Abstract
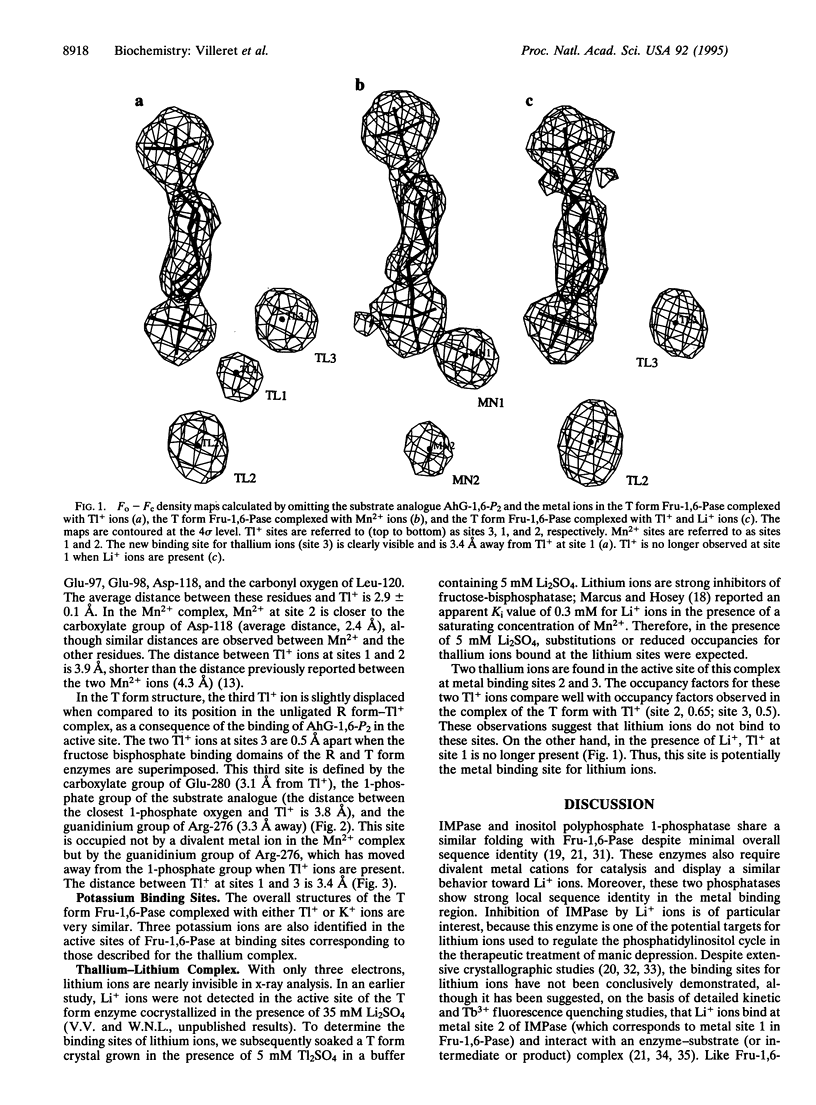
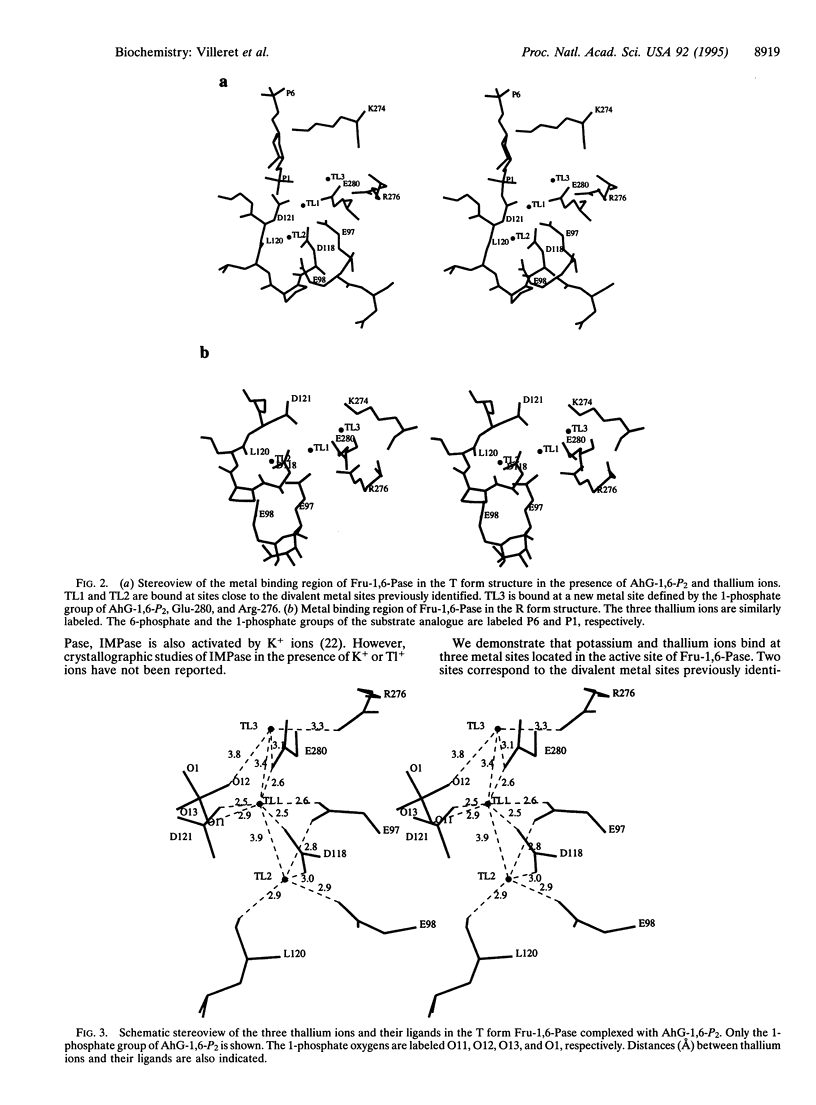
Fructose-1,6-bisphosphatase (Fru-1,6-Pase; D-fructose-1,6-bisphosphate 1-phosphohydrolase, EC 3.1.3.11) requires two divalent metal ions to hydrolyze alpha-D-fructose 1,6-bisphosphate. Although not required for catalysis, monovalent cations modify the enzyme activity; K+ and Tl+ ions are activators, whereas Li+ ions are inhibitors. Their mechanisms of action are still unknown. We report here crystallographic structures of pig kidney Fru-1,6-Pase complexed with K+, Tl+, or both Tl+ and Li+. In the T form Fru-1,6-Pase complexed with the substrate analogue 2,5-anhydro-D-glucitol 1,6-bisphosphate (AhG-1,6-P2) and Tl+ or K+ ions, three Tl+ or K+ binding sites are found. Site 1 is defined by Glu-97, Asp-118, Asp-121, Glu-280, and a 1-phosphate oxygen of AhG-1,6-P2; site 2 is defined by Glu-97, Glu-98, Asp-118, and Leu-120. Finally, site 3 is defined by Arg-276, Glu-280, and the 1-phosphate group of AhG-1,6-P2. The Tl+ or K+ ions at sites 1 and 2 are very close to the positions previously identified for the divalent metal ions. Site 3 is specific to K+ or Tl+. In the divalent metal ion complexes, site 3 is occupied by the guanidinium group of Arg-276. These observations suggest that Tl+ or K+ ions can substitute for Arg-276 in the active site and polarize the 1-phosphate group, thus facilitating nucleophilic attack on the phosphorus center. In the T form complexed with both Tl+ and Li+ ions, Li+ replaces Tl+ at metal site 1. Inhibition by lithium very likely occurs as it binds to this site, thus retarding turnover or phosphate release. The present study provides a structural basis for a similar mechanism of inhibition for inositol monophosphatase, one of the potential targets of lithium ions in the treatment of manic depression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic S. J., deMaine M. M. Mechanism of action of fructose 1,6-bisphosphatase. Adv Enzymol Relat Areas Mol Biol. 1982;53:45–82. doi: 10.1002/9780470122983.ch2. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Black W. J., Van Tol A., Fernando J., Horecker B. L. Isolation of ahighly active fructose diphosphatase from rabit muscle: its subunit structure and activation by monovalent cations. Arch Biochem Biophys. 1972 Aug;151(2):576–590. doi: 10.1016/0003-9861(72)90535-8. [DOI] [PubMed] [Google Scholar]
- Bone R., Frank L., Springer J. P., Atack J. R. Structural studies of metal binding by inositol monophosphatase: evidence for two-metal ion catalysis. Biochemistry. 1994 Aug 16;33(32):9468–9476. doi: 10.1021/bi00198a012. [DOI] [PubMed] [Google Scholar]
- Bone R., Frank L., Springer J. P., Pollack S. J., Osborne S. A., Atack J. R., Knowles M. R., McAllister G., Ragan C. I., Broughton H. B. Structural analysis of inositol monophosphatase complexes with substrates. Biochemistry. 1994 Aug 16;33(32):9460–9467. doi: 10.1021/bi00198a011. [DOI] [PubMed] [Google Scholar]
