, oestrogen has been suggested to play an important role in SLE pathogenesis. One proposed role is to induce B-cell activation, culminating in increased autoantibody production. Interleukin-21 (IL-21) has been shown to be crucial in the differentiation of activated B cells into plasma cells. We therefore hypothesized that oestrogen up-regulates IL-21 production and induces subsequent B-cell activation in SLE patients. Peripheral blood was obtained from 22 SLE patients and 16 healthy controls. Expression levels of IL-21 and its receptor in serum, peripheral blood mononuclear cells, and CD4+ T cells were higher in SLE patients than in healthy controls. Exposure of CD4+ T cells from SLE patients to 17β-oestradiol led to a dose- and time-dependent increase in IL-21 expression, which was abolished in the presence of mitogen-activated protein kinase (MAPK) (MAPK kinase, p38, Jun N-terminal kinase) inhibitors. B cells from healthy controls showed increased antibody production when they were co-cultured with oestrogen-treated CD4+ T cells from SLE patients. Treatment with IL-21 antibody abrogated the increased antibody production of the co-culture systems. This study revealed the association between oestrogen and IL-21 in SLE patients. Oestrogen up-regulates IL-21 expression of CD4+ T cells via MAPK-dependent pathways in SLE patients, which in turn induces increased antibody production by B cells.
Keywords: autoantibody, oestrogen, interleukin-21, mitogen-activated protein kinase, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects multiple organs. The disease is characterized by autoantibody production and immune complex deposition that results in tissue damage. Although the exact pathogenic mechanism has yet to be elucidated, certain environmental factors have been suggested to trigger abnormal immune responses in a genetically predisposed person. Among the genetic risk factors, the most important one is female sex. The female : male ratio of SLE patients has been reported to range from 7 to 20.1 Oestrogen has been proposed to be a prime candidate to explain this sex bias. It is not only because oestrogen is female specific, but also because various immune cells express oestrogen receptors, suggesting that oestrogen can exert an immune-modulating effect. Indeed, disease flare up has been reported in association with pregnancy,2 menstruation,3 and hormone replacement therapy after menopause.4 Therefore, the role of oestrogen in the pathogenesis of SLE has been investigated in several studies. We previously reported that oestrogen treatment induced a decrease in the DNA methyltransferase level and consequent hypomethylation in T and B cells of SLE patients.5 Oestrogen has also been reported to decrease T-cell and B-cell apoptosis, allowing autoreactive cells to persist for a long time.6,7 Furthermore, it enhances double-stranded DNA antibody production and IgG secretion in peripheral blood mononuclear cells (PBMCs) of SLE patients.8
Interleukin-21 (IL-21) is a common γ-chain family cytokine that exerts various effects on immune cells.9 The major role of this cytokine is to promote B-cell proliferation, plasma cell differentiation, and antibody production.10 More recently, it was proposed that IL-21 plays a significant role in the development of follicular helper T cells cells, which induce germinal centre formation,11 and in natural killer cell and T-cell proliferation12 and T helper 17 (Th17) differentiation.13 Considering that autoantibody production is a major pathogenic mechanism of SLE, it is conceivable that IL-21 is implicated in SLE. The role of IL-21 in SLE has been investigated mostly in murine models of SLE. In BSXSB.B6-Yaa+/J mice, mRNA expression of IL-21 was significantly increased compared with wild-type mice.14 Additionally, administration of IL-21R/Fc, a fusion protein that neutralizes IL-21, in BSXSB.B6-Yaa+/J mice resulted in diminished lymphocyte activation, decreased circulating IgG1 and proteinuria.15 In SLE in humans, the role of IL-21 is less clearly understood. Serum levels of IL-21 were reported to be elevated in SLE patients compared with healthy controls; however, IL-21 levels failed to reflect disease activity.16
Based on these concepts, we hypothesized that oestrogen might contribute to the pathogenesis of SLE by inducing increased expression of IL-21 in CD4+ T lymphocytes. Furthermore, we aimed to demonstrate that IL-21 induced by oestrogen stimuli can increase production of immunoglobulin by B cells.
Materials and methods
Study population
Twenty-two women with SLE (age range, 19–55 years) attending the rheumatology out-patient clinic of Seoul St Mary's Hospital of Korea and 16 healthy controls (all women; age range, 21–62 years) were recruited. Diagnosis of SLE was established according to the 1982 revised American Rheumatism Association criteria, and disease activity was evaluated by the SLE disease activity index (SLEDAI) score. The present study was approved by the Institutional Review Board of the Catholic University of Korea, and informed consent was obtained from all participants according to the Declaration of Helsinki.
Cell isolation
Twenty millilitres of heparinized blood was drawn from each individual. The PBMCs were prepared from heparinized blood by Ficoll–Hypaque (GE Healthcare Bio-Sciences, Uppsala, Sweden) density-gradient centrifugation. Anti-CD4 and anti-CD19 microbeads (Miltenyi Biotec, Auburn, CA) were used as recommended by the manufacturer. PBMCs were resuspended in 80 μl of fetal bovine serum staining buffer. Anti-CD4 microbeads (20 μl) were added and incubated for 15 min at 4°. The cells were then washed with PBS, and magnetically separated on an AutoMACS magnet (Miltenyi Biotec) fitted with a MACS MS column. Non-T cells were then incubated with anti-CD19 (20 μl) microbeads for 15 min at 4°. CD19+ B cells were isolated from non-T cells using AutoMACS. Cell purity was assessed by flow cytometric analysis of stained cells on a FACS Vantage sorter (BD Biosciences, Franklin Lakes, NJ). Most (> 95%) of the isolated cells had the CD4 T-cell or CD19+ B cells marker.
Cell stimulation and signal inhibition
Isolated cells at a density of 5 × 105 cells/500 μl were incubated with 10–1000 nm of 17β-oestradiol for 48 hr at 37°. For signalling inhibition, LY294002 [phosphatidyl inositol 3-kinase (PI3K) inhibitor; Calbiochem, Schwalbach, Germany], PD98059 [mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitor; Calbiochem), AG490 [signal transducer and activator of transcription 3 (STAT3) inhibitor; Calbiochem], SB203580 (p38 inhibitor; Calbiochem), SP600125 [Jun N-terminal kinase (JNK) inhibitor; Calbiochem], curcumin [activator protein 1 (AP-1) inhibitor; Sigma Aldrich, St Louis, MO] or BAY11 [nuclear factor-κB (NF-κB) inhibitor; BIOMOL Research Laboratories Inc, Plymouth Meeting, PA] were administered to cells 2 hr before oestrogen treatment. After culture, cells and supernatants were collected.
Measurement of IL-21 mRNA expression by real-time RT-PCR
Relative expression of specific mRNAs was quantified by real-time RT-PCR using SYBR Green I (Roche Diagnostics, Indianapolis, IN). The following sense and antisense primers were used: for IL-21, 5'- CTT ACC TGG CAA GAC CAG TAT GA-3' and 5'- GTA GAA GGC AGG GTC TTC GTA AT-3'; for β-actin, 5'- GGA CTT CGA GCA AGA GAT GG-3' and 5'- TGT GTT GGG GTA CAG GTC TTT G-3'.
ELISA of IL-21
Concentrations of IL-21 in the sera and the culture supernatants were measured by sandwich ELISA. Mouse IL-21 monoclonal antibody (R&D Systems, Minneapolis, MN) was added to a 96-well plate (Nunc, Roskilde, Denmark) and incubated overnight at 4°. The wells were treated with blocking solution (PBS containing 1% BSA and 0·05% Tween-20), samples and the standard recombinant IL-21 (R&D Systems) were added to the 96-well plate, and the plate was incubated. Biotinylated IL-21 polyclonal antibody (R&D Systems) was added, and the reactions were allowed to proceed. The plates were washed, 2000-fold diluted ExtrAvidin-alkaline phosphatase (Sigma Aldrich) was added, and reactions were allowed to proceed. The plates were washed, and p-nitrophenyl phosphate disodium salt (50 μl; Pierce Chemical Company, Rockford, IL) diluted in diethanolamine buffer was applied.