- Bone R., Springer J. P., Atack J. R. Structure of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10031–10035. doi: 10.1073/pnas.89.21.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hartman F. C., Barker R. An exploration of the active site of aldolase using structural analogs of fructose diphosphate. Biochemistry. 1965 Jun;4(6):1068–1075. doi: 10.1021/bi00882a014. [DOI] [PubMed] [Google Scholar]
- Hubert E., Villanueva J., Gonzalez A. M., Marcus F. Univalent cation activation of fructose 1,6-diphosphatase. Arch Biochem Biophys. 1970 Jun;138(2):590–597. doi: 10.1016/0003-9861(70)90385-1. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Liang J. Y., Zhang Y. P., Lipscomb W. N. Conformational transition of fructose-1,6-bisphosphatase: structure comparison between the AMP complex (T form) and the fructose 6-phosphate complex (R form). Biochemistry. 1991 May 7;30(18):4412–4420. doi: 10.1021/bi00232a007. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Thorpe C. M., Seaton B. a., Lipscomb W. N., Marcus F. Structure refinement of fructose-1,6-bisphosphatase and its fructose 2,6-bisphosphate complex at 2.8 A resolution. J Mol Biol. 1990 Apr 5;212(3):513–539. doi: 10.1016/0022-2836(90)90329-k. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Zhang Y. P., Liang J. Y., Lipscomb W. N. Crystal structure of the neutral form of fructose-1,6-bisphosphatase complexed with the product fructose 6-phosphate at 2.1-A resolution. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2989–2993. doi: 10.1073/pnas.88.8.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H. M., Zhang Y. P., Lipscomb W. N. Crystal structure of fructose-1,6-bisphosphatase complexed with fructose 6-phosphate, AMP, and magnesium. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5243–5247. doi: 10.1073/pnas.87.14.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtley M. E., Dix J. C. Activation of fructose diphosphatase by manganese, magnesium and cobalt. Arch Biochem Biophys. 1971 Dec;147(2):647–652. doi: 10.1016/0003-9861(71)90423-1. [DOI] [PubMed] [Google Scholar]
- Leech A. P., Baker G. R., Shute J. K., Cohen M. A., Gani D. Chemical and kinetic mechanism of the inositol monophosphatase reaction and its inhibition by Li+. Eur J Biochem. 1993 Mar 15;212(3):693–704. doi: 10.1111/j.1432-1033.1993.tb17707.x. [DOI] [PubMed] [Google Scholar]
- Liang J. Y., Huang S., Zhang Y., Ke H., Lipscomb W. N. Crystal structure of the neutral form of fructose 1,6-bisphosphatase complexed with regulatory inhibitor fructose 2,6-bisphosphate at 2.6-A resolution. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2404–2408. doi: 10.1073/pnas.89.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Hosey M. M. Purification and properties of liver fructose 1,6-bisphosphatase from C57BL/KsJ normal and diabetic mice. J Biol Chem. 1980 Mar 25;255(6):2481–2486. [PubMed] [Google Scholar]