Western blot
Peripheral blood mononuclear cells from healthy controls and SLE patients were stimulated with 1000 nm of 17β-oestradiol for 20 min. After incubation for 20 min, cell lysates were prepared from about 3 × 106 cells by homogenization in the lysis buffer and centrifuged at 14 000 g for 15 min. The protein concentration in the supernatant was determined using the Bradford method (BioRad, Hercules, CA). Protein samples (50 μg) were separated using 10% SDS–PAGE and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden). For Western blot hybridization, the membrane was pre-incubated with 0·5% skim milk in 0·1% Tween-20 in Tris-buffered saline (TTBS) at room temperature for 2 hr. The membranes were stained with primary antibodies to p-p38 (1 : 250), p-extracellular signal-regulated kinase (ERK) (1 : 250), p-JNK (1 : 250), p38 (1 : 250), ERK (1 : 250), JNK (1 : 250), (all from Cell Signaling Technology Inc., Danvers, MA) and β-actin (1 : 1000) (Sigma Aldrich) for 1 hr at room temperature. The horseradish peroxidase-conjugated secondary antibody was added for 1 hr at room temperature. The membrane was washed in TTBS, and the hybridized bands were detected using an enhanced chemiluminescent (ECL) detection kit and Hyperfilm-ECL reagents (Amersham Pharmacia). After phospho-protein Western blotting, the membrane was stripped and re-probed for total protein.
Measurement of immunoglobulin concentrations
Oestrogen-treated T cells (1 × 105) were co-cultured with B cells (1 × 105) and the cells were then treated with the IL-21 monoclonal antibody (2 μg/ml) (R&D Systems) for 3 days. Isolated T cells were cultured with oestrogen (100, 1000 nm) and IL-21 antibody (2 μg/ml) for 3 days, and then the supernatant was collected. Isolated B cells were cultured with the T-cell-cultured supernatant. To analyse immunoglobulin expression levels by B cells, IgG, IgG1 and IgG2 were measured in culture supernatants by ELISA. We used human IgG, IgG1 and IgG2a ELISA quantification kits (Bethyl Laboratories, Montgomery, TX).
Statistical analysis
The data are presented as means ± standard deviation (SD). Statistical analyses were performed using graphpad prism (Version 4 for Windows; GraphPad Software, San Diego, CA). When comparing the two groups, Student's t-test or Mann–Whitney U-test was used. For multiple comparisons, analysis of variance was used with Bonferroni's post hoc analysis. A value of P < 0·05 was deemed to be statistically significant.
Results
Interleukin-21 expression was increased in the serum and CD4+ T cells of patients with SLE
The clinical characteristics of the SLE patients were summarized in Table 1. Serum levels of IL-21 as determined by ELISA were significantly higher in SLE patients than in healthy controls (354·6 ± 34·58 versus 172·5 ± 18·36 pg/ml, respectively; P < 0·001). However, IL-21 serum levels did not correlate with disease activity as determined by the SLEDAI score. The mRNA expression of IL-21 and IL-21 receptor (IL-21R) in PBMCs and CD4+ T cells was assessed using real-time RT-PCR. The mRNA expression of IL-21 and IL-21R was significantly higher in PBMCs and CD4+ T cells from SLE patients than in those from healthy controls (Fig. 1).
Table 1.
Characteristics of the patients enrolled (n = 22)
| Age (year) | 35·4 ± 11·4 (19–55) |
| Disease duration (month) | 98·8 ± 79·6 (9–276) |
| Menopause | 2 (9·1%) |
| Hormone replacement therapy | 0 |
| Oral contraceptives | 0 |
| SLEDAI | 4·6 ± 6·0 (0–22) |
| ≥4 (active) | 10 (45·5%) |
| <4 | 12 (54·5%) |
| C3 (mg/dl) | 87·6 ± 33·6 |
| C4 (mg/dl) | 14·5 ± 7·2 |
| Prednisolone dose (mg/day) | 9·65 ± 9·62 (0-35) |
| Other immunosuppressants | |
| Hydroxychloroquine | 12 (54·5%) |
| Mycophenolate | 5 (27·8%) |
| Mizoribine | 2 (9·1%) |
| Azathioprine | 2 (9·1%) |
SLEDAI, SLE disease activity index.
Figure 1.

Increased interleukin-21 (IL-21) in sera and CD4+ T cells of patients with systemic lupus erythematosus (SLE). (a) The concentrations of IL-21 in sera isolated from 22 SLE patients and 16 healthy controls were analysed by ELISA. (b) Peripheral blood mononuclear cells (PBMCs) and CD4+ T cells were isolated from heparinized peripheral blood obtained from three patients and three healthy controls. The mRNA expression levels of IL-21 and IL-21 receptor were determined by RT-PCR. Results are expressed as means ± standard deviation (SD). (**P < 0·001).
Oestrogen treatment increased expression of IL-21 in CD4+ T cells from SLE patients in a dose- and time-dependent manner
To determine the effects of oestrogen on IL-21 production, CD4+ T cells from SLE patients were stimulated with various concentrations (10, 100 and 1000 nm) of 17β-oestradiol. After 48 hr, IL-21 mRNA expression by CD4+ T cells was measured using real-time RT-PCR. The level of IL-21 in culture supernatants as measured by ELISA increased with oestrogen treatment in a dose-dependent manner. When CD4+ T cells were stimulated with 1000 nm 17β-oestradiol for 24, 48 and 72 hr, respectively, the concentration of IL-21 in the supernatant increased in a time-dependent manner (Fig. 2).
Figure 2.
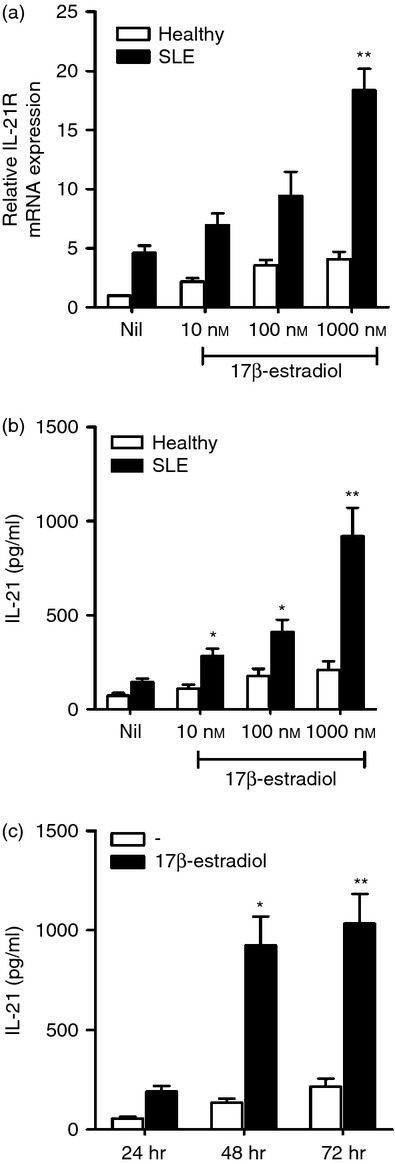
Oestrogen treatment increased interleukin-21 (IL-21) expression in CD4+ T cells of systemic lupus erythematosus (SLE) patients in a dose- and time-dependent manner. (a, b) Isolated CD4+ T cells were treated with 10, 100 and 1000 nm 17β-oestradiol for 48 hr. The mRNA expression of IL-21 was measured by RT-PCR (a). The concentration of IL-21 in the supernatant was determined by ELISA (b). (c) CD4+ T cells were cultured with 1000 nm 17β-oestradiol for 24, 48 and 72 hr. The concentration of IL-21 in the supernatant was determined by ELISA. Data are expressed as means ± SD of three independent experiments using cells obtained from three patients and three healthy controls. (*P < 0·05, **P < 0·01, versus untreated cells of SLE patients).
Synergistic effects of T-cell receptor stimulation on IL-21 production by oestrogen-pretreated CD4+ T cells from SLE patients
We investigated whether T-cell receptor stimulation in addition to oestrogen stimulation had synergistic effects on IL-21 production by CD4+ T cells. Isolated CD4+ T cells from SLE patients were cultured for 48 hr with 10, 100 and 1000 nm 17β-oestradiol in the presence or absence of 0·5 μg/ml CD3 antibody. The level of IL-21 was measured in the supernatant of each culture system by ELISA. The results showed that the CD3 antibody worked synergistically with oestrogen and increased IL-21 production by CD4+ T cells (Fig. 3). The level of IL-21 when stimulated only with 1000 mm 17β-oestradiol was 885 ± 254 pg/ml, whereas it increased to 2548 ± 4 pg/ml when the CD3 antibody was added (P < 0·05). In contrast, stimulation with 1000 nm testosterone instead of 17β-oestradiol failed to increase IL-21 production by CD4+ T cells.