- Nakashima K., Tuboi S. Size-dependent allosteric effects of monovalent cations on rabbit liver fructose-1,6-bisphosphatase. J Biol Chem. 1976 Jul 25;251(14):4315–4321. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. J Biol Chem. 1981 Apr 25;256(8):3619–3622. [PubMed] [Google Scholar]
- Pollack S. J., Atack J. R., Knowles M. R., McAllister G., Ragan C. I., Baker R., Fletcher S. R., Iversen L. L., Broughton H. B. Mechanism of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5766–5770. doi: 10.1073/pnas.91.13.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejwani G. A. Regulation of fructose-bisphosphatase activity. Adv Enzymol Relat Areas Mol Biol. 1983;54:121–194. doi: 10.1002/9780470122990.ch3. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981 May;78(5):2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeret V., Huang S., Zhang Y., Lipscomb W. N. Structural aspects of the allosteric inhibition of fructose-1,6-bisphosphatase by AMP: the binding of both the substrate analogue 2,5-anhydro-D-glucitol 1,6-bisphosphate and catalytic metal ions monitored by X-ray crystallography. Biochemistry. 1995 Apr 4;34(13):4307–4315. doi: 10.1021/bi00013a020. [DOI] [PubMed] [Google Scholar]
- Xue Y., Huang S., Liang J. Y., Zhang Y., Lipscomb W. N. Crystal structure of fructose-1,6-bisphosphatase complexed with fructose 2,6-bisphosphate, AMP, and Zn2+ at 2.0-A resolution: aspects of synergism between inhibitors. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12482–12486. doi: 10.1073/pnas.91.26.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J. D., Ponder J. W., Chen Z. W., Mathews F. S., Majerus P. W. Crystal structure of inositol polyphosphate 1-phosphatase at 2.3-A resolution. Biochemistry. 1994 Nov 15;33(45):13164–13171. doi: 10.1021/bi00249a002. [DOI] [PubMed] [Google Scholar]
- Zhang R., Fromm H. J. Mutation of arginine 276 to methionine changes Mg2+ cooperativity and the kinetic mechanism of fructose-1,6-bisphosphatase. Biochemistry. 1995 Jun 27;34(25):8190–8195. doi: 10.1021/bi00025a026. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang J. Y., Huang S., Ke H., Lipscomb W. N. Crystallographic studies of the catalytic mechanism of the neutral form of fructose-1,6-bisphosphatase. Biochemistry. 1993 Feb 23;32(7):1844–1857. doi: 10.1021/bi00058a019. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang J. Y., Huang S., Lipscomb W. N. Toward a mechanism for the allosteric transition of pig kidney fructose-1,6-bisphosphatase. J Mol Biol. 1994 Dec 16;244(5):609–624. doi: 10.1006/jmbi.1994.1755. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang J. Y., Lipscomb W. N. Structural similarities between fructose-1,6-bisphosphatase and inositol monophosphatase. Biochem Biophys Res Commun. 1993 Feb 15;190(3):1080–1083. doi: 10.1006/bbrc.1993.1159. [DOI] [PubMed] [Google Scholar]