Figure 3.

Synergistic effect of T-cell receptor (TCR) stimulation on interleukin-21 (IL-21) production by oestrogen-pretreated CD4+ T cells in systemic lupus erythematosus (SLE) patients. Isolated CD4+ T cells from SLE patients were cultured with 10, 100 and 1000 nm 17β-oestradiol for 48 hr in the presence of 0·5 μg/ml CD3 antibody. The concentration of IL-21 in the supernatant was determined by ELISA. Data are expressed as means ± SD of three independent experiments using cells obtained from three different patients. (*P < 0·05, versus cells treated with 1000 nm 17β-oestradiol only).
Oestrogen-induced IL-21 production is dependent on the MAPK signalling pathway
To identify the signalling molecules involved in oestrogen-induced IL-21-producing mechanisms, the effects of various inhibitors of signalling molecules were examined. Inhibitors of MEK (20 μm PD98509), p38 (10 μm SB203850), JNK (1 μm SP600125), PI3K (20 μm LY294002), STAT3 (50 μm AG490), NF-κB (50 μm BAY 11) and AP-1 (10 μm curcumin) were used. These inhibitors were added to CD4+ T cells 2 hr before oestrogen treatment and no cytotoxic effects on CD4+ T cells were observed with the experimental concentrations used. The inhibitor-treated CD4+ T cells were then cultured with 1000 nm 17β-oestradiol. After 48 hr, the level of IL-21 in the culture supernatant was measured by ELISA. Interestingly, oestrogen-induced increases in IL-21 production (635·0 ± 148·4 pg/ml) were abrogated with the MEK inhibitor (174·3 ± 53·7 pg/ml, P = 0·0072), p38 inhibitor (168·3 ± 42·5 pg/ml, P = 0·0064), and JNK inhibitor (327·7 ± 68·0 pg/ml, P = 0·031) (Fig. 4a). To confirm that the MAPK signalling pathway was involved in oestrogen-induced IL-21 expression, we investigated if 17β-oestradiol could activate MAPK. Treatment of 1000 nm of 17β-oestradiol increased the phosphorylated form of MAPK in CD4 T cells of SLE patients (Fig. 4b). In contrast, MAPK activation was not observed in the CD4 T cells from healthy controls with 17β-oestradiol treatment (data not shown).
Figure 4.

Signalling pathways involved in oestrogen-induced interleukin 21 (IL-21) production. (a) Isolated CD4+ T cells from systemic lupus erythematosus (SLE) patients were pre-treated with 20 μm PD98509, 10 μm SB203850, 1 μm SP600125, 20 μm LY294002, 50 μm AG490, 50 μm BAY 11, or 10 μm curcumin for 2 hr, and then cultured with 1000 nm of 17β-oestradiol for 48 hr. The concentration of IL-21 in the supernatant was measured by ELISA. Data are expressed as means ± SD of three independent experiments using cells obtained from three different patients. (b) CD4+ T cells from SLE patients were treated with 1000 nm 17β-oestradiol for 20 min. Phosphorylated forms of mitogen-activated protein kinases were measured by Western blot analysis. The representative Western blots of three independent experiments using cells from three different patients are shown. (*P < 0·05, **P < 0·01).
Increased antibody secretion by B cells co-cultured with oestrogen-stimulated CD4+ T cells
Finally, we investigated whether the oestrogen effects on CD4+ T cells could subsequently result in an increase in antibody production by B cells. B cells from healthy controls were co-cultured with oestrogen-stimulated CD4+ T cells and their supernatants, respectively. The levels of IgG, IgG1 and IgG2a were measured from the supernatant of each co-culture system using ELISA. The increased production of immunoglobulin by B cells was observed in both co-culture systems (Fig. 5). This effect was abolished when IL-21 blocking antibody was added.
Figure 5.
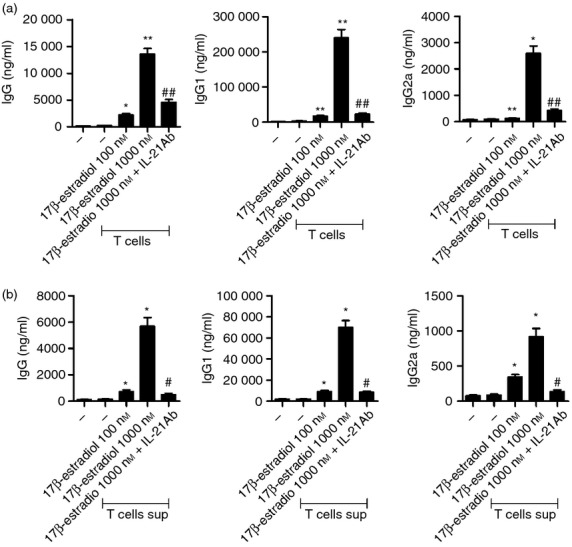
Increased antibody secretion by B cells co-cultured with oestrogen-stimulated CD4+ T cells. (a, b) CD4+ T cells from systemic lupus erythematosus (SLE) patients were treated with 1000 nm 17β-oestradiol for 48 hr. CD4+ T cells and culture supernatants were then separated by centrifugation. B cells isolated from the peripheral blood of healthy controls were cultured for 96 hr with oestrogen-pretreated T cells (a) and the culture supernatant (b), respectively, in the absence or presence of interleukin-21 (IL-21) blocking antibody. The concentrations of secreted IgG, IgG1 and IgG2a by B cells were measured by ELISA. Data are expressed as means ± SD of three independent experiments using cells from three patients and three healthy controls. (*P < 0·05, **P < 0·01, versus untreated cells, #P < 0·05, ##P < 0·01, versus 1000 nm 17β-oestradiol-treated cells).
Discussion
In the present study, we showed that oestrogen up-regulated IL-21 expression in CD4+ T cells from SLE patients, which in turn increased antibody production by B cells. This is the first study to determine the effects of oestrogen on IL-21 in SLE patients.
As IL-21 is known to participate in the process of antibody production by B cells, many researchers have sought to evaluate its contribution to the pathogenesis of SLE. In human SLE, the plasma level of IL-21 was reported to be significantly higher than that observed in healthy controls.16,17 Consistent with these findings, we observed a significant increase in IL-21 expression in PBMCs and CD4+ T cells from SLE patients compared with those from healthy controls.
We also demonstrated that mRNA expression of IL-21R was higher in CD4+ T cells and B cells from SLE patients compared with those from healthy controls. This suggests that immune cells of SLE patients might be more sensitive to IL-21. However, previous studies have reported contradictory results. Mitoma et al.18 showed that the expression of IL-21R in B cells from SLE patients was significantly lower than that in B cells from healthy controls. Another group demonstrated that no significant difference was found in IL-21R expression of lymphocytes between SLE patients and healthy controls.19 This discrepancy seems to be due to the difference in the method for determining the level of IL-21R expression.
When CD4+ T cells of SLE patients were treated with 17β-oestradiol, IL-21 expression was increased in a dose- and time-dependent manner. With regard to mRNA expression, it seems that the fold increase after oestradiol treatment was similar in both SLE patients and healthy controls. However, baseline production of IL-21 in SLE patients was five times as high as that in healthy controls, which suggested that the cells were already in an activated state. Taking this into account, it is plausible to suggest that SLE T cells were more responsive to oestrogen stimulation, although they showed the same threefold increase. Furthermore, the level of IL-21 in culture supernatant, which was measured by ELISA, showed a statistically significant dose-dependent increase with oestradiol treatment only in the T cells of SLE patients, not in those of healthy controls, which provided the more direct evidence. Collectively, the results support the theory that CD4+ T cells of SLE patients are more responsive to oestradiol stimulation in terms of IL-21 production.
One may raise the concern of the supraphysiological concentration of 17β-oestradiol (1000 nm) used in some experiments in our study. However, as shown in Fig. 2, the statistical significance was achieved even when the oestrogen concentration was within physiological levels (10−10 to 10−7 m). As the difference was greatest at 1000 nm, subsequent blocking experiments were conducted using 1000 nm 17β-oestradiol for a definite comparison.
Oestrogen affects various types of cells through its receptors. Two distinct types of pathways exist: the genomic and the non-genomic pathways. In the genomic pathway, oestradiol diffuses into the cell and binds to the intracellular receptor. Once oestrogen is bound to the receptor, the complex is translocated into the nucleus and controls transcription of certain genes either by directly binding to an oestrogen response element or recruiting other transcription factors such as NF-κB and AP-1.20 Conversely, in the non-genomic pathway, oestradiol binds to the membrane receptor and mediates a more rapid response, such as nitric oxide release. Such membrane receptors have been suggested to use various types of signal transduction pathways (e.g. ERK/MAPK, p38/MAPK, PI3K/AKT, and phospholipase C/protein kinase C).21 Recently, the genomic and non-genomic pathways have been reported to act in an integrated manner. For example, transcription factors including NF-κB and STAT are activated through signalling pathways that are involved in the non-genomic pathway, such as MAPK or PI3K, which can then affect the genomic pathway.22 To investigate the underlying mechanism of how oestrogen up-regulates IL-21, we used various signal inhibitors. In our study, MAPK (ERK, p38, JNK) inhibitors impeded the oestrogen-induced IL-21 increase, whereas inhibitors of NF-κB and AP-1 did not affect IL-21 production. This suggests that oestrogen-induced IL-21 expression is dependent on the MAPK pathway, but not on transcription factors such as NF-κB or AP-1. Further investigations are required to demonstrate whether IL-21 production is mediated by other transcription factors or co-activators that are activated by MAPK, or a non-genomic action of oestrogen dependent on MAPK recruiting another molecule to induce IL-21. Either way, oestrogen up-regulated the IL-21 expression in SLE patients. Given that IL-21 is implicated in the SLE pathogenesis, the finding might add a reasonable explanation for the female predominance in SLE.
In the present study, we demonstrated that simultaneous stimulation of T-cell receptor in addition to oestrogen treatment led to higher IL-21 expression. It is well known that activation of T-cell receptor induces T-cell activation and proliferation through various signalling pathways, including MAPK and PI3K pathways. Our results demonstrated that oestrogen-induced IL-21 production was dependent on the MAPK pathway. Therefore, it seems relevant to postulate that T-cell receptor ligation exerted an additional effect on MAPK pathway activation and resulted in a greater increase in IL-21 production.
Finally, we confirmed that increased IL-21 could induce immunoglobulin production by B cells. When oestrogen pre-exposed CD4+ T cells or culture supernatants were co-cultured with B cells from a healthy donor, B cells became activated and secreted immunoglobulin. This supports previous findings by Kanda et al.23 that showed that exposure of PBMCs to oestrogen led to increased IgG production. Interestingly, co-culture with only the supernatants was sufficient for B cells to produce immunoglobulin. This confirms that oestrogen-induced IL-21 can induce antibody production from B cells without the co-stimulation of T cells. However, immunoglobulin levels secreted by B cells that were co-cultured with T cells were twice as high as those from B cells cultured with T-cell supernatants only. This indicates the importance of the cell-to-cell interactive mechanism (e.g. CD40/CD40 ligand) between T and B cells in increasing antibody production as previously reported.14 Furthermore, this also suggests that oestrogen might enhance the development of IL-21-producing CD4+ cells. As a result, B cells that are co-cultured with T cells are exposed to more IL-21 and consequently produce more antibodies. As mentioned previously, the major cellular source of IL-21 is known to be follicular helper T cells, which are mainly found in secondary lymphoid organs. Recently, a subtype of Th17 cells was reported to act like follicular helper T cells in peripheral blood.24 Further, Th17 cells were reported to be increased in SLE patients compared with healthy controls.25 Therefore, it is plausible to assume that oestrogen-induced Th17-type CD4+ T cells act as sources of IL-21 in the peripheral blood of SLE patients. We plan to further investigate the role of oestrogen in SLE with respect to Th17.
Although we addressed the role of IL-21 in the pathogenesis of SLE as an enhancer of autoantibody production by B lymphocytes, IL-21 might make additional contributions. Interleukin-21 has also been implicated in other autoimmune diseases and various target cells have been identified. We recently reported that IL-21 promotes osteoclast differentiation from monocytes in patients with rheumatoid arthritis.26 Interleukin-21R expression was also reported in neurons in multiple sclerosis,27 and in keratinocytes in systemic sclerosis,28 suggesting that IL-21 may affect these non-immune cells. Hence, IL-21 appears to be an important cytokine in various autoimmune diseases and future research on this subject will be promising.
Acknowledgments
This work was supported by the Basic Science Research Programme through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (grant number 2009-0081791 and 2005-0048480).
Disclosures
The authors declare that they have no competing interests in relation to this manuscript.
References
- 1.Sekigawa I, Fujishiro M, Yamaguchi A, et al. A new hypothesis of the possible mechanisms of gender differences in systemic lupus erythematosus. Clin Exp Rheumatol. 2010;28:419–23. [PubMed] [Google Scholar]
- 2.Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 1991;34:1538–45. doi: 10.1002/art.1780341210. [DOI] [PubMed] [Google Scholar]
- 3.Jungers P, Kuttenn F, Liote F, et al. Hormonal modulation in systemic lupus erythematosus. Preliminary clinical and hormonal results with cyproterone acetate. Arthritis Rheum. 1985;28:1243–50. doi: 10.1002/art.1780281108. [DOI] [PubMed] [Google Scholar]
- 4.Buyon JP, Petri MA, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–62. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 5.Park MK, Park SH, Kwok SK, Cho ML, Kim HY. The effect of estrogen on the DNA methylation of B cells in patients with SLE. J Korean Rheum Assoc. 2007;14:23–30. [Google Scholar]
- 6.Kim WU, Min SY, Hwang SH, Yoo SA, Kim KJ, Cho CS. Effect of oestrogen on T cell apoptosis in patients with systemic lupus erythematosus. Clin Exp Immunol. 2010;161:453–8. doi: 10.1111/j.1365-2249.2010.04194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Solal JF, Jeganathan V, Hill L, Kawabata D, Rodriguez-Pinto D, Grimaldi C, Diamond B. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus. 2008;17:528–32. doi: 10.1177/0961203308089402. [DOI] [PubMed] [Google Scholar]
- 8.Kanda N, Tsuchida T, Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:328–37. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–98. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 10.Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, Ettinger R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell–B cell collaboration. J Immunol. 2007;179:5886–96. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 11.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–37. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozaki K, Spolski R, Ettinger R, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–71. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 15.Bubier JA, Bennett SM, Sproule TJ, Lyons BL, Olland S, Young DA, Roopenian DC. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1110:590–601. doi: 10.1196/annals.1423.063. [DOI] [PubMed] [Google Scholar]
- 16.Wong CK, Wong PT, Tam LS, Li EK, Chen DP, Lam CW. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J Clin Immunol. 2010;30:45–52. doi: 10.1007/s10875-009-9325-5. [DOI] [PubMed] [Google Scholar]
- 17.Kang KY, Kim HO, Kwok SK, et al. Impact of interleukin-21 in the pathogenesis of primary Sjögren's syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res Ther. 2011;13:R179. doi: 10.1186/ar3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitoma H, Horiuchi T, Kimoto Y, Tsukamoto H, Uchino A, Tamimoto Y, Miyagi Y, Harada M. Decreased expression of interleukin-21 receptor on peripheral B lymphocytes in systemic lupus erythematosus. Int J Mol Med. 2005;16:609–15. [PubMed] [Google Scholar]
- 19.Dolff S, Abdulahad WH, Westra J, Doornbos-van der Meer B, Limburg PC, Kallenberg CG, Bijl M. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R157. doi: 10.1186/ar3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassi E, Moutsatsou P. Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:317452. doi: 10.1155/2010/317452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–42. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 23.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol. 1999;103:282–8. doi: 10.1016/s0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- 24.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin MS, Lee N, Kang I. Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr Opin Rheumatol. 2011;23:444–8. doi: 10.1097/BOR.0b013e328349a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok SK, Cho ML, Park MK, et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2012;64:740–51. doi: 10.1002/art.33390. [DOI] [PubMed] [Google Scholar]
- 27.Tzartos JS, Craner MJ, Friese MA, Jakobsen KB, Newcombe J, Esiri MM, Fugger L. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. Am J Pathol. 2011;178:794–802. doi: 10.1016/j.ajpath.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Distler JH, Jungel A, Kowal-Bielecka O, et al. Expression of interleukin-21 receptor in epidermis from patients with systemic sclerosis. Arthritis Rheum. 2005;52:856–64. doi: 10.1002/art.20883. [DOI] [PubMed] [Google Scholar]


